Proton pump inhibitors an update of their clinical use and pharmacokinetics
- 格式:pdf
- 大小:297.54 KB
- 文档页数:18


埃索美拉唑回顾与临床应用研究进展张小康;马昌翰;白洁;张大军【摘要】埃索美拉唑是一种质子泵抑制剂,可长时间有效抑制胃酸的分泌,同时可在一定程度上保护胃黏膜,是治疗酸相关性疾病的首选药物,被广泛应用于治疗胃十二指肠溃疡、胃食管反流病、应激性溃疡和非甾体抗炎药不良反应的预防.同时发现埃索美拉唑还具有抗氧化、抗炎及抗肿瘤等多重药理活性.【期刊名称】《沈阳医学院学报》【年(卷),期】2016(018)001【总页数】4页(P51-54)【关键词】埃索美拉唑;质子泵抑制剂;消化系统疾病;抗肿瘤【作者】张小康;马昌翰;白洁;张大军【作者单位】沈阳医学院基础医学院临床医学专业2013级16班,辽宁沈阳110034;基础医学院临床医学专业2014级22班;基础医学院临床医学专业2012级9班;基础医学院化学教研室【正文语种】中文【中图分类】R975埃索美拉唑( esomeprazole)是奥美拉唑的S-异构体,也是( PPI)家族中第1个单一光学异构体,于2004年11月进入我国医保目录。
在众多PPI中,埃索美拉唑24 h内抑酸效果最为显著[1]。
同时,除了泌酸增加外,酸相关性疾病还可有其他发病机制,如由氧化应激、非甾体抗炎药( NSAID)以及幽门螺杆菌感染所造成的胃黏膜损伤等[2],而埃索美拉唑被广泛应用于治疗和维持胃食管反流病、胃溃疡,以及与抗生素联合应用根除幽门螺旋杆菌。
有研究指出,埃索美拉唑在治疗酸相关性疾病时,除了发挥抑制胃酸分泌的作用外,可能还有抗氧化、抗炎等作用[3]。
可与肿瘤细胞膜上的质子泵——V型ATP酶结合并抑制其活性,使肿瘤细胞内H+积聚并减弱适于肿瘤细胞生长的酸性微环境,进而起到抗肿瘤的作用。
本文将埃索美拉唑的药理学、药代动力学和药效学特性,及其临床应用进行综述。
埃索美拉唑为胃壁PPI的特异性抑制剂,通过特异性靶向作用机制减少胃酸分泌;为一弱碱,在壁细胞泌酸微管的低pH环境中浓集,转化为其活化形式次磺酰胺,然后通过二硫键与壁细胞H+-K+-ATP酶亚单位半胱氨酸残基上的巯基结合,使酶氧化失活,从而使壁细胞产生的H+不能被转运到胃腔,阻碍胃酸分泌的最后一步,对基础胃酸分泌和刺激的胃酸分泌均产生较强的抑制作用,进而达到升高胃内pH值的治疗效果[4]。

2023年幽门螺杆菌感染治疗基本手册英文版Basic Handbook for the Treatment of Helicobacter pylori Infection in 2023In the year 2023, the treatment of Helicobacter pylori infection remains a crucial aspect of healthcare. This handbook aims to provide essential guidelines for managing this common bacterial infection.DiagnosisDiagnosing Helicobacter pylori infection typically involves a combination of tests such as breath tests, blood tests, stool tests, and endoscopic procedures. These tests help in confirming the presence of the bacteria in the stomach.Treatment StrategiesThe primary treatment for Helicobacter pylori infection involves a combination of antibiotics, proton pump inhibitors (PPIs), andsometimes bismuth salts. The antibiotics are used to eradicate the bacteria, while PPIs help in reducing acid production in the stomach.Antibiotic TherapyAntibiotic therapy for Helicobacter pylori infection usually consists of a dual or triple therapy regimen. Common antibiotics used include clarithromycin, amoxicillin, metronidazole, and tetracycline. It is crucial to follow the prescribed dosage and duration to ensure successful eradication of the bacteria.Proton Pump InhibitorsProton pump inhibitors are commonly used in combination with antibiotics to treat Helicobacter pylori infection. These medications help in reducing acid production in the stomach, creating a more favorable environment for antibiotic therapy to work effectively.Bismuth SaltsIn some cases, bismuth salts may be included in the treatment regimen for Helicobacter pylori infection. These salts help in reducinginflammation in the stomach lining and promoting healing of the affected tissues.Lifestyle ModificationsIn addition to medication therapy, lifestyle modifications can also play a significant role in managing Helicobacter pylori infection. Avoiding spicy foods, alcohol, and tobacco can help in reducing symptoms and promoting faster recovery.Follow-UpAfter completing the prescribed treatment regimen, it is essential to undergo follow-up testing to confirm the eradication of Helicobacter pylori. This may involve repeat breath tests, blood tests, or endoscopic procedures to ensure successful treatment.ConclusionManaging Helicobacter pylori infection in 2023 involves a combination of antibiotics, proton pump inhibitors, and sometimes bismuth salts. Following the prescribed treatment regimen, makinglifestyle modifications, and undergoing follow-up testing are essential steps in achieving successful eradication of the bacteria.This handbook serves as a basic guide for healthcare professionals in the treatment of Helicobacter pylori infection in 2023.。

质子泵抑制剂作用机制质子泵抑制剂(Proton Pump Inhibitors,PPIs)是一类用于治疗胃酸相关疾病的药物,常见的质子泵抑制剂包括奥美拉唑(omeprazole)、兰索拉唑(lansoprazole)和雷贝拉唑(rabeprazole)等。
质子泵抑制剂通过抑制胃壁上特定细胞中的质子泵酶,从而减少胃酸的分泌,发挥其药效。
质子泵抑制剂的作用机制主要涉及质子泵酶的抑制和酸分泌的调节。
质子泵酶位于胃壁上的壁细胞中,负责将胃内的氢离子与氯离子结合,形成盐酸,这是胃酸的主要成分。
质子泵抑制剂能够特异性地与质子泵酶结合并抑制其活性,从而干扰氢离子和氯离子的结合,减少盐酸的产生。
具体来说,质子泵抑制剂在胃壁中被吸收后,进入壁细胞,通过被酸性质的胃内环境激活,转变成可与质子泵酶结合的活性态。
然后,质子泵抑制剂与质子泵酶结合,形成不可逆的复合物,抑制了酶的活性,导致质子泵酶无法将氢离子与氯离子结合,从而减少胃酸的分泌。
除了抑制质子泵酶的活性,质子泵抑制剂还能够调节酸分泌。
由于质子泵抑制剂与质子泵酶形成不可逆的复合物,使质子泵酶的再生需要合成新的酶蛋白,这需要一定的时间。
在此过程中,胃酸的分泌将被暂时抑制。
然而,由于胃壁的细胞是可更新的,新细胞会合成新的质子泵酶。
因此,质子泵抑制剂需要一段时间才能发挥全效,并使胃酸分泌恢复正常。
质子泵抑制剂作用机制主要通过抑制胃酸分泌来治疗胃酸相关疾病。
它们广泛用于胃溃疡、十二指肠溃疡、GERD(胃食管反流病)和功能性消化不良等消化系统疾病的治疗。
此外,质子泵抑制剂还可以与抗生素联用,用于治疗幽门螺杆菌引起的胃炎和胃溃疡。
总结起来,质子泵抑制剂通过抑制胃壁上的质子泵酶活性,减少胃内的盐酸分泌,适用于胃酸相关疾病的治疗。
其作用机制主要包括与质子泵酶结合形成不可逆的复合物,抑制胃酸的酸分泌,并通过调节胃壁细胞的再生过程,达到长效抑制胃酸的效果。

质子泵抑制剂的处方和审核要点2019年重庆市执业药师继续教育【最新版】目录一、质子泵抑制剂的概述二、质子泵抑制剂的适应证三、质子泵抑制剂的用法用量四、质子泵抑制剂的注意事项五、质子泵抑制剂的药物相互作用六、结语正文一、质子泵抑制剂的概述质子泵抑制剂(Proton Pump Inhibitors,PPIs)是一类抑制胃酸分泌的药物,具有抑酸作用强、特异性高、持续时间长等特点。
它们通过阻断胃酸分泌的最后通道,即胃壁细胞内的质子泵驱动细胞内 H+的交换,从而达到抑制胃酸分泌的目的。
与传统的胃酸抑制药物相比,PPIs 的作用位点不同,具有不同的特点,如夜间抑酸作用好、起效快、抑酸作用强、时间长、服用方便等。
二、质子泵抑制剂的适应证质子泵抑制剂被广泛应用于治疗消化性溃疡、胃食管反流病、上消化道出血和卓 - 艾综合征(又称胃泌素瘤)等酸相关性疾病及应激性溃疡的临床治疗和预防。
三、质子泵抑制剂的用法用量根据《质子泵抑制剂审方规则专家共识》,质子泵抑制剂的用法用量如下:1.慢性胃炎或功能性消化不良患者:以胃黏膜糜烂和/或以上腹痛、反酸、烧灼感等为主要症状的慢性胃炎患者;以上腹痛、灼烧感为主要症状的功能性消化不良患者。
所有 PPIs 口服制剂均可使用(常规用量,口服,每天 1 次)。
2.急性胰腺炎患者:所有 PPIs 注射剂均可使用(常规用量,静脉滴注,每天 2 次)。
3.胃食管反流病患者:伴有咽喉症状的胃食管反流病患者,特别是酸反流患者。
所有 PPIs 口服制剂均可使用(常规用量,口服,每天 2 次)。
四、质子泵抑制剂的注意事项1.质子泵抑制剂使用过程中可能出现不良反应,如头痛、恶心、呕吐、腹泻等,一般在用药过程中逐渐减轻或消失。
2.长期使用质子泵抑制剂可能会影响肠道菌群平衡,降低肠道菌群丰度和多样性,但口腔和上消化道共生菌的丰度显著增加。
3.质子泵抑制剂可能增加肠道感染或其他感染的风险。
五、质子泵抑制剂的药物相互作用质子泵抑制剂与其他药物的相互作用较少,但仍需注意以下情况:1.与氯吡格雷合用可能降低氯吡格雷的疗效。

注射用泮托拉唑钠在输液中的配伍稳定性研究摘要】目的:研究注射用泮托拉唑钠与几种临床常用输液配伍后的稳定性。
方法:分别在0.9%生理盐水、5%葡萄糖溶液、10%葡萄糖溶液、5%葡萄糖生理盐水中加入注射用泮托拉唑钠,并应用紫外线分光光度法测定配伍后0小时到6小时内泮托拉唑钠的含量;此外测定配额药物的PH值及配伍药液的颜色变化情况。
结果:泮托拉唑钠与0.9%生理盐水配伍较为稳定,但与其他三种注射液配伍后会出现PH值降低、泮托拉唑钠含量降低以及药液颜色改变等现象。
结论:临床采取0.9%生理盐水与注射用泮托拉唑钠配伍使用的稳定性较佳,与其他三种注射液配伍稳定性较差。
【关键词】泮托拉唑钠配伍稳定性【中图分类号】R96 【文献标识码】A 【文章编号】1672-5085(2014)23-0137-02泮托拉唑钠是一种苯并咪唑的衍生物,其作为新型的抗消化道溃疡药物,可以直接作用在患者的胃壁内,通过特异性地抑制H+-K+-ATP酶,来达到降低胃酸基础水平、减少胃酸分泌的目的[1]。
因该种药物具有起效快、作用强及副作用小等特点,现临床广泛应用该种药物治疗胃、十二指肠溃疡,急性内黏膜病变等。
伴随着该种药物临床的大量应用,关于其药物配伍的问题也就原来越多,我院多年临床实践显示,该种药物在与一些药液配伍后,会出现药液变色的情况。
因此,为指导临床合理用药,我院此次就泮托拉唑钠与4种临床常用的输液剂配伍后的情况进行了观察研究,现报道如下。
1 材料与方法1.1材料:包括药剂材料及仪器设备两个部分,其中药剂材料选取包括:国产泮托拉唑钠对照品(纯度99.9%),国产泮托拉唑钠注射剂(每支40mg),国产5%、10%葡萄糖注射液,5%葡萄糖生理盐水注射液及0.9%生理盐水注射液。
仪器包括:国产TU-22型紫外线分光光度计、PHS-3DC型精密数显酸度计、AE240型电子分析天平。
1.2方法1.2.1溶液的制备:称取40mg泮托拉唑钠对照品放置于100ml的量杯中,并加入蒸馏水至刻度后摇匀作为贮备液。


2023年幽门螺杆菌感染的标准治疗方法英文版Standard Treatment Methods for Helicobacter pylori Infection in 2023Helicobacter pylori (H. pylori) infection is a common bacterial infection that can cause various gastrointestinal issues. In 2023, the standard treatment methods for H. pylori infection typically involve a combination of antibiotics and acid-suppressing medications.The primary goal of treatment is to eradicate the H. pylori bacteria from the stomach, which can help alleviate symptoms and prevent complications such as ulcers and stomach cancer. The most commonly used antibiotics for H. pylori infection include clarithromycin, amoxicillin, and metronidazole.In addition to antibiotics, acid-suppressing medications such as proton pump inhibitors (PPIs) are often prescribed to reduce stomach acid production and create an environment that is less conducive to H.pylori growth. Commonly used PPIs include omeprazole, esomeprazole, and lansoprazole.Treatment regimens can vary depending on factors such as the patient's age, medical history, and the presence of antibiotic resistance. Triple therapy, which involves a combination of two antibiotics and a PPI, is a common first-line treatment option. Quadruple therapy, which includes two antibiotics, a PPI, and bismuth subsalicylate, may be used as a second-line treatment for cases of antibiotic resistance.It is important for patients to complete the full course of treatment as prescribed by their healthcare provider to ensure the successful eradication of H. pylori. Follow-up testing may be recommended after treatment to confirm that the infection has been cleared.Overall, the standard treatment methods for H. pylori infection in 2023 focus on a combination of antibiotics and acid-suppressing medications to successfully eradicate the bacteria and improve gastrointestinal health.。
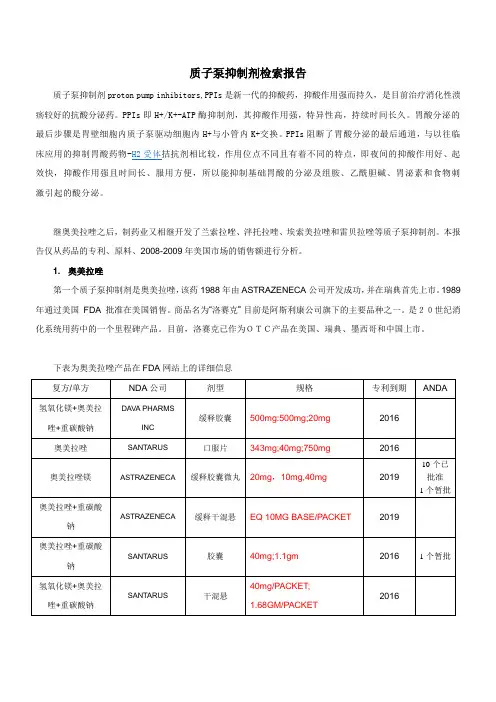
质子泵抑制剂检索报告质子泵抑制剂proton pump inhibitors,PPIs是新一代的抑酸药,抑酸作用强而持久,是目前治疗消化性溃疡较好的抗酸分泌药。
PPIs即H+/K+-ATP酶抑制剂,其抑酸作用强,特异性高,持续时间长久。
胃酸分泌的最后步骤是胃壁细胞内质子泵驱动细胞内H+与小管内K+交换。
PPIs阻断了胃酸分泌的最后通道,与以往临床应用的抑制胃酸药物-H2受体拮抗剂相比较,作用位点不同且有着不同的特点,即夜间的抑酸作用好、起效快,抑酸作用强且时间长、服用方便,所以能抑制基础胃酸的分泌及组胺、乙酰胆碱、胃泌素和食物刺激引起的酸分泌。
继奥美拉唑之后,制药业又相继开发了兰索拉唑、泮托拉唑、埃索美拉唑和雷贝拉唑等质子泵抑制剂。
本报告仅从药品的专利、原料、2008-2009年美国市场的销售额进行分析。
1. 奥美拉唑第一个质子泵抑制剂是奥美拉唑,该药1988年由ASTRAZENECA公司开发成功,并在瑞典首先上市。
1989 年通过美国FDA 批准在美国销售。
商品名为“洛赛克”目前是阿斯利康公司旗下的主要品种之一。
是20世纪消化系统用药中的一个里程碑产品。
目前,洛赛克已作为OTC产品在美国、瑞典、墨西哥和中国上市。
下表为奥美拉唑产品在FDA网站上的详细信息美国市场OTC产品中国市场OTC产品奥美拉唑行政保护结束后,国内有100 多家企业陆续申报了260 多个产品。
江苏常州第四制药厂、江苏联环制药厂等40 多家企业的产品上市,市场竞争愈加激烈。
OTC产品:奥美拉唑肠溶片,有16家企业生产;奥美拉唑肠溶胶囊,有103家企业生产。
无论处方药,还是OTC药品,以奥美拉唑原料计,其在美国市场销售额:2008年约为127亿美元,2009年约为120亿美元,增长率-4.87%,是质子泵抑制剂类中负增长的品种。
原料DMF:目前有五个厂家申报了DMF,其中西班牙两家,瑞典两家。
台湾1家,目前国内没有申报DMF 的厂家。

2023年幽门螺杆菌感染的基础治疗流程英文版Basic Treatment Protocol for Helicobacter pylori Infection in 2023Helicobacter pylori (H. pylori) infection is a common bacterial infection that affects the stomach lining. It is important to treat H. pylori infection to prevent complications such as ulcers and stomach cancer. The basic treatment protocol for H. pylori infection in 2023 involves a combination of antibiotics and acid-suppressing medications.1. Diagnosis: Diagnosis of H. pylori infection is usually confirmed through a breath test, stool test, or endoscopy. Once the infection is confirmed, treatment can begin.2. Antibiotics: The first-line treatment for H. pylori infection involves a combination of antibiotics to eradicate the bacteria. Commonly used antibiotics include clarithromycin, amoxicillin, andmetronidazole. These antibiotics are usually taken for a period of 7-14 days.3. Proton Pump Inhibitors (PPIs): In addition to antibiotics, proton pump inhibitors (PPIs) are often prescribed to reduce stomach acid production. PPIs help to create a more favorable environment for antibiotics to work effectively. Commonly used PPIs include omeprazole, esomeprazole, and lansoprazole.4. Bismuth Subsalicylate: Bismuth subsalicylate is another medication that is sometimes used in combination with antibiotics and PPIs to treat H. pylori infection. Bismuth subsalicylate helps to protect the stomach lining and enhance the effectiveness of antibiotics.5. Follow-Up Testing: After completing the initial treatment, it is important to undergo follow-up testing to confirm that the infection has been successfully eradicated. This may involve a repeat breath test, stool test, or endoscopy.6. Lifestyle Modifications: In addition to medication, making certain lifestyle modifications can help to improve the effectiveness of treatment and prevent reinfection. These may include avoiding tobacco, alcohol, and certain foods that can irritate the stomach lining.7. Consultation with a Healthcare Provider: It is important to consult with a healthcare provider before starting any treatment for H. pylori infection. Your healthcare provider can determine the most appropriate treatment plan based on your individual circumstances and medical history.In conclusion, the basic treatment protocol for H. pylori infection in 2023 involves a combination of antibiotics, proton pump inhibitors, and possibly bismuth subsalicylate. Follow-up testing and lifestyle modifications are also important components of treatment. It is important to work closely with a healthcare provider to ensure the most effective treatment for H. pylori infection.。

PPI之大不同人体胃黏膜壁细胞分泌小管膜上的H+/K+-ATP酶又称质子泵或酸泵,能选择性地抑制H+/K+-ATP酶的药物——质子泵抑制剂( proton-pump inhibitors,PPIs)已成为一代新型抑酸药物,对胃酸分泌均有显著的抑制作用,是目前治疗酸相关疾病(消化性溃疡、反流性食管炎等)的首选药物。
(一)分类及作用机制:PPIs 的分类,按照其结构特点可分2 种。
类型1: 苯并咪唑类衍生物( 结构式见图1) ,不可逆PPIs,也就是传统的质子泵抑制剂,代表药为:奥美拉唑、兰索拉唑、泮托拉唑、雷贝拉唑(部分可逆)。
作用机制:PPI为弱碱性化合物,在碱性环境中不易解离,为非活性状态的前药,可通过细胞膜进入到壁细胞分泌管内,遇到PH为2以下的酸性环境,PPI可转化为次磺酸活性体,与H+/K+-ATP酶不可逆结合从而使其失活,从而抑制胃酸的分泌,直到新的质子泵产生,壁细胞才能恢复泌酸功能,从而使pH值24h维持稳定。
由于PPIs是作用于壁细胞胃酸分泌的最后步骤,因此是目前已发现的作用最强的一类胃酸分泌抑制剂。
类型2: 咪唑吡啶替代物( 结构式见图2) ,可逆型PPIs(reversible proton pump inhibitions,RPPIs)新型质子泵抑制剂,代表药: 盐酸瑞伐拉赞(国内未上市),作用机制: 可竞争性抑制胃壁细胞上质子泵中高亲和部位的钾离子结合位点,抑制细胞浆中的H + 与胃分泌管中的K + 间的相互交换,而达到抗胃酸分泌的作用。
(二)药代动力学不同PPI药代动力学比较不同PPI在特殊人群中的药动学特点和剂量调整(三)药理学特点:不同PPI药理作用特点(四)相互作用PPI与药物相互作用主要有以下两种因素:1、抑制胃酸分泌,胃内PH值升高,影响其他药物吸收;固体食物的胃排空涉及一系列消化水解过程,PPI 抑制酸依赖的消化活性,从而延缓胃排空,影响药物的吸收、分布。
对所有的PPIs:①影响抗真菌药物吸收(伊曲康唑、酮康唑:伊曲康唑、酮康唑只有在上消化道的酸性环境中才能吸收);②影响铁剂吸收(铁剂以亚铁离子形式在十二指肠及空肠近端吸收,胃酸有助于铁吸收,PH升高,铁吸收减少)③增加地高辛吸收:降低胃液酸度, 可能减少地高辛水解,增加其吸收,加重地高辛中毒的危险2、通过肝药酶CYP450 ,影响经其代谢的其他药物。
质子泵抑制剂临床药理全解析作者:杜文琪来源:中国医学论坛报日期:2011-06-16梁茂植,教授,临床药理学、体内药物分析及药代动力学领域研究员,研究生导师,四川大学华西医院国家药品临床试验机构副主任兼?期临床试验研究室主任,临床药理研究室主任,中国药理学会临床药理专委会委员,中华医学会四川省分会第六届理事,中国药学会四川省分会临床药学专委会副主任委员,中华医学会四川省分会医院药学专业委员会委员,中国色谱学会四川省分会副秘书长,中国西部地区有机质谱委员会理事,国家食品药品监督管理局新药审评专家库成员,四川省国家食品药品监督管理局新药评审委员,四川省药品安全评价专家库成员。
主要研究方向为生物药物分析、手性药物代谢动力学和代谢物组学。
质子泵抑制剂(PPI)的诞生是消化系统疾病尤其酸相关疾病治疗史的一个里程碑。
尽管PPI在临床应用已有20年的历史,但临床医师对其的认识仍存在多种误区,如不同PPI制剂的个体差异性、代谢途径及PPI是否与氯吡格雷存在相互作用等,这些困惑或多或少对PPI临床合理应用造成了影响。
近日,本报记者就上述问题对临床药理专家、四川大学华西医院梁茂植教授进行了专访。
《中国医学论坛报》(以下简称《论坛报》):药物代谢特征与其临床疗效和副作用均有密切关系,请介绍一下PPI的代谢特征。
梁茂植:多数药物在体内的代谢转化主要在肝脏进行,可分为第一相代谢反应和第二相代谢反应。
第一相代谢反应包括氧化、去甲基化和水解反应。
药物经过第一相的氧化、去甲基化等代谢作用后,非极性脂溶性化合物变为极性和水溶性较高而活性较低的代谢物。
涉及的药物代谢酶95%以上是细胞色素P450(CYP)类酶,主要有CYP2C、CYP2D6、CYP2E1和CYP3A。
第二相反应是结合反应,指药物或其第一相代谢物与内源性结合剂的结合反应。
结合后药物毒性或活性降低、极性增加而易于被排出,以葡萄糖醛酸结合反应为最常见,此外的结合剂还有硫酸和谷胱甘肽。
质子泵抑制剂(PPIs)对氯吡格雷的影响吴雪梅,郑敬旭,刘茂柏福建医科大学附属协和医院临床药师培训基地摘要:尽管抗血小板药物可有效降低血栓事件的发生,但出血风险增加。
加用PPI可避免抗血小板治疗所导致的胃肠黏膜损害,但因PPI在肝脏的代谢具有与氯吡格雷相同的代谢途径,从而有可能降低氯吡格雷的抗血小板效益并导致不良心血管事件的危险增加。
最近的研究发现不同的质子泵抑制剂影响程度不同,泮托拉唑和埃索美拉唑对氯吡格雷作用影响较小,而奥关拉唑、雷贝拉唑和兰索拉唑对氯吡格雷的抗血小板活性抑制作用较大。
关键词:质子泵抑制剂;氯吡格雷;药物相互作用《不稳定性心绞痛和非ST段抬高心肌梗死诊断与治疗指南》推荐给予急性冠状动脉综合征(ACS)患者双重抗血小板治疗,即在应用小剂量阿司匹林的基础上加用氯吡格雷,二者联合应用可以明显减少心血管事件发生率,但同时增加消化道出血的风险。
临床上通常给予该类患者联用质子泵抑制剂(PPI)预防消化道出血。
而近期的一些研究表明,质子泵抑制剂影响氯吡格雷的抗血小板聚集作用,增加临床事件。
另外一些研究表明质子泵抑制剂不影响氯吡格雷的抗血小板聚集作用,目前对质子泵抑制剂是否会影响氯吡格雷的抗血小板聚集作用尚无统一观点。
1.氯吡格雷和PPIs体内作用的机制1.1氯吡格雷的体内作用机制氯吡格雷是噻吩并吡啶类二磷酸腺苷(ADP)受体拮抗剂,它是一种前体药物,须在体内经过一系列生物转化形成活性产物而发挥抗血小板作用。
氯吡格雷在体内通过氧化、水解两步连锁反应后形成一种硫醇衍生物,此衍生物与位于血小板上的ADP受体P2Y12不可逆结合,抑制纤维蛋白原受体GpⅡb/Ⅲa的活化,从而起到抑制血小板聚集的作用。
这一过程受细胞色素P450同工酶系统的影响,包括同工酶CYPlA2、CYP2B6、CYP2C9、CYP3A4、CYP2C19,而CYP2C19在这一过程中起主要作用[1]。
氯吡格雷的易损性在于它激活的方式,它激活的两步连锁反应过程都依赖于CYP2C19的功能。
奥美拉唑对溃疡性结肠炎的治疗效果溃疡性结肠炎是一种慢性炎症性肠病,主要影响结肠和直肠。
这种疾病会引起结肠黏膜的溃疡和炎症,导致腹泻、腹痛、便血等症状。
治疗溃疡性结肠炎的目标是减轻炎症、缓解症状、预防复发,并改善患者的生活质量。
奥美拉唑是一种常用的治疗溃疡性结肠炎的药物,下面将探讨奥美拉唑对该疾病的治疗效果。
奥美拉唑属于质子泵抑制剂(Proton Pump Inhibitors,PPIs)的一种,通过抑制胃酸分泌来减轻胃肠道的酸性刺激。
它通过与胃壁上的质子泵酶结合,阻断质子泵的酸性分泌,从而减少胃酸的产生。
这种药物不仅可以用于治疗胃溃疡和食道反流病,还可以用于治疗溃疡性结肠炎等炎症性肠病。
奥美拉唑在治疗溃疡性结肠炎中的主要作用是减轻炎症和改善症状。
炎症是溃疡性结肠炎的主要特征之一,奥美拉唑可以通过减少胃酸的分泌来降低结肠黏膜的酸性刺激,从而减轻炎症的程度。
此外,奥美拉唑还可以减少胃酸的反流,降低直肠黏膜的刺激,有助于改善腹泻和便血等症状。
研究表明,奥美拉唑在治疗溃疡性结肠炎中具有一定的疗效。
一项回顾性研究发现,奥美拉唑治疗能够显著减少腹泻和便血的发作次数,改善患者的生活质量。
另一项临床试验显示,奥美拉唑治疗组与安慰剂组相比,炎症指标(如C-反应蛋白、白细胞计数)显著下降,结肠黏膜的病理改变也有所减轻。
这些研究结果表明,奥美拉唑在治疗溃疡性结肠炎中具有抗炎和改善症状的作用。
然而,奥美拉唑并非治疗溃疡性结肠炎的唯一选择。
根据患者的具体情况和病情严重程度,医生可能会选择其他药物或治疗方法。
例如,对于轻度病例,初始治疗可以采用5-氨基水杨酸类药物或糖皮质激素短期治疗。
对于中度到重度的病例,可能需要使用免疫抑制剂或生物制剂来控制炎症和缓解症状。
此外,饮食调节、应激管理和手术治疗等也是治疗溃疡性结肠炎的重要手段。
需要注意的是,奥美拉唑在治疗溃疡性结肠炎中可能会出现一些副作用。
常见的副作用包括恶心、呕吐、腹胀、腹痛等消化系统症状。
1、药物(drug):药物是人类用来预防、治疗、诊断疾病、或为了调节人体功能,提高生活质量,保持身体健康的特殊化学品。
2、药物化学(medicinal chemistry):药物化学是一门发现与发明新药、研究化学药物的合成、阐明药物的化学性质、研究药物分子与机体细胞(生物大分子)之间相互作用规律的综合性学科,是药学领域中重要的带头学科以及极具朝气的朝阳学科。
4、非经典的抗精神病药物(atypical antipsychotic agents):近年来问世的一些抗精神病药物。
和传统的吩噻嗪类和氟哌啶醇药物不同,其拮抗多巴胺受体的作用较弱,可能是产生多巴胺和5-羟色胺受体的双相调节作用,其锥体外系的副反应较少,具有明显治疗精神病阳性和阴性症状的作用。
代表药物如氯氮平。
5、构效关系(structure- activity relationship,SAR):在同一基本结构的一系列药物中,药物结构的变化,引起药物活性的变化的规律称该类药物的构效关系。
其研究对揭示该类药物的作用机制、寻找新药等有重要意义。
1、拟胆碱药(cholinergic drugs):是一类具有与乙酰胆碱相似作用的药物。
按作用环节和机制的不同,主要可分为胆碱受体激动剂和乙酰胆碱酯酶抑制剂两种类型。
2、乙酰胆碱酯酶抑制剂(AChE inhibitors):又称为抗胆碱酯酶药(anticholinesterase drug),通过对乙酰胆碱酯酶的可逆性抑制,增强乙酰胆碱的作用。
不与胆碱受体直接作用,属于间接拟胆碱药。
在临床上主要用于治疗重症肌无力和青光眼,及抗早老性痴呆。
3、抗胆碱药(anticholinergic drugs):即胆碱受体拮抗剂(cholinoceptor antagonists),主要是阻断乙酰胆碱与胆碱受体的相互作用的药物。
4、非去极化型神经肌肉阻断剂(nondepolarizing neuromuscular blocking agents):又称非去极化型肌松药,属于N2胆碱受体拮抗剂。
REVIEW ARTICLEProton pump inhibitors:an update of their clinical use and pharmacokineticsShaojun Shi&Ulrich KlotzReceived:21January2008/Accepted:1July2008/Published online:5August2008#Springer-Verlag2008AbstractBackground Proton pump inhibitors(PPIs)represent drugs of first choice for treating peptic ulcer,Helicobacter pylori infection,gastrooesophageal reflux disease,nonsteroidal anti-inflammatory drug(NSAID)-induced gastrointestinal lesions(complications),and Zollinger-Ellison syndrome. Results The available agents(omeprazole/esomeprazole,lan-soprazole,pantoprazole,and rabeprazole)differ somewhat in their pharmacokinetic properties(e.g.,time-/dose-dependent bioavailability,metabolic pattern,interaction potential,genet-ic variability).For all PPIs,there is a clear relationship between drug exposure(area under the plasma concentration/ time curve)and the pharmacodynamic response(inhibition of acid secretion).Furthermore,clinical outcome(e.g.,healing and eradication rates)depends on maintaining intragastric pH values above certain threshold levels.Thus,any changes in drug disposition will subsequently be translated directly into clinical efficiency so that extensive metabolizers of CYP2C19 will demonstrate a higher rate of therapeutic nonresponse. Conclusions This update of pharmacokinetic,pharmacody-namic,and clinical data will provide the necessary guide by which to select between the various PPIs that differ—based on pharmacodynamic assessments—in their relative poten-cies(e.g.,higher doses are needed for pantoprazole and lansoprazole compared with rabeprazole).Despite their well-documented clinical efficacy and safety,there is still a certain number of patients who are refractory to treatment with PPIs(nonresponder),which will leave sufficient space for future drug development and clinical research. Keywords Proton pump inhibitors.Pharmacokinetics. Pharmacodynamics.Peptic ulcer.Reflux disease. Helicobacter pylori infectionIntroductionProton pump inhibitors(PPIs)are the mainstays in treating acid-related diseases.Since the introduction of omeprazole in1989,other PPIs became available,e.g.,lansoprazole (1995),pantoprazole(1997),rabeprazole(1999),and the S-enantiomer of omeprazole(2001).PPIs inhibit selectively and irreversibly the gastric H+/K+ATPase(the proton pump)that accomplishes the final step in acid secretion.All PPIs inhibit both basal and stimulated secretion of gastric acid,independent of the nature of parietal cell stimulation[1].PPIs undergo extensive hepatic metabolism by the cytochrome P450 (CYP)system,and CYP2C19polymorphisms have been shown to substantially influence the pharmacoki-netics,pharmacodynamics,and clinical outcome of PPIs [2,3].In addition,pharmacokinetic and pharmacody-namic differences between PPIs are reflected in their influence on both speed and degree of gastric acid suppression,which subsequently may affect their clinical efficacy[4].Eur J Clin Pharmacol(2008)64:935–951DOI10.1007/s00228-008-0538-yS.Shi:U.Klotz(*)Dr.Margarete Fischer-Bosch-Institut fürKlinische Pharmakologie,Auerbachstraße112,70376Stuttgart,Germanye-mail:ulrich.klotz@ikp-stuttgart.deS.Shi:U.KlotzUniversity of Tuebingen,Tuebingen,GermanyPresent address:S.ShiDepartment of Pharmacy of Union Hospital,Tongji Medical College,Huazhong University of Science and Technology, Wuhan,People’s Republic of ChinaPPIs are drugs of first choice for peptic ulcers(PU)and their complications(e.g.,bleeding),gastrooesophageal reflux disease(GERD),nonsteroidal anti-inflammatory drug(NSAID)-induced gastrointestinal(GI)lesions,Zol-linger-Ellison syndrome,dyspepsia,and eradication of Helicobacter pylori(H.pylori)with two antibiotics[5,6]. PPIs have demonstrated an excellent safety profile after approximately two decades of clinical use[7].There are some unspecific adverse events(AEs)such as headache, nausea,and diarrhea,but the main concern is emerging from the long-term suppression of acid secretion[8].During the last few years,much new data have been published on the pharmacological characteristics and therapeutic efficacy of PPIs.Therefore,it appears appro-priate to provide an update on the pharmacokinetics, pharmacodynamic action,and clinical use of PPIs. Pharmacokinetic propertiesPPIs are substituted benzimidazole derivatives and mem-brane-permeable,weak bases that accumulate in acid spaces of the active parietal cell as prodrugs.Here,they undergo acid-catalyzed conversion to the active sulfenic acid and sulfonamide derivatives.These bind covalently via disul-fide bridges to cysteine residues on the alpha subunit of the H+/K+ATPase,thus inhibiting acid secretion up to36h[1]. Whereas racemic PPI prodrugs possess a chiral center,the identical biological active principle(which is optically inactive)is formed from both enantiomers.The main pharmacokinetic parameters of all PPIs are compared in Table1[6,9].Omeprazole and esomeprazoleOmeprazole is administered as a racemic mixture of its two enantiomers,S-omeprazole(esomeprazole)and R-omepra-zole.Both prodrugs are acid labile and usually administered as encapsulated enteric-coated granules.After oral admin-istration,they are rapidly absorbed[9].They should be swallowed at least1h before eating.The bioavailability(F) of esomeprazole was significantly reduced when taken within15min before eating a high-fat meal compared with fasting conditions[10].When increasing the dose and after repeated administration of omeprazole or esomeprazole,the maximal plasma concentration(C max)and area under the concentration-time curve(AUC)increased in a nonlinear fashion[11],which is due to decreased first-pass elimina-tion and decreased systemic clearance(CL).Furthermore, due to a lower metabolic rate of esomeprazole compared with R-or racemic omeprazole,esomeprazole resulted in higher AUC values[11].Similarly,in patients with GERD, the C max and AUC values of esomeprazole increased on day7versus day1by80%and50%,respectively[12].Omeprazole and esomeprazole are extensively metabo-lized by CYP2C19and CYP3A4.Omeprazole is converted mainly to hydroxyl and5-O-desmethyl metabolites by CYP2C19and to the sulfone by CYP3A4[6].In vitro studies suggest that esomeprazole is predominantly metab-olized by CYP3A4and consequently is less dependent on CYP2C19,which recently could be substantiated by a clinical study[13].Approximately80%of each dose is excreted as metabolites in the urine[6].CYP2C19polymorphisms can significantly influence the metabolism of omeprazole and esomeprazole[3,14].Table1Pharmacokinetic properties of proton pump inhibitors(according to Klotz[6])Parameter Omeprazole Esomeprazole Lansoprazole Pantoprazole Rabeprazole t max[h]1–61–3.5 1.2–2.12–43–5F[%]25–40(↑upon multipledosing)50(acute dosing)70–80(chronic dosing)80–907752Linear pharmacokinetics No No Yes Yes Yesfu[%]0.050.050.030.020.04V[l/kg]0.13–0.350.22–0.260.40.15–t1/2[h]0.5–1.20.8–1.30.9–2.10.8–2.00.6–1.4CL[ml/min]400–620330(acute)160–250(chronic)400–65090–225–CL/F[ml/min]320310125600f e[%]Negligible Negligible Negligible Negligible Negligible Effect of age CL↓,t1/2(↑),F↑CL↔,t1/2↔CL↓,t1/2↑CL↔,t1/2↔CL↓,t1/2(↑) Renal insufficiency CL↔,t1/2(↔),F↔–CL↓↔,t1/2↑(↔)CL↔,t1/2↔CL(↑),t1/2↔Hepatic dysfunction CL↓,t1/2↑,F↑CL↓,t1/2↑CL↓,t1/2↑CL↓,t1/2↑,F↔CL↓,t1/2↑t max time to maximal plasma concentration,F oral bioavailability,fu fraction of drug unbound in plasma,V apparent volume of distribution,t1/2 elimination half life,CL systemic clearance,CL/F apparent oral clearance,f e fraction excreted in unchanged form into urine↑increase,↓decrease,↔no significant change,arrows in parentheses effects are equivocalAccording to metabolic rate(phenotype),individuals can be classified as homozygous extensive metabolizers(homEM), heterozygous extensive metabolizers(hetEM)and poor metabolizers(PM).The frequencies of these three sub-groups show a wide interethnic variation.The prevalence of PM ranges from1.2%to3.8%in Caucasian Europeans and up to23%in Asian Oceanian populations[15,16].PMs exhibit a3-to10-fold and hetEMs a2-to3-fold higher AUC(drug exposure)compared with homEMs[3,14].In a recent study,mean AUC ratios of omeprazole were 1:2.7:9.0after a single oral dose of40mg and1:1.7:4.3 after a single i.v.dose of20mg in six homEMs,eight hetEMs,and six PMs,respectively.Therefore,in PMs,F was higher than that in homEMs and hetEMs(87%vs.62% and41%;P<0.001)[17].Similarly,in homEMs,hetEMs, and PMs,the relative AUC ratios were1:2.8:7.5and t1/2 ratios1:1:1.7for omeprazole,indicating that disposition of omeprazole is greatly dependent on the CYP2C19genotype [18].Similar ratios were reported after repeated doses of omeprazole[19].Interestingly,the elderly EMs showed a wide variance in their CYP2C19activity and were phenotypically closer to the PMs than the young EMs compared with the young PMs.Thus,in the elderly,the CYP2C19genotype may not be as useful as phenotyping [20].The most frequently and extensively described variant alleles for PMs are CYP2C19*2and CYP2C19*3,which encode for nonfunctional proteins.Recently,the CYP2C19*17allele has been identified,which is associated with a very rapid metabolism phenotype and has a frequency of18%both in Swedes and Ethiopians but only 4%in Chines populations[21].Such subjects need higher doses of omeprazole for acid suppression[22,23].The pharmacokinetics of omeprazole was compared in 18healthy adults and in12children with GERD(mean age 6.1years).Oral clearance(CL/F)and apparent volume of distribution(V)in healthy adults(0.62L/h per kg and0.76 L/kg,respectively)were not significantly different from those in children with GERD(0.51L/h per kg and 0.66L/kg,respectively).Therefore,dosing on a milligram/ kilogram basis was recommended,with no further adjust-ments for treating GERD in children[24].In27children with GERD aged1–11years,the pharmacokinetic properties of esomeprazole were both dose(between5–20mg)and age dependent.The younger children(1–5years)showed a more rapid metabolism compared with older children(6–11years) [25].In28adolescent patients with GERD aged12–17 years,the mean AUC and C max values of esomeprazole were 3.5-fold higher with the40mg dose compared with the20 mg dose with single-and repeated-dose administration, confirming nonlinear pharmacokinetics[26].All PPIs cause increases in intragastric pH,which can affect absorption of concomitantly given drugs,such as ketoconazole,vitamin B12,and digoxin[27].In addition,omeprazole carries the potential for inhibiting the hepatic elimination of a wide range of drugs,including carbamaze-pine,diazepam,mephenytoin,methotrexate,nifedipine, phenytoin,warfarin,mefloquine,pyrimethamine,and sulfa-doxine[6,27,28].Likewise,inhibitors of CYP2C19or CYP3A4,e.g.,fluconazole[29]and fluvoxamine[30],can affect the metabolism of omeprazole.Furthermore,omepra-zole is a substrate and inhibitor of P-glycoprotein.So drug interactions could be also partly due to an inhibition of P-glycoprotein-mediated drug transport[31,32].On the contrary,St.John’s wort can induce both pathways in the metabolism of omeprazole and decreased its AUC(38–44%) and C max(38–50%)[33].As with smoking,omeprazole can dose dependently induce CYP1A2[6].In general,it can be expected that the interaction potential of esomeprazole is similar to that of racemic omeprazole[27,34].Triple therapy with omeprazole/clarithromycin/amoxicillin is widely used to eradicate H.pylori.The observed pharmacodynamic synergism of these drugs is at least partly due to pharmacokinetic interactions.The AUC of omepra-zole increased almost twofold after concomitant administra-tion of clarithromycin[35].Naproxen and rofecoxib did not interact with esomeprazole and visa versa.Apparently, esomeprazole can be used in combination with NSAIDs without the risk of pharmacokinetic interactions[36].LansoprazoleLansoprazole is rapidly absorbed and displays a linear increase in plasma concentrations over a dose range of15–60mg[37].Pharmacokinetics of repeated doses is similar to that of a single nsoprazole is extensively and rapidly(t1/2:1–2h)metabolized into sulfone and5-hydroxylated metabolites by CYP3A4and CYP2C19.A sulfide metabolite is also present in smaller amounts[6, 37].As CYP2C19is involved in the metabolism of lansoprazole,it is not surprising that PMs have a4.4-fold higher AUC than homEMs[3].The pharmacokinetics of lansoprazole(15mg/day)in children with GERD aged13–24months was comparable to those in older children and adults[38].Fluvoxamine treatment increased AUC of lansoprazole 3.8-fold(P<0.01)in homEMs and2.5-fold(P<0.05)in hetEMs,whereas in PMs,no difference was found in any pharmacokinetic parameter[39].A double-blind,random-ized controlled trial(RCT)showed that clarithromycin treatment significantly increased C max of lansoprazole 1.47-, 1.71-,and 1.52-fold and AUC 1.55-, 1.74-,and 1.80-fold in homEMs,hetEMs,and PMs,respectively. These very similar effects indicate that the drug interactions resulted from inhibition of CYP3A4[40].Surprisingly, grapefruit juice had no significant effect on the pharmaco-kinetics of lansoprazole[41].Lansoprazole did not demonstrate relevant interactions with theophylline,phenytoin,prednisone,warfarin,diaze-pam,oral contraceptives,ivabradine,or methotrexate[37, 42,43].In renal transplant recipients receiving tacrolimus and lansoprazole(30mg)or rabeprazole(20mg),elevated blood concentrations of tacrolimus were observed only in the very rare subgroup of subjects who are PM of CYP2C19and bearing also the CYP3A5*3/*3genotype [44].Absorption of atazanavir was significantly reduced when coadministered with lansoprazole,as evidenced by a 94%decline in AUC and by a96%decrease in C max[45].PantoprazolePantoprazole is rapidly absorbed after oral administration of enteric-coated tablets,which avoid degradation of the PPI by gastric acid[6].It hardly undergoes first-pass metabo-lism and has a bioavailability of approximately77%, independent of dose and food intake.Pantoprazole shows linear pharmacokinetics after both i.v.and oral administra-tion.It is completely metabolized by CYP2C19and CYP3A4.In a major pathway,pantoprazole undergoes O-demethylation followed by sulfate conjugation and sulfone/ sulfide formation[46].Pantoprazole has apparently no clinically relevant drug interactions,other than the class effect associated with elevated intragastric pH.No drug interactions have been demonstrated between pantoprazole and a wide range of drugs,such as theophylline,diazepam, carbamazepine,digoxin,and warfarin[46,47].Unlike omeprazole,administration of clarithromycin did not increase pantoprazole levels[35].RabeprazoleIn healthy subjects,mean F has been calculated to52% [48].C max and AUC values were linearly related to the dose,whereas t max and t1/2were dose independent. Rabeprazole’s metabolism is unique because of its reduc-tion to rabeprazole thioether via a nonenzymatic pathway with renal elimination of the metabolites.Both CYP2C19 and CYP3A4contribute only a small fraction to the overall metabolism[49].Thus,rabeprazole will be less susceptible to the influence of genetic polymorphisms of either CYP2C19or CYP3A4[50].In healthy Chinese subjects, the pharmacokinetics of rabeprazole was dependent to a certain degree on the CYP2C19genotype.AUC values for rabeprazole differed among the three genotype groups (homEM,hetEM,PM),with relative ratios of1.0:1.3:1.8 after a single dose and of1.0:1.1:1.7after repeated doses, respectively.These changes were much smaller than those observed for other PPIs[51].The modest differences in AUC of rabeprazole were also consistent with other studies [18,52].However,in12Chinese subjects receiving rabeprazole20mg b.i.d.,the mean AUC values of rabeprazole and rabeprazole thioether were higher(P< 0.001)in PMs than in EMs on day1.Similar results were observed on day4,which might be due to the high rabeprazole dose(40mg/day)used in this study[53].Pharmacokinetic properties of rabeprazole in children 12–16years old were similar to adults,and during multiple dosing,only headache and nausea were sometimes reported [54].As the CYP450-mediated pathways are secondary in rabeprazole metabolism,it has a low potential for drug interactions involving CYPs.Interaction studies with rabeprazole revealed no significant metabolic effect on theophylline,phenytoin,warfarin,or diazepam[27,49]. Clarithromycin or verapamil did not alter the pharmacoki-netics of rabeprazole,irrespective of the CYP2C19geno-types[55].Interestingly,fluvoxamine,an inhibitor of CYP1A2and CYP2C19,increased AUC of rabeprazole and rabeprazole thioether2.8-and5.1-fold in homEMs and 1.7-and 2.6-fold in hetEMs,respectively,whereas no difference was seen in PMs of CYP2C19[56].According to in vitro experiments all PPIs showed some inhibition of CYP2C9,2C19,and3A4;the inhibitory potency of rabeprazole was relatively lower than that of the other PPIs[57].Pharmacodynamic profileThe primary effect of PPIs is suppression of gastric acid secretion,which will determine healing rates in GERD and PU[58].Intragastric pH monitoring allows direct assess-ment of acid suppression,and it is a very useful measure with which to compare antisecretory therapies[59]. Typically,the antisecretory effects of PPIs have been reported as mean or median24-h intragastric pH or suppression of24-h intragastric acidity expressed as time above a predefined pH threshold.Intragastric pH value above3(PU)and4(GERD)have been defined as therapeutic targets[60].PPIs are regarded to be similarly effective,but their potency differs.Such differences may translate into a slight advantage for a particular PPI in a special clinical situation[61].Onset and degree of acid suppressionAll PPI prodrugs undergo accumulation and acid activation in the parietal cell,which relies on their pK a values.Based on in vitro experiments and pK a considerations,it can be estimated that rabeprazole will show the highest accumu-lation and faster conversion to the active form of the PPIs [1].This might affect the speed of acid suppression by PPIs.Apparently an earlier sustained inhibition of acidsecretion was seen with rabeprazole than with other PPIs [62].Comparing the antisecretory effects on the first day of dosing,rabeprazole achieved better acid control because the intragastric pH(3.4)and the time of pH>4during the 24-h postdose were significantly(P≤0.04)greater with rabeprazole than with lansoprazole,pantoprazole,or two formulations of omeprazole[63].In contrast,in72healthy volunteers,mean24-h pH and percentage of time for pH>4 were not significantly different between lansoprazole30mg and nsoprazole resulted in greater acid suppression during hours0–5on days1and5, whereas rabeprazole had greater suppression during hours 11–24on day5[64].In patients with heartburn,the intragastric pH profiles of all five PPIs were compared after giving once daily esomeprazole40mg,lansoprazole30mg,omeprazole20 mg,pantoprazole40mg,and rabeprazole20mg[65,66]. The results of day5suggest that intragastric pH control with esomeprazole40mg/day was superior to other PPIs [65].Similarly,with the same dosing schedule,esomepra-zole maintained intragastric pH>4for a longer time compared with all other PPIs on days1and5[66].In seven healthy homEMs,the median intragastric pH and percent time pH>4for24h increased dose dependently with omeprazole10,20,and40mg daily for7days,and10 and20mg b.i.d.were comparable with once-daily20and 40mg.Concerning percent time pH>4at nighttime, omeprazole20mg b.i.d.was superior(P<0.05)to40mg daily[67].Three RCTs indicated that a single dose of esomeprazole (40mg i.v.)was superior to omeprazole(40mg i.v.)in reducing stimulated acid secretion.However,control of intragastric pH was similar for esomeprazole and omeprazole at a high dose of80mg(over30min)+8mg/h(for23.5h) [68].Esomeprazole40mg i.v.resulted in11.8h with an intragastric pH>4compared with5.6h(P<0.0001)and 7.2h(P<0.001)for pantoprazole40mg i.v.as infusion or bolus injection,respectively,suggesting that esomeprazole was more potent than pantoprazole[69].Likewise,oral administration of esomeprazole40mg b.i.d.provided better and more consistent intragastric acid control than pantopra-zole40mg b.i.d.[70].In patients with GERD,esomeprazole (40mg daily or b.i.d.)was compared with lansoprazole(30 mg daily or b.i.d.).Mean time pH>4and mean24-h pH were highest for esomeprazole40mg b.i.d.,followed by lansoprazole30mg b.i.d.,esomeprazole40mg once daily, and lansoprazole30mg once daily[71].Based on three pooled open studies in80subjects,single doses of rabeprazole(20mg)and esomeprazole(40mg)were equivalent in their effects on24-h intragastric pH.After5 days’dosing,rabeprazole(20mg)maintained pH>4longer than20mg esomeprazole(62%vs56%;P=0.046),whereas the lower dose(10mg)of rabeprazole was slightly less effective(48%;P=0.035)[72].These results were consistent with two other RCTs[73,74].On a milligram basis,acid inhibition by rabeprazole was significantly superior to omeprazole.After the initial dose, rabeprazole(20mg)inhibited acid secretion by72%after 11h and by64%after23h compared with omeprazole 20mg(49%and38%,P<0.03).This advantage was maintained for8days of treatment[75].In addition,single and multiple doses of rabeprazole20mg produced greater acid suppression than oral or i.v.pantoprazole40mg[76,77].Furthermore,a reduced dose of rabeprazole(10mg b.i.d.)was comparable with the higher dosages of rabeprazole (20mg b.i.d.),to lansoprazole(30mg b.i.d.),and to omeprazole(20mg b.i.d.)for acid-suppressive efficacy [78,79].Overall,pharmacodynamic and clinical data indicated that on a milligram basis,rabeprazole can provide the greatest degree of acid suppression among the available PPIs.It also may provide a faster onset toward a maximal antisecretory effect than other drugs of this class.This can be of therapeutic relevance,as clinical outcome depends on extent and duration of secretory inhibition.Impact of CYP2C19polymorphism on acid suppression PPI-induced inhibition of acid secretion is closely related to AUC values[3,14].As EMs of CYP2C19have smaller AUC values than PMs,the genotype-dependent difference in pharmacokinetics will translate directly into a less pronounced acid suppression in EMs(about70%of Caucasian patients)compared with PMs(see Table2),so that EMs(and carriers of the CYP2C19*17allele)will demonstrate a higher rate of therapeutic nonresponse.This topic has been extensively investigated[80,81].In eight healthy EMs,rabeprazole(10mg/day)showed a faster onset of rising intragastric pH and a stronger inhibition of gastric acid secretion than did lansoprazole (30mg/day)or omeprazole(20mg/day)[82].In nine healthy,H.pylori-negative homEMs treated with rabepra-zole,omeprazole and lansoprazole once daily at reduced Table2Influence of CYP2C19genotype on intragastric pH(accord-ing to Klotz[3])Median pH over24hPPI PM hetEM homEMOmeprazole(20mg for7/8days) 5.7–6.6 4.4–5.5 3.1–4.1 Lansoprazole(30mg for8days) 5.4–6.2 3.5–5.0 3.1–4.5 Rabeprazole(20mg for8days) 5.8–6.0 4.6–5.0 3.8–4.8PPI proton pump inhibitor,PM poor metabolizers,hetEM heterozygous extensive metabolizers,homEM homozygous extensive metabolizersand standard doses for7days,the median values of the 24-h percent of time at pH>4was dose dependent but did not exceed65%under any of the regimens tested.Thus,to achieve effective acid suppression for the initial therapy of GERD,higher doses were needed in homEMs[83].The efficacy of omeprazole(10or20mg for7days)has been shown to be significantly stronger in PMs and hetEMs than in homEMs[84].Likewise,in31Korean patients with GERD receiving omeprazole20mg daily for28days, 24-h intragastric pH in PMs(5.3)was higher(P<0.005) than that in homEMs(2.8)and hetEMs(3.6)[85].In healthy volunteers(six homEMs,nine hetEMs,five PMs) treated with lansoprazole30mg b.i.d.,the median of 24-h intragastric pH in PMs(6.1)was higher(P<0.05)than those in homEMs(4.5)and hetEMs(5.0).In contrast,when lansoprazole30mg b.i.d.was given with famotidine20mg b.i.d.,the median intragastric pH was5.4,5.7,and6.1, respectively.Thus,acid inhibition by lansoprazole was significantly influenced by the CYP2C19genotype,but apparently this genetic influence could be offset by the concomitant use of the H2-receptor antagonist famotidine [86].The impact of the CYP2C19genotype on the efficacy of rabeprazole was less consistent.In H.pylori-negative GERD subjects given rabeprazole10mg/day for8weeks, the intragastric pH elevation was independent of CYP2C19 genotypes[87],which was consistent with another study [52].In contrast,two studies with rabeprazole20or40mg daily for8days demonstrated that acid inhibition by rabeprazole was dependent on the CYP2C19genotype [88,89].In Korean patients,pantoprazole40mg daily exhibited a variable acid inhibition that was significantly dependent on the CYP2C19genotype[90].As there is a significantly positive relationship between the extent and duration of elevated intragastric pH and the clinical efficacy of PPIs,it can be anticipated that the CYP2C19 genotype will have a significant impact on the therapeutic outcome of three PPIs(e.g.,omeprazole,lansoprazole,pantoprazole)that are predominantly metabolized by this polymorphic enzyme.Therapeutic usePeptic ulcerFor the management of PU(healing induction and remission maintenance),both suppression of gastric acid secretion and eradication of H.pylori are important mainstays.In general,there is little overall difference in PU healing rates among PPIs,and reported small differ-ences can be explained best by the variable dosage regimens applied[91–93].H.pylori eradication is accom-plished efficiently(80–90%eradication rates)by standard triple therapy with a PPI,amoxicillin,and clarithromycin or metronidazole[94–96](Table3),and there was no significant difference among the PPIs[97–101].A consensus on the optimal duration(7,10,or14days) of first-line triple therapy is still missing.However,many controlled studies and meta-analyses[102–108]indicated that extending this strategy beyond7days is very unlikely to be clinically useful,especially if taking antibiotic-induced AEs into account[101].There are even some data suggesting that a rabeprazole-based triple therapy for4 days will result in eradication rates around90%[109,110]. More recently,sequential treatment consisting of5days of therapy with a PPI and amoxicillin followed by5days of PPI+clarithromycin and tinidazole has attracted some favorable attention[111–114].As already outlined,the genotype of CYP2C19affects the pharmacokinetics and pharmacodynamics of most PPIs. In a recent meta-analysis of dual and triple therapies a, marked difference in the H.pylori eradication rates between PMs/hetEMs vs.homEMs was calculated,especially for omeprazole[115].Therefore,CYP2C19polymorphism is a major predictor of treatment failure for H.pylori eradication [116,117].Table3Recommendation of Helicobacter pylori eradication formulated in the Maastricht Consensus Report(adapted from Malfertheiner et al.[94])Choice StatementsFirst-choice treatment•PPI-amoxicillin-clarithromycin or metronidazole treatment remains the recommended first-choice treatmentin populations with less than15–20%clarithromycin resistance prevalence.•In populations with less than40%metronidazole resistance prevalence PPI-clarithromycin-metronidazole ispreferable.•Quadruple therapies are alternative first-choice treatments.Second-choice treatment•Bismuth-based quadruple therapies remain the best second-choice treatment.•If not available,a PPI,amoxicillin,or tetracycline and metronidazole are recommended.Third-choice treatment(rescuetreatment)•Rescue treatment should be based on antimicrobial susceptibility testing.。