Lineage Divergence at the First TCR-Dependent Checkpoint
- 格式:pdf
- 大小:2.74 MB
- 文档页数:12

恐龙进化鸟的过程英语作文The Avian Legacy: The Evolutionary Journey from Dinosaurs to Birds.The avian lineage, characterized by its unmistakable feathers and flight capabilities, traces its evolutionary roots deep into the realm of dinosaurs. The remarkable transformation from towering, thunderous giants to the graceful soarers of modern skies is a testament to the astonishing power of adaptation and the relentless march of time.Theropods: The Avian Precursors.The origins of birds lie within the theropod group, a diverse assemblage of carnivorous dinosaurs that roamed the earth during the Mesozoic Era. These bipedal creatures exhibited several key features that would later pave the way for avian evolution.Feathers: While feathers are commonly associated with birds, their origins can be traced back to theropods. Primitive feathers emerged as small, quill-like structures used for insulation and display.Hollow Bones: Theropods possessed hollow bones, a characteristic that reduced their skeletal weight and facilitated locomotion, particularly running and leaping.Three-Toed Feet: The feet of theropods featured three weight-bearing toes, an arrangement that enhanced their agility and became the foundation of the avian foot structure.The Rise of Proto-Birds.The gradual transition from theropods to birds began with the emergence of proto-birds, such as Archaeopteryx lithographica. This iconic creature, dating back to around 150 million years ago, possessed a captivating mosaic of reptilian and avian traits.Feathered Forelimbs: Archaeopteryx bore elongated, pennaceous feathers on its forelimbs, resembling the wings of modern birds.Long Tail: Unlike modern birds, Archaeopteryx retained a relatively long, reptilian tail, indicating an intermediate stage in the evolution of avian flight.Teeth and Claws: Archaeopteryx's jaws were equipped with small, serrated teeth, while its feet still bore sharp claws, vestiges of its theropod ancestry.The Advent of True Birds.The divergence of theropods into true birds, or Ornithurae, marked a significant milestone in avian evolution. Ornithurans, such as Confuciusornis sanctus, exhibited more advanced flight adaptations and reduced reptilian features.Flight Feathers: The forelimbs of ornithurans had evolved into fully functional wings, with distinct flightfeathers arranged in an aerodynamic configuration.Reduced Tail: The tail of ornithurans had shortened considerably, becoming a characteristic of modern birds' compact, streamlined bodies.Absence of Teeth: Ornithurans had lost their teeth, a specialization that allowed for a lighter skull and greater flight efficiency.From Ground to Sky.The evolution of birds from dinosaurs was not a linear progression but rather a complex evolutionary tapestry woven over millions of years. Natural selection favored the survival and reproduction of individuals with traits that enhanced their ability to escape predators, secure food, and endure environmental challenges.Arboreal Adaptations: Many proto-birds likely spent extended periods in trees, using their feathered forelimbs for balance and gliding.Predator Avoidance: Flight provided an effective means of evading larger predators, enabling proto-birds to access new food sources and habitats.Dietary Specialization: The loss of teeth led to ashift in diet towards smaller prey, such as insects, fruits, and seeds.The Legacy of Dinosaurs.Today, birds thrive in a myriad of habitats worldwide, carrying the remarkable legacy of their dinosaur ancestors. From the majestic eagles soaring high above to the tiny hummingbirds flitting through flowers, the avian lineage stands as a testament to the extraordinary power ofevolution's transformative dance.Through their remarkable journey, birds have not only inherited the traits that once defined their theropod predecessors but have also forged their own unique evolutionary path. As we marvel at the beauty and diversityof the avian world, let us never forget the incredible ancestry that shapes their existence.。
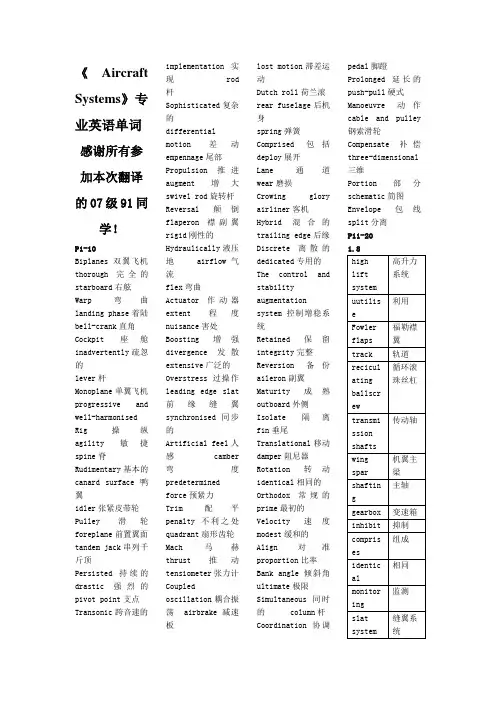
《Aircraft Systems》专业英语单词感谢所有参加本次翻译的07级91同学!P1-10Biplanes 双翼飞机thorough完全的starboard右舷Warp 弯曲landing phase着陆bell-crank直角Cockpit座舱inadvertently疏忽的lever杆Monoplane单翼飞机progressive and well-harmonised Rig操纵agility敏捷spine脊Rudimentary基本的canard surface鸭翼idler张紧皮带轮Pulley滑轮foreplane前置翼面tandem jack串列千斤顶Persisted持续的drastic强烈的pivot point支点Transonic跨音速的implementation实现 rod杆Sophisticated复杂的differentialmotion差动empennage尾部Propulsion推进augment增大swivel rod旋转杆Reversal颠倒flaperon襟副翼rigid刚性的Hydraulically液压地airflow气流flex弯曲Actuator作动器extent程度nuisance害处Boosting增强divergence发散extensive广泛的Overstress过操作leading edge slat前缘缝翼synchronised同步的Artificial feel人感camber弯度predeterminedforce预紧力Trim配平penalty不利之处quadrant扇形齿轮Mach马赫thrust推动tensiometer张力计Coupledoscillation耦合振荡 airbrake减速板lost motion滞差运动Dutch roll荷兰滚rear fuselage后机身spring弹簧Comprised包括deploy展开Lane通道wear磨损Crowing gloryairliner客机Hybrid混合的trailing edge后缘Discrete离散的dedicated专用的The control andstabilityaugmentationsystem控制增稳系统Retained保留integrity完整Reversion备份aileron副翼Maturity成熟outboard外侧Isolate隔离fin垂尾Translational移动damper阻尼器Rotation转动identical相同的Orthodox常规的prime最初的Velocity速度modest缓和的Align对准proportion比率Bank angle倾斜角ultimate极限Simultaneous同时的 column杆Coordination协调pedal脚蹬Prolonged延长的push-pull硬式Manoeuvre动作cable and pulley钢索滑轮Compensate补偿three-dimensional三维Portion部分schematic简图Envelope包线split分离P11-201.8P20-30Screwjack 螺旋千斤顶The aerodynamic load 气动载荷Displacement 排水量Velocity 速度The null position 复位Torque 扭矩Encompass 涵盖Algorithm 算法State-of-the-art现代Remainder 剩余部分Quiescent 静止的Jack ram 连杆Jam 卡住Exception 特例Retract 缩回Reduction 减速Rotary motion 旋转运动Bi-directional 双向的Windage 气流Rudimentary 基本的Hydrostatic 流体静力学的液体德尔Coincide 相符的Fixed displacement Hyd Pump 固定位移液压泵Spoiler 扰流板elevator 升降舵rudder 方向舵segregate 分开隔开P30-401.11.2airbusimplementation空客配置concorde 协和式飞机utilise/utilize利用tabulate 列成表格aileron 副翼roll spoiler 滚转扰流板tailplane trim 尾部配平slat 缝翼flap 襟翼reversionary mode继承模式hydraulic powersystem 液压动力系统actuator 做动器notation 符号注释ground spoilermode 着陆扰流板模式load alleviationmode 减轻负载模式conventional yawdamper function常规偏航阻尼功能certification证明tailplanehorizontalactuator 机尾水平做动器contemporary同时代的longitudinalstability margin纵向稳定裕度relaxed stability放宽静稳定性autopilot 自动驾驶仪standby mode 备用模式clutch 抓住抓紧flight controldata concentrator飞行控制数据聚集器maintenance 维修保养full-spanleading-edge slat满量程前缘缝翼trailing-edgeflap 后缘襟翼standalone unit单机独立单元subsume 把···归类包含lineage 家系,世系,系列1.12generalize 归纳authority 权限summarise 总结coordinationalgorithm 协调算法rudimentary 基本的初步的pedal 脚蹬踏板facilitate 使容易1.13derive 得到来自electro-hydrostatic actuator 电静液做动器electrical backuphydraulic actuator电备份液压做动器1.14venture 冒险敢于interface 分界面界面接口triple redundant三倍冗余module 模数模量舱deck 舱面coupler 连接器分配器耦合器flaperon 襟腹翼solenoid 螺线管energise 供给能量通电calculation 计算结果subsequent 随后的后来的simplex 单一的单纯的indefinitely 不明确地无限定地diapatch 派遣发出1.15generic 普通的一般的trajectory 轨道datum 数据equivalent 同等的panel 仪器板execute 执行vertical 垂直的3-dimensional 三维的terminal 最后的终点的MCDU 多功能控制显示单元FMS 飞行管理系统AFDS 自动驾驶仪指引系统FBW 电传系统2.1aviation aircraft航空飞机engine mounted fuel filter 发动机装用燃油滤清器upper wing sections 前翼部分the flight crew空勤人员的multi-valve control means 多阀控制方式jet turbine powered aircraft 喷气涡轮动力航空piston -engined aircraft 活塞式飞机sortie出击;突围。


小学上册英语第二单元期中试卷英语试题一、综合题(本题有100小题,每小题1分,共100分.每小题不选、错误,均不给分)1.What is the name of the famous tree in the Bible?A. Fig TreeB. Olive TreeC. Tree of KnowledgeD. Cedar Tree2.What is the name of the process by which plants lose water?A. AbsorptionB. RespirationC. TranspirationD. EvaporationC3.My mom enjoys gardening and planting ____ (vegetables).4.What do we call the act of traveling to different countries?A. ExploringB. AdventuringC. TouringD. VacationingC5.What is the name of the famous wizarding school in Harry Potter?A. DurmstrangB. BeauxbatonsC. HogwartsD. Ilvermorny6.The _____ (玩具枪) makes laser sounds.7.funding proposal) seeks financial support for projects. The ____8.What do you call a young ant?A. LarvaB. PupaC. WorkerD. Queen9.What do we celebrate on Christmas?A. New YearB. BirthdayC. HolidayD. Jesus' birthD10.The Doppler effect explains how sound changes as an object ______.11.Plants need __________ (阳光) to grow well.12.I drink _____ (water/coffee) with lunch.13. A bison is often seen in ______ (草原).14.The flowers in the vase are very _______ (花瓶里的花非常_______).15. A ________ (植物管理策略) is essential for success.16.________ (植物适应性分析项目) foster understanding.17.The ancient __________ (中国) invented gunpowder.18.My mom is a great __________ (家庭主妇).19.The __________ was an important period of historical change in Europe. (工业革命)20.What is the main gas in the air we breathe?A. OxygenB. NitrogenC. Carbon dioxideD. HeliumB21.What do you call a person who studies animals?A. ZoologistB. BiologistC. EcologistD. EthologistA22.The rabbit is ___. (hopping)23. A ____(land use) plan guides development.24.The __________ (历史的重要性) can’t be understated.25.The chemical formula for oleic acid is ______.26. A __________ can often be seen perched on a branch.27.The _______ (小瓢虫) has spots on its back.28.The macaw has colorful _________. (羽毛)29.We go swimming in the ___ (pool).30.The mountain is _____ (high/low).31. A _____ (植物生活) reflects the interdependence of all life forms.32.My favorite snack is ________ (薯片).33.I love to listen to ______ (音乐) while I study.34.Mountains usually have steep ______.35.ts are used in ______ for their medicinal properties. (某些植物因其药用特性而用于传统医学。

When it comes to weddings, the world is a vast tapestry of diverse traditions and customs. The East and the West, though separated by oceans and continents, both hold the celebration of marriage in high regard, yet the ways in which they do so are as different as the cultures themselves. Having attended both Eastern and Western weddings, I can attest to the unique charm and significance each has to offer.In the East, particularly in countries like China and India, weddings are often grand affairs that can last for days. The ceremonies are steeped in tradition and are deeply symbolic. For instance, in a traditional Chinese wedding, the bride and groom are adorned in red, the color of prosperity and good fortune. The ceremony might include a tea ceremony where the couple serves tea to their elders as a sign of respect and gratitude. This act is not just a ritual but a profound expression of filial piety and the continuity of family lineage.Contrastingly, Western weddings, especially in countries like the United States and the United Kingdom, tend to be more intimate and focused on the couple. The bride typically walks down the aisle to meet her groom, surrounded by the gaze of loved ones. The ceremony is often held in a church or other sacred place, symbolizing the sanctity of the union. The exchange of vows and rings is a pivotal moment, signifying the couples commitment to one another.One of the most striking differences between Eastern and Western weddings is the role of the family. In Eastern cultures, the family plays a central role in the wedding process. From the initial matchmaking to thedowry and the wedding banquet, the familys involvement is integral. Its not uncommon for entire villages to come together to celebrate the union, making it a community event as much as a family one.On the other hand, Western weddings often emphasize the couples autonomy. While family is certainly important, the focus is on the love and commitment between the bride and groom. The wedding is a celebration of their decision to join lives, and the ceremony is a personal and intimate affair.Cultural symbolism also varies greatly. In Eastern weddings, rituals are laden with meaning. For example, in a Hindu wedding, the couple circles a sacred fire while reciting vows, symbolizing their commitment to walk together through lifes journey. In contrast, Western weddings often include readings, prayers, and hymns that reflect the couples spiritual beliefs and the sanctity of their union.The reception, too, is a point of divergence. Eastern receptions are typically large, festive gatherings with an abundance of food, music, and dance. Its a time for joyous celebration and communal feasting. In contrast, Western receptions are often more structured, with speeches, the first dance, and cutting of the cake as highlights of the event.Despite these differences, there is a common thread that runs through all weddings, East and West: the celebration of love and the coming together of two hearts. Whether its the exchange of vows under a floweradorned arch in a Western church or the intricate rituals of an Eastern ceremony,the essence of the occasion remains the same.In my experience, attending a wedding is not just about witnessing the union of two people its about immersing oneself in the rich tapestry of a cultures traditions. Each wedding Ive attended has been a lesson in the diversity of human expression of love and commitment. Its a reminder that, despite our differences, we all share the universal desire to celebrate love and form lasting bonds.In conclusion, while Eastern and Western weddings differ in many ways, they are both beautiful expressions of the joyous occasion of marriage. Each has its own unique charm and significance, and to experience both is to appreciate the richness of our global community. As we continue to share and learn from one anothers customs, we enrich not only our understanding of different cultures but also our own lives.。

东西方文化差异对待老人的英语作文全文共3篇示例,供读者参考篇1The Differences in How the East and West Treat the ElderlyAs a student who has had the opportunity to learn about various cultures around the world, one of the most striking differences I've noticed is in how the elderly are viewed and treated in Eastern societies compared to Western ones. This divergence stems from the fundamentally different value systems and cultural traditions that have shaped these two broad regions over centuries.In many Eastern cultures, particularly those influenced by Confucian philosophy such as China, Japan, and Korea, there is a strong emphasis on filial piety – the virtue of respect and obedience towards one's parents and elderly family members. This deep-rooted value has its origins in ancient teachings that stress the importance of hierarchy, duty, and honoring one's ancestors.For instance, in Chinese culture, the elderly hold a revered position in the family structure. They are seen as the bearers ofwisdom, tradition, and the family's legacy. It is expected that younger generations will care for their aged parents and grandparents, often living together in multigenerational households. The elderly are consulted on major family decisions and their advice is highly valued.Similarly, in India, the concept of "Matri Devo Bhava, Pitri Devo Bhava" – which translates to "revere your mother as a goddess, revere your father as a god" – is deeply ingrained in the cultural psyche. The elderly are treated with the utmost respect and their blessings are considered auspicious for important life events.This reverence for the aged is also reflected in the way many Eastern languages use honorific titles and special pronouns when addressing or referring to elders, further reinforcing the societal norm of showing deference to them.In contrast, Western cultures, particularly those influenced by Judeo-Christian traditions and Enlightenment values, tend to place a greater emphasis on individualism, self-reliance, and nuclear family structures. While respect for the elderly is still expected, the level of veneration and duty towards them may not be as pronounced as in Eastern societies.In the United States and many European nations, for instance, it is common for the elderly to live independently or in assisted living facilities once they reach a certain age or level of frailty. The primary responsibility for their care often falls on professional caregivers or the state, rather than being an assumed duty of the younger family members.This cultural difference can sometimes lead to intergenerational tensions or conflicts, particularly among immigrant families trying to navigate the contrasting values of their Eastern heritage and Western adoptive home.However, it is important to note that these are broad generalizations, and there are exceptions and nuances within both Eastern and Western cultures. For example, some Western societies, such as those in Southern Europe, tend to have stronger multigenerational family ties and place a higher value on caring for the elderly within the family unit.Additionally, the rapid pace of modernization and urbanization in many Eastern countries has led to a gradual erosion of traditional values, with some younger generations prioritizing individual aspirations over filial obligations.Ultimately, how societies treat their elderly is a complex issue shaped by a multitude of factors, including economicrealities, social safety nets, and evolving cultural norms. As the world becomes increasingly interconnected, it is crucial for different cultures to learn from one another's strengths and find a balance between honoring tradition and adapting to changing circumstances.From a personal perspective, I believe that both Eastern and Western cultures have valuable lessons to offer when it comes to caring for the elderly. The Eastern emphasis on filial piety and reverence for elders can help foster stronger intergenerational bonds and ensure that the aged are treated with dignity and respect.At the same time, the Western focus on individual autonomy and professional care can help ensure that the elderly have access to high-quality services and are empowered to make decisions about their own well-being.Perhaps the ideal approach lies in finding a harmonious middle ground – one that upholds the cultural values of honoring and caring for the elderly, while also providing them with the independence and support they need to lead fulfilling lives in their later years.As the world's population continues to age, it is imperative that we have these important conversations and learn from oneanother's cultural wisdom to create societies that truly cherish and care for our elders.篇2The Contrasting Views on Aging: East vs WestAs a student interested in cross-cultural studies, I have long been fascinated by the dramatically different philosophies and attitudes that Eastern and Western societies have towards aging and the elderly. Having been raised in an Eastern culture myself, I was taught from a young age to respect and revere the elderly. However, after traveling and studying abroad in Western nations, I've come to realize just how starkly these two worlds diverge when it comes to their perceptions of old age.In the East, aging is seen through an almost celebratory lens – a Badge of Honor earned through decades of hard work, life experience, and perseverance. The elderly are esteemed as founts of wisdom, honored for their years, and valued for the guidance they can provide to younger generations. In countries like China, Japan, and India, it is considered disgraceful to shun or neglect one's aging parents or grandparents. The idea of packing off the elderly to nursing homes or assisted living facilities is viewed as a profound cultural taboo and moral failing.Instead, in these Eastern civilizations, the multi-generational family unit is the norm, with grandparents commonly living under the same roof as their children and grandchildren. The elderly are meant to be integrated fully into the family circle, their needs tended to by their offspring, who view this duty as a solemn obligation and privilege. Many of my Asian friends recount with pride how their grandmothers taught them traditional recipes, songs, or crafts – passing down an inextricable link to their heritage.This profound deference to the elderly is rooted in the core philosophies and belief systems of the East, which advocate filial piety and the preservation of family lineage above all else. Confucian and Buddhist teachings, which have held sway over cultures like China and Japan for millennia, place an immense value on respect for ancestors, deference to age and hierarchy, and maintaining strong intergenerational bonds. As a Chinese proverb states: "Having been cared for by them as children, we must return this favor in their old age."On the other side of the globe, however, Western attitudes towards aging and the elderly are notably different – and in many cases, jarringly opposed to those of the East. In nations like the United States, United Kingdom, and across much of Europe,youth is prized and glorified. We are endlessly bombarded with anti-aging product advertisements extolling us to battle the "scourge" of wrinkles, grey hairs, and other outward signs of growing old.Rather than being venerated, aging in the West is often viewed in a more skeptical, clinical light – as a deterioration of physical and mental faculties to be feared or overcome through medical and cosmetic interventions. This mindset stems largely from the West's long tradition of Individualism, where theself-determining individual reigns supreme over collective familial obligations. There is a greater sense that the elderly have "lived their life" and should now get out of the way and make room for the young and able-bodied.As such, the Western model typically embraces the widespread institutionalization of the elderly into nursing homes, retirement villages, and assisted living facilities once they reach an age where independent living becomes difficult. While often done with the best of intentions in terms of providing specialized care, this practice has also been criticized as a way for societies to quarantine and marginalize the aging – sweeping them to the fringes, out of sight and mind from the mainstream.During my time abroad, I was admittedly shocked to encounter retirement communities where there was little to no interaction between the elderly residents and younger generational cohorts. To my Eastern sensibilities, this sequestering of the aged away from the family unit represented a profound cultural disconnect – a deprivation of the warmth, vibrancy, and sense of purpose that multi-generationalco-existence can provide.Of course, I don't mean to paint an overly rosy picture of attitudes in the East either. With rapid urbanization and the erosion of traditional family structures in many Asian nations, there are increasing incidents of elder abandonment and neglect as well. The shift towards isolated nuclear family units often leaves little room for caring for aging parents – a sad reality I witnessed firsthand in my grandparents' twilight years.Nonetheless, even while contending with these modern challenges, the cultural backbone and deeply-ingrained values surrounding reverence for the elderly still remain remarkably intact across the Eastern world. I've seen how festivals like the Qingming tomb-sweeping holidays in China keep ancestor veneration alive. I've marveled at elderly Japanese couples stillholding hands, their familial devotion untarnished after decades of marriage.In the West, while there have been efforts to address ageism and bolster community services for seniors, the overall societal veneration of youth culture seems to carry the day. The elderly are too often cast aside to fend for themselves or shunted off to institutional facilities out of convenience or discomfort in dealing with the realities of aging and death. We could stand to take a page from the Eastern playbook in terms of re-integrating the elderly back into the warm embrace of the family collective.At the end of the day, while modernization has inevitably disrupted certain traditional norms, I believe both the East and West have wisdom to impart when it comes to caring for the aged with dignity and purpose. The East's deep-rooted filial philosophies remind us to cherish the elderly as vital fonts of our cultural lineage. But the West's medicalized view of aging as a new frontier to conquer could also potentially mitigate some of the suffering and indignities that come with old age.Perhaps an ideal solution lies in borrowing from the strengths of both value systems – creating a harmonic model where the elderly can age at home amid the loving care of their families, while simultaneously having access to cutting-edgehealthcare, community support networks, and quality-of-life resources. By bridging these cultural gaps surrounding attitudes towards aging, we may find newfound reverence and appreciation for those who have walked the path of life before us.篇3The Contrasting Attitudes: How East and West View the ElderlyAs a student hailing from an Asian country, I have always been intrigued by the stark differences between Eastern and Western cultures, particularly in their attitudes towards the elderly. Growing up in a society deeply rooted in Confucian values, I witnessed firsthand the reverence and respect accorded to the aged. However, upon interacting with peers from Western backgrounds, I realized that their perspectives on this matter diverged significantly from my own.In many Eastern cultures, the elderly are regarded as repositories of wisdom and experience, deserving of utmost veneration. This stems from the traditional belief that age equates to knowledge accumulated over a lifetime. Elders are seen as living embodiments of a community's history, custodiansof ancestral traditions, and beacons of guidance for the younger generation. Their counsel is sought on matters ranging from familial disputes to major life decisions, and their words carry immense weight.This reverence manifests itself in various cultural practices. For instance, in my home country, it is customary for the younger members of a family to greet their elders with a respectful bow or to address them using honorific titles. Caring for aging parents or grandparents is not merely a responsibility but an inherent duty, deeply ingrained in the fabric of our society. It is considered a grave dishonor to neglect or abandon one's elderly relatives, as they are viewed as the foundation upon which the family's legacy is built.In stark contrast, Western attitudes towards the elderly often prioritize independence and self-reliance. While respect for the aged is certainly present, it is tempered by a strong emphasis on individual autonomy and privacy. The elderly are encouraged to maintain their independence for as long as possible, residing in their own homes or retirement communities, rather than relying heavily on familial support.This divergence in perspectives can be traced back to the individualistic nature of Western societies, where personalfreedom and self-determination are highly valued. The elderly are celebrated for their ability to live independently, free from the burden of relying on others. Institutionalized care facilities, while not necessarily the norm, are more readily accepted as a viable option for those who require assistance.Additionally, the fast-paced lifestyle and mobile nature of Western societies can contribute to a perceived disconnect between generations. As families become more dispersed geographically, the close-knit multigenerational households that are prevalent in many Eastern cultures become less common. This physical separation can sometimes lead to a diminished sense of obligation towards caring for the elderly within the family unit.It is important to note, however, that these cultural differences are not absolute, and there are variations within both Eastern and Western societies. Globalization and cultural exchange have blurred the lines, with some Eastern countries adopting more Western-influenced attitudes towards aging, and vice versa. Additionally, individual families may deviate from societal norms based on their unique circumstances and personal values.Personally, I find both perspectives valuable and worthy of consideration. The Eastern emphasis on filial piety and respect for elders is admirable, recognizing the invaluable contributions and wisdom of the aged. However, the Western promotion of independence and self-determination also holds merit, allowing the elderly to maintain their dignity and autonomy for as long as possible.Perhaps the ideal lies in striking a balance, where the elderly are revered and their counsel sought, while also empowering them to live independently to the extent that they desire. Intergenerational bonds should be nurtured, fostering mutual understanding and respect between the young and old. Simultaneously, support systems should be in place to assist the elderly when needed, without compromising their sense of independence and agency.As the world becomes increasingly globalized, it is crucial for us to engage in cross-cultural dialogue and learn from the diverse perspectives on aging. By embracing the strengths of both Eastern and Western approaches, we can create a more compassionate and inclusive society that honors and uplifts the elderly, ensuring their well-being and dignity are preserved.。


物种概念及其界定龚佐山;买买提明·苏来曼【摘要】半世纪以来,物种概念的定义备受关注,不同研究方向的生物学家提出24种不同或至少有分歧的物种概念,根据其不同的物种概念,物种的界定和物种的数量会出现很大的差异.人们普遍认同:物种是进化分离的微居群谱系,但把谱系分离过程中获得的特征如生殖隔离、可鉴定性、单系统发生等视为鉴定物种次级特征却有不同的声音.该文提出统一的物种概念,把谱系进化分离作为物种界定的唯一而又必要的特征,把谱系分离过程中获得的次级特征作为界定谱系分离的证据.鉴于此,物种概念间的分歧就会化解.其一,物种概念化与物种界定明显分开,不再混淆;其二,谱系的次级特征只与物种界定有关,在某种程度上为谱系分离提供证据;第三,若能把合理解释的任何一个特征作为某物种客观存在的证据,这样更多的特征更能确定谱系分离;最后最重要的是,统一物种概念使我们解放思想,扬弃传统的物种界定标准,探求物种界定的新思路.%From the half century to now,the issue of species delimitation has long been confused by species conceptu alization, according to controversy species concepts,the boundaries and numbers of species could be great difference. Alternative species concepts agree in treating existence as a separately evolving metapopulation lineage as the primary defining property of the species category, but adopting different properties acquired by lineages during the course of divergence(e. G. Intrinsic reproductive isolation,diagnosability,monophyly)as secondary species criteria causes differ ent sounds. The paper gave a unified species concept treating a separately evolving metapopulation lineage as the only necessary property of species,and secondary species criteria as differentlines of evidence relevant to assessing lineage separation. This unified concept of species had several consequences for species delimitation as follows: first,the is sues of species conceptualization and species delimitation were clearly separated; the secondary species criteria were no longer considered relevant to species conceptualization but only to species delimitation. Second,all of the properties formerly treated as secondary species criteria were relevant to species delimitation to the extent that they provide evi dence of lineage separation. Third,if possible,the presence of any one of the properties was evidence for the existence of aspecies,though more properties and thus more lines of evidence are associated with a higher degree of corrobora tion;Lastly,the unified species made ones liberate minds from the traditional species criteria,not tied to those properties,and develop new methods of species delimitation.【期刊名称】《广西植物》【年(卷),期】2012(032)002【总页数】6页(P274-279)【关键词】物种概念;物种界定;统一【作者】龚佐山;买买提明·苏来曼【作者单位】新疆大学,生命科学与技术学院,乌鲁木齐市,830046;安徽省霍邱县第一中学,安徽,六安市,237400;新疆大学,生命科学与技术学院,乌鲁木齐市,830046【正文语种】中文【中图分类】Q111.21物种是生物学功能单位之一,尤其在系统生物学上,与基因、细胞、器官等各级结构和功能单位同等重要(de Queiroz,2005)。

男女性格的差异英语作文Gender Differences in Personality。
Introduction。
Throughout history, there has been an ongoing debate about the differences between men and women, including their personalities. While some argue that these differences are solely due to societal expectations and gender roles, others believe that there are inherent biological and psychological disparities between the two genders. This essay aims to explore the various factors that contribute to gender differences in personality, including biological, evolutionary, and social aspects.Biological Factors。
One of the primary explanations for gender differences in personality lies in biological factors. Research has shown that hormonal differences between men and women playa significant role in shaping their personalities. For instance, testosterone, which is more prevalent in men, has been linked to traits such as assertiveness, dominance, and risk-taking behavior. On the other hand, estrogen, found in higher levels in women, is associated with nurturing, empathy, and emotional sensitivity.These hormonal differences are believed to originate from evolutionary processes. Throughout history, men were traditionally the hunters and protectors, requiring traits such as aggression and competitiveness to succeed in these roles. Women, on the other hand, were primarily responsible for nurturing and caring for children, leading to the development of traits such as empathy and compassion. These biological differences, shaped by evolution, have contributed to the divergence in personality traits observed between men and women.Evolutionary Factors。

2024—2025学年度七年级英语下学期期末考试卷(含答案)(考试时间:90分钟试卷满分:120分,含听力)听力试题一、短对话理解(共8小题;每小题1. 5分,共12分)听下面8段短对话。
每段对话后有一道小题,从每题所给的A、B、C三个选项中选出最佳选项。
听完每段对话后,你将有10秒钟的时间来回答有关小题和阅读下一小题。
每段对话仅读一遍。
1. What is Sam doing?A. Playing football.B. Taking a walk.C. Watching TV.2. How far is Jasmine's home from her school?A. 3 kilometres.B. 5 kilometres.C. 7 kilometres.3. Where did Tom go on a trip?A. To Beijing.B. To Shenyang.C. To Shanghai.4. When is Kate's birthday?A. This Thursday.B. This Saturday.C. This Sunday.5. What animal is the story about?A. An elephant.B. A cat.C. A panda.6. What are the speakers mainly talking about?A. Pine trees.B. Chinese medicine.C. Furniture.7. Who is thin?A. Tyler.B. Tyler's daughter.C. Tyler's son.8. Why does Jack think Sunshine Cinema is the best?A. Because it's the cheapest.B. Because it has comfortable seats.C. Because it has the biggest screen.二、长对话理解(共12小题,每小题1. 5分;共18分)听下面4段长对话。

姓名,年级:时间:第六模块测评(时间:120分钟满分:150分)第一部分:听力(共两节,满分30分)第一节(共5小题;每小题1.5分,满分7.5分)听下面5段对话。
每段对话后有一个小题,从题中所给的A、B、C三个选项中选出最佳选项,并标在试卷的相应位置。
听完每段对话后,你都有10秒钟的时间来回答有关小题和阅读下一小题。
每段对话仅读一遍。
1.What is the relationship between the speakers?A.Strangers.B.Friends。
C.Brother and sister.2.What’s the man going to do?A.He is going to have something to eat.B.He is looking for a hotel。
C.He is trying to find the nearest street.3。
Where does the woman want to go?A。
The farm. B。
The factory. C。
The hospital。
4.What does the woman want to do?A。
She wants to take a bus.B.She wants to buy something。
C。
She wants to make friends with the man。
5.What is the woman probably going to do?A.She may be going to buy a book.B.She may be going to watch a play.C.She may be going to watch a football match.第二节(共15小题;每小题1.5分,满分22.5分)听下面5段对话或独白。
每段对话或独白后有几个小题,从题中所给的A、B、C三个选项中选出最佳选项,并标在试卷的相应位置。
普陀区2019学年第一学期高三英语质量调研英语试卷考生注意:1. 考试时间120分钟,试卷满分140分。
2. 本次考试设试卷和答题纸两部分。
所有答题必须涂(选择题)或写(非选择题)在答题纸上,做在试卷上一律不得分。
3. 答题前,务必在答题纸上填写准考证号和姓名,并将核对后的条形码贴在指定位置上, 在答题纸反面清楚地填写姓名。
I. Listening ComprehensionSection ADirections: In Section A, you will hear ten short conversations between two speakers. At the end of each conversation, a question will be asked about what was said. The conversations and the questions will be spoken only once. After you hear a conversation and the question about it, read the four possible answers on your paper, and decide which one is the best answer to the question you have heard.1. A. She is going to Thailand. B. She is going on vacation.C. She likes collecting postcards.D. She has traveled all over the world.2. A. To go out to have a cup of coffee. B. To enjoy the coffee in the office.C. To make a cup of coffee for him.D. To help him finish the program.3. A. In a civil court. B. In a cybercafé. C. At a sports club. D. At a theatre.4. A. Engineering. B. Geography. C. Math. D. Physics.5. A. 14:00. B. 17:00 C. 18:00. D: 19:00.6. A. The man will pick up Professor Rice at her office.B. The man didn’t expect his paper to be graded so soon.C. Professor Rice has given the man a very high grade.D. Professor Rice won’t see her student in her office.7. A. She had to be a liar sometimes. B. She is required to be slim.C. She had little chance for promotion.D. Her salary is not satisfactory.8. A. There was no park nearby.B. The woman hasn’t seen the film yet.C. The weather wasn’t ideal for a walk.D. It would be easier to go to the cinema.9. A. Dr. White comes from Greece.B. The woman couldn't understand Greek at all.C. The woman didn’t follow the professor’s explanation.D. Dr. White talked about the geography of Greece yesterday.10. A. It is more comfortable and convenient to take a bus.B. It is worth the money taking a plane to Vancouver.C. It is not always more expensive going by air.D. It is faster to go to Vancouver by bus.Section BDirections: In Section B, you will hear two short passages and one longer conversation, and you will be asked several questions on each of the passages and the conversation. The passages and the conversation will be read twice, but the questions will be spoken only once. When you hear a question, read the four possible answers on your paper and decide which one would be the best answer to the question you have heard.Questions 11 through 13 are based on the following passage.11. A. Babies have the ability to learn before birth.B. Newborn babies are influenced by mothers’ ability.C. Newborn babies can recognize the sounds of their mother.D. Babies only want food and to be kept warm and dry.12. A. By 18 months of age. B. By 6 months of age.C. By two years of age.D. By one year of age.13. A. They can recognize the different surroundings.B. They can identify the sounds of the mother tongue.C. They can imitate the sounds of the second language.D. They can differ the sounds of two different languages.Questions 14 through 16 are based on the following passage.14. A. To form an official league team. B. To join the Organization Earth.C. To win the world championship.D. To compete with Gree ce’s best teams.15. A. A luxurious life is no longer a dream.B. Life in the refugee camp is at times tense.C. The players care more about their racial identity.D. There are fewer fights between people of different races.16. A. Organization Earth is composed of refugees.B. The love for the football brings the refugees together.C. Greek government provides support for football training.D. Hope Refugee United has beaten the Greece’s best team.Questions 17 through 20 are based on the following conversation.17. A. A tourist guidebook. B. An annual traveler report.C. A travelling magazine.D. An airport ranking list.18. A. 3 weeks. B. 13 days. C. 31 hours. D. 3 hours.19. A. To illustrate the poor service.B. To state the cause of the delay.C. To praise the kindness of other passengers.D. To complain about the position of the Gate.20. A. They provide useless directions and services.B. They are completely indifferent to travelers’ needs.C. They are extremely caring a bout passengers’ safety.D. They provide the wrong address of the nearby hospital.II. Grammar and vocabularySection ADirections: After reading the passage below, fill in the blanks to make the passage coherent and grammatically correct. For the blanks with a given word, fill in each blank with the proper form of the given word; for the other blanks, use one word that best fits each blank.Surprise! A New PenguinA team of scientists in New Zealand recently came across the remains of a previously unknown species of , penguin—by mistake. The discovery of the Waitaha penguin species, which has been extinct for 500 years, is excitingnews for the scientific community ___1___ it gives new insight into how past extinction events can help shape thepresent environment.The researchers uncovered the Waitaha penguin remains while studying New Zealand’s rare yellow-eyedpenguin. The team wanted to investigate the effects ___2___ humans have had on the now endangered species. They studied centuries-old bones from ___3___ they thought were yellow-eyed penguins and compared them with the bones of modern yellow-eyed penguins. Surprisingly some of the bones were older than ___4___ (expect). Even more shockingly, the DNA in the bones indicated that they did not belong to yellow-eyed penguins. The scientists concluded that these very old bones ___5___ have belonged to a previously unknown species, which they named the Waitaha penguin.By studying the bones, scientists further concluded that the Waitaha penguin was once native ___6___ New Zealand. But after the settlement of humans on the island country, its population ___7___ (wipe) out.Based on the ages of the bones of both penguin species, the team discovered a gap in time between the disappearance of the Waitaha and the arrival of the yellow-eyed penguin. The time gap indicates that the extinction of the Waitaha penguin created the opportunity for the yellow-eyed penguin population ___8___ (migrate) to New Zealand.___9___ yellow-eyed penguins thrived (兴盛)in New Zealand for many years, that species now also faces extinction. The yellow-eyed penguin today is considered one of the world’s ___10___ (rare) species of penguin, with an estimated population of 7,000 that is now the focus of an extensive conservation effort in New Zealand.【答案】1. because/since/as2. that/ which3. what4. expected5. must6. to7. was wiped8. to migrate9. Though/ Although/While10. rarest【解析】本文是一篇说明文,介绍了科学家发现了已经灭绝了500年的怀塔哈企鹅物种以及这一发现的意义。
基因研究发现狗起源于中国-考研英语阅读Man’s Best Friend Came From China; Report Traces Origin of Dogs to East AsiaA new scientific research report has found strong evidence suggesting that man's best friend originated from China some 33,000 years ago.一项最新的科学研究发现了有力证据,证明人类最好的朋友——狗,起源于大约3.3万年前的中国The study, the findings of which were published in the science journal Cell Research, found that Chinese indigenous dogs represent an intermediate form between wolves and breed dogs, and that they have not experienced intense artificial selection.研究结果已发表在科学杂志《细胞研究》上。
该研究称,中国本地狗是狼与狗的中间体,没有经历过激烈的人工选择。
"Analyses of Chinese indigenous dogs therefore allow us to stratify the domestication process in dogs, and investigate the role of positive selection that occurred specifically during the first stage of domestication," said the report.报告称:“因此,对中国本地狗的分析让我们可以对狗的驯化过程进行分类,进而调查“正向选择”在驯化第一阶段发挥的具体作用”。
浙江大学生态学研究所---浙江大学植物科学研究所;教育部濒危动植物保护生物学重点实验室浙江大学国家濒危野生动植物种质基因保护中心植物系统进化与生物多样性研究室(浙江省卫生厅药用植物资源学重点学科)傅承新教授、邱英雄教授、赵云鹏副教授、姜维梅讲师、叶文博士后生命科学学院生物科学系,植物学课程组(《植物学及系统进化》浙江省优秀教学团队)傅承新教授、邱英雄教授、赵云鹏副教授、黄爱军副教授、姜维梅讲师、蒋金火讲师、叶文博士后2012年工作进展与总结I, 教学 Teaching1.完成授课《植物学及实验》甲、乙,大类课程,300多人优,良《普通生物学》大类课程,400多人,优,良《进化生物学》,专业,74人,《植物系统与进化》,硕、博士选修课程,13人《生命科学基础》医学求是班 18人 3周课程《植物学野外实习》,天目山实习,45人浙江大学-北卡《华东联合野外生物学实习》国际化课程,5-6月中国 18人浙江大学-北卡-哈佛《美国毕业外植物-生态野外实习》国际课程,7-8月美国 12人指导毕业论文9人(玛青、唐雨卉、王纵潮、徐文英;城市学院:5人裘琦箐、徐婧、方燕婷、张浩杰、施斌豪、张品)2.承担教学项目 Teaching Research Programs天目山国家生物学野外实习基地建设项目,国家基金委,2011-14;植物学建设10万元《植物系统与进化原理》研究生课程获校研究生示范课程项目资助,2009-2012,5万元。
《大类基础课植物学研究化、国际化的改革与实践》浙江大学教学成果奖培育项目1万元;《校内实习基地建设》项目,2012-2013, 3万元姜维梅老师继续参与中学生物学联赛的辅导工作;3.教学成果《植物系统与进化原理》研究生课程获校研究生示范课程项目10月结题获得优秀;《大类基础课植物学研究化、国际化的改革与实践》获浙江大学教学成果奖一等奖;《生物学野外实习基地建设共享探索与实践》获浙江大学教学成果一等奖,现已报浙江省, 本室是主要参与单位。
拼读乌龟的英语作文Title: The Enigmatic Tale of the Tortoise: A Linguistic Exploration。
In the annals of zoological taxonomy, few creatures embody the aura of mystique and ancient wisdom quite like the tortoise. This enigmatic reptile, with its armoredshell and deliberate gait, has captivated human imagination for centuries. In English, the term "tortoise" is not only a name but a portal into the realms of language, culture, and folklore.To understand the linguistic tapestry surrounding the word "tortoise," one must embark on a journey through time and space, traversing continents and epochs. The etymology of "tortoise" can be traced back to the Latin word "tortuca," which in turn derives from the Late Latin "tartaruchus," signifying a kind of turtle. This lineage reflects the enduring influence of Latin on the English language, a testament to the interconnectedness of humancivilizations.Across the English-speaking world, variations of the term "tortoise" abound, each imbued with its own nuances and connotations. In British English, the word "tortoise" typically refers to land-dwelling turtles, whereas in American English, it is often used interchangeably with "turtle" to denote both terrestrial and aquatic species. This subtle divergence highlights the fluidity of language and the cultural specificities that shape linguistic usage.Beyond its lexical significance, the tortoise occupies a prominent place in literature, mythology, and symbolism. From Aesop's fables to Lewis Carroll's whimsical tales, the tortoise serves as a recurring motif, symbolizing patience, wisdom, and endurance. In Chinese culture, the tortoise is revered as a symbol of longevity and prosperity, its shell adorned with intricate patterns representing the cosmic order.In scientific discourse, the tortoise is classified within the order Testudines, encompassing various speciescharacterized by their bony shell and slow metabolism. This taxonomical classification underscores the evolutionary adaptations that have enabled tortoises to thrive in diverse habitats, from arid deserts to lush rainforests. Despite their sluggish demeanor, tortoises exhibit remarkable resilience and adaptability, traits that have ensured their survival for millions of years.The cultural significance of the tortoise extends beyond its terrestrial existence, permeating the realms of art, philosophy, and spirituality. In Native American mythology, the World Turtle upholds the earth upon its shell, symbolizing the interconnectedness of all living beings. In Hindu cosmology, the tortoise incarnates as Kurma, the second avatar of the god Vishnu, supporting the celestial churning of the ocean of milk.In contemporary discourse, the plight of tortoises is emblematic of broader ecological concerns, includinghabitat loss, climate change, and poaching. Conservation efforts aimed at protecting endangered species such as the Galápagos giant tortoise underscore the urgent need forenvironmental stewardship and collective action. By raising awareness and fostering empathy for these ancient creatures, we can strive to safeguard the biodiversity of our planetfor future generations.In conclusion, the word "tortoise" serves as alinguistic gateway to explore the rich tapestry of human culture, history, and imagination. From its Latin roots to its global resonance, the tortoise embodies the enduring fascination with the natural world and our profound connection to all living beings. As we ponder the mysteries of the tortoise, let us heed the timeless wisdom it imparts and embrace our role as custodians of the earth's fragile ecosystems.。
感顿市安乐阳光实验学校Unit1 A land of diversity(2)重点单词1._______ n. 横渡;横越;十字路口;人行横道→cross n. 十字架v. 横渡;越过2._________ n. 申请人→apply v. 申请;请求→application n. 申请;申请书3.__________ n. 者;社会人adj. 者的→socialism n.→society n. 社会→social adj. 社会的4. vi. 发生;出现→occurrence n. 发生;出现5. vt. 指出;标示;表明;暗示→indicator n. 指示者;指示物→indicative adj. 指示的;暗示的→indication n. 指示;暗示;征兆;迹象6. adj. 显而易见的;显然的;表面上的→apparently adv. 显然地;显而易见地7.________ n. (公车)售票员;列车员;(乐队)指挥→conduct vt. 进行;实施;指挥;表现;传导8._________ n. 处罚;惩罚→punish vt. 处罚;惩罚9. adj. 公民的;国内的;民间的→civilization n. 文明;社会文明→civilize vt. 教化;开化;使文明10. vt. & vi. ;革新n. ;改造;改良→reformer n. 改革者;改良者重要短语1. ______________ 申请2. ______________ 组成3. ____________ 突然想到4. ____________ 除了5. _____________ 形状像……6. ___________区分7. __________许多,很多 8. ____________ 体制9. 背靠背_______________ 10.做评论____________11.与……合作或一起工作_________________12.用线画出范围;标出……界线_______________13.包括;吸收;理解;欺骗________________关键句型1.It didn’t ____________ me that...是的,我没想到……2.________________________________________ seemed as if it wouldtakeno time at all!从一个大国穿越到另一个大国看起来似乎毫不费时。
中英姓名文化差异作文英文Title: The Cultural Divergence in Chinese and English NamesNames are unique identifiers that carry profound cultural meanings and historical backgrounds. The differences between Chinese and English names reflect the rich cultural diversity between the two languages and their respective cultures.In the Chinese naming system, names often have multiple parts, with each part carrying specific meanings. Typically, a Chinese name consists of a surname followed by a given name. The surname, usually inherited from one's ancestors, represents one's family lineage and social status. The given name, on the other hand, is chosen by parents to carry their wishes and aspirations for the child. Common themes in Chinese given names include virtues, natural elements, and historical references, reflecting the cultural values and beliefs of the Chinese people.On the contrary, English names tend to be simpler and more straightforward. While they may also have historical and cultural significances, they are often chosen for their sounds or meanings that resonate with individuals or families. English names are typicallycomposed of a given name followed by a surname, with the surname being passed down through generations. Given names in English often have religious or biblical origins, or they may simply be popular choices at the time of birth.Another notable difference is the use of nicknames or pet names. In English culture, it is common for people to have nicknames that are derived from their given names or have personal meanings. These nicknames are often used informally among friends and family, adding a layer of familiarity and warmth to interpersonal relationships. In Chinese culture, however, nicknames are less common, and people are generally referred to by their formal names in most social settings. In conclusion, the differences in Chinese and English names reflect the rich cultural diversity between the two languages and their respective cultures. Chinese names are multi-faceted, carrying family lineages and deep cultural meanings, while English names are more straightforward and often chosen for personal preferences. Understanding these differences helps us appreciate the uniqueness and beauty of each naming system and the cultures they represent.。
The Journal of Immunology Lineage Divergence at the First TCR-Dependent Checkpoint: Preferential gd and Impaired ab T Cell Development in Nonobese Diabetic MiceNi Feng,Patricia Vegh,Ellen V.Rothenberg,and Mary A.YuiThefirst TCR-dependent checkpoint in the thymus determines ab versus gd T lineage fate and sets the stage for later T cell differentiation decisions.We had previously shown that early T cells in NOD mice that are unable to rearrange a TCR exhibit a defect in checkpoint enforcement at this stage.To determine if T cell progenitors from wild-type NOD mice also exhibit cell-autonomous defects in development,we investigated their differentiation in the Notch-ligand–presenting OP9-DL1coculture system,as well as by analysis of T cell development in vivo.Cultured CD4and CD8double-negative cells from NOD mice exhibited major defects in the generation of CD4and CD8double-positive ab T cells,whereas gd T cell development from bipotent precursors was enhanced.Limiting dilution and single-cell experiments show that the divergent effects on ab and gd T cell development did not spring from biased lineage choice but from increased proliferation of gd T cells and impaired accumulation of ab T lineage double-positive cells.In vivo,NOD early T cell subsets in the thymus also show characteristics indicative of defective b-selection,and peripheral ab T cells are poorly established in mixed bone marrow chimeras,contrasting with strong gd T as well as B cell repopulation.Thus,NOD T cell precursors reveal divergent,lineage-specific differentiation abnormalities in vitro and in vivo from thefirst TCR-dependent developmental choice point,which may have consequences for subsequent lineage decisions and effector functions.The Journal of Immunology,2011,186:826–837.T cells in all jawed vertebrates can be divided into two major subsets,those that use ab TCRs and those that use gdTCR.These two major lineages are broadly similar,both requiring successful TCR gene rearrangement to develop and using many of the same effector genes.Yet accumulating evidence shows that their development is promoted by different balances of transcription factors,activating signals,and inputs from Notch–Delta interaction.Whereas most of these effects have been seen in the artificial context of targeted mutant mice,in this study we present evidence that there are unexpectedly sharp,divergent ab-normalities in ab T and gd T cell developmental programming in the unmanipulated genetic background of the autoimmune-prone NOD mouse.All T cell lineages arise from the few multipotent precursor cells that migrate from bone marrow to the thymus,which provides the environmental signals,including Notch ligands and cytokines, needed for these progenitor cells to carry out the process of T lineage specification and commitment.These cells undergo ex-tensive proliferation and an ordered series of differentiation stages characterized by a progressive induction of T cell identity genes and loss of stem cell and other genes permitting alternative fate choices(1).These stages are defined by specific changes in cell surface markers.In mice,the early double-negative(DN)stages lack CD4and CD8,as well as rearranged TCR,and differentiate first from CD44+CD252c-Kit+DN1cells(or early thymic pre-cursors[ETPs])to CD44+CD25+c-Kit+DN2cells and then to CD442CD25+c-Kit lo DN3cells(2,3).TCR rearrangements are ongoing from the DN2through the DN3stage,and differentiation past thefirst T cell checkpoint at DN3requires successful rear-rangement and expression of a TCR b-chain or TCR g-and d-chains.If TCR b molecules are expressed,they pair with in-variant pre-T a molecules,triggering TCR signaling that results in b-selection(4–6).This is a process of rapid,major regulatory changes and extensive proliferation,coupled with effects on survival,culminating in differentiation to the CD4+CD8+double-positive(DP)stage.These DP cells undergo TCR a rearrange-ments and further selection and differentiation,based primarily but not solely on properties of the new ab TCR.T cells with strongly self-reactive TCRs are negatively selected and die, whereas cells with more moderate specificity for self-antigens differentiate into a variety of effector populations including CD4 or CD8single-positive T cells,as well as unconventional cells like regulatory T cells,NKT cells,and CD8aa cells,before exiting to the periphery(reviewed in Ref.7).Alternatively,if g and d TCR chains are rearranged before a TCR b-chain,gd TCR expression triggers an alternative differentiation program,gd-selection,that typically results in a lower level of proliferation and no upregu-lation of CD4or CD8(reviewed in Ref.8).The nature of the receptor itself does not necessarily determine whether a cell will undergo ab T or gd T cell programming,but the intensity and timing of that signal is crucial,with pre-T a/TCR b typically giving weaker and gd TCR stronger signals(9,10).Thus,b-and gd-selection represent thefirst of several stages at which survival and further differentiation of a cell is dependent upon character-Division of Biology,California Institute of Technology,Pasadena,CA91125 Received for publication August2,2010.Accepted for publication November9, 2010.This work was supported by grants from the Juvenile Diabetes Research Foundation International,the National Institutes of Health(AI64590to M.A.Y.),the Al Sherman Foundation,the Louis A.Garfinkle Memorial Laboratory Fund,the Vanguard Char-itable Endowment for Diabetes in Memory of Bently Pritsker,and the Albert Billings Ruddock Professorship(to E.V.R.).Address correspondence and reprint requests to Mary A.Yui,Division of Biology, MC156-29,California Institute of Technology,1200E.California Boulevard,Pasa-dena,CA91125.E-mail address:yui@Abbreviations used in this article:B6,C57BL/6;BMC,bone marrow cell;DL1, Delta-like1;DL4,Delta-like4;DN,double-negative;DP,double-positive;ETP,early thymic precursors;FSC,forward scatter;HSA,heat shock Ag;ic,intracellular;ISP, immature single-positive;SP,single-positive.CopyrightÓ2011by The American Association of Immunologists,Inc.0022-1767/11/$16.00 /cgi/doi/10.4049/jimmunol.1002630 by guest on November 27, 2015 / Downloaded fromistics of the cell’s TCR and associated signaling apparatus.Many T cell identity genes,including those needed for TCR signaling,are turned on in the DN2–DN3stages for first use at b -or gd -selection (1).For this reason,any fundamental genetically determined defect in T cells affecting signaling,survival,or proliferation could alter fate choices at this stage while also having an effect on,without being complicated by,subsequent lineage choices,positive and negative selection,and peripheral activation events.Autoimmune type 1diabetes results from abnormal responses to self-antigens by peripheral T cells,due to both a failure of T cell self-tolerance mechanisms and breakdown of active T cell sup-pression of these autoreactive cells (reviewed in Ref.11).A number of studies have reported that different T lineages from autoimmune diabetes-prone NOD mice have defective numbers and/or responses to various stimuli relative to cells from other nonautoimmune mouse strains as possible factors contributing to disease.The T cell lineages implicated in type 1diabetes include CD4,CD8,NKT,and regulatory ab T cells,as well as gd T intraepithelial lymphocytes (11–16).However,by the time mature T cells initiate and propagate an autoimmune response to pe-ripheral tissues,they have already undergone a sequence of prior developmental choices and signaling encounters with APCs that may have contributed cumulatively to their abnormalities.There-fore,to shed light on the possible origins of inappropriate re-sponses of T cells in NOD mice,we have turned to the earliest stage when the TCR response machinery is first used to dictate cell fate.This is during intrathymic development,at the checkpoint where commitment to the ab and gd T cell lineages is established.Although T cell populations in the thymi of NOD mice appear to be generally similar to those of other mouse strains in steady state (17,18),the early DN populations have not been investigated in great detail.Several studies have reported abnormalities in NOD thymic development.We previously reported that thy-mocytes from NOD.Prkdc scid /scid and NOD-Rag12/2mice appear to break through the b -selection checkpoint,resulting in the ab-errant development of DP cells (19).NOD thymi have also been shown to accumulate newly differentiated DP cells more slowly than conventional strains after dexamethasone-induced depletion of the DP compartment (20).Furthermore,a number of studies show defects in NOD DP cell responses to various stimuli,in-cluding responsiveness to Con A (21),apoptosis induced by glu-cocorticoids and gamma-irradiation (20)or anti-CD3ε(22),and responses to thymic selection signals (23–26).NOD mice also have defects in development of NKT cells from DP (17,27).All of these traits point to potential alterations in TCR signaling in NOD thymic T cells,which may have consequences for mature T cell activity (12).The nature of early T cell responses is set by the cellular signaling components,which in turn have effects on the thresholds for distinct responses including positive and nega-tive selection of ab TCR +DP cells (reviewed in Refs.7,28).The earliest stages of T cell development are difficult to assess in the presence of ongoing TCR rearrangements because they rep-resent only a small fraction of total thymocytes and because of the tremendous proliferation that occurs during differentiation from DN stages to DP,which can mask underlying developmental de-fects.In addition,steady-state proportions of thymic populations are affected by lineage decisions,cell death,and emigration (7).We have therefore used several approaches to compare the earliest stages of T cell development and lineage decisions in NOD to nonautoimmune C57BL/6(B6)mice.To investigate the intrinsic developmental potential of NOD T cell precursors,we took ad-vantage of the Notch-ligand–expressing OP9-DL1coculture sys-tem,which has been used extensively to elucidate the roles of Notch and other factors on the T cell developmental process inmice and humans (29–32).We also carried out detailed flow cy-tometric analyses of early T cell developmental stages as well as tracking of population dynamics by BrdU labeling in vivo and in mixed bone marrow chimeras.Our findings demonstrate that NOD early T cells exhibit impaired b -selection,whereas gd -selection is strongly enhanced.Materials and MethodsMiceNOD/ShiLtJ,C57BL/6J,BALB/cJ,NOD.Rag12/2,and B6.Rag12/2mice were obtained from The Jackson Laboratory (Bar Harbor,ME)and then bred and maintained in microisolator cages in our specific pathogen-free mouse colony in the Caltech Animal Resources facility (Pasadena,CA).The (NOD 3B6)F1.Rag12/2mice used for bone marrow chimeras were bred in our facility from the parent strains.Mice were used for these studies at 4–7wk of age.Euthanasia and animal care followed National Institutes of Health guidelines under protocols approved by the insti-tutional animal care and use committee.Ab staining,cell sorting,and flow cytometric analysisTo isolate DN populations,mice were sacrificed,their thymi were removed,and single-cell suspensions were made.Mature cells were depleted as previously described (33)by staining with biotinylated Abs to CD8a,TCR gd ,TCR b ,Gr1,Ter119,CD122,NK1.1,Dx5,and CD11c,after which the cells were incubated with streptavidin-coated magnetic beads and then passed through a magnetic column (Miltenyi Biotec,Auburn,CA).Eluted DN cells were either stained for direct flow cytometric analysis or for the sorting of specific DN populations into OP9cultures.ETP (CD252CD44hi c-Kit hi ),DN2(CD25+CD44hi c-Kit hi ),DN3a (CD25+CD44lo c-Kit lo CD27lo FSC lo ),and DN3b (CD25+CD44lo c-Kit lo CD27hi FSC hi )precursor cells,as well as gd T cells,were sorted from DN cells using a FACSAria with Diva software (Becton Dickinson Immunocy-tometry Systems,Mountain View,CA).For analysis of cocultures,cells were forcefully pipetted,filtered through nylon mesh to remove stromal cell clumps,and stained for CD45to identify input cells plus various combinations of lineage identifying Abs.Cells were analyzed using a FACSCalibur (Becton Dickinson Immunocytometry Systems)or MACS-Quant (Miltenyi)and FlowJo software (Tree Star,Ashland,OR).Abs were purchased from eBioscience (San Diego,CA).For intracellular staining,DN thymocytes were stained with surface markers,fixed and perme-abilized using Cytofix/Cytoperm Kit (BD),and stained with conjugated Abs to TCR b or TCR gd .Cell cultureOP9-DL1cocultures were carried out as previously described (33,34).For T cell development,thymic DN cells were placed on monolayers of OP9-DL1,supplemented with 2.5ng/ml IL-7and 5ng/ml Flt3L.After 7d,cultures were transferred to new plates with fresh OP9-DL1cells and the medium replaced,supplemented with 1ng/ml IL-7.Human forms of the cytokines were used and obtained from Peprotech (Rocky Hill,NJ).Tissue culture media and FCS were obtained from Invitrogen/Life Technologies (Carlsbad,CA).For bone marrow cultures,cells were column-depleted of mature cells using biotinylated Abs to CD3e,CD4,CD8,CD19,Gr-1,Ter119,CD11b,NK1.1as described earlier.Cells (105)were plated on OP9-DL1monolayers in 10-cm tissue culture flasks (Corning)with the addition of 10ng/ml IL-7and 5ng/ml Flt3L.After 6d,the bone marrow-derived cells,which are predominately DN1and DN2cells,were removed from the monolayers and replated on OP9-DL4cells with 2ng/ml IL-7.For proliferation assays,DN cell subsets were FACS sorted and then stained for 8min at 37˚C with 5m M CFSE (Cell Trace CFSE Proliferation Kit;Invitrogen),in accordance with the manufacturer’s instructions,before they were placed in culture.In vivo assaysFor BrdU pulse-labeling of thymocytes,B6and NOD mice were injected i.p.with 1mg BrdU in 0.2ml PBS twice 4h apart.Mice were sacrificed after 1,2,and 3d,and their thymocytes were stained with surface Abs followed by intracellular staining for BrdU–FITC in accordance with BrdU Flow Kit instructions (BD Biosciences).For mixed bone marrow chimeras,female 8-to 16-wk-old (NOD 3B6)F1.Rag12/2mice were irradiated with 400rad using a 137Cs source irradiator and retroorbitally injected with 53106bone marrow cells (BMCs)each from NOD and B6mice,mixed 1:1,and assayed between 5and 9wk after cell injection.To reduce the risk of infection,mice were housed using autoclaved cages,bedding,and food andThe Journal of Immunology827by guest on November 27, 2015/Downloaded fromwere treated with an antibiotic,Baytril (Bayer),in drinking water for several days before and 2wk after irradiation.RNA extraction and real-time quantitative PCR analysisRNA was isolated from sorted TCR gd +cells from B6and NOD mouse thymi and real-time quantitative PCR carried out as previously described (19).Briefly,cells were lysed in Qiazol (Qiagen,Valencia,CA),RNA isolated using RNAeasy extraction kits (Qiagen),and cDNA reverse transcribed using random hexamers and SuperscriptIII (Invitrogen)fol-lowing the manufacturer’s instructions.cDNA samples were diluted and mixed with gene-specific primers and SYBR GreenER (Invitrogen)and run on an ABI Prism 7900HT Sequence Detector (ABI,Mountain View,CA).Primers used for this study were synthesized by Eurofins MWG Operon (Huntsville,AL)and have been published previously (19,35,36)except for those for Sox13:forward,59-GAGAAGCTGCTGTCCAGT-GAC-39;reverse,59-GACCAAAAGCTGGAGTTCCTT-39.V g 1.1and J g 4primer sequences were obtained from Verykokakis et al.(37).Results were calculated using the D Ct method,normalizing all samples to Actb ex-pression.ResultsNOD DN cells are impaired in the generation and maintenance of ab T lineage DP cells,but not gd T cells,in OP9-DL1coculturesBecause steady-state thymic T cell populations reflect a combi-nation of intrinsic and extrinsic factors,proliferation,cell death,and emigration,which can mask developmental differences,we wished to assess the intrinsic developmental potential of T cell precursors from B6,NOD,and BALB/c mice under the same environmental conditions using the OP9-DL1coculture system (30,34).Immature DN thymocytes were purified by depletion of mature cells and placed in OP9-DL1coculture with IL-7and Flt3L.When analyzed on day 12of culture,B6and BALB/c DN cells were found to generate and maintain high percentages of DP cells as expected,whereas NOD DN cells produced a much lower proportion of DP cells under the same conditions (Fig.1A ,top panels ).In the same cultures,NOD DN cells produced a higher proportion of gd T cells relative to B6and BALB/c DN cells (Fig.1A ,bottom panels ).To assess the kinetics of stage-specific ab versus gd lineage development of NOD and B6DN cells,we tracked the devel-opment of purified intrathymic DN precursor subsets,including DN1/ETP (c-Kit hi CD252CD44hi ),DN2(c-Kit hi CD25+CD44hi ),and DN3a (c-Kit lo CD25+CD44lo CD27lo FSC lo )populations that retain both gd and ab T cell potential,as well as DN3b cells (c-Kit lo CD25+CD44lo CD27hi FSC hi ),which,as we previously showed for B6mice,have already completed TCR b rearrangement and pre-TCR signaling (34).Although NOD thymocytes also upreg-ulate CD27at b -selection,they do so to a lesser extent than B6cells (see later),so NOD and B6DN3b cells were enriched by sorting based on combined expression of relatively high levels of CD27and large size (high forward scatter [FSC],whereas DN3a cells were sorted based upon lower CD27and lower FSC (38).When precursor DN subsets from NOD and B6thymi were placed in OP9-DL1cocultures,neither B6nor NOD DN1/ETP and DN2cells generated DP cells by day 7(Fig.1B ).However,by day 14,B6DN1/ETP and DN2cell cultures contained abundant cells that had differentiated to the DP stage,whereas NOD DN1and DN2cells produced few if any DP cells.This is not due simply to a delay in differentiation,as NOD DP cell production did not increase with time (data not shown).In contrast,DN3cultures from both B6and NOD thymi began to express CD4and/or CD8by day 7of OP9-DL1coculture (Fig.1B ),a time point when few DN1or DN2cells from either strain had turned on CD4or CD8.Confirming the ab T cell lineage choice of the accumulated DP cells,all cultures that contained abundantDP cells,including B6and NOD DN3a and DN3b at day 7,as well as all B6DN subsets at day 14,clearly expressed surface TCR b ,whereas those NOD and B6cultures with few DP cells express very little TCR b (Fig.1C ).At day 7,the percentage of DP from NOD DN3a cells was moderately lower in comparison with that of B6DN3a cells,and NOD DN3b cells appeared to produce fewer cells overall and a lower proportion of DP cells.By day 14,the differences were even more pronounced,with a very low proportion of DP cells in NOD DN3a cultures and very few sur-viving cells in the DN3b cultures.This latter result was confirmed in further experiments when the ability of individual post–b -se-lection DN3b cells to generate DP cells was tested.Single DN3b cells were sorted from NOD and B6thymi and placed in OP9-DL1coculture for 7d.Although all individual NOD and B6DN3b cells proliferated and differentiated,fewer DP cells were generated in the NOD cultures (35.268.9,mean 6SEM)compared with that in B6cultures (179.5653.5)(p ,0.005,Mann–Whitney U test).These results suggest a possible loss of differentiation potential,proliferation,and/or viability in the generation of DP cells from DN cells sorted both before and after b -selection in the NOD thymus.To identify the stages at which the initial differentiation of the NOD DN cells might be blocked,the same cultures were stained with CD44and CD25at day 7(Fig.1D ).Most of the cells from cultures seeded with DN3a and DN3b cells from either B6or NOD mice had progressed to DN4,or later stages,as shown by the downregulation of CD25and CD44by day 7,in agreement with their predominately DP phenotype.Although both B6and the NOD DN1and DN2cells differentiated successfully to the DN3stage,the NOD DN1and DN2cell cultures produced a much lower proportion of DN4and later cells (Fig.1D ),suggestive of a partial block at the b -selection checkpoint from NOD DN1and DN2cells.In marked contrast to their poor production of DP cells,the DN1,DN2,and DN3a cultures from NOD thymocytes generated gd T cells very successfully (Fig.1E ).All of the NOD populations produced a higher proportion of gd T progeny than that of the corresponding B6precursors,with DN2cells producing the greatest differences:2.8%from B6and 21%from NOD DN2cultures on day 14.As expected,very few gd T cells were gen-erated from sorted DN3b cells from either strain,in agreement with our previously published results for B6mice (34).Thus,even though NOD DN1and DN2cells are extremely poor at passing through the b -selection checkpoint to generate DP ab T lineage cells in vitro,they are normal or enhanced in their ability to produce gd T lineage cells.These results show that NOD precursors exhibit a lineage-specific intrinsic defect in development when placed in OP9-DL1cultures.DN3cells from NOD mice,even those that had undergone b -selection in the thymus,generate fewer DP cells than do B6DN3cells,and the earliest DN1and DN2T cell precursors sorted from NOD thymi are profoundly defective in their ability to go through b -selection in vitro.However,these cells retain the ability to produce abundant TCR gd +cells.Prior thymic contact is not required for skewing of ab versus gd T cell fate from NOD cellsAlthough the highly skewed production of ab versus gd lineage cells from NOD DN cells appears intrinsic to these thymic-derived cells,two concerns could be raised.First,these cells might have already encountered biasing interactions in the thymus from which we isolated them.Second,the OP9-DL1cultures present the Delta-like 1(DL1)Notch ligand,whereas the Notch ligand ex-pressed in the normal thymus is Delta-like 4(DL4),which pro-828b -AND gd -SELECTION CHECKPOINT ABNORMALITIES IN NOD MICEby guest on November 27, 2015/Downloaded fromvides a somewhat different Notch signal to these cells (39–41).Therefore,we also tested the T lineage development of pre-thymically derived BMC progenitors in vitro,providing DL4as the source of Notch ligands rather than DL1.As shown in Fig.2A ,cells derived from precursor-enriched B6and NOD BMCs gen-erate both DP and gd T cells on OP9-DL4after 19–22d of culture.However,NOD progenitor cells again exhibited a poor differen-tiation of DP cells (Fig.2A ,left panels )and enhancement of gd T cell generation (Fig.2A ,right panels )in comparison with those from B6mice.Furthermore,when NOD and B6cells were co-cultured,similar differences in ab T versus gd T development were observed between the two strains (Fig.2B ).As shown in two independent cultures,NOD cells (CD45.1+)produced a lower proportion of DP cells and more gd T cells than those of cultured cells from B6mice (CD45.2+).These results confirm the profoundintrinsic and lineage-specific differences in T cell developmental programming between NOD and B6precursor cells.NOD DN2cells generate a higher absolute number of gd T cells and fewer DP cells than those generated by B6DN2cells in vitroSkewed production of gd T cells relative to DP cells could result from lineage-specific alterations in proliferation or survival as well as lineage choice itself.To distinguish between these possi-bilities,we focused on the DN2cells,which particularly differed between NOD and B6in terms of their abilities to generate DP versus gd T cells in OP9-DL1cocultures.First,we assessed the total cell numbers of DP and gd T cells generated from limited numbers of precursor cells in these cultures.Ten DN2cells from B6and NOD thymi were sorted directly into each of 24individualFIGURE 1.NOD DN cells generate and maintain few DP ab T cells while retaining gd T development in vitro.A ,Flow cytometric analyses showing the development of DP and gd T cells from magnetic bead-purified DN cells from B6,NOD,and BALB/c thymocytes after 12d of OP9-DL1coculture in 5ng/ml IL-7and Flt3L.Similar results were obtained from cultures harvested on day 8and when IL-7concentrations were dropped to 2ng/ml for the last 5d of culture or were maintained at 2ng/ml throughout the culture period (data not shown).B –E ,Development of FACS sorted DN1,DN2,DN3a,and DN3b (post b -selection)cells from B6and NOD thymi cultured in 2.5ng/ml IL-7and 5ng/ml Flt3L.B ,Flow cytometric plots showing CD4and CD8DP cell development at days 7and 14from each progenitor population.C ,Histograms showing levels of surface TCR b expression in the same cultures for B6and NOD cell populations at days 7and 14of culture.No data are shown for NOD DN3b cells on day 14because very few cells are remaining.D ,Flow cytometric plots of CD44versus CD25showing differences in initial DN differentiation in the same cultures at day 7.E ,gd T cell development in the same cultures at day 14.Percentages of cells in each gate are indicated.These results are representative of at least three independent experiments.The Journal of Immunology 829by guest on November 27, 2015/Downloaded fromOP9-DL1cultures and analyzed by flow cytometry on day 13.The percentage of gd T cells out of the total number of differentiated cells (calculated as 1003number of gd T/[number of gd T +number of DP])for each well is shown in Fig.3A (left panel ),and a highly significant difference was found between cultured B6and NOD DN2cells.Although DN2cells from NOD and B6thymi yielded comparable total numbers of CD45+cells per well,NOD cells produced significantly higher absolute numbers of gd T cells,as well as significantly fewer DP cells (Fig.3A ,right panels ).Thus,both poor DP cell differentiation and enhanced gd T cell development contribute to the NOD phenotype.To determine whether the differences in ab T versus gd T dif-ferentiation are the result of alterations in lineage choice,single-cell differentiation analyses were performed.Because commitment to the gd T cell lineage may occur as early as the DN2stage (42),we sought to determine if DN2cells from either mouse strain start with a biased frequency of cells precommitted to either lineage.For this experiment,single cells from two subsets of the DN2CD44+CD25+c-Kit hi population were tested separately based on their surface c-Kit levels,the less mature DN2a (CD44+CD25+c-Kit ++)cells and the more mature,T lineage-committed DN2b (CD44+CD25+c-Kit +)cells (33).Each well was scored for the presence and numbers of gd T and DP cells after 13d of OP9-DL1culture.Results in Fig.3B (left panel )show again that NOD DN2cells,both DN2a and DN2b,generally produce proportionately more gd T cells and fewer DP cells as a percentage of total differentiated cells than those of corresponding cells from B6mice.However,differences between B6and NOD were clearly not due to pre-mature commitment in cells from either strain as they exhibited similar percentages of bipotent cells (black bars)at each stage (Fig.3B ,right panel ).Almost all DN2a cells from both strains produced both gd T and DP cells.Although the majority of DN2b cells were still bipotent,individual DN2b cells from both NOD and B6micewere more likely than DN2a-stage cells to generate only DP cells,a trend that continued to the DN3a stage when few cells remained bipotent and most generated only DP cells,as shown previously for B6mice (34).Few single cells from either strain produced only gd T cells.Thus,the high proportion of gd T cells and low proportion of DP cells in NOD cultures is not a result of increased previous gd T lineage commitment of the precursors but rather the result of enhanced gd T cell production and reduced DP cell generation after the lineage choice is made.NOD gd T cells proliferate more rapidly than do B6gd T cells in vitroThe normal preponderance of ab -lineage DP cells over gd T cells in the thymus depends not only on the frequency of different se-lection events but also on the greater extent of net proliferation that is normally associated with the ab -lineage program after b -selection.To address the question of whether some of the dif-ferences in ab Τversus gd T cell development are attributable to a NOD defect in proliferation,unsorted thymocytes (“All”)as well as sorted DN1/ETP,DN2,DN3a,and DN3b populations were stained with CFSE and placed in OP9-DL1culture.NOD DN subsets showed similar or slightly faster proliferation rates than B6cells over the first 4d of culture (Fig.4A ),ruling out a generalized proliferation defect.Most prominently,the gd TCR-positive cells generated in the first 4d from NOD DN3a cultures undergo more rapid and extensive proliferation than that of gd TCR-positive cells from B6cell cultures,whereas the gd TCR-negative cells from NOD and B6cells proliferated more similarly (Fig.4B ).Thus,the high numbers of gd T cells generated from NOD DN cells result,at least in part,from their greater proliferation after surface TCR gd expression in comparison with B6gd T cells.Furthermore,the lower numbers of DP cells generated from NOD DN3cells is not due to an initial defect in proliferation after b-selection.FIGURE 2.NOD bone marrow progenitor cells also show attenuated in vitro development of DP ab T cells and enhanced gd T cell development.BMCs enriched for progenitors were isolated from NOD and B6mice and cultured on OP9-DL1and DL4cells as described in Materials and Methods .Flow cytometry plots show the gener-ation of CD4/CD8DP cells and gd T cells from B6and NOD bone mar-row cultures analyzed after 19and 22d (A )and two independent cultures established from mixing equal num-bers of NOD and B6BMCs,analyzed after 20d (B ).Results are representa-tive of at least three replicate wells from one of two independent experi-ments with similar results.830b -AND gd -SELECTION CHECKPOINT ABNORMALITIES IN NOD MICEby guest on November 27, 2015/Downloaded from。