On the Role of Grain-Boundary Films in Optimizing the Mechanical Properties of Silicon Carb
- 格式:pdf
- 大小:1.27 MB
- 文档页数:12

J I A N G S U U N I V E R S I T Y 材料强化与质量评定细晶强化的机理及其应用Fine-grain strengthening mechanism andits application学院名称:机械工程学院专业班级:机械1402学生姓名:XX指导教师姓名:XX指导教师职称:副教授2015年8 月细晶强化的机理及其应用摘要:通常金属是由许多晶粒组成的多晶体,晶粒的大小可以用单位体积内晶粒的数目来表示,数目越多,晶粒越细。
实验表明,在常温下的细晶粒金属比粗晶粒金属有更高的强度、硬度、塑性和韧性[1]。
因此,在实际使用中,人们常用细晶强化的方法来提高金属的力学性能。
关键词:定义、细晶强化机制、细化晶粒本质与途径、细晶强化新方法Fine-grain strengthening mechanism and itsapplicationAbstract: polycrystal metal is usually composed of many grain, grain size can be used to represent the number of grain per unit volume, the more the number, grain is fine. Experiments show that the fine grained metal at room temperature than coarse grain metal has higher strength, hardness, plasticity and toughness . Therefore, in the practical use, people often use fine-grain strengthening method to increase mechanical properties of the metal.Keywords:definition, fine-grain strengthening mechanism, refining grain essence new methods and ways, fine-grain strengthening1引言通过细化晶粒而使金属材料力学性能提高的方法称为细晶强化[2]。
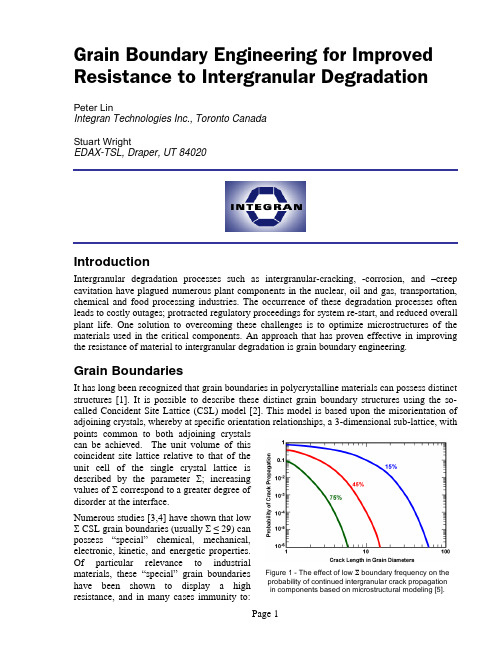
Grain Boundary Engineering for Improved Resistance to Intergranular Degradation Peter LinIntegran Technologies Inc., Toronto CanadaStuart WrightEDAX-TSL, Draper, UT 84020IntroductionIntergranular degradation processes such as intergranular-cracking, -corrosion, and –creep cavitation have plagued numerous plant components in the nuclear, oil and gas, transportation, chemical and food processing industries. The occurrence of these degradation processes often leads to costly outages; protracted regulatory proceedings for system re-start, and reduced overall plant life. One solution to overcoming these challenges is to optimize microstructures of the materials used in the critical components. An approach that has proven effective in improving the resistance of material to intergranular degradation is grain boundary engineering. Grain BoundariesIt has long been recognized that grain boundaries in polycrystalline materials can possess distinct structures [1]. It is possible to describe these distinct grain boundary structures using the so-called Concident Site Lattice (CSL) model [2]. This model is based upon the misorientation of adjoining crystals, whereby at specific orientation relationships, a 3-dimensional sub-lattice, with points common to both adjoining crystalscan be achieved. The unit volume of thiscoincident site lattice relative to that of the unit cell of the single crystal lattice isdescribed by the parameter ; increasingvalues of correspond to a greater degree ofdisorder at the interface.Numerous studies [3,4] have shown that lowCSL grain boundaries (usually 29) canpossess “special” chemical, mechanical,electronic, kinetic, and energetic properties.Of particular relevance to industrialmaterials, these “special” grain boundarieshave been shown to display a highresistance, and in many cases immunity to: Figure 1 - The effect of low boundary frequency on the probability of continued intergranular crack propagation in components based on microstructural modeling [5].(1) sliding, cavitation and fracture, (2) corrosion and stress corrosion cracking, (3) sensitization, and (4) solute segregation. Models have been developed to predict the effect of the special boundary population on these various properties. An example is shown in Figure 1.“Special” low CSL grain boundaries are found to naturally occur in all materials; their frequency of occurrence being strongly dependent upon the processing history of the material (e.g., casting, deformation, recrystallization heat treatment, etc.). Figure 2 shows an OIM map where the low CSL boundaries are highlighted in color along with a plot showing the distribution of the special boundaries. Generally, materials processing is undertaken without regard to resultant “grain boundary structure distributions”, thus producing component materials with highly variable populations of “special” grain boundaries ( 25%).Grain Boundary EngineeringGrain Boundary Engineering GBE®1 is a technique for optimizing the population of “special” boundaries in an effort to improve component material performance. The steps used in the general application of grain boundary engineering are two-fold.First, identify the effect of various thermomechanical processing steps used to make acomponent material on the “special” grain boundary population. OIM is the ideal tool for the statistical characterizations of grain boundarycharacter necessary for this procedure. It caneffectively measure the fraction of specialboundaries in materials.Second, from the insights gained, modify the thermomechanical processing route to produce materials with high “special” boundarypopulations. Materials can then be qualifiedby their “special” boundary population.Several successful examples showing the improvements achieved through grainboundary engineering are shown in Figures 3 through 7.1GBE® is a registered trademark of Integran Technologies Inc., Toronto, Canada. Figure 3 – Integranular cracking susceptibility in alloy 600 -a common nuclear steam generator tubing alloy. Samples stressed in 10% NaOH at 350°C for 3000 hours [6].Figure 2 – Extract of a map showing CSL boundaries and the corresponding distribution in a copper thin film.ConclusionsThe goal of materials engineering is to identifythe effect of materials forming processes on microstructure and in turn identify the rolemicrostructure plays on the properties of amaterial. Grain boundary engineering is a goodexample of this process. It is a very effectiveapproach for tailoring the thermomechanicalprocessing used to form materials to optimizetheir performance for applications whereprevention of intergranular degradation is acritical performance parameter.OIM is an enabling technology for grain boundary engineering. With the automation of EBSD, statistically relevant distributions of grainboundaries can be practically measured. Figure 5 – Creep resistance in alloy 625 – a super alloy used in gas turbine components. Samples creep tested under a 5 ksi tensile stress applied at 700°C.Figure 6 – Stress corrosion cracking in alloy 800, a nickel based superalloy. Cross sections were sensitized for1 hour at 600°C and exposed to a boiling solution of ferric sulphate-sulphuric acid for 120 hours.Figure 7 – Conventional and GBE® PbCaSn battery grids after 40 charge-discharge cycles (0 to 1.781 Vdc) in 1.28 s.g. H 2SO 4 at 70°C.Figure 4 – Intergranular penetration depth after 500hours exposure to Na 2So 4 at 850°C and improvementin room temperature fatigue resistance in alloy 738 –an advanced aerospace material.Bibliography[1] F. Hargreaves and R.J. Hills, “Work-Softening and a Theory of Intercrystalline Cohesion”,J. Inst. Met.41 (1929) 257.[2] M.L. Kronberg and F.H. Wilson, “Secondary Recrystallization in Copper”, Trans AIME185 (1949) 501-514.[3] K.T. Aust and J.W. Rutter, Trans TMS-AIME215 (1959) 119-126.[4] G. Palumbo and K.T. Aust, in Materials Interfaces: Atomic level structure and properties.(eds. D. Wolf and S. Yip) Chapman and Hall (1989) 190.[5] G. Palumbo, E. M. Lehockey, P. Lin, U. Erb and K. T. Aust, “Grain Boundary Engineeringfor Intergranular Fracture and Creep Resistance”, Microscopy and Microanalysis (eds.G.W. Bailey et al.) San Francisco Press (1996) 362-363.[6] G. Palumbo, E. M. Lehockey, P. Lin, U. Erb and K. T. Aust, “A Grain BoundaryEngineering Approach to Materials Reliability”, MRS Proceedings of the Symposium on Interfacial Engineering for Optimized Properties, Vol. 458, (1997) 273-383.。

Unit 1 Metals金属The use of metals has always been a key factor in the development of the social systems of man. Of the roughly 100 basic elements of which all matter is composed, about half are classified as metals. The distinction between a metal and a nonmetal is not always clear-cut. The most basic definition centers around the type of bonding existing between the atoms of the element, and around the characteristics of certain of the electrons associated with these atoms. In a more practical way, however, a metal can be defined as an element which has a particular package of properties.Metals are crystalline when in the solid state and, with few exceptions (e.g. mercury), are solid at ambient temperatures. They are good conductors of heat and electricity and are opaque to light. They usually have a comparatively high density. Many metals are ductile-that is, their shape can be changed permanently by the application of a force without breaking. The forces required to cause this deformation and those required to break or fracture a metal are comparatively high, although, the fracture forces is not nearly as high as would be expected from simple consideration of the forces required to tear apart the atoms of the metal.One of the more significant of these characteristics from our point of view is that of crystallinity. A crystalline solid is one in whichthe constituent atoms are located in a regular three-dimensional array as if they were locatedat the corners of the squares of a three-dimensional chessboard. The spacing of the atoms in the array is of the same order as the size of the atoms, the actual spacing being a characteristic of the particular metal. The directions of the axes of the array define the orientation of the crystal in space. The metals commonly used in engineering practice are composed of a large number of such crystals,在人类社会的发展中,金属的应用起着关键性的作用。

晶界特征分布和织构演变规律Crystallographic grain boundaries play a crucial role in determining the mechanical and physical properties of polycrystalline materials. Understanding the distribution and evolution of these boundaries, also known as grain boundary characteristics, is essential for predicting material behavior and optimizing processing conditions.晶界特征是指晶粒之间的原子排列畸变形成的界面,可以分为普通晶界和高角度晶界两种类型。
普通晶界由于原子位移小,结构匹配良好,因此稳定性较强。
高角度晶界则具有更大的原子位移和结构错配,使其不稳定易于迁移。
The distribution of grain boundaries within a polycrystalline material can vary depending on factors such as the processing method, initial microstructure, and annealing treatments. In general, materials processed through severe plastic deformation techniques such as equal channel angular pressing or high-pressure torsion tend to exhibit more equiaxed grains with a higher density of low-angle grain boundaries.织构演变是指材料在加工过程中由于外力的作用下出现晶粒取向调整的过程。

粮食作文素材英文英文:When it comes to the topic of grain, there are a lot of things to discuss. Grain is a staple food for many people around the world, and it plays an important role in our diets. In this article, I will discuss the importance of grain and its impact on our lives.First of all, grain is a major source of carbohydrates, which are essential for providing our bodies with energy. Carbohydrates are broken down into glucose, which is used by our cells as a source of fuel. Without enough carbohydrates in our diets, we can become tired and sluggish, and our bodies may not function properly.In addition to providing energy, grain is also a good source of fiber, which is important for maintaining a healthy digestive system. Fiber helps to keep us regular and can also help to lower cholesterol levels. Someexamples of high-fiber grains include oats, barley, and quinoa.Another benefit of grain is its versatility. Grain can be used in a variety of dishes, from bread and pasta to soups and stews. It can be cooked in many different ways, and it can be seasoned to suit a wide range of tastes. This makes grain a great choice for people who like to experiment with their cooking.However, it is important to note that not all grains are created equal. Some grains, such as white rice and white bread, have been processed and stripped of many of their nutrients. These grains are often referred to as "empty calories" because they provide little nutritional value. It is important to choose whole grains, which are more nutritious and provide more fiber, vitamins, and minerals.Overall, grain is an important part of our diets and plays a vital role in keeping us healthy. By choosing whole grains and incorporating them into our meals, we can enjoyall of the benefits that grain has to offer.中文:谈到粮食这个话题,有很多事情可以讨论。

地膜与粮食生产的故事英文回答:The Story of Plastic Film and Grain Production.I have always been fascinated by the story of plastic film and its impact on grain production. Plastic film, also known as agricultural mulch or plastic mulch, is a thin layer of plastic that is spread over the soil to improve crop growth and yield. It acts as a protective barrier, preventing weeds from growing, conserving soil moisture, and regulating soil temperature.One of the main benefits of plastic film is its ability to control weeds. Weeds compete with crops for nutrients, water, and sunlight, and can significantly reduce crop yields. By blocking sunlight from reaching the soil,plastic film inhibits weed growth, allowing the crops to thrive without competition. This results in higher yields and better quality grains.In addition to weed control, plastic film also helps conserve soil moisture. It acts as a barrier, reducing evaporation and preventing water loss from the soil. Thisis particularly important in arid and semi-arid regions where water scarcity is a major concern. By retaining moisture in the soil, plastic film ensures that the crops have access to sufficient water, even during dry periods. This promotes healthy growth and improves overall grain production.Furthermore, plastic film plays a crucial role in regulating soil temperature. It acts as an insulating layer, keeping the soil warm during cooler seasons and protectingit from extreme temperature fluctuations. This isespecially beneficial for crops that are sensitive to cold temperatures. By maintaining a stable and optimal soil temperature, plastic film creates a favorable environmentfor grain production.To illustrate the impact of plastic film on grain production, let me share a personal experience. I come froma farming family, and we have been using plastic film for many years. One year, we decided to conduct an experiment and compare the yield of crops grown with and withoutplastic film. We planted the same variety of grain in two adjacent fields, one covered with plastic film and theother left uncovered.The results were astonishing. The field with plasticfilm had significantly higher yields and healthier-looking crops compared to the uncovered field. The difference wasso noticeable that even our neighbors were impressed. The plastic film had effectively controlled weeds, conservedsoil moisture, and regulated soil temperature, creating the perfect conditions for grain production.中文回答:地膜与粮食生产的故事。
小学上册英语第5单元期末试卷英语试题一、综合题(本题有100小题,每小题1分,共100分.每小题不选、错误,均不给分)1.What do we call a baby goat?A. CalfB. KidC. FoalD. LambB2.I like to go ________ (购物) for new books.3.My cat enjoys climbing up on ______ (家具).4.The _____ (小鸟) builds its nest with twigs and leaves.5.The ______ teaches us how to be creative.6.The _______ plays a role in the life cycle of plants.7.I can ______ (培养) my skills through practice.8.The ________ (car) is red and fast.9. A ______ (植物的利用) can lead to innovative products.10._____ (annuals) need to be replanted each year.11.What is the largest organ in the human body?A. HeartB. SkinC. BrainD. Liver12. A _____ (植物观察者) enjoys studying flora in nature.13.I see a ______ (bird) in the tree.14.What shape is a stop sign?A. CircleB. TriangleC. SquareD. Octagon15.The elephant is the _______ (largest) land animal.16. A chemical reaction that occurs in the presence of oxygen is called a ______ reaction.17. A ___ (小狗) wags its tail when it is happy.18.The ________ was a famous explorer who mapped the Pacific Ocean.19.The cat loves to nuzzle its _______.anic chemistry is the study of carbon-containing _____.21.I am excited to learn about ______ (科学) experiments. They can be really fun and surprising!22.Which shape has three sides?A. SquareB. CircleC. TriangleD. Rectangle23. A ______ is a type of mixture where one substance dissolves in another.24.What do you call the place where you can see many animals?A. ParkB. ZooC. FarmD. Forest25.The main gas given off by decomposing matter is __________.26.I need to _____ (finish/start) my homework.27.What do we call a shape with four equal sides?A. RectangleB. SquareC. TriangleD. PentagonB28.The capital of Greece is ________.29.What is the capital of Ghana?A. AccraB. KumasiC. TakoradiD. TamaleA30.The __________ (文艺复兴) influenced art and science.31.I like to collect _______ (玩具) from different countries.32.Many plants produce ______ (种子) in the fall.33.What do you call a young meerkat?A. KitB. PupC. CalfD. Cub34.What do you call a person who teaches others?A. EducatorB. InstructorC. MentorD. All of the aboveD35.What do you call a person who studies space?A. BiologistB. AstronomerC. GeologistD. ChemistB36.What do we call the time of day when we eat dinner?A. BreakfastB. LunchC. DinnerD. Brunch37.The library is _____ (quiet/loud).38.The __________ (平原) is great for farming.39. A __________ (反应平衡) indicates the ratio of reactants to products.40.What do we call the main ingredient in a salad?A. DressingB. LettuceC. CroutonsD. CheeseB41.I enjoy making ________ (生日蛋糕) for friends.42. A substance that can accept protons is called a(n) _______.43.Many people visit the _____ for its historical significance.44.What do you call the natural satellite of the Earth?A. StarB. PlanetC. MoonD. AsteroidC45.I feel ______ when I read books.46.I want to _____ (make) a cake.47.What is the name of the famous Chinese philosopher?A. ConfuciusB. LaoziC. Sun TzuD. ZhuangziA48.I saw a _______ (小动物) at the nature reserve.49.I enjoy _____ with my friends. (playing)50.What do you call the sweet food made from milk and sugar that is often served as a dessert?A. PuddingB. CustardC. CreamD. GelatoB51.We have _____ (two/four) eyes.52.What do we call the first ten letters of the alphabet?A. A to JB. A to ZC. A to MD. A to N53.What do you call the story we read before bed?A. PoemB. NovelC. Bedtime storyD. JournalC54.The ______ (小鸡) pecks at the grain.55.The __________ (历史的丰富性) fosters appreciation.56.I enjoy attending events like __________. They allow me to meet new people and learn about various topics. I always leave feeling inspired.57.The gas released during fermentation is primarily _______.58.Chemical properties can be observed during a _____ reaction.59.My friends come over to . (我的朋友来。
粮食作文标题设计英文范文英文:Title: The Importance of Grain。
Grain is a staple food that has been consumed by humans for thousands of years. It is an essential source of carbohydrates, fiber, and protein, and is a key component of a healthy diet. In addition to its nutritional value, grain plays an important role in the economy and culture of many societies.From a nutritional perspective, grain is a complex carbohydrate that provides the body with energy. It is also a good source of fiber, which helps to regulate digestion and prevent constipation. Grain is also an important source of protein, which is essential for building and repairing tissues in the body.In terms of its economic and cultural significance,grain is a major commodity that is traded globally. It is a key ingredient in many food products, such as bread, pasta, and cereal. Grain is also an important component of many traditional dishes in various cultures around the world.For example, in China, rice is a staple food that is consumed daily by millions of people. It is used in avariety of dishes, such as stir-fry, fried rice, and congee. In Italy, pasta is a popular food that is made from wheat flour. It is used in dishes such as spaghetti, lasagna, and ravioli.In conclusion, grain is an important food source that provides both nutritional and cultural value. It isessential for maintaining a healthy diet and plays a significant role in the global economy and various cultures around the world.中文:标题,粮食的重要性。
IC2218 合金的回复激活能与再结晶激活能林一坚(上海市金属功能材料重点实验室, 200940)【摘要】经试验测得了IC2218 合金(含少量共格γ相的Ni3 Al (B ) 2Cr 基合金) 的回复激活能和再结晶激活能, 得到相同的数值: 230kJΠm ol 。
这与显微组织的观察结果相互印证, 即IC2218 合金的再结晶主要依靠小角晶界的运动, 而大角晶界则失去明显可动性。
由此进一步证实, IC2218 合金特别迟缓的再结晶动力学根源在于大角晶界失去了明显可动性。
以此为基础, 对文献报道中关于Ni3 Al (B) 再结晶研究结果的明显分歧作出了解释。
【关键词】IC2218 合金Ni3 Al 回复再结晶激活能THE ACTIV ATIO N ENERG Y OF RECO VER Y AN DRECR YST A LL IZATIO N IN IC2218 A LLOYLin Y ijian(Shanghai K ey Lab. of Functi onal Metallic Materials)【Abstract】T he activati on energy of recovery and recrystalliz ati on in IC2218 all oy (Ni3 Al (B ) 2Cr base all oy containing sm all am ount of coherent γphase) determ ined by our test were identical , both being 230k JΠm ol . T he result veri fied the m icrostructure observati on that the sm all angle boundar y m ovem ent palyed principal role during recrystalliz ati on while large angle boundaries appreciably l ost m obility. T herefore , it was confirm ed that the unusually sluggish recrystalliz ati on kinetics stemm ed from the appreciable m obility l ost by large angle boundaries. Based on the above result , an ex planati on was given about the inconsistent reports on recrystalliz ati on behavi or of Ni3 Al (B) in previ ous w ork.【K ey Words】IC2218 All oy , Ni3 Al , R ecovery , R ecrystalliz ati on , Activati on E nergy1 前言IC2218 合金是一个以Ni3 Al (B ) 2C r 为基含少量共格γ相的γ′(有序) +γ(无序) 两相合金。
晶界扩散表面涂覆工艺流程英文回答:Surface coating is a widely used technique in various industries, including the field of diffusion at grain boundaries. The process involves applying a thin layer of coating material onto the surface of a material to enhance its properties and protect it from corrosion, wear, orother forms of damage. In the context of grain boundary diffusion, the surface coating process plays a crucial role in controlling the diffusion behavior at the grain boundaries.The process of surface coating for grain boundary diffusion typically involves several steps. First, the surface of the material to be coated is cleaned thoroughlyto remove any contaminants or impurities that may hinderthe adhesion of the coating material. This step isessential to ensure a strong bond between the coating and the substrate. Cleaning can be done through various methods,such as chemical cleaning, mechanical cleaning, or a combination of both.Once the surface is clean, a suitable coating material is selected based on the specific requirements of the application. The choice of coating material depends on factors such as the desired properties, the compatibility with the substrate material, and the environmental conditions the coated material will be exposed to. Common coating materials used in grain boundary diffusion include metals, ceramics, polymers, and composites.After selecting the coating material, it is applied onto the surface using a suitable technique. There are several methods available for surface coating, including spraying, dipping, electroplating, and chemical vapor deposition. The choice of coating technique depends on factors such as the type of coating material, the desired thickness of the coating, and the complexity of the substrate geometry.Once the coating is applied, it is subjected to acuring or drying process to ensure its adhesion and stability. This step may involve heating the coated material to a specific temperature or exposing it to a controlled environment for a certain period. The curing process helps in the formation of strong bonds between the coating material and the substrate, enhancing thedurability and performance of the coated material.In summary, the surface coating process for grain boundary diffusion involves cleaning the surface, selecting a suitable coating material, applying the coating using a suitable technique, and curing the coating to ensure its adhesion and stability. This process is crucial in controlling the diffusion behavior at grain boundaries and enhancing the properties of the coated material.中文回答:晶界扩散表面涂覆工艺是各个行业广泛使用的一种技术,包括晶界扩散领域。
Mat. Res. Soc. Symp. Proc. Vol. 819 © 2004 Materials Research Society N1.1.1 On the Role of Grain-Boundary Films in Optimizing the Mechanical Properties ofSilicon Carbide CeramicsR. O. Ritchie,1,2 X.-F. Zhang 1 and L. C. De Jonghe 1,21 Materials Sciences Division, Lawrence Berkeley National Laboratory, and 2Department ofMaterials Science and Engineering, University of California, Berkeley, CA 94720ABSTRACTThrough control of the grain-boundary structure, principally in the nature of the nanoscale intergranular films, a silicon carbide with a fracture toughness as high as 9.1 MPa.m1/2 has been developed by hot pressing β-SiC powder with aluminum, boron, and carbon additions (ABC-SiC). Central in this material development has been systematic transmission electron microscopy (TEM) and mechanical characterizations. In particular, atomic-resolution electron microscopy and nanoprobe composition quantification were combined in analyzing grain boundary structure and nanoscale structural features. Elongated SiC grains with 1 nm-wide amorphous intergranular films were believed to be responsible for the in situ toughening of this material, specifically by mechanisms of crack deflection and grain bridging. Two methods were found to be effective in modifying microstructure and optimizing mechanical performance. First, prescribed post-annealing treatments at temperatures between 1100 and 1500o C were seen to cause full crystallization of the amorphous intergranular films and to introduce uniformly dispersed nanoprecipitates within SiC matrix grains; in addition, lattice diffusion of aluminum at elevated temperatures was seen to alter grain-boundary composition. Second, adjusting the nominal content of sintering additives was also observed to change the grain morphology, the grain-boundary structure, and the phase composition of the ABC-SiC. In this regard, the roles of individual additives in developing boundary microstructures were identified; this was demonstrated to be critical in optimizing the mechanical properties, including fracture toughness and fatigue resistance at ambient and elevated temperatures, flexural strength, wear resistance, and creep resistance.INTRODUCTIONThe nature of the grain boundaries, in particular the presence of nanoscale intergranular films, is of paramount importance in controlling the properties of many ceramics. This is especially important for silicon carbide ceramics, which despite offering many desirable properties, including a high melting temperature, low density, high elastic modulus and hardness, excellent wear resistance, and low creep rates, are rarely used structurally because of their poor toughness. Consequently, an imperative in the structural application of SiC is high fracture toughness, which for commercially available SiC is typically on the order of 3 MPa.m1/2, well below that of grain-elongated Si3N4 and yttria-stabilized ZrO2, for example.The toughening of ceramic materials can best be induced by crack bridging mechanisms(i.e., extrinsic toughening by crack-tip shielding), which can result from crack paths bridgedby unbroken reinforcement fibers in composite ceramics (ex situ toughening) [1], or by self-reinforcement from elongated grains in monolithic ceramics (in situ toughening) [1]. The latter approach was exploited in the present work by hot pressing SiC with Al, B and C additives (ABC-SiC [2]) to induce elongated grains decorated with brittle intergranular films.To achieve this, two strategies, specifically post-annealing heat treatments and changes in the additive content, were used to optimize the microstructure.In this paper, we review the microstructure and mechanical properties of in situ toughened SiC with particular emphasis on the role of the grain boundary films. Inparticular, atomic-resolution TEM in conjunction with nanoprobe chemical analyses were employed to determine the structure and chemistry of the grain boundaries. Such atomic-scale characterization was correlated with materials processing and mechanical testing with the objective of optimizing the structural performance of ABC-SiC.EXPERIMENTAL PROCEDURESSubmicron β-SiC powder was mixed with 3 wt% aluminum metal powder, 0.6 wt% boron, and 2 wt% carbon sintering. The slurry was stir dried, sieved, and uniaxially pressed at 35 MPa. The green bodies were hot pressed at 50 MPa at 1900°C for 1 hr in an Ar atmosphere. The final product, which was produced as 99% dense (3.18 g/cm3), 4 mm thick and 38 mm diameter disks of polycrystalline SiC, was compromise of predominantly 4H and 6H α-SiC phases, with a minor fraction of 3C β-SiC. Some as-hot-pressed ABC-SiC samples were further annealed in a tungsten mesh furnace under flowing Ar, at temperatures between 1000 and 1500o C, for times typically ranging from 72 to 168 hr. Structural and mechanical characterizations were performed for both as-hot-pressed and annealed samples.Structural and chemical analyses were carried out in a 200 kV Philips CM200 transmission electron microscope equipped with a windowless detector and corresponding X-ray energy-dispersive spectroscopy (EDS) system. A spatial-difference methodology was developed to determine the concentration of the impurities in the SiC grain boundaries using a nanoprobe with a diameter varied between 3 and 20 nm [3,4].Indentation hardness, bending strength, creep resistance, abrasive wear properties, R-curve fracture toughness, and cyclic fatigue-crack growth behavior at ambient and elevated temperatures were all evaluated for ABC-SiC. Experimental procedures for each of these property assessments are described in detail in the respective references quoted in this paper.RESULTSSome typical mechanical properties for as-processed ABC-SiC are summarized in Table 1. Of particular note is the fracture toughness, which has been measured to be as high as 7 to 9 MPa.m1/2; this is the highest K Ic value recorded for SiC to date.Table 1: Room temperature mechanical properties of as-processed ABC-SiCSample ABC-SiC4-Point Bending Strength (MPa) 691±12Vickers Hardness – 10 kg (GPa) 24.1Fracture Toughness (MPa.m1/2) 6.8-9.1 Using X-ray diffraction, 70 vol.% of the hot-pressed structure was identified as hexagonal 4H-SiC and the remaining 30% as cubic 3C-SiC. TEM studies revealed that the 3C-to-4H phase transformation, which is presumed to have occurred during hot pressing, promoted anisotropic grain growth [4], resulting in high area density of plate-like, elongated SiC grains (Fig. 1a); the length and width of these grains ranged from 3 to 11 µm and 1 to 3 µm, respectively, with an aspect ratio for 90 % of them between 2 and 5. Interlocking between the elongated SiC grains was often observed, as shown in Fig. 1b. Equiaxed 3C-SiC grains with a submicron size were also found in ABC-SiC. It is believed that the unusuallyhigh toughness of ABC-SiC results from a combination of crack deflection and principally frictional and elastic bridging by the elongated SiC grains in the wake of the crack tip [2,5]. To optimize this toughening mechanism, an understanding of the grain-boundary properties and structure is crucial; consequently, extensive TEM studies were focused on the nature of the grain-boundary microstructure.Fig. 1: (a) Bright-field TEM images showing elongated SiC grains in ABC-SiC, showing (b) the interlocked nature of the elongated grains.Fig. 2: (a) High-resolution electron micrograph showing a typical intergranular film between two SiC matrix grains. An amorphous structure is observed with a width of about 1 nm. (b) Distribution of amorphous grain-boundary widths determined by high-resolution electron microscopy. The grain-boundary width ranges from 0.75 nm to 2.75 nm, with a mean of about 1 nm.Fig. 2a shows a high-resolution TEM image of a typical grain-boundary area in as-pressed ABC-SiC. An amorphous intergranular film is seen between two adjacent SiC grains, about 1 nm in width. Using high-resolution electron microscopy, the statistical width of these films was determined; results are shown in Fig. 2b. Specifically, the amorphous grain-boundary films range in width from about 0.75 to 2.75 nm, with a mean of ~1 nm. This width is consistent with the values found in other ceramic materials [6,7], and with theoretical explanations developed by Clarke [8]. In some particularly wide films (e.g. ~2.75 nm), nanoscale crystallites were recognizable, indicating local ordering in the glassy films. Earlier work on SiC sintered with boron and carbon showed a solid-phase sintering procedure with no grain-boundary films being formed [9,10]. The formation of the current films was due to liquid-phase sintering promoted by the Al additives. The liquid phase allowed for densification of the SiC at temperatures roughly 200o C lower than Al-free compositions, i.e., at ~1900o C instead of ~2100o C. The amorphous intergranular films provide the preferred 1 µm a 0.5 µmb1 nm SiC SiCacrack path, which is an essential element for the development of crack bridging and hence toughening in ABC-SiC.Nanoprobe EDS analyses revealed that the grain-boundary films contained substantial Al, O, Si, and C. The oxygen came mainly from the SiO2 surface oxidation of the SiC starting powder, and is one of the features responsible for difference in the behavior of different starting powders. Boron was below the detectability limit at the grain boundaries and in the interior of the SiC grains. In fact, boron was largely involved in forming Al8B4C7 secondary phases. Other secondary phases identified in ABC-SiC include Al2OC-SiC, mullite, Al2O3, Al4C3, Al4O4C, and Al-O-C-B crystalline phases. These secondary phases were submicron in dimension and were present as triple-junction particles [3,13]. Al in solution in the SiC matrix grains was also detected.Effects of Post-annealingThe most significant phenomenon that we observed in the efforts of modifying microstructure of ABC-SiC was crystallization of amorphous intergranular films in post-annealing. While no significant change in the overall, large-scale microstructure was recognized, annealing at 1000o C for only 5-30 hrs was sufficient to transform about half number of the amorphous grain-boundary films into more ordered structures. Apparently, 1000o C was about the threshold temperature for activation of grain-boundary diffusion. Annealing at higher temperatures for prolonged hours fully crystallized the intergranular films [3]. For example, whereas about 90% of the grain boundaries were amorphous in as-hot-pressed samples, 86% of the intergranular films in the annealed material were found to be crystalline. Fig. 3a shows a high-resolution TEM image of a grain-boundary film crystallized after annealing at 1200o C for 500 hrs. In this image, the crystallized grain-boundary film is not readily distinguishable because the grain-boundary phase was strictly epitaxial with the 6H-SiC grain on the left-hand side. However, corresponding EDS detected substantial Al-O-Si-C segregations between the two SiC grains, confirming the existence of the boundary phase. An enlarged image from the framed area is shown in Fig. 3b for closer inspection. The two adjacent SiC grains in this image show very different orientations. Due to a large deviation from the [1120] type zone-axes, only (0004) lattice fringes, with the lattice spacing of 0.25 nm, could be resolved for the 4H-SiC grain on the upper-right side, while a two-dimensional [1120] zone-axis lattice can be recognized in the 6H-SiC grain on the other side of the grain boundary. Under the imaging conditions for Fig. 3, the black dots in the image correspond to cationic columns along the incident electron beam direction. Some black dots in the 6H-SiC grain were marked with black circles. The zigzag stacking of the dots correspond to the …ABCACBABCACB… hexagonal structure of the 6H-SiC. The closest layer spacing along the c-direction is 0.25 nm.The grain-boundary layer outlined in Fig. 3b can be distinguished by the abrupt change in the atomic arrangement. Some lattice points in the boundary region are marked with white circles. The projection distance between adjacent lattice dots in the grain-boundary layers is very close to that in the neighboring 6H-SiC grain, and the characteristic zigzag lattice arrangement is also apparent in the boundary layer. These observations would suggest a grain-boundary film structure similar to 6H-SiC. However, instead of the …ABCACBABCACB… periodic stacking for every six layers in the 6H-type structure, the stacking period in the grain-boundary phase contains only two layers in one period, resulting in …ABABAB… stacking with an 0.5 nm repeat length, within a grain-boundary width of about 1.25 nm. In conjunction with the quantitative analysis of the grain-boundary composition and computer image simulations, we concluded that one of the intergranular crystalline phases was aluminosilicate with a Al2OC-SiC solid solution composition and a2H-wurtzite structure (hexagonal unit cell, a = 3.1 Å, c = 5.0 Å) [3,12]. The crystallized structure usually has an epitaxial structural relationship with SiC matrix grains when the (0001) habit plane is available. As shown in Fig. 3, the typical grain-boundary width after crystallization remains about 1 nm.Fig. 3: (a) High-resolution electron microscopy image for a grain-boundary area in ABC-SiC annealed at 1200o C for 500 hrs. Amorphous intergranular film is crystallized. (b) Enlarged image from the framed area in (a). Atomic stacking in 6H-SiC grain on the bottom-left side is marked by black circles. 2H-wurtzite atomic stacking in grain-boundary film (GB) are marked by white circles. Note the epitaxial orientation relationships between the grain-boundary film and the 6H-SiC matrix grain.To further study the crystallization process in intergranular films at elevated temperatures, an ABC-SiC sample was heated in situ in a 300 kV JEOL 3010 transmission electron microscope equipped with a hot stage. Fig. 4 shows high-resolution images for the same intergranular film before and after in situ heating, respectively. The amorphous film prior to heating crystallized discretely after 25 hrs at 1200o C, as indicated by arrowheads in Fig. 4b. The crystallization tended to proceed epitaxially on the (0001) plane of the adjacent 6H-SiC matrix grain, with a 2H-wurtzite structure similar to that observed in ex situ annealed SiC samples. No discernable features could be identified as potential preferential sites for nucleation at the SiC/film interface. Presumably, local compositional fluctuations in the intergranular films serve as nucleation sites.Another significant consequence of the thermal treatment was coherent nano-precipitation within SiC matrix grains, as seen in Fig. 5a. Although Fig. 5a was taken from a sample annealed at 1400o C, the plate-like nanoprecipitates first formed at 1300o C. The precipitates were uniform in size and shape, and dispersed within the SiC matrix grains. The projected dimension of the precipitates after 1300o C annealing is ~4 × 1 nm 2 with a volumetric number density of 5×1022/m 3. The precipitates coarsened with annealing temperature, accompanied by decrease in the number density. Detailed high-resolution electron microscopy characterization and nanoprobe EDS analysis determined an Al 4C 3-based structure and composition for the nanoprecipitates [13]. A comparison of 6H-SiC and Al 4C 3 structures projected along the [0001] direction is shown in Fig. 5b. The similarity between the two structures caused coherent precipitation with the (0001) habit planes. The formation and coarsening of the precipitates at 1300-1600o C was a consequence of lattice-a 2 nm GBb 1 nmdiffusion-controlled nucleation and growth [13]. The diffusion of Al-rich species through the SiC lattice at 1300o C resulted in Al-enrichment in the boundary films, as revealed by EDS.Fig. 4: (a) High-resolution image of an amorphous intergranular film in as-hot-pressed ABC-SiC. (b) The same film as in (a) but after in situ heating in a transmission electron microscope at 1200o C for 25 hrs. Arrowheads indicate the discrete, crystallized boundary segments.Fig. 5: (a) Al 4C 3-based nanoprecipitate formed in a 6H-SiC grain annealed at 1400o C. The viewing directions are marked. (b) Atomic models for 6H-SiC and Al 4C 3 projected along the[0001] direction. The similarity between the two structures can be seen.The Al content in intergranular films as a function of annealing temperature was analyzed with EDS. The results are plotted in Fig. 6. It is apparent that Al solution in SiC grains decreased at 1100o C and especially above 1300o C, consistent with TEM observations that Al solutions formed nanoprecipitates exsolved from the SiC lattice. Not surprisingly, the Al site density in the grain boundaries (N Al GB ) changed as well upon annealing. Below 1200o C, the composition of intergranular films was virtually invariant, even while the intergranular films crystallized. The N Al GB value was doubled at 1300o C, which can be readily correlated with diffusion of the Al-rich chemical species into the grain-boundary films. The Al content in intergranular films after annealing at 1300o C is in agreement with Al 1.1Si 0.9OC, a solid solution between 2H-wurtzite Al 2OC and SiC [12]. At even higher annealing temperatures up to 1600o C, N Al GB changed marginally taking the standard deviation into account. SiC-6HAs-processedc 5 nm a 1200 oC-25 hrs SiC-6Hc b a = 3.33 ÅSi C AlAl 4C 3, [0001]Fig. 6: Plots of EDS-determined Al site density in grain-boundary films (N Al GB ) and in SiC matrix grains (Al/SiC, wt%) as a function of annealing temperature.The structural evolutions in intergranular films during thermal treatment demonstrated a profound influence on mechanical properties. It was found that the high strength, cyclic fatigue resistance, and particularly the fracture toughness of ABC-SiC at ambient temperature were not severely compromised at elevated temperatures. For example, the fatigue-crack growth properties up to 1300o C were essentially identical to those at 25o C. Fig. 7 illustrates the variation in fatigue-crack growth rates, da/dN , with applied stress-intensity range, ∆K , at a load ratio (minimum to maximum load) of R = 0.1 for ABC-SiC under different test conditions. It can be seen that at both 25o C and 1300o C, crack-growth rates display a marked sensitivity to the stress intensity but little effect is from change of loading frequency over the range 3 to 1000 Hz [14]. Mechanistically, the lack of a frequency effect in ABC-SiC is expected as crack advance occurs via predominantly intergranular cracking ahead of the tip, as shown in Fig. 8. Grain bridging in the crack wake is a common feature. However, the absence of a frequency effect at elevated temperatures is surprising, particularly since comparable materials, such as Si 3N 4, Al 2O 3 and silicide-matrix ceramics, show a marked sensitivity to frequency at above 1000o C [15-17]. In these later materials, softening of the intergranular films, grain-boundary cavitation and viscous-phase bridging are common. In contrast, TEM studies of regions in the immediate vicinity of the crack tip in ABC-SiC (e.g. Fig. 8) provided direct confirmation of fracture mechanisms which were similar at ambient and elevated temperatures, with no evidence for grain-boundary cavitation (creep) damage or viscous-phase bridging at temperatures as high as 1300o C. We conclude that the unique high-temperature mechanical characteristics of ABC-SiC appear to be a result of the thermal-induced crystallization of intergranular glassy films.The structural evolutions in grain boundary and matrix grains at elevated temperatures also benefit other mechanical properties of ABC-SiC. For example, the steady-state creep rate at high temperature of 1500o C was still impressively low (5 × 10-9/s at 100 MPa), and the creep rates at 1200o C in ABC-SiC was about three orders of magnitude slower than in single-crystal Ni-base superalloy tested under the same conditions [18]. In addition, 80% strength loss at 1300o C was restored by post-annealing [13]. Abrasive wear resistance was improved as well by formation of nanoprecipitates and by structural and compositional changes in grain boundaries after annealing [19]. These structural and mechanical characterization results demonstrate that the prescribed annealing is an effective way in tuning the microstructure and in turn optimizing the mechanical properties of ABC-SiC.1020304050020040060080010001200140016001800Annealing Temperature (o C)A l c o n t e n t i n I G F s (N A l GB (n m -3))0246810A lC o n t e n t i n S i C G r a i n s (w t %)Fig. 7: Cyclic fatigue-crack growth rates, da/dN , in ABC-SiC as a function of the applied stress-intensity range, ∆K , for the tests conducted at temperatures between 25 and 1300o C, load ratio R = 0.1, and frequencies between 3 and 1000 Hz.Fig. 8: TEM images of the intergranular crack profiles at the crack-tip in ABC-SiC at 1300o C under (a) cyclic loading (25 Hz, R = 0.1), and (b) static loading. Arrows indicate the direction of crack propagation. No evidence of viscous boundary layers or creep damage. Effects of Additive ContentA second effort in tailoring boundary microstructures of ABC-SiC was in adjusting the contents of the sintering additives. A series of samples were prepared by changing content of one of the three additives. For example, the Al content was 3 wt% in most ABC-SiC samples. This was then increased to 4 up to 7 wt%, while boron and carbon contents remained at 0.6 and 2 wt%, respectively. The samples were referred to as 3ABC-, 4ABC, up to 7ABC-SiC, according to the weight percentage of the Al content. Structural characterizations showed that Al variations between 3 and 7 wt% did not reduce the densification of SiC samples under the same processing conditions. However, changing the Al content did alter the microstructure, as illustrated in Fig. 9 (only images for the 3ABC- 5ABC-, and 7ABC-SiC are shown). Although all samples were composed of plate-like, elongated and equiaxed SiC grains, the size, aspect ratio and area density of the elongated grains varied significantly with increasing Al content. The length of the elongated grains was found to be at a maximum in the 5ABC-SiC, with the aspect ratio increasing almost linearly up to 6 wt% Al. Compared to 3ABC-SiC, the aspect ratios in 4ABC- to 7ABC-SiC are much higher, but the area density of the elongated grains continuously decreases. A distinct bimodal grain distribution is seen in the 5ABC-SiC, with elongated α-SiC grains and submicron-sized equiaxed β-SiC grains [20].10101010Stress Intensity Range, 10-7-8-9C r a c k G r o w t h R a t e , d a /d N (m /c y c l e )∆K (MPam 1/2)Fig. 9: Morphologies of the 3ABC-, 5ABC-, and 7ABC-SiC are shown in images in (a) to (c), respectively. Note the significant changes in dimension and area density of the elongated SiC grains. Note also the bimodal grain systems with 5ABC-SiC.Table 2: X-ray diffraction-determined polytipic SiC phases, corresponding volume fractionsand mechanical properties in 3ABC, 4ABC, 5ABC, 6ABC and 7ABC-SiCSamples Phase and Volume Fraction 4-Point Bending Strength (MPa) Toughness (MPa.m 1/2) Hardness*(GPa)3ABC-SiC 70% 4H 30% 3C691±12 6.8±0.3 24.1 4ABC-SiC 19% 6H, 23% 4H 58% 3C 561±13 6.3±0.7 23.9 5ABC-SiC 21% 6H, 11% 4H 67% 3C480±30 8.9±0.4 22.7 6ABC-SiC 24% 6H 76% 3C598±34 3.2±0.2 35.9 7ABC-SiC 20% 6H 80% 3C533±58 3.9±0.4 30.1 *Vickers indentation, Load = 10 kg. E = 430 GPa (for calculation).Changing of the Al content by 1 wt% not only caused a considerable change in the grain morphology, but also induced different degrees of 3C-to-4H and/or 6H-to-4H SiC phase conversion during hot-pressing. X-ray diffraction spectra were collected from polished surfaces of the 3ABC- to 7ABC-SiC samples and from the starting powder for comparison. The spectra were matched with standard 3C-, 4H-, 6H-, and 15R-SiC phase spectra; results are summarized in Table 2. It was found that the 3 wt% Al addition in the 3ABC-SiC was sufficient to transform all of the 20% preexisting 6H-phase seeds in 3C-dominanted starting powder into 4H-SiC, with more than 50% of the β-3C transforming into α-4H phase as well. However, a 1 wt% higher Al content resulted in a completely different phase composition by retarding the 3C-to-4H transformation. Further increases in Al monotonically decreased the extent of the 3C-to-4H transformation, and apparently inhibited the preexisting 6H phase to be transformed into 4H. These results imply that the 3C-to-4H transformation was mainly promoted by boron and carbon, and the transformation was retarded when boron and carbon were compensated by increasing the Al content. The experimental data also suggest close relationships between the phase composition and grain morphology, which would be expected if the β-to-α transformation promotes the grain elongation [4].In addition to the grain morphology, a factor that may have a determining influence on mechanical properties of dense, polycrystalline ceramic materials is the grain-boundary structure and composition. High-resolution TEM showed that about 90% of the intergranular films examined in the 3ABC-SiC processed an amorphous structure. In contrast, this fraction3ABC 5 µm a 5ABC b 7ABCcdropped to about 50% in the 5ABC-SiC, and all grain-boundary films examined in the 7ABC-SiC were crystalline in structure. EDS analyses indicated that increasing the Al content enhanced the Al concentration in the intergranular films, as well as in the bulk SiC grains, but the concentrations saturated in 5ABC-SiC. More than 5 wt% Al resulted in precipitation of excess free Al, as observed in the 6ABC-and 7ABC-SiC. It should be noted that crystalline boundaries were prevalent in as-hot-pressed 5ABC- to 7ABC-SiC without any post-treatment. It is plausible that Al facilitates the formation of crystalline intergranular films.These changes in microstructure can be expected to affect mechanical properties. Table 2 lists various mechanical properties for the sample series. The mechanical data illustrate the tradeoff mechanical performance which is often encountered in developing advanced ceramic materials. While the highest strength was obtained in the 3ABC-SiC, the hardness was at maximum for the 6ABC-SiC. As for toughness, it is clear that 5 wt% Al resulted in the best toughness whereas 6 wt% or higher Al additions significantly degraded the toughness so that the materials became extremely brittle. Cyclic fatigue tests revealed that the 3ABC- and particularly the 5ABC-SiC displayed excellent crack-growth resistance at both ambient (25°C) and elevated (1300°C) temperatures. Again, the crack propagation in both 3ABC- and 5ABC-SiC was intergranular, and crack bridging was observed in the crack wake. No evidence of viscous grain-boundary layers and creep damage, in the form of grain-boundary cavitation, was seen at temperatures up to 1300°C. The substantially enhanced toughness in the 5ABC-SiC was associated with extensive crack bridging from both interlocking grains, as in 3ABC-SiC, and uncracked ligaments, which only occurred in 5ABC-SiC. No toughening by crack bridging was apparent in 7ABC-SiC, concomitant with a transition from intergranular to transgranular fracture [21].Similar to the aluminum concentration variations, changes in boron and carbon contents also alter the microstructure and phase composition. Typical results are shown in Fig. 10. In this case, the Al content was fixed at 6 wt%, while either the B or the C concentrations were changed. The phase composition determined by X-ray diffraction is noted in each image. It is clear that at fixed Al (6 wt%) and B(0.6 wt%) contents, 1 wt% additional carbon promoted the formation of more elongated grains and enhanced the 3C-to-4H transformation (most of the 6H phase should originate from starting powder). This effect of carbon on 3C-to-4H transformation was also reported before by Sakai et al [22]. Growth of the equiaxed grains was found to be limited. With the Al and C contents kept constant, increasing the boron content from 0.6 wt% to 0.9 wt% largely increased the number density of elongated grains, but reduced their aspect ratio. In addition, boron promoted the 3C-to-4H and 6H-to-4H phase transformations more effectively than carbon. The positive effect of boron on the 6H-to-4H transformation is consistent with the previous observations of Huang et al [23].The systematic processing and characterizations of a series of ABC-SiC described above also allowed for the determination of the roles of Al, B, and C additives in developing the microstructures of silicon carbide. The observed effects may be summarized as follows: in terms of developing phase composition, boron is more effective in promoting the β-to-αphase transformations than carbon. Aluminum retards the β-to-α phase transformation, but promotes the 6H-to-4H transformation. As for the developing grain morphology, aluminum and carbon both promote anisotropic grain growth, whereas boron tends to coarsen the volume fraction, but reduce the aspect ratio, of the elongated grains. It should be noted that during processing, the combined roles of the Al, B and C additives often override their individual roles. For example, B and C together favor of the β-to-α phase transformation associated with grain elongation; however, the final microstructure does not necessarily have strongly elongated grains as B and C have opposite effects on anisotropic grain growth. Actually, the B:C ratio determines the final grain configuration. Aluminum has a different。