
High thermal conductivity of graphite ?ber silicon carbide composites for fusion reactor application
L.L.Snead
a,*
,M.Balden b ,R.A.Causey c ,H.Atsumi
d
a
Oak Ridge National Laboratory,Oak Ridge,TN 37831-6138,USA
b
Max-Planck-Institut f €u r Plasmaphysik,Boltzmannstrasse 2,D-85748Garching,Germany
c
Sandia National Laboratory,MS 9402Livermore,CA,USA d
Kinki University,Kowakae 3-4-1,Higashi-Osaka 577-8502,Japan
Abstract
The bene?ts of using CVI SiC/graphite ?ber composites as low tritium retaining,high thermal conductivity com-posites for fusion applications are presented.Three-dimensional woven composites have been chemically vapor in?l-trated with SiCand their thermophysical properties measured.One material used an intermediate grade graphite ?ber in all directions (Amoco P55)while a second material used very high thermal conductive ?ber (Amoco K-1100)in the high ?ber density direction.The overall void was less than 20%.Strength as measured by four-point bending was comparable to those of SiC/SiC composite.The room temperature thermal conductivity in the high conductivity di-rection was impressive for both materials,with values >70W/m K for the P-55and >420W/m K for the K-1100variant.The thermal conductivity was measured as a function of temperature and exceeds the highest thermal con-ductivity of CVD SiC currently available at fusion relevant temperatures (>600°C).Limited data on the irradiation-induced degradation in thermal conductivity is consistent with carbon ?ber composite literature.ó2002Elsevier Science B.V.All rights reserved.
1.Introduction
It is commonly accepted that carbon ?ber composites (CFC ?s)will have unacceptably high tritium reten-tion and signi?cant thermal conductivity reduction for application in fusion power reactors at intermediate temperatures (T <800°C).For higher temperature ap-plications (T >800°C)the thermal conductivity de-gradation is mitigated,but the mechanical property degradation is accelerated,setting CFC useful lifetime into the 10–20dpa range.Recently,the low thermal conductivity of silicon carbide ?ber/silicon carbide ma-trix (SiC f /SiC)composites,especially in the irradiated condition,raises questions regarding their viability as a ?rst wall material.The purpose of this paper is to dem-onstrate,through a basic understanding of the phenom-
enon of tritium retention and thermal conductivity,that a composite material can be engineered with signi?cantly reduced tritium retention with thermal conductivity and mechanical properties which satisfy the current fusion reactor concepts employing SiCcomposites.1.1.Tritium retention
Tritium retention in graphite and silicon carbide are similar in many ways.Both materials have tritium dif-fusion coe?cients that rise to measurable values only at very elevated temperatures [1–3],both materials have negative heats of solution for tritium [1,2],and both have tritium retention characteristics that depend on the crystallite size of the materials [2,4].The di?usion coef-?cient for tritium in graphite as given by Causey [2]is:D ?9:3?10à5exp eà2:8eV =kT Tm 2/s.The di?usion coe?cient for tritium in silicon carbide as given by Causey et al.[5]for beta silicon carbide is:D ?9:8?10à8exp eà1:89eV =kT Tm 2/s.Note the very high acti-vation energy for the di?usion,suggesting
migration
Journal of Nuclear Materials 307–311(2002)
1200–1204
https://www.doczj.com/doc/5b15039793.html,/locate/jnucmat
*
Corresponding author.Tel.:+1-8655749942;fax:+1-8652413650.
E-mail address:sneadll@https://www.doczj.com/doc/5b15039793.html, (L.L.Snead).
0022-3115/02/$-see front matter ó2002Elsevier Science B.V.All rights reserved.PII:S 0022-3115(02)01115-7
controlled by jumps from one trap site to another. Atsumi et al.[1]determined the solubility of hydrogen in graphite to be given by the expression S?6:4?10à5expet0:2eV=kTTatom fraction/atm1=2.Causey et al.[5]reported the solubility of tritium in beta silicon carbide to be1:7?10à7expet0:61eV=kTTatom frac-tion/atm1=2.A negative heat of solution(solubility that decreases with increasing temperature)is an indicator of the chemical a?nity of the hydrogen isotope to the host material.The inward permeation rate of tritium into these materials(product of di?usivity times/solubility) during exposure to hydrogen or tritium gas are among the lowest of all materials.
Atsumi et al.[4]have given direct proof of this in their study showing the apparent hydrogen solubility in graphite to vary inversely with the graphite perfection. Defects on the prism planes also provide a?nite number of very deep traps(%4.3eV)that capture some of the tritium as it migrates along the crystallites.The number of deep traps also varies indirectly with the lattice pa-rameter.Exposure to radiation damage decreases the graphite perfection(decreases the lattice parameter), increasing the apparent solubility and trap density [4,6,7].
It is possible that hydrogen also migrates along the crystal edges of silicon carbide.Evidence for this possi-bility comes from the study of Causey et al.[5]where beta silicon carbide samples with non-uniform crystal sizes were exposed to deuterium gas at elevated tem-peratures.Nuclear reaction pro?ling of the deuterium concentration showed enhanced retention on the sides of the samples with the smaller grains.Unlike graphite, radiation damage does not appear to a?ect the retention of hydrogen in beta silicon carbide[7].At the present time,the reason for this is not understood.However, this immunity to radiation e?ects on trapping gives sil-icon carbide a signi?cant advantage over graphite in minimizing tritium retention.
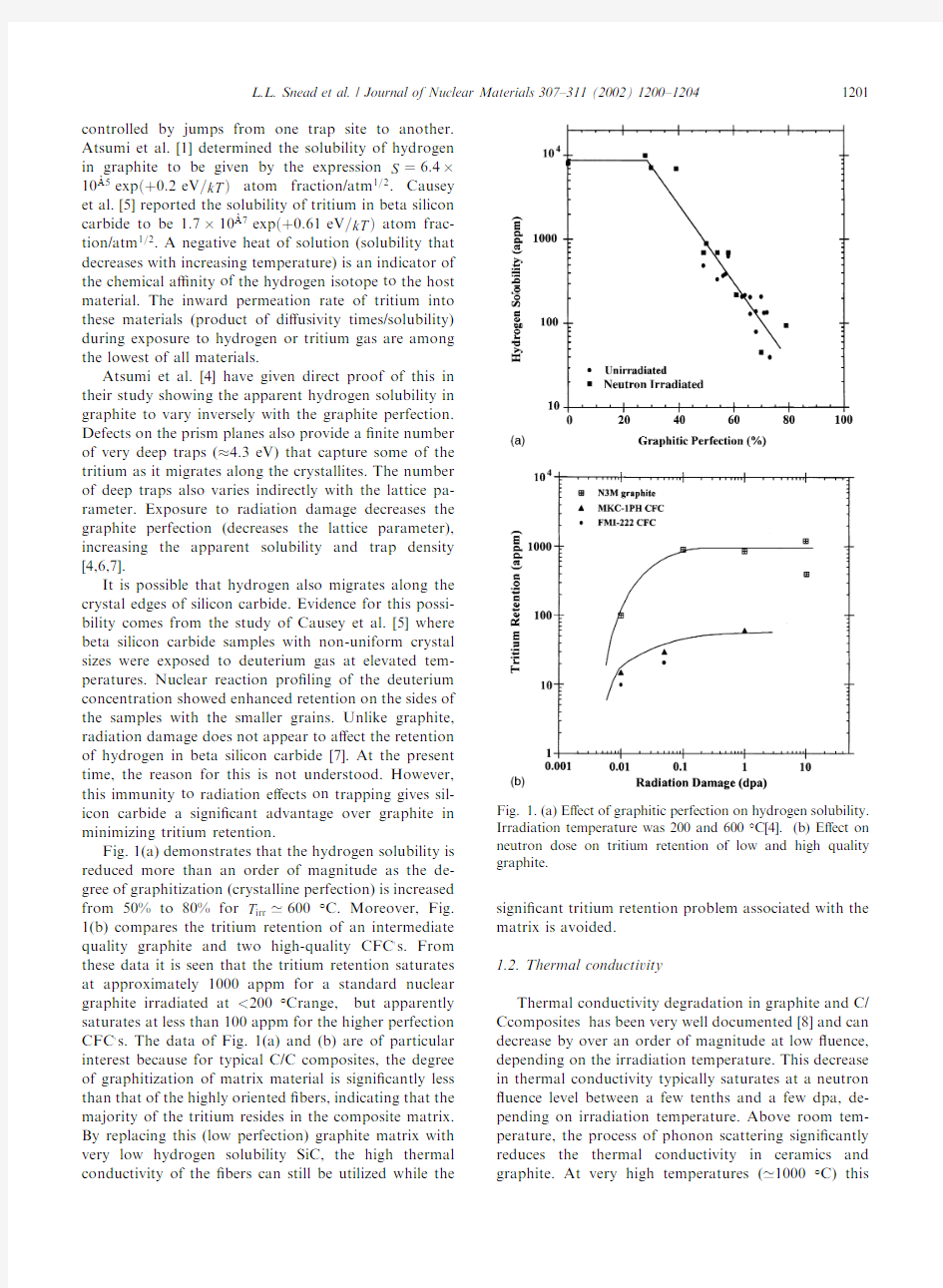
Fig.1(a)demonstrates that the hydrogen solubility is reduced more than an order of magnitude as the de-gree of graphitization(crystalline perfection)is increased
from50%to80%for T irr’600°C.Moreover,Fig. 1(b)compares the tritium retention of an intermediate quality graphite and two high-quality CFC?s.From these data it is seen that the tritium retention saturates at approximately1000appm for a standard nuclear graphite irradiated at<200°Crange,but apparently saturates at less than100appm for the higher perfection CFC?s.The data of Fig.1(a)and(b)are of particular interest because for typical C/C composites,the degree of graphitization of matrix material is signi?cantly less than that of the highly oriented?bers,indicating that the majority of the tritium resides in the composite matrix. By replacing this(low perfection)graphite matrix with very low hydrogen solubility SiC,the high thermal conductivity of the?bers can still be utilized while the signi?cant tritium retention problem associated with the matrix is avoided.
1.2.Thermal conductivity
Thermal conductivity degradation in graphite and C/ Ccomposites has been very well documented[8]and can decrease by over an order of magnitude at low?uence, depending on the irradiation temperature.This decrease in thermal conductivity typically saturates at a neutron ?uence level between a few tenths and a few dpa,de-pending on irradiation temperature.Above room tem-perature,the process of phonon scattering signi?cantly reduces the thermal conductivity in ceramics and graphite.At very high temperatures(’1000°C)
this Fig.1.(a)E?ect of graphitic perfection on hydrogen solubility. Irradiation temperature was200and600°C[4].(b)E?ect on neutron dose on tritium retention of low and high quality graphite.
L.L.Snead et al./Journal of Nuclear Materials307–311(2002)1200–12041201
process is as e?ective at reducing thermal conductivity as defects associated with high-dose neutron irradiation.A convenient way to compare the susceptibility of materials to irradiation-induced degradation in thermal conductivity is to compare their thermal defect resis-tances.This parameter is de?ned as follows:1=K rd ?1=K irr à1=K non àirr :
For both graphite [9]and most likely silicon carbide,the formation of vacancies and small vacancy clusters will dominate the phonon scattering,hence the thermal conductivity,in the temperature range from 200–1000°C.Fig.2shows the thermal defect resistance parameter for pyrolitic graphite and silicon carbide as a function of irradiation temperature [10–12].For both cases the neutron dose was su?cient to be above,or near,the point of saturation.Near room temperature the thermal defect resistance is about a factor of two less for the graphite,but as temperature is increases the defect re-sistance for the graphite rapidly decreases,while SiC slowly decreases with temperature.This is due to the limited interstitial mobility of SiC(T crit ’300K)[13,14]as compared to graphite (T crit ’20K)[15].To reinforce this point,assuming the data of Fig.2and that thermal defect resistance was dominant,the thermal conductivity at 1000°Cwould be ’100W/m K for graphite and ’20W/m K for silicon carbide.Clearly,the e?ect of irradi-ation on the thermal conductivity is more severe for SiC over the temperature range of interest to fusion.1.3.Mechanical properties
Though development of this system has been limited,some authors [16–23]have reported these materials to have similar or higher strength as compared to SiC
matrix/SiC?ber composites.Moreover,the ?exural fracture toughness as measured by the area under the curve beyond macroscopic matrix micro-cracking is su-perior for the graphite ?ber composites.A review on the subject of CVI SiC composites is given by Lara-Curzio [24].
2.Experimental
Composite preforms were composed of a three-dimensional,unbalanced weave of Amoco K1100and P55graphite ?bers.Both ?bers are pitch derived from the same precursor,but have di?erent graphitization tem-peratures,hence di?erent degrees of crystalline align-ment,perfection and thermal conductivity.The P55?ber was woven in 2-k tows in the x -and y -directions while six 2-k tows of K1100were stitched through the or-thogonal weave in the z -direction (de?ned as the K1100composite).A second perform with the same architec-ture used P-55?bers in all directions (de?ned as the P55composite).Preforms were rigidized with polymethyl-methacrylate.The overall ?ber volume fraction was 43.9%of which 7.1%was in the x -and y -directions and 85.7%was in the z direction.The graphite preform was machined into 4.45cm diameter,1.27cm thick discs which were mounted in graphite holders for in?ltration.The preform was then heated to 350°Cin an argon ?ow gas to burn out the PMMA binder.Preforms were then in?ltrated with SiCusing standard isothermal chemical vapor in?ltration and forced-?ow chemical vapor in?l-tration (FCVI)[25].Room temperature bend testing was carried out on 0:3?0:4?5:0cm 3bars with an upper and lower span of 1.9and 3.8cm,respectively.Thermal di?usivity was measured using a ?ash technique and conductivity calculated using the composite density and speci?c heat.A graphite standard was measured before each measurement ensuring the thermal di?usivity ap-paratus was operating to within a few percent error.The temperature dependent speci?c heat was calculated as-suming the rule of mixtures.Irradiation was carried out in the high ?ux isotope reactor in peripheral tube rabbit capsules at a ?ux of ’8?1018n/m 2(E >0:1MeV)and calculated sample temperatures between 400and 800°C.The samples were 6mm in diameter and thickness.The axis of the samples was along the composite high con-ductivity direction.Atmosphere of the irradiation cap-sules was static helium.
3.Results and discussion
The potential for this composite in fusion applica-tions is that it o?ers a high thermal conductivity com-posite with signi?cantly lower tritium retention than the advanced carbon/carbon composites (C/C ?s)
currently
Fig.2.Thermal defect resistance of pyrolitic graphite and sili-con carbide for doses above saturation [10–12].
1202L.L.Snead et al./Journal of Nuclear Materials 307–311(2002)1200–1204
considered for plasma facing components.The thermal conductivity of the composite system can be engineered to be isotropic or optimized for high conductivity in a single direction,as needed.The three-dimensional CVD SiC/K1100composite fabricated for this study yielded reasonably good strength and,from an analysis of the ?exure curves[26]and fracture surfaces,possessed a fair amount of toughness.In the major?ber direction,the four-point bend strength was(267?28)MPa which is comparable to CVI SiC/SiC?ber materials[24]. Strength of bend bars across the major?ber direction would be considerably reduced due to the reduced fraction of(P55)?bers in this orientation.
The thermal conductivity of both SiC/graphite pro-duced in this study is given in Fig.3.Also shown is the thermal conductivity of a very high quality monolithic CVD SiC materials produced by Morton(now Rohm Haas),and the transverse thermal conductivity of an FCVI SiC/Type S Nicalon composite.As expected,due
to the signi?cantly higher thermal conductivity of the graphite?bers(’950and’120W/m K at ambient for K1100and P55,respectively)as compared to the SiC ?ber(’15W/m K at ambient),the SiC/graphite com-posites have a much higher thermal conductivity in their major?ber direction.In their minor?ber direction,the thermal conductivity was’15W/m K,or about the same as the SiC/SiC composite.At approximately200°C,the thermal conductivity of the K1100composite exceeds that of the high quality CVD SiC while the P55 composite approaches this value at the highest temper-atures measured.The?lled points represent measured di?usivity data in Fig.3.
The e?ect of low-dose irradiation at400–800°Con the P55/SiCcomposite is given in Fig.4.Dimensional measurements yielded data consistent with irradiation-induced swelling of SiC.As expected,a large decrease in thermal conductivity has occurred for the three tem-perature/dose levels studied,with the largest decrease occurring for the higher dose,lower temperature speci-mens.As the dose is below that of saturation,and the data set is very limited,a quantitative comparison of the thermal conductivity degradation of the P55composite with models and previous data from the graphite liter-ature is not appropriate.However,from the data,the thermal defect resistance is comparable to,or perhaps larger,than expected from the literature on pyrolitic graphite.For the case of the0.5dpa irradiation,the thermal conductivity should be approaching saturation, indicating that the P55material may possess more than twice the’15W/m K minimum value of irradiated thermal conductivity called for in present day ARIES and TAURO design study.However,signi?cant further study on the e?ect of irradiation is required.In partic-ular,the thermal conductivity and the e?ects on non-isotropic swelling of the graphite?ber must be studied up to higher damage levels.As the thermal conductivity in these materials is carried primarily by the?bers, cracking of the?bers due to axial shrinkage and the e?ect of this on the thermal conductivity will need to be understood.
4.Conclusions
The potential of a silicon carbide matrix,graphite ?ber composite for special purpose application in fusion systems has been presented.It is speculated,based on previous work on tritium retention in graphite and SiC, that this system will have signi?cantly less tritium re-tention than present day high-quality CFC?s.For the two materials of this study,the thermal conductivity at the fusion relevant operating temperature of550°Cwas ’90W/m K(P55?ber composite)and150W/m
K Fig.4.E?ect of low-dose irradiation at400–800°Con the thermal conductivity of SiC/graphite
composite.
Fig.3.Temperature dependent thermal conductivity of non-
irradiated monolithic SiC,SiC/SiC and SiC/graphite compos-
ites.
L.L.Snead et al./Journal of Nuclear Materials307–311(2002)1200–12041203
(K1100?ber composite.)This considerably exceeds the thermal conductivity of the present day CVI SiC/SiC composites at that temperature(’12W/m K).The ef-fects of low-dose irradiation on thermal conductivity indicate a signi?cant reduction in thermal conductivity, as expected from the graphite radiation e?ects literature. However,these preliminary data indicate the thermal conductivity still signi?cantly exceeds the minimum value called for in design studies. Acknowledgements
The authors would like to thank Marie Williams for assistance with thermal di?usivity measurements. Research sponsored by the O?ce of Fusion Energy Sciences,US Department of Energy under contract DE-AC05-00OR22725with UT-Battelle,LLC. References
[1]H.Atsumi,S.Tokura,M.Miyake,J.Nucl.Mater.155–
157(1988)241.
[2]R.A.Causey,J.Nucl.Mater.162–164(1989)151.
[3]V.Malka,H.D.Rohrig,R.Hecker,in:Proc.Conf.Tritium
Technology in Fission,Fusion,and Isotope Applications, 1980,p.102.
[4]H.Atsumi,M.Iseki,T.Shikama,J.Nucl.Mater.212–215
(1994)1478.
[5]R.A.Causey,W.R.Wampler,J.R.Retelle,J.L.Kaae,J.
Nucl.Mater.203(1993)196.
[6]R.A.Causey,Fus.Technol.19(1991)1585.
[7]W.R.Wampler,R.A.Causey,Sandia Report SAND93-
2007,October1993.
[8]J.W.H.Simmons,Radiation Damage in Graphite,Vol.
102,Pergamon,1965.
[9]R.Taylor,K.E.Gilchrist,L.J.Poston,J.Phys.Chem.
Solids30(1969)2251.
[10]B.T.Kelly,Carbon9(1971)783.
[11]R.J.Price,J.Nucl.Mater.48(1973)47.
[12]D.J.Senor,G.E.Youngblood,C.E.Moore,D.J.Trimble,
G.A.Newsome,J.J.Woods,Fus.Technol.30(1996)
943.
[13]L.L.Snead,S.J.Zinkle,in:I.M.Robertson et al.(Eds.),
Microstructure Evolution During Irradiation,MRS Sym-posium Proceedings,Vol.439,Materials Research Society, Pittsburgh,1997,p.595.
[14]S.J.Zinkle,C.Kinoshita,J.Nucl.Mater.251(1997)200.
[15]J.W.Corbett,Electron Radiation Damage in Semiconduc-
tors and Metals,Academic Press,New York,1966.
[16]K.Nakano,J.Ceram.Soc.Jpn.100(4)(1992)472.
[17]T.Noda,J.Nucl.Mater.179–181(1991)379.
[18]E.Tani,K.Shobu,T.Watanabe,J.Ceram.Soc.Jpn.
(1992)596.
[19]H.Yoshida,J.Ceram.Soc.Jpn.100(4)(1992)454.
[20]G.Camus,L.Guillaumat,S.Baste,Compos.Sci.Technol.
57(1996)407.
[21]M.R.E?nger,D.S.Tucker,T.R.Barnett,Ceram.Eng.Sci.
Proc.17(1996)316.
[22]M.C.Halbig,Ceram.Eng.Sci.Proc.18(1997)547.
[23]https://www.doczj.com/doc/5b15039793.html,combe,J.M.Rouges,in:Space Programs and
Technologies Conference?90?,Huntsville,AL,1990. [24]https://www.doczj.com/doc/5b15039793.html,ra-Curzio,Comprehensive Composites Encyclopedia,
Vol.4.18,Elsevier,2000,p.533.
[25]T.M.Besmann,J.C.McLaughlin,H.T.Lin,J.Nucl.
Mater.219(1995)31.
[26]L.L.Snead,O.J.Schwarz,J.Nucl.Mater.219(1995)3.
1204L.L.Snead et al./Journal of Nuclear Materials307–311(2002)1200–1204
Preparation of Polylactide/Graphene Composites From Liquid-Phase Exfoliated Graphite Sheets Xianye Li,1Yinghong Xiao,2Anne Bergeret,3Marc Longerey,3Jianfei Che1 1Key Laboratory of Soft Chemistry and Functional Materials,Nanjing University of Science and Technology, Nanjing210094,China 2Jiangsu Collaborative Innovation Center of Biomedical Functional Materials,Jiangsu Key Laboratory of Biomedical Materials,College of Chemistry and Materials Science,Nanjing Normal University, Nanjing210046,China 3Materials Center,Ales School of Mines,30319Ales Cedex,France Polylactide(PLA)/graphene nanocomposites were pre-pared by a facile and low-cost method of solution-blending of PLA with liquid-phase exfoliated graphene using chloroform as a mutual solvent.Transmission electron microscopy(TEM)was used to observe the structure and morphology of the exfoliated graphene. The dispersion of graphene in PLA matrix was exam-ined by scanning electron microscope,X-ray diffrac-tion,and TEM.FTIR spectrum and the relatively low I D/I G ratio in Raman spectroscopy indicate that the structure of graphene sheets(GSs)is intact and can act as good reinforcement fillers in PLA matrix.Ther-mogravimetric analysis and dynamic mechanical analy-sis reveal that the addition of GSs greatly improves the thermal stability of PLA/GSs nanocomposites.More-over,tensile strength of PLA/GSs nanocomposites is much higher than that of PLA homopolymer,increasing from36.64(pure PLA)up to51.14MPa(PLA/GSs-1.0). https://www.doczj.com/doc/5b15039793.html,POS.,35:396–403,2014.V C2013Society of Plastics Engineers INTRODUCTION Polylactide(PLA),a renewable,sustainable,biode-gradable,and eco-friendly thermoplastic polyester,has balanced properties of mechanical strength[1],thermal plasticity[2],and compostibility for short-term commod-ity applications[3,4].It is currently considered as a promising polymer for various end-use applications for disposable and degradable plastic products[5–8].Never-theless,improvement in thermal and mechanical proper-ties of PLA is still needed to pursue commercial success. To achieve high performance of PLA,many studies on PLA-based nanocomposites have been performed by incorporating nanoparticles,such as clays[9,10],carbon nanotubes[11–13],and hydroxyapatite[14].However, research on PLA-based nanocomposites containing gra-phene sheets(GSs)or graphite nanoplatelets has just started[15–17].GSs exhibit unique structural features and physical properties.It has been known that GSs have excellent mechanical strength(Young’s modulus of1,060 GPa)[18],electrical conductivity of104S/cm[19],high specific surface area of2,630m2/g[20],and thermal sta-bility[21].Polymer nanocomposites based on graphene show substantial property enhancement at much lower fil-ler loadings than polymer composites with conventional micron-scale fillers,such as glass[22]or carbon fibers [23],which ultimately results in lower filler ratio and simple processing.Moreover,the multifunctional property enhancement of nanocomposites may create new applica-tions of polymers. However,the incorporation of graphene into PLA matrix is restricted by cost and yield.Although the weak interactions that hold GSs together in graphite allow them to slide readily over each other,the numerous weak bonds make it difficult to separate GSs homogeneously in sol-vents and polymer matrices[24].Many methods have been reported for exfoliation of graphite,such as interca-lation with alkali metals[25]or oxidation in strong acidic conditions[26–29].Recently,exfoliation of graphite in liquid-phase was found to be able to give oxide-free GSs with high quality and yield at relatively low cost[30–35]. Correspondence to:Y.H.Xiao;e-mail:yhxiao@https://www.doczj.com/doc/5b15039793.html, or J.F.Che; e-mail:xiaoche@https://www.doczj.com/doc/5b15039793.html, Contract grant sponsor:Specialized Research Fund for the Doctoral Program of Higher Education of China;contract grant number: 20123219110010;contract grant sponsor:Natural Science Foundation of Jiangsu Province of China;contract grant number:BK2012845;contract grant sponsors:Priority Academic Program Development of Jiangsu Higher Education Institutions(PAPD),contract grant sponsor:Financial support for short visit from Ales School of Mines,France. DOI10.1002/pc.22673 Published online in Wiley Online Library(https://www.doczj.com/doc/5b15039793.html,). V C2013Society of Plastics Engineers POLYMER COMPOSITES—2014
石墨烯材料 1.4石墨烯材料 纯净、完美的石墨烯是一种疏水材料,并且在大多数有机溶剂中也难于溶解。不过,对石墨烯进行复合和改性,如通过修饰,共价或非共价的方法将功能基团引入石墨烯平面,能使其溶解度显著提高H¨”。在没有分散剂的作用下,直接将疏永的石墨烯片分散在水中是很困难的。通过氨水调节pH值为10左右,用水合肼还原氧化石墨烯(GO)的办法,可以得到还原的石墨烯(rG0)。由于这利-石墨烯还含有少量的含氧基团,因而可在水溶液中分散。但这种分散能力依然是有限的,不超过O 5 mg/mL。除了水,一些有机溶剂,如乙醇、丙酮、二甲基亚砜和四氢呋喃也可以用来分散rGO。金属离子和功能基团同样可以用来修饰rGO片层。在KOH溶液中,用肼还原氧化石墨可得到钾离子修饰的石墨烯(hKlvlG),其能在水溶液中均匀分散。另外,将苯磺酸基团引入GO,还原后可得少量磺化的石墨烯,这种石墨烯在pH处于3-10的范围内时,浓度可达2mg/mL。 共价修饰石墨烯指的是用含有功能基团的分子与石墨烯表面的含氧基团的反应,如羧基、环氧基、羟基,包括平面内的碳碳双键。例如,分散在四氢呋喃,四氯化碳,1,2-二氯乙烷(EDC)qb的rGO,发现把其边缘的羧基修饰上十八胺时后,其稳定性增加[48-50。用异氰酸酯处理石墨烯时,表面的羟基和边缘的羧基会形成酰胺和氨基甲酸酯。氧化石墨烯的羧基与聚乙烯醇(P、後)的羟基酯化也实现了合成GO与聚合物的复合片层。另一方面,石墨烯表面的环氧基团可以接受亲核试剂(如离子液体1-(3-aminopropyl)-3-methylimidazolium bromide或APTS) 的进攻而发生开环反应。同样,rGO可以用重氮盐(如SDBS)共价功能化,使之在多种极性有机溶剂中具有很好的分散性。此外,由环加成反应将氮烯体系和碳碳双键连接,使苯基丙氨酸和迭氮三甲基硅烷等许多有机官能团引入石墨烯表面。与共价功能化相比,非共价功能化是基于rGO与稳定剂间的范德华力或相互作用。这种修饰不仅对石墨烯的结构破坏更小,而且为调控其溶解度和电子性质提供了便利。在氧化石墨烯的氨水溶液中,加入聚苯乙烯磺酸钠(PSS)后,再用水合肼还原,人们第一次制得了非共价修饰的可分散石墨烯。在这项工作中,PSS的疏水端与rGO发生吸附,阻碍了rGO的团聚。并且PSS 的另一端是亲水性的,这就使1<30.PSS在水中可以稳定分散。此外,通过与生物分子的
常熟理工学院学报(自然科学)Journal of Changshu Institute Technology (Natural Sciences )第26卷第10Vol.26No.102012年10月Oct.,2012 收稿日期:2012-09-05 作者简介:季红梅(1982—),女,江苏启东人,讲师,工学硕士,研究方向:无机功能材料.水热合成Fe 2O 3/石墨烯纳米 复合材料及其电化学性能研究 季红梅1,于湧涛2,王露1,王静1,杨刚1 (1.常熟理工学院化学与材料工程学院,江苏常熟215500;2.吉林石化公司研究院,吉林吉林132021) 摘要:利用水热法成功合成了Fe 2O 3/石墨烯(RGO )锂离子电池负极材料.导电性能良好的石墨烯网络起到连接导电性能极差的Fe 2O 3和集流体的作用.电化学性能测试表明,180℃下得到的 Fe 2O 3/RGO 具有良好的比容量和循环稳定性.在不同倍率充放电过程中,初始放电比容量为1023.6mAh/g (电流密度为40mA/g ),电流密度增加到800mA/g 时,放电比容量维持在406.6 mAh/g ,大于石墨的理论放电比容量~372mAh/g.在其他较高的电流密度下比容量均保持基本不变.该Fe 2O 3/RGO 有望成为高容量、低成本、低毒性的新一代锂离子电池负极材料.关键词:Fe 2O 3;石墨烯;负极材料中图分类号:TM911文献标识码:A 文章编号:1008-2794(2012)10-0055-05 自从P.Poizot [1]等报道过渡金属氧化物可以作为锂离子电池负极材料这一研究后,金属氧化物负极便逐渐引起人们的重视.铁的氧化物具有比容量大、倍率性能好和安全性能高等优点,且原料来源丰富、价格低廉、环境友好,因此是一类很有发展潜力的动力锂离子电池负极材料.Fe 2O 3作为一种常温下最稳定的铁氧化合物,理论容量为1005mAh/g ,远高于石墨类材料的理论比容量,已经成为锂离子电池负极材料的一个研究热点.近年来,石墨烯由于其高的电传导性,大的比表面积,良好的化学稳定性和柔韧性而被尝试用于与活性锂离子电池负极材料复合,提升材料的电化学性能.比如,Cui Y [2]课题组在溶剂热条件下两步法得到Mn 3O 4与石墨烯的复合材料,改善了Mn 3O 4的比容量和循环性能.Co 3O 4,Fe 3O 4等金属氧化物材料与石墨烯复合也有被研究,本课题组在石墨烯和金属氧化物材料复合方面也做了大量的工作[3].本文通过水热法一步合成Fe 2O 3/石墨烯纳米复合材料,并研究了其电化学性能,合成过程中采用三乙烯二胺提供反应的碱性环境,并控制Fe 2O 3的粒子生长.1 实验 1.1试剂和仪器 三乙烯二胺(C 6H 12N 2);无水三氯化铁(FeCl 3);石墨;硝酸钠(NaNO 3);浓硫酸(H 2SO 4);高锰酸钾(KMnO 4);双氧水(H 2O 2)和盐酸(HCl ),以上试剂均为分析纯.实验用水为去离子水.日本理学H-600型透射电子显微镜;日本理学D/max2200PC 型X 射线衍射仪;德国Bruker Vector 22红外光谱仪;日本JEOL-2000CX 透射电镜;美国Thermo Scientific Escalab 250Xi 光电子能谱仪;LAND 电池
石墨烯复合材料 石墨烯是单层碳原子通过sp2杂化形成的蜂窝点阵结构,属于二维原子晶体,此独特的空间结构,给石墨烯带来了优异的电学、力学、热学和比表面积大等性质。但是二维石墨烯由于片层之间具有较强的π-π作用和范德华力,使得石墨烯容易聚集形成石墨,限制了石墨烯在各个领域中的应用。因此,为了防止石墨烯的聚集和拓展石墨烯的应用,科研工作者将石墨烯与高分子或者无机纳米粒子进行复合,从而得到具有优异性能的复合材料。石墨烯的复合材料具有化学稳定性高、比表面积大,易回收等特点,在环境治理方面受到了科学家的青睐。 一、石墨烯复合材料的分类和制备 1、石墨烯-高分子复合材料 石墨烯-高分子复合材料,石墨烯的独特的结构和性能,对于改善高分子的导电性、热性能和吸附能力等方面有非常大的应用价值。制备石墨烯-高分复合材料最直接的方法是将高分子溶液与石墨烯的溶液混合,其中高分子和填充物在溶剂中的溶解能力是保证最佳分散度的重要因素。因此,在溶液混合时,可以将石墨基质表面功能化来提高它在多种溶剂中的溶解度。例如,异氰酸
苯酯修饰的GO在在聚苯乙烯的DMF溶液中表现出了较好的溶解度。 2、石墨烯-无机纳米粒子复合材料 无机纳米粒子存在着易于团簇的问题,并且选择合适的载体也是其广泛应用需要解决的问题。石墨烯具有多种优异的性能,并且具有较大的比表面积,可以成为无机纳米材料的载体。无机纳米粒子可以将易于团簇的石墨烯片层分开,防止团簇,从而两者形成石墨烯-无机纳米粒子新型的复合材料,这些材料广泛的应用于检测、催化和气体存储等方面。目前已报道的有负载的金属纳米粒子Ag、Au、氧化物纳米粒子ZnO和Fe3O4等。 3、其它石墨烯复合材料 石墨烯不仅仅可以和高分子、无机纳米材料复合,还可以同时结合高分子、纳米粒子和碳基材料中的一种或者两种,形成多元的含有石墨烯的复合材料。这类材料具有多功能性,用于超级电容器或者传感器等。 二、石墨烯复合材料在水治理的应用 1、吸附作用 碳材料中活性碳和碳纳米管被广泛的应用于水净化领域,将石墨烯与其它化合物进行复合,这些复合材料在吸附污染物上有非常高的效率,可以应用于染料、多芳香环烃和汽油的吸附。比如利用磁性-壳聚糖-石墨烯的复合材料可以大大提高去除溶液中的亚甲基蓝的效率,吸附能力达到
石墨烯复合材料的研究及其应用 任成,王小军,李永祥,王建龙,曹端林 摘要:石墨烯因其独特的结构和性能,成为物理化学和材料学界的研究热点。本文综述了石墨烯复合材料的结构和分类,主要包括石墨烯-纳米粒子复合材料、石墨烯-聚合物复合材料和石墨烯-碳基材料复合材料。并简述石墨烯复合材料在催化领域、电化学领域、生物医药领域和含能材料领域的应用。 关键词:石墨烯;复合材料;纳米粒子;含能材料 Research and Application of Graphene composites ABSTRACT: Graphene has recently attracted much interest in physics,chemistry and material field due to its unique structure and properties. This paper reviews the structure and classification of graphene composites, mainly inclouding graphene-nanoparticles composites, graphene-polymer composites and graphene-carbonmaterials composites. And resume the application of graphene composites in the field of catalysis, electrochemistry, biological medicine and energetic materials. Keywords: graphene; composites; nanoparticles; energetic materials 石墨烯自2004年曼彻斯特大学Geim[1-3]等成功制备出以来,因其独特的结构和性能,颇受物理化学和材料学界的重视。石墨烯是一种由碳原子紧密堆积构成的二维晶体,是包括富勒烯、碳纳米管、石墨在内的碳的同素异形体的基本组成单元。石墨烯的制备方法主要有机械剥离法,晶体外延法,化学气相沉积法,插层剥离法以及采用氧化石墨烯的高温脱氧和化学还原法等[4-10]。与碳纳米管类似,石墨烯很难作为单一原料生产某种产品,而主要是利用其突出特性与其它材料体系进行复合.从而获得具有优异性能的新型复合材料。而氧化石墨烯由于其特殊的性质和结构,使其成为制备石墨烯和石墨烯复合材料的理想前驱体。本文综述了石墨烯复合材料的结构、分类及其在催化领域、电化学领域、生物医药领域和含能材料领域的应用。
高分子/石墨烯纳米复合材料研究进展 高秋菊1,夏绍灵1,2* ,邹文俊1,彭 进1,曹少魁2 (1.河南工业大学材料科学与工程学院,郑州 450001;2.郑州大学材料科学与工程学院,郑州 450052 )收稿:2012-01-09;修回:2012-04- 24;基金项目:郑州科技攻关项目(0910SGYG23258- 1);作者简介:高秋菊(1984—),女,硕士研究生,主要从事高分子复合材料的研究。E-mail:gaoqiuj u2008@yahoo.com.cn;*通讯联系人,Tel:0371-67758722;E-mail:shaoling _xia@haut.edu.cn. 摘要: 石墨烯以其优异的力学、光学、电学和热学性能,得到日益广泛的关注和研究。本文介绍了石墨烯的结构、性能和特点,并对石墨烯的改性方法进行了概括。本文着重综述了高分子/石墨烯纳米复合材料的研究现状和进展,并介绍了高分子/石墨烯纳米复合材料的三种制备方法,即原位插层聚合法、溶液插层法和熔融插层法。此外,还对高分子/石墨烯纳米复合材料的应用前景进行了展望,并对石墨烯复合材料研究存在的问题和未来的研究方向进行了讨论。 关键词:石墨烯;高分子;纳米复合材料;研究进展 引言 石墨烯是以sp2 杂化连接的碳原子层构成的二维材料, 其厚度仅为一个碳原子层的厚度。这种“只有一层碳原子厚的碳薄片”,被公认为目前世界上已知的最薄、最坚硬、最有韧性的新型材料。石墨烯具 有超高的强度,碳原子间的强大作用力使其成为目前已知力学强度最高的材料。石墨烯比钻石还坚硬, 强度比世界上最好的钢铁还高100倍[1] 。石墨烯还具有特殊的电光热特性, 包括室温下高速的电子迁移率、 半整数量子霍尔效应、自旋轨道交互作用、高理论比表面积、高热导率和高模量、高强度,被认为在单分子探测器、集成电路、场效应晶体管等量子器件、功能性复合材料、储能材料、催化剂载体等方面有广泛 的应用前景[ 2] 。石墨烯是一种疏松物质,在高分子基体中易团聚,而且石墨烯本身不亲油、不亲水,在一定程度上也限制了石墨烯与高分子化合物的复合,尤其是纳米复合。因而,很多学者对石墨烯的改性进行了大量的研究,以提高石墨烯和高分子基体的亲和性,从而得到优异的复合效应。 1 石墨烯的改性方法 1.1 化学改性石墨烯 该方法基于改性Hummers法[3] 。首先,由天然石墨制得石墨氧化物, 再通过几种化学方法获得可溶性石墨烯。其化学方法包括:氧化石墨在稳定介质中的还原[4]、通过羧基酰胺化的共价改性[5] 、还原氧化石墨烯的非共价功能化[ 6]、环氧基的亲核取代[7]、重氮基盐的耦合[8] 等。此外,还出现了对石墨烯的氨基化[9]、酯化[10]、异氰酸酯[11] 改性等。用化学功能化的方法对石墨烯进行改性,不仅可以提高其溶解性 和加工性能,还可以增强有机高分子间的相互作用。1.2 电化学改性石墨烯 利用离子液体对石墨烯进行电化学改性已见报道[12] 。用电化学的方法,使石墨变成用化学改性石 墨烯的胶体悬浮体。石墨棒作为阴极,浸于水和咪唑离子液的相分离混合物中。以10~20V的恒定电 · 78· 第9期 高 分 子 通 报
石墨烯在复合材料中的应用 龚欣 (东南大学机械工程学院南京211189) 摘要:介绍了石墨烯与有机高聚物、无机纳米粒子以及其它碳基材料的复合物,同时展望了这些材料在相关领域中的应用前景. 关键词:石墨烯纳米复合材料 2004年至今, 关于石墨烯的研究成果已在SCI检索期刊上发表了超过2000篇论文, 石墨烯开始超越碳纳米管成为了备受瞩目的国际前沿和热点.基于石墨烯的纳米复合材料在能量储存、液晶器件、电子器件、生物材料、传感材料和催化剂载体等领域展现出许多优良性能,具有广阔的应用前景.目前研究的石墨烯复合材料主要有石墨烯/聚合物复合材料和石墨烯/无机物复合材料两类,其制备方法主要有共混法、溶胶-凝胶法、插层法和原位聚合法.本文将对石墨烯的纳米复合材料及其性能等方面进行简要的综述. 一、基于石墨烯的复合物 利用石墨烯优良的特性与其它材料复合可赋予材料优异的性质.如利用石墨烯较强的机械性能,将其添加到高分子中,可以提高高分子材料的机械性能和导电性能;以石墨烯为载体负载纳米粒子,可以提高这些粒子在催化、传感器、超级电容器等领域中的应用. 1.1 石墨烯与高聚物的复合物 功能化后的石墨烯具有很好的溶液稳定性,适用于制备高性能聚合物复合材料.根据实验研究,如用异氰酸酯改性后的氧化石墨烯分散到聚苯乙烯中,还原处理后就可以得到石墨烯-聚苯乙烯高分子复合物.该复合物具有很好的导电性,添加体积分数为1%的石墨烯时,常温下该复合物的导电率可达0.1S/M,可在导电材料方面得到的应用. 添加石墨烯还可显著影响高聚物的其它性能,如玻璃化转变温度(Tg)、力学和电学性能等.例如在聚丙稀腈中添加质量分数约1%的功能化石墨烯,可使其Tg 提高40℃.在聚甲基丙烯酸甲酯(PMMA)中仅添加质量分数0.05%的石墨烯就可以将其Tg提高近30℃.添加石墨烯的PMMA比添加膨胀石墨和碳纳米管的PMMA具有更高的强度、模量以及导电率.在聚乙烯醇(PVA)和PMMA中添加质量分数0.6% 的功能化石墨烯后,其弹性模量和硬度有明显的增加.在聚苯胺中添加适量的氧化石墨烯所获得的聚苯胺-氧化石墨烯复合物的电容量(531F/g)比聚苯胺本身的电容量(约为216F/g)大1倍多,且具有较大的拉伸强度(12.6MPa).这些性能为石墨烯-聚苯胺复合物在超级电容器方面的应用创造了条件. 石墨烯在高聚物中还可形成一定的有序结构.通过还原分散在Nafition膜中
河北工业大学 材料科学与工程学院 石墨烯及其纳米复合材料发展概况 专业金属材料 班级材料116 学号111899 姓名李浩槊 2015年01月05日
摘要 自从2004年,英国曼彻斯特大学物理学家安德烈·海姆和康斯坦丁·诺沃肖洛夫,成功地在实验中从石墨中分离出石墨烯,石墨烯因其优异的力学、电学和热学性能已经成为备受瞩目的研究热点。 石墨烯的碳原子排列与石墨的单原子层雷同,是碳原子以sp2混成轨域呈蜂巢晶格(honeycomb crystal lattice)排列构成的单层二维晶体。石墨烯可想像为由碳原子和其共价键所形成的原子尺寸网。石墨烯是世上最薄也是最坚硬的纳米材料,它几乎是完全透明的,只吸收2.3%的光;导热系数高达5300 W/(m·K),高于碳纳米管和金刚石,常温下其电子迁移率超过15000 cm2 /(V·s),又比纳米碳管或硅晶体高,而电阻率只约10-6Ω·cm,比铜或银更低,为世上电阻率最小的材料。因为它的电阻率极低,电子跑的速度极快,因此被期待可用来发展出更薄、导电速度更快的新一代电子元件或晶体管。由于石墨烯实质上是一种透明、良好的导体,也适合用来制造透明触控屏幕、光板,甚至是太阳能电池。 石墨烯的结构非常稳定,石墨烯内部的碳原子之间的连接很柔韧,当施加外力于石墨烯时,碳原子面会弯曲变形,使得碳原子不必重新排列来适应外力,从而保持结构稳定。这种稳定的晶格结构使石墨烯具有优秀的导热性。 但是,因为石墨烯片层之间存在很强的范德华力,导致其很容易堆积团聚,在一般溶剂中的分散性很差,所以其应用领域受到了限制。本文通过收集、查阅多篇有关石墨烯研究的论文,分析、整理了石墨烯及其纳米复合材料的制备技术发展及其应用的相关知识、理论。 关键词:石墨烯纳米材料制备复合材料
This article appeared in a journal published by Elsevier.The attached copy is furnished to the author for internal non-commercial research and education use,including for instruction at the authors institution and sharing with colleagues. Other uses,including reproduction and distribution,or selling or licensing copies,or posting to personal,institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article(e.g.in Word or Tex form)to their personal website or institutional repository.Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: https://www.doczj.com/doc/5b15039793.html,/copyright
研 究 生 课 程 论 文 (2016-2017学年第一学期) 石墨烯/高聚物复合材料流变学的研究进展 研究生:文睿
说明 1、课程论文要有题目、作者姓名、摘要、关键词、正文及参考文献。论文题目由研究生结合课程所学内容选定;摘要500字以下,博士生课程论文要求有英文摘要;关键词3~5个;参考文献不少于10篇,并应有一定的外文文献。 2、论文要求自己动手撰写,如发现论文是从网上下载的,或者是抄袭剽窃别人文章的,按作弊处理,本门课程考核成绩计0分。 3、课程论文用A4纸双面打印。字体全部用宋体简体,题目要求用小二号字加粗,标题行要求用小四号字加粗,正文内容要求用小四号字;经学院同意,课程论文可以用英文撰写,字体全部用Times New Roman,题目要求用18号字加粗;标题行要求用14号字加粗,正文内容要求用12号字;行距为2倍行距(方便教师批注);页边距左为3cm、右为2cm、上为2.5cm、下为2.5cm;其它格式请参照学位论文要求。 4、学位类别按博士、硕士、工程硕士、MBA、MPA等填写。 5、篇幅、内容等由任课教师提出具体要求。
石墨烯/高聚物复合材料流变学的研究进展 文睿 摘要:石墨烯具有独特的二维平面结构,其电导、热导和机械性能都非常优越,故常将其与高聚物掺杂以提高高聚物的电学、热学和气体阻隔性能。石墨烯/高聚物复合材料具有复杂的内部结构,很多作用机理尚不清楚,会对加工制备过程造成一定困难,而流变学是研究复合材料结构的重要工具,因此石墨烯/高聚物复合材料的流变学研究具有重要意义。本文综述了石墨烯/高聚物复合材料的熔体流变学、悬浮液流变学和电流变学的研究进展,并针对其中存在的问题提出了自己的看法。 关键词:石墨烯;高聚物;复合材料;流变学 1石墨烯/高聚物复合材料简介 石墨烯是sp2杂化的碳原子密致堆积形成的二维单片层蜂窝状晶格结构。严格说,石墨烯仅指代单片层石墨;但从广义上说,层数小于10的石墨层在物化性质上并不会表现出太大差异,因此,这些石墨层也可归入“石墨烯”的概念范畴。填充于石墨烯/高聚物复合材料中的石墨烯一般通过氧化还原法制备,因为此制备方法可使石墨烯表面接枝上极性基团,增大与基体材料的作用力,更有利于改善基体材料的性能。石墨烯/高聚物复合材料采用一般高聚物复合材料的制备方法即可制备,如:原位聚合法、熔融共混法、溶液共混法等,但不同制备方法得到的复合材料的石墨烯分散效果不同。由此可以看出,石墨烯/高聚物复合材料具有多层次的内部结构,而高聚物材料的流变行为正与内部结构相关,因此研究石墨烯/高聚物复合材料的流变学可以帮助推测石墨烯的分散度、石墨烯与基体材料的结合
石墨烯基聚合物复合材料的最新进展 摘要 本文综述了改性石墨烯以及构造石墨烯基复合材料的最新进展。由于加入少量的石墨烯就可以使某种性质产生巨大的提高,最近,石墨烯备受学术领域和工业领域科学家们的关注。人们对石墨烯/氧化石墨烯的改性以及利用这些材料与不同聚合物基体构造纳米复合材料进行了探索。人们运用一系列的方法利用不同聚合物构造添加石墨烯的聚合物基纳米复合材料。 对于改性的石墨烯基聚合物纳米复合材料中,添加非常少的石墨烯即可获得渗透阈值。本文阐述了科学文献中的具体例子,进而讨论了聚合物/石墨烯纳米复合材料的一般的结构、制备方法、以及性质。 1 引言 在过去的二十年中,纳米科学领域飞速发展,并且随着小型化在计算机、传感器、生物医学以及其他应用领域变得更为重要,纳米科技的重要性也会日益增加。这些学科的发展在很大程度上都依赖于合成不同材质、尺寸、形状的纳米材料以及把这些材料有效构造出复杂架构的能力。目前,由于纳米材料的结构特征,它们拥有广泛的应用领域。然而,材料学的科学家们正致力于测试在纳米科学与科技领域具有更合适尺寸的材料的得到改进的物理化学性质。在这个意义上,石墨烯以及石墨烯基聚合物纳米复合材料的发现是纳米科学领域的一个重要补充,在现代的科学与技术领域展示出重要作用。 丰田科研组发现的聚合物纳米复合材料曾在材料科学领域打开了新
的层面。特别是利用无机纳米材料作为添加剂制备聚合物/无机复合材料引起了越来越多的关注归因于其独特的性质以及在汽车,航天,建筑,电子等行业的无数的潜在应用。目前为止,大部分研究都集中在基于诸如层状的硅酸盐的蒙脱石类型以及合成陶土(分层的双羟基化合物)等天然来源的层状材料的聚合物纳米复合材料。然而,陶土的电导和热导率却非常的低。为了解决这些问题,诸如炭黑,EG,CNt和CNF等碳系的纳米填料被引入用以制备聚合物纳米复合材料。在这些材料中,CNT作为导电填料被证明是十分有效的。碳纳米管作为纳米填料的唯一缺点是成本过高。因此,大规模生产碳纳米管功能复合材料是十分困难的。正如Nicholas A.Kotov在自然杂志中发表论文所提出的问题:当碳纤维不能满足性能需要,而碳纳米管成本又太高的时候,科学家要到何处寻找一个考虑成本的问题的实用性的导电复合材料?答案应该是石墨烯。石墨烯被认为是拥有SP2的平面碳原子键合的密集堆积的蜂巢状晶格的单原子厚度的二维碳纳米填料。它被认为是具有巨大应用潜力的宇宙中最薄的材料。石墨烯被认为拥有显着的特性,如高导热性,优越的力学性能和优良的电子输运性。由于石墨烯这些固有的性质在无数的设备的中得到可能的利用,这些性质引起了科学家们的广泛兴趣。这些设备包括新一代高速射频逻辑器件,热和电纳米增强复合材料,超薄碳薄膜,电子电路,传感器,以及应用于太阳能电池的透明、可变电极。由于具有高的比表面积,长宽比,拉伸强度(TS),导热性和导电性,电磁屏蔽能力,灵活性,透明度以及低热膨胀系数,石墨烯由于其他的常规纳米填料(钠
石墨烯复合材料的制备及其性能研究进展
论文 题目: 石墨烯复合材料的制备 及其性能研究进展学生姓名: 学号: 院(系):化工与制药工程系专业班级: 指导教师: 职称: 201 年月
石墨烯复合材料的制备及其性能研究进展 摘要: 石墨烯以其优异的性能和独特的二维结构成为材料领域研究热点。本文综述了石墨烯的制备方法并分析比较了各种方法的优缺点, 简单介绍了石墨烯的力学、光学、电学及热学性能。基于石墨烯的复合材料是石墨烯应用领域中的重要研究方向, 本文详细介绍了石墨烯聚合物复合材料和石墨烯基无机纳米复合材料的制备及应用,以及石墨烯复合材料的展望。 关键词:石墨烯;制备;性能;复合材料
Research Progress on Preparation and properties of graphene composite materials Abstract: Graphene has become a hot research field of material for its excellent performance and unique two-dimensional structure. This paper summarizes the method for preparing graphene and compared the advantages and disadvantages of various methods,introduces the mechanics,graphene optical,electrical and thermal properties. Composite materials based on graphene is an important research direction in the field of application of graphene,this paper introduces the preparation and application of graphene polymer composites and graphene based inorganic nano composite material,and the prospect of graphene composite materials. Key words:graphene;preparation;properties;composite materials
石墨烯反应 1、加氢反应 2、与重氮化合物反应,之后与三氮唑化合物(苯并三氮唑)环加成反应 3、与碳烯反应 4、与芳炔化合物反应(生成芳基三氟甲磺酸三甲硅酯) 5、与四氰乙烯反应 6、与偶氮二甲亚胺反应 7、与氟化的苯基氮化合物反应
经还原的氧化石墨烯反应 1、与苯基重氮衍生物反应,之后与三氮唑化合物进一步反应 2、与氨基化合物反应(苯乙胺) 3、纳米颗粒负载 4、一 5、与苯基重氮衍生物反应,之后与2-溴丙酸乙酯反应,再与苯乙炔反应
1、催化石墨化,考虑其他催化剂的可能性 2、催化石墨化后直接添加到树脂中,考察合成材料的性能 3、催化石墨化后除去无定型碳添加到树脂中,考察合成材料的性能 4、催化石墨化后酸处理后制成石墨烯,再添加到树脂中 5、热固性树脂的种类 6、热塑性树脂的种类 尿醛树脂与石墨烯、氧化石墨、石墨复合 以氯化铵为固化剂,常温固化时间大概4-5小时。 脲醛树脂 尿素与甲醛反应得到的聚合物。又称脲甲醛树脂。英文缩写UF。加工成型时发生交联,制品为不溶不熔的热固性树脂。固化后的脲醛树脂颜色比酚醛树脂浅,呈半透明状,耐弱酸、弱碱,绝缘性能好,耐磨性极佳,价格便宜,但遇强酸、强碱易分解,耐候性较差。 基本介绍 脲醛树脂一般为水溶性树脂,较易固化,固化后的树脂无毒、无色、耐光性好,长期使用不变色,热成型时也不变色,可加入各种着色剂以制备各种色泽鲜艳的制品。 脲醛树脂坚硬,耐刮伤,耐弱酸弱碱及油脂等介质,价格便宜,具有一定的韧性,但它易于吸水,因而耐水性和电性能较差,耐热性也不高。 用途:可用于耐水性和介电性能要求不高的制品,如插线板、开关、机器手柄、仪表外壳、旋纽、日用品、装饰品、麻将牌、便桶盖,也可用于部分餐具的制造。 包装:大口塑料桶或铁桶,净重20kg。 储运:存于阴凉通风处,储存时间自生产日期起一个月,超过储存期,经检验合格后仍可使用。 它能在酸性介质中固化,常用的固化剂有氯化铵、草酸、邻苯二甲酸、硫脲等。产物需在中性条件下才能贮存。线性脲醛树脂以氯化铵为固化剂时可在室温固化。模塑粉则在130~160℃加热固化,促进剂如硫酸锌、磷酸三甲酯、草酸二乙酯等可加速固化过程。脲醛树脂主要用于制造模压塑料,制造日用生活品和电器零件,还可作板材粘合剂、纸和织物的浆料、贴面板、建筑装饰板等。由于其色浅和易于着色,制品往往色彩丰富瑰丽。 1、脲醛树脂胶的调制使用之前在胶料中要加入氯化铵,其作用是使胶料凝固速度加快,但 要注意用量适当,加少了固化时间太长,不利施工操作;加多了胶会发脆,胶接质量就不好。 根据经验,以100克脲醛树脂而言,在气温10℃以下时,一般应加2.5-3克的氯化铵,而在30℃的气温时,只要加入1历就够了。在胶合木板时,有时在胶料中还加入5-10%的黄豆粉或面粉(粉料不能有粒状,要过100目筛),目的是增加粘稠度,防止过量的胶液渗入木
石墨烯聚合物纳米复合材料的前沿与趋势 聚合物与其他塑料结合形成混纺纤维,与滑石粉及云母混合形成填充系统,和与其他非均质加固物进行模型挤压生产复合材料和杂化材料。这种简单的“混合搭配”方法使得塑料工程师们能够利用聚合物团生产一系列能够控制极端条件的有用的材料。在这种方法中最后加入的事石墨烯------人们早就了解到它的存在但是知道2004年才被制备与鉴定出的碳单原子层。英国曼彻斯特大学的Andre K.Geim和Konstantin S.Novoselov因为分离出碳单原子层而被授予诺贝尔物理学奖。他们的成就导致了聚合物纳米材料的蓝图发生了变化。人们已经长期熟知碳基材料,像金刚石,六方碳和石墨烯。但是聚合物纳米材料研究团体重新燃起的热情主要由于石墨烯可与塑料结合的特性以及它来自于廉价的先驱体。石墨烯的性价比优势在纳米复合材料、镀膜加工、传感器和存储装置的应用上正挑战着碳纳米管。接着,这些只能被想象出来的应用将会出现。事实上,Andre Geim说过“石墨烯对于它的名字来说就是一种拥有最佳性能的非凡的物质。”这能够在目前大量发表的文献中可以看出。石墨烯为什么能够这样引起人们的兴趣呢?本篇综述尝试去处理在石墨烯纳米复合材料新兴潮流中所产生的这类问题。这个工作的范围被石墨烯聚合物纳米复合材料(GPNC)研究员提出期望的发展潜力进行了拓展。 神奇的石墨烯 石墨烯被频繁引用的性能是它的电子传输能力。这意味着一个电子可以在其中不被散射或无障碍地通行。石墨烯的电子迁移率可达到20000cm2/Vs,比硅晶体管高一个数量级。一片最近的综述表明,以改良样品制备的石墨烯,电子迁移率甚至可以超过25000cm2/Vs。石墨烯是否缺少禁带以及大量合成纯石墨烯是否可行只有将来的研究可以解释。目前,非凡的电子传导性能使得石墨烯居于各类物质之首。所以,利用石墨烯代替硅作为基质的可能性将指日可待。虽然石墨烯的电子传导能力要比铜高得多,但是其密度只有铜的1/5。文献中大量记载了石墨烯的电子传导性能极其影响方面的细节。 由于它固有的特性人们开始对它在纳米复合材料的应用产生了兴趣。据预测,一个单层无缺陷的石墨烯薄膜的抗拉强度要比其他任何物质都要大。事实上,James Hone’s小组已经用原子力显微镜研究了独立的单层石墨烯薄膜的断裂强度。他们测得的平均断裂力为1700nN。他们还发现石墨烯这种物质可以抵挡超高的应力(约25%)。这些测量值使得这个团队计算出无缺陷石墨烯薄片的内在强度为45Nm-1。这儿的内在强度被规定为无缺陷的纯物质在断裂之前所能承受的最大应力。石墨烯如此卓越的是由于它相当于1.0Tpa的杨氏模量。在其他的特性中Paul McEuen和同事们只有一个原子厚度的石墨烯薄膜即可隔绝气体,包括氦气。即石墨烯在实际应用中可作为密闭的微室。石墨烯所表现出的热传导性能要比铜高出很多倍。这就意味着石墨烯能够很容易地进行散热。最近对大块石墨烯薄膜的研究表明其热传导系数是600W/(m.K)。石墨烯另外的一个特性是其具有高的比表面积,计算值为2630m2g-1,而碳纳米管仅为1315m2g-1,这使得石墨烯在储能装置应用上成为一个候选材料。Rod Ruoff’s小组通过改性的石墨烯演示了其具有的超高电容性能。对石墨烯的新奇属性的详细描述随处可见石墨烯与碳纳米管相比有一个截然相反的属性是其不含杂质(不含金属),这对构建可靠的传感器和储能装置来说是一个重要的优势。,更进一步,由于它形状与结构,石墨烯或许有更低的毒性,这也成为目前研究的主题。 独立的纳米材料的这些性质使得物理学家,化学家,和材料学家,不论作为理论学家还是实验学家,都为石墨烯的潜力而感到振奋。然而,最重要的问题是去区分炒作还是现实。