Biomass production in a 15-year-old poplar short-rotation coppice culture in Belgium
- 格式:pdf
- 大小:871.73 KB
- 文档页数:9

The Biogas Production in Wastewater Treatment Wastewater treatment is an essential process that aims to remove contaminants and pollutants from water before it is released back into the environment. However, this process generates a significant amount of organic waste that can be converted into biogas through anaerobic digestion. Biogas is a renewable energy source that can be used for heating, electricity generation, and transportation. In this essay, we will explore the benefits and challenges associated with biogas production in wastewater treatment plants.One of the primary benefits of biogas production in wastewater treatment is the potential for energy generation. The biogas produced can be used to generate electricity and heat, reducing the reliance on non-renewable sources of energy. This can help to reduce greenhouse gas emissions and mitigate the impacts of climate change. Additionally, the use of biogas can help to reduce operating costs for wastewater treatment plants, as they no longer need to purchase as much energy from external sources.Another benefit of biogas production in wastewater treatment is the potential for waste reduction. The organic waste generated during the treatment process can be converted into biogas, reducing the amount of waste that needs to be disposed of. This can help to reduce the environmental impact of wastewater treatment and improve the sustainability of the process.However, there are also challenges associated with biogas production in wastewater treatment plants. One of the main challenges is the variability of the organic waste generated during the treatment process. The composition of the waste can vary depending on factors such as the time of day, the season, and the type of wastewater being treated. This can make it difficult to optimize the anaerobic digestion process and maximize biogas production.Another challenge is the potential for odors and air emissions associated with the anaerobic digestion process. The production of biogas can release odorous compounds and greenhouse gases such as methane and carbon dioxide. These emissions can be a nuisancefor nearby residents and can contribute to air pollution. Therefore, it is essential to have proper odor control and gas management systems in place to mitigate these impacts.In addition to these challenges, there are also economic considerations associated with biogas production in wastewater treatment plants. While biogas can help to reduce operating costs, there are also significant capital costs associated with the construction and operation of anaerobic digestion systems. The cost of these systems can vary depending on factors such as the size of the plant, the type of technology used, and the local regulatory environment.Despite these challenges, biogas production in wastewater treatment plants has significant potential to reduce greenhouse gas emissions, improve the sustainability of the treatment process, and generate renewable energy. To maximize the benefits of biogas production, it is essential to optimize the anaerobic digestion process, implement effective odor control and gas management systems, and carefully consider the economic feasibility of the technology. With proper planning and management, biogas production can be a valuable tool for improving the sustainability of wastewater treatment and reducing the environmental impact of the process.。


Biodiesel from MicroalgaeRenéH. Wijffelswww.bpe.wur.nlContentsHype cycle of biofuelsTruth of microalgaeDesign of a photobioreactorFeasibility of production of biodiesel from microalgaeHype cycle of biofuelsBiodiesel from microalgae BotryococcusHydrocarbons (average C34)Concentration high (40ĉ70%) Other microalgae20ĉ60% lipidsBiodiesel •High productivities112 344MicroalgaePE 3%; 40% lipids; NLPE 9% ; 80% lipids; NL 1 892Jatropha5 950Oil palm1 250Rapeseed386Sunflower446Soybeans172CornOil ProductivitiesL / ha /year Feedstock18 800Where we Where to•No competition for agricultural landBiomass productivityThese values were calculated for the highest PE values reported for each reactor50100150200250300P onds Tubular Flat panel Theoreticalmaximum B i o m a s s p r o d u c t i v i t y (t o n h a -1 y r -1)E indhoven BonairePotential050001000015000200002500030000SoybeansRapeseedPalm oilJatrophaMicroalgaeMicroalgaeOil Productivity (L ha -1yr -1)Sources: Eindhoven; 2% PE solar ; 20% oil contentBonaire; 2% PE solar 40% oil contentPromisesTheoretical maximum Bonaire: 115,000 L/ha/year Present technology: 27,000 L/ha/year Bioking: 930,000 L/ha/year Green Fuels Technology: 49,000 L/ha/year Petrosun: 150,000 L/ha/year Solix Biofuels: 150,000 L/ha/year Global Green Solutions: 65,200 L/ha/year Green Star Products: 300,000 L/ha/yearDesign of a photobioreactorMaximum use of lightInside of the reactor should be dark Run reactor at high light intensityPhotoĉinhibition at high light intensities Supply of CO2Produced oxygen is toxicPossible designsOpen systemsBubble columnsTubular reactorTubular reactor + dilution of light Flat panelsOpen systems: raceway pondsCyanotech(Hawaii), 75 ha OpenMixing via paddle wheelsSystem that is used mostLow investments costsLimitation in CO2supplyProductivity 20 tonnes. haĉ1. yearĉ1Biomass concentration <0.5 g/lHigh costs for harvestingBubble columnClosedMixing via air and CO2Productivity 50 tonnes. haĉ1. yearĉ1 Biomass concentration 2 g/lHard to scale up (forest)ECN, 70 lWageningen Univ, 70 l Mario Tredici, Univ. FlorenceTubular reactorClosedMixing via air and CO2Productivity 60 tonnes. haĉ1.Algatechnology, Israel yearĉ1High surface/volume ratioBiomass concentration 3 g/lAccumulation oxygenScalableTechnogrow/LGem, MadeTubular reactor + dilution of light+++Tubular reactorDilution of light1.2 haKlötze(Germany)Productivity 80 tonnes. haĉ1.yearĉ1Bioprodukte Prof. Steinberg Produktionsĉund Vertriebs GmbHFlat panel reactorIntensive mixingShort lightĉdark periodsHigh biomassconcentrations (>15 g/l)Productivity 100 ton. haĉ1.Wageningen University yearĉ1Scalable?BenĉGurion Universiteit, IsraëlTechnical and economical feasibilityRaceway ponds Horizontal TubularFlat panelTubular PhotobioreactorDegasser Stack Harvest Nutrient Monitor and Stack 25 %Headspacegas CO 2Solar collectorCentrifugePumptankBiomassInletTpH DOControlUnitgas /CO2Biomass production costs horizontal tubular reactor 1 ha plant10.62 /kg biomass100 ha plant4.02 /kg biomass150 /GJ Present value10 /GJ Centrifuge w estfalia separator AG Centrifuge Feed Pump Medium Filter UnitMedium Feed pump Medium preparation tank Harvest broth storage tank Seaw ater pump station Automatic Weighing Station w ith Silos Culture circulation pump Installations costs Instrumentation and control PipingBuildings Polyethylene tubes P hotobioreactor Culture mediumCarbon dioxide Media Filters Air filtersPow er Labor Payroll charges Maintenance General plant overheadsSensitivity analysis-100-90-80-70-60-50-40-30-20-100Culture medium for freeCO2 for freeBoth CO2 and medium for freeDilution rate 10% v/v per dayP hotosynthetic E fficiency 5%CO2 incentive (15 € / ton CO2)No centrifugationP E 5%; CO2 and medium for free, CO2 incentive Mixing w ith 10* less energyMixing 10*, P E 5%, CO2 incentive, mdium and CO2 free Curacao P E 5%, CO2 Incentive, medium and CO2 free% Decrease in production costBiomass production costCentrifuge w estfalia separator AG Centrifuge Feed P ump Medium Filter UnitMedium Feed pump Medium preparation tank Harvest broth storage tank Seaw ater pump station Automatic Weighing Station w ith Silos Culture circulation pump Installations costs Instrumentation and control PipingBuildings P olyethylene tubes P hotobioreactor Culture mediumCarbon dioxide Media Filters Air filtersPow er Labor Payroll charges Maintenance General plant overheads4.02 €/ kg biomass 10.62 €/ kg biomass0.4 €/ kg biomass 15 €/GJ89% decrease1 ha 100 ha potentialFlat panel reactorFlat panel reactor0.75 mLight Intensity missing the reactor•Sun InclinationNL 21st June–61.5º1.5 m63º0.75 mCost analysisBiomass production costlaboroverheads maintenanceFixed Capital Charges21.34%All others 7.77%Utilities 57.40%Raw material 13.49%Centrifuge westfalia separator AG Centrifuge Feed Pump Medium Filter Unit Medium Feed pump Medium preparation tank Harvest broth storage tank Seawater pump stationAutomatic Weighing Station with Silos Culture circulation pump Installations costsInstrumentation and control Piping BuildingsPolyethylene tubes Photobioreactor Culture medium Carbon dioxide Media Filters Air filters Power LaborPayroll chargesBiomass production costP o w e r 57%Raceway ponds1 pondLenght: 100 mWidth: 10 mCulture Depth: 0.20 mArea/ pond1000 m2Volume /pond200 m3 N.B.T technologies, IsraelFixed Capital Charges60.64%A ll others 15.77%Utilities 7.35%Raw material 16.24%Cost analysisBiomass production cost14 %CentrifugeBiomass production costInstallation costsBuilding costsMaintenance½of considered for PBR 7 %PowerComparison of systems (100ha)Circulation pump46%Air blowers 24% Centrifuge 15 %%Main contributor to biomassproduction cost4.024.035.70€/ kg DWBiomass production cost 341938647M€/ha Investment2967157692180180m 3Culture volume 303010%Daily dilution rate 0.0340.030.2m Light path351.5%Photosynthetic Efficiency 414163632071ton /yearBiomass ProductionHorizontaltubular reactorFlat panelRacewaypond UnitsResearch topics to realize economical feasibility Combine with nutrient removal waste streamsReduce energy input for mixingIncrease photosynthetic efficiencyIncrease lipid productivityGrow algae in biofilmsBiorefinary approach:make value from proteinBuild up experiencewww.bpe.wur.nlSolix Biofuels。

Continuous high-solids anaerobic co-digestion of organic solid wastes under mesophilic conditionsDong-Hoon Kim a ,Sae-Eun Oh b ,⇑a Wastes Energy Research Center,Korea Institute of Energy Research,102,Gajeong-ro,Yuseong-gu,Daejeon 305-343,Republic of KoreabDepartment of Environmental Engineering,Hanbat National University,San 16-1,Duckmyoung-dong,Yuseong-gu,Daejeon,Republic of Koreaa r t i c l e i n f o Article history:Received 4November 2010Accepted 17May 2011Available online 18June 2011Keywords:Dry anaerobic digestion Methane Food waste Paper waste Livestock waste Ammoniaa b s t r a c tWith increasing concerns over the limited capacity of landfills,conservation of resources,and reduction of CO 2emissions,high-solids (dry)anaerobic digestion of organic solid waste (OSW)is attracting a great deal of attention these days.In the present work,two dry anaerobic co-digestion systems fed with differ-ent mixtures of OSW were continuously operated under mesophilic conditions.Dewatered sludge cake was used as a main seeding source.In reactor (I),which was fed with food waste (FW)and paper waste (PW),hydraulic retention time (HRT)and solid content were controlled to find the maximum treatability.At a fixed solid content of 30%total solids (TS),stable performance was maintained up to an HRT decrease to 40d.However,the stable performance was not sustained at 30d HRT,and hence,HRT was increased to 40d again.In further operation,instead of decreasing HRT,solid content was increased to 40%TS,which was found to be a better option to increase the treatability.The biogas production rate (BPR),CH 4produc-tion yield (MPY)and VS reduction achieved in this condition were 5.0m 3/m 3/d,0.25m 3CH 4/g COD added ,and 80%,respectively.Reactor (II)was fed with FW and livestock waste (LW),and LW content was increased during the operation.Until a 40%LW content increase,reactor (II)exhibited a stable perfor-mance.A BPR of 1.7m 3/m 3/d,MPY of 0.26m 3CH 4/g COD added ,and VS reduction of 72%was achieved at 40%LW content.However,when the LW content was increased to 60%,there was a significant perfor-mance drop,which was attributed to free ammonia inhibition.The performances in these two reactors were comparable to the ones achieved in the conventional wet digestion and thermophilic dry digestion processes.Ó2011Elsevier Ltd.All rights reserved.1.IntroductionIn order to mitigate the effects of climate change,the Kyoto Pro-tocol was announced in 1997,dictating that industrial countries should reduce their total greenhouse gas (GHG)emissions by 5.2%from the 1990level by the end of 2012.This target can only be met with a significant transition from fossil fuels to alternative energy sources that are cheap,renewable,and nonpolluting (Saxe-na et al.,2009).Tidal,geothermal,hydroelectric,and wind power could be suitable candidates in some countries;however,they are not expected to become the dominant energy sources of the fu-ture (Zidansek et al.,2009).Meanwhile,biomass is spreading throughout the world,and thereby is not subject to world price fluctuations or supply uncertainties,in contrast with imported fuels.In addition,it is a carbon neutral resource in its life cycle (Fortman et al.,2008).Anaerobic digestion (AD)is a biological process in which organic matter is degraded and converted to clean biogas under anaerobic conditions.The produced CH 4can then be utilized for heat or electricity generation,replacing fossil fuels and thereby reducing carbon dioxide emissions (Salminen and Rintala,2002).EU policies concerning renewable energy have set forward the task of supplying 5%of the European energy demands from AD biogas by year 2020(Holm-Nielson et al.,2009).In addition,the effluent sludge may be composted or used for soil conditioning depending on its character-istics (Vallini et al.,1993).Nearly all kinds of organic solid waste (OSW)could be the feedstock for AD,but the performance is largely dependent on the types of waste.Food waste (FW)and paper waste (PW)are generally the main waste streams of OSW in urban area,while FW and livestock waste (LW)are main ones in rural areas.In South Korea,the generation of FW reached 15,142tons/d,accounting for 40.7%of municipal solid waste in 2008.Most FW are currently recycled as animal feed and compost,but the demand for them is low due to their poor quality (Kim et al.,2009).PW (35.1%)is the largest source of flammable wastes,and most of them are disposed in landfills.LW is both organic and nitrogen rich,but contains a large amount of pathogens.In South Korea,around 90%of LW is now composted,which requires a lot of oxygen and emits CO 2during the process (MOE 2009).All the above-mentioned OSW have high organic content,making them suitable feedstocks of AD.However,as conventional0956-053X/$-see front matter Ó2011Elsevier Ltd.All rights reserved.doi:10.1016/j.wasman.2011.05.007Corresponding author.Tel.:+82428211263;fax:+82428211476.E-mail address:saeun@hanbat.ac.kr (S.-E.Oh).AD proceeds under a slurry state(<5%TS,Total Solids),a large amount of external water is required foraccordingly not only increase the energyheating and feed slurry pumping,but alsoeffluent that should be dewatered(come these drawbacks,dry digestion orcan be employed in which solids withfed to the reactor(Bolzonella et al.,2003).During the1990s,dry digestion prevailedseveral commercialized dry digestionDebare,1992),KOMPOGAS(Willinger et(Laclos et al.,1997),were developed.such as agricultural residues,sludge cake,ted.Recently,with increasing concernslandfill,conservation of resources,anddry digestion is garnering increasedsearch has been limited to batch tests orstart-up period,and all the systems havemophilic conditions based onbeing favorable in terms of hydrolysis andster-Carneiro et al.,2007;Shuguang et al.,et al.,2008a,b).The experience ofin terms of performance stability for athe maximum treatability of the system,andat mesophilic conditions(30–40°C)wouldtive,thereby enhancing economic viability.In the present work,two dry anaerobicwith different mixtures of OSW weremesophilic conditions.The anaerobickinds of waste sources is adigestibility and biogas production bytary effects,which offset the lack ofsubstances(Kim et al.,2007).In theand PW,hydraulic retention time(HRT)and the solid contentswere controlled tofind maximum treatability,while the reactor (R(II))fed with FW and LW,LW ratio and HRT were controlled dur-ing the operation.2.Materials and methods2.1.Feedstock and seeding sourceFW collected from a school cafeteria,and PW comprised of toi-let paper,newspaper,and copy paper were shredded by a hammer crusher(TOP-03H)and cut crusher(TOP-03-CC),respectively,to a diameter of less than5mm.Both crushers were manufactured by the Korean Mechanics Engineering Corp.LW collected from a stor-age tank of a local livestock wastewater treatment plant was di-rectly used as a feedstock.The characteristics of FW and LW are arranged in Table1.In R(I) operation,the mixing ratio of FW and PW was set at7:3on a weight basis and water was added to adjust the TS concentration. The volatile solids content(VS/TS),total nitrogen(TN),and chem-ical oxygen demand(COD)concentration of the mixed waste was94.5±0.9%(VS/TS),0.014±0.004g N/g TS,and1.09±0.10g COD/g TS,respectively.As a seeding source,we used a mixture of dewatered sludge cake and anaerobic digester sludge,taken from the same local wastewater treatment plant.The characteristics of these two dif-ferent types of sludge are presented in Table2.Dewatered sludge cake and anaerobic digester sludge were mixed at a4:1ratio ona volume basis,resulting in initial TS and VS concentrations of17.3%and7.6%,respectively.2.2.Reactor operationAs shown in Fig.1,a horizontal-type cylindrical reactor was used for dry AD.The total volume of the reactor was60L with a diameter and length of320mm and750mm,respectively.The broth was agitated by four impellers at25rpm.Thirty liters of seeding source was added to the reactor,and purged with N2for 10min in order to provide anaerobic conditions.As an adaptation period,there was no substrate injection forfive days in both reactors.To R(I),0.4L of substrate(30%TS),corresponding to100days of HRT(Phase I-1),was fed daily.There was no sludge waste dis-charge until the inside sludge volume reached an effective volume of40L.In further operation,HRT was decreased to60(Phase I-2), 40(Phase I-3),and30d(Phase I-4)at afixed solid content of30% TS.As a decline in the system performance was observed in Phase I-4,HRT was increased to40d(Phase I-5)again for performance recovery.Solid content was subsequently increased to40%TS (Phase II)and50%TS(Phase III)at40d of HRT.Just as in the R(I)operation,initially,0.4L of substrate com-prised of mainly FW was fed daily without sludge waste until the inside sludge volume reached an effective volume.After that,we began adding LW at a ratio of10%(Phase I)and20%(Phase II)onTable1Characteristics of food waste and livestock waste.Item Unit Food waste Livestock wasteTotal solids(TS)g TS/L208±10212±8Volatile solids(VS)g VS/L189±8153±11Total COD g COD/L240±20256±19Total nitrogen(TN)g N/L 6.9±0.516.2±1.0Ammonia g NH4–N/L0.5±0.1 3.4±0.2pH– 4.0±0.28.0±0.1Table2Fig.1.Schematic of anaerobic dry digestion system.1944 D.-H.Kim,S.-E.Oh/Waste Management31(2011)1943–1948a weight basis.HRT was decreased to60LW content increased to40%(Phase IV)further operation.In both reactors,For an efficient injection of solid-typepump was turned on after it(=Q)wasside sludge(=5Q).All systems weretrolled(35±1°C)room.2.3.AnalysisMeasured biogas production wasditions of temperature(0°C)and pressurecontents of CH4,N2,and CO2wereraphy(GC,Gow Mac series580)using adetector and a1.8Â3.2mmporapak Q(80/100mesh)with helium as aatures of injector,detector,and column50°C,respectively.Volatile fatty acidswere analyzed by a high performance(HPLC)(Finnigan Spectra SYSTEM LC,an ultraviolet(210nm)detector(UV1000,Thermo Electron)andan100Â7.8mm Fast Acid Analysis column(Bio-Rad Lab.)using 0.005M H2SO4as a mobile phase.The liquid samples were pre-treated with a0.45l m membranefilter before injection to the HPLC.The concentrations of TS,VS,COD,alkalinity,pH,TN,and ammonia were measured according to Standard Methods(APHA, 1998).3.Results and discussion3.1.Start-up of dry ADThe adaptation of seeding inoculum to the feedstock and oper-ating conditions is an important issue in AD(Fdez-Guelfo et al., 2010).Especially in dry AD,as high-solids are fed to the reactor,successful:CH4production was observed from thefirst day,and the biogas production was stabilized within30d(Fig.2and Fig.5).However,in employing this strategy,special care should be taken with regard to ammonia inhibition,since dewatered sludge cake is rich in nitrogen,which is degraded into ammonia during the digestion,and nitrogen removal reaction is negligible during AD process.In this study,the ammonia concentration dur-ing the start-up period did not exceed3500mg NH4–N/L,and as the substrate was continuously supplied,its concentration gradu-ally decreased to a range of1500–2500mg NH4–N/L.It is strongly recommended that if the dewatered sludge cake contains a high concentration of ammonia,it should be removed by stripping or applying some other methods prior to seeding.Or,toxicity ofFig. 2.Daily biogas production in Reactor I(fed with FW and PW)at various operating conditions.Fig.3.Change of CH4content and VS concentration in reactor(I)(fed with FW and PW)at various operating conditions.Fig.4.Change of pH and alkalinity in reactor(I)(fed with FW and PW)at various operating conditions.2.0,3.5,5.0,7.5,and10.0kg TS/m3/d,respectively.With an SLR increase,biogas production increased.However,during Phase I-4, increased biogas production was not sustained.From the180th day,a drastic biogas production drop was observed along with a decrease of CH4content in the produced biogas(Fig.3).CH4con-tent was in a range of50–55%till Phase I-3but it dropped below 50%in Phase I-4Also,the VS concentration in the reactor clearly showed an increasing trend.In addition,as shown in Fig.4,both the pH and alkalinity concentration,which were maintained at over7.5and8000mg CaCO3/L,respectively,significantly dropped. The total organic acids concentration was lower than150mg/L un-til Phase I-3,but it increased to1800mg/L,at which point most of the acids consisted of propionic acid(1600mg/L)and butyric acid (200mg/L).This indicates that the balance was broken between the production of acids and their consumption by methanogenesis at Phase I-4.The accumulation of acids decreased the pH,resulting in a total system failure.The growth rate of the acidogenic bacteria is much higher than that of methanogenic archea,and the bacteria can be active at a weak acidic condition,whereas the archea can-not.Therefore,the balance between the generation of acids and their conversion to CH4is important in a single-stage anaerobic digestion process(Ward et al.,2008).During further operation,HRT was increased again to40d (Phase I-5)in order to see the performance recovery.The produc-tivity of biogas recovered,and all important parameters including CH4content,VS concentration,pH,and alkalinity clearly showed a recovering trend.In Phase II,instead of controlling HRT,solid content was in-creased to40%TS as a means to increase SLR.At this time,stable biogas production was observed without showing a decreasing in CH4content,pH,and alkalinity.Atfirst glance,the VS concentra-tion in the reactor seemed increasing,but this did not indicate a decrease of VS reduction efficiency since higher solid content sub-strate was fed.VS reduction efficiency achieved in treating both a 30%TS and40%TS of substrate reached around80%.However,a further increase in substrate concentration to50%TS(Phase III)re-increase of biogas production in Phase II,and as solid loading increased with an HRT decrease(Phase III),biogas production gradually increased and achieved stable performance from the 150th day onwards.At an initial stage of Phase IV,biogas produc-tion seemed to decrease,but it soon recovered.However,at Phase V,when the LW content increased to60%,there was a drastic drop in biogas production.Also,CH4content,which was maintained at over50%,was decreased to40%.The performance failure during Phase V might have resulted from the inhibitory effect of free ammonia(NH3).As shown in Fig.6,the total ammonia concentration gradually increased from 2000to7000mg NH4–N/L,as the operation went on.LW contained a substantial nitrogen source that would be converted to ammonia via AD.It is well known that NH3has much higher toxicity than the ammonia ion form(NH4+)since NH3can directly penetrate cells, thereby hindering the metabolism(Angelidaki and Ahring,1993; Kadam and Boone,1996).NH3concentration depends on pH and temperature,as shown in Eq.(1),and NH3inhibition on AD gener-ally starts from150mg NH3–N/L(Calli et al.,2005).In Phase IV, although NH3concentration reached700mg NH3–N/L,the perfor-mance was stable,which was attributed to the adaptability.When anaerobic microorganisms are gradually exposed to high concen-trations of ammonia,they can adapt and perform normal activities (Calli et al.,2005;Sung and Liu,2003).According to Calli et al. (2005),by gradual increases of ammonia concentration,80%of COD removal was maintained until800mg NH3–N/L.In Phase V, as LW ratio increased to60%,the total ammonia concentration did not vary,but as the pH increased to8.3(Fig.7),NH3concentra-tion reached around1000mg NH3–N/L.At this highly toxic condi-tion,not only methanogens but also acidogens were inhibited, considering the increasing tendency of VS concentration in the reactor.½Free ammonia ¼½Total ammonia Â1þ10ÀpHÀ0:09018þ2729:92TðKÞ8><>:9>=>;À1ð1ÞFig.5.Daily biogas production in reactor(II)(fed with FW and LW)at various operating conditions.6.Ammonia concentration change in reactor(II)(fed with FW and LW) various operating conditions.1946 D.-H.Kim,S.-E.Oh/Waste Management31(2011)1943–1948enough compared to HRT,and therefore,the representing perfor-mance was obtained under these conditions.In R(I),the average biogas production rate (BPR),CH 4content,and VS reduction were 5.0±0.1m 3/m 3/d,53.7±1.2%,and 79.8±2.3%,respectively.The average CH 4production yield (MPY),which is an important parameter determining the success of an AD system,was 0.27±0.01m 3CH 4/kg TS added .Based on the input COD,MPY was 0.25m 3CH 4/kg COD added ,indicating that 71%of the energy content in the OSW was converted to clean bio-energy,CH 4.In R(2),the average BPR,CH 4content,VS reduction,and MPY were 1.7±0.1m 3/m 3/d,54.3±2.5%,71.5±1.9%,and 0.26m 3CH 4/kg COD added ,respectively.All performances achieved in these two reactors were compara-ble with the ones achieved in the conventional wet digestion pro-cess (Mata-Alvarez et al.,2000),and thermophilic dry digestion process.Montero et al.(2009)operated continuous thermophilic dry digestion system treating synthetic OSW,and achieved an 80%of VS removal efficiency and 0.26m 3CH 4/kg COD added of MPY.In terms of energy saving,mesophilic operation is more favorable than thermophilic operation,and especially in treating LW that might cause ammonia inhibition,mesophilic operation is superior.If R(II)was operated under thermophilic conditions (55°C),the NH 3concentration would have reached around 2000mg NH 3–N/L at Phase IV (pH 8.0,total ammonia =7000mg NH 4–N/L),which was more than two times higher than that of mesophilic operation.4.ConclusionsForm the continuous operation of two mesophilic-dry anaerobic co-digestion systems treating OSW comprised of FW,PW,and LW,the following conclusions were drawn.(1)In R(I)fed with FW and PW,during Phase I (30%TS),biogasproduction increased as HRT decreased till 40d.But at 30d of HRT,this production increase was not sustained,and a decreasing trend appeared in terms of CH 4content,pH,and alkalinity concentration.The VS concentration was also increased,indicating the inhibition of solid hydrolysis.The performance was recovered when HRT was increased to 40d again.Instead of controlling HRT,the substrate solid content was increased to 40%TS during further operation,which was found to be a better option to increase thetreatability.At this condition,stable performance was achieved with an average BPR of 5.0m 3/m 3/d,MPY of 0.25m 3CH 4/g COD added ,and VS reduction of 80%.A further increase of the substrate concentration to 50%TS resulted in a drastic decrease in performance.(2)In R(II)fed with FW and LW,the LW ratio was graduallyincreased during the operation.Until a 40%LW content increase,R(II)exhibited a stable performance with an aver-age BPR of 1.7m 3/m 3/d,MPY of 0.26m 3CH 4/g COD added ,and VS reduction of 72%.However,in Phase V when LW ratio was increased to 60%,there was a significant performance drop,which was attributed to NH 3inhibition.AcknowledgementsThis work was supported by New &Renewable Energy Infra-structure Development Program (Grant No.2008-N-BI18-P-01-000)under the Korea Ministry of Knowledge Economy (MKE).ReferencesAngelidaki,I.,Ahring,B.K.,1993.Thermophilic anaerobic digestion of livestockwaste:the effect of ammonia.Appl.Environ.Biotechnol.38,560–564.APHA,AWWA,WEF,1998.Standard methods for the examination of water andwastewater.20th ed.Baltimore.American Public Health Association.2,pp.57–59.Bolzonella, D.,Innocenti,L.,Pavan,P.,Traverso,P.,Cecchi, F.,2003.Semi-drythermophilic anaerobic digestion of the organic fraction of municipal solid waste:focusing on the start-up phase.Bioresour.Technol.86,123–129.Calli,B.,Metroglu,B.,Inanc,B.,Yenigun,O.,2005.Effects of high free ammoniaconcentrations on the performances of anaerobic bioreactors.Process Biochem.40,1285–1292.Fdez-Guelfo,L.A.,Alvarez-Gallego,C.,Marquez,D.S.,Garcia,L.I.R.,2010.Start-up ofthermophilic-dry anaerobic digestion of OFMSW using adapted modified SEBA inoculum.Bioresour.Technol.101(23),9031–9039.Fernandez,J.,Perez,M.,Romero,L.I.,2008.Effect of substrate concentration on drymesophilic anaerobic digestion of organic fraction of municipal solid waste (OFMSW).Bioresour.Technol.99,6075–6080.Fortman,J.L.,Chhabra,S.,Mukhopadhyay,A.,Chou,H.,Lee,T.S.,Steen,E.,Keasling,J.D.,2008.Biofuel alternatives to ethanol:pumping the microbial wall.Trends Biotechnol.26(7),375–381.Forster-Carneiro,T.,Perez,M.,Romero,L.I.,Sales, D.,2007.Dry-thermophilicanaerobic digestion of organic fraction of the municipal solid waste.Bioresour.Technol.98,3195–3203.Forster-Carneiro,T.,Perez,M.,Romero,L.I.,2008a.Anaerobic digestion of municipalsolid wastes:dry thermophilic performance.Bioresour.Technol.99,8180–8184.Forster-Carneiro,T.,Perez,M.,Romero,L.I.,2008b.Thermophilic anaerobicdigestion of source-sorted organic fraction of municipal solid waste.Bioresour.Technol.99,6763–6770.Holm-Nielson,J.B.,Seadi,T.A.,Oleskowicz-Popiel,P.,2009.The future of anaerobicdigestion and biogas utilization.Bioresour.Technol.100,5478–5484.Kadam,P.C.,Boone,D.R.,1996.Influence of pH on ammonia accumulation andtoxicity in halophilic,methylotrophic methanogens.Appl.Environ.Microbiol.62,4486–4492.Kim,D.H.,Kim,S.H.,Shin,H.S.,2009.Hydrogen fermentation of food waste withoutinoculum addition.Enzyme Microb.Technol.45,181–187.Kim,H.W.,Han,S.K.,Oh,S.E.,Shin,H.S.,2007.Response surface optimization ofsubstrates for thermophilic anaerobic codigestion of sewage sludge and food waste.J.Air Waste Manage.57,309–318.Laclos,H.F.,Desbois,S.,Saint-Joly,C.,1997.Anaerobic digestion of municipal solidorganic waste:VALORGA Full-scale plant in Tilburg,the Netherlands.Wat.Sci.Tech.36(6–7),457–462.Mata-Alvarez,J.,Mace,S.,Llabres,P.,2000.Anaerobic digestion of organic solidwastes.An overview of research achievements and perspectives.Bioresour.Technol.74,3–16.Ministry of Environment,2009.The state of waste generation and treatment in2008.Seoul.Montero,B.,Garcia-Morales,J.L.,Sales,D.,Solera,R.,2009.Analysis of methanogenicactivity in a thermophilic dry anaerobic reactor:use of fluorescent in situ hybridization.Waste Manage.29,1144–1151.Radwan, A.M.,Sebak,H.A.,Mitry,N.R.,El-Zanati, E.A.,Hamad,M.A.,1993.Dryanaerobic fermentation of agricultural residues.Biomass Bioenergy 5(6),495–499.Salminen, E.,Rintala,J.,2002.Anaerobic digestion of organic solid poultryslaughterhouse wate –A review.Bioresour.Technol.83,13–26.Saxena,R.C.,Adhikari,D.K.,Goyal,H.B.,2009.Biomass-based energy fuel throughbiochemical routes.Renew.Sust.Energ.Rev.13(1),167–178.Fig.7.Change of pH and VS concentration in reactor (II)(fed with FW and PW)at various operating conditions.31(2011)1943–19481947Shuguang,L.,Tsuyoshi,I.,Masao,U.,Masahiko,S.,2007.Start-up performances of anaerobic mesophilic and thermophilic digestions of organic solid wastes.J.Environ.Sci.19,416–420.Six,W.,De Baere,L.,1992.Dry anaerobic conversion of municipal solid waste by means of the DRANCO process.Wat.Sci.Tech.25,295–300.Sung,S.,Liu,T.,2003.Ammonia inhibition on thermophilic anaerobic digestion.Chemosphere53,43–52.Vallini,G.,Cecchi,F.,Pavan,P.,Pera,A.,Mata-Alvarez,J.,Bassetti,A.,1993.Recovery and disposal of theorganic fraction of MSW by means of combined anaerobic and aerobic biotreatments.Wat.Sci.Tech.27(2),121–132.Vavilin,V.A.,Vasiliev,V.B.,Rytov,S.V.,1995.Modeling of gas pressure effects on anaerobic digestion.Bioresour.Technol.52,25–32.Ward, A.J.,Hobbs,P.J.,Holliman,P.J.,Jones, D.L.,2008.Optimization of the anaerobic digestion of agricultural resources.Bioresour.Technol.99(17), 7928–7940.Willinger,A.,Wyder,K.,Metzler,A.C.,1993.KOMPOGAS-a new system for the anaerobic treatment of source separated waste.Wat.Sci.Tech.27(2),153–158. Zidansek,A.,Blinc,R.,Jeglic,A.,Kabashi,S.,Bekteshi,S.,Slaus,I.,2009.Climate changes,biofuels and the sustainable future.Int.J.Hydrogen Energy34,6980–6983.1948 D.-H.Kim,S.-E.Oh/Waste Management31(2011)1943–1948。
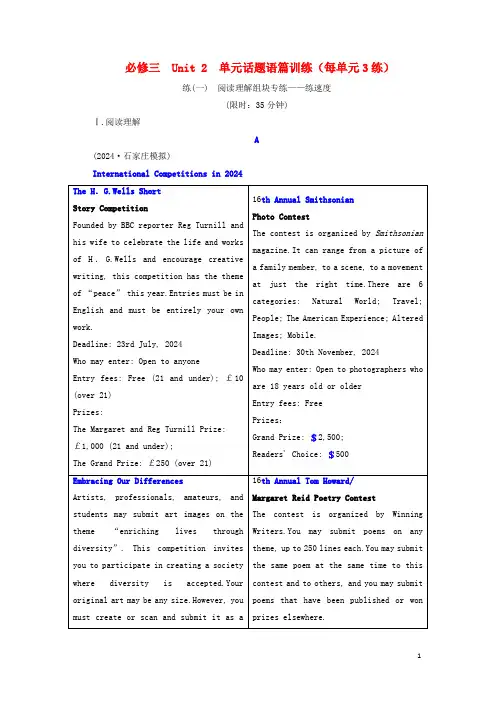
必修三 Unit 2 单元话题语篇训练(每单元3练)练(一) 阅读理解组块专练——练速度(限时:35分钟)Ⅰ.阅读理解A(2024·石家庄模拟)International Competitions in 2024A.Creating the story in English.B.Having the work scanned.C.Paying money for the entry.D.Submitting the work by July.解析:选A 细微环节理解题。
依据The H.G.Wells Short Story Competition部分中的“Entries must be in English and must be entirely your own work”可知,写故事竞赛要求参赛者的作品是用英语写的原创作品,故选A。
2.What is special about “16th Annual Smithsonian Photo Contest”?A.It gives a theme. B.It offers a grand prize.C.It has an age limit. D.It has two categories.解析:选C 细微环节理解题。
依据16th Annual Smithsonian Photo Contest部分中的“Who may enter: Open to photographers who are 18 years old or older”并结合其他三个竞赛的“Who may enter”的信息可知,这个竞赛对参赛者的年龄有肯定的限制,故选C。
3.Who may get Margaret Reid Prize after winning the contest?A.Short story writers. B.Photographers.C.Art designers. D.Poem writers.解析:选D 推理推断题。

Corresponding Author: Mariela González, Dpto. Botánica, Facultad de Ciencias Naturales y Oceanográficas, Universidad de Concepción, Casilla 160-C, Concepción, Chile. Telephone: (56-41) 204785 - Fax: (56-41) 246005 - e-mail: mgonzale@udec.cl Received: March 26, 2003. Accepted: September 12, 2003INTRODUCTIONSince the 1930s, the freshwater microalga Haematococcus pluvialis Flotow (Volvocales, Chlorophyceae) has been widely recognized by its ability to accumulate large amounts of the ketocarotenoid astaxanthin (Elliot, 1934),but interest in this alga has been renewed in recent years due to the increasing demand for natural pigments of vegetable origin to be used as a substitute for their synthetic counterparts. Astaxanthin is used as a source of pigmentation for fish in aquaculture (especially salmonids) and for eggs in the poultry industry, but it is also recognized as having a higher antioxidant activity than other carotenoids (Meyers, 1994; Miki,Optimization of biomass, total carotenoids and astaxanthin production in Haematococcus pluvialis Flotow strain Steptoe (Nevada, USA) under laboratory conditionsANA S CIFUENTES, MARIELA A GONZÁLEZ, SILVIA VARGAS, MARITZA HOENEISEN and NELSON GONZÁLEZDepartamento de Botánica, Facultad de Ciencias Naturales y Oceanográficas, Universidad de Concepción, Casilla 160-C, Concepción, ChileABSTRACTThe microalga Haematococcus pluvialis Flotow is one of the natural sources of astaxanthin, a pigment widely used in salmon feed. This study was made to discover optimal conditions for biomass and astaxanthin production in H.pluvialis from Steptoe, Nevada (USA), cultured in batch mode. Growth was carried out under autotrophic (with NaNO 3, NH 4Cl and urea) and mixotrophic conditions (with 4, 8, 12 mM sodium acetate) under two photon flux densities (PFD) (35 and 85 µmol m -2 s -1). The carotenogenesis was induced by 1) addition of NaCl (0.2 and 0.8 %),2) N-deprivation and 3) high PFD (150 µmol m -2 s -1). Total carotenoids were estimated by spectrophotometry and total astaxanthin by HPLC. Ammonium chloride was the best N-source for growth (k=0.7 div day -1, 228-258 mg l -1and 2.0 x 105 - 2.5 x 105 cells ml -1 at both PFD, respectively). With increasing acetate concentration, a slight increment in growth occurred only at 85 µmol m -2 s -1. Light was the best inductive carotenogenic factor, and the highest carotenoid production (4.9 mg l -1, 25.0 pg cell -1) was obtained in cultures pre-grown in nitrate at low light.The NaCl caused an increase in carotenoid content per cell at increasing salt concentrations, but resulted in a high cell mortality and did not produce any increment in carotenoid content per volume compared to cultures grown at 150 µmol m -2 s -1. The highest carotenoid content per cell (22 pg) and astaxanthin content per dry weight (10.3 mg g -1)(1% w/w) were obtained at 85 µmol m -2 s -1 with 0.8% NaCl.Key terms: astaxanthin, carotenogenesis, growth, Haematococcus pluvialis .1991; Kobayashi et al., 1997a). Only a few microorganisms (some species of bacteria,lichens, fungi and microalgae) have been reported to synthesize astaxanthin (Johnson and Schroeder, 1995; Armstrong, 1997), and H. pluvialis is one of the most challenging to study, since it is capable of accumulating the highest proportion of astaxanthin in relation to its dry weight: 1.5 to 5.0% w/w (Johnson and Schroeder 1995; Krishna and Mohanty, 1998). Despite this physiological advantage, which has been extensively studied, the published results have discouraged, to some extent, the commercial production of astaxanthin from Haematococcus. This alga exhibits some unfavorable characteristics when compared to other microalgae successfully cultivatedBiol Res 36: 343-357, 2003344CIFUENTES ET AL. Biol Res 36, 2003, 343-357at commercial scale (i.e.,Dunaliella spp., Spirulina spp.) These concerns are mainly related to its slow growth rate and its complex life cycle, exhibiting motile and non-motile cells, solitary and/or grouped in palmella stages (Elliot, 1934; Triki et al., 1997; Lee and Ding, 1994) which is not yet well understood.To date, it is well known that the accumulation of astaxanthin in H. pluvialis is associated preferentially with a morphological transformation of green motile vegetative cells to deep-red non-motile cysts, and many research efforts have sought to enhance the relatively slow growth rate of the motile vegetative cells: 0.5 – 0.7 div day-1 (Zlotnik et al., 1993; Chaumont and Thèpenier, 1995; Fan et al., 1994; Barbera et al., 1993; Gong and Chen, 1997; Hagen et al., 2001; Orosa et al., 2001), exceptionally 0.9 div day-1 (Grunewald et al., 1997; Fan et al., 1994; Hagen et al., 2000). Other research efforts have focused on the low maximal cell densities exhibited by this alga at different culture conditions: 1.5 - 2.5 x 105 cells ml-1 (Hagen et al., 1993; Lee and Ding, 1994, 1995; Harker et al., 1996b; Grünewald et al., 1997), more exceptionally 5.5 x 105 cells ml-1 or higher (Kobayashi et al., 1993; Spencer, 1989; Bubrick, 1991; Kakizono et al., 1992). From this point of view, any factor tending to enhance and/or to maintain vegetative growth would not allow astaxanthin cell accumulation, and any astaxanthin inductive condition tends to constrain the algal growth. This pattern of cell growth and carotenoid accumulation has generated two productive strategies for growing Haematococcus: one, in a single step using a suitable medium (sub-optimum) for both biomass and astaxanthin production, where astaxanthin is accumulated while cells are growing, and the other, in two consecutive steps, the first under optimal conditions for vegetative growth followed by another for astaxanthin production in non-growing cells.There exists a great debate concerning the function of secondary carotenoids in H. pluvialis and where and when they are synthesized. Numerous studies have suggested a strict relationship between astaxanthin accumulation and the formation of resting cells (cysts) (Boussiba and Vonshak, 1991; Kobayashi et al., 1991). Other works, however, have clearly shown astaxanthin accumulation in the motile vegetative stage (Lee and Ding, 1994; Chaumont and Thèpenier, 1995; Grünewald et al., 1997; Sun et al., 1998; Hagen et al., 2000). Currently, commercial production of astaxanthin by H. pluvialis has been reported based on both strategies using a two-step culture [Cyanotech Corporation and Aquasearch Inc., Microbio Resources Inc., and Algatec Inc. (Bubrick, 1991)] and/ or a single step process [Microgaia Inc. (Olaizola, 2000)] and both processes are carried out under photoautotrophic conditions. Although mixotrophic metabolism in this alga has been studied and documented (Borowitzka et al., 1991; Kobayashi et al., 1992; Gong and Chen, 1997), and heterotrophic growth has been reported in some strains of H. pluvialis (Kobashayi et al., 1992; Hata et al., 2001), these conditions have not been applied in cultures at commercial scale.The present study was aimed at optimizing the phototrophic requirements for growth of H. pluvialis in relation to the nitrogen source and the addition of sodium acetate to estimate mixotrophic growth in batch cultures at different photon flux densities. From the various factors inducing astaxanthin production reported in the literature, the addition of salt, the deprivation of nitrogen in the medium and the higher irradiance, were examined here. The strain under study (from Steptoe, Nevada, USA) has not been studied before, and these results constitute the first set of data to compare it with other strains of H. pluvialis. Ranges of optimal culture conditions found for different strains may show great disparity. Results obtained by our working group on different strains of Dunaliella salina reinforce this assertion (Cifuentes et al., 1992, 1996a, b, 2001; Gómez et al., 1999).MATERIALS AND METHODSOrganism, growth medium and maintenance conditions of the inocula Haematococcus pluvialis (Flotow) strain Steptoe (Nevada, USA) was donated in345CIFUENTES ET AL. Biol Res 36, 2003, 343-357unialgal condition by Dr. Ralph Lewin in1989, and since that date has beenmaintained in the Microalgal CultureCollection at the University of Concepción,Concepción, Chile. The alga was initiallygrown in Bristol medium (Starr and Zeikus,1987) in a static continuous culture regime(batch mode) under the followingconditions: temperature of 23°±2ºC, photonflux density (PFD) of 35 µmol m-2s-1,photoperiod of 16:8 (L:D), without aerationbut manually agitated twice a day. Thestable growth parameters obtained underthese conditions were: Ni = 103 cells ml-1,N7days = 5 x 104 cells ml-1, k= 0.8 div day-1,v= 10 ml vinocule = 200 µl. In these conditions,the inocula for the experiments consisted of motile vegetative cells (98%) and cysts (2%).Optimum nitrogen source for growth Three sources of nitrogen were tested in the medium: namely, sodium nitrate, ammonium chloride, and urea at concentrations of 2.9 mM. The cultures were grown in 500 ml Erlenmeyer flasks with 200 ml of medium, under two different continuous PFD: 35 and 85 µmol m-2s-1 (supplied by fluorescent cool daylight lamps), at 23°±2ºC, without aeration and agitated manually twice a day. The pH of the medium was adjusted to 6.0 after being autoclaved, and the initial cell density was 2 x 103 cells ml-1. During growth, the pH was not modified except in the cultures growing with ammonium. To these, drops of NaOH 1 N were added every other day in order to maintain the pH between 5.0 and 6.0, because of the acidification from algalmetabolism (NH+4 H+ + N-algal). The pHreached values as low as 3.5, between one control and another, but the healthy condition of the cultures was not affected. The algal dry weight was determined after 13 days of cultivation by filtering 20-ml aliquots through Millipore filters of 5 µm pore size, washing in distilled water, and drying at 100ºC to constant weight (24 h). Cell density was also estimated in each nitrogen source in order to have estimations on a cell and volumetric basis. The relative number (%) of motile vegetative cells and cysts was also registered. The experiments were carried out in triplicate. Mixotrophic growth with addition of sodium acetateThe concentrations of the sodium acetate assayed were 4, 8 and 12 mM, with an initial pH adjusted to 6.0. Each of these acetate concentrations was tested at two continuous photon flux densities, 35 and 85 µmol m-2 s-1,and the cultures grew from an initial cell density of 103 cells ml-1 (about 1 ml of a seven-day culture was inoculated into 50 ml medium in 125 ml Erlenmeyer flasks). The flasks were incubated for 14 days at 23°±2ºC, without aeration. In order to compare mixo- and autotrophic growth and to discover if heterotrophic growth occurs in this strain, cultures with addition of sodium acetate were established in darkness under the same previously described conditions. The pH of all treatments was measured after inoculating the alga and adjusting it to a value of 6.0 every three days. Cell density and algal dry weight were determined as growth indices at the end of the experiment.INDUCTION OF ASTAXANTHIN SYNTHESISa) By nitrogen deprivation and by exposure to high PFDWhen the experiments to determine the best nitrogen source for growth were finished (15 days) and the aliquots for algal dry weight (20 ml) and cell density (3 ml) removed, the cultures with the best growth (in sodium nitrate and ammonium chloride with pH adjusted during growth between 5.0 - 6.0 range, both grown at the two PFD) were mixed in single cultures: four cultures, corresponding to the two nitrogen sources and the two PFD, were obtained. The volume of each of these cultures was uniformly distributed into eight tubes (each with 20 ml); four of them were kept at higher PFD (150 µmol m-2 s-1) than during growth (35 µmol m-2 s-1), and the remaining346four tubes were assayed for nitrogen deprivation. This was achieved by successive centrifugation (three times at 1500 rpm for five min) and re-suspensionof the algal pellet in fresh NaNO3-deprivedBristol medium (with isosmotic exchangeof NaNO3 by KCl). The induction periodfor all the cultures lasted 12 days. Totalcarotenoids and chlorophyll “a” contentand the relative number of cysts after theinduction period were estimated in eachreplica.b) By salt stressInitially, the alga was cultured in threebottles of five-liter capacity for 11 dayswith three liters of medium reaching a meancell density of 1.6 x 105 cells ml-1. Unlikethe previous experiments of this study, theinoculum for these cultures consisted of98% cysts, and it was taken from a two-month-old culture at a stationary phase ofgrowth. The bottles were maintained at19°±2ºC, under a continuous PFD of 35µmol m-2s-1 (supplied by cool white fluorescent tubes) and continuous aerationgiven with air filtered through Milliporefilters of 0.2 µm pore size. The experimentalprocedure was as following: from bottleNº1 and Nº2, volumes of 200 ml of the algalsuspension were transferred into 500 mlErlenmeyer flasks and cultured with 0.0%,0.2% and 0.8% NaCl at 85 and 35 µmol m-2 s-1, respectively,in triplicate. In order to study the effect of a higher PFD (than that utilized during growth) and the natural aging of the cultures, one set of three flasks (from bottle Nº 3) was kept at 150 µmol m-2 s-1 without addition of salt. The 21 flasks were incubated at 19°± 2ºC without aeration and manually shaken twice a day. At the end, both the growth period (day 11) and the carotenogenesis induction period (day 20, induction period of nine days), algal dry weight and total pigment content (total carotenoids and chlorophyll “a”) were determined in 25 ml and 10 ml filtered aliquots, respectively. The total astaxanthin contents, both on a volumetric and on cell dry weight basis, were determined by HPLC analysis.Analytical MethodsGrowth rate was determined by cell counting using 1 ml Utermohl chambers and a Zeiss inverted microscope, according to Guillard (1973).Raw extracts of pigments were obtained by grinding the algal cell pellet, utilizing either a manual tissue homogenizer (for vegetative cells) or liquid nitrogen in a mortar (for cysts). Then, the pigments were extracted with 90% acetone and left overnight at 4ºC in darkness. The extracts were centrifuged and analyzed by spectrophotometry, according to Strickland and Parsons (1973). The total carotenoid concentrations calculated by this method (at 480 nm) are equivalent to using the extinction coefficient = 2500 (Davies, 1976). The total chlorophyll concentration was calibrated as chlorophyll “a” for the major chlorophyll component. Astaxanthin analysis was carried out by HPLC using equipment with automatic injector pumps, UV variable detector, reverse-phase column RP-18 Lichrocart 250-4 and integrator. Pigments were eluted at a flux rate of 0.5 ml/min, with a solvent system of acetonitrile-dichloromethane-methanol in the proportion 70:20:10 v/v at environmental temperature. Astaxanthin was detected at 480 nm and was identified by its retention time and absorption spectra with standard substance (Sigma).Anaerobic saponification of astaxanthin estersThe esters were dissolved in CH2Cl2and 1% KOH in CH3OH was added under N2. After hydrolysis, the solution was neutralized with 1 % aq. NH4Cl, and astaxanthin extracted with diethyl ether (Grung et al., 1992).Statistical analysisThe data were subjected to statistical analysis, utilizing a factorial design, performing analysis of variance (ANOVA), analysis of covariance and multiple-CIFUENTES ET AL. Biol Res 36, 2003, 343-357347comparisons tests (Tukey, Scheffe, Fisher´s least significant difference method) with the computational program STATISTICA. Differences were considered to be significant at a probability of 5% (p ≤0.05). RESULTSOptimum nitrogen source for growth of H. pluvialis strain SteptoeThe best nitrogen source for growth in this strain was clearly the ammonium chloride when the pH was not permitted to attain values lower than 4.0 (Fig.1). When cultivated in sodium nitrate, the growth parameters, i.e., maximum cell density and cell dry weight, were lower than in ammonium chloride, but clearly higher than in urea, where the growth of the alga was deficient (Table I). In the cultures grown with ammonium, the pH fluctuations due to the acidification of the medium and the modification of the pH whenever it reached 4.0-4.5, raising it to 6.0, did not affect the algal growth but, on the contrary, permitted these cultures to exhibit the highest cell densities (2.0 x 105 and 2.5 x 105 cells ml-1) and algal dry weights (228.0 and 257.5 mg l-1) at 35 and 85 µmol m-2 s-1, respectively. These values were significantly different (p≤ 0.05) from the biomass values obtained in nitrate (at 85 µmol m-2 s-1) and/or in urea at both PFD.In all the nitrogen sources assayed, the relative number of motile vegetative cells during growth was high (≥ 85%) and higher in ammonium (95%) than in the other sources, a condition that supported very healthy cells, showing a deep green color and a very thin translucent cell wall. Due to the pH decrease in ammonium, which needed a strict modification during growth, in the subsequent experiments of this study sodium nitrate was used as the nitrogen source for growth.Mixotrophic growth with addition of sodium acetateThe addition of sodium acetate did not produce any increase in growth at either PFD assayed,when compared to the control cultures (grown without acetate) (Table II). Although a slight increase in the maximal cell densities (from 1.6 x 105 to 1.8 x 105 cells ml-1) and in the algal dry weight (from 183 to 204 mg l-1) occurred at increasing acetate concentrations at 85 µmol m-2s-1, the values were not significantly different (p ≤ 0.05) from those obtained in the autotrophic condition. At 35 µmol m-2 s-1, the inverse occurred, i.e., the strain grew slower than in the control cultures, and there was a decrease in growth at higher acetate concentration, from 0.43 div day-1 (4.0 mM) to 0.21 div day-1 (12.0 mM). Maximal cell densities and dry weight at 12.0 mM acetate (2.5 x 104 cells ml-1, 29 mg l-1) were lower by one order of magnitude than the densities and dry weights achieved in the control (2.5 x 105 cells ml-1, 298 mg l-1). The differences in biomass obtained in the range of acetate concentration at this PFD (35 µmol m-2 s-1) were significantly different (p ≤ 0.05) from each other and compared to the control cultures without acetate. On the other hand, the addition of acetate caused a significant increase in the relative amount of cysts, from 0.42% (control cultures) to 8.7% (12 mM acetate) at 35 µmol m-2 s-1 (p≤0.05). A minor increase of cysts was found at 85 µmol m-2 s-1, ranging from 1.9% in Figure 1: Growth of Haematococcus pluvialis (Steptoe strain) with different nitrogen sources (sodium nitrate, ammonium chloride and urea) and under different photon flux densities (35 and 85 µmol m-2 s-1), without aeration.CIFUENTES ET AL. Biol Res 36, 2003, 343-357348control cultures to 3.0 % in 12 mM acetate. In darkness, cell density showed no change during the 11 days growth period, but the relative number of cysts increased to 13.9%. Although the pH was fixed to a value of 6.0 every three days, the variations of this parameter differed just slightly among the different acetate concentrations.INDUCTION OF ASTAXANTHIN SYNTHESISa) By nitrogen deprivation and by exposure to high PFDThe best inductive factor for carotenoid accumulation, both per volume and per cell, was exposure to high PFD (150 µmol m-2 s-1) in those cultures pre-grown in nitrate under 35 µmol m-2s-1.By contrast, nitrogen deprivation in the nitrate grown cultures did not produce any increment in total carotenoids, at any of the PFD assayed (Table III).The carotenoid content in cultures grown in nitrate at 35 µmol m-2 s-1 and subjected to high light (150 µmol m-2 s-1) increased from1.7 to 4.88 mg l-1 and from 10 to 25 pg cell-1,a highly significant increment (p≤0.05) when compared to the increment in carotenoid content obtained under all other treatments. The carotenoid content in cultures grown in nitrate at 85 µmol m-2 s-1 and subjected to high light increased much less, both per volume unit (from 2.0 to 2.9 mg l-1 ) and per cell ( from 15 to 17.9 pg cell-1), and the final carotenoid content per volume unit was not significantly different (p ≤0.05) from the carotenoid accumulated by the cultures grown in ammonium and subjected to N-deprivation (Table III). The cultures grown in ammonium, where the maximal cell number was two fold higher compared to the cultures grown in nitrate, did not survive in the high PFD treatment (without pH control) and cells died on day four of the induction period. On the contrary, nitrogen deprivation produced an increment in total carotenoids by a factor 1.6 and 1.4 in cultures grown in ammonium under 85 µmol m-2 s-1 (3.20 mg l-1) and 35 µmol m-2s-1 (2.85 mg l-1), respectively. This last increment in total carotenoids was very similar to that observed in the cultures grown in nitrate under 85 µmol m-2 s-1 and subjected to high PFD (from 2.0 mg l-1 to 2.9 mg l-1).The total carotenoid content (per liter and per cell) obtained in the N-deprived cells grown in ammonium at 35 and/or 85CIFUENTES ET AL. Biol Res 36, 2003, 343-357TABLE IMaximum cell density (Nmax ), exponential growth rate (t ≤ 7 days) (kmax), mean growth rate(t = 13 days) (kmean ), cell dry weight per volume unit (mg l-1) and per cell (pg cell-1), andrelative number of motile vegetative cells and cysts in H. pluvialis cultures grown in Bristol medium with different nitrogen sources under two continuous PFD (35 and 85 µmol m-2 s-1), temperature of 23°±2ºC, without aeration and manually shaken twice a day, for 13 days, from Ni= 2 x 103 cells ml-1 and pHi= 6.0. (Values are the means of three replicates) Parameter Nitrogen source (A) and PFD (B) (µ mol m-2 s-1)A NH4Cl NaNO3UreaB358535853585N max (cells ml-1) 2.0 x105 2.5 x105 1.7 x105 1.4 x105 5.2 x104 4.5 x104k max (div day-1)0.720.700.680.590.470.44k mean (div day-1)0.510.530.490.470.360.34Dry weight per228.0257.5194.3170.056.042.0 volume unit (mg l-1)Dry weight per cell11401030114312101077933 (pg cell-1)Final pH 3.4 3.57.77.8 6.6 6.8% motile cells959490898885% cysts 4.6 5.8 5.0 6.6 4.9 5.5349cellular mortality (p ≤ 0.05), being the greatest at the highest salt concentration (45% cell loss at 0.8% NaCl under both PFD) (Table IV). On the contrary, an increase both in cell density and in algal dry weight,in the assays without NaCl, occurred at the three given PFD. This meant that these cultures continue growing as during the experimental inductive period. A significant value (p ≤ 0.05) for the highest algal yield (dry weight and cell density) was obtained at the highest PFD (150 µmol m -2 s -1) without NaCl (208 mg l -1, 1.8 x 105 cells ml -1).Although there was an increment in the total carotenoid content, both per volume and per cell unit (and a concomitant decrease in the chlorophyll content) at increasing salinity under both PFD assayed, the values obtained were considered very low. The best carotenogenic condition by addition of salt was obtained at 85 µmol m -2 s -1with 0.8%NaCl, raising the total carotenoids per volume unit by a factor of 2.8 with respect to the initial value. In absolute total carotenoid content, cells accumulated 1.72 mg l -1 and 22 pg cell -1 with a carotenoid to chlorophyll ratio of 4.2. The increment in total carotenoids produced in this condition was significantly different from the increments estimated in the other conditions, except for the increments observed at 85 µmol m -2 s -1CIFUENTES ET AL. Biol Res 36, 2003, 343-357TABLE IIGrowth (N cells ml -1 and k div day -1) and algal dry weight (a.d.w. mg l -1) of H. pluvialis cultured during 11 days (from Ni= 5 x 103 cells ml -1), in Bristol medium with addition of sodium acetate (4, 8 and 12 mM) and under two PFD (35 and 85 µmol m -2 s -1). Controls in autotrophic and heterotrophic growth were kept without addition of acetate under the two PFD and with 12 mM acetate in darkness, respectively. The relative amount of cysts (%) at the end of thegrowth period was also determined. (Values are the means of three replicates)35 µmol m -2 s -185 µmol m -2 s -1darkness sodium acetate without sodium 4.08.012.0without sodium 4.08.012.012.0concentration (mM)acetate acetate pH variation 6.0 – 6.85 6.0 – 7.09 6.0 – 8.00 6.0 – 8.42 6.0 – 6.78 6.0 – 7.50 6.0 – 8.42 6.0 – 8.73 6.0 – 8.42N cells ml -1 2.5 x105 1.4 x1059.7 x104 2.5 x104 1.5 x105 1.6 x105 1.7 x105 1.8 x105 5.1 x103% cysts 0.42 1.02 1.38.7 1.9 2.6 2.3 3.013.9k div day -10.510.430.390.210.450.450.460.470.0014a.d.w. mg l -129815711029169183195204n.d.µmol m -2 s -1 was not significantly different (p ≤0.05) from the contents exhibited by N-deprived cells cultivated in nitrate at either PFD (Table III).The chlorophyll content was much higher in the cultures grown in ammonium than in nitrate under both PFD (2.3 and 1.6 mg l -1versus 0.9 and 0.7 mg l -1 under 35 and 85µmol m -2 s -1, respectively), and this content remained high in those cultures that survived the carotenogenesis period promoted by nitrogen deprivation (3.5 mg l -1 and 4.0 mg l -1, under 35 and 85 µmol m -2 s -1, respectively). The carotenoid to chlorophyll ratio was always less than 1,even before the induction period.The relative number of cysts was very similar in all growth conditions at the beginning of the carotenogenesis induction period (4.4 % - 5.8%), but at the end it was much higher in those cultures induced by high PFD (ca . 33%) than under nitrogen deprivation (12.8% - 18.5% in ammonium and nitrate, both under 85 µmol m -2 s -1,respectively).b) By salt stressThe results obtained after stressing the cells by addition of salt showed a significant350without NaCl and at 85 µmol m-2s-1 with 0.2% NaCl (p ≤ 0.05), in which the carotenoid content increased by a factor of 2.6 and 2.7, respectively. Under 35 µmol m-2 s-1 and with 0.8% NaCl, the total carotenoid content increased less (by a factor of 1.3 and 2.3, for carotenoids per volume and per cell, respectively). The carotenoid to chlorophyll ratio in this condition was almost two fold higher than that estimated before the addition of salt (it varied from 0.8 to 1.5).In relation to astaxanthin content, the highest amounts were obtained without NaCl under 85 and 150 µmol m-2 s-1: 1.07 and 0.77 mg l-1, respectively, corresponding to 68% and 44% of the total carotenoids estimated by spectrophotometry. The maximum astaxanthin content per dry weight, 10.25 mg g-1 (1.0 % w/w), was registered under 85 µmol m-2 s-1 with 0.8% NaCl, and it was significantly different from all other treatments (p ≤ 0.05). DISCUSSIONThe culture of H. pluvialis, both in laboratory conditions and for commercial purposes, has received much attention, even though research on the factors controlling growth and astaxanthin accumulation have yielded controversial results, postponing the management of the cause-effect association within reliable certitude. In part, this lackCIFUENTES ET AL. Biol Res 36, 2003, 343-357TABLE IIIAlgal dry weight (a.d.w.mg l -1), total carotenoid content per volume (carmg l-1), on a dry weightbasis (carmg g -1), per cell (carpg cell-1), chlorophyll “a” (chl “a”) per volume unit (chl “a”mg l-1),cell density (Ncells ml -1), color of extract and relative number (%) of cysts in H. pluvialiscultured in NaN03 and NH4Cl , under two PFD (35 y 85 µmol m-2 s-1) and then subjected tocarotenogenesis induction by nitrogen deprivation and by exposure to high PFD (150 µmol m-2 s-1) for 12 days. (Values are the means of three replicates (B=before and A= after induction);n.d.=not determined because of culture death)NaNO3 (35)NaNO3 (85)NH4Cl (35)NH4Cl (85)-N high PFD-N high PFD-N high PFD-N high PFDa.d.w.mg l-1B194.0170.0228.0258.0A173.0237.0209.0190.0279.0n.d.315.0n.d.car mg l-1B 1.70 2.04 1.98 2.06A 1.84 4.88 1.91 2.87 2.85n.d. 3.19n.d.car mg g-1B8.812.08.78.0A10.620.69.1415.110.2n.d.10.1n.d.chl “a” mgl-1B0.870.65 2.33 1.64A 1.41 1.09 1.060.59 3.53n.d. 4.05n.d.car chl-1B 1.97 3.210.92 1.43A 1.30 4.60 1.80 5.120.82n.d.0.80n.d.car pg cell-1B10.015.010.08.0A12.225.010.617.911.4n.d.10.3n.d.N cells ml-1B 1.7 x 105 1.4 x 105 2 x 105 2.5 x 105A 1.5 x105 1.95 x 105 1.8 x105 1.6 x105 2.5 x105n.d. 3.1 x105n.d.color of extract B slight yellow-orange bright yellow-orange greenish lemon yellow lemon yellowA Bright bright slight slight grass-colorless grass-colorlessorange-green orange-red orange orange-red green green% cysts B5,44,44,65,8A15,433,618,533,33,8n.d.12,8n.d.。

Extraction of Fucoxanthin from Undaria Pinnatifida using enzymatic pre-treatment followed by DME & EtoH co-solvent extraction Jagan M Billakanti*1, Owen Catchpole1, Tina Fenton1 and Kevin Mitchell11Industrial Research Limited, Integrative Bioactive Technologies, 69 Gracefield Road, PO Box 31310, Lower Hutt5040, New Zealand.Corresponding author: j.billakanti@; Phone: (+64) 4 931 3285; Fax: (+64) 4 566 6004 ABSTRACTThe brown seaweed (Undaria pinnatifida) has been recognized as a potential source of biologically active lipid compounds and in particular, fucoxanthin. Fucoxanthin has been reported to have anti-cancer, anti-obesity and anti-inflammatory effects. Like other carotenoids, fucoxanthin is a fat soluble compound and usually requires organic solvents for its extraction. The extraction of fucoxanthin poses a number of challenges since it is unstable with respect to high and low pH, and also to light; and the extraction of fucoxanthin from dry materials gives poor yields. This study was carried out to improve the yields of total lipids and fucoxanthin from Undaria pinnatifida using an enzyme-assisted extraction process followed by dimethyl ether (DME) extraction. Enzymatic pre-treatment was employed to degrade cell wall polysaccharides to oligosaccharides prior to the extraction of fucoxanthin from the residual biomass using near critical dimethyl ether with and without ethanol as a co-solvent. The residual biomass was separated from hydrolyzed polysaccharides by filtration or centrifugation. Optimal enzyme pre-treatment conditions were achieved using 0.05% (g/g dry weight) alginase lyase, a pH of 6.2, temperature of 37°C, reaction time of 2 hrs and 5% biomass solid content (dry basis). Lipids extracted by DME & ethanol co-solvent after the enzymatic pre-treatment of Undaria pinnatifida were recovered at >95% of the yield obtained using classical solvent extraction techniques, a 15-20% improvement over non-enzyme treated wet biomass. Similarly, the amount of fucoxanthin in the enzyme pre-treated (0.64% g/g of extract) biomass showed >50% improvement over non-treated biomass (0.30%, g/g of extract). Thus, an enzyme-assisted DME extraction process appears to be a good method for recovering biologically active fucoxanthin from Undaria pinnatifida with improved yields.Keywords: Fucoxanthin, brown seaweed, dimethyl ether extraction, Alginase lyase, Undaria pinnatifida INTRODUCTIONUndaria pinnatifida is a brown seaweed and also known as Wakame. This is the most commonly consumed dietary seaweed in Japan due in part to its potential health promoting benefits [1-5]. Undaria kelp is present in almost all marine regions of the world, and is an invasive pest species in New Zealand marine aquaculture operations [6-7]. Undaria seaweed is a good source of natural bioactive compounds such as complex and neutral lipids rich in essential ω-3 fatty acids, carotenoids (fucoxanthin), dietary fiber, proteins, vitamins, polyphenolic compounds, sulphated polysaccharides and fucoidans [8-9]. Fucoxanthin isolated from brown seaweed has been reported to have several health benefits including anti-obesity, anti-diabetic and anti cancer properties [4-5]. Therefore, brown seaweed is considered as very interesting source of natural compounds with numerous biological activities that could potentially be used as functional ingredients in many industrial applications. Fucoxanthin is a fat soluble carotenoid compound that usually requires organic solvents for its extraction. In addition, fucoxanthin is sensitive to light, temperature, pH and strong acidic & alkaline conditions. Therefore, extraction procedures for fucoxanthin must be carried out under a controlled environment to minimize the degradation and hydrolysis of carotenoids [10-11]. The most common method of extraction is by using liquid organic solvents such as toluene, hexane, dimethyl sulfoxide (DMSO), acetone, methanol and combinations thereof [11-15]. However, many of these solvents are not suitable for the production of food ingredients or nutraceuticals and removal of solvents at elevated temperatures can also damage functional properties of bioactive compounds [16-18]. In addition, recovery yields of these methods varied widely and not suitable for industrial applications due to the presence of large quantities of cell wall polysaccharides, which greatly reduce the extraction efficiencies. Supercritical fluid extraction (SFE) using CO2provides an alternative technology with potentially better efficiencies and improved recoveries. The extract obtained from SFE contains fewer polar impurities than by conventional organic solvent extraction. Because SFE has a favorable criticaltemperature and pressure that enables heat liable bioactive compounds recovery [18]. However, one of the main drawbacks of conventional SFE using CO2 is the need to process dry feed materials. Bioactive compounds present in brown seaweed are greatly reduced by conventional (air) drying methods and freeze-drying is very expensive. In order to avoid the loss of bioactive compounds in brown seaweed the materials should be processed in its native state. Recent developments in SFE using near critical dimethyl ether (DME) facilitates the processing of wet feed materials [17]. In addition, recent research into the recovery of bioactive compounds from seaweed using microwave-assisted or enzyme assisted extraction techniques appeared to give improved yields over classical methods [19-21]. Interestingly, enzymatic digestion of algal cell wall polysaccharides prior to the extraction gains more attention due to its potency in proving the recovery yields of bioactive compounds.In this report, we have investigated the use of enzyme-assisted DME and DME-EtOH co-solvent extraction of bioactive lipids and carotenoids from Undaria pinnatifida seaweed, and compared this with SFE-CO2and DME extraction of dry seaweed. The enzyme and DME extraction process could be a potential alternative method to achieve improved yields of industrially useful bioactive compounds from brown seaweed.MATERIALS AND METHODSUndaria pinnatifida seaweed material used in this study was obtained by Tai Tipu, Malborough, New Zealand. The seaweed was water washed and ground and then frozen. Frozen seaweed (wet) was stored in the freezer (-20°C) until it used in the experiments. Freeze and air dried seaweed was also supplied by Tai Tipu. Until unless stated, all chemicals and solvents used in this study were analytical grade. Fucoxanthin and a mixture of carotenoid standards were purchased from DHI Lab, Denmark. Alginase lyase enzyme was purchased from Sigma Aldrich, Australia. LIPID CONTENT ESTIMATIONTotal lipid (TL) content of Undaria pinnatifida seaweed was estimated using the Bligh and Dyer [22] method with modifications necessary for dealing with seaweed. Typically, 10 grams of brown seaweed (wet) was transferred into a 50 mL Falcon tube and mixed thoroughly for an hour with dH2O, MeOH and CHCl3 (1:1:1). The phases were the separated and lipids were extracted in the chloroform phase by phase separation using centrifugation at 4000 rpm for 5 min, and the chloroform layer containing the lipids was then recovered. The residual biomass was re-extracted a further 2-3 times using the same procedure until the maximum lipid extraction achieved. All solvent + extract solutions were pooled together and the solvent was removed by rotary evaporation at 45°C until complete dryness. Total lipid content was estimated by subtracting the empty flask weight and lipid content was represented in wt% (g/g of wet seaweed).CAROTENOID COMPOUNDS ANALYSISSample solutions were prepared by accurately weighing samples and dissolving in ethanol to a known volume, typically, 100 mg of sample made up to 20.0 ml in a volumetric flask. Sample solutions were then analysed directly by HPLC method following membrane filtration (0.20 µm). A flow rate of 0.15 mL/min was employed and an injection volume of 5 µL was used. Solvent A was 20mM ammonium acetate (prepared in DI water) and solvent B methanol. Initial solvent conditions were 75% solvent B. These conditions were held for 0.5 min followed by a linear increase to 100% solvent B at 39 min following injection. Solvent composition was held at this level for 10 min before being changed back to the initial conditions over 1 min and the column was then allowed to equilibrate for 10 min before a subsequent injection was made. The analysis was carried out using a Waters Acuity UPLC equipped with diode array detector and column oven. Chromatography was carried out using a Waters Acquity UPLC BEH C18 1.7 µm 2x150mm column, Waters Acquity UPLC BEH C18 1.7 µm VanGuard Pre-Column and oven temperature of 60ºC.Samples were maintained at 15ºC prior to analysis in the auto sampler compartment. Peak areas in chromatograms recorded at 449 nm were used for quantification following subtraction of a blank run chromatogram generated by running the above gradient with an ethanol blank solution being injected. The system was calibrated using standard solutions of carotenoid compounds of known concentrations purchased from DHI Lab, Denmark. Individual carotenoids were identified by comparison of retention times and on line UV-Vis. absorption spectra with those of the authentic standards. Fucoxanthin content in the brown seaweed extracts was estimated using the above HPLC method and represented in wt% (g/g lipid extract basis).ETHANOL EXTRACTION METHODEthanol was used as a solvent for recovering lipid soluble compounds from brown seaweed because EtOH was used because of it is a food grade (FG) solvent. Generally, 10 grams of brown seaweed (wet) was mixed with 90 mL of EtOH (FG) for an hour at room temperature and centrifuged at 4000 rpm for 20 minutes. The ethanol phase was separated and transferred into a weighed round bottom flask and residual biomass was re-extracted twice more with another 90 mL of EtOH each time. All EtOH extracts were pooled together and lipid-rich extract was concentrated by rotary evaporation (50ºC) until dryness. Some EtOH extracts were re-extracted using CHCl3: MeOH: dH2O solvent system to remove any non-lipid materials present and thus to determine the lipid content.PACKED BED EXTRACTION USING DME AND CO2Extraction of lipids and carotenoids from wet brown seaweed was carried out using near critical dimethylether (DME); and DME+ ethanol so-solvent system for achieving better recovery yields. The seaweed (wet or dry) is packed in a basket which has porous plates at the top and bottom. The basket is then placed in the 2L extraction vessel and lipid extraction was carried out with DME or DME+EtOH (FG) co-solvent (10% mass ratio) at a pressure of 40 bar and temperature of 60ºC (Fig. 1), or CO2 and CO2+EtOH co-solvent (10% mass ratio) at 300 bar and 60ºC. The DME or CO2(and co-solvent) flowed upwards through the bed, before passing through a pressure reduction valve and into the separator. The extract and co-solvent is recovered from the separator (held at ~ 6 bars for DME or ~ 55 bar for CO2 and at the same temperature) at regular time intervals. The DME or CO2 is then recycled via a condensing heat exchanger and compressor (Haskel AG72). The co-solvent flow is stopped after a given extraction time period, and the extraction continued until the amount of extract recovered per time interval drops to almost zero. The plant is then depressurized, the extracted solids recovered and then the separator and control valve rinsed with solvent to recover any additional extract. The total recovered extract and ethanol is then rotary evaporated to recover the lipid-rich extract and to remove any solvent. Lipid extracts were analysed for fucoxanthin quantities in each extract by HPLC method and ω-3 fatty acids by GC. Lipid profiles were determined by TLC..ENZYME PRE-TREATMENT PRIOR TO EXTRACTION OF FUCOXANTHINAlginase lyase enzyme selected for this work has the ability to hydrolyze specific polymer bonds present in the intact cell wall of brown seaweed, which assists in obtaining the maximum recovery of fucoxanthin. There are several factors that directly influence the enzyme performance during the digestion process. These factors were adjusted to find the optimal reaction conditions in small scale experiments. The incubation time-temperature combination of Alginase lyase treatment was one of the most important factors to be optimized. During the brown seaweed cell wall polysaccharide digestion, Alginase lyase hydrolysis was carried out at mild reaction conditions to minimize the loss of fucoxanthin, which can be greatly influenced by pH, temperature and light. Typical polysaccharide hydrolysis was performed at 5% (w/v) solids, pH 6.2, 37°C, 2 hrs and 0.05% (w/w) enzyme at continuous mixing conditions. Hydrolyzed polysaccharide (water soluble compounds) materials was separated from the seaweed biomass by centrifugation at 4000 rpm for 20 minutes and stored at 4°C until further processing.EXTRACTION USING ENZYME DIGESTION AND CONTINUOUS DME EXTRACTIONA combined extraction method was derived using firstly enzyme digestion as above (without the final centrifugation step) followed by direct DME processing of the enzyme digest solution. Typically, brown seaweed was defrosted and mixed with water to a final solids content of 5%. The resultant seaweed slurry was adjusted to pH 6.2, temperature 37°C (water bath) and then enzymatic hydrolysis was initiated by the addition of 0.05% (w/w) Alginase lyase enzyme. Lipid soluble compounds from the enzyme treated brown seaweed biomass were then extracted using a continuous DME+EtOH co-solvent extraction method directly from the enzyme digest slurry. An empty, pre-weighed extraction basket was placed into the extraction vessel, the vessel was then closed, and then the system was pressurized with DME up to the desired operating pressure of 40 bar and temperature of 60°C. The slurry was pumped into the top of the extraction vessel through a nozzle, while simultaneously DME or DME with FG ethanol at a 10 % addition rate was passed upwards thyrough the extraction vessel and basket. The DME or DME + cos-solvent simultaneously extracted the lipids and water from the enzyme digest solution, giving a dry residual biomass (and enzyme) that collected in the bottom of the basket. The DME, ethanol, water and lipid extract then passes through the pressure reduction valve as per usual, and the water, ethanol and lipid are collected at regular time intervals from the separator (held at ~ 6 bar and 60°). The DME is then recycled via a condensing heat exchanger and compressor (Haskel AG72). The slurry feed pump is stopped at a desired throughput, and then the co-solventflow is stopped after a further 30 minutes. The extraction carries on until the amount of extract recovered per time interval drops to almost zero. The plant is then depressurized, the extracted solids recovered and then the separator and control valve rinsed with solvent to recover any additional extract. The total recovered extract, water and ethanol is then rotary evaporated to recover the lipid-rich extract and to remove any solvent. Lipid extracts were analysed for fucoxanthin by HPLC and ω-3 fatty acids by GC. Lipid profiles were determined by TLCAlgae(wet)Aqueousextractsupercritical extraction technology.RESULTS AND DISCUSSIONLIPID EXTRACTION OF WET UNDARIA PINNATIFIDA SEAWEED USING ETHANOLTotal lipid extraction of wet Undaria pinnatifida (wet) was performed by repeated extraction of brown seaweed biomass using CHCl3: MeOH: H2O (1:1:1) solvent system and lipid content was determined to be 0.64% (w/w of wet seaweed or 6.4% on dry weight basis). Initial extracts (3 cycles using CHCl3: MeOH: H2O) appeared to contain a higher lipid content than expected and re-extraction of initial extracts using CHCl3-MeOH removed approximately10-20% of non-lipid materials. This indicates that repeated extraction caused the extraction of some non-lipid materials into the chloroform-rich phase. Hence re-extraction of initial extract using the same solvent system was routinely performed to remove non-lipid extracts. Residual biomass of CHCl3-MeOH extracted Undaria seaweed appeared slightly green and this indicates that complete lipid extraction was not achieved even after repeated extraction and could be because of intact cell wall polysaccharides present in brown seaweed.Lipid soluble compounds can be extracted from brown seaweed using food grade EtOH. Lipid extract (after re-extraction with CHCl3-MeOH) obtained from EtOH extraction (3 cycles) was 0.3-0.35%, which accounts for 40-50%of feed material lipid content (Table 1). The residual biomass after exhaustive EtOH extraction appeared to retain a significant green colour (due to chlorophyll), and this is indicative of incomplete lipid extraction. Increasing the number of EtOH extraction cycles did not improve recovery. During the EtOH extraction of Undaria seaweed, large amounts (30-50%) of non-lipid materials were also extracted into the EtOH phase. On the other hand, pre-treatment of Undaria using Alginase lyase digested the cell wall polysaccharides into oligosaccharides and hydrolyzed oligosaccharides were easily separated from the seaweed biomass by centrifugation. Enzymatic hydrolysis of Undaria seaweed helped to remove approximately 30-40% of non-lipid solids from feed materials. Lipid extraction followed by EtOH extraction (3 times) and CHCl3-MeOH partitioning showed improved recovery yields (40-50% improvement over untreated, lipid yield 0.57 %). Enzyme pre-treated extracts also showed significantly reduced levels of non-lipid materials in the EtOH extract. However, processing large volumes of Undaria seaweed using this combined method for industrial application will require huge amounts of EtOH and significant costs for ethanol recovery and reuse. Both extracts, with and without enzyme pre-treatment contained similar fucoxanthin concentrations (Table 1), but fucoxanthin recovery increased more than 40% using the enzyme pre-treatment.LIPID EXTRACTION OF WET UNDARIA PINNATIFIDA SEAWEED USING DMEIn order to improve the recovery of bioactive compounds from brown seaweed and overcome some of the limitations of ethanol as a solvent, DME extraction with and without EtOH co-solvent was employed and achieved good lipid yields (0.55%). The residual biomass after DME-EtOH co-solvent extraction was almost completely dry, a light green/brown colour, and contained a small amount of lipids (lipid extract from the residue was a light green/yellow colour, and contained more polar than neutral lipids). The DME-EtOH co-solvent extraction method gave better lipid yields (40-50%) compared to EtOH (Table 1 & 2). However, around 40 % of the total extract was non-lipids (Table 2). DME-EtOH co-solvent SFE extraction was further improved using enzyme pre-treatment. Undaria seaweed (wet biomass) was pre-treated with Alginate lyase enzyme, which digests complex polysaccharides into oligosaccharides, prior to the extraction of bioactive compounds using DME-EtOH co-solvent extraction. Enzymatic hydrolysis of Undaria seaweed helped to remove approximately 30-40% solids from the feed materials (mainly polysaccharides) by means of centrifugation, which accounts for 50% improvement over untreated material. Lipid extraction of enzyme treated seaweed biomass using DME-EtOH co-solvent extraction gave a total lipid yield of 0.68%; and the crude extract contained only 20% of non-lipid materials. In addition, this method achieved almost complete fat soluble bioactive compounds recovery (6.8% g/g dry seaweed) from Undaria pinnatifida seaweed (residual biomass contains only 0.175%, g/g dry seaweed of lipids and mostly polar lipids). On the other hand, fucoxanthin concentration in the enzyme pre-treated lipid extract contain >50% than that of untreated DME-EtOH extract (Table 2). Removal of cell wall polysaccharides from Undaria seaweed by enzyme pre-treatment not only improved the recovery of lipids and fucoxanthin, it also reduced the amount of biomass (≈40-50%) to be processed in DME-EtOH co-solvent extraction, which results in less solvent usage and this potentially provides economic benefits in industrial applications.Table 1: Summary of lipid extracts obtained from Undaria pinnatifida seaweed (wet) by different extraction methods. Lipid extracts given in this table were re-extracted using CHCl3: MeOH: dH2O after initial extraction with respected solvent system. Until unless stated, all extracts were repeated and average values are given. SW-seaweed.Extraction method Seaweed(g, wet)Lipid extract(g)Lipid extract(g/g wet SW)Fucoxanthin(g/g lipid extract)Fucoxanthin(mg/g wet SW)CHCl3: MeOH: dH2O 20 0.128 0.0064 0.0130 0.083 EtOH only 200 0.624 0.00312 0.0129 0.040 EtOH & Enzyme 200 1.148 0.00574 0.0131 0.075 Table 2: Summary of lipid extracts obtained from DME extraction with EtOH co-solvent SFE technologyExtraction method Seaweed(g, wet)EtOHextract (g)Lipidextract (g)Lipid extract(g/g wet SW)Fucoxanthin(g/g EtOH extract)Fucoxanthin(mg/g wet SW)DME+EtOH 620 5.73 3.44 0.0055 0.0030 0.028Enzyme/DME+EtOH800 6.86 5.48 0.0068 0.0064 0.055Note*: EtOH extract is the concentrate of DME-EtOH extract and lipid extract is then obtained from the re-extraction of the DME-EtOH extract using CHCl3: MeOH: dH2O solvent. Fucoxanthin content analysis was carried out on EtOH extract. SW-seaweed.CONCLUSIONIn this paper, the extraction of lipid soluble bioactive compounds from Undaria pinnatifida seaweed using enzyme-assisted DME-EtOH co-solvent extraction has been successfully demonstrated at a laboratory scale. Results indicate that enzyme pre-treatment improved the yield of fucoxanthin by >50% and total lipid by >10% over untreated feed materials; and that a continuous process for extraction of the enzyme digest solution is possible using DME + EtOH co-solvent system. Enzymatic digestion of intact cell wall polysaccharides assisted in release of lipid soluble compounds from brown seaweed. In addition, enzyme pre-treatment also helped to remove 30-40% polysaccharides from feed materials and this could greatly reduce the amount of solvents used per gram of seaweed processed. The proposed enzyme assisted continuous DME-EtOH extraction method is a potential alternative to ethanol for the extraction of fucoxanthin from Undaria pinnatifida seaweed that also gives good yields of complex lipids rich in ω-3. ACKNOWLEDGEMENTSThe authors thank MSI for providing funding for this work through grant CO8X0305.REFERENCES1.SE-KWON, K., RATIH, P., Advances in Food and Nutrition Research, Academic Press, Vol 64, 2011, p. 1112.HAYATO, M., MASASHI, H., TOKUTAKE, S., KATSURA, F., KAZUO, M., Biochem &mun, Vol 332, 2005, p 392.3.PRABHASANKAR, P., GANESAN, P., BHASKAR, N., HIROSE, A., NIMISHMOL, S., LALITHA, R G.,HOSOKAWA, M., MIYASHITA, K., Food Chem. Vol 115, 2009, p.5014.RYZA OIL & FAT CHEMICAL CO., LTD. ver.2.0 SJ5.MAEDA, H., TSUKUI, T., SASHIMA, T., HOSOKAWA, M., MIYASHITA, K., Asia Pac J Clin Nutr. Vol 17,2008, p. 1966.PARSONS, M. J., Landcare research contract report: LC 9495/61, 19947.KEIJI, I., KANJI, H., Food Rev. Int. Vol. 5, 1989, p.1018.SERGE, M., JOËL, F., Tren in Food Sci. & Tech., Vol 4, 1993, p. 1039.YOHEI, I., MASAYUKI, K., MAI, W., CHIE, M., HIROYUKI, K., SHIGERU, I., YASUHIRO, K.,KAZUHIKO, S., Bioch Biophys. Acta (BBA) - Bioenergetics, Vol 1777, 2008, p. 35110.J.T.O. KIRK., Plant Sci.Let., Vol 9, 1977, p. 37311.ARKOYO AND ELIFI A.S., Makara, Tech. Vol 15, 2011, p.512.SIEW-LING, H., POOI-YI, C., KWAN-KIT, W., CHING-LEE, W., Aus. J. Bas & App. Sci., Vol 4, 2010, p.458013.NOVIENDRI, D., JASWIR, I., SALLEH, H.M., TAHER, M., MIYASHITA, K., RAMLI, N., J. Med. Plan. Res.,Vol 5, 2011, p. 2405.14.MISE, T., UEDA, M., YASUMOTO, T. Adv. J. Food Sci. and Tech., Vol 3, 2011, p.7315.JARLE, A. H., SYNNØVE, L.J., Phytochem. Vol 28, 1989, p. 279716.YA, F. S., SANG, M. K., WON, J. L., BYUNG-HUN, U., J. Biosci. Bioeng. Vol 111, 2011, p. 23717.OWEN, C., JASON, R., YIN, Z., KRISTINA, F., JOHN, G., MIKHAIL, V., ANDREW, M., EDUARD, N.,KEVIN, M., J. Sup. Flu., Vol 53, 2010, p.3418.MYONG-KYUN, R., SALIM, U., BYUNG-SOO, C. Biotech. Bioproc. Eng. Vol 13, 2008, p.72419.W.A.J.P. WIJESINGHE., YOU-JIN, J., Fitoterapia, Vol 83, 2012, p. 620.PAULA, M., ROBERTO, E. A., Eur. J. Lipid Sci. Technol. Vol 113, 2011, p.53921.GACESA, P., Int.J. Bioche, Vol 24, 1992, p.54522.BLIGH, E. G., DYER, W. J., Can.J. Biochem. Physiol. Vol37, 1959, p.911。

江苏省泰州中学、宿迁中学、宜兴中学2023-2024学年高三上学期12月调研测试英语试卷学校:___________姓名:___________班级:___________考号:___________一、阅读选择Of Special Interest to FreshmanFreshman SeminarsFreshman Seminars are small classes just for freshmen, with some of York’s most distinguished teachers. Some seminars provide an introduction to a particular field of study; others take an interdisciplinary (跨学科的) approach to a variety of topics. All seminars provide a friendly environment for developing relationships with teachers and other students.STARSSTARS (Science, Technology, and Research Scholars) provides undergraduates of every year with an opportunity to combine research and course-based study. The program offers research opportunities and support to students historically disadvantaged in the fields of natural science and quantitative reasoning, such as racial and ethnic minorities, women, and the physically challenged. More than 100 students each year participate in STARS, during the academic year or over the summer months.Academic AdvisingAcademic Advising is a collective effort by the residential colleges, academic departments and various offices connected to York University Dean’s (院长的) office. Students’ primary academic advisors are their residential college deans, to whom they may always turn for academic and personal advice. The deans live in residential colleges and supervise the advising networks in the college. Each academic department has a director of undergraduate studies (DUS) who can discuss with students the department’s course offerings and requirements for majors.Perspectives on Science and EngineeringPerspectives on Science and Engineering is a lecture and discussion course for about 75 selected freshmen who have exceptionally strong backgrounds in science or mathematics. The yearlong course explores a broad range of topics, exposes students to questions at the frontiers of science, and connects the first-year students to York’s Scientific Community. 1.An African female freshman seeking opportunities of research is most likely to choose__________.A.Academic Advising B.Freshman SeminarsC.Perspectives on Science and Engineering D.STARS2.Which of the following is TRUE about the residential colleges?A.Directors of academic departments live with students there.B.The college deans serve as the central figures in an advising network.C.Directors of undergraduate studies of most majors work together there.D.The college deans engage in scientific research with selected freshmen.3.Which freshman may have priority to attend Perspectives on Science and Engineering?A.A medalist of the International Mathematical Olympiad.B.The one who has already got a novel published.C.The one who has designed an original engineering project.D.An applicant for York’s Scientific Community.Soaring to 29, 035 feet, the famous Mount Everest had long been considered unclimbable due to the freezing weather, the obvious potential fall from cliffs and the effects of the extreme high altitude, often called “mountain sickness.” But that was to be changed by Edmund Hillary.When he was invited to join the British Everest expedition in 1953, Edmund Hillary was a highly capable climber. The glacier-covered peaks in his hometown in New Zealand proved a perfect training ground for the Himalaya. It was his fourth Himalayan expedition in just over two years and he was at the peak of fitness.On May 28, 1953, Edmund Hillary and Tenzing Norgay, an experienced Sherpa (夏尔巴人), set out and reached the South Summit by 9 a.m. next day. But after that, the ridge (山脊) slightly fell before rising suddenly in a rocky spur (尖坡) about 17 meters high just before the true summit. The formation is difficult to climb due to its extreme pitch because a mistake would be deadly. Scratching at the snow with his ax, Hillary managed to overcome this enormous obstacle, later to be known as the Hillary Step.At 11: 30 a. m., the two men found themselves standing at the top of the world. “Not until we were about 50 feet of the top was I ever completely convinced that we were actually going to reach the summit.” Hillary later recounted, “Of course I was very, very pleased to be on the summit, but my first thought was a little bit of surprise. After all, this is the ambition ofall mountaineers.”Emerging as the first to summit Mount Everest, Hillary continued by helping explore Antarctica, and establishing the Himalayan Trust (信托基金), through which he provided a number of beneficial services to the Himalayan peoples. He also left a sizeable legacy that mountain climbers have chased ever since. As a young climber said, “It was not just Hillary and Tenzing that reached the summit of Mount Everest. It was all of humanity. Suddenly, all of us could go.”4.What made Edmund Hillary a capable climber on the 1953 expedition?A.His undisputed reputation.B.His previous training on Mount Everest.C.His remarkable physical condition.D.His exceptional ability to adapt to the cold.5.What does the Hillary Step refer to?A.A steep spur of rock Hillary conquered.B.An ax Hillary used to scratch snow.C.A mistake Hillary avoided making.D.A sudden fall of a ridge Hillary skipped. 6.What was Hillary’s initial feeling upon reaching the summit of Mount Everest?A.Overwhelming joy.B.A touch of astonishment.C.Complete disbelief.D.Enormous pride.7.What was the impact of Hillary’s achievement on mountaineering?A.It led to friendly regulations for mountaineering.B.It left financial benefits for climbers to pursue.C.It enabled him to give back to his hometown.D.It opens up possibilities for other climbers.Farming is destroying the planet, but there could be a much more environmentally friendly way to feed ourselves: using renewable energy to turn carbon dioxide into food. “This is becoming a reality,” says Pasi Vainikka at Solar Foods, a company that is building the first commercial-scale factory that will be able to make food directly from CO2.There can be no doubt that immediate attention to find greener ways to grow food is required. Conventional agriculture, including organic farming, causes damage to the environment in many ways. It requires a lot of land, leading to habitat loss and deforestation. It is also the source of a third of all greenhouse gas emissions and releases other pollutants. It isn’t very efficient, either. Crops typically transform less than 1 percent of light energy intousable biomass (生物量).Instead, Solar Foods plans to avoid photosynthesis (光合作用) altogether, and grow bacteria that use hydrogen as their source of energy. At the factory, renewable electricity will be used to split water to produce hydrogen and oxygen. The hydrogen will be added to large containers, where the bacteria grow, along with CO2 and ammonia (氨气). The end result will be a yellow powder called Solein.Solein is made of bacterial cells and is up to 70 percent protein. It can be used as an ingredient in all kinds of foods. “We are aiming at replacing animal-sourced proteins, which we think have the highest environmental impact,” says Vainikka.Compared with plant crops, Solein will use 100 times less water per kilogram of protein produced, 20 times less land and emit a fifth as much CO2, according to Solar Foods. There are other benefits, too: factories could be situated anywhere in the world and production won’t be affected by weather conditions.“With Solar Foods and other companies scaling up their systems, this is truly beginning a new era of agriculture,” says Dorian Leger at Connectomix Bio in Germany. “I think these trends are exciting and will help bend the carbon curve as well as lead to improved global food supply security.”8.What is the author’s purpose in mentioning conventional agriculture in paragraph 2?A.To demonstrate its influence on crops.B.To compare different farming methods.C.To highlight the urgent need for alternatives.D.To provide an example ofagricultural types.9.What is mainly presented in paragraph 3 concerning Solein?A.Its production process.B.Its storage condition.C.Its ingredient materials.D.Its investment potential.10.All of the following are the features of Solein except __________.A.it is protein-rich B.its production is weather sensitiveC.it is resource-efficient D.its production is location-independent 11.Which statement would Dorian Leger probably agree with?A.The use of Solein may help reduce carbon emissions.B.Solein will dominate the agricultural development.C.Solein can help achieve global food safety.D.The prospect of Solein remains to be seen.Many people have participated into lots of virtual meetings these years. Some research shows this adjustment might not impact workplace productivity to any great degree. A new study, though, suggests otherwise.In the study, 602 participants were randomly paired and asked to come up with creative uses for a product. They were also randomly selected to work together either in person or virtually. The pairs were then ranked by assessing their total number of ideas, as well as those concepts’ degree of novelty, and asked to submit their best idea. Among the groups, virtual pairs came up with significantly fewer ideas, suggesting that something about face-to-face interaction generates more creative ideas. The findings could stiffen employers’ resolve to urge or require their employees to come back to the office.“We ran this experiment based on feedback from companies that it was harder to innovate with remote workers,” said lead researcher Melanie Brucks. “Unlike other forms of virtual communication, like phone calls or e-mail, videoconferencing copies the in-person experience quite well, so I was surprised when we found meaningful differences between in-person and video interaction for idea generation.”When random objects were placed in both the virtual and physical rooms, the virtual pairs of participants spent more time looking directly at each other rather than letting their look wander about the room and taking in the entire scene. Eyeing one’s whole environment and noticing the random objects were associated with increased idea generation. On platforms, the screen occupies our interactions. Our look wavers less. “Looking away might come across as rude,” said Brucks, “so we have to look at the screen because that is the defined context of the interaction, the same way we wouldn’t walk to another room while talking to someone in person.”Like most educators, Brucks has primarily taught virtually in the past three years, and she did notice some benefits of the approach as well. Her students were more likely to take turns speaking and her shyer students spoke up more often, rid of the anxiety that comes from addressing a large classroom. Brucks found that one solution to improving virtual idea generation might be to simply turn off the camera, for her students felt “freer” and more creative when asked to do so. And this may be sound advice for the workplace.12.What does the underlined word “stiffen” in Paragraph 2 most probably mean?A.challenge.B.revise.C.strengthen.D.shake.13.At first, lead researcher Melanie Brucks might think that _________.A.Creative ideas may emerge from casual thoughts.B.The feedback from companies seems questionable.C.Participants should make eye contact in an online meeting.D.Videoconferencing can’t compare with in-person communication.14.What can we learn about Brucks’ students?A.They progressed in focusing attention.B.They relieved anxiety by speaking up.C.They displayed talent for public speaking.D.They took advantage of virtual learning.15.Which of the following would be the best title for the passage?A.Brainstorming Online Limits Creativity B.Interacting In Person Boosts Efficiency C.Grouping Randomly Increases Productivity D.Maintaining Teamwork Improves InnovationFor most, the first thing that likely comes to mind when thinking about vitamin C isHere are the primary benefits of vitamin C when applied topically to the skin.It protects skin cells from environmental damage. Vitamin C’s main function in skin is that of a powerful antioxidant, protecting us from cell damage caused by free radicals (自由基). “ 17 ,” Dr. Mack explains, and they are highly reactive. Vitamin C destroys these free radicals by donating electrons, preventing them from damaging skin.18 . Vitamin C is also one of the gold standards for evening skin tone and boosting radiance. “Vitamin C is a well-known skin brightener, preventing melanin production and eventually fading dark spots, resulting in a better skin tone,” Dr. Mack explains.It firms skin and promotes collagen (胶原蛋白) production. Our skin is made of collagen and elastin, which are proteins that give it structure and flexibility. 19 . “Daily application of vitamin C helps to maintain the completeness of the collagen that we have, prevents rapid breakdown with age and promotes collagen production,” Dr. Mack says.It works with other antioxidants for enhancing UV (紫外线) protection. 20 . A study in the Journal of Investigative Dermatology showed that the combination of vitamin C and E not only offered improved stability of vitamins C and E, but also improved skin’s UVprotection.A.It brightens skinB.It helps lower the risk of heart disease and depressionC.Vitamin C works cooperatively with vitamin E to reduce UV damage in skinD.Free radicals are produced by the body when exposed to radiation in sunlightE.But this popular vitamin has several benefits for skin when applied topically, tooF.Free radicals are believed to be related to heart disease, cancer and the ageing process G.As we age, the production of these proteins decreases and our skin looks and feels less firm二、完形填空Learning to ask for what I needed was the win I hadn’t initially set my sights on, but 35 wanting the most.21.A.completely B.temporarily C.roughly D.highly 22.A.return B.relearn C.recollect D.recycle 23.A.insurance B.attendance C.assistance D.avoidance 24.A.grateful B.forgetful C.regretful D.powerful 25.A.sensibility B.possibility C.essence D.weight 26.A.forced B.accomplished C.started D.abandoned 27.A.extraordinary B.usual C.plain D.similar 28.A.stuck B.beat C.confused D.hurt 29.A.substitute B.suit C.jewelry D.invention 30.A.abusing B.using C.recording D.struggling 31.A.help B.sorrow C.strategies D.actions 32.A.more than B.other than C.less than D.rather than 33.A.incident B.tradition C.attraction D.difference 34.A.disorder B.disbelief C.disadvantage D.disability 35.A.sent up B.ended up C.tore up D.looked up三、语法填空阅读下面短文,在空白处填入1个适当的单词或括号内单词的正确形式。

中考英语新能源的未来发展趋势单选题40题1.New energy sources are renewable and _____.A.pollutingB.pollutedC.non-pollutingD.pollution答案:C。
本题考查形容词的用法。
新能源是可再生的且无污染的,A 选项“污染的”,B 选项“被污染的”,C 选项“无污染的”,D 选项“污染”是名词。
所以选C。
2.Solar energy is a kind of new energy that is _____.A.expensive and limitedB.cheap and unlimitedC.dangerous and rareD.costly and scarce答案:B。
本题考查对太阳能特点的了解。
太阳能是一种便宜且无限的新能源,A 选项“昂贵且有限”,B 选项“便宜且无限”,C 选项“危险且稀少”,D 选项“昂贵且稀少”。
所以选B。
3.Wind energy is generated by _____.A.windmillsB.fossil fuelsC.nuclear powerD.coal mines答案:A。
本题考查风能的产生方式。
风能是由风车产生的,B 选项“化石燃料”,C 选项“核能”,D 选项“煤矿”都与风能无关。
所以选A。
4.Hydro energy comes from _____.A.rivers and lakesB.oil wellsC.gas stationsD.mines答案:A。
本题考查水能的来源。
水能来自河流和湖泊,B 选项“油井”,C 选项“加油站”,D 选项“矿山”都与水能无关。
所以选A。
5.Biomass energy is produced from _____.A.plants and animalsB.mineralsC.metal oresD.petroleum答案:A。

保护环境英语演讲稿(5篇)第1篇:保护环境英语演讲稿DEMAND for graduates in the UK with skills related to sustainability is booming. Academia is waking up to the need to train specialists in green architecture, chemistry and engineering.Dean Millar founded the world’s first ever BSc course in Renewable Energy at Exeter University after working as an industry consultant.He said: “A lot of panies were plaining that there was a lack of bright young people suitably trained to work in the renewables business.”Now his course has around 90 students learning the ins and outs of biomass, tidal, solar and offshore wind power.Their career prospects are incredibly bright. Employment opportunities are not just limited to the energy sector, but segue into the regulatory and, increasingly, investment sectors as well.“The big boys are moving in. The returns to be had are significant, especially in terms of wind development,” said Millar. “Financial firms need expertise across the board so they can make sensible investment decisions.”Students on York University’s Master’s course in Green Chemistry are ideally placed to take advantage of the growing demand in industry for graduates with waste minimization skills.Professor James Clark is founder of York’s Green Chemistry Centre for Excellenc e and the world’s leading green chemistry journal, Green Chemistry. He said: “We are now talking to producers of automobiles, electronics, furniture and pharmaceuticals, people who need to use chemistry but don’t think of themselves as chemists.”As waste production legislation es increasingly strict, the traditional emphasis on making as much of a chemical as possible is shifting towards minimizing the waste that process creates. “A lot of chemicals are made from petroleum, but we are developing sustainable ways to make carbon, typically from biomass such as dead plants and trees,” said professor Clark.All of last year’s graduates quickly found employment, some receiving multiple job offers in a variety of different fields.Li Li, a 26-year-old Chinese postgraduate student, graduated from York in 2006. She said:“I hadn’t heard about green chemistry in China. It was a new area and I was curious. Now the government back home needspeople who know about these principles.”Deep in the heart of Wales, 400 students at the Centre for Alternative Technology’s (CAT) Graduate School of the Environment are busy designing and building everything from high insulation wood housing to wind power turbines. With energy costs rising, building firms are increasingly looking to minimize the energy wasted heating and lighting homes, a service CAT students are well-equipped to provide.A new institute is being built to meet the burgeoning demand for places and a distance learning facility will be launched next September so foreign students can plete CAT courses from home.The expertise being taught on these courses is essential to a continually expanding range of panies and government authorities. Yet there is a larger goal at stake; the students are at the forefront of the battle against climate change. In the words of Dean Millar, “If we’re going to save the planet, we need to start turning these people out in big numbers.”第2篇:保护环境英语演讲稿Win Competition of Environment Protection for BeijingAlthough the 2008 Olympics are still three years away,another special petition has already started in Beijing. This time, the petitors are not the athletes from all over the world, but the people living in Beijing. The special petition is not held in a stadium, but in every street and every corner of Beijing. I suppose some of you may have already guessed what the special petition is. Yes, it is the petition of protecting our environment and creating a green Beijing for the 2008 Olympic Games.Someone may ask who is our rival in this petition? The modern Los Angeles, the charming Sydney, or the historic Athens? No, none of them. The real rival is ourselves. It is our bad habits of neglecting to protect environment in our daily life.Several years ago, I was very lucky to have an opportunity to live in the United States for about two years. I not only enjoyed the beautiful environment there, but also appreciated the American people's active way of protecting their environment. Now, whenever the environment protection is mentioned, a beautiful view of California will arise in my mind: white clouds flying acrothe blue sky, green grassplot sprinkled with colorful flowers and small animals playing happily among the trees.I remember that at the beginning of my ing to America, I often went to my father's working place, the United StatesGeological Survey, to have fun. Each time I found a lot of people riding bicycles to their offices. Among the cyclists, an old man with white hair attracted my attention. Curiously, I asked my father,“ Why does the old man ride his bike to work every day? Doesn't he have enough money to buy a car?" Father laughed, "No, not because of money. Actually, he is one of the greatest scientists in the world. He can afford to buy a motorcade if he likes. He is just an environmentalist and usually doesn't drive unlegoing shopping, or in bad weather. In America, there are a lot of environmentalists, who actively protect their environment. For example, in Palo Alto city we are living now, there is even a bicycle-to-work day on May 19th every year to encourage people to decrease air pollution caused by cars".Later, I also learnt another interesting fact of environment protection there. In some states of America, in order to decrease air pollution, save energy and reduce traffic jams, state governments encourage people to take buses to work or to share a car among several people. They even set special "diamond lanes" in some main streets, which are only for the vehicles with 2 or more people.The positive actions of American people and the effectivemeasures the American government takes in environment protection fully won my respects and deeply affected my consciousnein environment protection.When I was back in China, people often asked me: " What do you think of America?" I always bolt out:" Wonderful, especially the beautiful environment." Frankly speaking, after several years, the faces of my American teachers and friends have gradually faded away from my mind, but the blue sky, green graand lovely animals in California often arise in my mind, and became my dream of visiting there again.The 2008 Olympics provide us with the opportunity to publicize and practise environment protection in Beijing. Is it possible for Beijing to Is it possible for Beijing to e as beautiful as California? The answer is "yes", but the dream needs every Beijing citizen's full support and active moves to plish.From now on, if every student who is driven to school can take bus or ride bicycle to school once a week, if every car owner goes to work in a car pool once a week, we can make a difference. If everyone can actively protect the environment in our daily life, the blue sky, green graand lovely animals in California will appear in Beijing.Tiny streams can bine into a vast ocean, small trees can together be an immense forest. Beijing is often described as a beautiful and aged picture. If every Beijing citizen adds a trait of green on the picture, the whole Beijing will e an ocean of green. Let us unite together to win the petition of environment protection in Beijing, and present the world a big gold medal. That is "Green Beijing, Great Olympics ".第3篇:保护环境英语演讲稿Hello! I wonder whether everybody knows the meaning of these two words of " energy-conservation " and " low carbon "? I assume as a matter of course and know. Does " energy-conservation " save the energy? Does " low carbon " reduce carbon emission? Yes, it is really simple. We often chat about them. But, do you really understand them? Have they really taken root on your bottom of heart deeply?Once, the Earth mother left our rich energy to cause us to be jubilant, sighed on Earth's energy inexhaustible, inexhaustible, now, the newest statistics indicated, the petroleum will dry up after 60 years, the coal also might supply the humanity to use for 250 years; Once, developed first, the environment question the situation which neglected is often occurred, now, the sustainabledevelopment, was together harmoniously with the nature the biggest topic.Not difficult to see, the environment question in is taken unceasingly by the people.For all this, the environment question was still stern, the energy conservation reduced the platoon, the low-carbon lives imminently.At the Copenhagen climate congress, this affects the human destiny the question slowly to be unable to reach the agreement actually.In the life, is driving the great displacement automobile, purchases including the fluorine air conditioning, the refrigerator, including the phosphorus laundry powder, turns on the air conditioning the low temperature also one side to bind in the summer the quilt, the daylight lamp is being long all night clearly, water cock water drop sound day and night not rest.These influence environment phenomenon mon occurrence.This is rebels with ours position. American President Kennedy has said: Do not have to ask the country can make any for us, must ask first oneself can make any for the country.The low-carbon life needs everybody to participation!The low-carbon life first is one kind of life manner.So long as wants, each person may be able to achieve! The electricity saving, saves gas and oil, the solar terms, saving water, the tree-planting,makes use of waste, by step generation of vehicle.The intravenous drip, in life each aspect, all may choose the low-carbon life the manner.The low-carbon life is also representing one kind healthily, the more natural life style.Little eats counter-season food, the generation by works as season food; Little uses the disposable product, the generation by the duplicated things; Little rides an overhead traveling crane, little sits one time the elevator, the generation rides the bicycle, crawls the staircase, while falls the low-carbon withdrawal, we will have a healthier body and mind.We believed, so long as everybody works as one, participation together, humanity's tomorrow certainly will be able to be happier! Schoolmates, today, your low-carbn.第4篇:保护环境英语演讲稿as we all know, the challenge and opportunity always, confused with hope, in the 21st century, is also twin human facing challenges period. special performance in this aspect of the relation between man and nature. human deforestation, blind development farming, damaging the vegetation, causes soil erosion, desertification, any natural resources, make the underground mining mineral deposits in the increasinglyexhausted, wastewater and waste, exhaust gas emission, make continuous natural environment seriously deteriorating.human nature is excessive to take, suffer the nature ruthleretaliation: hurricane, rainstorm, storms, floods and drought, insect, heat, forest fires, earthquake, etc., the world because of drought disaster equipoised reasons caused the transference refugees until 2025 reach 100m. painful ecological lesson, has attracted the attention of the world and all mankind, ioc mission inspection bid cities, improving the eco-environment is one important content. china has issued a lot of environmental protection policies and regulations. our country also attaches great importance to the protection of environment education, the "building a green campus activities", in the country for grandeur.students, our school is always in advocating "green wanli", which is one of important content protection beautify our campus. we often see many students on campus each picking up litter, with our own hands to protect a beautiful campus. however, we also often see some we don't want to see their scenario: the playground, stairs, corridor have the pa-pe-r scraps, the profession. again see the lawn, some classmates in trample, the school's green belts, sometimes found to have deep foot-prints,some places even gone became trail.my fellow students, we is the 21st century master, awareneof environmental protection is modern signs. we must have the era of responsibility. responding to global, focus on the side, based on the campus. many curved waist, don't pick up peel confetti throws anywhere disorderly vomit, more step, don't crogreen belts, grassland. "kindnesmall and not for, is a sin to steal a pin," starts from me, since the childhood, starts from the side with, starting from now. to protect the earth mother, purify the campus.so it is high time that we should do something to change this situation. as a citizen, we can do much for it. for example, we could use disposable goods less. and we are supposed to save resources such as water, electricity and so on. also, we ought to plant more trees to reduce the greenhouse effect. in addition, we could try our best to publicize the knowledge of environment protection. because we only have one world。

植物氮素吸收过程研究进展王平;陈举林【摘要】氮是植株生长发育所需求的第一大矿质营养元素,主要通过根系从土壤吸收获得.因此,了解植物吸收氮素的过程及影响因素对明确氮素吸收机理,提高氮素吸收利用,促进植物生长具有重要意义.土壤中的氮素主要以无机态氮(NO3-、NH4+)和小分子有机态氮的形式存在,其中植物所能吸收利用的主要是NO3-态氮素.该研究介绍了植物对氮素的需求、NO3-吸收过程及其影响因素的研究进展.这对认识氮素吸收机理、提高氮素吸收效率具有重要的意义.【期刊名称】《安徽农业科学》【年(卷),期】2016(000)001【总页数】3页(P33-35)【关键词】氮素需求;氮素吸收;硝态氮【作者】王平;陈举林【作者单位】泰安市农业科学研究院,山东泰安271000;山东农业大学,山东泰安271000;泰安市农业科学研究院,山东泰安271000【正文语种】中文【中图分类】S501氮素是植物生长需要的重要元素之一,是蛋白质、核酸、磷脂等植物生长发育所必需物质的组成成分,是植物需求量最大的营养元素。
氮在植物生命活动中占有重要的地位,因而具有“生命的元素”之称[1]。
1994年美国著名植物遗传育种家、诺贝尔和平奖获得者Borlang博士断言,全世界作物产量增加的50%来自化肥的施用,其中氮肥占1/2以上。
植物从外界环境获得氮素主要是通过3条途径,即通过把吸收的硝态氮)还原转化为生命体可用的有机氮,通过固氮菌对N2的固定,直接吸收土壤中的铵或有机氮。
高等植物能直接吸收利用的土壤氮素为、铵态氮)和水溶性有机氮化物[2-4]。
植物对氮素的吸收能力是反映其生长状况的重要指标。
根系对土壤氮素的吸收、利用、转运能力决定了氮素营养对植物生长的满足程度,直接影响地上部生长状况,对植物生长发育具有重要的作用[5-6]。
笔者对植物氮素的吸收过程及影响因素做一综述。
氮的需求就是植物从土壤中吸收氮,用于满足植物生长和同化合成新组织所需求的氮量。
Biochar Production from BiomassBiochar is a high-carbon, fine-grained product that is obtained by heating organic materials in a low or zero oxygen environment. This practice is called pyrolysis, and it results in a carbon-rich product that has many valuable properties. Biochar has been gaining recognition recently for its potential to help mitigate climate change, improve soil health, and provide a sustainable source of energy. In this article, we will explore the process of biochar production from biomass, as well as some of its benefits and applications.Biomass is any organic matter derived from living or recently living organisms that can be used as fuel or for industrial production. This includes wood, grasses, crops, agricultural residues, animal manure, and many other materials. The first step in biochar production is selecting the appropriate biomass feedstock, which will determine the properties and quality of the final product. The ideal feedstock should be abundant, affordable, and sustainable, and it should have a high carbon-to-ash ratio. This means that it should contain a relatively high amount of carbon and a low amount of inorganic minerals, which can lead to ash buildup and reduce the quality of the biochar.After selecting the feedstock, the next step is to prepare it for pyrolysis. This typically involves drying it to reduce the moisture content and chopping it into small pieces to increase its surface area and promote even heating. The prepared biomass is then loaded into a pyrolysis kiln, which can vary in size and design depending on the scale and purpose of the operation. The kiln is typically heated to a temperature between 350–600°C, which allows for the thermal decomposition of the organic matter and the release of volatile gases, some of which can be captured for further use as biofuels or chemicals.As the biomass decomposes, the carbon-rich residue (biochar) is left behind. The quality of the biochar depends on several factors, including the feedstock, pyrolysis temperature, heating rate, and residence time. Generally, a slower heating rate and longer residence time can lead to better-quality biochar with higher carbon content, while highertemperatures can lead to more efficient production but lower-quality biochar. The resulting biochar can then be cooled, sieved, and packaged for use in various applications.One of the most promising applications of biochar is in agriculture. Biochar has been shown to improve soil fertility, water retention, and nutrient availability by increasing the soil's ability to hold moisture and store carbon. It also provides a stable habitat for beneficial microorganisms that can enhance plant growth and suppress plant pathogens. In addition, biochar can assist with the management of animal manure and other agricultural wastes by reducing odor and nutrient runoff, as well as providing a source of renewable energy.Another potential application of biochar is in the production of biofuels. Biochar can be used as a catalyst or adsorbent in the production of biofuels from lignocellulosic biomass, such as wood chips or agricultural residues. It can also serve as a fuel source in itself, either through combustion or gasification. Biochar combustion can provide a source of heat and power, while gasification can produce a syngas mixture that can be further processed into fuels or chemicals.In conclusion, biochar production from biomass is a promising technology that has the potential to provide numerous benefits to society, from mitigating climate change to improving soil health and providing renewable energy. While there are still many challenges and uncertainties associated with biochar production, ongoing research and development efforts are helping to refine the technology and explore its full potential. With the right policies and investments, biochar could become a valuable tool in the transition towards a more sustainable and resilient future.。
英文原文Sludge reduction during brewery wastewater treatment by hydrolyzation-food chain reactor systemAbstract: During brewery wastewater treatment by a hydrolyzation-food chain reactor (FCR) system, sludge was recycled to the anaerobic segment. With the function of hydrolyzation acidification in the anaerobic segment and the processes of aerobic oxidation and antagonism, predation,interaction and symbiosis among microbes in multilevel oxidation segment, residual sludge could be reduced effectively. The 6-month dynamic experiments show that the average chemical oxygen demand (COD) removal ratio was 92.6% and average sludge production of the aerobic segment was 8.14%, with the COD of the influent at 960–1720 mg/L and hydraulic retention time (HRT) of 12 h.Since the produced sludge could be recycled and hydrolyzed in the anaerobic segment, no excess sludge was produced during the steady running for this system. Keywords hydrolyzation, multilevel oxidation, excesssludge, reduction1.IntroductionDuring the 1980s, the main brewery wastewater treatment locally and abroad was the aerobic technique, then the hydrolytic-aerobic techniques showed up in the late 1980s. Currently, the main technology for brewery wastewater treatment are the activated sludge process, contact oxidation process, and hydrolytic-aerobic techniques. Although these techniques have some advantages of their own, they all have a problem with sludge disposal [1]. The sludge production is about 60% of the chemical oxygendemand (COD) removal amount for conventional activated sludge technology, and about 30% for conventional biofilm method [2]. The cost of sludge disposal had become an economic burden of the sewage plant. The sludge produced may bring about secondary pollution.Therefore, the study on water treatment processes that can lead to sludge reduction is becoming one of the important issues in sewage treatment.This study adopted principles of cleaner production. With the hydrolyzation-acidification in anaerobic segments, residual sludge could be translated into soluble organicmatter and small organic molecules, then enter the aerobic segment as organic load. A series contact oxidation system for food chain reactor (FCR) was applied in the aerobic segment to form amanual biogeocenose and food chain. Based on biological theory, the longer the food chain is, the moreenergy lost, and thus less energy that can be used for growth of the organisms, and less biomass left in the ecosystem as a result. Therefore, prolonging the food chain and strengthening the predation of microzoans in the food chain are both effective in sludge reduction. ‘‘Zero Discharge’’ of residual sludge was achieved during the brewery wastewater treatment by a hydrolyzation-FCR system. This study explored the mechanism of sludge reduction during the hydrolyzation process and multilevel oxidation process。
2017年第36卷第12期 CHEMICAL INDUSTRY AND ENGINEERING PROGRESS·4575·化 工 进展葡萄糖化学催化异构制备果糖研究进展张雄1,徐志祥1,李雪辉1,关建郁1,龙金星1,2(1华南理工大学化学与化工学院,广东 广州 510640;2广东省新能源和可再生能源研究开发与应用重点实验室,广东 广州 510640)摘要:生物质是自然界中唯一含有固定碳的可再生资源,因此其被认为是替代化石资源制备五羟甲基糠醛、乙酰丙酸等高附加值生物化学品的最佳原料。
而生物质炼制过程中葡萄糖异构制备果糖的反应是实现上述过程的非常关键的步骤。
此外,葡萄糖的异构也是食品科学中的重要过程。
基于上述背景,本文综述了近年来葡萄糖化学催化异构制备果糖过程催化剂研究进展,重点介绍了Brönsted 碱、固体碱、金属盐以及分子筛等传统酸碱催化剂以及金属有机骨架和离子液体等新型催化材料在此过程中的催化行为。
此外,还对葡萄糖异构的酸催化机理和碱催化机理进行了简要介绍,对其研究方法进行了总结。
针对当前葡萄糖化学催化异构过程中存在的诸如催化剂水热稳定性差、过程效率低、果糖选择性低等技术难题,提出设计新型水热稳定催化剂以及基于化工过程强化的理念,构建葡萄糖催化异构与产物原位分离相耦合的新体系等可能的解决措施。
与此同时,高效、高选择性的新型催化材料和催化体系的开发、系列对葡萄糖异构具强化作用的新型绿色过程的构筑以及多手段联合(例如原位技术、二维核磁和计算机模拟相结合)的现代分析方法在葡萄糖化学催化异构机理研究方面的应用将会受到越来越多的关注。
关键词:葡萄糖;果糖;异构;催化剂中图分类号:TQ032.4;Q532 文献标志码:A 文章编号:1000–6613(2017)12–4575–11 DOI :10.16085/j.issn.1000-6613.2017-0516Chemical isomerization of glucose into fructoseZHANG Xiong 1,XU Zhixiang 1,LI Xuehui 1,GUAN Jianyu 1,LONG Jinxing 1,2(1School of Chemistry and Chemical Engineering ,South China University of Technology ,Guangzhou 510640,Guangdong ,China ;2Key Laboratory of Renewable Energy ,Guangzhou Institute of Energy Conversion Chinese Academyof Sciences ,Guangzhou 510640,Guangdong ,China )Abstract :Biomass is the unique carbon-containing renewable material on the globe. It has long been considered as the most promising alternative for the fossil fuel in the production of versatile biochemical ,such as 5-hydromethylfurfuran and levulinic acid. It should be noted that the isomerization of glucose to fructose is the most crucial process in above mentioned bio-refining. Furthermore ,the isomerization of glucose is very important in the food science as well. Therefore ,it has been widely investigated. In this paper ,an overview on the recent achievement and progress on the chemocatalytic isomerization of glucose to fructose was presented. Both conventional acid-base catalysts (such as Brönsted base ,solid base ,metal salt and molecular sieve ) and novel catalyst materials (for example ,metal organic frameworks and ionic liquids ) were intensively summarized. The acid and alkali catalytic mechanism for glucose isomerization were briefly introduced ,and the current第一作者:张雄(1991—),男,硕士研究生。
湖北省高中名校2024届高三上学期第二次联合测评英语试卷学校:___________姓名:___________班级:___________考号:___________ 一、阅读理解There are always the same number of restaurants on The Top 100, but every year offers more-more quality, more variety, more surprises. What never changes is how a restaurant gets on the list-wonderful food well served in an inviting setting, among which are some Chinese restaurants.Long Island PekinLong Island Pekin reminds us of the first Chinese foods many of us fell in love with: the roast duck and pork, the to me in and fried rice, the steamed dumplings and pot stickers. There are more than a dozen dumplings and buns to start the meal, three types of noodles and then meats roasted in the “hung oven”, including Peking duck.New Fu RunNew Fu Run has been serving the cuisine of Dongbei in Great Neck since 2017. Dongbei cooking tends to be earthier than Cantonese and not as spicy as Sichuan. Treat your taste buds (味蕾) to a cold starter of country-style beef with cucumber and the signature dish-Cumin Lamb Chop. Longing for something more familiar? Call three days in advance and New Fu Run will prepare a three-course meal based on Peking Duck.Splendid NoodleIt’s always a splendid occasion when there’s a chef twisting, pounding and swinging ropes of wheat noodles in the air. The most sought-after Chinese noodle houses have an open kitchen where the acrobatics (杂技) are on display. The Stony Brook shop offers this wavy noodle as well as a couple cold noodle dishes in light sesame sauce.1.What qualifies a restaurant to get on the list of The Top 100?A. Unique dishes.B. A splendid occasion.C. Great food and environment.D. More quality, variety and surprises.2.What do Long Island Pekin and New Fu Run have in common?A. They serve spicy food.B. They offer Peking DuckC. They have a cooking display.D. They require advance reservations. 3.Where is this text most probably taken from?A. A cuisine book.B. A restaurant guideC. A restaurant marketing plan.D. An online food delivery platform.My kids sit in Gee’s living room and carefully lift antique Christmas ornaments (装饰品) out of a delicate cardboard box. They gasp when they discover a tiny stuffed cat. Gee stands beside them, quietly explaining each treasure. “Ella, the story is that Tom and I built our ornament collection piece by piece during each year’s after-Christmas sale.” she tells me. She smiles as we leave with the box. Her precious treasures, gathered over a lifetime, have found a new home.We first met Tom and Gee in the early days of our marriage. Someone had been returning our garbage cans to the garage each garbage day, and Jim and I had wondered who. Then one day we spotted him: an elderly man who lived across the street.I baked cookies and left them on a stool outside the garage with a thank-you note. When we got home from work that day, a typed letter had replaced the gift. The letter was from Tom and explained how he had come to walk the neighborhood on garbage day, returning cans for people he barely knew. A few years after we’d moved in, Tom died. We photocopied that letter and attached it to one of our own for Gee. We told her how special Tom had been to us. She wrote back and told us she still talked to Tom every day. When Gee invited us over to look through Christmas ornaments, I realized how hard it must be to part with that box, a piece of Tom.Th ese days, we’re piling up boxes of our own. We’re planning a move. The house that seemed so huge six years ago is filled to capacity with furniture and books and toys and of course people. We know it’s time to go, and yet we can’t seem to stick the For Sal e sign up on the lawn. Gaining a third bedroom and maybe an office sometimes seems like an awful trade for all we stand to lose.The moving boxes are still neatly packed in our basement, but Jim and I agree to wait until January. This Christmas, we’ll decorate our tree with Gee’s ornaments, out of the box that is labeled in Tom’s handwriting. Maybe I’ll talk to him just as Gee still does.4.In which way did Gee and Tom build their ornament collection?A. They developed it through donations.B. They accumulated it from antique shops.C. They gathered it from their Christmas gifts.D. They acquired it through years of purchase.5.What do we know about Tom?A. He left his good deed a mystery.B. He meant significantly to Jim and Ella.C. He worked for the cleaning department.D. He volunteered to guard the neighborhood.6.What can we learn from the text?A. It is very tough for Gee to give away the box.B. Ella and Jim are eager to move to the new house.C. Ella thinks it is a good deal to gain an extra room.D. Ella’s Christmas tree is labeled in Tom’s handwriting.7.Which word best describes the author’s attitude to Gee and Tom?A. Faithful.B. Sympathetic.C. Affectionate.D. Tolerant.Farming is destroying the planet, but there could be a much more environmentally friendly way to feed ourselves: using renewable energy to turn carbon dioxide into food. “This is becoming a reality,” says Pasi Vainikka at Solar Foods, a company that is building the first commercial-scale factory that will be able to make food directly from CO2.There can be no doubt that immediate attention to find greener ways to grow food is required. Conventional agriculture, including organic farming, causes damage to the environment in many ways. It requires a lot of land, leading to habitat loss and deforestation. It is also the source of a third of all greenhouse gas emissions and releases other pollutants. It isn’t very efficient, either. Crops typically transform less than 1 percent of light energy into usable biomass (生物量).Instead, Solar Foods plans to avoid photosynthesis (光合作用) altogether, and grow bacteria that use hydrogen as their source of energy. At the factory, renewable electricity will be used to split water to produce hydrogen and oxygen. The hydrogen will be added to large containers, where the bacteria grow, along with CO2 and ammonia (氮气). The end result will be a yellow powder called Solein.Solein is made of bacterial cells and is up to 70 percent protein. It can be used as an ingredient in all kinds of foods. “We are aiming at replacing animal-sourced proteins, which we think have the highest environmental impact,” says Vainikka.Compared with plant crops, Solein will use 100 times less water per kilogram of protein produced, 20 times less land and emit a fifth as much CO2, according to Solar Foods. There are other benefits, too: factories could be situated anywhere in the world and production won’t be affected by weather conditions.“With Solar Foods and other companies scaling up thei r systems, this is truly beginning a new era of agriculture,” says Dorian Leger at Connectomix Bio in Germany. “I think thesetrends are exciting and will help bend the carbon curve as well as lead to improved global food supply security.”8.What is the au thor’s purpose in mentioning conventional agriculture in paragraph 2?A. To demonstrate its influence on crops.B. To compare different farming methods.C. To provide an example of agricultural types.D. To highlight the urgent need for alternatives.9.What is mainly presented in paragraph 3 concerning Solein?A. Its storage condition.B. Its production process.C. Its ingredient materialsD. Its investment potential.10.All of the following are the features of Solein except __________.A. it is protein-richB. it is resource-efficientC. its production is weather-sensitiveD. its production is location-independent11.Which statement would Dorian Leger probably agree with?A. The prospect of Solein remains to be seen.B. Solein can help achieve global food safety.C. Solein will dominate the agricultural development.D. The use of Solein may help reduce carbon emissions.People in long-term pain are often offered antidepressants, but a review has found little evidence to support using most such drugs in this way.It is estimated that about 1 in 5 people have ongoing pain, with a variety of causes and in various locations. However, treatment options are limited. While opioid-based medicines (阿片类药物) are effective for new-onset pain, they can be addictive when used long term. Anti-inflammatory drugs (抗炎药物) can treat pain, but can damage organs with extended use.This may be why some doctors offer antidepressants as treatment for long-term pain, even though the drugs generally aren’t licensed fo r such use. Some people with chronic (慢性的) pain are also depressed or anxious, so doctors could see the medicines as primarily helping with these conditions. but antidepressants are also thought to have a separate painkilling effect. The mechanism is unknown, but one idea is that it stems fromFerreira and his colleagues conducted a review with a detailed breakdown of the existing evidence, making a thorough analysis of the outcomes of 156 randomized trials involving more than 25. 000 participants. They looked at the effectiveness of eight types of antidepressant at treating 22 pain conditions. The team discovered there was no good evidence for the effectiveness of most of the drugs. Only one class showed evidence of effectiveness, but even this class reduced pain only modestly, by less than 10 points on a scale of 0 to 100.The analysis comes to different conclusions than a 2021 review by the National Institute for Health and Care Excellence (NICE), which said antidepressants were the only class of medicines that doctors should consider for chronic pain, although this should only be after discussing the potential benefits and harms. The difference in conclusions may be because the latest analysis considered each pain condition separately, says Ferreira.Cathy Stannard, who advised on the NICE gu idelines, says the new review doesn’t mean antidepressants should be ruled out. “Some people will get a useful benefit and there’s no way of predicting who. “But there is unlikely to be any “magic bullet” for chronic pain, says Stannard.12.What contributes to the use of antidepressants to treat long-term pain?A. They have a dramatic painkilling effect.B. They have gained approval for treating it.C. They are believed to possess no side effects.D. They are thought to be multifunctional drugs.13.What does the underlined word “dampening” in paragraph 3 mean?A. Worsening.B. Stimulating.C. Relieving.D. Causing14.How did Ferreira and his team carry out the review?A. By analyzing the results of previous studies.B. By observing patients’ react ions to antidepressants.C. By conducting interviews with over 25, 000 participants.D. By repeating the trials of antidepressants on participants.15.What can be a suitable title for the text?A. Antidepressants rarely ease painB. The painkilling effect of antidepressantsC. How to tackle long-term painful conditionsD. Antidepressants: “magic bullets” for chronic pain二、七选五16.How to stop procrastinating (拖延) and make a start Procrastinating is as much about fear as it is about motivation. Perhaps you have avoided paying bills, outlining a proposal or asking someone out. ① Fortunately, here are four tips to get you moving again.Little steps for big tasks.Most proc rastinators tell themselves, “I’ll wait until I’m in the mood. “Let’s face it: you’re never going to feel like balancing your cheque-book or cleaning up the mess on your desk. These are boring, unpleasant tasks. ② One way to do this is to break your job into steps that can be accomplished bit by bit.③Those who procrastinate often assume that successful people achieve their goals without frustration, self-doubt and failure. This is unrealistic. Highly productive people know that life is frustrating. They assume they’ll encounter obstacles; when they do, they persevere until they overcome them.Tune out negative thoughts.When you’re avoiding a task, it may be because you’re feeding yourself unrealistic, negative messages. ④Once you change your negative thoughts, energy returns.Give yourself credit.⑤But if you never allow yourself to feel satisfied with your efforts, you’ll soon find it pointless to try. So, no matter how small the achievement is, give yourself credit. Then you can tackle your toughest task.However, if you are sure that there are good reasons why you avoid doing something, you may need to re-evaluate your goals.A. Never give up.B. Expect difficulties.C. Sometimes you simply have to get yourself goingD. We usually think of rewards as coming from the outside.E. By writing them down, you have a chance of ridding them.F. Be here now, and don’t worry about things you have to do in the future.G. Whatever the thing, putting it off often becomes a bigger problem than actually doing it.三、完形填空(15空)There was a time in my life when I lost everything. Infected by a rare bacterium, I lostlost everything else.had nowhere to live and had to move back in with my parents.and true integrity.When bad things happen, it doesn’t mean that better things aren’t down the line. We just17.A. rose up B. rolled up C. ended up D. turned up18.A. temper B. patience C. cooperation D. touch19.A. status B. wealth C. fame D. career20.A. purpose B. trust C. loss D. shame21.A. conflict B. crisis C. divorce D. departure22.A. poverty B. despair C. confusion D. trouble23.A. hope B. relief C. courage D. strength24.A. fight B. respond C. twist D. heal25.A. declined B. performed C. returned D. changed26.A. accidentally B. cautiously C. quickly D. deliberately27.A. compared B. discussed C. integrated D. simplified28.A. entered B. influenced C. benefited D. shaped29.A. friendly B. flexible C. strategic D. romantic30.A. revealed B. marked C. preserved D. recorded31.A. get close to B. hold on to C. let go of D. take notice of四、短文填空32.The opening ceremony of the Hangzhou Asiad took place on Saturday evening at the Hangzhou Olympic Sports Center Stadium. Over 100 million people ①____________ (join) on digital platforms watched lighting up the cauldron () “The collective participation of people in the lighting of the cauldron symbolizes the vision of a human community with a ②____________ (share) future, “Sha Xiaolan, chief directo r and producer for the Hangzhou Asian Games opening ceremony, said at ③____________ press conference. “I want to thank my team ④____________ their outstanding efforts, “Sha said, adding “Tonight we took one step further’.”AR technologies were ⑤____________ (main) featured at the opening ceremony, including the AR-generated dome (that displayed flying sports ⑥____________ (equip) and the AR-generated waterfall that served as a backdrop.“It was the first time that 3D dual aerial performance technology ⑦____________ (use) in a major stadium, and it was very challenging. It was great team efforts ⑧____________ contributed to the presentation of the energetic and surging momentum,” Meng, the executive director, commented.The production team also extended their gratitude to the performers, of ⑨____________ the majority are university students. Executive director Gao Yan said, “They volunteered their time and efforts ⑩____________ (make) the Hangzhou Asian Games a success.”五、书面表达33.假定你是李华,你校于上周五举行了趣味运动会,你的新西兰朋友Terry很想了解该活动,请你给他写一封邮件,内容包括:1. 趣味运动会情况:2. 你的感受和收获。
See discussions, stats, and author profiles for this publication at: https:///publication/268520412Biomass production in a 15-year-old poplar short-rotation coppice culture in BelgiumCONFERENCE PAPER · JANUARY 2011CITATIONSREADS5726 AUTHORS, INCLUDING: Stefan Paula Patrick Vanbeveren University of Antwerp14 PUBLICATIONS 38 CITATIONS SEE PROFILEAll in-text references underlined in blue are linked to publications on ResearchGate, letting you access and read them immediately.Available from: Stefan Paula Patrick Vanbeveren Retrieved on: 17 February 2016Aspects of Applied Biology 112, 2011 Biomass and Energy Crops IVBiomass production in a 15-year-old poplar short-rotation coppice culture in BelgiumBy S Y DILLEN, S VANBEVEREN, N AL AFAS, I LAUREYSENS, S CROES and R CEULEMANS Antwerp University, Biology Department, Research Group Plant and Vegetation Ecology, Universiteitsplein 1, 2610 Wilrijk, Belgium Summary Biomass production of a 15-year-old multiclonal poplar short-rotation coppice (SRC) in Flanders, Belgium, is presented in this study. A wide range of 17 clonal varieties from six different parentages were planted in 1996 and no irrigation, fertilizers or fungicides were applied after the establishment year. After 15 years or four rotations, clones from pure species displayed significantly higher yields than hybrid clones. Specifically, clone Wolterson (Populus nigra) and clones Columbia River, Fritzi Pauley and Trichobel (P. trichocarpa) proved to be good candidates for SRC in temperate regions of Northwestern Europe. During the fourth rotation, lowest biomass yields were observed for the D×T and T×D clones, despite fast juvenile growth for the latter. Trends observed in the dynamics of biomass production during earlier rotations continued in the fourth rotation though differences among clones became more pronounced after 15 years of low-input SRC. For some clones, re-sprouting from root suckers likely affected stool survival values during the fourth rotation. Key words: Bioenergy, clone, Populus spp., stool survival, number of shoots Introduction Short-rotation coppice (SRC) systems are carefully tended plantations of fast-growing perennial species with good coppice and resprout capacity such as poplar and willow (Ceulemans & Deraedt 1999; Dillen et al., 2010). Poplar and willow SRC have been extensively studied since the oil crisis in the 1970s as a substitute to fossil fuels, i.e. bioenergy (Dickmann, 1996). Some decades later, their potential to mitigate climate change has generated renewed interest. However, recent studies reported that adverse environmental effects may outweigh the benefits of their mitigation potential (Sevigne et al., 2011). The environmental impacts of SRC are evaluated through life cycle assessment, although a widely accepted and uniform methodological approach is still lacking (Njakou Djomo et al., 2011). SRC toward bioenergy production is characterized by high planting density and relatively short rotations, i.e. 2–5 years. The complete life span of poplar or willow SRC systems is believed to be 20–25 years but life expectancy can be markedly affected by plantation maintenance, planting density in relation to harvest frequency and presence of pathogens (Sims et al., 2001). Experiments covering the complete life span of SRC are scarce. Nevertheless, long-term experiments are essential to gain insight into biomass potential, energy costs, greenhouse gas balance and environmental impacts of the SRC system throughout its full life cycle. 99In this study, we documented biomass production of a 15-year-old multiclonal poplar SRC. The plantation was maintained as a low energy input system; no fertilization, irrigation or fungicides were applied after the establishment year. During 15 years, including four rotations, stool survival, number of shoots and biomass production were frequently estimated for the 17 poplar clones (Al Afas et al., 2008). We built on earlier work and compared actual yields with yields from earlier rotations (Laureysens et al., 2004, 2005; Al Afas et al., 2008). To study the dynamics of biomass production of a poplar SRC over 15 years we estimated effects of clone and year as well as their mutual interactions interactions on stool survival, number of shoots and biomass production. Material and Methods Site and experimental design The plantation was established on a former household waste land filled with a mixture of sand, clay and rubble from nearby areas in Boom, Flanders, Belgium (51°05’N, 04°22’E). In April 1996, 25 cm-long hardwood cuttings from selected clones were planted at an initial planting density of 10 000 trees per hectare according to a double row design system with alternating interrow distances of 0.75 and 1.5 m and a spacing of 0.9 m between cuttings within rows. The 17 clones were distributed using a randomized block design with three replicate plots per clone. Each plot consisted of 100 trees or 10 rows by 10 columns, but only a core of six rows by six columns, i.e. 36 assessment trees, was sampled to avoid border effects. Plant material Clones were a mixture of hybrids and pure species: one Populus nigra L. clone (N) Wolterson; three P. trichocarpa Torr. & Gray clones (T) Columbia River, Fritzi Pauley and Trichobel; six P. trichocarpa × P. deltoides Bartr. clones (T×D) Beaupré, Boelare, Hazendans, Hoogvorst, Raspalje and Unal; three P. deltoides × P. trichocarpa (D×T) clones IBW1, IBW2 and IBW3; three P. deltoides × P. nigra clones (D×N) Gaver, Gibecq and Primo; and one P. trichocarpa × P. balsamifera L. clone (T×B) Balsam Spire. Management regime After planting, weeds were controlled by mechanical weeding and herbicides (Laureysens et al., 2004, 2005; Al Afas et al., 2008). All saplings were cut in December 1996 to obtain a multi-stem coppice in the next growing season. Mechanic weed control and/or herbicides were also applied during the first growing season after each harvest. No irrigation, fertilizers or fungicides were applied after the establishment year. A schematic overview of the coppice regime, rotation cycles and maintenance of the plantation is given in Fig. 1. Biomass estimation Survival of stools (%), number of shoots and shoot diameter were assessed among the 36 assessment trees at the end of the growing season of years 1997–2003, 2005, 2006 and 2010. Shoot diameter (D) was measured at 22 cm above ground level using a digital caliper (Mitutoyo, type CD-15DC, UK). When D exceeded 3 cm, the average of two perpendicular D measurements was further used in calculations (Pontailler et al., 1997). At regular intervals, a selection of shoots representative of the shoot diameter frequency distribution was randomly harvested from stumps, i.e. 5–30 shoots per clone (Pellis et al., 2004; Laureysens et al., 2004; Al Afas et al., 2008). Shoots were weighed after being dried at 105°C in a drying oven until constant mass was reached. For the years during which harvests were realized, above-ground woody biomass production was estimated by means of allometric power relationships between shoot dry mass and shoot diameter per clone and per year (M = a Db, with a and b as regression coefficients, and M as shoot dry mass; Pontailler et al., 1997). Biomass production in 1997 and 100Fig. 1. Schematic overview of coppice regime, rotation cycles and management of the 15-year-old poplar short-rotation coppice at Boom, Belgium (1996–2010).1998 was estimated using the allometric power equations of 1999, and biomass production of 2005, 2006 and 2010 using the equations of 2003 (Al Afas et al., 2008). At the end of each rotation, the harvested biomass was weighed (fresh and/or dry weight). Statistical analyses Analyses were performed in R Statistical Computing Environment (Language Environment Version 2.12.1). Means were calculated with their standard error (SE). Clonal and rotation effects on survival, number of shoots and biomass were tested using a repeated measures analysis of variance (ANOVA). The following model was used: Y = µ + Cl + Yr<Rt> + Rt + Cl × Rt + Cl × Yr<Rt> + ε where Y is stool survival, number of shoots or biomass production; clone (Cl), rotation (Rt) and year (Yr; nested within rotation) were treated as fixed effects; ε is the residual error. Post-hoc evaluation was done by Tukey’s HSD test. All differences were considered significant at P ≤ 0.05. Pearson correlation coefficients (r) among traits and Spearman rank coefficients (ρ) among years were calculated from clonal means. Results Biomass production Significant clonal variation was observed for stool survival, number of shoots and biomass production (Table 1). Significantly higher biomass production was observed for N and T clones compared to the D×T ad T×D clones. Clone Wolterson (N) was the most productive clone and 101Table 1. Tests of fixed effects of the repeated measures three-way ANOVA model for stool survival, number of shoots and biomass production Clone Stool survival Number of shoots Biomass production F16,353 = 55.9*** F16,325 = 27.8*** F16,322 = 21.9*** Rotation F3,353 = 106.1*** F3,325 = 113.6*** F3,322 = 206.7*** Year F7,353 =4.8*** F6,325 =113.6*** F6,322 =61.9*** Clone × year F112,353 = 0.19ns F95,325 = 2.1*** F95,322 = 1.1ns Clone × rotation F 48,353 = 5.56*** F 47,325 = 5.6*** F 47,322 = 4.6***Year was treated as nested factor within rotation. Significance levels are indicated as follows: *** P ≤ 0.001; ** P ≤ 0.01; * P ≤ 0.05; ns non-significant.Fig. 2. Time course of survival (%), number of shoots and biomass production (ton ha-1) during four rotation cycles of the short-rotation coppice with 17 clones belonging to six parentages. Means ± standard102error. T = Populus trichocarpa; B = P. balsamifera; D = P. deltoides; N = P. nigra.displayed lowest stool mortality and most vigorous resprouting in the 15-year-old SRC (Fig. 2). T clones showed high biomass yields by growing few but large shoots. On the other hand, very low biomass yields were obtained for D×T and T×D clones after 15 years (Fig. 2). Overall, significant clone × rotation interactions were observed (Table 1; Fig. 2). The T×D clones had vigorous juvenile growth and high biomass production during the first years but due to high mortality rates, T×D biomass production dropped dramatically from the second growing season onward (Fig. 2). Clone Hoogvorst (T×D) did not survive the third rotation. As opposed to T×D clones, D×N clones slowly established and had low growth rates during the first growing season (Fig. 2). But after the first rotation, biomass production of the D×N clones steadily increased to intermediately and highly ranked biomass values compared to other clones in the fourth and third rotations respectively. Surprisingly, stool survival of some clones was higher in the fourth than in the third rotation (Fig. 2). Explanations for this unexpected observation are given in the Discussion. Correlations among traits Significant correlations were found among traits in 2010, i.e. third year of fourth rotation. Obviously, high biomass production was associated with higher stool survival (r = 0.67 and P ≤ 0.01). Overall, clones producing a higher number of shoots tended to have higher biomass production (r = 0.56 and P ≤ 0.05). However, different growth strategy, i.e. few but larger shoots, resulted in large biomass yields for T clones. Finally, high stool survival was significantly correlated to stronger resprout capacity, or a higher number of shoots (r = 0.66 and P ≤ 0.01) According to Spearman rank coefficients across years, clonal stability of biomass production was generally highest within rotations (cf. Al Afas et al., 2008). Across rotations, the first rotation is not representative for the subsequent rotations (Table 2). Some changes in clonal biomass rankings occur between the second and the fourth rotation, but Spearman rank coefficients suggested high clonal stability between the last two rotations (Table 2). Table 2. Spearman rank coefficients calculated from clonal means of biomass production between fourth and earlier rotations of the 15-year-old poplar SRC 4th rotation 20092008 1 rotationst1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 20072010 ns ns ns ns ns 0.53* 0.68**2nd rotation3rd rotation0.74*** 0.88***Years without biomass measurements are in italic. Significance levels are indicated as follows: *** P ≤ 0.001; ** P ≤ 0.01; * P ≤ 0.05; ns non-significant.103Discussion The 15-year-old poplar SRC was planted with commercially available clonal varieties at the time of establishment, except for the IBW clones (D×T) which were clones in the observation phase. Striking clonal differences in biomass production were observed after four rotations. Some clones displayed the highest biomass levels in comparison with previous rotations. The pure species clones Wolterson, Columbia River, Fritzi Pauley and Trichobel yielded 7.9–10.3 ton ha-1 yr-1 in the fourth rotation. Yet, strikingly different growth strategies were maintained by these promising N and T clones. Wolterson produced numerous shoots after coppicing, while the T clones displayed high apical dominance and produced few, but large shoots. The N and T clones accommodated the growth strategy typical of their respective parentage, P. nigra and P. trichocarpa (Marron et al., 2010). Other clones did not survive the third rotation (Hoogvorst) or showed extremely low yields as Beaupre, Boelare and the IBW clones. Consequently, the IBW clones were not commercialized. The poor results the D×T and many of the T×D clones can be largely explained by their high susceptibility to leaf rust (Melampsora larici-populina). As discussed in Al Afas et al. (2008), a severe rust attack in 2001 caused high mortality. None of the D×T and T×D clones completely recovered and biomass production of these clones continued to decrease, even several years after the major leaf rust infestation. Plots with high mortality as a result of the rust attack were overgrown with tall weeds, as weed control was only applied the first year after each harvest. The tall weeds have likely reduced growth of poplar resprouts by competing for light, water and/ or nutrients in high-mortality plots (Sage et al., 1999). Al Afas et al. (2008) raised the question whether hybrids between P. deltoides and P. trichocarpa lose their resprout capacity and growth vigour after several harvests. Poplar hybrids are widely planted because of their vigorous juvenile growth. They often outperform the pure species at early age and assure rapid establishment of the plantation (Hayes 1952; Stettler et al., 1996). Consistently decreasing stool survival over the first three rotations has been demonstrated for the studied SRC by Laureysens et al. (2003) and Al Afas et al. (2008). Rooting difficulties during the first years and between-shoot competition for light during canopy closure were believed to affect stool survival. Unexpectedly, higher stool survival was observed in the fourth rotation for several clones of T, D×N and T×B parentage. Root sprouts from neighboring trees may have occupied some of the open areas in the field. These new shoots could not always be distinguished from originally planted individuals. The performance of some clones varied substantially over different rotations and years. Spearman rank coefficients demonstrated that productive clones in the first and second rotations did not per se displayed high biomass production during the fourth rotation. The clonal ranking of biomass in the fourth rotation was similar to that of the third rotation though differences among clones were more pronounced; highly productive clones as Wolterson and Fritzi Pauley reached peak biomass levels while biomass production of T×D and D×T clones was lowest after 15 years. In conclusion, this study highlights the need for long-term experiments to evaluate clonal performance over the complete life cycle of SRC systems. Clone × year or clone × rotation interactions likely affect the choice of clonal varieties to be grown in SRC systems. From this long-term experiment, clones from pure species are preferred over hybrids. The wide range of clones reduced the risks of the severe rust attack in 2001. Acknowledgements This study was supported by Research Foundation Flanders (FWO/G.0108.97), European Commission (AL/95/121/SWE), European Research Council (ERC Adv. Grant, POPFULL), 104Center of Excellence ECO (University of Antwerp), Province of Antwerp and City Council of Boom. The project has been carried out in close cooperation with Eta-com B., supplying the grounds and part of the infrastructure. All plant materials were kindly provided by the Research Institute for Nature and Forest (Geraardsbergen, Belgium) and by the Forest Research, Forestry Commission (UK). We gratefully acknowledge everyone who helped with biomass production measurements over the three rotations. O. El Kasmioui and S.Y. Dillen are Research Associates of the Research Foundation-Flanders (F.W.O.-Vlaanderen, Belgium). References Al Afas N, Marron N, Van Dongen S, Laureysens I, Ceulemans R. 2008. Dynamics of biomass production in a poplar coppice culture over three rotations (11 years). Forest Ecology and Management 255: 1883-1891. Ceulemans R, Deraedt W. 1999. Production physiology and growth potential of poplars under short-rotation forestry culture. Forest Ecology and Management 121:9–23. Dickmann D I. 1996. Silviculture and biology of short-rotation woody crops in temperate regions: then and now. Biomass and Bioenergy 30:696–705. Dillen S Y, Marron N, El Kasmioui O, Calfapietra C, Ceulemans R. 2011. Poplar. In Energy Crops, Chapter 14, DOI: 10.1039/9781849732048-00275 (In press). Eds N G Halford and A Karp. Cambridge, UK: Royal Society of Chemistry. Hayes H K. 1952. Development of the heterosis concept. In Heterosis. Ed. J W Gowen. Ames, Iowa: Iowa State. University College Press. Laureysens I, Deraedt W, Indeherberge T, Ceulemans R. 2003. Population dynamics in a 6-year-old coppice culture of poplar. I. Clonal differences in stool mortality, shoot dynamics and shoot diameter distribution in relation to biomass production. Biomass and Bioenergy 24:81–95. Laureysens I, Bogaert J, Blust R, Ceulemans R. 2004. Biomass production of 17 poplar clones in a short rotation coppice culture on a waste disposal site and its relation to soil characteristics. Forest Ecology and Management 187:295–309. Laureysens I, Pellis A, Willems J, Ceulemans R. 2005. Growth and production of a short rotation coppice culture of poplar. III. Second rotation results. Biomass and Bioenergy 29:10–21. Marron N, Storme V, Dillen SY, Bastien C, Ricciotti L, Salani F, Sabatti M, Rae AM, Ceulemans R, Boerjan W. 2010. Genomic regions involved in productivity of two interspecific poplar families in Europe. 2. Biomass production and its relationships with tree architecture and phenology. Tree Genetics and Genomes 6:533–554. Njakou Djomo S N, El Kasmioui O, Ceulemans R. 2011. Energy and greenhouse gas balance of bioenergy production from poplar and willow: a review. GCB Bioenergy 3:181–197. Pellis A, Laureysens I, Ceulemans R. 2004. Growth and production of a short rotation coppice culture of poplar. I. Clonal differences in leaf characteristics in relation to biomass production. Biomass and Bioenergy 27:9–19. Pontailler J Y, Ceulemans R, Guittet J, Mau F. 1997. Linear and non-linear functions of volume index to estimate woody biomass in high density young poplar stands. Annals of Forest Science 4:335–345. Sage R B. 1999. Weed competition in willow coppice crops: the cause and extent of yield losses. Weed Research 39:399–411. Sevigne E, Gasol CM, Brun F, Rovira L, Pagés J M, Camps F, Rieradevall J, Gabarrell. 2011. Water and energy consumption of Populus spp. bioenergy systems : A case study in Southern Europe. Renewable and Sustainable Energy Reviews 15:1133–1140. Sims R E H, Maiava T G, Bullock B T. 2001. Short rotation coppice tree species selection for woody biomass production in New Zealand. Biomass and Bioenergy 20:329–335. Stettler R F, Zsuffa L, Wu R. 1996. The role of hybridization in the genetic manipulation of 105Populus. In Biology of Populus and Its Implications for Management and Conservation. Eds R F Stettler, H DBradshaw Jr, P E Heilman and T M Hinckley. Ottawa: NRC Research Press.106。