化学专业英语
- 格式:doc
- 大小:50.50 KB
- 文档页数:4

专业英语词汇Unit 1TEXT A : Chemical Reactions and Group Reactionscustomary a. 通常的,惯例的handle n.柄vt.触摸handling n.处理,管理derive vt.取得,得到,衍生oxidate vt.使氧化oxidation n.satisfactory a.令人满意的,符合要求的rapid a.快的,迅速的,动作快的combustion n.燃烧somewhat pron. ad. 一点点,几分,有点effort n.努力commercial a.商业的,商务的undesirable a不.合需要的,不受欢迎的,讨厌的retard vt.延迟,放慢,使停滞transformer n.变压器transform vt.改变,转变automotive a.自动的,机动的,汽车的cracked裂化的sluge n.软泥,淤泥stiff a.硬的,强烈的extent 广度,程度distillation n.蒸馏distill vt.vi.unrefined a.未精致,未提炼的acidity n.酸味,酸性acidify vt. Vi.Involve vt. 包缠,卷缠Fell=followingIndividual a.个人的,个体的Presumable a可.假定的,可推测的Destruction n.破坏,毁灭Overall n。
a.全面的,综合的Exceed 超过,胜过Isolate vt.隔离,孤立,使离析iso—构词成分“均匀”“异构”“苯”Analyse vt. 分析,分解Carbonyl 羰基Carboxyl羧基Hydroxyl羟基Decomposition分解Alkyl烷基,烃基Ketone 酮Aldehyde n.醛Yield vt. 出产,产出Explosive a. 爆炸Vapor n.蒸汽, vi.蒸发Propagation 繁殖,增殖;传播Dehydrate vt.使脱水Acet 构词成分Acetaldehyde乙醛Resin n.树脂Resinous a.树脂的Carboxylic a.羟基的Substantial a.物质的,实质的Susceptible a易.受感动的,敏感的Analogous a.类似的,相似的( to)Response n.作答,回答,响应,反应Readily ad.乐意地,很快地Readiness n准.备就绪,愿意Extent n.广度长度Steric 空间的,位的Likewise ad.同样的,照样地;也,又Suffer vt.遭受,经历Progressive a进.步的,长进的,渐次的Adjacent a.邻近的,紧挨着的Terminal a.末端的,终点的MethyleneBromide n.溴化物Substitute n.代替物(人),代用品substitution n.代替,替换Remote a相.隔较远的Acetone n.丙酮Ether n.醚,乙醚Correspond vi.符合,一致;相当,相应Reservation n保.留,预定Tend vi.走向,趋向。

☆常用: ppm: parts per millionppb: parts per billion pH: potential of hydrogen1. 化合物的命名:规则:金属(或某些非金属)元素+阴离子名称(1)MgCl2 magnesium [mæɡ’ni:zj əm] chloride (2)NaNO2 sodium nitrite [‘naitrait](3)KNO3 potassium[p ə’tæsi əm] nitrate [‘naitreit] (4)硝酸 nitric acid(5)NaHCO3 sodium hydrogen carbonate练习:▪ FeBr2 ▪ (NH4)2SO4 ▪ NH4H2PO4▪KMnO4▪亚硫酸▪sulfurous acid▪H2S▪NO2 有机物命名▪Hydrocarbon▪{Aliphatic hydrocarbon; Aromatic Hydrocarbon}▪Aliphatic hydrocarbon (脂肪烃)▪{Alkane (烷); Alkene(烯); Alkyne(炔)}▪Alcohol 醇▪Aldehyde 醛▪Ketone [‘ki:təun] 酮▪Carboxylic acid 羧酸▪Aromatic hydrocarbon(芳香烃)▪{benzene (苯) hydroxybenzene(酚) quinone(醌)无机物中关于数字的写法mono-, di-, tri-, tetra-, penta- hexa-, hepta-, octa-, nona-, deca-一,二,三,四,五,六,七,八,九,十有机物中关于数字的写法meth-, eth-, prop-, but-, pent-, hex-,甲乙丙丁戊已hept-, oct-, non-, dec-, cyclo-, poly-庚辛壬葵环聚练习▪甲烷乙炔▪丙酮丁醇▪戊烷己烯▪庚醛辛烷▪2-甲基壬酸 3,5-二乙基癸醇Lithium [‘liθiəm] n.锂Beryllium [bə’riljəm] n.铍(Be)Sodium [‘səudiəm] n.钠Potassium [pə’tæsiəm] 钾Rubidium [ru:’bidiəm] 铷Caesium [‘si:ziəm] 铯Nucleus[‘nju:kli s] 原子核,是nuclear的复数Halogen[‘hælədʒən] 卤素general chemistry 普通化学positive[‘pƆzətiv] ion 阳离子orbital electron 轨道电子effective nuclear charge 有效核电荷atomic radius 原子半径,raddi的复数ionic radius 离子半径negative ion 阴离子electron cloud 电子云Van der Waals non-bounded radius单质分子晶体中相邻分子间两个非键合原子核间距离的一半称为范德华半径metallic [mi’tælik] character[‘kæriktə] 金属特性electropositive [I’lektrəu’pɔzətiv] a.带正电的Ionization [‘aiənai’zeiʃən] energy 电离能carbon 碳 germanium[dʒə:’meiniəm] 锗tin [tin] 锡 lead [led] 铅sodium[‘səudiəm] 钠 magnesium[mæɡ’ni:zjəm] 镁silicon [‘silikən] 硅 chlorine [’klɔ:ri:n] 氯nonmetallic [‘nɔnmi’tælik]adj.n.非金属的,非金属Electronegativity 电负性Metallic oxide 金属氧化物Metallic hydroxide [hai’drɔksaid] 金属氢氧化物Hydroxyl [hai‘drɔksil] ions 氢氧根离子insoluble[in’sɔljubl] 不溶解的Ionic [ai‘ɔnik] adj. 离子的Transition element 过渡元素Basicity [bə’sisiti] n. 碱性,碱度Oxyacid [,ɔksi’æsid] 含氧酸Carbonate [‘kɑ:bəneit] 碳酸盐Nitrate [‘naitreit] 硝酸盐Sulphate [‘sʌlfeit] 硫酸盐 = sulfateAmphoteric [,æmfə’terik] adj.两性的Acid [‘æsid] n. adj.alkali [‘ælkəlai] n.adj.Hydration [hai’dreiʃən] 水合作用Hydrolyze [‘haidrəlaiz] vi. 水解Oxysalt [‘ɔksisɔ:lt] 含氧酸盐Complex 络合物,复合物句子理解1) Metals are electropositive and have a tendency to loss electrons, if suppliedwith energy: M M+ + e. 金属是电正性的,如果供给能量,有失去电子的趋势。




unite 1. Inorganic chemistry1.1 what is chemistry(1). 重点专业词汇讲解:Chemical: adj . 化学的、化学药品Transformation: 变化,化学转变,转化Dye: n. 染料染色,或者vt. 染Charcoal: ['t ? ck??l] 木炭Cellulose : 纤维素细胞的['selj?l??z; ] Fat:n. 脂肪肥肉adj . 肥大的alkalis :碱adj . 碱性的glycerin: 甘油丙三醇alkalis: n. 碱金属alloy: 合金使成合金bronze:青铜色的n. 青铜(铜和锡的合金)brass:[br a s] n.黄铜(铜和锌)要求学生会区别黄铜及青铜的不同翻译Poison:毒物毒药t.毒害放毒下毒Proton:n. 质子Nulei: n.核(nucleus 的复数形式)['njukl ??s]Identical : adj . 同一的Chirality n.手性手征和Handeness的区别Amino acid :n. 氨基酸Ala nine: n.丙氨酸2. 课文中重点词组(phrase)Chemical change:化学变化physical change:物理变化Explore: 探险研究research investigate studyIsolate: 分离chemical bonds 化学键chemical reaction :化学反应Natural substance 天然物质Coke : 焦炭carbon monoxide 一氧化碳Carbon Dioxide 二氧化碳Chemical bond 化学键fundamental principle 基本原理The periodic table of elements :元素周期表numbers of protons 质子数atomic number 原子序数covalent bonds 共价键positive 正阳性negative 负阴性3. 课文中重点句子The first and most important principle is that chemical substances are made up of molecules in which atoms of various elements are linked in well-defined ways. 需要着重给学生讲解第一条也是最重要的原理是化学物质是有分子组成的,分子中的不同元素的原子是以一定的方式连接在一起的。

化学专业英语1、化学专业英语:一、无机化学术语1、periodic table 元素周期表2、electronic structure电子构型3、wavelength波长4、frequency频率5、wave number波数6、diffraction衍射7、quantum量子8、quantized量子化9、quantum theory量子理论10、photoelectric effect光电效应11、photon光子12、quantum mechanics量子力学13、Heisenberg uncertainty principle海森堡测不准原理14、momentum动量15、angular momentum角动量16、ground state基态17、excited states激发态18、quantum number量子数19、atomic orbital原子轨道20、the four quantum numbers四个量子数21、electron configuration电子构型22、Pauli exclusion principle泡利不相容原理23、Hund’s principle洪特规则24、paramagnetism顺磁性25、diamagnetism反磁性26、period周期27、noble gas惰性气体28、Representative elements代表性元素29、Transition elements过渡元素30、Metals金属31、nonmetals非金属32、semiconducting elements半导体元素33、chemical bond化学键34、valence electrons价电子35、Lewis symbol路易斯符号36、Chemical stability化学稳定性37、octet rule八隅体规则38、chemical reactivity化学反应性39、metallic bonding金属键40、ionic bonding 离子键41、Lewis structures路易斯结构42、nonbonding electron pairs(lone pairs)非成键电子对43、covalent bonding共价键44、single单键45、multiple(double,triple) and coordinate(donor atom and acceptor atom) covalent bond配位键46、resonance共振47、resonance hybrid共振杂化48、nonpolar and polar covalent bond非极性和极性共价键49、dipole偶极50、network covalent substances51、bond dissociation energy键解离能52、lattice energy点阵能,晶格能53、atomic radii原子半径54、effective nuclear charge有效核电荷55、screening effect屏蔽效应56、Scanning 扫描57、Lanthanide contraction镧系收缩58、isoelectronic ions等电子离子59、ionization energy电离能60、noble gas configuration惰性气体构型61、electron affinity电子亲和能62、pseudo-noble gas configuration稀有气体原子实63、polarization of an ion离子极化64、electronegativity电负性65、electronegative atom电正性原子66、electropositive atom电负性原子67、Oxidation numbers氧化值68、Oxidation state氧化态69、molecular geometry分子几何70、bond axis键轴71、valence bond theory价键理论72、hybridization杂化73、isomers异构体74、structural isomers结构异构75、delocalized electrons离域电子76、dipole moment偶极矩77、London bond色散力78、nuclide核素79、nucleons核子80、mass defect质量缺陷81、nuclear binding energy核结合能82、nuclear fusion核聚变83、nuclear fission核裂变84、radioactivity放射性85、radionuclides放射性核素86、magic number幻数87、bombardment reaction轰击反应88、antineutrino反中微子89、neutrino中微子90、positron正电子(阳电子)91、electron capture电子捕获92、chain reaction链式反应93、crtical mass临界质量94、nuclear reaction 核反应95、thermonuclear reactions热核反应96、breeder reactor增殖反应97、hydration水合98、solvation溶剂化99、chemical equilibrium化学平衡100、hydrolysis水解101、hydrates水合物102、efflorescence风化物103、hygroscopic 吸湿104、deliquescence潮解105、electrolytes电解质106、strong(weak)electrolytes强电解质107、nonelectrolytes非电解质108、acidic(alkaline)aqueous solution109、polyprotic acids多元酸110、neutralization中和反应111、complex ion络合离子112、ligands配体113、hard water 硬水114、carbonate hardness碳酸盐硬度115、water softening水软化116、permanent hardness永久硬度117、ion exchange离子交换118、fossil fuels化石燃料119、oxidation氧化120、reduction还原121、oxidation-reduction(redox)reactions氧化还原反应122、oxidizing agent氧化剂123、heavy water重水124、absorption吸附125、acidic anhydride(oxide)酸性酸酐126、basic anhydride(oxide)碱性酸酐127、amphoteric两性128、allotropes同素异形体129、acid salt酸式盐130、oxidizing anion氧化性阴离子131、disproportionation reaction歧化反应132、oxidizing acids氧化性酸。

专业英语词汇Unit 1 TEXT A:Chemical Reactions and Group Reactions customary a. 通常的,惯例的handle n.柄vt.触摸handling n.处理,管理derive vt.取得,得到,衍生oxidate vt.使氧化oxidation n.satisfactory a.令人满意的,符合要求的rapid a.快的,迅速的,动作快的combustion n.燃烧somewhat pron. ad. 一点点,几分,有点effort n.努力commercial a.商业的,商务的undesirable a.不合需要的,不受欢迎的,讨厌的retard vt.延迟,放慢,使停滞transformer n.变压器transform vt.改变,转变automotive a.自动的,机动的,汽车的cracked 裂化的sluge n.软泥,淤泥stiff a.硬的,强烈的extent 广度,程度distillation n.蒸馏distill vt.vi.unrefined a.未精致,未提炼的acidity n.酸味,酸性acidify vt. Vi.Involve vt.包缠,卷缠Fell=followingIndividual a.个人的,个体的Presumable a.可假定的,可推测的Destruction n.破坏,毁灭Overall n。
a.全面的,综合的Exceed 超过,胜过Isolate vt.隔离,孤立,使离析iso—构词成分“均匀”“异构”“苯”Analyse vt. 分析,分解Carbonyl 羰基Carboxyl 羧基Hydroxyl 羟基Decomposition 分解Alkyl 烷基,烃基Aldehyde n.醛Yield vt. 出产,产出Explosive a. 爆炸Vapor n.蒸汽,vi.蒸发Propagation 繁殖,增殖;传播Dehydrate vt.使脱水Acet 构词成分Acetaldehyde 乙醛Resin n.树脂Resinous a.树脂的Carboxylic a.羟基的Substantial a.物质的,实质的Susceptible a.易受感动的,敏感的Analogous a.类似的,相似的(to)Response n.作答,回答,响应,反应Readily ad.乐意地,很快地Readiness n.准备就绪,愿意Extent n.广度长度Steric 空间的,位的Likewise ad.同样的,照样地;也,又Suffer vt.遭受,经历Progressive a.进步的,长进的,渐次的Adjacent a.邻近的,紧挨着的Terminal a.末端的,终点的MethyleneBromide n.溴化物Substitute n.代替物(人),代用品substitution n.代替,替换Remote a.相隔较远的Acetone n.丙酮Ether n.醚,乙醚Correspond vi.符合,一致;相当,相应Reservation n.保留,预定Tend vi.走向,趋向。

1、Beginning in the late seventeenth century with the work of Robert Boyle, who proposed the presently accepted concept of an element, numerous investigations produced a considerable knowledge of the properties of elements and their compounds.早在17义耳就开始了这项工作,他提出了现在公认的元素概念,大量的研究使我们对元素极其化合物的性质有了相当的了解。
In modern form, the law states that the properties of the elements are periodic functions of their atomic numbers. In other words, when the elements are listed in order of increasing atomic number, elements having closely similar properties will fall at definite intervals along the list.用现代的话说,这个规律叙述了元素的性质是它们的原子序数的周期性函数。
换句话说,当元素按照原子序数逐渐递增的顺序列表(排列时),性质非常接近的元素将占据表格中具有一定间隔的位置.Thus it is possible to arrange the list of elements in tabular form with elements having similar properties placed in vertical columns.于是,将具有类似性质的元素排成纵列,从而把元素排成表格形式是可能的。
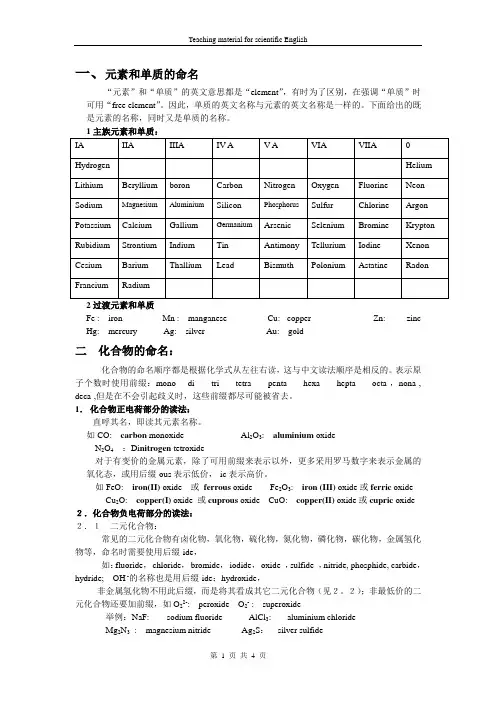
一、元素和单质的命名“元素”和“单质”的英文意思都是“element”,有时为了区别,在强调“单质”时可用“free element”。
因此,单质的英文名称与元素的英文名称是一样的。
下面给出的既是元素的名称,同时又是单质的名称。
2过渡元素和单质Fe : iron Mn : manganese Cu: copper Zn: zinc Hg: mercury Ag: silver Au: gold二化合物的命名:化合物的命名顺序都是根据化学式从左往右读,这与中文读法顺序是相反的。
表示原子个数时使用前缀:mono-di -tri- tetra -penta- hexa-hepta- octa-,nona-, deca-,但是在不会引起歧义时,这些前缀都尽可能被省去。
1.化合物正电荷部分的读法:直呼其名,即读其元素名称。
如CO: carbon monoxide Al2O3: aluminium oxideN2O4:Di nitrogen tetroxide对于有变价的金属元素,除了可用前缀来表示以外,更多采用罗马数字来表示金属的氧化态,或用后缀-ous表示低价,-ic表示高价。
如FeO: iron(II) oxide 或ferrous oxide Fe2O3: iron (III) oxide或ferric oxide Cu2O: copper(I) oxide 或cuprous oxide CuO: copper(II) oxide或cupric oxide 2.化合物负电荷部分的读法:2.1二元化合物:常见的二元化合物有卤化物,氧化物,硫化物,氮化物,磷化物,碳化物,金属氢化物等,命名时需要使用后缀-ide,如:fluoride,chloride,bromide,iodide,oxide ,sulfide ,nitride, phosphide, carbide,hydride; OH -的名称也是用后缀-ide:hydroxide,非金属氢化物不用此后缀,而是将其看成其它二元化合物(见2。

专业英语词汇Unit 1 TEXT A:Chemical Reactions and Group Reactionscustomary a. 通常的,惯例的handle n.柄vt.触摸handling n.处理,管理derive vt.取得,得到,衍生oxidate vt.使氧化oxidation n.satisfactory a.令人满意的,符合要求的rapid a.快的,迅速的,动作快的combustion n.燃烧somewhat pron. ad. 一点点,几分,有点effort n.努力commercial a.商业的,商务的undesirable a不.合需要的,不受欢迎的,讨厌的retard vt.延迟,放慢,使停滞transformer n.变压器transform vt.改变,转变automotive a.自动的,机动的,汽车的cracked 裂化的sluge n.软泥,淤泥stiff a.硬的,强烈的extent 广度,程度distillation n.蒸馏distill vt.vi.unrefined a.未精致,未提炼的acidity n.酸味,酸性acidify vt. Vi.Involve vt.包缠,卷缠Fell=followingIndividual a.个人的,个体的Presumable a可. 假定的,可推测的Destruction n.破坏,毁灭Overall n。
a.全面的,综合的Exceed 超过,胜过Isolate vt.隔离,孤立,使离析iso—构词成分“均匀”“异构”“苯”Analyse vt. 分析,分解Carbonyl 羰基Carboxyl 羧基Hydroxyl 羟基Decomposition 分解Alkyl 烷基,烃基Aldehyde n.醛Yield vt. 出产,产出Explosive a. 爆炸Vapor n.蒸汽,vi.蒸发Propagation 繁殖,增殖;传播Dehydrate vt.使脱水Acet 构词成分Acetaldehyde 乙醛Resin n.树脂Resinous a.树脂的Carboxylic a.羟基的Substantial a.物质的,实质的Susceptible a易.受感动的,敏感的Analogous a.类似的,相似的(to)Response n.作答,回答,响应,反应Readily ad.乐意地,很快地Readiness n准. 备就绪,愿意Extent n.广度长度Steric 空间的,位的Likewise ad.同样的,照样地;也,又Suffer vt.遭受,经历Progressive a进. 步的,长进的,渐次的Adjacent a.邻近的,紧挨着的Terminal a.末端的,终点的MethyleneBromide n.溴化物Substitute n.代替物(人),代用品substitution n.代替,替换Remote a相.隔较远的Acetone n.丙酮Ether n.醚,乙醚Correspond vi.符合,一致;相当,相应Reservation n保.留,预定Tend vi.走向,趋向。
化学专业英语(修订版)译文目录:1、THE ELEMENTS AND THE PERIODIC TABLE(元素和周期表)........................................6、THE CLASSIFICATION OF INORGANIC COMPOUNDS(无机化合物的分类).................................................................................................................................................................................7、The Nomenclature of Inorganic Compounds(无机化合物的命名) ....................................................9、The coordination complex(配位化合物) ...........................................................................................10、Alkanes(烷烃) ..............................................................................................................................................11、Unsaturated Compounds不饱和化合物...............................................................................................12、The Nomenclature of Cyclic Hydrocarbons(环烃的命名) ...........................................................13、Subsitutive Nomencalture(取代基命名法)......................................................................................14、The Compounds Containing Oxygen(含氧化合物).......................................................................15、Preparation of A Carboxylic Acid by the Grignard Method (格式法制备羧酸) .....................................................................................................................................................18、Synthesis of alcohols and design of organic synthesis (醇的合成及有机合成的设计)........................................................................................................................................22、Polymers(聚合物).................................................................................................................................1、THE ELEMENTS AND THE PERIODIC TABLE(元素和周期表)在原子核中质子的数目被称为原子序数,或质子数,Z。
(完整版)化学专业英语一、基础词汇篇1. 原子与分子Atom(原子):物质的基本单位,由质子、中子和电子组成。
2. 化学反应Reactant(反应物):参与化学反应的物质。
Product(物):化学反应后的物质。
Catalyst(催化剂):能改变化学反应速率而本身不发生永久变化的物质。
3. 物质状态Solid(固体):具有一定形状和体积的物质。
Liquid(液体):具有一定体积,无固定形状的物质。
Gas(气体):无固定形状和体积的物质。
4. 酸碱盐Acid(酸):在水溶液中能电离出氢离子的物质。
Base(碱):在水溶液中能电离出氢氧根离子的物质。
Salt(盐):由酸的阴离子和碱的阳离子组成的化合物。
5. 溶液与浓度Solution(溶液):由溶剂和溶质组成的均匀混合物。
Solvent(溶剂):能溶解其他物质的物质。
Solute(溶质):被溶解的物质。
Concentration(浓度):溶液中溶质含量的度量。
二、专业术语篇1. 有机化学Organic Chemistry(有机化学):研究碳化合物及其衍生物的化学分支。
Functional Group(官能团):决定有机化合物化学性质的原子或原子团。
Polymer(聚合物):由许多重复单元组成的大分子化合物。
2. 无机化学Inorganic Chemistry(无机化学):研究不含碳的化合物及其性质的化学分支。
Crystal(晶体):具有规则排列的原子、离子或分子的固体。
OxidationReduction Reaction(氧化还原反应):涉及电子转移的化学反应。
3. 物理化学Physical Chemistry(物理化学):研究化学现象与物理现象之间关系的化学分支。
Chemical Bond(化学键):原子间相互作用力,使原子结合成分子。
Thermodynamics(热力学):研究能量转换和物质性质的科学。
4. 分析化学Analytical Chemistry(分析化学):研究物质的组成、结构和性质的科学。
完整版)化学专业英语Teaching Material for Scientific EnglishI。
Naming of XXX1.Naming of XXXThe English word for both "元素" and "单质" is "element"。
To distinguish een the two。
"free element" may be used when emphasizing "单质"。
Therefore。
the English names for XXX are the same。
The following are the names of elements that are also names of free elements:Group IA:XXXXXXSodiumGroup IIA:XXXMagnesiumGroup IIIA: Boron AluminumGroup IVA: Carbon Silicon GermaniumGroup VA: Nitrogen PhosphorusGroup VIA: Oxygen Sulfur XXXXXXPoloniumGroup VIIA: Fluorine Chlorine Bromine IodineXXXGroup 0: XXXNeon ArgonXXX Xenon RadonGroup IA: Potassium CalciumGroup IIA:RubidiumCesiumFranciumGroup IIIA:GalliumIndiumXXXGroup IVA:ArsenicXXXXXXXXXLead2.Naming of CompoundsCompounds are named from left to right according to their chemical formula。
1, oxygen ['ɔksidʒən] n. 氧气,氧2, alkenes n. 烯属烃3, methyl ['mi:θail, 'miθil] n. 甲基;木精4, alkanes ['ælkeinz] n. 烷类,链烷烃;烷属烃(alkane的复数)5, turpentine ['tə:pəntain] n. 松节油;松脂 vt. 涂松节油 vi. 提取松节油6, benzene ['benzi:n]n. [化]苯7, chlorine ['klɔ:ri:n]n. 氯(17号化学元素)8, nickle alloy nickle alloy: 而非不含金之镍合金 | 而非不含金的镍合金9, brominate ['brəumineit]vt. 用溴处理,溴化10, haloalkane n. [有化] 卤代烷11, decolourise [di:'kʌləraiz]vt. 使…脱色;将…漂白 vi. 漂白(等于decolorize)12, copper ['kɔpə]n. 铜;铜币;[俚]警察 adj. 铜的 vt. 镀铜于13, hydrolysis [hai'drɔlisis]n. [化]水解作用14, derived adj. 导出的;衍生的,派生的 v. 得到;由…而来;推断(derive的过去分词)15, detergent [di'tə:dʒənt]n. 清洁剂;去垢剂16, sheet [ʃi:t]n. 薄片,纸张;床单;薄板 vt. 盖上被单;覆盖;使成大片 vi. 成片流动;大片落下adj. 片状的17, glycol ['ɡlaikɔl]n. 乙二醇;甘醇;二羟基醇18, bromide ['brəumaid]n. 溴化物;庸俗的人;陈词滥调19, styrene ['stairi:n, 'sti-]n. [有化] 苯乙烯,苏合香烯20, insulation [,insju'leiʃən, 'insə-]n. 绝缘;隔离,孤立21, antiseptics [,ænti'septiks]n. 防腐剂(antiseptic的复数)22, hydration [hai'dreiʃən]n. [化学] 水合作用23, halogenation [,hælədʒi'neiʃən]n. 卤化,加卤作用24, adhesive [əd'hi:siv]n. 粘合剂;胶黏剂 adj. 带粘性的;粘着的25, clay [klei]n. 泥土;粘土;肉体;似黏土的东西 vt. 用黏土处理26, sugarcane ['ʃugə,kein]n. [作物] 甘蔗;糖蔗27, fermentation [,fə:men'teiʃən]n. 发酵28, molasses [mə'læsiz]n. 糖蜜,糖浆29, intermediate [,intə'mi:djət, -dieit]vi. 起媒介作用 adj. 中间的,中级的 n. 中间物;媒介30, volatile ['vɔlətail]adj. 爆炸性的;不稳定的;挥发性的;反覆无常的 n. 挥发物;有翅的动物31, carbonisation n. 碳化,碳化作用32, liquefaction [,likwi'fækʃən]n. 液化;熔解33, synthesis ['sinθisis]n. 综合,合成;综合体34, optimum ['ɔptiməm]adj. 最适宜的 n. 最佳效果;最适宜条件35, usage ['ju:zidʒ]n. 用法;使用;惯例36, empirical [em'pirikəl]adj. 经验主义的,完全根据经验的37, endothermic [,endəu'θə:mik,-məl]adj. [热] 吸热的;温血的38, radical ['rædikəl]adj. 根本的;激进的;彻底的 n. 激进分子;原子团;根数;基础39, prefix [,pri:'fiks, 'pri:fiks]n. 前缀vt. 加前缀;将某事物加在前面40, initiator [i'niʃieitə]n. 发起人,创始者;教导者;启动程序;引爆器41, dimer ['daimə]n. [有化] 二聚物;二量体42, activation [,ækti'veiʃən]n. 激活;活化作用43, propagation [,prɔpə'ɡeiʃən]n. 传播;繁殖;增殖44, rigid ['ridʒid]adj. 严格的;僵硬的,死板的;坚硬的;精确的45, amorphous [ə'mɔ:fəs]adj. 无定形的;无组织的;非晶形的46, viscous ['viskəs]adj. 粘性的;黏的47, bracket ['brækit]n. 支架;括号;墙上凸出的托架 vt. 括在一起;把…归入同一类;排除48, caustic ['kɔ:stik]adj. [化]腐蚀性的;苛性的;刻薄的;[物]焦散的n. [医]腐蚀剂;[化]苛性钠;[物]焦散曲线49, moderate ['mɔdərət, 'mɔdəreit]adj. 适度的,中等的;有节制的;稳健的,温和的vi. 变缓和,变弱 vt. 节制;减轻50, brittle ['britl]adj. 易碎的,脆弱的;易生气的51, stiffiness n. 硬度52, gutter ['ɡʌtə]n. 排水沟;槽;贫民区 vi. 流;形成沟vt. 开沟于…;弄熄 adj. 贫贱的;粗俗的;耸人听闻的53, upholstery [ʌp'həulstəri]n. 家具装饰用品业;垫衬物54, curtain ['kə:tən]n. 窗帘;幕 vt. 遮蔽;装上门帘55, ultraviolet [,ʌltrə'vaiələt]adj. 紫外的;紫外线的 n. 紫外线辐射,紫外光56, decomposition [,di:kɔmpə'ziʃən]n. 分解,腐烂;变质57, cassettes n. 盒式录音带(cassette的复数)58, cassette [kæ'set]n. 盒式磁带;暗盒;珠宝箱;片匣59, disposable [dis'pəuzəbl]adj. 可任意处理的;可自由使用的;用完即可丢弃的60, autoclave ['ɔ:təkleiv]n. 高压灭菌器;高压锅 vt. 用高压锅烹饪 vi. 用高压锅烹饪。
精心整理一、元素和单质的命名
“元素”和“单质”的英文意思都是“element”,有时为了区别,在强调“单质”时可用“freeelement”。
因此,单质的英文名称与元素的英文名称是一样的。
下面给出的既是元素的名称,同时又是单质的名称。
或用后缀-ous表示低价,-ic表示高价。
如FeO:iron(II)oxide或ferrous oxideFe2O3:iron(III)oxide或ferric oxide
Cu2O:copper(I)oxide或cuprous oxide CuO:copper(II)oxide或cupric oxide
2.化合物负电荷部分的读法:
2.1二元化合物:
常见的二元化合物有卤化物,氧化物,硫化物,氮化物,磷化物,碳化物,金属氢化物等,命名时需要使用后缀-ide,
如:fluoride,chloride,bromide,iodide,oxide,sulfide,nitride,phosphide,carbide,hydride;OH-的名称也是用后缀-ide:hydroxide,
非金属氢化物不用此后缀,而是将其看成其它二元化合物(见2。
2);非最低价的二元化合
物还要加前缀,如O22-:peroxideO2-:superoxide
举例:NaF:sodiumfluoride AlCl3:aluminiumchloride
Mg2N3:magnesiumnitride Ag2S:silversulfide
CaC2:calciumcarbide Fe(OH)2:iron(II)hydroxide
有些物质常用俗称,如NOnitricoxideN2Onitrousoxide
2.2非金属氢化物
除了水和氨气使用俗称water,ammonia以外,其它的非金属氢化物都用系统名称,命名规则根据化学式的写法不同而有所不同。
对于卤族和氧族氢化物,H在化学式中写在前面,因此将其看成另一元素的二元化合物。
举例:HFhydrogenfluorideHClhydrogenchloride
HBrhydrogenbromideHIhydrogeniodide
CH4
H
高某酸
举例:
H
HPO3
正盐:根据化学式从左往右分别读出阳离子和阴离子的名称。
如FeSO4iron(II)sulfateKMnO4potassiumpermanganate
酸式盐:同正盐的读法,酸根中的H读做hydrogen,氢原子的个数用前缀表示。
如NaHCO3:sodiumhydrogencarbonate或sodiumbicarbonate
NaH2PO4:sodiumdihydrogenphosphate
复盐:同正盐的读法,并且阳离子按英文名称的第一个字母顺序读。
如KNaCO3:potassiumsodiumcarbonate
NaNH4HPO4:ammoniumsodiumhydrogenphosphate
水合盐:结晶水读做water或hydrate
如AlCl3.6H2O:aluminumchloride6-water或aluminumchloridehexahydrate
AlK(SO4)212H2Oaluminiumpotassiumsulphate12-water
三物理性质(physicalproperties)
colour:colorless,red-brown,violet-black,purple-black,paleyellow,darkbrown
smell:odorless,pungent,penetrating,offensive,choking,bitter,sour,sweet
state:solid,liquid,gas,gaseous,oily,crystalline,uncrystalline,molten,fused
solubility:soluble,insoluble,slightlysoluble,verysoluble,
density:heavy,light,lessdense,denser,greatlydenser,slightlydenser,
aboutthesamedense
hardness:hard,soft,ductile,malleable
toxicity:toxic,poisonous
meltingpoint,boilingpoint:high,low
1
;
;2
3读法:
yst.
yst.
3.3Reactionbetweennitrogenandhydrogenathightemperatureandpressurewiththepresenceofacatalystgivesa mmonia.
Athightemperatureandpressure,reactionofnitrogenwithhydrogeninthepresenceofacatalysttakesplace.
六化学计算(ChemicalCalculation)
1化学术语:
atomicmass/weight;molecularweight;amount(ofsubstance);mole;numberofmoles;molarmass;molarvolume ;concentration;molarity;excessagent;limitingagent;reactant;product;yield;
2数学术语:
+-×÷
运算名称addition subtraction mulplication division
动词读法add substract(ed)·frommultiply(ied)·bydivide(d)·by
介词读法plus minus times over
运算结果sum difference product quotient
0.001o/zeropointooone
2/3twothirds
=equals/isequalto
≈isapproximatelyequalto
<lessthan
>greaterthan
x2xsquared;x3xcubed;x-10xtotheminustenthpower
100o
()
1
烧瓶
玻棒
石蕊
角匙
研,棒
2
3
confirmativetest;inquirytest;qualitativeanalysis;quantitativeanalysis;measurement/determinationon 4实验操作:
collectgas(overwater;upwarddisplacementofair;downwarddelivery)
bubblegasthrough;drygas;suckbac。