Anti-allodynic effects of intrathecally and intracerebroventricularly administered
- 格式:pdf
- 大小:336.28 KB
- 文档页数:8

MicroRNA 则可能参与神经系统的生理或病理过程。
miR-396可能参与阿尔兹海默大鼠脑内淀粉样蛋白的产生与蓄积,也可能参与神经系统肿瘤的增殖、转移及凋亡过程[16]。
本研究显示实验1组与实验2组麻醉后7d 海马组织的miR-96相对表达量显著高于对照组(P <0.05),实验2组显著高于实验1组(P <0.05),表明七氟醚的应用能促进miR-96的表达。
4结论综上所述,七氟醚能通过上调认知障碍大鼠海马组织miR-96的表达,抑制血清IGF -1的表达,从而损伤大鼠的认知功能,且七氟醚暴露8h 较暴露4h 对其损伤更明显,但具体机制尚有待进一步研究。
参考文献[1]Liu B ,Ou G ,Chen Y ,et al.Inhibition of protein tyrosine phosphatase1B protects against sevoflurane -induced neurotoxicity mediated by ERstress in developing brain [J ].Brain Res Bull ,2018,(146):28-39.[2]赵丹.七氟醚-瑞芬太尼静吸复合麻醉对子宫肌瘤腹腔镜术后认知功能的影响效果观察[J ].中国实用医药,2018,13(30):137-138.[3]张奕文,陈汉文,张忠其,等.依托咪酯对老年患者腹腔镜下结直肠癌术后早期认知功能及血浆皮质醇的影响[J ].广州医科大学学报,2018,46(2):23-26.[4]Ju X ,Jang Y ,Heo JY ,et al.Anesthesia affects excitatory /inhibitorysynapses during the critical synaptogenic period in the hippocampus of young mice :Importance of sex as a biological variable [J ].Neurotoxi-cology ,2018,(70):146-153.[5]Ayers D ,Scerri C.Non -coding RNA influences in dementia [J ].Noncoding RNA Res ,2018,3(4):188-194.[6]Martinez B ,Peplow PV.MicroRNAs as diagnostic and therapeutic toolsfor Alzheimer 's disease :advances and limitations [J ].Neural Regen Res ,2019,14(2):242-255.[7]赵春梅,刘强,王振海.HSE 模型小鼠的水迷宫实验观察[J ].宁夏医科大学学报,2012,34(2):115-117,封4.[8]Zhou Y ,Deng J ,Chu X ,et al.Role of Post -Transcriptional Control ofCalpain by miR-124-3p in the Development of Alzheimer's Disease [J ].J Alzheimers Dis ,2019,67(2):571-581.[9]蒋琦亮,郭震,唐炜,等.七氟醚与丙泊酚对行体外循环下心脏手术患者实验室指标及术后认知障碍的影响[J ].临床和实验医学杂志,2018,17(12):1326-1329.[10]Azimaraghi O ,Nezhad Sistani M ,Abdollahifar MA ,et al.Effects of re-peated exposure to different concentrations of sevoflurane on the neonatalmouse hippocampus [J ].Rev Bras Anestesiol ,2019,69(1):58-63.[11]Bi C ,Cai Q ,Shan Y ,et al.Sevoflurane induces neurotoxicity in thedeveloping rat hippocampus by upregulating connexin 43via the JNK /c -Jun /AP -1pathway [J ].Biomed Pharmacother ,2018,108:1469-1476.[12]刚绍鹏,方开云,马熠,等.异氟醚和七氟醚对老年患者术后认知功能的影响[J ].临床麻醉学杂志,2018,34(2):153-155.[13]Mihara R,Takasu A ,Maemura K ,et al.Prolonged severe hemorrhagicshock at a mean arterial pressure of 40mmHg does not lead to brain damage in rats [J ].Acute Med Surg ,2018,5(4):350-357.[14]李永莉,赵婧,刘黎明,等.胰岛素样生长因子-1对全脑缺血大鼠学习记忆能力的影响[J ].中国医药导报,2018,15(31):8-11.[15]Hasegawa K ,Kamiya H ,Morimoto Y.Sevoflurane inhibits presynapticcalcium influx without affecting presynaptic action potentials in hipp-ocampal CA1region [J ].Biomed Res ,2018,39(5):223-230.[16]Swarbrick S ,Wragg N ,Ghosh S ,et al.Systematic Review of miRNA asBiomarkers in Alzheimer's Disease [J ].Mol Neurobiol ,2019,2(8):114-118.(收稿日期:2018-11-09)DOI :10.3969/j.issn.1671-4695.2019.06.003文章编号:1671-4695(2019)06-0568-05大黄酚对IgA 肾病大鼠肾损伤和免疫反应的调控作用龚豪黄丽张庆红李涛史秀岩(十堰市太和医院肾病内科湖北十堰442000)基金项目:湖北省自然科学基金(编号:2015CFA076)【摘要】目的探讨大黄酚对IgA 肾病大鼠肾损伤和免疫反应的影响。

抑郁症与糖尿病共病的运动干预机制--瘦素抵抗假说刘微娜;季浏【摘要】Diabetic patients with depression phenomenon is attracting more and more attention , long‐term use of tricyclic antidepressants and SSRIs will increase the risk of diabetes .Exercise promotes recovery of depressive patients and patients with diabetes ,but the mechanisms under‐lying the exercise intervention still remain unknown .For diabetes patients with co‐morbid de‐pression ,focus should be on downstream of leptin signaling and leptin resistance .JAK2/STAT3 pathway is involved in leptin signaling via leptin receptor (LepRb) phosphorylation , and inflammatory cytokines (ICK) induces IKKβ/NFκB activation .JAK2/STAT3 and IKKβ/NFκB pathway are inhibited by the suppressor of cytokine signaling 3 (SOCS3 ) in leptin re‐sistance .Therefore ,the current study puts forward that LepRb/ICK‐SOCS3 pathway mediates leptin resistance in exercise intervention for comorbidity of depression and diabetes .The pro‐spective findings may contribute to our understanding of the comorbidity between depression and diabetes ,and suggest a new target or guide for pharmacological research and development against metabolic diseases .%糖尿病患者罹患抑郁症现象正吸引越来越多的关注,常用抗抑郁药又会增加糖尿病风险。

异甘草素对创伤性脑损伤大鼠血清细胞因子的影响杨永明;荔志云;季玮【摘要】ObjectiveTo observe the effects of isoliquiritigenin on levels of serum IFNγ, MCP-1, IL-2, IL-4 and IL-13 in rats after traumatic brain injury (TBI). Methods: Forty-five rats were randomly divided into sham-operated, model and treatment groups with 15 rats in each group. The TBI model was established with the modi-fied Feeney's method. In the rats in the sham-operated group the skull was just window-opened but without any blow. After the operation, the animals in treatment group were given isoliquiritigenin, while the rats in the sham-operated and model groups were given the same volume of normal saline. On day 5 after TBI, the serum cytokines were measured and brain water content was determined. The morphology of neurons in hippocampus was observed. Results:When compared with the model group, the level of IFNγ in the treatment group was de-creased ( <0.05), while the levels of MCP-1,IL-2,IL-4 and IL-13 in the treatment group were increased ( <0.05). The water content of brain in the treatment group was decreased compared to that in the model group( <0.05). And compared with the model group, the morphology of hippocampus was improved ( <0.05).Conclu-sion:Isoliquiritigenin can promote brain tissue rehabilitation in rats after TBI, perhaps mediated by modulation of cytokines.%目的:观察异甘草素对创伤性脑损伤大鼠血清干扰素γ(IFNγ)、单核细胞趋化蛋白-1(MCP-1)、白介素-2(IL-2)、IL-4和IL-13的影响。

·4037··指南·共识·【编者按】 2型糖尿病(T 2DM )是一种终身性代谢性疾病,目前没有被治愈的证据,但经研究证实,针对超重和肥胖的T 2DM 患者采取强化生活方式干预、药物治疗(包括强化胰岛素治疗和口服降糖药治疗)以及代谢手术治疗可以实现T 2DM 缓解。
什么是T 2DM 缓解?近期美国糖尿病协会(ADA )发布的《2021 共识报告:缓解2型糖尿病的定义和解释》,将停用降糖药物至少3个月后,糖化血红蛋白<6.5%作为T 2DM 缓解的诊断标准;当糖化血红蛋白不能反映真实血糖水平,可以用空腹血糖<7.0 mmol/L 或通过动态葡萄糖监测计算估计的糖化血红蛋白<6.5%作为诊断T 2DM 缓解的替代标准。
目前我国T 2DM 呈高发趋势,患者承受着心理、身体、社会、经济等多方面的压力,若能实现糖尿病缓解,对于患者及其家庭,乃至整个社会的意义重大。
因此,由邹大进教授、张征教授、纪立农教授牵头组织国内专家,结合国内外研究证据及ADA 的共识报告,制定了一部符合我国糖尿病患者健康需求的《缓解2型糖尿病中国专家共识》,以期指导临床医生规范开展T 2DM 缓解诊疗工作,帮助T 2DM 患者获得安全、有效且经济的干预措施。
缓解2型糖尿病中国专家共识《缓解2型糖尿病中国专家共识》编写专家委员会【摘要】 2型糖尿病(T2DM)是一种以高血糖为特征的进展性疾病,一直被认为需要长期使用降糖药物治疗。
近年来大量研究结果显示,通过生活方式干预、药物治疗以及代谢手术能够促进合并超重和肥胖的T2DM 缓解,使患者在较长时间内免于使用降糖药物。
T2DM 缓解有助于减轻患者心理负担、增强患者依从健康生活方式的信心,提升患者生活质量,远期还可以延缓疾病进展速度,降低终生并发症的发生风险。
为帮助我国临床医生规范开展在超重和肥胖T2DM 患者中缓解T2DM 相关的临床诊疗工作,促进相关研究的发展,使患者获得安全、有效的干预措施,特制定《缓解2型糖尿病中国专家共识》。


他汀类药物减少神经细胞内β-淀粉样多肽的聚集李晓晴;王力;王力锋【期刊名称】《神经损伤与功能重建》【年(卷),期】2012(007)001【摘要】Objective: To investigate the effect of Statins on intracellular Amyloid β(Aβ) aggregation. Methods; SH-SY5Y cells were divided into groups control, transfection, Atorvasta-tin and Simvastatin. The control group received no treatment, and the other 3 groups were trans-fected by plasmid pcDNA3. 0-YFP-C99 to construct Alzheimer's disease (AD) cell models. The Atorvastatin group and Simvastatin group were added Atorvastatin and Simvastatin at different concentration (5,10,20,50,100μmol/L) respectively. The Aβ changes in all the groups were observed with fluorescence microscope and quantitatively analyzed with ELISA assay and Western blot before and 12,24,48 and 72 h after culture. Results: In the pathogenic process of AD, A initially accumulated within the cells. By observation with fluorescence microscopy, intracellular Aβ was significantly reduced by statin treatment in a dose-and time-dependent manner. TheAβ reduction was statistically significant among the groups by 20 βmol/L statin treatment for 24 h (P<0. 05). Conclusion; Aβ aggregation occurs initially in the AD cell model. Different types of statins could decrease A|3 accumulation in a dose- and time-dependent manner.%目的:探讨他汀类药物对β淀粉样多肽(Aβ)生成的影响.方法:将入神经母细胞瘤SH-SY5Y细胞分为对照组、转染组、阿托伐他汀干预组和辛伐他汀干预组,对照组不予任何处理,其它3组通过pcDNA3.0-YFP-C99质粒转染构建阿尔茨海默病(AD)细胞模型,阿托伐他汀干预组和辛伐他汀干预组分别加入不同浓度(5、10、20、50、100 μmol/L)的阿托伐他汀、辛伐他汀.4组均在培养前和培养12、24、48、72 h时,通过荧光显微镜定性观察,ELISA和Western blot定量分析细胞内Aβ的变化.结果:荧光显微镜下观察他汀类药物能显著减少细胞内Aβ聚集.不同浓度的他汀类药物和细胞培养时间均能减少Aβ,呈浓度-时间依赖性.他汀类药物浓度为20 μmol/L和细胞培养24 h时,各组间的Aβ减少有统计学意义(P<0.05).结论:不同种类的他汀类药物能减少细胞内Aβ聚集,并且具有浓度-时间依赖性.【总页数】4页(P9-12)【作者】李晓晴;王力;王力锋【作者单位】首都医科大学附属北京安贞医院神经科,北京100029;首都医科大学附属北京安贞医院神经科,北京100029;首都医科大学附属北京安贞医院神经科,北京100029【正文语种】中文【中图分类】R741;R741.02【相关文献】1.胰岛淀粉样多肽与甘丙肽在家兔和大鼠脊神经节细胞内分布与共存的免疫组织化学研究 [J], 王雪岷;周长满;苏慧慈;张远强;鄂玲玲;单文戈2.大鼠缺血性脑损伤致星形神经胶质细胞内β-淀粉样蛋白聚集与β-分泌酶增加相关 [J], 熊曼;孙凤艳3.利拉鲁肽对人胰岛淀粉样多肽聚集性及胰岛细胞毒性的影响 [J], 赵夕琛;靳育静;孙晓薇;李代清;朱铁虹4.胰岛淀粉样多肽与5-羟色胺、多巴胺在大鼠和家兔背根神经节细胞内共存的免疫组织化学研究 [J], 王雪岷;周长满;苏慧慈;张远强;单文戈;鄂玲玲5.姜黄素通过激活PPARγ和抑制BACE1减少神经细胞内β淀粉样蛋白的产生 [J], 斯露;张雄;张红梅;李昱因版权原因,仅展示原文概要,查看原文内容请购买。

•878•中国老年学杂志2019年2月第39卷神经元的能量代谢耦联作用⑴)等有关,其具体机制有待深入研究。
4参考文献1Nalivaeva NN,Beckett C,Belyaev ND,et al.Are amyloid-degrading enzymes viable therapeutic targets in Alzheimer*s disease]J〕?J Neu-rochem,2012;120(si):167-85.2Shadfar S,Hwang CJ,Lim MS,et al.Involvement of inflammation in Alzheimer*s disease pathogenesis and therapeutic potential of anti-inflammatory agents[J].Arch Pharmacal Res,2015;38(12):2106-19. 3Sawikr Y,Yarla NS,Peluso l,et al.Neuroinflammation in Alzheimer' s disease:the preventive and therapeutic potential of polyphenolic nu-traceuticals(J].Adv Protein Chem Struct Biol,2017;108:33-57.4Laird FM,Cai H,Savonenko AV,ei al.BACE1,a major determinant of selective vulnerability of the brain to amyloid-beta amyloidogenesis, is essential for cognitive,emotional,and synaptic functions]J〕.J Neu-rosci,2005;25(50):11693.5郭义.实验针灸学〔M〕.北京:中国中医药出版社,2008:360.6Liddelow SA,Barres BA.Reactive astrocytes:production,function, and therapeutic potentialf J].Immunity,2017;46(6):957-67.7Kidana K,Tatebe T,Ito K,e/al.Loss of kallikrein-related peptidase7 exacerbates amyloid pathology in Alzheimer's disease model mice (JJ.Embo Molec Med,2018;10(3):e8184.8王琦,周海霞.阿尔茨海默病中0淀粉样蛋白对星形胶质细胞的作用〔J〕•中国老年学杂志,2011;31(18):3643-6.9王玮瑶,占素扬,孙传博,等.L-NMMA对阿尔茨海默病模型大鼠海马齿状回区谷氨酸含量的影响〔J〕.中国老年学杂志,2018;38(9):2197-9.10Funato H,Yoshimura M,Yamazaki T,et al.Astrocytes containing amyloid beta-protein(Abeta)-positive granules are associated with Abeta40-positive diffuse plaques in the aged human brain〔J〕.Am J Pathol,1998;152(4):983-92.11张雪梅,柯开富,方小霞,等.Api42诱导阿尔茨海默病模型大鼠海马内细胞因子表达变化的研究〔J〕.天津医药,2013;41(8):789-92.12D'Anna L,Aburumeileh S,Fabris M,et al.Serum interleukin-10levels correlate with cerebrospinal fluid amyloid beta deposition in Alzheimer disease patients〔J〕.Neurodegen Dis,2017;17(4-5): 227-34.13赵姝,李克深.益智醒脑方联合西医治疗对阿尔茨海默病患者脑脊液炎性因子及神经递质的影响〔J〕.中国免疫学杂志,2018;34(5):699-702.14金涛.星形胶质细胞与阿尔茨海默病〔J〕.国外医学•老年医学分册,2001;22(4):185-7.15Kriegstein A,Alvarez-Buylla A.The glial nature of embryonic and a-dult neural stem cells[J].Ann Rev Neurosci,2009;32:149-84.16艾潇琳,方芳.内源性干细胞在中枢神经系统退行性疾病中的研究进展〔J〕•华西医学,2018;33(3):348-53.17徐磊,王晓冬.星形胶质细胞诱导分化为神经细胞的研究进展〔J〕.神经解剖学杂志,2018;34(3):409-12.18罗程,夏贞焰,赵永华.脑内神经元-星形胶质细胞能量代谢偶联研究进展〔J〕•重庆医学,2018,47(9):1244-7.[2018-12-04修回〕(编辑张慧)复方健耳剂干预小鼠老年性耳蜗感音神经细胞凋亡径路陈思仲I唐俊波'宣伟军I韦璃龙2宣毅'(广西中医药大学1第一临床医学院耳鼻咽喉头颈外科,广西南宁530023;2瑞康医学院制药厂;3 School of Engineering,Tufts University)〔摘要〕目的探讨中药复方健耳剂干预老年性耳蜗感音神经细胞凋亡作用可能径路。

米诺环素抑制大鼠脊髓背角胶状质神经元的超极化激活电流朱梦叶;刘娜娜;彭斯聪;李凌超;张达颖;柳涛【期刊名称】《南方医科大学学报》【年(卷),期】2015(035)008【摘要】Objective To investigate the effect of minocycline on hyperpolarization-activated current (Ih) in the substantia gelatinosa (SG) neurons in rat spinal dorsal horn. Methods In vitro spinal cord transverse slices were prepared from 3-5-week-old male Sprague-Dawley rats. Using whole-cell patch clamp technique, Ih currents were recorded before and after bath application of minocycline (1-300 μmol/L) to the SG neuro ns. Results Ih currents were observed in nearly 50% of the recorded neurons, and were blocked by Ih blocker CsCl and ZD7288. Minocycline rapidly and reversibly reduced the amplitude of Ih and decreased the current density in a concentration-dependent manne r with an IC50 of 34μmol/L. Conclusion Minocycline suppresses the excitability of SG neurons through inhibiting the amplitude and current density of Ih and thereby contributes to pain modulation.%目的研究米诺环素对大鼠脊髓背角胶状质(SG)神经元超极化激活电流(Ih)的影响.方法选取3~5周龄雄性SD大鼠,制作离体脊髓横切片,应用全细胞膜片钳技术记录SG神经元Ih,灌流不同浓度米诺环素(1~300μmol/L)并观察其对Ih的影响.结果记录的SG神经元中约50%可记录到Ih,该电流被Ih阻断剂CsCl和ZD7288阻断.米诺环素可减低Ih幅值、减小Ih电流密度,此效应呈可逆性及剂量依赖性,半数有效抑制浓度(IC50)为34μmol/L.结论米诺环素具有抑制脊髓背角SG神经元Ih的作用,从而降低SG神经元兴奋性,对疼痛的调控具有重要的意义.【总页数】7页(P1155-1161)【作者】朱梦叶;刘娜娜;彭斯聪;李凌超;张达颖;柳涛【作者单位】南昌大学第一附属医院疼痛科,江西南昌 330006;南昌大学第一附属医院儿科,江西南昌 330006;南昌大学第一附属医院儿科,江西南昌 330006;南昌大学第一附属医院疼痛科,江西南昌 330006;南昌大学第一附属医院疼痛科,江西南昌 330006;南昌大学第一附属医院儿科,江西南昌 330006【正文语种】中文【相关文献】1.腰5脊神经结扎大鼠腰4背根神经节神经元超极化激活电流的变化 [J], 梅其勇;邢国刚;李杰;万有2.淫羊藿总黄酮抑制大鼠脊髓背角神经元甘氨酸激活的全细胞电流 [J], 程新萍;朱峰;周可青3.大鼠脊髓背角胶状质神经元的去极化反跳及调控机制 [J], 李凌超;张达颖;彭斯聪;吴静;蒋昌宇;柳涛4.大鼠脊髓背角胶状质神经元去极化反跳参与大鼠\r慢性神经病理性疼痛 [J], 李凌超;朱梦叶;吴艳盈;张达颖5.大鼠背根节神经元后超极化中Ca^(2+)激活K^+电流的蜂毒明肽敏感成分I_(AHP) [J], 陈丽敏;胡三觉;韦耿泽因版权原因,仅展示原文概要,查看原文内容请购买。

中闺新药与临床杂志(ChinJNewDrugsCliaRem),2000年11月,19(6):495498新型非苯二氯蕈类镇静催跟药扎栗警隆李年芳,顾牛范(卜海市精神卫生中心,上海200030)e关毽诃】礼来普隆;GABA-苯并二氨革受体;催眠药和镇静药;药理学;药物动力学f摘要】失眠是一秽常见鲍癌状,镇静催隈药是主要姆治疗手提。
新莺哉来普隆与苯=氯革娄镇静催眠药不离。
它舆寄对受俸作用选择性强、起教快、不曼反应步等特点。
本文就扎采普隆的药理作用、临床应用等作一鲸述。
【中圉挣粪号】R971.3[文献标识秘】A[文章编号】1007—7669(2承J0)06.0495。
04失眠是一种常见的症状,火多数词查发现成年入中发生率在5%~45%之间H’引。
在老年人中失眠更普遍。
正因如此,人们越来越重视其治疗问题。
镇静催眠药是治疗失眠蛙常用的药物,目前用得较多的必苯二蒸蕈豢药物,这类药物作用明显,假可能会gl起一定程度的药物婊竣及寤醉症状。
近几年,新型菲苯二氮摹类镇静催眠药得到较快发曩。
已用于幅床的新药有睦吼垣(zolpidem)及佐匿壳隆(zopiclone)。
由美国惠氐医药公司开发的孛L来普隆(zalepion)为更新的一种镇静催眠药。
药理作用扎来普隆的化学名为N.{3一[3-氰基吡唑(I,5一a)噻啶一7一y1]苯1.N。
乙基己魁胺{N。
[3-3一cyanopyrazolof1,5-a)pyrimidin一7+啦】Ncthytaeimamide}。
结构式冕缀t。
在中枢神经系统,存在2种苯二氮苹(Rz)受体i亚整,即Bzl,Bz2或者埘1,嗡受体。
Bz】受体主要、位于与镇静作用有关的大脑区域,H品受体主要集中于与认知、记忆、精神运动作用有关的区域13J。
、大多数苯二氮革类药物对受体的作熙并无选择性,j这榉蘸不可避免地;l起耱秘神经系统不良反应。
掰。
型扮非苯二二氮革类镇静摧瞩药不具祷苯二氮蕈类药。
静的一些不丧蕊虚,这类药物造择髓作沼予丫_氨基、丁馥一受俸复合俸(GABk"受体复台体)上的BZ受鼙】扎来警盛的分手绪棒式体。
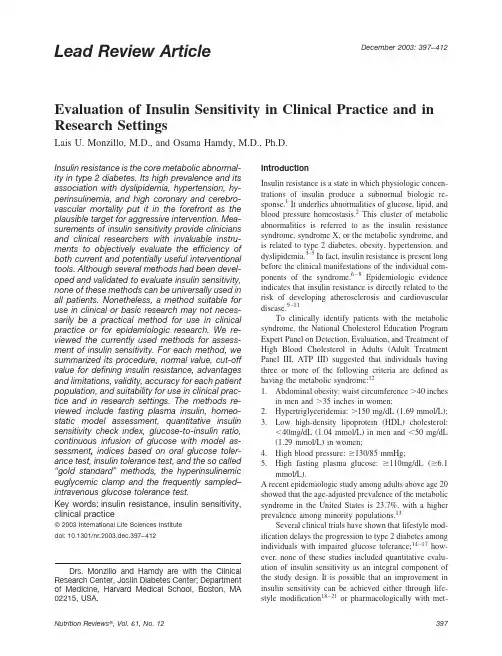
December2003:397–412 Lead Review ArticleEvaluation of Insulin Sensitivity in Clinical Practice and in Research SettingsLais U.Monzillo,M.D.,and Osama Hamdy,M.D.,Ph.D.Insulin resistance is the core metabolic abnormal-ity in type2diabetes.Its high prevalence and its association with dyslipidemia,hypertension,hy-perinsulinemia,and high coronary and cerebro-vascular mortality put it in the forefront as the plausible target for aggressive intervention.Mea-surements of insulin sensitivity provide clinicians and clinical researchers with invaluable instru-ments to objectively evaluate the efficiency of both current and potentially useful interventional tools.Although several methods had been devel-oped and validated to evaluate insulin sensitivity, none of these methods can be universally used in all patients.Nonetheless,a method suitable for use in clinical or basic research may not neces-sarily be a practical method for use in clinical practice or for epidemiologic research.We re-viewed the currently used methods for assess-ment of insulin sensitivity.For each method,we summarized its procedure,normal value,cut-off value for defining insulin resistance,advantages and limitations,validity,accuracy for each patient population,and suitability for use in clinical prac-tice and in research settings.The methods re-viewed include fasting plasma insulin,homeo-static model assessment,quantitative insulin sensitivity check index,glucose-to-insulin ratio, continuous infusion of glucose with model as-sessment,indices based on oral glucose toler-ance test,insulin tolerance test,and the so called “gold standard”methods,the hyperinsulinemic euglycemic clamp and the frequently sampled–intravenous glucose tolerance test.Key words:insulin resistance,insulin sensitivity, clinical practice©2003International Life Sciences Institutedoi:10.1301/nr.2003.dec.397–412IntroductionInsulin resistance is a state in which physiologic concen-trations of insulin produce a subnormal biologic re-sponse.1It underlies abnormalities of glucose,lipid,and blood pressure homeostasis.2This cluster of metabolic abnormalities is referred to as the insulin resistance syndrome,syndrome X,or the metabolic syndrome,and is related to type2diabetes,obesity,hypertension,and dyslipidemia.3–5In fact,insulin resistance is present long before the clinical manifestations of the individual com-ponents of the syndrome.6–8Epidemiologic evidence indicates that insulin resistance is directly related to the risk of developing atherosclerosis and cardiovascular disease.9–11To clinically identify patients with the metabolic syndrome,the National Cholesterol Education Program Expert Panel on Detection,Evaluation,and Treatment of High Blood Cholesterol in Adults(Adult Treatment Panel III,ATP III)suggested that individuals having three or more of the following criteria are defined as having the metabolic syndrome:121.Abdominal obesity:waist circumferenceϾ40inchesin men andϾ35inches in women;2.Hypertriglyceridemia:Ͼ150mg/dL(1.69mmol/L);3.Low high-density lipoprotein(HDL)cholesterol:Ͻ40mg/dL(1.04mmol/L)in men andϽ50mg/dL(1.29mmol/L)in women;4.High blood pressure:Ն130/85mmHg;5.High fasting plasma glucose:Ն110mg/dL(Ն6.1mmol/L).A recent epidemiologic study among adults above age20 showed that the age-adjusted prevalence of the metabolic syndrome in the United States is23.7%,with a higher prevalence among minority populations.13Several clinical trials have shown that lifestyle mod-ification delays the progression to type2diabetes among individuals with impaired glucose tolerance;14–17how-ever,none of these studies included quantitative evalu-ation of insulin sensitivity as an integral component of the study design.It is possible that an improvement in insulin sensitivity can be achieved either through life-style modification18–21or pharmacologically with met-Drs.Monzillo and Hamdy are with the Clinical Research Center,Joslin Diabetes Center;Department of Medicine,Harvard Medical School,Boston,MA 02215,USA.formin22,23or thiazolidinediones.24–26The Food and Drug Association(FDA)has not approved either of these pharmacologic compounds for treatment of insulin resis-tance in nondiabetic individuals;however,the diagnosis of type2diabetes,hypertension,and dyslipidemia man-dates aggressive appropriate treatment with antidiabetic, blood pressure–lowering,and lipid-lowering agents aimed at reducing cardiovascular morbidity and mortal-ity.The rapidly growing epidemic of obesity and con-sequent insulin resistance has increased the interest in finding quantitative,accurate,and easy methods to eval-uate insulin sensitivity in both clinical research and clinical practice.Such a tool is not only useful for early identification of insulin resistance but also to assess the degree of success in treating this syndrome and its consequences.This review will summarize our current knowledge of the available methods used to evaluate insulin sensitivity in humans.The components of each method,its indications,and its limitations are discussed. Fasting Plasma Insulin ConcentrationOne of the most practical ways to estimate insulin resis-tance from the clinical perspective is to measure plasma insulin concentration after an overnight fast.As it is inexpensive and easy to do,it has been used in several population-based studies.27–30Very high plasma insulin values reflect the presence of insulin resistance.Despite the relatively good correlation between fasting plasma insulin and insulin sensitivity derived from the hyperin-sulinemic euglycemic clamp,measures of fasting plasma insulin explain no more than5to50%of the variability in insulin action seen in nondiabetic subjects.31,32This is because plasma insulin levels depend not only on insulin sensitivity,but also on insulin secretion,distribution,and degradation.33Moreover,with the development of diabetes,fasting plasma insulin levels tend to decrease owing to beta cell dysfunction.Therefore,plasma insulin levels in diabetic patients are valid reflection of both target tissue insulin resistance and diminishing insulin production.34This explains why fasting plasma insulin levels may accu-rately predict insulin sensitivity among normoglycemic patients than among those with impaired glucose toler-ance(IGT)or type2diabetes.32,35,36Another limitation to using fasting plasma insulin to predict insulin resis-tance is cross-reactivity between insulin and proinsulin. Proinsulin levels are high among insulin-resistant sub-jects with type2diabetes and IGT,37,38but not in people who are insulin resistant and normoglycemic.39 The commonly used radioimmunoassay(RIA) method has a lower specificity and sensitivity,and a higher interassay coefficient of variation,when com-pared with the two-site monoclonal antibody-based in-sulin assay methods(immuno-radiometric[IRMA],im-muno-enzymometric[IEMA],and immuno-fluorimetric [IFMA])methods.40,41The presence of anti-insulin an-tibodies in type1and type2diabetic patients,who are treated with human or animal insulin,can interfere with both the RIA and two-site monoclonal assay,unless removal of anti-insulin antibodies and antibody-bound insulin is performed.41,42The normal range for insulin levels using RIA is3to 32mU/L.43,44However,there is no defined cut-off value indicating insulin resistance.This lack of consensus stems partly from the various means used to define abnormal.In a population-based study examining the association between insulin levels and cardiovascular risk,Lindahl et al.8defined insulin resistance as a plasma insulin levelϾ7.2mU/ing the hyperinsulinemic euglycemic clamp as the reference standard,McAuley et al.45found that a fasting insulinϾ12.2mU/L predicted insulin resistance among normoglycemic adults. Laakso,32also using the hyperinsulinemic clamp in nor-moglycemic adults,arrived at a cut-off of18mU/L. Finally,defining the abnormal range as the upper10% percentile,Ascaso et al.46defined insulin resistance in nondiabetic individuals when plasma insulin levels were equal or greater than16.7mU/l(Table1).While these variations illustrate how study designs influences what insulin level is determined to represent insulin resistance, the lack of established standards for insulin assay proce-dures further complicates the issue.47Another limitation for measurement of fasting plasma insulin is the pulsatile mode of insulin secretion (pulses with a periodicity of10–15minutes,and ultra-dian oscillations periods of1to3hours).The periodicity, amplitude,and ultradian oscillations of insulin pulsesparison of Fasting Plasma Insulin Values and Insulin Assays Used to Assess Insulin Sensitivity in Different StudiesStudy Year Population Insulin Assay Insulin Resist Value Lindahl et al.81993General population RIAϾ7.2mU/L McAuley et al.452001General population RIAϾ12.2mU/L Laakso et al.321992Normoglycemic RIAϾ18mU/L Ascaso et al.462001Normoglycemic RIAՆ16.7mU/L RIAϭradioimmunoassay.vary in the fasting state,and are altered in IGT and in type2diabetes.41Because of these limitations,fasting plasma insulin levels are of limited value for clinical purposes,but have some utility as a research tool in population-based studies.The Homeostasis Model Assessment(HOMA)Because fasting insulin per se does not provide an accu-rate measure of insulin sensitivity in diabetic patients, efforts have been made to incorporate fasting plasma glucose in a formula to arrive at a better estimate of insulin-sensitivity.HOMA was developed by Matthews et al.48as a method for estimating insulin sensitivity from fasting serum insulin(FI)and fasting plasma glu-cose(FG)using the following mathematic formula: HOMA Insulin Resistance(HOMA IR)ϭFIϫFG/22.5FI is measured inU/mL and FG is measured in mmol/L.Low HOMA IR indicates high insulin sensitiv-ity,whereas high HOMA IR indicates low insulin sensi-tivity.In their original report,Matthews et al.found HOMA IR ranges between1.21and1.45in normal sub-jects and between 2.61and 2.89in insulin-resistant diabetic subjects.48However,further epidemiologic studies performed in the general population reported higher HOMA IR values of2.1,442.7,31and3.8.46 Because fasting insulin is a major component of the HOMA IR calculation,all previously mentioned limita-tions should apply to this formula.Three samples for fasting plasma insulin should be drawn5minutes apart to avoid errors that may arise owing to the pulsatile nature of insulin secretion.However,most studies use only one basal insulin measurement to calculate HOMA IR.HOMA IR correlates well with the glucose disposal rate derived from the hyperinsulinemic euglycemic clamp.49–53In addition,two authors found a good cor-relation between the HOMA IR and the insulin sensitivity index(S i)derived from the frequently sampled intrave-nous glucose tolerance test(FSIVGT).54,55By contrast, Anderson et al.35failed to demonstrate a good correla-tion between the two.Furthermore,some of the studies that initially demonstrated significant correlation be-tween the HOMA IR and the clamp-derived insulin sen-sitivity used a low insulin infusion rate of20 mU⅐m2Ϫ1⅐minuteϪ1during the clamp,which might not have completely suppressed the hepatic glucose pro-duction and may have created an error in calculating theglucose uptake by peripheral tissues.51,52One of the limitations of HOMA IR is the model assumption that insulin sensitivity in the liver and pe-ripheral tissues are equivalent,whereas it is known that they can differ considerably in the same individual.50 Furthermore,some data suggest that the accuracy of HOMA IR is limited by hyperglycemia.Those studies that demonstrated good correlations between HOMA IR and the clamp-derived insulin sensitivity in diabetic patients tended to enroll patients without significant hyperglyce-mia.48–50,52,53Mari et al.56failed to show a significant correlation between HOMA IR and clamp in type2dia-betic patients with higher glucose levels(mean basal plasma glucose of205mg/dL).In addition,Anderson et al.35and Brun et al.57found that the correlation between HOMA IR and S i derived from the FSIVGT weakened as glycemia increased.These results suggest a non-linear relationship between S i and HOMA IR.The coefficient of variation(CV)for HOMA IR is as high as31%,48which limits its use in clinical practice and clinical research.47Optimizing sample size and in-sulin assay method reduceHOMA IR CV to8to12%.49,51 In conclusion,HOMA IR is mostly useful for the evaluation of insulin sensitivity in euglycemic individu-als and in persons with mild diabetes;however,this index appears to offer little or no advantage over the fasting insulin concentration alone.31,45,58In patients with severe hyperglycemia or in lean diabetic patients with beta cell dysfunction,the HOMA IR may not be accurate.Its usefulness should therefore be restricted to large population-based studies that require a simple method to assess insulin sensitivity.Quantitative Insulin Sensitivity Check Index (QUICKI)QUICKI is another mathematic model available to esti-mate insulin sensitivity.59QUICKIϭ1/[log(I0)ϩlog(G0)],where I0is the fasting plasma insulin level inU/mL, and G0is the fasting plasma glucose level in mg/dL.The mean QUICKI for lean,obese,and obese-diabetic sub-jects are0.382,0.331,and0.304,respectively.59Al-though other studies have found a similar range for a normal healthy population of0.372and0.366,60,61one study showed a wider range between0.265and0.518.62 The mathematic difference between the QUICKI and the HOMA IR is that the former uses the reciprocal of the logarithm of both glucose and insulin to account for the skewed distribution of fasting insulin values.As expected,there is very good correlation between QUICKI and HOMA IR,63especially when the HOMA IR is log-transformed.59,62,64,65Although two studies failed to demonstrate any real advantage of QUICKI when compared with log HOMA IR,62,65other studies argue that QUICKI has the advantage of being applied to wider ranges of insulin sensitivity.61,63,64QUICKI was also shown to correlate well with the FSIVGT66and the hyperinsulinemic eu-glycemic clamp.58However,the correlation is weakerwhen insulin levels were low,as seen in non-obese insulin-sensitive subjects and diabetic patients with di-minished insulin production;59,60,62,65,67,68this is be-cause low insulin levels lead to variability in determined insulin concentrations and because of the oscillatory pattern of insulin secretion in healthy individuals.Other limitations to this mathematic method include its limited applicability for type1diabetic patients owing to lack of endogenous insulin secretion,59and its inaccuracy if conducted following exercise training.67In conclusion,the QUICKI may be a useful and simple tool for assessing insulin sensitivity in epidemi-ologic settings;it may offer some advantage over the HOMA IR,especially in obese and diabetic individuals with relatively preserved beta cell function.However, the model needs validation in a wider range of subjects with different glucose tolerance patterns in order to confirm its reliability for use in clinical practice and in research settings.Fasting Plasma Glucose-to-Insulin Ratio(G/I)G/I is another mathematic method that uses fasting plasma insulin and fasting plasma glucose to estimate insulin sensitivity.The higher the ratio,the more insulin-resistant an individual is.The index generally correlates well with other indi-ces of insulin sensitivity.1,45,69–75It correlated with in-sulin sensitivity indices derived from the oral glucose tolerance test(OGTT,rϭ0.82,PϽ0.05),1,71and FSIVGT(rϭ0,76,PϽ0.001).1,69,72Vuguin et al.72 found that a fasting G/I ratioϽ7provided87%sensitiv-ity and89%specificity for identifying low insulin sen-sitivity in young girls with premature adrenarche.In another study of white nondiabetic women with polycys-tic ovarian syndrome(PCOS),Legro et al.69found the G/I ratio to be the best screening test for insulin resis-tance.The authors showed that a cut-offϽ4.5provided an87%positive predictive value and94%negative predictive value in screening for insulin resistance in PCOS.G/I ratio was found to correlate well with HOMA IR(rϭ0.83,PϽ0.01),fasting insulin(rϭ0.95, PϽ0.001),73and QUICKI(rϭ0.91,PϽ0.0001)74in healthy individuals.Data on the correlation between G/I ratio and insulin sensitivity derived from the euglycemic clamp procedure are inconsistent;whereas two studies found a significant correlation,1,45another did not.50 Adding to the previously mentioned problems that in-clude precision of insulin assay,pulsatile pattern of insulin secretion,and cross reactivity with proinsulin,the major problem with using the G/I ratio is its inaccuracy in diabetic patients owing to defects in insulin secretion and high plasma fasting glucose.1,50,70,76In subjects with normoglycemia,G/I ratio offered little advantage over the1/insulin measure76or fasting insulin.45Moreover,it provides indirect information on whole-body sensitivitybut not on the effect of insulin in peripheral tissues.1Inconclusion,this index,like the previously describedindices,should be limited to the nondiabetic population.For research purposes,its superiority over the fastinginsulin is questionable.Continuous Infusion of Glucose with Model Assessment(CIGMA)Because of the inaccuracy that may result from low basalinsulin concentrations,an alternative mathematic methodwas proposed.This method assesses insulin sensitivitythrough the evaluation of the near–steady state glucoseand insulin concentrations after a continuous infusion ofglucose with model assessment.77This procedure mim-ics postprandial glucose and insulin concentrations.CIGMA not only provides information about glucosetolerance and insulin sensitivity,but also about beta celling a mathematic model of glucose ho-meostasis,glucose and insulin values are compared withknown physiologic data of glucose and insulin kinetics inresponse to glucose infusion that are derived fromhealthy lean subjects with no family history of diabetes.The glucose and insulin values used for CIGMA areobtained during the last15minutes of the60-minutecontinuous glucose infusion(5mg glucose⅐kg idealbody weightϪ1⅐minuteϪ1).Samples are collected at five-minute intervals,to avoid the oscillatory variation ininsulin concentration.The average is then compared withpredicted values from the computer model.The medianvalue for normal subjects is1.35and for diabetic patientswith mild hyperglycemia is4.0.77Although CIGMA has been used in several studiesto evaluate insulin resistance,78–83few studies havecompared CIGMA with other insulin sensitivity indices.In elderly normoglycemic patients,CIGMA significantlycorrelated with mean fasting plasma insulin concentra-tions.84Hermans et al.55compared CIGMA,HOMA IR,FSIVGT,and the insulin tolerance test(ITT),in subjectswith glucose tolerance ranging from normal to frankdiabetes.They found that CIGMA and HOMA IR wereable to discriminate differences in insulin sensitivityamong subjects as well as the FSIVGT and better thanthe ITT.Among the four methods,CIGMA was the bestdiscriminatory test in precision analysis.It is worthmentioning that CIGMA in this study derived from a2-hour test(compared with the original1-hour CIGMA).Other studies have also reported data from2-hourCIGMA.85,86Data aiming to validate CIGMA against the clamp-derived insulin sensitivity index are scarce.In the orig-inal article,CIGMA was shown to correlate well with theeuglycemic hyperinsulinemic clamp(rϭ0.87,P Ͻ0.0001)77in normal subjects and in diabetic patientswith mild hyperglycemia.However,the relationship be-tween CIGMA and the clamp was nonlinear for diabetic patients with severe insulin resistance.Nijpels et al.70 studied90subjects,most of them with normal or im-paired glucose tolerance,and found a modest correlation between CIGMA and the clamp-derived insulin sensitiv-ity(rϭ0.66;PϽ0.05).The CV of CIGMA ranges between17%84and20%.77There are two main advantages of CIGMA over HOMA IR.First,the insulin values that are measured in CIGMA are much higher than those in HOMA IR owing to the glucose stimulus;therefore,the high insulin inter-assay CV(10–15%)41,47that is problematic at low insu-lin a concentration is avoided.55Second,higher insulin concentration in CIGMA stimulates peripheral glucose uptake producing a steady-state glucose concentration, which is a better reflection of the peripheral insulin sensitivity.Although CIGMA is more physiologic,practical, cheaper,and less invasive than the FSIVGT and clamp procedure,the model incorrectly assumes that levels of insulin resistance at the liver and peripheral tissues are equal.Furthermore,in insulin-deficient subjects,where the insulin response is insufficient to stimulate glucose uptake,the interpretation of CIGMA is difficult.33As CIGMA is a procedure and not a simple test such as fasting insulin or the HOMA IR,its use in clinical practice is limited.Moreover,due to insufficient data comparing CIGMA against the“gold standard”euglycemic hyper-insulinemic clamp,its use in research settings should also be viewed with caution.The Oral Glucose Tolerance Test(OGTT) Because oral glucose tolerance is in part determined by sensitivity of peripheral tissues to insulin,the OGTT has been used to evaluate insulin release and the sensitivity of the peripheral tissue to the insulin action.Being a less costly and less labor-intensive procedure compared with the FSIVGT and the euglycemic clamp,the OGTT has been considered a practical method for epidemiologic studies,58for population screening,and for large-scale intervention trials.50,63,87Several indices to estimate in-sulin sensitivity have been derived from the four samples of insulin and glucose(0,30,60,and120minutes)taken after ingestion of75grams of glucose(Table2). Insulin Sensitivity Indices Based on the OGTT Levine et al.88was one of thefirst authors to use the product of the area under the curve for glucose(AUC G) and the area under the curve for insulin(AUC I)during the OGTT to derive an estimate of insulin sensitivity. Later,AUC I was used alone as an estimate.31,36,89Cederholm and Wibell Index90SIϭM/G⅐log I,where Mϭglucose load/120ϩ(0-h plasma glucose concentration–2-h plasma glucose concentration)ϫ1.15ϫ180ϫ0.19ϫbody weight/120;where Gϭmean plasma glucose concentration,and Iϭmean serum insulin.A normal reference value is79Ϯ14.Gutt et al.Index91ISI0,120ϭMCR/log MSI(mean serum insulin),uses the fasting(0min)and120min post-load insulin and glucose concentrations,where MCR(metabolic clear-ance rate)is m/MPG(mean plasma glucose),where mϭ(75000mgϩ[0min glucose–120min glucose]ϫ0.19ϫbody weight)/120min.The reference range for lean controls was89Ϯ39,for obese58Ϯ23,for IGT 46Ϯ12,and for diabetic patients23Ϯ19.Avignon et al.Index92Sibϭ108/(IϫGϫVD)⅐(normal rangeϭ11.99Ϯ1.43)Si2hϭ108/(I2hϫG2hϫVD)⅐(normal rangeϭ1.79Ϯ0.33), where Iϭfasting insulin,Gϭfasting plasma glucose, G2h and I2hϭplasma glucose and insulin at the second hour of the OGTT,and VDϭvolume distribution(150 mL/kg of body weight).An additional insulin sensitivity index(S i M)was derived by the average of the2,after multiplying S i b by a correcting factor:SiMϭ[(0.137ϫSib)ϩSi2h]/2(normal rangeϭ1.71Ϯ0.24). Matsuda et al.Index50ISI(composite)ϭ10,000/͙(FPGϫFPI)ϫ(GϫI), where FPGϭfasting plasma glucose,FPIϭfasting plasma insulin,and Gϭmean plasma glucose,and Iϭmean plasma insulin concentration.Belfiore et al.Index93ISIϭ2/(INSpϫGLYp)ϩ1,where INSp and GLYp are the insulinemic and glycemic areas of the person under study recorded during OGTT. Reference value in normal controls was around1,butmarkedly reduced in the obese and obese-diabetic sub-groups.Stumvoll et al.Index94MCR est(OGTT)ϭ18.8Ϫ0.271BMIϪ0.0052ϫI120Ϫ0.27ϫG90, where MCR est stands for metabolic clearance rate estimate derived from the OGTT,BMIϭbody mass index,I120ϭplasma insulin at120minutes OGTT,and G90ϭplasma glucose at90minutes OGTT.Mari et al.Index56OGIS180ϭ[637106(G(120)Ϫ90)ϩ1]Cl ogtt, where OGIS180ϭoral glucose insulin sensitivity,G120ϭplasma glucose at2h OGTT,andCl ogttϭ289DoϪ104[G(180)ϪG(120)/60]G(120)ϩ14.0103G(0)440I(120)ϪI(0)ϩ270, where Clϭglucose clearance in mL⅐minϪ1⅐mϪ2, Doϭoral glucose dose in g/m2,G(120)ϭplasmaTable2.OGTT-derived Indices to Estimate Insulin Sensitivity and their Correlation with the Euglycemic Hyperinsulinemic Clamp or Frequently Sampled Intravenous Glucose Tolerance Test(FSIVGT)in Various PopulationsFormulae Subjects Correlation with 1.AUC I NGT Euglycemic clamp89rϭ0.61,Pϭ0.001IST31rϭ0.79,PϽ0.001AUC II30min I2hrG30min G2hr NGT,IGT ITT36rϭϪ0.51,PϽ0.001rϭϪ0.43,PϽ0.001rϭϪ0.39,PϽ0.001rϭϪ0.28,Pϭ0.01rϭϪ0.38,PϽ0.0012.SIϭMGϫlog INGT,IGT,DMEuglycemic clamp90rϭ0.62,PϽ0.00013.ISI0,120ϭMCR/log MSI NGT,IGT,DM Euglycemic clamp91rϭ0.63,PϽ0.001 4.Sibϭ108/(f Iϫf GϫVD)NGT,IGT,DM FSIVGT92Si2hϭ108(I2hϫG2hϫVD)rϭ0.90,PϽ0.0001 SiMϭ[(0.137ϫSib)ϩSi2h]/25.ISI(Comp)ϭ10,000͙(FPGϫFPI)ϫ(GϫI)NGT,IGT,DMEuglycemic clamp50rϭ0.73,PϽ0.00016.ISIϭ2(INSpϫGLYp)ϩ1NGT,O,ODMEuglycemic clamp93rϭ0.96,PϽ0.0017.MCRestϭ18.8Ϫ0.271BMIϪ0.0052ϫI120Ϫ0.27ϫG90NGT,IGT Euglycemic clamp94rϭ0.80;PϽ0.000058.OGIS180ϭ[637106(G{120}Ϫ90)ϩ1]Cl ogtt L,O,IGT,DM Euglycemic clamp56rϭ0.73;PϽ0.0001 AUC Iϭarea under the insulin curve,NGTϭnormal glucose tolerance,IGTϭimpaired glucose tolerance,I30minϭ30minutes post-load insulin,I2hrϭ2hours post-load insulin,G30minϭ30minutes post-load glucose,G2hrϭ2hour post-load glucose, ITTϭinsulin tolerance test,SIϭinsulin sensitivity,Mϭglucose uptake rate in mg⅐minϪ1,Gϭmean glucose concentration,Iϭmean insulin concentration,DMϭtype2diabetes,ISI0,120ϭindex of insulin sensitivity from fasting and120minutes post OGTT insulin and glucose concentrations,MCRϭmetabolic clearance rate,MSIϭmean serum insulin,Sibϭinsulin sensitivity in the basal state,Si2hϭinsulin sensitivity at the second hour,f Iϭfasting insulin concentration,f Gϭfasting glucose concentration,VDϭ150mL/kg of body weight,SiMϭinsulin sensitivity index,ISI(Comp)ϭcomposite whole-body insulin sensitivity index,FPGϭfasting plasma g glucose,FPIϭfasting plasma insulin,Gϭglucose,Iϭinsulin,ISIϭinsulin sensitivity index,INSpϭinsulinemic area,GLYpϭglycemic area,MCRestϭmetabolic clearance rate estimate,OGISϭoral glucose insulin sensitivity,Doϭoral dose glucose,Cl ogttϭglucose clearance.glucose at120minutes OGTT,G(180)ϭplasma glucose at180minutes OGTT,G(0)ϭfasting plasma glucose, I(120)ϭinsulin levels at120minutes,and I(0)ϭfasting insulin.Reference values in lean controls ranged300–600mL⅐minϪ1⅐mϪ2.As shown in Table2,the insulin sensitivity mea-sures derived from these formulas correlate well with insulin sensitivity determined by the euglycemic clamp50,89,90,93and FSIVGT.93However,the correlation was weaker in type2diabetic patients50,92,94and in the IGT group.36,58Belfiore et al.93advocate that their for-mula should not be used in type2diabetic patients with significant insulin deficiency.On the other hand,Mari et al.formula(OGIS),56showed a positive correlation with the clamp data in type2diabetic patients(rϭ0.49,P Ͻ0.002).In addition to the inadequacy of this method in insulin deficient states,other problems should be consid-ered.First,during the oral glucose tolerance test suppres-sion of hepatic glucose production is minimal,confound-ing interpretation of the plasma glucose level.Thus,it is impossible to differentiate among whole-body,periph-eral,or hepatic insulin sensitivity separately using data from the OGTT.49Second,the insulin level achieved in response to an oral glucose load involves gut hormones, neural stimulation,and of course the integrity of the pancreatic beta cells.68For example it has been shown that after75grams of glucose,obese subjects exhibit insulin hypersecretion,95while type2diabetes patients show a blunted response.96Third,glucose homeostasis in the postprandial state depends partly on the suppression of glucagon secretion and partly on the rate of entry of ingested glucose into the circulation.This rate is deter-mined by the rate of gastric emptying and splanchnic glucose uptake.60,61Fourth,the OGTT is poorly repro-ducible.Several studies show only about50to65% reproducibility of the results of an OGTT.63,97,98 Despite these limitations,the OGTT may be used in clinical settings to assess insulin action and in large-scale clinical and epidemiologic studies.However,the glucose and insulin excursions in the OGTT should be inter-preted with caution in populations with varying glucose tolerance.The Insulin Tolerance Test(ITT)ITT was one of thefirst methods developed to assess insulin sensitivity in vivo.99In this method,afixed bolus of regular insulin(0.1U/kg body weight)is given intra-venously after an8-to10-hour fast.The plasma glucose decrement over60minutes is then measured.The faster the decline in glucose concentration,the more insulin sensitive the subject is.The slope of the linear decline in plasma glucose(K ITT)can be calculated by dividing 0.693by the plasma glucose half-time(50%from base-line,Figure1).100K ITTϭ0.693/t1/2ϫ100,where t1/2represents the half-life of plasma glucose decrease.Normal K ITT isϾ2.0%/minute and values Ͻ1.5are considered abnormal.This method gives an indirect estimate of overall insulin sensitivity.It has been shown to correlate with the euglycemic clamp(rϭ0.811,PϽ0.001)101in several studies.101–104Some of the drawbacks of this method include the supraphysi-ologic insulin dose used,102and also the fact that the test does not differentiate peripheral versus hepatic insulin resistance.A major limitation of this test is the risk of hypo-glycemia,particularly in normoglycemic subjects and in elderly diabetic patients.Moreover,hypoglycemia trig-gers counterregulatory hormonal responses,which may interfere with insulin sensitivity.A lower insulin dose method of0.05units/kg,or shortening the test to15 minutes was suggested as an attempt to decrease the risk of hypoglycemia.105–107The lower dose ITT has also been shown to correlate well with the clamp.105How-ever,some studies failed to demonstrate reduction of the risk of hypoglycemia in insulin sensitive sub-jects.55,108,109They also showed a higher CV(16and 31%)in comparison to the conventional dose ITT(6–9% CV).101,103,104,110The shorter version101,103evolved from the notion that the counterregulatory hormone re-sponse occurs only after20minutes of the insulin infu-sion.111–113The short ITT yielded a good correlation with the euglycemic clamp101,103,105and has been used in most of the recent studies.114–117In conclusion,the ITT should be used with great caution in insulin sensitive individuals because of the increased risk of hypoglycemia,even when thesmallerFigure1.Calculation of the KITT(percentage decline in plasma glucose concentration per minute)in nondiabetic subjects.100 The time(t1⁄2)required for the plasma glucose concentration to decline by50%(i.e.,from90to45mg/dL)was25minutes.From the equation,KITTϭ0.693/t1⁄2ϫ100,the K rate was determined to be2.77%.。

DOI:10.13325/ki.acta.nutr.sin.2008.04.025营养学报2008年第30卷第4期 397大豆异黄酮联合叶酸对β淀粉样肽介导的神经毒性的影响—对大鼠学习记忆损伤的拮抗作用肖 荣,李 丽,余焕玲,张 杰,向 丽,段一凡(首都医科大学公共卫生与家庭医学学院,北京 100069)【摘 要】目的 探讨大豆异黄酮(SIF)单独或联合叶酸(FA)拮抗β淀粉样肽(Aβ)致大鼠学习记忆损伤的神经保护作用及其机制。
方法(1)神经毒性研究 Wistar大鼠随机分为4组,每组15只,分别于侧脑室注射生理盐水、Aβ25-35、Aβ31-35和Aβ1-40。
于术后7d,Morris水迷宫试验检测大鼠学习记忆能力;术后14d,免疫组织化学法检测大脑Aβ阳性表达细胞数及脑组织中抗氧化相关指标,观察不同Aβ片段的神经毒性。
(2)干预研究 实验分为5组(对照、Aβ模型、SIF、FA、SIF与SIF+FA干预组),每组15只,分别灌胃0.5%羧甲基纤维素钠(CMC-Na)、0.5%的CMC-Na、SIF(160 mg/kg bw •d)、FA (0.7mg/kg bw •d)和SIF(160 mg/kg bw •d)+FA(0.7mg/kg bw •d),14d后,对照组于侧脑室注射生理盐水,Aβ模型、SIF、FA、SIF+FA干预组于侧脑室注射Aβ1-40,检测指标同毒性实验。
结果 (1)与对照组相比,Aβ25-35、Aβ1-40组大鼠平均逃避潜伏期和总路程显著延长;Aβ25-35、Aβ31-35和Aβ1-40组大鼠大脑皮质Aβ阳性表达细胞数显著增加,Aβ31-35和Aβ1-40组大鼠海马CA1区Aβ阳性表达细胞数显著增加;脑组织中AOC、GSH水平显著降低。
(2)与模型组相比,SIF、FA和SIF+FA干预组大鼠平均逃避潜伏期显著缩短;大脑皮质和海马CA1区Aβ阳性表达细胞数显著减少;脑组织中AOC和GSH水平显著增高。
胰岛素抵抗与海马、内嗅皮层体积对轻度认知功能障碍的临床意义宋艳;谈跃【期刊名称】《西南国防医药》【年(卷),期】2012(022)011【总页数】2页(P1266-1267)【关键词】胰岛素抵抗;海马;内嗅皮层;认知功能障碍【作者】宋艳;谈跃【作者单位】650032,昆明,昆明医学院研究生部;昆明医学院附属第二医院;昆明医学院附属第二医院【正文语种】中文【中图分类】R742轻度认知功能障碍(mild cognitive impairment,MCI)是指老年人有轻度认知功能及记忆功能的损害,是介于正常衰老与阿尔茨海默病(Alzheimer disease,AD)之间的状态。
相较于普通衰老人群而言,MCI向AD的转化率明显增高〔1〕。
相关研究表明,MCI向AD转化的年转化率为6% ~25%〔2〕,而糖尿病患者MCI的发生率高达32.7%〔3〕。
在影响认知功能的众多因素中,胰岛素抵抗受到的关注日益增多,其与认知功能相关联的海马、内嗅皮层的关系,为临床MCI向AD发展提供了新的切入点。
现对胰岛素抵抗、海马、内嗅皮层体积变化的研究进展综述如下。
1 胰岛素抵抗与MCI胰岛素抵抗(insulin resistance,IR)是指各种原因使胰岛素作用的靶组织(主要为肝、脂肪、血管内皮细胞等)对胰岛素促进其摄取和利用的效率和作用降低,即对胰岛素的生物学反应低于正常水平。
在IR早期,胰岛β细胞为维持血糖的正常水平而代偿性分泌过多的胰岛素(即高胰岛素血症)。
IR是普遍存在于多种生理和病理状态中的现象,如青年期、老年、妊娠,均可有不同程度的胰岛素敏感指数的降低,即IR。
但当出现体重增加、血糖、血压升高、血脂紊乱、高尿酸血症时,IR的发生率和严重程度均明显增加,引起各种代谢异常及IR综合征(包括IR、肥胖、高血压、高TG和低HDL-C)的发生,该综合征实质上是一系列心脑血管疾病危险因子的聚集状态,患者是心脑血管疾病的高危人群。
英夫利昔单抗治疗伴有葡萄膜炎的慢性囊样黄斑水肿Markomichelakis N.N.;Theodossiadis P.G.;Pantelia E.;陈立军【期刊名称】《世界核心医学期刊文摘:眼科学分册》【年(卷),期】2005(000)004【摘要】To assess the efficacy of the anti- TNF monoclonal antibody infliximab in uveitis patients without clinically evident ocular inflammation and impaired visual acuity because of chronic cystoid macular edema (CME). Prospective, noncomparative, interventional case series. Patients with refractory CME (14 eyes, mean duration of 14 months), associated with intermediate uveitis (n=6), Adamantiades- Behcet disease (n=2), adult- type vascular pseudotumor (n=1), and HLAB27+ - related uveitis (n =1) received an intravenous infliximab infusion (5 mg/kg); five patients were retreated after 1 month. Macular thickness, measured by ocular cohe rence tomography, was reduced from 428 ± 138 μ m to 219 ± 51 μ m at 2 months postbaseline (P=. 000 1), while visual acuity increased from 0.41 ± 0.18 to 0.83 ± .0.17 (P < .000 01). Anatomic and functional improvement was sustained at 6 months in all. No ocular or extra- ocular side effects were noted. These promising results suggest that TNF may play an important pathogenetic role in chronic CME, thus, a controlled trial is warranted.rights reserved.【总页数】2页(P20-21)【作者】Markomichelakis N.N.;Theodossiadis P.G.;Pantelia E.;陈立军【作者单位】【正文语种】中文【中图分类】R774.5【相关文献】1.中西医结合治疗慢性前葡萄膜炎黄斑水肿 [J],2.用不同剂量的曲安奈德治疗葡萄膜炎所致黄斑囊样水肿的效果探析 [J], 吴爱温3.抗VEGF药物治疗葡萄膜炎并发黄斑囊样水肿和脉络膜新生血管研究进展 [J], 姚蕾;宫媛媛4.益气调血方药治疗葡萄膜炎黄斑囊样水肿的临床观察 [J], 王琦妙;庞雅菊;李岩;李晴;杨光;韩梅;巩鸿霞;王欣5.中西医结合治疗慢性前葡萄膜炎性黄斑水肿 [J], 孙建西;黎莉因版权原因,仅展示原文概要,查看原文内容请购买。
富血小板血浆在膝关节疾病治疗中的应用刘永辉赵烨王向阳郭马珑崔宏勋【摘要】富血小板血浆(platelet-rich-plasma,PRP)是利用全血各成分沉降系数不同特性离心而得到的高浓度血小板血浆,由于其富含多种促进组织修复的生长因子,且制作、使用便捷而被广泛运用到骨科领域,尤其是近几年在治疗膝关节疾病疗效方面备受关注。
本文就其治疗膝关节骨性关节炎、半月板、交叉韧带损伤及膝关节滑膜炎方面做一综述,为临床治疗膝关节常见疾病提供参考。
【关键词】富血小板血浆;膝关节骨性关节炎;半月板损伤;交叉韧带损伤;膝关节滑膜炎【Abstract】Platelet-rich-plasma is a high-concentration platelet plasma obtained by centrifugation with different characteristics of sedimentation coefficients of all components of the whole blood.It is widely used in the field of orthopedics because it is rich in a variety of growth factors to promote tissue repair and is easy to make and use,especially in the treatment of knee diseases in recent years.In this paper,the treatment of knee osteoarthritis,meniscus,cruciate ligament injury and knee synovitis are reviewed to provide reference for clinical treatment of common knee diseases.【Key words】Platelet-rich-plasma;Knee osteoarthritis;Meniscus injury;Cruciate ligament injury;Knee synovitis富血小板血浆(platelet-rich-plasma,PRP)是自体外周血离心而得到以血小板和白细胞为主的血浆,研究发现[1],PRP中含有转化生长因子β(transforming growth factor-β,TGF-β),成纤维细胞生长因子(fibroblast growth factor,FGF),血小板衍化生长因子(platelet-derived growth factor,PDGF),血管内皮生长因子(vascular endothelial growth factor,VEGF)等多种细胞因子和介质,通过PRP注射到受损组织等方式能够促进损伤组织的修复与再生[2]。
Peptides32(2011)1262–1269Contents lists available at ScienceDirectPeptidesj o u r n a l h o m e p a g e:w w w.e l s e v i e r.c o m/l o c a t e/p e p t i d esAnti-allodynic effects of intrathecally and intracerebroventricularly administered 26RFa,an intrinsic agonist for GRP103,in the rat partial sciatic nerve ligation modelTatsuo Yamamoto∗,Rika Miyazaki,Toshihiko Yamada,Tomonari ShinozakiDepartment of Anesthesiology,Graduate School of Medical Science,Kumamoto University,1-1-1Honjo,Kumamoto-shi,Kumamoto860-8556,Japana r t i c l e i n f oArticle history:Received20October2010Received in revised form13March2011 Accepted14March2011Available online23March2011Keywords:Dorsal root ganglionGPR103Neuropathic pain Immunohistochemistry a b s t r a c t26RFa and QRFP are endogenous ligands of GPR103.26RFa binding sites are widely distributed in the brain and the spinal cord where they are involved in processing pain.In the present study,the effects of intrathecal and intracerebroventricular applications of26RFa on the level of mechanical allodynia induced by partial sciatic nerve ligation were examined in rats.The level of mechanical allodynia was measured using von Freyfilaments.Intrathecal and intracerebroventricular injection of26RFa attenuated the level of mechanical allodynia.26RFa has been reported to activate not only GPR103but also neuropep-tide FF2receptor and the effect of intrathecally and intracerebroventricularly administered26RFa was not antagonized by BIBP3226,an antagonist of neuropeptide FF receptor.Immunohistochemical exami-nation revealed that QRFP-like immunoreactivity(QRFP-LI)was expressed mainly in the small to medium sized neurons in the L5dorsal root ganglion(DRG)and that partial sciatic nerve injury increased the per-centage of QRFP-LI positive neurons.7days after the nerve injury,QRFP-LI positive neurons in the L5DRG ipsilateral to the partial sciatic nerve injury were larger than those in the L5DRG ipsilateral to the sham operation.These data suggest that(1)exogenously applied26RFa modulates nociceptive transmission at the spinal and the supraspinal brain in the neuropathic pain model,(2)the mechanism26RFa uses to produce an anti-allodynic effect may be mediated by the activation of GPR103,and(3)partial sciatic nerve ligation affects the expression of QRFP-LI in the dorsal root ganglion.©2011Published by Elsevier Inc.1.IntroductionGPR103(also referred to as SP9155or AQ27)is known as an orphan G protein-coupled receptor.GPR103shows similarities with orexin,neuropeptide FF(NPFF),and cholecystokinin recep-tors.Recently,two endogenous ligands for GPR103were identified, 26RFa(also referred to as P518)and QRFP(also referred to as 43RFa)[9].26RFa is a novel26-residue RFamide peptide,initially isolated from a frog brain extract[4].QRFP is an N-terminally elongated form of26RFa.Although the role of GPR103and its endogenous ligands,26RFa and QRFP,is not fully understood, intravenous administered26RFa has been reported to cause a biphasic change in blood pressure and an increase in heart rate in urethane-anaesthetized rats[8].It has been reported that intrac-erebroventricular(ICV)administration of26RFa stimulates feedingAbbreviations:NPFF,neuropeptide FF;LI,like immunoreactivity;IT,intrathe-cal;ICV,intracerebroventricular;DRG,dorsal root ganglion;NPY,neuropeptide Y; ANOVA,analysis of variance;IP,intraperitoneal.∗Corresponding author.Tel.:+81963735275;fax:+81963639697.E-mail address:yamamotot@fc.kuh.kumamoto-u.ac.jp(T.Yamamoto).behavior and locomotion activity and that QRFP plays an important role in energy homeostasis[12,14,18,27].Recently,Bruzzone et al.[1]reported that,in rats,26RFa bind-ing sites are widely distributed in the brain and the spinal cord, whereas the expression of GPR103mRNA is more discrete,notably in the mid brain,the pons,the medulla oblongata,and the spinal cord.In the spinal cord,a very strong expression of GPR103mRNA was detected in the superficial layers of the dorsal horn and a high density of26RFa binding sites was also observed in the superficial layers of the dorsal horn[1].In addition,many26RFa binding sites have also been observed in various nuclei of the brain involved in processing pain such as the parafascicular thalamic nucleus,the locus coeruleus,the dorsal raphe nucleus,and the parabrachial nucleus and these data strongly suggest that26RFa may modu-late transmission of nociceptive stimuli[1,2].The authors have reported that intrathecal(IT)injection of26RFa inhibited the agi-tation behavior induced by paw formalin injection and attenuated the level of mechanical allodynia induced by paw carrageenan injection and that these effects of26RFa may be mediated by the activation of spinal GPR103[23].The authors also reported that ICV injection of26RFa attenuated the agitation behavior induced by paw formalin injection[22].However,IT or ICV injection of0196-9781/$–see front matter©2011Published by Elsevier Inc. doi:10.1016/j.peptides.2011.03.008T.Yamamoto et al./Peptides32(2011)1262–1269126326RFa had no effect on thermal nociceptive pain[22,23].Formalin induced agitation behavior and carrageenan induced mechanical allodynia are known to be an inflammatory pain model and these data strongly suggest that the GPR103–26RFa system is involved in nociceptive transmission from spinal cord to brain during inflam-mation.Sensory nerve injury may induce neuropathic pain that is con-stant,intermittent,or paroxysmal,with a burning,sharp,or aching sensation.Neuropathic pain is intractable pain and neuropathic pain is thought to be refractory to standard analgesics such as non-steroidal anti-inflammatory drugs and opioids.It has been suggested that both inflammatory pain and neuropathic pain,but not thermal nociceptive pain,are mediated,at least partly,by the sensitized systems of the spinal cord[11,24,25].Thus,it is pos-sible that activation of GPR103in the brain or in the spinal cord may produce an analgesic effect in the neuropathic pain models. In the present study,the authors examined the effect of either IT or ICV injection of26RFa on mechanical allodynia in the partial sciatic nerve ligation model,a neuropathic pain model[17],in the rat.The authors previously reported that QRFP-like immunore-activity(QRFP-LI)is expressed in the rat dorsal root ganglion (DRG)[23].It has been reported that,in DRG,peripheral nerve injury decreased the expression of substance P and calcitonin gene related peptide and increased the expression of cholecys-tokinin,galanin,neuropeptide Y(NPY)and vasoactive intentinal polypeptide[5,10,19,20].In the present study,the authors exam-ined whether partial sciatic nerve ligation affects the expression of QRFP-LI in the L5DRG or not.2.Materials and methodsThe following investigations were performed according to a protocol approved by the Institutional Animal Care Committee of Kumamoto University,Kumamoto,Japan.Male Sprague–Dawley rats weighing250–300g werefitted with long-term IT catheters and ICV cannulae and examined for the effects of the agents on the level of mechanical allodynia induced by partial sciatic nerve ligation.In the present study,the effect of QRFP was not examined because QRFP is insoluble in water.The manufacturer recommends that100g of QRFP is dissolved in50l of60%acetonitrile in water with0.1%dimethyl sulfoxide and then the solution is diluted up with any desired buffer.When10g of QRFP was injected IT or ICV, the animal showed abnormal behavior and could not be examined for the effect of QRFP.2.1.IT catheters and ICV cannulaeChronic IT catheters were inserted,during halothane anesthesia, by passing a PE-10catheter through an incision in the atlanto-occipital membrane to a position8cm caudal to the cisterna at the level of lumbar enlargement[21].The catheter was externalized on the top of the skull and sealed with a steel wire,and the wound was closed with3-0silk sutures.Implantation of the injection cannula into the right lateral ven-tricle was performed stereotaxically under halothane anesthesia.A stainless steel thin-wall guide cannula(24gauge,0.64mm outer diameter,15mm long)was stereotaxically placed through a burr hole(0.5mm caudal to coronal suture and1mm lateral to sagi-tal suture;3mm deep to the dura)and affixed to the skull with stainless steel screws and cranioplastic cement.All animals displayed normal feeding and drinking behaviors post-operatively.Rats showing neurological deficits were not stud-ied.2.2.Partial sciatic nerve ligationAnesthesia was induced by inhalation of5%halothane,main-tained at a concentration of2–3%,as needed.After a local incision, the biceps femoralis of the right leg was bluntly dissected at mid-thigh to expose the sciatic nerve.The right sciatic nerve was then carefully mobilized,with care taken to avoid undue stretching.Par-tial sciatic nerve injury was created by a tight ligation of1/3to1/2 of the right sciatic nerve[17].An8-0silicon-treated silk suture was inserted into the sciatic nerve just proximal to the sciatic trifurca-tion with a3/8curved,reversed-cutting mini-needle,and tightly ligated so that the dorsal1/3to1/2of the nerve thickness was trapped in the ligature.Incisions were closed,layer-to-layer,with 3-0silk sutures,and the rats were allowed to recover from the anesthetic.After surgery,the animals were individually maintained in plastic cages with solidfloors covered with3–6cm sawdust. All animals postoperatively displayed normal feeding and drinking behavior.2.3.Assessments of mechanical allodyniaMechanical thresholds were measured using von Freyfila-ments with logarithmically incremental stiffnesses(0.41,0.70, 1.20,2.00,3.63,5.50,8.50and15.1g)(Stoelting,Wood Dale,IL) to calculate the50%probability thresholds for mechanical paw withdrawal[3].Beginning with the2.00g probe,filaments were applied to the plantar surface of a hind paw for6–8s,in a step-wise ascending or descending order following negative or positive withdrawal responses,respectively,until six consecutive responses were noted.50%probability thresholds were calculated according to the method suggested by Dixon[6].In a case where continuous negative or positive responses were observed to the limits of the stimulus set,values of15.00g or0.25g were assigned,respectively.2.4.Mechanical nociceptive testThe mechanical nociceptive threshold was evaluated by mea-suring the frequency of foot withdrawals in response to a 29g von Freyfilament.The rats were placed in a clear plas-tic cage(10cm×20cm×24cm)on an elevated meshfloor(grid: 5mm×5mm).To initiate a test,a rat was placed in the box and allowed5–10min to habituate.The frequency of paw withdrawal to repetitive applications of a29g von Freyfilament to the center of the footpad was tested in an unrestrained rat standing on four legs. The authors chose a29g von Freyfilament because preliminary study revealed that ten applications of a29g von Freyfilament to the foot pad induced about three or four withdrawal responses in the untreated normal rat.Thus,both the analgesic and the hyper-algesic effects of drugs can be evaluated with this method.During one trial,the von Freyfilament was repetitively applied to the hind-paw10times with an inter-stimulus interval of5s.The number of paw withdrawal responses was counted.2.5.ImmunohistochemistryAn immnohistochemical study was performed in animals with partial sciatic nerve injury and in sham-operated animals whose sciatic nerves were only mobilized.After anesthetizing rats with 60mg/kg of sodium pentobarbital intraperitoneally,surgery pro-ceeded with sternotomy,transcardiac aortic needle cannulation, and perfusion with50ml of saline,followed by500ml of4% paraformaldehyde in0.1M phosphate buffer(pH=7.4).L5DRG ipsilateral to either the nerve injury or the sham operation was removed and postfixed in the samefixative solution overnight at 4◦C.After storing in0.01M phosphate buffered saline(PBS)con-taining20%sucrose for6h at room temperature,the DRG was1264T.Yamamoto et al./Peptides32(2011)1262–1269sectioned at a thickness of10m,for QRFP immunohistochemistry, on a cryostat.Sections were mounted onto gelatin-coated slides.After immersion in PBS containing5%normal horse serum and0.3%Triton X-100for1h,the sections were incubated with rabbit antibody to QRFP(1:1000;Phoenix Pharmaceuticals Inc., Burlingame,CA)and mouse antibody to NeuN(1:1000,Chemi-con,Temecula,CA)diluted with PBS containing5%normal horse serum and0.3%Triton X-100for20h at4◦C.In the present study, the authors examined the expression of QRFP-LI,but not26RFa-LI,because the specific antibody to26RFa,which is applicable to immunohistochemical study,could not be obtained.A neuron was defined as a NeuN-LI positive cell.The authors previously demonstrated that the antibody to QRFP,which was used in this experiment,was completely neutralized by QRFP,and this indi-cated that the QRFP antibody used in the present study is specific to QRFP[23].The sections were then incubated at room temperature for3h with Alexa488-conjugated donkey anti-rabbit IgG(1:100; Molecular Probes,Eugene,OR,USA)and Alexa546-conjugated goat anti-mouse IgG(1:200;Molecular Probes)in PBS containing5%nor-mal horse serum and0.3%Triton X-100,and cover slipped with PermaFluor Aqueous(Shandon,Pittsburgh,PA).2.6.Behavioral analysisThe general behavior of each rat was carefully observed and tested.Motor functions were evaluated by the performance of two specific behavioral tasks,as follows.(1)The placing/stepping reflex: this response was evoked by drawing the dorsum of either hind paw over the edge of a tabletop.In normal animals,this stimulus elicits an upward lifting of the paw onto the surface of the table, called stepping.Animals with any degree of hind limbflaccidity will demonstrate an altered or absent reflex.(2)The righting reflex:an animal placed horizontally with its back on the table will normally show an immediate coordinated twisting of the body around its longitudinal axis to regain its normal position on its feet.Animals displaying ataxic behavior will show a decreased ability to right themselves.To quantify the evaluation of motor functions,both tasks were scored on a scale of0–2in which0=absence of function and2=normal motor functions.Animals that were able to perform the motor tasks but did so more slowly than normal animals were assigned a score of1.2.7.DrugsThe IT administered drugs were delivered in a total volume of 10l followed by10l of saline toflush the catheter.The ICV administered drugs were delivered in a total volume of2l.The agents used in this study were dissolved in saline.26RFa(molec-ular weight=2820)was purchased from Phoenix Pharmaceuticals Inc.BIBP3226(molecular weight=474)was purchased from Sigma (St.Louis,MO).Although BIBP3226has been reported to be a selec-tive non-peptide NPY Y1receptor antagonist[16],BIBP3226has also been reported to have antagonistic effects on neuropeptide FF1(NPFF1)and NPFF2receptors expressed in CHO cells[13]. More recently,Fang et al.[7]reported that intravenous injection of BIBP3226antagonized the effect of NPFF on arterial blood pres-sure in rats.These data suggested that BIBP3226has the property of a mixed antagonist to NPY Y1and NPFF receptors.2.8.Experimental protocol2.8.1.Dose–response studyA preliminary study performed in the authors’laboratory revealed that the maximum level of mechanical allodynia was observed between7and14days after the nerve injury.Thus, drugs were administered7days after the nerve injury.IT catheters were inserted4days before the nerve injury.ICV cannulae were implanted3days after the nerve injury.ICV cannulae were some-times plugged7days after implantation.If ICV cannulae were implanted4days before the nerve injury,the authors sometimes could not inject drugs through the ICV cannulae.Before and7days after the nerve injury,the mechanical threshold of the right hind paw was measured as control data.Then26RFa was administered IT(30g(n=5);10g(n=5);3g(n=5))or ICV(30g(n=5); 10g(n=6);3g(n=5)),and the right hind paw was tested at5, 15,30,45,60and90min after the drug administration.To obtain control data,vehicle(saline)was injected IT(n=5)or ICV(n=5).26RFa has been reported to activate not only GPR103but also NPFF2receptor[1].To test whether the effect of IT or ICV admin-istered26RFa on an anti-allodynic effect was produced by an interaction between26RFa and spinal NPFF2receptor or central NPFF2receptor,10g of BIBP3226was administered IT or ICV 10min before the IT or ICV injection of30or10g of26RFa(IT study:30g:n=5;10g:n=4;ICV study:30g:n=5;10g: n=4).10g of BIBP3226was also injected IT(n=5)or ICV(n=5). The motor function was examined just after each measurement of mechanical threshold.As mentioned above,QRFP could not be injected IT or ICV because of its insolubility in saline.To examine the role of intrinsic QRFP in the maintenance of mechanical allodynia induced by nerve injury,rabbit antibody to QRFP(0.25l;Phoenix Pharmaceuticals Inc.,Burlingame,CA),which was also used for the immunohisto-chemical study,was administered IT or ICV,and the right hind paw was tested at5,15,30,45and60min after the drug administration.2.8.2.Mechanical nociceptive test in the sham operated ratsBefore the drug administration,baseline measurements of the response frequency were made.30g of26RFa was administered IT(n=4)or ICV(n=4)and the response of the sham-operated paw to10applications of a29g von Freyfilament was measured at5,15, 30,45and60min after the drug administration.To obtain control data,saline was administered IT(n=5)or ICV(n=5).2.8.3.Immunohistochemical studyThe sections were examined using afluorescent microscope (Axioskop2,Carl Zeiss,Germany).For the quantification of the immunohistochemical study,five sections from each rat were randomly selected.The investigator responsible for the immuno-histochemical study was blinded to the operation(partial sciatic nerve ligation or sham operation)that the animals received.The number of neurons and the number of QRFP-LI positive neurons in the right L5DRG was counted7days after the par-tial sciatic nerve ligation(n=5)or the sham operation(n=5).The proportion of QRFP-LI positive neurons to the total neurons of L5 DRG was calculated.The immunohistochemical examination of the nerve-injured rats proceeded concurrently with that of the sham-operated rats.The areas of the QRFP-LI positive neurons were measured7days after the partial sciatic nerve ligation(n=5)or the sham operation (n=5)using a computer-assisted imaging analysis system(AxioVi-sion,version2.05,Carl Zeiss,Germany).Only neurons with clearly visible nuclei were used for quantification.2.9.Statistical analysis2.9.1.Dose–response studyTo determine whether partial sciatic nerve ligation induced significant mechanical allodynia,the50%probability threshold before the nerve injury was compared with that7days after the nerve injury with a Mann–Whitney rank sum test.To analyze the time–effect curves,one way repeated measured analysis of variance(repeated measured ANOVA)with the Dunnet’s multipleT.Yamamoto et al./Peptides32(2011)1262–12691265comparison test was used.To analyze the dose–response effect of drugs on a mechanical threshold,the area under the curve(AUC) was calculated by use of the trapezoidal rule over the entire time course of the time–effect curve.To analyze the dose-dependency, one-way ANOVA with Dunnet’s multiple comparison test was used. To analyze the antagonistic effect of BIBP3226on the effect of 26RFa,the AUC of the BIBP3226+26RFa time–effect curve was com-pared with the AUC of the26RFa time effect curve with a t-test. 2.9.2.Mechanical nociceptive testTo analyze the effects of drugs on the response frequency,the AUC of the entire time–effect curve was calculated by use of the trapezoidal rule over the entire time course of the response fre-quency.The effect of26RFa on the AUC was analyzed by t-test. 2.9.3.Immunohistochemical studyThe immunohistochemical study data are arranged in2×2or 2×7contingency tables.The most common procedure for analyz-ing contingency table data is by using the chi-square statistic[26]. Thus,to compare the proportion of QRFP-LI positive neurons in the total neurons of L5DRG in the nerve injured rats with that in the sham-operated rats(2×2table)or to compare the distribution of areas of QRFP-LI positive neurons in the partial sciatic nerve ligation rats with that in the sham-operated rats(2×7table),the chi-square test was used.Wherever appropriate,results are expressed as mean±SEM. Critical values that reached a p<0.05level of significance were considered statistically significant.3.Results3.1.Behavioral analysisAfter IT or ICV injection of26RFa,all animals scored2(normal motor function)in the placing/stepping reflex and righting reflex tests.No scratching or biting behavior was observed after the IT or ICV injection of26RFa.3.2.Anti-allodynic Effects of26RFaThe50%probability threshold7days after partial sciatic nerve ligation(IT study(n=44):1.56±0.15g;ICV study(n=43): 1.65±0.05g)is significantly less than the pre-surgery50% probability threshold level(IT study:14.4±0.3g;ICV study: 15.0±0.00g)(p<0.001by Mann–Whitney Rank Sum test)and this suggested that partial sciatic nerve ligation induced significant mechanical allodynia7days after nerve injury.7days after nerve injury,the50%probability threshold level in the IT study group was not different from that in the ICV study group(p>0.5by t-test). 3.2.1.Effects of IT injection of26RFaThere were no differences in mean threshold differences after grouping the animals(data not shown,p>0.5by ANOVA).IT injection of30g of26RFa increased the50%probability threshold5min after the drug administration and the effect lasted for45min(p<0.05by repeated measured ANOVA,Fig.1)and there was no statistically significant differences between the50%proba-bility threshold60min after26RFa administration and the pre-drug 50%probability threshold(Fig.1).IT injection of26RFa significantly increased AUC as compared with the saline-treated rats at doses of 10and30g(p<0.001by ANOVA,Fig.2).The AUCs in the26RFa(10 or30g)+BIBP3226(10g)treated rats were not different from those in the26RFa(10or30g)treated rats,respectively(p>0.4 by t test,Fig.2).BIBP322610g itself had no effect on the level of AUC as compared with that in the saline treated rats(BIBP32265%probabilitythreshold(g)µgTime (min)5%probabilitythreshold(g)26RFa 30 µgµgFig.1.Effects of IT injection of30g of26RFa and saline(IT injection study,top) and effects of ICV administration of30g of26RFa and saline(ICV injection study, bottom)on the time course of the50%probability threshold(g)of the right hind paw (sciatic nerve injured paw).The effect of coadministration of BIBP3226and26RFa is also presented.26RFa,BIBP3226or saline was administered IT or ICV7days after partial sciatic nerve ligation.Ordinate:50%probability threshold(g);abscissa:time after drug administration(min).Each line represents the group mean and SEM of five rats.*p<0.05as compared with the pre-drug(time=0)level.treated rats:95.8±12.3;saline treated rats:101±8.8,p>0.7by t test).3.2.2.Effects of ICV injection of26RFaThere were no differences in mean threshold differences after grouping the animals(data not shown,p>0.4by ANOVA).ICV injection of30g of26RFa increased the50%probabil-ity threshold5min after the drug administration and their effects lasted for60min after the drug administration(p<0.05by repeated measured ANOVA,Fig.1),and there was no statistically significant difference between50%probability threshold90min after26RFa ICV injection and pre-drug50%probability threshold(Fig.1).ICV injection of26RFa significantly increased the AUC as compared with the saline-treated rats at doses of10g and30g(p<0.001 by ANOVA,Fig.2).There was no difference between AUCs in the 26RFa(10or30g)+BIBP3226(10g)treated rats and those in the26RFa(10or30g)treated rats,respectively(p>0.4by t test, Fig.2).BIBP322610g itself had no effect on the AUC as com-pared with that in the saline treated rats(BIBP3226treated rats: 119±17.4;saline treated rats:107±15.2,p>0.6).3.2.3.Effects of an antibody to QRFP on mechanical allodyniaIT or ICV injection of an antibody to QRFP had no effect on the AUC as compared with IT or ICV saline-treated rats,respectively(IT antibody to QRFP(n=5):142±20.7; IT saline(n=5):101±8.8;ICV antibody to QRFP(n=5): 152±19.4;ICV saline(n=5):107±15.2,p>0.1by t test, Fig.3).1266T.Yamamoto et al./Peptides 32(2011)1262–1269A U CDose (µg)A U CFig.2.Dose–response curves for IT injected 26RFa (IT injection study,top)and ICV injected 26RFa (ICV injection study,bottom).Ordinate:area under the curve (AUC);abscissa:dose of 26RFa (g).AUC was calculated by use of trapezoidal rule over the entire time course of the time effect curve.26RFa or saline was administered,either IT or ICV,7days after the partial sciatic nerve ligation.26RFa increased the AUC in a dose-dependent manner in either the IT injection study or the ICV injection study.Pre-treatment with 10g of BIBP3226had no effect on the anti-allodynic effect of either 30g or 10g of 26RFa in either IT injection study or ICV injection study.Each line represents the group mean and SEM of five or six rats.*p <0.05as compared with the saline treated rats.3.2.4.Effects of 26RFa on the mechanical nociceptive testIT or ICV injection of 30g of 26RFa had no effect on the mechan-ical nociceptive test as compared with IT or ICV saline treated rats,respectively (AUC of IT study:26RFa treated rats:2440±240,saline treated rats:2160±142;AUC of ICV study:26RFa treated rats:2530±200,saline treated rats:2620±340,p >0.3,by t test,Fig.4).3.3.Immunohistochemical StudyIn the rats with partial sciatic nerve ligation,665neurons out of 3155neurons (21.1%)in the L5DRG ipsilateral to the nerve injury were immunoreactive for QRFP.In the rats with the sham operation,574neurons out of 3534neurons (16.2%)in the L5DRG ipsilateral to the sham operation were immunoreactive for QRFP.Proportion of QRFP-LI positive neurons in total neurons of L5DRG in the nerve injured rats was significantly larger than that in the sham operated rats (Fig.5,p <0.001by chi-square test).The QRFP-LI was observed mainly in small-to medium sized neurons in the rats with the sham operation (Figs.5and 6).7days after the nerve injury,QRFP-LI positive neurons in the L5DRG ipsi-50 % p r o b a b i l i t y t h r e s h o l d (g )123456Time (min)50 % p r o b a b i l i t y t h r e s h o l d (g )123456Fig.3.Effects of IT injection or ICV injection of an antibody to QRFP on the time course of the 50%probability threshold (g)of the right hind paw (sciatic nerve injured paw).Ordinate:50%probability threshold (g);abscissa:time after antibody to QRFP or saline administration (min).Each line represents the group mean and SEM of five rats.lateral to the partial sciatic nerve ligation were larger than those in the L5DRG ipsilateral to the sham operation (p <0.005by qui-square test,Figs.5and 6).4.DiscussionIt has been clearly demonstrated that either IT or ICV injec-tion of 26RFa attenuated the level of mechanical allodynia induced by partial sciatic nerve ligation at doses of 10and 30g.The anti-allodynic effect of IT administered 26RFa lasted 45min and the antiallodynic effect of ICV administered 26RFa lasted 60min.Either IT or ICV injection of 26RFa had no effect on the mechanical nociceptive test in the sham-operated rats.This suggested that IT or ICV injected 26RFa elevated the mechan-ical nociceptive threshold only when the sciatic nerve was injured.Primeaux et al.[15]reported that ICV injection of 10and 15g of 26RFa increased food intake in rats.Kampe et al.[12]also reported that centrally administered 10and 50g of 26RFa increased food intake in rats.These data strongly suggest that 30g of 26RFa is not a high dose in rats and that 30g of 26RFa is needed to produce an effect when 26RFa is administered ICV.In the present study,IT catheters were implanted 4days before the nerve injury and ICV cannulae were implanted 3days after the。