Palladium
- 格式:doc
- 大小:58.00 KB
- 文档页数:6

化学元素钯的符号是Pd。
钯(Palladium,化学符号:Pd)是一种化学元素,原子序数为46,位于周期表的第5周期、第Ⅷ族(也称作8B族),属于过渡金属。
钯呈银白色,具有光泽,延展性好,是铂族金属的一员,与铂、铑、钌、铱和锇等元素在物理和化学性质上有许多相似之处。
钯的主要物理性质包括:
- 密度约为12.023 g/cm³。
- 熔点相对较低,在铂族金属中熔点最低,约为1555°C。
- 沸点较高,约2927°C。
- 在常温下耐腐蚀性能优良。
钯的化学性质稳定,常见氧化态为+2和+4,但最常见的是+2状态。
它能形成多种配合物,并且对许多气体有良好的吸附能力,如氢气,因此被广泛应用于催化反应,尤其是在汽车尾气净化系统中的催化剂。
钯在工业上用途广泛,除了用作催化剂外,还用于制造珠宝首饰、牙科材料、电子电气工业部件以及作
为投资产品(如钯金条或金币)。
此外,由于其优越的化学稳定性及导电性能,钯也被用于制作各种精密电阻器和其他电子元件。

化学及化工专业词汇英语翻译(P-Z)package 包装packed absorption tower 填充吸收塔packed column 填充塔packed dyeing 填充染色packed reaction column 填充反应塔packed rectification tower 填充精馏塔packed tower 填充塔packing 填充物packing density 填充密度packing paper 包装纸padding 表面染色paddle agitator 桨式混合机paddle mixer 桨式混合机paint 涂料paint hardner 镀金粘料paint remover 油漆去除剂paint stripper 油漆去除剂pair annihilation 对湮没pair creation 正负电子对产生pair ion 离子对pale crepe 苍铍胶palladium 钯palladium asbestos 钯石棉palladium black 钯黑palladium chloride 氯化钯palladium nitrate 硝酸钯palladium oxide 氧化钯palladium sponge 海绵钯palm kernel oil 棕榈仁油palm oil 棕榈油palmarosa oil 掌玫油palmitate 棕榈酸盐palmitic acid 棕榈酸palmitin 棕榈精palmitone 棕榈酮pan mill 碾磨盘panchromatic film 全色胶片panchromatic plate 全色干片pancreatin 胰酶pancreozymin 肠促胰酶素pangamic acid 潘氨酸pantothenic acid 泛酸papain 木瓜酶papaverine 罂粟碱paper chromatography 纸色谱分析法paper electrophoresis 纸电泳paper filter 纸滤器paper industry 造纸工业paper machine 造纸机paper pulp 纸浆paper stencilling 刻花纸paper yarn 纸绳para aminohippuric acid 对氨基马尿酸para hydrogen 仲氢para isomer 对位异构体para molecule 仲分子para position 对位para red 对位红para rubber 三叶橡胶paraaldehyde 仲醛parabanic acid 仲班酸parabola 抛物线paracasein 副酪朊parachor 等张比容paraconic acid 仲康酸paracyanogen 仲氰paraffin 石蜡paraffin base crude 石蜡基原油paraffin hydrocarbon 烷属烃paraffin oil 石蜡油paraffin stock 石蜡原油paraffin wax 石蜡paraformaldehyde 仲甲醛parafuchsine 副品红paragenesis 共生paragonite 钠云母paraldol 仲醛醇parallel flow 并流平行流parallel reaction 平行反应paralysis 麻痹paralyzer 麻痹药paramagnetic resonance 顺磁共振paramagnetic substance 顺磁质paramagnetism 顺磁性parameter 参数paramine 对胺paramorphism 同质异晶paranitroaniline red 对位红pararosaniline 副蔷薇胺parastrophe 对位取代化合物parathion 硝苯硫磷酯paraxanthine 副黄嘌呤paraxylene 对二甲苯parchment 羊皮纸parchment paper 仿羊皮纸parent name 母体名parent substance 母体parinol 苯吡醇paris green 巴黎绿paris white 巴黎胡粉白parity 奇偶性parkerizing 磷化处理parpon 帕尔庞partial combustion 部分燃烧partial condensation 部分冷凝partial condenser 分馏柱partial molar quantity 偏摩尔数量partial molar volume 偏摩尔体积partial pressure 分压partial reaction 部分反应partial valence 余价participation of neighboring group 邻基参与particle 粒子particle counting 粒子计数法particle diameter 颗粒直径particle electrophoresis 粒子电泳particle induced x ray emission 粒子诱导 x 射线发射particle scattering factor 粒子散射因数particle size 粒度particle size analysis 粒径分析particle thickness technique 质点厚度测量技术parting 分离parting agent 分离剂partition 配分partition chromatography 配分色层法partition coefficient 配分系数partition function 配分函数partition wall 隔墙passivation 钝化酌passivation potential 钝化电势passive state 钝态passivity 钝态paste 糊剂paste mixer 惮机paste paint 厚漆paste resin 糊状尸pasteboard 纸板pasted plate 涂浆的板pasteurization 巴氏杀菌pasteurizer 巴氏灭菌器pasting 糊化patchouli oil 绿叶油patent 专利pattern 图样pattern recognition 模式辫认peak 峰值peak inverse voltage 最大反向电压peak load 峰值负荷peak potential 顶点电位peanut oil 花生油pearl 珍珠pearl ash 珍珠灰pearl essence 珍珠粉pearl polymerization 成珠聚合pearl white 锌钡白pearlite 珍珠岩peat 泥炭peat tar 泥煤焦油pebble mill 砾磨机pectic acid 果胶酸pectic substances 果胶质pectin 果胶pectinase 果胶酶pectization 凝结pectocellulose 果胶纤维素peeling 剥落peep hole 窥孔pegmatite 伟晶岩pelargonaldehyde 壬醛pelargonic acid 正壬酸pelargonidin 花葵素pellet 粒状pellet cooler 颗粒冷却器pelletizer 造粒机pellotine 佩落碱pendulum mill 摆旋式研磨机penetrability 穿透性penetrant 渗透剂penetrating agent 渗透剂penetration 穿透;渗入度penetration complex 透入配合物penetration test 贯入度试验penetrator 针入度计penetrometer 透度计penicillase 青霉素酶penicillin 青霉素pentacarbonyl iron 五羰基铁pentacene 并五苯pentachlorophenol 五氯酚pentadiene 戊二烯pentaerythritol 季戊四醇pentaerythritol tetranitrate 季戊四醇四硝酸酯pentamethylene 环戊烷pentamethylenediamine 戊甲撑二胺pentane 戊烷pentane thermometer 戊烷温度计pentanol 戊醇pentasol 成氯醇pentene 戊烯penthrit 季戊四醇四硝酸酯pentlandite 镍黄铁矿pentobarbital 戊巴比妥pentosan 多缩戊糖pentose 戊糖pentose phosphate cycle 戊糖磷酸循环pentose phosphate pathway 戊糖磷酸途径pentyl alcohol 戊醇pentyne 戊炔peppermint 薄荷peppermint oil 薄荷油pepsin 胃蛋白酶pepsinogen 胃蛋白酶原peptidase 肽酶peptide 肽peptide bond 肽键peptide linkage 肽键peptide synthetase 肽合成酶peptidoglycan 肽聚糖peptization 胶溶酌peptized fuel 胶溶燃料peptizer 胶溶剂peptizing agent 胶溶剂peptone 胨peracetic acid 过乙酸peracid 过酸peralcohol 过醇perbenzoic acid 过苯酸perborate 过硼酸盐perboric acid 过硼酸perbromate 过溴酸盐perbromic acid 过溴酸percarbonate 过碳酸盐percent grafting 接枝率perchlorate 高氯酸盐perchloric acid 高氯酸perchloride 高氯化物perchloroethane 六氯乙烷percolation 沥滤percolator 渗滤器percussion cap 雷管percussion powder 雷管火药perfect analysis 完全分析perfect black body 完全黑体perfect combustion 完全燃烧perfect crystal 完整晶体perfect elasticity 完全弹性perfect fluid 理想铃perfect gas 完美气体perfect mixing 完全混合perfect solution 完美溶液perfluorocyclobutane 过氟化环丁烷perforated pipe distributor 喷淋器多孔管分布器perforated plate 多孔板perforated plate distillation 多孔板蒸馏perforated plate tower 孔板塔performance testing 性能试验performic acid 过甲酸perfume 香料perfume extracting solvent 香料抽出溶剂perfume industry 香料工业perhydride 过氢化物perhydrol 强双氧水periclase 方镁石perilla oil 紫苏子油period 周期periodate 高碘酸盐periodic acid 高碘酸periodic law 周期律periodic precipitation 间歇沉淀periodic reaction 周期反应periodic system of elements 元素周期系periodic table 周期表periodide 高碘化物periphery 边界periplogenin 杠柳甙元perissad 奇价元素peritectic 包晶peritectic point 包晶点perkin reaction 佩金反应permanence 持久性permanent current 持恒电流permanent dipole 永久偶极子permanent expansion 永久膨胀permanent gas 永久气体permanent hardness 永久硬度permanent magnet 永磁铁permanent set 永久变形permanganate 高锰酸盐permanganate bleaching 高锰酸盐漂白permanganate titration 高锰酸盐滴定法permanganic acid 高锰酸permanganometry 高锰酸盐滴定法permeability 渗透性permeable membrane 可透膜permeameter 渗透计permeametry 渗透测定法permeation 渗透permissible error 容许误差permissible explosive 安全炸药permitted explosive 安全炸药permselective membrane 选择性渗透膜permselectivity 选择渗透性permutation 置换permutite 人造沸石perovskite 钙钛矿peroxidase 过氧物酶peroxide 过氧化peroxide effect 过氧化物效应peroxide index 过氧化值peroxide number 过氧化值peroxo acid 过氧基酸peroxy acid 过酸peroxydisulfuric acid 过硫酸perpetual motion 永久运动perphenazine 佩吩嗪perrhenate 高铼酸盐perrhenic acid 高铼酸perruthenate 过钌酸盐persalt 过酸盐persian red 铬红persistent current 持恒电流personal error 人为误差persorption 吸混酌persulfate 过硫酸盐persulfide 过硫化物persulfuric acid 过硫酸peru balsam 秘鲁香脂pesticide 杀虫剂pestle 杵棒pethidine 哌替啶pethidine hydrochloride 盐酸哌替啶petri dish 陪替氏培养皿petrochemical industry 石油化学工业petrochemical reaction 石油化学反应petrochemicals 石油化学产品petrochemistry 石油化学petrolatum 矿脂petroleum 石油petroleum acid 石油酸petroleum asphalt 石油沥青petroleum benzine 石油醚petroleum chemicals 石油化学产品petroleum chemistry 石油化学petroleum coke 石油焦炭petroleum distillation 石油蒸馏petroleum emulsion 石油乳剂petroleum ether 石油醚petroleum gas 石油气体petroleum grease 石油润滑脂petroleum industry 石油工业petroleum isomerization process 石油异构化过程petroleum jelly 矿脂petroleum pitch 石油沥青petroleum pitch coke 石油沥青焦炭petroleum product 石油产品petroleum refinery 石油加工厂petroleum refining 石油提炼petroleum refining industry 石油炼制工业petroleum resin 石油尸petroleum wax 石油蜡ph 氢指数ph electrode ph 电极ph indicator ph 指示剂ph meter ph 计ph standard solution ph 标准溶液ph test paper ph 试验纸ph value ph 值phanerocrystalline 显晶质pharmaceutical chemistry 药物化学pharmaceutics 制药学pharmacology 药理学pharmacy 药学phase 相phase average 相平均phase boundary 相界phase boundary potential 相界电位phase change 相转移phase contrast microscope 相差显微镜phase diagram 相图phase equilibrium 相平衡phase modulation 相位灯phase rule 相律phase sensitive detection 相敏检波phase separation 相分离phase shifter 移相器phase solubility 相溶解度phase solubility analysis 相溶度分析phase space 相空间phase titration 相滴定phase trajectory 相位轨道phase transfer catalyst 相转移催化剂phase transition 相转移phellandren 菲兰烯phenacetin 非那巍phenacite 硅铍石phenacyl alcohol 苯甲酰甲醇phenacyl bromide 苯甲酰甲基溴phenadon 非那酮phenamine 非那明phenanthrene 菲phenanthrenequinone 菲醌phenanthroline 二氮杂菲phenanthroline indicator 菲绕啉指示剂phenazine 吩嗪phenetidin 氨基苯乙醚phenetidine 氨基苯乙醚phenetole 苯乙醚phenobarbital 苯巴比妥phenocryst 斑晶phenol 酚phenol ether 苯酚醚phenol formaldehyde resin 苯酚甲醛尸phenol lignin 苯酚木素phenol red 酚红phenolase 酚酶phenolate 酚盐phenolic acid 酚酸phenolic resin 酚尸phenolic resin varnish 酚醛清漆phenolphthalein 酚酞phenolsulfonic acid 苯酚磺酸phenoplast 酚醛塑料phenosafranine 酚藏花红phenothiazine 吩噻嗪phenoxazine 吩恶phenoxide 酚盐phenoxyacetic acid 苯氧基乙酸phenyl acetate 醋酸苯酯phenyl ether 苯基醚phenyl isocyanate 异氰酸苯酯phenyl salicylate 萨罗phenylacetaldehyde 苯乙醛phenylacetamide 苯乙酰胺phenylacrylic acid 苯基丙烯酸phenylalanine 苯丙氨酸phenylbutazone 苯基丁氮酮phenylcinchonic acid 辛可芬phenylene 苯撑phenylenediamine 撑二氨phenylethyl acetate 醋酸苯乙酯phenylethyl alcohol 苯基乙醇phenylethyl isobutyrate 异丁酸苯乙酯phenylethyl propionate 丙酸苯乙酯phenylethylacetic acid 苯乙基醋酸phenylhydrazine 苯肼phenylmagnesium bromide 溴化苯镁phenylmagnesium chloride 氯化苯镁phenylmagnesium halide 卤化苯镁phenylmagnesium iodide 碘化苯镁phenylmercuric acetate 核酸汞phenylmercuric chloride 氯化苯汞phenylmorpholine 苯替吗啉phenylphenol 苯基苯酚phenylpiperazine 苯基哌嗪pheromone 信息素phlogiston 燃素phlorizin 根皮甙phloroglucinol 根皮山梨醇phonochemistry 声化学phonon 声子phorone 佛尔酮phosgene 光气phosphamide 磷酰胺phosphamidon 磷酰胺酮phosphatase 磷酸酶phosphate 磷酸盐phosphate glass 磷酸盐玻璃phosphatic ferfilizer 磷肥phosphatidic acid 磷脂酸phosphatizing 磷酸盐处理phosphide 磷化物phosphine 磷化氢phosphite 亚磷酸盐phosphocreatine 磷酸肌酸phosphoenolpyruvate 磷酸烯醇丙酮酸phospholipid 磷脂phosphomolybdate 磷钼酸盐phosphomolybdic acid 磷钼酸phosphomonoesterase 磷酸单酯酶phosphoprotein 磷蛋白phosphor 磷光体phosphor bronze 磷青铜phosphor salt 磷盐phosphorescence 磷光phosphoretted hydrogen 磷化氢phosphoric acid 磷酸phosphoric acid anhydride 磷酐phosphorimetric analysis 磷光分析phosphorimetry 磷光分析phosphorite 磷钙土phosphoro organic insecticide 磷有机杀虫剂phosphorolysis 磷酸解phosphoroscope 磷光计phosphorous acid 亚磷酸phosphorous anhydride 亚磷酸酐phosphorus 磷phosphorus chloride 氯化磷phosphorus nitride 氮化磷phosphorus oxychloride 磷酰氯phosphorus pentabromide 五溴化磷phosphorus pentachloride 五氯化磷phosphorus pentasulfide 五硫化二磷phosphorus sesquisulfide 三硫化四磷phosphorus tribromide 三溴化磷phosphorus trichloride 三氯化磷phosphorus trifluoride 三氟化磷phosphorus triiodide 三碘化磷phosphoryl chloride 磷酰氯phosphorylase 磷酸化酶phosphotungstic acid 磷钨酸photobiochemistry 光生物化学photocatalysis 光催化酌photocell 光电池photochemical catalysis 光催化photochemical equilibrium 光化学平衡photochemical reaction 光化反应photochemistry 光化学photochromic reaction 彩色反应photochromism 光致变色现象photochromy 彩色照相术photoconductor 光电导体photocurrent 光电流photodetachment 光致分离photodimerization 光二聚酌photodissociation 光解酌photodosimetry 照相剂量学photoelasticity 光弹性photoelectric cell 光电池photoelectric color comparator 比色仪photoelectric colorimeter 光电比色计photoelectric colorimetry 光电比色法photoelectric effect 光电效应photoelectric fraction collector 光电分级收集器photoelectric pyrometer 光电高温计photoelectric spectrophotometer 光电分光光度计photoelectric tube 光电管photoelectrochemistry 光电化学photoelectron 光电子photoemission 光电发射photofission 光致裂变photogel 摄影煤photographic characteristic curve 照相特性曲线photographic chemistry 照相化学photographic contrast 照相对比度photographic densitometer 照相密度计photographic density 照相底片密度photographic developer 显象剂photographic development 照相显相photographic emulsion 照相乳胶photographic emulsion layer 感光层photographic film 胶片photographic material 照相材料photographic negative 照相底片photographic paper 印相纸photographic plate 照相底片photographic positive 照相正片photographic process 照相法photography 照相photogyration 照相底片photohomolysis 光同种溶解photoionization 光致电离photoisomer 光异构体photolithography 照相平版印刷photoluminescence 光致发光photolysis 光分解photomeson 光介子photometer 光度计photometric titration 光度滴定photon 光子photonuclear reaction 光致核反应photooxidation 光氧化photophoresis 光泳现象photophosphorylation 光磷酸化photopigment 光色素photopolymer 光聚合物photopolymerization 光聚合photoreactive chlorophyll 光反应性叶绿素photoreduction 光致还原photorespiration 光呼吸photosensitive glass 光敏玻璃photosensitive polymer 光敏聚合物photosensitive resin 感光尸photosensitivity 光敏性photostructural transformation 光结构转变photosynthesis 光合酌phototropy 光致变色现象phototube 光电管phthalaldehyde 酞醛phthalaldehydic acid 苯醛酸phthalamide 酞酰胺phthalate 邻苯二甲酸盐phthalazine 酞嗪phthalein 酞phthalein dye 酞类染料phthalic acid 酞酸phthalic anhydride 酞酐phthalide 苯酞phthalimide 酞亚胺phthalocyanine 酞菁phthalocyanine blue 酞菁蓝phthalonic acid 酞酮酸phthalonitrile 酞腈phthalyl chloride 酞酰氯phthalylsulfathiazole 邻苯二甲酰基磺胺噻唑phylloquinone 叶绿醌physical adsorption 物理吸附physical chemistry 物理化学physical chemistry of high polymer 高聚物物理化学physical development 物理显影physical property 物理性质physical ripening 物理成熟physico chemical analysis 物理化学分析法physiological acidic fertilizer 生理酸性肥料physiological chemistry 生理化学physiological salt solution 生理盐溶液physiological solution 生理溶液physisorption 物理吸附physostigmine 毒扁豆碱phytase 植酸酶phytic acid 肌醇六磷酸phytin 非汀phytochemistry 植物化学phytochrome 光敏素phytol 植醇phytosterol 植物甾醇phytotoxin 植物毒素picene 二萘品苯picoline 皮考啉picolinic acid 皮考啉酸picral 苦醛腐蚀溶液picramic acid 苦氨酸picrate 苦味酸盐picric acid 苦味酸picrolonate 苦酮酸盐picrolonic acid 苦酮酸picrotoxin 苦毒piezochemistry 高压化学piezoelectric effect 压电效应piezoelectric vibrator 压电振子piezoelectricity 压电piezometer 压力计pig iron 生铁pigment 颜料pigmentation 着色pilocarpine 匹鲁卡品pilot cell 领示电池pilot ion 领示离子pilot plant 中间工厂pimaric acid 海松酸pimaricin 匹马菌素pimelic acid 庚二酸pimple 小突起pinacol 频哪醇pinacolin 特己酮pinacolin rearrangement 邻二叔醇重排酌pinacone 频哪醇pinane 蒎烷pincette 小钳子pinch cock 弹簧夹pinch effect 夹紧效应pine oil 松释pine resin 松脂pine tar oil 松焦油pinene 蒎烯pinic acid 蒎酸pink salt 锡盐pinoline 松香烃pipe furnace 管式炉pipe line 管路pipe still 管式蒸馏装置pipecoline 哌可啉piperazine 哌嗪piperic acid 胡椒酸piperidine 氮己烷piperine 胡椒碱piperonal 胡椒醛piperonylic acid 胡椒基酸piperylene 戊间二稀pipet 吸移管pipet support 吸移管架pisang wax 香蕉蜡pisanite 铜绿矾piston compressor 活塞压缩机piston pump 活塞泵pitch 沥青pitch coke 沥青焦炭pitchblende 沥青铀矿pitot tube 皮托管pitting 点腐蚀pivalic acid 特戊酸placer gold 砂金plain water 淡水planck's constant 普朗克常数planck's law of radiation 普朗克辐射定律planckian radiator 完全黑体plane curve 平面曲线plane of polarization 偏光面plane of symmetry 对称面plant base 植物盐基plant chemistry 植物化学plant fermentation 植物发酵plant growth regulator 植物生长第剂plant sterol 植物甾醇plasma 等离子体;血浆plasma chemistry 等离子体化学plaster of paris 烧石膏plasterboard 石膏板plastic clay 塑粘上plastic deformation 塑性变形plastic flow 塑性怜plastic refractory 耐火塑料plastic state 塑性状态plasticator 塑炼机plasticity 可塑度plasticizer 增塑剂plasticizing efficiency 可塑效率plasticizing oil 增塑油plastics 塑料plastification 增塑plastigel 塑性凝胶plastisol 塑溶胶plastomer 塑料plastometer 塑性计plastometry 塑性测定法plate 板plate efficiency 板效率plate glass 板玻璃plate rectification column 板极整六plate theory 塔板理论platelet 血小板plating 镀锌platinized asbestos 披铂石棉platinocyanide 铂化物platinum 铂platinum black 铂黑platinum chloride 氯化铂platinum dish 白金碟platinum electrode 铂电极platinum foil 铂箔platinum oxide 氧化铂platinum plating 镀铂platinum rhodium 铂铑合金platinum sponge 铂棉platinum tetrachloride 四氯化铂platinum wire 铂丝platinzation 镀铂pleochroism 多色性pleomorphism 多形现象plexiglass 普列克斯玻璃plumbate 铅酸盐plumbism 铅中毒plumbite 亚铅酸盐plumbite process 铅酸钠精制过程plumbite solution 博士溶液plunger 柱塞plunger pump 柱塞泵plus strand 正链plutonium 钚plutonium bromide 溴化钚plutonium hydride 氢化钚plutonium oxide 氧化钚plutonium reactor 钚反应堆ply 层plywood 层板pneumatic conveying 气动输送pneumatic conveying drying 气辽燥pneumatic conveyor 气动输送机pneumatic drier 气辽燥器pneumatic separation 气力分选pneumatic stirring 气动搅拌podophyllin 足叶草硷point charge 点电荷point group 点群point of neutralization 中和点poise 泊poiseuille's law 泊肃叶定律poison 毒poison gas 毒气poisoning 中毒poisson's coefficient 泊松系数poisson's distribution 泊松分布poisson's ratio 泊松比polar adsorption 极性吸附polar axis 极轴polar bond 极性键polar compound 极性化合物polar coordinates 极坐标polar molecule 极性分子polar solvent 极性溶剂polarimeter 偏光计polarimetric analysis 旋光分析polarimetry 旋光分析polarity 极性polarizability 极化率polarization 极化polarization analysis 偏光解析polarization capacity 极化额polarization colorimeter 偏光比色计polarization curve 极化曲线polarization potential 极化电势polarization resistance 极化阻力polarized light 偏光polarizer 起偏器polarizing microscope 偏光显微镜polarogram 极谱图polarograph 极谱仪polarographic analysis 极谱分析polarographic cell 极谱池polarographic maximum 极谱极大polarographic wave 极谱波polarography 极谱法pole 极polishing 磨光pollution 沾污pollution load 污染负荷polonium 钋poly 聚polyacid 缩多酸polyacidic base 多价碱polyacrylamide 聚丙烯酰胺polyacrylate 聚丙烯酸酯polyacrylic acid 聚丙烯酸polyacrylonitrile 聚丙烯腈polyaddition reaction 加聚合反应polyalcohol 多元醇polyallomer 聚异质同晶体polyamide 聚酰胺polyamino acid 聚氨基酸polyampholyte 聚两性电解质polyatom 多原子polyatomic molecule 多原子分子polyazo dye 多偶氮染料polybasic acid 多元酸polybutadiene 聚丁二烯polybutadiene rubber 聚丁二烯橡胶polycarbonate 聚碳酸酯polychloroprene 聚氯丁二烯polycondensate 缩聚物polycondensation 缩聚polycrystal 多晶体polycyclic 多环的polycyclic compound 多环化合物polycyclic hydrocarbon 多环烃polydisperse sol 多分散胶polydispersity 多分散性polyelectrolyte 聚合电解质polyene 多烯polyester 聚酯polyester fiber 聚酯纤维polyester resin 聚酯尸polyether 聚醚polyethylene 聚乙烯polyethylene glycol 聚乙二醇polyethylene oxide 聚氧化乙烯polyethylene terephthalate 聚乙烯对苯二甲酸酯polyformaldehyde 聚甲醛polyfunctionality 多功能性polyglycol 聚乙二醇polyhalide 多卤化物polyhalogenide 多卤化物polyhexamethylene adipamide 聚己二酰己二胺polyhydric alcohol 多元醇polyisobutylene 聚异丁烯polyisoprene 聚异戊二烯polymer 聚合物polymer analogue 聚合物系类polymer blend 共混聚合物polymer gasoline 聚合汽油polymer homologue 同系聚合物polymer isomer 聚合物异构体polymeric plasticizer 高分子增塑剂polymerization 聚合polymerization accelerator 聚合促进剂polymerization degree 聚合度polymerization inhibitor 聚合抑制剂polymerization initiator 聚合引发剂polymerization retarder 聚合抑制剂polymerized oil 聚合油polymery 聚合现象polymethacrylate 聚甲基丙烯酸酯polymethyl methacrylate 聚甲基丙烯酸甲酯polymethylene 聚甲烯polymorphic transition 多形转变polymorphism 多形现象polynaphthene 多环烷polynitroethylene 聚硝基乙烯polynuclear complex 多核配合物polynuclear hydrocarbon 多环烃polynucleotide 多核甙酸polyolefin 聚烯烃polyolefine 聚烯烃polyoxyethylene 聚乙二醇polyoxymethylene 聚氧化甲烯polypeptide 多肽polyphase equilibrium 多相平衡polyphenol 多元酚polyphosphate 多聚磷酸酯polypropylene 聚丙烯polypropylene oxide 聚环氧丙烷polyprotic base 多价碱polysaccharide 多糖polystyrene 聚苯乙烯polystyrol 聚苯乙烯polysulfide 多硫化合物polysulfide rubber 聚硫橡胶polysulfonamide 聚磺酰胺polysulfone 聚砜polyterpene 聚萜烯polytetrafluoroethylene 聚四氟乙烯polythene 聚乙烯polythionic acid 连多硫酸polythiourea 聚硫脲polyurea 聚脲polyurethane 聚氨酯polyurethane foam 聚氨酯泡沫体polyurethane resin 聚氨酯尸polyvinyl acetate 聚醋酸乙烯酯polyvinyl alcohol 聚乙烯醇polyvinyl butyral 聚乙烯醇缩丁醛polyvinyl carbazole 聚乙烯咔唑polyvinyl chloride 聚氯乙烯polyvinyl chloroacetate 聚氯醋酸乙烯酯polyvinyl ether 聚乙烯醚polyvinyl fluoride 聚氟乙烯polyvinylamine 聚乙烯胺poppy seed oil 罂粟油population 群体population inversion 粒子数反转porcelain 瓷器porcelain clay 瓷土porcelain crucible 瓷坩埚porcelain earth 瓷土porcelain enamel 搪瓷porcelain glaze 瓷釉porcelain mortar 瓷乳钵pore 微孔porosity 多孔性porous ion exchange resin 多孔离子交换尸porous pot 素瓷器素烧瓶porphin 卟吩porphyrin 卟啉portland cement 波特兰水泥position representation 位置表象positional isomer 位置异构物positive catalyst 正催化剂positive displacement meter 正排量计positive displacement pump 正排量泵positive electrode 阳电极positive ion 阳离子positive lens 会聚透镜positive maximum 正极大positive plate 阳极板positive valency 正化合价positron 阳电子postignition 后点火postprecipitation 后沉淀pot 罐pot life 可用时间potash 草碱potash alum 茂potash bulb 钾球potash fusion 钾熔化potash glass 钾玻璃potash lye 钾碱液potash mica 钾云母potash soap 钾皂potassic fertilizer 钾肥料potassium 钾potassium acetate 醋酸钾potassium alum 茂potassium aluminate 铝酸钾potassium antimonyl tartrate 酒石酸氧锑钾potassium arsenate 砷酸钾potassium arsenite 亚砷酸钾potassium bicarbonate 碳酸氢钾potassium bisulfate 硫酸氢钾potassium bisulfite 亚硫酸氢钾potassium bromate 溴酸钾potassium bromide 溴化钾potassium carbonate 碳酸钾potassium chlorate 氯酸钾potassium chloride 氯化钾potassium chromate 铬酸钾potassium dichromate 重铬酸钾potassium dihydrogenphosphate 磷酸二氢钾potassium feldspar 钾长石potassium ferricyanide 铁氰化钾potassium ferrocyanide 亚铁氰化钾potassium fluoride 氟化钾potassium hexacyanoferrate 亚铁氰化钾potassium hydrogen oxalate 草酸氢钾potassium hydrogen phthalate 氢酞酸钾potassium hydroxide 氢氧化钾potassium hypochlorite 次氯酸钾potassium iodate 碘酸钾potassium iodide 碘化钾potassium nitrate 硝酸钾potassium nitrite 亚硝酸钾potassium perchlorate 高氯酸钾potassium periodate 高碘酸钾potassium permanganate 过锰酸钾potassium peroxodisulfate 过硫酸钾potassium persulfate 过硫酸钾potassium phosphate 磷酸钾potassium polysulfide 多硫化钾potassium primary phosphate 磷酸二氢钾potassium pyroantimonate 焦锑酸钾potassium pyrophosphate 焦磷酸钾potassium pyrosulfate 焦硫酸钾potassium rhodanide 硫氰酸钾potassium silicate 硅酸钾potassium sodium carbonate 碳酸钠钾potassium sodium tartrate 罗谢尔盐potassium sulfate 硫酸钾potassium sulfide 硫化钾potassium sulfite 亚硫酸钾potassium tartrate 酒石酸钾potassium thiocyanate 硫氰酸钾potassium thiosulfate 硫代硫酸钾potassium xanthate 黄原酸钾potato starch 马铃薯淀粉potential 势potential barrier 势垒potential difference 电势差potential energy 位能potential gradient 电势梯度potential well 势阱potentiometer 电势计potentiometric analysis 电势分析potentiometric cell 电位滴定池potentiometric titration 电势滴定potentiostatic electrolysis 静电位电解potter's clay 塑粘上pottery 陶器potting 浇灌pour point 怜点pour point depressant 降凝剂powder 粉powder metallurgy 粉末冶金powdered catalyst 粉状催化剂powdered coal 粉煤powdery yeast 粉状酵母power 力power consumption 功率消耗power factor 功率因数pozzolanic cement 火山灰水泥practical units 实用单位prandtl number 普朗特数praseodymium 镨praseodymium acetate 醋酸镨praseodymium bromate 溴酸镨praseodymium bromide 溴化镨praseodymium carbonate 碳酸镨praseodymium chloride 氯化镨praseodymium hydroxide 氢氧化镨praseodymium molybdate 钼酸镨praseodymium nitrate 硝酸镨praseodymium oxalate 草酸镨praseodymium oxychloride 氯氧化镨praseodymium selenate 硒酸镨praseodymium sesquioxide 二氧化二镨praseodymium sulfate 硫酸镨praseodymium sulfide 硫化镨precious metal 贵金属precious stone 宝石precipitability 沉淀性precipitant 沉淀剂precipitate 沉淀物precipitated barium carbonate 沉淀碳酸钡precipitated sulfur 沉淀硫precipitating agent 沉淀剂precipitation 沉淀;降水precipitation agent 沉淀剂precipitation analysis 沉淀分析precipitation membrane 沉淀膜precipitation reaction 沉降反应precipitation titration 沉淀滴定precipitation value 沉淀值precipitator 沉淀器precision 精度precision balance 精密天平precision instrument 精密仪器precleaning 预净化precooling 预冷却precure 早期硫化precursor 前驱物质predissociation 预离解predrying 预干燥pregnenolone 孕烯醇酮preheater 预热器preheating 预热preheating furnace 预热炉preheating oven 预热炉preliminary cleaning 预净化preliminary drying 预干燥preparation 制法preparation of a surface 表面处理preparative gas chromatography 制备级气相色谱法prepared paint 低涂料prepolymer 预聚合物preservation 保存preservatives 保存剂preserve hydrogen with rare earths 用稀土金属储存氢press 压机press cake 滤饼press cure 加压硫化press filter 压滤机press vulcanization 加压硫化pressed glass 压制玻璃pressed yeast 压缩酵母pressing 压制pressure 压力pressure base factor 基压系数pressure distillation 加压蒸馏pressure filtration 加压过滤pressure gage 压力计pressure loss 压力损失pressure medium 压力介质pressure regulator 压力第器pressure sensitive adhesive 压敏粘着剂pressure sensitive dye 压敏色素pressure still 加压蒸馏器pressure tank 压力罐pretreatment 预处理prevulcanization 早期硫化primary air 一次空气primary alcohol 伯醇primary amine 伯胺primary carbon atom 伯碳原子primary cell 原电池primary phase 初晶相primary salt 一代盐primary winding 初级线圈prime coat 底涂primer 底层涂料primidone 普里米酮priming coat 底涂priming paint 底层涂料priming tube 雷管primitive cell 初基胞primitive lattice 初基点阵principal axis of inertia 惯性轴principal component 知组分principal fermentation 知发酵酌principal quantum number 挚子数principal reaction 知反应principal series 值列principal valence 汁子价principle of corresponding states 对应状态原理principle of energy dissipation 能量耗散原理principle of least action 最小酌原理principle of maximum overlapping 最大重迭原则principle of superposition 迭加原理prins reaction 普林斯反应printing 印刷printing ink 印刷墨printing paper 印刷纸prism 棱镜prism for infrared ray 红外线棱镜prism spectrograph 棱镜光谱仪prismatic spectrum 棱镜光谱pristane 姥鲛烷probability 概率probability amplitude 概率幅probability density 概率密度probability distribution 概率分布probability space 概率空间probable error 概率误差procainamide 普鲁卡因胺procaine 普鲁卡因procaine base 普鲁卡因碱process 工程process analyzer 工程分析计process monitoring 工程监视process steam 加工蒸汽process variable 工艺变量processing 加工processing aid of textile 织物加工助剂producer 发生炉producer gas 发生炉煤气producer gas reaction 发生炉气体反应product 产品product of distillation 蒸馏产物production 生产production of pairs 正负电子对产生proflavine 前黄素progesterone 孕酮prolactin 促乳泌素prolamin 醇溶朊prolidase 脯肽酶proline 脯氨酸promazine 普马嗪promedol 二甲哌替啶promethazine 普鲁米近promethium 钜prominence 日珥promotor 促进剂pronamide 拿草特propadiene 丙二烯propagation 增长反应propagation rate 传播速率propanal 丙醛propane 丙烷propanoic acid 丙酸propanol 丙醇propargyl alcohol 炔丙醇propargyl bromide 炔丙基溴propargylic acid 丙炔酸propeller 螺旋桨propeller agitator 螺旋桨式搅拌器propeller stirrer 螺旋桨式搅拌器propene 丙烯proper value 本盏proper vibration 固有振动propiolic acid 丙炔酸propionaldehyde 丙醛propionamide 丙酰胺propionic acid 丙酸propionic anhydride 丙酸酐propionitrile 丙腈propiophenone 苯丙酮propolis 蜂巢腊胶proportion 比例proportional counter 正比计数管proportional intensification 比例加强proportional limit 比例极限proportional reduction 比例缩减proportioning pump 计量泵propyl acetate 醋酸丙酯propyl alcohol 丙醇propyl aldehyde 丙醛propyl bromide 丙基溴propyl chloride 丙基氯propyl gallate 没食子酸丙酯propyl mercaptan 丙硫醇propyl propionate 丙酸丙酯propyl sulfide 二丙硫propylamine 丙胺propylbenzene 丙基苯propylene 丙烯propylene glycol 丙二醇propylene oxide 环氧丙烷propylidene 亚丙烷propyliodone 丙碘酮propylthiouracil 丙硫氧嘧啶propyne 丙炔prosthetic group 辅基protactinium 镤protamine 精蛋白protease 朊酶protease inhibitor 蛋白酶抑制剂protection 保护。

氢气脱氧钯触媒简介氢气脱氧钯触媒(Palladium Catalyst for Hydrogen Deoxygenation)是一种重要的催化剂,用于将含氧化合物中的氧原子转化为水,同时生成氢气。
该催化剂广泛应用于石油化工、有机合成和环境保护等领域。
催化原理氢气脱氧钯触媒基于钯(Pd)元素的催化活性。
在反应过程中,含氧化合物与催化剂表面的活性位点发生作用,将其中的氧原子转移至钯表面,并与来自反应物或溶液中的质子结合形成水。
同时,被还原的钯表面上会生成吸附态的质子(H+),这些质子与溶液中的水分子结合形成分子状态下的H2。
催化剂制备目前常见的制备方法包括湿法沉积、共沉淀法和溶胶-凝胶法等。
其中,湿法沉积是一种较为常见且简单易行的方法。
具体步骤如下: 1. 将适量的钯盐溶解于溶剂中,形成钯离子溶液。
2. 将活性载体(如活性炭、氧化铝等)浸入钯离子溶液中,使其充分吸附钯离子。
3. 将浸渍后的载体进行干燥和煅烧处理,使钯盐还原为金属钯,并与载体表面发生物理或化学吸附。
应用领域石油化工在石油加工过程中,氢气脱氧钯触媒常用于催化裂化、重整和加氢脱硫等反应。
例如,在催化裂化过程中,该催化剂可将含有酚、醛、酮等含氧杂质的烃类原料转化为无氧杂质的高纯度产品。
有机合成在有机合成领域,氢气脱氧钯触媒广泛应用于还原反应。
例如,在药物合成中,该催化剂可将含有羰基(如酮、醛等)的有机物还原为对应的醇。
此外,它还可以用于邻位选择性还原、不对称还原和环戊二烯的转化等反应。
环境保护氢气脱氧钯触媒在环境保护领域具有重要的应用价值。
例如,它可以用于水处理过程中的污染物去除,如有机废水中的酚类、醛类和酮类化合物。
此外,该催化剂还可用于废气中有机物的催化燃烧和二氧化碳的还原等反应。
催化剂性能改进为了提高氢气脱氧钯触媒的催化性能,研究人员不断探索新的改进方法。
以下是一些常见的改进策略: 1. 调控钯粒子尺寸:通过控制制备条件和添加助剂等手段,可以调节钯粒子的尺寸,从而影响其表面活性位点密度和反应活性。

Palladium Z2原理1. 概述Palladium Z2是一种新型的材料,具有独特的物理和化学性质。
近年来,Palladium Z2在电子、能源和催化领域备受关注,其特殊的原理成为研究的热点之一。
本文旨在探讨Palladium Z2的原理,从结构、电子性质、磁性和应用等方面进行深入分析,以期为相关领域的研究和应用提供参考。
2. 结构Palladium Z2是一种层状结构的材料,具有Pd和Z两种元素交替排列的结构。
Pd原子和Z原子在晶格中交替排列,形成多层结构。
Palladium Z2的晶格结构稳定,具有优异的机械性能和热稳定性,这为其在应用中的稳定性提供了基础。
3. 电子性质Palladium Z2的电子性质也备受关注。
研究表明,Palladium Z2具有特殊的电子结构,其中具有两个与费米面(Fermi surface)相关的驻点(nodal points),这些驻点对材料的电子输运性能和热电性能具有重要影响。
Palladium Z2的电子结构还表现出一些奇特的性质,如非常规的拓扑性质和量子自旋霍尔效应,这些性质为材料的应用提供了新的可能性。
4. 磁性除了电子性质,Palladium Z2的磁性也是研究的重点之一。
研究发现,Palladium Z2在一定条件下可以表现出自旋极化和磁性序。
这些特殊的磁性性质不仅可以用于磁性存储和磁传感器等领域,还可以为研究者提供新的材料评台,用于探索自旋电子学等新领域。
5. 应用Palladium Z2由于其独特的物理和化学性质,在多个领域都具有潜在的应用价值。
在电子领域,Palladium Z2可以作为新型的拓扑绝缘体应用于量子计算和自旋电子学中;在能源领域,Palladium Z2具有优异的热电性能,可用于制备高效的热电材料;在催化领域,Palladium Z2可以作为催化剂用于催化反应中。
Palladium Z2还具有潜在的应用于磁性存储、磁传感器等领域。

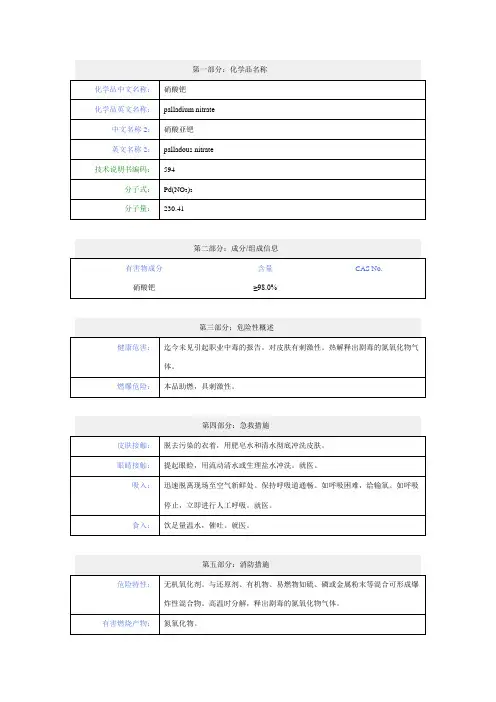
第一部分化学品及企业标识化学品中文名:氯化钯(π-肉桂基)二聚物化学品英文名:Palladium(II)(pi-cinnamyl) Chloride DimerPalladiumCAS No.:12131-44-1分子式:C18H12Cl2Pd2产品推荐及限制用途:用于进一步制备钯配合物催化剂。
第二部分危险性概述紧急情况概述造成皮肤刺激。
造成严重眼刺激。
GHS危险性类别皮肤腐蚀 / 刺激类别 2严重眼损伤 / 眼刺激类别 2标签要素:象形图:警示词:警告危险性说明:H315 造成皮肤刺激H319 造成严重眼刺激防范说明●预防措施:—— P264 作业后彻底清洗。
—— P280 戴防护手套/穿防护服/戴防护眼罩/戴防护面具。
●事故响应:—— P302+P352 如皮肤沾染:用水充分清洗。
—— P332+P313 如发生皮肤刺激:求医/就诊。
—— P362+P364 脱掉沾染的衣服,清洗后方可重新使用——P305+P351+P338 如进入眼睛:用水小心冲洗几分钟。
如戴隐形眼镜并可方便地取出,取出隐形眼镜。
继续冲洗。
—— P337+P313 如仍觉眼刺激:求医/就诊。
●安全储存:—— P403+P233 存放在通风良好的地方。
保持容器密闭。
—— P405 存放处须加锁。
●废弃处置:—— P501 按当地法规处置内装物/容器。
物理和化学危险:无资料。
健康危害:造成皮肤刺激。
造成严重眼刺激。
环境危害:无资料。
第三部分成分/组成信息√物质混合物第四部分急救措施急救:吸入:如果吸入,请将患者移到新鲜空气处。
皮肤接触:脱去污染的衣着,用肥皂水和清水彻底冲洗皮肤。
如有不适感,就医。
眼晴接触:分开眼睑,用流动清水或生理盐水冲洗。
如有不适感,就医。
食入:饮水,禁止催吐。
如有不适感,就医。
对保护施救者的忠告:将患者转移到安全的场所。
咨询医生。
出示此化学品安全技术说明书给到现场的医生看。
对医生的特别提示:无资料。
第五部分消防措施灭火剂:用水雾、干粉、泡沫或二氧化碳灭火剂灭火。


钯碳还原炔基钯碳还原炔基(Palladium-Catalyzed Carbon-Carbon Bond Formation)这一反应是有机化学合成中的一项重要反应,主要用于构建碳碳键。
钯碳还原催化剂具有高效、高选择性,和求组物质易于获取等特点,因此在有机合成和药物化学领域得到了广泛的应用。
该反应以亚氨基炔(Terminal Alkynes)和双键化合物(Alkenes)为反应物,通过钯催化下的还原作用,形成顺式或反式烯烃。
1.反应机理钯碳还原炔基反应具有复杂的反应机理。
它的反应不仅经过一个钯的还原氧化循环(Palladium Reduction-Oxidation Cycle),还涉及到一些加成,消除,原子转移和重排等反应。
本文主要介绍含氧基团的烯烃与炔烃的钯碳还原反应机理和影响因素。
(1) 蒯碳还原炔基反应中的过渡态炔烃基团(C≡C)在钯催化剂或锂盐辅助下与钯化合物(PdX, X= L, Cl, OAc等)发生配位作用形成内配合物1。
接着,Pd0与炔烃内配合物反应生成配位基团(L)的PdII配合物2。
在PdII配合物的催化下,炔基中的末端氢离子化生成催化剂和烯丙基烷基阳离子中间体3,该中间体很容易被水或醇等试剂捕捉。
在此中间体3的基础上进行加成反应,可以得到闭环产物或重排产物。
(2) “姓名反应”机理:除了上述机理,还有一种较复杂的“姓名反应”(Name Reaction)机理,该机理得名于反应物中母骨架的结构。
该反应共包含四步,包括内配位物的形成、内配位物的氧化加成、配对基团的消除和内部重排。
由于该过程中间体间的影响,反应的产物可能不仅仅是加成、消除和重排的产物,而是一系列的反应产物和中间体。
2. 影响因素(1) 钯催化剂的选择钯催化剂对反应的活性有着重要的影响,目前主要的钯催化剂有钯化合物、钯离子和有机钯化合物。
钯复合物的选择范围最广,催化活性最高,但成本相对较高。
相对来说,无机钯盐和有机钯化合物成本更低,但催化活性较差。

palladium 金属元素英文简写Palladium is a chemical element with the symbol "Pd" and an atomic number of 46. It belongs to the platinum group metals and is known for its excellent catalytic properties, high corrosion resistance, and low electrical resistance. In this article, we will delve into the characteristics, applications, and significance of palladium.Palladium is a steel-white metal with a lustrous appearance. It is ductile, malleable, and has a relatively low melting point. The element was discovered in 1803 by William Hyde Wollaston, an English chemist, while he was examining platinum ore. Wollaston named it after the asteroid Pallas, which had been discovered two years earlier.One of the remarkable properties of palladium is its ability to absorb and store hydrogen gas. This property makes it an essential component in catalytic converters, which are used in automobiles to convert harmful exhaust gases into less harmful emissions. As a catalyst, palladium facilitates chemical reactions by accelerating the rate of reaction without being consumed in the process.Apart from its role in catalytic converters, palladium has various other applications. It is widely used in the electronics industry for making capacitors, resistors, and connectors due to its low electrical resistance and high thermal conductivity. Palladium-based compounds are also used in dentistry for making dental crowns and bridges, as they offer durability and biocompatibility.The jewelry industry also values palladium for its attractive appearance and resistance to tarnish. It is often used as a substitute for platinum in the manufacturing of fine jewelry. Additionally, palladium is employed in the production of white gold, an alloy of gold and palladium, which gives a silver-white color to the metal.Furthermore, palladium plays a vital role in the field of medicine. It is used in diagnostic equipment, such as imaging agents for magnetic resonance imaging (MRI) scans. Palladium-based compounds also exhibit potential anticancer activity, showing promise in the development of new cancer medications. Researchers continue to explore its medicinal applications and the possible use of palladium in drug delivery systems.From an economic standpoint, palladium holds significant value due to its scarcity. It is mainly extracted as a byproduct of platinum mining, making its supply limited. Palladium prices are subject to market demand, particularly in the automotive industry, which accounts for a substantial portion of its consumption. In recent years, the demand for palladium has surged due to tighter emission regulations and the increased production of hybrid and electric vehicles.To ensure the responsible sourcing and production of palladium, industry organizations, such as the Responsible Jewellery Council and the Initiative for Responsible Mining Assurance, have developed standards and certifications. These initiatives aim to promote sustainable practices and minimize the environmental and social impacts associated with palladium extraction and processing.In conclusion, palladium, with its symbol "Pd," is a versatile chemical element with a wide range of applications. Its unique properties make it indispensable in various industries, including automotive, electronics, jewelry, and medicine. As demand continues to grow, it is crucial to emphasize responsible sourcing and sustainable practices to ensure the long-term availability and environmental impact of this valuable metal.。

TCPA/Palladium 问与答一、什么是 TCPA,什么又是 Palladium?答: TCPA 是 Trusted Computing Platform Alliance 的缩写,由 Intel 公司发起的一个组织。
该组织的目标是致力于促成新一代具有安全、信任能力的硬件运算平台。
而 Palladium 是微软新一代的**作系统里的计划之一,这个计划可以运用 TCPA 的平台来具体实现所谓的保防功能。
二、请用白话来说清楚,倒底 TCPA/Palladium 是在干嘛?答:最明显的用途,是把「版权控管保护」手段,简称DRM,做进你的个人计算机里,无处不在。
包括你自己打的 Microsoft 文件,全部被这套完美计划给加密了。
就好比美国片商把 DVD 片用 CSS 来保护一样。
只是,这回做进计算机里去了。
藉由它,唱片音乐公司可以卖给你「只能播放三次」或是「只能在你生日播放」的 CD 。
所有一切想得到的智财产品行销包装手法,都将会发生。
TCPA/Palladium 会让你难以使用未经授权的软件,盗版软件可以在远程被侦测并且移除它。
它也可以让使用租赁软件的计价变得方便好用。
比尔盖兹早就梦想着如何让中国人民付钱给微软,TCPA/Palladium 正是梦的实现。
还有很多其它的可能性…政府可以让公务人员所处理的机密性文件无法给新闻媒体开启解读;拍卖网站可以限制你必须从它们所信任的代理服务器上来竞标,你再也无法在竞标过程里耍小聪明;想在计算机游戏里作弊?难上加难。
除了这些正面的规范性用途,也是有黑暗面的手段:远程督查、删除盗版音乐软件的功能,也可以被检警甚至是软件设计公司自己拿来删除他们认为具有威胁性的文件。
这些文件可以是政府认定的成人\\*\\*甚或是政治批评。
软件设计公司也可以设计出你难以摆脱换用的软件产品;例如 M$ Office Word 可以把你打的文件统统以只有微软有金钥可打开的加密方法来加密。

钯纳米颗粒熔点
钯(Palladium)是一种化学元素,其熔点是在摄氏1,552-1,554度(华氏2,826度)。
请注意,这是钯的常压下的熔点。
在纳米颗粒的情况下,由于纳米材料的尺寸效应,其熔点可能有所改变。
纳米颗粒具有特殊的物理和化学性质,与大尺寸的材料相比,其熔点、熔化行为等性质可能呈现出不同的特征。
纳米颗粒的性质通常受到颗粒大小、形状、表面性质等因素的影响。
由于这些因素,纳米颗粒可能表现出比宏观材料更高的表面能、更高的活性等特点,而这些特性在实际应用中可以得到利用,例如在催化、传感等领域。
请注意,有关纳米颗粒的性质,特别是纳米钯颗粒的熔点的具体数值,可能因制备方法、纳米颗粒的形态和尺寸分布等因素而有所不同。
化学及化工专业词汇英语翻译package 包装packed absorption tower 填充吸收塔packed column 填充塔packed dyeing 填充染色packed reaction column 填充反应塔packed rectification tower 填充精馏塔packed tower 填充塔packing 填充物packing density 填充密度packing paper 包装纸padding 表面染色paddle agitator 桨式混合机paddle mixer 桨式混合机paint 涂料paint hardner 镀金粘料paint remover 油漆去除剂paint stripper 油漆去除剂pair annihilation 对湮没pair creation 正负电子对产生pair ion 离子对pale crepe 苍铍胶palladium 钯palladium asbestos 钯石棉palladium black 钯黑palladium chloride 氯化钯palladium nitrate 硝酸钯palladium oxide 氧化钯palladium sponge 海绵钯palm kernel oil 棕榈仁油palm oil 棕榈油palmarosa oil 掌玫油palmitate 棕榈酸盐palmitic acid 棕榈酸palmitin 棕榈精palmitone 棕榈酮pan mill 碾磨盘panchromatic film 全色胶片panchromatic plate 全色干片pancreatin 胰酶pancreozymin 肠促胰酶素pangamic acid 潘氨酸pantothenic acid 泛酸papain 木瓜酶papaverine 罂粟碱paper chromatography 纸色谱分析法paper electrophoresis 纸电泳paper filter 纸滤器paper industry 造纸工业paper machine 造纸机paper pulp 纸浆paper stencilling 刻花纸paper yarn 纸绳para aminohippuric acid 对氨基马尿酸para hydrogen 仲氢para isomer 对位异构体para molecule 仲分子para position 对位para red 对位红para rubber 三叶橡胶paraaldehyde 仲醛parabanic acid 仲班酸parabola 抛物线paracasein 副酪朊parachor 等张比容paraconic acid 仲康酸paracyanogen 仲氰paraffin 石蜡paraffin base crude 石蜡基原油paraffin hydrocarbon 烷属烃paraffin oil 石蜡油paraffin stock 石蜡原油paraffin wax 石蜡paraformaldehyde 仲甲醛parafuchsine 副品红paragenesis 共生paragonite 钠云母paraldol 仲醛醇parallel flow 并流平行流parallel reaction 平行反应paralysis 麻痹paralyzer 麻痹药paramagnetic resonance 顺磁共振paramagnetic substance 顺磁质paramagnetism 顺磁性parameter 参数paramine 对胺paramorphism 同质异晶paranitroaniline red 对位红pararosaniline 副蔷薇胺parastrophe 对位取代化合物parathion 硝苯硫磷酯paraxanthine 副黄嘌呤paraxylene 对二甲苯parchment 羊皮纸parchment paper 仿羊皮纸parent name 母体名parent substance 母体parinol 苯吡醇paris green 巴黎绿paris white 巴黎胡粉白parity 奇偶性parkerizing 磷化处理parpon 帕尔庞partial combustion 部分燃烧partial condensation 部分冷凝partial condenser 分馏柱partial molar quantity 偏摩尔数量partial molar volume 偏摩尔体积partial pressure 分压partial reaction 部分反应partial valence 余价participation of neighboring group 邻基参与particle 粒子particle counting 粒子计数法particle diameter 颗粒直径particle electrophoresis 粒子电泳particle induced x ray emission 粒子诱导x 射线发射particle scattering factor 粒子散射因数particle size 粒度particle size analysis 粒径分析particle thickness technique 质点厚度测量技术parting 分离parting agent 分离剂partition 配分partition chromatography 配分色层法partition coefficient 配分系数partition function 配分函数partition wall 隔墙passivation 钝化酌passivation potential 钝化电势passive state 钝态passivity 钝态paste 糊剂paste mixer 惮机paste paint 厚漆paste resin 糊状尸pasteboard 纸板pasted plate 涂浆的板pasteurization 巴氏杀菌pasteurizer 巴氏灭菌器pasting 糊化patchouli oil 绿叶油patent 专利pattern 图样pattern recognition 模式辫认peak 峰值peak inverse voltage 最大反向电压peak load 峰值负荷peak potential 顶点电位peanut oil 花生油pearl 珍珠pearl ash 珍珠灰pearl essence 珍珠粉pearl polymerization 成珠聚合pearl white 锌钡白pearlite 珍珠岩peat 泥炭peat tar 泥煤焦油pebble mill 砾磨机pectic acid 果胶酸pectic substances 果胶质pectin 果胶pectinase 果胶酶pectization 凝结pectocellulose 果胶纤维素peeling 剥落peep hole 窥孔pegmatite 伟晶岩pelargonaldehyde 壬醛pelargonic acid 正壬酸pelargonidin 花葵素pellet 粒状pellet cooler 颗粒冷却器pelletizer 造粒机pellotine 佩落碱pendulum mill 摆旋式研磨机penetrability 穿透性penetrant 渗透剂penetrating agent 渗透剂penetration 穿透;渗入度penetration complex 透入配合物penetration test 贯入度试验penetrator 针入度计penetrometer 透度计penicillase 青霉素酶penicillin 青霉素pentacarbonyl iron 五羰基铁pentacene 并五苯pentachlorophenol 五氯酚pentadiene 戊二烯pentaerythritol 季戊四醇pentaerythritol tetranitrate 季戊四醇四硝酸酯pentamethylene 环戊烷pentamethylenediamine 戊甲撑二胺pentane 戊烷pentane thermometer 戊烷温度计pentanol 戊醇pentasol 成氯醇pentene 戊烯penthrit 季戊四醇四硝酸酯pentlandite 镍黄铁矿pentobarbital 戊巴比妥pentosan 多缩戊糖pentose 戊糖pentose phosphate cycle 戊糖磷酸循环pentose phosphate pathway 戊糖磷酸途径pentyl alcohol 戊醇pentyne 戊炔peppermint 薄荷peppermint oil 薄荷油pepsin 胃蛋白酶pepsinogen 胃蛋白酶原peptidase 肽酶peptide 肽peptide bond 肽键peptide linkage 肽键peptide synthetase 肽合成酶peptidoglycan 肽聚糖peptization 胶溶酌peptized fuel 胶溶燃料peptizer 胶溶剂peptizing agent 胶溶剂peptone 胨peracetic acid 过乙酸peracid 过酸peralcohol 过醇perbenzoic acid 过苯酸perborate 过硼酸盐perboric acid 过硼酸perbromate 过溴酸盐perbromic acid 过溴酸percarbonate 过碳酸盐percent grafting 接枝率perchlorate 高氯酸盐perchloric acid 高氯酸perchloride 高氯化物perchloroethane 六氯乙烷percolation 沥滤percolator 渗滤器percussion cap 雷管percussion powder 雷管火药perfect analysis 完全分析perfect black body 完全黑体perfect combustion 完全燃烧perfect crystal 完整晶体perfect elasticity 完全弹性perfect fluid 理想铃perfect gas 完美气体perfect mixing 完全混合perfect solution 完美溶液perfluorocyclobutane 过氟化环丁烷perforated pipe distributor 喷淋器多孔管分布器perforated plate 多孔板perforated plate distillation 多孔板蒸馏perforated plate tower 孔板塔performance testing 性能试验performic acid 过甲酸perfume 香料perfume extracting solvent 香料抽出溶剂perfume industry 香料工业perhydride 过氢化物perhydrol 强双氧水periclase 方镁石perilla oil 紫苏子油period 周期periodate 高碘酸盐periodic acid 高碘酸periodic law 周期律periodic precipitation 间歇沉淀periodic reaction 周期反应periodic system of elements 元素周期系periodic table 周期表periodide 高碘化物periphery 边界periplogenin 杠柳甙元perissad 奇价元素peritectic 包晶peritectic point 包晶点perkin reaction 佩金反应permanence 持久性permanent current 持恒电流permanent dipole 永久偶极子permanent expansion 永久膨胀permanent gas 永久气体permanent hardness 永久硬度permanent magnet 永磁铁permanent set 永久变形permanganate 高锰酸盐permanganate bleaching 高锰酸盐漂白permanganate titration 高锰酸盐滴定法permanganic acid 高锰酸permanganometry 高锰酸盐滴定法permeability 渗透性permeable membrane 可透膜permeameter 渗透计permeametry 渗透测定法permeation 渗透permissible error 容许误差permissible explosive 安全炸药permitted explosive 安全炸药permselective membrane 选择性渗透膜permselectivity 选择渗透性permutation 置换permutite 人造沸石perovskite 钙钛矿peroxidase 过氧物酶peroxide 过氧化peroxide effect 过氧化物效应peroxide index 过氧化值peroxide number 过氧化值peroxo acid 过氧基酸peroxy acid 过酸peroxydisulfuric acid 过硫酸perpetual motion 永久运动perphenazine 佩吩嗪perrhenate 高铼酸盐perrhenic acid 高铼酸perruthenate 过钌酸盐persalt 过酸盐persian red 铬红persistent current 持恒电流personal error 人为误差persorption 吸混酌persulfate 过硫酸盐persulfide 过硫化物persulfuric acid 过硫酸peru balsam 秘鲁香脂pesticide 杀虫剂pestle 杵棒pethidine 哌替啶pethidine hydrochloride 盐酸哌替啶petri dish 陪替氏培养皿petrochemical industry 石油化学工业petrochemical reaction 石油化学反应petrochemicals 石油化学产品petrochemistry 石油化学petrolatum 矿脂petroleum 石油petroleum acid 石油酸petroleum asphalt 石油沥青petroleum benzine 石油醚petroleum chemicals 石油化学产品petroleum chemistry 石油化学petroleum coke 石油焦炭petroleum distillation 石油蒸馏petroleum emulsion 石油乳剂petroleum ether 石油醚petroleum gas 石油气体petroleum grease 石油润滑脂petroleum industry 石油工业petroleum isomerization process石油异构化过程petroleum jelly 矿脂petroleum pitch 石油沥青petroleum pitch coke 石油沥青焦炭petroleum product 石油产品petroleum refinery 石油加工厂petroleum refining 石油提炼petroleum refining industry 石油炼制工业petroleum resin 石油尸petroleum wax 石油蜡ph 氢指数ph electrode ph 电极ph indicator ph 指示剂ph meter ph 计ph standard solution ph 标准溶液ph test paper ph 试验纸ph value ph 值phanerocrystalline 显晶质pharmaceutical chemistry 药物化学pharmaceutics 制药学pharmacology 药理学pharmacy 药学phase 相phase average 相平均phase boundary 相界phase boundary potential 相界电位phase change 相转移phase contrast microscope 相差显微镜phase diagram 相图phase equilibrium 相平衡phase modulation 相位灯phase rule 相律phase sensitive detection 相敏检波phase separation 相分离phase shifter 移相器phase solubility 相溶解度phase solubility analysis 相溶度分析phase space 相空间phase titration 相滴定phase trajectory 相位轨道phase transfer catalyst 相转移催化剂phase transition 相转移phellandren 菲兰烯phenacetin 非那巍phenacite 硅铍石phenacyl alcohol 苯甲酰甲醇phenacyl bromide 苯甲酰甲基溴phenadon 非那酮phenamine 非那明phenanthrene 菲phenanthrenequinone 菲醌phenanthroline 二氮杂菲phenanthroline indicator 菲绕啉指示剂phenazine 吩嗪phenetidin 氨基苯乙醚phenetidine 氨基苯乙醚phenetole 苯乙醚phenobarbital 苯巴比妥phenocryst 斑晶phenol 酚phenol ether 苯酚醚phenol formaldehyde resin 苯酚甲醛尸phenol lignin 苯酚木素phenol red 酚红phenolase 酚酶phenolate 酚盐phenolic acid 酚酸phenolic resin 酚尸phenolic resin varnish 酚醛清漆phenolphthalein 酚酞phenolsulfonic acid 苯酚磺酸phenoplast 酚醛塑料phenosafranine 酚藏花红phenothiazine 吩噻嗪phenoxazine 吩恶phenoxide 酚盐phenoxyacetic acid 苯氧基乙酸phenyl acetate 醋酸苯酯phenyl ether 苯基醚phenyl isocyanate 异氰酸苯酯phenyl salicylate 萨罗phenylacetaldehyde 苯乙醛phenylacetamide 苯乙酰胺phenylacrylic acid 苯基丙烯酸phenylalanine 苯丙氨酸phenylbutazone 苯基丁氮酮phenylcinchonic acid 辛可芬phenylene 苯撑phenylenediamine 撑二氨phenylethyl acetate 醋酸苯乙酯phenylethyl alcohol 苯基乙醇phenylethyl isobutyrate 异丁酸苯乙酯phenylethyl propionate 丙酸苯乙酯phenylethylacetic acid 苯乙基醋酸phenylhydrazine 苯肼phenylmagnesium bromide 溴化苯镁phenylmagnesium chloride 氯化苯镁phenylmagnesium halide 卤化苯镁phenylmagnesium iodide 碘化苯镁phenylmercuric acetate 核酸汞phenylmercuric chloride 氯化苯汞phenylmorpholine 苯替吗啉phenylphenol 苯基苯酚phenylpiperazine 苯基哌嗪pheromone 信息素phlogiston 燃素phlorizin 根皮甙phloroglucinol 根皮山梨醇phonochemistry 声化学phonon 声子phorone 佛尔酮phosgene 光气phosphamide 磷酰胺phosphamidon 磷酰胺酮phosphatase 磷酸酶phosphate 磷酸盐phosphate glass 磷酸盐玻璃phosphatic ferfilizer 磷肥phosphatidic acid 磷脂酸phosphatizing 磷酸盐处理phosphide 磷化物phosphine 磷化氢phosphite 亚磷酸盐phosphocreatine 磷酸肌酸phosphoenolpyruvate 磷酸烯醇丙酮酸phospholipid 磷脂phosphomolybdate 磷钼酸盐phosphomolybdic acid 磷钼酸phosphomonoesterase 磷酸单酯酶phosphoprotein 磷蛋白phosphor 磷光体phosphor bronze 磷青铜phosphor salt 磷盐phosphorescence 磷光phosphoretted hydrogen 磷化氢phosphoric acid 磷酸phosphoric acid anhydride 磷酐phosphorimetric analysis 磷光分析phosphorimetry 磷光分析phosphorite 磷钙土phosphoro organic insecticide 磷有机杀虫剂phosphorolysis 磷酸解phosphoroscope 磷光计phosphorous acid 亚磷酸phosphorous anhydride 亚磷酸酐phosphorus 磷phosphorus chloride 氯化磷phosphorus nitride 氮化磷phosphorus oxychloride 磷酰氯phosphorus pentabromide 五溴化磷phosphorus pentachloride 五氯化磷phosphorus pentasulfide 五硫化二磷phosphorus sesquisulfide 三硫化四磷phosphorus tribromide 三溴化磷phosphorus trichloride 三氯化磷phosphorus trifluoride 三氟化磷phosphorus triiodide 三碘化磷phosphoryl chloride 磷酰氯phosphorylase 磷酸化酶phosphotungstic acid 磷钨酸photobiochemistry 光生物化学photocatalysis 光催化酌photocell 光电池photochemical catalysis 光催化photochemical equilibrium 光化学平衡photochemical reaction 光化反应photochemistry 光化学photochromic reaction 彩色反应photochromism 光致变色现象photochromy 彩色照相术photoconductor 光电导体photocurrent 光电流photodetachment 光致分离photodimerization 光二聚酌photodissociation 光解酌photodosimetry 照相剂量学photoelasticity 光弹性photoelectric cell 光电池photoelectric color comparator 比色仪photoelectric colorimeter 光电比色计photoelectric colorimetry 光电比色法photoelectric effect 光电效应photoelectric fraction collector 光电分级收集器photoelectric pyrometer 光电高温计photoelectric spectrophotometer光电分光光度计photoelectric tube 光电管photoelectrochemistry 光电化学photoelectron 光电子photoemission 光电发射photofission 光致裂变photogel 摄影煤photographic characteristic curve照相特性曲线photographic chemistry 照相化学photographic contrast 照相对比度photographic densitometer 照相密度计photographic density 照相底片密度photographic developer 显象剂photographic development 照相显相photographic emulsion 照相乳胶photographic emulsion layer 感光层photographic film 胶片photographic material 照相材料photographic negative 照相底片photographic paper 印相纸photographic plate 照相底片photographic positive 照相正片photographic process 照相法photography 照相photogyration 照相底片photohomolysis 光同种溶解photoionization 光致电离photoisomer 光异构体photolithography 照相平版印刷photoluminescence 光致发光photolysis 光分解photomeson 光介子photometer 光度计photometric titration 光度滴定photon 光子photonuclear reaction 光致核反应photooxidation 光氧化photophoresis 光泳现象photophosphorylation 光磷酸化photopigment 光色素photopolymer 光聚合物photopolymerization 光聚合photoreactive chlorophyll 光反应性叶绿素photoreduction 光致还原photorespiration 光呼吸photosensitive glass 光敏玻璃photosensitive polymer 光敏聚合物photosensitive resin 感光尸photosensitivity 光敏性photostructural transformation 光结构转变photosynthesis 光合酌phototropy 光致变色现象phototube 光电管phthalaldehyde 酞醛phthalaldehydic acid 苯醛酸phthalamide 酞酰胺phthalate 邻苯二甲酸盐phthalazine 酞嗪phthalein 酞phthalein dye 酞类染料phthalic acid 酞酸phthalic anhydride 酞酐phthalide 苯酞phthalimide 酞亚胺phthalocyanine 酞菁phthalocyanine blue 酞菁蓝phthalonic acid 酞酮酸phthalonitrile 酞腈phthalyl chloride 酞酰氯phthalylsulfathiazole 邻苯二甲酰基磺胺噻唑phylloquinone 叶绿醌physical adsorption 物理吸附physical chemistry 物理化学physical chemistry of high polymer 高聚物物理化学physical development 物理显影physical property 物理性质physical ripening 物理成熟physico chemical analysis 物理化学分析法physiological acidic fertilizer 生理酸性肥料physiological chemistry 生理化学physiological salt solution 生理盐溶液physiological solution 生理溶液physisorption 物理吸附physostigmine 毒扁豆碱phytase 植酸酶phytic acid 肌醇六磷酸phytin 非汀phytochemistry 植物化学phytochrome 光敏素phytol 植醇phytosterol 植物甾醇phytotoxin 植物毒素picene 二萘品苯picoline 皮考啉picolinic acid 皮考啉酸picral 苦醛腐蚀溶液picramic acid 苦氨酸picrate 苦味酸盐picric acid 苦味酸picrolonate 苦酮酸盐picrolonic acid 苦酮酸picrotoxin 苦毒piezochemistry 高压化学piezoelectric effect 压电效应piezoelectric vibrator 压电振子piezoelectricity 压电piezometer 压力计pig iron 生铁pigment 颜料pigmentation 着色pilocarpine 匹鲁卡品pilot cell 领示电池pilot ion 领示离子pilot plant 中间工厂pimaric acid 海松酸pimaricin 匹马菌素pimelic acid 庚二酸pimple 小突起pinacol 频哪醇pinacolin 特己酮pinacolin rearrangement 邻二叔醇重排酌pinacone 频哪醇pinane 蒎烷pincette 小钳子pinch cock 弹簧夹pinch effect 夹紧效应pine oil 松释pine resin 松脂pine tar oil 松焦油pinene 蒎烯pinic acid 蒎酸pink salt 锡盐pinoline 松香烃pipe furnace 管式炉pipe line 管路pipe still 管式蒸馏装置pipecoline 哌可啉piperazine 哌嗪piperic acid 胡椒酸piperidine 氮己烷piperine 胡椒碱piperonal 胡椒醛piperonylic acid 胡椒基酸piperylene 戊间二稀pipet 吸移管pipet support 吸移管架pisang wax 香蕉蜡pisanite 铜绿矾piston compressor 活塞压缩机piston pump 活塞泵pitch 沥青pitch coke 沥青焦炭pitchblende 沥青铀矿pitot tube 皮托管pitting 点腐蚀pivalic acid 特戊酸placer gold 砂金plain water 淡水planck's constant 普朗克常数planck's law of radiation 普朗克辐射定律planckian radiator 完全黑体plane curve 平面曲线plane of polarization 偏光面plane of symmetry 对称面plant base 植物盐基plant chemistry 植物化学plant fermentation 植物发酵plant growth regulator 植物生长第剂plant sterol 植物甾醇plasma 等离子体;血浆plasma chemistry 等离子体化学plaster of paris 烧石膏plasterboard 石膏板plastic clay 塑粘上plastic deformation 塑性变形plastic flow 塑性怜plastic refractory 耐火塑料plastic state 塑性状态plasticator 塑炼机plasticity 可塑度plasticizer 增塑剂plasticizing efficiency 可塑效率plasticizing oil 增塑油plastics 塑料plastification 增塑plastigel 塑性凝胶plastisol 塑溶胶plastomer 塑料plastometer 塑性计plastometry 塑性测定法plate 板plate efficiency 板效率plate glass 板玻璃plate rectification column 板极整六plate theory 塔板理论platelet 血小板plating 镀锌platinized asbestos 披铂石棉platinocyanide 铂化物platinum 铂platinum black 铂黑platinum chloride 氯化铂platinum dish 白金碟platinum electrode 铂电极platinum foil 铂箔platinum oxide 氧化铂platinum plating 镀铂platinum rhodium 铂铑合金platinum sponge 铂棉platinum tetrachloride 四氯化铂platinum wire 铂丝platinzation 镀铂pleochroism 多色性pleomorphism 多形现象plexiglass 普列克斯玻璃plumbate 铅酸盐plumbism 铅中毒plumbite 亚铅酸盐plumbite process 铅酸钠精制过程plumbite solution 博士溶液plunger 柱塞plunger pump 柱塞泵plus strand 正链plutonium 钚plutonium bromide 溴化钚plutonium hydride 氢化钚plutonium oxide 氧化钚plutonium reactor 钚反应堆ply 层plywood 层板pneumatic conveying 气动输送pneumatic conveying drying 气辽燥pneumatic conveyor 气动输送机pneumatic drier 气辽燥器pneumatic separation 气力分选pneumatic stirring 气动搅拌podophyllin 足叶草硷point charge 点电荷point group 点群point of neutralization 中和点poise 泊poiseuille's law 泊肃叶定律poison 毒poison gas 毒气poisoning 中毒poisson's coefficient 泊松系数poisson's distribution 泊松分布poisson's ratio 泊松比polar adsorption 极性吸附polar axis 极轴polar bond 极性键polar compound 极性化合物polar coordinates 极坐标polar molecule 极性分子polar solvent 极性溶剂polarimeter 偏光计polarimetric analysis 旋光分析polarimetry 旋光分析polarity 极性polarizability 极化率polarization 极化polarization analysis 偏光解析polarization capacity 极化额polarization colorimeter 偏光比色计polarization curve 极化曲线polarization potential 极化电势polarization resistance 极化阻力polarized light 偏光polarizer 起偏器polarizing microscope 偏光显微镜polarogram 极谱图polarograph 极谱仪polarographic analysis 极谱分析polarographic cell 极谱池polarographic maximum 极谱极大polarographic wave 极谱波polarography 极谱法pole 极polishing 磨光pollution 沾污pollution load 污染负荷polonium 钋poly 聚polyacid 缩多酸polyacidic base 多价碱polyacrylamide 聚丙烯酰胺polyacrylate 聚丙烯酸酯polyacrylic acid 聚丙烯酸polyacrylonitrile 聚丙烯腈polyaddition reaction 加聚合反应polyalcohol 多元醇polyallomer 聚异质同晶体polyamide 聚酰胺polyamino acid 聚氨基酸polyampholyte 聚两性电解质polyatom 多原子polyatomic molecule 多原子分子polyazo dye 多偶氮染料polybasic acid 多元酸polybutadiene 聚丁二烯polybutadiene rubber 聚丁二烯橡胶polycarbonate 聚碳酸酯polychloroprene 聚氯丁二烯polycondensate 缩聚物polycondensation 缩聚polycrystal 多晶体polycyclic 多环的polycyclic compound 多环化合物polycyclic hydrocarbon 多环烃polydisperse sol 多分散胶polydispersity 多分散性polyelectrolyte 聚合电解质polyene 多烯polyester 聚酯polyester fiber 聚酯纤维polyester resin 聚酯尸polyether 聚醚polyethylene 聚乙烯polyethylene glycol 聚乙二醇polyethylene oxide 聚氧化乙烯polyethylene terephthalate 聚乙烯对苯二甲酸酯polyformaldehyde 聚甲醛polyfunctionality 多功能性polyglycol 聚乙二醇polyhalide 多卤化物polyhalogenide 多卤化物polyhexamethylene adipamide 聚己二酰己二胺polyhydric alcohol 多元醇polyisobutylene 聚异丁烯polyisoprene 聚异戊二烯polymer 聚合物polymer analogue 聚合物系类polymer blend 共混聚合物polymer gasoline 聚合汽油polymer homologue 同系聚合物polymer isomer 聚合物异构体polymeric plasticizer 高分子增塑剂polymerization 聚合polymerization accelerator 聚合促进剂polymerization degree 聚合度polymerization inhibitor 聚合抑制剂polymerization initiator 聚合引发剂polymerization retarder 聚合抑制剂polymerized oil 聚合油polymery 聚合现象polymethacrylate 聚甲基丙烯酸酯polymethyl methacrylate 聚甲基丙烯酸甲酯polymethylene 聚甲烯polymorphic transition 多形转变polymorphism 多形现象polynaphthene 多环烷polynitroethylene 聚硝基乙烯polynuclear complex 多核配合物polynuclear hydrocarbon 多环烃polynucleotide 多核甙酸polyolefin 聚烯烃polyolefine 聚烯烃polyoxyethylene 聚乙二醇polyoxymethylene 聚氧化甲烯polypeptide 多肽polyphase equilibrium 多相平衡polyphenol 多元酚polyphosphate 多聚磷酸酯polypropylene 聚丙烯polypropylene oxide 聚环氧丙烷polyprotic base 多价碱polysaccharide 多糖polystyrene 聚苯乙烯polystyrol 聚苯乙烯polysulfide 多硫化合物polysulfide rubber 聚硫橡胶polysulfonamide 聚磺酰胺polysulfone 聚砜polyterpene 聚萜烯polytetrafluoroethylene 聚四氟乙烯polythene 聚乙烯polythionic acid 连多硫酸polythiourea 聚硫脲polyurea 聚脲polyurethane 聚氨酯polyurethane foam 聚氨酯泡沫体polyurethane resin 聚氨酯尸polyvinyl acetate 聚醋酸乙烯酯polyvinyl alcohol 聚乙烯醇polyvinyl butyral 聚乙烯醇缩丁醛polyvinyl carbazole 聚乙烯咔唑polyvinyl chloride 聚氯乙烯polyvinyl chloroacetate 聚氯醋酸乙烯酯polyvinyl ether 聚乙烯醚polyvinyl fluoride 聚氟乙烯polyvinylamine 聚乙烯胺poppy seed oil 罂粟油population 群体population inversion 粒子数反转porcelain 瓷器porcelain clay 瓷土porcelain crucible 瓷坩埚porcelain earth 瓷土porcelain enamel 搪瓷porcelain glaze 瓷釉porcelain mortar 瓷乳钵pore 微孔porosity 多孔性porous ion exchange resin 多孔离子交换尸porous pot 素瓷器素烧瓶porphin 卟吩porphyrin 卟啉portland cement 波特兰水泥position representation 位置表象positional isomer 位置异构物positive catalyst 正催化剂positive displacement meter 正排量计positive displacement pump 正排量泵positive electrode 阳电极positive ion 阳离子positive lens 会聚透镜positive maximum 正极大positive plate 阳极板positive valency 正化合价positron 阳电子postignition 后点火postprecipitation 后沉淀pot 罐pot life 可用时间potash 草碱potash alum 茂potash bulb 钾球potash fusion 钾熔化potash glass 钾玻璃potash lye 钾碱液potash mica 钾云母potash soap 钾皂potassic fertilizer 钾肥料potassium 钾potassium acetate 醋酸钾potassium alum 茂potassium aluminate 铝酸钾potassium antimonyl tartrate 酒石酸氧锑钾potassium arsenate 砷酸钾potassium arsenite 亚砷酸钾potassium bicarbonate 碳酸氢钾potassium bisulfate 硫酸氢钾potassium bisulfite 亚硫酸氢钾potassium bromate 溴酸钾potassium bromide 溴化钾potassium carbonate 碳酸钾potassium chlorate 氯酸钾potassium chloride 氯化钾potassium chromate 铬酸钾potassium dichromate 重铬酸钾potassium dihydrogenphosphate 磷酸二氢钾potassium feldspar 钾长石potassium ferricyanide 铁氰化钾potassium ferrocyanide 亚铁氰化钾potassium fluoride 氟化钾potassium hexacyanoferrate 亚铁氰化钾potassium hydrogen oxalate 草酸氢钾potassium hydrogen phthalate 氢酞酸钾potassium hydroxide 氢氧化钾potassium hypochlorite 次氯酸钾potassium iodate 碘酸钾potassium iodide 碘化钾potassium nitrate 硝酸钾potassium nitrite 亚硝酸钾potassium perchlorate 高氯酸钾potassium periodate 高碘酸钾potassium permanganate 过锰酸钾potassium peroxodisulfate 过硫酸钾potassium persulfate 过硫酸钾potassium phosphate 磷酸钾potassium polysulfide 多硫化钾potassium primary phosphate 磷酸二氢钾potassium pyroantimonate 焦锑酸钾potassium pyrophosphate 焦磷酸钾potassium pyrosulfate 焦硫酸钾potassium rhodanide 硫氰酸钾potassium silicate 硅酸钾potassium sodium carbonate 碳酸钠钾potassium sodium tartrate 罗谢尔盐potassium sulfate 硫酸钾potassium sulfide 硫化钾potassium sulfite 亚硫酸钾potassium tartrate 酒石酸钾potassium thiocyanate 硫氰酸钾potassium thiosulfate 硫代硫酸钾potassium xanthate 黄原酸钾potato starch 马铃薯淀粉potential 势potential barrier 势垒potential difference 电势差potential energy 位能potential gradient 电势梯度potential well 势阱potentiometer 电势计potentiometric analysis 电势分析potentiometric cell 电位滴定池potentiometric titration 电势滴定potentiostatic electrolysis 静电位电解potter's clay 塑粘上pottery 陶器potting 浇灌pour point 怜点pour point depressant 降凝剂powder 粉powder metallurgy 粉末冶金powdered catalyst 粉状催化剂powdered coal 粉煤powdery yeast 粉状酵母power 力power consumption 功率消耗power factor 功率因数pozzolanic cement 火山灰水泥practical units 实用单位prandtl number 普朗特数praseodymium 镨praseodymium acetate 醋酸镨praseodymium bromate 溴酸镨praseodymium bromide 溴化镨praseodymium carbonate 碳酸镨praseodymium chloride 氯化镨praseodymium hydroxide 氢氧化镨praseodymium molybdate 钼酸镨praseodymium nitrate 硝酸镨praseodymium oxalate 草酸镨praseodymium oxychloride 氯氧化镨praseodymium selenate 硒酸镨praseodymium sesquioxide 二氧化二镨praseodymium sulfate 硫酸镨praseodymium sulfide 硫化镨precious metal 贵金属precious stone 宝石precipitability 沉淀性precipitant 沉淀剂precipitate 沉淀物precipitated barium carbonate 沉淀碳酸钡precipitated sulfur 沉淀硫precipitating agent 沉淀剂precipitation 沉淀;降水precipitation agent 沉淀剂precipitation analysis 沉淀分析precipitation membrane 沉淀膜precipitation reaction 沉降反应precipitation titration 沉淀滴定precipitation value 沉淀值precipitator 沉淀器precision 精度precision balance 精密天平precision instrument 精密仪器precleaning 预净化precooling 预冷却precure 早期硫化precursor 前驱物质predissociation 预离解predrying 预干燥pregnenolone 孕烯醇酮preheater 预热器preheating 预热preheating furnace 预热炉preheating oven 预热炉preliminary cleaning 预净化preliminary drying 预干燥preparation 制法preparation of a surface 表面处理preparative gas chromatography 制备级气相色谱法prepared paint 低涂料prepolymer 预聚合物preservation 保存preservatives 保存剂preserve hydrogen with rare earths 用稀土金属储存氢press 压机press cake 滤饼press cure 加压硫化press filter 压滤机press vulcanization 加压硫化pressed glass 压制玻璃pressed yeast 压缩酵母pressing 压制pressure 压力pressure base factor 基压系数pressure distillation 加压蒸馏pressure filtration 加压过滤pressure gage 压力计pressure loss 压力损失pressure medium 压力介质pressure regulator 压力第器pressure sensitive adhesive 压敏粘着剂pressure sensitive dye 压敏色素pressure still 加压蒸馏器pressure tank 压力罐pretreatment 预处理prevulcanization 早期硫化primary air 一次空气primary alcohol 伯醇primary amine 伯胺primary carbon atom 伯碳原子primary cell 原电池primary phase 初晶相primary salt 一代盐primary winding 初级线圈prime coat 底涂primer 底层涂料primidone 普里米酮priming coat 底涂priming paint 底层涂料priming tube 雷管primitive cell 初基胞primitive lattice 初基点阵principal axis of inertia 惯性轴principal component 知组分principal fermentation 知发酵酌principal quantum number 挚子数principal reaction 知反应principal series 值列principal valence 汁子价principle of corresponding states对应状态原理principle of energy dissipation 能量耗散原理principle of least action 最小酌原理principle of maximum overlapping最大重迭原则principle of superposition 迭加原理prins reaction 普林斯反应printing 印刷printing ink 印刷墨printing paper 印刷纸prism 棱镜prism for infrared ray 红外线棱镜prism spectrograph 棱镜光谱仪prismatic spectrum 棱镜光谱pristane 姥鲛烷probability 概率probability amplitude 概率幅probability density 概率密度probability distribution 概率分布probability space 概率空间probable error 概率误差procainamide 普鲁卡因胺procaine 普鲁卡因procaine base 普鲁卡因碱process 工程process analyzer 工程分析计process monitoring 工程监视process steam 加工蒸汽process variable 工艺变量processing 加工processing aid of textile 织物加工助剂producer 发生炉producer gas 发生炉煤气producer gas reaction 发生炉气体反应product 产品product of distillation 蒸馏产物production 生产production of pairs 正负电子对产生proflavine 前黄素progesterone 孕酮prolactin 促乳泌素prolamin 醇溶朊prolidase 脯肽酶proline 脯氨酸promazine 普马嗪promedol 二甲哌替啶promethazine 普鲁米近promethium 钜prominence 日珥promotor 促进剂pronamide 拿草特propadiene 丙二烯propagation 增长反应propagation rate 传播速率propanal 丙醛propane 丙烷propanoic acid 丙酸propanol 丙醇propargyl alcohol 炔丙醇propargyl bromide 炔丙基溴propargylic acid 丙炔酸propeller 螺旋桨propeller agitator 螺旋桨式搅拌器propeller stirrer 螺旋桨式搅拌器propene 丙烯proper value 本盏proper vibration 固有振动propiolic acid 丙炔酸propionaldehyde 丙醛propionamide 丙酰胺propionic acid 丙酸propionic anhydride 丙酸酐propionitrile 丙腈propiophenone 苯丙酮propolis 蜂巢腊胶proportion 比例proportional counter 正比计数管proportional intensification 比例加强proportional limit 比例极限proportional reduction 比例缩减proportioning pump 计量泵propyl acetate 醋酸丙酯propyl alcohol 丙醇propyl aldehyde 丙醛propyl bromide 丙基溴propyl chloride 丙基氯propyl gallate 没食子酸丙酯propyl mercaptan 丙硫醇propyl propionate 丙酸丙酯propyl sulfide 二丙硫propylamine 丙胺propylbenzene 丙基苯propylene 丙烯propylene glycol 丙二醇propylene oxide 环氧丙烷propylidene 亚丙烷propyliodone 丙碘酮propylthiouracil 丙硫氧嘧啶propyne 丙炔prosthetic group 辅基protactinium 镤protamine 精蛋白protease 朊酶protease inhibitor 蛋白酶抑制剂protection 保护protective action 保护酌protective agent 保护剂protective coating 保护涂层protective colloid 保护胶体protective glass 保护玻璃protective material 保护剂protective number 保护量protein 蛋白质protein biosynthesis 蛋白质的生合成protein fiber 蛋白质纤维protein metabolism 蛋白质代谢protein plastic 蛋白质塑料protein refractometer 蛋白折射计protein wave 蛋白波proteolysis 蛋白质水解proteolytic enzyme 蛋白水解酶proteose 朊间质prothoate 发果prothrombin 凝血酶原protic solvent 质子性溶剂protium 氕protocatechuic acid 原儿茶酸protolysis 光解酌proton 质子。
化学品安全技术说明书1.化学品及企业标识产品名称Palladium(0-5%)on Calcium Carbonate(poisoned withLead)商品名称钯催化剂,林德拉催化剂生产厂家西安凯立新材料股份有限公司企业地址西安市经济技术开发区泾渭新城泾勤路西段6号710201电话号码************紧急服务电话************传真号码************E-mail******************/****************2.危险性概述GHS分类物理性危害未分类健康危害未分类环境危害未分类GHS标签元素图标或危害标志无信号词无信号词危险描述无防范说明无其他没有分类的危险粉末或颗粒状与空气混合可能引起粉尘爆炸3.成分/组成信息单一物质/混合物混合物化学名(中文名)钯碳酸钙催化剂(0-5%)(铅毒化)百分比…CAS编码7440-05-3分子式Pd/CaCO34.急救措施吸入将受害者移到新鲜空气处,保持呼吸通畅,休息。
若感不适请求医/就诊。
皮肤接触立即去除/脱掉所有被污染的衣物。
用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触用水小心清洗几分钟。
如果方便,易操作,摘除隐形眼镜。
继续清洗。
如果眼睛刺激,求医/就诊。
食入若感不适,求医/就诊。
漱口。
紧急救助者的防护救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
5.消防措施合适的灭火剂干粉,干砂不适用的灭火剂水特殊危险性小心,燃烧或高温下可能分解产生毒烟。
特定方法从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具灭火时,一定要穿戴个人防护用品6.泄漏应急处理个人防护措施和用具,紧急措施使用个人防护用品。
远离溢出物/泄露处并处在上风处。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施防止进入下水道。
控制和清洗的方法和材料清扫收集粉尘,封入密闭容器。
钯pá形声。
字从金,从巴,巴亦声。
本字指金属的“耙”。
本字列在《汉语大字典》袖珍本第1791页。
又读:bǎ。
指近代发现的化学元素palladium。
元素名称:钯英文名称:palladium化学符号:Pd ,第五周期Ⅷ族铂系元素的成员元素类型:金属元素化合价:+2和+4原子序数:46质子数:46中子数:62摩尔质量:106.42g/mol化学元素结构晶体结构:晶胞为面心立方晶胞,每个晶胞含有4个金属原子。
晶胞参数:a = 389.07 pmb = 389.07 pmc = 389.07 pmα = 90°β = 90°γ = 90°电子层排布:2-8-18-18-0核电荷数:46电子层:K-L-M-N-O外围电子层排布:4d10电离能(kJ /mol) :M - M+ 805M+ - M2+ 1875M2+ - M3+ 3177M3+ - M4+ 4700M4+ - M5+ 6300M5+ - M6+ 8700M6+ - M7+ 10700M7+ - M8+ 12700M8+ - M9+ 15000M9+ - M10+ 17200物理基本数据熔点:1554 ℃沸点:2970 ℃比热:244J密度:12.02g/cm3(20℃)莫氏硬度:4.75声音在其中的传播速率:3070 m/S元素含量:在太阳中的含量:0.003ppm、太平洋表面:0.000000019ppm、地壳中含量:0.0006ppm物理性质钯是银白色过渡金属,较软,有良好的延展性和可塑性,能锻造、压延和拉丝。
块状金属钯能吸收大量氢气,使体积显著胀大,变脆乃至破裂成碎片。
常温下,1体积海绵钯可吸收900体积氢气,1体积胶体钯可吸收1200体积氢气。
加热到40~50℃,吸收的氢气即大部释出,广泛地用作气体反应,特别是氢化或脱氢催化剂,还可制作电阻线、钟表用合金等。
化学性质主要化合物二氯化钯(PdCl2)、四氯钯酸钠(Na2PdCl4)和二氯四氨合钯(Pd(NH3)4Cl2)。
第一部分化学品及企业标识化学品中文名:双三苯基磷二氯化钯化学品英文名:Dichlorobis(triphenylphosphine)palladiumCAS No.:13965-03-2分子式:C36H30Cl2P2Pd产品推荐及限制用途:作为催化剂。
第二部分危险性概述紧急情况概述可能对水生生物造成长期持续有害影响。
GHS危险性类别危害水生环境——长期危险类别 4标签要素:象形图:警示词:警告危险性说明:H413 可能对水生生物造成长期持续有害影响防范说明●预防措施:—— P264 作业后彻底清洗。
—— P280 戴防护手套/穿防护服/戴防护眼罩/戴防护面具。
—— P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾。
—— P271 只能在室外或通风良好处使用。
●事故响应:—— P302+P352 如皮肤沾染:用水充分清洗。
—— P332+P313 如发生皮肤刺激:求医/就诊。
—— P362+P364 脱掉沾染的衣服,清洗后方可重新使用——P305+P351+P338 如进入眼睛:用水小心冲洗几分钟。
如戴隐形眼镜并可方便地取出,取出隐形眼镜。
继续冲洗。
—— P337+P313 如仍觉眼刺激:求医/就诊。
—— P304+P340 如误吸入:将人转移到空气新鲜处,保持呼吸舒适体位。
—— P312 如感觉不适,呼叫解毒中心/医生●安全储存:—— P403+P233 存放在通风良好的地方。
保持容器密闭。
—— P405 存放处须加锁。
●废弃处置:—— P501 按当地法规处置内装物/容器。
物理和化学危险:无资料。
健康危害:无资料。
环境危害:可能对水生生物造成长期持续有害影响。
第三部分成分/组成信息√物质混合物第四部分急救措施急救:吸入:如果吸入,请将患者移到新鲜空气处。
皮肤接触:脱去污染的衣着,用肥皂水和清水彻底冲洗皮肤。
如有不适感,就医。
眼晴接触:分开眼睑,用流动清水或生理盐水冲洗。
如有不适感,就医。
食入:饮水,禁止催吐。
Palladium-Catalyzed Carbon-Carbon Coupling Reactions2.1. Matsuda-Heck Reaction2.1.1. Olefinic Coupling PartnerKikukawa and Matsuda were the pioneers in studying thereactivity of diazonium salts with transition metals. Thenew method emerged as a complementary strategy to theMeerwein reaction6 for the arylation of unactivated olefinsand also as an improvement on the method proposed in theearly studies with aryl halides that Mizoroki and Heckseparately conducted.20Kikukawa和Matsuda是研究重氮盐与过渡金属盐反应的先驱者。
The olefins that were arylated werestyrene, cyclopentene, allylic alcohols, and ethyl acrylate.The in situ reduction of LiPd(II)Cl3 by sodium formate orPd2(dba)3 as a source of Pd(0) in aqueous acetonitrile wasused as a catalytic system. The reaction occurred at roomtemperature with sodium acetate as the base until productionof nitrogen gas ceased. The same authors extended themethodology to the arylation of ethylene based on acomparative study of a variety of monosubstituted arenediazoniumsalts21,22 (Scheme 5).Some exceptions were o andp-nitrobenzenediazonium salts, which only gave nitrobenzeneas the main reaction product. It seems that thereaction was also sensitive to the steric effects since mesitylenediazonium salt did not produce the correspondingstyrene. A specific application by the same authors wasvinylation of a benzocrown ether23The detailedstudy of acrylonitrile was taken up again in 2001 when Caiet al.24 published a paper that investigated both the arylationof acrylonitrile and acrylamide with various arenediazonium tetrafluoroborates using Pd(OAc)2 in EtOH at 80 ︒C. Thecorresponding (E)-cinnamonitrile and (E)-cinnamamide derivativeswere obtained in yields of between 78% and 89%.In 2001, another paper published by Cai et al.25 reported yields of up to 63% for the arylation of alcohols 11 and 12 using Pd(OAc)2 asthe catalyst in EtOH at 60 ︒C. More recently, Muzart,Roglans et al.26 achieved the arylation of several allylicalcohols with arenediazonium tetrafluoroborates with Pd2-(dba)3 in MeOH, obtaining the corresponding protectedaldehydes 16 from primary alcohols 11 and 12 and ketones19 from secondary alcohols 17 and 18 (Scheme 7).One of the main limitations of olefin arylations bydiazonium salts is the instability of some of these salts atroom temperature. Studying the reaction of arylamines with styrene in the presence of palladium salts, Fujiwara et al.27 found that addition of an equimolar amount of tert-butylnitrite to the reaction mixture considerably improved the yields of the corresponding stilbenes. Inspired by Matsuda’s success in obtaining aryl-substituted olefins by a Pd-catalyzed reaction of the olefin and arenediazonium salts,19 Fujiwara described for the first time a direct domino diazotizationstyrene arylation by amines in the presence of palladium salts (Scheme 8). However, in the method used by Fujiwara, stoichiometric amounts of Pd(OAc)2 were required in orderto obtain good yields (conditions i in Scheme 8). At the same time, Matsuda et al.28 described the arylation of severalolefins by arylamines (the examples with styrene are givenin Scheme 8) using alkyl nitrite and catalytic amounts ofPd2(dba)3. In this case it was necessary to add a mixture of monochloroacetic acid and acetic acid to improve the results (conditions ii in Scheme 8).The “one-pot” diazotization-arylation reactionwas also subsequently employed by other authors. Beller etal.29 described the direct synthesi s of substituted styrenes starting from several anilines and ethylene. The diazotization- arylation process took place in the presence oft BuONO, acetic acid, and a catalytic amount (5 mol %) ofPd(OAc)2 at room temperature and with ethylene under atmospheric pressure (Scheme 9).Sengupta33 and Goeldner34 proposed an alternative to overcome the problem of the instability of arenediazonium salts based on the study of the effect of their counteranionson the Matsuda-Heck reactions. Sengupta et al.33 generated several arenediazonium salts in situ with different counteranions (OAc, ClO4, F, CH3SO3, BF4, CF3CO2) by acidolysisof 1-aryltriazenes with the corresponding acid and studiedtheir utility in the Matsuda-Heck reaction with ethyl acrylate. With the exception of acetic acid, all acids produced clean arylation processes in high yields (73-97%). For all cases studied, the more economical diazonium perchloratesand fluorides gave better results than the corresponding isolated arenediazonium tetrafluoroborates. Goeldner et al.34 described an efficient, mild procedure for the synthesis of arenediazonium trifluoroacetates under anhydrous conditions. The method permitted production of large quantities of an extensive series of aniline derivatives. Their application inthe arylation of ethyl acrylate was studied in depth, and an interesting alternative to arenediazonium tetrafluoroborates was found.A very recent contribution in this area is due to Barbero,Fochi et al.35 and Dughera,36 which use arenediazoniumo-benzenedisulfonimides (Figure 1) as an interesting alternative to the more usual arenediazonium tetrafluoroborates.These compounds are easy to prepare and isolate and are highly stable.37 Furthermore, benzenedisulfonimide is easily recovered for reuse at the end of the reaction. The authorsuse this kind of arenediazonium salt in Matsuda-Heck35 andStille36 (see section 2.4.1.) coupling reactions.It should be noted that in most cited cases reactions withchloro-, bromo-, or iodo-substituted arenediazonium salts displayed a high degree of chemoselectivity, indicating thesuperior reactivity of the diazonium nucleofuge over chloride, bromide, and even iodide. This fact illustrates one of the advantages of working with arenediazonium salts as arylating reagents in Matsuda-Heck reactions and shows that thearyl-nitrogen bond is more reactive to zerovalent palladiumthan aryl-halogen bonds. Sengupta38 and Xu39 have taken advantage of the above-mentioned differential coupling ofthe nucleofuges.Sengupta38 described a stepwise assemblycarried out on a 4-iodo-2-methylbenzenediazonium salt toprepare unsymmetrical divinylbenzene derivatives (Scheme11). As shown in Scheme 11, the different regioisomers were obtained by switching the order in which the olefins wereadded.Xu et al.39 also described the synthesis of unsymmetrical divinylbenzenes taking advantage of the superior reactivityof arenediazonium salts as compared to aroyl chlorides.40Several carboxybenzenediazonium salts were used to arylate methyl acrylate using Li2PdCl4-CuCl as a catalytic systemin a methanolic solution at room temperature in 10-30 min.The corresponding alkenylated benzoic acids were transformed into their benzoyl chlorides, and this functionalitywas used to run a second Pd-catalyzed arylation process.Since it is known that arenediazonium salts in the presenceof Cu(I) can give a Meerwein reaction, the authors ran the reaction in the absence of Li2PdCl4 to confirm that thisreaction did not occur here. However, the authors do notexplain why CuCl is required (Scheme 12).Two- and 4-fold Matsuda-Heck reactions of bisdiazoniumsalts 20 and 21 and tetrakis-diazonium salt 22 were describedby Sengupta et al.41,42 as a further demonstration of thegreater reactivity of arenediazonium salts. Matsuda-Heckadducts 23 and 24 were obtained after 1 h with 60-83%yields when Pd(OAc)2 was used as a catalyst in EtOH at 80︒C. Another specific demonstration of the superior reactivityof arenediazonium salts over aryl halides as electrophiles in Matsuda-Heck reactions was given by Geneˆt et al.43 onstudying the arylation of perfluoroalkenes.2.2. Suzuki-Miyaura ReactionIt was not until 20 years after the first utilization of the arenediazonium salts in a palladium-catalyzed reactions thatthese electrophiles were applied in Suzuki-Miyaura crosscouplings. This late discovery was made independently, but simultaneously, in the laboratories of Geneˆt et al.83 and Sengupta at al.84 Moderate to good yields of biaryls wereobtained by coupling of arenediazonium tetrafluoroboratesand arylboronic acids in the presence of catalytic amountsof Pd(OAc)2 and in the absence of both an added base andligands.After the ini tial discovery the Geneˆt group performed anintensive study to improve the reaction by modifying theboronic counterpart.85 The search for a more nucleophilic organoborane moiety led them to test potassium aryltrifluoroborates, whose easy preparation had been previouslyreported by Vedejs et al.86 in the Suzuki-Miyaura coupling.These very stable, water-resistant, and easily isolated nucleophiles were shown to be more efficient and reactive thanthe corresponding organoboronic acids, leading to higherproduct yields in shorter time periodsGeneˆt et al.83b showed that alkenyl boronic acids couldalso be effectively coupled to arenediazonium tetrafluoroborates using Pd(OAc)2 in 1,4-dioxane at room temperature.Stilbene and styrene derivatives were obtained in good tomoderate yields (Scheme 31).An elegant step forward was proposed by Geneˆt etal.85b,89 in describing an efficient synthesis for potassium vinyltrifluoroborate and studying its reactivity toward arenediazonium salts under palladium catalysis. Good yieldsof differently substituted styrenes were obtainedAs a logical follow-up on the diversification of substrates,Geneˆt et al.85b tried to extend the diazonium-trifluoroborate coupling to formation of sp2-sp carbon-carbon bonds.2.3. Carbonylative CouplingKikukawa, Matsuda et al.97 pioneered the use of diazoniumsalts in carbonylation reactions. They first reported the Pdcatalyzed reaction of arenediazonium tetrafluoroborates withCO in the presence of sodium acetate, or other sodium carboxylates, in acetonitrile.Kikukawa, Matsuda etal.99 were again the pioneers in the Pd-catalyzed carbonylationof arenediazonium salts in the presence of tincompounds, and their first communication appeared in1982,99a the same year that arylboronic acids were describedfor the first time1002.4.1. Stille Cross-CouplingConventional Stille couplings17 with diazonium salts haveattracted much less attention than both Suzuki-Miyauracross-couplings (section 2.2) and carbonylative couplingsinvolving organostannanes (section 2.3).Kikukawa, Matsuda et al.79 reported methylations withMe4Sn to give toluenes 103, arylations, as well as a vinylationwith CH2dCH-SnBu3 that produced styrene (Scheme 47). Methylation of diazonium salts, reported for the first timein this study, was carried out using arenediazonium tetrafluoroboratesor hexafluorophosphates with different substituents,giving moderate to good yields of substituted toluenes 103in 2 h (Scheme 47).2.4.2. Carbon Heteroatom CouplingBy analogy with carbonylations, Keim et al.104 preparedsulfinic acids 104 by reaction of diazonium tetrafluoroboratesin an atmosphere of SO2 and hydrogen in the presence of10 mol % Pd/C (Scheme 48). As a reducing agent, theauthors demonstrated that hydrogen was superior to silanes,which had been used by Kikukawa et al.98 in reductive carbonylation processes.Borylation of aromatics was achieved by Strongin et al.105by palladium-catalyzed carbon-boron bond formation from arenediazonium tetrafluoroborates. Thus, arenediazoniumsalts reacted with both halves of bis(pinacolato)diborane 105in the presence of PdCl2(dppf) in refluxing methanol to afford boronic esters 106 (Scheme 49).Two processes which do not fit any of our previousheadings involving arenediazonium salts in palladium crosscoupling reactions are shown in Scheme 50. A ternarycoupling between norbornadiene, arenediazonium tetrafluoroborates, and tin or boron compounds as formal donors ofPh- or Ph-CtC-produced norbornenes 107 in moderateto good yields.107The second very different case is that of the reaction of benzenediazonium tetrafluoroborate with silyl enol ethers inthe presence of Pd(PPh3)4, NaBPh4 using pyridine as asolvent to afford a 74% yield of R-phenyl ketone. However,the reaction was more efficient in the absence of thepalladium catalyst, demonstrating that arylation proceededvia a radical mechanism promoted by pyridine.108Concluding Remarksarenediazonium saltsare attractive partners in palladium-catalyzed cross-coupling reactions. In particular, Heck reactions involving arenediazonium salts have been extensively developed and widelyused in the synthesis of natural products and other complex organic compounds. There are fewer publications involvinguse of these electrophiles in Suzuki-Miyaura, carbonylative, Stille, and carbon-heteroatom cross-couplings. A series of advantages of working with arenediazonium salts as electrophiles has been observed. Arenediazonium salts derivefrom inexpensive aromatic anilines, and the corresponding tetrafluoroborates have the additional advantage of beingeasily prepared in large quantities.Thesuperior reactivity of the diazonium nucleofuge over halideshas also permitted the chemoselectivity of the crosscouplings, which has resulted in interesting applications beingdeveloped. Moreover, the cross-couplings do not seem to be sensitive to the electronic nature of the substituents in the arenediazonium salts, which is an important drawback when aryl halides are used.。