PPF_Development_Guide
- 格式:pdf
- 大小:1.05 MB
- 文档页数:52

Guideline for the Admission of Dietary Supplement Ingredients to the USP–NF Monograph Development ProcessG1.12-00Background The purpose of this Guideline is to set forth the criteria that USP Expert Committee uses to determine whether or not a dietary ingredient qualifies for admission to the process for the development of a quality monograph. The Guideline establishes a Class A and Class B system that categorizes dietary ingredients according to their level of safety concern, and describes the process by which the ingredients are classified. The USP Dietary Supplements Admission Evaluations Joint Standard Setting Subcommittee (DS AE JS3) makes a determination whether or not USP should proceed with monograph development.Subsequent approval of the monograph for inclusion into the United States Pharmacopeia and National Formulary (USP–NF ) is determined by the USP Balloting Process.Selection and Prioritization of Dietary IngredientsThe initial selection and prioritization of dietary ingredients for admission into the USP-NF compendial monograph development process is based on several considerations, including but not limited to the following:1. Extent of use, based upon market sales or other factors2. Historical use3. Knowledge of chemical composition4. Existence of other pharmacopeial standards5. Evidence of benefit6. Interest from a governmental body7. Potential risks to health associated with its use.Classification SystemUSP classifies dietary ingredients as follows:Class A: Admitted to the USP-NF compendial monograph development process Ingredients for which the available evidence does not indicate a serious risk to health* or other public health concern that precludes development of a USP-NF monograph and that could be approved for inclusion in the compendia with or without a cautionary label statement**.Guideline for the Admission of Dietary Supplement Ingredients to theUSP–NF Monograph Development ProcessG1.12-00Class B: Not admitted into the compendial monograph development process Ingredients for which the available evidence indicates a serious risk to health* or other public health concern that precludes development of a monograph for inclusion in the USP-NF .*Serious risk to health means that the use of the Ingredients could:(A) result in: (i) death; (ii) a life–threatening experience; (iii) inpatient hospitalization; (iv) a persistent orsignificant disability or incapacity; or (v) a congenital anomaly or birth defect; or (B) require, based on reasonable medical judgment, a medical or surgical intervention to prevent an outcome described under subparagraph (A).** Cautionary Label StatementIn certain cases the DSAE JS3 may recommend that monograph development proceed under Class A only if a cautionary label statement is included in the monograph. In such cases the monograph will not proceed to ballot until after the DSAE JS3 has had the opportunity to review the draft monograph to ensure that a suitable cautionary label statement is included. Examples of cases of approved monographs where cautionary label statements have been included are provided below. ArticleCautionary label statement that was included Black Cohosh Dosage forms prepared with this article should bear the followingstatement: Discontinue use and consult a healthcare practitioner ifyou have a liver disorder or develop symptoms of liver trouble,such as abdominal pain, dark urine, or jaundice.St. John’s Wort The label bears a statement indicating that “Rare cases of allergicreactions and photosensitivity have been reported with the use ofSt. John’s Wort. St. John’s Wort interacts with numerousmedications. Check with your healthcare provider before using.”Salix Species BarkThe label bears a statement indicating “Not for use in children,women who are pregnant or nursing, or by persons with knownsensitivity to aspirin”.Guideline for the Admission of Dietary Supplement Ingredients to theUSP–NF Monograph Development ProcessG1.12-00Comprehensive USP Admission EvaluationFor comprehensive USP admission evaluation, USP researches and evaluates diverse sources of safety information to determine the classification for dietary ingredients. Some articles may be exempt from comprehensive evaluation as outlined under “Criteria for Exemption from Comprehensive USP Admission Evaluation” below .The sources of information evaluated by USP include, but are not limited to the following:1. Human data: Although dietary ingredients are not required to undergo controlled clinicaltrials before they are marketed,1 the safety profile of an ingredient may be evaluatedusing the following information:a) Clinical safety studies: Sufficiently powered prospective observational studies,clinical trials, dose-escalation studies, systematic reviews, or retrospective meta-analysis of clinical studies provide useful information to evaluate the safety of aningredient.b) Other clinical studies: Although clinical studies may be limited by the small numberof study participants, observation of adverse events under controlled studyconditions generates useful safety information on the ingredient. Information fromrandomized, placebo-controlled, double-blind clinical studies, are valuable.c) Postmarket surveillance: Premarket safety studies sometimes are limited by thenumber of study subjects. When products are in wide use, detection of adverseevents provides a strong surrogate for safety monitoring in the general populationand in consumers who have chronic conditions. Postmarket surveillance alsoprovides valuable information about an ingredient’s safety profile in vulnerablepopulations, e.g., in pregnancy, lactation, the elderly, children, or prescriptionmedication users. Epidemiological reports might be helpful if the dietary ingredients are widely used as a traditional preparation.d) Adverse events: An adverse event associated with a supplement may be reportedby a healthcare practitioner (HCP) in a peer-reviewed journal or may be reportedby the HCP or a consumer to the local Poison Control Center or the United States1 Dietary Supplement Health and Education Act 1994.Available athttps:///About/DSHEA_Wording.aspx Accessed February 09, 2016Guideline for the Admission of Dietary Supplement Ingredients to theUSP–NF Monograph Development ProcessG1.12-00(U.S.) Food and Drug Administration’s (FDA) primary adverse event reportingportal, MedWatch. Since December 22, 2007, dietary supplement manufacturersand distributors are required to submit serious adverse event reports to FDAMedWatch. Valuable information about adverse events also is available from other international regulatory agencies such as Health Canada, the British Medicines and Healthcare Products Regulatory Agency (MHRA), and the Australian TherapeuticGoods Administration (TGA).e) Supplement interactions: Interactions with prescription drugs have significant safetyimplications because of their effects on bioavailability or induction/inhibition ofmetabolizing enzymes. Such interactions may lead to synergism or antagonism ofintended effects.f) Data from other relevant products: When long term safety information is not welldocumented, particularly for new dietary ingredients (NDIs) (ingredients introduced into the market after Oct. 15, 1994), any additional publicly available informationmay be useful in establishing the ingredient’s safety profile. Knowledge aboutchemical constituents may be used during investigations of equivalency amongcommercial dietary supplement products and their traditional counterparts. 2. Non-human data (pharmacological data): Carefully planned and justified in vivo animalstudies and in vitro experiments that investigate potential risks to human health.Information from animal experiments may bridge the knowledge gap regarding the safety of dietary supplement use.a) Reproductive toxicity: Animal experimental data regarding genotoxicity,reproductive and developmental toxicity, and carcinogenicity provide valuablesafety information for the use of the dietary ingredient by pregnant and lactatingmothers. In vitro studies such as the Ames test provide insights into likelygenotoxicity.b) Studies in experimental animals: Studies may provide insights into the mechanismof action of a substance, its purported efficacy, and its effect(s) on target organs. In vitro cell culture studies may provide information about effects at the cellular leveland molecular mediators involved in the observed effects.Guideline for the Admission of Dietary Supplement Ingredients to theUSP–NF Monograph Development ProcessG1.12-00c) Pharmacokinetics: Information about the absorption kinetics, bioavailability,accumulation in vital organs, plasma maximum concentration (C max ), time for half-life (t 1/2), and elimination kinetics, or marker compound kinetics identifies the dose or duration mediated toxicity of a dietary ingredient.d) Safety margin: Information from animal studies regarding the effective dose(ED 50), lethal dose (LD 50), no observed adverse effect level (NOAEL), and lowest observed adverse effect level (LOAEL) are used to determine the relative safetymargin in the use of the dietary ingredient.e) Presence of toxic constituents (or structurally related compounds with establishedtoxicity): Knowledge of chemical components of a dietary ingredient aids in safetyevaluation by identifying potentially toxic constituents, components containingtoxicophores, or constituents known to mimic or modulate endogenousintermediates. Structure-based computational prediction models and in silicoanalysis may be used if available, to make better determinations on toxicity whenaddressing uncertainty with safety data (such as equivocal evidence or missinginformation) from conventional in vivo or in vitro studies.3. Contemporaneous extent of use globally and in the U.S., including patterns of misuse,abuse, and fluctuations of use: Information collected from dietary supplement trade publications and regulatory bulletins helps generate signals of value. Informationregarding the extent of use (global market information) provides insights into the safety profile.4. Historical use: Traditional use documented in authoritative texts—including the context ofuse, dose, duration of use, method of preparation, and traditional cautions—provides valuable information about deviations of the commercial preparations from the traditional use, if any, and unexpected adverse effects. Traditional medical systems such asAyurveda and Traditional Chinese Medicine provide useful information about historical use.5. Regulatory status in the U.S. and other countries:a) Regulatory actions: Information about regulatory actions (including product recallsand safety alerts) from international regulatory agencies may indicate the extent ofadverse event reports, the incidence and methods of detection ofadulteration/contamination, the regional or global nature of adverse events, anddietary supplement–prescription drug interactions.Guideline for the Admission of Dietary Supplement Ingredients to theUSP–NF Monograph Development ProcessG1.12-00b) Regulatory status: The regulation of dietary supplements in the U.S. differs fromother countries. In some European countries, dietary supplement ingredients areregulated as OTC drugs or prescription drugs, which may require registration orpre-market approval. In the U.S. these same ingredients may be regulated asdietary supplements and thus do not require pre-marketing approval by FDA unless they are NDIs. Thus, the intervention of the HCP and the consumption patterns ofthe dietary ingredients by consumers are different in the U.S. and other countries.Accordingly, the qualitative and quantitative information on the safety profileobtained from different countries provides valuable information.c) GRAS/NDI status: FDA may be notified of Generally Recognized as Safe (GRAS)status for some dietary ingredients and the intended conditions of use. GRASnotifications to FDA of dietary ingredients may provide information about availablescientific data and basis of a product’s safety. Similarly, NDI notifications to FDAprovide information about the basis for a product’s safety.6. Existence of pharmacopeial monographs in other pharmacopeias may provide criticalinformation about the standards of purity, adulterants/contaminants, dosage, and caution statements intended to ensure the safe use of dietary ingredients. Examples of such authoritative information include but are not limited to, the World Health Organization (WHO), the European Scientific Cooperative on Phytotherapy (ESCOP), the German Commission E, Indian Pharmacopeia, Pharmacopoeia of the People’s Republic of China, and Health Canada monographs.In analyzing information from the above sources for a dietary ingredient, the limitations of dietary supplement specific issues (detailed in Gardiner et al., 20082,3) are considered. Reports of serious adverse events may be analyzed with an appropriate causality algorithm (such as the WHO-UMC system for standardized case causality assessment). The “odds ratio” for a serious adverse reaction and the signal of safety concern may be estimated from the extent of use, “number needed to harm” or proportional representation ratio.Commensurate with the signal of safety concern, USP may communicate the admission evaluation and classification through publications in peer–reviewed journals, public2 Gardiner P, Sarma DN, Low Dog T, Barrett ML, Chavez ML, Ko R, Mahady GB, Marles RJ,Pellicore LS, and Giancaspro GI. 2008. The state of dietary supplement adverse event reporting in the United States. Pharmacoepidemiology and Drug Safety. 17: 962–970. 3 Dietary Supplements: A Framework for Evaluating Safety. Institute of Medicine & National Research Council; The National Academies Press: Washington, DC, 2005.Guideline for the Admission of Dietary Supplement Ingredients to theUSP–NF Monograph Development ProcessG1.12-00communications, Pharmacopeial Forum notices, USP Dietary Supplement Compendium, or other appropriate means.The AE JS3 will determine the appropriate classification based on the totality of the evidence obtained above for each of the parameters examined. Criteria for Exemption from Comprehensive USP Admission EvaluationA comprehensive literature search and review may not be necessary for dietary ingredients for which a GRAS or an NDI notification was submitted to the FDA, for review and notobjected to by the Agency. USP Admission Classification for such dietary ingredients may be based on the information from the GRAS or NDI notifications, which usually contain themajority of the information that USP assesses as described above. A comprehensive review may be performed for dietary ingredients for which a GRAS or an NDI notification does not contain sufficient information to make an admission evaluation, or where the candidatedietary ingredient for admission is not exactly the same or equivalent to the subject of the NDI or GRAS notification. The following attributes will be reviewed to determine whether an exemption from a comprehensive admission evaluation applies:1. Equivalency of the candidate dietary ingredient to the subject of the NDI or GRASnotification. To determine equivalency, information on the following attributes will be reviewed:a. Identity of the ingredient;b. Physical characteristics of the ingredient i.e., the form of the ingredient reviewedwhether liquid, powder or other, including the particle size characteristics (micro ornanoparticle); and,c. The chemical profile, including the extraction procedure and compounds present inthe ingredient.2. Daily Intake: The maximum amount of the candidate dietary ingredient recommended orsuggested for use in a dietary supplement as determined from publicly availablepublications (for example, from the NIH Office of Dietary Supplements and NationalGuideline for the Admission of Dietary Supplement Ingredients to theUSP–NF Monograph Development ProcessG1.12-00Library of Medicine Dietary Supplement Label Database (DSLD)4), should not exceed the intake level stipulated in the GRAS or NDI notification.This Guideline is intended to allow USP to evaluate safety issues depending on the particular dietary ingredient involved, the level of available safety data, and other relevantconsiderations. For example, even where a dietary ingredient is classified as Class A and is eligible for admission into the USP-NF monograph development process, there may be specific safety concerns that will require future monitoring. USP monitors safety information for all dietary ingredients for which dietary supplement monographs have been developed on an ongoing basis for possible re–evaluation of an article’s admission status.USP’s evaluation of a dietary ingredient under this Guideline is performed for the sole purpose of determining admission into the compendial monograph development process and should not be relied upon as any finding about the intrinsic safety or effectiveness of the dietary ingredient under review.This Guideline supersedes any previous guideline issued by USP on safety criteria and admission classification for dietary supplements. 4 ODS-NLM Dietary Supplement Label Database (DSLD). Available at/dsld/ (Accessed February 09, 2016).。

psfgen User’s GuideVersion1.3.3Justin Gullingsrud and Jim PhillipsSeptember14,2005Theoretical Biophysics GroupUniversity of Illinois and Beckman Institute405N.MathewsUrbana,IL618011Creating PSF Structure FilesThe psfgen structure building tool consists of a portable library of structure andfile manipulation routines with a Tcl interface.Current capabilities include•reading CHARMM topologyfiles•reading psffiles in X-PLOR/NAMD format•extracting sequence data from single segment PDBfiles•generating a full molecular structure from sequence data•applying patches to modify or link different segments•writing NAMD and VMD compatible PSF structurefiles•extracting coordinate data from PDBfiles•constructing(guessing)missing atomic coordinates•deleting selected atoms from the structure•writing NAMD and VMD compatible PDB coordinatefilesWe are currently refining the interface of psfgen and adding features to create a complete molecular building solution.We welcome your feedback on this new tool.2Ordinary Usagepsfgen is currently distributed in two forms.One form is as a standalone program implemented as a Tcl interpreter which reads commands from standard output.You may use loops,variables,etc.as you would in a VMD or NAMD script.You may use psfgen interactively,but we expect it to be run most often with a scriptfile redirected to standard input.The second form is as a Tcl package which can be imported into any Tcl application,including VMD.All the commands available to the standalone version of psfgen are available to the Tcl package;using psfgen within VMD lets you harness VMD’s powerful atom selection capability,as well as instantly view the result of your structure building scripts.Examples of using psfgen both with and without VMD are provided in this document.Generating PSF and PDBfiles for use with NAMD will typically consist of the following steps:1.Preparing separate PDBfiles containing individual segments of protein,solvent,etc.before runningpsfgen.2.Reading in the appropriate topology definitionfiles and aliasing residue and atom names found in thePDBfile to those found in the topologyfiles.This will generally include selecting a default protonation state for histidine residues.3.Generating the default structure using segment and pdb commands.4.Applying additional patches to the structure.5.Reading coordinates from the PDBfiles.6.Deleting unwanted atoms,such as overlapping water molecules.7.Guessing missing coordinates of hydrogens and other atoms.8.Writing PSF and PDBfiles for use in NAMD.22.1Preparing separate PDBfilesMany PDBfiles in the PDB databank contain multiple chains,corresponding to protein subunits,water, and other miscellaneous groups.Protein subunits are often identified by their chain ID in the PDBfile.In psfgen,each of these groups must be assigned to their own segment.This applies most strictly in the case of protein chains,each of which must be assigned to its own segment so that N-terminal and C-terminal patches can be applied.You are free to group water molecules into whatever segments you choose.Chains can be split up into their own PDBfiles using your favorite text editor and/or Unix shell com-mands,as illustrated in the BPTI example below.If you are using VMD you can also use atom selections to write pieces of the structure to separatefiles:#Split a file containing protein and water into separate segments.#Creates files named myfile_water.pdb,myfile_frag0.pdb,myfile_frag1.pdb,...#Requires VMD.mol load pdb myfile.pdbset water[atomselect top water]$water writepdb myfile_water.pdbset protein[atomselect top protein]set chains[lsort-unique[$protein get pfrag]]foreach chain$chains{set sel[atomselect top"pfrag$chain"]$sel writepdb myfile_frag${chain}.pdb}2.2Deleting unwanted atomsThe delatom command described below allows you to delete selected atoms from the structure.It’sfine to remove atoms from your structure before building the PSF and PDBfiles,but you should never edit the PSF and PDBfiles created by psfgen by hand as it will probably mess up the internal numbering in the PSFfile.Very often the atoms you want to delete are water molecules that are either too far from the solute,or else outside of the periodic box you are trying to prepare.In either case VMD atom selections can be used to select the waters you want to delete.For example:#Load a pdb and psf file into both psfgen and VMD.resetpsfreadpsf myfile.psfreadpdb myfile.pdbmol load psf myfile.psf pdb myfile.pdb#Select waters that are more than10Angstroms from the protein.set badwater1[atomselect top"name OH2and not within10of protein"]#Alternatively,select waters that are outside our periodic cell.set badwater2[atomselect top"name OH2and(x<-30or x>30or y<-30or>30or z<-30or z>30)"]#Delete the residues corresponding to the atoms we selected.foreach segid[$badwater1get segid]resid[$badwater1get resid]{delatom$segid$resid}#Have psfgen write out the new psf and pdb file(VMD’s structure and#coordinates are unmodified!).writepsf myfile_chopwater.psfwritepdb myfile_chopwater.pdb33BPTI ExampleTo actually run this demo requires•the program psfgen from any NAMD distribution,•the CHARMM topology and parameterfiles top_all22_prot.inp and par_all22_prot.inp from https:///research/amackere/research.html,and•the BPTI PDBfile6PTI.pdb available from the Protein Data Bank at /by searching for6PTI and downloading the complete structurefile in PDB format.Building the BPTI structureIn this demo,we create thefiles bpti.psf and bpti.pdb in the output directory which can then be used for a simple NAMD simulation.#File:bpti_example.tcl#Requirements:topology file top_all22_prot.inp in directory toppar#PDB file6PTI.pdb in current directory#Create working directory;remove old output filesmkdir-p outputrm-f output/6PTI_protein.pdb output/6PTI_water.pdb#(1)Split input PDB file into segments}grep-v’^HETATM’6PTI.pdb>output/6PTI_protein.pdbgrep’HOH’6PTI.pdb>output/6PTI_water.pdb#(2)Embed the psfgen commands in this scriptpsfgen<<ENDMOL#(3)Read topology filetopology toppar/top_all22_prot.inp#(4)Build protein segmentsegment BPTI{pdb output/6PTI_protein.pdb}#(5)Patch protein segmentpatch DISU BPTI:5BPTI:55patch DISU BPTI:14BPTI:38patch DISU BPTI:30BPTI:51#(6)Read protein coordinates from PDB filepdbalias atom ILE CD1CD;#formerly"alias atom..."coordpdb output/6PTI_protein.pdb BPTI#(7)Build water segmentpdbalias residue HOH TIP3;#formerly"alias residue..."segment SOLV{auto nonepdb output/6PTI_water.pdb}4#(8)Read water coordinaes from PDB filepdbalias atom HOH O OH2;#formerly"alias atom..."coordpdb output/6PTI_water.pdb SOLV#(9)Guess missing coordinatesguesscoord#(10)Write structure and coordinate fileswritepsf output/bpti.psfwritepdb output/bpti.pdb#End of psfgen commandsENDMOLStep-by-step explanation of the script:(1)Split input PDBfile into segments.6PTI.pdb is the originalfile from the Protein Data Bank.It contains a single chain of protein and some PO4and H2O HETATM records.Since each segment must have a separate inputfile,we remove all non-protein atom records using grep.If there were multiple chains we would have to split thefile by hand.Create a secondfile containing only waters.(2)Embed the psfgen commands in this script.Run the psfgen program,taking everything until “ENDMOL”as input.You may run psfgen interactively as well.Since psfgen is built on a Tcl interpreter, you may use loops,variables,etc.,but you must use$$for variables when inside a shell script.If you want, run psfgen and enter the following commands manually.(3)Read topologyfile.Read in the topology definitions for the residues we will create.This must match the parameterfile used for the simulation as well.Multiple topologyfiles may be read in since psfgen and NAMD use atom type names rather than numbers in psffiles.(4)Build protein segment.Actually build a segment,calling it BPTI and reading the sequence of residues from the stripped pdbfile created above.In addition to the pdb command,we could specify residues explicitly.Both angles and dihedrals are generated automatically unless“auto none”is added (which is required to build residues of water).The commands“first”and“last”may be used to change the default patches for the ends of the chain.The structure is built when the closing}is encountered,and some errors regarding thefirst and last residue are normal.(5)Patch protein segment.Some patch residues(those not used to begin or end a chain)are applied after the segment is built.These contain all angle and dihedral terms explicitly since they were already generated.In this case we apply the patch for a disulfide link three separate times.(6)Read protein coordinates from PDBfile.The samefile used to generate the sequence is now read to extract coordinates.In the residue ILE,the atom CD is called CD1in the pdbfile,so we use“pdbalias atom”to define the correct name.If the segment names in the pdbfile match the name we gave in the segment statement,then we don’t need to specify it again;in this case we do specify the segment,so that all atoms in the pdbfile must belong to the segment.(7)Build water segment.Build a segment for the crystal waters.The residue type for water depends on the model,so here we alias HOH to TIP3.Because CHARMM uses an additional H-H bond we must disable generation of angles and dihedrals for segments containing water.Then read the pdbfile.5(8)Read water coordinates from PDBfile.Alias the atom type for water oxygen as well and read coordinates from thefile to the segment SOLV.Hydrogen doesn’t show up in crystal structures so it is missing from this pdbfile.(9)Guessing missing coordinates.The tolopogyfile contains default internal coordinates which can be used to guess the locations of many atoms,hydrogens in particular.In the output pdbfile,the occupancy field of guessed atoms will be set to0,atoms which are known are set to1,and atoms which could not be guessed are set to-1.Some atoms are“poorly guessed”if needed bond lengths and angles were missing from the topologyfile.Similarly,waters with missing hydrogen coordinates are given a default orientation.Write structure and coordinatefiles.Now that all of the atoms and bonds have been created,we can write out the psf structurefile for the system.We also create the matching coordinate pdbfile.The psf and pdbfiles are a matched set with identical atom ordering as needed by NAMD.Using generatedfiles in NAMD.Thefiles bpti.pdb and bpti.psf can now be used with NAMD,but the initial coordinates require minimization first.The following is an example NAMD configurationfile for the BPTI example.#NAMD configuration file for BPTI#molecular systemstructure output/bpti.psf#force fieldparatypecharmm onparameters toppar/par_all22_prot.inpexclude scaled1-41-4scaling1.0#approximationsswitching onswitchdist8cutoff12pairlistdist13.5margin0stepspercycle20#integratortimestep1.0#outputoutputenergies10outputtiming100binaryoutput no#molecular systemcoordinates output/bpti.pdb#outputoutputname output/bptidcdfreq10006#protocoltemperature0reassignFreq1000reassignTemp25reassignIncr25reassignHold300#scriptminimize1000run200004Building solvent around a proteinThe following script illustrates how psfgen and VMD can be used together to add water around a protein structure.It assumes you already have a psf and pdbfile for your protein,as well as a box of water which is large enough to contain the protein.For more information on how atomselections can be used within VMD scripts,see the VMD User’s Guide.proc addwater{psffile pdbfile watpsf watpdb}{#Create psf/pdb files that contain both our protein as well as#a box of equilibrated water.The water box should be large enough#to easily contain our protein.resetpsfreadpsf$psffilereadpsf$watpsfcoordpdb$pdbfilecoordpdb$watpdb#Load the combined structure into VMDwritepsf combine.psfwritepdb combine.pdbmol load psf combine.psf pdb combine.pdb#Assume that the segid of the water in watpsf is QQQ#We want to delete waters outside of a box ten Angstroms#bigger than the extent of the protein.set protein[atomselect top"not segid QQQ"]set minmax[measure minmax$protein]foreach{min max}$minmax{break}foreach{xmin ymin zmin}$min{break}foreach{xmax ymax zmax}$max{break}set xmin[expr$xmin-10]set ymin[expr$ymin-10]set zmin[expr$zmin-10]set xmax[expr$xmax+10]set ymax[expr$ymax+10]set zmax[expr$zmax+10]#Center the water on the protein.Also update the coordinates held#by psfgen.set wat[atomselect top"segid QQQ"]7$wat moveby[vecsub[measure center$protein][measure center$wat]]foreach atom[$wat get{segid resid name x y z}]{foreach{segid resid name x y z}$atom{break}coord$segid$resid$name[list$x$y$z]}#Select waters that we don’t want in the final structure.set outsidebox[atomselect top"segid QQQ and(x<=$xmin or y<=$ymin\or z<=$zmin or x>=$xmax or y>=$ymax or z>=$xmax)"]set overlap[atomselect top"segid QQQ and within 2.4of(not segid QQQ)"]#Get a list of all the residues that are in the two selections,and delete#those residues from the structure.set reslist[concat[$outsidebox get resid][$overlap get resid]]set reslist[lsort-unique-integer$reslist]foreach resid$reslist{delatom QQQ$resid}#That should do it-write out the new psf and pdb file.writepsf solvate.psfwritepdb solvate.pdb#Delete the combined water/protein molecule and load the system that#has excess water removed.mol delete topmol load psf solvate.psf pdb solvate.pdb#Return the size of the water boxreturn[list[list$xmin$ymin$zmin][list$xmax$ymax$zmax]]}5List of Commands•topology<file name>Purpose:Read in molecular topology definitions fromfile.Arguments:<file name>:CHARMM format topologyfile.Context:Beginning of script,before segment.May call multiple times.•pdbalias residue<alternate name><real name>Purpose:Provide translations from residues found in PDBfiles to proper residue names read in from topology definitionfiles.Proper names from topologyfiles will be used in generated PSF and PDB files.This command also exists under the deprecated name alias.Arguments:<alternate name>:Residue name found in PDBfile.<real name>:Residue name found in topologyfile.Context:Before reading sequence with pdb.May call multiple times.•segment<segment ID>{<commands>}Purpose:Build a segment of the molecule.A segment is typically a single chain of protein or DNA, with default patches applied to the termini.Segments may also contain pure solvent or lipid.Arguments:<segment ID>:Unique name for segment,1–4characters.<commands>:Sequence of commands in Tcl syntax to build the primary structure of the segment,8including auto,first,last,residue,pdb,etc.Context:After topology definitions and residue aliases.May call multiple times.Structure informa-tion is generated at the end of every segment command.•auto[angles][dihedrals][none]Purpose:Override default settings from topologyfile for automatic generation of angles and dihedrals for the current segment.Arguments:angles:Enable generation of angles from bonds.dihedrals:Enable generation of dihedrals from angles.none:Disable generation of angles and dihedrals.Context:Anywhere within segment,does not affect later segments.•first<patch name>Purpose:Override default patch applied tofirst residue in segment.Default is read from topology file and may be residue-specific.Arguments:<patch name>:Single-target patch residue name or none.Context:Anywhere within segment,does not affect later segments.•last<patch name>Purpose:Override default patch applied to last residue in segment.Default is read from topology file and may be residue-specific.Arguments:<patch name>:Single-target patch residue name or none.Context:Anywhere within segment,does not affect later segments.•residue<resid><resname>Purpose:Add a single residue to the end of the current segment.Arguments:<resid>:Unique name for residue,1–5characters,usually numeric.<resname>: Residue type name from topologyfile.Context:Anywhere within segment.•pdb<file name>Purpose:Extract sequence information from PDBfile when building segment.Residue IDs will be preserved,residue names must match entries in the topologyfile or should be aliased before pdb is called.Arguments:<file name>:PDBfile containing known or aliased residues.Context:Anywhere within segment.•mutate<resid><resname>Purpose:Change the type of a single residue in the current segment.Arguments:<resid>:Unique name for residue,1–5characters,usually numeric.<resname>:New residue type name from topologyfile.Context:Within segment,after target residue has been created.•patch<patch residue name><segid:resid>[...]Purpose:Apply a patch to one or more residues.Patches make small modifications to the structure of residues such as converting one to a terminus,changing the protonation state,or creating disulphide bonds between a pair of residues.Arguments:<patch residue name>:Name of patch residue from topology definitionfile.<segid:resid>:List of segment and residue pairs to which patch should be applied.Context:After one or more segments have been built.•regenerate[angles][dihedrals]Purpose:Remove all angles and/or dihedrals and completely regenerate them using the segment automatic generation algorithms.This is only needed if patches were applied that do not correct angles and bonds.Segment andfile defaults are ignored,and angles/dihedrals for the entire molecule are regenerated from scratch.9Arguments:angles:Enable generation of angles from bonds.dihedrals:Enable generation of dihedrals from angles.Context:After one or more segments have been built.•multiply<factor><segid[:resid[:atomname]]>[...]Purpose:Create multiple images of a set of atoms for use in locally enhanced sampling.The beta column of the output pdbfile is set to1...<factor>for each image.Multiple copies of bonds,angles, etc.are created.Atom,residue or segment names are not altered;images are distinguished only by beta value.This is not a normal molecular structure and may confuse other tools.Arguments:<factor>:<segid:resid:atomname>:segment,residue,or atom to be multiplied.If:resid is omitted the entire segment is multiplied;if:atomname is omitted the entire residue is multiplied.May be repeated as many times as necessary to include all atoms.Context:After one or more segments have been built,all patches applied,and coordinates guessed. The effects of this command may confuse other commands.•delatom<segid>[resid][atom name]Purpose:Delete one or more atoms.If only segid is specified,all atoms from that segment will be removed from the structure.If both segid and resid are specified,all atoms from just that residue will be removed.If segid,resid,and atom name are all specified,just a single atom will be removed. Arguments:<segid>:Name of segment.<resid>:Name of residue(optional).<atom name>:Name of atom(optional).Context:After all segments have been built and patched.•resetpsfPurpose:Delete all segments in from the structure.The topology definitions and aliases are left intact.Arguments:Context:After one or more segments have been built.•psfcontext[context][new][delete]Purpose:Switches between complete contexts,including structure,topology definitions,and aliases. If no arguments are provided,the current context is returned.If<context>or new is specified,a new context is entered and the old context is returned.If delete is also specified,the old context is destroyed and“deleted<old context>”is returned.An error is returned if the specified context does not exist or if delete was specified and the specified context is the same as the current context. Arguments:<context>:Context ID returned by psfcontext.Context:At any time.•writepsf[charmm][x-plor]<file name>Purpose:Write out structure information as PSFfile.Arguments:charmm:Use CHARMM format(numbers for atom types).x-plor:Use X-PLOR format(names for atom types),the default format required by NAMD.<file name>:PSFfile to be generated.Context:After all segments have been built and patched.•readpsf<file name>Purpose:Read in structure information from PSFfile and adds it to the structure.It is an error if any segments in the PSFfile already exist.Arguments:<file name>:PSFfile in X-PLOR format(names for atom types).Context:Anywhere but within segment.•pdbalias atom<residue name><alternate name><real name>Purpose:Provide translations from atom names found in PDBfiles to proper atom names read in10from topology definitionfiles.Proper names from topologyfiles will be used in generated PSF and PDBfiles.This command also exists under the deprecated name alias.Arguments:<residue name>:Proper or aliased residue name.<alternate name>:Atom name found in PDBfile.<real name>:Atom name found in topologyfile.Context:Before reading coordinates with coordpdb.May call multiple times.•coord<segid><resid><atomname><{x y z}>Purpose:Set coordinates for a single atom.Arguments:<segid>:Segment ID of target atom.<resid>:Residue ID of target atom.<atomname>:Name of target atom.<{x y z}>:Coordinates to be assigned.Context:After structure has been generated.•coordpdb<file name>[segid]Purpose:Read coordinates from PDBfile,matching segment,residue and atom names. Arguments:<file name>:PDBfile containing known or aliased residues and atoms.<segid>:If specified override segment IDs in PDBfile.Context:After segment has been generated and atom aliases defined.•guesscoordPurpose:Guesses coordinates of atoms for which they were not explicitly set.Calculation is based on internal coordinate hints contained in toplogy definitionfiles.When these are insufficient,wild guesses are attempted based on bond lengths of1˚A and angles of109◦.Arguments:None.Context:After stucture has been generated and known coordinates read in.•writepdb<file name>Purpose:Writes PDBfile containing coordinates.Atoms order is identical to PSFfile generated by writepsf(unless structure has been changed).The Ofield is set to1for atoms with known coordinates, 0for atoms with guessed coordinates,and-1for atoms with no coordinate data available(coordinates are set to0for these atoms).Arguments:<file name>:PDBfile to be written.Context:After structure and coordinates are complete.11。



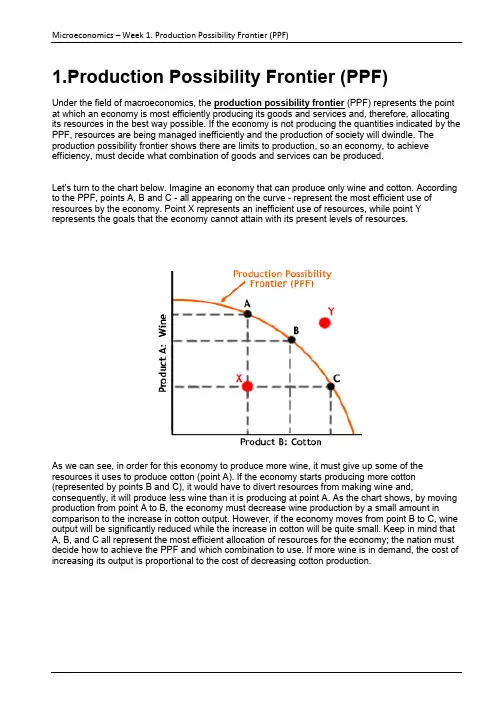
1.Production Possibility Frontier (PPF)Under the field of macroeconomics, the production possibility frontier (PPF) represents the point at which an economy is most efficiently producing its goods and services and, therefore, allocating its resources in the best way possible. If the economy is not producing the quantities indicated by the PPF, resources are being managed inefficiently and the production of society will dwindle. The production possibility frontier shows there are limits to production, so an economy, to achieve efficiency, must decide what combination of goods and services can be produced.Let's turn to the chart below. Imagine an economy that can produce only wine and cotton. According to the PPF, points A, B and C - all appearing on the curve - represent the most efficient use of resources by the economy. Point X represents an inefficient use of resources, while point Y represents the goals that the economy cannot attain with its present levels of resources.As we can see, in order for this economy to produce more wine, it must give up some of the resources it uses to produce cotton (point A). If the economy starts producing more cotton (represented by points B and C), it would have to divert resources from making wine and, consequently, it will produce less wine than it is producing at point A. As the chart shows, by moving production from point A to B, the economy must decrease wine production by a small amount in comparison to the increase in cotton output. However, if the economy moves from point B to C, wine output will be significantly reduced while the increase in cotton will be quite small. Keep in mind that A, B, and C all represent the most efficient allocation of resources for the economy; the nation must decide how to achieve the PPF and which combination to use. If more wine is in demand, the cost of increasing its output is proportional to the cost of decreasing cotton production.Point X means that the country's resources are not being used efficiently or, more specifically, that the country is not producing enough cotton or wine given the potential of its resources. Point Y, as we mentioned above, represents an output level that is currently unreachable by this economy. However, if there were changes in technology while the level of land, labour and capital remained the same, the time required to pick cotton and grapes would be reduced. Output would increase, and the PPF would be pushed outwards. A new curve, on which Y would appear, would represent the new efficient allocation of resources.When the PPF shifts outwards, we know there is growth in an economy. Alternatively, when the PPF shifts inwards it indicates that the economy is shrinking as a result of a decline in its most efficient allocation of resources and optimal production capability. A shrinking economy could be a result of a decrease in supplies or a deficiency in technology.An economy can be producing on the PPF curve only in theory. In reality, economies constantly struggle to reach an optimal production capacity. And because scarcity forces an economy to forgo one choice for another, the slope of the PPF will always be negative; if production of product A increases then production of product B will have to decrease accordingly.2. Opportunity CostOpportunity cost is the value of what is foregone in order to have something else. This valueis unique for each individual. You may, for instance, forgo ice cream in order to have an extra helping of mashed potatoes. For you, the mashed potatoes have a greater value than dessert. But you can always change your mind in the future because there may be some instances when the mashed potatoes are just not as attractive as the ice cream. The opportunity cost of an individual's decisions, therefore, is determined by his or her needs, wants, time and resources (income).This is important to the PPF because a country will decide how to best allocate its resources according to its opportunity cost. Therefore, the previous wine/cotton example shows that if the country chooses to produce more wine than cotton, the opportunity cost is equivalent to the cost of giving up the required cotton production.Let's look at another example to demonstrate how opportunity cost ensures that an individual will buy the least expensive of two similar goods when given the choice. For example, assume that an individual has a choice between two telephone services. If he or she were to buy the most expensive service, that individual may have to reduce the number of times he or she goes to the movies each month. Giving up these opportunities to go to the movies may be a cost that is too high for thisperson, leading him or her to choose the less expensive service.Remember that opportunity cost is different for each individual and nation. Thus, what is valued more than something else will vary among people and countries when decisions are made about how to allocate resources.3. Trade, Comparative Advantage and Absolute Advantage Specialisation and Comparative AdvantageAn economy can focus on producing all of the goods and services it needs to function, but this may lead to an inefficient allocation of resources and hinder future growth. By using specialization, a country can concentrate on the production of one thing that it can do best, rather than dividing up its resources.For example, let's look at a hypothetical world that has only two countries (Country A and Country B) and two products (cars and cotton). Each country can make cars and/or cotton. Now suppose that Country A has very little fertile land and an abundance of steel for car production. Country B, on the other hand, has an abundance of fertile land but very little steel. If Country A were to try to produce both cars and cotton, it would need to divide up its resources. Because it requires a lot of effort to produce cotton by irrigating the land, Country A would have to sacrifice producing cars. The opportunity cost of producing both cars and cotton is high for Country A, which will have to give up a lot of capital in order to produce both. Similarly, for Country B, the opportunity cost of producing both products is high because the effort required to produce cars is greater than that of producing cotton. Each country can produce one of the products more efficiently (at a lower cost) than the other. Country A, which has an abundance of steel, would need to give up more cars than Country B would to produce the same amount of cotton. Country B would need to give up more cotton than Country A to produce the same amount of cars. Therefore, County A has a comparative advantage over Country B in the production of cars, and Country B has a comparative advantage over Country A in the production of cotton.Now let's say that both countries (A and B) specialize in producing the goods with which they have a comparative advantage. If they trade the goods that they produce for other goods in which they don't have a comparative advantage, both countries will be able to enjoy both products at a lower opportunity cost. Furthermore, each country will be exchanging the best product it can make for another good or service that is the best that the other country can produce. Specialization and trade also works when several different countries are involved. For example, if Country C specializes in the production of corn, it can trade its corn for cars from Country A and cotton from Country B. Determining how countries exchange goods produced by a comparative advantage ("the best for the best") is the backbone of international trade theory. This method of exchange is considered an optimal allocation of resources, whereby economies, in theory, will no longer be lacking anything that they need. Like opportunity cost, specialization and comparative advantage also apply to the way in which individuals interact within an economy.Absolute AdvantageSometimes a country or an individual can produce more than another country, even though countries both have the same amount of inputs. For example, Country A may have a technological advantage that, with the same amount of inputs (arable land, steel, labour), enables the country to manufacture more of both cars and cotton than Country B. A country that can produce more of both goods is said to have an absolute advantage. Better quality resources can give a country an absolute advantage as can a higher level of education and overall technological advancement. It is not possible, however, for a country to have a comparative advantage in everything that it produces, so it will always be able to benefit from trade.。

术创新PCR技术在食品微生物检测中的应用与发展魏明斌(黑龙江农业经济职业学院食品药品工程系黑龙江牡丹江157041)摘 要:随着社会经济发展与人民生活水平的提高,食品安全问题受到了社会和人民群众的广泛关注,特别是我国现阶段的发展,使食品安全问题成为备受舆论监督的焦点,可见,食品安全问题在我国已经成为关系到人民身体健康、社会稳定、经济民生的重要问题,而经济市场食品日趋多样化和个性化的发展也对食品安全检测技术提出了更高的目标与要求。
本文从对PCR技术的原理、所需的原料和检测食品微生物的注意事项等几个问题进行阐述,并指出了PCR技术在食品微生物检测中应用的重要意义;然后又从食品食源性菌群检测、食品微生物的有效成分鉴定和转基因食品的鉴定等3个领域的PCR技术应用进行了研究;最后,对PCR技术对食品微生物检测应用发展进行了展望。
关键词:P CR技术微生物检测食品安全DNA聚合酶中图分类号:T S207.3文献标识码:A文章编号:1674-098X(2022)09(a)-0026-03食品安全是我国民生基础性工程的重要组成部分。
当前,在社会经济生活快速发展的今天,食品安全已经为人们所高度关注,特别是食品行业处于快速发展阶段,生产、加工、运输和销售等环节都有可能遭受来自各种环境下的污染,而这之中微生物污染在食品安全中可能尤为严重。
食品的微生物污染可能不仅会导致人的身体受到伤害,严重者甚至可能出现危及生命的情况,因此,对食品的微生物检测工作刻不容缓。
有效的PCR技术可以对食品中的危害物质进行详细的检测,并可以做到及时发现、准确掌握、精准处理,适时做好人们的身体保健工作。
在检测食品的微生物时,在众多的检测技术中,PCR技术更加快捷、方便、易操作,且在我国的食品检测技术中占据了主导地位,成为我国食品检测的关键技术之一,对解决好我国的食品安全起到了重要的作用[1]。
1 PCR技术的相关介绍1.1 PCR技术的概念PCR技术是在20世纪70年代由Khorana提出的原始的设想,而这种技术理论在当时看来是不太可能实现的,直到20世纪80年代才被美国的科学家Kary Mullis正式研究出来[2]。

WHO 第992号技术报告附录3 非无菌工艺验证Annex 3Guidelines on good manufacturing practices: validation,Appendix 7: non?sterile process validationBackgroundThe appendices of the Supplementary guidelines on good manufacturing practices:validation currently comprise the following:Appendix 1. Validation of heating, ventilation and air-conditioning systemsAppendix 2. Validation of water systems for pharmaceutical useAppendix 3. Cleaning validationAppendix 4. Analytical method validationAppendix 5. Validation of computerized systemsAppendix 6. Qualification of systems and equipmentAppendix 7. Non-sterile process validation – revised text reproduced in this Annex1. Background and scope 背景和范围2. Glossary 术语3. Introduction 概述4. Process design 工艺设计5. Process qualification 工艺确认6. Continued process verification 持续工艺确认7. Change management 变更管理References 参考文献1. Background and scope背景和范围Further to the Supplementary guidelines on good manufacturing practices: validation, as published in the World Health Organization (WHO) Technical Report Series, No. 937 (1), additional guidelines to support current approaches to good manufacturing practices (GMP) are published here. These guidelines are intended to further support the concept of process validation linked to quality risk management (QRM) and quality by design principles as described by WHO and the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH).WHO第937号技术报告中公布了GMP增补指南:验证,此外还公布了一些指南来支持现行的GMP实施方法。

PP-Pure®High Purity Polypropylene (PP) Piping Specification PART 1: GENERAL1.1 SummaryFurnish a complete high purity pigmented PP piping system to include pipe, fittings, specialty fittings and valves.1.2 ReferencesThe following standards apply to products used within this section.ASTM D 1598 ASTM D 1599 DIN 8077ASTM D 2122 ASTM D 2657ASTM D 2837 ASTM D 4101DVS 2207-11 ISO 15494The system design shall meet the requirements of ASME/ANSI B31.3 for design criteria where temperature and pressure fall within the limits of the code.1.3 DefinitionsPolypropylene - PPR grey: Random copolymer polypropylene with pigmentation.PP-Pure®: Asahi/America’s pigmented polypropylene high purity piping system.1.4 System Description and Pressure RatingSystem shall be a PP-Pure® system made of uniform pipe and fitting resin. System pressure ratings shall be based on continuous use of 50 years. PP-Pure® pipe, fittings, and valves shall be based on a Standard Dimensional Ratio (SDR) of 11, 1/2” - 12” (20 - 315mm). Pressure rating for pipe and fittings, unless otherwise noted, shall be rated in accordance with ASTM D2837 and DIN 8077 for hydrostatic design basis. Components are rated to 150psi for all SDR 11 material and 150psi for all applied valves at 68° F.1.5 System Performance RequirementsSystem performance requirements shall handle the following:Operating Pressure: (TBD by Engineer/Project Owner)Operating Temp: (TBD by Engineer/Project Owner)Test Pressure: (TBD by Engineer/Project Owner)Media: (TBD by Engineer/Project Owner)1.6 SubmittalsSubmit the following:A. Product data for the system specified; relative to materials, dimensions of individual components, profiles and finishes.B. Product certificates signed by manufacturer of PP-Pure® piping product, stating compliance to stated requirements.C. Welder certificates, certifying that welders comply with the installation procedures as outlined by ASTM D-2657 & DVS 2207-11. All required training should be scheduled and completed at job start-up.D. Qualification of firms supplying PP-Pure®: Firms must have a minimum of five years experience in design, installationand operation of thermoplastic high purity piping systems.1.7 Quality AssuranceObtain components from a single source having responsibility and accountability to answer and resolve problems regarding proper installation, compatibility, performance, and acceptance.1.8 Delivery, Storage and HandlingA. Deliver all PP-Pure® pipe to arrive on-site double bagged for protection inside polyethylene static-free, non-tear bags forcleanroom applications. See section 2.3.B for quantities per bag.B. Deliver all PP-Pure® fittings to arrive on-site double bagged in boxes, when possible, layered with bubble packing orexpanding Styrofoam to prevent damage.C. Store products on elevated platforms in a dry location with protection from the environment. Failure to protect pipe fromdamage during the project may result in longer start up times, pressure failures or premature breakage.D. Lift, support and transport PP-Pure® piping per manufacturers’ recommendations.1.9 WarrantyWarranty period is one year after date of substantial completion. Asahi/America is not responsible for failures due to installation error or neglect.PART 2: PRODUCTS2.1 ManufacturersSubject to compliance with requirements products which may be incorporated in the work include: The PP-Pure® system as supplied by Asahi/America, Inc. of Lawrence, Massachusetts, 800-343-3618. Produced by Alois Gruber GmbH AGRU of Bad Hall, Austria. 2.2 MaterialPipe, valves and fittings shall be made from resin produced by one supplier. The resin shall meet or exceed the requirements outlined for a random copolymer resin according to DIN 16774 and ASTM D 4101-96a. MFI shall be 1.25 g/10min per 230/5. Resin must be approved for contact with foodstuff as per the FDA CFR, Title 21 (2001) 177.1520, and shall be certified as USP Class VI.Manufacturer shall test all lots to ensure the melt flow index is within allowable range.Traceability of all molded and extruded components must be molded into or otherwise printed on the outside of the pipingcomponent.2.3 PipeA. ProductionAll pipe shall be produced on a dedicated extruder completely within a dedicated clean area. Dimensions and tolerances shall exceed ISO 15494 requirement. Surface finish smoothness is as follows:Size (inch) Size (mm) Result1/2 - 1-1/4 20 - 40 Ra = 39.3µ” (1.0µm)1-1/2 - 12 50 - 315 Ra = 31.5µ” (0.8µm)B. PackagingAll pipes shall have capped ends. Pipe shall be sleeved in a PE bag and heat-sealed on both sides. Pipe is then packed into a second PE bag and heat sealed on both sides. The following chart designates quantities of pipe per second PE bag:Size (inch) Size(mm)Quantity PerTube1/2 20 Five 3/4 25 Four 1 32 Three 1-1/4 40 One 1-1/2 50 One 2 63 One 2-1/2 75 One 3 90 One4 110 One6 160 One8 200 One9 225 One10 250 One12 315 OneC. Pressure RatingAll pipe shall meet the requirements of Section 1.4.2.4 FittingsA. ProductionAll standard fittings 1/2” - 12” (20 - 315mm) shall be injected molded. All fittings are to be molded with equipment in a clean environment. After secondary machining all fittings shall be cleaned for a minimum of one hour in an automated hot DI rinse. The DI rinse water shall be 70° C with resistivity above 18MΩand TOC ≤10PPB. Dimensions and tolerances shall exceed ISO 15494 requirement and shall be molded with a central injection gate. Surface finish smoothness is as follows:Size (inch) Size (mm) Result1/2 - 5 20 - 140 Ra = 11.8µ” (0.3µm)6 - 12 160 - 315 Ra = 19.7µ” (0.5µm)B. PackagingAll molded fittings are to be packaged in a class 100 cleanroom immediately after the cleaning process. All machined fittings are to be packaged in a class 1000 cleanroom. Fittings are to be double bagged, purged with clean dry class 100 air or nitrogen in PE/Nylon composite bags. Bags are to be silicone free and anti-static.C. Specialty FittingsSpecialty fittings are to include restraint fittings, butt fusion instrumentation fittings, sanitary connections, etc. Specialty fittings shall be machined or molded of a compatible resin to the PP-Pure® pipe and fittings and shall be packaged according to the requirements of section 2.4.B.D. Pressure RatingAll fittings, unless otherwise noted, shall meet the requirements of Section 1.4.2.5 ValvesAll valves shall be produced in the same manner as high purity fittingsA. Type-342 Spigot Diaphragm Valve:1/2” - 2” (20mm - 63mm) shall be the Type-342 of the PP-Pure® system. The valves shall be molded using a compatible resin with options for EPDM backed PTFE or EPDM diaphragms. Valve bodies are to be unibody, molded design with a full 150psi rating at 70° F. All metal nuts and bolts must be capped or covered to reduce metal exposure. Top works must include integral lockout device on the handle and position indicator.B. Type-343 Zero Dead Leg Valve:1/2” x 1/2” - 2” x 1” (20mm x 20mm - 63mm x 32mm) reduced dead leg (zero dead leg) valves shall be Type-343 style from the PP- Pure®system. Valves shall be made of PP-Pure®resin. Valve bodies are to be unibody, molded design with a full 150psi rating at 68° F (20° C). All metal nuts and bolts must be capped or covered to reduce metal exposure. Top works must include integral lockout device on the handle and position indicator.C. Flanged Diaphragm Valves:1/2” - 10” (20mm - 250mm) shall be Type-14, Type-15 or Type-G pigmented polypropylene unibody with flanges molded as part of the body. Diaphragm shall be a two-piece style with PTFE and separate EPDM backing. Top works shall include a position indicator and travel stop. Supplied cleaned and double bagged.D. Butterfly Valves:1-1/2” - 12” (50mm - 315mm) shall be Type-57P manual gear operated valves with a polypropylene disc and ANSI 150# bolt pattern. Liners shall be available in EPDM or FKM materials. Supplied cleaned and double bagged.E. Ball Valve:PP-Pure® ball valves shall be available 1/2” - 2” (20mm - 63mm). O-rings are to be FKM or EPDM. Ball valves shall be offered in spigot ends. Supplied cleaned and double bagged.F. Ball Check Valves:1/2” - 4” (20mm - 110mm) shall be polypropylene ball type check with FKM or EPDM seat and seals. Ball check valves shall be offered in spigot ends. Supplied cleaned and double bagged.G. V182 Pressure Regulating Valve with Gauge Guard:1/2” - 4” (20mm - 110mm) shall be polypropylene V182 with EPDM/EPDM or PTFE/FKM diaphragms/seals. 1/2” - 1-1/2”(20mm - 50mm) shall be pressure rated to 150psi at 68° F. 2” - 3” (63mm - 90mm) shall be pressure rated to 90psi at 68° F. 4”(110mm) shall be pressure rated to 60psi at 68° F.H. V186 Back Pressure Regulating Valve:1/2” - 2-1/2” shall be polypropylene V182 with EPDM/EPDM or PTFE/FKM diaphragms/seals. 1/2” - 1-1/2” shall be pressure rated to 150psi at 68° F. 2” shall be pressure rated to 90psi at 68° F.I. Flow Meters:Available in polysulphone (PSU) and polyamide (PA) Frank M123 and M335 flow meters shall be supplied with spigot ends suitable for high purity welding. Flow ranges available up to 135 gallons-per-minute with custom ranges available.2.6 Pipe/Valve Hangers and SupportsA. Support SpacingDesign pipe supports and anchors in accordance with Asahi/America’s recommended support spacing for SDR 11 polypropylene pipe. See Section 4.3 for recommended support spacing for PP-Pure® piping systems.B. Supports and HangersUse Asahi/America’s recommended support types per document Asahi/America Engineering Design Guide. Metallic supports and clamps shall not come directly into contact with plastic piping systems.2.7 Joining EquipmentPP-Pure® installations shall be performed by factory certified and trained installers in accordance with manufacturer’s ISOprocedures, ASTM D 2657 and DVS 2207-11. Date of certification or re-certification shall not exceed two years from the beginning of project. Available joining techniques are as follows:A. Butt FusionProper equipment selection should be based on pipe size and site conditions. Butt fusion equipment should be designed and tested to provide reliable welds. All equipment should utilize electronically controlled heating elements for accurate welding temperatures.Tools should also incorporate planing units to face ends prior to heating. Butt fusion equipment supplied shall weld joints based on force or pressure and not mechanical stops.B. Non-Contact Butt FusionProper equipment selection should be based on installation requirements and line sizes. Tool shall be fully automatic (SP series).SP-S Series:Tool shall be made available in the following modelsSP 110-S 1/2” - 4” (20mm - 110mm)SP 250-S 4” - 10” (110mm - 250mm)SP 315-S 4” - 12” (110mm - 315mm)Tools shall possess electronic planer and infrared heating element. Tools will utilize and measure the welding pressures to join material and not mechanical stops. To avoid improper welded joints, tool shall automatically operate clamps and control joining force. Tools shall possess the following features:1. Computer control and automatic fusion.2. Touch screen for tool operation and parameter selection.3. Restricted access through use of barcode or RFID cards.4. Automatic label printouts after each weld.5. Ability to display and graph weld processes as weld is proceeding.6. Memory storage of welds7. Magnetic clamps to reduce change out time from one size to another.8. Vertical and horizontal adjustment for pipe alignment.C. Beadless FusionSP-B Series:SP 110-B: 1/2” - 4” (20mm - 110mm)Tools shall possess electronic planer and heating element. To avoid improper welded joints, tool shall automatically operate clamps, heater movement, heater closing and control joining force. Tools shall possess identical features as:1. Computer control and automatic fusion.2. Touch screen for tool operation and parameter selection.3. Restricted access through use of RFID cards.4. Automatic label printouts after each weld.5. Ability to display and graph weld processes as weld is proceeding.6. Memory storage of welds7. Magnetic clamps to reduce change out time from one size to another.8. Vertical and horizontal adjustment for pipe alignment.PART 3: EXECUTION3.1 Piping AssemblyA. FacilitiesSubassembly and fabrication work should be conducted in a separate, temporary clean room located within the building.Cleanroom should be equipped with the following to provide a clean installation:1. Provide laminar flow HEPA filters in room ceiling to reach a level of class 10,000.2. The quantity of filters should be determined by providing a minimum of 60 room air changes per hour.3. Nitrogen should be available for purging the pipelines with a positive pressure if the assemblies expand beyond the bounds of theroom.B. ToolsAll fusion tools utilized are to be dedicated for clean build only, and should be kept separate. Special attention should be given to the fusion tools to prevent the possibility of contaminating a weld. The contractor shall lease or purchase all necessary weldingequipment from the manufacturer. At the end of the installation, any necessary equipment needed on-site should be sold to the owner. Contractor is responsible for proper maintenance and care of the fusion tools during construction.C. CertificationInstallers shall be pre-qualified as per section 2.7. Manufacturer shall provide on-site training in the assembly and installation of the PP-Pure® piping system as needed.D. Installation of Flanged ConnectionsFor each flange connection, only Asahi/America seal clean expanded PTFE (e-PTFE) gaskets should be used. Ensure that each gasket is centered according to manufacturer’s recommendation. Apply the recommended backing rings and torques. Ensure correct support of piping in the area of flange connection.3.2 TestingA. InspectionPrior to pressure testing, the system shall be examined for the following items:1. Pipe shall be completed per drawing layout with all pipe and valve supports in place.2. Pipe, valves, and equipment shall be supported as specified, without any concentrated loads on the system.3. Pipe shall be in good conditions, void of any cracks, gouges or deformation.4. Pipe flanges shall be properly aligned. All flange bolts should be checked for correct torques.5. All diaphragm valve bonnet bolts shall be checked for correct torques.6. All joints should be reviewed for appropriate welding technique:Non-Contact: Identity labels shall identify weld certification by the print “welding parameters OK”. Joints should have twobeads 360° around the joint.Butt: To have two beads, 360° around the joint.Manufacturer to supply inspection procedures beyond above recommendations. If any deficiencies appear, the quality control manager shall provide directions for repair.B. Pressure Test1. Test fluid should be deionized water, with quality level set by quality control engineer. In all cases test must be donehydrostatically. Air test is not allowed.2. Filling the system: Open all valves and vents to purge the system of air. Slowly inject the water into the system, making sure thatair does not become trapped in the system.3. Begin pressurizing the system in increments of 10psi. Bring the system up to 100psi and hold. Allow system to hold pressure fora minimum of two hours and up to a recommended 12 hours. Check pressure gauge after one hour. Due to natural creep effects onplastic piping, the pressure will have decreased. If drop is less than 10%, pump the pressure back up. At this time, the system may be fully pressurized to desired test pressure.4. If after one hour the pressure has decreased more than 10%, test is considered a failure. Note the 10% value may need to begreater for larger systems, or systems experiencing significant thermal changes.5. Test is to be witnessed by quality control engineer and certified by the contractor.3.3 Cleaning of PP-Pure® Piping SystemSystem shall be cleaned at completion of project according to requirements set by owner.PART 4: APPENDICESDisclaimer: This information is provided for convenience. For additional information, please consult Asahi/America’s engineering design guide or contact our engineering staff at 781-321-5409.4.1 Material PropertiesTable 1 - Material Properties of PP-Pure ® PPPropertiesCondition Standard Units PP-R P h y s i c a l Density 23° C (73.4° F) ISO 1183 g/cm 3 0.91 Melt Flow Rate230° C/5 kg 190° C/5 kg ISO 1133 g/10min 1.25 0.5 M e c h a n i c a l P r o p e r t i e sTensile stress at yield 50 mm/min ISO 527 MPa 25 Elongation at yield 50 mm/min ISO 527 % 12 Elongation at break 50 mm/min ISO 527 % >300 Impact strength unnotched23° C (73.4° F) ISO 179/1eUkJ/m 2no break 0° C (32° F) no break -20° C (-4° F)40 Impact strength notched23 °C (73.4° F)ISO 179/1eAkJ/m 220 0° C (32° F) 3.5 -20° C (-4° F)2 Ball indentation hardness according to RockwellISO 2039-1 MPa 45 Flexural strength (3.5% flexural stress)ISO 178MPa 20 Modulus of elasticityISO 527 MPa 900 T h e r m a l P r o p e r t i e s Vicat-Softening point VST/B/50 ISO 306 °C °F 65 149 Heat deflection temperature HDT/BISO 75 °C °F 70 158 Linear thermal expansion coefficientDIN 53752 K -1 x 10-4 1.5 Thermal conductivity 20° C (68° F)DIN 52612 W/(m x K)0.24 FlammabilityUL94 DIN 4102 94-HB B2 E l e c t r i c a l P r o p e r t i e s Specific volume resistance VDE 0303 Ω x cm >1016 Specific surface resistanceVDE 0303 Ω>1013 Dielectric constant 1 MHzDIN 53483 2.3 Dielectric strength VDE 0303 kV/mm70 G e n e r a lPhysiologically nontoxic EEC 90/128 Yes FDAYes USP Class VI Yes UV ResistanceNo ColorPP-Pure ® GreyRAL 7032 PolyPure ®Natural4.2 Pressure RatingPermissible operating pressure for PP-Pure® piping systems based on years of operation and temperature. These tables are for water, a safety correction factor shall be applied for chemical service. Consult Asahi/America Engineering staff for chemical recommendation. Additionally, a system reduction factor of 0.8 shall be used for influences such as welding, joints, flange, and bending loads for aboveground installations and 1.0 should be used for below ground installation.Table 2 - Permissible Operating Pressures for PP-Pure® PP (psi)Temperature 1 Year 5 Years10 Years25 Years 50 Years100Years PP 150SDR 11PP 150SDR 11PP 150SDR 11PP 150SDR 11PP 150SDR 11PP 150SDR 11° C ° F10 50 306 289 281 271 265 258 20 68 261 245 239 231 225 219 30 86 222 209 203 196 190 - 40 104 189 176 171 164 160 - 50 122 160 148 144 138 135 - 60 140 135 125 120 116 112 - 70 158 113 104 102 87 74 - 80 176 94 84 71 57 - - 90 194 78 55 46 - - - 95 203 67 45 - - - -4.3 Support SpacingTable 3 - PP-Pure® PP Support Spacing (feet)Pipe Size 68° F/20° C 86° F/30° C104° F/40° C122° F/50° C140° F/60° C158° F/70° C176° F/80° Cmm inch20 1/2 1.7 1.7 1.6 1.5 1.5 1.4 1.425 3/4 2.0 1.9 1.8 1.8 1.7 1.7 1.632 1 2.3 2.3 2.2 2.2 2.1 2.0 1.840 1-1/4 2.7 2.6 2.6 2.5 2.3 2.3 2.250 1-1/2 3.1 3.0 3.0 2.8 2.7 2.6 2.563 2 3.6 3.5 3.4 3.3 3.2 3.1 3.075 2-1/2 3.8 3.7 3.6 3.4 3.3 3.2 3.190 3 4.1 3.9 3.8 3.7 3.6 3.4 3.3110 4 4.6 4.4 4.3 4.2 3.9 3.7 3.4125 4-1/2 4.9 4.8 4.7 4.4 4.2 3.9 3.7140 5 5.2 5.0 4.9 4.7 4.4 4.2 3.9160 6 5.5 5.4 5.2 4.9 4.7 4.4 4.2180 7 5.8 5.7 5.4 5.2 4.9 4.7 4.4200 8 6.2 5.9 5.7 5.4 5.2 4.9 4.7225 9 6.5 6.3 6.0 5.8 5.5 5.3 4.9250 10 6.9 6.6 6.4 6.2 5.9 5.7 5.3280 11 7.3 7.0 6.8 6.5 6.3 6.0 5.7315 12 10.3 10.0 9.7 9.4 8.9 8.5 8.0For gases and fluids with different densities, the conversion factors shown below should be used.LL=LL AA∗ff1f1 – conversion factor (See Table 4)L – new support distance [mm]L A – permissible support distance (See Table 3)Table 4 - External Support Spacing Correction Factors basedon Operating Media Density for PPMaterial SDRConversion factor f1Media density [g/cm3]Gases Water Other Media <0.01 1.00 1.25 1.50PP-Pure® 11 1.30 1.0 0.96 0.92。

Internal auditing practices and internal control systemFaudziah Hanim Fadzil; Haron.Hasnah; Jantan.Muhamad.Internal auditing has undergone dramatic changes that have expanded its scope in a way that allows it to make greater contributions to the organization it serves. Internal auditing is also performed in diverse legal and cultural environments; within organizations that vary in purpose, size, and structure; and also by persons within or outside the organization. Furthermore, the internal auditing profession also walks a tightrope between serving as a management consultant and an independent professional. A survey done by the Malaysian Institute of Corporate Governance (MICG), The Institute of Internal Auditors Malaysia (Π AM) and Ernst and Young concluded that internal auditors are best placed to understand and appreciate the business processes of a company and they act as management consultant to reduce risks. Internal auditors also help run a company more efficiently and effectively to increase shareholders' value.As this is the case, internal auditors need to be out in front, leading the business units with regards to the internal control system and also focusing on strategic business objectives. The internal auditors also need to establish themselves as vital cogs in their organizations, rather than as observers who watch from the periphery and wait for events to impact them (Sawyer and Vinten, 1996).One issue that has emerged related to the internal auditing practices is; "what is a proper and sound measurement of the internal auditing practices?" Barrett (1986) notes, "effectiveness (of internal audit) can be described, but it is difficult to quantify and in the final analysis, effectiveness is determined by the perception of auditees". In the company environment, management is the most important auditee of the internal audit department since effectiveness of the internal auditing practices can be described through the expectations of management with regard to the internal auditing practices. The management will expect the internal auditors to perform their internal auditing practices to a certain level that is complying with the Standards for the Professional Practice ofInternal Auditors (SPPIA) (now known as the Professional Practice Framework (PPF)), since it can be easily described. Compliance with SPPIA is therefore an indication of the effectiveness of the Internal Audit Department.The auditing profession, both internal and external, has come under increasing scrutiny since the highly publicised collapse of energy trader, Enron (Vinten, 2003) in the USA last year. The uncovering of alleged irregularities at Technology Resources Industries Berhad (TRI) has highlighted the need for greater corporate governance in Malaysia.TRI had issued false invoices between 1998 and 1999, which amounts to MYR260 million (USD 68 million). According to Dato' Samad Alias, the President of the Malaysian Institute of Accountants (MIA), "This could be the largest accounting fraud in Malaysian history". The false invoices was discovered by Telekom Malaysia, who took control of the TRI in June 2002, after conducting several internal audits, when Arthur Andersen was TRI's auditor. "We had to rely on the information prepared by the company's accountants", said Dato' Abdul Samad, who was then head of the Andersen's Malaysian office. If TRI was to restate 1998 and 1999 accounts, its net book value will reduce as much as MYR 198 million (Financial Times, 2002). The case has certainly shed a light on the importance of the role of internal auditors, which was only recognised and emphasised after irregularities were discovered.Internal auditors are often described as both a business partner and a policeman because of his work as a business partner with client management and also because he acts as an independent reviewer of management. As a business partner, the internal auditor is expected to provide expertise to assist an organization in meeting its objectives while as a policeman; an internal auditor is often thought of as an adversary looking for flaws. As such, the extent of the internal auditing practices plays an important factor on these roles. This study will provide empirical evidence whether compliance with internal auditing practices will lead to a better internal control system.The development of SPPIASPPIA are the criteria by which the operations of an internal auditing department are evaluated and measured and intended to represent the internal auditing practices, as it should be. It is also meant to serve the entire profession of internal auditing, in all types of organisations where internal auditors are found. It was first developed in June 1978, at the Internal Auditors International Conference in San Francisco. The SPPIA comprise of five general standards of:(1) independence;(2) professional proficiency;(3) scope of work;(4) performance of audit work; and(5) management of the internal audit department.This framework allowed the future expansion of the internal auditing practices until the new framework was released in January 2002.A new framework, called PPF was released in January 2002[1] and it consisted of three sets of standards: attribute, performance, and implementation standards. The attribute standards address the attributes of organisations and individuals performing internal audit services while the performance standards describe the nature of internal audit services and provide quality criteria against which performance of these services can be measured. The implementation standards on the other hand provide guidance applicable in specific types of engagements. However, this new framework is not utilised in this study because of the timing of its release.Internal control system. The internal control system plays an important role in the internal auditing practices since the internal auditors might be considered as being specialists in management control (Chambers et al, 1987). Internal auditing practices appraise the effectiveness of internal control systems, which is a definition of internal auditing and which also includes an appraisal of the actions by management to correct situations, which are at variance with planned outcomes. The definition of internal control systems reveals that it is not fundamentally different from management control, which has an essential component of control such as planning, organising, staffing and directing (Chambers et al, 1987). Senior management and the audit committee normally expect that the CAE will perform sufficient audit work and gather other available information during the year so as to form a judgement about the adequacy and effectiveness of the control processes. The CAE should then communicate that overall judgement about the organisation's system of controls to the senior management and the audit committee. This is necessary since internal auditors play an intermediary role and assist in the discharge of the oversight function of audit committee. If the above internal audit function is not available, the management needs to apply other monitoring processes in order to assure itself and the board that the system of internal control system is functioning as intended. In thesecircumstances, the board of the company will need to assess whether such processes provide sufficient and objective assurance or regular review and appraisal of the adequacy and the integrity of the internal control systems in the company.Bursa Listing Requirements has established industry taskforce which formulated the "Statement on Internal Control: Guidance For Directors of Public Listed Companies" to fulfil the above circumstances. The aim of this guidance is to assist listed companies in making disclosures in their annual reports on the state of internal control, in compliance with the Listing Requirements of the KLSE. Pursuant to the requirements of the Code in relation to the Internal Audit Function, in May 2001, the securities Commission appointed the HAM to establish a separate industry taskforce to formulate these Guidelines to assist the board of public listed companies in effectively discharging their responsibilities in relation to establishing an Internal Audit Function. Risk assessment, control environment, control activities, information and communication and monitoring are five important characteristics in this guideline.The roles of internal auditors. In the revised statement of responsibilities of internal auditing issued by the Institute of Internal Auditors (IIA) (2000) as part of the standards framework, the section on objectives states:The objective of internal auditing is to assist all members of management in the effective discharge of their responsibilities by furnishing them with analyses, appraisals, recommendations and pertinent comments concerning the activities reviewed. The internal auditor is concerned with any phase of business activity where he can be of service to management. This involves going beyond accounting and financial records to obtain a full understanding of the operations under review (p. 3).Sawyer and Vinten (1996) noted four benefits managers have gained from internal auditing assistance. These benefits were providing managers with the bases for judgement and action, helping managers by reporting weaknesses in control and performance and in recommending improvements, providing counsel to managers and boards of directors on the solutions of business problems, and supplying information that is timely, reliable and useful to all levels of management.Additionally, the statement sets forth the types of services that should be performed and the kinds of activities carried on by the internal audit function in attaining the overall objective. Internal auditors should first review and appraise the soundness and adequacy of the accounting, financial, and other operating controls, and promote effective controlsat reasonable cost. secondly, the internal auditors should ascertain the extent of compliance with established policies, plans, procedures, laws and regulations, which could have a significant impact on the company's operations. Then the internal auditors review the means of safeguarding assets and when appropriate, verify the existence of such assets and appraise the economy and efficiency with which resources are employed. Lastly, the internal auditors review operations or programs to ascertain whether results are consistent with established objectives and goals and whether the operations or programs are being carried out as planned.Reviewing and evaluating the adequacy and effectiveness of an organisation's internal control system and the quality of performance in carrying out assigned responsibilities is representative of several primary core activities of internal audit work. The purpose of the review of the adequacy of the internal auditing is to ascertain whether the established system provides reasonable assurance that the organisation's objectives and goals will be met efficiently and economically.Adequate control is considered to be present if administrative management has planned and organised in a manner, which provides reasonable assurance that the organisation's objectives and goals will be achieved efficiently and economically. Reasonable assurance is provided when cost-effective actions are taken to restrict deviations, such as improper or illegal acts, to a tolerable level.The role of internal auditing in the review of effectiveness of the system of internal control is to ascertain whether the system is functioning as intended. Effective control is present when the administrative management directs the system in such as way as to provide reasonable assurance that the organisation's objectives and goals will be achieved. The purpose of the review for quality of performance is to ascertain whether the organisation's objectives and goals have been achieved.The primary objectives of an organisation's system of internal control are to provide administrative management with reasonable assurance that financial information is accurate and reliable; the organisation complies with policies, plans, procedures, laws, regulations and contracts; assets are safeguarded against loss and theft; resources are used economically and efficiently; and established objectives and goals for operations or programs can be met. Internal auditing focuses on an evaluation of this system or framework of internal control.A second type of audit work that internal auditors are guided to perform is reviewing the accuracy and reliability of financial and operating information and the means used to identify, measure, classify and reportsuch information. Information systems provide data for decision-making, control, and compliance with external requirement. Therefore, internal auditors should examine information systems and determine whether financial and operating records and reports contain accurate, reliable, timely, complete and useful information, and controls over record keeping and reporting are adequate and effective.The performance of reviews of the systems established to ensure compliance with policies, plans, procedures, laws, regulations and contracts represents a third element of audit activity described by the standards. Administrative management is responsible for establishing the systems designed to ensure compliance with such requirements as laws, rules, regulations, policies and procedures. The internal auditors role is to determine whether the systems designed by management are adequate and effective and whether the activities audited are complying with the appropriate requirements.Further, as described by the standards, the internal auditor's role includes providing appraisals with recommendations regarding administration management established objectives and goals for operations and programs.。
CMMI 基本介绍V2.0目录1组织成熟度级别和类别 (3)2通用目标和通用实践 (5)3RD 需求开发REQUIREMENTS DEVELOPMENT (7)4REQM 需求管理REQUIREMENTS MANAGEMENT (9)5PP 项目策:划PROJECT PLANNING (10)6PMC 项目监督和控制PROJECT MONITORING AND CONTROL (12)7RSKM 风险管理RISK MANAGEMENT (14)8SAM 供应商协议管理SUPPLIER AGREEMENT MANAGEMENT (15)9CM 配置管理CONFIGURATION MANAGEMENT (16)10PPQA 过程和产品质量保证PROCESS AND PRODUCT QUALITY ASSURANCE (17)11MA 度量和分析MEASUREMENT AND ANALYSIS (18)12DAR 决策分析和解决DECISION ANALYSIS AND RESOLUTION (19)13TS 技术解决方案TECHNICAL SOLUTION (20)14PI 产品集成PRODUCT INTEGRATION (21)15VER 验证VERIFICATION (22)16VAL 确认VALIDATION (24)17OPF 组织过程聚焦ORGANIZATIONAL PROCESS FOCUS (25)18OPD 组织过程定义ORGANIZATIONAL PROCESS DEFINITION 27 19OT 组织培训ORGANIZATIONAL TRAINING (28)20IPM 集成项目管理INTEGRATED PROJECT MANAGEMENT 29 21OPP 组织过程性能ORGANIZATIONAL PROCESS PERFORMANCE (31)22QPM 量化项目管理QUANTITATIVE PROJECT MANAGEMENT 32 23CAR 因果分析和解决CAUSAL ANALYSIS AND RESOLUTION 33 24OPM 组织性能管理ORGANIZATIONAL PERFORMANCE MANAGEMENT (34)1组织成熟度级别和类别2通用目标和通用实践3RD 需求开发Requirements Developme nt4REQM 需求管理RequirementsMan ageme nt目的:管理项目的产品及产品组件需求,并标识出这些需求与项目策划及工作产品之间的不5PP 项目策划Project Planning6 PMC 项目监督和控制ProjectMonitoring and Control目的:目的在于了解项目的进度,以便项目在执行性能严重偏离项目策划时,可采取适当的纠正措施。
WHO第961号技术报告附件7 药物生产技术转移指南World Health OrganizationWHO Technical Report Series, No. 961, 2011WHO第961号技术报告附件7 药物生产技术转移指南Annex 7附件7WHO guidelines on transfer of technology in pharmaceutical manufacturingWHO药物生产技术转移指南1. Introduction介绍2. Scope范围3. Glossary术语4. Organization and management组织和管理5. Production: transfer (processing, packaging and cleaning)生产:转移(工艺、包装和清洁)6. Quality control: analytical method transfer质量控制:分析方法转移7. Premises and equipment厂房设施和设备8. Documentation文件9. Qualification and validation确认和验证References参考文献1.Introduction介绍These guiding principles on transfer of technology are intended to serve as a framework which can be applied in a flexible manner rather than as strict rigid guidance. Focus has been placed on the quality asp ects, in line with WHO’s mandate.本指南中关于技术转移的原则意在作为一个框架,以不同方式应用,而不是一个需要严格遵守的指南。
【行业资讯】ISPE发布第三版技术转移指南(中英对照版)Good Practice Guide: Technology Transfer 3rd Edition 良好实践指南:技术转移第三版Transfer of manufacturing processes and analytical procedures between facilities or laboratories is a necessary part of pharmaceutical development and commercialization. Technology transfers take the outputs of process or method development activities and transfer the knowledge to a different location where a process or analytical procedure will be operated.在工厂或实验室之间转移生产工艺和分析程序是药品开发和商业化的必要部分。
技术转移获取工艺或方法开发活动的结果,并将知识转移到工艺或者方法执行的不同位置。
This Guide presents industry good practices for successful and efficient execution of technology transfer projects. It intends to achieve a balance between risk management and cost effectiveness while aligning with applicable regulatory expectations.本指南介绍了成功和有效地执行技术转移项目的良好做法。
它旨在实现风险管理和成本效益之间的平衡,同时符合适用的监管预期。
Minister Date Name of organisationor individual Purpose of meetingThe Rt Hon Iain Duncan Smith Secretary of S Jul-15Sister Rita Lee, Lalley Centre Discuss Welfare ReformThe Rt Hon Iain Duncan Smith Secretary of S Sep-15Board of Deputies of British JeEmployers guide to Judaism Launch The Rt Hon Iain Duncan Smith Secretary of S Sep-15Strategic Defence Initiatives Introductory Meeting with CEOThe Rt Hon Iain Duncan Smith Secretary of S Sep-15CISCO Work & Pensions ProjectJustin Tomlinson MP Minister for Disabled P Jul-15Capita Introductory Meeting with CEO Justin Tomlinson MP Minister for Disabled P Jul-15Parkinsons UK/MIND PIP RulesJustin Tomlinson MP Minister for Disabled P Jul-15Deaf Council Access to work CapJustin Tomlinson MP Minister for Disabled P Jul-15FA Mars Just Play Awards Just Play ProgrammeJustin Tomlinson MP Minister for Disabled P Jul-15Attitude is everything Introductory Meeting with CEO Justin Tomlinson MP Minister for Disabled P Jul-15Burdus Ltd Disability ConfidentJustin Tomlinson MP Minister for Disabled P Jul-15Paralympic Legacy Advisory G Introductory Meeting with co-chair Justin Tomlinson MP Minister for Disabled P Jul-15Google Introductory Meeting with directors Justin Tomlinson MP Minister for Disabled P Jul-15National Union of British Sign Access to work fundingJustin Tomlinson MP Minister for Disabled P Jul-15Disability Charities ConsortiumRegular catch up meetingJustin Tomlinson MP Minister for Disabled P Jul-15Employment Related Services Supporting Jobseekers with disabilities Justin Tomlinson MP Minister for Disabled P Jul-15Carers UK Carers AllowanceJustin Tomlinson MP Minister for Disabled P Jul-15Business Disability Forum Introductory Meeting with directors Justin Tomlinson MP Minister for Disabled P Jul-15IntoWork Convention Discuss Labour MarketJustin Tomlinson MP Minister for Disabled P Jul-15Hearing Dogs Introductory MeetingJustin Tomlinson MP Minister for Disabled P Jul-15Institute of Personal DevelopmDisability ConfidentJustin Tomlinson MP Minister for Disabled P Jul-15Shaw Trust/Whizz Kids Encouraging disabled to workJustin Tomlinson MP Minister for Disabled P Jul-15Atos Progress and CapacityJustin Tomlinson MP Minister for Disabled P Jul-15Industry Injuries Advisory Cou Annual MeetingJustin Tomlinson MP Minister for Disabled P Jul-15Equality & Human Rights Com Working in PartnershipThe Rt Hon Lord Freud Minister of State for Jul-15Payments Council Richer DataThe Rt Hon Lord Freud Minister of State for Jul-15Retailcure Credit UnionsThe Rt Hon Lord Freud Minister of State for Sep-15Peter Seymour Richer DataThe Rt Hon Lord Freud Minister of State for Sep-15Local Govt Association Universal Credit & Support The Rt Hon Lord Freud Minister of State for Sep-15Council of Mortgage lenders M ortgage InterestThe Rt Hon Lord Freud Minister of State for Sep-15President of Employment Trib Fit for WorkBaroness Altmann CBE Minister of State for Jul-15NEST Private Pensions Baroness Altmann CBE Minister of State for Jul-15Retirement Solutions Private Pensions Baroness Altmann CBE Minister of State for Jul-15Lloyds Banking Group Private Pensions Baroness Altmann CBE Minister of State for Jul-15TPR Private Pensions Baroness Altmann CBE Minister of State for Jul-15Pensions Ombudsman Private Pensions Baroness Altmann CBE Minister of State for Jul-15ACA Private PensionsLCPAONIFOABaroness Altmann CBE Minister of State for Jul-15SERCO Labs New state pension digita Baroness Altmann CBE Minister of State for Aug-15Pension Regulator Quarterly meeting Baroness Altmann CBE Minister of State for Aug-15PPF Introductory Meeting Baroness Altmann CBE Minister of State for Sep-15Gleneagles Financial ServicesThe Rt Hon Priti Patel Minister of State for E Jul-15Working links Roundtable; Work ProgrammeERSA, A4E, Avanta, G4SIngeus, InterserveLearndirect, MaximusNewcastle College GroupPertempsPeople Development GrpProspects, RehabReed in PartnershipJobfilt, Seetec, SercoShaw TrustThe Rt Hon Priti Patel Minister of State for E Jul-15Maximus Introductory Meeting CEO The Rt Hon Priti Patel Minister of State for E Jul-15CITB Support for NEETSThe Rt Hon Priti Patel Minister of State for E Jul-15British Retail Consortium Partnership WorkingThe Rt Hon Priti Patel Minister of State for E Jul-15British services Ass Partnership Working。
指南解读:PPF的提出及诊疗方法2022年5月1日,美国胸科学会(ATS)、欧洲呼吸学会(ERS)、日本呼吸学会(JRS)和拉丁美洲胸科协会(ALAT)联合发布了《成人特发性肺纤维化(更新)和进行性肺纤维化:ATS/ERS/JRS/ALAT 官方临床实践指南》[1](以下简称ATS/ERS/JRS/ALAT指南)。
该指南提出了进行性肺纤维化(PPF)这一概念,本文将提炼其中的重点内容,以飨读者。
PPF的提出及诊断此前,进行性纤维化性间质性肺病(PF-ILD)更多被临床用来描述ILD纤维化进展后表现出的共同特征。
此次指南委员会通过讨论最终达成共识,以PPF替代PF-ILD,并定义为已知或未知病因、存在肺纤维化影像学证据的ILD患者(IPF除外),并在既往1年内发生以下3项标准中的至少2项,且不能用其他原因解释:在所有PPF定义标准中,指南委员会特别指出,FVC和胸部高分辨计算机断层扫描(HRCT)是相对特异性较高的指标。
而生理学进展中的另一项指标——一氧化碳弥散量(经血红蛋白校正)虽然特异性相对较低,但这一指标在预测多种纤维化性肺疾病患者的死亡率方面,是一个一致性高且强有力的预测因子。
PPF的治疗方法超过90%的指南委员会专家建议使用尼达尼布治疗经纤维化ILD (IPF除外)标准治疗失败的PPF患者。
这一治疗建议是基于已发布的里程碑式临床Ⅲ期试验——INBUILD研究的结论。
本研究纳入了663例PF-ILD(IPF除外)患者,经过长达52周的试验后,研究结果显示,尼达尼布组患者的FVC下降率为每年-80.8mL,安慰剂组为每年-187.8mL,组间差异为每年107.0mL[2],其第二次锁库的数据显示,尼达尼布可降低PF-ILD患者首次急性加重或死亡风险33%[3]。
在临床上,特发性非特异性间质性肺炎(iNSIP)和慢性过敏性肺炎(HP)是较常见的两类ILD疾病,也都很容易进展为PPF。
因此临床医生还应该加强对这两类疾病患者的鉴别,一旦有PPF表现,尽早考虑使用尼达尼布进行抗纤维化治疗,延缓疾病进展。
PPFPost Processing Framework Guidelines for application developers June 30, 2003Contact Persons: Daniel-Alexander Heller (PPF)Contents:1PPF Overview (4)2Preparation (5)2.1Necessary Classes (5)The implementation steps are explained using the example of a demo application. The demo application and all mentioned classes are available in all systems (transaction SPPFDEMO, development class SPPF_DEMO). (5)2.1.1Application Class (5)2.1.2Application-Specific Processing (9)2.2Customizing (15)2.2.1Define New Application (15)2.2.2Defining the Action Profile (16)2.2.3Define Action Definitions for the Action Profile (17)2.2.4Details for the Action Definition (18)2.2.5Assignment of Processing Types for an Action Definition (20)2.3Determination and Merging of Actions (23)2.3.1Determination (Condition Configuration) (23)2.3.2Action Merging (28)3Interaction Between Application and PPF at Runtime (29)3.1Calling the PPF (29)3.2Processing Actions (32)3.2.1Immediate Processing (32)3.2.2Processing with Document Posting (32)3.2.3Later Processing (32)3.2.4Manual Triggering of Processing in the Dialog (32)3.3User Interface at Runtime (33)3.3.1Standard User Interface (33)3.3.2Connection of Generic Object Services (GOS) (35)3.4Transaction Concept (36)3.4.1Overview (36)3.4.2Object Pool (36)4Administration User Interface (38)5Extendibility (39)5.1Business Application Add Ins (BAdIs) (39)5.1.1Exit for the printer determination (PRINTER_DETERM_PPF) (39)5.1.2Exit After Generated Action (TRIGGER_EXECUTED) (39)The BADI has the application names as the filter value. The action is also transferred and can deliver its processing status (successful, with error) or other information. (39)5.1.3Exit for Context Extension (CONTEXT_EXTEND_PPF) (39)5.1.4Exit for Completion of Processing Options (COMPLETE_PROC_PPF) (40)5.1.5Extend Container for Condition Evaluation (CONTAINER_PPF) (40)5.1.6Exit for Execution of Actions (EXEC_METHODCALL_PPF) (40)5.1.7Exit for Getting Possible Partner Functions of an Application (GET_PARTN_ROLES_PPF ) (41)5.1.8Exit for Double Clicking on Values in the Display (GRID_CLICK_PPF) (41)5.1.9Exit for Checking if Deletion of Action Profile is allowed (CONTEXT_DELETE_PPF) (41)5.1.10Exit for evaluation of schedule conditions (EVAL_SCHEDCOND_PPF) (41)5.1.11Exit for evaluation of start conditions (EVAL_STARTCOND_PPF) (41)5.1.12Exit for Adding further data to workflow container (WF_CONT_MODIFY_PPF) (41)5.2PPF Interface (42)5.2.1Connection of Your Own Processing Options (42)5.2.2Connection of a Logic for the Determination (42)5.2.3Connection to a Separate Logic for Action Merging (42)6Appendix (44)6.1Description of Interfaces (44)6.1.1CL_MANAGER_PPF (44)6.2Class Diagram (47)6.2.1Customizing Classes (47)6.2.2Runtime Classes (48)6.2.3Service Classes (49)6.3Sequence Diagrams (50)6.3.1Calling the PPF (50)6.3.2Method DETERMINE Part 1 (51)6.3.3Method DETERMINE Part 2 (52)1 PPF OverviewThe Post Processing Framework (PPF) is a tool for the generic execution of functions and processes. It provides the applications with a uniform interface to any actions. Actions can be outputs in the traditional sense such as print, fax, mail, or XML but functions such as the triggering of workflows or any method call can also be triggered. Which actions this eventually are, is determined by an individually configurable or a self-programmable determination technology, depending on the application document data. Execution of the action can also take place depending on the data of the application document.Therefore, the PPF automatically generates actions from document data (for example, delivery notes or order confirmations, generation of an item in the document, creation of a subsequent document).PPF additionally provides uniform action administration. There is status management and a processing log for every action.The PPF itself was programmed with ABAP objects. In addition, various object services were used. However, you do not have to program your PPF application in an object-oriented manner to use the PPF. Overview graphic:2 Preparation2.1 Necessary ClassesCreate the following classes in the Class Builder:∙ A persistent class (in the sense of Object Services) that represents the application object (for example: CL_BOOK_PPF)∙ A partner class that represents a partner of an application object (for example:CL_BOOK_PARTNER_PPF)∙ A context class that encapsulates all information for the PPF (Example: CL_DEMO_CONTEXT_PPF)∙ A processing class provided Smart Forms are used (Example: CL_PROCESSING_DEMOBOOK_PPF) ∙ A BADI implementation provided the processing method call is used∙Workflow template provided a workflow is to be triggeredThe implementation steps are explained using the example of a demo application. The demo application and all mentioned classes are available in all systems (transaction SPPFDEMO, development class SPPF_DEMO).The classes to be implemented by the application are shaded in gray in the following graphics. All other classes are provided by the PPF.2.1.1 Application ClassThe PPF expects a persistent class so it can handle different types of application objects.In most cases, the application object is not implemented as a persistent class, or it is a BOR object. You must then create a persistent proxy class that does nothing other than refer to the actual application object. The key of the application object also appears as an attribute of the proxy class. The application object implements the interface IF_LOCK_PPF and thus both methods for locking and unlocking the application object. These two methods are important so that an action does not operate on documents that are already locked. If the interface is not implemented, this can result in actions being executed twice under certain circumstances. Generally, the interface must be implemented.When using the workflow conditions, a BOR object is necessary in addition to the persistent application object since the schedule condition and start condition is defined on attributes of the BOR object (more on this in the relevant sections).The implementation of IF_LOCK_PPF is important so that the action does not change documentdata for a document that is being processed. The implementation also prevents the same actionbeing executed multiple times in parallel processing.Generation of the object at runtime:DATA:* reference to application/proxy objectappl_object TYPE REF TO cl_book_ppf,book_id TYPE CHAR10.* create a persistent application object via the class agent of it’s co-class* object services create 2 service classes for any persistent classca class contains all persistency services, e.g. creation of persistent objectcreate application object, key (book_id) must be set beforeappl_object ?=ca_book_ppf=>agent->if_os_factory~create_persistent_by_key(i_key = book_id ).Partner classCreate a partner class for the document partner that implements the interface IF_PARTNER_PPF. The document partners are collected in a collection and transferred to the PPF. The class of the partner collection already exists.A partner consists of a partner function, partner number, and the data assigned from the central address management (CAM: ZAV “Zentrale Adressverwaltung” in German): Person number, address number, and address type. This data must be made available for every document partner by the application.The PPF determines the relevant communication data for fax processing or mail processing using the address numbers transferred.Generation of a partner object at runtime and appendage to the partner collection:DATA:* reference to partner objectpartner TYPE REF TO cl_book_partner_ppf,* reference to partner collectionpartner_coll TYPE REF TO cl_partner_coll_ppf,* create partner collectionCREATE OBJECT partner_coll.* create first partner objectCREATE OBJECT partnerEXPORTING ip_partner_role = 'LF'ip_partner_no = '1234567890'ip_partner_text = 'Vendor Meyer'ip_zav_addressno = '0000015762'ip_zav_persno = '0000015763'ip_zav_addr_type = '3'.* append partner to partner collectionCALL METHOD partner_coll->add_element( partner ).Context classThe context class encapsulates all the application data necessary for the PPF: ∙Application name∙Reference to the application object (instance of the application class or proxy object)∙Reference to a partner collection∙Further application attributes (fields that can be included as sort fields in the management table of actions)Note when creating the context class that this is not marked as final. The customer should potentially have the possibility to extend this using further attributes. This is only possible provided the class has not been marked as final.The context class redefines the method GET_VALUE_OF_ATTRIBUTE of class CL_CONTEXT_PPF. This serves dynamic attribute accesses that are not yet supported in the ABAP standard. For the redefinition, simply copy the coding from the template and set it again in the method. This step is necessary so that the method is executed for your action profile class itself and can access its attributes.Fill the context instance at runtime:DATA:* reference to context objectcontext TYPE REF TO cl_demo_context_ppf.* create context objectcreate object context.* set context attributecontext->applctn = 'BOOK'. ”Declared in Customizing (see chapter 2.2)context->name = 'BOOK'. ”Declared in Customizing (see chapter 2.2)context->appl = appl_object.context->partner = partner_coll.* additional context fields*.these fields can be used as sort fields for a later processing of the actions* the fields can be overtaken into the database table for actions (PPFTTRIGG)context->ID = ‘1234’.context->creator = sy-uname.....2.1.2 Application-Specific Processing2.1.2.1 Using Smart Forms Processing OptionsIf Smart Forms are used for the action, you must program a processing class. Here, the PPF helps you as much as possible.Smart Forms are function modules whose interface consists of a general part that is independent from the relevant Smart Form and an application-specific part. Rather, the PPF cannot directly call the function module. However, it fills the general part of the interface.Your processing class must also inherit the processing method EXEC_SMART_FORMS from the PPF class CL_SF_PROCESSING_PPF that serves as the copy template and sets the general parameter of the Smart Forms function module. You only need to fill the specific parameter.You must also set the language of your Smart Form here. If your application object involves a BOR object then you can link the action (Smart Form) with your BOR object, or optically archive the action using Archive Link.The copied processing methods in the example application are as follows:METHOD process_smart_form .* function nameDATA: function_name TYPE rs38l_fnam,dummy(254) TYPE c.* get the function name for this smart formCALL FUNCTION 'SSF_FUNCTION_MODULE_NAME'EXPORTINGformname = ip_smart_form* VARIANT = ' '* DIRECT_CALL = ' 'IMPORTINGfm_name = function_nameEXCEPTIONSno_form = 1no_function_module = 2OTHERS = 3.IF sy-subrc <> 0.* add an error message to processing protocolMESSAGE i015(sppf_media) WITH ip_smart_form.CALL METHOD cl_log_ppf=>add_messageEXPORTINGip_problemclass = '1'ip_handle = cp_application_log.EXIT.ENDIF.*------------------- get application specific data---------------------- DATA: lo_book TYPE REF TO cl_book_ppf,ls_book TYPE ppftbook....* cast imported object so that we can refer to its attributeslo_book ?= io_appl_object.* get key fields of application objectls_book-id = lo_book->get_id( ).ls_book-author = lo_book->get_author( ).ls_book-title = lo_book->get_title( ).ls_book-pagecount = lo_book->get_pagecount( ).ls_book-creator = lo_book->get_creator( ).ls_book-datecreate = lo_book->get_datecreate( ).ls_book-datechange = lo_book->get_datechange( ).ls_book-status = lo_book->get_status( ).ls_book-isbn = lo_book->get_isbn( ).*----------------------------------------------------------------------- *---------- is_mail_appl_obj -------------------------------------------* fill this parameter if your application object is a BOR object* the output (smart form) will be linked with the BOR object* is_mail_appl_obj-LOGSYS =* is_mail_appl_obj-OBJTYPE =* is_mail_appl_obj-OBJKEY =* is_mail_appl_obj-DESCRIBE =*-----------------------------------------------------------------------*-----------fill archive parameters for archive link ------------------- IF is_output_options-tdarmod = '2' ORis_output_options-tdarmod = '3'.* archive_index_tabREAD TABLE ct_archive_index_tab INTO ls_archive_index INDEX 1.* just fill the id of your actual BOR object* ------>* ls_archive_index-object_id = 'ID_OF_YOUR_BOR_OBJECT'.* ------>IF ls_archive_index-object_id IS INITIAL.DELETE ct_archive_index_tab INDEX 1.ELSE.MODIFY ct_archive_index_tab FROM ls_archive_index INDEX 1.ENDIF.ENDIF.*-----------------------------------------------------------------------*-----------language of smart form-------------------------------------- * determine here the language of the smart form and set it* default language is the system language* is_control_parameters-langu = language_of_my_smart_form.*-----------------------------------------------------------------------* call function to process smart formCALL FUNCTION function_nameEXPORTINGarchive_index = is_archive_indexarchive_parameters = is_archive_parameterscontrol_parameters = is_control_parametersmail_appl_obj = is_mail_appl_objmail_recipient = is_mail_recipientmail_sender = is_mail_senderoutput_options = is_output_optionsuser_settings = ip_user_settings*-------- additional fields have to be filled by the application-------- is_book = ls_book*-----------------------------------------------------------------------IMPORTINGdocument_output_info = es_document_output_infojob_output_info = es_job_output_infojob_output_options = es_job_output_optionsEXCEPTIONSoutput_canceled = 1parameter_error = 2OTHERS = 3.IF sy-subrc <> 0.* add an error message to processing protocolCASE sy-subrc.WHEN 1.MESSAGE e016(sppf_media)....ENDCASE.CALL METHOD cl_log_ppf=>add_messageEXPORTINGip_problemclass = '1'ip_handle = cp_application_log.ENDIF.* get error tableCALL FUNCTION 'SSF_READ_ERRORS'IMPORTINGerrortab = et_error_tab.ENDMETHOD.The return parameter is evaluated centrally using the PPF. You can add your own entries to the processing log and should also do this so that the application specific processing routines are also logged. A handle on the application log is transferred for this as the parameter: ip_application_log. Entries can simply be made using the interface of the service class CL_LOG_PPF:Example:MESSAGE i015(sppf_media) WITH ip_smart_form.CALL METHOD cl_log_ppf=>add_messageEXPORTINGip_problemclass = '1'ip_handle = ip_application_log.2.1.2.2 Usage of Processing Method CallThe method calls are created using BADI implementations. The relevant BADI definition (transaction SE18) is called EXEC_METHODCALL_PPF. You can create an implementation for this definition (transactionSE19). The interface looks as follows:FLT_VAL Importing Type PPFDFLTVAL Parameter FLT_VAL of method EXECUTE IO_APPL_OBJECT Importing Type Ref To OBJECT Reference to application objectIO_PARTNER Importing Type Ref To CL_PARTNER_PPF Message partnerIP_APPLICATION_LOG Importing Type BALLOGHNDL Handle on application logIP_PREVIEW Importing Type CHAR1 Preview flagII_CONTAINER Importing Type Ref To IF_SWJ_PPF_CONTAINER Container with parameter valuesIP_ACTION Importing Type PPFDTT Name of action definitionRP_STATUS Returning Type PPFDTSTAT PPF: Output statusThe filter value is created when the BADI implementation is created and is therefore freely definable. A description for the filter value should also be entered. For processing, you receive a reference to its application object and hence have access to the document data. In addition, the document partner, a reference to an application log (processing log), and a container with parameters are transferred. The parameter values can be determined in Customizing. After processing, the processing status must be set correspondingly (processed successfully = 1, processed with errors = 2). If the status is not explicitly set, the PPF sets the status to processed with errors = 2.In the BADI-Builder (transaction SE19), this can appear as follows:2.1.2.3 Use of the Processing WorkflowNo application specific coding is needed here.2.1.2.4 Use of the Processing of External SendingThe processing of external sending, like Smart Forms, also needs an application-specific logic for data retrieval and for calling the Smart Form. Creation no longer takes place using a method implementation, but rather using a BADI implementation. The BADI is called DOC_PERSONALIZE_BCS. The interface is very similar to the method for Smart Forms processing:FLT_VAL Importing Type BCSDFLTVAL BCS: Filter value for BADI DOC_PERSONALIZE_BCSIS_ARCHIVE_PARAMETERS Importing Type ARC_PARAMS ImageLink structureIS_CONTROL_PARAMETERS Importing Type SSFCTRLOP Smart Forms: Control structureIS_OUTPUT_OPTIONS Importing Type SSFCOMPOP SAP Smart Forms: Options smart composer (transfer)IO_APPL_OBJECT Importing Type Ref To OBJECT Application objectIP_SMART_FORM Importing Type TDSFNAME Smart Forms: Form nameIS_MAIL_APPL_OBJ Importing Type SWOTOBJID Structure for object IDIS_MAIL_RECIPIENT Importing Type SWOTOBJID Structure for object IDIS_MAIL_SENDER Importing Type SWOTOBJID Structure for object IDIP_USER_SETTINGS Importing Type TDBOOL Selection field (yes or no)IP_APPLICATION_LOG Importing Type BALLOGHNDL Application log: Log handleIO_PERSONALIZE_DATA Importing Type Ref To CL_PERSONALIZE_DATA_BCS Service class for the personalization of a mail ES_DOCUMENT_OUTPUT_INFO Exporting Type SSFCRESPD Smart Forms: Return of document informationES_JOB_OUTPUT_INFO Exporting Type SSFCRESCL Smart Forms: Return when form printing is completedES_JOB_OUTPUT_OPTIONS Exporting Type SSFCRESOP Smart Forms: Return when starting form printing ET_ERROR_TAB Exporting Type TSFERROR SAP Smart Forms: Runtime errorCT_ARCHIVE_INDEX_TAB Changing Type TSFDARA SAP Smart Forms: Table with archive indicesC_DOCUMENT_TITLE Changing Type TDTITLE Document titleThe logic for calling the Smart Forms is the same as the processing above, for an example, see the implementation BCS_PROC (transaction SE19).2.2 CustomizingIn Customizing (transaction SPPFC) you make your classes known to the PPF and define the determination of the outputs: You assign the possible action definitions (for example, delivery note, order acknowledgment, and so on) to your application. You must define a determination technology for each output type (see section “Determination and Merging of Actions“).The definition in Customizing only determines the framework, that is, all potential actions and their possible processing options are declared. This definition can be used in an additional Customizing step to define conditions (see 2.3 onwards). The actions only appear when the conditions have been maintained. Definition in Customizing alone does not lead to the generation of actions.Transaction SPPFC should never be added to the IMG directly. It can be called in a separateapplication specific transaction when the parameter is set for the application.2.2.1 Define New ApplicationAn application that wants to use the PPF must be entered in Customizing of the PPF. In the initial screen, you only enter a name and a description. In addition, you can enter the date profile of the application and the BOR object or the business class here. The date profile and the object type are required for the definition of the schedule condition. The conditions are based on attributes of the object type and date rules of the date profile can also be integrated. Here you must also enter the name of your context class.Since Basis Release 6.10, the application is maintained in a separate transaction (SPPFCADM).Application name Description Date profile ofapplicationObject typeof theapplication2.2.2 Defining the Action ProfileAn action profile is a collection area for actions in a specific application context (for example, leasing actions, actions for contracts, and so on).You can transfer all relevant application data to the PPF using an instance of the context class. If you maintained the context class at the application level, it does not have to be entered again here when it is created from new. It is copied as the default value. The same applies for the date profile and object type. The composite profile flag specifies that a profile is used as composite profile. A composite profile can be inserted in other action profiles. Thus the actions from the composite profile can be used in the current profile.Name of the action profileDescriptionName of the context classBusiness Object Type and Date Profile for Time RulesActions from this composite profile are also availableUsed as composite profile2.2.3 Define Action Definitions for the Action ProfileAn action profile can be assigned various action definitions. An action definition is the smallest business unit that is to be output or processed –for example, if all delivery notes belong to the ’delivery note’ action definition, whether they should now be printed, faxed, or output as normal.You can deactivate an action using the action setting. If the action is set to inactive, it is ignored for action determinations. It can therefore be ’deactivated’.The actions in the document can be sorted using the sort function.By double-clicking on the action definition, you can display the detailed settings.Name of the action definition Description DeactivateactionSort for thedisplay2.2.4 Details for the Action DefinitionYou can configure an action definition in various ways:∙Processing time (immediate processing, when the document is posted, later using a selection report)∙Processing times that are not permitted: Here you can enter processing times that make no sense for this action. For example, Send order confirmation -> Process time immediate. The action orderconfirmation may be processed after document editing is complete or using the selection report. The time immediate is entered here as a time that is not allowed.∙Schedule automatically: If the flag is set, the action is scheduled automatically provided the schedule condition is fulfilled. If it is not set, the action appears in the worklist and can be scheduled manually by the agent for document editing.∙Sorting in the display (in which order should the actions in the document for runtime be displayed, provided a user interface is included)∙Automatic scheduling (X = action is to be scheduled, SPACE = action should be put in the worklist, that is, the agent can schedule it manually)∙Delete after processing (the action is executed and then deleted)∙Can be changed in the dialog (after automatic determination, should you be allowed to make changes and repeat the action definition manually?)∙Can be executed in the dialog (the action can take place during document editing, even though no posting has yet taken place)∙Display on the toolbar: Specifies whether the action is to be displayed on the GOS toolbar (provided the service is used)∙Partner function: Default function of the partner to which the action goes – moved in the manual creation of an action∙Partner-dependent: specifies whether a partner determination is to be performed∙Selection of a determination technology, that is to be used for this action definition (see section below on determination)∙Selection of a technology for merging of actions: (see section below on action merging)∙Sort field 1 – 3: Application specific data that is supplied in the action profile class. Display or processing can be sorted according to this data∙The action description delivers details on the action and can be displayed by the agent for document editingSettings in detailThe search help for the partner function field can only deliver values if the application creates and delivers a BAdI implementation for GET_PARTN_ROLES_PPF. Here you can use theexample implementation for the demo application (GET_ROLES_BOOK_PPF) to get moreinformation.2.2.5 Assignment of Processing Types for an Action DefinitionActions always occur using the execution of a processing. Currently, Smart Forms are supported as print, faxor mail, plus the triggering of workflows and any method call. The new processing external sending canreplace the existing Smart Forms processing and additionally offers further extended functions.Add a separate configuration for every processing. The settings made here serve as default values and canbe overridden in the conditions.2.2.5.1 Smart Forms Processing OptionsIn the Smart Forms processing options, enter the name of the Smart Form used and a processing class witha processing method, that you have programmed (see section “processing class“). Provided the application supports optical archiving using Archive Link, the archive parameter archive object type and document typemust be entered. These must first be created in Archive Link Customizing. You can reach Archive Link Customizing using the transactions (oac2, oac3, oaco). The option archive copies optically archives allmultiple outputs, that is, it also archives identical multiple archives.Assignment ofpossible processingoptions to an actiondefinitionDetailed settingsfor Smart Formsprocessing2.2.5.2 Method Call ProcessingThis processing enables the execution of any action. A BAdI implementation is called. This enables the creation of a subsequent document or the creation of an item in the document, for example. The method can be entered using the F4 help. All active BAdI implementations for BAdI definition EXEC_METHODCALL_PPF are displayed there.The processing parameters are freely definable and can be provided with values. Static values are used here. The values can be changed again in the configuration of conditions.Example: Create method subsequent offer, processing parameter: Type of subsequent offerMake sure that the implementation was activated, otherwise it is not displayed in the F4 Help.2.2.5.3 Workflow ProcessingWorkflow templates can also be started using actions. The workflow template is added to it. The business object must be defined as an input parameter in the container definition of the workflow template. The name of the parameter must be BUSINESSOBJECT. Using Display/Change, the assigned workflow template can be edited and a new workflow template can be created using the Create button. The F4 help for the assignment only displays the workflow templates that support the assigned business object in the action profile.2.2.5.4 External Sending ProcessingThe processing of external sending can take the place of the existing Smart Forms processing. It completely covers its functions and additionally makes it possible to send an attachment, send the outputs to copy recipients and in further releases it is also possible to send SMSs.The Customizing settings are similar to the Smart Forms processing options. The name of a Smart Form is added, a format logic (as implementation of BAdI DOC_PERSONALIZE_BCS) and as the optimizationparameter the type of document personalization can be specified. In recipient-dependent personalization, the formatting of the document takes place for every recipient, since recipient-dependent data is to be replaced. When personalization is not recipient dependent (an identical text or mail to all recipients), document formatting only takes place once for all recipients.。