
Conjugation vs hyperconjugation in molecular structure of acrolein
Svitlana V.Shishkina a ,?,Anzhelika I.Slabko b ,Oleg V.Shishkin a ,c
a
Division of Functional Materials Chemistry,SSI ‘Institute for Single Crystals’,National Academy of Science of Ukraine,60Lenina Ave.,Kharkiv 61001,Ukraine b
Department of Technology of Plastic Masses,National Technical University ‘Kharkiv Polythechnic Institute’,21Frunze Str.,Kharkiv 61002,Ukraine c
Department of Inorganic Chemistry,V.N.Karazin Kharkiv National University,4Svobody Sq.,Kharkiv 61077,Ukraine
a r t i c l e i n f o Article history:
Received 4August 2012
In ?nal form 16November 2012Available online 29November 2012
a b s t r a c t
Analysis of geometric parameters of butadiene and acrolein reveals the contradiction between the Csp 2–Csp 2bond length in acrolein and classical concept of conjugation degree in the polarized molecules.In this Letter the reasons of this contradiction have been investigated.It is concluded that the Csp 2–Csp 2bond length in acrolein is determined by in?uence of the bonding for it p –p conjugation and antibonding n ?r ?hyperconjugation between the oxygen lone pair and the antibonding orbital of the single bond.It was shown also this bond length depends on the difference in energy of conjugative and hyperconjuga-tive interactions.
ó2012Elsevier B.V.All rights reserved.
1.Introduction
Butadiene and acrolein belong to the most fundamental mole-cules in the organic chemistry.They are canonical objects for the investigation of phenomena of p –p conjugation between double bonds and polarization of p -system by heteroatom [1].According to many experimental [2–16]as well as theoretical studies [13,17–23]the molecular structure of butadiene is determined by conjugation between p -orbitals of two double bonds and may be described as superposition of two resonance structures (Scheme 1).The presence of zwitterionic structure causes the shortening of the central single Csp 2–Csp 2bond as compare with similar unconjugated bond [24].
Acrolein differs from butadiene by presence of the oxygen atom instead terminal methylene group.According to classical concepts of organic chemistry such replacement should causes polarization of p -system due to presence of highly polar C @O bond [25].This leads to signi?cant increase of the contribution of the zwitterionic resonance structure (Scheme 1)re?ecting strengthening of conju-gation between p -systems of double bonds.Therefore the central Csp 2–Csp 2bond must be shorter in acrolein as compared with one in butadiene.
However numerous investigations of acrolein by experimental [26–28]and theoretical methods [26,27,29–36]demonstrate an opposite situation:the Csp 2–Csp 2bond length varies within the range 1.469?1.481?in acrolein as compared with 1.454?1.467in butadiene.Based on these data one can conclude that conjugation between double bonds in acrolein is weaker than in butadiene.At that time the rotation barrier obtained from quan-tum-chemical calculations is higher in acrolein [26,27],con?rming stronger conjugation between double bonds.Thus,results of experimental and theoretical investigations demonstrate the con-tradiction between the strengthening of the conjugation in acrolein as compare with butadiene and the values of the Csp 2–Csp 2bond length in these molecules.
Recently such illogical situation was observed also in derivatives of cyclohexene containing conjugated endocyclic and exocyclic double bonds [37].It was assumed that elongation of the Csp 2–Csp 2bond in cycloxen-2-enone as compare with one in 3-methylene-cyclohexene is caused by the in?uence of n ?r ?hyperconjugation.
In this Letter we demonstrate the results of the investigation of intramolecular interactions in butadiene and acrolein which ex-plain the experimentally observed contradiction between the length of the Csp 2–Csp 2bond and degree of conjugation in acrolein.2.Method of calculations
The structures of all investigated molecules were optimized using second-order M?ller-Plesset perturbation theory [38].The standard aug-cc-pvtz basis set [39]was applied.The character of stationary points on the potential energy surface was veri?ed by calculations of vibrational frequencies within the harmonic approximation using analytical second derivatives at the same level of theory.All stationary points possess zero (minima)or one (saddle points)imaginary frequencies.The veri?cation of the calculation method was performed using optimization of butadi-ene and acrolein by MP2/aug-cc-pvqz,CCSD(T)/cc-pvtz and CCSD(T)/6-311G(d,p)methods [40].
The geometry of saddle points for the rotation process was lo-cated using standard optimization technique [41].The barrier of the rotation in all molecules was calculated as the difference be-tween the Gibbs free energies at 298K of the most stable s-trans
0009-2614/$-see front matter ó2012Elsevier B.V.All rights reserved.https://www.doczj.com/doc/755430757.html,/10.1016/j.cplett.2012.11.032
Corresponding author.Fax:+3805723409339.
E-mail address:sveta@https://www.doczj.com/doc/755430757.html, (S.V.Shishkina).
conformer and saddle-point conformation.All calculations were performed using the G AUSSIAN 03program [42].
The intramolecular interactions were investigated within the Natural Bonding Orbitals theory [43]with N BO 5.0program [44].Calculations were performed using B3LYP/aug-cc-pvtz wave func-tion obtained from single point calculations by G AUSSIAN 03program.The conjugation and hyperconjugation interactions are referred to as ‘delocalization’corrections to the zeroth-order natural Lewis structure.For each donor N BO (i )and acceptor N BO (j ),the stabiliza-tion energy E (2)associated with delocalization (‘2e-stabilization’)i ?j is estimated as
E e2T?D E ij ?q i
F ei ;j T2
2j à2i
;
where q i is the donor orbital occupancy,e j and e i are the diagonal elements (orbital energies),and F (i,j )is the off-diagonal N BO Fock matrix element.
3.Results and discussion
The equilibrium geometry of s-trans and s-cis conformers of butadiene and acrolein calculated by MP/aug-cc-pvtz method (Ta-ble 2)agrees very well with obtained earlier results [2–23,25–34]and data of higher and more computationally expensive methods (Table 1).It can be noted that the C–C bond length in acrolein
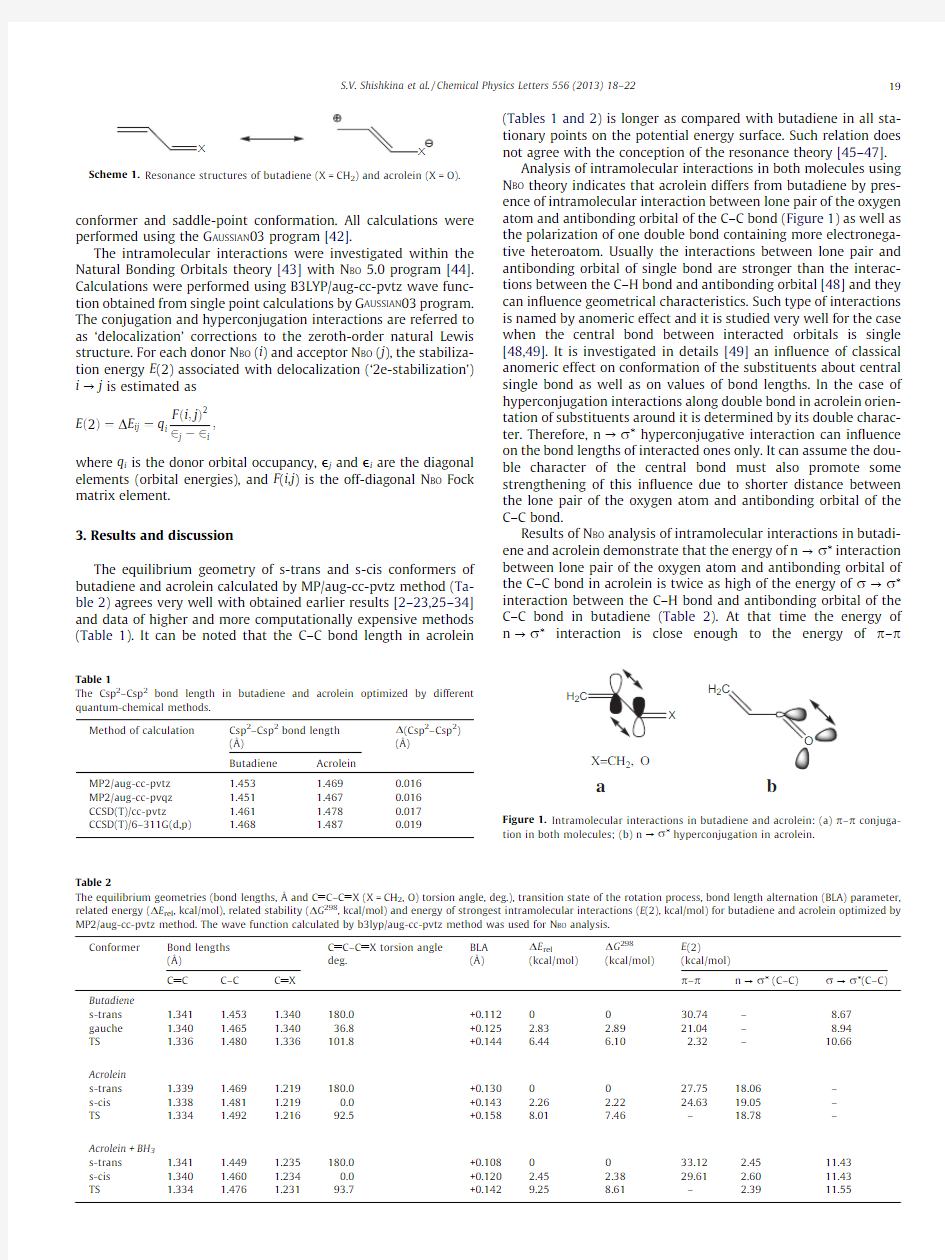
(Tables 1and 2)is longer as compared with butadiene in all sta-tionary points on the potential energy surface.Such relation does not agree with the conception of the resonance theory [45–47].Analysis of intramolecular interactions in both molecules using N BO theory indicates that acrolein differs from butadiene by pres-ence of intramolecular interaction between lone pair of the oxygen atom and antibonding orbital of the C–C bond (Figure 1)as well as the polarization of one double bond containing more electronega-tive https://www.doczj.com/doc/755430757.html,ually the interactions between lone pair and antibonding orbital of single bond are stronger than the interac-tions between the C–H bond and antibonding orbital [48]and they can in?uence geometrical characteristics.Such type of interactions is named by anomeric effect and it is studied very well for the case when the central bond between interacted orbitals is single [48,49].It is investigated in details [49]an in?uence of classical anomeric effect on conformation of the substituents about central single bond as well as on values of bond lengths.In the case of hyperconjugation interactions along double bond in acrolein orien-tation of substituents around it is determined by its double charac-ter.Therefore,n ?r ?hyperconjugative interaction can in?uence on the bond lengths of interacted ones only.It can assume the dou-ble character of the central bond must also promote some strengthening of this in?uence due to shorter distance between the lone pair of the oxygen atom and antibonding orbital of the C–C bond.
Results of N BO analysis of intramolecular interactions in butadi-ene and acrolein demonstrate that the energy of n ?r ?interaction between lone pair of the oxygen atom and antibonding orbital of the C–C bond in acrolein is twice as high of the energy of r ?r ?interaction between the C–H bond and antibonding orbital of the C–C bond in butadiene (Table 2).At that time the energy of n ?r ?interaction is close enough to the energy of p –p
Table 2
The equilibrium geometries (bond lengths,?and C @C–C @X (X =CH 2,O)torsion angle,deg.),transition state of the rotation process,bond length alternation (BLA)parameter,related energy (D E rel ,kcal/mol),related stability (D G 298,kcal/mol)and energy of strongest intramolecular interactions (E (2),kcal/mol)for butadiene and acrolein optimized by MP2/aug-cc-pvtz method.The wave function calculated by b3lyp/aug-cc-pvtz method was used for N BO analysis.Conformer
Bond lengths (?)C @C–C @X torsion angle deg.
BLA (?)
D E rel
(kcal/mol)D G 298
(kcal/mol)
E (2)
(kcal/mol)
C @C
C–C C @X p –p
n ?r ?(C–C)r ?r ?(C–C)
Butadiene s-trans 1.341 1.453 1.340180.0+0.1120030.74–8.67gauche 1.340 1.465 1.34036.8+0.125 2.83 2.8921.04–8.94TS 1.336
1.480
1.336
101.8
+0.144
6.44
6.10
2.32
–
10.66
Acrolein s-trans 1.339 1.469 1.219180.0+0.1300027.7518.06–s-cis 1.338 1.481 1.2190.0+0.143 2.26 2.2224.6319.05–TS
1.334 1.492 1.2169
2.5+0.1588.017.46–18.78–
Acrolein +BH 3s-trans 1.341 1.449 1.235180.0+0.1080033.12 2.4511.43s-cis 1.340 1.460 1.2340.0+0.120 2.45 2.3829.61 2.6011.43TS
1.334 1.476 1.23193.7+0.1429.258.61–
2.3911.55
Table 1
The Csp 2–Csp 2bond length in butadiene and acrolein optimized by different quantum-chemical methods.Method of calculation
Csp 2–Csp 2bond length (?)D (Csp 2–Csp 2)(?)
Butadiene
Acrolein MP2/aug-cc-pvtz 1.453 1.4690.016MP2/aug-cc-pvqz 1.451 1.4670.016CCSD(T)/cc-pvtz
1.461 1.4780.017CCSD(T)/6–311G(d,p)
1.468
1.487
0.019
S.V.Shishkina et al./Chemical Physics Letters 556(2013)18–2219
conjugation between two double bonds.Therefore it can assume that the in?uence of p–p conjugation and n?r?hyperconjuga-tion on the C–C bond length should be comparable.However two strongest intramolecular interactions in the acrolein differ from each other:p–p conjugation between double bonds causes the shortening of the C–C bond in contrary to n?r?hyperconjugation which leads to the elongation of the C–C bond owing to the popu-lation of its antibonding orbital.
Taking into account this situation it is possible to conclude that length of the Csp2–Csp2single bond in acrolein is determined by balance of two opposite factors namely p–p conjugation and n?r?hyperconjugation which may be considered as bonding and antibonding interactions for this bond(Figure1).In this case the length of the Csp2–Csp2bond in acrolein depends on the con-tribution of each of these factors.The changing of the delocaliza-tion of the structures due to in?uence of intramolecular interactions can be analyzed easier by mean of the bond length alternation(BLA)parameter(Table2).The analysis of BLA shows the presence of n?r?hyperconjugative interaction in acrolein what results in the increasing of alternation of double bonds as compare with butadiene.
Clear estimation of in?uence of both interactions on geometri-cal parameters of molecule may be performed by comparison of properties of single C–C bond and BLA parameter in equilibrium s-trans conformation and in situations where one or both intramo-lecular interactions are absent.
It is well known that p–p conjugation between double bonds decreases appreciably up to disappearing(in acrolein)in the tran-sition state for the rotation around single bond process(Figure2). The data of N BO analysis for butadiene and acrolein in the transition state con?rm this evidence(Table2).As expected the absence of p–p conjugation results in the elongation of the Csp2–Csp2bond and increasing of BLA as compare with equilibrium geometry.At that time single C–C bond remains longer in the transition state for acrolein as compare with one for butadiene’s transition state.
1.492
1.469
1.4491.476
π?π is present n σ* is present
π?π is absent
n σ* is absent without π?π
without n σ?
Figure2.In?uence of p–p?conjugation and n?r?hyperconjugation on the C–C
bond length in acrolein.
Table3
The energy(E(2),kcal/mol)of the conjugative(bonding)and hyperconjugative(antibonding)intramolecular interactions in?uencing the Csp2–Csp2bond length in butadiene, acrolein and its complex with BH3.
Molecule Bonding interactions E(2)
(kcal/mol)Antibonding interactions E(2)
(kcal/mol)
Butadiene
s-trans BD(2)C1-C2–BD(2)C3-C430.74BD(1)C1-H5–BD(1)C2-C38.67 BD(1)C2-H7–BD(1)C3-H87.68BD(1)C4-H9–BD(1)C2-C38.67 gauche BD(2)C1-C2–BD(2)C3-C421.04BD(1)C1-H5–BD(1)C2-C38.94 BD(1)C2-H7–BD(1)C3-C4 5.36BD(1)C4-H9–BD(1)C2-C39.01
BD(1)C3-H8–BD(1)C1-C2 5.36
TS BD(1)C1-C2–BD(1)C3-C4 3.5BD(1)C1-H5–BD(1)C2-C310.66 BD(1)C1-C2–BD(2)C3-C4 3.46BD(1)C4-H9–BD(1)C2-C310.66
BD(1)C3-C4–BD(2)C1-C2 3.46
BD(2)C1-C2–BD(2)C3-C4 2.32
BD(1)C3-H8–BD(2)C1-C29.57
BD(1)C2-H7–BD(2)C3-C49.57
Acrolein
s-trans BD(1)C1-C2–BD(1)C3-O4 2.73BD(1)C1-H5–BD(1)C2-C38.25 BD(2)C1-C2–BD(2)C3-O427.75LP(2)O4–BD(1)C2-C318.06
BD(1)C2-H7–BD(1)C3-H8 5.95
s-cis BD(2)C1-C2–BD(2)C3-O424.63BD(1)C1-H5–BD(1)C2-C38.76 BD(1)C2-H7–BD(1)C3-O4 4.05LP(2)O4–BD(1)C2-C319.05
BD(1)C3-H8–BD(1)C1-C2 5.01
TS BD(1)C1-C2–BD(2)C3-O4 2.98BD(1)C1-H5–BD(1)C2-C39.07 BD(1)C3-O4–BD(2)C1-C2 4.48LP(2)O4–BD(1)C2-C318.78
BD(1)C3-H8–BD(2)C1-C2 5.85
BD(1)C2-H7–BD(2)C3-O4 6.16
Acrolein+BH3
s-trans BD(1)C1-C2–BD(1)C3-O4 3.07BD(1)C1-H6–BD(1)C2-C38.09 BD(2)C1-C2–BD(2)C3-O433.12BD(1)C2-C3–BD(1)O4-B511.43
BD(1)C2-H8–BD(1)C3-H9 5.97LP(1)O4–BD(1)C2-C3 2.45 s-cis BD(1)C1-C2–BD(1)C3-H9 4.98BD(1)C1-H6–BD(1)C2-C38.51 BD(2)C1-C2–BD(2)C3-O429.61BD(1)C2-C3–BD(1)O4-B511.43
BD(1)C2-H8–BD(1)C3-O4 4.41LP(1)O4–BD(1)C2-C3 2.60 TS BD(1)C1-C2–BD(1)C3-O40.55BD(1)C1-H6–BD(1)C2-C39.05 BD(1)C1-C2–BD(2)C3-O4 3.01BD(1)C2-C3–BD(1)O4-B511.55
BD(2)C1-C2–BD(1)C3-O4 4.90LP(1)O4–BD(1)C2-C3 2.39
BD(1)C3-H9–BD(2)C1-C2 6.13
BD(1)C2-H8–BD(2)C3-O4 6.88
20S.V.Shishkina et al./Chemical Physics Letters556(2013)18–22
It is additional argument about the in?uence of n?r?hypercon-jugation on the C–C bond length through the C@O double bond.
In contrary to p–p conjugation n?r?hyperconjugation is present in all stationary points on the potential energy surface for acrolein(Table2).But this interaction can be shielded by for-mation of dative bond involving lone pair of the oxygen atom and unoccupied orbital of Lewis acid,for example,BH3.The formed O–B bond has r-character and the energy of its interaction with antibonding orbital of the central C–C bond is very close to the en-ergy of similar C–H?r?(C–C)interaction in butadiene(Table2). The absence of n?r?hyperconjugation results signi?cant short-ening of the Csp2–Csp2bond and decreasing of BLA in all stationary points for acrolein.It is more interesting that the C–C bond in acro-lein becomes shorter and p–p conjugation becomes stronger as compare with ones in butadiene in the case of absence of n?r?hyperconjugative interaction(Table2)what agrees well with the resonance theory.This evidence is con?rmed also by values of BLA parameter.
It is very interesting the situation when both strong intramolec-ular interactions are absent namely acrolein with shielded by BH3 lone pair in the transition state for the rotation process.In absence of p–p conjugative and n?r?hyperconjugative interactions the C–C bond length is almost equal to mean value for length of this bond for s-trans and s-cis conformers of acrolein with both interac-tions(Table2).This fact con?rms that the C–C bond length in acro-lein in the equilibrium state is determined by balance of p–p conjugation and n?r?hyperconjugation.
Taking into account the opposite in?uence of two types of intra-molecular interactions on the C–C bond one may assume that its length depends on the difference in energy of bonding and antibonding interactions for this bond.In such case all bonding for C–C bond and antibonding for it interactions(Table3)must be taken into account.Specially,this is important for transition states where p–p conjugative interaction is minimal and r(c-H)–p interaction appears instead it.This interaction has bond-
ing for Csp2–Csp2bond character and it is weaker as compare with p–p interaction.Analysis of relation between C–C bond length and total energy of intramolecular interaction in?uencing on it demon-strates good correlation between them(Figure3)with correlation coef?cient aboutà0.93.
The barrier of the rotation around ordinary C–C bond is also sensitive to intramolecular interactions.The absence of n?r?hyperconjugation in acrolein results the increase of conjugation in molecule what leads to the increase of the rotation barrier (Table2).
4.Conclusions
Results of quantum-chemical calculations demonstrate the structure of acrolein does not correspond to conventional views about in?uence of the polarization of p-system by the oxygen atom.According to classic viewpoint this effect should lead to in-crease of conjugation between double bonds and shortening of central single C–C bond as compared to butadiene.However,anal-ysis of intramolecular interactions shows that the geometry of acrolein is determined by counteraction of p–p conjugation and n?r?hyperconjugation.The energies of these interactions are very close but ones in?uence on the C–C bond lengths in opposite directions.Conjugation promotes the shortening of the central sin-gle bond due to the overlapping of the p-orbitals of two double bonds.In the contrary the n?r?hyperconjugation causes the elongation of the C–C bond due to the population of its antibonding orbital.The absence of conjugation in the transition state for the rotation about the C–C bond process results in the elongation of the single bond in conjugated system.In turn the shielding of n?r?hyperconjugation by the formation of dative bond between lone pair of oxygen atom and vacant orbital of Lewis acid causes the shortening of the C–C bond in acrolein.The C–C bond length correlates well with the difference between two strong intramolec-ular interactions.The absence of both interactions does not almost change the C–C bond length.Thus,these data clearly indicate that molecular structure of conjugated systems containing heteroatoms is determined by not only p–p conjugation but also by n?r?hyperconjugation.
References
[1]F.A Carey,R.J.Sundberg,Advanced Organic Chemistry.Part A:Structure and
Mechanisms,Springer,Virginia,2007.
[2]Yu.N.Panchenko,Yu.A.Pentin,V.I.Tyulin,V.M.Tatevskii,Opt.Spectrosc.13
(1962)488.
[3]A.R.H.Cole,G.M.Mohay,G.A.Osborne,Spectrochim.Acta23A(1967)909.
[4]K.Kuchitsu,T.Fukuyama,Y.Morino,J.Mol.Struct.1(1967–1968)463.
[5]R.L.Lipnick,E.W.Garbisch Jr.,J.Am.Chem.Soc.95(1973)6370.
[6]Yu.N.Panchenko,Spectrochim.Acta31A(1975)1201.
[7]Yu.N.Panchenko,A.V.Abramenkov,V.I.Mochalov,A.A.Zenkin,G.Keresztury,
G.J.Jalsovszky,J.Mol.Spectrosc.99(1983)288.
[8]W.Caminati,G.Grassi,A.Bauder,Chem.Phys.Lett.148(1988)13.
[9]M.E.Squillacote,T.C.Semple,P.W.Mui,J.Am.Chem.Soc.107(1985)6842.
[10]Y.Furukawa,H.Takeuchi,I.Harada,M.Tasumi,Bull.Chem.Soc.Jpn.56(1983)
392.
[11]B.R.Arnold,V.Balaji,J.W.Downing,J.G.Radziszewski,J.J.Fisher,J.Michl,J.Am.
Chem.Soc.113(1991)2910.
[12]J.Saltiel,J.-O.Choi,D.F.Sears Jr.,D.W.Eaker,F.B.Mallory,C.W.Mallory,J.Phys.
Chem.98(1994)13162.
[13]K.W.Wiberg,R.E.Rosenberg,J.Am.Chem.Soc.112(1990)1509.
[14]J.Saltiel,D.F.Sears Jr,A.M.Turek,J.Phys.Chem.A105(2001)7569.
[15]M.S.Deleuze,S.Knippenberg,J.Chem.Phys.125(2006)104309-1.
[16]P.Boopalachandran,N.C.Craig,https://www.doczj.com/doc/755430757.html,ane,J.Phys.Chem.A116(2012)271.
[17]H.Guo,M.Karplus,J.Chem.Phys.94(1991)3679.
[18]R.Hargitai,P.G.Szalay,G.Pongor,G.Fogarasi,J.Mol.Struct.(THEOCHEM)112
(1994)293.
[19]G.R.De Maré,Yu.N.Panchenko,J.V.Auwera,J.Phys.Chem.A101(1997)3998.
[20]J.C.Sancho-García,A.J.Pérez-Jiménez,F.Moscardó,J.Phys.Chem.A105(2001)
11541.
[21]N.C.Craig,P.Groner,D.C.McKean,J.Phys.Chem.A110(2006)7461.
[22]D.Feller,K.A.Peterson,J.Chem.Phys.126(2007)114105.
[23]D.Feller,N.C.Craig,A.R.Maltin,J.Phys.Chem.A112(2008)2131.
[24]D.Feller,N.C.Craig,J.Phys.Chem.A113(2009)1601.
[25]H.-B.Burgi,J.D.Dunitz,Structure Correlation,vol.2,VCH,Weinheim,1994.
[26]K.B.Wiberg,R.E.Rosenberg,P.R.Rablen,J.Am.Chem.Soc.113(1991)2890.
[27]K.B.Wiberg,P.R.Rablen,M.Marquez,J.Am.Chem.Soc.114(1992)8654.
[28]K.Kuchitsu,T.Fukuyama,Y.Morino,J.Mol.Struct.1(1967–1968)463.
[29]G.Celebre,M.Concistré,G.DeLuca,M.Longeri,G.Pileio,J.W.Emsley,Chem.
Eur.J.11(2005)3599.
[30]R.J.Loncharich,T.R.Schwartz,K.N.Houk,J.Am.Chem.Soc.109(1987)14.
[31]G.R.DeMare,Yu.N.Panchenko,A.J.Abramenkov,J.Mol.Struct.160(1987)327.
S.V.Shishkina et al./Chemical Physics Letters556(2013)18–2221
[32]G.R.DeMare,Can.J.Chem.63(1985)1672.
[33]Y.Osamura,H.F.Schaefer III,J.Chem.Phys.74(1981)4576.
[34]C.E.Bolm,A.Bauder,Chem.Phys.Lett.88(1982)55.
[35]B.Mannfors,J.T.Koskinen,L.-O.Pietil?,L.Ahjopalo,J.Mol.Struct.(THEOCHEM)
393(1997)39.
[36]J.I.García,J.A.Mayoral,L.Salvatella,X.Assfeld,M.F.Ruiz-López,J.Mol.Struct.
(THEOCHEM)362(1996)187.
[37]S.V.Shishkina,O.V.Shishkin,S.M.Desenko,J.Leszczynski,J.Phys.Chem.A112
(2008)7080.
[38]C.M?ller,M.S.Plesset,Phys.Rev.46(1934)618.
[39]R.A.Kendall,T.H.Dunning Jr.,R.J.Harrison,J.Chem.Phys.96(1992)6792.
[40]W.H.Hehre,L.Radom,P.V.R.Schleyer,J.A.Pople,Ab initio Molecular Orbital
Theory,Wiley,New York,1986.
[41]P.Culot,G.Dive,V.H.Nguyen,J.M.Ghuysen,Theor.Chim.Acta82(1992)189.[42]M.J.Frisch et al.,G AUSSIAN,Inc.,Wallingford CT,2004.
[43]F.Weinhold,in:P.V.R.Schleyer,N.L.Allinger,T.Clark,J.Gasteiger,P.A.
Kollman,H.F.Schaefer III,P.R.Schreiner(Eds.),Encyclopedia of Computational Chemistry,vol.3,John Wiley&Sons,Chicheste,UK,1998.1792–1792. [44]E.D.Glendening,J.K.Badenhoop,A.E.Reed,J.E.Carpenter,J.A.Bohmann,C.M.
Morales,F.Weinhold,N BO5.0Theoretical Chemistry Institute,University of Wisconsin,Madison,WI,2001.
[45]E.D.Glendening,F.Weinhold,https://www.doczj.com/doc/755430757.html,put.Chem.19(1998)593.
[46]E.D.Glendening,F.Weinhold,https://www.doczj.com/doc/755430757.html,put.Chem.19(1998)610.
[47]E.D.Glendening,J.K.Badenhoop,F.Weinhold,https://www.doczj.com/doc/755430757.html,put.Chem.19(1998)628.
[48]A.J.Kirby,Stereoelectronic Effects,Oxford University Press,New York,1996.
[49]I.V.Alabugin,K.M.Gilmore,P.W.Peterson,WIREs Computational Molecular
Science1(2011)109.
22S.V.Shishkina et al./Chemical Physics Letters556(2013)18–22
Heavy Oil Development Technology of Liaohe Oilfield Han Yun (Scientific Research Information Department Exploration&Development Research Institute,Liaohe Oilfield Company) Liaohe Oilfield,the largest heavy oil production base in China,features in various reservoir types,deep burial,and wide range of crude oil viscosity.For many years,a series of technologies have been developed for different oil products and reservoir types of the oilfield,of which water flooding,foam slug drive,steam stimulation,steam drive,and SAGD are the main technologies. After continuous improvement,they have been further developed and played an important role in the development of heavy oil in the oilfield. Liaohe Oilfield is abundant in heavy oil resources,46%of the total proved reserves of Liaohe Oilfield Company. Horizontally the resources concentrates in the West Depression and the southern plunging belt of the Central Uplift in Liaohe Rift. Vertically,it is mainly distributed in Paleocene Shahejie Formation(ES). The distinctive geological feature of Liaohe 0ilfield is manifested in three aspects:first,the heavy oil reservoirs are deeply buried and 80%of them are buried more than 900m deep;second,the heavy oil viscosity ranges widely.For most of the reservoirs.the dead oil viscosity ranges in 100~100000mPa·s with the maximum 650000mPa·s.Third the reservoir types are various with complicated oil—water relationship,most of the reservoirs are edge water and bosom water reservoirs and there are also edge water reservoirs,top water reservoirs and bosom water reservoirs.For more than 20 years of development,Liaohe Oilfield has developed series of heavy oil development technologies for different oil products and different types of reservoirs,such as water flooding, foam slug drive,steam stimulation steam drive and SAGD.The most difficult issues have been overcome in the development of the super
超详细中英文论文参考文献标准格式 1、参考文献和注释。按论文中所引用文献或注释编号的顺序列在论文正文之后,参考文献之前。图表或数据必须注明来源和出处。 (参考文献是期刊时,书写格式为: [编号]、作者、文章题目、期刊名(外文可缩写)、年份、卷号、期数、页码。参考文献是图书时,书写格式为: [编号]、作者、书名、出版单位、年份、版次、页码。) 2、附录。包括放在正文内过份冗长的公式推导,以备他人阅读方便所需的辅助性数学工具、重复性数据图表、论文使用的符号意义、单位缩写、程序全文及有关说明等。 参考文献(即引文出处)的类型以单字母方式标识,具体如下: [M]--专著,著作 [C]--论文集(一般指会议发表的论文续集,及一些专题论文集,如《***大学研究生学术论文集》[N]-- 报纸文章 [J]--期刊文章:发表在期刊上的论文,尽管有时我们看到的是从网上下载的(如知网),但它也是发表在期刊上的,你看到的电子期刊仅是其电子版 [D]--学位论文:不区分硕士还是博士论文 [R]--报告:一般在标题中会有"关于****的报告"字样 [S]-- 标准 [P]--专利 [A]--文章:很少用,主要是不属于以上类型的文章 [Z]--对于不属于上述的文献类型,可用字母"Z"标识,但这种情况非常少见 常用的电子文献及载体类型标识: [DB/OL] --联机网上数据(database online) [DB/MT] --磁带数据库(database on magnetic tape) [M/CD] --光盘图书(monograph on CDROM) [CP/DK] --磁盘软件(computer program on disk) [J/OL] --网上期刊(serial online) [EB/OL] --网上电子公告(electronic bulletin board online) 很显然,标识的就是该资源的英文缩写,/前面表示类型,/后面表示资源的载体,如OL表示在线资源 二、参考文献的格式及举例 1.期刊类 【格式】[序号]作者.篇名[J].刊名,出版年份,卷号(期号)起止页码. 【举例】 [1] 周融,任志国,杨尚雷,厉星星.对新形势下毕业设计管理工作的思考与实践[J].电气电子教学学报,2003(6):107-109. [2] 夏鲁惠.高等学校毕业设计(论文)教学情况调研报告[J].高等理科教育,2004(1):46-52. [3] Heider, E.R.& D.C.Oliver. The structure of color space in naming and memory of two languages [J]. Foreign Language Teaching and Research, 1999, (3): 62 67. 2.专著类
五、外文资料翻译 Stress and Strain 1.Introduction to Mechanics of Materials Mechanics of materials is a branch of applied mechanics that deals with the behavior of solid bodies subjected to various types of loading. It is a field of study that i s known by a variety of names, including “strength of materials” and “mechanics of deformable bodies”. The solid bodies considered in this book include axially-loaded bars, shafts, beams, and columns, as well as structures that are assemblies of these components. Usually the objective of our analysis will be the determination of the stresses, strains, and deformations produced by the loads; if these quantities can be found for all values of load up to the failure load, then we will have obtained a complete picture of the mechanics behavior of the body. Theoretical analyses and experimental results have equally important roles in the study of mechanics of materials . On many occasion we will make logical derivations to obtain formulas and equations for predicting mechanics behavior, but at the same time we must recognize that these formulas cannot be used in a realistic way unless certain properties of the been made in the laboratory. Also , many problems of importance in engineering cannot be handled efficiently by theoretical means, and experimental measurements become a practical necessity. The historical development of mechanics of materials is a fascinating blend of both theory and experiment, with experiments pointing the way to useful results in some instances and with theory doing so in others①. Such famous men as Leonardo da Vinci(1452-1519) and Galileo Galilei (1564-1642) made experiments to adequate to determine the strength of wires , bars , and beams , although they did not develop any adequate theo ries (by today’s standards ) to explain their test results . By contrast , the famous mathematician Leonhard Euler(1707-1783) developed the mathematical theory any of columns and calculated the critical load of a column in 1744 , long before any experimental evidence existed to show the significance of his results ②. Thus , Euler’s theoretical results remained unused for many years, although today they form the basis of column theory. The importance of combining theoretical derivations with experimentally determined properties of materials will be evident theoretical derivations with experimentally determined properties of materials will be evident as we proceed with
华北电力大学科技学院 毕业设计(论文)附件 外文文献翻译 学号:121912020115姓名:彭钰钊 所在系别:动力工程系专业班级:测控技术与仪器12K1指导教师:李冰 原文标题:Infrared Remote Control System Abstract 2016 年 4 月 19 日
红外遥控系统 摘要 红外数据通信技术是目前在世界范围内被广泛使用的一种无线连接技术,被众多的硬件和软件平台所支持。红外收发器产品具有成本低,小型化,传输速率快,点对点安全传输,不受电磁干扰等特点,可以实现信息在不同产品之间快速、方便、安全地交换与传送,在短距离无线传输方面拥有十分明显的优势。红外遥控收发系统的设计在具有很高的实用价值,目前红外收发器产品在可携式产品中的应用潜力很大。全世界约有1亿5千万台设备采用红外技术,在电子产品和工业设备、医疗设备等领域广泛使用。绝大多数笔记本电脑和手机都配置红外收发器接口。随着红外数据传输技术更加成熟、成本下降,红外收发器在短距离通讯领域必将得到更广泛的应用。 本系统的设计目的是用红外线作为传输媒质来传输用户的操作信息并由接收电路解调出原始信号,主要用到编码芯片和解码芯片对信号进行调制与解调,其中编码芯片用的是台湾生产的PT2262,解码芯片是PT2272。主要工作原理是:利用编码键盘可以为PT2262提供的输入信息,PT2262对输入的信息进行编码并加载到38KHZ的载波上并调制红外发射二极管并辐射到空间,然后再由接收系统接收到发射的信号并解调出原始信息,由PT2272对原信号进行解码以驱动相应的电路完成用户的操作要求。 关键字:红外线;编码;解码;LM386;红外收发器。 1 绪论
Inventory management Inventory Control On the so-called "inventory control", many people will interpret it as a "storage management", which is actually a big distortion. The traditional narrow view, mainly for warehouse inventory control of materials for inventory, data processing, storage, distribution, etc., through the implementation of anti-corrosion, temperature and humidity control means, to make the custody of the physical inventory to maintain optimum purposes. This is just a form of inventory control, or can be defined as the physical inventory control. How, then, from a broad perspective to understand inventory control? Inventory control should be related to the company's financial and operational objectives, in particular operating cash flow by optimizing the entire demand and supply chain management processes (DSCM), a reasonable set of ERP control strategy, and supported by appropriate information processing tools, tools to achieved in ensuring the timely delivery of the premise, as far as possible to reduce inventory levels, reducing inventory and obsolescence, the risk of devaluation. In this sense, the physical inventory control to achieve financial goals is just a means to control the entire inventory or just a necessary part; from the perspective of organizational functions, physical inventory control, warehouse management is mainly the responsibility of The broad inventory control is the demand and supply chain management, and the whole company's responsibility. Why until now many people's understanding of inventory control, limited physical inventory control? The following two reasons can not be ignored: First, our enterprises do not attach importance to inventory control. Especially those who benefit relatively good business, as long as there is money on the few people to consider the problem of inventory turnover. Inventory control is simply interpreted as warehouse management, unless the time to spend money, it may have been to see the inventory problem, and see the results are often very simple procurement to buy more, or did not do warehouse departments . Second, ERP misleading. Invoicing software is simple audacity to call it ERP, companies on their so-called ERP can reduce the number of inventory, inventory control, seems to rely on their small software can get. Even as SAP, BAAN ERP world, the field of
在广州甚至广东的住宅小区电气设计中,一般都会涉及到小区的高低压供配电系统的设计.如10kV高压配电系统图,低压配电系统图等等图纸一大堆.然而在真正实施过程中,供电部门(尤其是供电公司指定的所谓电力设计小公司)根本将这些图纸作为一回事,按其电脑里原有的电子档图纸将数据稍作改动以及断路器按其所好换个厂家名称便美其名曰设计(可笑不?),拿出来的图纸根本无法满足电气设计的设计意图,致使严重存在以下问题:(也不知道是职业道德问题还是根本一窍不通) 1.跟原设计的电气系统货不对板,存在与低压开关柜后出线回路严重冲突,对实际施工造成严重阻碍,经常要求设计单位改动原有电气系统图才能满足它的要求(垄断的没话说). 2.对消防负荷和非消防负荷的供电(主要在高层建筑里)应严格分回路(从母线段)都不清楚,将消防负荷和非消防负荷按一个回路出线(尤其是将电梯和消防电梯,地下室的动力合在一起等等,有的甚至将楼顶消防风机和梯间照明合在一个回路,以一个表计量). 3.系统接地保护接地型式由原设计的TN-S系统竟曲解成"TN-S-C-S"系统(室内的还需要做TN-C,好玩吧?),严格的按照所谓的"三相四线制"再做重复接地来实施,导致后续施工中存在重复浪费资源以及安全隐患等等问题.. ............................(违反建筑电气设计规范等等问题实在不好意思一一例举,给那帮人留点混饭吃的面子算了) 总之吧,在通过图纸审查后的电气设计图纸在这帮人的眼里根本不知何物,经常是完工后的高低压供配电系统已是面目全非了,能有百分之五十的保留已经是谢天谢地了. 所以.我觉得:住宅建筑电气设计,让供电部门走!大不了留点位置,让他供几个必需回路的电,爱怎么折腾让他自个怎么折腾去.. Guangzhou, Guangdong, even in the electrical design of residential quarters, generally involving high-low cell power supply system design. 10kV power distribution systems, such as maps, drawings, etc. low-voltage distribution system map a lot. But in the real implementation of the process, the power sector (especially the so-called power supply design company appointed a small company) did these drawings for one thing, according to computer drawings of the original electronic file data to make a little change, and circuit breakers by their the name of another manufacturer will be sounding good design (ridiculously?), drawing out the design simply can not meet the electrical design intent, resulting in a serious following problems: (do not know or not know nothing about ethical issues) 1. With the original design of the electrical system not meeting board, the existence and low voltage switchgear circuit after qualifying serious conflicts seriously hinder the actual construction, often require changes to the original design unit plans to meet its electrical system requirements (monopoly impress ). 2. On the fire load and fire load of non-supply (mainly in high-rise building in) should be strictly sub-loop (from the bus segment) are not clear, the fire load and fire load of non-qualifying press of a circuit (especially the elevator and fire elevator, basement, etc.
https://www.doczj.com/doc/755430757.html,/finance/company/consumer.html Consumer finance company The consumer finance division of the SG group of France has become highly active within India. They plan to offer finance for vehicles and two-wheelers to consumers, aiming to provide close to Rs. 400 billion in India in the next few years of its operations. The SG group is also dealing in stock broking, asset management, investment banking, private banking, information technology and business processing. SG group has ventured into the rapidly growing consumer credit market in India, and have plans to construct a headquarters at Kolkata. The AIG Group has been approved by the RBI to set up a non-banking finance company (NBFC). AIG seeks to introduce its consumer finance and asset management businesses in India. AIG Capital India plans to emphasize credit cards, mortgage financing, consumer durable financing and personal loans. Leading Indian and international concerns like the HSBC, Deutsche Bank, Goldman Sachs, Barclays and HDFC Bank are also waiting to be approved by the Reserve Bank of India to initiate similar operations. AIG is presently involved in insurance and financial services in more than one hundred countries. The affiliates of the AIG Group also provide retirement and asset management services all over the world. Many international companies have been looking at NBFC business because of the growing consumer finance market. Unlike foreign banks, there are no strictures on branch openings for the NBFCs. GE Consumer Finance is a section of General Electric. It is responsible for looking after the retail finance operations. GE Consumer Finance also governs the GE Capital Asia. Outside the United States, GE Consumer Finance performs its operations under the GE Money brand. GE Consumer Finance currently offers financial services in more than fifty countries. The company deals in credit cards, personal finance, mortgages and automobile solutions. It has a client base of more than 118 million customers throughout the world
Foreign material: Chemical Industry 1.Origins of the Chemical Industry Although the use of chemicals dates back to the ancient civilizations, the evolution of what we know as the modern chemical industry started much more recently. It may be considered to have begun during the Industrial Revolution, about 1800, and developed to provide chemicals roe use by other industries. Examples are alkali for soapmaking, bleaching powder for cotton, and silica and sodium carbonate for glassmaking. It will be noted that these are all inorganic chemicals. The organic chemicals industry started in the 1860s with the exploitation of William Henry Perkin’s discovery if the first synthetic dyestuff—mauve. At the start of the twentieth century the emphasis on research on the applied aspects of chemistry in Germany had paid off handsomely, and by 1914 had resulted in the German chemical industry having 75% of the world market in chemicals. This was based on the discovery of new dyestuffs plus the development of both the contact process for sulphuric acid and the Haber process for ammonia. The later required a major technological breakthrough that of being able to carry out chemical reactions under conditions of very high pressure for the first time. The experience gained with this was to stand Germany in good stead, particularly with the rapidly increased demand for nitrogen-based compounds (ammonium salts for fertilizers and nitric acid for explosives manufacture) with the outbreak of world warⅠin 1914. This initiated profound changes which continued during the inter-war years (1918-1939). Since 1940 the chemical industry has grown at a remarkable rate, although this has slowed significantly in recent years. The lion’s share of this growth has been in the organic chemicals sector due to the development and growth of the petrochemicals area since 1950s. The explosives growth in petrochemicals in the 1960s and 1970s was largely due to the enormous increase in demand for synthetic polymers such as polyethylene, polypropylene, nylon, polyesters and epoxy resins. The chemical industry today is a very diverse sector of manufacturing industry, within which it plays a central role. It makes thousands of different chemicals which
英语毕业论文引用和参考文献格式 英语专业毕业论文引用和参考文献格式采用APA格式及规。 一、文中夹注格式 英语学位论文引用别人的观点、方法、言论必须注明出处,注明出处时使用括号夹注的方法(一般不使用脚注或者尾注),且一般应在正文后面的参考文献中列出。关于夹注,采用APA格式。 (一)引用整篇文献的观点 引用整篇文献(即全书或全文)观点时有两种情况: 1.作者的姓氏在正文中没有出现,如: Charlotte and Emily Bronte were polar opposites, not only in their personalities but in their sources of inspiration for writing (Taylor, 1990). 2. 作者的姓氏已在正文同一句中出现,如: Taylor claims that Charlotte and Emily Bronte were polar opposites, not only in their personalities but in their sources of inspiration for writing (1990). 3. 如果作者的姓氏和文献出版年份均已在正文同一句中出现,按APA的规不需使用括号夹注,如: In a 1990 article, Taylor claims that Charlotte and Emily Bronte were polar opposites, not only in their personalities but in their sources of inspiration for writing. 4. 在英文撰写的论文中引用中文著作或者期刊,括号夹注中只需用汉语拼音标明作者的姓氏,不得使用汉字,如:(Zhang, 2005) (二)引用文献中具体观点或文字 引用文献中某一具体观点或文字时必须注明该观点或者该段文字出现的页码出版年份,没有页码是文献引用不规的表现。 1.引用一位作者的文献 (1)引用容在一页,如: Emily Bronte “expressed increasing hostility for the world of human relationships, whether sexual or social” (Taylor, 1988:11). (2)引用容在多页上,如: Newmark (1988:39-40) notes three characteristically expressive text-types: (a) serious imaginative literature (e.g. lyrical poetry); (b) authoritative statements (political speeches and documents, statutes and legal documents, philosophical and academic works by acknowledged authorities); (c) autobiography, essays, personal correspondence (when these are personal effusions).
International Journal of Minerals, Metallurgy and Materials Volume 17, Number 4, August 2010, Page 500 DOI: 10.1007/s12613-010-0348-y Corresponding author: Zhuan Li E-mail: li_zhuan@https://www.doczj.com/doc/755430757.html, ? University of Science and Technology Beijing and Springer-Verlag Berlin Heidelberg 2010 Preparation and properties of C/C-SiC brake composites fabricated by warm compacted-in situ reaction Zhuan Li, Peng Xiao, and Xiang Xiong State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China (Received: 12 August 2009; revised: 28 August 2009; accepted: 2 September 2009) Abstract: Carbon fibre reinforced carbon and silicon carbide dual matrix composites (C/C-SiC) were fabricated by the warm compacted-in situ reaction. The microstructure, mechanical properties, tribological properties, and wear mechanism of C/C-SiC composites at different brake speeds were investigated. The results indicate that the composites are composed of 58wt% C, 37wt% SiC, and 5wt% Si. The density and open porosity are 2.0 g·cm–3 and 10%, respectively. The C/C-SiC brake composites exhibit good mechanical properties. The flexural strength can reach up to 160 MPa, and the impact strength can reach 2.5 kJ·m–2. The C/C-SiC brake composites show excellent tribological performances. The friction coefficient is between 0.57 and 0.67 at the brake speeds from 8 to 24 m·s?1. The brake is stable, and the wear rate is less than 2.02×10?6 cm3·J?1. These results show that the C/C-SiC brake composites are the promising candidates for advanced brake and clutch systems. Keywords: C/C-SiC; ceramic matrix composites; tribological properties; microstructure [This work was financially supported by the National High-Tech Research and Development Program of China (No.2006AA03Z560) and the Graduate Degree Thesis Innovation Foundation of Central South University (No.2008yb019).] 温压-原位反应法制备C / C-SiC刹车复合材料的工艺和性能 李专,肖鹏,熊翔 粉末冶金国家重点实验室,中南大学,湖南长沙410083,中国(收稿日期:2009年8月12日修订:2009年8月28日;接受日期:2009年9月2日) 摘要:采用温压?原位反应法制备炭纤维增强炭和碳化硅双基体(C/C-SiC)复合材