Rat Models of Traumatic Spinal Cord Injury to Assess Motor Recovery
- 格式:pdf
- 大小:131.55 KB
- 文档页数:11

山东医药2021年第61卷第17期间充质干细胞外泌体修复脊髓损伤作用机制的研究进展马麟,张晓勃,赵光海,巩朝阳,张海鸿兰州大学第二医院,兰州730030摘要:脊髓损伤(SCI)通常会导致不可逆的神经退行性改变并影响终生,但目前缺乏有效治疗策略。
间充质干细胞外泌体可通过促进血管生成、促进轴突生长、调节炎症反应、调节免疫反应及抑制细胞凋亡等方式修复SCI,或可能成为SCI患者治疗的新选择。
关键词:间充质干细胞;外泌体;脊髓损伤;脊髓修复;作用机制doi:10.3969/j.issn.1002-266X.2021.17.024中图分类号:R744.9;R651.2文献标志码:A文章编号:1002-266X(2021)17-0089-03脊髓损伤(SCI)是一种破坏性神经退行性疾病,临床上目前缺乏对该病有效的治疗方法,而纳米技术和再生医学策略为新型疗法的开发带来了希望。
干细胞可通过替代丢失或受损的细胞为神经元提供营养支持,改善脊髓的微环境,从而促进受损轴突再生,加快SCI修复[1]。
间充质干细胞(MSCs)可来自骨髓、脂肪、脐带血和胎盘等多种组织,具有归巢、增殖、分化、分泌和免疫调节的功能,是动物研究和人类临床试验中最常用的干细胞[2]。
外泌体是释放到细胞膜外的纳米级囊泡,含有大量复杂分子如蛋白质、脂类和各种核酸,而这些分子的特性与它们的来源细胞有关[3]。
MSCs外泌体(MSCs-Exo)的生物学功能与MSCs相似,但MSCs-Exo更稳定,不会引发机体免疫排斥反应;其具有易分离的特点,故可用于将遗传物质或药物转运至靶细胞;并且尺寸相对较小,故能渗透血脑屏障到达中枢神经系统损伤部位[4-5]。
因此,MSCs-Exo是无细胞治疗的合适选择。
多项研究显示,MSCs-Exo在SCI修复中有巨大潜力。
本文就MSCs-Exo修复SCI的作用机制综述如下。
1MSCs-Exo通过促进血管生成修复SCI血管生成是SCI修复的关键,局部血管丢失与血脑屏障损伤引起的破坏可导致缺血和炎症反应,从而引发脊髓神经组织的综合性损伤[6]。

【⼿法康复】颅骶疗法(CraniosacralTherapy,CST)--调节⾝体机能的⼿法介绍颅骶疗法(Craniosacral Therapy,CST)是⼀种轻柔的⾮⼊侵式的⼿法触诊疗法,通过触摸⼈体中轴颅骶系统的不同部位,改变脑脊液的流动节律和流量,直接调节脑和脊髓的功能状态,使中枢神经系统与⾝体其他系统恢复正常联系和⾃然运动,可⽤以评估(诊断)和修正(治疗)⼈体中轴颅骶系统的失衡和约束,治疗机体的多种疾病和创伤,以及解除情感或⼼理的困扰。
历史颅骶疗法起源于美国,Andrew Taylor Still, M.D. (1828-1917) ,创⽴了整⾻疗法(Osteopathy)威廉·G·萨瑟兰(1873-1954),颅骶疗法(Craniosacral Therapy)创始⼈要先由美国的⼀位医⽣ Andrew Taylor Still, M.D. (1828-1917) 的医疗⼯作说起。
Still是家中九兄弟姊妹中的三哥,他的⽗亲是⼀位医⽣兼传道⼈,也是Still的⽼师。
Still 在⼆⼗五岁时结了婚,后来更举家搬到Kansas。
1861年,正值是美国的内战时期,Still 正在医院⾥担任服务员并因此看见了战争的恐怖。
这时也是脊膜炎 (Spinal Meningitis) 横⾏的时期,他有三个孩⼦是死于脊膜炎,第⼀任妻⼦死于难产,第⼆任妻⼦的⼥⼉也死于肺炎。
Still 眼见⾃⼰作为医⽣对亲⼈逝世的⽆助及内战带给⼈的伤害,他认为有需要有⼀种更好的治疗⽅法。
Still 惊讶于动物⾝体结构和功能的完美配合,这带领他对解剖学和⽣理学作更深⼊的研究,并对结构和功能的关系有更深⼊的理解,最终这些研究都为他⽇后所创⽴的⼀个新的治疗系统打下了基础。
经过了好些⽇⼦后,A.T. Still 越来越对当时的⼀般的医疗⽅式反感,例如截肢⼿术及过量使⽤药物。
当然 Still 对当时医疗⽅式的看法受到不少反对声⾳的攻击,包括教会认为他的⼿法治疗是亵渎神灵、他家⼈亦对他质疑医疗⽅法的态度感到⼗分尴尬。
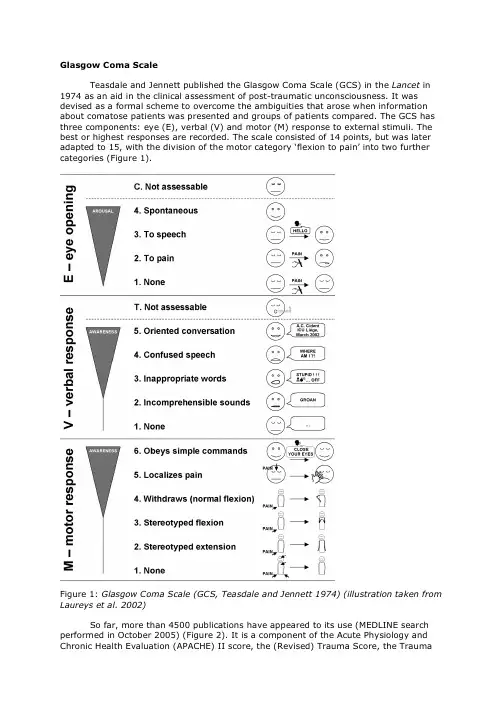
Glasgow Coma ScaleTeasdale and Jennett published the Glasgow Coma Scale (GCS) in the Lancet in 1974 as an aid in the clinical assessment of post-traumatic unconsciousness. It was devised as a formal scheme to overcome the ambiguities that arose when information about comatose patients was presented and groups of patients compared. The GCS has three components: eye (E), verbal (V) and motor (M) response to external stimuli. The best or highest responses are recorded. The scale consisted of 14 points, but was later adapted to 15, with the division of the motor category ‘flexion to pain’ into two further categories (Figure 1).Figure 1: Glasgow Coma Scale (GCS, Teasdale and Jennett 1974) (illustration taken from Laureys et al. 2002)So far, more than 4500 publications have appeared to its use (MEDLINE search performed in October 2005) (Figure 2). It is a component of the Acute Physiology and Chronic Health Evaluation (APACHE) II score, the (Revised) Trauma Score, the Traumaand Injury Severity Score (TRISS) and the Circulation, Respiration, Abdomen, Motor, Speech (CRAMS) Scale, demonstrating the widespread adoption of the scale.Figure 2: Number of scientific papers making reference to the Glasgow Coma Scale (from Laureys et al. 2005).The presence of spontaneous eye opening “indicates that the arousal mechanisms of the brainstem are active” (Teasdale and Jennett 1974). Preserved arousal does not imply the presence of awareness. Patients in a vegetative state have awakened from their coma but remain completely unaware of their environment and self. Most comatose patients who survive will eventually open their eyes, regardless of the severity of their cerebral injuries (Jennett 1972). Indeed, less than 4% of head-injured patients never open their eyes before they die (Bricolo et al. 1980). The eye opening in response to speech tests the reaction “to any verbal approach, whether spoken or shouted, not necessarily the command to open the eyes” (Teasdale and Jennett 1974). Again, this response is observed in vegetative patients who can be awakened by non-specific auditory stimulation. In these patients it is recommended to differentiate between a reproducible response to command and to non-sense speech. Eye opening in response to pain should be tested by a stimulus in the limbs, because the grimacing associated with supraorbital or jaw-angle pressure may cause eye closure.After arousing the patient the presence of verbal responses indicates the restoration of a high degree of interaction with the environment (i.e. awareness). An oriented conversation implies awareness of the self (e.g., the patient can answer the question: “What is your name?”) and environment (e.g., the patient correctly answers the questions: “Where are we?” and “What year/month is it?”). Confused speech is recorded when the patient is capable of producing language, for instance phrases and sentences, but is unable to answer the questions about orientation. When the patient presents intelligible articulation but exclaims only isolated words in a random way (often swear words, obtained by physical stimulation rather than by a verbal approach) this is scored as “inappropriate speech”. Incomprehensible sounds refer to moaning and groaning without any recognizable words. This rudimentary vocalization does not necessitate awareness and is thought to depend upon subcortical functioning as it can be observed in anencephalic children and vegetative patients.The motor response first assesses whether the patient obeys to simple commands, given in verbal, gestural or written form. A non-specific sound stimulus may induce a reflex contraction of the patient’s fingers or alternatively such a reflex response can result from the physical presence of the examiner’s fingers against the palm of the patient (i.e., grasping reflex). Before accepting that the patient is truly obeying commands, it is advised to test that the patient will also release and squeeze again to repeated commands. If there is no response a painful stimulus is applied. First, pressure is applied to the fingernail bed with a pencil. If flexion is observed stimulation is then applied to other sites (applying pressure to the supraorbital ridge, pinching the trapezium or rubbing the sternum) to differentiate between localization (i.e., a stimulus at more than one site causes a limb to move so as to attempt to remove it by crossing the midline), withdrawal flexion (i.e., a rapid flexion of the elbow associated with abduction of the shoulder) or ‘abnormal’ flexion (i.e., a slower stereotyped flexion of the elbow with adduction of the shoulder that can be achieved when stimulated at other sites). Stereotyped flexion responses are the most common of the motor reactions observed in severely brain-injured patients; they are also the most enduring (Born 1988). Extensor posturing is more easily distinguished and is usually associated with adduction, internal rotation of the shoulder and pronation of the forearm. The term ‘decerebrate rigidity’ should be avoided because it implies a specific physioanatomical correlation. Abnormal flexion and extension motor responses often co-exist (Bricolo et al. 1977). It is important to appreciate that it is the best response that should be scored and that abduction movements reflect some residual awareness while stereotyped postures do not. The presence of asymmetrical responses are significant in indicating that there is a focal as well as a diffuse disturbance of brain function, and this should be noted separately. The side showing the impaired response locates the site of the focal brain damage and the level of the best response of the better side reflects the extent of general depression in brain function. The scale of responses to pain is applicable to the movements of the arms. The movements of the legs are not only more limited in range, but may take place on the basis of a spinal withdrawal reflex (e.g., in brain death, a spinal reflex may still cause the legs to flex briskly in response to pain applied locally (Ivan 1973)).It is very tempting to sum the three components of the GCS (E-V-M) into a total score, ranging from 3 to 15. However, given the increased use of intubation, ventilation and sedation of patients with impaired consciousness before arrival at specialists units, and even before arrival at hospital (Marion and Carlier 1994), patients might wrongly being scored as GCS 3/15 rather than being more appropriately reported as impossible to assess or score. In a recent study of 1005 patients with head injuries in European centers, assessment of each of the three components of the GCS was possible only in 61% of patients before hospital, in 77% on arrival at the first hospital, in 56% on arrival in the neurosurgical unit, and in 49% of ‘post-resuscitation (Murray et al. 1993). The inappropriate scoring of absent responsiveness as 3 has led to some data indicating that the mortality of patients with a score of 3 is apparently lower than that of those with a score of 4. Summing GCS components has also been criticized on a purely mathematical basis. Because there are only four units assigned to the eye responses, versus five to the verbal and six to the motor responses, the scale incorporates a numerical skew toward motor response. This problem can be tackled by weighting individual scores for eye, verbal and motor responses in such a way that each has a minimum contribution of one and a maximum of five (Bhatty and Kapoor 1993). This approach, however, is too complicated for practical use. Moreover, this effort to provide mathematical parity for the three components has abutted against studies that have stressed the particular importance of the motor portion of the GCS. Indeed, the motor score is more important than either of the other two components in predicting the magnitude of neurologic injury for patients with severe head injury (Jagger et al. 1983). While verbal and eye scores are more pertinent in patients who are not, in fact, comatose. It is a widespread but erroneous usage to define mild brain injury as a summed score ranging from 13-15, moderate injury, 9-12, and severe injury, 3-8. Indeed, in the persistent vegetative state, patients open their eyes spontaneously (E4) and may make moaning sounds (V2) or flex abnormally to pain (M3), while their condition hardly reflects “moderate” brain injury. Forclinical purposes, summation of the GCS is too imprecise (Bozza Marrubini 1984). To achieve a total score of 6 to 12 there are more than 10 simple combinations of variables, each with very different clinical profiles. In Glasgow, patients are always described by the three separate responses and never by the total (Teasdale et al. 1983). It is, therefore, good practice to communicate the GCS in terms such as “patient scored E2, VT, M4” and only sum its three components for research applications.PitfallsUntrained or inexperienced observers produce unreliable scoring of consciousness (Rowley and Fielding 1991). In one study, one out of five ICU workers were mistaken when asked to make judgments as to whether patients were ‘conscious’ or ‘unconscious’, (Teasdale and Jennett 1976). Consciousness needs considerable skill to evaluate and the observer should be aware of the pitfalls encountered at ICU settings. It is also well known that the preceding score of the patient frequently influences the examinator when rating the patient’s present state of consciousness. It therefore is recommended to score in a “blinded” manner.Obviously, problems arise when the eyes are swollen shut, either following periorbital edema, direct ocular trauma, facial injury, craniotomy, cranial nerve VII injury or neuromuscular blockade. In these circumstances the enforced closure of the patient’s eyes should be recorded on his chart by marking “C” (= eyes closed) (Teasdale 1975). In deep coma, flaccid eye muscles will show no response to stimulation yet the eyes remain open if the lids are drawn back. Such opening should be recorded as unresponsive. It is important to stress that although opening of the eyes implies arousal, it does not necessarily mean that the patient is aware.Continued speechlessness may be due to causes other than unawareness (e.g., neuromuscular blockade, intubation via the oropharynx or through a tracheostomy, fractured mandible or maxillae, edematous tongue, deafness, foreign language, dysphasia, confusion or delirium). The evaluation of verbal responses is also biased when patients are sedated, alcohol or drug intoxicated or too young to speak. The use of early intubation and administration of neuromuscular paralyzing agents in the pre-hospital phase of care has rendered verbal and motor responses unmeasurable in these cases. Early treatment was uncommon when the GCS was first described, but has since gained greater acceptance. The FOUR (Wijdicks et al. 2005) and RLS85 (Starmark et al. 1988) which do not include a verbal response criterion, are the most notable alternative for scoring intubated patients. Several other techniques have been proposed to designate the verbal score in intubated patients. Some have proposed to assign an arbitrary score of one point to all intubated patients (Marshall et al. 1983). Others have created a pseudo-score by averaging the testable scores and adding this calculated score to the sum in lieu of the verbal score (Grahm et al. 1990). Linear regression predication of the verbal scores based on the other two scores has also been utilized (Meredith et al. 1998). The best alternative is to report separate responses, using a non-numerical designation of “T” (= intubated) when the verbal score cannot be assessed and not to sum the responses (Marion and Carlier 1994). The patient’s verbal response may also be impaired as a result of a single focal lesion of the speech areas in the dominant hemisphere, that is, aphasia. The assessment of such a patient’s language ability requires a specialized evaluation (e.g., written instructions and written replies in the case of motor dysphasia). The level of verbal response should still be indicated but an appropriate note may be made that the impairment is considered to be due to dysphasia (“D”= dysphasia) (Teasdale 1975). Motor responses cannot be reliably monitored in cases of spinal cord, plexus or peripheral nerve injury or in the presence of splint or immobilization devices. As previously stated, one must take care not to interpret a grasp reflex or postural adjustment as a response to command.In most scoring systems, awareness is assessed as the level of obeying to commands. This approach cannot be applied to cases where the patient is clinically or pharmacologically paralyzed yet alert (e.g., locked-in syndrome, severe polyneuropathy or use of neuromuscular blocking agents) or those with psychogenic unresponsiveness. It is important to stress that special effort should be made to identify and exclude theserare causes of pseudo-coma. The GCS has also been critiqued for lacking reliability in monitoring levels of consciousness in patients with moderate brain injury (Segatore and Way 1992). More detailed scales are recommended for the assessment of awareness in these patients (Malkmus et al. 1980; Majerus and Van der Linden 2000). Finally, as consciousness is a subjective first person experience, we remain with the theoretical limitation to the certainty of our clinical assessment of consciousness (since it is in another person that the clinician has to infer the presence or absence of conscious experience) (Bernat 1992).Even if the GCS is the most widely used and validated tool to evaluate the state of consciousness, it also is the most frequently misused. One study showed that 51% of patients were incorrectly assessed (Crossman et al. 1998). It is important to stress that for clinical use, patients should be communicated by the three separate scores (E, V, M and R) and never by the total sum. If eye or verbal responses cannot be evaluated, this should be indicated by marking a “C” (eyes closed) or “T” (intubated), respectively. References (text adapted from Laureys et al. 2002)Bernat, J. L. (1992). "The boundaries of the persistent vegetative state." J Clin Ethics 3(3): 176-80.Bhatty, G. B. and N. Kapoor (1993). "The Glasgow Coma Scale: a mathematical critique."Acta Neurochir 120(3-4): 132-5.Born, J. D. (1988). "The Glasgow-Liège Scale. Prognostic value and evaluation of motor response and brain stem reflexes after severe head injury." Acta Neurochir 95:49-52.Bozza Marrubini, M. (1984). "Classifications of coma." Intensive Care Med 10(5): 217-26.Bricolo, A., S. Turazzi, A. Alexandre and N. Rizzuto (1977). "Decerebrate rigidity in acute head injury." J Neurosurg 47(5): 680-9.Bricolo, A., S. Turazzi and G. Feriotti (1980). "Prolonged posttraumatic unconsciousness: therapeutic assets and liabilities." J Neurosurg 52(5): 625-34.Crossman, J., M. Bankes, A. Bhan and H. A. Crockard (1998). "The Glasgow Coma Score: reliable evidence?" Injury 29(6): 435-7.Grahm, T. W., F. C. Williams, Jr., T. Harrington and R. F. Spetzler (1990). "Civilian gunshot wounds to the head: a prospective study." Neurosurgery 27(5): 696-700; discussion 700.Ivan, L. P. (1973). "Spinal reflexes in cerebral death." Neurology 23(6): 650-2. Jagger, J., J. A. Jane and R. Rimel (1983). "The Glasgow coma scale: to sum or not to sum?" Lancet 2(8341): 97.Jennett, B. (1972). "Prognosis after severe head injury." Clin Neurosurg 19: 200-7. Laureys, S., S. Majerus and G. Moonen (2002). Assessing consciousness in critically ill patients. 2002 Yearbook of Intensive Care and Emergency Medicine. J. L. Vincent.Heidelberg, Springer-Verlag: 715-727.Laureys, S., S. Piret and D. Ledoux (2005). "Quantifying consciousness." Lancet Neurol 4(12): 789-90.Majerus, S. and M. Van der Linden (2000). "Wessex Head Injury Matrix and Glasgow/Glasgow-Liège Coma Scale: A validation and comparison study."Neuropsychological Rehabilitation 10(2): 167-184.Malkmus, D., B. Booth and C. Kodimer (1980). Rehabilitation of the Head-Injured Adult: Comprehensive Cognitive Management., Professional Staff Association of Rancho Los Amigos Hospital.Marion, D. W. and P. M. Carlier (1994). "Problems with initial Glasgow Coma Scale assessment caused by prehospital treatment of patients with head injuries:results of a national survey." J Trauma 36(1): 89-95.Marshall, L. F., D. P. Becker, S. A. Bowers, C. Cayard, H. Eisenberg, C. R. Gross, R. G.Grossman, J. A. Jane, S. C. Kunitz, R. Rimel, K. Tabaddor and J. Warren (1983)."The National Traumatic Coma Data Bank. Part 1: Design, purpose, goals, andresults." J Neurosurg 59(2): 276-84.Meredith, W., R. Rutledge, S. M. Fakhry, S. Emery and S. Kromhout-Schiro (1998). "The conundrum of the Glasgow Coma Scale in intubated patients: a linear regressionprediction of the Glasgow verbal score from the Glasgow eye and motor scores." J Trauma 44(5): 839-44; discussion 844-5.Murray, L. S., G. M. Teasdale, G. D. Murray, B. Jennett, J. D. Miller, J. D. Pickard, M. D.Shaw, J. Achilles, S. Bailey and P. Jones (1993). "Does prediction of outcome alter patient management? [see comments]." 341(8859): 1487-1491.Rowley, G. and K. Fielding (1991). "Reliability and accuracy of the Glasgow Coma Scale with experienced and inexperienced users." Lancet 337(8740): 535-8. Segatore, M. and C. Way (1992). "The Glasgow Coma Scale: time for change." Heart Lung 21(6): 548-57.Starmark, J. E., D. Stalhammar, E. Holmgren and B. Rosander (1988). "A comparison of the Glasgow Coma Scale and the Reaction Level Scale (RLS85)." J Neurosurg69(5): 699-706.Teasdale, G. (1975). "Acute impairment of brain function-1. Assessing 'conscious level'."Nurs Times 71(24): 914-7.Teasdale, G. and B. Jennett (1974). "Assessment of coma and impaired consciousness. A practical scale." Lancet 2(7872): 81-4.Teasdale, G. and B. Jennett (1976). "Assessment and prognosis of coma after head injury." Acta Neurochir (Wien) 34(1-4): 45-55.Teasdale, G., B. Jennett, L. Murray and G. Murray (1983). "Glasgow coma scale: to sum or not to sum." Lancet 2(8351): 678.Wijdicks, E. F., W. R. Bamlet, B. V. Maramattom, E. M. Manno and R. L. McClelland (2005). "Validation of a new coma scale: The FOUR score." Ann Neurol 58(4):585-93.。

施马伦贝格病毒英国利物浦大学兽医病理学教授马尔科姆·贝内特(Malcolm Bennett)表示:“施马伦贝格病毒对经济带来的影响较大,到了羊羔出生高峰季节,农场主的损失将会更严重。
目前出现疫情的农场中,大约有10%到50%的羔羊患病,这或将沉重打击英国刚刚复苏的经济。
Schmallenberg virusSchmallenberg virus is the informal name given to an orthobunyavirus related to Shamonda virus, which has not been given a formal name as of January 2012, initially reported in November 2011 to cause fetal congenital malformations and stillbirths in cattle, sheep, and goats.[1]It appears to be transmitted by midges(Culicoides spp.) which are likely to have been most active in causing the infection in the northern hemisphere summer and autumn of 2011, with animals subsequently giving birth from late 2011.[1]The virus is named after Schmallenberg, in North Rhine-Westphalia, Germany, from where the first definitive sample was derived.[1]It has also been detected in Lower Saxony in Germany [2], as well as in the Netherlands, Belgium, France, Luxembourg, Italy and the United Kingdom.[3]The virus has been recognised by the European Commission's Standing Committee on the Food Chain and Animal Health[1] and theFriedrich-Loeffler-Institut (German Research Institute for Animal Health)[2]. A risk assessment in December 2011 did not consider it likely to be a threat to human health[4], as other comparable viruses are not zoonotic[2].It was confirmed as present in the UK on 22 January 2012, having been formally identified in four sheep farms in Norfolk, Suffolk and East Sussex.[5]By 27 February 2012, the disease was reported in other counties in the south of England including the Isle of Wight, Wiltshire, West Berkshire, Gloucestershire, Hampshire and Cornwall. [6]It is likely that it was carried to Eastern England by midges from mainland Europe,[5] a possibility previously identified as a risk by DEFRA.[5]Contents[hide]∙ 1 Symptoms∙ 2 Diagnosis∙ 3 References∙ 4 External linksSymptomsThe virus causes two different profiles of Schmallenberg:∙Fever of short duration, diarrhoea and reduced production of milk in cowsThese symptoms have occurred during the period when the disease vectors (mosquitos, sandflies, midges) are active, during the summer and autumn of 2011, mainly affecting cattle.∙Stillbirths and birth defects in sheep, cattle and goatsCongenital malformations in newborn sheep, goats and calves are the most obvious symptoms. In many cases, the mother apparently has not presented symptoms of illness. These cases have occurred from December 2011, especially in sheep. The major malformations observed were: scoliosis, hydrocephalus, arthrogryposis, hypoplasia of the cerebellum and an enlarged thymus.[7]DiagnosisBlood samples from live animals with suspicious symptoms are taken for analysis. Dead or aborted fetuses suspected of having the virus are sampled by taking a piece of the brain or spleen for analysis. The samples are tested with the RT-PCR for Schmallenberg virus that has been developed by the Friedrich-Loeffler Institute in Germany.[7]References1.^ a b c d New Animal Virus Takes Northern Europe by Surprise, Kai Kupferschmidt, (AAAS), 13 January 2012, accessed 16 January 20122.^ a b c Schmallenberg Virus: New Orthobunyavirus in cattle, updated 10th January2012, accessed 16 January 20123.^http://web.oie.int/wahis/public.php?page=weekly_report_index&admin=04.^Risk assessment: New Orthobunyavirus isolated from infected cattle and smalllivestock ─potential implications for human health, European Center for DiseasePrevention and Control, 22 December 2011, accessed 17 January 20125.^ a b c Carrington, Damian (23 January 2012). "Schmallenberg virus confirmed onfarms in the UK". Guardian./science/2012/jan/23/schmallenberg-virus-confirmed-uk-farms. Retrieved 23 January 2012.6.^BBC News - Schmallenberg livestock virus hits 74 farms in England.7.^ a b Programa nacional de vigilancia epidemiológica frente al virus de Schmallenberg.Ministerio de Agricultura Alimentación y Medio Ambiente (España), 2012.Consultado el 8 de febrero de 2012Schmallenberg virus: questions and answers (来自下述网址:/)The rapid spread of a new virus on livestock farms in England has alarmed farmers and animal health experts. Here we answer some of the key questions about Schmall enberg virus.It was named Schmallenberg virus after the German town, 70 miles east of Cologne, where it was first found Photo: EPAWhat is Schmallenberg virus?From last August, a mysterious disease began appearing in adult cattle in Holland and Germany. Later, in November, sheep, cattle and goats in those countries, as well as in Belgium, began suffering stillbirths and giving birth to deformed young.A new virus was identified as the cause of both conditions in December. It was named Schmallenberg virus after the German town, 70 miles east of Cologne, where it was first found. It is related to a family of viruses found mainly in Asia, Africa and Australia, but not previously seen in Europe. It is not yet clear how the disease may have arrived in Europe, but one theory is that it was carried by imported animals or may haveevolved in Europe from similar viruses that were brought here by imported animals.What animals are affected and what are the symptoms?Related Articl es∙Mystery virus kills thousands of lambs25 Feb 2012∙Fears of 'catastrophe' as new virus hits farms26 Feb 2012∙Town at ground zero worries about farming and tourism25 Feb 2012The disease has been reported in cattle, sheep and goats, but it affects the animals differently. Adult cows suffer fever, reductions in milk yield and diarrhoea, which can affect their body weight and so their value. Adult cattle tend to recover after several days, however, and it is not lethal. There are no clinical symptoms in adult sheep.The virus has been found to cause cattle, sheep and goats to abort late in their pregnancies, or has resulted in stillbirths. Calves and lambs have been born with severe malformations of the limbs, damage to the spinal cord and fused joints. Some animals born without deformations can have problems with their nervous system.How has it spread?It is thought that it has been spread by insects such as midges and mosquitoes, which carry the virus and infect livestock when they bite. The species of insects that is responsible for transmitting the virus has yet to be identified and it is unclear if the virus can be spread from animal to animal.Experts believe it is most likely that it was brought to the UK by infected midges that were blown across the sea from the Continent, probably during the autumn months, when cases were first being noted there. As it does not seem to affect adult sheep, but rather their offspring, the effects are being noted only now, in the lambing season. Infected midges blownacross the sea were also blamed for introducing the Bluetongue virus to the UK in 2007.However, it cannot be discounted that the Schmallenberg virus could have been introduced by imported livestock.How many British farms have confirmed outbreaks?There are currently 74 farms where animals have been confirmed to have been infected with the virus. Five of the cases have been in cattle herds, and 69 have been in sheep.Currently infections have been contained in the south of the country, mainly clustered in the south-east in the areas closest to the Continent. The counties with the highest number of cases are all coastal: Norfolk, Suffolk, East Sussex and Kent.Are there any treatments available?There is currently no treatment or vaccine available for this disease. It is a newly identified virus and researchers are attempting to learn more about it, how it is spread, and how it may be treated.It could take at least 18 months before a vaccine is available, and even then authorities may be reluctant to use it, as it can then make monitoring the spread of the disease difficult. Infected adult cattle have been found to recover rapidly after infection. Animals born with abnormalities usually need to be put down.How does it compare to previous outbreaks of disease in animals, such as Foot and Mouth and Bluetongue?The outbreak of Schmallenberg virus in Europe and the UK has yet to reach the same scale as that of Foot and Mouth, in 2001, and Bluetongue, in 2007The Bluetongue virus, which was also spread by midges, killed millions of sheep, cattle, deer and goats across Europe. The Schmallenberg virus is different in one key respect in that it does not seem to cause severe symptoms in adult animals.Foot and Mouth disease is highly infectious, spreads easily through direct contact between animals and can even become airborne. It is, however, rarely fatal, but causes blisters in the mouth and feet of livestock. This can permanently affect the growth and milk production of infected animals, decimating the value of herds. It can also leave animals sterile and, invery rare cases, it can be caught by humans. Due to the harm that it could cause to herds of cattle, strict biosecurity measures were implemented to prevent humans and vehicles from spreading Foot and Mouth from farm to farm during the 2001 outbreak. Herds of cattle were also culled, leading to burning piles of carcases, in an attempt to prevent the disease from being passed on.Why are farmers so concerned about Schmallenberg?It is a new virus, so the long-term consequences to infected animals are not yet known, creating a great deal of unease.The damage to calves and lambs can impact on attempts to produce new stock, and delivering deformed young can be traumatic for farmers. The number of cases of infected animals is expected to continue growing. Sheep infected last autumn are now just starting to give birth but most cows will begin to give birth later in the year, as they have a gestation period of nine months.The cases in cows so far in the UK have been in calves that have aborted after six months, so the full scale of the disease in cattle will not become clear until later in the year. When the weather warms up and midges become more prevalent, the virus may spread further around the country.What measures can be taken to control its spread?There are currently no control measures in place and the Department for Environment, Food and Rural Affairs does not consider Schmallenberg virus to be a "notifiable disease", where farmers are required by law to report infections to the authorities, as not enough is known about the virus yet to change its statusIn the Bluetongue outbreak, the Government implemented strict controls over the movement of animals around infected farms in an attempt to control the spread. They also introduced a voluntary vaccination process. The year after the first outbreak, Bluetongue spread further north but did not become a major problem in Scotland, perhaps because the midges there were not able to carry the virus.Is there a risk to humans?According to the Veterinary Laboratories Agency, there is unlikely to be a risk to human health, but this is not yet certain. Farmers and veterinary surgeons have been advised to take hygiene precautions when working with infected animals.Although several members of the group of related viruses can affect humans, the ability to do so is thought to be due to a gene sequence which is not present in Schmallenberg virus.As this is a new virus, however, virologists are conducting work to find out if it may cause any health problems in humans.。



关于极限运动备受争议的英语作文Extreme sports have been a topic of much debate and controversy in recent years. On one hand, they offer a thrilling and adrenaline-fueled experience for participants, pushing the boundaries of human physical and mental capabilities. However, the inherent risks associated with these activities have raised concerns about their safety and the potential harm they pose to both participants and spectators.One of the primary arguments in favor of extreme sports is the sense of personal accomplishment and fulfillment they can provide. Individuals who engage in these activities often report a deep sense of satisfaction and pride in their achievements, whether it's conquering a challenging rock-climbing route, performing a death-defying stunt on a motorcycle, or soaring through the air during a skydive. The sense of freedom and liberation that comes with pushing one's limits can be incredibly empowering and transformative for participants.Moreover, extreme sports can foster a strong sense of community and camaraderie among enthusiasts. The shared experience of facing and overcoming daunting challenges can create a powerful bondbetween individuals, as they support and encourage one another in their pursuits. This sense of belonging and shared purpose can be particularly meaningful for those who may feel isolated or disconnected from mainstream society.However, the risks associated with extreme sports are undeniable. These activities often involve high speeds, dangerous maneuvers, and the potential for catastrophic injuries or even death. Participants may face the possibility of broken bones, spinal cord injuries, traumatic brain injuries, and other life-altering consequences. The emotional and financial toll on individuals, families, and communities can be immense, as they grapple with the aftermath of such incidents.Furthermore, the impact of extreme sports extends beyond the participants themselves. Spectators and bystanders may also be put at risk, as events and competitions often take place in public spaces or involve the use of equipment that could potentially cause harm to onlookers. The potential for collateral damage and the strain on emergency services and medical resources are also significant concerns.Another aspect of the debate surrounding extreme sports is the ethical and moral implications. Some argue that the pursuit of personal thrill and adrenaline rush at the expense of one's ownsafety and the safety of others is inherently selfish and irresponsible. They contend that individuals who engage in these activities are not only jeopardizing their own well-being but also placing an undue burden on society, particularly in terms of the resources required for rescue and medical care.Proponents of extreme sports, on the other hand, argue that individuals should have the autonomy to make their own choices and take calculated risks. They assert that the freedom to pursue one's passions and push the boundaries of human potential is a fundamental human right, and that the benefits of these activities, both personal and societal, outweigh the risks.Ultimately, the debate surrounding extreme sports is a complex and multifaceted issue that involves considerations of individual liberty, public safety, ethical responsibilities, and the broader impact on society. While there are valid arguments on both sides, it is clear that finding a balance between the thrill and excitement of these activities and the need to ensure the well-being and safety of all involved is a critical challenge that must be addressed.As with any controversial topic, it is essential to approach the discussion with an open and nuanced perspective, considering the diverse perspectives and experiences of all stakeholders. Only through thoughtful dialogue, evidence-based policymaking, and acommitment to fostering a culture of responsible risk-taking can we hope to find a way forward that respects the desires of extreme sports enthusiasts while also prioritizing the safety and well-being of all members of the community.。

山东医药2019年第59卷第23期信号通路的激活有关。
参考文献:[1]Takenami T,Wang G,Nara Y,et al.Intrathecally administeredropivacaine is less neurotoxic than procaine,bupivacaine,and levobupivacaine in a rat spinal model[J].Can J Anesthes,2012, 33(5):456.[]申华素,崔静静,徐贯杰•局麻药脊髓神经毒性的研究进展[J].实用疼痛学杂志,2013(1):58-62.[3]苑振亭,高培平,刘成刚•临床用药指南与评价[M].北京:金盾出版社,2016:351.[4]Ji J,Yan X,Li Z,et al.Therapeutic effects of intrathecal versusintravenous monosialoganglioside against bupivacaine-induced spinal neurotoxicity in rats[J].Biomed Phamaco Ther,2015,69(6):311-316.[5]Yin KJ,Kim GM,Lee JM,et al.JNK activation contributes toDP5induc-tion and apoptosis following traumatic spinal cord injury [J].Neurobio Dis,2005,20(3)881-889.[]张利亮,祁莉娜,姚泽宇•长链非编码RNA-Paupar在局麻药致神经毒性过程中的作用研究[J].四川大学学报(医学版),2017,48(6):873-876.[7]Martini AC,Stefania F,Koepp J,et al.Inhibition of spinal c-Jun-NH2-terminal kinase(JNK)improves locomotor activity of spinal cord injured rats[J].Nerosci Lett,2016,621:54-61.[8]Xu F,Li T,Zhang B.An improved method for protecting and fixing the lumbar catheters placed in the spinal subarachnoid space of rats[J].J Neurosci Meth,2009,183(2):114-118.[9]Basso DM,Beattie MS,Bresnahan JC.A sensitive and reliable locomotor rating scale for open field testing in rats[J] .J Neurotru-am,1995,12(1):1-21.[10]Liu B,Ji J,Feng Q,et al.Monosialoganglioside protects againstbupivacaine-induced neurotoxicity caused by endoplasmic reticulum stress in rats[J].Drug Des Dev Ther,2019,13(3):707718.[11]Chen L,Li Q,Wang H,et al.Paeoniflorin attenuated bupiva-caine-induced neurotoxicity in SH-SY5Y cells via suppression of the p38MAPK pathway[J].J Cell Biochem,2018,2018:27964.[12]Sovolyova N,Healy S,Samali,et al.Stressed to death-mechanisms of ER stress-induced cell death[J].Biological Chemistry, 2014,395(1):1-13.[13]Lauricella M,Emanuele S,Anneo AD,et al.JNK and AP-1mediate apoptosis induced by bortezomib in HepG2cells via FasL/ caspase-8and mitochondria-dependent pathways[J] .Apoptosis, 2006,11(4)607-625.[14]Filho BT,Araujo FF,Higino LD,et al.The effect of monosialog-anglyoside(GM-1)administration in spinal cord injury[J].Acta Ortop Bras,2016,24(3):123-126.[15]Su D,Ma J,Yang J,et al.Monosialotetrahexosy-1ganglioside attenuates diabetes-associated cerebral ischemia/reperfusion injury through suppression of the endoplasmic reticulum stress-induced apoptosis[J] .J Clin Neurosci,2017,41:54-59.(:2019-04-27)-作者•编者•读者•《山东医药》对医学名词及术语的一般要求医学名词应使用全国科学技术名词审定委员会公布的名词。

Advanced Trauma Life SupportAdvanced Trauma Life Support (ATLS) is a training program for doctors and Advanced Practice/Critical Care Paramedics in the management of acute trauma cases, developed by the American College of Surgeons. The program has been adopted worldwide in over 40 countries,[1] sometimes under the name of Early Management of Severe Trauma (EMST), especially outside North America. Its goal is to teach a simplified and standardized approach to trauma patients. Originally designed for emergency situations where only one doctor and one nurse are present, ATLS is now widely accepted as the standard of care for initial assessment and treatment in trauma centers. The premise of the ATLS program is to treat the greatest threat to life first. It also advocates that the lack of a definitive diagnosis and a detailed history should not slow the application of indicated treatment for life-threatening injury, with the most time-critical interventions performed early. However, there is mixed evidence to show that ATLS improves patient outcomes.[1][2][3][4][5][6]Primary SurveyThe first and key part of the assessment of patients presenting with trauma is called the primary survey. During this time, life-threatening injuries are identified and simultaneously resuscitation is begun. A simple mnemonic, ABCDE, is used as a memory aid for the order in which problems should be addressed.A AirwayB BreathingC CirculationD DisabilitiesE Expose/EnvironmentA - Airway Maintenance with Cervical Spine ProtectionThe first stage of the primary survey is to assess the airway. If the patient is able to talk, the airway is likely to be clear. If the patient is unconscious, he/she may not be able to maintain his/her own airway. The airway can be opened using a chin lift or jaw thrust. Airway adjuncts may be required. If the airway is blocked (e.g, by blood or vomit), the fluid must be cleaned out of the patient's mouth by the help of sucking instruments.B - Breathing and VentilationThe chest must be examined by inspection, palpation, percussion and auscultation. Subcutaneous emphysema and tracheal deviation must be identified if present. Life-threatening chest injuries, including tension pneumothorax, open pneumothorax, flail chest and massive haemothorax must be identified and rapidly treated. Flail chest, penetrating injuries and bruising can be recognised by inspection.C - Circulation with Hemorrhage ControlHemorrhage is the predominant cause of preventable post-injury deaths. Hypovolemic shock is caused by significant blood loss. Two large-bore intravenous lines are established and crystalloid solution given. If the patient does not respond to this, type-specific blood, or O-negative if this is not available, should be given. External bleeding is controlled by direct pressure. Occult blood loss may be into the chest, abdomen, pelvis or from the long bones.D - Disability (Neurologic Evaluation)During the primary survey a basic neurological assessment is made, known by the mnenomic AVPU (alert, verbal stimuli response, painful stimuli response, or unresponsive). A more detailed and rapid neurological evaluation is performed at the end of the primary survey. This establishes the patient's level of consciousness, pupil size and reaction, lateralizing signs, and spinal cord injury level.The Glasgow Coma Scale is a quick method to determine the level of consciousness, and is predictive of patient outcome. If not done in the primary survey, it should be performed as part of the more detailed neurologic examination in the secondary survey. An altered level of consciousness indicates the need for immediate reevaluation of the patient's oxygenation, ventilation, and perfusion status. Hypoglycemia and drugs, including alcohol, may influence the level of consciousness. If these are excluded, changes in the level of consciousness should be considered to be due to traumatic brain injury until proven otherwise.E - Exposure / Environmental controlThe patient should be completely undressed, usually by cutting off the garments. It is imperative to cover the patient with warm blankets to prevent hypothermia in the emergency department. Intravenous fluids should be warmed and a warm environment maintained. Patient privacy should be maintained.Secondary SurveyWhen the primary survey is completed, resuscitation efforts are well established, and the vital signs are normalizing, the secondary survey can begin.The secondary survey is a head-to-toe evaluation of the trauma patient, including a complete history and physical examination, including the reassessment of all vital signs. Each region of the body must be fully examined. X-rays indicated by examination are obtained.If at any time during the secondary survey the patient deteriorates, another primary survey is carried out as a potential life threat may be present.The person should be removed from the hard spine board and placed on a firm mattress as soon as reasonably feasible as the spine board can rapidly cause skin breakdown and pain while a firm mattress provides equivalent stability for potential spinal fractures.[7]Alternatives to ATLSAnaesthesia Trauma and Critical Care (ATACC) is an international trauma course based in the United Kingdom. It is an advanced trauma course and represents the next level for trauma care and trauma patient management post ATLS certification. Accredited by two Royal Colleges and numerous emergency services, the course runs numerous times per year for candidates drawn from all areas of medicine and trauma care.[8] Specific injuries, such as major burn injury, may be better managed by modified ATLS protocols such as EMSB (Emergency Management of Severe Burns: a training course and protocols developed by the Australian and New Zealand Burn Association (ANZBA) and also adopted by the British Burn Association).[9][10]EvidenceAs of 2008 no evidence exist as to whether or not ATLS training improved outcomes.[11]HistoryATLS has its origins in the United States in 1976, when orthopaedic surgeon Dr. James K. Styner, piloting a light aircraft, crashed his plane into a field in Nebraska. His wife was killed instantly and three of his four children sustained critical injuries. He carried out the initial triage of his children at the crash site. Dr. Styner had to flag down a car to transport him to the nearest hospital; upon arrival, he found it closed. Even once the hospital was opened and a doctor called in, he found that the emergency care provided at the small regional hospital where they were treated was inadequate and inappropriate.[12]Upon returning to work, he set about developing a system for saving lives in medical trauma situations. Styner and his colleague Paul 'Skip' Collicott, with assistance from Advanced Cardiac Life Support personnel and the Lincoln Medical Education Foundation, produced the initial ATLS course which was held in 1978. In 1980, the American College of Surgeons Committee on Trauma adopted ATLS and began US and international dissemination of the course. Styner himself recently recertified as an ATLS instructor, teaching his Instructor Candidate course in the UK and then in the Netherlands.Since its inception, ATLS has become the standard for trauma care in American emergency departments and advanced paramedical services. Since emergency physicians, paramedics and other advanced practitioners use ATLS as their model for trauma care it makes sense that programs for other providers caring for trauma would be designed to interface well with ATLS. The Society of Trauma Nurses has developed the Advanced Trauma Care for Nurses (ATCN) course for Registered Nurses. ATCN meets concurrently with ATLS and shares some of the lecture portions. This approach allows for medical and nursing care to be well coordinated with one another as both the medical and nursing care providers have been trained in essentially the same model of care. Similarly, the National Association of Emergency Medical Technicians has developed the Prehospital Trauma Life Support (PHTLS) course for basic Emergency Medical Technicians (EMT)s and a more advanced level class for Paramedics. The International Trauma Life Support committee publishes the ITLS-Basic and ITLS-Advanced courses for prehospital profesionals as well. This course is based around ATLS and allows the PHTLS-trained EMTs to work alongside paramedics and to transition smoothly into the care provided by the ATLS and ATCN-trained providers in the hospital.See also•Trauma team•Basic Life Support•Advanced Life Support•Advanced Cardiac Life Support•Pediatric Advanced Life Support•Definitive Surgical Trauma Skills•ABC (medicine)•List of emergency medicine coursesFurther reading•American College of Surgeons (2008). Atls, Advanced Trauma Life Support Program for Doctors. Amer College of Surgeons. ISBN 978-1-880696-31-6.External links•Advanced Trauma Care for Nurses [13]•Definitive Surgical Trauma Skills [14]•About ATLS [15]References[1]Bouillon, B., Kanz, K.G., Lackner, C.K., Mutschler, W., & Sturm, J. The importance of Advanced Trauma Life Support (ATLS) in theemergency room [Article in German]. Unfallchirurg, 107(10), 844-850.[2]Hedges, J.R., Adams, A.L., & Gunnels, M.D. ATLS practices and survival at rural level III trauma hospitals, 1995-1999. PrehospitalEmergency Care, 6(3), 299-305.[3]Sethi, D.D., Habibula, S., & Kelly, A.M. Advanced trauma life support training for hospital staff. Cochrane Database of Systematic Reviews2003, Issue 3. Art. No.: CD004173. DOI: 10.1002/14651858.CD004173.pub2.[4]van Olden, G.D., Meeuwis, J.D., Bolhuis, H.W., Boxma, H., & Goris, R.J. (2004, November). Clinical impact of advanced trauma lifesupport. American Journal of Emergency Medicine, 22(7), 522-525.[5]Barsuk, D., Ziv, A., Lin, G., Blumenfeld, A., Rubin, O., Keidan, I., Munz, Y., & Berkenstadt, H. (2005, March). Using advanced simulationfor recognition and correction of gaps in airway and breathing management skills in prehospital trauma care. Anesthesia and Analgesia, 100(3), 803-809.[6]Roettger, R. H., Taylor, S. M., Youkey, J. R., & Blackhurst, D. W. (2005, August). The general surgery model: A more appealing andsustainable alternative for the care of trauma patients. The American Surgeon, 71(8), 633-638.[7]Amal Mattu; Deepi Goyal; Barrett, Jeffrey W.; Joshua Broder; DeAngelis, Michael; Peter Deblieux; Gus M. Garmel; Richard Harrigan;David Karras; Anita L'Italien; David Manthey (2007). Emergency medicine: avoiding the pitfalls and improving the outcomes. Malden, Mass: Blackwell Pub./BMJ Books. pp. 60. ISBN 1-4051-4166-2.[8]Anaesthesia Trauma and Critical Care ()[9]/emsb.htm[10].au/go/education-and-training/courses/external-provider-courses/emsb[11]Jayaraman S, Sethi D (2009). "Advanced trauma life support training for hospital staff". Cochrane Database Syst Rev (2): CD004173.doi:10.1002/14651858.CD004173.pub3. PMID 19370594.[12]Carmont MR (2005). "The Advanced Trauma Life Support course: a history of its development and review of related literature".Postgraduate medical journal81 (952): 87–91. doi:10.1136/pgmj.2004.021543. PMID 15701739.[13]/education/atcn[14]/education/courses/surgical_trauma.html[15]/trauma/atls/about.htmlArticle Sources and Contributors5 Article Sources and ContributorsAdvanced Trauma Life Support Source: /w/index.php?oldid=359050281 Contributors: Andreas Carter, Anna Lincoln, Atacc1, Autoload, Balancer, BigrTex,Biophysiscool, Blackhawk charlie2003, Brendanconway, Bunnyhop11, Carbonix, Cburnett, Ckshayin, CliffC, Couki, Dan100, Dancinginblood, Daniel575, Dantheman531, Daveb, Drravikanojia, Edward, Fingers-of-Pyrex, Flowersofnight, Galaxiaad, Graham87, GrigoriX2, Howard224, Ian4298, Idmdave, Jakednb, Jmh649, Johan Malmgren, Mandarax, Matt2501, Obsidianearth, Quadell, Rainbowbriteuk, Ravindar bethi, Rhcastilhos, Rjwilmsi, Rsabbatini, Sapient, Serrin, Starburns, StefanB sv, Trevor Wennblom, Twirligig, World Perspective, Wouterstomp, Wuzur, Xnike315x,59 anonymous editsLicenseCreative Commons Attribution-Share Alike 3.0 Unported/licenses/by-sa/3.0/。


神外杂志英-中文目录(2022年10月) Neurosurgery1.Assessment of Spinal Metastases Surgery Risk Stratification Tools in BreastCancer by Molecular Subtype按照分子亚型评估乳腺癌脊柱转移手术风险分层工具2.Microsurgery versus Microsurgery With Preoperative Embolization for BrainArteriovenous Malformation Treatment: A Systematic Review and Meta-analysis 显微手术与显微手术联合术前栓塞治疗脑动静脉畸形的系统评价和荟萃分析mentary: Silk Vista Baby for the Treatment of Complex Posterior InferiorCerebellar Artery Aneurysms点评: Silk Vista Baby用于治疗复杂的小脑下后动脉动脉瘤4.Targeted Public Health Training for Neurosurgeons: An Essential Task for thePrioritization of Neurosurgery in the Evolving Global Health Landscape针对神经外科医生的有针对性的公共卫生培训:在不断变化的全球卫生格局中确定神经外科手术优先顺序的一项重要任务5.Chronic Encapsulated Expanding Hematomas After Stereotactic Radiosurgery forIntracranial Arteriovenous Malformations: An International Multicenter Case Series立体定向放射外科治疗颅内动静脉畸形后的慢性包裹性扩张血肿:国际多中心病例系列6.Trends in Reimbursement and Approach Selection for Lumbar Arthrodesis腰椎融合术的费用报销和入路选择趋势7.Diffusion Basis Spectrum Imaging Provides Insights Into Cervical SpondyloticMyelopathy Pathology扩散基础频谱成像提供了脊髓型颈椎病病理学的见解8.Association Between Neighborhood-Level Socioeconomic Disadvantage andPatient-Reported Outcomes in Lumbar Spine Surgery邻域水平的社会经济劣势与腰椎手术患者报告结果之间的关系mentary: Prognostic Models for Traumatic Brain Injury Have GoodDiscrimination But Poor Overall Model Performance for Predicting Mortality and Unfavorable Outcomes评论:创伤性脑损伤的预后模型在预测death率和不良结局方面具有良好的区分性,但总体模型性能较差mentary: Serum Levels of Myo-inositol Predicts Clinical Outcome 1 YearAfter Aneurysmal Subarachnoid Hemorrhage评论:血清肌醇水平预测动脉瘤性蛛网膜下腔出血1年后的临床结局mentary: Laser Interstitial Thermal Therapy for First-Line Treatment ofSurgically Accessible Recurrent Glioblastoma: Outcomes Compared With a Surgical Cohort评论:激光间质热疗用于手术可及复发性胶质母细胞瘤的一线治疗:与手术队列的结果比较12.Functional Reorganization of the Mesial Frontal Premotor Cortex in Patients WithSupplementary Motor Area Seizures辅助性运动区癫痫患者中额内侧运动前皮质的功能重组13.Concurrent Administration of Immune Checkpoint Inhibitors and StereotacticRadiosurgery Is Well-Tolerated in Patients With Melanoma Brain Metastases: An International Multicenter Study of 203 Patients免疫检查点抑制剂联合立体定向放射外科治疗对黑色素瘤脑转移患者的耐受性良好:一项针对203例患者的国际多中心研究14.Prognosis of Rotational Angiography-Based Stereotactic Radiosurgery for DuralArteriovenous Fistulas: A Retrospective Analysis基于旋转血管造影术的立体定向放射外科治疗硬脑膜动静脉瘘的预后:回顾性分析15.Letter: Development and Internal Validation of the ARISE Prediction Models forRebleeding After Aneurysmal Subarachnoid Hemorrhage信件:动脉瘤性蛛网膜下腔出血后再出血的ARISE预测模型的开发和内部验证16.Development of Risk Stratification Predictive Models for Cervical DeformitySurgery颈椎畸形手术风险分层预测模型的建立17.First-Pass Effect Predicts Clinical Outcome and Infarct Growth AfterThrombectomy for Distal Medium Vessel Occlusions首过效应预测远端中血管闭塞血栓切除术后的临床结局和梗死生长mentary: Risk for Hemorrhage the First 2 Years After Gamma Knife Surgeryfor Arteriovenous Malformations: An Update评论:动静脉畸形伽玛刀手术后前2年出血风险:更新19.A Systematic Review of Neuropsychological Outcomes After Treatment ofIntracranial Aneurysms颅内动脉瘤治疗后神经心理结局的系统评价20.Does a Screening Trial for Spinal Cord Stimulation in Patients With Chronic Painof Neuropathic Origin Have Clinical Utility (TRIAL-STIM)? 36-Month Results From a Randomized Controlled Trial神经性慢性疼痛患者脊髓刺激筛选试验是否具有临床实用性(TRIAL-STIM)?一项随机对照试验的36个月结果21.Letter: Transcriptomic Profiling Revealed Lnc-GOLGA6A-1 as a NovelPrognostic Biomarker of Meningioma Recurrence信件:转录组分析显示Lnc-GOLGA6A-1是脑膜瘤复发的一种新的预后生物标志物mentary: The Impact of Frailty on Traumatic Brain Injury Outcomes: AnAnalysis of 691 821 Nationwide Cases评论:虚弱对创伤性脑损伤结局的影响:全国691821例病例分析23.Optimal Cost-Effective Screening Strategy for Unruptured Intracranial Aneurysmsin Female Smokers女性吸烟者中未破裂颅内动脉瘤的最佳成本效益筛查策略24.Letter: Pressure to Publish—A Precarious Precedent Among Medical Students信件:出版压力——医学研究者中一个不稳定的先例25.Letter: Protocol for a Multicenter, Prospective, Observational Pilot Study on theImplementation of Resource-Stratified Algorithms for the Treatment of SevereTraumatic Brain Injury Across Four Treatment Phases: Prehospital, Emergency Department, Neurosurgery, and Intensive Care Unit信件:一项跨四个治疗阶段(院前、急诊科、神经外科和重症监护室)实施资源分层算法的多中心、前瞻性、观察性试点研究的协议26.Risk for Hemorrhage the First 2 Years After Gamma Knife Surgery forArteriovenous Malformations: An Update动静脉畸形伽玛刀手术后前2年出血风险:更新Journal of Neurosurgery27.Association of homotopic areas in the right hemisphere with language deficits inthe short term after tumor resection肿瘤切除术后短期内右半球同话题区与语言缺陷的关系28.Association of preoperative glucose concentration with mortality in patientsundergoing craniotomy for brain tumor脑肿瘤开颅手术患者术前血糖浓度与death率的关系29.Deep brain stimulation for movement disorders after stroke: a systematic review ofthe literature脑深部电刺激治疗脑卒中后运动障碍的系统评价30.Effectiveness of immune checkpoint inhibitors in combination with stereotacticradiosurgery for patients with brain metastases from renal cell carcinoma: inverse probability of treatment weighting using propensity scores免疫检查点抑制剂联合立体定向放射外科治疗肾细胞癌脑转移患者的有效性:使用倾向评分进行治疗加权的反向概率31.Endovascular treatment of brain arteriovenous malformations: clinical outcomesof patients included in the registry of a pragmatic randomized trial脑动静脉畸形的血管内治疗:纳入实用随机试验登记处的患者的临床结果32.Feasibility of bevacizumab-IRDye800CW as a tracer for fluorescence-guidedmeningioma surgery贝伐单抗- IRDye800CW作为荧光导向脑膜瘤手术示踪剂的可行性33.Precuneal gliomas promote behaviorally relevant remodeling of the functionalconnectome前神经胶质瘤促进功能性连接体的行为相关重塑34.Pursuing perfect 2D and 3D photography in neuroanatomy: a new paradigm forstaying up to date with digital technology在神经解剖学中追求完美的2D和三维摄影:跟上数字技术的新范式35.Recurrent insular low-grade gliomas: factors guiding the decision to reoperate复发性岛叶低级别胶质瘤:决定再次手术的指导因素36.Relationship between phenotypic features in Loeys-Dietz syndrome and thepresence of intracranial aneurysmsLoeys-Dietz综合征的表型特征与颅内动脉瘤存在的关系37.Continued underrepresentation of historically excluded groups in the neurosurgerypipeline: an analysis of racial and ethnic trends across stages of medical training from 2012 to 2020神经外科管道中历史上被排除群体的代表性持续不足:2012年至2020年不同医学培训阶段的种族和族裔趋势分析38.Management strategies in clival and craniovertebral junction chordomas: a 29-yearexperience斜坡和颅椎交界脊索瘤的治疗策略:29年经验39.A national stratification of the global macroeconomic burden of central nervoussystem cancer中枢神经系统癌症全球宏观经济负担的国家分层40.Phase II trial of icotinib in adult patients with neurofibromatosis type 2 andprogressive vestibular schwannoma在患有2型神经纤维瘤病和进行性前庭神经鞘瘤的成人患者中进行的盐酸埃克替尼II期试验41.Predicting leptomeningeal disease spread after resection of brain metastases usingmachine learning用机器学习预测脑转移瘤切除术后软脑膜疾病的扩散42.Short- and long-term outcomes of moyamoya patients post-revascularization烟雾病患者血运重建后的短期和长期结局43.Alteration of default mode network: association with executive dysfunction infrontal glioma patients默认模式网络的改变:与额叶胶质瘤患者执行功能障碍的相关性44.Correlation between tumor volume and serum prolactin and its effect on surgicaloutcomes in a cohort of 219 prolactinoma patients219例泌乳素瘤患者的肿瘤体积与血清催乳素的相关性及其对手术结果的影响45.Is intracranial electroencephalography mandatory for MRI-negative neocorticalepilepsy surgery?对于MRI阴性的新皮质癫痫手术,是否必须进行颅内脑电图检查?46.Neurosurgeons as complete stroke doctors: the time is now神经外科医生作为完全中风的医生:时间是现在47.Seizure outcome after resection of insular glioma: a systematic review, meta-analysis, and institutional experience岛叶胶质瘤切除术后癫痫发作结局:一项系统综述、荟萃分析和机构经验48.Surgery for glioblastomas in the elderly: an Association des Neuro-oncologuesd’Expression Française (ANOCEF) trial老年人成胶质细胞瘤的手术治疗:法国神经肿瘤学与表达协会(ANOCEF)试验49.Surgical instruments and catheter damage during ventriculoperitoneal shuntassembly脑室腹腔分流术装配过程中的手术器械和导管损坏50.Cost-effectiveness analysis on small (< 5 mm) unruptured intracranial aneurysmfollow-up strategies较小(< 5 mm)未破裂颅内动脉瘤随访策略的成本-效果分析51.Evaluating syntactic comprehension during awake intraoperative corticalstimulation mapping清醒术中皮质刺激标测时句法理解能力的评估52.Factors associated with radiation toxicity and long-term tumor control more than10 years after Gamma Knife surgery for non–skull base, nonperioptic benignsupratentorial meningiomas非颅底、非周期性良性幕上脑膜瘤伽玛刀术后10年以上与放射毒性和长期肿瘤控制相关的因素53.Multidisciplinary management of patients with non–small cell lung cancer withleptomeningeal metastasis in the tyrosine kinase inhibitor era酪氨酸激酶抑制剂时代有软脑膜转移的非小细胞肺癌患者的多学科管理54.Predicting the growth of middle cerebral artery bifurcation aneurysms usingdifferences in the bifurcation angle and inflow coefficient利用分叉角和流入系数的差异预测大脑中动脉分叉动脉瘤的生长55.Predictors of surgical site infection in glioblastoma patients undergoing craniotomyfor tumor resection胶质母细胞瘤患者行开颅手术切除肿瘤时手术部位感染的预测因素56.Stereotactic radiosurgery for orbital cavernous hemangiomas立体定向放射外科治疗眼眶海绵状血管瘤57.Surgical management of large cerebellopontine angle meningiomas: long-termresults of a less aggressive resection strategy大型桥小脑角脑膜瘤的手术治疗:较小侵袭性切除策略的长期结果Journal of Neurosurgery: Case Lessons58.5-ALA fluorescence–guided resection of a recurrent anaplastic pleomorphicxanthoastrocytoma: illustrative case5-ALA荧光引导下切除复发性间变性多形性黄色星形细胞瘤:说明性病例59.Flossing technique for endovascular repair of a penetrating cerebrovascular injury:illustrative case牙线技术用于血管内修复穿透性脑血管损伤:例证性病例60.Nerve transfers in a patient with asymmetrical neurological deficit followingtraumatic cervical spinal cord injury: simultaneous bilateral restoration of pinch grip and elbow extension. Illustrative case创伤性颈脊髓损伤后不对称神经功能缺损患者的神经转移:同时双侧恢复捏手和肘关节伸展。
Degenerative Disc Disease in Dogs狗锥间盘突出What is a disc, and what is its purpose"椎间盘及其功能The spinal cord脊髓 is one of the most important and sensitive organ systems in the body. If it is damaged, the nerve cells do not regenerate but are replaced with fibrous or scar tissue. Spinal cord injuries usually result in permanent,irreversible damage. To protect it from damage, the spinal cord runs through a bony canal within the spine and is surrounded by protective bone everywhere e*cept the junction of the vertebrae. These junctions are filled by rubber-like cushions called intervertebral discs.椎间盘The individual vertebrae and intervertebral discs allow the back to move up and down and sideways without allowing contact between the bones of the spinal column. 脊柱This e*treme protection of the spinal cord reflects its importance and fragility.脊髓是动物体内最重要、最敏感的器官系统之一,一旦损坏,脑神经细胞将不能修复再生,从而变为纤维和疤痕组织。
·综述·小胶质细胞在脊髓损伤中的作用机制研究进展夏宇,丁璐,邓宇斌作者单位中山大学附属第七医院科研中心深圳518107基金项目国家自然科学基金项目(No.82071362)收稿日期2022-04-25通讯作者邓宇斌dengyub@摘要脊髓损伤(spinal cord injury ,SCI )是由于外力或非外力作用造成脊柱骨、韧带及神经结构的破坏,并伴随着损伤部位以下躯干与四肢的感觉运动功能障碍,其致残率高。
小胶质细胞作为中枢神经系统固有的免疫细胞,在SCI 后接受损伤信号,发挥分泌因子及吞噬作用,同时和神经元、星形胶质细胞、少突胶质细胞及其它细胞与非细胞成分发生反应。
目前研究显示,小胶质细胞具有多态性和多功能性,参与SCI 的病理生理过程,包括炎症、疤痕形成和疼痛。
本综述结合前期课题组星形胶质细胞研究基础,通过总结近年来小胶质细胞在SCI 过程中功能的研究文献,为SCI 疾病进展研究提供新的思路与方向。
关键词脊髓损伤;小胶质细胞;星形胶质细胞中图分类号R741;R741.02;R744文献标识码A DOI 10.16780/ki.sjssgncj.20220376本文引用格式:夏宇,丁璐,邓宇斌.小胶质细胞在脊髓损伤中的作用机制研究进展[J].神经损伤与功能重建,2023,18(10):593-596.脊髓损伤(spinal cord injury ,SCI )是由于外力或非外力作用造成脊柱骨、韧带及神经结构的破坏,并伴随着损伤部位以下躯干与四肢的感觉运动功能障碍,每年全球约70万例新发病例,致残率高[1,2]。
神经功能障碍是导致SCI 高残障率的基础。
除了神经元的死亡、突触连接的丢失等原发性损伤,小胶质细胞作为胶质细胞的一员参与激活炎症级联反应,造成继发性损伤[3]。
1小胶质细胞的定义小胶质细胞作为中枢神经系统(central nervous system ,CNS )固有的免疫细胞,是神经组织中唯一来源于中胚层的细胞[4]。
脊髓损伤对男性性功能影响的临床观察李广伟1,王少波2,赵文奎3,白忠旭1,王红军1,孙晓智1(1.郑州市第二人民医院骨科,河南郑州450000;.北京大学第三医院骨科,北京 100191;.北京大学第三医院疼痛科,北京 100191)摘要:目的探讨脊髓损伤对男性性功能的影响。
方法对2017年12月至2019年5月在郑州市第二人民医院诊治的39例外伤性脊髓损伤患者进行回顾性分析。
伤后所有患者均手术处理。
患者均为男性;年龄28〜46岁,平均 年龄(37.5土5.5)岁。
采用勃起功能国际指数(international mdex of erectile function-5 ,IIEF-5)评定勃起功能,神经脊髓功能则用美国脊柱损伤协会(American spinal injury association,ASIA)制定的脊髓损伤神经学分类标准进行ASIA 分级.ASIA 运动及感觉评分进行评价,观察脊髓损伤前后脊髓神经功能受损及性功能的影响。
术后行脊髓神经功能及性功能恢复的对比研究,并对性功能障碍患者进一步测定其反射性勃起和精神性勃起的情况。
结果所有患者随访时间14〜31个月,平均(21.9土5.2)个月。
脊髓损伤后除运动感觉受损外,性功能也同时受损,与伤前比较差异具 有统计学意义(P <0.05)。
IIEF -5评分由伤前的(22.9土 1.4)分降为伤后的(11.3土2.7)分,术后提高到(17.9土5.2)分。
ASIA 感觉评分术前为(116.9土50.0)分,术后提高到(174.6土 36.0)分;运动评分术前为(60.5土 14.3)分,术后提高到(83.0土 13.8)分,感觉评分与运动评分均较术前明显改善,差异有统计学意义(P <0.05)。
结论外伤性脊髓损伤除导致常见的运动感觉神经功能受损外,对男性性功能有明显的影响。
治疗时间、受损节段、损伤程度、恢复时间等因素对性功能障碍的程度和恢复均有影响。
急性颈髓损伤水电解质紊乱的分析与治疗张勤安1母心灵1 王少华2孙宜保3 杨勇3【摘要】目的:探讨急性颈髓损伤(CSCI)后出现的水电解质紊乱的异常情况及治疗。
方法:回顾性分析40例CSCI患者水电解质紊乱及处理方法。
结果:CSCI导致患者水电解质紊乱是常见的并发症,对于这种并发症临床医生应该正确掌握补液方法,及时纠正水电解质紊乱对提高急性颈髓损伤患者救治水平起重要作用。
【关键词】颈髓损伤;电解质紊乱;低血钠症Water and Electrolyte Disorders and Their Supporting Treatment during AcuteCervical spinal cord injury(CSCI)Qin an Zhang1,Xin ling Mu1,Shao hua Wang2,Yi Bao Sun3,Yang Yong3【Abstract】Objective :To explore water-electrolytes imbalance and Giving Appropriate Measures.in patients with cervical spinal Cervical spinal cord injury(CSCI).Methods :Some 40 patients were retrospectively analyzed for water-electrolytes Na+ imbalance,and Giving Appropriate Measures.Results :hyponatremia of water-electrolytes imbalance is as very complication in acute complete CSCI patients, Clinical doctors can actively respond to Giving Appropriate Measures. 【Author,s Adderss】orthopaedic hospital spinalⅡDpartment of Zhengzhou,450052【Key words】Cervical spinal cord injury(CSCI);water-electrolytes imbalance: hyponatremia.急性颈髓损伤(cervical spinal cord injury,CSCI)是一种在临床较为常见、严重的创伤,颈髓损伤的同时严重的低钠血症是急性颈髓损伤常见的并发症,但其发生机制尚不明确。
神经调控英语NeuromodulationThe field of neuromodulation has emerged as a revolutionary approach to addressing a wide range of neurological and psychiatric disorders. Neuromodulation, a term that encompasses various techniques aimed at altering the activity of the nervous system, has the potential to transform the way we understand and treat a multitude of complex conditions.At the heart of neuromodulation lies the principle of targeted intervention. By directly influencing the neural pathways and circuits responsible for specific functions or dysfunctions, clinicians and researchers can achieve remarkable outcomes. This approach offers a promising alternative to traditional pharmacological or surgical interventions, often providing more precise and personalized treatment options.One of the primary applications of neuromodulation is in the field of pain management. Chronic pain, a debilitating condition that affects millions of individuals worldwide, has long been a challenge for healthcare providers. Neuromodulation techniques, such as spinalcord stimulation and peripheral nerve stimulation, have demonstrated remarkable success in managing various types of chronic pain, including neuropathic pain, failed back surgery syndrome, and complex regional pain syndrome. By selectively modulating the neural pathways responsible for pain transmission, these interventions can effectively alleviate symptoms and improve the quality of life for patients.Beyond pain management, neuromodulation has also made significant strides in the treatment of neurological disorders. Conditions like Parkinson's disease, essential tremor, and dystonia have been the focus of extensive research and clinical trials utilizing deep brain stimulation (DBS) – a technique that involves the implantation of electrodes into targeted regions of the brain. By precisely delivering electrical impulses to specific neural structures, DBS has been shown to improve motor function, reduce tremors, and alleviate other debilitating symptoms associated with these neurological disorders.In the realm of psychiatric disorders, neuromodulation has also emerged as a promising approach. Conditions such as treatment-resistant depression, obsessive-compulsive disorder, and post-traumatic stress disorder have been the subject of numerous studies exploring the potential of techniques like transcranial magnetic stimulation (TMS) and vagus nerve stimulation (VNS). These non-invasive or minimally invasive interventions aim to modulate the neural circuits involved in the regulation of mood, cognition, and emotional processing, offering new hope for individuals who have not responded well to traditional pharmacological or psychotherapeutic treatments.The advancements in neuromodulation have also extended to the field of rehabilitation and neurological recovery. Patients who have suffered from strokes, spinal cord injuries, or traumatic brain injuries often face significant challenges in regaining lost functions and independence. Neuromodulation techniques, such as transcranial direct current stimulation (tDCS) and epidural spinal cord stimulation, have demonstrated the ability to enhance neuroplasticity, facilitate motor learning, and improve functional outcomes in these populations.Importantly, the field of neuromodulation is not limited to the treatment of specific disorders; it also holds promise for enhancing cognitive and sensory capabilities in healthy individuals. Researchers are exploring the use of non-invasive brain stimulation techniques to improve memory, attention, and decision-making, as well as to enhance sensory perception and motor skills. These applications, often referred to as "neuroenhancement," raise intriguing ethical and societal considerations that require thoughtful discussion and regulation.As the field of neuromodulation continues to evolve, it is essential to acknowledge the complexities and challenges that accompany these advancements. The precise targeting of neural structures, the optimization of stimulation parameters, and the long-term safety and efficacy of these interventions are all areas that require ongoing research and clinical evaluation. Additionally, the integration of neuromodulation into existing healthcare systems and the development of accessible and affordable treatment options are crucial considerations for ensuring equitable access to these innovative therapies.Despite these challenges, the potential of neuromodulation to transform the lives of individuals living with neurological and psychiatric conditions is undeniable. By harnessing the remarkable plasticity and adaptability of the nervous system, clinicians and researchers are poised to unlock new frontiers in the understanding and management of a wide range of complex disorders. As the field continues to evolve, the promise of neuromodulation holds the potential to revolutionize the way we approach neurological and mental health care, offering hope and improved quality of life for those in need.。
中南大学学报(医学版)J Cent South Univ (Med Sci)2019, 44(4) htt p://377脊髓哺乳动物雷帕霉素靶蛋白信号通路参与大鼠外周神经损伤诱发的痛觉过敏杨文茜1,2,郭曲练1,程智刚1,王云姣1,白念岳1,贺正华1 ( 1. 中南大学湘雅医院麻醉科,长沙 410008;2. 湖南省肿瘤医院,中南大学湘雅医学院附属肿瘤医院麻醉科,长沙 410013 )[摘要] 目的:探讨哺乳动物雷帕霉素(rapamycin ,RAPA)靶蛋白(mammalian target of RAPA ,mTOR)信号通路是否通过激活脊髓背角星形胶质细胞参与外周神经损伤诱发的大鼠痛觉过敏。
方法:取健康雄性Sprague-Dawley(SD)大鼠30只,随机分为6组(n =5):1 d 组(D1组)、4 d 组(D4组)、7 d 组(D7组)、14 d 组(D14组)、正常组、假手术组。
其中D1,D4,D7,D14组建立坐骨神经慢性压榨损伤(chronic constriction injury ,CCI)模型,Normal 组不做处理,Sham 组仅暴露坐骨神经。
于CCI 术后第1,4,7,14天分别测定各组大鼠左后肢机械缩足阈值(paw withdrawal mechanical threshold ,PWMT)和热缩足潜伏期(paw withdrawal thermal latency ,PWTL)。
D1,D4,D7,D14组分别于CCI 术后第1,4,7,14天,假手术组和正常组于相应第14天采集腰段脊髓。
采用免疫组织化学法观察mTOR 在大鼠脊髓的分布,采用real-time PCR 和蛋白质印迹法检测CCI 大鼠腰段脊髓mTOR mRNA 和蛋白的表达。
另取雄性SD 大鼠30只,完成鞘内置管后,随机分为6组(n =5):空白组、CCI 组、早给药组(CCI+early RAPA 组)、早溶剂组[CCI+early 二甲基亚砜(dimethylsulfoxide ,DMSO)组]、晚给药组(CCI+later RA PA 组)、晚溶剂组(CCI+later DMSO 组)。
Rat Models of Traumatic Spinal Cord Injury to Assess Motor Recovery Stephen M.Onifer,Alexander G.Rabchevsky,and Stephen W.ScheffAbstractDevastating motor,sensory,and autonomic dysfunctions render long-term personal hardships to the survivors of trau-matic spinal cord injury(SCI).The suffering also extends to the survivors’families and friends,who endure emotional, physical,and financial burdens in providing for necessary surgeries,care,and rehabilitation.After the primary me-chanical SCI,there is a complex secondary injury cascade that leads to the progressive death of otherwise potentially viable axons and cells and that impairs endogenous re-covery processes.Investigations of possible cures and of ways to alleviate the hardships of traumatic SCI include those of interventions that attenuate or overcome the sec-ondary injury cascade,enhance the endogenous repair mechanisms,regenerate axons,replace lost cells,and reha-bilitate.These investigations have led to the creation of laboratory animal models of the different types of traumatic human SCI and components of the secondary injury cas-cade.However,no particular model completely addresses all aspects of traumatic SCI.In this article,we describe adult rat SCI models and the motor,and in some cases sensory and autonomic,deficits that each produces.Impor-tantly,as researchers in this area move toward clinical trials to alleviate the hardships of traumatic SCI,there is a need for standardized small and large animal SCI models as well as quantitative behavioral and electrophysiological assess-ments of their outcomes so that investigators testing various interventions can directly compare their results and corre-late them with the molecular,biochemical,and histological alterations.Key Words:compression;contusion;demyelination; excitotoxicity;free radicals;inflammation;ischemia; laceration IntroductionT raumatic spinal cord injury(SCI1)in the United States happens to approximately11,000persons each year (Spinal Cord Injury Information Network,www. ).While the majority of these injuries occur to the cervical spinal cord,devastating motor,sen-sory,and autonomic dysfunctions below the injury render long-term hardships to the survivors of all levels of SCI—cervical,thoracic,lumbar,and sacral.The suffering also extends to survivors’families and friends,who endure emo-tional,physical,and financial burdens in providing for nec-essary surgeries,care,and rehabilitation.Based on imaging and histology of injured human spinal cords,Bunge and colleagues classified each traumatic SCI as(1)a contusion evolving to cavity formation,(2)a massive compression,or(3)a laceration(Bunge et al.1993, 1997).The most frequent contusion injuries are focal spinal cord compression and render both an intact glial limitans and pia surrounding varying extents of intact white and gray matter.Fluid-filled cavities or cysts evolve from the hem-orrhage into the spinal cord parenchyma and where tissue has degenerated.In contrast,the glial limitans and pia are cut in massive compression and laceration injuries.Massive compression injuries occur over substantial lengths of the spinal cord and include ceration injuries are focal and,similar to massive compression injuries,result in the formation over time of a connective tissue mass in the spinal cord.After the primary mechanical SCI,there is a complex secondary injury cascade(Tator and Fehlings1991)that leads to the progressive death of otherwise potentially vi-able axons and cells and that impairs endogenous recovery processes.Investigations of possible cures and ways to al-leviate the hardships of traumatic SCI include those of in-terventions that attenuate or overcome the secondary injury cascade,enhance the endogenous repair mechanisms,re-generate axons,replace lost cells,and rehabilitate(Ander-son et al.2005a;Blight and Tuszynski2006;Bradbury and McMahon2006;Kleitman2004;Rabchevsky and Smith 2001;Tator2006;Thuret et al.2006).These investigationsStephen M.Onifer,Ph.D.,is an Assistant Professor,Department of Anatomy and Neurobiology,and Scientist III;and Alexander G.Rabchev-sky,Ph.D.,is an Associate Professor,Department of Physiology,both at the Spinal Cord and Brain Injury Research Center,University of Kentucky, Lexington,KY.Stephen W.Scheff,Ph.D.,is a Professor,Department of Anatomy and Neurobiology,Sanders-Brown Center on Aging101, University of Kentucky,Lexington,KY.Address correspondence and reprint requests to Dr.Stephen M.Onifer, Spinal Cord and Brain Injury Research Center,Biomedical and Biological Sciences Research Building,B365,University of Kentucky,741South Limestone Street,Lexington,KY40536-0509,or email smonif2@ .1Abbreviations used in this article:BBB scale,Basso,Beattie,and Bresna-han open-field locomotor rating scale;EMG,electromyographic;IH,Infi-nite Horizon;MIER,magnetically evoked interenlargement response; OSU,Ohio State University;SCI,spinal cord injury;SSEP,somatosensory evoked potential;tcMMEP,transcranial magnetic motor evoked potential.have led to the creation of laboratory animal models of traumatic human SCI.In this article,we describe adult rat SCI models and the motor,and in some cases sensory and autonomic,deficits that each produces.Rats have been chosen to study trau-matic SCI not only because they are readily available but also because the morphological,biochemical,and func-tional changes that occur after SCI are similar to those seen in humans(Fleming et al.2006;McTigue et al.2000;Metz et al.2000;Norenberg et al.2004).The rats are always anesthetized for the SCI and other surgical procedures,after which they require specialized veterinary care,the extent of which depends on both the location and severity of the injury(Santos-Benito et al.2006).Female rats are prefer-able because of the relative ease of manual bladder empty-ing after SCI,resulting in less frequent urinary tract infections.Assessments of Motor Deficits and Recovery in Adult Rats with SCIA number of assessments of behavioral and electrophysi-ological function are available for adult rats(for reviews, see Basso2004;Kesslak and Keirstead2003;Nichols et al. 2005;Webb and Muir2005).Importantly,most of these assessments evaluate the sensorimotor function rather than sensory or motor functions individually.Some are quanti-tative while others are not.We recommend the use of quan-titative assessments even though they require training the rats to criterion.The completion of assessments before SCI helps in establishing baseline values for each rat.We,like others,also recommend a battery of assessments whenever appropriate.For information purposes,we briefly discuss commonly used quantitative and semiquantitative assess-ments for adult rat models of traumatic SCI.Detailed infor-mation about these assessments is available in the reviews indicated above and the references throughout this section.BehaviorBecause rats prehend food with their mouths then manipu-late it with their forepaws,pellet retrieval tests are useful to assess reach,grasp,and retrieval behaviors after cervical SCI(Anderson et al.2005b;Nash et al.2002;Onifer et al. 1997b;Schrimsher and Reier1992;Weidner et al.2001). Importantly,it is necessary to train the rats used in post-SCI studies to prehend with their e of the“Staircase Test”platform and chamber(Montoya et al.1991)enables cervical spinal cord-injured rats to avoid having to support themselves on three dysfunctional limbs while reaching for, grasping,and retrieving pellets(Onifer et al.1997b).Inves-tigators have examined forelimb and forepaw usage for sup-port during spontaneous vertical exploration in a cylinder after cervical SCI(Liu et al.1999b;Soblosky et al.2001; Webb and Muir2004).There are also studies of forelimb grip strength in which the cervical-SCI rat is pulled away while grasping a bar(Anderson et al.2005b;Onifer et al. 1997b).Other studies evaluate forelimb and hindlimb plac-ing and footfalls while the rat walks or locomotes on a horizontal(Onifer et al.2005;Pearse et al.2005)or inclined (Li et al.2003)grid,horizontal ladder(Soblosky et al.2001; Webb and Muir2003,2004),beam(Jeffery and Blakemore 1997;Kim et al.2001),or rope(Anderson et al.2005b;Kim et al.2001).Many of the current models of SCI utilize the Basso, Beattie,and Bresnahan open-field locomotor rating scale (BBB scale1;Basso et al.1995)to assess functional out-come.The BBB scale has a range from zero(no hindlimb movements)to21(normal coordinate gait),using paw placement,joint movement,and truncal stability as impor-tant factors in determining the level of functional recovery. Scores in the0to7range focus primarily on hip,knee,and ankle joint movement,the8to13range keys in on paw placement and coordination,and scores of14to21rely heavily on trunk stability,tail position,and paw placement. Tarlov and Klinger(1954)developed one of the original open-field behavior tests for SCI and it involved a rather simplistic observational assessment of the animal’s locomo-tor ability.A modified version of the Tarlov scale(Gale et al.1985)consisting of six levels of motor movement(from 0for no hindlimb movement to5for normal walking)ap-pears extensively in the literature(Haghighi et al.2000; Voda et al.2005).Other analyses of locomotion include footprint analysis(Cheng et al.1997;Kunkel-Bagden et al. 1993),the CatWalk-assisted gait analysis(Gensel et al. 2006;Hamers et al.2001;Hendriks et al.2006),and kine-matics(Broton et al.1996;Collazos-Castro et al.2006). Several studies correlate these data to electromyographic (EMG1)recordings made at the same time from electrodes implanted in limb muscles(Ballermann et al.2006;Kaegi et al.2002;Thota et al.2005).Numerous studies have used the inclined plane to evalu-ate functional outcome in rats after SCI(Rivlin and Tator 1977).The device consists of a hinged board raised and lowered to different angles.The object is for the rat to maintain itself on the board for5seconds as the angle is gradually increased at5o intervals.The assigned score is the maximum angle of the plane that the rat can maintain for5 seconds without sliding off onto a padded surface.Unin-jured rats achieve scores of approximately80o. ElectrophysiologyIn both humans and animals with traumatic SCI,electro-physiology is a valuable tool for investigation of the neural substrates underlying deficits and functional recovery as revealed by behavioral testing.Terminal electrophysiology procedures can be useful in experimental animals(e.g., Massey et al.2006),but it is much more advantageous to reproduce the procedure a number of times after SCI (Nashmi et al.1997)and without anesthesia.The transcra-nial magnetic motor evoked potential(tcMMEP1)procedure involves noninvasive magnetic stimulation at the unanes-thetized rat’s skull and the recording of evoked potentials with EMG electrodes temporarily inserted into hindlimb muscles(Fishback et al.1995;Linden et al.1999).While the tcMMEP procedure assesses supraspinal axon conduc-tion,magnetically evoked interenlargement(MIER1)and somotosensory evoked potential(SSEP1)procedures are ef-fective for the evaluation of propriospinal and sensory spi-nal axon conduction,respectively,in unanesthetized rats. The MIER procedure involves noninvasive magnetic stimu-lation at the rat’s hip or knee and the recording of evoked potentials with EMG electrodes temporarily inserted into forelimb and masseter muscles(Beaumont et al.2006).The SSEP procedure involves electrical stimulation of the paws with electrodes temporarily inserted into them,and the re-cording of evoked potentials from electrodes previously im-planted in the cranium over the somatosensory cortex (Onifer et al.2005).All three procedures take only a few minutes and cause only slight pain and distress and so are done in unanesthetized rats.Rat Models of Traumatic SCIWeight DropThe first well-documented animal experimentation of SCI was that described by Allen(1911),which used a weight-drop technique on dogs.The approach was quite simple:A laminectomy was performed on the anesthetized animal, exposing the dorsal(posterior)surface of the spinal cord.A given weight was dropped from a known height,down a vented guide tube positioned perpendicular to the laminec-tomy site.As the weight struck the exposed spinal cord, either directly or through an impounder plate that rested on the exposed cord(and may have diffused the injury over a wider area),the underlying tissue was subjected to a dy-namic compression.Depending on the weight and the height,different amounts of force were applied to the cord and resulted in varying degrees of SCI.The shape and di-ameter of the impounder surface were important variables in the injury outcome(Gerber and Corrie1979;Koozekanani et al.1976)as was the resistance encountered during the “free fall”(Dohrmann and Panjabi1976;Dohrmann et al. 1978).While Allen’s original work utilized dogs,a weight-drop technique was later adapted for the anesthetized rat (Panjabi and Wrathall1988;Wrathall et al.1985).Because of the difference in size of the impactor tip,the technique was initially thought to be too unreliable in the rodent(Khan and Griebel1983),with consistency observed only at the high severity end of the spectrum.Important variables that made the Wrathall model work were stabilization of the spinal column before the injury and a strain gauge,used to measure force,mounted on a“C”ring attached to an im-pounder resting on the exposed spinal cord dura.Many of the subsequent weight-drop models that were scaled down for producing cervical(Onifer et al.1997b;Schrimsher and Reier1992;Soblosky et al.2001)or thoracic SCI in the rat offered limited information on the biomechanical properties of the injury,although they did allow for reproducibility and graded levels of functional outcome(Wrathall et al.1985).It is extremely important that an injury model have the capability of generating different degrees of injury severity and functional outcomes.If injuries are too moderate,spon-taneous recovery occurs very rapidly and it is difficult to assess potential therapeutic outcomes.Injuries that are too severe result in extremely limited functional outcome and again can mask potentially useful therapeutic strategies.In an improvement over the Wrathall model,Khan and col-leagues(1999)compared functional outcomes after injury in both rats and cats.While both species showed injury-related declines in behavioral and electrophysiological func-tion,the time course of some spontaneous recovery was significantly shorter for the cats.As with earlier weight-drop models,little information concerning the biomechani-cal parameters of the SCI was available.One of the most popular weight-drop models is the New York University(NYU)/MASCIS device.This rather so-phisticated device was developed by Gruner(1992)and consists of dropping a10-gram weight from6.25,12.5,25, or50mm directly onto the exposed dorsal spinal cord dura. An electrical circuit determines cord surface before the injury,thus eliminating concerns about a preload injury, common with models that use an impounder plate.One of the major advantages to this model is the monitoring of injury parameters such as impact velocity and tissue dis-placement,providing a mechanism to eliminate injured ani-mals that do not meet preestablished criteria.The device has greatly reduced the risk of multiple injuries because of drop-weight“bounce.”After the injury,all of the rats demonstrate a severe loss of locomotor activity.Basso and colleagues(Basso et al. 1996)used the BBB scale to describe graded locomotor outcomes following different thoracic injury severities with this device.With the exception of the6.25-mm drop,all groups showed severe loss of locomotor movement in the hind legs.However,most of the groups showed spontane-ous improvement over3to4weeks,after which locomotor ability reached a plateau.Investigators have reported graded motor deficits and recovery as found with grooming,paw preference,the CatWalk,and horizontal ladder tests,as well as kinematics and the BBB scale for rats following cervical or lumbar injury severities with this device(Collazos-Castro et al.2005;Gensel et al.2006;Magnuson et al.2005). Aneurysm Clip CompressionAs mentioned above,several research groups considered the rat too small for the weight-drop method originally designed by Allen.To overcome this problem and to more closely model the ventral(anterior)compression normally observedin the human clinical condition,investigators effected SCI through sustained compression with specially modified Kerr-Lougheed aneurysm clips(Rivlin and Tator1978). This model entails exposing the rat spinal cord and applying the aneurysm clip with one blade under the ventral surface of the cord and the other over the dorsal surface.The clip, calibrated for a known compression force,is rapidly re-leased and allowed to compress the cord for a predeter-mined amount of time(e.g.,60seconds)before release and removal.In the initial study,this type of injury resulted in a quite dramatic and sustained deficit as measured with an inclined plane task.Some investigators have applied com-pression force–calibrated aneurysm clips vertically(later-ally)and assessed behavior outcome(von Euler et al. 1997a).In both cases,results indicated a high correlation of all neurological outcomes(BBB scale,inclined plane,beam walk)to the compression force.Calibrated Forceps CompressionAs an alternative to the use of aneurysm clips that produce a very focal injury,Blight(1991)developed a moderate-severity injury technique in the guinea pig using modified forceps.This compression injury produced a considerably larger volume of tissue compression and displacement than the aneurysm clips,with the added advantage of a special spinal column stabilized support.A detailed study in the rat (Gruner et al.1996)carefully monitored functional recovery using a modified Tarlov scale following a wide range of spinal cord compressions.The results demonstrated signifi-cant differences in the temporal pattern of the behavioral recovery when comparing the rat and the guinea pig.A delayed functional loss in the guinea pig was not observed in the rat,indicating possible significant species differences. ContusionIn an attempt to gain more control over the injury severity and more closely monitor biomechanical properties,several laboratories began to experiment with controlled pneumatic compression models(Anderson1982;Kearney et al.1988). One such model that used rats reported no indications of changes in functional outcome(Narayana et al.1999).A report by Noyes(1987)described a new electromechanical spinal cord impactor,now known as the Ohio State Univer-sity(OSU1)device.This model injures the spinal cord by means of a solenoid-controlled air cylinder mounted on a rigid frame with a tip that impacts the exposed dorsal spinal cord.The spinal column is firmly secured with no im-pounder plate on the exposed spinal cord.A companion study(Somerson and Stokes1987)demonstrated the func-tional outcome following three different levels of injury using the Tarlov scale.Additional studies(Behrmann et al. 1992;Bresnahan et al.1987)more carefully described func-tional recovery patterns in rats based on thoracic injury severity in terms of tissue displacement and post-trauma recovery time using the Tarlov scale,inclined plane task, grid walking,and footprint analysis.As with the weight-drop studies,functional deficits appeared immediately fol-lowing the trauma and showed spontaneous recovery that plateaued around2to3weeks following injury.Reports describe graded motor deficits and recovery found with forelimb grip strength,inclined plane,and grid-walk tests as well as footprint analysis and the BBB scale for rats fol-lowing different cervical injury severities with this device (Pearse et al.2005).Although the lack of commercial avail-ability of the OSU device limits its experimental usage by other laboratories,this sophisticated model provides excel-lent control over the biomechanical aspects of the injury (i.e.,velocity,depth of compression,force)that can effec-tively dictate the severity of the injury.A relatively new,commercially available kinetic contu-sion device uses force rather than tissue displacement to injure the rat spinal cord(Scheff et al.2003).Similar to the OSU device,this model requires exposure of the dorsal surface of the spinal cord and the rigid stabilization of the spinal column.One of the unique features of this comput-erized device,first produced by Infinite Horizon(IH1),is the ability to inflict reproducible injuries without touching the spinal cord before the hit.Some experimenters believe that any device that touches the exposed spinal cord before a more severe compression could cause a priming injury and thus alter the intended injury outcome.The IH instrumen-tation senses the surface of the exposed spinal cord and continues to displace spinal tissue until it attains a prede-termined force,at which point the impactor tip immediately withdraws.Initial behavioral and electrophysiological char-acterization studies used the BBB scale and the tcMMEP procedure to describe the loss and reacquisition of hind-limb function and motor axon conduction following a wide range of injury severities(Cao et al.2005;Scheff et al. 2003).A subsequent paper,evaluating creatine as a neuro-protective agent,compared injury with the IH device to the NYU/MASCIS weight-drop model(Rabchevsky et al. 2003)and found that both models produced significant SCI and resulted in very similar functional recovery profiles.An advantage of the IH device is that it enables monitoring of various biomechanical parameters(i.e.,velocity,force,tis-sue compression/displacement)and thus the early elimina-tion from the study of animals that do not meet preset standards.LacerationOf the many traumatic SCI models,the most frequently used methods of injury include complete transection,focal myelotomy(incision),dorsal or lateral hemisection,lateral overhemisection,resection,or aspiration lesions that defini-tively sever spinal cord axons of passage.Although these types of laceration injuries are not typically seen clinically, they can effectively disconnect both ascending and descend-ing axonal pathways at designated levels of SCI.This ap-proach allows the study of mechanisms that govern the inhibition or successful regeneration of axons across or around laceration injuries as well as of the resulting func-tional deficits and potential recovery.Importantly,the se-verity and level of SCI entirely dictate the applicability of quantitative assessments for impaired motor,sensory,and/ or autonomic functions.One of the many syndromes that occur after both com-plete and incomplete human SCI at thoracic level6(T6)or above is a condition called autonomic dysreflexia(Karlsson 1999;Rabchevsky2006).The most common trigger of this arduous hypertensive disorder is painful distension of the bladder or bowel,which elicits massive discharge of unin-hibited sympathetic neurons in the injured spinal cord dis-connected from brainstem control.The inflation of a balloon catheter in the distal colon of unanesthetized rats induces autonomic dysreflexia2weeks after complete tran-section of the cervical(C)or high thoracic spinal cord (Cameron et al.2006;Chau et al.2000;Krassioukov et al. 2002;Krassioukov and Weaver1995;Osborn et al.1989, 1990).While there is no perception of noxious stimuli,the rats experience autonomic cardiopathophysiology that stems from post-traumatic intraspinal plasticity(Rabchev-sky2006).Unlike complete spinal cord transection injuries,the re-producibility of partial spinal cord laceration injuries is somewhat inconsistent.Such injuries include focal myeloto-mies at spinal level C4,with the subsequent use of a pellet retrieval test to examine the effects of damage to axon path-ways in the dorsolateral and ventrolateral funiculi on fore-limb reach,grasp,and retrieval behaviors(Schrimsher and Reier1993).Unilateral SCI of distinct ascending and de-scending spinal pathways at similar levels is useful to in-vestigate locomotor and sensory deficits as well as compensatory recovery using electromechanical assess-ments(Webb and Muir2002,2003,2004).An alternative to unilateral SCI is more extensive dorsal-ventral and ventral-dorsal laceration SCI(Schucht et al.2002).Among the be-havioral tests utilized with these lesions are a forepaw usage task,the BBB scale,analyses of footslips during ladder or grid walking,quantitative kinetic measurements of ground reaction forces during locomotion,and Von Frey filament testing for tactile sensation.The recently created Vi-braKnife TM creates discrete myelotomies and dorsal hemi-sections of the midcervical spinal cord that elicit differential alterations in forelimb SSEPs and in upper extremity sen-sorimotor deficits(Onifer et al.2005).After various discrete thoracic spinal cord hemisection injuries,use of the MIER procedure demonstrates differential alterations in ascending propriospinal axon pathway conduction that significantly correlate both with white matter sparing in the lateral fu-niculus and with hindlimb function assessed by the BBB scale(Beaumont et al.2006).Combinatorial injuries,consisting of a thoracic spinal cord dorsal hemisection and unilateral transection of a pyramidal tract,show that new intraspinal circuitry forms spontaneously in the injured spinal cord of adult rats (Bareyre et al.2004).In particular,transected hindlimb cor-ticospinal tract axons sprouted into the cervical spinal cord gray matter to contact long propriospinal neurons that bridged the lesion and extended processes to lumbar moto-neurons.This created a new intraspinal circuit relaying supraspinal input to its original spinal targets.Electrophysi-ological and behavioral testing,as well as retrograde tran-synaptic tract tracing that revealed progressive changes in cortical representations over time,confirmed functionality.Complete resection injuries and discrete hemisection as-piration lesions of the thoracic spinal cord have also been used to test various“bridge”interface approaches by graft-ing neural tissues,alone or in combination with biosynthetic guidance channels,to promote successful graft survival and axonal regeneration in the injured adult rat spinal cord (Aguayo et al.1981;Bregman et al.1993;Cheng et al. 1996;David and Aguayo1981;Iannotti et al.2003;Jake-man and Reier1991;Reier et al.1986;Xu et al.1995,1997, 1999).Outcome measures for such interventions include histological and axonal tract tracing methods to assess tissue integrity,cytoarchitecture and neural connectivity between graft and host,the combined behavior score,the modified Tarlov scale,and forelimb functional recovery tasks. Chemical-mediated SCIProcedures involving a variety of chemical-mediated inju-ries throughout the adult rat spinal cord can be useful to model specific aspects of the secondary injury cascade that occur after the initial traumatic SCI and to address questions about spinal cord circuitry.In contrast to the previously described models,chemical-mediated SCI is a targeted ap-proach that may not include all the components of traumatic SCI.The clinical relevance of findings from experiments that evaluate treatments in these models must take this into account.Vascular damage occurs after traumatic SCI and results in hemorrhage,reduced blood flow,and ischemia(poor oxygen and nutrient supply)(Casella et al.2002;Fleming et al.2006;Loy et al.2002a;Norenberg et al.2004;Tator and Fehlings1991).There is evidence that ischemia causes spi-nal cord damage and then dysfunction in both humans and rats after occlusion of the descending thoracoabdominal aorta(for review,Black and Cambria2006;Zhang et al. 2000).It is also possible to produce ischemia in the rat spinal cord by combining intravenous injection of the pho-tosensitive dyes rose bengal(Watson et al.1986)or ery-throsin B(Cameron et al.1990;Hao et al.1991)with irradiation of the exposed vertebrae to produce vascular thrombosis.An alternative is to bathe the dura-covered spi-nal cord with rose bengal then use irradiation(Garcia-Alias et al.2003;Verdu et al.2003).Irradiation duration is an ef-fective way to grade the ensuing motor behavioral and axon conduction deficits(Cameron et al.1990;Garcia-Alias et al. 2003;Prado et al.1987;Wiesenfeld-Hallin et al.1993)。