羧甲基纤维素
- 格式:pdf
- 大小:633.22 KB
- 文档页数:9

羧甲基纤维素的常见规格一、粘度粘度是羧甲基纤维素(CMC)溶液的一种重要物理性质,反映了其流动性。
一般来说,高粘度的CMC具有更好的增稠和稳定性能。
粘度范围因不同的生产方法和应用需求而有所不同,常见的CMC粘度范围在500-20000厘泊(cps)之间。
二、取代度取代度是指CMC分子中羧甲基基团的数量与总可取代基团数量之比,通常以"DS"表示。
DS值对CMC的溶解性、粘度、透明度、稳定性等性能有重要影响。
一般来说,DS值越高,CMC的溶解性越好,粘度和稳定性也越高。
常见的CMC取代度范围在0.3-0.9之间。
三、粒度粒度是指CMC颗粒的大小。
粒度大小对CMC的溶解速度、混合均匀度以及应用性能有一定影响。
一般来说,较细的CMC颗粒具有更好的溶解性和混合均匀度。
常见的CMC粒度范围在100-300目之间。
四、溶解性溶解性是指CMC在水中或其他溶剂中的溶解能力。
CMC的溶解性受到取代度、聚合度、粒度、温度等因素的影响。
良好的溶解性是CMC能够广泛应用的关键因素之一。
CMC通常在热水或碱性溶液中溶解,并根据不同的取代度和粒度等规格,表现出不同的溶解速率和溶解程度。
五、粘合强度粘合强度是指CMC作为粘合剂时,对不同材料表面的粘附能力。
粘合强度受到CMC的取代度、分子量、粘度、添加量等因素的影响。
良好的粘合强度是CMC作为粘合剂的重要性能指标之一,它能够提高材料的粘附力和整体性能。
六、稳定性稳定性是指CMC在不同环境条件下的性能保持能力。
CMC的稳定性受到温度、pH值、紫外线等因素的影响。
在加工、储存和使用过程中,CMC需要保持良好的稳定性,以确保其性能的稳定性和持久性。
七、灰分灰分是指CMC中的无机杂质含量,如钠、钙等。
灰分含量过高会影响CMC的纯度和透明度,同时也会影响其应用性能。
因此,对于食品、医药等应用领域,需要控制CMC的灰分含量在较低水平。
常见的CMC灰分含量一般在0.5%以下。


羧甲基纤维素——离子交换剂1.引言1.1 概述羧甲基纤维素是一种具有离子交换能力的材料,具有广泛的应用潜力。
它可以通过对纤维素进行化学修饰得到,使其表面具有羧基官能团。
这种化学修饰不仅能够增强纤维素的稳定性和机械强度,还能赋予其离子交换能力。
离子交换是指离子间的相互转移,通过固体表面上带有特定功能团的材料与溶液中的离子进行相互吸附和解吸附的过程。
羧甲基纤维素作为一种离子交换剂,具有很高的吸附容量和选择性,可以用于各种离子的去除和回收。
羧甲基纤维素的制备方法有多种,包括化学修饰法、原位聚合法等。
其中,化学修饰法是最常用的方法,通过将羧甲基功能团引入纤维素分子结构中,使其具有离子交换性能。
羧甲基纤维素的应用领域非常广泛,可以用于水处理、废水处理、离子交换树脂等领域。
本文旨在对羧甲基纤维素作为离子交换剂的优势进行详细探讨,并探究其在环境保护中的潜在应用。
通过深入了解羧甲基纤维素的定义、特性、制备方法和应用,我们可以更好地认识和利用这一材料,为环境保护和资源回收做出积极贡献。
1.2文章结构【1.2 文章结构】本文主要分为引言、正文和结论三个部分。
下面将简要介绍每个部分的内容。
1. 引言部分:在引言部分,首先会对羧甲基纤维素进行概述,介绍其起源、性质以及已知的特点。
接下来,将对整篇文章的结构进行概括和介绍,明确各个部分的内容和目的。
最后,明确本文的主要目的,即探讨羧甲基纤维素作为离子交换剂的潜力和应用。
2. 正文部分:正文部分将包括两个主要内容:羧甲基纤维素的定义和特性,以及羧甲基纤维素的制备方法和应用。
2.1 羧甲基纤维素的定义和特性:这一部分将详细介绍羧甲基纤维素的定义,解释其由何种成分组成以及其中的化学结构。
同时,还会涵盖羧甲基纤维素的主要特性,如其吸附能力、离子交换能力等。
2.2 羧甲基纤维素的制备方法和应用:在这一部分,将详细介绍羧甲基纤维素的制备方法,包括从原料的选择到制备步骤的具体过程。
此外,还将探讨羧甲基纤维素在不同行业的应用,如环境保护、水处理、催化剂等。

羧甲基纤维素一、名称:1. 化学名称:羧甲基纤维素钠,又称羧甲基纤维素2. 英文全称:Carboxymethyl Cellulose3. 英文简称:CMC二、分子式:[C6H7O2(OH)2CH2COONa]n三、制备:CMC 的主要化学反应是纤维素和碱生成碱纤维素的碱化反应以及碱纤维素和一氯乙酸的醚化反应。
碱化: [C6H7O2(OH) 3] n + nNaOH→[C6H7O2(OH) 2ONa ] n + nH2O醚化: [C6H7O2(OH) 2ONa ] n + nClCH2COONa →[C6H7O2(OH) 2OCH2COONa ] n + nNaCl三、物理性质:外观为白色或微黄色絮状纤维粉未或白色粉未,无嗅无味,无毒;易溶于冷水或热水,形成胶状,溶液为中性或微碱性,不溶于乙醇、乙醚、异丙醇、丙酮等有机溶剂,可溶于含水60%的乙醇或丙酮溶液。
有吸湿性,对光热稳定,粘度随温度升高而降低,溶液在PH值2~10稳定,PH低于2,有固体析出,PH值高于10粘度降低。
变色温度227℃,炭化温度252℃,2%水溶液表面张力71mn/n。
常用钠盐。
白色絮状粉末,无臭,无味,无毒。
易溶于水,形成透明胶状液,溶液呈中性。
对光、热稳定。
有吸湿性。
不溶于酸、甲酚、乙醇、丙酮、氯仿、苯等,难溶于甲醇、乙醚。
有羧甲基取代基的纤维素衍生物,用氢氧化钠处理纤维素形成碱纤维素,再与一氯醋酸反应制得。
构成纤维素的葡萄糖单位有3个可被置换的羟基,因此可获得不同置换度的产品。
平均每1g干重导人1mmol羧甲基者,在水及稀酸中不溶解,但能膨润,用于离子交换层析。
羧甲基pKa在纯水中约为4,在0.5mol/L NaCl中约为3.5,是弱酸性阳离子交换剂,通常于pH4以上用于中性和碱性蛋白质的分离。
40%以上羟基为羧甲基置换者可溶于水形成稳定的高黏度胶体溶液。
制药业选用适当黏度CMC作片剂的黏合剂、崩解剂,混悬剂的助悬剂等。

羧甲基纤维素(Carboxymethyl Cellulose,简称CMC)是一种重要的纤维素衍生物,其钠盐(羧甲基纤维素钠)被广泛用作食品添加剂、乳化剂、增稠剂等。
质谱(LCMS)是一种分析化学技术,用于分析化合物。
在这里,LCMS被用于分析羧甲基纤维素的结构和性质。
LCMS分析羧甲基纤维素的过程主要包括以下步骤:
1. 样品制备:将羧甲基纤维素样品溶解在适当的溶剂中,通常为水或有机溶剂。
2. 色谱分离:通过液相色谱(LC)对羧甲基纤维素分子进行分离。
色谱柱通常选择合适的凝胶柱,如羟丙基-Sephadex G-75。
3. 质谱分析:通过串联质谱仪(MS)对经过色谱分离后的化合物进行质谱分析。
质谱分析可以帮助确定羧甲基纤维素的分子量、结构以及取代度等性质。
4. 数据处理:将质谱分析得到的数据进行处理,得到羧甲基纤维素的详细结构信息。

羧甲基纤维素羧甲基纤维素含量分析毒性使用限量食品添加剂最大允许使用量最大允许残留量标准羧甲基纤维素试剂级价格羧甲基纤维素CAS号: 9004-32-4英文名称: Carboxymethyl cellulose英文同义词: b10;cmc2;s75m;7h3sf;carbo;cmc7h;cmc7m;cmc41a;cmc4h1;cmc4m6中文名称: 羧甲基纤维素中文同义词: 纤维素胶;酸甲基纖維;羧甲基纤维素;羧甲纤维素钠;羧甲基纤维素钠;羧甲基纤维素钠盐;羧甲基醚纤维素钠盐;羧甲基纤维素1M6;羧甲基纤维素钠(碱性);羧甲基纤维素(IM6)CBNumber: CB5209844分子式: C6H7O2(OH)2CH2COONa分子量: 0MOL File: Mol file羧甲基纤维素化学性质熔点: 274 °C (dec.)密度: 1,6 g/cm3溶解度: H2O: 20 mg/mL, solubleform : low viscosity水溶解性: solubleMerck : 14,1829稳定性: Stable. Incompatible with strong oxidizing agents.EPA化学物质信息: Cellulose, carboxymethyl ether, sodium salt(9004-32-4)安全信息危险类别码: 40安全说明: 24/25WGK Germany : 1RTECS号: FJ5950000F : 3羧甲基纤维素MSDSCarboxymethyl cellulose羧甲基纤维素性质、用途与生产工艺含量分析羧甲基纤维素钠的百分含量按100减去下述氯化钠和乙醇酸钠的百分含量而得。
氯化钠含量精确称取试样约5g,移人一250m1烧杯,加水50ml和30%过氧化氢5ml,在蒸汽浴上加热20min,偶尔搅拌一下,至完全溶解。
冷却,采用硫酸银和硫酸汞一硫酸钾电极,并不停搅拌,加水100ml和硝酸10ml,然后用0.05mol/L硝酸银滴定至电位终点。

一、概述:羧甲基纤维素(Sodium Carboxymethyl Cellulose)简称CMC,属表面活性胶体的高分子化合物,是一种无臭、无味、无毒的水溶性纤维素衍生物,一般使用的是其钠盐,故其全名应叫羧甲基纤维素钠,即CMC—Na。
二.产品特性:1.CMC为白色或微黄色纤维颗粒状粉末,无味、无臭、无毒,易溶于水,并形成透明粘稠胶体,溶液呈中性或微碱性。
可长期保存不变质,在低温及日光照射下也是稳定的。
但因温度急剧变化,溶液酸碱性变化。
在紫外线照射下以及微生物的影响,也会引起水解或氧化,溶液粘度下降,甚至溶液腐败,溶液如需长期保存,可选则适宜的防腐剂,如甲醛、苯酚、苯甲酸、有机汞化合物等。
2.CMC与其它高分子电解质相同,溶解时,首先产生澎涨现象,粒子间相互粘附形成皮膜或粘胶团,致使不能分散,而是溶解迟缓。
因此,在配制其水溶液时,如能先使粒子均匀润湿,能显著增加溶解速度。
3.CMC具有吸湿性,在大气中CMC的平均水份随空气温度增加而增加,随空气温度上升而减少,在室温平均温度80%--50%时,平衡水份在26%以上,产品水份为10%以下。
因此产品包装及存放应注意防潮。
4.锌、铜、铅、铝、银、铁、锡、铬等重金属盐类,能使CMC水溶液发生沉淀,沉淀除盐基性的醋酸铅外,仍可重溶于氢氧化钠或氢氧化铵溶液内。
5.有机的或无机的酸类,对本产品的溶液,也会起沉淀现象,沉淀现象因酸的种类及浓度而有所不同,一般在PH2.5以下即发生沉淀,加碱中和后可以回复。
6.钙、镁及食盐等盐内,对CMC溶液不起沉淀作用,但影响降低粘度。
7.CMC与其它水溶性胶类及软化剂、树脂等均有相溶性。
8.CMC抽成的薄膜,在室温下浸渍于丙酮、苯、醋酸丁酯、四氯化碳、蓖麻油、玉米油、乙醇、乙醚、二氯乙烷、石油、甲醇、醋酸甲酯、甲基乙基酮、甲苯、松节油、二甲苯、花生油等二十四小时内可无变化。
9.本产品外形为细粉或粗粒,或仍如纤维状,只因加工不同而异与其物理化学性能无关系。

羧甲基纤维素的制备英文回答:Carboxymethyl cellulose (CMC) is a cellulose derivative that is widely used in various industries due to its unique properties. It is prepared by the reaction of cellulose with sodium chloroacetate, which introduces carboxymethyl groups onto the cellulose backbone. The synthesis of CMC involves several steps.First, cellulose is typically derived from natural sources such as wood pulp or cotton fibers. It is important to note that cellulose is a linear polymer composed of glucose units linked by β-1,4-glycosidic bonds. The starting material, cellulose, needs to be in the form of a fine powder or fibers for the subsequent reactions.Next, the cellulose is treated with sodium hydroxide (NaOH) to activate the hydroxyl groups on the cellulose chain. This step is crucial as it increases the reactivityof cellulose towards the carboxymethylation reaction. The cellulose is then washed to remove any impurities and excess NaOH.After the activation step, the cellulose is reacted with sodium chloroacetate (NaClO2CH2COONa) in the presence of an alkaline catalyst such as sodium hydroxide. This reaction is carried out under controlled conditions of temperature and time. The carboxymethylation reaction involves the nucleophilic substitution of the hydroxyl groups on the cellulose chain by the carboxymethyl groups from sodium chloroacetate.Once the reaction is complete, the product is neutralized to remove any unreacted sodium hydroxide or other impurities. The neutralization can be achieved by adding an acid such as hydrochloric acid (HCl) or acetic acid (CH3COOH) to the reaction mixture. The resulting CMC is then filtered, washed, and dried to obtain the final product.中文回答:羧甲基纤维素(CMC)是一种纤维素衍生物,由于其独特的性质,在各个行业广泛应用。

羧甲基纤维素成分全文共四篇示例,供读者参考第一篇示例:羧甲基纤维素(Carboxymethyl cellulose,CMC)是一种以天然纤维素为基础的半合成阻滞因子,由纤维素在碱性条件下与氯酸钠反应得到。
羧甲基纤维素是一种对水溶性高分子化合物,具有增稠、乳化、减少热量等多种作用。
羧甲基纤维素在食品工业中被广泛应用,可以用作增稠剂、稳定剂、乳化剂、抗结冰剂等。
其作为增稠剂在食品加工中起到了很好的作用,可以增加食品的粘度和稠度,提高口感和质感。
在饮料、果酱、奶制品等食品中,羧甲基纤维素可以起到增稠和稳定作用,使得食品更加美味和持久。
除了在食品工业中的应用,羧甲基纤维素在医药和化妆品行业也有着广泛的应用。
在医药方面,羧甲基纤维素可以用作胶囊的成型剂,使得制成的胶囊更加坚固和耐嚼。
在化妆品方面,羧甲基纤维素可以用作乳液、面霜、洗发水等产品的稳定剂和乳化剂,保持产品的质感和稳定性。
在日常生活中,羧甲基纤维素也有着一些应用。
比如在墙纸、涂料等建筑材料中,羧甲基纤维素可以起到增稠、增加粘度的作用,提高涂料的附着力和覆盖性。
在纺织品、皮革制品等行业中,羧甲基纤维素也可以用作染料的增稠剂和分散剂,提高染色的效率和均匀性。
第二篇示例:羧甲基纤维素是一种在食品、医药、化妆品等多个领域中被广泛使用的成分。
它是一种水溶性的纤维素,具有许多重要的功能和优点。
在本文中,我们将探讨羧甲基纤维素的来源、生产方法、性质以及在不同领域的应用。
羧甲基纤维素的来源主要是天然植物材料,如木质纤维、粮食等。
羧甲基纤维素是通过化学方法通过纤维素水解、脱水和羧甲基化反应合成的。
其结构中含有大量的羧基和甲基,这使得它具有较好的水溶性和胶凝性。
在生产方法中,羧甲基纤维素是通过反应剂与纤维素进行反应制备而成的。
羧甲基纤维素的性质决定了它在多个领域中的广泛应用。
在食品行业中,羧甲基纤维素可用作增稠剂、胶凝剂和稳定剂,用于改善食品的口感和质地。
在医药领域,羧甲基纤维素可用作缓释剂、粘合剂和包衣剂,用于药品的制备和包装。
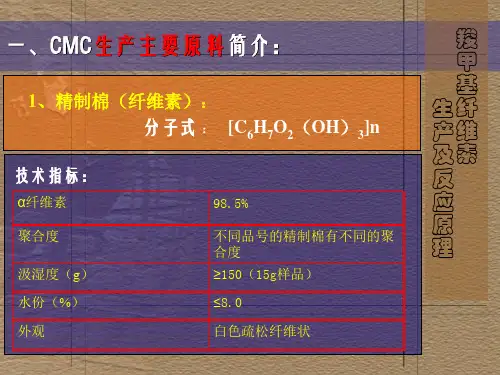

羧甲基纤维素及其盐类
羧甲基纤维素(CMC)是一种纤维素衍生物,其羧甲基(-CH2-COOH)与构成纤维素主链的吡喃葡萄糖单体的部分羟基结合。
常用的是它的钠盐,即羧甲基纤维素钠。
羧甲基纤维素是由纤维素与氯乙酸在碱催化下反应合成的。
这种反应包括两个步骤:第一步是碱化,即天然纤维素与氢氧化钠反应;第二步是醚化,即碱化后的纤维素与氯乙酸钠反应。
羧甲基纤维素钠(CMC)属阴离子型纤维素醚类,外观为白色或微黄色絮状纤维粉末或白色粉末,无臭无味,无毒。
它易溶于冷水或热水,形成具有一定粘度的透明溶液。
该溶液为中性或微碱性,不溶于乙醇、乙醚、异丙醇、丙酮等有机溶剂,可溶于含水60%的乙醇或丙酮溶液。
羧甲基纤维素的应用非常广泛。
例如,在眼科学中,CMC被用作人工泪液,用于治疗干眼症。
此外,它还常用于制药、食品、化妆品、石油、粘蚊剂、造纸和纺织等行业。
在石油和纺织工业中,它通常用作粘胶剂或浆料。
在食品工业中,CMC可以作为增稠剂、稳定剂或乳化剂。
请注意,羧甲基纤维素及其盐类的应用和制备方法可能会因不同的应用领域和需求而有所不同。
在使用羧甲基纤维素及其盐类时,应遵循相关的安全指南和规范,以确保产品的质量和安全性。
羧甲基纤维素的物理性能羧甲基纤维素(Carboxymethyl Cellulose,CMC)是一种重要的环保型高分子材料,被广泛应用于食品、制药、化妆品、石油、纺织、造纸等行业。
CMC的物理性能对其应用有着重要的影响。
本文着重探讨CMC的物理性能,包括流变性质、热性能、透明度和机械性能等方面。
流变性质CMC的流变性质是传递声波或电磁波的介质性质,以及在加工过程中其流动性能的表现。
在实际应用中,CMC往往是在水、酒精、乙醇等溶剂中使用,并在调整其浓度范围内调整黏度。
CMC的黏度和浓度呈线性关系,在同等浓度下,不同的羟丙基纤维素具有不同的黏度。
在流变测试中,CMC表现出荡流性质,其流变学模型为半寿命钝化模型。
热性能CMC的热性能表现为其热稳定性、玻璃化温度和热分解行为。
研究表明,CMC 的热稳定性与其羧基取代度、分子量和结晶性有关。
在一定的取代度和分子量下,CMC的结晶程度和热稳定性呈正相关关系。
CMC的玻璃化温度通常在140℃-200℃之间,同时,CMC的热分解温度也较高,在400℃以上。
透明度在CMC的应用中,其透明度是比较关键的物理性能之一,特别是在食品和药品等领域。
CMC的透明度与赤铁矿矿物油、羟基苯甲酸酯等添加剂的使用量有着很大的关系。
同时,CMC的透明度还与温度、PH值、离子强度、旋光度等因素有关。
机械性能CMC的机械性能是指其在外界力作用下,发生形变和破坏的性质。
CMC在干态状态下的机械强度较低,但在水溶液中能显著提高机械强度,这与CMC分子链的聚集状态和水分子的熔融作用有关。
同时,CMC的吸水性和溶胀性也是影响其机械性能的因素之一。
综上所述,CMC作为一种重要的高分子材料,其物理性能的好坏直接影响着其应用范围和效果。
在实际应用中,需要根据其具体的用途和要求,选择合适的CMC类型和制备方法,以达到最优的效果。
羧甲基纤维素螯合金属离子概述说明以及解释1. 引言1.1 概述羧甲基纤维素(CMC)是一种常见的离子聚合物,在化工、环境科学和生物医学领域广泛应用。
羧甲基纤维素具有类似于纤维素的多糖结构,其分子中含有大量的羧酸基团。
这些羧酸基团可以与金属离子形成稳定的螯合络合物。
本文将探讨羧甲基纤维素螯合金属离子的作用机制以及其在环境污染治理和生物医学领域中的应用。
1.2 文章结构本文包括以下几个部分:引言、羧甲基纤维素螯合金属离子的作用机制、羧甲基纤维素螯合金属离子在环境污染治理中的应用、羧甲基纤维素螯合金属离子在生物医学领域中的研究进展以及结论部分。
1.3 目的本文旨在通过对羧甲基纤维素螯合金属离子进行概述和解释,深入探讨其作用机制,并分析其在环境污染治理和生物医学领域中的应用。
通过对近年来相关研究成果的总结,展望羧甲基纤维素螯合金属离子未来可能面临的挑战和发展方向。
最终旨在为相关领域的科研工作者提供参考,推动羧甲基纤维素螯合金属离子在实际应用中的进一步发展。
2. 羧甲基纤维素螯合金属离子的作用机制2.1 羧甲基纤维素的结构特点羧甲基纤维素是一种具有羧基官能团的水溶性聚合物。
其结构特点主要包括含有大量羧酸(-COOH)官能团和部分亲水性的纤维素骨架。
这使得羧甲基纤维素在水中具有较好的溶解性和可调控的表面活性。
2.2 金属离子与羧甲基纤维素之间的螯合反应机制羧甲基纤维素与金属离子之间发生螯合反应,主要通过羧酸官能团上的羟基(-OH)与金属离子形成配位键。
这种配位键可以通过共价键或者氢键形式存在。
在螯合反应中,金属离子和羧甲基纤维素之间的相互作用强度和选择性受多个因素影响,包括:1) 金属离子电荷:带正电荷的金属离子更容易与负电荷的羧酸官能团发生反应,从而形成较为稳定的螯合配合物。
2) 羧酸官能团的数目和空间排布:羧甲基纤维素中含有多个羧酸官能团,这些官能团的数目和排布方式会影响金属离子与之结合的程度和稳定性。
羧甲基纤维素成分-概述说明以及解释1.引言1.1 概述羧甲基纤维素是一种重要的纤维素衍生物,具有广泛的应用价值。
它是通过羟甲基化纤维素制备而成的,被普遍应用于纺织、造纸、涂料和医药等领域。
羧甲基纤维素具有良好的可溶性和可降解性,能够在水中形成胶体溶液,并且在一定条件下能够发生凝胶化反应。
这使得羧甲基纤维素成为许多行业中必不可少的一种功能性材料。
羧甲基纤维素的制备方法多种多样,常见的制备方法包括酸催化法、酵素法和化学合成法等。
在制备过程中,羟甲基化纤维素通过与羧酸反应,形成羧甲基纤维素。
制备过程的选择和优化对羧甲基纤维素的性质和应用具有重要影响。
羧甲基纤维素在纺织行业中有着广泛的应用。
它可以作为染料和功能性助剂的载体,提高染料的吸附性和稳定性,同时改善纺织品的耐洗涤性能。
此外,羧甲基纤维素还能够增强纺织品的附着力和抗皱性能,提升织物的质量和品质。
在造纸行业中,羧甲基纤维素作为纸浆增稠剂和纸张强度剂,能够改善纸浆的流变性和增加纸张的强度。
同时,羧甲基纤维素还可以作为表面施胶剂,提高纸张的润湿性和印刷性能。
在涂料行业中,羧甲基纤维素常用作稳定剂和乳化剂,能够提高涂料的黏稠度和延展性,同时改善涂料的流变性和干燥性能。
此外,羧甲基纤维素还具有优良的乳化、增稠和稳定性能,使得涂料具有更好的使用效果和持久性。
此外,羧甲基纤维素还有着广泛的医药应用。
它可以用作药物缓释剂和胶囊材料,能够控制药物的释放速率和提高药物的稳定性。
同时,羧甲基纤维素还能够增强药物的吸附性和生物可降解性,减少药物的副作用。
总之,羧甲基纤维素作为一种重要的纤维素衍生物,在各个领域都具有着重要的应用价值。
随着科学技术的不断进步,羧甲基纤维素的研究和应用前景将会更加广阔。
在未来的发展中,人们可以通过优化制备方法和改进性能,进一步拓宽羧甲基纤维素的应用范围,实现更多领域的创新与发展。
文章结构部分内容可按照以下方式编写:1.2 文章结构本文将按照以下结构进行论述羧甲基纤维素成分的相关内容:第一部分是引言部分,主要包括概述、文章结构和目的三个方面。
中文名羧甲基纤维素英文名Cellulose CM别名羧甲基醚纤维素羧甲纤维素英文别名carboxy-methyl cellulosecarboxymethyl cellulosecellulose carboxymethyl ethercmc-4lfcarbosecarboximethylcellulosumcarboxymethyl cellulose ethercarboxymethylated cellulose pulpcarboxymethylcellulosecarboxymethylcellulosumcarmellosecarmellosumcarmelosacellulose gum 7hcellulose carboxymethylatecellulose, (carboxymethyl)cellulose, ether with glycolic acidcelluloseglycolic acidcolloresinecroscarmellosecroscarmellosumcm-celluloseFEMA No. 2239DuodcelGlycocel TAhexose - acetic acid (1:1)CAS 9000-11-7177317-30-5191616-54-3196886-89-2204336-41-4化学式C8H16O8分子量240.206inchiInChI=1/C6H12O6.C2H4O2/c7-1-3(9)5(11)6(12)4(10)2-8;1-2(3)4/h1,3-6,8-12H,2H2;1H3,( H,3,4)沸点527.1°C at 760 mmHg闪点286.7°C蒸汽压 2.59E-13mmHg at 25°C羧甲基纤维素- 性质羧甲基纤维素是最具有代表性的离子型纤维素醚,通常使用的是它的钠盐,故亦称羧甲基纤维素钠。
甲基纤维素,和羧甲基纤维素甲基纤维素是一种纤维素甲基醚。
白色或浅黄或浅灰色小颗粒、纤丝状或粉末。
无臭无味,其中约27%~32%的羟基以甲氧基的形式存在。
不同级别的甲基纤维素具有不同的聚合度,其范围为50~1000;而其分子量(平均数)的范围在10000~220000Da之间,其取代度被定义为甲氧基的平均数,甲氧基则连接于链上的每一个葡萄糖酐单元。
理化性质外观MC为白色或类白色纤维状或颗粒状粉末,无臭。
性状在无水乙醇、乙醚、丙酮中几乎不溶。
在80~90℃的热水中迅速分散、溶胀,降温后迅速溶解,水溶液在常温下相当稳定,高温时能凝胶,并且此凝胶能随温度的高低与溶液互相转变。
具有优良的润湿性、分散性、粘接性、增稠性、乳化性、保水性和成膜性,以及对油脂的不透性。
所成膜具有优良的韧性、柔曲性和透明度,因属非离子型,可与其他的乳化剂配伍,但易盐析,溶液在pH在2-12范围内稳定。
视密度:0.30-0.70g/cm3,密度约1.3g/cm3。
工业上甲基纤维素的理论取代度DS为1.5~2.0,松散密度0.35~0.55g/cm3。
质量指标编辑播报外观:灰白色纤维状至粉米状凝胶温度(2%水溶液):50~55℃甲氧基含量:26%~33%水不溶物:≤2.0%取代度(DS):1.3~2.0水分:≤5.0%黏度(20℃,2%水溶液):15~4000mPa·s应用用作水溶性胶黏剂的增稠剂,如氯丁胶乳的增稠剂。
也可用作氯乙烯、苯乙烯悬浮聚合的分散剂、乳化剂和稳定剂等。
DS=2.4~2.7的MC溶于极性有机溶剂,可阻止溶剂(二氯甲烷一乙醇混合物)的挥发。
鉴别1、取该品1%的水溶液适量,置试管中,沿管壁缓缓加0.035%蒽酮的硫酸溶液2mL,放置,在两液界面处显蓝绿色环。
2、取该品1%的水溶液10mL,加热,溶液产生雾状或片状沉淀,冷却后,沉淀溶解。
3、取该品1%的水溶液适量,倾倒在玻璃板上,俟水分蒸发后,形成一层有韧性的膜。
1. IntroductionOsteoarthritis, characterized by progressively degen-eration or loss of articular cartilage, is the most common form of arthritis. Tissue engineering has shown great promise as a strategy to develop bio-logical substitutes to restore, replace or regenerate the defective tissue [1]. Scaffolds made of poly-meric biomaterials offer support for cell attach-ment, proliferation and differentiation [2, 3]. Encap-sulation of living cells within a semi-permeable membrane is a simple one-step procedure with char-acteristics of homogenous cell distribution and excel-lent cell viability [4, 5]. Its unique self-assembled capability makes it suitable for injectable scaffolds for in situ tissue regeneration. Recent advances in microfluid designs have brought the field of micro -fluidics to the forefront of the preparation of micro-or nano-gels for cell encapsulation [6–8].Many naturally derived biomaterials have been used for encapsulation, such as sodium alginate [9] and agarose [10]. However, these biomaterials have limited ability to support cell attachment, growth and differentiation, resulting in low cell viability and growth [11]. Carboxymethyl cellulose (CMC) is a water-soluble, biodegradable and biocompatible derivative of cellulose. Its hydrophilic carboxylic or hydroxyl groups serve as active sites for preparing CMC gels. Physical-crosslinking CMC gels viasupermolecular [12] and ionic [13] interaction are simple to be produced, but usually questioned by their reversibility.Chemical-crosslinking provides CMC gels a more stable three-dimensional network. For example, divinyl sulfone [14], epichlorohydrin [15], aldehy-des [16, 17], fumaric acid [18] and citric acid [19] have been used as crosslinkers to form CMC and CMC composite gels. Monomers with double bond, such as N-isopropyl acrylamide [20] and partially neutralized acrylic acid/rectorite [21], have been ini-tiated by ammonium persulfate and coupled onto CMC backbones via methylene bisacrylamide (crosslinker). These chemical crosslinkers are lim-ited for the applications in biomedical or pharmaceu-tical areas because of their toxicity or the stimula-tory reaction to the encapsulated bioactive molecules or live cells. A bio-based carbodiimide crosslinker has been used to prepare chitosan/CMC microgels [22], indicating a very low efficiency of crosslink-ing reaction. Another synthetic route is to form CMC gels via functional groups of both components with-out any crosslinker, for example, hydrazide-func-tionalized CMC/aldehyde-functionalized dextran, suggested by Kesselman et al. [23]. They have intro-duced the two reactive streams into a continuous oil solution simultaneously through two separate inlets of a microfluidics device. However, the premixing of the reactive streams before the formation of droplets often resulted in the blockage of small opening of the microfluidics.Enzymatic reaction has been extensively studied owing to its low toxicity, mild reaction, stereo-chemistry, and high reaction velocity, high enantio-, regio- and chemo-selectivity [24]. Horseradish per-oxidise/hydrogen peroxide (HRP/H2O2) is a com-mon enzymatic system, where peroxidases (oxi-doreductases) catalyzes the oxidation of donors using H2O2, resulting in polyphenols linked at the aromatic ring by C–C and C–O coupling of phenols [25]. DeV older et al.[26] have prepared hydrogels of alginate grafted with pyrrole groups through a HRP-activated crosslinking reaction for drug release system. We designed a special microfluidic device to prepare monodisperse CMC-based microdroplets [27]. In this work, we synthesized 4-hydroxybenzy-lamine modified CMC (CMC-Ph) with different molecular weight and prepared CMC-Ph microgels through an enzymatic reaction. The properties of CMC-Ph that influenced the formation of microgels were studied. Moreover, cells were encapsulated in CMC-Ph microgels to study the biocompatibility of CMC-Ph and the microfluidic approach through the enzymatic crosslinking reaction.2. Experimental2.1. Materials1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC), N-hydroxysulfosuccinimide (NHS), HRP (250units/mg), lecithin, 4-hydroxyben-zylamine, 1-hydroxybenzotriazole hydrate (HOBT) and 2-(4-morpholino)ethanesulfonic acid (MES) were obtained from Qiyun Biotech (China). Aqueous H2O2(30%, w/w) and liquid paraffin were purchased from Dalu Chemical Reagent (China). All reagents were of analytical grade and used as received. CMC with M w of 1.0!105(CMC10), 2.0!105(CMC20) and 3.0!105(CMC30) were purchased from Jingchun Chemical Reagent (China). CMC-Ph was synthe-sized according to our previous study [27]: Briefly, CMC and 4-hydroxybenzylamine were dissolved in MES buffer (50mM, pH6.0) at 1.0 and 0.6%, respectively. NHS, HOBt and EDC were added at a weight ratio of 1:0.26:0.68:0.70 (CMC:NHS:HOBt: EDC). The solution was magnetic stirred for 24hours and dialyzed against deionized water using an ultrafiltration membrane (molecular weight cut-off= 3500Da) at 25°C for 4days. The resultant polymer solution was enriched by a rotary evapora-tor (100rpm) at 50°C and lyophilized.2.2. Preparation of CMC-Ph microgelsA co-flowing microfluidic device with an inner diameter of 260µm and an outer diameter of 510µm was used for preparing CMC-Ph microparticles. The continuous liquid paraffin phase was prepared as follows: In brief, 250mL of liquid paraffin con-taining 1.25mL H2O2was magnetically stirred for 12hours at 25°C and centrifuged at 2000rpm for 10minutes. Lecithin was dissolved at 3.0% (w/v) into the upper liquid paraffin containing H2O2. CMC-Ph aqueous solution containing HRP as the dispersed phase was injected into the microfluidic device using a micro-syringe pump (Baoding Longer TS-1B/W0109-1B, China), and the continuous phase was injected into an inlet in a perpendicular direction. The particles flew along the channel and finally collected.2.3. Preparation of cell-laden CMC-PhmicrogelsThe lyophilized CMC-Ph was sterilized by expo-sure to epoxyethane vapor. CMC-Ph (~0.2g) was dissolved in 4mL of Dulbecco’s modified Eagle medium (DMEM, Hyclone, USA). HRP was then dissolved at 1mg/mL in DMEM, and the solution was kept at 37°C. The chondrocytic cell line A TDC5 (Sigma, USA) of sixth passage was trypsinized at a density of 1!107cells/mL. 1mL of cell suspension was added into the CMC-Ph solution containing HRP, which injected as the dispersed phase at a rate of 50µL/min. The continuous liquid paraffin phase was injected at a rate of 10mL/min. The resulted microgels were collected and centrifuged at 2000rpm for 5min. Phosphate buffer saline (PBS, pH= 7.4, Gibco, USA) was added to the tube, fol-lowed by centrifuging at 2000rpm for 5min twice. The collected cell-laden CMC-Ph microgels were removed into 6-well cell culture dishes (Corning, USA). Cells enclosed in microgels were incubated in DMEM supplemented with 10% (v/v) fetal bovine serum (FBS, Gibco, USA) in a humidified atmos-phere at 37°C under 5% CO2. The medium was exchanged for fresh medium every 2days, and a new cell culture dish was replaced every 4 days till cell-culturing for 40days. The morphology of the cell-laden CMC-Ph microgels was evaluated under an inverted light microscope (Nikon ECLIPSE TS100, Japan).2.4. CharacterizationA Bruker (Germany) Vertex 70 Fourier transform infrared spectrometry (FTIR) was used to obtain infrared analyses of 4-hydroxybenzylamine, CMC and CMC-Ph using KBr pellet method. The spectra comprised 64scans at a resolution of 1cm–1in 4000~400cm–1spectral range.1H nuclear magnetic resonance (NMR, Drx-400 Bruker, Germany) spectra were achieved at 400MHz using deuterated water (D2O) as solvent in the pres-ence of tetramethylsilane as an internal standard. Graft density of phenols in CMC-Ph was calculated by measuring the absorbance at 275nm of CMC-Ph solutions using a Thermo (USA) Evolution 300 UV-VIS spectrometer. The absorbance being measured was compared with a 4-hydroxybenzylamine stan-dard curve. The graft density of phenols then calcu-lated from the ratio of phenols to 100repeat units of CMC. The data was the mean of five samples.Molecular weight and polydispersity index (PDI) of CMC and CMC-Ph were determined by a Water (USA) 515–410gel permeation chromatography (GPC). Weight-average (M w) and number-average (M n) molecular weight were expressed with respect to polyethyleneglycol standards. Rheological behavior of CMC-Ph solutions with different concentrations was measured using a TA (USA) ARES/RFS rotational viscometer at 16 and 30°C, respectively. The solutions were prepared by mass using a XS105DU balance (Mettler Toledo, Switzerland) with a precision of 10–5g. The employed shear rate varied from 0.01 to 250s–1, and viscosity and stress were identified. Stereomicroscope images of the microdroplets in the liquid paraffin were obtained with a SMZ-DM200 stereomicroscope (Optec, China) with a digital CCD camera to estimate the size in number-average diam-eters and coefficients of variation (CV, defined as the ratio of standard deviation to the mean) by ana-lyzing images of 100particles in liquid paraffin phase.3. Results and discussion3.1. CMC-Ph3.1.1. FTIR spectrumIn Figure1a, the absorption bands at 1450, 1501, 1561 and 1611cm–1were ascribed to the benzene skeleton vibration. The peaks at 842, 1261 and 1386cm–1were attributed to the C–H out-of-plane bending vibration of benzene ring, C–O stretching vibration and O–H in-plane bending vibration of phenol, respectively. The absorption peak at 1642cm–1was associated to N–H scissoring vibra-tion of free amine. The broad association peak at 3423cm–1would be the stretching vibration of C–H, O–H and N–H, in which a hydrogen bond formed between a hydrogen atom and O–H group or N–H group. In FTIR spectrum of CMC-Ph (Figure1b, curve(2)), the absorption bands at 1045, 1323 and 1415cm–1were attributed to the –C=O stretching vibration, in-plane bending vibration of O–H and C–H scissoring vibration of methylene of CMC, respectively (Figure1b, curve(1)). The characteris-tic absorption bands of 4-hydroxybenzylamine were traced, including the C–H out-of-plane bending vibration of benzene ring (827cm–1), C–O strong stretching vibration of phenol (1257cm–1), benzene skeleton vibration (1504 and 1456cm–1) and N–H scissoring vibration of free amine (1644cm–1). Theband with a peak at 1579cm–1 was the IR absorption of the –O–stretching vibration of CMC (1592cm–1, Figure1b, curve(1)) and the benzene skeleton vibra-tion (1561 and 1611cm–1).3.1.2. 1H NMR spectraFigure2 illustrates 1H NMR spectra along with schematics of their chemical structures, on which proton assignments are indicated. 4-Hydroxybenzy-lamine (Figure2a) showed chemical shifts (") at 6.60 and 7.06ppm of aromatic protons (a) and (b), respectively, and at 3.81ppm of aliphatic protons (c). The spectra of CMC (Figure2b) and CMC-Ph (Figure2c) showed " of anomeric protons at 4.15 and 4.42ppm, and sugar ring protons between 3.11 and 3.99ppm for both macromolecules [28]. The signals at 7.21 and 6.84 ppm of CMC-Ph spectrum were attributed to the aromatic protons of 4-hydrox-ybenzylamine being grafted with CMC (CMC-g-Ph, 1a and 1b). The strong inductive effect being caused by the neighboring carbonyl groups decreased the electron density of the benzene ring, so that " of aromatic protons decreased compared with those of 4-hydroxybenzylamine (Figure2a, (a) and (b)). The signals at 1.79~2.02ppm (2a), 0.96~1.19ppm (2b) and 2.75ppm (2c) were ascribed to the byproduct being formed between CMC and EDC (CMC-EDC). EDC and phenolic groups of 4-hydroxybenzylamine reacted to form CMC-g-Ph-EDC, resulting in a change in proton shift of the aromatic protons at 7.51 and 7.35ppm (3a and 3b) [29].3.1.3. Graft density of phenolsThe CMC-Ph aqueous solution showed a specific absorbance with a peak at 275nm of 4-hydroxy-benzylamine. We also tested the dialysis solution at four days, the UV absorption was not detected, so that the UV absorption of the CMC-Ph solution would be ascribed to the grafted 4-hydroxybenzy-lamine. Based on the standard curve, the amount of the grafted 4-hydroxybenzylamine per 100units of CMC (grafting density) was calculated (Table1). It fell in the range of 17~23, and increased as M w of CMC increased. Usually, the flexibility of polymer increased with the increasing molecular weight, so that CMC with higher M w or M n was favorable to collide effectively with small molecules via chang-ing conformation, leading to a higher reaction prob-ability of CMC with 4-hydroxybenzylamine.3.1.4. Molecular weightThe molecular weight and the polydispersity (PI) of the molecular weight were also shown in Table1. M w, M n and PI of CMC-Ph were higher than those of CMC. The difference of molecular weight between CMC and CMC-Ph increased as the molecular weight of CMC increased, perhaps owing to a slight cross -linking between hydroxyl groups of 4-hydroxyben-zylamine and carboxyl groups of CMC under EDC/ NHS reactive system.Figure 1.FTIR spectra of 4-hydroxybenzylamine(a), CMC(b-(1)) and CMC-Ph(b-(2))3.1.5. ViscosityRheological behavior of the CMC-Ph solution with the different molecular weight has been determined by analyzing the influence of shear rate on viscos-ity. Flow curve was a straight line passing through the origin, and the stress increased lineally with the shear rate at 30°C, respectively (Figure3 (a2), (b2), (c2). An increase in the molecular weight produced an increase in the viscosity (Figure3). For example, the viscosity was 5.4, 14.8 and 25.5mPa·s, respec-tively for CMC10-Ph, CMC20-Ph and CMC30-Ph at 30°C. As the molecular weight of CMC-Ph increased, the intermolecular volume decreased and thus the molecular interaction of CMC-Ph increased. The CMC-Ph with high molecular weight possessed low ability to rearrange and move past each other, soFigure 2.1H NMR spectra of 4-hydroxybenzylamine(a), CMC(b) and CMC-Ph(c) with protons assignedthat the internal resistance to flow increased. There-fore, the viscosity increased with the increasing molecular weight of CMC-Ph.At 16°C, the viscosity of CMC 30-Ph decreased sig-nificantly with the increasing shear rate. When the shear rate was 215s –1, the viscosity was 9.9, 19.1 and 26.6mPa·s, respectively for CMC 10-Ph, CMC 20-Ph and CMC 30-Ph. In general, an increase in tempera-ture allows quicker molecules motion and thus less energy is needed to flow CMC-Ph solution, result-ing in a decrease in the viscosity. When the shear rate was 215s –1, the difference between viscosity meas-uring at 16 and 30°C was 4.45, 4.31 and 1.08mPa·s for CMC 10-Ph, CMC 20-Ph and CMC 30-Ph, respec-tively. The less difference of CMC 30-Ph was per-haps owing to the decreasing viscosity as the shear rate increased. As the shear rate was 215s –1, the viscosity ratio of CMC 30-Ph to CMC 10-Ph at 16°C was 2.7, which reached 4.7 when temperature went up to 30°C.3.2. CMC-Ph microgelsPrior to the microfluidic preparation of CMC-Ph microgels, we examined the gelation of the disperse fluid as the presence of H 2O 2. The CMC-Ph gels were formed after a contact time of 1min. In the microfluidic device, the viscous disperse fluid was extruded into the immiscible continuous liquid flowing in the same direction. The disperse fluid flowed and snapped off at the orifice. H 2O 2in the continuous liquid surrounded the CMC-Ph droplets containing HRP. Thereafter, diffusion of H 2O 2from the continuous fluid to the disperse phase triggered the gelation reaction to bind the phenols groups.Because H 2O 2was separated from HRP, the enzy-matic reaction cannot occur before the droplets for-mation, thus avoiding the blockage of the inner opening. The concentration of H 2O 2in the continu-ous phase (0.82mmol/L) would not bring severe harmful effect on the encapsulated contents, but it was enough for gelation of the microdroplets. The CMC-Ph microgels were intact while immersed in DMEM for 40days. This diffusion-controlled cross-linking was very important for improving the integrity and encapsulation efficiency of micropar-ticles.We prepared the CMC-Ph microdroplets with dif-ferent molecular weight while using the 1% CMC-Ph containing HRP (1mg/mL). As the flow rate of the disperse phase (Q d ) was fixed at 10µL/min, the effect of the flow rate of the continuous phase (Q c )on the radius of microdroplets was shown in Fig-ure 4a. The increased flow rate of the continuous phase produced a stronger shear force, thus resulted in a decreasing radius of the CMC-Ph microdroplets.Figure 3. Viscosity of 1.0% CMC-Ph solution being meas-ured at 16°C: (a 1)CMC 30-Ph; (b 1)CMC 20-Ph;(c 1)CMC 10-Ph, and at 30°C: (a 2)CMC 30-Ph;(b 2)CMC 20-Ph; (c 2)CMC 10-PhFigure 4.Dependence of flow rates on the size of the microdroplets obtained by using 1.0% CMC 10-Ph (triangle), CMC 20-Ph (circle) and CMC 30-Ph (square): (a)effect of Q c on the radius of microdroplets at a constant Q d (10µL/min);(b)effect of Q d on the radius of microdroplets at a fixed Q c (10mL/min)When using a fixed Q c(10mL/min), the radius of the CMC-Ph microdroplets increased with an increas-ing flow rate of the disperse fluid (Figure4b). In general, the CMC-Ph solution with higher molecu-lar weight produced larger microdroplets due to higher viscosity.3.3. Cell-laden CMC-Ph microgelsThe injectable microsphere scaffolds should sup-port cells adhesion, migration, and proliferation, and more important, maintain the differentiated phenotype of the cells within the scaffold. The cell-laden microgels would facilitate gas exchange, nutrient diffusion, and waste metabolism. ATDC5 is a prechondrogenic stem cell line, and reproduces the differentiation stages of chondrocytes during endochondral bone formation [30]. The ATDC5-laden microgels being cultured up to 40days were shown in Figure5. The microgels presented round morphology that was very important for mechanical stability under the compressive forces in the body. The living cells (light dots) were distributed sepa-rately in the microgels (Figure5a). Some of the cell-laden microgels broke with the damaged border. The microgel did not maintain the round morphol-ogy any more (Figure5b). Some of the cells released from the broken microgels and can stick to the cell culture dishes at 40days of culturing (Figure5c), showing high viability.4. Conclusions4-Hydroxybenzylamine modified CMC with differ-ent molecular weight was synthesized through EDC/ NHS coupling agents. Uniform CMC-Ph micropar-ticles were obtained in the co-flowing microfluidic devices. The radius was tuned in the range of (100~ 500)µm by changing the flow rates of the disperse phase and the continuous phase, respectively. Thecells encapsulated in CMC-Ph microgels were still living at 40days of culturing. The microfluidic approach to the preparation of the cell-laden micro-gels will provide a potential method of fabricating scaffolds for tissue engineering, especially in the defect with an irregular-shape and/or a minimally invasive approach. The CMC-Ph microgels with the different molecular weight along with the different encapsulating content may also been used to pre-pare the injectable microsphere scaffolds, having a gradient mechanical property, a gradient encapsu-lating content, and a gradient releasing property. AcknowledgementsThis study was financially supported by the Basic Research Project of China (2012CB619105), the National Natural Science Foundation of China (51072056, 51173053), Guang-dong Natural Science Foundation (9451063201003024), Guangdong Provincial Program for Excellent Talents in Universities, and Key Laboratory of Biomaterials of Guang-dong Higher Education Institutes of Jinan University.Figure 5.Photographs of the cell-laden microgels up to 40days of culturing: (a)living cells distributedseparately at 40days; (b)broken cell-laden micro-gels at 5days; (c)cells from the broken microgelssticking to the dishes at 40daysReferences[1]Langer R., Vacanti J. P.: Tissue engineering. Science,260, 920–926 (1993).DOI:10.1126/science.8493529[2]Hutmacher D. W.: Scaffolds in tissue engineering boneand cartilage. Biomaterials, 21, 2529–2543 (2000).DOI:10.1016/S0142-9612(00)00121-6[3]Ke Y., Wu G., Wang Y. J.: PHBV/PAM scaffolds withlocal oriented structure through UV polymerization for tissue engineering. BioMed Research International, 2014, 157987/1–157987/9 (2014).DOI:10.1155/2014/157987[4]Lanza R. P., Hayes J. L., Chick W. L.: Encapsulated celltechnology. Nature Biotechnology, 14, 1107–1111 (1996).DOI:10.1038/nbt0996-1107[5]Orive G., Hernández R. M., Gascón A. R., Calafiore R.,Chang T. M. S., De V os P., Hortelano G., Hunkeler D., Lacík I., Shapiro A. M. J., Pedraz J. L.: Cell encapsula-tion: Promise and progress. Nature Medicine, 9, 104–107 (2003).DOI:10.1038/nm0103-104[6]Dendukuri D., Doyle P. S.: The synthesis and assem-bly of polymeric microparticles using microfluidics.Advanced Materials, 21, 4071–4086 (2009).DOI:10.1002/adma.200803386[7]Lazarus L. L., Riche C. T., Marin B. C., Gupta M.,Malmstadt N., Brutchey R. L.: Two-phase microfluidic droplet flows of ionic liquids for the synthesis of gold and silver nanoparticles. ACS Applied Materials and Interfaces, 4, 3077–3083 (2012).DOI:10.1021/am3004413[8]Ke Y.: Microfluidic-assisted fabrication of nanoparti-cles for nanomedicine application. Recent Patents on Nanomedicine, 1, 109–122 (2011).DOI:10.2174/1877912311101020109[9]Sakai S., Kawakami K.: Both ionically and enzymati-cally crosslinkable alginate–tyramine conjugate as materials for cell encapsulation. Journal of Biomedical Materials Research Part A, 85, 345–351 (2008).DOI:10.1002/jbm.a.31299[10]Batorsky A., Liao J., Lund A. W., Plopper G. E., Stege-mann J. P.: Encapsulation of adult human mesenchy-mal stem cells within collagen-agarose microenviron-ments. Biotechnology and Bioengineering, 92, 492–500 (2005).DOI:10.1002/bit.20614[11]Zimmermann H., Hillgärtner M., Manz B., Feilen P.,Brunnenmeier F., Leinfelder U., Weber M., Cramer H., Schneider S., Hendrich C., V olke F., Zimmermann U.: Fabrication of homogeneously cross-linked, functional alginate microcapsules validated by NMR-, CLSM- and AFM-imaging. Biomaterials, 24, 2083–2096 (2003).DOI:10.1016/S0142-9612(02)00639-7[12]Liu H., Huang S., Li X., Zhang L., Tan Y., Wei C., LvJ.: Facile fabrication of novel polyhedral oligomeric silsesquioxane/carboxymethyl cellulose hybrid hydro-gel based on supermolecular interactions. Materials Letters, 90, 142–144 (2013).DOI:10.1016/j.matlet.2012.09.030[13]Zhang H., Tumarkin E., Peerani R., Nie Z. H., SullanR. M. A., Walker G. C., Kumacheva E.: Microfluidic production of biopolymer microcapsules with con-trolled morphology. Journal of the American Chemical Society, 128, 12205–12210 (2006).DOI:10.1021/ja0635682[14]Butun S., Ince F. G., Erdugan H., Sahiner N.: One-stepfabrication of biocompatible carboxymethyl cellulose polymeric particles for drug delivery systems. Carbo-hydrate Polymers, 86, 636–643 (2011).DOI:10.1016/j.carbpol.2011.05.001[15]Chang C., He M., Zhou J., Zhang L.: Swelling behav-iors of pH- and salt-responsive cellulose-based hydro-gels. Macromolecules, 44, 1642–1648 (2012).DOI:10.1021/ma102801f[16]Rao K. M., Mallikarjuna B., Rao K. S. V. K., Prab-hakar M. N., Rao K. C., Subha M. C. S.: Preparation and characterization of pH sensitive poly(vinyl alco-hol)/sodium carboxymethyl cellulose IPN micros-pheres for in vitro release studies of an anti-cancer drug.Polymer Bulletin, 68, 1905–1919 (2012).DOI:10.1007/s00289-011-0675-9[17]Patenaude M., Hoare T.: Injectable, mixed natural-synthetic polymer hydrogels with modular properties.Biomacromolecules, 13, 369–378 (2012).DOI:10.1021/bm2013982[18]Akar E., Altını#ık A., Seki Y.: Preparation of pH- andionic-strength responsive biodegradable fumaric acid crosslinked carboxymethyl cellulose. Carbohydrate Polymers, 90, 1634–1641 (2012).DOI:10.1016/j.carbpol.2012.07.043[19]Gorgieva S., Kokol V.: Synthesis and application ofnew temperature-responsive hydrogels based on car-boxymethyl and hydroxyethyl cellulose derivatives for the functional finishing of cotton knitwear. Carbohy-drate Polymers, 85, 664–673 (2011).DOI:10.1016/j.carbpol.2011.03.037[20]Ekici S.: Intelligent poly(N-isopropylacrylamide)-car-boxymethyl cellulose full interpenetrating polymeric networks for protein adsorption studies. Journal of Materials Science, 46, 2843–2850 (2011).DOI:10.1007/s10853-010-5158-0[21]Wang W. B., Wang A. Q.: Preparation, swelling, andstimuli-responsive characteristics of superabsorbent nanocomposites based on carboxymethyl cellulose and rectorite. Polymers for Advanced Technologies, 22, 1602–1611 (2011).DOI:10.1002/pat.1647[22]Dhar N., Akhlaghi S. P., Tam K. C.: Biodegradable andbiocompatible polyampholyte microgels derived from chitosan, carboxymethyl cellulose and modified methyl cellulose. Carbohydrate Polymers, 87, 101–109 (2012).DOI:10.1016/j.carbpol.2011.07.022[23]Kesselman L. R. B., Shinwary S., Selvaganapathy P.R., Hoare T.: Synthesis of monodisperse, covalently cross-linked, degradable ‘smart’ microgels using micro -fluidics. Small, 8, 1092–1098 (2012).DOI:10.1002/smll.201102113[24]Kobayashi S., Uyama H., Kalra B.: Enzymatic poly-merization. Chemical Reviews, 101, 3793–3813 (2001).DOI:10.1021/cr990121l[25]Kurisawa M., Chung J. E., Yang Y. Y., Gao S. J., UyamaH.: Injectable biodegradable hydrogels composed ofhyaluronic acid–tyramine conjugates for drug delivery and tissue engineering. Chemical Communications, 34, 4312–4314 (2005).DOI:10.1039/B506989K[26]DeV older R., Antoniadou E., Kong H. J.: Enzymati-cally cross-linked injectable alginate-g-pyrrole hydro-gels for neovascularization. Journal of Controlled Release, 172, 30–37 (2013).DOI:10.1016/j.jconrel.2013.07.010[27]Ke Y., Liu G. S., Guo T., Zhang Y., Li C., Xue W., WuG., Wang J., Du C.: Size controlling of monodispersecarboxymethyl cellulose microparticles via a microflu-idic process. Journal of Applied Polymer Science, 131, 40663 (2014).DOI:10.1002/app.40663[28]Darr A., Calabro A.: Synthesis and characterization oftyramine-based hyaluronan hydrogels. Journal of Mate-rials Science: Materials in Medicine, 20, 33–44 (2009).DOI:10.1007/s10856-008-3540-0[29]Castillo J. J., Torres M. H., Molina D. R., Castillo-León J., Svendsen W. E., Escobar P., Martínez F.: Mon-itoring the functionalization of single-walled carbon nanotubes with chitosan and folic acid by two-dimen-sional diffusion-ordered NMR. Carbon, 50, 2691–2697 (2012).DOI:10.1016/j.carbon.2012.02.010[30]Atkinson B. L., Fantle K. S., Benedict J. J., Huffer W.E., Gutierrez-Hartmann A.: Combination of osteoin-ductive bone proteins differentiates mesenchymal C3H/ 10T1/2 cells specifically to the cartilage lineage. Jour-nal of Cellular Biochemistry, 65, 325–339 (1997).DOI:10.1002/(SICI)1097-4644(19970601)65:3<325:: AID-JCB3>3.0.CO;2-U。