Collagen matrix as a tool in studying fibroblastic cell behavior
- 格式:pdf
- 大小:1.28 MB
- 文档页数:38

GREENTECH S.A Biopôle Clermont-Limagne - 63360 Saint-Beauzire – France1GREENTECH S.A Biopôle Clermont-Limagne - 63360 Saint-Beauzire – France2TAMANOLINTRODUCTIONFirst sign of skin ageing is skin thinning. This loss is about 6% per decade in relation to initial relative thickness at birth.Nevertheless, these modifications are inequal according to each one and also depend on environmental factors.The inequal loss of different type of macromolecules of skin matrix can explain the decrease of skin smoothly, flexibility, firmness and hydration as well as wrinkles appearance.Significant changes can be observed at collagen matrix level.Skin ageing is an ineluctable physiological phenomenon, slowly progressive which can be translated particularly by a scarcity and disappearance of reticular dermis collagen beams, deep dermis located under papillar dermis which is just under epidermis.As one grows, fibroblast are losing their reproductive abilities and then collagen synthesis is getting limited.Ratio between collagen type III and collagen type I keeps increasing.Compounds application aimed at stimulating collagen synthesis must both play a preventive function as regards to skin ageing and help to improve skin microdepth.Such a stimulative activity must also improve healing which must allow prevention of dermis fibers breaking and for instance stretch marks appearance.GREENTECH S.A Biopôle Clermont-Limagne - 63360 Saint-Beauzire – France3TAMANOLPLANT DESCRIPTIONTAMANOL is the native name of the plant in TAHITI. Considered as sacral tree, it was planted around “marae” (places of royal worship and its trunk was reserved to confection of big idoles which was decorated with the nicest feathers). The olders were saying that, during human sacrifices, Gods were coming without being seen, were laying down under their shadow in order to attend to ceremony.TAMANU is a tree coming from Tropical Asia. At present, it can be found in wet tropical forests in Africa,East India, Thaïland, Indochina and more specifically in Polynesia.It is growing natively on shores coral sands.This tree with its 10 to 15 meters’ high and its thick trunk is covered with black, rugged and cracked bark.This fruit is an orange drupe which flavour reminds apple one. It contains oleaginous seeds from which oil is extracted when fruits, after reaching a certain degree of maturity, are harvested and stored in an air and dry place during 3 months.Tamanol, unfit for human consumption, presents on the other hand, by topic applications, interesting healing properties. It is particularly used traditionnally by native populations to treat skin deseases and cracks.Tamanol healing properties are very interesting in all ageing problems which are associated with degradation and rigidification of collagen fibers at dermis level.Dermis constitutes a support tissue which one gives tonus and consistance to skin. Wrinkles and stretch marks are as many imperfections damaging skin architecture.An Active likely to stimulate collagen synthesis constitutes an excellent candidate to prevent ageing.P H Y T O B I O A C T I V E S T a m a n o l GREENTECH S.A Biopôle Clermont-Limagne - 63360 Saint-Beauzire – France4TAMANOLINCI NAME : CALOPHYLLUM INOPHYLUM OILANALYTICAL CHARACTERISTICSTamanol is extracted by cold pressure from fruit almonds of Callophyllum Inophylum. The extraction fis not so easy. From extraction quality depends oil physiological quality.This oil, unlike most plant oils, doesn’t exist in mature fruit when that one falls down from the tree. It is created during almond dessication.Organoleptical characteristics :Colour : green Aspect : Viscous Density to 20°C : 0,920 - 0,940Refraction index: 1,4750 - 1,4820Iode Index: 100 - 115Peroxyde index : < 20,0 meqLipidic fraction : 98 - 99,5%Unsaponifiable : 0,5 - 2%Lipidic fraction is mainly constituted by :Neutral lipid : 90 - 92%Glycolipid : 5 - 7%Phospholipids : 1 - 2%Composition in fatty acidPalmitic acid 15 - 17%Palmitoleic acid 0.5 - 1%Stearic acid 8 - 16%Oleic acid 30 - 50%Linoléic acid 25 - 40%Arachidonic acid 0.5 - 1%Gadoleic acid 0.5 - 1%The fraction to be said unsaponifiable is made up of :- hydrocarbures with linear long chain and/or branched out sometimes unsaturated - Fatty alcohols - sterols (sitosterol and stigmasterol and methyl sterols)- Xanthone - Coumarinic derived - Calophyllic, isocalophyllic, isopetalic, chapelieric, pseudobrasilic acids - triterpenic compounds specially from friedeline familyGREENTECH S.A Biopôle Clermont-Limagne - 63360 Saint-Beauzire – France5TAMANOLCOSMETIC PROPERTIESDERMIS ARCHITECTUREDermis is constituted by two kinds of fix cells, fibroblasts and migratory cells which are the cells being involved in cellular defense.Fibroblasts synthesize all macromolecules which take part in the extracellular matrix constitution as collagen and elastin. Fibroblasts are surrounded with wealth of non mineral and non cartilaginous substances which are made up for a large part of collagen. Their activity is heavy during healing phenomenon.Actually, several types of collagen fibers are found and gathered in more or less voluminous beams. We can see in dermis, collagens of type I, III and IV, collagen of Type I being accounted for a large part. Each collagen fiber is constituted of tropollagen elementory molecules which itself is made up of three chains of several hundred of amino-acids, each one rolled up between itself as rope strands.Cohesion between collagen molecules is due, at the beginning of their formation, to electrostatic force, buttheir stability is weak and are easily soluble. Then, during collagen development a new kind of link arises :intra and intermolecular binding. They prevent fibers from sliding ones on the others, make them insoluble and decrease their sensitivity to enzyms, that constitutes one of the main characteristics of skin ageing.Then, a stimulation of native collagen has the effect of limiting ageing marksGREENTECH S.A Biopôle Clermont-Limagne - 63360 Saint-Beauzire – France6TAMANOL CYTOXICITYProduct cytotoxicity has been assessed on human fibroblasts cultures established from skin biopsies,according to explants methods.Considering its lipophile character, Tamanol has first been made soluble in Ethanol then diluted in culture environment with wanted concentration.Ethanol final concentration is 1%, concentration for which no cytotoxicity has been observed.After incubation of 72 hours, concentrations inferior or equal to 100µg/ml no lead significant changes of cellular response beside MTT.This dose has been accepted as maximal dose in order to objectivize the product.PROMOTING EFFECT OF TAMANOL ON COLLAGEN SYNTHESISThe study has been carried out on human fibroblasts.Three Tamanol concentrations have been studied.Rates of Collagen Type I secreted by cells in incubation environment have been measured by ELISA method after 3 days and 6 days culture, in absence and presence of ascorbic acid.Stimulation of collagen synthesis in presence of ascorbic acidAscorbic acid is used as a point of reference in order to stimulate collagen synthesis. In fact, Vitamin C is known to entail a significant increase in collagen synthesis by fibroblasts. Vitamin C actually activates the prolyl-hydroxylase enzym and raises quantity of coding messenger ARN for protocollagens polypeptidic chains .The aim of this study is to determine the Tamanol promoting effect on the initial collagen synthesis associated with the presence of ascorbic acid.GREENTECH S.A Biopôle Clermont-Limagne - 63360 Saint-Beauzire – France7A 16% increase in collagen rate is registered to treated cells level during 6days.Stimulation of collagen synthesis in absence of ascorbic acidDuring skin ageing, changes in skin appearance, particularly its firming and elasticity are essentially due to dermic compartment changes. A global decrease in rate of collagen Type I and coding genes expression for collagen type I and III can be observed when one’s getting older.In absence of ascorbic acid, Tamanol carries out a significant stimulation of collagen Type I biosynthesis.1234Collagen stimulation (%)0.001%0.005%0.01%TAMANOL Concentration Collagen synthesis stimulation by Tamanol is carried out according to concentration. After 3 days of incubation, it is 31%.After 6 incubation days, stimulation is 37%.The Tamanol ability to stimulate collagen synthesis, sets this active as a dermic regenerating from which exceptional properties can be put forward in the field of skin firming and healing.GREENTECH S.A Biopôle Clermont-Limagne - 63360 Saint-Beauzire – France8HEALING EFFECT OF TAMANOLThe main function of fibroblasts is to synthetize and put down around him the various extracellular constituants as collagen, structural and adhesive glycoproteins, elastin, proteoglycan as well as their degradation enzyms.The fibroblasts’ mobility and adhesion properties result from interaction between internal - actine or myosine cytoskeleton which can be found in cells - membranar receptors and extracellular proteins.In vitro fibroblasts can carry on a traction on their suport and form for instance a collagen gel which is used in equivalent-dermis rebuilding.This system of living artificial dermis (collagen lattice or equivalent dermis) enables to study the product action in physiological environment reproducing in vitro the existing matrix-cells interactions in vivo.Within collagen matrix, fibroblasts regain a differenciation close to the one they’ve got in vivo. The equivalent dermis is a selected pharmacological model for skin biology studies and particularly adapted for healing pharmacology given that this real tissue is progressively reorganizing.In a first time, Tamanol regenerating effect has been assessed by cultivated fibroblasts dynamic properties in an equivalent dermis.GREENTECH S.A Biopôle Clermont-Limagne - 63360 Saint-Beauzire – France9It appears that equivalent dermis contraction of treated batches is much more significant than the one registered on the control.123456D2D3D4These results underscore a 50% stimulation of fibroblasts contractil power in Tamanol presence.The Tamanol healing effect could be ratified thanks to its ability to raise lattice contraction inhibitiongenerated by corticoïds. In fact, hydrocortisone slows down equivalent dermis contraction.2468D2D3D4The fact of adding up Tamanol allows to raise lattice contraction inhibition generated by hydrocortisone.After 4 days of incubation, contractil power is 25% more higher on treated batches.Treatment by Tamanol enables to restore initial contractil power as in these conditions, it is equivalent to control without hydrocortisone.GREENTECH S.A Biopôle Clermont-Limagne - 63360 Saint-Beauzire – France10CONCLUSIONSThe tamanol ability to stimulate collagen synthesis appearsas a very favourable ingredient to inquired dermicregenerating effect in order to fight against ageing and to compensate decrease in collagen rate which occurs with age.Tamanol stimulates fibroblasts contractil power insuringthen a better final cohesion to equivalent dermis.The ability to oppose to direct action of corticoides on fibroblasts retraction confers on tamanu very interesting properties in healing “field” and skin tissues’ strenghtening.In this way, tamanol tackles dermic degeneration troublesobserved during ageing and constitutes an efficientprevention mean to protect skin youth reserve.GREENTECH S.A Biopôle Clermont-Limagne - 63360 Saint-Beauzire – France11COSMETIC APPLICATIONSREGENERATING CREAMSANTI-AGEING CREAMSSUN CREAMSAFTER SUN CREAMSANTI-STRETCH MARKSGREENTECH S.A Biopôle Clermont-Limagne - 63360 Saint-Beauzire – France12TOXICOLOGICAL STUDIES Ocular irritation assessment by HET-CAM methodTamanol ocular irritation is assessed on four chicken embryonic eggs of about 60 g incubated to 37% during a period of 10 days’period.Oil is deposited to chorio-allantoïdian membrane surface. After a contact of 20 seconds, membrane is rinsed with 5ml Nacl 0,9%.The appearance of injection, haemorrhage and coagulation phenomenon is sought during 5 minutes.TESTSConcentration Score/ eggs Average score Classification TAMANOL0% in parafine 0/0/0/00.0Practically non irritant Betaïne sulfo Lauryl 3%17/15/17/1716.0irritantTamanol can be considered as practically non irritant at ocular level.Skin irritation assessment by MTT methodSkin explants from healthy experimental subject are obtained during plastic surgery operation. After adipose tissue elimination, skin samples are put in survival in a suitable culture environment.Products to be tested are deposited to skin explant surface and left in contact during 20 hours.A reference tensio-active (the sodium sulfate lauryl at 20 mg/ml is also tested in parallel as cytotoxic control.A cellular viability test is carried out in order to determine the assessed product toxicity according to a viability pourcentage.TESTConcentration Average D.O.Viability percentage de in relation to control MTT classification Classification from histological analysis TAMANOL10%0.59285Non irritant Non irritantLauryl sulfate 20mg/ml 0,12519rritant 0,697100Tamanol can be classified as a non irritant product at skin level.。

Discovery Labware, Inc ., Two Oak Park, Bedford, MA 01730, Tel: 1.978.442.2200 (U.S.)**************************/lifesciencesFor Research Use Only. Not for use in diagnostic or therapeutic procedures.For a listing of trademarks, visit /lifesciences/trademarks© 2013 Corning IncorporatedGUIDELINES FOR USEPRODUCT: Corning ® Matrigel ® hESC-qualified Matrix, 5 mL vialCATALOG NUMBER: 354277BACKGROUND: Basement membranes are continuous sheets of specialized extracellular matrix that arefound at the dermal-epidermal junction, at the base of all lumen-lining epitheliathroughout the digestive, respiratory, reproductive and urinary tracts and that underlieparenchyma of endocrine and exocrine glands.Corning Matrigel hESC-qualified Matrix is a soluble basement membrane extract ofthe Engelbreth-Holm-Swarm (EHS) tumor that gels at room temperature to form agenuine reconstituted basement membrane.1 The major components of CorningMatrigel hESC-qualified Matrix are laminin, collagen IV, entactin and heparan sulfateproteoglycan.2-3 Growth factors, collagenases, plasminogen activators and otherundefined components have also been reported in Corning Matrigel hESC-qualifiedMatrix.4-5STEM CELLS: Historically, human embryonic stem (hES) cell derivation and culturing techniquesutilized serum and/or mouse embryonic fibroblast (MEF) feeder layers.6 An idealenvironment for hES cell research consists of both a cell culture surface specificallyqualified for hES cells, and a serum-free, defined medium. Corning Matrigel hESC-qualified Matrix and STEMCELL Technologies’ mTeSR™1 (developed underlicense from the WiCell Research Institute),7 a high quality surface and mediumcombination, create the first complete environment to support feeder-independentexpansion of hES cells.Corning Matrigel hESC-qualified Matrix is an optimized surface for your stem cellresearch. It has been qualified as mTeSR1-compatible by STEMCELL Technologies,eliminating the need for time-consuming screening, while providing thereproducibility and consistency essential for your hES cell research. When coupledwith a variety of culture media, Corning Matrigel hESC-qualified Matrix has beenwidely accepted as an alternative substrate for the culture of hES cells as well ashuman induced pluripotent stem (iPS) cells.8-11 The mTeSR1 formulation is definedand serum-free, and has been designed to maintain and expand hES cells in anundifferentiated state when used with Corning Matrigel hESC-qualified Matrix as asubstrate. It does not require any further addition of growth factors or supplements.The mTeSR1 formulation and Corning Matrigel hESC-qualified Matrix have beenshown to be a successful combination for culturing different hES cell lines for up to20 passages.12 Cells maintained in mTeSR1 express high levels of pluripotencymarkers such as Oct-3/4 and SSEA-3, and pluripotency of cells maintained inmTeSR1 has also been demonstrated by the ability of these cells to differentiate intoall three germ layers in the teratoma assay.7,13SOURCE:Engelbreth-Holm-Swarm (EHS) Mouse TumorDiscovery Labware, Inc ., Two Oak Park, Bedford, MA 01730, Tel: 1.978.442.2200 (U.S.)**************************/lifesciencesFor Research Use Only. Not for use in diagnostic or therapeutic procedures.For a listing of trademarks, visit /lifesciences/trademarks© 2013 Corning IncorporatedFORMULATION:Dulbecco's Modified Eagle's Medium with 50 µg/mL gentamycin.Corning ® Matrigel ® hESC-qualified Matrix is compatible with all culture media.STORAGE :Stable when stored at -20°C. Store aliquots in either the -20°C or -70°C freezer until ready for use. Freeze thaws should be minimized by aliquotting into one time use aliquots. DO NOT STORE IN FROST-FREE FREEZER. KEEP FROZEN .EXPIRATION DATE :The expiration date for Corning Matrigel hESC-qualified Matrix is lot specific and can be found on the product Certificate of Analysis.CAUTION: It is extremely important that Corning Matrigel hESC-qualified Matrix and allcultureware or media coming in contact with Corning Matrigel hESC-qualifiedMatrix should be pre-chilled/ice-cold since Corning Matrigel hESC-qualified Matrixwill start to gel above 10°C.RECONSTITUTION AND USE: Color variations may occur in frozen or thawed vials of Corning Matrigel hESC-qualified Matrix, ranging from straw yellow to dark red due to the interaction of carbondioxide with the bicarbonate buffer and phenol red. This is normal, does not affectproduct efficacy, and will disappear upon equilibration with 5% CO 2.Thaw Corning Matrigel hESC-qualified Matrix by submerging the vial in ice in a 4°Crefrigerator, in the back, overnight. Once Corning Matrigel hESC-qualified Matrix isthawed swirl vial to ensure that material is evenly dispersed. Spray top of vial with 70%ethanol and air dry. Keep product on ice and handle using sterile technique. Dispensematerial into appropriate aliquots in pre-cooled tubes, switching tips wheneverCorning Matrigel hESC-qualified Matrix is clogging the tip and/or causing the pipet tomeasure inaccurately and refreeze immediately. Gelled Corning Matrigel hESC-qualified Matrix may be re-liquified if placed at 4°C in ice for 24-48 hours.DILUTION FACTOR: The dilution is calculated for each lot based on the protein concentration. To use withSTEMCELL Technologies’ mTeSR™1 medium, prepare aliquots according to thedilution factor provided on the Certificate of Analysis. The volume of the aliquots istypically between 270-350 µL.To Use: Add one aliquot of Corning Matrigel hESC-qualified Matrix to 25 mL ofDMEM/F-12 to coat four 6-well plates (1 mL/well) or three 100 mm dishes (8mL/dish). Incubate the cultureware at room temperature (15-25ºC) for at least 1 hourbefore use. Aspirate the remaining liquid from cultureware just before use. Ensurethat the tip of the pipet does not scratch the coated surface. Plates/dishes are nowready to use.NOTE: For more details on specific applications of Corning Matrigel matrix visit support page at/lifesciences for technical bulletins/application notes, protocols, and frequently asked questions.Discovery Labware, Inc ., Two Oak Park, Bedford, MA 01730, Tel: 1.978.442.2200 (U.S.)**************************/lifesciencesFor Research Use Only. Not for use in diagnostic or therapeutic procedures.For a listing of trademarks, visit /lifesciences/trademarks© 2013 Corning IncorporatedREFERENCES: 1. Kleinman HK, et al, Basement membrane complexes with biological activity, Biochemistry , 25:312 (1986).2. Kleinman HK, et al, Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan fromthe EHS sarcoma, Biochemistry , 21:6188 (1982).3. Bissell DM, et al, Support of cultured hepatocytes by a laminin-rich gel. Evidence for a functionally significantsubendothelial matrix in normal rat liver, J Clin Invest , 79(3):801 (1987).4. Vukicevic S, et al, Identification of multiple active growth factors in basement membrane Matrigel suggests caution ininterpretation of cellular activity related to extracellular matrix components, Exp Cell Res , 202:1 (1992).5. McGuire PG, and Seeds NW, The interaction of plasminogen activator with a reconstituted basement membrane matrix andextracellular macromolecules produced by cultured epithelial cells, J Cell Biochem , 40:215 (1989).6. Thomson JA, et al, Embryonic stem cell lines derived from human blastocysts, Science , 282:1145 (1998).7. Ludwig TE, et al, Feeder-independent culture of human embryonic stem cells, Nat Methods , 3(8):637 (2006).8. Xu C, et al, Feeder-free growth of undifferentiated human embryonic stem cells, Nat Biotechnol , 19:971 (2001).9. Xu C, et al, Immortalized fibroblast-like cells derived from human embryonic stem cells support undifferentiated cellgrowth, Stem Cell , 22:972 (2004).10. Drukker M, et al, Isolation of primitive endoderm, mesoderm, vascular endothelial and trophoblast progenitors from humanpluripotent stem cells, Nat Biotechnol ., 30(6):531 (2012).11. Hammerick, KE, et al, Elastic properties of induced pluripotent stem cells, Tissue Eng Part A , 17(3-4):495 (2011).12. Ludwig TE, et al, Derivation of human embryonic stem cells in defined conditions, Nat Biotechnol , 24:185 (2006).13. Amit M, et al, Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential forprolonged periods of culture, Dev Biol , 227:271 (2000).California Proposition 65 NoticeWARNING: This product contains a chemical known to the state of California to cause cancer.Component: ChloroformNOTE: Human embryonic stem cell research may be restricted in your national jurisdiction. Prior to the use of this product for hESC research, please consult your applicable laws regarding such activities.RELATED PRODUCT: mTeSR™1 Maintenance Medium for Human Embryonic Stem Cells 500 mL (1 kit) Cat. No. 05850. Please visit for more information.STEMCELL Technologies, Inc.tel:800.667.0322,fax:800.567.2899,e-mail:*****************,mTeSR is a trademark of WiCell Research Institute.__________________________________________To place an order in the U.S., contact Customer Service at:tel:800.492.1110,fax:978.442.2476;email:***********************.For technical assistance, contact Technical Support at:tel:800.492.1110,fax:978.442.2476;email:***********************.Outside the U.S., contact your local distributor or visit /lifesciences to locate your nearest Corning office.。

Table of ContentsIntroduction . . . . . . . . . . . . . . . . . . . . . . . . . . . .1 Selecting the Right T ranswell Membraneand Pore Size . . . . . . . . . . . . . . . . . . . . . . . . . .2 Selecting the Right T ranswell System . . . . . .4Using Transwell Permeable Supports . . . . . .5 Helpful Hints . . . . . . . . . . . . . . . . . . . . . . . . . .5 General Directions for Use . . . . . . . . . . . . . . .6Technical Assistance . . . . . . . . . . . . . . . . . . . . .7Ordering Information . . . . . . . . . . . . . . . . . . . .8 Polyester (PET) T ranswell Inserts . . . . . . . . .8 Collagen-Coated T ranswell-COL Inserts . . .8 Polycarbonate T ranswell Inserts . . . . . . . . . .9 Snapwell™Inserts . . . . . . . . . . . . . . . . . . . . . .9 HTS T ranswell-24 Systems . . . . . . . . . . . . . .10 HTS T ranswell-96 Systems . . . . . . . . . . . . . .11 6, 12, and 24 Well Cell Culture Plates . . . . .12IntroductionCell and tissue culture techniques are becoming increasingly important for basic and applied life science research. The development of new culture vessels and cell attachment substrates is currently being driven by the need to produce an environ-ment that resembles the in vivo state as closely as possible to enable the growth of specialized cell types. Consequently, using permeable supports with microporous membranes have become a standard method for culturing these cells. These permeable supports have allowed significant improvements in culturing polarized cells since these permeable supports permit cells to uptake and secrete molecules on both their basal and apical surfaces and thereby carry out metabolic activities in a more natural fashion.Membrane filters have been used as cell growth substrates since the transfilter metanephric induc-tion studies of Grobstein (Nature, 172:860-872; 1953). Adapted over the years to a variety of cell types and numerous applications, permeable sup-ports treated for cell growth are now recognized as providing significant advantages over solid, impermeable cell growth substrates. For epithelial and other cell types, the use of permeable supportsTranswell®Permeable SupportsSelection and Use GuideLifeSciencesin vitro allows cells to be grown and studied in a polarized state under more natural conditions. Cellular differentia-tion can also proceed to higher levels resulting in cells that morphologically |and functionally better represent their in vitro counterparts.Cellular functions such as transport, adsorp-tion and secretion can also be studied since cells grown on permeable supports provide convenient, independent access to apical and basolateral plasma mem-brane domains. The use of permeable support systems for cell culture has proven to be an invaluable tool in the cell biology laboratory. Selecting the Right Transwell ®Membrane and Pore Size T ranswell permeable supports are avail-able in three membrane materials: poly-carbonate (PC), polyester (PET), and collagen-coated polytetrafluoroethylene (PTFE). See T able 1 for additional infor-mation on these membrane characteristics. ◗Polyester T ranswell-Clear inserts fea-ture a microscopically transparent poly-ester membrane that is tissue culture treated for optimal cell attachment and growth. T ranswell-Clear inserts provide better cell visibility under phase con-trast microscopy and allow assessment of cell viability and monolayer forma-tion. ◗Polycarbonate T ranswell inserts are available in a variety of pore sizes rang-ing from 0.1 µm to 12.0 µm. Most are treated for optimal cell attachment.◗T ranswell-COL inserts have a transpar-ent (when wet), collagen-treated PTFE membrane that promotes cell attach-ment and spreading and allow cells to be visualized during culture. The T ranswell-COL membrane has an equimolar mixture of types I and III collagen derived from bovine placentas.Unlike traditional coating techniques that result in occluding film layers,Corning’s proprietary coating process results in a biologically stable collagen that covers every fibril of the filter matrix, thereby retaining the porosity of the membrane.Selecting Pore Sizes Selecting the correct pore size for experiments using T ranswell permeable supports is also very important. T able 2reviews common permeable support applications along with recommended pore sizes. The smallest pore size T ranswell membranes (0.1 µm) are pri-marily used in drug transport studies.Cell invasion, chemotaxis and motility studies are usually done in T ranswell membranes with 3.0 µm or larger pores.The ability of cells to migrate through pores of a membrane is dependent on the cell line used and the culture conditions,as well as the pore size. Cell migration will not occur with pores smaller than 3.0 µm. For critical experiments, Corning suggests experimenting with appropriate controls with a range of pore sizes to determine which size works best with your cell cultures and your specific application. As an alternative, followSEM of the surface of a0.4 µm pore polycarbonatemembraneSEM of a PTFE membraneshowing its pore structureThe polyester Transwell-Clearinserts in a 6 well plate showthe clarity of the membraneThese 12 mm diameterTranswell inserts have apolycarbonate membraneMD).recommendations in published scientific literature. For additional application and use information, please refer to the T ranswell ®Bibliography on the T echnical Information section of the Corning Life Sciences web site that lists over 800literature references using T ranswell permeable supports. Chemical Compatibility All of the T ranswell membranes are compatible with histological fixatives including methanol and formaldehyde.The polyester T ranswell membranes have the best overall chemical resistance.These membranes (but not the poly-styrene housings) are compatible with many alcohols, amines, esters, ethers,ketones, oils and some solvents including many halogenated hydrocarbons and DMSO, but are not recommended for use with strong acids and bases.Pore Density Of the three types of T ranswell mem-branes, only the collagen-coated PTFE membrane does not have a defined pore density because it is a tortuous path mem-brane. The two membranes with nominally defined pore densities are polycarbonate and polyester. The polyester T ranswell membranes do not have as high a pore density as the polycarbonate T ranswell membranes but have better optical clarity as a result. The nominal pore densities for Corning ®polycarbonate and poly-ester membranes are given in T able 3.Selecting the Right Transwell System T ranswell permeable support units come in three basic designs:◗T raditional T ranswell plate inserts that are used individually in 6, 12 and 24 well plates or 100 mm dishes;◗HTS T ranswell-24 and HTS T ranswell-96 insert systems that are mounted in special holders to allow for automation and ease of handling;◗Snapwell ™inserts for use in diffusion or Ussing chambers.More detailed information on each of these products is found below and in the ordering section.24.5 mm Transwell-COL insert being placed into a 6 well microplate.75 mm Transwell insert and 100 mm dish bottomTraditional Transwell ®Permeable Supports T ranswell inserts are available in four membrane diameters: 6.5 mm (24 well plate), 12 mm (12 well plate), 24 mm (6 well plate) and 75 mm (100 mm dish)formats. See T able 4 for cell growth areas provided by these sizes. Several membrane types and a large selection of pore sizes are available with each of these units. The patented self-centering design prevents medium from wicking between the sides of the insert and the well wall. The hanging design keeps the T ranswell membrane about a millimeter off the bottom of the well.This prevents co-cultured cell monolay-ers in the bottom of the well from being scratched or disturbed when the insert is moved. Windows or openings in the sides of the T ranswell insert allow access to the bottom compartment.HTS Transwell Systems The HTS T ranswell systems are arrays of either 24 or 96 individual T ranswell inserts connected by a rigid, robotics-friendly holder that enables all of the T ranswell-24 or T ranswell-96 inserts to be handled as a single unit. This makes HTS T ranswell systems ideal tools for running automated, high throughput drug transport (Caco-2 cells) or cell toxicity studies. The HTS T ranswell-96 culture system consists of 4 parts: a 96 well insert sup-port plate with a choice of either 1.0 µm pore polyester or 0.4 µm pore polycar-bonate membranes; a Reservoir Plate with a removable media stabilizer for feeding cultures; a 96 well Receiver Plate for use in assays; and two lids to minimize evaporation and protect against contami-nation. Each well insert has a 0.143 cm 2membrane area and large apical and basolateral access ports for feeding and sampling.The HTS T ranswell-24 culture system is available with a treated polycarbonate membrane in either 0.4 µm or 3.0 µm pore sizes and provides an excellent sub-strate for cell attachment, growth, and differentiation. An open culture reservoir plate is used to reduce liquid handling during cell feeding (medium can be exchanged all at once). Once the cell layers are confluent, the HTS T ranswell-24 insert is transferred to a standard Corning ®24 well microplate for running experiments. Snapwell ™Inserts The Snapwell insert is a modified T ranswell culture insert that contains a 12 mm diameter tissue culture treated polycarbonate or clear polyester mem-brane supported by a detachable ring.These inserts are primarily used for transport and electrophysiological studies.Once cells are grown to confluence, this ring-supported membrane can be placed into either vertical or horizontal diffusion or Using chambers. Chambers are avail-able from Harvard Apparatus: ing Transwell Permeable Supports Helpful Hints 1.Cell morphology and cell densities on permeable supports are influenced by filter pore rger pore sizes may permit some cell types to migrate through the pores on the permeable support.3.Cells grown on permeable supports are often sensitive to initial seeding densi-ty for good cell attachment. On first use, try bracketing a range of seeding densities for optimum growth.4.Cell attachment and spreading may be improved by preincubating permeable supports in medium prior to seeding.5.Cells requiring extracellular matrix coatings on plastic substrates will also require them on permeable supports.6.The T ranswell-Clear insert contains a transparent tissue culture treated polyester membrane that allows easy viewing of cells using phase contrast microscopy.The HTS-96 System is ideal for high throughput transportstudies.HTS Transwell Systems are designed for use withrobotics.Snapwell inserts are designed for use with diffusion or Ussing chambers.7.The T ranswell ®-COL insert contains a PTFE membrane that has been treated with an equimolar mixture of types I and III bovine placental collagens.This results in a biologically stabilized collagen matrix covering the fibrils of the filter membrane. These T ranswell inserts are excellent for the growth of cells requiring a biological coating.General Directions for Use 1.T ranswell inserts are used by first adding medium to the multiple well plate well, then adding the T ranswell insert, and then adding the medium and cells to the inside compartment of the T ranswell insert. Recommended medium volumes are shown in T able 5. 2.An initial equilibrium period may be used to improve cell attachment by adding medium to the multiple well plate well and then to the T ranswell insert. The plate should then be incu-bated for at least one hour or even overnight at the same temperature that will be used to grow the cells. The cells are then added in fresh medium to the T ranswell insert and returned to the incubator.3.The medium level can be checked periodically and fresh medium added as required.4.T ranswell inserts have three openings for standard pipette tips to allow sam-ples to be added or removed from the lower compartment.Add the medium to the culture plate first,then add the medium and cells tothe Transwell insert.The three side wall openings for pipette tip access can be seen in this 24 mm poly-carbonate membrane Transwell insert.The porous bottom of the insert provides independent access to both sides of a cell monolayer giving researchers a versatile tool to study cell transport and other metabolic activities in vitro.5.Cell monolayers may be fixed and stained while in the T ranswell® insert using standard cytological techniques.Avoid using solvents that dissolve poly-styrene or the polycarbonate or poly-ester membrane materials. Processing steps may be carried out by sequential-ly moving the T ranswell insert through a series of multiple well plate wells containing the appropriate reagents.Protocols for fixing and staining T ranswell inserts are available on the Corning Life Sciences web site.6.If it is necessary to remove cells from T ranswell membranes, we recommend rinsing both the T ranswell insert and the plate well. Then the dissociating solution should be added to both the well and the T ranswell insert and incu-bated until the cells begin to come off.A protocol, Trypsinization Procedure for Transwell ®Inserts , for removing cells from T ranswell inserts is available in the technical section of the Corning Life Sciences web site.7. Corning recommends using a Micromatic 8-channel Aspirator (Corning Cat. No. 3389) for removing medium from HTS Transwell-96systems. These aspirators are designed to remove medium and solutions from the upper wells without damaging the sensitive cell monolayers.8.The polycarbonate or polyester mem-brane with the fixed and stained cells attached may be removed from the T ranswell insert by carefully cutting around the membrane edges with a scalpel.9.The collagen-coated PTFE membrane is fragile and requires careful handling during removal. A wetted cellulosic membrane filter should be placed in direct contact with the underside of the T ranswell insert membrane before it is cut out with a scalpel. The wetted,more rigid, cellulosic filter will serve as a support for the collagen-coated membrane.Technical Assistance For additional product or technical infor-mation, please visit /lifesciences or call 1.800.492.1110.Customers outside the United States,please call at 1.978.635.2200.The distance between the tips of the Micromatic ™8-Channel Aspirator (Corning Cat.No.3389) and the membrane layer of the HTS Transwell-96 insert has been optimized to prevent damage to the cell monolayerduring medium removal.Fixed and crystal violet stained CHO-K1 cells on a 3 µm PET membraneOrdering Information Polyester (PET) Membrane Transwell ®-Clear Inserts T ranswell-Clear inserts feature a thin, microscopically transparent polyester membrane that is tissue culture treated for optimal cell attachment and growth. T ranswell-Clear inserts provide excellent cell visibility under phase contrast microscopy and allow assess-ment of cell viability and monolayer formation. T ranswell-Clear inserts are available sterile and preloaded in 6, 12 and 24 multiple well plates. All plates come with lids.Cat. Membrane Growth Surface Membrane Inner Inserts/ No.Diameter* (mm)Area* (cm 2)Pore Size (µm)Packaging Case 3470 6.50.330.412 inserts/24 well plate 243472 6.50.33 3.012 inserts/24 well plate 24346012 1.120.412 inserts/12 well plate 24346212 1.12 3.012 inserts/12 well plate 24345024 4.670.4 6 inserts/6 well plate 24345224 4.67 3.0 6 inserts/6 well plate 24*Values are reported as nominal and may vary due to inherent variability of the manufacturing process. T o insure success, we recommend that researchers validate their methods independent from our reported values.Collagen-Coated Transwell-COL Inserts T ranswell-COL inserts have a transparent, collagen-treated PTFE membrane that promotes cell attachment and spreading and allows cells to be visualized during culture.The T ranswell-COL membrane has an equimolar mixture of types I and III collagen derived from bovine placentas. Unlike traditional coating techniques that result in occluding film layers, Corning’s proprietary coating process results in a biologically stable collagen that covers every fibril of the filter matrix, thereby retaining the porosity of the membrane. T ranswell-COL inserts are sterile and individually blister packed.Appropriate multiple well plates are included in each case. All plates come with lids.Membrane Growth Membrane Multiple Cat. Diameter* Surface Pore Size Inner Well Inserts/ No.(mm)Area* (cm 2)(µm)Packaging Plate Case 3495 6.50.330.4Individually wrapped 2-24 well 243496 6.50.33 3.0Individually wrapped 2-24 well 24349312 1.120.4Individually wrapped 2-12 well 24349412 1.12 3.0Individually wrapped 2-12 well 24349124 4.670.4Individually wrapped 4-6 well 24349224 4.67 3.0Individually wrapped 4-6 well 24*Values are reported as nominal and may vary due to inherent variability of the manufacturing process. T o insure success, we recommend that researchers validate their methods independent from our reported values.24 mm Transwell-Clear InsertPolycarbonate Membrane Transwell ®Inserts These T ranswell inserts feature a thin, translucent polycarbonate membrane available in six pore sizes ranging from 0.1 µm to 12.0 µm. All are treated for optimal cell attachment.They are supplied sterile and come preloaded in multiple well plates or dishes. The polycarbonate membrane is compatible with most organic fixatives and stains. All plates come with lids.Membrane Growth Membrane Cat.Diameter* Surface Pore Size Inner Inserts/ No.(mm)Area* (cm 2)(µm)Packaging Case 3413 6.50.330.412 inserts/24 well plate 483415 6.50.33 3.012 inserts/24 well plate 483421 6.50.33 5.012 inserts/24 well plate 483422 6.50.338.012 inserts/24 well plate 48340112 1.120.412 inserts/12 well plate 48340212 1.12 3.012 inserts/12 well plate 48340312 1.1212.012 inserts/12 well plate 48341224 4.670.4 6 inserts/6 well plate 24341424 4.67 3.0 6 inserts/6 well plate 24342824 4.678.0 6 inserts/6 well plate 24341975440.4 1 insert/100 mm dish 1234207544 3.0 1 insert/100 mm dish 12*Values are reported as nominal and may vary due to inherent variability of the manufacturing process. T o insure success, we recommend that researchers validate their methods independent from our reported values.Snapwell ™Inserts The Snapwell insert is a modified T ranswell culture insert that contains a 12 mm diameter tissue culture treated membrane supported by a detachable ring. Once cells are grown to confluence, this ring-supported membrane can be placed into either ver-tical or horizontal diffusion or Ussing chambers. Chambers are available from Harvard Apparatus: . Snapwell inserts are provided sterile and pre-loaded in 6 well plates. All plates come with lids.Membrane Growth Membrane Cat. Diameter Surface Pore Size Membrane Inner Inserts/No.(µm)*Area* (cm 2)(µm)Material Packaging Case 340712 mm 1.120.4Polycarbonate 6 inserts/6 well plate 24380112 mm 1.120.4Clear Polyester 6 inserts/6 well plate 24380212 mm 1.12 3.0Polycarbonate 6 inserts/6 well plate 24*Values are reported as nominal and may vary due to inherent variability of the manufacturing process. T o insure success, we recommend that researchers validate their methods independent from our reported values.Snapwell Inserts withpolycarbonate (lower) and polyester (upper) membranesHTS Transwell ®-24Systems The HTS T ranswell-24 System has an array of 24 wells with permeable inserts connected by a rigid, robotics-friendly tray that enables all 24 T ranswell supports to be handled as a single unit. The individually packaged product consists of two individually wrapped HTS T ranswell-24 units loaded into open reservoirs and includes two 24 well plates.The bulk packaged products consist of 12 HTS T ranswell-24 units loaded into 24 well plates only. Open reservoirs can be purchased separately.◗Choice of either 0.4 µm polyester membrane or 0.4 µm and 3.0 µm pore polycarbonate membrane ◗Cell growth area is 0.33 cm 2/well ◗Choice of either individual or bulk packaging ◗HTS T ranswell-24 Systems are all tissue culture treated and sterile Cat. Membrane Pore Size No.Description Material (µm)Qty/Cs 3396HTS T ranswell-24 System: insert tray in a PC 0.42reservoir plate with lid, 1/pack; plus a separate 24 well receiver plate with lid, 1/pack 3379HTS T ranswell-24 System: insert tray in a reservoir PET 0.42plate with lid, 1/pack; plus a separate 24 well receiver plate with lid, 1/pack 3397HTS T ranswell-24 System, Bulk Packed: PC 0.412insert trays in 24 well plates with lids, 12/pack 3378HTS T ranswell-24 System, Bulk Packed: insert trays PET 0.412in 24 well plates with lids, 12/pack 3398HTS T ranswell-24 System: insert tray in a PC 3.02reservoir plate with lid, 1/pack; plus a separate 24 well receiver plate with lid, 1/pack 3399HTS T ranswell-24 System, Bulk Packed: PC 3.012insert trays in 24 well plates with lids, 12/pack 3395HTS T ranswell-24 Reservoir (Feeder) Plate and lid, NA NA 48not treated, 12/packHTS Tranwell-24 System showing both the culture reservoir and the 24 well microplate.HTS Transwell ®-96 SystemsThe HTS T ranswell-96 System has an array of 96 wells with permeable inserts connected by a rigid, robotics-friendly tray that enables all 96 inserts to be handled as a single unit.Each HTS T ranswell-96 System includes 1 integral tray containing 96 individual inserts in a reservoir plate (no wells) with a removable media stabilizer for cell growth steps,plus a 96 well receiver plate for growth or assay steps and 2 lids.◗HTS T ranswell-96 insert membranes are all tissue culture treated and sterile ◗Choice of either a 0.4 µm polyester membrane or 0.4 µm and 3.0 µm pore poly-carbonate membranes◗Cell growth area is 0.143 cm 2/well which is 20 to 50% greater than competitive devices ◗Large apical and basolateral access ports for easier filling and sampling ◗Removable media stabilizer reduces media spills during handling◗Automation optimized design with multichannel feeder ports, improved gripping surface and optional bar coding◗Corning offers the Micromatic ™8-Channel Aspirator (Corning Cat. No. 3389) to help safely vacuum aspirate medium or buffers from the apical portion of the HTS T ranswell-96 inserts.Cat. Membrane Pore Size No.Description Material (µm)Qty/Cs3380HTS T ranswell-96 System: insert tray in aPET 1.01reservoir plate with lid 1/pack; plus a separately packed 96 well receiver plate with lid, 1/pack3392HTS T ranswell-96 System, Bulk Packed: insert trays PET 1.05in a reservoir plates with lids, 5/pack; plus separately packed 96 well receiver plates with lids, 5/pack3381HTS T ranswell-96 System: insert tray in a reservoir PC 0.41plate with lid, 1/pack; plus a separately packed 96 well receiver plate with lid, 1/pack3391HTS T ranswell-96 System, Bulk Packed: insert trays PC 0.45in reservoir plates with lids, 5/pack; plus separately packed 96 well receiver plates with lids, 5/pack 3382HTS T ranswell-96 Receiver Plate with lid, NA NA 10not treated, 10/pack3383HTS T ranswell-96 Reservoir (Feeder) Plate with NANA10removable media stabilizer and lid, not treated, 10/pack3389Micromatic 8-Channel Aspirator for NA NA 1HTS T ranswell-96 Systems, AutoclavableHTS Tranwell-96 Systemshowing the culture reservoir with removable media stabilizer (top),the 96 well insert tray (middle) and the 96 well receiver plate(bottom).Using the Micromatic8-Channel Aspirator (Corning Cat.No.3389)reduces cell death ordetachment caused by too rapid removal of solutions or media during vacuum aspiration.6,12,and 24 Well Cell Culture PlatesThese multiple well plates are treated for optimal cell attachment, sterilized by gamma radiation and are certified nonpyrogenic. All plates have a uniform footprint and a raised bead to aid in stacking. Alphanumeric labels provide individual well identification. The 6.5, 12, and 24.5 mm T ranswell inserts are designed to automatically center themselves when placed into the appropriate culture plate.Cat. Number Well GrowthNo.of Wells Diameter* (mm)Surface Area* (cm 2)Qty/PkQty/Cs3506634.89.551003516634.89.515035121222.1 3.8510035131222.1 3.815035272415.6 1.9510035132415.6 1.9150*Values are reported as nominal and may vary due to inherent variability of the manufacturing process. T o insure success, we recommend that researchers validate their methods independent from our reported values.Corning and Transwell are registered trademarks of Corning Incorporated,Corning,NY.Discovering Beyond Imagination,Flame of Discovery design,and Snapwell are trademarks of Corning Incorporated,Corning,NY.Micromatic is a registered trademark of Popper and Sons,New Hyde Park NY.Corning Incorporated,One Riverfront Plaza,Corning,NY 14831-0001© 2005 C o r n i n g I n c o r p o r a t e d P r i n t e d i n U S A 5/05 P O D C L S -C C -007W R E V 2Corning Incorporated Life Sciences45 Nagog Park Acton,MA 01720t 800.492.1110t 978.635.2200f /lifesciencesWorldwide Support OfficesA S I A Australiat 61 2-9416-0492f 61 2-9416-0493Chinat 86 21-3222-4666f 86 21-6288-1575Hong Kong t 852-2807-2723f 852-2807-2152Indiat 91 11 341 3440f 91 11 341 1520Japant 81 (0) 3-3586 1996/1997f 81 (0) 3-3586 1291/1292Koreat 82 2-796-9500f 82 2-796-9300Singapore t 65 6733-6511f 65 6861-2913Taiwant 886 2-2716-0338f 886 2-2716-0339E U R O P E Francet 0800 916 882f 0800 918 636Germanyt 0800 101 1153f 0800 101 2427The Netherlands & All OtherEuropean Countries t 31 (0) 20 659 60 51f 31 (0) 20 659 76 73United Kingdom t 0800 376 8660f 0800 279 1117L AT I N A M E R I C A Brasilt (55-11) 3089-7419f (55-11) 3167-0700Mexicot (52-81) 8158-8400f (52-81) 8313-8589For additional product or technical information, please visit /lifesciences or call 800.492.1110. Customers outside the United States, please call +1.978.635.2200or contact your local Corning sales office listed below.Corning offers a variety of multiple well plate designs and sizes.。

TISSUE ENGINEERINGVolume 10, Number 9/10, 2004©Mary Ann Liebert, Inc.Modulation of Angiogenic Potential of Collagen Matrices by Covalent Incorporation of Heparin and Loading with VascularEndothelial Growth FactorG.C.M. STEFFENS, Ph.D.,1C. YAO, M.D.,1,2P. PRÉVEL, M.D.,1,2M. MARKOWICZ, M.D.,2P. SCHENCK, Ph.D.,3E.M. NOAH, Ph.D., M.D.,2and N. PALLUA, Ph.D., M.D.2ABSTRACTOne of the prominent shortcomings of matrices for tissue engineering is their poor ability to sup-port angiogenesis. We report here on experiments to enhance the angiogenic properties of collagen matrices. Our aim is to achieve this goal by covalently incorporating heparin into collagen matri-ces and by physically immobilizing angiogenic vascular endothelial growth factor (VEGF) to the heparin. The immobilization of heparin was performed with 1-ethyl-3-(3-dimethylaminopropyl)-car-bodiimide (EDC) and N -hydroxysuccinimide (NHS). Carboxyl groups on the heparin are activated to succinimidyl esters, which react with amino functions on the collagen to zero length cross-links.This modification leads—in addition to the incorporation of heparin—to gross changes in in vitro degradation behavior and water-binding capacity. As a first approach to testing angiogenic capa-bilities, endothelial cells were exposed to nonmodified and heparinized collagen matrices. This ex-posure leads to an increase in endothelial cell proliferation. The increase can be further enhanced by loading the (heparinized) collagen matrices with VEGF. Evaluation of the angiogenic potential of heparinized matrices was further investigated by exposing them to the chorioallantoic membrane of chicken embryos and to the subcutaneous tissue of rats. Both approaches show that heparinized matrices have substantially increased angiogenic potential. In particular, the loading of heparinized matrices with VEGF invokes a further increase in angiogenic potential. It is apparent that the phys-ical binding of VEGF to heparin allows for a release that is beneficial to angiogenesis. By varying the heparin and EDC/NHS concentrations during the modification process and by varying the load-ing with VEGF, the angiogenic potential as well as the degradation behavior can be adapted to ob-tain matrices that fulfill specific angiogenic requirements in the field of tissue engineering.1502INTRODUCTIONENHANCEMENT OF THE ANGIOGENIC POTENTIALof im-plantable (bio)materials has received much atten-tion.1Because the angiogenic potential of most synthetic and natural materials is insufficient, many attempts havebeen made to enhance angiogenic potential either by changing the physicochemical parameters or by supple-mentation with angiogenic factors. Angiogenesis is the sprouting of new capillaries from preexisting vasculature and represents a complex multistep process that requires the adhesion, proliferation, and differentiation of endo-1Institute of Biochemistry 2Department of Plastic, Hand, and Burn Surgery, RWTH Aachen University, Aachen, Germany.3Dr. Suwelack Skin and Health Care, Billerbeck, Germany.thelial cells. Endothelial cell growth, proliferation, and differentiation depend on recruitment of specific integrins (a v 3) on the cell surface. These integrins must bind to their proper ligands in order to initiate distinct second-messenger pathways that finally lead to transformation into the angiogenic phenotype of endothelial cells.2In the present study, the angiogenic properties of ma-trices made of collagen are investigated. Collagen repre-sents a suitable substrate for cell attachment: it is bio-compatible and degrades into harmless products that are metabolized or excreted.3Collagen can be formed into three-dimensional matrices that are being applied as tis-sue substitutes or scaffolds for tissue regeneration.4The properties of collagen can be modified in many different ways.5,6Several authors claim that the covalent incorporation of glycosaminoglycans modifies the prop-erties of collagenous material. Thus Silver et al .7Wissink et al .,8,9and Tsai et al .10covalently linked heparin to col-lagenous films whereas Yannas and Burke 11and Pieper et al .12,13covalently incorporated chondroitin sulfate into collagen matrices. These modifications had positive ef-fects on in vivo blood compatibility and the proliferation of endothelial cells, respectively. Loading of heparinized collagen films with basic fibroblast growth factor (bFGF)in general leads to even stronger effects on the prolifer-ation of endothelial cells.14In our study we investigated the effects of the cova-lent incorporation of heparin into three-dimensional col-lagen matrices by 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide/N -hydroxysuccinimide (EDC/NHS). More-over, we report on the effects exerted by loading vascu-lar endothelial growth factor (VEGF) into these heparinized collagen matrices. Together these modifica-tions lead to a variety of collagen matrices in which the following parameters can be modulated: (1) angiogenic potential, (2) resistance to in vitro degradation with col-lagenase, and (3) moisture-binding capacity. These prop-erties may thus be modulated in order to adapt the col-lagenous matrices to specific requirements in the field of tissue engineering.MATERIALS AND METHODSCollagen matricesCollagen matrices were produced by Dr. Suwelack Skin & Health Care (Billerbeck, Germany). The matri-ces were obtained through lyophilization of collagen sus-pensions containing primarily collagen type I. The porous structure is nondirected and the pore sizes vary from 15to 25 m; the overall porosity amounts to ϳ98%.The collagen matrices were cut into cubes of either 5ϫ5ϫ5 mm (3.8–4.2 mg) or 10ϫ10ϫ10 mm (22–24 mg) and into circular specimens (diameter, 10mm; thickness, 2 and 5 mm).HEPARIN/VEGF-MODIFIED ANGIOGENIC POTENTIAL Heparin immobilizationHeparin immobilization was performed essentially as described in Hinrichs e t al .15and Wissink e t al .8Car-boxylic acid groups of heparin (Hep-COOH) were acti-vated with EDC and NHS. Heparin (sodium salt, 170 USP units/mg), EDC, and NHS were purchased from Sigma-Aldrich (St. Louis, MO). A typical modification experi-ment was performed as follows: 1 mg of heparin was ac-tivated with 1 mg of EDC per 0.6 mg of NHS in 500 L of 0.05 M 2-morpholinoethanesulfonic acid (MES) buffer (pH 5.6) for 10 min at 37°C, cubic collagen matrices (size, 5ϫ5ϫ5 mm) were immersed into the reagent so-lution, and the solution was evacuated to remove air from the matrices. After a reaction period of 4 h at 37°C, un-der gentle shaking, the heparinized collagen sponges were washed with 0.1 M Na 2HPO 4(pH 9.2) (2 h), 4 M NaCl (four times in 24 h) and deionized water (five times in 24 h). Finally, modified sponges were frozen at Ϫ80°C overnight (16 h), lyophilized, and stored at room tem-perature. Collagen matrices according to these parame-ters are designated H1E1 (1 mg of heparin and 1 mg of EDC per 0.6 mg of NHS per 500 L). The modification parameter H1E2 refers to 1 mg of heparin and 2 mg of EDC per 1.2 mg of NHS, and H0E0 refers to a collagen matrix that underwent all the procedures, except that no heparin and EDC/NHS were added.Determination of immobilized heparinThe amount of immobilized heparin was determined by toluidine blue assay.15,16Cubic collagen matrices (size, 5ϫ5ϫ5 mm; weight, 3.8–4.2 mg) were incu-bated with 5 mL of an aqueous solution of toluidine blue (0.1 M HCl, NaCl [2 mg/mL], and toluidine blue zinc chloride double salt, [0.4 mg/mL]; Sigma) for 4 h at room temperature, resulting in complexation of toluidine blue with heparin. Specimens were then washed five times with distilled water (10 mL/sample) in 24 h. Subse-quently, toluidine blue complexed to heparin was solu-bilized with 5 mL of a 1:4 (v/v) mixture of 0.1 M NaOH and ethanol. Absorbance of the resulting solution was de-termined at 530 nm after 1:5 dilution with the sodium hydroxide–ethanol solution. Standard curves were ob-tained according to the heparin solution assay protocol of Hinrichs et al .15Determination of free amino groups in heparinized collagen matricesThe residual number of free primary amino groups af-ter heparin immobilization was determined with trini-trobenzene sulfonic acid (TNBS). Collagen specimens were immersed in a mixture of 1 mL of 4 wt% NaHCO 3(pH 9) and 1 mL of 0.5% TNBS, and incubated overnight at 40°C. To this 3 mL of 6 M HCl was added and the so-lution was incubated at 60°C for another 1.5 h. After cool-1503ing to room temperature, 20 mL of anhydrous ethyl etherwas added for extraction of the excess TNBS and TNP-␣-amino acids17; this procedure was repeated at least five times. After 1:10 dilution with distilled water the ab-sorbance of the resulting solution was determined at 345nm. A control was prepared applying the same procedurewith the exception that HCl was added before the addi-tion of TNBS. The number of amino groups per 1000amino acids was obtained with the following formula: Free amino groupsϭ(absorbance at 345 nm)(0.05 L)MW/(1.46ϫ10,000 L/mol и cm)bx where MW is the molecular mass of the collagen (g/mol), 1.46ϫ10,000 L/mol иcm is the molar absorptivity of TNP-lysine, b is the cell path length (cm), and x is the sample weight (g).17In vitro degradation with collagenaseThe degree of degradation of heparinized collagensponges was determined by measuring sample weightsbefore and after degradation by collagenase fromClostridium histolyticum(type I; Worthington Biochem-icals, Lakewood, NJ). The original weights of specimens were determined after lyophilization. Modified and non-modified matrices were immersed in a solution contain-ing 40 or 200 units of collagenase in 1 mL of (PBS) buffer (pH 7.2) and incubated at 37°C under gentle shaking for the desired period of time. Degradation was stopped at specified time intervals by addition of 0.2 mL of a 0.25 M EDTA solution and the samples were cooled on ice for 10 min. Subsequently, the samples were washed with 5 mL of PBS buffer (pH 7.2, three times for 15 min each) and demineralized water (three times for 15 min each), frozen at Ϫ80°C (overnight), and lyophilized. After lyophilization, the weights of the residual samples were determined and the percentage of degradation at the spec-ified time interval t is calculated as follows: Degradation (%)ϭ(original weight Ϫ residualweight at time t)/(original weight/100) Moisture uptakeFor the analysis of moisture uptake the initial dryweights (W dry) of collagen matrix specimens (size, 5ϫ5ϫ5 mm; weight, 3.8–4.2 mg) were determined. Afterimmersion in PBS for 2 h at 37°C the weights of thewet specimens (W wet) were determined. Before theweighing process the wet matrices were brought intocontact with a piece of filter paper to remove looselybound moisture. Moisture binding was calculated ac-cording to the formulaMoisture uptake (mg/mg)ϭ(W wetϪW dry)ᎏᎏW drySTEFFENS ET AL.Exposure to endothelial cellsEndothelial cells were isolated from human umbilicalcords (human umbilical vascular endothelial cells[HUVECs] and cultivated in endothelial cell basalmedium (ECBM). For exposure experiments the secondpassage was used.In each well of a 6-well plate 100,000 HUVECs wereallowed to adhere for 1 day in 2 mL of ECBM (Cell-Systems, St. Katharinen, Germany). On day 2 collagenmatrices (cubes, 10ϫ10ϫ10 mm in size) modified ac-cording to the specified parameters were placed in thewells and the wells were filled with 2 mL of mediumwithout VEGF. Some of the matrices were loaded witha sterile solution (20 L) containing 100 ng ofrhVEGF165(R&D Systems, Minneapolis, MN). For ref-erence, one well of each plate was filled with ECBMonly. The cells were allowed to proliferate for 5 days,and on day 7 the matrices were removed and theHUVECs were trypsinized with EDTA–trypsin. Cellswere counted microscopically in a Neubauer chamber.To highlight changes in proliferation, cell numbers pres-ent on day 1 were subtracted from the observed numbersof cells.Chorioallantoic membrane assayFertilized chicken eggs were obtained from Bücher-hof (Horbach-Aachen, Germany). The chorioallantoicmembrane assay (CAM assay) was performed essen-tially as described in Zwadlo-Klarwasser e t al.18He-parinized and cross-linked collagen matrices were pre-pared according to the modification proceduresdescribed above and sterilized by immersion in 70%ethanol for 24 h. Sub-sequently they were equilibratedwith either PBS or serum-free ECBM under sterile con-ditions. In selected experiments 300 ng of rhVEGF165(R&D Systems) was loaded onto modified and non-modified matrices before exposure to chorioallantoicmembrane. Circular specimens of the various collagenmatrices (diameter, 12 mm; thickness, 2 mm) werecarefully placed on the chorioallantoic membrane andthe eggs were further incubated at 37°C for 7 days. Atthe end of this period, the chorioallantoic membraneswere either fixed in situ with 4% buffered formalin, ex-cised, and mounted on a slide, or the collagen speci-mens were dissected together with the surroundinggranulation tissue and processed for histological char-acterization.Quantitation of capillaries was carried out by countingthem at 50-fold magnification in three nonoverlappingareas. The average number of small vessels (diametersmaller than 20–40 m) in the defined areas was takenas an index for angiogenic potential after subtraction ofthe number of capillaries counted in the absence of anymatrix.1504Animal model experimentsCollagen matrices (diameter, 10 mm; thickness, 5 mm)modified according to the parameters described abovewere implanted in four dorsal subcutaneous pockets ofLewis rats, each implant 1 cm from the skin incision andwith 4 cm between them. The variously modified colla-gen matrices were implanted in animals either loadedwith 300 ng of recombinant rat VEGF 165or nonloaded.After 14 days of implantation the animals were killed andthe matrices were explanted. Explants were extensivelywashed with 1 mL of water for about 24 h and the hemeprotein content was determined spectrophotometrically atthe Soret band (absorbance, 410 nm).RESULTSIn an attempt to increase the angiogenic potential of collagen matrices by covalent incorporation of heparin and subsequent physical immobilization of VEGF, a se-ries of experiments was performed to evaluate heparin content after exposure of collagen matrices to heparin activated with 1-ethyl-3-(3-dimethylamino-propyl)-car-bodiimide (EDC)/N -hydroxysuccinimide (NHS). The ex-tent of heparin immobilization was determined with o -toluidine blue; this dye specifically binds to carboxyl groups.15After removal of excess dye by extensive wash-ing with water, the bound dye molecules were released by hydrolysis with NaOH and ethanol followed by quan-tification by absorption spectrophotometry.Figure 1 gives an overview of a number of properties of collagen matrices prepared according to a restrictedset of modification parameters (the modification param-eters are described in detail in Materials and Methods).In brief, the designation H1E0.5 refers to 1 mg of hep-HEPARIN/VEGF-MODIFIED ANGIOGENIC POTENTIAL arin and 0.5 mg of EDC (0.3 mg of NHS) being present in 500 L of the activation solution.The results of Fig. 1 show that the amount of heparinimmobilization clearly correlates with the concentrationof cross-linking reagents EDC and NHS in the solutionfor activating the carboxyl groups of heparin. The resultsobtained with the modification parameters H1E0 showthat adsorptive binding of heparin is negligible, appar-ently all physically bound heparin is removed during theextensive washing procedure. The amounts of immobi-lized heparin vary from 7 to about 40 g of heparin permilligram of collagen. It appears that with 2 mg of EDCa plateau is reached, and higher concentrations ofEDC/NHS do not lead to higher heparin-binding densi-ties.In addition to the covalent immobilization of the hep-arin to collagen, an additional cross-linking of collagen is likely to occur.14,19This additional cross-linking sub-stantially affects the resistance against degradation by collagenase.5,14We therefore subjected the same set of collagen matrices to in vitro degradation experiments.The results of these experiments are also shown in Fig.1. Whereas matrices modified at relatively low EDC con-centrations are substantially degraded, degradation per-centages with 40 units of collagenase for 2 h at 37°C vary from 60 to 30%. Matrices modified with EDC concen-trations equal to or greater than 1 mg of EDC per 500-L reaction volume clearly withstand degradation and the corresponding degradation percentages are on the or-der of 10% or less. A sharp increase in resistance againstdegradation was observed by increasing the EDC con-centration from 0.2 to 1.0%.To better understand the binding and cross-linking mechanisms we also investigated the number of available free ␣-amino groups (i.e., the number of available lysines and hydroxylysines) in the modified and non-modified1505FIG. 1.Extent of heparin immobilization and in vitro degradation as a function of increasing EDC/NHS-to-heparin weight ra-tios. Collagen matrices were modified and designated according to parameters specified in Materials and Methods. Degradation was carried out with 40 units of collagenase in 1 mL of PBS buffer for 2 h at 37°C. The columns show the mean values, and the error bars represent the corresponding standard deviations (n ϭ5).collagen matrices. Available amino groups were deter-mined with trinitrobenzenesulfonic acid (TNBS).17These results are given in Fig. 2. Within the set of modification parameters a continuous decrease of the number in free amino groups is observed, surprisingly, a sharp decrease of the number in free amino groups—as might be ex-pected from the degradation experiments—is not ob-served. The number of 35 free amino groups in non mod-ified collagen (H0E0) nicely corresponds to the number of lysines and hydroxylysines as deduced from the DNA-derived amino acid composition and amino acid analysis investigations.5The number of 21 free amino groups in the collagen matrix H1E4 demonstrates that in this case about 14 lysines and hydroxylysines per 1000 amino acids of collagen are involved in heparin binding and cross-linking.Another property of the modified collagen matrices that may be affected by heparin binding and/or additional cross-linking is the capability to take up moisture. The effects of the modification procedures on moisture up-take can also be deduced from Fig. 2: the moisture-bind-ing capacity of collagen matrices H1E2, H1E3, and H1E4 is almost twice as high as the moisture uptake of non-modified matrices. Above a concentration of 2 mg of EDC per 500-L reaction volume the moisture uptake seems to have reached a plateau.The angiogenic potential of the modified matrices was investigated by three different approaches. The first ap-proach focused on the change in proliferation rates of en-dothelial cells when they contact nonmodified and he-parinized collagen matrices. Figure 3 shows the results of a series of cell culture experiments with human um-bilical endothelial cells exposed to nonmodified (H0E0)STEFFENS ET AL. and heparinized (H1E0.2–H1E2) collagen matrices. To test the possible beneficial effect of loading VEGF onto these matrices, matrices were loaded with 50 ng of VEGF as indicated. The results clearly demonstrate that both the modification and the loading with VEGF have a sub-stantial impact on proliferation.The proliferation rate increases with increasing EDC/NHS concentrations and reaches a plateau at a con-centration of 1 mg of EDC per 500-L reaction volume. The additional effect exerted by loading with VEGF is clearly more prominent for collagen matrices modified by incorporation of heparin. The greatest effect is ob-served for H1E1 collagen matrices loaded with 50 ng of VEGF.As a second approach to evaluating the angiogenic po-tential of heparinized collagen matrices, we exposed the chorioallantoic membrane of chicken embryo18,20to non-modified and modified collagen matrices. Angiogenic potential was deduced from the density of microvessels (number per area) induced by the nonmodified and mod-ified collagen matrices. Microvessel densities already ob-served in the absence of any collagen matrix (control) were subtracted from the number of capillaries induced in the presence of the modified matrices. Figure 4 shows the number of observed capillaries and the increase in capillary density. The induction of angiogenesis increases with larger EDC-to-heparin ratios.In another set of experiments nonmodified (H0E0) and heparinized (H1E1) matrices were loaded with VEGF be-fore being exposed to the chorioallantoic membrane. Fig-ure 5 shows the observed number of capillaries and the calculated increases induced by loading H0E0 and H1E1 matrices with VEGF.1506FIG. 2.Free amino groups per 1000 amino acids and moisture uptake as a function of increasing EDC/NHS-to-heparin weight ratios. Collagen matrices were modified and designated according to parameters specified in Materials and Methods. Free amino groups were determined with trinitrobenzenesulfonic acid (TNBS). Columns show the mean values, and error bars represent the corresponding standard deviations (nϭ5).The effects on angiogenic potential were also investi-gated in animal model experiments with rats. Nonmodi-fied and heparinized specimens were either loaded with 300 ng of VEGF or nonloaded and subcutaneously im-planted in pockets prepared on the back of Lewis rats.The specimens were explanted after 14 days and evalu-ated for their heme protein contents. These experiments were restricted to nonmodified (H0E0) and collagen ma-trices modified according to the parameters H1E1.Figure 6 shows the hemoglobin contents of the corre-sponding explants. Heparinized matrices demonstrated substantially increased vascularization, which could beHEPARIN/VEGF-MODIFIED ANGIOGENIC POTENTIALfurther enhanced by loading the heparinized matrices with VEGF. In the case of nonmodified collagen matrix the additional beneficial effect of VEGF loading remains relatively small.DISCUSSIONThe covalent incorporation of heparin into collagen matrices has been investigated in order to develop colla-gen matrices with enhanced angiogenic potential. Figures 1 and 2 compile a series of biochemical and biophysical1507FIG. 3.Induction of proliferation of human umbilical vein endothelial cells (HUVECs) by exposure to nonmodified (H0E0)and modified (H1E0.2–H1E2) collagen matrices. Matrices were either nonloaded or loaded with 50 ng of rhVEGF 165. The ex-periments were carried out as described in Materials and Methods. The increase in the number of cells was obtained by sub-tracting the number of cells at the start of the experiment (100,000). Columns show the mean values, and error bars represent the corresponding standard deviations (n ϭ3).FIG. 4.Angiogenic effect exerted by implantation of collagen matrices into the chorioallantoic membrane of chicken embryos.Collagen matrices were modified and designated according to parameters specified in Materials and Methods. Capillaries were counted as described in Materials and Methods. The increase in number of capillaries was obtained by subtracting the number of capillaries in the control experiments. Columns show the mean values, and error bars represent the corresponding standard deviations (n ϭ5).characterizations of modified matrices. The immobiliza-tion of heparin almost linearly increases with the con-centration of EDC/NHS in the reaction mixture. Values vary from 10 to about 40 g/mg of collagen and appear to reach a maximum at concentrations in excess of 1 mg of EDC per 500-L reaction volume. Resistance to in vitro degradation with collagenase sharply increases at EDC concentrations in excess of 0.2 mg of EDC per 500-L reaction volume. We explain this observation as fol-lows: at relatively low concentrations of heparin, most of the EDC/NHS is used for the activation of carboxyl groups on the heparin molecules, and thus fewer EDC/NHS molecules are available for the additional cross-linking of collagen fibrils. When the EDC/NHS concentration exceeds 0.2 mg/500-L reaction volume,more cross-linking molecules remain available for addi-tional cross-linking of collagen fibrils. This process leads to a sharp decrease in the degradation percentages. In-creasing heparin-to-EDC ratios lead to both lower hep-arin incorporation and lower additional cross-linking;thus H2E1 incorporates less heparin and is degraded more rapidly (data not shown).The number of free amino groups consistently decreases with increasing EDC/NHS concentrations, the largest re-duction of free amino groups being observed on increasing the EDC/NHS concentration from 3 to 4 mg/500-L reac-tion mixture. This may be explained by the fact that in this case only a small amount of EDC/NHS is consumed for the activation of heparin, and thus all the extra EDC/NHS molecules are available for cross-linking of collagen.The evaluation of angiogenic potential by three indepen-dent approaches shows that the heparinized matrices induce greater angiogenic effects. The exposure of HUVECs to he-parinized matrices clearly leads to higher proliferation rates,and loading of these matrices with VEGF further increases the proliferation rates. The most prominent effect was ob-served with collagen matrices modified according to the pa-rameters H1E1 and loaded with VEGF (Fig. 3).Similar effects were observed by exposing chicken em-bryo chorioallantoic membrane to heparinized collagen matrices. Again H1E1 collagen matrices loaded with VEGF induced significantly greater angiogenic effects (Figs. 4 and 5).Subcutaneous implantation of nonmodified and he-1508FIG. 5.Angiogenic effects of loading 100 ng of rhVEGF 165into nonmodified (H0E0) and heparinized (H1E1) collagen ma-trices. Angiogenic effects were evaluated by implanting collagen matrices—either loaded or nonloaded—into chicken embryo chorioallantoic membranes. Capillaries were counted as described in Materials and Methods. The increase in the number of cap-illaries was obtained by subtracting the number of capillaries in the control experiments. Columns show the mean values, and error bars represent the corresponding standard deviations (n ϭ5).FIG. 6.Angiogenic potential of modified and nonmodified collagen matrices as evaluated by subcutaneous implantation in rats. Collagen matrices were modified and designated accord-ing to parameters specified in Materials and Methods. Matrices were either loaded with 300 ng of rhVEGF 165or nonloaded.Angiogenesis was evaluated by determining the hemoglobin content of matrices, which were explanted after 14 days.Columns show the mean values, and error bars represent the corresponding standard deviations (n ϭ2).parinized matrices in animal model experiments allowed for alternative in vivo evaluation with closer proximity to the final clinical indication. Angiogenic potential was evaluated by determining the hemoglobin absorbance of wash solutions of each specimen, explanted after 14 days.As evaluated by this approach, heparinized matrix H1E1loaded with VEGF again showed the greatest increases in angiogenic effect.Because the applied approaches do not allow state-ments on the quality of the vasculature within the im-plants we are currently evaluating explants from the in vivo experiments by immunohistochemistry. Further-more, it would be worth investigating to what extent and by which parameters the additional cross-linking is in-fluencing the angiogenic outcome of the modifications described in this article.ACKNOWLEDGMENTSThis work was supported by grant 0312692 from the Bundesministerium für Bildung und Forschung (Berlin,Germany) to Dr. Suwelack Skin and Health Care AG (Billerbeck, Germany) and by grant TV B 47 from the Interdisciplinary Centre for Clinical Research Biomat of the Medical Faculty of RWTH Aachen University (Aachen, Germany).REFERENCES1.Nomi, M., Atala, A., Coppi, P.D., and Soker, S. Principles of neovascularization for tissue engineering. Mol. Aspects Med. 23,463, 2002.2.Hall, H., Baechi, T., and Hubbell, J.A. Molecular proper-ties of fibrin-based matrices for promotion of angiogene-sis in vitro . Microvasc. Res. 62,315, 2001.3.Friess, W. Collagen: Biomaterial for drug delivery. Eur. J.Pharm. Biopharm. 45,113, 1998.4.Yannas, I.V., Burke, J.F., Orgill, D.P., and Skrabut, E.M.Wound tissue can utilize a polymeric template to synthe-size a functional extension of skin. Science 215,174, 1982.5.Zeeman, R., Cross-linking of collagen-based materials [Ph.D. thesis]. University of Twente, Enschede, The Netherlands, 1998.6.Zeeman, R., Dijkstra, P.J., van Wachem, P.B., van Luyn,M.J., Hendriks, M., Cahalan, P.T., and Feijen, J. Succes-sive epoxy and carbodiimide cross-linking of dermal sheep collagen. Biomaterials 20,921, 1999.7.Silver, F.H., Yannas, I.V., and Salzman, E.W. Glycosami-noglycan inhibition of collagen induced platelet aggrega-tion. Thromb. Res. 13,267, 1978.8.Wissink, M.J., Beernink, R., Pieper, J.S., Poot, A.A., En-gbers, G.H., Beugeling, T., van Aken, W.G., and Feijen, J.Immobilization of heparin to EDC/NHS-crosslinked colla-gen: Characterization and in vitro evaluation. Biomaterials 22,151, 2001.9.Wissink, M.J., Beernink, R., Poot, A.A., Engbers, G.H.,Beugeling, T., van Aken, W.G., and Feijen, J. ImprovedHEPARIN/VEGF-MODIFIED ANGIOGENIC POTENTIAL endothelialization of vascular grafts by local release of growth factor from heparinized collagen matrices. J. Con-trol. Release 64,103, 2000.10.Tsai, C.C., Chang, Y., Sung, H.W., Hsu, J.C., and Chen,C.N. Effects of heparin immobilization on the surface char-acteristics of a biological tissue fixed with a naturally oc-curring crosslinking agent (genipin): An in vitro study. Bio-materials 22,523, 2001.11.Yannas, I.V., and Burke, J.F. Design of an artificial skin. I.Basic design principles. J. Biomed. Mater. Res. 14,65, 1980.12.Pieper, J .S., van Wachem, P.B., van Luyn, M.J.A.,Brouwer, L.A., Hafmans, T., Veerkamp, J.H., and van Kup-pevelt, T.H. Attachment of glycosaminoglycans to col-lagenous matrices modulates the tissue response in rats.Biomaterials 21,1689, 2000.13.Pieper, J.S., Hafmans, T., Veerkamp, J.H., and van Kup-pevelt, T.H. Development of tailor-made collagen–glycos-aminoglycan matrices: EDC/NHS crosslinking, and ultra-structural aspects. Biomaterials 21,581, 2000.14.Wissink, M.J., van Luyn, M.J., Beernink, R., Dijk, F., Poot,A.A., Engbers, G.H., Beugeling, T., van Aken, W.G., and Feijen, J. Endothelial cell seeding on crosslinked collagen:Effects of crosslinking on endothelial cell proliferation and functional parameters. Thromb. Haemost. 84,325, 2000.15.Hinrichs, W.L.J., ten Hoopen, H.W.M., Wissink, M.J.B.,Engbers, G.H.M., and Feijen, J. Design of a new type of coating for the controlled release of heparin. J. Control. Re-lease 45,163, 1997.16.Sano, S., Kato, K., and Ikada, Y. Introduction of functional groups onto the surface of polyethylene for protein immo-bilization. Biomaterials 14,817, 1993.17.Bubnis, W.A., and Ofner, C.M., III. The determination of ⑀-amino groups in soluble and poorly soluble proteinaceous materials by a spectrophotometric method using trini-trobenzenesulfonic acid. Anal. Biochem. 207,129, 1992.18.Zwadlo-Klarwasser, G., Görlitz, K., Hafemann, B., Klee,D., and Klosterhalfen, B. The chorioallantoic membrane of the chick embryo as a simple model for the study of the angiogenic and inflammatory response to biomaterials. J.Mater. Sci. Mater. Med. 12,195, 2001.19.van Wachem, P.B., Plantinga, J .A., Wissink, M.J .,Beernink, R., Poot, A.A., Engbers, G.H., Beugeling, T., van Aken, W.G., Feijen, J., and van Luyn, M.J. In vivo bio-compatibility of carbodiimide-crosslinked collagen matri-ces: Effects of crosslink density, heparin immobilization,and bFGF loading. J. Biomed. Mater. Res. 55,368, 2001.20.Soker, S., Machado, M., and Atala, A. Systems for thera-peutic angiogenesis in tissue engineering. World J. Urol.18,10, 2000.Address reprint requests to:G.C.M. Steffens, Ph.D.Department of Biochemistry and Molecular CellBiologyInstitute of Biochemistry RWTH Aachen UniversityPauwelsstrasse 3052074 Aachen, GermanyE-mail:gcm.steffens@post.rwth-aachen.de1509。

TPO 24Animal fossils usually provide very little opportunity to study the actual animal tissues because in fossils the animals' living tissues have been largely replaced by minerals. Thus, scientists were very excited recently when it appeared that a 70-million-year-old fossil of Tyrannosaurus rex (T. rex), a dinosaur, might still contain remains of the actual tissues of the animal. The discovery was made when researchers deliberately broke open the T. rex's leg bone, thereby exposing its insides to reveal materials that seem to be remains of blood vessels, red blood cells, and collagen matrix.First, the breaking of the fossilized leg bone revealed many small branching channels inside, which probably correspond to hollows in the bones where blood vessels were once located. The exciting finding was the presence of a soft, flexible organic substance inside the channels. This soft substance may very well represent the remains of the actual blood vessels of T. Rex.Second, microscopic examination of the various parts of the inner bone revealed the presence of spheres that could be the remains of red blood cells. Tests showed that the spheres contained iron - a material vital to the role of red blood cells in transporting oxygen to tissues. Moreover, the spheres had dark red centers (substances with iron tend to be reddish in color) and were also about the size of red blood cells.Third, scientists performed a test on the dinosaur leg bone that showed that it contained collagen. Collagen is a fibrous protein that is a main component of living bone tissue, in which it forms a so-called collagen matrix. Collagen (or its chemical derivatives) is exactly the kind of biochemical material that one would expect to find in association with bone tissue.As much as we would like to have the remains of actual dinosaur tissue, there are sound reasons for being skeptical of the identifications made in the reading.First, the soft, flexible substance inside the bone channels isn't necessarily the remains of blood vessels. It is much more likely to be something else. Like what? You might say. Well, long after an organism is died, bacteria sometimes colonize hollows, empty areas in bones, like the channels that once held blood vessels. When bacteria lived inside bones, they often leave behind traces of organic material. What the researchers in the reading are identifying as blood vessels might just be traces of soft and moist residue left by bacteria colonies.All right. What about the iron-filled spheres? Well, the problem is that scientists found identical reddish spheres in fossils of other animals found in the same place. That includes fossils of primitive animals that did not have any red blood cells when they were alive. Clearly, if these spheres appear in organisms that did not have any red blood cells, then the spheres cannot be the remains of red blood cells. The spheres probably have a very different origin. They are probably just pieces of reddish mineral.Third, the collagen. The problem is that we have never found collagen in animal remains that are older than one hundred thousand years. Collagen probably cannot last longer than that. Finding collagen from an animal that lived seventy million years ago would really contradict our ideas about how long collagen can last. It is just too improbable. The most likely explanation for the presence of collagen is that it doesn't come from the T.rex, but from another much more recent source. For example, human skin contains collagen, so the collagen may have come from the skin of the researchers who are handling the bone.In the reading passage, it talks about the fossils related to animal tissues, however, the professor holds different ideas.To begin with, in the reading material, we learn that the lots of tiny branching channels are found inside of the leg bone fossil. The reading material argues that soft tissue is expected to be found in the channels. However, the professor thinks that the channels are more likely to be left by bacteria. In addition, in the reading passage, it talks about that spheres are found in inner bone and red blood cells can be remained. And in the reading passage, it gives evidence that the sphere have red centers. However the professor indicates that there is no evidence show that creature at that time had red cells and the red centers should be something else.As a final point, in the reading passage, it talks about that collagen is found on the leg bone. Collagen is actually a biochemical material, which is expected to be an association with bone tissue.However, the professor says that collagen is not likely to live as long as 7 million year. It may be some recent material. For instant, it may be from the hand skin of the researchers.In conclusion the professor does not agree with the reading passage.TPO 25In 1938 an archaeologist in Iraq acquired a set of clay jars that had been excavated two years earlier by villagers constructing a railroad line. The vessel was about 2,200 years old. Each clay jay contained a copper cylinder surrounding an iron rod. The archaeologist proposed that vessel were ancient electric batteries and even demonstrated that they can produce a small electric current when filled with some liquids. However, it is not likely that the vessels were actually used as electric batteries in ancient times.First of all, if the vessels were used as batteries, they would probably have been attached to some electricity conductors such as metal wires. But there is no evidence that any metal wires were located near the vessels. All that has been excavated are the vessels themselves.Second, the copper cylinders inside the jars look exactly like copper cylinders discovered in the ruins of Seleucia, an ancient city located nearby. We know that the copper cylinders from Seleucia were used for holding scrolls of sacred texts, not for generating electricity. Since the cylinders found with the jars have the same shape, it is very likely they were used for holding scrolls as well. That no scrolls were found inside the jars can be explained by the fact that the scrolls simply disintegrated over the centuries.Finally, what could ancient people have done with the electricity that the vessels were supposed to have generated? They had no devices that replied on electricity. As batteries, the vessels would have been completely useless to them.Your reading says that these vessels were not used as batteries in ancient times, but the arguments used in the reading are not convincing. The battery explanation could very well be correct.First, about the absence of wires or other conductors, Remember, vessels were discovered by local people, not archaeologists. These people might have found other material located near the jars. But since they were not trained archaeologists, they may not have recognized the importance of that material. So materials serving as wires or conductors might have been overlooked as uninteresting or even thrown away. We’ll never know.Second, it is true that the copper cylinders in the vessels are similar to cylinders used to hold scrolls, but that does not really prove anything. It’s possible that the copper cylinders were originally designed to preserve scrolls. And that some ancient inventor later discovered that if you use them together with iron rods and some liquid in a clay vessel, they will produce electricity. That’s how the first ancient battery could have been born. In other words, the copper cylinders could have been originally used for one purpose, but then adapted for another purpose.Finally, there’s the question of the possible uses of the battery in the ancient world. Well, the battery could produce a mild shock or tingling sensation when someone touched it. This could very well have been interpreted as evidence of some invisible power. You can easily see how people could convince others that they had magical powers through the use of the battery. Also, the battery could have been used for healing. Modern medicine uses mild electric current to stimulate muscles and relieve aches and pains. Ancient doctors may have used the batteries for the same purpose.In the reading material, the author states that the vessels found in Iraq in 1938 were not actually used as electric batteries in ancient times. However in the listening material, the professor refutes that the argument is unconvincing as it was used as batteries.First, according to the reading passage, the author suggests that if they were used as batteries, they would have been attached to some electricity conductors. However in the listening, the professor claims that we should remember that the discovery was made by local people along with some other materials. As they were not trained as archaeologist, they could not recognize the importance of some certain excavations.Perhaps they were overlook as something uninterested and then thrown away. Second, the author in the reading material mentions that the vessels were likely used for holding scrolls. Unfortunately the professor argues that it could not prove anything. It is possible that the vessels may be originally designed to scrolls. However ancient inventor then discovered that if the vessels were used with iron rod and some liquid, it could generate the electricity. So the copper cylinders may be originally used for one purpose but adapted for another purpose.Finally, the author of the reading passage asserts that the vessels would have been completely useless to ancient people as they had no devices that replied on electricity. In the contrary in obviously contradicts with the listening passage in which the professor contends that the battery could generate some mild shock and this also interprets evidence of some invisible power that how people convince others they had the magic power. Also it could be used for healing. In modern society doctors would use batteries to stimulate muscles and release pains. In ancient times people could also do that.In conclusion, according to the listening material, the argument that the vessels could not be used as batteries is unwarranted.TPO 26The zebra mussel, a freshwater shellfish native to Eastern Europe, has long been spreading out from its original habitats and has now reached parts of North America. There are reasons to believe that this invasion cannot be stopped and that it poses a serious threat to freshwater fish populations in all of North America.First, the history of the zebra mussel's spread suggests that the invasion might be unstoppable. It is a prime example of an invasion made possible by human transportation. From the zebra mussel’s original habitats in Eastern Europe, ships helped spread it out along new canals built to connect Europe’s waterways. The mussel can attach itself to a ship’ s bottom or can survive in the water—called "ballast water"—that the ship needs to take on to properly balance its cargo. By the early nineteenth century, the mussel had spread to the whole of Europe. It was later carried to the east coast of North America in the ballast water of ships traveling from Europe. The way ships have spread the zebra mussel in the past strongly suggests that the species will soon colonize all of North America.Moreover, once zebra mussels are carried to a new habitat, they can dominate it. They are a hardy species that does well under a variety of conditions, and they have a high rate of reproduction. Most important, however, zebra mussels often have no predators in their new habitats, and species without natural predators are likely to dominate their habitats.Finally, zebra mussels are likely to cause a decline in the overall fish population in habitats where they become dominant. The mussels are plankton eaters, which means that they compete for food with many freshwater fish species.Contrary to what you just read, there are ways to control the zebra mussel's spread. What's more, it is not so clear that the mussel is a serious threat to fish populations.True, the spread of zebra mussels couldn't be controlled in the past, but that's because people didn't have enough knowledge. In fact, there are effective ways to stop ships from carrying the mussels to new locations. Here's an example. The way zebra mussels usually travel across the ocean is that a ship takes on some fresh "ballast water" in Europe and then empties that water into American waterways when it arrives. Full of zebra mussels, but the ship can be required to empty out the freshwater and refill with ocean water while still out in the ocean. Salt water will kill the mussels.Second, it's true that zebra mussels often don't have predators in their new habitats, but that's only in the beginning. What's been happening in Europe is that local aquatic birds sooner or later notice there's a new food source around and change their habits to exploit it. They switch from whatever they were eating before to eating zebra mussels. And birds can eat a lot of mussels. So zebra mussels aren't so likely to dominate their new habitats after all.Finally, even in habitats where zebra mussels become dominant, is the overall fish population likely to decrease. It's true that zebra mussels may have a negative impact on fish that eat plankton. But on other fish, they can have a positive impact. For example, the mussels generate nutrients that are eaten by fish that feed near the bottom of the lake or river. So bottom-feeding fish populations may increase, even if plankton-eating fish population decrease.Contrary to what is argued in the passage, the lecture illustrates how zebra mussels are not likely to become a serious threat to freshwater fish populations in North America.First and foremost, new knowledge of the zebra mussel has shed light on new ways to prevent their invasion, even though people in the past have not been able to stop the spread of zebra mussels effectively. For instance, although a large amount of zebra mussels spread to North America by staying in the ballast water of a ship, people can now get rid of them before the ship gets to the shore – if the ballast water is emptied halfway of the journey and refreshed with sea water, the zebra mussels can be exterminated as soon as they get exposed to salt water.Furthermore, zebra mussels are not likely to dominate a new habitat for a long period of time. The lecture agrees that zebra mussels may have no predators and reproduce rapidly in the beginning, but it would not be long before predators notice this new source of food and therefore prevent its domination.Finally, zebra mussels would not cause the decline of overall fish population. While zebra mussels would most likely cause the decline of plankton eaters, as the passage suggests, they would also provide nutrients for bottom-feeding fish and eventually cause the population of those fish to increase.TPO 33Carved stone balls are a curious type of artifact found at a number of |locations in Scotland. They date from the late Neolithic period, around 4,000 years ago. They are round in shape; they were carved from several types of stone; most are about 70 mm in diameter; and many are ornamented to some degree. Archaeologists do not agree about their purpose and meaning, but there are several theories.One theory is that the carved stone balls were weapons used in hunting or fighting. Some of the stone balls have been found with holes in them, and many have grooves on the surface. It is possible that a cord was strung through the holes or laid in the grooves around the ball. Holding the stone balls at the end of the cord would have allowed a person to swing it around or throw it.A second theory is that the carved stone balls were used as part of a primitive system of weights and measures. The fact that they are so nearly uniform in size -at 70 mm in diameter-suggests that the balls were interchangeable and represented some standard unit of measure. They could have been used as standard weights to measure quantities of grain or other food, or anything that needed to be measured by weight on a balance or scale for the purpose of trade.A third theory is that the carved stone balls served a social purpose as opposed to a practical or utilitarian one. This view is supported by the fact that many stone balls have elaborate designs. The elaborate carving suggests that the stones may have marked the important social status of their owners.None of the three theories presented in the reading passage are very convincing.First, the stone balls as hunting weapons, common Neolithic weapons such as arrowheads and hand axes generally show signs of wear, so we should expect that if the stone balls had been used as weapons for hunting of fighting, they too would show signs of that use. Many of the stone balls would be cracked or have pieces broken off. However, the surfaces of the balls are generally well preserved, showing little or no wear or damage.Second, the carved stone balls maybe remarkably uniform in size, but their masses vary too considerably to have been used as uniform weights. This is because the stone balls were made of different types of stone including sandstone, green stone and quartzite. Each type of stone has a different density. Some types of stone are heavier than others just as a handful of feathers weighs less than a handful of rocks. Two balls of the same size are different weights depending on the type of stone they are made of. Therefore, the balls could not have been used as a primitive weighing system.Third, it's unlikely that the main purpose of the balls was as some kind of social marker.A couple of facts are inconsistent with this theory. For one thing, while some of the balls are carved with intricate patterns, many others have markings that are extremely simple, too simple to make the balls look like status symbols. Furthermore, we know that in Neolithic Britain, when someone died, particularly a high-ranking person, they were usually buried with their possessions. However, none of the carved stone balls have been actually found in tombs or graves. That makes it unlikely that the balls were personal possessions that marked a person's status within the community.The author in the reading passage explores three major functions of the carved stone balls. However, in the lecture, the professor respectively contradicts all his assertions by using three specific points as supports.First, even though the reading passage suggests that the stone balls were weapons because of the holes and grooves on their surface, the professor argues that the stone balls didn’t show signs of use, which means they are neither cracked nor broken and thus cannot be used as weapons.Second, despite the statement in the reading passage that the stone balls were used as primitive weighing system due to their uniform size, the professor contends that their masses vary too considerably from each other. Therefore, the balls could not function as weighing system.Third, the author asserts that the stone balls served a social purpose owing to their elaborate designs while the professor proves that this claim is indefensible by pointing out that the balls were carved with not only intricate patterns but also simple ones, besides, none of the balls were found in the ancient tombs or graves. Consequently, it’s impossible that the balls were social markers.TPO 34A huge marine mammal known as Steller’s sea cow once lived in the waters around Bering Island off the coast of Siberia. It was described in 1741 by Georg W. Steller, a naturalist who was among the first European to see one. In 1768 the animal became extinct. The reasons for the extinction are not clear. Here are three theories about the main cause of the extinction.First, the sea cows may have been overhunted by groups of native Siberian people. If this theory is correct, then the sea cow population would have originally been quite large, but hundreds of years off too much hunting by the native people diminished the number of sea cows. Sea cows were a good source of food in a harsh environment, so overhunting by native people could have been the main cause of extinction.Second, the sea cow population may have become extinct because of ecosystems disturbances that caused a decline in their main source of food, kelp (a type of sea plant). Kelp populations respond negatively to a number of ecological changes. It is possible that ecological changes near Bering island some time before 1768 caused a decrease of the kelp that the sea cows depend on.Third, the main cause of extinction of the sea cows could have been European fur traders who came to the island after 1741. It is recorded that the fur traders caught the last sea cow in 1768. It thus seems reasonable to believe that hunting by European fur traders, who possessed weapons that allowed them to quickly kill a large number of the animals, was the main cause of the sea cow’s extinction.Now I want to tell you about what one company found when it decided that it would turn over some of its new projects to teams of people, and make the team responsible for planning the projects and getting the work done. After about six months, the company took a look at how well the teams performed.On virtually every team, some members got almost a “free ride”… they didn’t contribute much at all, but if their team did a good job, they nevertheless benefited from the recognition the team got. And what about group members who worked especially well and who provided a lot of insight on problems and issues? Well … the recognition for a job well done went to the group as a whole, no names were named. So it wont surprise you to learn that when the real contributors were asked how they felt about the group process, their attitude was just the opposite of what the reading predicts.Another finding was that some projects just didn’t move very quickly. Why? Because it took so long to reach consensus; it took many, many meetings to build the agreement among group members about how they would move the project along. On the other hand, there were other instances where one or two people managed to become very influential over what their group did. Sometimes when those influencers said “that will never work” about an idea the group was developing, the idea was quickly dropped instead of being further discussed. And then there was another occasion when a couple influencers convinced the group that a plan of theirs was “highly creative.” And even though some members tried to warn the rest of the group that the project was moving in directions that might not work, they were basically ignored by other group members. Can you guess the ending to this story? When the project failed, the blame was placed on all the members of the group.The writer and the professor all talk about the possible reasons for the extinction of sea cows. While the passage raises three theories about the main cause of the extinction, the lecture has differing views.According to the author, sea cows may have been over hunted by groups of native Siberian people, which could have been the main cause of the extinction. Not as the author puts it, the professor argues that over hunting for food could not be the main cause. He mentions that the sea cow was such a massive creature that could feed a small village for a month, and the population of native Siberian people was not very large. So, it was unlikely the native people hunted too much sea cow, the population of which would have originally been quite large.In the passage, it is said that a decline in their main source of food,caused by ecosystems' disturbance, may be the second explanation. On the contrary, the lecture suggests that no real evidence indicated the sea cows had little kelp to eat. The ecosystems' disturbances really happened before 1768, but it caused other part of marine creatures decreased, not sea cows' main source of food, kelp.At the points out the main cause could have been European fur traders, who were recorded catching sea cows. Quite the opposite, the speaker claims that it was not the real reason. Since the population was already small before Eropeanfur traders came to the island, there could have been something else decline a large number of the sea cows. And that is what the professor thinks the real reason of the extinction.。
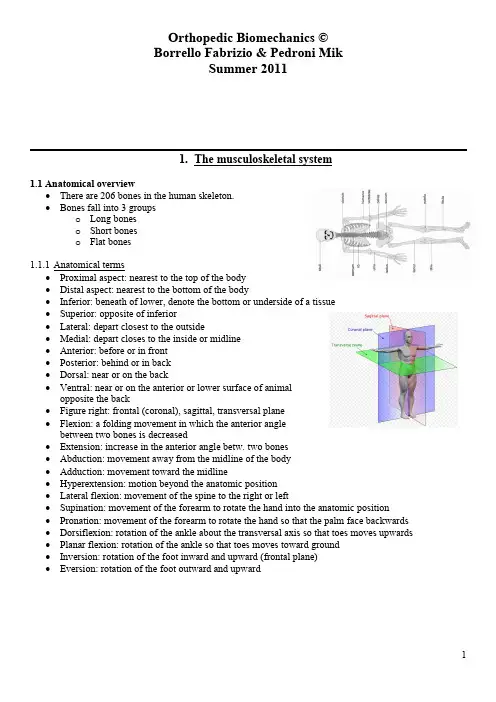

Biomedical Materials and EngineeringBiomedical engineering is a multidisciplinary field that combines the principles of engineering, biology, and medicine to design and develop solutions for healthcare problems. Biomedical materials are essential components of biomedical engineering that serve as the building blocks for medical devices, implants, and tissue engineering scaffolds. Biomedical materials and engineering play a crucial role in advancing healthcare innovation.Biomedical materials can be classified into two categories: natural and synthetic. Natural materials are derived from biological sources, such as collagen, chitosan, and silk. These materials have excellent biocompatibility, biodegradability, and bioactive properties, making them ideal for tissue engineering and regenerative medicine applications. Synthetic materials, on the other hand, are man-made and include polymers, ceramics, and metals. These materials can be tailored to achieve specific mechanical, chemical, and biological properties, making them ideal for medical devices and implants.Biomedical engineering focuses on designing and developing medical devices that improve patient outcomes and quality of life. Medical devices, such as pacemakers, artificial joints, and heart valves, are examples of how biomedical engineering has revolutionized healthcare. Biomedical materials play a key role in the design and development of medical devices that are safe, effective, and reliable. Polymeric materials, such as polyurethane and silicone, are commonly used in medical devices due to their biocompatibility, flexibility, and durability.Tissue engineering is another area where biomedical materials and engineering have made significant advances. Tissue engineering involves creating functional tissues for replacement or repair of damaged or diseased tissues and organs. Tissue engineering scaffolds are typically made from natural materials that mimic the extracellular matrix and provide a template for cell attachment, growth, and differentiation. Biomedical materials, such as collagen and hyaluronic acid, have been extensively studied for tissueengineering applications and have shown promising results in regenerating tissues such as skin, bone, and cartilage.Nanotechnology is a rapidly growing area of biomedical engineering that has enormous potential for improving healthcare. Nanoparticles, nanotubes, and nanofibers offer unique properties that can be tailored for various biomedical applications. For example, nanoparticles can be used as drug carriers to deliver drugs to specific targets in the body, while nanofibers can be used as tissue engineering scaffolds. Biomedical materials made from nanotechnology have the potential to revolutionize drug delivery, cancer treatment, and regenerative medicine.Biomaterials play a critical role in ensuring the safety and efficacy of medical devices and implants. Before a medical device or implant is approved for use, it undergoes rigorous testing to ensure that it meets safety and efficacy standards. Biomedical materials are tested for biocompatibility, degradation, mechanical properties, and durability. In addition, regulatory agencies such as the U.S. Food and Drug Administration (FDA) provide guidelines for the development, testing, and approval of medical devices and implants.In conclusion, biomedical materials and engineering have made significant contributions to healthcare innovation. Biomedical materials are essential components of medical devices, tissue engineering scaffolds, and regenerative medicine. Biomedical engineering has revolutionized healthcare by designing and developing medical devices that improve patient outcomes and quality of life. Advances in nanotechnology have enormous potential for improving drug delivery, cancer treatment, and regenerative medicine. Biomedical materials and engineering play a crucial role in ensuring the safety and efficacy of medical devices and implants. It is an exciting time for biomedical engineering and materials science, and the possibilities for future innovation are endless.。

IntroductionThe respiratory system, an intricate network of organs and structures responsible for the exchange of gases between the body and the environment, plays a vital role in maintaining life-sustaining processes. Despite its remarkable efficiency and resilience, this system is susceptible to a wide array of disorders that can significantly impact an individual's health and quality of life. This essay presents a comprehensive, multifaceted analysis of common respiratory disorders, examining their etiology, pathophysiology, clinical manifestations, diagnostic approaches, treatment strategies, and preventive measures, thereby offering a holistic understanding of these conditions.I. Etiology and Risk FactorsA. Environmental Factors1. Air Pollution: Exposure to particulate matter, ozone, nitrogen dioxide, and sulfur dioxide can trigger or exacerbate respiratory disorders, particularly asthma, chronic obstructive pulmonary disease (COPD), and lung cancer. Long-term exposure to indoor air pollutants, such as tobacco smoke, mold, and volatile organic compounds, also poses significant risks.2. Occupational Hazards: Workers in industries involving asbestos, silica, coal dust, or other toxic substances face heightened risks of developing occupational lung diseases like asbestosis, silicosis, and pneumoconiosis.B. Lifestyle Factors1. Smoking and Tobacco Use: Cigarette smoking remains the leading cause of preventable respiratory morbidity and mortality worldwide, contributing to the development of COPD, lung cancer, and various respiratory infections.2. Alcohol Consumption: Excessive alcohol intake can weaken the immune system, increasing susceptibility to respiratory infections, and exacerbating existing respiratory conditions.C. Genetic and Immunological Factors1. Genetic Predispositions: Certain genetic polymorphisms can increase the risk of developing respiratory disorders, such as alpha-1 antitrypsin deficiencyin COPD or mutations in the CFTR gene in cystic fibrosis.2. Immune System Dysfunction: Conditions like primary immunodeficiency, autoimmune diseases, or allergic hypersensitivity can predispose individuals to recurrent or severe respiratory infections, bronchiectasis, or interstitial lung diseases.II. PathophysiologyA. Obstructive Lung Diseases1. Asthma: Characterized by chronic inflammation, bronchial hyperresponsiveness, and reversible airflow obstruction due to bronchoconstriction, mucus production, and airway remodeling.2. COPD: A progressive disorder characterized by persistent airflow limitation resulting from chronic bronchitis (inflammation and excessive mucus production) and/or emphysema (alveolar wall destruction).B. Restrictive Lung Diseases1. Pulmonary Fibrosis: The abnormal accumulation of collagen and extracellular matrix proteins in the interstitium, leading to reduced lung compliance and impaired gas exchange.2. Pleural Disorders: Conditions affecting the pleura, such as pleurisy, pleural effusion, or pleural thickening, can restrict lung expansion and impair ventilation.C. Infectious Respiratory Disorders1. Acute Respiratory Infections: Viral (e.g., influenza, COVID-19) and bacterial (e.g., pneumonia) infections that can range from mild self-limiting illnesses to severe, life-threatening conditions.2. Tuberculosis: A chronic infectious disease caused by Mycobacterium tuberculosis, characterized by granulomatous inflammation and tissue destruction in the lungs.III. Clinical ManifestationsA. Symptoms1. Dyspnea: Shortness of breath, often exacerbated by physical exertion oremotional stress.2. Cough: Persistent or productive cough, which may be dry, wheezy, or accompanied by sputum production.3. Chest Pain or Tightness: Discomfort or pressure in the chest, which may worsen with deep breathing, coughing, or physical activity.4. Wheezing, Rhonchi, or Crackles: Auscultatory findings indicative of airway obstruction, secretions, or fluid accumulation.B. Signs1. Tachypnea, Hypoxemia, or Cyanosis: Rapid breathing, low blood oxygen saturation, or bluish discoloration of the skin, respectively, indicating inadequate ventilation or gas exchange.2. Clubbing, Barrel Chest, or Inspiratory Muscle Use: Physical signs suggesting chronic lung disease, such as finger clubbing, increased anterior-posterior chest diameter, or accessory muscle use during breathing.IV. Diagnostic ApproachesA. Medical History and Physical Examination: Crucial for identifying risk factors, symptom patterns, and characteristic physical signs.B. Laboratory Tests: Blood tests (e.g., complete blood count, C-reactive protein, immunoglobulin levels), sputum examination, or serological tests for specific pathogens or antibodies.C. Imaging Studies1. Chest Radiography: Initial imaging modality for detecting lung abnormalities, pleural effusions, or infiltrates.2. Computed Tomography (CT): Provides detailed information on lung parenchyma, airways, and vasculature, useful for diagnosing interstitial lung diseases, bronchiectasis, or lung nodules.3. Pulmonary Function Tests (PFTs): Assess lung volumes, airflow rates, diffusion capacity, and bronchodilator responsiveness, crucial for diagnosing and monitoring obstructive and restrictive lung diseases.D. Bronchoscopy and Biopsy: Direct visualization of the airways andcollection of bronchial washings, brushings, or biopsy specimens for histopathological, microbiological, or molecular analysis.E. Sleep Studies: Polysomnography or home sleep apnea testing to diagnose sleep-disordered breathing, such as obstructive sleep apnea.V. Treatment StrategiesA. Pharmacotherapy1. Bronchodilators: β2-agonists, anticholinergics, and methylxanthines for relieving airway obstruction in asthma and COPD.2. Anti-inflammatory Agents: Corticosteroids, leukotriene modifiers, and biologic therapies targeting specific inflammatory pathways in asthma and COPD.3. Antibiotics and Antivirals: For treating respiratory infections, guided by culture and sensitivity results or viral PCR testing.4. Antifibrotic Agents: Pirfenidone and nintedanib for slowing disease progression in idiopathic pulmonary fibrosis.5. Oxygen Therapy: Long-term oxygen therapy for patients with chronic hypoxemia due to advanced lung disease.B. Non-pharmacological Interventions1. Smoking Cessation Counseling and Pharmacotherapy: Essential for preventing disease progression and improving outcomes in smokers with respiratory disorders.2. Pulmonary Rehabilitation: Exercise training, education, and behavioral interventions to improve exercise tolerance, symptoms, and quality of life in patients with chronic respiratory diseases.3. Sleep Apnea Management: Continuous positive airway pressure (CPAP) therapy, oral appliances, or surgical interventions for treating obstructive sleep apnea.C. Surgical and Interventional Procedures1. Thoracic Surgery: Lobectomy, pneumonectomy, or segmentectomy for treating localized lung cancer or severe bullous emphysema.2. Endobronchial Interventions: Bronchoscopic techniques, such as balloondilation, stent placement, or electrocautery, for managing airway obstructions or fistulas.3. Lung Volume Reduction Surgery or Endobronchial Valve Placement: For selected patients with advanced emphysema to improve lung function and symptoms.VI. Preventive MeasuresA. Public Health Interventions1. Air Quality Improvement: Implementing policies to reduce air pollution, promote clean energy, and regulate industrial emissions.2. Vaccination Programs: Childhood immunizations against pertussis, pneumococcus, and influenza, as well as targeted vaccination campaigns for high-risk populations (e.g., pneumococcal and influenza vaccines for elderly individuals and those with chronic respiratory diseases).B. Personal Health Behaviors1. Smoking Avoidance and Cessation: Educating individuals about the harms of smoking and providing support for quitting.2. Healthy Lifestyle: Encouraging regular physical activity, balanced diet, adequate sleep, and stress management to maintain overall health and reduce respiratory disease risk.3. Occupational Safety: Ensuring proper protective equipment and ventilation systems in workplaces with respiratory hazards.ConclusionRespiratory disorders encompass a diverse array of conditions, each with distinct etiologies, pathophysiologies, clinical presentations, and management strategies. A comprehensive understanding of these disorders necessitates a multifaceted approach, considering environmental, lifestyle, genetic, and immunological factors alongside the complex interplay of inflammation, structural changes, and impaired gas exchange within the respiratory system. Effective diagnosis and treatment rely on a combination of thorough medical assessment, advanced diagnostic tools, tailored pharmacotherapy, non-pharmacological interventions, and, when appropriate, surgical orinterventional procedures. Ultimately, preventive measures at both the public health and individual level play a pivotal role in reducing the burden of respiratory diseases and improving global respiratory health.。

托福TPO24综合写作阅读+听力原文+满分范文【雷哥托福整理】考过的同学会发现托福综合作文分数不高,很大程度上是受我们听力实力的影响,我们很多托福考生的听力分数只有16分上下的时候,对于托福综合作文的听力妥妥的是束手无策,而且很多托福考生还感觉自己都听懂了,那也只能说明你听懂了大意,但是听力里面要的是每一个细节!请注意,是每一个细节!雷哥托福小托君给大家分享TPO1-33综合作文部分的阅读和听力文本全集与综合作文的满分作文,以及满分作文的解析。
如果自己的托福综合作文分数如果可以很给力的话,就已经搞定了15分的分数,可以极大地缓解托福独立作文的压力。
如何使用这个文件呢?做托福TPO模考之后,可以根据这里面的听力的文本,来检验自己的听力内容是否抓的足够好,尤其是要看写的够不够全!在托福考试前来做跟读,口语实力不够,那么做跟读,仔细地来模仿ETS官方素材,是一个很好的提高自己口语的方式。
熟悉托福考试的专业词汇。
不少托福考生之所以在听力考试里面不够给力,是因为对于里面的专业词汇不够熟悉。
毕竟在托福考试过程中,如果核心词汇都不懂的话,那么在听力部分只能是束手就擒了。
TPO24 综合写作听力+阅读原文ReadingAnimal fossils usually provide very little opportunity to study the actual animal tissues, because in fossils the animals' living tissues have been largely replaced by minerals. Thus, scientists were very excited recently when it appeared that a70-million-year-old fossil of Tyrannosaurus rex (T. rex), a dinosaur, might still contain remains of the actual tissues of the animal. The discovery was made whenresearchers deliberately broke open the T. rex’s leg bone, thereby exposing its insides to reveal materials that seem to be remains of blood vessels, red blood cells, and collagen matrix.First, the breaking of the fossilized leg bone revealed many small branching channels inside, which probably correspond to hollows in the bones where blood vessels were once located. The exciting finding was the presence of a soft, flexible organic substance inside the channels. This soft substance may very well represent the remains of the actual blood vessels of T. rex.Second, microscopic examination of the various parts of the inner bone revealed the presence of spheres that could be the remains of red blood cells. Tests showed that the spheres contained iron-a material vital to the role of red blood cells in transporting oxygen to tissues. Moreover, the spheres had dark red centers (substances with iron tend to be reddish in color) and were also about the size of red blood cells.Third, scientists performed a test on the dinosaur leg bone that showed that it contained collagen. Collagen is a fibrous protein that is a main component of living bone tissue, in which it forms a so-called collagen matrix. Collagen (or its chemical derivatives) is exactly the kind of biochemical material that one would expect to find in association with bone tissue.ListeningAs much as we would like to have the remains of actual dinosaur tissue, there are sound reasons for being skeptical of the identifications made in the reading.First, the soft, flexible substance inside the bone channels isn’t necessarily the remains of blood vessels. It is much more likely to be something else. Like what? You might say. Well, long after an organism is died, bacteria sometimes colonize hollows, empty areas in bones, like the channels that once held blood vessels. When bacterialived inside bones, they often leave behind traces of organic material. What the researchers in the reading are identifying as blood vessels might just be traces of soft and moist residue left by bacteria colonies.All right. What about the iron-filled spheres? Well, the problem is that scientists found identical reddish spheres in fossils of other animals found in the same place. That includes fossils of primitive animals that did not have any red blood cells when they were alive. Clearly, if these spheres appear in organisms that did not have any red blood cells, then the spheres cannot be the remains of red blood cells. The spheres probably have a very different origin. They are probably just pieces of reddish mineral.Third, the collagen. The problem is that we have never found collagen in animal remains that are older than one hundred thousand years. Collagen probably cannot last longer than that. Finding collagen from an animal that lived seventy million years ago would really contradict our ideas about how long collagen can last. It is just too improbable. The most likely explanation for the presence of collagen is that it doesn’t come from the T.rex, but from another much more recent source. For example, human skin contains collagen, so the collagen may have come from the skin of the researchers who are handling the bone.由于篇幅有限,托福综合写作满分范文,在雷哥托福微信公众号获取。

【托福写作备考】TPO24综合写作文本与解析TPO 24以下是综合写作的阅读材料:Animal fossils usually provide very little opportunity to study the actualanimal tissues because in fossils the animals’ living tissues have been largelyreplaced by minerals. Thus, scientists were very excited recently when itappeared that a 70-million-year-old fossil of Tyrannosaurus rex (T. rex), adinosaur, might still contain remains of the actual tissues of the animal. Thediscovery was made when researchers deliberately broke open the T. rex’s legbone, thereby exposing its insides to reveal materials that seem to be remainsof blood vessels, red blood cells, and collagen matrix.译文:动物化石很难帮助人们研究动物的软组织,这是因为化石里面的动物活性组织大部分都被矿物质代替了。
最近,发现一个7千万年前的霸王龙化石中可能存在真正的动物软组织。
这令科学家们非常兴奋。
科学家小心翼翼地打开了霸王龙腿骨的时候,发现里面存在着可能为血管、血红细胞和胶原蛋白基质的物质。
First, the breaking of the fossilized leg bone revealed many small branchingchannels inside, which probably correspond to hollows in the bones where bloodvessels were once located. The exciting finding was the presence of a soft,flexible organic substance inside the channels. This soft substance may verywell represent the remains of the actual blood vessels of T. rex.译文:首先,打开腿骨化石之后,科学家发现了内部有一些管状分支,这些管状分支可能是骨内血管存在的地方。
NOX-4调控PI3K信号通路参与TGF-β1诱导肺癌细胞表达Ⅰ型胶原蛋白董年;余垭妮;吴登敏;王蓓蓓;应赵建;裘丹萍;董莉;陈成水【摘要】目的:研究NADPH氧化酶4(NOX-4)调控PI3K信号通路在转化生长因子β1(TGF-β1)诱导肺癌细胞表达Ⅰ型胶原蛋白(collagen Ⅰ)的作用及分子机制.方法:体外培养人肺癌A549细胞,予TGF-β1刺激后,观察NOX家族和collagen 家族的mRNA和蛋白表达的变化,以及PI3K class Ⅰ催化亚基的表达和PI3K信号通路活化的变化;NOX-4抑制剂二亚苯基碘鎓(DPI)预先处理肺癌细胞,观察TGF-β1刺激后collagen Ⅰ的mRNA和蛋白表达的变化以及PI3K class Ⅰ催化亚基表达和PI3K信号通路活化.结果:TGF-β1可以诱导肺癌细胞中NOX-4和colla-gen Ⅰ的mRNA和蛋白表达升高,并诱导PI3K class Ⅰ催化亚基中PIK3CD表达升高和PI3K信号通路的活化. NOX-4抑制剂DPI可以抑制TGF-β1诱导的collagen Ⅰ表达升高;抑制NOX-4 并不影响TGF-β1 诱导的PI3K催化亚基PIK3CD表达,但可以降低TGF-β1诱导PI3K信号通路的活化程度.结论:NOX-4经调控PI3K信号通路的活化参与了TGF-β1诱导肺癌细胞表达collagen Ⅰ的分子机制. TGF-β1/NOX-4/PI3K信号通路轴在肺癌细胞collagen Ⅰ的表达中发挥了调控作用.%AIM:To investigate the regulatory effect of NADPH oxidase-4 (NOX-4) on PI3K signaling path-way in transforming growth factor-β1 (TGF-β1)-induced collagen type Ⅰ (collagen Ⅰ) synthesis from lung cancer cells and the mechanisms. METHODS:Human lung cancer A549 cells were cultured in vitro and stimulated with TGF-β1. The ex-pression of NOX family and collagen family at mRNA and protein levels as well as the PI3K class Ⅰ catalytic subunits and the activation of PI3K signaling pathway wasmeasured. A549 cells were pre-treated with NOX-4 inhibitor diphenyleneiodo-nium (DPI), and the expression of collag en Ⅰ at mRNA level as well as the PI3K class Ⅰ catalytic subunits and the activa-tion of PI3K signaling pathway was measured upon TGF-β1 stimulation. RESULTS:TGF-β1 stimulated the expression of NOX-4 and collagen Ⅰ at mRNA and protein levels as well as the expression of PIK3CD and the activation of PI3K signaling pathway at a dose- and time-dependent manner. NOX-4 inhibitor DPI partly reversed TGF-β1-induced collagen Ⅰ expres-sion. Inhibition of NOX-4 down-regulated the degree of TGF-β1-stimulated activation of PI3K signaling pathway without effect on the expression of PIK3CD. CONCLUSION:NOX-4 participates in TGF-β1-induced collagen Ⅰ synthesis from lung cancer cells via regulating the activation of PI3K signaling pathway. TGF-β1/NOX-4/PI3K signaling pathway axis acts as a regulatory role in collagen Ⅰ synthesis from lung cancer cells.【期刊名称】《中国病理生理杂志》【年(卷),期】2018(034)006【总页数】6页(P1014-1019)【关键词】转化生长因子β1;NADPH氧化酶4;PI3K/Akt信号通路;Ⅰ型胶原蛋白;肺癌【作者】董年;余垭妮;吴登敏;王蓓蓓;应赵建;裘丹萍;董莉;陈成水【作者单位】温州医科大学附属第一医院呼吸与危重症医学科,浙江温州325000;温州医科大学附属第一医院呼吸与危重症医学科,浙江温州325000;温州医科大学附属第一医院呼吸与危重症医学科,浙江温州325000;温州医科大学附属第一医院呼吸与危重症医学科,浙江温州325000;温州医科大学附属第一医院呼吸与危重症医学科,浙江温州325000;温州医科大学附属第一医院呼吸与危重症医学科,浙江温州325000;温州医科大学附属第一医院呼吸与危重症医学科,浙江温州325000;温州医科大学附属第一医院呼吸与危重症医学科,浙江温州325000【正文语种】中文【中图分类】R329.28;R734.2肿瘤微环境由间质细胞和细胞外基质(extracellular matrix,ECM)共同构成,在肿瘤的免疫逃避、浸润转移和化疗耐药等恶性生物学行为方面发挥了重要作用,是肺癌5年生存率居高不下的原因之一[1]。
角膜缘干细胞在眼科的应用进展宋曲园1 综述,马雅玲2 审校 (1.宁夏医学院,宁夏 银川 750004;2.宁夏医学院附属医院眼科,宁夏 银川 750004)[关键词] 角膜缘干细胞;载体;移植中图分类号:R772.2 文献标识码:A 文章编号:1004-0412(2007)04-0445-03 角膜是位于眼球前部的透明组织,起着眼球保护膜和“透光窗”的重要功能。
角膜上皮的更新及创伤愈合过程,有赖于角膜缘干细胞的增殖、移行。
当角膜缘干细胞缺失,会使角膜上皮增殖能力丧失、屏障功能下降,致使眼部出现不同程度的病理改变。
角膜缘干细胞(L i m bal stem cells,LSCs)的发现是近几十年来眼科学最重要的进展之一,其移植技术也是最先应用于眼科的成体干细胞移植技术。
本文从LSCs定位鉴定、培养载体、临床应用三方面对其在眼科的应用进展进行阐述。
1 L SC s的发现、定位及鉴定 1971年Davanger等[1]发现角膜上皮有由周边向中心移动的特性,据此提出角膜缘干细胞存在的假说。
1986年Scher mer等[2]首次通过实验证明角膜上皮干细胞位于角膜缘基底层,尤其是Vogt栅栏区乳头状结构中的角膜缘基底细胞层;且增生方式为:干细胞→短暂扩充细胞→有丝分裂后细胞→终级分化细胞。
目前关于角膜缘干细胞存在于角膜缘的实验证据有:1.1 角蛋白的测定:角膜上皮表达3对角蛋白。
其中相对分子质量为64000的碱性角蛋白(K3)在角膜上皮细胞独特表达,AE5单克隆抗体是针对K3角蛋白的特异性抗体,其在结膜上皮表达阴性,而在角膜缘上皮细胞、角膜上皮表达阳性[2]。
表明角膜缘基底细胞含有角膜上皮干细胞,提示角膜上皮再生的源头位于角膜缘。
1.2 角膜缘上皮细胞的克隆化培养:可通过细胞克隆化形成的3种细胞形态来判断细胞的增殖能力,即全克隆(代表极高的增殖力)、旁克隆(增殖力较弱)、部分克隆(全克隆至旁克隆的过渡)。
2024届上海市静安区高三下学期二模英语试题(5)一、听力选择题1. When should the meeting have started?A.At 9:00 a.m.B.At 10:00 a.m.C.At 11:00 a.m.2.A.Sally is not fond of any party.B.Sally dislikes the man.C.Sally is afraid to gain weight.D.Sally wants to lose weight in the winter.3. When does the conversation take place?A.In the morning.B.At noon.C.In the evening.4. What is the man doing?A.Telling good news.B.Keeping a secret.C.Planning a vacation.5. What will the man do later?A.See a doctor.B.Go to the beach.C.Have dinner with his parents.二、听力选择题6. 听下面一段较长对话,回答以下小题。
1. What do we know about the area?A.Lightning strikes mostly in December.B.The people there worry about getting hit.C.The area is near the Andes Mountains.2. What did NASA call the area?A.The Never-Ending Storm of Catatumbo.B.The Lightning Capital of the World.C.The Light of Venezuela.3. How many people are struck by lightning near the Catatumbo River?A.One in three per year.B.One in 12,000 per year.C.80% of people who live there.4. What does the man say in the end?A.He’s scared of storms.B.He’ll never visit Venezuela.C.He’d be careful if he lived in the area.7. 听下面一段较长对话,回答以下小题。
M E DPOR®Oculoplastic surgeryMEDPOR ®biomaterialyears of proven clinical history30+MEDPOR has been a trusted name in the industry since 1985, with hundreds of thousands of procedures performed, and hundreds of published clinical reports in reconstructive, cranial, Oculoplastic surgery, and cosmetic applications.Our MEDPOR product line provides you an array of porous polyethylene solutions for your reconstruction and augmentation needs. We understand that bio-compatibility characteristics of implants are paramount to help surgeons achieve positive patient outcomes. The omni-directional pore structure of our polyethylene implants may increase implant acceptance by allowing the patient‘s native tissue to integrate with the implant. In addition to our comprehensive line of stock MEDPOR implants, we offer CT-based patient specific implants,putting the implant design in your hands.• MEDPOR is easy to work with. The materialcan be trimmed with a blade in the sterile field, carved and feathered intra-operatively for an excellent final fit.• No pre-placing of fixation plates. MEDPOR can be easily drilled and fixated and is designed to accept screws and plates without cracking, giving the surgeon more flexibility in fixation options and placement.• MEDPOR surgical implants can be cut with a varietyof surgical instruments. Implants may require fittingto the defect area at the time of surgery. The implant edges can be delicately shaped and feathered for a smooth transition from the implant to the patient’s own bony contour.• MEDPOR surgical implants are provided sterile and should not be resterilized.• Do not place or carve the implant on surgical drapes, surgical clothing or any other surface that may contaminate the implant with lint and other particulate matter.MEDPOR spheres provide surgeons with porous, biocompatible materials for orbital reconstruction following enucleation and evisceration procedures. The interconnecting, omni-directional pore structure of the MEDPOR biomaterial may allow for vascularization and soft tissue ingrowth. Healthy extra-ocular muscles can be sutured directly to the implant or to an overlying tissue wrap.Smooth Surface Tunnel (SST-EZ) spheres have a smooth, porous anterior surface and suture tunnels to allow easy rectus muscle attachment without the use of an implant wrap. Theredesigned suture holes and curved tunnels of the new MEDPOR SST-EZ may allow for easier insertion of ophthalmic needles typically used to attach the extra-ocular muscles tothe implant. Both suture arms from one muscle are passed through each tunnel. Each muscle end can be drawn to within 3mm of the implant anterior apex or allowed to hang back at the desired attachment location.SpheresSmooth Surface Tunnel spheres (SST-EZ)MEDPOR Oculoplastic surgery CAT# Description Size (mm)80008SST-EZ sphere 1680010SST-EZ sphere 1880012SST-EZ sphere 2080014SST-EZ sphere22CAT# Description Size (mm) diameter6316Sphere 146326Sphere 166327Sphere 186317Sphere 206322Sphere22The orbital volume sizer set makes it easy to evaluate post-enucleation orbital volume to select the appropriate size implant. The set contains five (5) sizes of stainless steel spheres with attached handles that are assembled in a convenient sterilizable tray.CAT# Description Size (mm)9805Sizer set with tray14, 16, 18, 20, 22SPHERESSSTSST-EZMEDPOR CAT# Description A (mm) B (mm)Thickness 9541Regular – left 22317.009542Regular – right 22317.009543Large – left 28407.509544Large – right28407.50The MEDPOR enophthalmos wedge mimics the contour of the orbital floor and is designed to provide volume to restore the orbit to its normal shape and size.Ocular conformersCAT# Description A (mm) B (mm)ThicknessOcular conformers are designed to be used after surgery to prevent closure or adhesions during the healing process. Conformers are small, acrylic-cup shaped devices whose inner surfaces are shaped to approximate the curvature of the orbit. MEDPOR Ocular conformers are supplied sterile in both vented and non-vented styles.NON-VENTEDANON-VENTEDVENTEDBCollagen implant designed for use as a soft tissue patch to reinforce soft tissue where weakness exists and for the surgical repair of damaged or ruptured soft tissue membranes in plastic and reconstructive surgery of the face and head.Biomaterial for excellent resultsCross-linked - ENDURAGen biomaterial is made up of acellularcross-linked porcine dermal collagen with its constituent elastin fibers.1Structural architecture - collagen matrix has a structural architecture comparable to human tissue, which may offer a natural scaffold for fibroblast infiltration and vascularization.1Tough but flexible - readily conforms to anatomical shapes and may provide surgeons with the flexibility to meet their patient’s individualrequirements; may be cut, shaped and sutured.Long-lasting - the enzymatic digestion and cross-linking manufacturing process makesENDURAGen Implants resistant to breakdown and absorption, potentially allowing the surgeon to effect a durable repair or reconstruction for soft tissue contouring and/or reinforcement procedures.1Moist - implants are supplied sterile in double wrapped, heat sealed packet and require no hydration prior to use.Uniform - consistent thickness throughout the implant.CAT# Thickness (mm) A (cm) B (cm)892210.502589223 1.001489224 1.0025892251.0038ENDURAGen ®MEDPOROculoplastic surgery Actual size2cm x 5cm x 0.5mmBAMEDPOR TITAN®ConfigurationsMTMTitanium mesh embedded within porous, high-density polyethylene.MTBTitanium mesh embedded withina porous polyethylene matrixwith a solid, barrier surface onone side, potentially allowing forfibrovascular ingrowth only onthe porous side of the implant.BTBTitanium mesh embeddedwithin solid, non-poroushigh-density polyethylene.The smooth barrier surfacecan prevent fibrovascularingrowth.Combines high-density polyethylene and titanium mesh in a single implant for increased flexibility, shape retention, radiographic visualization and strength2.MEDPOR Oculoplastic surgeryImplants designed using CT-scan data to approximate the anatomy of the orbital floor& medial wall to enhance the effectiveness and efficiency of reconstruction. MEDPORcoating minimizes sharp edges even if the plates require modification, and the superior,non-porous barrier side helps prevent tissue ingrowth along the aspect of the globe.Plate A B C DLarge L/R 36mm(1.4 in.)37mm(1.4 in.)17mm(0.6 in.)1.2mmSmall L/R 32mm(1.2 in.)35mm(1.4 in.)13mm(0.5 in.)1.2mmCatalog # Description81041MEDPOR TITAN 3D Orbital floor, MTB left small 81042MEDPOR TITAN 3D Orbital floor, MTB right small 81043MEDPOR TITAN 3D Orbital floor, MTB left large 81044MEDPOR TITAN 3D Orbital floor, MTB right large 01-01820Plate holding forcepMEDPOROculoplastic surgery US Patent 7,655,047TITANOrbital Floor and Wall (OFW)CAT# Description A (mm) B (mm)Thickness81020MTM 50760.8581021MTM 38500.8581022MTM 3850 1.5081023MTM 5076 1.5081024BTB 38500.6081025BTB 50760.6081026MTB 3850 1.0081027MTB 5076 1.0081028MTB 3850 1.6081029MTB50761.60CAT# Description A (mm) B (mm) C (mm)Thickness81034MAX MTM 4241 1.00.8581035MAX MTB - Left 4241 1.0 1.081036MAX MTB - Right42411.01.0CAT# Description A (mm) B (mm) C (mm)Thickness81030MTM 42410.50.8581031MTB - Left 42410.5 1.081032MTB - Right 42410.5 1.081033BTB42410.50.6MEDPOR Available in two configurations, with or without a BARRIERTITAN fanCAT# Description A (mm) B (mm)Thickness81049MTM 406181050MTB4061MEDPOR TITAN Orbital floor and WallCAT# Description A (mm) B (mm)Thickness83020Micro thin sheet 38500.2583022Micro thin sheet 38500.358438Micro thin sheet 30500.4083029Micro thin sheet 38500.4583030Micro thin sheet 50760.45MEDPOR biomaterial sheets provide the surgeon with options for craniofacial reconstruction and augmentation.6330Sheet 3850 1.506331Sheet 5076 1.508662Sheet 76127 1.509562Sheet38503.007210Ultra thin sheet 38500.857212Ultra thin sheet 50760.857214Ultra thin sheet 761270.85A 0.25mm 0.35mm 0.40mm0.45mm 0.85mm 1.50mm 3.00mmThicknessBMEDPOROculoplastic surgery 9567Inferior 2/3 orbit - left 108759568Inferior 2/3 orbit - right 108759569Complete orbit – left 93759570Complete orbit - right9375A BBComplete OrbitABABBARRIER sheetsCAT# Description A (mm) B (mm)Thickness8305Orbital floor implant 3850 1.009305Orbital floor implant 3850 1.608312Rectangle 5076 1.009312Rectangle50761.60MEDPOROculoplastic surgery Inferior orbital rimCAT# Description A (mm) B (mm) C (mm)9429Inferior orbital rim - left4318 3.29430Inferior orbital rim - right4318 3.2MEDPOR extended orbital rim implants are designed to provide thesurgeon with an option for augmenting the inferior rim.CAT# Description A (mm) B (mm) C (mm)9539Orbital rim - extended left4740 6.39540Orbital rim - extended right4740 6.3The MEDPOR midface contour implant is designed to aid in reconstruction or augmentation of the midface. The shell-type design of the implant allows the surgeon to carve portions of the implant most appropriate for each patient.The MEDPOR Midface contour implant is packaged with a sterile silicone template.CAT# Description A (mm) B (mm) C (mm)83007Midface contour implant - left6040483008Midface contour implant - right6040492-83007Midface contour implant - left6541492-83008Midface contour implant - right65414The MEDPOR inferior orbital rim implant can provide up to 5mm of anterior projection and is designed to be trimmed to meet the needs of the individual patient. A small flange allows it to rest on the most anterior aspect of the orbital floor. This flange allows for positioning of the implant and a possible area for screw fixation to the skeleton.MEDPOROculoplastic surgeryThe MEDPOR midface rim is designed to augment areas of bony concavities of the midface, including the inferior orbital rim and malar.Midface rimCAT# Description A (mm) B (mm) C (mm)83003Midface rim - left 4728383004Midface rim - right47283The MEDPOR Inferior Medial Orbital Rim Implant (IMORI) is designed to wrap over the inferior orbital rim and extend superiorly and inferiorly medial to the inferior orbital nerve.Inferior Medial Orbital Rim Implant (IMORI)CAT# Description A (mm) B (mm) C (mm)87003Inferior medial orbital rim - left 2526 2.5087004Inferior medial orbital rim - right25262.50CACLRABACBLThe MEDPOR orbital rim onlay implants are designed to augment the inferior and lateral orbital rims and increase the anterior rim projection.CAT# Description A (mm) B (mm) C (mm)81001Orbital rim onlay - left 40408.4581002Orbital rim onlay - right40408.45MEDPOR implants are built from patient CT data and offer you the ability to design an implant that fits your patients re-constructive or augmentation needs.Each MEDPOR implant kit contains two (2) identical sterile implants and one (1) sterile host bone model (defect area). The host bone model is provided as a preoperative guide to demonstrate orientation and fit of the customized implant(s).Individual designed implantsMEDPOR Oculoplastic surgery CAT# Description54440110MEDPOR patient specific - small 54440210MEDPOR patient specific - medium 54440310MEDPOR patient specific - large 54440410MEDPOR patient specific - XL 54440510MEDPOR patient specific midface54440710MEDPOR patient specific midface augmentationiD SolutionsTMIndividually designed. Personalized care.Facial iD - Reconstruction and augmentation®Stryker Craniomaxillofacial Kalamazoo, MI 49002 USA t: 269 389 5346toll free: 800 962 6558 f: 877 648 7114/cmfCraniomaxillofacialThis document is intended solely for the use of healthcare professionals. A surgeon must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Stryker does not dispense medical advice and recommends that surgeons be trained in the use of any particular product before using it in surgery.The information presented is intended to demonstrate a Stryker product. A surgeon must always refer to the package insert, product label and/or instructions for use, including the instructions for cleaning and sterilization (if applicable), before using any Stryker product. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your Stryker representative if you have questions about the availability of Stryker products in your area.Stryker Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following trademarks or service marks: BARRIER, BTB, Facial iD, iD Solutions, IMORI, MAX, MEDPOR, MEDPOR TITAN, MTB, MTM, OFW , OZ, SST-EZ, Stryker, TITAN. All other trademarks are trademarks of their respective owners or holders. Devices may not have been licensed in accordance with Canadian Law.ENDURAGen is a registered trademark of Tissue Science Laboratories Limited.References:1: “ENDURAGen Collegen Implent - Ideal Biomaterial for Ideal Results” Tissue Science Laboratories Manufacterer’s Technical Statement, pp2 and 5, 2005.2: Holck, D., Foster J., and Dahl T., “Custom Shaped Porous Polyethylene-Titanium Mesh Orbital Implants for Internal Orbital Floor/Medial-Wall Fracture Repair”CMF-BR-91_Rev. 2_22950Copyright © 2019 Stryker Printed in the USA。
Product Information SheetSoluble Collagen Quantification Assay Kit FluorometricCS0006Product DescriptionCollagen is one of the most abundant proteins in connective tissues and internal organs of mammals. Collagen provides the tensile strength of the extracellular matrix (ECM) and is classified into several structurally and genetically distinct types. Although different types of collagen exist, they are all composed of molecules with three polypeptide chains that are arranged in a triple helical conformation. Slight differences in the primary structure (amino acid sequence) establish differences between the types.1-4 The Soluble Collagen Quantification Assay Kit provides a simple and sensitive procedure for measuring soluble collagen in various sample types. The kit does not require the use of perchloric acid. The amount of soluble collagen is determined based on an enzymatic reaction, where collagen is specifically digested into peptides. Subsequently, the collagen peptides are labeled with a fluorescent probe. The fluorescence intensity, measured at λex = 375 nm / λem = 465 nm, is proportional to the amount of soluble collagen in the sample.This kit can be used to quantify soluble collagen5 extracted from tissues (such as muscle or heart), tissue culture cell lysate and medium, serum samples, collagen in food, and purified collagens of various sources. This kit can detect purified collagen types I, II, III, IV and V.Precautions and DisclaimerFor R&D use only. Not for drug, household,or other uses. Please consult the Safety DataSheet for information regarding hazards andsafe handling practices.Storage/StabilityThe kit is shipped on dry ice. Upon receipt, store all components at –20 °C, protected from light. Upon thawing, the Assay Buffer and 10× Collagen Standard should be stored at 2-8 °C. The unopened kit is stable for 2 years as supplied. ComponentsThis kit contains sufficient reagents for200 fluorometric tests in 96-well plates.ComponentComponentNumber AmountCap Color/ComponentInformation Assay Buffer CS0006A 50 mLWhite cap/bottle10× CollagenStandard CS0006B 300 µLYellow cap/vial 5× DigestEnzyme CS0006C 1 mL Red cap/ vial 30× Probe CS0006D 600 µL Brown vial 10×DevelopmentSolutionCS0006E 600 µL Brown vial Component InformationAssay Buffer (Component CS0006A): Ready-to-use. Upon thawing, store at 2-8 °C.10× Collagen Standard (Component CS0006B): Contains a 2 mg/mL Collagen Type I solution. Upon thawing, store at 2-8 °C.5× Digest Enzyme (Component CS0006C): Store at –20 °C. To avoid multiple freeze/thaw cycles, it is recommended to prepare aliquots upon thawing, and store the aliquots at –20 °C. Keep on ice while in use. 30× Probe (Component CS0006D): Prior to use, vortex thoroughly. To avoid multiple freeze/thaw cycles, it is recommended to prepare aliquots upon thawing, and store the aliquots at –20 °C, protected from light.10× Development Solution (Component CS0006E): Prior to use, vortex thoroughly. To avoid multiple freeze/thaw cycles, it is recommended to prepare aliquots upon thawing, and store the aliquots at–20 °C, protected from light.Equipment Required (Not Provided)•96-well black flat-bottom plates•Fluorescence (λex = 375 nm / λem = 465 nm) plate reader•0.5 M acetic acid (if required;see "Sample preparation" below)•0.5 M NaOH (if required;see "Sample preparation" below) ProcedureSummary (flowchart)Add samples Add standardsBackground controls: Add 20 µL Assay BufferAdd 20 µL 1× digest enzymeworking solution(1 hour at 37 °C)Add 75 µL 1× probe working solution(10 minutes at 37 °C)Add 25 µL 1× development working solution(10-15 minutes at 37 °C) Read at λex = 375 nm / λem = 465 nmGeneral Notes•All samples, background controls, and standards should be run in duplicate.• A fresh set of standards should be prepared for each set of assays.•Briefly centrifuge vials before opening.•All reagents except the 5× Digest Enzyme should be equilibrated to room temperature before use.The 5× Digest Enzyme should be kept on icewhile in use.•For convenience, an Excel-based calculation sheet is available on the Product Detail Page.Use this sheet to calculate the amounts ofreagents required, as well as to calculate thetest results. •All assays (samples, standards, and blank) require 80 µL of sample for each reaction (well).Therefore, bring the volume to 80 µL if required.When required, samples should be diluted inAssay Buffer. For unknown samples, it issuggested to test several sample dilutions toensure that the readings are within the linearrange of the standard curve.• A background control should be included for each tested sample (see details below).•The kit’s optimal pH range is 7.5-8.0. If samples do not fall within this pH range, it is suggested to adjust the pH.Sample preparationAdherent cells:1.Remove culture medium.2.Trypsinize cells.3.Collect the harvested cells by centrifugation.4.Wash cells in PBS.5.Pellet cells by centrifugation and aspirate PBS.6.Resuspend cell pellet in 1 mL ice-cold 0.5 M aceticacid per ~1 × 107 cells.7.Collagen can be extracted by sonicating the lysateon ice for several sonication cycles, to achieve ahomogeneous preparation. To ensure theavailability of soluble collagen for the assay, it isimportant to keep samples chilled during thesonication procedure.8.Transfer the sample to a microfuge tube. Vortexthoroughly. Incubate at 4 °C overnight withgentle agitation.9.Centrifuge the sample at 10,000 × g for15 minutes at 4 °C. Transfer the supernatant to anew microfuge tube.10.Neutralize the sample by adding an equal volumeof 0.5 M NaOH to the supernatant.Secreted collagen from cell culture:Note: These samples can be assayed directly.1.Collect a sample of culture medium. If the cellsare in suspension, centrifuge to remove the cells, and collect the supernatant.2.Centrifuge at 10,000 × g for 15 min at 4 °C topellet any cells and/or debris.3.Collect the supernatant, to be used in the assay.CS0006pis Rev 09/22Serum samples:•Note: These samples can be diluted.•Typical dilutions are in the range of 10-20 fold in Assay Buffer. However, it is suggested to testseveral sample dilutions to ensure that thereadings are within the linear range of thestandard curve.Soft tissues:1.Soft tissue samples should be rinsed withice-cold ultrapure water or PBS to remove anyresidual blood.2.Blot dry.3.Dissociate the tissue with scissors to obtain small,fine pieces.4.Add 1 mL of ice-cold 0.5 M acetic acid per~100 mg of the dissociated tissue.5.To extract the collagen, the dissociated tissue canbe sonicated on ice for several sonication cycles,to achieve a homogeneous preparation.6.To ensure the availability of soluble collagen forthe assay, it is important to keep samples chilledduring the sonication procedure.7.The sample should be transferred to a microfugetube, vortexed thoroughly, and incubated at 4 °Covernight with gentle agitation.8.Centrifuge the homogenate at 10,000 × g for15 minutes at 4 °C.9.Transfer the supernatant to a newmicrofuge tube.10.Neutralize the sample by adding an equal volume(such as 1 mL) of 0.5 M NaOH to the supernatant. Collagen standard curve preparation:Dilute the 10× Collagen Standard (yellow cap vial)10-fold to a final concentration of 0.2 mg/mL: 20 µLof the 10× Collagen Standard with 180 µL of ultrapure water, to prepare a 1× collagen standard. Add 0, 1, 2, 4, 6, 8, and 10 µL of the 1× collagen standard into a 96-well plate, to generate 0 (blank), 0.2, 0.4, 0.8, 1.2, 1.6, and 2 µg/well standards. Complete the volume to 80 µL with Assay Buffer (see Table 1). Table 1. Preparation of Collagen Standards* 1× collagenstandardvolumeAssayBuffervolumeFinal collagenamount per well0 µL 80 µL 0 µg (blank)1 µL 79 µL 0.2 µg2 µL 78 µL 0.4 µg4 µL 76 µL 0.8 µg6 µL 74 µL 1.2 µg8 µL 72 µL 1.6 µg10 µL 70 µL 2 µg* Work in duplicateDigest Enzyme1.Dilute the 5× Digest Enzyme (red cap vial) 5-foldin Assay Buffer to prepare a 1× digest enzymeworking solution, according to Table 2. 20 µL ofthe 1× digest enzyme working solution is required for each reaction (well).Note: Include a sample background control (byreplacing the digest enzyme with Assay Buffer)for each sample. The standard curve does notrequire a background control. Multiply thevolumes in Table 2 according to the number ofwells in the assay.Table 2. Preparation of 1× digest enzyme working solution, per one well5×DigestEnzymevolumeAssayBuffervolume1× DigestEnzymeworkingsolutionfinal volume Sample andstandards4 µL 16 µL 20 µLSamplebackgroundcontrol (forsamplesonly)–20 µL –2.Add 20 µL of the 1× digest enzyme workingsolution to each of the standard and sample wells.3.Add 20 µL of sample background control(Assay Buffer) to each of the sample background control wells.4.Mix well. Incubate for 60 minutes at 37 °C.Probe1.Immediately prior to use, dilute the 30× Probe30-fold in Assay Buffer, to prepare a 1× probeworking solution, according to Table 3. 75 µL ofthe 1× probe working solution is required for each reaction (well). Multiply the volumes in Table 3according to the number of wells in the assay. Table 3. Preparation of 1× probe working solution, per one well*30× Probe volumeAssayBuffervolume1× probe workingsolution finalvolume2.5 µL 72.5 µL 75 µL* Protect from light2.Add 75 μL of the 1× probe working solution toeach of the standard and sample wells, including sample background control wells.3.Mix well. Incubate for 5 minutes at 37 °C,protected from light.Development solution1.Immediately prior to use, dilute the 10×Development Solution 10-fold in ddH2O to preparea 1× development working solution, according toTable 4. 25 µL of the 1× development workingsolution is required for each reaction (well).Multiply the volumes in Table 4 according to thenumber of wells in the assay.Table 4. Preparation of 1× development working solution, per one well*10× Development Solution volume ddH2Ovolume1× Developmentworking solutionfinal volume2.5 µL 22.5 µL 25 µL* Protect from light2.Add 25 µL of the 1× development workingsolution to each of the standard and sample wells, including sample background control wells.3.Mix well. Incubate for 10-15 minutes at 37 °Cwith gentle shaking, protected from light. MeasurementMeasure fluorescence intensity at:λex = 375 nm / λem = 465 nmResultsCalculations•An Excel-based calculation sheet is available at the Product Detail Page. Use this sheet tocalculate the test results. •If the Excel-based calculation sheet at the Product Detail Page is not used, calculations should beperformed as follows:1.Subtract the blank value (no standard) from allstandards values2.Plot the fluorescence measured for each standardagainst the standard amount per well. Determine the linear regression equation. Use the following equation to calculate the collagen amount in the sample:µg collagen = {[(Sample) – (Sample backgroundcontrol)] / Slope} × DFWhere:Sample = fluorescence intensity units of the sample Sample background control = fluorescence intensity units of the sample background control (no Digest Enzyme)Slope = The standard curve slope, obtained from the linear regression equationDF = Sample Dilution Factor (if sample is not diluted, the DF value is 1)Note: If the sample was neutralized (see "Sample preparation" above), then a 2-fold dilution should be accounted for, in addition to any dilution of the sample, if applicable.Example:For 100 mg of tissue in 1 mL acetic acid, plus 1 mL of NaOH, for a total volume of 2 mL, 10 µL (0.5 mg tissue) was assayed using the kit.•Mean sample = 16,588 RFU•Mean sample background control = 761 RFU •Slope = 10,689•DF = 2 (to obtain the result in values of µg collagen per mg tissue)•[(16,588 – 761) / 10,689] × 2 = 2.96 µg collagen per mg tissueThe life science business of Merck operates as MilliporeSigma in the U.S. and Canada.Merck and Sigma-Aldrich are trademarks of Merck KGaA, Darmstadt, Germany or its affiliates. All other trademarks are the property of their respective owners. Detailed information on trademarks is available via publicly accessible resources.References1. Tanzer, M.L., Science , 180(4086), 561-566(1973). 2. Bornstein, P., and Sage, H., Ann. Rev. Biochem.,49, 957-1003 (1980). 3. Kruegel, J., and Miosge, N., Cell. Mol. Life Sci.,67(17), 2879-2895 (2010). 4. Khoshnoodi, J. et al ., Microsc. Res. Tech., 71(5),357-370 (2008). 5. Feng, D. et al ., J. Biol. Chem ., 295(39),13640-13650 (2020).NoticeWe provide information and advice to our customers on application technologies and regulatory matters to the best of our knowledge and ability, but without obligation or liability. Existing laws and regulations are to be observed in all cases by our customers. This also applies in respect to any rights of third parties. Our information and advice do not relieve ourcustomers of their own responsibility for checking the suitability of our products for the envisaged purpose. The information in this document is subject to change without notice and should not be construed as acommitment by the manufacturing or selling entity, or an affiliate. We assume no responsibility for any errors that may appear in this document.Technical AssistanceVisit the tech service page at /techservice .Terms and Conditions of SaleWarranty, use restrictions, and other conditions of sale may be found at /terms .Contact InformationFor the location of the office nearest you, go to /offices .。
This article was downloaded by: [Ocean University of China]On: 31 March 2015, At: 19:57Publisher: Taylor & FrancisInforma Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House,37-41 Mortimer Street, London W1T 3JH, UKClick for updatesCell Adhesion & MigrationPublication details, including instructions for authors and subscription information:/loi/kcam20Collagen matrix as a tool in studying fibroblastic cell behaviorJi ří KantaaaDepartment of Medical Biochemistry , Medical Faculty in Hradec Králové, Charles University in Prague, Czech RepublicAccepted author version posted online: 03 Mar 2015.Disclaimer: This is a version of an unedited manuscript that has been accepted for publication. As a service to authors and researchers we are providing this version of the accepted manuscript (AM). Copyediting,typesetting, and review of the resulting proof will be undertaken on this manuscript before final publication of the Version of Record (VoR). During production and pre-press, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal relate to this version also.PLEASE SCROLL DOWN FOR ARTICLEA cc ep t ed M a n u s c ri ptCollagen matrix as a tool in studying fibroblastic cell behaviorJi ří KantaDepartment of Medical Biochemistry, Medical Faculty in Hradec Králové, Charles Universityin Prague, Czech RepublicKeywords : extracellular matrix, collagen, fibroblasts, myofibroblasts, cell culture, cell proliferation, integrins, metalloproteinases, fibronectin, substrate stiffnessAbbreviations : AP-1, activator protein 1; ECM, extracellular matrix; ERK, extracellular signal-regulated kinase; FAK, focal adhesion kinase; GT, granulation tissue; HSC, hepatic stellate cells; JNK, c-Jun N-terminal kinase; MFB, myofibroblasts; MKL1, megakaryoblastic leukemia1; MMP, metalloproteinases; NF-κB, nuclear factor kappa B; PI3K/Akt, phosphatidylinositide3-kinase/Ak strain transforming; PEG, polyethylene glycol; α-SMA, alpha-smooth muscle actin; 3D, three-dimensional; TGF β-1, transforming growth factor beta 1; TIMP, tissue inhibitor of metalloproteinases; TNF-α, tumor necrosis factor αCorrespondence to: Ji ří Kanta; Email: kanta@lfhk.cuni.czD o w n l o a d e d b y [O c e a n U n i v e r s i t y o f C h i n a ] a t 19:57 31 M a r c h 2015A cc ep t ed M a n u s c ri ptType I collagen is a fibrillar protein, a member of a large family of collagen proteins. It is present in most body tissues, usually in combination with other collagens and other components of extracellular matrix. Its synthesis is increased in various pathological situations, in healing wounds, in fibrotic tissues and in many tumors. After extraction from collagen-rich tissues it is widely used in studies of cell behavior, especially those offibroblasts and myofibroblasts. Cells cultured in a classical way, on planar plastic dishes, lackthe third dimension that is characteristic of body tissues. Collagen I forms gel at neutral pHand may become a basis of a 3D matrix that better mimics conditions in tissue than plastic dishes.D o w n l o a d e d b y [O c e a n U n i v e r s i t y o f C h i n a ] a t 19:57 31 M a r c h 2015A cc ep t ed M a n u s c ri ptIntroductionCells in a tissue are surrounded with other cells and with ECM, which is a network containing proteins, glycoproteins, proteoglycans and glycosaminoglycans. ECM provides chemical and mechanical signals whose effects are interdependent. Chemical signals may originate in thechemical structure of ECM components or may be provided by cytokines and growth factorsstored in the ECM and released under certain circumstances. ECM is more pliable than hardplastic surface and its mechanical properties contribute to diversity of physiological and pathological situations in the tissue. ECM is degradable and cells can migrate through it.1,2 Cell culture on thin collagen film covering plastic substrate is useful in studies of some aspects of cell behavior, e.g. interaction with integrins,3 but the contact with 3D environment makes the cells in tissue behave differently than the cells in conventional tissue culture on stiff plastic dishes do, as far as their morphology, differentiation, migration, and proliferation is concerned. The cells surrounded with an appropriate scaffold, usuallycollageneous, acquire tissue-like phenotype not observed in cells in monolayer. Mechanicalsignals from the ECM to the cells and contractile forces to the ECM are transmitted byprotein complexes called focal adhesions. Three-dimensional matrix adhesions differ fromadhesions on 2D substrates in protein composition and biological activity.4 Force applied tointegrins is transmitted through focal adhesions to the cytoskeleton.5 The cells may integrate global signals coming from the entire surface and sense the spatial organization of activated adhesions.6Fibroblasts and myofibroblasts are cells involved in the healing of various tissues.Fibroblasts in the tissue surrounding the wound are activated and migrate into the provisional matrix containing fibrin and plasma fibronectin. Fibrin is a major component ofD o w n l o a d e d b y [O c e a n U n i v e r s i t y o f C h i n a ] a t 19:57 31 M a r c h 2015A cc ep t ed M a n u s c ri ptthe provisional matrix formed during wound healing and enables migration of inflammatory cells and fibroblasts. Collagen production becomes the main fibroblast function and the provisional matrix is gradually replaced with a collagenous ECM.7,8,9 A part of fibroblasts may differentiate to protomyofibroblasts and further to MFB characterized by prominent stress fibers that contain α-SMA associated with non-muscle myosin. These proteins endow MFBwith high contractional force that is combined with synthetic abilities of the MFB.10,11 Thewound contracts and the provisional matrix is replaced with GT that is gradually convertedto a scar. Most cells then die by apoptosis. However, the reparative process may be dysregulated and result in fibrosis. The most abundant ECM component providing a scaffold that binds other proteins and proteoglycans is collagen I.12,13Solid tumors contain stroma that resembles GT in many aspects. The stroma is highlyvascularized. Fibrin is formed by clotting extravasated fibrinogen and together with other plasma proteins it gives rise to a provisional matrix. Fibroblasts settle in the matrix and produce collagen I and other ECM components. Cancer-associated fibroblasts promote thegrowth of cancer cells and vice versa they respond to signals from epithelial cells byincreased synthesis of collagen and other fibrogenic factors.14,15Three-dimensional matrices used as a model to study cell behavior in the tissue-likeenvironment in normal and pathological situations are therefore often made of collagen.2,16Fibrin gels can also be used to provide 3D environment for cells but they have much smaller influence on cell behavior and their effects may be opposite to those of collagen.17,18Various models of collagen matrix aiming to mimic in vivo situations will be discussedin this review. Fibroblastic cells transferred from plastic to collagen gel change their morphology and functions and in response they modify their environment. Secretion of MMP by the cells has a particular role in these interactions. The contact of cells with collagenD o w n l o a d e d b y [O c e a n U n i v e r s i t y o f C h i n a ] a t 19:57 31 M a r c h 2015A cc ep t ed M a n u s c ri ptis mediated by specific receptors, integrins. Plasma fibronectin interacts both with collagen and cells in vivo and it is therefore often added to collagen matrix in vitro.Collagen matrix modelsRodent tail tendons contain almost pure collagen I that can be extracted with dilutedacids.19,20,21 Collagen forms gel when its solution is neutralized. The concentration of 1 to 2mg collagen/ml is frequently used to form matrix. Collagen contained in bovine skin is more crosslinked and its extraction requires the use of pepsin. This enzyme cleaves off telopeptides, the nonhelical amino acid sequences at the C- and N-ends of the collagen molecule. The lack of telopeptides may interfere with gel formation and change the properties of the gel. The pores in gel made of acid-extracted rat tail collagen are 1 – 2 µm in diameter. The pores in the gel from pepsin-digested collagen are larger and allow easier migration of the cells.16 Cells can be seeded on top of the gel or be suspended in collagensolution. The cells on collagen may be covered with a second layer of collagen gel to add thecells the third dimension. Fibroblasts placed between two collagen layers migrate intothem.22,23Fibroblasts cultured for a few weeks in the presence of L-ascorbic acid 2-phosphateform a multilayered structure surrounded by hydroxyproline-rich ECM.24 The self-produced dermis-like structure formed in the presence of ascorbic acid in a long term culture contains collagens I and VI.25Fibroblasts embedded in collagen gel cause its contraction. When collagen gel formed in tissue culture dishes remains attached to the walls of the dish, fibroblasts can contract it only in the vertical direction. When the gel is detached from the dish immediatelyD o w n l o a d e d b y [O c e a n U n i v e r s i t y o f C h i n a ] a t 19:57 31 M a r c h 2015A cc ep t ed M a n u s c ri ptafter gelation, it floats in the culture medium and contracts in all directions. The resulting matrix is called floating. The gel may be maintained under tension for 1 or more days before it is detached. This type of matrix is called stressed or stress-released. Contraction of floating collagen matrices gives rise to mechanically relaxed tissue resembling dermis, attached matrices resemble granulation tissue.26,27,28 The shape of liver MFB growing on hard plasticand on collagen gels is shown in Figs. 1 – 3. The cells respond to changing stiffness andtension of the substrate.Collagen properties can be modified by crosslinking collagen molecules and fibrils.Collagen I is a substrate of transglutaminase that introduces ɛ-(γ-glutamyl) lysine cross-links into its molecule at 37o C.29,30 Fibroblast attachment, spreading and proliferation is enhanced on collagen polymerized as a result of transglutaminase treatment.31 Collagen can also be crosslinked by 0.2% glutaraldehyde and used as a matrix for cell culture. No toxicity to fibroblasts is observed when collagen is treated with this glutaraldehyde concentration.32Mechanical properties of the matrix play a significant role in determining cellbehavior. The stiffness of isotropic material is characterized by Young ’s modulus (elasticmodulus). Its unit is Pascal (Pa). The stiffness of soft tissues is low; the stiffness of liver is 0.1to 1 kPa, dermis 1 – 5 kPa and fibrotic tissues 20 to 100 kPa. Young ’s modulus of theprovisional matrix in healing wounds (0.01 to 0.1 kPa) is comparable to that of collagen gel.33Collagen fibrils in 0.2-2.0% gel are similar to those in tumor ECM.34Collagen stiffness can be increased when a portion of liquid is removed byultracentrifugation,34 gel compression 35 or by evaporating the solvent.36 Fibroblasts seeded in matrix containing 20 or 40 mg collagen/ml are viable, migrate and proliferate. They reach a density similar to that found in human dermis.36,37D o w n l o a d e d b y [O c e a n U n i v e r s i t y o f C h i n a ] a t 19:57 31 M a r c h 2015A cc ep t ed M a n u s c ri ptThe differences between cell growth on hard plastic surface and on soft collagen gel suggest that the rigidity of the substrate plays a crucial role. Cells can be placed between two sheets of collagen-coated polyacrylamide gel. The stiffness of polyacrylamide gel can be controlled by changing the percentage of the crosslinking compound, bis-acrylamide, in the reaction mixture and can be adjusted to correspond to the physiological stiffness of tissues.This treatment makes fibroblasts that are well spread in 2D culture change their morphologyinto bipolar or stellate characteristic of fibroblasts in vivo.38 Increased rigidity affects not onlystress fibers formation but also integrin expression on the cell surface.39,40Polyethylene glycol (PEG) can be covalently bonded to collagenous matrix extracted from porcine heart. PEG gels retain fibrillar structure, are more resistant to enzymatic degradation and do not inhibit metabolic activity of incorporated fibroblasts.41Interaction of fibroblasts with collagen gelThe cells can be plated on the surface of collagen gel or incorporated into it to form a tissue-like structure.19,20 Fibroblasts are rounded when they are embedded into collagen gel; theyadopt stellate morphology within a few hours and they are spindle-shaped later.42 Fibroblastand MFB morphology in 3D matrix is comparable to that in their original tissue but muchdifferent from polygonal appearance they adopt on a planar substratum.18,43 Fibroblasts on collagen gel aggregate; this tendency decreases with increasing collagen concentration.18,44Fibroblasts embedded in collagen remodel surrounding matrix. They have few celladhesions on their surface but they produce dendritic extensions that interact with collagen fibrils.45,46 Fibroblasts align the flexible collagen meshwork around themselves and hold collagen fibrils in place. The fibrils are then stabilized by noncovalent interactions that do notD o w n l o a d e d b y [O c e a n U n i v e r s i t y o f C h i n a ] a t 19:57 31 M a r c h 2015A cc ep t ed M a n u s c ri ptrequire cell presence.47,48 Fibroblasts in attached matrices develop isometric tension. The forces they generate do not depend on the stiffness of the substrate.49 The forces produced by fibroblasts not expressing α-SMA increase rapidly within the first six hours after embedding the cells into collagen.50When collagen matrix is attached to the walls of the culture dish, distinct actin stressfibers that develop in fibroblasts can be visualized by phalloidin staining.51,52 Whenfibroblasts are embedded in collagen layer cast on polyacrylamide gels, actin fibers stainingwith rhodamine phalloidin appear if Young ’s modulus is adjusted to 1.6 to 3.6 kPa. Stress fibers formation is facilitated by cell-cell contact. Direct linkage of the cytoskeleton stress fibers mediated by cell surface cadherins maintains tension between neighbouring cells.39,53 When tension generated by the cells reaches a critical level, α-SMA that is at first diffusely distributed in the cytosol is incorporated to preexisting β-actin-containing stress fibers.54 Transcription factor MKL1 attached to globular actin (G-actin) is released after G-actin polymerization and translocated to the nucleus. It binds to the α-SMA gene promoter andinitiates α-SMA expression. Matrix stiffening also activates the small GTPase RhoA and Rhokinase (ROCK) that control the balance between polymerized and depolymerized actin.55These changes correspond to the increasing tension in the forming GT in healing wounds.Cytoplasmic actin microfilament system containing α-SMA characterizes MFB.56Profibrogenic cytokine TGF-β1 and smad signaling are involved in gel contraction byfibroblasts derived from normal skin or hypertrophic scars.57 TGF-β-pretreated fibroblasts cause significantly more rapid gel contraction.58 The degree of substrate stiffness determined by underlying polyacrylamide gel modulates TGF-β-induced transdifferentiation of fibroblasts.59 Both substrate stiffness and the presence of TGF-β are required for the differentiation of liver portal fibroblasts to MFB.60D o w n l o a d e d b y [O c e a n U n i v e r s i t y o f C h i n a ] a t 19:57 31 M a r c h 2015A cc ep t ed M a n u s c ri ptGeneration of the threshold tension necessary for α-SMA incorporation requires formation of large, …supermature “ adhesion sites.54 Contractility of both muscles and non-muscle cells is dependent on the interaction of actin and myosin. Non-muscle myosin II is closely associated with actin stress fibers in cochlear fibrocytes and the contraction of collagen matrix can be prevented by an inhibitor of myosin II function.61 Aging dermalfibroblasts lose the ability of force generation in collagen gel which may be caused bydecreased expression of myosin light chain kinase and Rho kinase.62Increasing collagen concentration supports cell proliferation and suppresses apoptosis.63 Fibroblasts migrate along collagen concentration gradient to the stiffer regions of a collagen construct. This effect is called durotaxis.64 α-SMA-positive MFB appear in wound GT when Young ’s modulus is about 20 kPa.54 The stiffness of collagen matrices containing 1-2 mg collagen is about 50 Pa and the stiffness of plastic used in tissue culture is about 1 GPa.2Fibroblasts, MFB and other cells involved in wound healing are affected by intrinsicforces produced by the ECM and extracellular fluid.65 The final outcome of ECM remodelingis determined both by tissue stiffness and by mechanical loading of the tissue, i.e. the forceapplied to tissue. Mechanical forces influence both cell proliferation and gene expression.Prolonged mechanical loading may result in higher tissue stiffness.66,67Most cells grow only if their surfaces are attached to the ECM. The attachment ofcells to ECM molecules is mediated by integrins. These receptors consisting of subunits α and β link ECM with the actin cytoskeleton and transmit signals from the outside to the cell and vice versa.68 Integrins α1β1, α2β1, α10β1 and α11β1 are collagen receptors.69 The engagement of integrins leads to the activation of signaling cascades, focal adhesion kinase (FAK), extracellular signal-regulated protein kinase (ERK) and Rho GTPases.70,71 DiscoidinD o w n l o a d e d b y [O c e a n U n i v e r s i t y o f C h i n a ] a t 19:57 31 M a r c h 2015A cc ep t ed M a n u s c ri ptdomain receptors (DDR) 1 and 2 represent another family of cell-surface receptors. They are activated by collagens and regulate cell proliferation and ECM synthesis.They are expressed on fibroblasts in healing wounds and in tumors.72,73…Synthetic “ phenotype of fibroblasts in mechanically stressed collagen matricesAttached matrix . Physiological levels of tissue stiffness function as a brake on fibroblastproliferation and collagen I synthesis. Fibrotic diseases are accompanied by tissue stiffening which is no longer regarded as a mere consequence of the disease; it has become clear that it may drive the whole process.74Fibroblasts switch between proliferative and quiescence phenotypes. Fibroblasts in attached gels assume a …synthetic “ phenotype.52 They proliferate and synthesize collagen. The number of cells in attached gels increases rapidly while the culture in floating gels regresses. However, the cells remaining in the floating gels are viable and divide at the samerate when they return to standard culture conditions. Fibroblasts in attached gel are bipolar,fibroblasts in floating gel are stellate.75 DNA synthesis measured by 3H-thymidineincorporation into DNA is almost one order of magnitude higher in the cells on plastic than inthe cells in attached matrix and about two orders higher than in the cells in floating matrix.76Fibroblast proliferation is proportional to collagen concentration in the matrix when the gel is compressed and its Young ’s modulus increased.77 DNA synthesis in attached gel is dependent on the ERK pathway. This signaling pathway is disrupted when the gels are released.78 The cytoskeleton is then disorganized and DNA synthesis is inhibited. However, these two events are independent because only DNA synthesis in adhering gel is affected by an ERK inhibitor.79D o w n l o a d e d b y [O c e a n U n i v e r s i t y o f C h i n a ] a t 19:57 31 M a r c h 2015A cc ep t ed M a n u s c ri ptThe release of attached matrix from the walls of the culture dish 24 hours after casting, (stress-released matrix), induces secretion of cytokines IL-6 and IL-8 by the embedded fibroblasts. The response of cells to TGF-β1 and TNF-α changes, growth inhibition is less severe in stress-released matrix than in the attached one. The signaling networks that include these cytokines are modified.80Collagen synthesis is downregulated on both RNA and protein levels when fibroblastsare transferred from plastic to 3D collagen matrix.81 Both total protein synthesis andcollagen synthesis measured by 3H-proline incorporation is several times higher in attached gels than in floating gels.75,82 The expression of fibrillar collagens I and III in liver portal fibroblasts increases on a stiff substrate in paralel with α-SMA, while the expression of net-forming collagen IV decreases.60 Tensile strength also controls the expression of collagen type XII that is associated with collagen fibrils.83 Collagen α1(I) mRNA synthesis and steady-state level is decreased in fibroblasts transferred from plastic into collagen gel. No change is observed in the expression of fibronectin mRNA.84 Total protein synthesis and collagensynthesis are high in the cells on plastic and in attached fibrin gel but low in floating collagenand fibrin gels. Mechanical forces seem to play a dominant role in this case.85Floating matrix . Contraction of freely-floating matrix by cells is dependent on α-SMAexpression.86,87 Fibroblasts in relaxed collagen gel lose stress fibers and focal adhesions anddo not proliferate. They form dendritic extensions that have microtubule cores and actin rich-tips.88,89 The extent of contraction is dependent on initial collagen concentration; lower density gels contract more rapidly. The release of mechanical tension triggers fibroblast apoptosis.90,91 This effect is specific of collagen, apoptosis is not observed in contractile fibrin gels.92 Signal transduction from the ECM is disturbed.93 Ribosomal RNA content is lower in collagen matrices than in the cells in monolayer.94 mRNA expression of TGF-β1 increases inD o w n l o a d e d b y [O c e a n U n i v e r s i t y o f C h i n a ] a t 19:57 31 M a r c h 2015A cc ep t ed M a n u s c ri ptthe order of plastic, attached matrix, stress-relaxed matrix and floating matrix. The expression of collagenase mRNA is higher in collagen matrix than in the cells on plastic.95 The release of stressed matrix is followed by a burst of c-fos expression and ERK 1/2 kinase activation.96Cell survival, collagen synthesis and degradation are regulated by integrins.Antibodies to α2β1 integrins prevent the contraction and reduce apoptosis.97,98,99 Integrinα11 mRNA and protein are up-regulated in attached collagen gel and down-regulated infibroblasts grown in floating gel.100 Rat liver MFB utilize α1β1 integrin for collagen matrix contraction as α2 subunit is not expressed in HSC, their precursors in vivo.101 The expression of α2 subunit in fibroblasts cultured in collagen gel is dependent on NF-κB activity that is induced by contact of the cells with collagen.102 The expression of the two receptors is regulated differentially and their functions are not identical. α1β1 mediates downregulation of collagen gene expression and α2β1 mediates induction of collagenase.103 Both of them are able to mediate gel contraction but their expression in vitro depends on theenvironment and in vivo on the physiological state of the tissue.101 Matrix contraction maybe enhanced by collagen V that binds integrin αv β3.104Increased synthesis of collagen is observed in the skin of α1-null mice. Col1(I) mRNAlevels in both granulation tissue and fetal fibroblasts are higher in the cells isolated from α1-null animals and embedded in collagen gel than in wild-type cells. Integrins α1β1 provide a feedback inhibition of collagen synthesis.105 Blocking of β1 subunit by a monoclonal antibody, which abrogates phoshorylation of Akt/protein kinase B, protects cells from contraction-induced apoptosis. Phosphatidyl-inositol 3-kinase (PI3K)/Akt signaling is a regulator of cell survival. Downregulation of PI3/Akt survival signal results in apoptosis.D o w n l o a d e d b y [O c e a n U n i v e r s i t y o f C h i n a ] a t 19:57 31 M a r c h 2015A cc ep t ed M a n u s c ri ptConstitutive expression of phophatidylinositol 3-kinase (PI3K) protects fibroblasts from both apoptosis and anoikis.106Secretion of metalloproteinasesMMP are a family of zinc-dependent proteinases that are secreted to the extracellular spaceor localized to the cell surface. They are collectivelly able to cleave all components of ECMand they can modify other biologically active molecules. A subgroup of collagenases comprises MMP-1, -8, -13 and -14 that degrade fibrillar collagens. Gelatinases MMP-2 and -9 cleave denatured collagen and collagen type IV.107Both mechanical forces and the chemical nature of collagen play an important role in regulating MMP expression. Collagenase mRNA expression and activity are higher in fibroblasts cultured on type I collagen gel when compared to cells on plastic.108 Releasing stress by treating fibroblasts on plastic with cytochalasin D that disrupts cell cytoskeletonenhances expresion and secretion of MMP-1, -2, -3, -13 and membrane-bound MMP-14. Theactive form of MMP is more strongly expressed in cells cultured in floating matrices than incells in monolayer.109 Increased expression of MMP-2 and its inhibitor TIMP-2 mRNAs isobserved in fibroblasts when collagen gel with cultured cells is prestrained.110 Contact ofhuman MFB with collagen I gel results in the activation of proMMP-2 that is not observed in the cells grown on plastic or plastic coated with a thin layer of collagen I or IV, laminin or Matrigel. The induction of MMP-2 by accumulating collagen I may contribute to the remodeling of ECM in fibrotic liver.111,112 Increased expression of the active form of MMP-2 on collagen gel is accompanied by up-regulation of MT1-MMP protein. Metalloproteinase MT1-MMP (MMP-14) is known to activate MMP-2.113D o w n l o a d e d b y [O c e a n U n i v e r s i t y o f C h i n a ] a t 19:57 31 M a r c h 2015A cc ep t ed M a n u s c ri ptCollagen degradation is more rapid in floating matrices than in attached gels.114 Active forms of MMP-1 and MMP-2 can be detected around human HSC cultured on collagen gel by in situ zymography and in the culture medium, respectively.115 The expression of MMP-13 increases in rat liver MFB when the cells are embedded in attached collagen gel. However, the observed collagen degradation is a result of a joint action of a fewproteinases.18Collagen contraction is enhanced by MMP activity and impaired by MMP inhibition.MMP activity is stimulated in floating gel.116,117,118 Gel contraction by fibroblasts is greatly accelerated when the matrix is treated with plasmin that may activate MMP-1 secreted by the cells. Fibroblasts in healing wounds are in close proximity to keratinocytes that produce plasminogen activator in response to cytokines. Plasmin may play a role in provisional matrix remodeling.119 Contraction of floating collagen matrix gives rise to a mechanically relaxed tissue resembling dermis. The cells in floating matrix show low capacity to synthesize DNA and proliferate, decreased responsiveness to growth factors and decreased ability tosynthesize collagen.26 Three-dimensional matrix, especially at higher stiffness, impedes cellproliferation and migration. The cells may secrete proteinases and degrade adjacent matrixto create space for these activities. Interconnected multicellular networks are formed in gelswith low Young ’s modulus.120Integrins α1β1 and α2β1 have different functions in the regulation of MMPexpression. MMP-1 mRNA level in fibroblasts cultured in retracting collagen gel is higher than that in cells on plastic. The diference can be eliminated when α2β1 integrin is blocked by a specific antibody. In contrast, the difference is much larger when α1 subunit is blocked. Changes in MMP expression are paralleled by changed expression of Ets-1 transcription factor.103,121 The induction of MMP-13 in periodontal ligament fibroblasts is dependent onD o w n l o a d e d b y [O c e a n U n i v e r s i t y o f C h i n a ] a t 19:57 31 M a r c h 2015。