Kinetics of transesterification in rapeseed oil to biodiesel fuel as treated in supercritical
- 格式:pdf
- 大小:96.03 KB
- 文档页数:6

碳酸钾催化菜籽油与甲醇反应制备生物柴油的研究张福建;姜绍通;潘丽军;刘文玉【摘要】以菜籽油和甲醇为原料,在碳酸钾作用下催化制备生物柴油,考察了反应温度、醇油物质的量比、催化剂用量对生物柴油产率的影响,建立了动力学模型,并利用该模型对试验数据进行拟合,估算出动力学参数:频率因子A为13.8,活化能△E为20.6 kJ/mol,并发现反应常数与催化剂用量呈线性关系,线性关系式可表示为κ=κ0+κwW.%To prepare biodiesel with rapeseed oil and methanol catalyzed by potassium carbonate, the effect of reaction temperature, mass ratio of methanol to oil and catalyst dosage on biodiesel yield was investigated. A kinetics model was established and the model was used to fit the experimental data, and the kinetics parameters were estimated. Results:The frequency factor A is 13.8, activation of energyΔE is 20.6kJ/mol. There is a linear relationship between reaction rate constant and catalyst dosage, and the linear relationship can be denoted as k = k0 + kw W.【期刊名称】《中国粮油学报》【年(卷),期】2011(026)003【总页数】4页(P52-55)【关键词】菜籽油;生物柴油;碳酸钾;动力学【作者】张福建;姜绍通;潘丽军;刘文玉【作者单位】合肥工业大学,合肥,230009;合肥工业大学,合肥,230009;合肥工业大学,合肥,230009;合肥工业大学,合肥,230009【正文语种】中文【中图分类】TQ645.1生物柴油是来源于天然油脂的可再生燃料,近年来其正受到越来越多的关注[1]。

* *化学实验室常用物品英文名称1、常用仪器试管test tube试管架test rack试管夹test tube holder玻璃棒glass rod滴管medicine dropper小滴管dropper烧杯beaker不锈钢杯stainless-steel beaker酒精灯alcohol burner酒精喷灯blast alcohol burner本生灯Bunsen burner量杯measuring cup量筒measuring cylindermeasuring/graduated flask 漏斗funnel分液漏斗separatory funnel布氏漏斗Busher funnel赫氏漏斗Hirsch funnel长颈漏斗long-stem funnel无颈漏斗stemless funnel滤纸filter paper华特门滤纸Whatman (filter) paper 广口瓶wide-mouth bottle烧瓶flask平底烧瓶florence flask碘量瓶iodine flask锥形瓶Erlenmeyer flaskconical flask容量瓶volumetric flaskmeasuring flask 移液管(one-mark) pipette移液管架pipette rack洗耳球rubber suction bulb刻度移液管graduated pipettes吸液管pipette滴定管burette滴定管夹burette clamp滴定架burette holder滴定台support holder酸氏滴定管Geiser burette (stopcock)碱氏滴定管Mohr burette for use with pinchcock石蕊试纸litmus paper石蕊litmuspH值指示剂PH indicatorpH计pH meter广泛ph试纸universal ph indicator paper三口烧瓶3-neck round bottom flask蒸馏装置distilling apparatus蒸馏烧瓶distilling flask滴液漏斗dropping adapter加装温度计的瓶塞reducing adapter 减压蒸馏头vacuum-distilling adapter克氏蒸馏头Claisen distilling adapter球型冷凝管Allihn condenser* *直型冷凝管West condenser空气冷凝管air condenserLiebig condenser 蛇型冷凝管Grahams condenser 蒸馏头three-way adapter分馏柱fractionating column接液管105 degree bent adapter adapter曲颈甑retort坩埚crucible (pot)melting pot坩埚钳crucible clampcrucible tong天平balance , scale分析天平analytical balance电子天平electronic balance台秤platform balance游码crossbeams and sliding weights称量瓶weighing bottle称量纸weighing paper研钵mortar研杵pestle玛瑙研钵agate mortar2、其他常用器皿及仪器洗瓶plastic wash bottle试剂瓶reagent bottles塞子stopper橡胶塞rubber stopper玻璃塞Pennyhead stopper玻璃活塞stopcpck药匙lab spoon镊子forceps升降台lab jack铁架台iron support圆形漏斗架cast-iron ring万能夹extension clamp蝴蝶夹double-buret clamp螺旋夹screw clamp石棉网asbestos-free wire gauze 三角架tripod搅拌装置stirring device搅拌棒stirring rod表面皿watch glass 蒸发皿evaporating dish瓷蒸发皿porcelain旋转蒸发器rotary evaporator trap 冷凝器condenser玻璃活塞stopcock止水夹flat jaw pinch cock弹簧节流夹pinch clamp橡胶管rubber tubing恒温循环仪Thermostatic circulator 真空泵vacuum pump真空阀vacuum traps多头抽真空装置vacuum manifold 抽滤瓶filter flasksuction flask离心机centrifuge离心管centrifuge tube电炉heater马弗炉furnace烘箱oven微波炉电热套heating mantle电动搅拌器power basic stirrer磁力搅拌器magnetic stirrer转子rotor水浴bath沸石boiling stone剪刀scissor烤钵cupel石蜡封口膜parafilm卵形瓶matrass比重瓶specific gravity bottle提勒熔点管Thiele melting point tube水银温度计(mercury-filled) thermometer温度计thermometer倒置显微镜Inverted microscope 擦镜纸wiper for lens玻璃片glass plate盖玻片cover glass载玻片slide闪点仪flash point tester折光仪refractometer紫外灯ultraviolet illumination狭槽slot秒表stopwatch空气过滤器air filter(laminar flow cabinet)药柜cupboard 衣服挂钩coat hanger口罩respirator防毒面具respirator、gasmask 消毒器Sterilizer消毒剂disinfector空调机air conditioner摇床shaker螺丝钉screw螺丝刀screw driver螺栓,插销bolt锁紧螺母nut, cap nut齿槽,插口socket扳手shifting spanner锤子hammer钉子nail插头plug电极electrode电泳槽gel tank微细胞;小电泳槽minicell复印机duplicatorcopying machine复印纸copy paper复写纸carbon paper关节joint计时器timer闹钟alarm clock订书机staplerU形钉staple3、有关化学反应的词汇分析analysis 分馏fractionation吸热反应endothermic reaction放热反应exothermic reaction热力学thermodynamics动力学kinetics沉淀precipitate, precipitation沉淀,沉降sediment, sedimentation 蒸馏distill, distillation煅烧calcine氧化oxidize, oxygenate碱化alkalinization中和neutralize, neutralization滴定titration氢化hydrogenate水合,水化hydrate脱水dehydrate发酵fermentation溶解solution燃烧combustion熔解fusion, melting碱性alkalinity同分异物现象isomerism水解hydrolysis电解electrolysis电解质溶液electrolyte电极electrode阳极,正极anode阴极,负极cathode催化catalyse, catalyze催化剂catalyst催化作用catalysis氧化oxidization, oxidation还原剂reducer分解dissolution, decomposition 合成synthesis可逆的reversible 不可逆的irreversible仪器设备apparatus产物product1.有机化合物的官能团和重要的基团官能团functional group双键double bond三键triple bond烃基hydroxy group琉基mercapto硫轻基sulfhydryl group羰基carbonyl group氨基amino group亚氨基imino group硝基nitro group亚硝基nitroso group氰基cyano group羧基carboxyl group磺基sulpho group烷基alkyl group苯基phenyl group卡基benzyl group芳基aryl group烯基allyl group烷氧基alkoxyl group酰基acyl group活性亚甲基active methylene group2.有机化合物的类型烃hydrocarbon石蜡paraffin脂肪烃aliphatic hydrocarbon烷烃alkane烯烃alkene炔烃alkyne共扼二烯烃conjugated diene脂环烃alicyclic hydrocarbon螺环化合物spiro compound桥环化合物bridged ring compound芳烃aromatic hydrocarbon非苯芳烃nonbenzenoid aromatic hydrocarbon 稠环芳烃condensed aromatics卤代烃halohydrocarbon醇alcohol酚phenol醚ether环氧化合物epoxide 冠醚crown ether 硫醇thiol硫酚thiophenol硫醚sulfide二硫化物disulfide亚磺酸sulfinic acid 磺酸sulfonic acid 亚砜sulfoxide砜sulfone醛aldehyde酮ketone半缩醛hemiacetaI 半缩酮hemiketal 缩醛acetal缩酮ketal西佛碱shiff's base肟oxime腙hydrozone缩氨脲semicarbazoneα,β-不饱和酮α,β--unsaturated ketone 醌quinone羧酸carboxylic acid酰卤acid halide酸酐acid anhydride酯ester酰胺amide內酯lactone内酰胺lactam月青nitrile取代酸substituted acid羟基酸hydroxy acid醇酸alcoholic acid酚酸phenolic acid酮酸keto acidB-酮酸酯B-ketone ester乙酰乙酸乙醋ethyl acetoacetate亚硝基化合物nitroso compound硝基化合物njtro compound亚胺imine胺amine伯胺primary amine仲胺secondary amine叔胺tertiary amine季铵盐quaternary ammonium salt季铵碱quaternary ammonium hydroxide 重氮盐diazonium salt偶氮化合物azo compound胍guanidine氨基酸amino acid磷phosphine磷酸酯phosphate亚磷酸酯phosphite膦酸酯phosphonate膦酸phosphonic acid3.杂环化合物吡咯pyrrol* * 呋喃furane噻吩thiophone吲哚indole卟吩porphine咪唑imidazole噻唑thioazole吡啶pyridine喹啉quinoline异喹啉isoquinoline吡喃鎓盐pyrylium salts黄酮flavone嘧啶pirimidine嘌呤purine4.有机天然产物肽peptide多肽polypeptide核酸nucleic acid核苷nucleoside核苷酸nucleotide生物碱alkaloid碳水化合物carbohydrate单糖monosaccharide醛糖aldoses酮糖ketosesD-核糖ribose D-2-脱氧核糖deoxyribose 葡萄糖glucose果糖fructose糖脎osazone糖苷glucoside低聚糖oligosaccharide 麦芽糖maltose蔗糖sucrose纤维二糖cellobiose环糊精cyclodextrin多糖polysaccharide淀粉starch纤维素cellulose类脂lipid萜类化合物terpenoid甾族化合物steroid脂肪fat油oil脂肪酸fatty acid甘油三羧酸酯triglyceride磷脂phospholipid磷脂酸phosphalidic acid蜡wax5.有机化合物的结构理论价键理论valence-bond theory分子轨道理论molecular orbital theory 共振论resonance theory凯库勒式Kekule formula路易斯式Lewis formulaσ键σbondπ键πbond键能bond energy键角bond angle键长bond Iength成键轨道bonding orbital反键轨道antibonding orbital最高已占轨道HOMO highest occupied molecular orbital 最低末占轨道LUMO lowest unoccupied molecular orbital 诱导效应inductive effect共轭效应conjugated effectπ,π-共轭π,π- conjugationp,π-共轭p,π- conjugation超共轭作用hyperconjugation离域能delocalization energy共振能resonance energy给电子基团electron donating group吸电子基团electron withdrawing group芳性aromaticity休克尔规律Huckel's rule两性离子Zwitterion6.有机化学中的同分异构异构体isomer构造constitution构型configuration构象conformation构造异构constitutional isomerism立体异构stereo isomerism构型异构configurational isomerism顺反异构cis-trans isomerism次序规则sequence ruIe同侧Zugammen Z异侧Entgegen E顺式cis反式trans对映异构enantiomerism = 光学异构旋光异构optical isomerism旋光性optical activity旋光度optical rotation比旋光度specific rotation对称面plane of symmetry对称中心center of symmetry对称轴axis of symmetry手性chirality手性分子chiral molecules对映异构体,对映体enantiomer 非对映体diastereomer外消旋体raceme左旋体leveisomer右旋体dextroisomer内消旋体mesomer费歇尔投影式Fischer projection相对构型relative configuration绝对构型absolute configurationR -构型R -configurationS -构型S -configuration赤式erythro苏式threo外消旋化racemization拆分resolution光学纯度Optical Purity对映体过量百分数enantiomeric excess立体专一性反应stereospecific reaction 立体选择性反应stereoselective reaction不对称合成asymmetric synthesis构象异构conformational isomerism构象分析conformational analysis锯架式perspective formula纽曼投影式Newman projection formula椅式chair form船式boat form直立键a键axial bond平伏键e键equatorial bond互变异构tautomerism酮式keto-form烯醇式enol-form差向异构化epimerization变旋现象mutamerism哈武斯式Haworth form7.有机反应的名称取代反应substitution reaction加成反应addition reaction马尔科夫尼可夫规律Markovnikov rule 共轭加成conjugate addition消去反应elemination reaction查依采夫规律Saytzeff rule霍夫曼规律Hofmann rule硼氢化反应hydroboration催化加氢catalytic hydrogenation聚合反应polymerization单体monomer聚合物polymer硝化反应nitration卤化反应halogenation磺化反应sulfonation烷基化反应alkylation酰基化反应acylation酯化反应esterification酯交换反应transesterification脱羧反应decarboxylation氯甲基化反应chloromethylation傅列德尔-克拉夫茨反应Friedel-Crafts reaction 格利雅反应Grignard reaction 格利雅试剂(格氏试剂) Grignard reagent赖默-梯曼反应Reimer-Tiemann reaction 卤仿反应haloform reaction水解反应hydrolysis reaction醇解反应alcoholysis reaction氨解反应ammonolysisi reaction皂化saponification插烯作用vinylogy缩合condensation克莱森缩合Claisen condensation安息香缩合benzoin condensation羟醛缩合aldol condensation列弗尔马茨基反应Reformatsky reaction迈克尔反应Michael reaction诺文格尔反应Knoevenagel reaction加布里反应Gabriel reaction乙酰乙酸乙酯合成法acetoacetic ester synthesis 丙二酸酯合成法malonic ester synthesis 威廉逊合成法William Son synthesis海森堡试验Hinsberg test重氮化反应diazotization reaction偶联反应coupling reaction脱氨基反应deamination reaction维悌希反应Wittig reaction氧化反应oxidation reaction还原反应reduction reaction周环反应pericyclic reaction环加成反应cycloaddition reaction电环化反应electrocyclic reaction坎尼扎罗反应Cannizzaro reaction 齐齐巴宾反应Chichibabin reaction 狄尔斯-阿德尔反应Diels-alder reaction斐林试剂Fehling reagent托伦试剂Tollens reagent沃克还原Wolff-Kishner reduction 罗森蒙德还原Rosenmund reduction 克莱门森还原Clemmenson reduction考普重排Cope rearrangement霍夫曼重排Hofmann rearrangement 嚬哪醇重排pinacol rearrangement弗里茨重排Fries rearrangement克莱森重排Claisen rearrangement二烯体diene亲二烯体dienophile分子轨道对称守恒原理conversation of orbital symmetry8.有机反应机理均裂homolytic异裂heterolytic活性中间体active intermediate碳正离子carbocation碳负离子carbanion烯醇负离子enolate anion自由基,游离基free radical卡宾,碳烯carbene氮烯nitrene速度决定步骤rate-determining step哈蒙特假定Hammond postulate能线图energy profile过渡状态transition state邻基参与neighboring group participation动力学控制kinetic control热力学控制thermodynamic control离去基团leaving group底物substrate亲电试剂electrofphile亲核试剂nucleophile亲电加成反应electrophilic addition亲电取代反应electrophilic substitution定位规律orientation rule亲核取代反应nucleophilic substitutionSN2 反应机理SN2 reaction mechanismSN1 反应机理SN1 reaction mechanism瓦尔登转化Walden inversion亲核加成反应nucleophilic addition亲核加成-消去反应nucleophilic addition-elimination reaction 消去反应机理elimination reaction mechanismE1 反应机理E1 reaction mechanismE2 反应机理E2 reaction mechanism反式消去anti elimination重排反应机理rearrangement reaction mechanism 自由基反应free radical reaction链引发chain initation链增长chain propagation链终止chain termination9.有机化合物的光谱红外光谱IR Infrared spectra傅立叶变换Fourier Transform指纹区finger print region吸收频率absorption frequency紫外光谱UV Ultraviolet spectra电子跃迁elctronic transition吸光度absorbance摩尔消光系数molar extinction coefficient发色团chromophore助色团auxochrome核磁共振NMR Nuclear Magnetic Resonance1HNMR 谱1HNMR spectra13CNMR 谱13CNMR spectra屏蔽效应shielding effect化学位移chemical shift自旋偶合spin-spin coupling自旋裂分spin-spin splitting偶合常数coupling constant质子去偶proton spin decoupling 质子偏共振去偶proton off-resonance decoupling质谱Mass Spectra(MS)电子流轰击election impact (EI)快原子轰击fast atom bombarment (FAB)分子离子峰molecular ion peak同位素峰isotopic peak基峰base peak质荷比(m/z) mass-to-charge ratio10.分子间作用力氢键hydrogen bond色散力dispersion force 范德华力Van Der Waals force 偶极-偶极作用力dipole-dipole interraction force11.物理性质熔点melting point沸点boiling point密度density溶解度solubility偶极矩dipole moment12.有机化合物的酸碱性酸性acidity碱性basicity<HTML>本站材料仅为本院教师与学生教学所用,请勿它用!</HTML>化学用语中英文对照organic chemistry 有机化学inorganic chemistry 无机化学derivative 衍生物series 系列acid 酸hydrochloric acid 盐酸sulphuric acid 硫酸nitric acid 硝酸aqua fortis 王水fatty acid 脂肪酸organic acid 有机酸hydrosulphuric acid 氢硫酸hydrogen sulfide 氢化硫alkali 碱,强碱ammonia 氨base 碱hydrate 水合物hydroxide 氢氧化物,羟化物hydracid 氢酸hydrocarbon 碳氢化合物,羟anhydride 酐alkaloid 生物碱aldehyde 醛oxide 氧化物phosphate 磷酸盐acetate 醋酸盐methane 甲烷,沼气butane 丁烷salt 盐potassium carbonate 碳酸钾soda 苏打sodium carbonate 碳酸钠caustic potash 苛性钾caustic soda 苛性钠ester 酯gel 凝胶体analysis 分解fractionation 分馏endothermic reaction 吸热反应 exothermic reaction 放热反应precipitation 沉淀to precipitate 沉淀to distil, to distill 蒸馏distillation 蒸馏to calcine 煅烧to oxidize 氧化alkalinization 碱化to oxygenate, to oxidize 脱氧,氧化 to neutralize 中和to hydrogenate 氢化to hydrate 水合,水化to dehydrate 脱水fermentation 发酵solution 溶解combustion 燃烧fusion, melting 熔解alkalinity 碱性isomerism, isomery 同分异物现象 hydrolysis 水解electrolysis 电解electrode 电极anode 阳极,正极cathode 阴极,负极catalyst 催化剂catalysis 催化作用oxidization, oxidation 氧化reducer 还原剂dissolution 分解synthesis 合成reversible 可逆的refining 炼油refinery 炼油厂cracking 裂化separation 分离fractionating tower 分馏塔fractional distillation 分馏distillation column 分裂蒸馏塔polymerizing, polymerization 聚合reforming 重整purification 净化hydrocarbon 烃,碳氢化合物crude oil, crude 原油petrol 汽油(美作:gasoline)LPG, liquefied petroleum gas 液化石油气LNG, liquefied natural gas 液化天然气octane number 辛烷数,辛烷值vaseline 凡士林paraffin 石蜡kerosene, karaffin oil 煤油gas oil 柴油lubricating oil 润滑油asphalt 沥青benzene 苯fuel 燃料natural gas 天然olefin 烯烃high-grade petrol, high-octane petrol 高级汽油,高辛烷值汽油 plastic 塑料chemical fiber 化学纤维synthetic rubber 合成橡胶solvent 溶剂product 化学反应产物flask 烧瓶apparatus 设备PH indicator PH值指示剂,氢离子(浓度的)负指数指示剂matrass 卵形瓶litmus 石蕊litmus paper 石蕊试纸graduate, graduated flask 量筒,量杯reagent 试剂test tube 试管burette 滴定管retort 曲颈甑still 蒸馏釜cupel 烤钵crucible pot, melting pot 坩埚pipette 吸液管filter滤管stirring rod 搅拌棒element 元素body 物体compound 化合物atom 原子gram atom 克原子atomic weight 原子量atomic number 原子数atomic mass 原子质量molecule 分子electrolyte 电解质ion 离子anion 阴离子cation 阳离子electron 电子isotope 同位素isomer 同分异物现象polymer 聚合物symbol 复合radical 基structural formula 分子式valence, valency 价monovalent 单价bivalent 二价halogen 成盐元素bond 原子的聚合mixture 混合combination 合成作用compound 合成物alloy 合金metal 金属metalloid 非金属有机化学常用基团中英文对照Common control group of Organic Chemistry in Chinese and English甲基(缩写Me)——methyl 乙基(缩写Et)——ethyl丙基(缩写Pr)——propyl丁基(缩写Bu)——butyl戊基——amyl,pentyl己基——hexyl庚基——heptyl辛基——octyl壬基——nonyl癸基——decyl十一烷基——hendecyl十二烷基——dodecyl十六(烷)基/鲸蜡基——cetyl 十七(烷)基——heptadecyl 十八(烷)基——octodecyl苯基(缩写Ph)——phenyl苯甲基/苄基——phenmethyl 苯乙基——phenylethyl三价苯基——phenenyl乙氧苯基——phenelyl,ethoxyphenyl 萘基——naphthyl菲基——phenanthryl菲啶基——phenanthridinyl乙烯基(缩写Vi)——vinyl,ethenyl乙炔基——ethinyl己烯基——hexenyl庚烯基——heptenyl喹啉基——quinolyl吩噻嗪基——phenothiazinyl醌基——quinonyl,benzoquinonyl 甲酸基——formyl乙酰基、醋酸基——acetyl丙烯酰——acryloyl,acrylyl氨基/胺基——amidocyanogen,羰基——carbonyl羧基——carboxyl羟基/氢氧基——hydroxyl酯烷基——ester alkyl烷基——alkyl芳基——aryl环戊基——cyclopentyl环己基——cyclohexyl硝基——nitro-Bunsen burner 本生灯product 化学反应产物flask 烧瓶apparatus 设备PH indicator PH值指示剂,氢离子(浓度的)负指数指示剂matrass 卵形瓶litmus 石蕊litmus paper 石蕊试纸graduate, graduated flask 量筒,量杯 reagent 试剂test tube 试管burette 滴定管retort 曲颈甑still 蒸馏釜cupel 烤钵crucible pot, melting pot 坩埚pipette 吸液管filter滤管stirring rod 搅拌棒element 元素body 物体compound 化合物atom 原子gram atom 克原子atomic weight 原子量 atomic number 原子数 atomic mass 原子质量 molecule 分子electrolyte 电解质ion 离子anion 阴离子cation 阳离子electron 电子isotope 同位素isomer 同分异物现象polymer 聚合物symbol 复合radical 基structural formula 分子式valence, valency 价 monovalent 单价bivalent 二价halogen 成盐元素bond 原子的聚合mixture 混合combination 合成作用 compound 合成物alloy 合金metal 金属metalloid 非金属Actinium(Ac) 锕Aluminium(Al) 铝Americium(Am) 镅Antimony(Sb) 锑Argon(Ar) 氩 Arsenic(As) 砷Astatine(At) 砹Barium(Ba) 钡Berkelium(Bk) 锫Beryllium(Be) 铍Bismuth(Bi) 铋Boron(B) 硼Bromine(Br) 溴Cadmium(Cd) 镉 Caesium(Cs) 铯 Calcium(Ca) 钙Californium(Cf) 锎 Carbon(C) 碳Cerium(Ce) 铈Chlorine(Cl) 氯Chromium(Cr) 铬 Cobalt(Co) 钴Copper(Cu) 铜Curium(Cm) 锔Dysprosium(Dy) 镝 Einsteinium(Es) 锿 Erbium(Er) 铒Europium(Eu) 铕 Fermium(Fm) 镄 Fluorine(F) 氟Francium(Fr) 钫Gadolinium(Gd) 钆 Gallium(Ga) 镓Germanium(Ge) 锗Gold(Au) 金Hafnium(Hf) 铪 Helium(He) 氦Holmium(Ho) 钬 Hydrogen(H) 氢 Indium(In) 铟Iodine(I) 碘Iridium(Ir) 铱Iron(Fe) 铁Krypton(Kr) 氪Lanthanum(La) 镧Lead(Pb) 铅Lithium(Li) 锂Lutetium(Lu) 镥Magnesium(Mg) 镁 Manganese(Mn) 锰 Mendelevium(Md) 钔 Mercury(Hg) 汞Molybdenum(Mo) 钼 Neodymium(Nd) 钕 Neon(Ne) 氖Nickel(Ni) 镍Niobium(Nb) 铌 Nitrogen(N) 氮 Nobelium(No) 锘 Osmium(Os) 锇 Oxygen(O) 氧Palladium(Pd) 钯 Phosphorus(P) 磷 Platinum(Pt) 铂 Plutonium(Pu) 钚Potassium(K) 钾Praseodymium(Pr) 镨 Promethium(Pm) 钷 Protactinium(Pa) 镤 Radium(Ra) 镭Radon(Rn) 氡Rhenium(Re) 铼Rhodium(Rh) 铑Rubidium(Rb) 铷Ruthenium(Ru) 钌Scandium(Sc) 钪 Selenium(Se) 硒 Silicon(Si) 硅Silver(Ag) 银Sodium(Na) 钠 Strontium(Sr) 锶 Sulphur(S) 锍Tantalum(Ta) 钽 Technetium(Tc) 锝 Tellurium(Te) 碲Thallium(Tl) 铊 Thorium(Th) 钍 Tin(Sn) 锡Thulium(Tm) 铥 Titanium(Ti) 钛 Tungsten(W) 钨 Uranium(U) 铀 Vanadium(V) 钒 Xenon(Xe) 氙Ytterbium(Yb) 镱Zinc(Zn) 锌Zirconium(Zr) 锆organic chemistry 有机化学inorganic chemistry 无机化学derivative 衍生物series 系列acid 酸hydrochloric acid 盐酸sulphuric acid 硫酸nitric acid 硝酸aqua fortis 王水 fatty acid 脂肪酸organic acid 有机酸hydrosulphuric acid 氢硫酸hydrogen sulfide 氢化硫 alkali 碱,强碱ammonia 氨base 碱hydrate 水合物hydroxide 氢氧化物,羟化物 hydracid 氢酸hydrocarbon 碳氢化合物,羟 anhydride 酐alkaloid 生物碱aldehyde 醛oxide 氧化物phosphate 磷酸盐acetate 醋酸盐methane 甲烷,沼气butane 丁烷salt 盐potassium carbonate 碳酸钾 soda 苏打sodium carbonate 碳酸钠 caustic potash 苛性钾caustic soda 苛性钠ester 酯gel 凝胶体analysis 分解fractionation 分馏endothermic reaction 吸热反应 exothermic reaction 放热反应 precipitation 沉淀to precipitate 沉淀to distil, to distill 蒸馏distillation 蒸馏to calcine 煅烧。

CHEMICAL INDUSTRY AND ENGINEERING PROGRESS 2017年第36卷第8期·2748·化 工 进展甲基苯基碳酸酯歧化反应研究进展付嫱1,3,欧阳春1,2,曾毅1,2,王公应1,2(1中国科学院成都有机化学研究所,四川 成都 610041;2中国科学院成都有机化学有限公司,四川 成都610041;3中国科学院大学,北京 100049)摘要:碳酸二甲酯(DMC )与苯酚通过酯交换法合成碳酸二苯酯(DPC )是一条清洁生产路线,该法一般分两步进行,研究发现,其中第二步甲基苯基碳酸酯(MPC )的歧化反应大都不能彻底进行,极大地制约了目标产物DPC 的生成,因此,MPC 歧化反应作为DMC 与苯酚发生酯交换反应生成DPC 的关键步骤,对其进行深入的研究有着重要意义。
本文综述了MPC 歧化反应热力学、动力学、催化剂以及合成工艺方面的研究进展,尤其对MPC 歧化反应的动力学问题和平衡限制问题进行了详细讨论,指出打破平衡限制的合理途径是采用减压歧化工艺,而高选择性地生成DPC 取决于催化剂的性能,多相催化剂的研究开发应作为今后主要的研究方向。
最后对国内研究现状进行了评价和展望,旨在加快我国聚碳酸酯行业的工业化进程。
关键词:甲基苯基碳酸酯;热力学;动力学;催化剂;反应工艺中图分类号:TQ225.5 文献标志码:A 文章编号:1000–6613(2017)08–2748–08 DOI :10.16085/j.issn.1000-6613.2016-2321Progresses in the research on disproportionation of methyl phenylcarbonateFU Qiang 1,3,OUYANG Chun 1,2,ZENG Yi 1,2,WANG Gongying 1,2(1Chengdu Institute of Organic Chemistry ,Chinese Academy of Sciences ,Chengdu 610041,Sichuan ,China; 2ChengduOrganic Chemicals Co .,Ltd .,Chinese Academy of Sciences ,Chengdu 610041,Sichuan ,China ;3University of ChineseAcademy of Sciences ,Beijing 100049,China )Abstract :The transesterification of dimethyl carbonate (DMC )and phenol to diphenyl carbonate (DPC )is considered as a clean production route ,and this route is usually carried out through two steps. However ,in the second step the disproportionation of methyl phenyl carbonate (MPC )usually cannot react thoroughly ,which ,to a certain degree ,have deeply limited the production of the target product DPC. Therefore ,the disproportionation of MPC is a key step to synthesize DPC in the transesterification reaction from DMC and phenol and further research of it is of great significance. The reaction thermodynamic ,dynamics ,catalysts and the synthesis process of the disproportionation of MPC are mainly reviewed. Problems on dynamics and equilibrium constraint of the disproportionation are discussed in details. The proper way to break the equilibrium constraint is to reduce the distillation pressure in the disproportionation and the high selectivity of DPC relies on the property of the catalyst. The main research of the catalyst are supposed to be directed to heterogeneous catalyst. Finally ,the perspective and evaluation of domestic researches are given.Key words :methyl phenyl carbonate ;thermodynamics ;kinetics ;catalyst ;process conditions第一作者:付嫱(1992—),女,硕士研究生。

Rap1b上调Twist1促进肝癌细胞的侵袭迁移能力的开题报告一、背景与意义肝癌是全球癌症死亡人数第二大的原因,其患病率和死亡率不断上升,是一个具有高度危险性和严重性的疾病。
肝癌的侵袭和转移是导致其死亡的主要原因之一。
因此,深入研究肝癌细胞的侵袭和转移机制,寻找治疗肝癌的新靶点和药物,对于改善肝癌患者的预后和生存率,具有重要的临床意义。
扭曲因子1(Twist1)是一种转录因子,广泛参与胚胎发育、上皮间充质转化和肿瘤发生、发展等过程。
近年来的研究发现,Twist1在多种癌症中高表达,并促进癌细胞的侵袭、转移和耐药等生物学行为。
此外,前期研究还发现,Rap1b是一种小GTP酶,参与多个信号通路的调控,与癌症的发生和发展密切相关。
但Rap1b在肝癌细胞的侵袭和转移中的作用和调节机制还不清楚,需要进一步探究。
二、研究问题本项目旨在探讨Rap1b调控Twist1促进肝癌细胞侵袭和转移的分子机制,并寻找治疗肝癌的新靶点和策略。
具体研究问题如下:1. Rap1b的表达与Twist1在肝癌组织中的表达是否相关?2. Rap1b是否通过调控Twist1促进肝癌细胞的侵袭和转移?3. Rap1b调控Twist1参与何种信号通路的调节?4. Rap1b/Twist1信号通路是否可以作为治疗肝癌的新靶点和策略?三、研究方案和方法本项目将从肝癌组织和细胞水平开展研究。
1. 肝癌组织样本的采集和检测在符合纳入标准的患者中,采集肝癌组织样本和正常对照组织样本,通过免疫组化技术检测Twist1和Rap1b的表达水平,并分析二者之间的相关性。
2. 细胞实验选取常见的肝癌细胞株HepG2和Huh7作为研究对象,通过siRNA干扰和cDNA重组技术,分别沉默和过表达Rap1b和Twist1,并检测细胞的侵袭和转移能力。
同时,采用Western blot和Real-time PCR等技术,检测相关信号通路的变化和分子机制。
四、预期结果通过本项目的研究,我们预计可以:1. 确定Rap1b和Twist1在肝癌组织中的表达情况以及二者之间的相关性;2. 验证Rap1b通过调控Twist1促进肝癌细胞的侵袭和转移,探明其参与的信号通路及相关分子机制;3. 探索Rap1b/Twist1信号通路是否可以作为治疗肝癌的新靶点和策略。



BST聚合酶的结构引言BST聚合酶(BST-1)是一种酶,它在人体中广泛存在,并且在多种生物学过程中发挥重要作用。
本文将详细介绍BST聚合酶的结构,包括其组成、空间结构和功能。
组成BST聚合酶是由一组蛋白质亚基组成的复合物。
目前已知的BST聚合酶复合物主要由两个亚基组成:BST-1A和BST-1B。
这两个亚基在结构和功能上有所差异,但它们共同参与了BST聚合酶的活性。
BST-1A亚基是一种酶,它能够催化底物的聚合反应。
BST-1B亚基则起到调控和稳定BST聚合酶的作用。
这两个亚基之间通过非共价键相互结合,形成一个稳定的复合物。
空间结构BST聚合酶的空间结构是由其亚基的序列决定的。
根据X射线晶体学和核磁共振等技术的研究,科学家们揭示了BST聚合酶的三维结构。
BST-1A亚基是一个由多个螺旋和折叠区域组成的蛋白质。
这些结构区域相互作用,形成一个具有特定功能的活性中心。
BST-1B亚基则是一个较小的蛋白质,通过与BST-1A亚基的特定区域结合,起到调控和稳定的作用。
BST聚合酶的空间结构具有一定的稳定性和可塑性。
它可以适应不同的环境和底物,从而发挥多样化的功能。
功能BST聚合酶在人体中发挥着多种重要的功能。
首先,它参与了蛋白质合成过程中的聚合反应。
BST-1A亚基能够催化氨基酸的连接,从而形成多肽链。
这一过程对于蛋白质的合成和功能发挥至关重要。
此外,BST聚合酶还参与了DNA和RNA的合成过程。
它能够催化核苷酸的连接,从而形成DNA和RNA的链。
这一过程对于基因表达和遗传信息传递起着重要作用。
除了参与生物分子的合成,BST聚合酶还具有调控和信号传导的功能。
它可以与其他蛋白质相互作用,从而参与细胞信号传导通路的调控。
这一过程对于细胞的正常功能和生理过程至关重要。
应用由于BST聚合酶在人体中发挥着重要的生物学功能,它在医学和生物技术领域具有广泛的应用前景。
首先,BST聚合酶作为一种酶,在药物研发中具有重要意义。

离子液体在酶催化应用中的研究进展张社利;闫月荣;范云场【摘要】简要介绍了离子液体的性质,论述了离子液体作为反应介质在脂肪酶、纤维素酶、蛋白酶等催化中的应用研究进展;阐述了离子液体对酶活性影响的机理。
提出了离子液体在酶催化应用中所存在的问题。
%The properties of ILs were briefly introduced .The development of the use of ILs as reaction media in the catalysis of lipases ,cellulases and proteases was discussed .The mechanisms of the effects of ILs on the enzyme activity wereexpatiated .The problems of the applications of ILs in enzyme catalysis were introduced .【期刊名称】《应用化工》【年(卷),期】2016(045)009【总页数】4页(P1760-1762,1766)【关键词】离子液体;酶催化;脂肪酶;纤维素酶;蛋白酶【作者】张社利;闫月荣;范云场【作者单位】焦作师范高等专科学校理工学院,河南焦作 454000;焦作师范高等专科学校理工学院,河南焦作 454000;河南理工大学化学化工学院,河南焦作454003【正文语种】中文【中图分类】TQ426.97离子液体具有挥发性差、毒性低等特点,被视为环境友好型溶剂,其在酶催化领域的应用也引起了广大科研人员的兴趣。
离子液体对酶活性的影响主要体现在霍夫梅斯特效应、氢键作用、疏水作用等。
简要总结了离子液体在脂肪酶、纤维素酶、蛋白酶等催化中的应用。
最后提出了离子液体在酶催化中的应用所存在的问题。
酶作为生物催化剂不仅具有一般催化剂的优点,还具有专一性、温和性、高效性、低能耗、化学品用量少、产生的废弃物少等特点,广泛应用于有机合成、医药、污水处理及食品等行业。

生物质能源综述摘要:生物质能源作为一种能够进行物质生产的可再生能源正日益受到世界各国的青睐和重视,发展生物质能源对于缓解能源危机、保护国家安全等都有着极其重要的意义,从生物质能源的概念入手,综述和探讨了国内外生物质能源的发展状况,展望了中国生物质能源开发的广阔前景,并进一步提出了生物质能源今后发展的方向与措施。
关键词:生物质能源;开发;利用Abstract: Aiming at the grave significance of biomass energy to economic development,this paper, starting from the concept of biomass energy,synthesized and discussed the national and international development,reviewed the expansive foreground,and brought forward the orientation and measures for the future development in the end.Key words: biomass energy;exploitation;utilization0 前言20世纪70年代以来,面对常规矿物能源的日益枯竭和环境的逐渐恶化,世界许多国家将目光逐渐转移到了具备可再生、环保、可转化等优点的生物质能源上。
改革开放以后,中国也逐步迈上了发展生物质能源的轨道。
进入21世纪,谁能把握住生物质能源开发利用的先机,谁将在未来的国际竞争中立于不败之地。
因此,应该提高对发展生物质能源重要性的认识,为顺利开展生物质能源的开发利用创造有利环境。
1 生物质能源的概念生物质是一种通过大气,水,大地以及阳光有机协作产生的可持续性资源。
生物质如果没有通过能源或物质方式被利用,将被微生物分解成水,二氧化碳以及热能散发掉。

顶空气相色谱-质谱法测定土壤中5种乙酸酯类化合物郭佳惠;汪凌佳;曾珍;唐占谱;陈峰【摘要】建立顶空气相色谱-质谱法测定土壤中5种乙酸酯类化合物的方法.对仪器工作条件进行优化,平衡温度为70℃,平衡时间为20 min,氯化钠用量为2.0 g.选用DB-624毛细管柱(30 m×0.32 mm,1.8μm),在柱流量1.5 mL/min条件下采用电子轰击电离源,全扫描定性,选择离子外标法定量.当土壤取样量为2.0 g时,5种乙酸酯类化合物的含量在1.0~20.0 mg/kg范围内与其色谱峰面积呈良好的线性,线性相关系数大于0.999,方法检出限为0.22~0.32 mg/kg.在3个不同加标水平下,土壤样品加标回收率为88.5%~107.0%,测定结果的相对标准偏差为3.0%~11.0%(n=6).该方法检测快速,灵敏度高,满足土壤样品中5种乙酸酯类化合物快速测定要求.【期刊名称】《化学分析计量》【年(卷),期】2019(028)004【总页数】4页(P26-29)【关键词】乙酸酯类化合物;气相色谱-质谱法;顶空;土壤【作者】郭佳惠;汪凌佳;曾珍;唐占谱;陈峰【作者单位】杭州市环境监测中心站,杭州 310007;杭州市环境监测中心站,杭州310007;杭州市环境监测中心站,杭州 310007;杭州市环境监测中心站,杭州310007;杭州市环境监测中心站,杭州 310007【正文语种】中文【中图分类】O657.7乙酸酯类化合物是一类用途广泛的精细化工产品,具有优异的溶解性、速干性,是重要的有机化工原料和工业溶剂,广泛应用于催化剂、萃取剂、涂料香料、化妆品、表面活性剂、木材粘结剂、人造皮革加工、纺织加工、胶卷和火药制造等领域[1-8]。
乙酸酯类化合物属于易挥发低毒类物质,对眼、鼻、咽喉有刺激作用[9-10],我国对空气中乙酸酯类有限值要求。
我国土壤标准体系中尚无乙酸酯类的排放标准和检测方法,国内外学者也很少对土壤中乙酸酯类的检测方法进行报道,多数研究主要针对水和空气中的乙酸酯类[11-15]。
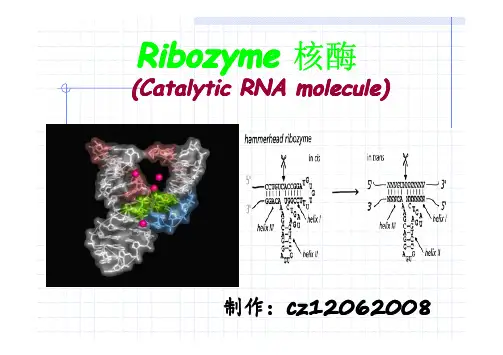
RibozymeRibozyme 核酶(Catalytic RNA molecule)制作:cz120620081.1.What is a Ribozyme?What is a Ribozyme? Ribozymes are true enzymes.. Ribozymes are true enzymesA ribozyme is an RNA molecule(分子) that is capable(能力)of catalyzing (催化)a chemical reaction.NOT PROTEIN(蛋白)discovered First Ribozyme discovered First Ribozyme上世纪80年代,美国科罗拉多大学博尔德分校的Thomas Cech(The intron inTetrahemena isthe pre-rRNA of Tetrahemena isThe intron in the pre-rRNA ofSidnery Altan n(The M1 self-spliced)和美国耶鲁大学的Sidnery Altain ribonuclease P is catalytic)各自独立地发现RNA具有RNA in ribonucleaseRNA生物催化功能.从而改变了生物催比剂的传统概念。
1989 Nobel PrizeIn ChemistrySid Altman Tom CechI型内含子剪接型核酶II型内含子 锤头核酶剪切型核酶发夹核酶 自体催化 丁型肝炎病毒(HDV)核酶 RNaseP RNaseP 异体催化 2.核酶的分类通过既剪又接的方式除去内含子(Intron)自身催化的反应是只切不接。
异体催化能剪切所tRNA前体的5‘端How many ribozyme ?the hammerhead ribozyme (plant virus)the hammerhead ribozyme (plant virus)锤头状核酶(来源:植物病毒)- the hairpin ribozyme (plan virus)- the hairpin ribozyme (plan virus)发夹状核酶- hepatitis delta ribozyme (human virus)- hepatitis delta ribozyme (human virus)丁型肝炎核酶(来源:人类病毒)- neurospora VS ribozyme (mitochondrial RNA)- neurospora VS ribozyme (mitochondrial RNA)孢VS核酶(来源:线粒体RNA)- group I and group II intron ribozyme (rRNA and mt RNA)- group I and group II intron ribozyme (rRNA and mt RNA) 内含子I和II核酶(来源:rRNA和MT RNA)- RNAse P (tRNA maturation)核糖核酸酶P(来源:tRNA的成熟)- Ribosome (translation)核糖体(翻译)- Spliceosome (splicing)剪接(拼接)Ribozym Ribozym Sequenced Sequenced Sequenced Size (nt) Size (nt) Size (nt) Activity Activity Activity SourceSource (核酶) (测序) (大小) (功能) (来源)Group I intron >1>1’’500 210 self-splicing via 3self-splicing via 3self-splicing via 3’’-OH -OH eukayotic pre-mRNA,eukayotic pre-mRNA, of G; transesterificationof G; transesterification organelles, few bacteria Group II intron >700 500 self-splicing via 3 self-splicing via 3’’-OH organelles, few prokaryotes organelles, few prokaryotesof A; transesterificationof A; transesterification Hammerhead 11 40 self-cleavage via self-cleavage via plant virusesplant viruses Hepatitis delta virus Hepatitis delta virus 2 90 transesterification hepatitis B virus hepatitis B virus hepatitis B virus (RNAgen.)(RNAgen.)Hairpin 1 70 (2 70 (2’’,3,3’’-cyclic phosphate) satellite RNA of plant virussatellite RNA of plant virus RNAse P >500 >500 300 300 pre-tRNA processing pre-tRNA processing pre-tRNA processing prokaryotes and organelles prokaryotes and organelles prokaryotes and organellesvia hydrolysis (3via hydrolysis (3via hydrolysis (3’’-OH) of eukaryotes of eukaryotes of eukaryotesSpliceosomepre-mRNA splicing via eukaryotic pre-mRNAs (U2 + U6 snRNAs) (U2 + U6 snRNAs) 70; 5070; 50 180; 100 ransesterification (3 180; 100 ransesterification (3’’OH)Ribosome Ribosome >900>900 2 2’’600 peptidyl transfer (amide) prokaryotes and eukaryotes (23S rRNA)(23S rRNA)Naturally occurring ribozymes and ribonucleoprotein enzymes (2002)peptidyl transfer (amide)转移肽(酰胺) self-splicing 自我剪接self-cleavage self-cleavage 自我裂解 22’,3,3’’-cyclic phosphate -cyclic phosphate 2',3' -环磷酸 transesterification transesterification 酯交换 prokaryotes and eukaryotes prokaryotes and eukaryotes prokaryotes and eukaryotes原核生物和真核生物 pre-tRNA processing via hydrolysis (3pre-tRNA processing via hydrolysis (3’’-OH)处理经水解的前氨酰- tRNA (3' - OH )天然核酶及核糖酶3.结构(Structure )As with proteins, we consider...Primary:GGCCGAACUGGUA一级Secondary二级:Tertiary三级:3.1 3.1 Primary StructurePrimary Structure 一级结构Limited to ribonucleotides 核苷酸 (base 碱基 + ribose 核糖 + phosphate 磷酸):Common Bases:常见碱基Uncommon Bases:罕见碱基N N N N O N N N NN NN N O N N N OO A G C UN N O O N NN N O Pseudouridine假尿苷Inosine 肌苷 etc...3.2 3.2 Secondary Structure Secondary Structure (二级结构)Watson-Crick Base Pairing 沃森-克里克碱基配对Helix Formation 螺旋形成B-DNA RNARNA usually assumesA-form helices A-form helices……DNA 常常假设A-型螺旋Small pore along helical axis 沿螺旋轴形成个小孔“Rungs Rungs”” stack obliquely to axis 阶梯式的斜上升的中心轴A-DNA3′5′-5BVC U GYGG A A-5BVI10GAAACCUUAAHA403050202-2 发卡型核酶二级结构Secondary Structure (二级结构)Conserved base-pairing interactions result in...保守的碱基配对相互作用的导致:• Three Three ““stem stem”” regions 三个结构域• Uridine-containing turn旋转而封闭的尿苷• AnAn ““augmenting helix augmenting helix”” joining stems II and III结构域II 与 III 连接形成的增强螺旋结构vs.Ribozyme vs.vs. tRNA Phe 核酶 苯丙氨酸tRNAfolding折叠The hammerhead ribozyme (锤头状核酶) - discovered in small RNA satellites of small viruses (1986)是小病毒携带的小的RNA- replication by rolling circle mechanism通过不断循环机制进行复制折叠Secondary structureRNAse P(核糖核酸酶P) is ais a ribozyme RNAse P cleaves the 5’’ end of pre-RNAse P cleaves the 5tRNAs(核糖核酸酶P切割5'前体tRNA的末段)It is composed of 12 kDa P protein and about 400 nt long RNA(它是由12 kDa蛋白质和长约400元的RNA组成)The catalytic activity lies entirely within RNA part(催化活性中心位于RNA上)is a ribozyme RNAse P(核糖核酸酶P) is aEnzyme is efficient without P protein but in high salt conditions(在高的盐条件,酶在没有P蛋白时还是有活性的)P protein or high salt is thought to screen the repulsive electrostatic interactions between RNAse P RNA and substrate pre-tRNA(P 蛋白质或高盐被认为是核糖核酸酶P RNA 与前体tRNA表面之间静电排斥反应的屏障。
肉桂酸合成实验报告1. 引言本实验旨在合成肉桂酸,并通过实验验证反应条件对合成产率的影响。
肉桂酸是一种常用的有机合成原料,广泛应用于食品、香料和医药等行业。
通过本实验可以了解合成肉桂酸的反应途径及反应条件的选择。
2. 实验原理肉桂酸的合成反应如下所示:苯乙烯 + 二氧化碳 + 乙醇→ 肉桂酸本实验采用的反应是苯乙烯与二氧化碳在乙醇催化下发生的加成反应。
乙醇作为溶剂具有良好的溶解性能和催化活性,利于反应的进行。
3. 实验步骤3.1 实验器材及试剂准备•三口烧瓶•还原钠•无水乙醇•苯乙烯•二氧化碳气源3.2 反应条件的选择和优化在本实验中,反应条件的选择对合成产率至关重要。
我们对反应时间、温度和催化剂的用量进行了优化。
- 反应时间:根据文献资料,反应时间的常用范围为2-4小时。
我们选取了3小时的反应时间,并在此基础上进一步优化。
- 温度:温度对反应速率和产率有一定影响。
我们选择了80℃作为初始温度,并通过逐渐升高温度来寻找最佳条件。
- 催化剂用量:催化剂对反应速率和产率有重要影响。
我们选取了不同催化剂用量进行了试验,并分析了其影响。
3.3 实验操作1.在三口烧瓶中加入适量的还原钠和无水乙醇,制备催化剂。
2.将苯乙烯加入反应瓶中,并通入CO2气体,同时加入催化剂。
3.控制反应的时间和温度,反应结束后进行冷却。
4.过滤得到产物,进行结晶和干燥。
4. 结果与分析4.1 反应条件的选择和优化结果经过反复试验和优化,我们得出以下结果: - 反应时间:3小时为最佳反应时间,此时反应固定,产率达到最高。
- 温度:合成肉桂酸的最佳温度为100℃,在该温度下反应速率较快且产率较高。
- 催化剂用量:催化剂用量为2 mol%时,产率最高。
4.2 产物的分析对于合成得到的肉桂酸产物,我们进行了以下分析: - 红外光谱分析:通过对产物的红外光谱图进行解析,证明了所得产物的结构为肉桂酸。
- 薄层色谱分析:使用薄层色谱技术对产物进行分离和鉴定,结果显示所得产物纯度较高。
RibozymeRibozyme 核酶(Catalytic RNA molecule)制作:cz120620081.1.What is a Ribozyme?What is a Ribozyme? Ribozymes are true enzymes.. Ribozymes are true enzymesA ribozyme is an RNA molecule(分子) that is capable(能力)of catalyzing (催化)a chemical reaction.NOT PROTEIN(蛋白)discovered First Ribozyme discovered First Ribozyme上世纪80年代,美国科罗拉多大学博尔德分校的Thomas Cech(The intron inTetrahemena isthe pre-rRNA of Tetrahemena isThe intron in the pre-rRNA ofSidnery Altan n(The M1 self-spliced)和美国耶鲁大学的Sidnery Altain ribonuclease P is catalytic)各自独立地发现RNA具有RNA in ribonucleaseRNA生物催化功能.从而改变了生物催比剂的传统概念。
1989 Nobel PrizeIn ChemistrySid Altman Tom CechI型内含子剪接型核酶II型内含子 锤头核酶剪切型核酶发夹核酶 自体催化 丁型肝炎病毒(HDV)核酶 RNaseP RNaseP 异体催化 2.核酶的分类通过既剪又接的方式除去内含子(Intron)自身催化的反应是只切不接。
异体催化能剪切所tRNA前体的5‘端How many ribozyme ?the hammerhead ribozyme (plant virus)the hammerhead ribozyme (plant virus)锤头状核酶(来源:植物病毒)- the hairpin ribozyme (plan virus)- the hairpin ribozyme (plan virus)发夹状核酶- hepatitis delta ribozyme (human virus)- hepatitis delta ribozyme (human virus)丁型肝炎核酶(来源:人类病毒)- neurospora VS ribozyme (mitochondrial RNA)- neurospora VS ribozyme (mitochondrial RNA)孢VS核酶(来源:线粒体RNA)- group I and group II intron ribozyme (rRNA and mt RNA)- group I and group II intron ribozyme (rRNA and mt RNA) 内含子I和II核酶(来源:rRNA和MT RNA)- RNAse P (tRNA maturation)核糖核酸酶P(来源:tRNA的成熟)- Ribosome (translation)核糖体(翻译)- Spliceosome (splicing)剪接(拼接)Ribozym Ribozym Sequenced Sequenced Sequenced Size (nt) Size (nt) Size (nt) Activity Activity Activity SourceSource (核酶) (测序) (大小) (功能) (来源)Group I intron >1>1’’500 210 self-splicing via 3self-splicing via 3self-splicing via 3’’-OH -OH eukayotic pre-mRNA,eukayotic pre-mRNA, of G; transesterificationof G; transesterification organelles, few bacteria Group II intron >700 500 self-splicing via 3 self-splicing via 3’’-OH organelles, few prokaryotes organelles, few prokaryotesof A; transesterificationof A; transesterification Hammerhead 11 40 self-cleavage via self-cleavage via plant virusesplant viruses Hepatitis delta virus Hepatitis delta virus 2 90 transesterification hepatitis B virus hepatitis B virus hepatitis B virus (RNAgen.)(RNAgen.)Hairpin 1 70 (2 70 (2’’,3,3’’-cyclic phosphate) satellite RNA of plant virussatellite RNA of plant virus RNAse P >500 >500 300 300 pre-tRNA processing pre-tRNA processing pre-tRNA processing prokaryotes and organelles prokaryotes and organelles prokaryotes and organellesvia hydrolysis (3via hydrolysis (3via hydrolysis (3’’-OH) of eukaryotes of eukaryotes of eukaryotesSpliceosomepre-mRNA splicing via eukaryotic pre-mRNAs (U2 + U6 snRNAs) (U2 + U6 snRNAs) 70; 5070; 50 180; 100 ransesterification (3 180; 100 ransesterification (3’’OH)Ribosome Ribosome >900>900 2 2’’600 peptidyl transfer (amide) prokaryotes and eukaryotes (23S rRNA)(23S rRNA)Naturally occurring ribozymes and ribonucleoprotein enzymes (2002)peptidyl transfer (amide)转移肽(酰胺) self-splicing 自我剪接self-cleavage self-cleavage 自我裂解 22’,3,3’’-cyclic phosphate -cyclic phosphate 2',3' -环磷酸 transesterification transesterification 酯交换 prokaryotes and eukaryotes prokaryotes and eukaryotes prokaryotes and eukaryotes原核生物和真核生物 pre-tRNA processing via hydrolysis (3pre-tRNA processing via hydrolysis (3’’-OH)处理经水解的前氨酰- tRNA (3' - OH )天然核酶及核糖酶3.结构(Structure )As with proteins, we consider...Primary:GGCCGAACUGGUA一级Secondary二级:Tertiary三级:3.1 3.1 Primary StructurePrimary Structure 一级结构Limited to ribonucleotides 核苷酸 (base 碱基 + ribose 核糖 + phosphate 磷酸):Common Bases:常见碱基Uncommon Bases:罕见碱基N N N N O N N N NN NN N O N N N OO A G C UN N O O N NN N O Pseudouridine假尿苷Inosine 肌苷 etc...3.2 3.2 Secondary Structure Secondary Structure (二级结构)Watson-Crick Base Pairing 沃森-克里克碱基配对Helix Formation 螺旋形成B-DNA RNARNA usually assumesA-form helices A-form helices……DNA 常常假设A-型螺旋Small pore along helical axis 沿螺旋轴形成个小孔“Rungs Rungs”” stack obliquely to axis 阶梯式的斜上升的中心轴A-DNA3′5′-5BVC U GYGG A A-5BVI10GAAACCUUAAHA403050202-2 发卡型核酶二级结构Secondary Structure (二级结构)Conserved base-pairing interactions result in...保守的碱基配对相互作用的导致:• Three Three ““stem stem”” regions 三个结构域• Uridine-containing turn旋转而封闭的尿苷• AnAn ““augmenting helix augmenting helix”” joining stems II and III结构域II 与 III 连接形成的增强螺旋结构vs.Ribozyme vs.vs. tRNA Phe 核酶 苯丙氨酸tRNAfolding折叠The hammerhead ribozyme (锤头状核酶) - discovered in small RNA satellites of small viruses (1986)是小病毒携带的小的RNA- replication by rolling circle mechanism通过不断循环机制进行复制折叠Secondary structureRNAse P(核糖核酸酶P) is ais a ribozyme RNAse P cleaves the 5’’ end of pre-RNAse P cleaves the 5tRNAs(核糖核酸酶P切割5'前体tRNA的末段)It is composed of 12 kDa P protein and about 400 nt long RNA(它是由12 kDa蛋白质和长约400元的RNA组成)The catalytic activity lies entirely within RNA part(催化活性中心位于RNA上)is a ribozyme RNAse P(核糖核酸酶P) is aEnzyme is efficient without P protein but in high salt conditions(在高的盐条件,酶在没有P蛋白时还是有活性的)P protein or high salt is thought to screen the repulsive electrostatic interactions between RNAse P RNA and substrate pre-tRNA(P 蛋白质或高盐被认为是核糖核酸酶P RNA 与前体tRNA表面之间静电排斥反应的屏障。
胶原活化和基质金属蛋白酶相关基因胶原是人体中最丰富的蛋白质之一,它在细胞外基质中起着支撑和保护组织的重要作用。
胶原的稳定和合成受到多种因素的调控,其中包括胶原活化和基质金属蛋白酶相关基因的表达和功能。
胶原活化是指胶原在细胞外基质中的转化和修饰过程。
胶原蛋白的合成和分泌是由细胞内的基因调控和信号转导途径控制的。
胶原合成的关键基因包括胶原前体蛋白基因和胶原后期修饰酶基因。
胶原前体蛋白基因编码胶原的前体蛋白质,这些蛋白质在细胞内合成后,会进一步转运到细胞外基质,并在那里进行后期修饰。
胶原后期修饰酶基因编码的酶能够将胶原前体蛋白质进行修饰,包括蛋白质的剪切、交联等,从而使其具有稳定的结构和功能。
基质金属蛋白酶是一类能够降解细胞外基质中胶原蛋白的酶。
这些酶在细胞外基质的动态平衡中起着重要的调控作用。
基质金属蛋白酶的活性和表达受到多种因素的调控,其中包括基因表达、酶的激活和抑制等。
基质金属蛋白酶的基因编码了这些酶的蛋白质结构,它们在蛋白质合成和转运过程中起着重要的作用。
此外,细胞信号转导途径的激活也可以影响基质金属蛋白酶的表达和活性,从而进一步调控细胞外基质的降解过程。
胶原活化和基质金属蛋白酶相关基因在多种生理和病理过程中发挥重要作用。
例如,在组织修复和再生过程中,胶原合成和降解的平衡是维持组织结构和功能的关键。
一些疾病,如肿瘤和炎症,胶原活化和基质金属蛋白酶相关基因的异常表达和功能改变会导致组织结构的破坏和疾病的发展。
因此,深入研究胶原活化和基质金属蛋白酶相关基因的调控机制,对于理解这些生理和病理过程具有重要意义。
近年来,随着基因组学和生物信息学的发展,研究人员能够更深入地挖掘和分析胶原活化和基质金属蛋白酶相关基因的功能和调控机制。
通过大规模的基因表达谱分析和功能筛选,已经鉴定出了许多与胶原活化和基质金属蛋白酶相关的基因。
这些基因包括胶原前体蛋白基因家族、胶原后期修饰酶基因家族以及基质金属蛋白酶基因家族等。
Kinetics of transesteri®cation in rapeseed oil to biodiesel fuel as treated insupercritical methanolD.Kusdiana,S.Saka*Department of Socio-Environmental Energy Science,Graduate School of Energy Science,Kyoto University,Yoshida Honmachi,Sakyo-ku,Kyoto606-8501,JapanReceived28December1999;accepted3August2000AbstractA kinetic study in free catalyst transesteri®cation of rapeseed oil was made in subcritical and supercritical methanol under different reaction conditions of temperatures and reaction times.Runs were made in a bath-type reaction vessel ranging from2008C in subcritical temperature to5008C at supercritical state with different molar ratios of methanol to rapeseed oil to determine rate constants by employing a simple method.As a result,the conversion rate of rapeseed oil to its methyl esters was found to increase dramatically in the supercritical state, and reaction temperature of3508C was considered as the best condition,with the molar ratio of methanol in rapeseed oil being42.q2001 Elsevier Science Ltd.All rights reserved.Keywords:Kinetics of transesteri®cation;Supercritical methanol;Methyl esters;Biodiesel fuel1.IntroductionTransesteri®cation of vegetable oils with simple alcohol has long been a preferred method for producing biodiesel fuel[1±3].Generally speaking,there are two methods of transesteri®cation reaction.One is the method using a cata-lyst and the other is without the help of a catalyst.The former method has a long story of development and now biodiesel fuel produced by this method is in the market in some countries such as North America,Japan and some west European countries.However,there are at least two problems associated with this process;the process is relatively time consuming and puri®cation of the product for catalyst and saponi®ed products are necessary.The®rst problem due to the two phase nature of vegetable oil/methanol mixture requires vigorous stirring to proceed in the transesteri®cation reac-tion.To solve this problem,Boocock et al.reported that the use of a simple ether such as tetrahydrofuran can make this two phase nature into one phase of its mixture and that methyl esters can be produced in less than15min depending on the catalyst concentration[4].Yet,the catalyst problem cannot be solved for puri®cation.Therefore,this conven-tional process still requires a high production cost and energy.The overall process,thus,includes transesteri®ca-tion reaction,recovery of unreacted methanol,puri®cation of methyl esters from catalyst and separation of glycerin as a co-product from saponi®ed products.The latter method involves uncatalyzed transesteri®ca-tion of vegetable oil in supercritical methanol as recently reported by Saka and Kusdiana[5].The supercritical state of methanol is believed to solve the two phase nature of oil/ methanol mixture to form a single phase due to a decrease in dielectric constant of methanol in supercritical state[6].As a result,the reaction was found to be complete in a very short time within2±4min,as described in their previous work[5].In addition,because of non-catalytic process,the puri®cation of products after transesteri®cation reaction is much simpler and environmentally friendly,compared with the conventional commercial method in which all the catalyst and saponi®ed products have to be removed for biodiesel fuel.Some researchers have reported kinetics for both acid-and alkali-catalyzed transesteri®cation reactions.Dufek and coworkers studied the acid-catalyzed esteri®cation and transesteri®cation of9(10)-carboxystearic acid and its mono-and di-methyl esters[7].Freedman et al.reported transesteri®cation reaction of soybean oil and other vegeta-ble oils with alcohols[8],and examined in their study were the effects of the type of alcohol,molar ratio,type and amount of catalyst and reaction temperature on rateFuel80(2001)693±6980016-2361/01/$-see front matter q2001Elsevier Science Ltd.All rights reserved. PII:S0016-2361(00)00140-X /locate/fuel*Corresponding author.Tel./fax:181-75-753-4738.E-mail address:saka@energy.kyoto-u.ac.jp(S.Saka).constants and kinetic order.Noureddin and Zhu applied the effects of mixing of soybean oil with methanol on its kinetics of transesteri®cation[9].Recently,Diasakov et al. reported kinetics on uncatalytic transesteri®cation reaction of soybean oil[10].However,the kinetic study of vegetable oil in supercritical medium without catalyst has not yet been presented.Therefore,in this study,kinetics of transesteri®cation of rapeseed oil to biodiesel fuel was studied as treated in super-critical methanol without using any catalyst.We reported the effects of molar ratio and reaction temperature on methyl ester formation followed by a proposed simple method for the kinetics of the transesteri®cation reaction.2.Materials and methodsThe rapeseed oil from Nacalai Tesque was used in this work as a vegetable oil.In addition,various methyl esters from fatty acids such as palmitic,stearic,oleic,linoleic and linolenic acids were also obtained from Nacalai Tesque and used as a standard.The supercritical methanol biomass conversion system employed in this work is described in a previous work[5]. In brief,the rapeseed oil and methanol with a different molar ratio was fed to the reaction vessel made of Inconel-625and it was shaken to mix.Subsequently,the entire reaction vessel was quickly immersed into the tin bath preheated at a designated reaction temperature and kept for a set time interval for subcritical and supercritical treatments of methanol(200±5008C).It was,subsequently,moved into the water bath to stop the reaction.The temperature and pressure inside the vessel were monitored during the treat-ment to con®rm that a subcritical or supercritical condition was achieved.For a subcritical treatment,the temperature was too low for tin bath to be melt.Therefore,oil bath was used for the treatments at200and2308C with a temperature controller.The treated liquid was then removed from the reaction vessel and allowed to settle for about30min.After the product was separated to be two portions,the upper and lower,each portion was evaporated at908C for20min to remove methanol.The obtained products were analyzed on its composition by using the high performance liquid chromatography (HPLC)(Shimadzu,LC-10AT)which consists of the column(STR ODS-II,25cm in length£4.6mm in inner diameter,Shinwa Chem.Ind.Co.)and refractive index detector(Shimadzu,RID-10A)operated at408C with 1.0ml/min¯ow rate of methanol as a carrier solvent.The sample injection volume was20m l and peak identi®cation was made by comparing in the retention time between the sample and the standard compound as in a previous work [5].3.Results and discussionThe supercritical experiments of methanol with rapeseed oil were carried out with a batch-type of reaction vessel. Therefore,the temperature and the pressure inside the reac-tion vessel are different in different reaction conditions.Fig. 1shows a typical relationship between the maximum reac-tion temperature and pressure inside the reaction vessel during the treatment.Since a critical point of methanol is 2398C(T c)in temperature and8.09MPa(P c)in pressure, supercritical state of methanol can be achieved in the shadowed zone in Fig.1.3.1.Effect of molar ratio of methanol to rapeseed oil The molar ratio of methanol to rapeseed oil is one of the most important variables affecting the yields of methyl esters converted.The stoichiometry of the transesteri®ca-tion of rapeseed oil requires three molecules of methanol to react with one molecule of rapeseed oil.Encinar[11] stated in the conventional commercial process with alkaline catalyst that the yield of methyl esters increases as the molarD.Kusdiana,S.Saka/Fuel80(2001)693±698694ratio of methanol to Cynara oil rises and that the optimal ratios for its transesteri®cation result between4.05and5.67. For its molar ratio less than4.05,the reaction is reported to be incomplete,whereas at higher than5.67,it becomes dif®cult to separate glycerin from methanol as a by-product. Another worker[12]further noted that a98%conversion of vegetable oils could be made to the methyl esters at the molar ratio of6,but that even higher molar ratio up to45 was necessary when the oil contained a large amount of free fatty acids.However,as the molar ratio decreased to the theoretical value of3,its conversion was decreased down to82%.In this work,therefore,the effect of the molar ratio of methanol to rapeseed oil was studied in the range between 3.5and42on the yield of methyl esters formed for super-critical methanol treatments,assuming that the average molecular weight of rapeseed oil is806as triglycerides. Fig.2shows the obtained HPLC chromatograms of rape-seed oil as treated in various molar ratios for4min under supercritical conditions.In the previous study[5],it was demonstrated that the intensive peak in the chromatogram observed in the short retention times(3±10min)are methyl esteri®ed compounds,while in the longer retention times, intermediates such as monoglycerides and diglycerides appeared(10±20min).Therefore,from Fig.2,it is apparent that the conversion state of rapeseed oil is different as various molar ratios of methanol were applied to the trans-esteri®cation reaction of the rapeseed oil.With a higher molar ratio of methanol applied,the methyl esteri®ed compounds are increased with a decrease in the intermedi-ate compounds.Fig.3shows the content of methyl esters produced as different supercritical treatments were carried out at 3508C.For a molar ratio of42in methanol,almost complete conversion was achieved in a yield of95%of methyl esters, whereas for the lower molar ratio of6or less,incomplete conversion was apparent with the lower yield of methyl esters.These lines of evidence,therefore,indicate that the higher molar ratios of methanol result in the better transes-teri®cation reaction,due perhaps to the increased contact area between methanol and triglycerides.3.2.Effect of temperature on methyl esters formationTo determine the effect of temperature on methyl esters formation,transesteri®cation reactions of rapeseed oil were carried out with a®xed molar ratio of42in methanol,the best condition found in Fig.3,at various temperatures ranging from200to5008C.Fig.4shows the obtained HPLC chromatograms of rapeseed oil as treated in various conditions of temperatures and reaction times,while the content of methyl esters obtained is shown in Fig.5,in which the obtained experimental data are shown by the symbols,whereas the simulated curves are shown by the lines as discussed later.At temperatures of200and2308C,the relatively low conversion to methyl esters is evident in Figs.4and5due to the subcritical state of methanol.In these conditions, methyl esters formed are at most about68and70%at200 and2308C,respectively,at3600s(1h)treatment.These results are in good accordance with those already reported [10].At a temperature of2708C,the conversion rate is still low which might be related with the stability of supercritical condition.As can be seen in Fig.1,maximum pressure reached in this treatment is14MPa,still in the transition between subcritical and supercritical state of methanol. However,at3008C,a considerable change in the conversion rate can be seen with about80%of methyl esters produced in240s.As observed in the previous study[5],at3508C, 240s treatment resulted in a high conversion of rapeseed oil to methyl esters with its yield of95%.An important result here is that the composition of methyl esters yielded is very similar with that prepared by the conventional commercial process with alkaline catalyst.D.Kusdiana,S.Saka/Fuel80(2001)693±698695At even higher temperature of4008C,the transesteri®cation reaction is essentially completed for120s to convert almost all rapeseed oil to their methyl esters.However,in such a high reaction temperature,new peaks in the shorter reten-tion time(3±4min)are dominating in the HPLC chromato-grams as shown in Fig.4and the previous study[5].This indicates that decomposition reaction takes place at temperature above4008C due to the thermal degradation. As a result,the transesteri®cation reactions of rapeseed oil to methyl esters proceed appropriately at temperature of 3508C under supercritical condition of methanol without any catalyst used.3.3.Kinetics of rapeseed oil to methyl estersTo correlate experimental data and to quantify the temperature and reaction time effects observed above,the experimental results were analyzed further in terms of the kinetics of rapeseed oil to methyl esters.As mentioned earlier,the model is based on overall reaction.Since the molar ratio of methanol to rapeseed oil was®xed to be42, the concentration of methanol was not taken into account,as reported by other researchers[10,12].Diasakov[10]proposed the thermal transesteri®cation reaction to be divided into3steps.Triglycerides react with methanol to produce diglycerides,and then diglyc-erides react to produce monoglycerides.Finally monoglyc-erides react with methanol to give glycerin as a by-product. At each reaction step,one molecule of methylated compounds is produced for each molecule of methanol consumed.As a result,six different rate constants of the reaction are reported for the whole reaction.Due to reality that®nal products for the whole reaction in the transesteri®cation reaction for biodiesel fuel production are methyl esters with glycerin,we de®ned a simpler math-ematical model for this reaction by ignoring the intermedi-ate reactions of diglycerides and monoglycerides,so the3 steps can be simpli®ed to be one step asfollows:TGd t1where[TG]refers to the content of vegetable oil used in this study.In this supercritical methanol method,three species were de®ned as methyl esters(ME),glycerin(GL)and unmethyl esteri®ed compounds(uME)which include trigly-cerides,diglycerides,monoglycerides and unreacted free fatty acids.Therefore,Eq.(1)can be modi®ed to beRate 2duMEd t2 or2d uMEd tk uME 3where[uME]refers to the content of the species,excluding methyl esters and glycerin,that result or remain after the supercritical treatment was carried out.Assuming that the initial concentration of uME,at time t 0;is uME,0and that it falls down to uME,t at some later time t,the integration gives2uME;tuME;0d uMEu MEktd t 4D.Kusdiana,S.Saka/Fuel80(2001)693±698696and2ln uME;tuME;0kt 5orK ln uME;t 2ln uME;0t6Fig.6shows the correlation between the content of unmethyl esterifed compounds and reaction times.As mentioned previously,unmethyl esteri®ed compounds are de®ned as other compounds obtained from the upper portion excluding®ve types of methyl esters,such as methyl palmi-tate,methyl oleate,methyl stearate,methyl linoleate and methyl linolenate.The straight line was determined to®t the data in order to adopt the®rst order rate equation. Based on the results in Fig.6,the rate constant was obtained for each reaction temperature as shown in Table 1and the corresponding Arrhenius plot for this method is presented in Fig.7.It is evident that at subcritical tempera-ture below2398C,the reaction rates are so low but much higher at supercritical state,with the rate constant increased by a factor of about85at the temperature of3508C. Liquid methanol is a polar solvent and has hydrogen bondings between OH oxygen and OH hydrogen to form methanol clusters.Because the degree of hydrogen bonding decreases with increasing temperature,the polarity of methanol would decrease in supercritical state.This means that supercritical methanol has a hydrophobic nature with the lower dielectric constant.As a result,non-polar triglycerides can be well solvated with supercritical metha-nol to form a single phase of vegetable oil/methanol mixture.This phenomenon with the high temperature con-ditions seems to be likely to promote transesteri®cation reaction of rapeseed oil.The simulation was made on a relationship between the formation of methyl esters and reaction times,based on Eqs.(3)and(6)to examine the®tness of the experimental results, as shown in Fig.5.In this®gure,the simulated curves are shown by lines and the experimental data are represented by symbols.In the subcritical temperature,simulated curves are somewhat different from those of experimental data. This would be because at the longer treatment,the conver-sion rate is low due to the equilibrium reaction approached. However,at the supercritical state,the simulated curves®t well with the experimental results in all cases.Therefore,a simple method proposed to determine the rate constants in transesteri®cation must be valid.4.Concluding remarksA highly ef®cient transesteri®cation process has been described and the proposed kinetics in transesteri®cation of rapeseed oil has been proven to®t very well with those of experimental data.A reaction temperature of3508C with the molar ratio of methanol being42were considered as the best condition for a free-catalyst process of biodiesel fuel production.The supercritical methanol method,therefore, offers a potentially low cost method with simpler technol-ogy for producing an alternative fuel for compression igni-tion engines.The considerable yield of methyl esters by the environmentally friendly method renders this technique ideally suited for industrialization.References[1]Serdari A,Lois E,Stournas S.Ind Engng Chem Res1999;38:3543.[2]Aksoy HA,Karaosmanoglu BF,Yatmaz HC,Civelekoglu H.Fuel1990;69:600.[3]Schwab AW,Bagby MO,Freedman B.Fuel1987;66:1372.[4]Boocock DGB,Konar SK,Mao V,Lee C,Buligan S.JAOCS1998;75:1167.[5]Saka S,Dadan K.Biodiesel fuel from rapeseed oil as prepared insupercritical methanol.Fuel2001;80:225.[6]Deslandes N,Bellenger V,Jaf®ol F,Verdu J.Appl Polym Sci1998;69:2663.[7]Dufek EJ,Butter®eld RO,Frankel EN.JAOCS1972;49:302.[8]Freedman B,Butter®eld RO,Pryde EH.JAOCS1986;63:1375.[9]Noureddin H,Zhu D.JAOCS1997;74:1457.D.Kusdiana,S.Saka/Fuel80(2001)693±698697 Table1The rate constant of transesteri®cation reactionReaction condition k(s21)Temperature(8C)Pressure(MPa)20070.000223090.0003270120.0007300140.0071350190.0178385650.0249431900.05034871050.0803[10]Diasakov M,Loulodi A,Papayannakos N.Fuel1998;77:1297.[11]Encinar JM,Gonzalez JF,Sabio E,Ramino MG.Ind Engng ChemRes1999;38:2927.[12]Freedman B,Pryde CH,Mounts TL.JAOCS1984;61:1638.[13]Barrow GM.Physical chemistry.Tokyo:McGraw-Hill Kokusha Ltd,1973(p.419).[14]Steinfeld JI,Francisco JS,Hase WL.Chemical kinetics and dynamics.New York:Prentice Hall,1989(p.6).D.Kusdiana,S.Saka/Fuel80(2001)693±698 698。