
Review
Circular dichroism,a powerful tool for the assessment of absolute
con?guration of ?avonoids
Desmond Slade
a,*
,Daneel Ferreira
a,b
,Jannie P.J.Marais
a
a
National Center for Natural Products Research,Research Institute of Pharmaceutical Sciences,School of Pharmacy,
The University of Mississippi University,MS 38677,USA
b
Department of Pharmacognosy,School of Pharmacy,The University of Mississippi,University,MS 38677,USA
Received 27August 2004;received in revised form 24January 2005
Available online 16March 2005
Abstract
Circular dichroism is a powerful tool for establishing the absolute con?guration of ?avonoids and proanthocyanidin analogues.It has been utilized to study the con?guration of ?avanones,dihydro?avonols (3-hydroxy?avanones),?avan-3-ols,?avan-4-ols,?a-van-3,4-diols,?avans,iso?avans,iso?avanones,pterocarpans,6a-hydroxypterocarpans,rotenoids,12a-hydroxyrotenoids,neo?avo-noids,3,4-dihydro-4-arylcoumarins,4-aryl?avan-3-ols,auronols,homoiso?avanones,proanthocyanidins,and various classes of bi?avonoids.Results relevant to the correlation of circular dichroic data and the absolute con?guration of the diastereoisomers of some of the above classes of compounds will be discussed.ó2005Elsevier Ltd.All rights reserved.
Keywords:Circular dichroism;Absolute con?guration;Flavonoids;Flavanones;Dihydro?avonols;3-Hydroxy?avanones;Flavan-3-ols;Flavan-4-ols;Flavan-3,4-diols;Flavans;Iso?avans;Iso?avanones;Pterocarpans;6a-Hydroxypterocarpans;Rotenoids;12a-Hydroxyrotenoids;Neo?avonoids;3,4-Dihydro-4-arylcoumarins;4-Aryl?avan-3-ols
Contents 1.Introduction ..........................................................................21782.Flavonoids ...........................................................................21793.Flavanones ...........................................................................21794.Dihydroflavonols (3-hydroxyflavanones)......................................................21805.
Flavan-3-ols ..........................................................................21815.1.Introduction ..........................................................................21815.2.The 1L b transition......................................................................21835.3.The 1L a transition ......................................................................21856.
Flavan-4-ols...............................................................................21856.1.2,4-cis -Flavan-4-ols .....................................................................21856.2.2,4-trans -Flavan-4-ols ...................................................................21887.
Flavan-3,4-diols........................................................................21897.1.The 1L b transition......................................................................21897.2.The 1L a transition ......................................................................
2191
0031-9422/$-see front matter ó2005Elsevier Ltd.All rights reserved.doi:10.1016/j.phytochem.2005.02.002
*
Corresponding author.Tel.:+16629155928;fax:+16629155587.E-mail address:dslade@https://www.doczj.com/doc/2118394745.html, (D.Slade).
https://www.doczj.com/doc/2118394745.html,/locate/phytochem
Phytochemistry 66(2005)
2177–2215
PHYTOCHEMISTRY
8.Flavans (2192)
9.Isoflavans (2192)
10.Isoflavanones (2194)
11.Pterocarpans (2195)
11.1.Pterocarpans (2195)
11.2.6a-Hydroxypterocarpans (2199)
12.Rotenoids (2201)
13.Neoflavonoids (2205)
13.1.3,4-Dihydro-4-arylcoumarins (2205)
13.2.4-Arylflavan-3-ols (2205)
14.Conclusion (2211)
Acknowledgment (2211)
References (2211)
1.Introduction
Plane or linearly polarized light can be viewed as the vector sum of left and right circularly polarized light of equal amplitude and phase.When an optically active medium is traversed by plane polarized light in the wavelength range in which the chromophore of an opti-cally active molecule absorbs,the plane of polarization is rotated at an angle,a,and the optically active matter absorbs the left and right hand circularly polarized light di?erently.The resulting light is therefore elliptically polarized and the medium exhibits circular dichroism (CD).If e l and e r are the molecular extinction coe?cients for the left(l)and right(r)polarized light,respectively, the di?erence,known as the di?erential dichroic absorp-tion(D e=e làe r),is a measure of the intensity of the CD.Molecular ellipticity[H]is related to D e by a simple equation:[H]=3300D e.(Velluz et al.,1965).
Any optically active compound will exhibit an optical rotatory dispersion(ORD)curve,which gives the opti-cal activity(U)in the function of wavelength(k in nm).If the compound under investigation has no chro-mophores that absorb in the wavelength range being used,the ORD curve is plain or normal,in other words, the curve does not show any extremum.Anomalous ORD curves,however,exhibit a maximum or a mini-mum,or both,and are observed when the molecule pos-sesses an absorption band in the region being investigated.These anomalous dispersion e?ects are known as Cotton e?ects(CEs)(Crabbe′,1967a).
The ORD and CD methods permit analogous con?g-urational conclusions and the data can be mutually and directly interchanged.The two curves can be mutually derived from each other in good approximation by the Kronig–Kramers transform(Velluz et al.,1965). Although the information obtained by ORD and CD is essentially identical,ORD curves are more complicated than CD curves.Since a maximum or minimum in CD corresponds to two peaks with zero transition in ORD, smaller Cotton e?ects may be easily obscured by positive or negative e?ects in the region of a strong Cotton e?ect.
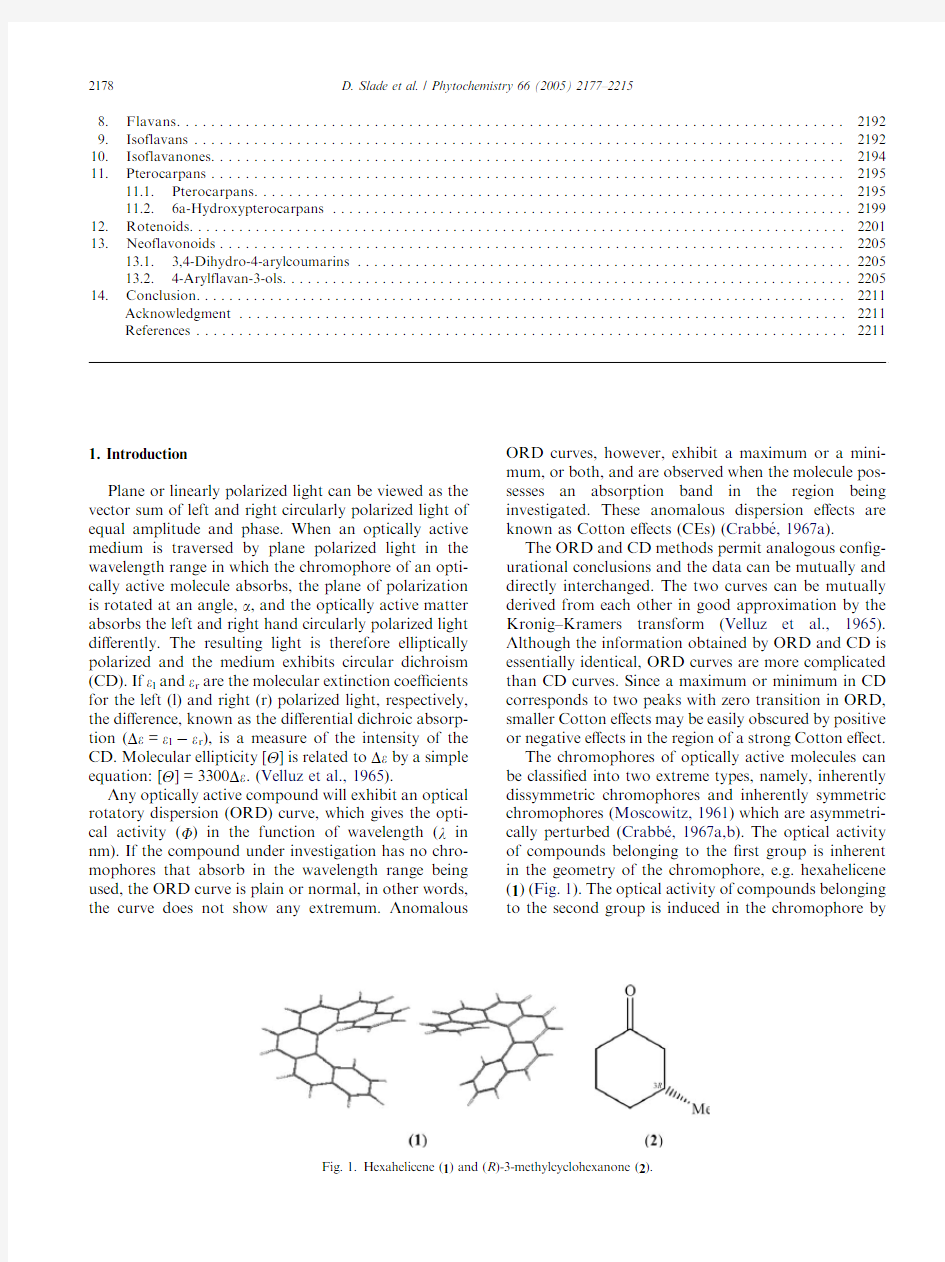
The chromophores of optically active molecules can be classi?ed into two extreme types,namely,inherently dissymmetric chromophores and inherently symmetric chromophores(Moscowitz,1961)which are asymmetri-cally perturbed(Crabbe′,1967a,b).The optical activity of compounds belonging to the?rst group is inherent in the geometry of the chromophore,e.g.hexahelicene (1)(Fig.1).The optical activity of compounds belonging to the second group is induced in the chromophore
by
Fig.1.Hexahelicene(1)and(R)-3-methylcyclohexanone(2).
2178 D.Slade et al./Phytochemistry66(2005)2177–2215
its chiral environment,e.g.(R)-3-methylcyclohexanone (2)(Fig.1).
The two main uses of ORD and/or CD in natural product research is for the determination of the absolute con?guration and conformation of a molecule.
Chirality sector rules are formulated for various chro-mophores and are used to determine the absolute con?g-uration if the conformation of a molecule is known or vice versa.The octant rule(Mo?tt et al.,1961)for sat-urated alkyl ketones and aldehydes was the?rst sector rule used,and along with the exciton chirality rule (Harada and Nakanishi,1983),remains the most widely known and most successful of the chirality rules.Sector rules focus on chromophores and relate the CD Cotton e?ect associated with such chromophores to the chirality of the extrachromophoric environment,the chirality of the chromophore,or both(Lightner,2000).
2.Flavonoids
Polyphenols are important secondary metabolites of plants that can be divided into major classes based on their carbon skeleton,e.g.?avonoids and phenolic acids. Flavonoids can be divided into a number of structural classes according to the oxidation state of the C3unit.
A large number of?avonoids are known due to the modi?cation of the C6.C3.C6skeleton by hydroxyl-ation,methoxylation,glycosylation,other modi?ca-tions,and combinations thereof(Strack,1997).
Although the absolute con?guration of?avonoids has been elucidated by optical activity and ORD mea-surements since the early?fties,the more convenient, and in many cases,easier CD method has become more popular since the middle sixties(Djerassi,1967).CD is now routinely used in the analysis of optically active ?avonoids(Le′vai,1998), e.g.?avanones(Ga?eld, 1970),dihydro?avonols(Ga?eld,1970;Nonaka et al., 1987;Wu et al.,2003),?avan-3-ols(Korver and Wilkins, 1971;Van Rensburg et al.,1999),?avan-4-ols(Snatzke et al.,1973a),?avan-3,4-diols(Ferreira et al.,2004),?a-vans(Antus et al.,2001),iso?avans(Versteeg et al., 1999),iso?avanones(Paiva et al.,1994),pterocarpans (Szarvas et al.,2000),6a-hydroxypterocarpans(Van Aardt et al.,2001),rotenoids(Dewick,1994),12a-hydroxyrotenoids(Tahara et al.,1990),neo?avonoids (Nonaka and Nishioka,1982),3,4-dihydro-4-aryl-coumarins(Fan et al.,1999),4-aryl?avan-3-ols(Van der Westhuizen et al.,1981;Van Zyl et al.,1993),auro-nols(Bekker et al.,2001),homoiso?avanones(Amschler et al.,1996),proanthocyanidins(Barrett et al.,1979; Botha et al.,1981a;Calzada et al.,1999),and various classes of bi?avonoids(Mayer,2004).
The aim of this review is to demonstrate the utility of CD in the establishment of the absolute con?guration and conformation of the biologically important group of?avonoids.
3.Flavanones
The?avanones are characterized by two structural features that are important in determining their absolute con?guration,namely,the absence of the C2–C3double bond found in?avones and the presence of the C2ste-reocenter.The majority of naturally occurring?ava-nones have the C2-phenyl group in the a-orientation and is therefore designated as S,e.g.(3)(Bohm,1998).
Utilization of CD or ORD in conjunction with NMR spectroscopic data to de?ne the absolute con?guration of?avanones was done by Ga?eld(1970).
The UV absorptions of?avanones at270–290nm (maximum)and320–330nm(in?ection)have been as-signed to the p!p*and n!p*acetophenone chromo-phore transitions,respectively.The modi?ed octant rule (Snatzke,1965a),de?ning the relationship between the chirality of a,b-unsaturated ketones and the sign of their high wavelength CE,was extended to aryl ketones(ace-tophenones)(Snatzke,1965b).Thus,?avanones with2S con?guration possessing a conformation with P-helicity of the heterocyclic ring and having a C2equatorial aryl group[Figs.2and4(a);Ga?eld,1970]will exhibit a po-sitive CE at the n!p*absorption band and a negative CE at the p!p*absorption band.
The advantage of using the n!p*absorption band for con?gurational assignment is that the sign of this transition is independent on the substitution pattern of the aromatic ring system(Snatzke et al.,1973a), although it must be remembered that the n!p* transition at longer wavelength tends to diminish
with Fig.2.(2S)-?avanones(3).
D.Slade et al./Phytochemistry66(2005)2177–22152179
increasing amounts of the opposite enantiomers (Gaf-?eld,1970;Li et al.,2002).
The large coupling constant (J 2,3)between H2and H3(ax)of the heterocyclic ring indicates that all natural ?avanones are in the thermodynamically favored con-formation with the C2-aryl group equatorial (Fig.2;Clark-Lewis,1968).This implies that all laevorotatory
?avanones possess a 2S con?guration (Antus et al.,1994;Garo et al.,1998).
A good example of the use of this information can be found in the identi?cation of obo?avanone A (4)and obo?avanone
B (5)(Fig.3and Table 1;Dumontet et al.,2004).
4.Dihydro?avonols (3-hydroxy?avanones)
Dihydro?avonols are intermediates in the biosyn-thetic pathway to ?avonols and anthocyanidins via ?a-van-3,4-diols.They possess chiral centers at C2and C3,and therefore have four possible stereoisomers.The (2R ,3R )isomer is the most common in nature,
Table 1
Con?gurational assignment at C2of obo?avanone A (4)and obo?avanone B (5)using CD data Compound CE (nm)Con?guration at C2
p !p *n !p *Obo?avanone A (4)290à334+(S )Obo?avanone B (5)
290
+
334
à
(R )
2180 D.Slade et al./Phytochemistry 66(2005)2177–2215
but there are cases where all four isomers co-occur (Nonaka et al.,1987;Bohm,1998).
The assignment of absolute con?guration to dihydro?avonols proceeds in two steps.The ?rst step is to identify the relative con?guration of the C2and C3substituents from NMR coupling constants (J 2,3)as either trans or cis .For the trans -isomers,the ther-modynamically more stable conformation is when H2and H3are diaxial and therefore the absolute con?gu-ration has to be (2R ,3R )or (2S ,3S ).In the cis con?g-uration,the thermodynamically more stable conformation is when H2is axial and H3is equatorial,and therefore the absolute con?guration has to be (2R ,3S )or (2S ,3R ).In the second step,CD data is used to determine the absolute con?guration at C2.A posi-tive n !p *CE at high wavelength (ca.300–340nm)indicates a 2R con?guration and a negative n !p *CE indicates a 2S con?guration (Table 2;Ga?eld,1970).As for the ?avanones,it should be emphasized that the sign of the n !p *transition depends on the helicity of the heterocyclic ring,which,in conjunction with the relative con?guration and the equatorial ori-entation of the C2-aryl group,establishes the absolute con?guration.
It should further be emphasized that (2R ,3R )-dihydro?avonols and (2S )-?avanones are homochiral due to a change in the Cahn–Ingold–Prelog priority when going from H at C3in the ?avanones to OH in the dihydro?avonols.The fact that (2R ,3R )-dihydro-
?avonols and (2S )-?avanones indeed have the same het-ero-ring helicities and thus similar signs of their n !p *transition CEs is demonstrated in Fig.4.
The CEs due to the positive n !p *transitions in their CD spectra were used in the determination of the absolute con?guration of phellodensin-A (6)and phello-densin-C (7)(Fig.5and Table 3;Wu et al.,2003)as both being (2R ,3R ).
5.Flavan-3-ols 5.1.Introduction
The chroman chromophore (8)(Fig.6)is present in various naturally occurring O -heterocycles.The chro-man derivatives belong to the benzene chromophores with a chiral second sphere.The achiral benzene A-ring chromophore is chirally perturbed by the fused chiral O -heterocyclic ring (second sphere)and the substituents of the heterocyclic ring (third sphere).This gives rise to the observed CEs at ca.260–280nm (1L b band)and ca.200–240nm (1L a band).The chirality (conformation)of the heterocyclic ring can be deduced from the CD spectrum if the relationship between the helicity of the nonaro-matic ring and the sign of the 1L b band CD is known.The absolute con?guration can then be assigned from NMR spectroscopic experiments that give the relative con?guration between the substituents at the chiral centers.
Table 3
Con?gurational assignment at C2and C3of phellodensin-A (6)and phellodensin-C (7)using 1H NMR and CD data Compound NMR:J 2,3CE at n !p *(ca.300and 340nm)Absolute con?guration Phellodensin A (6)trans Positive (2R ,3R )Phellodensin C (7)
trans
Positive
(2R ,3R )
Table 2
Dihydro?avonol C2and C3-geometry and con?guration NMR:J 2,3Result CE at n !p *(ca.300–340nm)Result Absolute con?guration trans (2R ,3R )or Positive 2R (2R ,3R )(2S ,3S )Negative 2S (2S ,3S )cis
(2R ,3S )or Positive 2R (2R ,3S )(2S ,3R )
Negative
2S
(2S ,3R )
D.Slade et al./Phytochemistry 66(2005)2177–2215
2181
A helicity rule for the benzene chromophore of chiral tetralin(9),tetrahydroisoquinoline(10)(Snatzke and Ho,1971),isochroman(11)(Antus et al.,1983)and 1,4-benzodioxan(12)(Antus et al.,1991)derivatives was developed and can be formulated as follows:if there is no pseudoaxial substituent at the benzylic C4and the benzene A-ring is not further substituted,P-helicity of the hetero-ring(half-chair conformation)leads to a po-sitive and M-helicity to a negative CE within the1L b band(Fig.6).Sector rules were developed for third sphere contributions,namely,the nonaromatic ring sub-stituents(Luche et al.,1972;Dornhege and Snatzke, 1970;Schulte and Snatzke,1989;Hagishita and Kuriy-ama,1982).
In homochiral compounds the signs of the second and third sphere contributions to the1L b band CE de-pend on the substitution pattern of the benzene ring (Snatzke et al.,1972,1973b).The‘‘spectroscopic mo-ments’’(q)of di?erent achiral substituents,whose signs and magnitudes have been investigated(Platt,1949, 1951;Petruska,1961),can be used to explain the change in sign of the1L b band CE together with the electric transition moment vector(l=R q),which is the sum of the spectroscopic moments(Fig.7).
It is therefore clear that as a?rst step it has to be determined which form of Snatzke?s helicity rule is valid for these chromophores,and since the benzene rings are substituted in most of these natural products,the in?u-ence of the achiral substituents on the chiroptical prop-erties also has to be determined for each chromophore to apply their CD correctly for con?gurational assignment.
Antus et al.(2001)reported that methoxy and/or hy-droxyl groups at C2,C3,C5and/or C7do not change the chroman helicity rule,so that substituted chroman derivatives can also be analyzed using this technique.
Simple aromatic compounds usually show three ma-jor electronic transitions:two strong absorption bands at ca.180–190and200–240nm,and a weak transition, usually showing considerable?ne structure,at ca.260–280nm(Table4).The weak1L b transition at ca.260–280nm is formally forbidden in symmetrical aromatic compounds,but in any dissymmetric aromatic system, the p!p*transition will become optically active (Crabbe′,1967b).
Flavan-3-ols(catechins),e.g.(17),have two aromatic chromophores,namely,the benzene A-and B-rings, with the absorption bands of these chromophores found at ca.280nm(1L b transition)and ca.240nm(1L a tran-sition).The presence of two aromatic chromophores gives two bands in each region(Korver and Wilkins, 1971).The?avan-3-ols have two stereocenters and therefore four possible diastereomers,namely,(2R,3S)-2,3-trans,(2S,3R)-2,3-trans,(2R,3R)-2,3-cis,and
2182 D.Slade et al./Phytochemistry66(2005)2177–2215
(2S,3S)-2,3-cis exist.According to Snatzke et al. (1973b),the A-ring chromophore forms the?rst sphere, the heterocyclic C-ring forms the second sphere,and the B-ring chromophore and the C3-substituent form the third sphere.
5.2.The1L b transition
The C-ring(second sphere)of?avan-3-ols(17)is the closest chiral sphere to the A-ring chromophore (?rst sphere),and therefore determines not only the sign of the CE within each absorption band,but also a signi?cant part of the magnitude.The sign of the CE of the aromatic A-ring is determined by the chirality of the heterocyclic C-ring,with the absolute con?guration at C3having only a minor in?uence, which is similar to the situation found for tetralins (Dornhege and Snatzke,1970).The chirality of the C-ring is determined by the preference of the C2-aryl B-ring for an equatorial position as established by NMR spectroscopic analysis(Fig.8;Clark-Lewis, 1968).
The CD spectra of?avan-3-ols(17)should therefore be explicable in terms of the helicity rules proposed for the chroman(3,4-dihydro-2H-pyran)ring system.
Table4
The?rst three UV absorption bands of aromatic systems Wavelength(nm)Band 260–280a
1L
b
1A
1g !1B2u
1A!1L
b
B
200–240p
1L
a
1A
1g !1B1u
1A!1L
a
E2
180–190b
1B
a
1A
1g !1E1u
1A!1B
E1
D.Slade et al./Phytochemistry66(2005)2177–22152183
P -helicity of the C-ring with its preferred half-chair/C2-sofa conformation (Porter et al.,1986)should lead to positive CEs within the 1L b transition,and M -helicity to negative CEs (Snatzke et al.,1973b )as was recorded for a series of tetralin derivatives (Dornhege and Sna-tzke,1970).Flavan-3-ols with 2R and 2S absolute con-?guration,respectively,display P -and M -helicity,respectively (Fig.8).
However,CD data (Korver and Wilkins,1971;Van Rensburg et al.,1999)indicates that 2R and 2S absolute con?gurations give rise to negative and positive CEs,respectively,for chromophore A in the 1L b region (Figs.9and 10;Table 5).This result is opposite to that found for the tetralins,which conforms to the helicity rules.A possible reason for this might be the loss of C 2v symme-try in going from the tetralin to the chroman chromo-
2184 D.Slade et al./Phytochemistry 66(2005)2177–2215
phore.An n !p *transition from the p z -orbital of the C-ring oxygen to the p *-orbital of the A-ring might also contribute to the inversion of the rule (Korver and Wil-kins,1971).
The A-conformation for 2,3-cis -?avan-3-ols is a rela-tively stable conformation which leads to a reduced amplitude of the long wavelength CE due to the inver-sion of helicity in going from an E-to an A-conforma-tion (Figs.8and 10;Van Rensburg et al.,1999).
5.3.The 1L a transition
The (2R ,3S )-and (2S ,3R )-trans enantiomers have CEs of opposite sign and the (2R ,3R )-and (2S ,3S )-cis enantiomers have CEs of the same sign for the 1L a tran-sition (ca.240nm)as compared to the 1L b transition (ca.280nm).Positive and negative CEs in the 240nm region seem to indicate 3S and 3R absolute con?gurations,respectively (Table 6;Figs.9and 10).Exceptions to this rule occur when the A-ring is devoid of hydroxylation (Van Rensburg et al.,1999).
A new ?avan-3-ol glucoside was isolated from barley (Hordeum vulgare L.)and identi?ed as (2R ,3S )-catechin-7-O -b -D -glucopyranoside (18)using NMR,FAB-MS,UV and CD.A J 2,3coupling constant of 7.4Hz con-?rmed a 2,3-trans con?guration and a negative CE at 280nm indicated a 2R con?guration (Fig.11;Friedrich and Galensa,2002).
6.Flavan-4-ols
6.1.2,4-cis-Flavan-4-ols
The NMR spectra and respective J 2,4coupling con-stants of (+)-(2R ,4R )-4-amino?avan hydrochloride
(19),(+)-(2R ,4R )-4-acetamido?avan (20)(Bogna
′r et al.,1970),and (2R ,4R )-?avan-4-ol benzoate (21)(Bolger et al.,1966)established their relative con?gurations as cis (Fig.12)with the dihydropyran C-ring having either a half-chair or a sofa conformation.
Table 5
Flavan-3-ol C2-and C3-geometry and con?guration CE at 1L b (ca.280nm)Result Helicity NMR:J 2,3Absolute con?guration Negative 2R P trans (2R ,3S )cis (2R ,3R )Positive
2S
M
trans (2S ,3R )cis
(2S ,3S )
Table 6
Flavan-3-ol CEs Absolute con?guration NMR:J 2,3Helicity CE 1L a
(ca.240nm)CE at 1L b (ca.280nm)(2R ,3S )trans P Positive Negative (2R ,3R )cis P Negative Negative (2S ,3R )trans M Negative Positive (2S ,3S
)
cis
M
Positive
Positive
D.Slade et al./Phytochemistry 66(2005)2177–22152185
With a half-chair conformation of the C-ring (Fig.13),C4–OH would be forced into the unfavorable w -equatorial (pseudo-equatorial)position (H2and H4diaxial),while in the other possible half-chair conforma-tion,the C2-phenyl group has to adopt the equally unfa-vorable axial position (H2and H4diequatorial).The boat conformation can also be discarded by conforma-tional analysis.In the sofa conformation,C4–OH is ori-ented in such a way that peri -interaction is avoided,and therefore the sofa conformation seems more probable than the half-chair conformation (Fig.14;Snatzke et al.,1973a ).
The CE of tetralins and tetrahydroisoquinolines can be interpreted by di?erentiating between second-and third-sphere contributions,the former generally being
stronger than the latter (Dornhege and Snatzke,1970;Barry et al.,1971;Luche et al.,1972;Snatzke and Ho,1971;Snatzke et al.,1972).Tetralin and tetrahydroiso-quinoline derivatives without A-ring substitution have positive CEs for both the 1L a and 1L b transitions if the chiral C-ring has P -helicity.For derivatives with A-ring substitution,the same rule may apply,or the inverse may hold,depending on the substitution pattern.
Flavonoids,in contrast to the tetralins and tetrahy-droisoquinolines that use two carbons,utilize one
2186 D.Slade et al./Phytochemistry 66(2005)2177–2215
oxygen and one carbon to connect the chiral C-ring to the benzene A-ring.Also,with a sofa conformation, the chirality of the second sphere is no longer as strong as for tetralins and tetrahydroisoquinolines with their half-chair conformations.The second-sphere contribu-tions of the CE will thus be smaller,and with the chiral C4–OH next to the A-ring chromophore,third-sphere contributions may become even stronger(Snatzke et al.,1973a).
The(2R,4R)-cis diastereomer is expected to have a negative second-sphere contribution for the chroman (A-ring)chromophore when the sofa conformation is assumed(Fig.14).The contribution of the C-ring chro-mophore,which depends strongly on its preferred con-formation,to the CD is expected to be one or two orders of magnitude smaller,although it can still con-tribute like any other substituent to the third-sphere ef-fects for the A-ring chromophore.
It was experimentally found that epimerization at C4 changes the sign of the1L b band and therefore it can be assumed that C2is a good approximation for the sym-metry of the chroman molecule and that the plane of the benzene A-ring is a nodal plane.The(2R,4R)-cis dia-stereomer(22)is expected to have a positive contribu-tion from C4–OH for the CEs in both the1L a and1L b bands(Fig.15).For the1L b band and with a sofa con-formation of the chiral ring,the in?uence of the second-sphere is much smaller than that of the C4–OH group next to the A-ring chromophore,i.e.,the third-sphere contribution(Snatzke et al.,1973a).
Although the second-sphere contributions are smaller than that of the third-sphere,the helicity rule for the cis-enantiomers is the same as that for the?avan-3-ols(Ta-bles6and7)and inverse to the rule for the tetralins.
Antus et al.(2001)treated the2,4-cis enantiomers as having a half-chair conformation(Fig.13)with their helicity governed by the equatorial orientation of the C2-phenyl group,but came to the same conclusion: P-/M-helicity of the O-heterocyclic ring in the chroman chromophore is re?ected by a negative/positive CE within the1L b band transition of the benzene ring when the O-heterocyclic ring adopts a half-chair conformation and there is no substituent on the fused aromatic ring (Table7).
Table7
Flavan-4-ol CEs
Absolute con?guration NMR:J2,4Helicity CE1L a(ca.240nm)CE at1L b(ca.280nm) (2R,4R)cis M Positive Positive
(2S,4S)cis P Negative Negative
(2R,4S)trans M Negative Negative
(2S,4R)trans P Positive Positive
D.Slade et al./Phytochemistry66(2005)2177–22152187
6.2.2,4-trans-Flavan-4-ols
NMR spectroscopic analysis indicates that2,4-trans-?avan-4-ols have either a half-chair(Fig.16)or a sofa conformation(Fig.17);conformational analysis cannot di?erentiate between these two forms.Second-sphere helicity and third-sphere contribution of the C4–OH group for the(2R,4S)-2,4-trans enantiomers(23/25) (Fig.15)are both predicted to show negative CEs in the1L a and1L b bands for the half-chair and the sofa conformation.The absolute value of the rotational strength of the more reliably measurable1L b band is practically the same for the two epimers(2R,4R)-2,4-cis-?avan-4-ol(22/24)and(2R,4S)-2,4-trans-?avan-4-ol (23/25).It can therefore be concluded that the dihydro-pyran C-ring of the trans-compounds prefers the sofa conformation with C2out of the plane of the benzene A-ring,although the half-chair conformation could not be excluded totally(Snatzke et al.,1973a).
Antus et al.(2001)concluded that the2,4-trans-?a-van-4-ols follows a di?erent rule to that of the2,4-cis-?avan-4-ols(half-chair/sofa conformation)(Figs.13 and14)due to the axial C4–OH that forces the O-het-erocyclic ring into a?at sofa conformation(Fig.17) which makes the chiral third-sphere(substituent at C4) contribution overrule the second one(helicity of the O-heterocyclic ring).Thus,P-/M-helicity is re?ected by a positive/negative CE within the1L b band transition of the benzene ring when the O-heterocyclic ring adopts a sofa conformation and there is no substituent on the fused aromatic ring(Fig.17and Table7).
The CD data for three?avan-4-ols is tabulated in Table8(Fig.15;Snatzke et al.,1973a).
Table8
CD spectra data of?avan-4-ols.Solvent:acetonitrile
Flavan-4-ol k max(D e)
(2R,4R)-cis283(+1.22),276(+1.28),227(+2.23)
(2R,4S)-trans282(à1.17),276(à1.17),226(à7.62)
(2S,4R)-trans282(+1.44),276(+1.41),226(+0.80) 2188 D.Slade et al./Phytochemistry66(2005)2177–2215
7.Flavan-3,4-diols
The?avan-3,4-diols and?avan-3-ols are the bioge-netic precursors to the proanthocyanidins(condensed tannins)(Ferreira et al.,1999).The majority of?avan-3,4-diols have been isolated from the bark or wood of Acacia species(Haslam,1982).
The relative con?guration of?avan-3,4-diols(26)is readily accessable from the3J H,H values of their C-ring three-bond proton coupling constants(Clark-Lewis et al.,1964).Small but signi?cant di?erences in the coupling constants of2,3-cis-3,4-trans and 2,3-cis-3,4-cis analogues allow assignment of the relative con?gurations via appropriate NOE experiments.
7.1.The1L b transition
The1L b band of C-ring substituted?avans at ca. 280nm is usually exploited for con?gurational assign-ment since it is less prone to mixing with other transi-tions or overlapping than the1L a band at shorter wavelengths(Antus et al.,2001).The sign and a sub-stantial part of the magnitude of the CE within each
D.Slade et al./Phytochemistry66(2005)2177–22152189
absorption band are determined by the chiral sphere closest to the A-ring chromophore,i.e.,the C-ring of the ?avan-3,4-diols.Accordingly,the absolute con?gu-ration at C3will have a minor in?uence on the sign of the CE of this transition.Thus,the spectra of ?avan-3,4-diols with their chiral second-sphere (C-ring)and the preference of the B-ring for an equatorial orienta-tion,should be explicable in terms of the ‘‘inversed’’helicity rules proposed for the dihydropyran ring system (Korver and Wilkins,1971;Nel et al.,1999;Van Rens-burg et al.,1999;Snatzke et al.,1973a;Majer et al.,1995;Antus et al.,2001).P -Helicity (2R con?guration)of the C-ring with its preferred half-chair/C2-sofa con-formation (Clark-Lewis et al.,1964;Porter et al.,1986)should lead to negative CEs within the 1L b transi-tion,and M -helicity (2S con?guration)to positive ones (Figs.18–20;Table 9).
The E-conformer of the all-trans analogues,namely,(2R ,3S ,4R )-and (2S ,3R ,4S )-2,3-trans -3,4-trans -?avan-3,4-diol,experiences allylic strain between C4–OH and H5.This results in a conformational change to alleviate the strain and leads to a relatively stable inversed half-chair/C2sofa A-conformation (Porter et al.,1986)with M -as opposed to P -helicity for the 2R -all-trans -?avan-3,4-diols,and vice versa for the 2S analogues (Fig.21).The net e?ect of the conforma-tional change is a reduced amplitude of the 1L b CE for the all-trans analogues compared to,e.g.,the 2,3-trans -3,4-cis analogues.
The A-conformer of the 2,4-cis -diaxial analogues,namely,(2R ,3R ,4R )-and (2S ,3S ,4S )-2,3-cis -3,4-cis -?a-van-3,4-diol,and (2R ,3S ,4R )-and (2S ,3R ,4S )-2,3-trans -3,4-trans -?avan-3,4-diol,may be stabilized via hydrogen bonding between C4–OH and the aromatic B-ring (Fig.22).Once C4–OH is derivatized,hydrogen bonding and hence stabilization of an A-conformer are eliminated and the amplitude of the 1L b band CE in-creases relative to that of the 1L a transition (Ferreira et al.,2004).
The E-conformer of the all-cis analogues,namely,(2R ,3R ,4R )-and (2S ,3S ,4S )-2,3-cis -3,4-cis -?avan-3,4-diol,is stabilized by hydrogen bonding between the axial C3–OH and the O -heteroatom of the C-ring (Fig.23;Clark-Lewis and Williams,1967).
Table 9
Flavan-3,4-diol CEs CE at 1L b (ca.280nm)Helicity C2NMR:J 2,3C3NMR:J 3,4C4Absolute con?guration Positive
M
S
cis S cis 4S 2S ,3S ,4S trans 4R 2S ,3S ,4R trans
R cis 4R 2S ,3R ,4R trans 4S 2S ,3R ,4S Negative
P
R
cis R cis 4R 2R ,3R ,4R trans 4S 2R,3R,4S trans
S
cis 4S 2R ,3S ,4S trans
4R
2R ,3S ,4R
2190 D.Slade et al./Phytochemistry 66(2005)2177–2215
7.2.The1L a transition
Ferreira et al.(2004)reported that the sign of the1L a CE(ca.240nm)is the same as that for the1L b CE(ca. 280nm)for the all-trans-,all-cis-and2,3-cis-3,4-trans-?avan-3,4-diols,except for the tetraacetate derivative of (+)-mopanol A.The sign of the1L a CE(ca.240nm)seems to be opposite to the1L b CE(ca.280nm)for the2,3-trans-3,4-cis-?avan-3,4-diols,except for2,3-trans,3,4-cis-leuco-cyanidin.It was also concluded that the CEs of the compounds under investigation did not consistently obey the DeAngelis–Wildman aromatic quadrant rule for cor-relating the1L a CE sign with the absolute C4con?gura-tion(DeAngelis and Wildman,1969).
D.Slade et al./Phytochemistry66(2005)2177–22152191
8.Flavans
Only a few?avans(27)have been reported to occur naturally,probably due to the fact that they are not readily visible on chromatograms and they have simple spectroscopic features compared to most other?avo-noid classes(Bohm,1998).
Flavans arise from a double reduction of a?avanone. Many?avans co-occur with the?avanone of identical substitution pattern.ORD and CD studies have re-vealed that all natural?avans have the2S absolute con-?guration,as would be expected from the?avanone origin.Many of the natural?avans are listed as racemic due to a rotation of zero at589nm.The low speci?c rotation of?avans makes this a precarious assumption and their con?guration can only be elucidated by study-ing their full CD data(Porter,1988).
Antus et al.(2001)established that?avans have a half-chair conformation with the C2-phenyl group equa-torial and that they follow the by now familiar rule of P-/M-helicity of the O-heterocyclic ring in the chroman chromophore leading to negative/positive CEs within the1L b band.This implies that a negative1L b CE is the result of2S absolute con?guration and a positive 1L
b
band the result of2R absolute con?guration(Fig. 24;Table10).
The?avan glycoside(28),isolated from Buckleya lanceolata Miq.leaves,was reported by Sashida et al. (1976)and its absolute2S con?guration determined by CD spectroscopy(Fig.25).
9.Iso?avans
Equol(7,40-dihydroxyiso?avan),whose con?guration was established as3S(Kurosawa et al.,1968),is possibly the only?avonoid that was?rst obtained as an animal metabolite(Wong,1975).Iso?avans(29)from plant ori-gin always have an oxygen at C20and they almost never have oxygenation at C5(Bohm,1998).
Optical rotation measurements alone are not su?-cient to assign absolute con?guration to iso?avans. (3S)-iso?avans exhibit a negative CE in the260–300nm region of their ORD curves,but ORD and NMR measurements are markedly in?uenced by the preferred conformation of the molecule in solution (Kurosawa et al.,1978).
The favored conformation of the O-heterocyclic ring of iso?avans is expected to be the half-chair form,as for most other?avonoids and by analogy with cyclohexene and based on the minimization of torsional strain(Fig.
26).It is,however,not possible to state if the E-or A-conformer is of lower free energy,since the axial C3-phenyl(A-conformer)does not result in the familiar
Table10
Flavan CEs
CE at1L b(ca.280nm)Helicity Absolute con?guration
Positive M(2R)
Negative P(2S)
2192 D.Slade et al./Phytochemistry66(2005)2177–2215
destabilizing 1,3-diaxial interactions of axially substi-tuted cyclohexane and cyclohexene systems (Eliel et al.,1965).The vicinal coupling constants (J 2,3and J 3,4)for the iso?avans are,however,consistent with the values expected from the half-chair conformation (Emsley et al.,1968)with the C3-phenyl group equato-rial (Kurosawa et al.,1968).
When using chiroptical methods for the establish-ment of absolute con?guration of iso?avonoids,care should be taken to avoid problems caused by solvational e?ects upon the observed optical activity.The e?ects can be explained in terms of solvational and/or conforma-tional equilibrium changes (Verbit and Clark-Lewis,1968)and the problem can be alleviated by using the same solvent system for all the compounds under investigation.
Plain ORD curves above ca.325nm have been used to identify the absolute con?guration of iso?avans (Suginome,1966),but this has led to incorrect assign-ment of C3con?guration.ORD curves in the region of electronic absorption,however,gives a di?erent pic-ture (Verbit and Clark-Lewis,1968),because the sign of the CE is a more accurate indicator of absolute con-?guration than the sign of the plain dispersion curve
(Crabbe
′,1965).A study of the available ORD and CD data of iso?-avans (Kurosawa et al.,1968,1978;Verbit and Clark-Lewis,1968;Donnelly et al.,1973;Woodward,1980;Yahara et al.,1985;Clark-Lewis,1962;Quaglia et al.,1991;Clark-Lewis et al.,1965;Hamburger et al.,1988;Versteeg et al.,1999)revealed that they follow an inverse helicity rule for the chroman chromophore compared to the ?avonoids discussed thus far.This means that P -/M -
helicity of the O -heterocyclic ring results in positive/neg-ative CEs in the 1L b transition region (260–320nm)and negative/positive CEs in the 1L a transition region (220–260nm)(Fig.26and Table 11).
Although this rule is helpful in assigning absolute con?guration to iso?avans,20,60-disubstitution of the B-ring leads to di?erent conformations of the O -hetero-cyclic ring due to steric e?ects (Fig.27)and therefore to atypical ORD and CD curves (Wong,1975).The NMR spectra of these compounds also re?ect this conforma-tional e?ect (Pelter and Amenechi,1969).
Table 11
Iso?avan CEs CE at 1L b
(ca.260–320nm)CE at 1L a
(ca.220–260nm)Helicity Absolute con?guration Positive Negative P (3R )Negative
Positive
M
(3S )
D.Slade et al./Phytochemistry 66(2005)2177–22152193
Versteeg et al.(1999)synthesized six iso?avans and their enantiomers (31a/b-36a/b ),and used authentic 3S -and 3R -vestitol (30a and 30b )derivatives to estab-lish the absolute con?guration at C3of the synthetic iso?avans (Fig.28).(3S )-iso?avans with oxygenation at both the A-and B-rings (34a ,35a and 36a )display positive and negative CEs in the 240(1L a )and 270–280nm (1L b )regions,respectively,and conversely for the 3R -enantiomers (34b ,35b and 36b )(Fig.29).The
7-deoxy 3S -iso?avans with mono-(31a )and di-oxygen-ation (32a and 33a )on the B-ring displayed negative CEs in both the 230–240(1L a )and 270–290nm (1L b )re-gions,and conversely for the 3R -enantiomers (31b ,32b and 33b )(Fig.30).
10.Iso?avanones
(3R )-Sophorol (20,7-dihydroxy-40,50-methylenedi-oxyiso?avanone)was for many years the only natural optically active iso?avanone (37)known.Since racemi-zation may occur under mild conditions,it was assumed that extraction and puri?cation techniques were respon-sible for the observed isolation of optically inactive forms (Dewick,1982).They show absorption at 200–240and 260–300nm (p !p *)and 320–352nm (n !p *).A disproportionate number of the natural iso?avanones possess oxygenation at C20(Wong,1975).CD data is essential to assign absolute con?guration to iso?avanones (Dewick,1994).The octant rule modi-?ed for the cyclic arylketones (Snatzke,1965a )predicts a positive CE for the n !p *carbonyl transition for (3R )-iso?avanones with the B-ring in the favored equa-torial position (Fig.31).The equatorial orientation of the B-ring can be veri?ed by NMR spectroscopic analy-sis with the coupling constant between the trans -diaxial H2b and H3of ca.11Hz (Gale?et al.,1997).Although assignments have been made using other absorption re-gions (Russell,1997;Yahara et al.,1989),e.g.,1L a ,1L b and p !p *,they seem to contradict each other and it is advisable to only use the n !p *carbonyl transition for assignments (Table 12).
Gale?et al.(1997)used CD to establish the absolute con?guration of two prenylated iso?avanones (38and
2194 D.Slade et al./Phytochemistry 66(2005)2177–2215
39)as 3R using the n !p *carbonyl absorption band (Fig.32).
11.Pterocarpans
Pterocarpans (40),6a-hydroxypterocarpans (41)(Fig.33),rotenoids (42),and 12a-hydroxyrotenoids (43)(Fig.34)are members of the tetracyclic iso?avonoid subgroup of ?avonoids (Bohm,1998).
11.1.Pterocarpans
Pterocarpans contain two chiral centers,but only the (6a R ,11a R )-cis and (6a S ,11a S )-cis con?gurations are found in nature (Dewick,1982,1994;Szarvas et al.,2000).The trans -fused B/C-ring system (45)has been synthesized in racemic form (Van Aardt et al.,2001).It is accepted that all laevorotatory pterocarpans have the (6a R ,11a R )absolute con?guration,and dextrorota-tory ones the (6a S ,11a S )con?guration (Wong,1975;Dewick,1982,1988,1994).
The preference in nature for the cis con?guration over the trans has been established by computational
Table 12
Iso?avanone CEs Absolute con?guration CE at n !p *(ca.320–352nm)(3R )Positive (3S )
Negative
D.Slade et al./Phytochemistry 66(2005)2177–22152195
studies that prove that a trans-fused B/C-ring system is energetically less favorable than the cis-fused system (Scho¨ning and Friedrichsen,1989).Although two strain-free conformations can exist,the preferred one is that in which the pyran B-ring approximates to a half-chair with the D-ring quasi-equatorial,making H6b quasi-axial and H6a quasi-equatorial(Fig.35; Friedrichsen and Scho¨ning,1990).In other words,the preferred conformation is a6a,11a-di-pseudo-axially substituted arrangement with the B-ring in a half-chair form,giving a truly staggered conformation along the C6–C6a axis.This served as con?rmation of what had earlier been found through the study of1H NMR coupling constants(Pachler and Underwood,1967).
The UV spectra of simple pterocarpans generally dis-play a major peak at ca.285–310nm and a smaller band at ca.280–287nm,while their ORD spectra show multi-ple CEs in the250–350nm region,which may also be used to assign absolute con?guration(Cook et al., 1978).
The chroman(A-plus B-ring)and2,3-dihydroben-zo[b]furan(C-plus D-ring)chirally perturbed achiral chromophores are concurrently present in the cis annu-lated pterocarpans.The CD bands of these compounds
2196 D.Slade et al./Phytochemistry66(2005)2177–2215
第二节分子的立体构型 知识点一形形色色的分子 1. 分子的立体构型 (1)概念:指多原子构成的共价分子中的原子的空间关系问题。由于多原子构成的分子中一定存在共价键,共价键的方向性使得分子中的原子按一定的空间结构排列,形成了分子的构型。如3原子分子的构型有直线型(CO2)和V(H2O)型两种。 (2)作用:分子构型对物质的活泼性、极性、状态、颜色和生物活性等性质都起决定性作用。 特别提醒:双原子均为直线型,不存在立体构型。 2.形形色色的分子 不同分子,构型不同。常见分子立体构型如下表: 知识点二价层电子对互斥模型 1.价层电子对互斥理论(VSEPR模型) (1)内容:分子中的价层电子对(包括σ键电子对和中心原子上的孤对电子)由于相互排斥作用,尽可能而趋向于彼此远离以减小斥力,分子尽可能采用对称的空间构型。电子对之间夹角越大,排斥力越小。 (2)VSEPR模型特征:用有区别的标记表示分子中的孤对电子和成对电子,如H2O、NH3的VSEPR 模型特征为: 2.利用价层电子对互斥理论判断分子的空间构型 (1)VSEPR模型把分子分成以下两大类 ①中心原子上的价电子都用于成键。在这类分子中,由于价层电子对之间的相互排斥作用,它们趋向于尽可能的相互远离,成键原子的几何构型总是采取电子对排斥最小的那种结构。它们的立体结构可用中心原子周围的原子数来预测。如:
②中心原子上有孤对电子的分子或离子。对于这类分子,首先建立四面体模型,每个键占据一个方向(多重键只占据一个方向),孤对电子也要占据中心原子周围的空间,并参与互相排斥。 (2)价层电子对数的计算 ①σ键电子对数的计算 σ键电子对数可由分子式确定,中心原子有几个σ键,就有几对σ键电子对。如H2O分子中σ键电子对数为,NH3分子中σ键电子对数为。 ②孤电子对数的计算 中心原子上的孤电子对数=1/2(a-xb) a为中心原子的价电子数; x为与中心原子结合的原子数; b为与中心原子结合的原子最多能接受的电子数。 如:如何确定CO2-3和NH+4的中心原子的孤电子对数 阳离子:a为中心原子的价电子数减去离子的电荷数(绝对值),故NH+4中中心原子为N,a=5-1,b=1,x=4,所以中心原子孤电子对数=1/2(a-xb)=1/2(4-4×1)=0。 阴离子:a为中心原子的价电子数加上离子的电荷数(绝对值),故CO2-3中中心原子为C:a=4+2,b=2,x=3,所以中心原子孤电子对数=1/2(a-xb)=1/2(6-3×2)=0。 ③中心原子的价层电子对数=σ键电子对数+1/2(a-xb)。 例1:下列分子中心原子的价层电子对数是3的是( ) A.H2O B.BF3C.CH4D.NH3 【解析】H2O中O的价层电子对数=2+1/2(6-2×1)=4 BF3中B的价层电子对数=3+1/2(3-3×1)=3 CH4中C的价层电子对数=4+1/2(4-4×1)=4 NH3中N的价层电子对数=3+1/2(5-3×1)=4。 (3)分子立体构型的确定 依据价层电子对互斥模型,判断出分子中中心原子的孤电子对数,再利用中心原子的成键电子对数,两者结合,就可以确定分子较稳定的立体构型。举例说明如下表:
百度文库 第二节分子的立体结构(学案) 【学习目标】 1、认识共价分子的多样性和复杂性; 2、初步认识价层电子对互斥模型; 3、能用 VSEPR 模型预测简单分子或离子的立体结构;理解价层电子对互斥模型和分子 空间构型间的关系。 4、认识杂化轨道理论的要点 5、进一步了解有机化合物中碳的成键特征 6、能根据杂化轨道理论判断简单分子或离子的构型 7、进一步增强分析、归纳、综合的能力和空间想象能力 【重点知识】:分子的立体结构;利用价层电子对互斥模型、杂化轨道理论模型预测分子的立体结构。 【回顾思考】 1举例说明什么叫化学式? 2举例说明什么叫结构式? 3举例说明什么是结构简式? 4举例说明什么是电子式? 5举例说明什么价电子? (第一课时) 一、形形色色的分子 【阅读课本】 认真阅读课本35 到 37 页“二、价层电子对互斥理论”处。在阅读过程中勾出你认为重要 的句子、词语、规律等,如发现新问题请写在课本中相应地方。认真读图2-8、 2-9、2-10、2-11、 2-12 和 36 页的知识卡片等去认识分子的多样性,自己动手制作几种分子的模型体验 分子的空间构型。然后思考下列问题。 【阅读思考1】 完成下表 化学式结构式键角分子的立体构型备注CO 2 H2O CH2O NH 3 CH 4 P4 1、原子数相同的分子,它们的空间结构相同吗? 2、请你利用身边的易得材料参照课本35、36 页内容制作CO2、H2O、NH 3、CH2 O、CH4
分子的球辊模型(或比例模型) ;并用书面用语描述它们的分子构型。 3、你如何理解分子的空间结构? 4、写出 CO 2、 H 2O 、NH 3、 CH 2O 、CH 4 的电子式; 5、观察上述分子的电子式,分析 H 、 C 、N 、 O 原子分别可以形成几个共价键,你知道 原因吗? 6、如何计算分子中中心原子的价层电子对?(成 σ键电子对、未成键电子对) 二、价层电子对互斥理论 【阅读课本】 认真阅读课本 37 到 39 页“三、杂化轨道理论简介 ”处。在阅读过程中勾出你认为重要的 句子、词语、规律等,如发现新问题请写在课本中相应地方。认真读图 2-15、表 2-4、 2-5, 对比价层电子对互斥模型和分子构型。然后思考下列思考问题。 【阅读思考 2】 1、中心原子:指出下列分子的中心原子: H O CO 2 NH 3 CH 4 BF 3 CH O 2 2 2、价层电子对: ( 1)根据上表中分子的电子式,指出下列分子里中心原子的价层电子对数目: H 2O CO 2 NH 3 CH 4 BF 3 CH 2O ( 2)根据你对价层电子对现有的知识,价层电子对可分为哪几类?如果计算? (二)认识 VSEPR 模型 1、VSEPR 模型(用于预测分子的立体构型) 结合 CH 4 、 CH 2O 的立体结构的球棍模型理解 VSEPR 模型(重点是从键角的 角度理解价层电子对的相互排斥) 【思考】 VSEPR 模型和分子的空间构型一样吗? 2、分类 第一类:中心原子的价层电子对全部为成键电子对。如: CH 4 CO 2 等。 价层电子对的排斥力:价层电子对相同,排斥力相同; 价层电子对不同,叁键>双键>单键 判断方法: 分子的立体结构 σ键电子对数 立体结构 范例 ABn 2 直线型 CO 2
选修三第二章 第2节 分子的立体构型 第2节 分子的立体构型 一、常见分子的空间构型 1.双原子分子都是直线形,如:HCl 、NO 、O 2、N 2 等。 2.三原子分子有直线形,如CO 2、CS 2等;还有“V ”形,如H 2O 、H 2S 、SO 2等。 3.四原子分子有平面三角形,如BF 3、BCl 3、CH 2O 等; 有三角锥形,如NH 3、PH 3等; 也有正四面体,如P 4。 4.五原子分子有正四面体,如CH 4、CCl 4等,也有不规则四面体,如CH 3Cl 、CH 2Cl 2、CHCl 3。 另外乙烯分子和苯分子都是平面形分子。 二、价层电子对互斥理论(Valance Shell Electron Pair Repulsion Theory )简称VSEPR 适用AD m 型分子 1、理论模型 分子中的价电子对(包括成键电子对和孤电子对),由于相互排斥作用,而趋向尽可能彼此远离以减小斥力,分子尽可能采取对称的空间构型。 2、用价层电子对互斥理论推断分子或离子的空间构型的一般步骤: (1)确定中心原子A 价层电子对数目 法1.经验总结 中心原子的价层电子对数= 2 1 (中心离子价电子数+配对原子提供电子总数) 对于AB m 型分子(A 为中心原子,B 为配位原子),计算方法如下: n =中心原子的价电子数+每个配位原子提供的价电子数×m 2 注意:①氧族元素的氧做中心时:价电子数为 6, 如 H 2O ,H 2S ;做配体时:提供电子数为 0,如在 CO 2 中。 ②如果讨论的是离子,则应加上或减去与离子电荷相应的电子数。如PO - 34中P 原子价层电子数5+(0 ×4)+3 = 8;NH +4 中N 原子的价层电子数5+(1×4)-1 = 8。 ③结果为单电子时视作有一个电子对。 例:IF 5 价层电子对数为 21 [7+(5×1)] = 6对 正八面体(初步判断) N H +4 价层电子对数为21 [5+(4×1)-1] = 4对 正四面体 PO - 34 价层电子对数为21[5+(0×4)+3] = 4对 正四面体 NO 2 价层电子对数为2 1 [5+0] = ?→? 对 平面三角形 法2. 确定中心原子A 价层电子对数目-----普遍规则 中心原子A 价层电子对数目=成键电子对数+孤对电子数 (VP = BP + LP ) VP 是价层电子对,BP 是成键电子对(BOND ),LP 是孤对电子对(LONE PAIR ) VP = BP + LP =与中心原子成键的原子数+中心原子的孤对电子对数LP =配位原子数+LP Lp = 2 1 (中心原子价电子数—配位原子未成对电子数之和) IF 5 Lp =21 [7-(5×1)] = 1 构型由八面体?→? 四方锥 NH +4 Lp =21 [(5-1)-(4×1)] = 0 正四面体 PO - 34 Lp =2 1[(5+3)-(4×2)] = 0 正四面体
AutoCAD的多文档设计环境,让非计算机专业人员也能很快掌握并使用。使用AutoCAD 进行二维绘图,对具有机械制图基础的人来说,是比较容易掌握的;但对三维建模,特别是自学者,却总觉得不知从何下手。本篇AutoCAD教程就教大家由三视图绘制三维实体图时的整个建模过程的步骤和方法。 一、分析三视图,确定主体建模的坐标平面 在拿到一个三视图后,首先要做的是分析零件的主体部分,或大多数形体的形状特征图是在哪个视图中。从而确定画三维图的第一步——选择画三维图的第一个坐标面。这一点很重要,初学者往往不作任何分析,一律用默认的俯视图平面作为建模的第一个绘图平面,结果很容易给后续建模造成混乱。 图1 此零件主要部分为几个轴线平行的通孔圆柱,其形状特征为圆,特征视图明显都在主视图中,因此,画三维图的第一步,必须在视图管理器中选择主视图,即在主视图下画出三视图中所画主视图的全部图线。
图2 此零件的特征图:上下底板-四边形及其中的圆孔,主体-圆筒及肋板等,都在俯视图,故应在俯视图下画出三视图中的俯视图。 下图是用三维图模画三维图,很明显,其主要结构的形状特征――圆是在俯视方向,故应首先在俯视图下作图。
图3 二、构型处理,尽量在一个方向完成基本建模操作 确定了绘图的坐标平面后,接下来就是在此平面上绘制建模的基础图形了。必须指出,建模的基础图形并不是完全照抄三视图的图形,必须作构型处理。所谓构型,就是画出各形体在该坐标平面上能反映其实际形状,可供拉伸或放样、扫掠的实形图。 如上文图1所示零件,三个圆柱筒,按尺寸要求画出图4中所示6个绿色圆。与三个圆筒相切支撑的肋板,则用多段线画出图4中的红色图形。其它两块肋板,用多段线画出图中的两个黄色矩形。
精品教育 -可编辑- 选修三第二章 第2节 分子的立体构型 第2节 分子的立体构型 一、常见分子的空间构型 1.双原子分子都是直线形,如:HCl 、NO 、O 2、N 2 等。 2.三原子分子有直线形,如CO 2、CS 2等;还有“V ”形,如H 2O 、H 2S 、SO 2等。 3.四原子分子有平面三角形,如BF 3、BCl 3、CH 2O 等; 有三角锥形,如NH 3、PH 3等; 也有正四面体,如P 4。 4.五原子分子有正四面体,如CH 4、CCl 4等,也有不规则四面体,如CH 3Cl 、CH 2Cl 2、CHCl 3。 另外乙烯分子和苯分子都是平面形分子。 二、价层电子对互斥理论(Valance Shell Electron Pair Repulsion Theory )简称VSEPR 适用AD m 型分子 1、理论模型 分子中的价电子对(包括成键电子对和孤电子对),由于相互排斥作用,而趋向尽可能彼此远离以减小斥力,分子尽可能采取对称的空间构型。 2、用价层电子对互斥理论推断分子或离子的空间构型的一般步骤: (1)确定中心原子A 价层电子对数目 法1.经验总结 中心原子的价层电子对数= 2 1 (中心离子价电子数+配对原子提供电子总数) 对于AB m 型分子(A 为中心原子,B 为配位原子),计算方法如下: n =中心原子的价电子数+每个配位原子提供的价电子数×m 2 注意:①氧族元素的氧做中心时:价电子数为 6, 如 H 2O ,H 2S ;做配体时:提供电子数为 0,如在 CO 2 中。 ②如果讨论的是离子,则应加上或减去与离子电荷相应的电子数。如PO - 34中P 原子价层电子数5+ (0×4)+3 = 8;NH +4 中N 原子的价层电子数5+(1×4)-1 = 8。 ③结果为单电子时视作有一个电子对。 例:IF 5 价层电子对数为 21 [7+(5×1)] = 6对 正八面体(初步判断) N H +4 价层电子对数为 21 [5+(4×1)-1] = 4对 正四面体 PO - 34 价层电子对数为 21[5+(0×4)+3] = 4对 正四面体 NO 2 价层电子对数为2 1 [5+0] = 2.5?→? 3对 平面三角形 法2. 确定中心原子A 价层电子对数目-----普遍规则 中心原子A 价层电子对数目=成键电子对数+孤对电子数 (VP = BP + LP ) VP 是价层电子对,BP 是成键电子对(BOND ),LP 是孤对电子对(LONE PAIR ) VP = BP + LP =与中心原子成键的原子数+中心原子的孤对电子对数LP
第1章认识钢筋混凝土结构 习题 一、单选题。 1.不属于钢筋混凝土结构的是( D ) A .框架结构 B.框架剪力墙结构 C.框支剪力墙结构 D.砖混结构 2.混凝土的( D )主要与其密实度及内部孔隙的大小和构造有关。 A .抗冻性 B .抗侵蚀性 C . 抗老化 D . 抗渗性 3.( B )框架结构建筑说法正确的是。 A.适合超高层建筑 B.节点处应力集中 C.空间利用率低 D.墙体承重 二、多选题。 1 .属于框架结构建筑的构件有( A B C D ) A .框架柱 B.框架梁 C. 板 D.楼梯 E.构造柱 2 .框支剪力墙结构构件有( A B C D ) A.框支柱 B.框支梁 C.板 D.楼梯 E.构造柱 第二章柱平法识图规则 习题 选择题 1.在基础内的第一支柱箍筋到基础顶面的距离是多少(B) A.50 B.100 C.3d( d为箍筋直径) D.5d( d为箍筋直径) 2.抗震中柱顶层节点构造,能直锚时,直锚长度为(D) A.1 2d B. L aE C.伸至柱顶 D.伸至柱顶,≥L aE 3.柱箍筋加密区的范围包括( C ) A.有地下室框架结构地下室顶板嵌固部位向上Hn/6 B.底层刚性地面上500mm C.无地下室框架结构基础顶面嵌固部位向上Hn/3 D.搭接范围 4.某框架三层柱截面尺寸300×600mm 2 ,柱净高3.6 m ,该柱在楼面处的箍筋加密区高度应为(C)。 A.400 B.500 C.600 D.700 5.上层柱和下层柱纵向钢筋根数相同,当上层柱配置的钢筋直径比下层柱钢筋直径粗时,柱的纵筋搭接区域应在( C)。 A.上层柱 B.柱和梁相交处 C.下层柱 D.不受限制 6.抗震框架边柱顶部的外侧钢筋采用全部锚入顶层梁板中的连接方式时,该外侧钢筋自底起锚入顶层梁板中的长度应不少于( C )。 https://www.doczj.com/doc/2118394745.html,E B.0.4 L aE C. 1.5LaE D. 2LaE 7.下列关于柱平法施工图制图规则论述中错误的是(C )。 A.柱平法施工图系在柱平面布置图上采用列表注写方式或截面注写方式。 B.柱平法施工图中应按规定注明各结构层的楼面标高、结构层高及相应的结构层号。 C.注写各段柱的起止标高,自柱根部往上以变截面位置为界分段注写,截面未变但配筋改变处无须分界。
《第二节 分子的立体构型》 1.能说明CH 4分子的5个原子不在同一平面而为正四面体构型的是( ) A .两个键之间夹角为109°28′ B . C —H 键为极性共价键 C .4个C —H 键的键能、键长相同 D .碳的价层电子都形成共价键 2.(2010年扬州高二检测)下列说法中正确的是( ) A .NO 2、SO 2、BF 3、NCl 3分子中没有一个分子中原子的最外层 电子都满足了8电子稳定结构 B .P 4和CH 4都是正四面体分子且键角都为109°28′ C .NH 4+ 的电子式为[H ··N ··H ··H]+,离子呈平面正方形结构 D. NH 3分子中有一对未成键的孤电子对,它对成键电子的排斥作 用较强 3.(2010年泉州高二检测)用价层电子对互斥理论预测H 2S 和BF 3的立体构型,两个结构都正确的是( ) A .直线形;三角锥形 B .V 形;三角锥形 C .直线形;平面三角形 D .V 形;平面三角形 4.(2010年普宁高二检测)有关乙炔分子中的化学键描述不. 正确的是( ) A .两个碳原子均采用sp 杂化方式 B .两个碳原子均采用sp 2杂化方式 C .每个碳原子都有两个未杂化的2p 轨道形成π键 D .两个碳原子形成两个π键 5.下列有关苯分子中的化学键描述正确的是( ) A .每个碳原子的sp 2杂化轨道中的一个形成大π键 B .每个碳原子的未参与杂化的2p 轨道形成大π键 C .每个碳原子的三个sp 2杂化轨道与其他两个碳原子和一个氢原 子形成三个σ键 D .每个碳原子的未参加杂化的2p 轨道与其他原子形成σ键 6.下列对二氧化硫与二氧化碳的说法中正确的是( ) A .都是直线形结构 B .中心原子都采取sp 杂化 C .硫原子和碳原子上都没有孤电子对 D .SO 2为V 形结构,CO 2为直线形结构 7.下列分子中的中心原子的杂化轨道类型相同的是( ) A .CO 2与SO 2 B .CH 4与NH 3 C .BeCl 2与BF 3 D .C 2H 4与C 2H 2 8.膦(PH 3)又称磷化氢,在常温下是一种无色、有大蒜臭味的有毒气体,电石气的杂质中常含有磷化氢。它的分子构型是三角锥形。则下列关于PH 3的叙述正确的是( ) A .PH 3分子中有未成键的孤电子对 B .PH 3是空间对称结构 C .PH 3是一种强氧化剂 D .PH 3分子中的P —H 键间夹角是90° 9.已知:①红磷在氯气中燃烧可以生成两种化合物——PCl 3和PCl 5,氮与氢也可形成两种化合物——NH 3和NH 5。 ②PCl 5分子中,磷原子的1个3s 轨道、3个3p 轨道和1个 3d 轨道发生杂化形成5个sp 3d 杂化轨道,PCl 5分子呈三角双锥形
第三章立体化学 Stereochemistry Contents 3.1 对称性与对称因素(2) 1、平面对称因素 2、中心对称因素 3、简单轴对称因素 4、反射对称因素 3.2 旋光性与对映异构(4) 1、物质的旋光性 2、旋光性与分子手性 3.3 含手性碳原子化合物的立体化学 1、含一个手性碳原子的分子 2、含两个不同手性碳原子的分子 3、含两个相同手性碳原子的分子 4、含假不对称碳原子的分子 3.4 不含手性碳原子化合物的立体化学 1、丙二烯型化合物 2、联苯型化合物 3、含其它不对称原子的化合物 4、环状化合物的光活异构体 3.5 外消旋体的拆分 *3.6 反应中的立体化学 在有机化学中广泛存在着同分异构现象。1832年,柏采利里乌斯根据实验事实建议:把具有相同组成而具有不同性质和晶形的物质称同分异构物质。1861年,布特列洛夫的结构学说说明了产生同分异构的原因是由于化学结构的不同;1874年,范特荷夫和勒贝尔的碳四面体概念奠定了立体化学基础,说明了物质的旋光性。 构造异构(Constitution isomerism):有机分子中,原子相互连接的次序不同而产生的异构现象。 立体异构(Stereoisomerism):分子中原子的连接次序相同,但空间的排布方式不同而呈现的异构现象。 碳胳异构:碳链不同 构造异构—位置异构:官能团的位置不同 官能团异构:官能团不同 互变异构:不同官能团迅速互变达到平衡,如烯醇与 酮式结构的互变 同分异构—— 顺反异构:双键或环的存在使分子中某 些原子在空间排布不同 构型异构—对映异构:分子具有手性 立体异构—— 构象异构:由于C—C键旋转而使分子在空间中呈现 不同形象 立体异构涉及到分子的几何形象,而分子的几何形象对其物理性质和化学性质有时具有非常惊人的影响。如碳的不同同素异形体,无定形碳、石墨、金刚石、足球烯,具有完全不同的几何形象,其外观、物理和化学性质完全不同。分子几何形象的微少差别对自然界及生命现象都起着难以估计的影响,绝大多数生命及生理化
第三章曲面立体 第一节曲线与曲面 ●1.曲线:圆、椭圆、抛物线、双曲线 ●2.曲面:回转曲面、非回转曲面 ●直纹曲面、曲纹曲面 ●3.曲面立体的形成:回转曲面立体 ●圆的投影 ●1。圆面是投影面的平行面: ●2.圆面是投影面的垂直面 ●3.圆面是投影面的倾斜面 ●平行—反应实形,圆 ●垂直—积聚的线,线长=直径 ●倾斜—椭圆。长轴:圆面内的平行线直径 ●短轴:垂直圆内平行线的最大斜度线 ●1.曲线 柱状屋面 柱状面是由一直母线沿两曲导线移动,同时又平行于一导平面形成的曲面见图3-44a,该柱状面是直母线A D沿着两曲导线A B C和D E F移动且平行于导平面而形成的。 曲面立体 表面由平面与曲面围成,或全部由曲面围成的立体称为曲面立体。 常见曲面是回转面,它是由一直线或曲线以一定直线为轴线回转形成。 由回转曲面组成的立体,称回转体,如圆柱体、圆锥体、球体等。 3.曲面立体的形成 ? 第二节曲面立体的投影及其表面求点的投影 ?1.圆柱的投影 ?圆柱表面点的投影 ?2.圆锥的投影 ?圆锥表面点的投影 ?3.球体的投影 ?球体表面点的投影 (一)圆柱体 圆柱体是由顶面、底面和圆柱面所组成。圆柱面是由一条直母线绕与它平行的轴线回转而成。圆柱面上任意一条平行于轴线的直线,称为圆柱面的素线。曲面体表面上的点和平面体表面上的点相似。为了作图方便,在求曲面体表面上的点时,可把点分为两类: –特殊位置的点,如圆柱、圆锥的最前、最后、最左、最右、底边,
球体上平行于三个投影面的最大圆周上等位置上的点,这样的点可直接利用线上点的方法求得。 圆等 圆锥面是由一直母线绕着与它相交的轴线旋转而成。 在圆锥面上通过锥顶S的任一直线称为圆锥面的素线。
巧用3Ds MAX阵列建模方法制作化学三维结构图像 摘要在开展化学多媒体教学的过程中,我们经常要用三维结构图像和动画模拟一些化学中的微观结构。但在制作多媒体课件时,我们往往觉得3Ds MAX非常深奥、难学。本文谈谈自己通过大量的实践和研究掌握的一些化学三维结构图像和动画制作技巧。 关键词 3Ds MAX 三维球棍模型甲烷 在3Ds MAX中制作化学三维结构图像和动画的基础是三维结构的建模。建模方法很多,有对象建模、放样建模、布尔建模、次对象建模、网格建模和面片建模等。本人根据大量的实践得出,中学化学的三维结构建模只要几步即可完成。下面介绍自创的建模方法:阵列法。 一、用阵列法创建三维球棍模型 1.前期准备——数据处理 3Ds MAX是一个可视化工具,正确显示模型各部分的比例,可以使模型看起来合理、易理解。因此,在建模前须了解各原子的半径及键长。有关数据如下(单位:nm): 若以rH为8个单位,即原数据的250倍,键长再乘以2,则有: 以创建甲烷(CH4)三维球棍模型为例,甲烷分子是正四面体结构,碳原子位于正四面体的中心,氢原子在正四面体的四个顶点,C-H键长0.109nm ,键角109.5° 2.制作过程--创建甲烷三维球棍模型 下面介绍用3Ds MAX 7.0 中文版创建甲烷三维球棍模型的全过程: (1)选取命令面板(图1)左上角的创建命令,单击标准 基本体按钮。单击对象类型栏球体按钮,再单击键盘输 入项,打开参数项卷展栏,输入参数(如图2),可创建碳原 子模型(其中参数X、Y、Z分别为三维空间的坐标系,半径为 球体的半径)。输入参数完毕,单击创建按钮,即完成碳原子 的建模。
第三章场景设计的空间构成 ?第一节形的基本概念 ?第二节造型的规律是造型艺术、平面艺术、立体构成的基本常识和规律 ?第三节造型的方法 ?第四节动画场景中空间表现★ ?学习目标:了解场景造型的空间构成 ?学习重点:形的基本概念和场景在空间中的表现 ?学习难点:场景在空间中的表现 第一节形的基本概念 宇宙间有形的事物千变万化,我们可以把它们解构成点、线、面、体四种基本的造型要素。 一、点 1、点的基本概念:概念都是分几何学和造型两个方面的 2、点的主要特征: 3、点的构成方式: 4、点与形的关系: 二、线 1、线的基本概念:几何学分几何学 2、线的主要特征:造型中和造型两个方面 3、线的种类: 4、线的构成方式: 5、线的形态: 6、线与形的关系: 7、线在动画场景造型中的作用: 三、面 1、面的基本概念:概念都是分几何学和造型两个方面的 2、面的主要特质:在平面构成中,正方形、三角形、圆形被称为三个基本形。 3、面的种类 四、体 1、体的基本概念:概念都是分几何学和造型两个方面的 2、体的主要特征:体的存在特性在于其所具有的体积、重量和容量的共同关系。 3、体的类型:体可以分为半立体、点立体、线立体、面立体和块立体等多种主要类型。 4、体的类型特征: 第二节造型的规律(形式美法则)---简答题
一、风格统一:和谐的美感效果 二、单纯:最有力的形态效果 三、节奏:有秩序、有规律变化的视觉效果 四、平衡:不偏不倚的稳定感觉效果 第三节造型的方法 一、相近法:协调的形式感效果 二、对比法:强烈、明快效果 三、重复法:连续视觉效果 四、平衡法:庄重、严谨效果 五、比例法:优美的比例效果黄金分割 第四节动画场景中空间表现★ 一、动画场景的空间概念 二、动画场景的组成要素 三、动画场景的分类 动画场景分为内景和外景。课本P5 场景一般分为:内景、外景和内外结合景。 四、动画场景的空间结构 1、单一空间结构 2、纵向多层次空间结构 3、横向排列空间结构 4、垂直组合空间结构 5、复合式组合空间结构 五、动画场景空间形式的心理感受 1、高而直 2、高而宽 3、圆形 4、三角形 5、四方形 六、塑造场景空间的方法 动画场景空间大致可分为两类:物理空间和心理空间。物理空间和心理空间各自的含义 1、引力感法 2、强化景深法:直线透视、利用前景遮挡、利用光影 3、空间融合 4、多层次综合设置法 5、角色调度法 6、人景接触法 7、镜头组接法:宫崎骏标志性镜头语言---“三镜头式” 七、场景空间在动画影片中的作用 1、塑造生动感 2、营造神秘感 3、制造危机感 八、场景空间象征隐喻
1.导体:镀锡软铜导体。 2.绝缘:辐照交联低烟无卤绝缘料。结构 1.导体:镀锡软铜导体。 2.绝缘:辐照交联低烟无卤绝缘料。 3.隔离层:包带。 4.护套:辐照交联低烟无卤护套料。结构 1.导体:镀锡软铜导体。 2.绝缘:辐照交联低烟无卤绝缘料。 3.屏蔽:镀锡铜丝屏蔽层。 4.隔离层:包带(可选)。 5.护套:辐照交联低烟无卤护套料。
1.导体:镀锡软铜导体。 2.绝缘1:辐照交联低烟无卤绝缘料1。 3.绝缘2:辐照交联低烟无卤绝缘料2。结构 1.导体:镀锡软铜导体。 2.绝缘1:辐照交联低烟无卤绝缘料1。 3.绝缘2:辐照交联低烟无卤绝缘料2。 4.隔离层:包带。 5.护套:辐照交联低烟无卤护套料。结构 1.导体:镀锡软铜导体。 2.绝缘1:辐照交联低烟无卤绝缘料1。 3.绝缘2:辐照交联低烟无卤绝缘料2。 4.屏蔽:镀锡铜丝屏蔽层。 5.隔离层:包带(可选)。 6.护套:辐照交联低烟无卤护套料。
1.导体:镀锡软铜导体。 2.绝缘:辐照交联低烟无卤绝缘料。结构 1.导体:镀锡软铜导体。 2.绝缘:辐照交联低烟无卤绝缘料。 3.隔离层:包带。 4.护套:辐照交联低烟无卤护套料。结构 1.导体:镀锡软铜导体。 2.绝缘:辐照交联低烟无卤绝缘料。 3.屏蔽:镀锡铜丝屏蔽层。 4.隔离层:包带(可选)。 5.护套:辐照交联低烟无卤护套料。结构
1.导体:镀锡软铜导体。 2.绝缘1:辐照交联低烟无卤绝缘料1。 3.绝缘2:辐照交联低烟无卤绝缘料2。结构 1.导体:镀锡软铜导体。 2.绝缘1:辐照交联低烟无卤绝缘料1。 3.绝缘2:辐照交联低烟无卤绝缘料2。 4.屏蔽:镀锡铜丝屏蔽层。 5.护套:辐照交联低烟无卤护套料。结构 1.导体:镀锡软铜导体。 2.绝缘1:辐照交联低烟无卤绝缘料1。 3.绝缘2:辐照交联低烟无卤绝缘料2。 4.屏蔽:镀锡铜丝屏蔽层。 5.隔离层:包带(可选) 6.护套:辐照交联低烟无卤护套料。结构 1.导体:镀锡软铜导体。 2.绝缘1:辐照交联低烟无卤绝缘料1。 3.绝缘2:辐照交联低烟无卤绝缘料2。
上海交通大学 硕士学位论文 序列内窥镜图像的三维结构重建 姓名:罗肖 申请学位级别:硕士 专业:生物医学工程 指导教师:秦斌杰 20090101
序列内窥镜图像的三维结构重建 摘要 基于内窥镜的微创手术作为外科手术领域的一个重要发展方向,在临床手术中有广泛的应用。但在内窥镜手术中,医生只能获得二维图像,无法感知三维的真实场景以及内窥镜视野在手术空间中的相对位置。针对这两个问题,本文提出了基于序列单目内窥镜图像的三维结构重建方法,跟踪内窥镜的运动轨迹和重建当前内窥镜视野的三维结构。本文主要内容如下: 1.在内窥镜图像畸变校正方面,本文采用摄像机的非线性模型对内窥镜进行标定,确定其镜头的内参数和畸变参数,并以此完成对内窥镜图像的畸变矫正; 2.在内窥镜图像特征提取和特征跟踪方面。本文讨论了基于KLT的特征跟踪算法并对内窥镜图像进行了特征跟踪匹配。针对KLT算法对图像尺度变化和亮度变化较为敏感等缺点,提出了基于SIFT的特征跟踪算法。该算法对SIFT算子进行优化,结合帧间运动估计的块匹配方法对序 第I页
列图像进行特征跟踪,并且基于运动一致性约束提出了一种简便的误匹配剔除策略,取得了较好的特征跟踪效果; 3.在得到序列图像的匹配特征点对后,利用基于多视图矩阵的迭代分解算法和线性三角形法从运动中恢复出结构,得到了内窥镜当前场景的射影重建结果; 4.针对手术过程中可能发生内窥镜摄像机变焦等情形,本文引入了对内窥镜焦距等内参数进行自定标的方法,得到内窥镜的运动轨迹和内窥镜视野的三维欧氏结构,再现了当前内窥镜视野的真实场景。 本文对采自上海市第六人民医院的一组鼻腔内窥镜图像进行实验,成功取得了内窥镜摄像机的运动轨迹和鼻腔的三维场景,验证了基于单目内窥镜图像进行三维结构重建方法的可行性。 关键词:透视模型,特征点跟踪,SIFT描述子,多视图矩阵,自标定,欧氏重建 第II页
第二节分子的立体构型 1、下列分子的立体结构模型正确的是() 2、下列分子的立体构型为正四面体形的是() ①P4②NH3③CCI4④CH4⑤H2S⑥C O2 A.①③④⑤B.①③④⑤⑥ C.①③④D.④⑤ 3、(l)硫化氢(H2S)分子中,两个H一S键的夹角接近90o,说明H2S分子的立体构型为___________ (2)二氧化碳(CO2)分子中,两个C一O键的夹角是180 o,说明CO2分子的立体构型为___________。 (3)能说明CH4分子不是平面四边形,而是正四面体结构的是________(双选) a.两个键之间的夹角为109 o28' b.C一H键为极性共价键 c.4个C一H键的键能、键长都相等 d.二氯甲烷(CH2Cl2)只有一种(不存在同分异构体) 4、用价层电子对互斥理论判断SO3的立体构型为() A.正四面体形B.V形 C.三角锥形D.平面三角形 5、若AB n型分子的中心原子A上没有未用于形成共价键的孤电子对,运用价层电子对互斥理论,判断下 列说法中正确的是() A.若n=2,则分子的立体构型为V形 B.若n=3,则分子的立体构型为三角锥形 C.若n=4,则分子的立体构型为正四面体形 D.以上说法都不正确 6、用价层电子对互斥理论分别预测H2S和BF3的立体结构,两个结论都正确的是() A.直线形三角锥形B.V形三角锥形 C.直线形平面三角形D.V形平面三角形 7、下列分子或离子中,价层电子对互斥模型为四面体形,但分子或离子的立体构型为V形的是() 8、以下有关杂化轨道的说法中错误的是() A.第ⅠA族元素成键时不可能有杂化轨道 B.杂化轨道既可能形成σ键,也可能形成π键 C.孤电子对有可能参加杂化 D.s轨道和p轨道杂化不可能有sp4出现 9、下列分子的立体构型,可以用sp杂化方式解释的是()
从2005年以后,Autodesk开始发力Revit(后文简称RVT)推行BIM 概念,先今RVT已然有当初AutoCAD在国内辉煌的势头。在国内,无论 BIM技能考试、各类BIM设计大赛、BIM培训机构、BIM论坛,都以RVT 为主要软件平台;同时RVT格式的BIM模型在设计院、业主单位、施工 单位之间的交互已有一定的规模。 经过多版本升级与迭代,RVT功能愈加强大,二次开发接口(API)和文档也已经逐步完善。国内有多家公司在做基于Revit的深度应用二次开发,全面覆盖RVT 建模和应用模方面的不足。除BIM之外,甚至有的RVT插件可以将PDMS工程直接拉入RVT中展示: 图1 某插件在Revit中显示PDMS三维工厂模型的设备和管道 RVT提供从三维设计到图纸管理一系列功能,在实际应用中也存在一些问题: 1. 对硬件环境要求较高,模型较大时操作不流畅(Autodesk公司完全有实力解决该问题)。 2.数据组织方式不同于成熟的数据库模式。实际应用中需要建立RVT族库,或借助特定的软件自动从已有的数据转换。 3.一些细节尚待完善。如生成的结构平面视图一些情况下消隐处理不符合国内习惯;RVT 中一些样式无法调整达到国内施工图要求的效果或需要深度定制。 4. 没有有效的手段依据RVT模型自动生成施工图。现阶段DWG还是出版、交付及归档标准。 在设计院结构专业的实际应用中,RVT的上述问题尤为突出:一方面生 产、管理及交付均有强烈的三维结构模型需求;另一方面因结构设计的 专业性与反复以及交付的紧迫性,不得不走最为成熟的先二维后三维路 线——三维结构模型沦为副产品。正是RVT在实际应用中存在的上述问 题,使得设计院结构专业暂时较难以三维结构模型为核心展开工作。
第三章小结 一、基本体及其投影特点 1、平面体 (1)棱柱体:两底面平行,侧棱面⊥底面。 1)棱柱体投影特点:一个投影反映底面的真形,另两个投影为矩形+棱线。 2)表面上点的投影特性: 侧棱线上的点:积聚为底面投影的各顶点; 侧棱面上的点:积聚为底面投影的各底边; 底面上的点:积聚为侧棱面投影的矩形上/下边上。 (2)棱锥体: 1)棱锥体投影特点:一个投影反映底面的真形,另两个投影为三角形+棱线。 2)表面上点的投影特性: 底面上的点:积聚为侧棱面投影的三角形底边上。 2、回转体 基本概念: 1)回转面:母线绕轴旋转一周形成的面。 2)转向轮廓线:从投影方向看去,回转面可见部分与不看见部分的分界线。正面投影的转向轮廓线称为正转向轮廓线;侧面投影的转向轮廓线称为侧转向轮廓线。 (1)圆柱体:两底面平行,回转面⊥底面。 1)圆柱体投影特点:一个投影为圆,另两个投影为矩形。 2)表面上点的投影特性: 转向轮廓线上的点:积聚在另两个投影的对称中心线上; 回转面上的点:积聚在圆周上。 (2)圆锥体: 1)圆锥体投影特点:一个投影为圆,另两个投影为等腰三角形。 2)表面上点的投影特性: 转向轮廓线上的点:积聚在另两个投影的对称中心线上; 回转面上的点:积聚在圆周内。注意:可根据点或轮廓线的(不)可见性,初步判定其位置。 二、绘制基本体表面上点的投影 基本依据:基本体表面点的投影特性。 基本思路:对于特殊点:根据其特性得到;对于非特殊点:借助特殊点作辅助线得到。 具体方法如下: 1、平面体 最特殊的点:棱线上的点。 (1)棱柱体:先初步判断点的位置(棱线上?侧棱面上?底面上?),然后根据相应的投影特性得出其投影。 (2)棱锥体:①先在已知投影中标出锥体顶点和底面各顶点,并初步判断点的位置;②根据标注的顶点,可得到各棱线上点的投影;③对于侧棱面上的点,可借助棱线上的点做辅助线得到。辅助线做法有两种:一种是过锥体顶点和该点已知投影作辅助线,交三角形底边于一点;另一种是过该点已知投影作底边的平行线,与棱线相交于一(或两)点。所求点的投影便在该辅助线上。 2、回转体 最特殊的点:转向轮廓线上的点。 (1)圆柱体:初步判断点的位置,根据其投影特性即可得到其投影。(2)圆锥体:对于回转面上点的投影,可借助转向轮廓线或底面圆周上的点做辅助线得到。有两种方法: ①素线法:过锥体顶点和该点已知投影作辅助线,交三角形底边(或圆周线)于一点,所求点的投影便在该辅助线上。 ②纬圆法:分两种情况:若点的已知投影在等腰三角形内,则先过该投影做三角形底边的平行线,交三角形腰于一(或两)点,接着过该交点做底圆投影对称轴的垂线,得到一垂足,然后以底圆的圆心为圆心,过该垂足作一辅助圆,所求点的投影便在该圆上。。若点的已知投影在底圆内,则先做过该点的辅助圆,与对称轴相交,再做该交点在三角形中的投影(在腰上),然后过该投影做三角形底边的平行线,所求点的投影
第三课《立体构成——线的立体构成》时间:第三周教学目的:使学生通过学习线的立体构成的基本概念和技能,提高对形式美规律的认识,培养造型的创造能力。 教学方法:讲授 教学用具:立体构成成品,PPT 教学重点与难点: 重点:发挥学生的想像力,创作出富有情趣的、奇妙的画面,渗透设计意识,合理利用材料。 难点:制作得新颖、生动观,富于个性。 复习: 立体构成 立体构成的构成元素: 点、线、面、块 导入新课: 展示线立体构成成品,引起学生兴趣 讲授新课: 一、定义
立体构成中的线是有决定长度特征的材料实体,通常称这种材料为线材。 用线型材料构成的立体形态成为线立体构成。 二、作用 线材在立体构成中起到了框架和造型的作用。现代高层建筑大多采用直线形态构建,用直线形态构建的斜拉桥承载力更大。尽管直线易使人的视觉产生疲劳,但从视觉感知的效果来看,用直线构建的形态更容易感知。 三、线的材料分类 线材因材料强度的不同可分为硬质线材和软质线材(硬线材和软线材). 常见的硬质线材有:条状的木材、金属、塑料、玻璃等;硬线材的性质特点是不易变形,可切削,可连接直线、折线。 软质线材有:毛、棉、丝、麻以及化纤等软线和较软的金属丝.软线材的性质特点是易变形,可拉,可缠绕,打结等。 1、硬线构成:即硬质线材的构成. 硬质线材的强度较好,有比较好的自身支持力,但柔韧性和可塑性较差.因此,硬质线材的构成可以不依靠支架,多以线材排列、叠加、组合的形式构成,再用粘结材料进行固定.硬线构成具有强烈的空间感、节奏感和运动感.
2、软线构成:又叫软质线材的构成. 软质线材的材料强度较弱,没有自身支持力,柔韧性和可塑性好.所以,软质线材通常要框架来支持立体形态,对框架的依赖软线构成可分为有框架构成和无框架构成. A\有框架构成先用硬质线材制作框架,再在框架上定接线点,然后用软质线材按照接线点的位置连接. B\无框架构成是利用软质线材的编结、层排、堆积进行构成如软挂壁、织物等. 四、硬线材构成 1、连续构造 2、垒积构造 3、线层结构 4、框架结构 (1)、连续构造 选择有一定硬度的金属丝或其它线性材料,构成时不限定范围,以连续的线使其自由构成,产生连续的空间效果。表现对象可以是抽象的,也可以是具像的。 (2)、垒积构造 把硬线材料一层层堆积起来,相互间没有固定的连接点,可以任意改变的立体构成,叫垒积构成。
武汉理工大学艺术与设计学院 交通工具设计系 《立体构成》讲稿 工业设计0801 0802 主讲:胡国堂 二零一零年二月
《立体构成》讲稿 第一章立体构成概述 第二章立体构成的造型要素(重点、难点)第三章立体构成的形式要素(重点、难点)第四章立体构成材料 第五章线材构成 第六章面材立体构成(重点、难点) 第七章单体集聚构成(难点)方式。 第八章立体构成在设计领域中的运用 知识点:一、形态与形状的区别 二、包豪斯构成理论 三、基本素质和技能训练 四、立体构成的基础特性 五、线材构成的特点 六、仿生结构的内容和加工方法
第一章立体构成概述 第一节立体构成概念及起源 概念: 立体构成是现代设计领域中一门基础造型课,也是一门艺术创作设计课。在立体造型中首先需要明确一个概念,即形态与形状的区别(知识点一),平面造型中我们称平面的形为形状,这个形状是物象的外轮廓。在立体造型中形状是指立体物在某一距离、角度、环境条件下所呈现的外貌,而形态是指立体物的整个外貌。即形状是形态的诸多面向中的一个面向,形态则是诸多形状构成的统和体。形态是立体造型全方位的印象,是形与神的统一。 作为研究形态创造与造型设计的独立学科。立体构成所涉及的学科有建筑设计、室内设计、工业造型、雕塑、广告等设计行业。除在平面上塑造形象与空间感的图案及绘画艺术外,其它各类造型艺术都应划归立体艺术与立体造型设计的范畴。它们的特点是,以实体占有空间、限定空间、并与空间一同构成新的环境、新的视觉产物。由此,人们给了它们一个最摩登的称谓:"空间艺术"。 立体构成也称为空间构成。立体构成是以一定的材料、以视觉为基础,以力学为依据,将造型要素,按照一定的构成