水的粘度0-40℃
- 格式:docx
- 大小:22.79 KB
- 文档页数:4

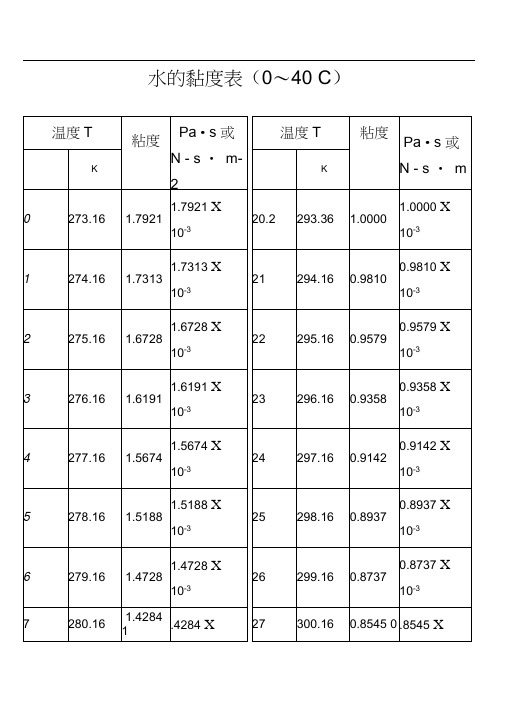
水的粘度计算表水的黏度表(0~40℃)温度T粘度μPa·s或N·s·m-2温度T粘度μPaN·K ℃K273.16 1.7921 1.7921×10-320.2 293.36 1.00001.000010-3274.16 1.7313 1.7313×10-321 294.16 0.98100.981010-3275.16 1.6728 1.6728×10-322 295.16 0.95790.957910-3276.16 1.6191 1.6191×10-323 296.16 0.93580.935810-3 1.5674×0.9142281.16 1.3860 1.3860×10-328 301.16 0.83600.836010-3282.16 1.3462 1.3462×10-329 302.16 0.81800.818010-3283.16 1.3077 1.3077×10-330 303.16 0.80070.800710-3284.16 1.2713 1.2713×10-331 304.16 0.78400.784010-3285.16 1.2363 1.2363×10-332 305.16 0.76790.767910-3286.16 1.2028 1.2028×10-333 306.16 0.75230.752310-3水的物理性质12.31 988.1 209.30 4.174 64.78 54.94 4.49 67.7 19.932 983.2 251.12 4.178 65.94 46.88 5.11 66.2 31.164 977.8 292.99 4.178 66.76 40.61 5.70 64.3 47.379 971.8 334.94 4.195 67.45 35.65 6.32 62.6 70.136 965.3 376.98 4.208 67.98 31.65 6.95 60.7 101.33 958.4 419.10 4.220 68.04 28.38 7.52 58.8 143.31 951.0 461.34 4.238 68.27 25.89 8.08 56.9 198.64 943.1 503.67 4.250 68.50 23.73 8.64 54.8 270.25 934.8 546.38 4.266 68.50 21.77 9.17 52.8 361.47 926.1 589.08 4.287 68.27 20.10 9.72 50.7 476.24 917.0 632.20 4.312 68.38 18.63 10.3 48.62320.88 840.3 943.70 4.614 64.55 12.46 14.8 33.8 2798.59 827.3 990.18 4.681 63.73 11.97 15.9 31.6 3347.91 813.6 1037.49 4.756 62.80 11.47 16.8 29.1 3977.67 799.0 1085.64 4.844 61.76 10.98 18.1 26.7 4693.75 784.0 1135.04 4.949 60.84 10.59 19.7 24.2 5503.99 767.9 1185.28 5.070 59.96 10.20 21.6 21.9 6417.24 750.7 1236.28 5.229 57.45 9.81 23.7 19.5 7443.29 732.3 1289.95 5.485 55.82 9.42 26.2 17.2 8592.94 712.5 1344.80 5.736 53.96 9.12 29.2 14.7 9877.96 691.1 1402.16 6.071 52.34 8.83 32.9 12.3 11300.3 667.1 1462.03 6.573 50.59 8.53 38.2 10.0F3Viscosity decreases with pressure(at temperatures below 33°C)Viscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and the volume of these voids reduces, so normally increasing pressure increases the viscosity.Water's pressure-viscosity behavior [534] can be explained by the increased pressure (up to about 150 MPa) causing deformation, so reducing the strength of the hydrogen-bonded network, which is also partially responsible for the viscosity. This reduction in cohesivity more than compensates for the reduced void volume. It is thus a direct consequence of the balance between hydrogen bonding effects andthe van der Waals dispersion forces [558] in water; hydrogen bonding prevailing at lower temperatures and pressures. At higher pressures (and densities), the balance between hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining hydrogen bonds are stronger due to the closer proximity of the contributing oxygen atoms [655]. Viscosity, then, increases with pressure. The dashed line (opposite) indicates the viscosity minima.The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength.Self-diffusion is also affected by pressure where (at low temperatures) both the translational and rotational motion of water anomalously increase as the pressure increases.。

水的粘度(0~40℃)水的物理性质F3 Viscosity decreases with pressure (at temperatures below 33°C)Viscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and the volume of these voids reduces, so normally increasing pressure increases the viscosity.Water's pressure-viscosity behavior [534] can be explained by the increased pressure (up to about 150 MPa) causing deformation, so reducing the strength of the hydrogen-bonded network, which is also partially responsible for the viscosity. This reduction in cohesivity more than compensates for the reduced void volume. It is thus a direct consequence of the balance between hydrogen bonding effects and the van der Waals dispersion forces [558] in water; hydrogen bonding prevailing at lowertemperatures and pressures. At higher pressures (and densities), the balance between hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining hydrogen bonds are stronger due to the closer proximity of the contributing oxygen atoms [655]. Viscosity, then, increases with pressure. The dashed line (opposite) indicates the viscosity minima.The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength.Self-diffusion is also affected by pressure where (at low temperatures) both the translational and rotational motion of water anomalously increase as the pressure increases.。
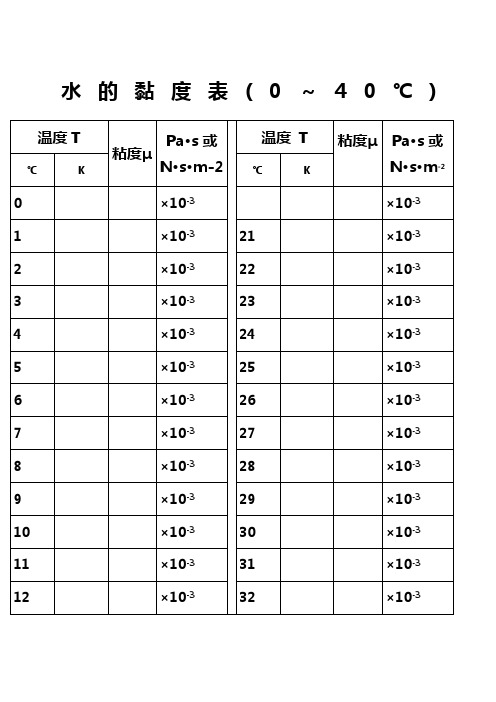
水的黏度表(0~40℃)水的物理性质F3??? Viscosity decreases with pressure(at temperatures below 33°C)Viscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and the volume of these voids reduces, so normally increasing pressure increases the viscosity.Water's pressure-viscosity behavior [] can be explained by the increased pressure (up to about 150 MPa) causing deformation, so reducing the strength of the hydrogen-bonded network, which is also partially responsible for the viscosity. This reduction in cohesivity more than compensates for the reduced void volume. It is thus a direct consequence of the between hydrogen bonding effects and the van der Waals dispersion forces [] in water; hydrogen bonding prevailing at lower temperatures and pressures. At higher pressures (and densities), the between hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining are stronger due to the closer proximity of the contributing oxygen atoms []. Viscosity, then, increases with pressure. The dashed line (opposite) indicates the viscosity minima.The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength.Self-diffusion is also affected by pressure where (at low temperatures) both the translational and rotational motion of water anomalously increase as the pressure increases.。

水的粘度计算表WTD standardization office【WTD 5AB- WTDK 08- WTD 2C】水的黏度表(0~40℃)水的物理性质350360 109370 264F3??? Viscosity decreases with pressure(at temperatures below 33°C)Viscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and the volume of these voids reduces, so normally increasing pressure increases the viscosity.Water's pressure-viscosity behavior [] can be explained by the increased pressure (up to about 150 MPa) causing deformation, so reducing the strength of the hydrogen-bonded network, which is also partially responsible for the viscosity. This reduction in cohesivity more than compensates for the reduced void volume. It is thus a direct consequence ofthe between hydrogen bonding effects and the van der Waals dispersion forces [] in water; hydrogen bonding prevailing at lower temperatures and pressures. At higher pressures (and densities), the between hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining are stronger due to the closer proximity of the contributing oxygen atoms []. Viscosity, then, increases with pressure. The dashed line (opposite) indicates the viscosity minima.The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength.Self-diffusion is also affected by pressure where (at low temperatures) both the translational and rotational motion of water anomalously increase as the pressure increases.。
水的黏度表(0〜40 C)水的物理性质F3 Viscosity decreases with p ressure(at temp eratures below 33Water's p ressure-viscosity behavior [534] can be explained by the in creased p ressure (up to about 150 MPa) caus ing deformatio n, so reduci ng the stre ngth of the hydroge n-bon ded n etwork, which is also p artially res pon sible for the viscosity. This reduct ion in cohesivity more tha n compen sates for the reduced void volume. It is thus a direct con seque nee of the bala nee betwee n hydroge n bonding effects and the van der Waals dis persion forces [558] in water; hydroge n bonding p revaili ng at lower temp eratures and p ressures. At higher p ressures (and den sities), the bala nee betwee n hydroge n bonding effects and the van der Waals dis persi on forces is tipped in favor of the dis persion forces and the rema ining hydroge n bonds are stron ger dueViscous flow occurs by molecules movi ng through the voids that exist betwee n them. As the p ressure in creases, the volume decreases and the volume ofthese voids reduces, so no rmally in creas ing p ressure in creases the viscosity.|:|k-二 _r 13ireSC去*. i i screr- 丁" \ . / . 一 '气:rJ J:V .; r "舄■ 3 口二K nPV ■■L T 三n 曲 •■ 5 M r丐 町寸 -;J 百* "T N ; 【I bl■呻口 " 口寸津a “ d c i 0 290八rao 800 i wooPressure, MPa g亠C)Co©4— □]J%一MJs」气1□ u古气 a15•” ”〕阳"1■ \■ID% ;:s' ¥口『 屮n◎ 9 r奇* =' f f- ::[丄 备IT记|B - 3 D■i电-'uO丰759勺;】I -一 11 L. Pto the closer p roximity of the con tribut ing oxyge n atoms [655]. Viscosity, the n, in creases with p ressure. The dashed line (opp osite) in dicates the viscosity mi ni ma.1 .a』IflOXThe variati on of viscosity with p ressure and temp erature has bee n used as evide neethat the viscosity is determ ined more by the exte nt of hydroge n bonding rather tha nhydroge n bonding stre ngth.Self-diffusio n is also affected by p ressure where (at low temp eratures) both the tran slatio nal and rotati onal moti on of water ano malously in crease as the p ressure in creases.1血200 oPr^sure ; MPa 75X、” _50^ 山:30°C 20弋5'10X2.2 X51 ---- 护乞fOr,QR 牡m 护,/a"-1- yiy二 h--------- 0c ,宀;:u 占14^ra_ 7^6^-*=0。
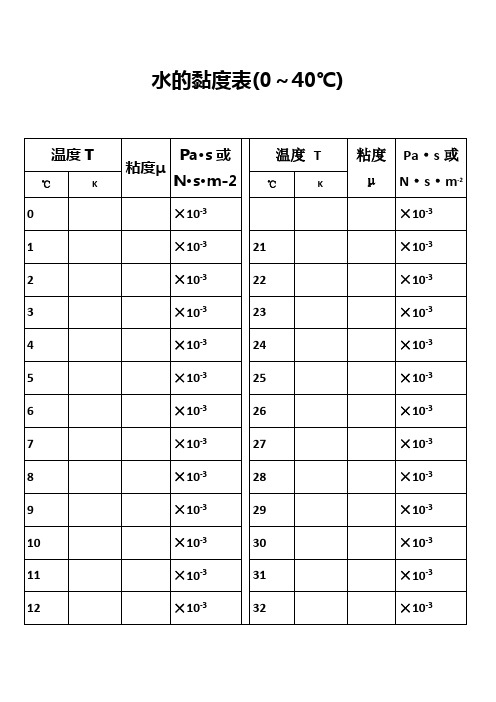
水的黏度表(0~40℃)水的物理性质F3 Viscosity decreases with pressure(at temperatures below 33°C)Viscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and the volume of these voids reduces, so normally increasing pressure increases the viscosity.Water's pressure-viscosity behavior [534] can be explained by the increased pressure (up to about 150 MPa) causing deformation, so reducing the strength of the hydrogen-bonded network, which is also partially responsible for the viscosity. This reduction in cohesivity more than compensates for the reduced void volume. It is thus a direct consequence of the balance between hydrogen bonding effects and the van der Waals dispersion forces [558] in water; hydrogen bonding prevailing at lower temperatures and pressures. At higher pressures (and densities), the balance between hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining hydrogen bonds are stronger dueto the closer proximity of the contributing oxygen atoms [655]. Viscosity, then, increases with pressure. The dashed line (opposite) indicates the viscosity minima.The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength.Self-diffusion is also affected by pressure where (at low temperatures) both the translational and rotational motion of water anomalously increase as the pressure increases.。
水的粘度对照表水的粘度是指水流动时所表现出的阻力大小,也就是水的黏度。
水的黏度值与温度有关,在不同温度下的水黏度值也是不同的。
以下是水的粘度对照表,列出了不同温度下水的粘度值。
一、水在0℃以下的黏度:水在0℃以下始终处于冰冻状态,此时无法测量其粘度值。
二、水在0℃~5℃的黏度:水在0℃~5℃范围内属于冷水,虽然温度较低但黏度并不高,粘度值约为0.00153 Pa·s。
三、水在5℃~10℃的黏度:水在5℃~10℃的温度下,黏度值开始升高,约为0.00131 Pa·s。
四、水在10℃~15℃的黏度:水在10℃~15℃的温度下,黏度值约为0.00114 Pa·s。
五、水在15℃~20℃的黏度:水在15℃~20℃的温度下,黏度值逐渐升高,约为0.001 Pa·s。
六、水在20℃~25℃的黏度:水在20℃~25℃的温度下,黏度值约为0.00089 Pa·s。
七、水在25℃~30℃的黏度:水在25℃~30℃的温度下,黏度值约为0.00081 Pa·s。
八、水在30℃~35℃的黏度:水在30℃~35℃的温度下,黏度值逐渐升高,约为0.00075 Pa·s。
九、水在35℃~40℃的黏度:水在35℃~40℃的温度下,黏度值约为0.0007 Pa·s。
十、水在40℃以上的黏度:水在40℃以上的温度下,黏度值不断升高,约为0.00064 Pa·s。
总结来说,水的黏度随着温度的升高而升高,但这种变化趋势并不是线性的,大致呈现出曲线形态。
在一定温度范围内,水的黏度值并不会随着温度的升高而在同样的比例增加,因为水的黏度受到温度、压力、浓度等多方面的影响,因此需要对不同条件下的水进行具体的测试才能得到精确的黏度值。
水的粘度(0-40℃)水的粘度(0-40℃)概述粘度是液体流动时抵抗流动的程度的物理属性。
对于水而言,其粘度在不同温度下会有所变化。
本文将介绍水的粘度在0-40℃温度范围内的变化规律。
粘度的定义粘度是指液体在受力作用下流动时的内阻力大小。
粘度可分为动力粘度和运动粘度两种,表示液体内阻力的大小。
单位为帕斯卡秒(Pa·s)或厘泊(cp)。
温度对水粘度的影响温度是影响水粘度的重要因素之一。
随着温度的升高,水的粘度通常会下降。
这是因为温度升高会导致水分子的运动加剧,分子间的相互作用力减弱,从而使得水分子更容易流动。
水的粘度变化曲线下表展示了水的粘度在0-40℃温度范围内的变化情况。
- 温度(℃) - 粘度(cP) --- 0 - 1.792 -- 5 - 1.519 -- 10 - 1.307 -- 15 - 1.139 -- 20 - 1.002 -- 25 - 0.891 -- 30 - 0.797 -- 35 - 0.717 -- 40 - 0.650 -分析与讨论根据上述数据,可以观察到温度越高,水的粘度越低。
在0-40℃的温度范围内,水的粘度几乎是一个随温度线性递减的趋势。
粘度对于许多工程和科学领域都具有重要的影响。
例如,在化工过程中,了解粘度的变化规律可以帮助工程师选择合适的管道尺寸和流体泵能力,以确保流体能够正常流动。
在石油勘探中,了解油井流体的粘度可以帮助预测油井的生产能力和流动性。
,对于科学研究来说,了解水的粘度变化规律也是非常重要的。
水作为大自然中最为普遍的液体之一,其粘度的测量和理解对于许多领域的研究都有着重要的意义。
本文介绍了水的粘度在0-40℃温度范围内的变化规律。
来说,随着温度的升高,水的粘度逐渐降低,呈线性递减趋势。
了解水的粘度变化规律对于工程应用和科学研究都具有重要意义。
水的粘度计算表 Revised as of 23 November 2020水的黏度表(0~40℃)水的物理性质F3 Viscosity decreases with pressure(at temperatures below 33°C)Viscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and the volume of thesevoids reduces, so normally increasing pressure increases the viscosity.Water's pressure-viscosity behavior [] can be explained by the increased pressure (up to about 150 MPa) causing deformation, so reducing the strength of the hydrogen-bonded network, which is also partially responsible for the viscosity. This reduction in cohesivity more than compensates for the reduced void volume. It is thus a direct consequence of the between hydrogen bonding effects and the van der Waals dispersion forces [] in water; hydrogen bonding prevailing at lower temperatures and pressures. At higher pressures (and densities), the between hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining are stronger due to the closer proximity of the contributing oxygen atoms []. Viscosity, then, increases with pressure. The dashed line (opposite) indicates the viscosity minima.The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength.Self-diffusion is also affected by pressure where (at low temperatures) both the translational and rotational motion of water anomalously increase as the pressure increases.。
水的黏度表(0~40℃)水的物理性质F3 Viscosity decreases with pressure(at temperatures below 33°C)Viscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and the volume of these voids reduces, sonormally increasing pressure increases the viscosity.37021040.9450.51892.4340.31933.73 5.692640.48 6.80Water's pressure-viscosity behavior [534] can be explained by the increased pressure (up to about 150 MPa) causing deformation, so reducing the strength of thehydrogen-bonded network, which is also partially responsible for the viscosity. This reduction in cohesivity more than compensates for the reduced void volume. It is thus a direct consequence of thebalance between hydrogen bonding effects and the van der Waals dispersion forces [558] in water; hydrogen bonding prevailing at lower temperatures and pressures. At higher pressures (and densities), the balance between hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining hydrogen bonds are stronger due to the closer proximity of the contributing oxygen atoms [655]. Viscosity, then, increases with pressure. The dashed line (opposite) indicates the viscosity minima..The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent of hydrogen bonding rather than hydrogen bonding strength.Self-diffusion is also affected by pressure where (at low temperatures) both the translational and rotational motion of water anomalously increase as the pressure increases.如有侵权请联系告知删除,感谢你们的配合!精品。