(2004)High-rate flame synthesis of vertically aligned carbon nanotubes using electric field control
- 格式:pdf
- 大小:840.96 KB
- 文档页数:10

产品说明书超净工作台的优点是操作方便自如,比较舒适,工作效率高,预备时间短,开机30分钟以上即可操作,基本上可随时使用。
在工厂化生产中,接种工作量很大,需要经常长久地工作时,超净台是很理想的设备。
超净工作台由三相电机作鼓风动力,功率145~260W左右,将空气通过由特制的微孔泡沫塑料片层叠合组成的“超级滤清器”后吹送出来,形成连续不断的无尘无菌的超净空气层流,即所谓“高效的特殊空气”,它除去了大于0.3μm的尘埃、真菌和细菌孢子等等。
超净空气的流速为24~30m/min,这已足够防止附近空气可能袭扰而引起的污染,这样的流速也不会妨碍采用酒精灯或本生灯对器械等的灼烧消毒。
工作人员就在这样的无菌条件下操作,保持无菌材料在转移接种过程中不受污染。
但是万一操作中途遇到停电,暴露在未过滤空气中的材料便难以幸免污染。
这时应迅速结束工作,并在瓶上作出记号,内中的材料如处于增殖阶段,则以后不再用作增殖而转入生根培养。
如为一般性生产材料,因极其丰富也可弃去。
如处于生根过程,则可留待以后种植用。
超净台电源多采用三相四线制,其中有一零线,连通机器外壳,应接牢在地线上,另外三线都是相线,工作电压是380V。
三线接入电路中有一定的顺序,如线头接错了,风机会反转,这时声音正常或稍不正常,超净台正面无风(可用酒精灯火焰观察动静,不宜久试),应及时切断电源,只要将其中任何两相的线头交换一下位置再接上,就可解决。
三相线如只接入两相,或三相中有一相接触不良,则机器声音很不正常,应立即切断电源仔细检修,否则会烧毁电机。
这些常识应在开始使用超净台时就向工作人员讲解清楚,免除不应造成的事故与损失。
超净工作台进风口在背面或正面的下方,金属网罩内有一普通泡沫塑料片或无纺布,用以阻拦大颗粒尘埃,应常检查、拆洗,如发现泡沫塑料老化,要及时更换。
除进风口以外,如有漏气孔隙,应当堵严,如贴胶布,塞棉花,贴胶水纸等。
工作台正面的金属网罩内是超级滤清器,超级滤清器也可更换,如因使用年久,尘粒堵塞,风速减小,不能保证无菌操作时,则可换上新的。

镁铝合金直接燃烧法合成AlN晶体镁铝合金直接燃烧法合成AlN晶体谢晓;隋颖;黄晓昱;朱晨光【摘要】以球形镁铝合金(Al12Mg17)颗粒为原料,在空气中直接燃烧合成氮化铝(AlN)晶体.实验样品堆积在直径为1 cm的区域内,使用乙烷火焰点燃.使用高速摄像仪记录燃烧合成过程.借助XRD和SEM对原料和产物的组成及结构进行分析,并使用TG-DSC分析合金的热力学性质.结果表明:镁铝合金中的铝可以全部转化为AlN 晶体.合金的点火温度约为494.4℃,一旦点燃,不需要外界热源的持续加热,样品可持续燃烧.燃烧开始后,合金颗粒中镁快速汽化,与空气中氧发生优先反应,并耗掉颗粒周围的氧气,使氮气进入液态铝表层,生成氮化铝.燃烧产物有明显分层,检测结果表明上层产物为白色氧化镁,下层产物为黑色氮化铝晶体.合成过程中,镁对氮化铝的形成起着积极的促进作用.【期刊名称】《无机材料学报》【年(卷),期】2019(034)004【总页数】5页(P439-443)【关键词】AlN;镁铝合金;燃烧【作者】谢晓;隋颖;黄晓昱;朱晨光【作者单位】南京理工大学化工学院,南京 210094;南京理工大学化工学院,南京210094;南京理工大学化工学院,南京 210094;南京理工大学化工学院,南京210094【正文语种】中文【中图分类】TQ174氮化铝具有优良的性能,被广泛应用在半导体,陶瓷以及光电等领域[1-3]。
传统合成氮化铝的方法主要包括碳热还原法、直接氮化法和自蔓延高温合成法等[4]。
对于直接氮化法和自蔓延高温合成法,由于氮气渗透能垒的存在,反应往往需要在高压下进行。
并且,反应对氮化温度有着严格的要求[5-7]。
对于碳热还原法[8],虽然能获得较为纯净的氮化铝,但是其还原温度一般在1000 ℃以上,并且由于在合成过程中使用了过量的碳粉,碳热还原法制得的氮化铝还需进行脱碳处理,合成耗时且工艺复杂,对设备要求较高。
近几年的研究发现,当纳米铝粉在空气中燃烧时,产物中会含有大量的氮化铝。

A High Energy Resolution X-ray Spectrometer using SDDCHEN Er Lei 1, 2, a , FENG Chang Qing 1, 2, b *,YE Chun Feng 1, 2, and LIU Shu Bin 1, 21Modern Physics Department, University of Science and Technology of China2The State Key Laboratory of Particle Detection and Electronics, USTCa ****************,b ***************.cn*Correspondingauthor.Tel.**************.E-mailaddress:***************.cn.Keywords: Spectrometer, X-ray, Silicon Drift Detector, high energy resolution, FWHM.Abstract. A high energy resolution X-ray spectrometer based on Silicon Drift Detector is described in this paper. The spectrometer consists of the SDD detector module, the analog electronics for shaping and filtering and the digital electronics for peak detection and data transfer. The system can working at room temperature as a thermo electric cooler (Peltier Element) is integrated into the SDD chip. The dynamic range is about 1 keV to 10 keV. Test results indicated high energy resolution Full Width at Half Maximum (FWHM), which is better than 160 eV @ 5.9 keV with the incoming photon of radioisotopes (55Fe).IntroductionThe radiation of X-rays are widely used in many fields (such as airport security, X-ray astronomy, X-ray medical, X-ray crystallography, X-ray microscopic and so on) based on its properties [1]. X-rays with photon energies above 5 - 10 keV are called hard X-rays, while those with lower energy are called soft X-rays. A high energy resolution of Soft X-ray Detection (SXD) system is very necessary anduseful in scientific experiments and to explor the origin of the universe. Photon energy (keV)D e t e c t i o n e f f i c i e n c y (%)Fig. 1 SDD detector and its absolution efficiencySilicon Drift Detectors (SDD) are the state-of-the-art X-ray detectors based on silicon substrates. As shown in Fig. 1 is the used detector KETEK VITUS SDD H30 [2] and its absolution efficiency curve (about 70% at 1 keV and 98% at 10 keV), while the detector have 30 mm 2 active area with 8 µm Be window. An incoming photon will generate a number of electrons and holes dependent on its energy.A followed readout electronics based on the SDD will be discribed in the following sections. Joint International Mechanical, Electronic and Information Technology Conference (JIMET 2015)Structure of the readout electronicsA m p l i t u d e (V )A m p l i t u d e (m V )As shown in Fig. 2, is the structure of the readout electronics. The SDD detector is surpported bythe High Voltage Module and Temperature Controller to guarantee a good working environment, while the statues of the voltage and temperature were monitored by the Field Programmable Gate Array (FPGA) simultaneously. The output of the SDD is buffered by a low-noise & high-gain Pre-Amp, which is a voltage step well between ± 2 V because of the reset signal. The output of the Pre-Amp is followed by the CR-RC 2 [3] filter and shaper, after then the quasi-Gaussian output is fed to the Peak Hold module and then be digitalized by a 14-bit Analog to Digital Converter (ADC) (3 MHz sample rate). The digitized results is buffred and packaged in the FPGA, then be readout to remote PC. Detailes will be listed bellow.Silicon Drift DetectorU BACK(-65V)U OR (-130V)U IR(-20V)GND X-rayEntrance windowAnode FETC FBAs shown in Fig. 3 is the schematic of the SDD chip. The anode of the SDD is connected to a Field Effect Transistor (FET) and Feed Back Capacitance (C FB ) which forms the first part of a Charge Sensitive Amplifier (CSA). A certain rise time is dependent on the location of interaction of the X-ray with the SDD chip, while a certain amplitude is dependent on the energy of the incoming photon. SDDs require High Voltages Power Supply (low noise stabilized, U OR ‘-130V ’, U IR ‘-25V ’, U BACK ‘-65V ’,), Preamplifier Module (ultra-low-noise, charge-sensitive, ramped reset type, high-gain) andTemperature Controller Module (Peltier Element based thermo electric cooler) to guarantee a good working enviroment, meanwhile it is necessary to read out the temperature sensor of the SDD. Peak-Hold moduleOne kernel part of the readout electronics is the Peak Hold method, which combine with Analog to Digital Converter (ADC) for peak detection. The peak of the analog quasi-Gaussian output is detected and held by the Peak Hold module, then be injected to ADC for digitization. A high performance peak-hold chip (PH300) was chosen, which was designed for satellite instrumentation (equally useful in laboratory and commercial applications for its stability and characteristics).Detected peakFig. 4 The Function and Timing diagram of PH300 [4]PH300 is a peak-hold device, to track an analog input and keep the maximum amplitude as a peak voltage on a hold capacitor. As shown in Fig. 4, the input (marked as ‘IN’) is sensed by the error amplifier when the Gate is open (High), which is controlled by Transistor-Transistor Loigc (TTL) standard. The hold capacitor is charged though a charging diode and a hold resistor during the rise time of the signal (called charging mode marked as ‘C’). Then the device goes into the hold mode (marked as ‘H’) as soon as the input reaches V max and starts to decay. After completing the sample of the peak, the circuit was in fast discharge (was configured in the application) mode (marked as ‘D’), the hold capacitor is discharged through a large current for a short period of time. Finally, the circuit was ready for reciving a new peak, which is called the tracking mode (marked as ‘T’).The control signal is generated by the FPGA, which is converted from 3.3 V standard to 5V standard according to a schmitt CMOS 16-bit Bidirectional MultiPurpose Transceiver (UT54ACS164245S). The ‘GATE’ was set as HIGH in the application. The ‘PKDT’ was the output of th e PH300 when the peak is found and in the holding mode. The ‘RAMP’ is the input which starts from the rise time of the ‘IN’, and set as LOW when in the discharge mode. The ‘OUT’ from PH300 is sampled by a 14-bit ADC (AD9243) with 3 MHz samping rate. The results is buffered and packaged in FPGA, then transmitted to remote PC for further analyzing.Test resultsIn order to evaluate the X-ray spectrometer designed in this paper, a test platform was set up, as shown in Fig. 5. The test system is mainly consists of the signal source (AFG 3252), the radioisotope (55Fe), the SDD module and shaping board (marked as ‘A’), the digital board (marked as ‘B’) and power supply module.Firstly we measure the electronics linearity of the peak hold and ADC module, a series of quasi-Gaussian (generate by signal source AFG3252) were conducted with increasing amplitudemeasured by the readout electronics. The input amplitude ranges from 50 mV to 2500 mV with 300mV interval, which is equivalent to the dynamic range (1 keV to 10 keV).Fig. 5 Test platfrom of the soft X-ray spectrometerA linear curve is shown in Fig. 6, with an integral non-linearity less than 1%.After the X-ray spectrometer were assembled, including both the detector and electronics properties. We use the radioisotope (55Fe) as the input of the incoming photon [5].C ODE Input(mV) Fig. 6 The linear curve of the Peakhold and ADC circutC o u n t s Code Fig. 7 The test spectrum using the radioisotope 55FeAs shown in Fig. 7, the result of energy spectrum indicates that the energy resolution Full Width at HalfMaximum(FWHM)************************(usingX-rayastheincomingphotonfor SDD).SummaryA high energy resolution of soft X-ray spectrometer based on SDD is designed in this paper. Which achives a dynamic range of about 1 keV to 10 keV. The energy resolution (FWHM) is better than 160 *********.AcknowledgmentThis work was supported by the National Natural Science Funds of China (Grant No. 11205154).References[1] X-rays. NASA. Retrieved November 7, 2012. /ems/11_xrays.html.[2] KETEK GmbH. VITUS Silicon Drift Detectors. User’s Manual.[3] Wulleman J. Detector-noise suppression by appropriate CR-(RC)n shaping [J]. Electronics Letters, 1996, 32(21): 1953-1954.[4] AMP-TEK. PH300 Peak Hold Detector. PH300 Specifications.[5] Zhang F, Wang H Y, Peng W X, et al. High resolution solar soft X-ray spectrometer. Chinese Physics C. DOI: 10.1088/1674-1137/36/2/008.。

三聚氰胺多磷酸盐合成原理Melamine phosphate is a commonly used flame retardant, which is synthesized through the reaction between melamine and phosphoric acid. 三聚氰胺磷酸盐是一种常用的阻燃剂,通过三聚氰胺和磷酸反应合成。
This synthesis process involves several steps and is crucial in the production of flame-retardant materials. 这个合成过程涉及到几个步骤,在阻燃材料的生产中至关重要。
The first step in the synthesis of melamine phosphate involves the reaction between melamine and phosphoric acid. 三聚氰胺磷酸盐的合成的第一步涉及三聚氰胺和磷酸之间的反应。
Melamine, a nitrogen-rich compound, reacts with phosphoric acid in the presence of heat to form melamine phosphate. 三聚氰胺是一种富含氮的化合物,在热的作用下与磷酸反应形成三聚氰胺磷酸盐。
The reaction is typically carried out in a controlled environment to ensure the desired product is obtained. 反应通常在受控环境中进行,以确保获得所需的产物。
This may involve precise control of temperature, pressure, and reaction time. 这可能涉及对温度、压力和反应时间的精确控制。

聚磷酸三聚氰胺合成工艺流程英文回答:Polyphosphoric acid melamine (PPAM) is a type of flame retardant that is commonly used in various industries such as textiles, plastics, and coatings. The synthesis of PPAM involves several steps and requires the use of specific reagents and conditions.Firstly, the raw materials needed for the synthesis of PPAM are melamine and phosphoric acid. Melamine is a white crystalline powder that is widely used in the production of plastics and resins. Phosphoric acid, on the other hand, is a colorless liquid that is commonly used in the production of fertilizers and detergents.The synthesis of PPAM begins by dissolving melamine in phosphoric acid. This is typically done by adding melamine to a reactor vessel containing phosphoric acid and stirring the mixture until a homogeneous solution is obtained. Thereaction is exothermic, meaning that it releases heat, soit is important to control the temperature during this step.Once the melamine is fully dissolved in the phosphoric acid, the mixture is heated to a specific temperature and held at that temperature for a certain period of time. This step is known as the condensation reaction, and it iscrucial for the formation of the desired PPAM product.During the condensation reaction, the melaminemolecules react with the phosphoric acid to form a polymer network. This network is responsible for the flameretardant properties of PPAM. The reaction is typically carried out at temperatures ranging from 150 to 200 degrees Celsius, and the reaction time can vary from a few hours to several days, depending on the desired properties of thefinal product.After the condensation reaction is complete, the resulting PPAM product is usually in the form of a viscous liquid. This liquid can then be further processed to obtain the desired physical form, such as a powder or a solid.This can be achieved by methods such as spray drying or extrusion.In summary, the synthesis of polyphosphoric acid melamine involves dissolving melamine in phosphoric acid, heating the mixture to a specific temperature, and allowing the condensation reaction to occur. The resulting productis a flame retardant material that can be used in various applications.中文回答:聚磷酸三聚氰胺(PPAM)是一种常用的阻燃剂,广泛应用于纺织、塑料和涂料等多个行业。


第15卷 第1期强激光与粒子束Vol.15,No.1 2003年1月HIGH POWER LASER AND PAR TICL E B EAMS Jan.,2003 文章编号:100124322(2003)012009720418Me V质子辐照对Ti Ni形状记忆合金R相变的影响Ξ王治国, 祖小涛, 封向东, 刘丽娟, 林理彬(四川大学物理系教育部辐射物理及技术重点实验室,四川成都610064) 摘 要: 研究了用HZ2B串列加速器的18MeV质子辐照对TiNi形状记忆合金R相变的影响,辐照在奥氏体母相状态下进行。
示差扫描量热法(DSC)表明,辐照后R相变开始温度T s R和逆马氏体相变结束温度T f A随辐照注量的增加而降低。
当注量为1.53×1014/cm2时,T s R和T f A分别下降6K和13K,辐照未引起R相变结束温度T f R和逆马氏体相变开始温度T s A的变化。
表明辐照后母相(奥氏体相)稳定。
透射电镜(TEM)分析表明辐照后没有引起合金可观察的微观组织变化。
辐照对R相变开始温度T s R和逆马氏体相变结束温度Af的影响可能是由于质子辐照后产生了孤立的缺陷团,形成了局部应力场,引起晶格有序度的下降所造成的。
关键词: TiNi形状记忆合金;质子辐照;R相变;示差扫描量热法;TEM 中图分类号:TG139.6 文献标识码:A TiNi形状记忆合金是目前应用最为广泛,也最成功的一种智能材料,集传感功能与驱动功能于一体,在核反应堆和太空等核辐射环境下用作传感与驱动元件已引起了关注[1,2]。
TiNi合金中R相变具有热滞后小,响应速度快的特点,在实际应用中得到了广泛的应用[3]。
在以前的研究中利用R相变得到了具有双向记忆效应的弹簧,伸缩率可达25%[4]。
由于核辐射会对形状记忆合金相变特性产生影响,因而研究其改变规律及机理对形状记忆合金在辐射环境下应用的可靠性和可行性是十分必要的。
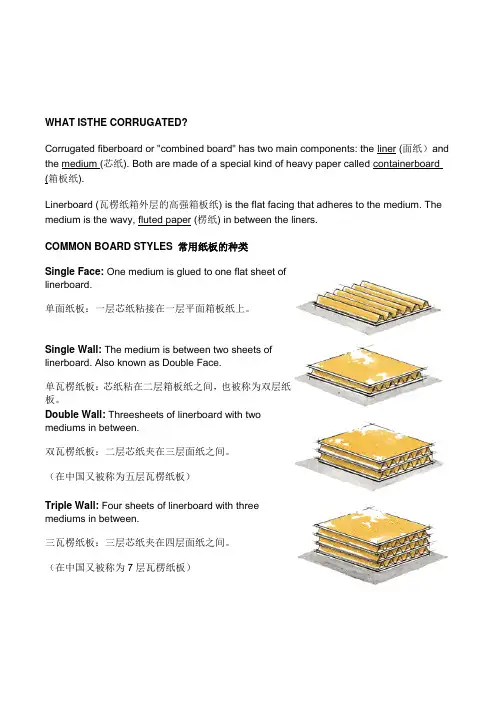
WHAT ISTHE CORRUGATED?Corrugated fiberboard or "combined board" has two main components: the liner (面纸)and the medium (芯纸). Both are made of a special kind of heavy paper called containerboard (箱板纸).Linerboard (瓦楞纸箱外层的高强箱板纸) is the flat facing that adheres to the medium. The medium is the wavy, fluted paper (楞纸) in between the liners.COMMON BOARD STYLES 常用纸板的种类Single Face: One medium is glued to one flat sheet oflinerboard.单面纸板:一层芯纸粘接在一层平面箱板纸上。
Single Wall: The medium is between two sheets oflinerboard. Also known as Double Face.单瓦楞纸板:芯纸粘在二层箱板纸之间,也被称为双层纸板。
Double Wall: Threesheets of linerboard with twomediums in between.双瓦楞纸板:二层芯纸夹在三层面纸之间。
(在中国又被称为五层瓦楞纸板)Triple Wall: Four sheets of linerboard with threemediums in between.三瓦楞纸板:三层芯纸夹在四层面纸之间。
(在中国又被称为7层瓦楞纸板)Flutes 楞形Architects have known for thousands of years that an arch(拱) with the proper curve (曲线) is the strongest way tospan a given space (跨越一个给定的空间). The inventorsof corrugated fiberboard applied this same principle to paperwhen they put arches in the corrugated medium(瓦楞芯纸).These arches are known as flutes (楞)and when anchoredto the linerboard with adhesive, they resist bending andpressure from all directions.When a piece of combined board is placed on its end, the arches form rigid columns, capable of supporting a great deal of weight. When pressure is applied to the side of the board, the space in between the flutes acts as a cushion(垫子)to protect the container's contents. The flutes also serve as an insulator (绝缘体, 绝热器,保温层), providing some product protection from sudden temperature changes. At the same time, the vertical linerboard provides even more strength and protects the flutes from damage.Flutes come in several standard shapes or flute profiles (A, B, C, E, F, etc.). A-flute (A楞)was the first to be developed. B-flute was the next and is much smaller than A-flute. C-flute followed and is between A and B in size. It is also the most common large flute profile.E-flute is smaller than B and F-flute is smaller yet. Due to variances in flute sizes between manufacturers, FBA no longer publishes flute guidelines.In addition to these five most common profiles, new flute profiles—both larger and smaller than those listed here—are being created for more specialized boards. Generally, larger flute profiles deliver greater vertical compression strength (垂直压力)and cushioning, while smaller profiles provide better resistance to process and printing crush.Different flute profiles can be combined in one piece of combined board. For instance, in a triple wall board, one layer of medium might be A-flute while the other two layers might beC-flute. Mixing flute profiles in this way allows designers to manipulate the compression strength, cushioning strength (减震强度、缓冲强度) and total thickness of the combined board.Corrugated BasicsWhat is Corrugated?Corrugated boxes are easy to recognize. Corrugated is made ofpaper and has an arched layer, called "fluting," between smoothsheets, called "liner." The corrugated most commonly used tomake boxes has one layer of fluting between two smoothsheets. But there are many types of corrugated available, eachwith different flute sizes and thicknesses(厚度).Corrugated is an extremely durable, versatile, economical and lightweight material used for custom-manufactured shipping containers(运输容器), packaging and point-of-purchase displays(销售展示包装,即所谓POP展示包装), in addition to numerous non-traditional applications ranging from pallets(货盘、托盘)to children's toys to furniture.Why Corrugated?Corrugated. It's not just a brown box.Corrugated is a complete, high-performance material design, manufacturing and delivery system. Corrugated is the preferred packaging material because it is:1. Durable2. Versatile3. Lightweight4. Environmentally Friendly5. High-Tech6. Customizable7. Protective8. Graphically Appealing9. Cost-EffectiveIf it's not just a cardboard box, what is it?A High-Tech Engineered Material.What may come as a big surprise to many is that the ever-present corrugated "cardboard box" is high-tech:1. Ongoing R&D (研发)programs continuously improve such characteristics asstrength-to-weight ratios(强度与重度的比率), printability, moisture barriers (潮气阻隔) and recyclability.2. Corrugated components, designs and end products are manufactured on sophisticated,automatic equipment that reduces costs and ensures consistent performance.3. The vast majority of corrugated products are designed and prototyped with advanced,computer-aided design (CAD)and manufacturing systems, providing customers with the best and most cost-effective solutions to their packaging challenges.Infinitely Customizable.Corrugated offers thousands of possible combinations of board types, flute sizes (caliper), basis weight, adhesives, treatment and coatings, including flame retardant and static control protection.Corrugated is the only rigid shipping container and packaging medium that can be cut and folded into an infinite variety of shapes and sizes and direct-printed with high-resolution color graphics (including lithography, flexography and silk screening). And corrugated is not just for displays and boxes. Other uses include low-cost, one-way recyclable pallets, retail bulk bins, and lightweight castles that children can build themselves.There are hundreds of basic designs and thousands of adaptations, each chosen on the basis of proven experience and the proposed use of the product.Corrugated is routinely custom-designed to fit specific product protection, shelf space and shipping density requirements (including inner packaging that prevents shifting).Tenaciously Protective.Corrugated combines structural rigidity with superior cushioning qualities. Containers, packages and pallets nest products in an optimally protective environment, so even heavy or fragile contents arrive undamaged.Corrugated offers excellent tear, tensile and burst strength to withstand shipping pressures.It resists impact, drop and vibration damage while offering uniform stacking and weight distribution so the load stays put, regardless of the form of transportation.Corrugated can be designed to contain flowable, granular or loose bulk products and even hazardous materials. It is also used to ship liquids and fresh foods, with the addition of removable plastic or waxed liners which serve as moisture barriers.All this from a material that is lightweight, low-cost and recyclable.Graphically Appealing.Corrugated containers and packaging are mobile billboards that create product image wherever they travel. Corrugated displays are eye-catching modular units that can be set up quickly and recycled at the end of a promotion.Corrugated is a very flexible medium that accommodates a wide range of printing options to support the end-use requirement:1. Offset lithography and rotogravure (high-volume).2. Flexography or letterpress (shorter runs)3. Silk screening (displays)4. Corrugated can be direct printed in plant or manufactured with high-end process colorgraphics.Preeminently Cost-Effective.One of the least expensive containers ever developed, the overall cost of corrugatedshipping containers is usually between one percent and four percent of the value of the goods they carry.The cost of labor and tools required to produce, fill, and move the container is low. The cost of shipping is low, due to lower weights and higher fill densities than alternative packaging.The trend toward light weighting will continue to drive down shipping costs. Low raw material costs and mass production of corrugated containers makes them particularly cost-efficient.The ultimate contribution to cost reduction is when corrugated is used as an all-in-one shipping, storage, advertising and display medium - a growing trend both in warehouse and other retail stores.Environmentally Responsible.Corrugated, made from a natural renewable resource, has a great environmental record. Corrugated is frequently manufactured using high percentages of secondary fiber (including old corrugated containers, kraft, old newspapers and even straw), thereby diverting these materials from the municipal solid waste stream.In 2004, more than 24 million tons of corrugated were recovered and recycled in the U.S. -- that's 73 percent of all containerboard produced in the same year. Corrugated has the best recycling rate of any packaging material used today.In addition, the use of corrugated constructions with high-performance linerboard has led to a significant overall reduction in basis weight and a significant source reduction of raw materials.Water-based inks are now used almost exclusively for printing graphics on corrugated containers, avoiding the use of lead-based inks and solvents which pollute the air and the water used to wash down printing equipment between color changes.Box DimensionsDimensions are given in the sequence of length, width and depth. Internationally, the words length, breadth and height may be used to express these dimensions. The dimensions of a box are described based on the opening of an assembled box, which can be located on the top or the side, depending on how it is to be filled. The opening of a box is a rectangle; that is, it has two sets of parallel sides. The longer of the two sides is considered its length, the shorter of the two sides is considered its width. The side perpendicular to length and width is considered the depth of the box.Dimensions can be specified for either the inside or the outside of the box. Accurate inside dimensions must be determined to ensure the proper fit for the product being shipped or stored. At the same time, palletizing and distributing the boxes depends on the outside dimensions. The box manufacturer should be informed as to which dimension is most important to the customer.。

烈焰飞雪合成公式(Energy-saving synthetic formula)Tien mine:Formula 1: step *4== (70% *1 Tien lightsaber mine successfully synthesized probability)Formula 2: Dragon *3== *1 (70% chance Tien ore successfully synthesized)Formula 3: millet huge bow *4== Tien ore *1 (70% chance of successful synthesis)Formula 4: *4== *1 (70% lightsaber Xuantian mine likely successfully synthesized)Formula 5: beam's terror rhinoceros gun *2+ dragon car *3== Tien mine. *1 (70% chance of successful synthesis)Formula 6: death dragon car *2+ lives with rhinoceros Gun + beam's gun with Rhino mine *1 (70% probability = Tien synthesized)Ming Shan series forging composite formula (NPC: alchemists)The night mountain waterfall dragon knife sword = death + knife + night mountain waterfall has destroyed the synthetic spectrum of pure gold (+ 3) (high success probability)The night mountain waterfall arc = spear gun + de Qi Star Silver sable waterfall Gun + night mountain synthetic spectrum + pure gold (3) (a higher probability of success)The night mountain waterfall at the Ming De = bow bow + bow + waterfall arc containing chapter Ming mountain synthetic spectrum + pure gold (3) (a higher probability of success)The night mountain waterfall arc = crossbow crossbow + de icing Lonza waterfall crossbow + night mountain synthetic spectrum + pure gold (3) (a higher probability of success)Ming Shan shield = water dragon shield + body Qi Cang Feng Ming Shan xingdun + + synthetic spectra of pure gold (3) (a higher probability of success)Hai min mountain waterfall De Zhen Jun = rhinoceros gun with rhinoceros artillery + night mountain synthetic spectrum + pure gold (3) (a higher probability of success)Deep landslide dragon gun = benglong gun out waterfall Zhen Jun min mountain + + pure gold spectrum synthesis (3) (a higher probability of success)Ming Shan sword = dragon scales +40-50 +50-60 blue knife equipment equipment +60-70 equipment + blue blue pure silver (3) (low probability of success)Ming Shan spear gun +40-50 = an ermine blue equipment +50-60 equipment +60-70 blue blue equipment + pure silver (3) (low probability of success)Ming Ming Kou mountain bow bow +40-50 = blue +50-60 blue +60-70 equipment equipment equipment to prepare pure blue + silver (3)(low probability of success)The night mountain crossbow crossbow +40-50 blue = Lonza +50-60 equipment +60-70 equipment blue blue equipment + pure silver (3) (low probability of success)The night mountain shield shield +40-50 blue beryl = +50-60 equipment +60-70 equipment blue blue equipment + pure silver (3) (low probability of success)The night mountain with Rhino gun gun +40-50 Blue Rhino = hack equipment +50-60 equipment +60-70 blue blue equipment + pure silver (3) (low probability of success)Deep landslide dragon gun = benglong gun +40-50 equipment +50-60 equipment +60-70 blue blue blue equipment + pure silver (3) (low probability of success)Solid series:Solid gold step cloud shoe = step cloud shoe + copper gold jade *6+The strong mark = + fine copper *6+ jade Mohist markStrong Qinglong wrist wrist + copper *6+ = Qinglong jadeStrong Suzaku Suzaku Bracers = wrist + copper *6+ jadeThe strong storm storm + COPPER NECKLACE Necklace = *6+ jadeStrong Kuafu boots boots + = Kuafu copper *6+ jadeNoriko strong Paramecium = moment copper emerald *6+ + sub wayStrong Chang Chang + copper amulet amulet = *6+ jade72 Life SeriesVector Dance Dance Dragon = life BanZhi vector + *6+ jade BanZhi iron ore concentrate *2Vector dance life dance dragon vector = wrist wrist + *6+ *2 iron ore fine jadeVector Dance Dance Dragon vector = Life Necklace Necklace + fine jade *2 *6+ iron oreVector dance life dance dragon boots boots = vector + *6+ *2 emerald ore concentrateLife's Dragon's BanZhi = + fine jade BanZhi iron ore *6+ *2Life's wrist ='s wrist + *6+'s iron ore concentrate jade *2Life's necklace ='s Dragon Necklace + fine jade *2 *6+ iron oreLife's boots ='s Dragon boots + fine jade mine *6+ *2The life of Wu Sheng Wu Sheng's pull pull that = + fine iron ore *6+ jade *2The life of Wu Sheng Wu Sheng's wrist = wrist + *6+ *2 jade ore concentrateThe life of Wu Sheng Wu Sheng's necklace Necklace = + fine jade *2 *6+ iron oreThe life of Wu Sheng Wu Sheng's boots boots = *6+ + fine iron ore jade *275 golden clothes synthesis formula:Yu Lun [male counsellor]= Xuanwu armor *3+ + Acura Huben mark Mohist stone *3+ huntian treasures (20% probability of successful synthesis)Dragon riding]= Qinglong [male war armor *3+ Qinglong wrist + Acura Huben stone *3+ huntian treasures (20% probability of successful synthesis)Herd [male archer]= Suzaku Suzaku *3+ armored wrist + Acura Huben stone *3+ huntian treasures (20% probability of successful synthesis)Into the cloud]= Xuanwu armor 1*3+ [female counselors imprint + gourmet ice Mohist rock *3+ huntian treasures (20% probability of successful synthesis)Qing Luan [female war riding]= Qinglong armor 1*3+ Qinglong wrist + gourmet ice rock *3+ huntian treasures (20% probability of successful synthesis)Phoenix [female archer]= Suzaku Suzaku 1*3+ armored wrist + gourmet ice rock *3+ huntian treasures (20% probability of successful synthesis)75 level artifact synthesis formula:Pan Ying: Formula 1: with the sword +72 Hou Huben warrior jewelry + gourmet stone + gourmet dry stone bag + Jingwei stone (10% success rate)Formula 2: Tien Qinglong mine + wrist + gourmet Huben stone + gourmet dry stone bag + Jingwei stone (10% probability of successful synthesis)Vulcan: Formula 1: propagation of weak +72 bow archer Huben stone + jewelry + gourmet gourmet dry stone bag + Hou Yi arrow (10% success rate) formula 2: Tien mine + Huben rosefinch wrist + gourmet stone + gourmet dry stone bag + Hou Yi arrow (10% probability of successful synthesis)A surname: Formula 1: Dragon Sword +72 warrior jewelry + Huben stone + Acura Acura venom tube + Kuafu boots (10% probability of successful synthesis)Formula 2: the Yan chapter Ge +72 soldiers jewelry Huben stone + + Acura Acura venom tube + Kuafu boots (10% probability of successful synthesis)Formula 3: Tien Qinglong mine + wrist + gourmet Huben stone + best venom tube boots (10% + Kuafu probability synthesis success)The blue sky with Rhino gun: Formula 1: Tien ore + Huben mark + gourmet Mohist stone + gourmet dry stone bag + Jingwei stone (10% success rate) formula 2: death dragon car + Acheron gun +72 soldiers armed with Rhino jewelry + Huben gourmet stone + gourmet dry bag + Jingwei stone (stone 10%)80 class honours seriesThe statue of the statue of PA = honor pull down payment + pure silver *6+ *4 jade BanZhiThe statue of the statue of the PA = honor wrist wrist + pure silver drop gold jade *6+ *4The statue of the statue of PA = honor Necklace drop Gold Necklace + pure silver jade *6+ *4The statue of the statue of honor boots = PA + pure silver drop Golden Boot *6+ jade *4Guiguzi honorary = Guiguzi down gold fingerstall fingerstall + pure silver jade *6+ *4Guiguzi honorary = Guiguzi down gold wrist wrist + pure silver jade *6+ *4Guiguzi honorary = drop gold necklace Necklace GUI + pure silver jade *6+ *4The GUI honor boots down GUI = *6+ jade + pure silver boot *4Break an empty broken down pull that honor = gold silver *6+ *4 jade + pure pullBreak an empty honor = break an empty drop gold wrist wrist + pure silver jade *6+ *4Break an empty broken down empty honor Necklace = Gold Necklace + pure silver jade *6+ *4Break down = honor boots break an empty empty *6+ *4 silver jade + pure gold85 gold equipment synthesis formula:Dragon Statue down gold wrist: PA + + Qinglong mine Tien wrist wrist + Diamond + + (100% probability of oblivion stone jade dragon successfully synthesized): PA down Dragon Statue fingerstall fingerstall + gold + + ore of Wu Sheng Tien BanZhi diamond + fine iron ore *3+ jade (100% probability)Dragon Statue Drop Necklace: PA Gold Necklace + + blast Necklace + Tien ore diamond *2+ pure gold jade *3+ (100% success rate) dragon boots: PA statue down the Golden Boot + boots + + Kua Fu Tien ore diamond *3+ jade + pure silver (100% probability of successful synthesis)The wrist: break an empty drop gold wrist + + + diamond mine Tien rosefinch Bracers + oblivion stone + jade (100% probability of successful synthesis) herd fingerstall: break an empty drop's gold ore + + BanZhi Tien vector dance + Diamond+ fine iron ore BanZhi jade *3+ (100% chance): breaking down empty Necklace herd Gold Necklace + + + diamond mine Tien Chang of *2+ pure gold jade *3+ (100% success rate) herd boots: break an empty drop + + torque Tien Golden Boot ore + Diamond + pure silver Paramecium *3+ jade (100% probability of successful synthesis)Yu Lun GUI wrist: drop gold wrist + + + diamond mine Tien Mohist imprint + oblivion stone + jade (100% success rate)Yu Lun fingerstall: drop's gold GUI's ore + + Tien fingerstall fingerstall + Diamond + fine iron ore *3+ jade (100% probability): Guiguzi down feather fiber Necklace Gold Necklace + + blast Necklace + Tien ore diamond *2+ pure gold jade *3+ (100% success rate)Yu Lun boots: Guiguzi down + + gold mine Xuantian boot step cloud shoe + Diamond + pure silver jade *3+ (100% chance of successful synthesis)85 Life SeriesLife = dragon dragon wrist wrist + *6+ *6 pure gold jadeLife = dragon dragon BanZhi + pure gold *6+ *6 jade BanZhiLife long necklace = Dragon Necklace + pure gold jade *6+ *6Life dragon boots = dragon boots + pure gold jade *6+ *6Life = herd herd wrist wrist + *6+ *6 pure gold jadeLife = herd herd BanZhi + pure gold *6+ *6 jade BanZhiLife = herd herd Necklace Necklace + pure gold jade *6+ *6 Life = herd herd boots boots + pure gold jade *6+ *6Life = Yu Lun Lun Yu wrist wrist + *6+ *6 pure gold jade Life = Yu Lun Lun Yu BanZhi + pure gold *6+ *6 jade BanZhiLife = Yu Lun Lun Feather Necklace Necklace + pure gold jade *6+ *6Life = Yu Lun Lun Yu boots boots + pure gold jade *6+ *695 golden clothes synthesis formula:The star = herd + strong Joojak wrist + hammer rogue + Wu Weishi + flame stone + Pearl (30% probability of successful synthesis)Dragon = dragon + strong Qinglong wrist + hammer rogue + Wu Weishi + flames of the stone + Pearl (30% probability of successful synthesis)The Qianmo = strong imprint + + Lun Yu Wu Weishi + + hammer rogue flames stone + Pearl (30% probability of successful synthesis)The Suzaku strong wrist + + = Huofeng + + Weishi hammer rogue Wuhan snow stone + Pearl (30% probability of successful synthesis)Aokage = Qinglong qingluan + strong wrist + + + hammer rogue Wu Weishi snow stone + Pearl (30% probability of successful synthesis)The snow = cloud + strong cutting mark + + + Weishi hammer rogue Wuhan snow stone + Pearl (30% probability of successful synthesis)95 level artifact synthesis formulaNote: the artifact 1. weapons and seal failure to exercise every.2. releasing a weapon can only have a chance).Summer dragon sparrow (Feng Yin): Night mountain sword + with sword + + Hou Tien ore hematite + Diamond *2 (25% chance of successful synthesis)Li Quan (seal): Night Mountain Dragon Sword Spear + + + Diamond + hematite ore Tien *2 (25% chance of successful synthesis)Painting (seal): Night mountain shadow bow + bow + + mine fan weak Tien hematite + Diamond *2 (25% chance of successful synthesis)The benglong gun (Feng Yin): deep landslide dragon Gun + Acheron benglong Gun + + + hematite ore Xuantian diamond *2 (25% success rate)95 artifact:Results: after releasing releasing items will be divided into 5 grades: broken; ordinary; excellent; epic; legendSoldier: the lifting of the seal formula: Summer dragon sparrow (seal) + + + Darksteel secret blacksmith hammer hematite + jadeKnight: the lifting of the seal (seal) formula: Stephen Li + Darksteel + secret blacksmith hammer + hematite + jadeArcher: the lifting of the seal (seal) formula: draw Darksteel + + secret blacksmith hammer + hematite + jadeCounselor: the lifting of the seal formula: Sky Dragon gun (seal) collapse Darksteel + + + + secret blacksmith hammer hematite jadeThe flames of stone, snow stone get waySynthesis of NPC: (synthetic alchemist certain probability of success)Synthetic formula:Fire stone = emerald + good Huben stone + good + liangpin venom tube ice rock + good dry stone bag + refined Huxin round mirror + hematite + Blue CrystalFire stone = emerald + grade Huben stone + grade + rock + ice venom tube top grade dry stone bag + famous Huxin round mirror + hematite + Blue CrystalFire stone = emerald + gourmet Huben stone + gourmet gourmet ice rock venom tube + + gourmet dry stone bag + best supporting circle mirror + hematite + Blue CrystalSnow stone = diamond + good Huben stone + good + liangpin venom tube ice rock + good dry stone bag + refined Huxin round mirror + hematite + Blue CrystalSnow stone = diamond + grade Huben stone + grade + rock + ice venom tube top grade dry stone bag + masters Hu Xinyuan mirror + hematite + Blue CrystalSnow stone = diamond + gourmet Huben stone + gourmet gourmet ice rock venom tube + + gourmet dry stone bag + best supporting circle mirror + hematite + Blue CrystalNote: number 1, more than synthetic materials are a. 2 more than the synthetic formula has a certain probability of failure.The evil gun:The world really need Gun + dry stone bag + top Hu dress Band = evil blue sky gun "(synthetic success rate 100%)The blue sky with rhino gun:Formula 1: Tien ore + Huben stone mark + Mohist gourmet gourmet + dry stone bag + Jingwei stone (10% probability of successful synthesis)Formula 2: death dragon car + Acheron gun +72 soldiers armedwith Rhino jewelry + Huben gourmet stone + gourmet dry stone bag + Jingwei stone (10% probability of successful synthesis)。

经改色粉—红色高温高压合成钻石谱学特征及呈色机理研究经改色粉—红色高温高压合成钻石谱学特征及呈色机理研究摘要:本文研究了经改色粉合成的红色高压高温合成钻石的谱学特征及其呈色机理。
首先介绍了经改色粉高压高温合成钻石的原理和方法,在此基础上进行了谱学分析,发现改色粉处理可以显著影响合成钻石的谱线强度、形状和位置。
随着改色处理条件的不同,合成钻石的各种谱学参数也有所变化。
接下来,利用X射线衍射和红外光谱等技术对合成钻石的结构和化学成分进行了分析。
结果表明,经过改色处理后合成钻石的晶体结构和化学成分都有一定程度的改变。
进一步通过电子顺磁共振(EPR)分析,研究了合成钻石中色心的形成机制。
实验结果表明,红色高压高温合成钻石中色心主要由顺磁离子交换引起,同时还可能受到多个因素的影响。
最后,通过对红色高压高温合成钻石的颜色机理进行深入研究,建立了合成钻石颜色与其组成、缺陷类型、浓度等因素之间的关系模型,并探讨了钻石颜色的影响因素和控制方法。
关键词:经改色粉,红色高压高温合成钻石,谱学特征,呈色机理,电子顺磁共振,颜色机理。
Abstract:This paper studies the spectral characteristics and coloring mechanism of red high-pressure and high-temperature synthetic diamonds synthesized by the improved color powder. Firstly, the principle and method of high-pressure and high-temperature synthesis of diamonds by the improved color powder are introduced. On this basis, spectral analysis iscarried out, and it is found that the color-changing treatment can have a significant impact on thespectral line intensity, shape, and position of synthetic diamonds. With different color-changing treatment conditions, various spectral parameters of synthetic diamonds also change. Next, the structure and chemical composition of the synthesized diamond are analyzed using techniques such as X-raydiffraction and infrared spectroscopy. The results show that the crystal structure and chemical composition of the synthesized diamond have changed to a certain extent after the color-changing treatment. Furthermore, the formation mechanism of color centers in synthetic diamonds is studied by electronic paramagnetic resonance (EPR) analysis. The experimental results show that the color centers in red high-pressure and high-temperature synthetic diamonds are mainly caused by paramagnetic ion exchange and may also be affected by multiple factors.Finally, by studying the color mechanism of red high-pressure and high-temperature synthetic diamonds in depth, a relationship model between the color of synthetic diamonds and their composition, defect type, concentration, and other factors is established, and the factors affecting and controlling the diamondcolor are discussed.Keywords: Improved color powder, red high-pressure and high-temperature synthetic diamonds, spectral characteristics, coloring mechanism, electronic paramagnetic resonance, color mechanismThe color of synthetic diamonds is closely related to their composition, defect type, concentration, and other factors. A relationship model has been established to understand the mechanism of red high-pressure and high-temperature synthetic diamonds in depth. This model helps to understand the factors that affect and control diamond color.One of the key factors that affects diamond color is the concentration of impurities. For example, nitrogen impurities are responsible for the yellow and brown hues that are commonly seen in diamonds. However, in high-pressure and high-temperature synthetic diamonds, nitrogen impurities are removed through a purificationprocess, resulting in a pure, colorless diamond.On the other hand, the introduction of certain impurities can also give rise to vivid, fancy colors in diamonds. The improved color powder method is one such example where small quantities of transition metal impurities are introduced during diamond growth. The spectral characteristics of these impurities can then be used to control the color of the resulting diamond.Another important factor that affects diamond color is the type of defects present in the crystal lattice. For example, the presence of nitrogen-vacancy defects can give rise to a pink or red hue. These defects can be intentionally created through the addition of specific impurities or through ion irradiation.Electronic paramagnetic resonance is a useful technique for studying the defects and impurities present in diamonds. By analyzing the spectra obtained using this technique, researchers can gain insights into the electronic structure of the defects and impurities and how they affect the diamond's optical properties.In conclusion, the color of synthetic diamonds isinfluenced by a variety of factors, including impurity concentration, defect type and concentration, and growth conditions. Understanding these factors and their interactions is key to controlling and optimizing the color of synthetic diamondsScientists and engineers are constantly working to improve the properties of synthetic diamonds,including their color. One application for colored synthetic diamonds is the jewelry industry, where they can be used as affordable alternatives to natural diamonds or to create unique and vibrant pieces that cannot be found in natural diamonds.Another potential application for colored synthetic diamonds is in electronics. The unique electronic properties of diamond, combined with its optical transparency, make it a promising material for use in high-performance electronic devices such as high-power transistors and radiation detectors. Color centers can also be used as qubits in quantum computing, since they can exhibit long spin relaxation times and robustness against noise.Research into the color of synthetic diamonds is ongoing, as scientists and engineers seek to improve the quality and range of colors available. Byunderstanding the fundamental physical properties that underlie diamond color, researchers can develop new growth techniques and synthesis strategies that lead to structurally and chemically homogeneous diamond crystals with controlled impurities and defects. These materials can then be tailored for specific applications in electronics, photonics, and other fields.Overall, the study of synthetic diamond color provides a fascinating glimpse into the complex interplay between structure and function in materials science.It also highlights the potential for innovative and transformative applications of diamond-based materials in a wide range of fields. As research in this area continues, it is likely that we will see even more exciting developments in the color and properties of synthetic diamonds in the years to comeThe study of synthetic diamond color is just one facet of the larger field of materials science. This fieldis dedicated to understanding the properties and behavior of a wide range of materials, from metals and semiconductors to plastics, ceramics, and composites. Materials science plays a crucial role in modern technology, as it provides the foundation for the development of new materials and products that arestronger, lighter, more durable, and more efficient than their predecessors.One area of particular interest in materials scienceis the development of new materials for renewable energy technologies. Solar cells, wind turbines, and other renewable energy devices require materials that can convert sunlight, wind, or other sources of energy into usable electrical power. Diamond-based materials may play a role in this area, as they have unique electronic and optical properties that could be useful for solar cells, sensors, and other energy-related applications.The study of synthetic diamond color also has implications for the field of photonics, which is dedicated to the development of new technologies that harness the properties of light. Photonics has applications in a wide range of fields, from telecommunications and data storage to medical imaging and defense. Diamond-based materials may be useful in this area, as they have exceptional optical properties, including high transparency, low absorption, and high thermal conductivity.In addition to their potential applications in renewable energy and photonics, diamond-basedmaterials also have important medical applications. Synthetic diamonds have been used as medical implants due to their biocompatibility and resistance to corrosion. Diamond-based coatings have also been developed that can reduce friction and wear in medical devices, such as joint replacements.Finally, the study of synthetic diamond color highlights the importance of interdisciplinary research in materials science. Understanding the properties and behavior of materials requires expertise in physics, chemistry, mathematics, engineering, and a range of other fields. By bringing together scientists and engineers with diverse backgrounds and skillsets, researchers can approach complex problems from multiple perspectives and develop innovative solutions that would be impossible to achieve otherwise.In conclusion, the study of synthetic diamond color provides a fascinating glimpse into the complex world of materials science. This field holds incredible potential for developing new materials with unique properties that can be harnessed for a wide range of applications, from renewable energy and photonics to medicine and beyond. As research in this area continues, we are likely to see even more excitingdevelopments in both the color and properties of synthetic diamonds, as well as in the broader field of materials science as a wholeIn conclusion, materials science is a rapidly growing field with incredible potential for developing new materials with unique properties. Synthetic diamonds are just one example of the fascinating research being conducted in this area, with exciting developments in color and properties. As we continue to explore the possibilities of materials science, we can expect to see even more groundbreaking advancements that have the potential to revolutionize industries and change our world。

专利名称:Flame synthesis of metal salt nanoparticles, in particular calcium and phosphatecomprising nanoparticles发明人:Wendelin Jan Stark,Sotiris-EmmanuelPratsinis,Marek Maciejewski,Stefan FridolinLoher,Alfons Baiker申请号:US10592913申请日:20040315公开号:US20070196259A1公开日:20070823专利内容由知识产权出版社提供摘要:Described is a method for the production of metal salts, wherein the cationic metal is preferably selected from Group I to IV metals and mixtures thereof and the anionic group is selected from phosphates, silicates, sulfates, carbonates, hydroxides, fluorides and mixtures thereof, and wherein said method comprises forming a mixture of at least one metal source that is a metal carboxylate with a mean carbon value per carboxylate group of at least 3 and at least one anion source into droplets and oxiding said droplets in a high temperature environment, preferably a flame. This method is especially suited for the production of calcium phosphate biomaterials such as hydroxyapatite (HAp,Cal0(P04)6(OH)2) and tricalcium phosphate (TCP,Ca3(P04)2) that exhibit excellent biocompatibility and osteoconductivity and therefore are widely used for reparation of bony or periodontal defects, coating of metallic implants and bone space fillers.申请人:Wendelin Jan Stark,Sotiris-Emmanuel Pratsinis,Marek Maciejewski,StefanFridolin Loher,Alfons Baiker地址:Zurich CH,Zurich CH,Zurich CH,Zurich CH,Opfikon CH 国籍:CH,CH,CH,CH,CH更多信息请下载全文后查看。
1 研究背景聚苯硫醚(PPS)是一种新型高性能热塑性树脂,具有优良的耐热性、阻燃性、绝缘性和耐腐蚀性,属于特种工程塑料[1-2]。
经过复合改性后,PPS转变为特殊的工程塑料,具有不同的性能和用途类别。
它是迄今为止全球最具成本效益的特种工程塑料,被誉为世界上使用最广泛的特种工程塑料。
PPS由于产能的增加和成本的降低,被列入通用工程塑料排名,成为继聚碳酸酯、聚酯、聚甲醛、尼龙、聚苯醚之后的第六大工程塑料[3]。
PPS具有优良的耐高温、耐腐蚀、耐辐射、阻燃和均衡的物理机械性能,同时还具有优良的尺寸稳定性和优良的电气性能。
至今已经已有几十年的发展史,现为国内外最大的特种工程塑料和第六大通用工程塑料,在汽车、电子电气、纺织、环保、建材等行业得到广泛应用[4]。
其应用领域如图1所示。
2 PPS 主要合成方法目前PPS有多种合成方法,可用于工业生产的主要有硫化钠法和硫磺溶液法。
日本武裕曾采用硫溶液法,后因硫磺溶液法缺陷不易解决而作废。
因此,目前国内外生产厂家多采用间歇式硫化钠法合成PPS树脂。
除这2种方法外,合成PPS 的方法还有氧化聚合法、对卤代苯硫酚盐自缩聚法、硫化氢法等。
其中,国内厂家已有硫化氢法的尝试,但由于存在反应速度过快等缺陷,目前基本未采用该方法。
线性高相对分子质量聚苯硫醚树脂的合成研究李沃源浙江新和成股份有限公司 浙江 绍兴 312500摘要:聚苯硫醚(PPS)具有超高耐热性,可在220℃以下长期使用,具有良好的耐化学性、高强度、天然阻燃性,与聚醚酰亚胺(PEI),可熔性聚四氟乙烯(PFA),聚醚醚酮(PEEK)等同类特种工程塑料相比,且性价比较高,因此被广泛用于汽车、电子、水处理、塑钢等领域,制成不同性能、不同等级的特殊工程塑料,是世界上迄今为止成本效益最高、被称为最大的特种工程塑料。
分析了目前较为常见的PPS合成方法,经过综合比较,硫化钠法合成PPS产品质量好,工艺短,副产物少。
为此重点对硫化钠法合成PPS工艺以及合成工艺中影响因素进行了详细阐述。
Halogenated Flame Retardants in Consumer Products:Do the Fire Safety Benefits Justify the Health and Environmental Risks?Arlene D. Blum1 and Linda Birnbaum21 University of California, Berkeley, Green Science Policy Institute, 1492 Olympus Avenue, Berkeley, California 947082 U.S. National Institute of Environmental Health Sciences and National Toxicology Program, P.O. Box 12233 Mail Drop B2-01, Research Triangle Park, North Carolina 27709IntroductionBeginning in the 1970’s, increasingly severe flammability standards in the United States were met with brominated or chlorinated flame retardants without consideration of potential adverse health or environmental impacts. Since then a series of toxic, persistent, and/or bioaccumulative halogenated flame retardants have been removed from use, only to be replaced by others with similar properties (Blum 1977, , 2007, Gold 1978). The continued use of certain halogenated flame retardants in consumer products should be questioned as current research suggests they have the potential to contribute to serious long term health problems, while providing only limited fire safety benefits.In 1977 the U. S. Consumer Product Safety Commission (CPSC) banned brominated Tris [tris (2,3-dibromopropyl) phosphate] from children’s sleepwear after it was found to be a mutagen, a carcinogen, and absorbed into children’s bodies (CPSC 1977). The main replacement for brominated Tris was chlorinated Tris or TDCP, [tris (1,3-dichloro-2-propyl) phosphate]. After also being found to be a mutagen, chlorinated Tris was removed from use in sleepwear in 1978, but is currently used in furniture and juvenile product foam (nursing pillows, baby carriers, high chairs, etc.) to meet California Technical Bulletin 117 (TB117). Recent studies show chlorinated Tris and other organohalogen flame retardants can migrate from products into dust, a likely route of human exposure (Wu et al. 2007, Stapelton et al. 2009). The CPSC estimates the lifetime cancer risk from Tris-treated furniture foam is up to 300 cancer cases/million (Babich 2006).TB117 is a unique California flammability standard that requires polyurethane foam to withstand exposure to a small open flame for twelve seconds. From its implementation in 1975 until 2004, this standard was primarily met with penta-brominated diphenyl ether (pentaBDE). PentaBDE and other polybrominated diphenyl ethers (PBDEs) are structurally similar to known human toxicants polybrominated biphenyls (PBBs), polychlorinated biphenyls (PCBs), dioxins and furans (Figure 1). In addition to having similar mechanisms of toxicity in animal and human studies (Birnbaum et al. 2003), PBDEs also persist and bioaccumulate in humans and animals (Hites 2005). In 1999, 98% of global pentaBDE usage was in North America, in large part to meet TB117 (Hale et al. 2003). PentaBDE was banned in California in 2003; eight other states and the European Union (EU) followed suit (Blum 2007). In 2004 Chemtura, the sole U.S. manufacturer, voluntarily ceased production, and in 2009 pentaBDE and octaBDE were listed as persistent organic pollutants under the Stockholm Convention (UNEP 2009). Late in 2009, after negotiations with the U.S. EPA, the three manufacturers of decaBDE agreed to cease production within three years. However decabrominated diphenylethane (DBDPE), a major substitute for decaBDE, is similar in structure, persistence and bioaccumulation (Betts 2008). PBDEs continue to increase in humans, animals, and the food supply, moving from consumer products in homes into dust and the environment (Shaw & Kurunthachalam 2009).According to the furniture and the polyurethane foam industry, all furniture sold in California and about 30% of furniture sold in the U.S. and in Canada outside of California complies with TB117 (Luedeka, Batson). A major replacement for pentaBDE, used in furniture and baby product foam today, is Firemaster 550, produced by Chemtura. In 2004, the U.S. EPA requested health information on Firemaster 550 based on its predicted reproductive, neurological, and developmental toxicity and persistent degradation products (EPA Furniture Flame Retardancy Partnership. 2005). While awaiting the test results and their evaluation during the past five years, the EPA has allowed Firemaster 550 to continue to be used.Firemaster 550 components include: (1) triphenyl phosphate (highly eco-toxic); (2) triaryl phosphate isopropylated (probable reproductive toxin); (3) 2-ethylhexyl-2,3,4,5-tetrabromobenzoate; (4) Bis (2-ethylhexyl) tetrabromophthalate (Stapleton et al. 2008). The components have been found in dust, sewage sludge (Klosterhaus et al. 2008) and sediment in California as well as in marine mammals near flame retardant production facilities in China (Lam 2009).Thus, a series of toxic or untested brominated and chlorinated flame retardant chemicals continue to be used in consumer products in close contact with humans, without adequate consideration of their health and environmental impact. The present study investigates flammability regulations for some consumer products in North America, and discusses the fire safety benefits they provide, the toxicity of the chemicals that have been used to meet them, and how alternative strategies can reduce fire hazard without adding potential persistent organic pollutants to consumer products.MethodsMethods consisted of reviewing the literature and interviewing leaders in industry, government, and the private sector on the following topics: major uses of halogenated flame retardant chemicals; exposure and health impacts; regulations leading to the uses of flame retardants; fire safety data showing impacts of flame retardant chemicals in consumer products; and policy decisions regarding flame retardant chemicals Results and DiscussionMajor uses of halogenated flame retardant chemicalsThe major uses of halogenated flame retardant chemicals in North America are in 1) electronics, 2) building insulation, 3) transportation, and 4) home furnishings. The chemicals are commonly used at levels up 5 % of the weight of polyurethane foam and 15% of the weight of the plastic of electronic housings (Allen et al. 2008).Exposure and health impactsHalogenated flame retardants are the predominant class of toxic chemical found in human biomonitoring studies (Houlihan et al. 2005). They are semi-volatile and can form thin films on walls and windows (Weschler & Nazaroff 2008). Toddlers have much higher body burdens of pentaBDE than their mothers (Toms et al. 2008). Californians have higher levels in their house dust and body fluids than residents of other states (Zota et al. 2008).Many halogenated flame retardants have been shown to cause cancer, immune and endocrine disruption, and adverse reproductive and neurodevelopmental effects in animals (Birnbaum et al. 2003). In humans, these substances are associated with reproductive abnormalities (Meeker & Stapleton 2009), diabetes (Lim et al. 2008), thyroid dysregulation (Turyk et al. 2008, Meeker et al. 2009), cognitive changes (Roze et al. 2009, Herbstman et al. 2010), and cryptorchidism (undescended testicles) (Main et al. 2007). Since the1970s, brominated flame retardants have increased in use and, at present, PBDE levels in marine biota and people from North America are the highest in the world, reflecting the unique flammability standards leading to the use of these compounds in the U.S. (Shaw & Kurunthachalam 2009).Halogenated flame retardants also pose recycling and end of life problems. When products treated with these chemicals are exported to developing countries, they are often burned in the open, leading to the production of brominated and chlorinated dioxins and furans (Zennegg et al. 2009, Wong et al. 2007) and the release of PBDEs, other BFRs and toxics (Wong et al. 2007). When landfilled, they can leach into water and soil (Danon-Schaeffer et al. 2008) and make their way into food (Melber & Kielhorn 1998). Fire safety data showing impacts of flame retardant chemicals in consumer productsThe use of flame retardant chemicals in consumer products has not been shown to reduce fire deaths in the peer-reviewed literature. U.S. National Fire Protection Association (NFPA) data does not show a greater reduction in the rate of fire deaths in California than in other states that do not have furniture flammability standards (Figure 2).Reducing ignition sources can prevent fires without adding potentially hazardous chemicals to consumer products. A 60% decrease in fire deaths in the United States since 1980 parallels the decrease in per capita cigarette consumption (Diekman & Ballesteros 2008, Ahrens 2008), increased enforcement of improved building, fire, and electrical codes; and the increased use of smoke detectors and sprinklers. An estimated 65% of reported home fire deaths in 2000-2004 resulted from fires in homes without working smoke alarms (Ahrens 2008).Recent laws mandating fire-safe cigarettes and a voluntary industry standard for fire-safe candles promise further reductions in fire death and injury. The European Union and 44 U.S. states have passed legislation requiring fire-safe cigarettes.Regulations leading to the uses of flame retardants and how they are metTB 117 for filling materials in upholstered furniture and juvenile products is primarily met by the addition of halogenated chemicals. California TB 133, a severe flammability standard for furniture for use in public occupancies, is met by the use of higher density foam which is less flammable and the flame retardant melamine, often mixed with chlorinated Tris or TDCP. The severe new U.S. flammability standard for mattresses, CFR 1633, is met by a barrier technology where flame-retardant polymeric fabrics are wrapped around the foam to serve as a barrier to ignition. The CPSC estimates that this standard will prevent 78% of deaths from fires that originate in mattresses (CPSC Release 2006). A related technology could be used to protect the foam inside furniture from ignition. Other design alternatives, such as making electronics of metal, glass, or ceramics instead of plastics, can reduce flammability without chemicals (Betts 2008). Policy decisions regarding flame retardant chemicalsPrior to implementing new flammability regulations leading to halogenated chemicals in consumer products, decision makers should consider health and environmental hazards of the chemicals and materials likely to be used, as well as proven fire safety benefits.Flammability regulations can be designed to be met without flame retardant chemicals. For example, the CPSC is moving forward with a staff draft federal furniture standard that addresses fire safety without the use of added chemicals in polyurethane foam (CPSC 2008). A previous CPSC draft flammability standard, similar to TB117, was removed from consideration due in part to health and environmental concerns (Moore 2007). Strategies to reduce fire hazard without potential adverse health impacts include newtechnologies and materials, product design, and green chemistry. Reducing the use of untested halogenated compounds with a potential to be persistent organic pollutants will protect human and animal health and the global environment.Figure 2. Residential Fire Death Rates 1981 -2005The Research described was reviewed by the National Institute of Environmental Health Sciences, and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does the mention of trade names or commercial products constitute an endorsement or recommendation.ReferencesAhrens M. 2008. 2008. Fire Technology 44.Ahrens M. 2008. National Fire Protection Association, Quincy, MA. May.Allen JG, Mcclean MD, Stapleton HM, Webster TF. 2008. Environ. Sci. Technol 42 4222-4228. Figure 1. PBDEs are structurally similar to PBB, PCBs, dioxins andfurans.Babich MA. 2006. CPSC Staff Preliminary Risk Assessment of Flame Retardant (FR) Chemicals in Upholstered Furniture Foam.Betts KS. 2008. Environmental Health Perspectives 116(5): A211.Birnbaum LS, Staskal DF, Diliberto JJ. 2003. Environnent International 29(6): 855.Blum A. 1977. Science (4273):17-23.Blum A. 2007. Science 318: 194.California Bureau of Thermal Insulation and Home Furnishings. 1990. Technical Bulletin 117, 1975. CPSC.Standard for the Flammability of Residential Upholstered Furniture.2008. Federal Register 16 CFR Part 1634. Mar 4, 2008.US Consumer Product Safety Commission Release #06-091 February 16, 2006.CPSC 1977. /cpscpub/prerel/prhtml77/77030.html.Danon-Schaeffer MN, Grace JR, Ikonomou MG. 2008. Organohalogen Compounds 70 365-368. Diekman ST, Ballesteros MF, Berger LR, Kegler SR. 2008. Inj Prev 14: 228.Gold MD 1978. Science (4343):785-7.Hale RC, Alaee M, Manchester-Neesvig JB, Staplton HM, Ikonomou MG. 2003. Environment International 29:6, 771-779.Herbstman JB, Sjödin A, Apelberg BJ, Witter FR, Halden RU, Patterson DG, Jr., Panny SR, Needham LL, Goldman LR. 2008. Environ Health Perspect 116(10): 1376.Hites RA. 2005. Environ SciTechnol 38(4):945.Houlihan J, Kropp T, Wiles R, Gray S, Campbell C. 2005. Environmental Working Group.The Coalition for Fire Safe Cigarettes. 2010..EPA, Furniture Flame Retardancy Partnership (EPA 742-R-05-002A, September, 2005).Klosterhaus S, Konstantinov A, Stapleton HM. 2008. Presentation at the 10th Annual Workshop on Brominated Flame Retardants. Victoria, British Columbia, Canada.Lam JCW, Lau RKF, Margaret BM, Lam PKS. 2009. ES&T, 43(18): 6944.Lim JS, Lee DH, Jacobs DR Jr. 2008. Diabetes Care 31(9):1802.Luedeka R, Polyurethane Foam Ass. and Batson R. American Home Furnishings Ass. Personal communication.Main KM, Kiviranta H, Virtanen HE, Sundqvist E, TuomistoJT, Tuomisto J, Vartiainen T, Skakkebæk NE, Toppari1 J. 2007. Environ Health Perspect 115(10): 1519.Meeker JD, Johnson PI, Camann D, Hauser R. 2009. Science of the Total Environment 407(10): 3425. Meeker JD, Stapleton HM. 2009. Environ Health Perspect. doi: 10.1289/ehp.Melber C, Kielhorn J. 1998. Environmental Health Criteria 205.Moore TH. 2007. /pr/moore122707.html.Roze E, Meijer L, Bakker A, Van Braeckel KNJA, Sauer PJJ, Bos AF. 2009. Environ Health Perspect doi: 10.1289/ehp.0901015 (available at /).Shaw SD, Kurunthachalam K. 2009. Reviews on Environmental Health 24: 157.Stapleton HM, Allen JG, Kelly SM, Konstantinov A, Klosterhaus S, Watkins D, McClean MD, Webster TF. 2008. Environ SciTechnol 42(18): 6910.Stapelton, HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, Webster, TF. 2009. ES& T43: 7490. Toms LM, Harden F, Paepke O, Hobson P, Ryan JJ, Mueller JF. 2008. Environ SciTechnol 42(19): 7510. Turyk ME, Persky VW, Imm P, Knobeloch L, Chatterton R, Jr., Anderson HA. 2008. EHP 116(12): 1635. UNEP (2009) Report of the Conference of the Parties of the Stockholm Convention on Persistent Organic Pollutants on the work of its fourth meeting. UNEP/POPS/COP.4/38.Weschler CJ, Nazaroff WW. 2008. Atmospheric Environment. 42(40): 9018.Wong MH, Wu SC, Deng WJ, Yu XZ, Luo Q, Leung AOW, Wong CSC, Luksemburg WJ, Wong AS. 2007. Environmental Pollution 149 (2007) 131e140.Wu N, Herrmann T, Paepke O, Tickner J, Hale R, Harvey E, La Guardia M, McClean MD, Webster TF. 2007. Environ. Sci. Technol 2007 41(5): 1584.Zennegg M, Yu X, Wong MH, Weber R. 2009. Organohalogen Compounds 71 2263-2267.Zota AR, Rudel RA, Morello-Frosch RA, Brody JG. 2008. Environ Sci Technol 42(21): 8158.。
第27卷,第8期光谱学与光谱分析v ol -27,N n 8,pp l 457—14602 O O 7年8月Spectroscopy a n d spect 瞄AnaIysfsAugust ,2007爆炸性物质太赫兹时间分辨光谱测量张亮亮。
2,张存林2,赵跃进1,刘小华11.北京理工大学光电工程系.北京1000812.首都师范大学物理系,北京100037摘要利用自由空间电光取样方法,研究了四种炸药在太赫兹(THz)频段的光学特性。
通过太赫兹时间 分辨光谱测量,作者得到了四种炸药DN T(2,4_二硝基甲苯)、钝化的RI)x(黑索今)、HM x(奥克托金)和 TNT(2,4,6一三硝基甲苯)的透射光谱,进而计算得出它们在o 2~2.5 THz 频段的吸收系数和折射率。
作者发现,2,4一DNT 在1.08 THz ,HMx 在1.82 THz存在显著的吸收尖峰,RDx 在此颤段存在多个吸收蟑, T NT 的吸收谱线相对其他三种样品比较平缓,这种共振吸收一般认为是由分子间相互作用或声子共振模式 引起的。
四种炸药对太赫兹波独特的吸收性质说明,太赫兹时同分辨光谱测量技术在炸药特征识别及安全检测领域具有潜在应用价值。
作者对致癌物质偶氮苯进行了太赫兹光谱研究,发现了国产偶氮苯和进口偶 氮苯在太赫兹渡段均存在特征吸收峰,可用于物质鉴别。
关毽词爆炸物;太赫兹;飞秒激光;电光取样;时间分辨光谱中图分类号:0434.1 文献标识码:A 文章编号:1000—0593(2007)08一1457一04高于TNT ,探测其蒸汽浓度可以发现隐藏的地雷或未爆炸引言 的军火f(4)HMx ,环四次甲基四硝胺[钝感的],俗称奥克托金,分子式G H8 N8Q ,白色细腻粉末压片,是生产R DX由于爆炸性物质在安全检测和环境控制方面的重要地 的副产品,由于爆炸速率极高而与T NT 等混合作为形状填 位,其光谱和成像研究是目前的焦点o q 。
原子吸收测火焰法原子化器高度英文回答:The height of the atomizer in atomic absorption flame spectrometry is an important parameter that affects the performance of the technique. The atomizer is responsiblefor converting the analyte into atomic vapor, which is then excited and subsequently absorbed by the atoms in the flame.The height of the atomizer is typically adjusted to optimize the sensitivity and stability of the measurement.If the atomizer is positioned too low, the sample may notbe efficiently vaporized, leading to lower sensitivity. On the other hand, if the atomizer is positioned too high, the sample may be over-vaporized, resulting in a decrease in sensitivity and an increase in background noise.To determine the optimal height of the atomizer,several factors need to be considered, including the typeof flame, the nature of the analyte, and the instrumentused. Different flames, such as air-acetylene or nitrous oxide-acetylene, may require different atomizer heights to achieve the best performance.For example, in a flame photometer, which is commonly used for the determination of alkali and alkaline earth metals, the atomizer height can be adjusted to ensure that the flame is stable and the analyte is efficiently vaporized. If the atomizer is too low, the flame may flicker or go out, leading to inaccurate results. Conversely, if the atomizer is too high, the flame may become too hot, causing excessive background noise and interfering species.In atomic absorption spectroscopy, the atomizer height is typically adjusted by trial and error. The height is gradually increased or decreased, and the resulting signal intensity is monitored. The optimal height is reached when the signal is maximized and the noise is minimized.中文回答:原子吸收测火焰法中的原子化器高度是影响该技术性能的重要参数。
The Joy of Learning: Embracing the Adventure of KnowledgeIn the vast expanse of human endeavor, few pursuits are as rewarding as the quest for knowledge. Learning is not merely an obligation or a means to an end; it is a journey filled with discovery, growth, and transformation. The joy of learning lies in its ability to ignite curiosity, foster creativity, and deepen our understanding of the world around us. This essay explores the multifaceted pleasures of learning and how embracing this adventure can enrich our lives in ways both expected and surprising. Curiosity as the SparkAt the heart of learning is curiosity—the innate desire to explore, question, and understand. Curiosity is the spark that ignites the flame of knowledge, propelling us forward into realms unknown. It is the child asking “why” until the mysteries of the universe begin to unravel, the scientist hypothesizing and experimenting to uncover truths hidden beneath the surface, and the artist seeking inspiration in the beauty and chaos of life. When we allow curiosity to guide us, learning becomes an exciting expedition, where each answer leads to a myriad of new questions, and every discovery opens doors to previously unseen vistas.The Thrill of MasteryLearning also brings the exhilaration of mastery. As we delve deeper into a subject, we develop skills, gain insights, and gradually master the material. This process is akin to climbing a mountain, where each step taken, each obstacle overcome, leads to a greater view. The satisfaction of solving a complex problem, articulating a coherent argument, or mastering a challenging piece of music is unparalleled. Mastery is not just about achieving competence; it is the realization of our potential, the embodiment of our dedication, and the tangible evidence of our growth. Connecting the DotsOne of the most delightful aspects of learning is the realization of connections between seemingly disparate pieces of knowledge. As we accumulate information and insights, patterns emerge, revealing the intricate web of relationships that underpins the universe. From mathematics to literature, physics to philosophy, every discipline contributes to our understanding of the whole. This synthesis of knowledge fosters a sense of interconnectedness, reminding us that nothing exists in isolation and that every piece of the puzzle contributes to the grand design.The Social Fabric of LearningLearning is not a solitary endeavor. It thrives in the company of others, whether through collaborative projects, discussions, or simply the shared enthusiasm for a subject. Engaging with peers, mentors, and experts broadens our perspectives, challenges our assumptions, and enriches our understanding. The exchange of ideas, the act of teaching others, and the collective pursuit of knowledge create a vibrant tapestry of human interaction, where learning becomes a communal celebration of intellect and imagination.The Continuous JourneyFinally, the joy of learning lies in its perpetual nature. Unlike fleeting pleasures, the quest for knowledge is a lifelong adventure. It adapts to our evolving interests, accommodates our changing circumstances, and remains a constant source of fulfillment. Learning teaches us resilience, adaptability, and the courage to embrace the unknown. It equips us with the tools to navigate life’s challenges, to appreciate its complexities, and to contribute meaningfully to the world.In conclusion, the joy of learning is a multifaceted experience that touches upon our deepest desires for exploration, mastery, connection, and growth. It is a voyage that begins with a spark of curiosity, gains momentum through the thrill of mastery, finds resonance in the interconnectedness of knowledge, flourishes in the company of others, and endures as a lifelong commitment. As we embark on this adventure, let us remember that the true joy of learning lies not in the destination but in the journey itself—a journey that enriches our minds, expands our hearts, and connects us to the infinite possibilities of the human experience.。
能让人一看就吸引住的英语作文题目The Allure of the Unknown: Exploring the Enigmatic Appeal of Mysteries.In the realm of human experience, mysteries have long held an enduring fascination, drawing us like moths to a flickering flame. From ancient riddles to modern scientific enigmas, the allure of the unknown beckons us to delve into the unexplored depths, to unravel secrets that have remained hidden for centuries.The Intriguing Nature of Uncertainty.Uncertainty, the very essence of mysteries, plays a paradoxical role in their appeal. It simultaneously tantalizes and terrifies us, creating a cognitive itch that demands to be scratched. The absence of clear answers leaves our minds racing with possibilities, fueling our imagination and sparking an insatiable curiosity.The thrill of the chase, the adrenaline rush that accompanies the process of discovery, is an intrinsic partof the allure of mysteries. As we piece together clues, eliminate possibilities, and approach the elusive truth, a sense of achievement washes over us, rewarding us for our intellectual endeavors.The Promise of Enlightenment.Mysteries offer more than just fleeting moments of excitement. They have the potential to expand our knowledge, challenge our beliefs, and reshape our understanding of the world. By confronting enigmatic phenomena, we embark on a journey of intellectual growth, pushing the boundaries of our comprehension.Solving a mystery often involves a synthesis ofdifferent perspectives, a cross-pollination of ideas that can lead to transformative insights. By unraveling the unknown, we not only satisfy our curiosity but also enrich our mental landscape, broadening our horizons and deepening our understanding of the complexities of existence.The Emotional Resonance of Secrets.Mysteries tap into our primal fear of the unknown, an instinct that has kept humans alive throughout history. The uncertainty and potential danger associated with enigmatic phenomena can trigger a mix of emotions, from awe and wonder to trepidation and suspense.This emotional resonance makes mysteries incredibly effective in storytelling, whether in literature, film, or even real-life crime investigations. By crafting narratives around unsolved crimes, supernatural occurrences, or puzzling historical events, storytellers can evoke powerful emotional responses in their audiences, keeping them on the edge of their seats from beginning to end.The Cultural Significance of Mysteries.Mysteries have played a pivotal role in human culture for millennia. From ancient myths and legends to modern scientific enigmas, they have woven themselves into thefabric of our collective consciousness. By exploring the unknown, we not only satisfy our curiosity but also connect with our past and lay the groundwork for our future.Mysteries inspire artists, fuel scientific discoveries, and shape our beliefs and values. They remind us that there is always more to learn, more to discover, and more to understand about the world around us.Conclusion.The allure of mysteries lies in their ability to captivate our minds, ignite our imaginations, and challenge our preconceptions. They offer a glimpse into the unknown, promising both excitement and enlightenment. Whether we seek to unravel ancient secrets or uncover scientific truths, the pursuit of mysteries is an endeavor that enriches our lives, expands our knowledge, and ultimately brings us closer to understanding the enigmatic tapestry of existence.。
High-rate flame synthesis of vertically aligned carbonnanotubes using electric field controlWilson Merchan-Merchan a ,Alexei V.Saveliev b ,Lawrence A.Kennedyb,*aDepartment of Materials Sciences and Engineering,University of Illinois at Chicago,Chicago,IL 60607,USAbDepartment of Mechanical and Industrial Engineering,University of Illinois at Chicago,851South Morgan Street,Chicago,IL 60607,USAReceived 31July 2003;accepted 17December 2003AbstractThe electric field controlled synthesis of carbon nanomaterials on a Ni-based catalytic support positioned at the fuel side of the opposed flow oxy-flame is studied experimentally.Carbon nanomaterials formed on the probe surface are comparatively analyzed for two characteristic operational modes:a grounded probe mode and a floating probe mode.In a grounded mode a number of various carbon nanostructures are formed depending on the probe location in flame.Observed nanoforms include multi-walled carbon nanotubes (MWNTs),MWNT bundles,helically coiled tubular nanofibers,and ribbon-like coiled nanofibers with rectan-gular cross-section.The presence of various carbon nanoforms is attributed to the space variation of flame parameters,namely flame temperature and concentration of chemical species.It is found that the presence of an electric potential (floating mode operation)provides the ability to control the nanostructure morphology and synthesis rate.A thick layer (35–40l m)of vertically aligned carbon nanotubes (VACNTs)is found to be formed on the probe surface in the floating potential mode.This layer is characterized by high uniformity and narrow distribution of nanotube diameters.Overall,the electric field control method demonstrates sta-bilization of the structure in a wide flame region while growth rate remains dependent on flame location.Ó2004Elsevier Ltd.All rights reserved.Keywords:A.Carbon nanotubes;B.Catalyst support;C.Scanning electron microscopy1.IntroductionIn recent years great effort has been devoted to the study of synthesis of carbon nanotubes in flames.Since the discovery of nanotubes in 1991by Iijima [1]con-ventional methods such as arc plasma discharges [2,3],pulsed laser vaporization [4],and chemical vapor deposition (CVD)[5]have dominated the field.Re-cently,it has been demonstrated in a number of publi-cations that flames can be applied as a relatively inexpensive,robust,pyrolysing carbon source for growing tubular nanomaterials [6–14].Mainly studies in co-flow diffusion flames with the introduction of a cat-alyst in the form of nano-aerosol or in the form of solid support have been reported.Yuan et al.[6]analyzed flame-based growth of nanotubes on a Ni/Cr support in laminar co-flow methane–air diffusion flames.Forma-tion of entangled MWNTs with a diameter range of 20–60nm was reported.In related work,Yuan and co-workers [7]used a catalytic support in the form of a stainless steel grid to produce MWNTs in an ethylene–air diffusion flame.The electroplating of the grid with cobalt resulted in synthesis of aligned MWNTs.The metal catalyst dispersed on TiO 2substrate was used by Vander Wal [8]to generate MWNTs in ethylene/air and acetylene/air diffusion co-flow flames.It was demon-strated that the structure of the obtained nanotubes and nanofibers strongly depends on the catalytic particle shape and chemical composition.Recently,Vander Wal and co-workers [9,10]also reported that single-walled carbon nanotubes (SWNTs)can be grown in premixed co-flow flames by seeding the fuel line with ferrocene and compositions of metal nitrates serving as catalyst precursors for the formation of nanotubes.It was demonstrated that ferrocene and Fe nanoparticles can yield bundles of self-assembled single-wall nanotubes with diameters as small as two nanometers.The rich premixed flame synthesis of carbon nanotubes using supported catalyst [11]was optimized by selection of optimal flame conditions including fuel composition and*Corresponding author.Tel.:+1-312-996-2400;fax:+1-312-996-8664.E-mail address:lkennedy@ (L.A.Kennedy).0008-6223/$-see front matter Ó2004Elsevier Ltd.All rights reserved.doi:10.1016/j.carbon.2003.12.086Carbon 42(2004)599–608fuel-to-air ratio.The studied fuels include methane, ethane,ethylene,acetylene,and propane.A recent work by Height and co-workers[12]used premixed co-flow flames to grow SWNTs.A detailed characterization of SWNT growth was given with emphasis onflame posi-tion andflame air-to-fuel ratio.The non-catalytic for-mation of carbon nanotubes was reported in opposed flow oxygen enrichedflame studies[13].The strong potential of theseflames for carbon nanomaterial syn-thesis was recently proven employing a catalytic probe [14].If compared with CVD and plasma methods,a typi-calflame is a reacting medium characterized by strong thermal and chemical non-uniformity.It is not surpris-ing,that a number offlame studies show a high mor-phological and growth rate sensitivity of formed carbon nanomaterials to theflame location.An efficient control method is required to improve uniformity and produc-tivity offlame based synthesis and utilization of elec-tromagneticfield control is one of the promising approaches.The electricfield control is successfully tested in chemical vapor deposition(CVD)and plasma synthesis studies[15–21]as reported recently by several authors:Kuzuya et al.[15]applied an electricfield for effective control of producing helically coiled carbon materials using CVD;Avigal et al.[16]grew short aligned nanotubes by positively biasing the substrate during the nanotube formation in CVD;Srivastava et al.[17]showed that nanotubes that are grown under the influence of an electricfield in plasma can permanently maintain their alignment when the electricfield force is removed;Lee et al.[18]using CVD and magneticfields showed that it is possible to grow aligned carbon na-notubes at any angle with respect to the substrate sur-face;Ural et al.[19]used CVD to grow carbon nanotubes with and without the influence of electric fields to show the ability of electricfields to grow aligned carbon nanotubes;Colbert et al.[20]used an arc plasma discharge to show that an electricfield is essential for nanotube growth by stabilizing the open tip and pre-venting it from closure;Srivastava et al.[21]used plas-ma varying voltages to study the size,length,and alignment of carbon nanotubes.To our knowledge,electricfields have not been ap-plied to control growth of carbon nanotubes inflames prior to this study.In this article the carbon nanoforms formed on a catalytic probe in opposedflow oxy-flame are comparatively analyzed for various probe potentials. It is shown that implementation of an electricfield control allows to increase their structural uniformity and growth rate.2.ExperimentalFig.1shows the schematic of the experimental setup employed in the present study.A counter-flow burner forms two opposite streams of gases;the fuel(methane seeded with4%of acetylene)is supplied from the top nozzle and the oxidizer(50%O2+50%N2)is introduced from the bottom nozzle.The fuel and oxidizerflows impinge against each other to form a stable stagnation plane,with a diffusionflame established from the oxi-dizer side.The introduction of co-flowing nitrogen through a cylindrical annular duct around the outer edge of the oxidizer nozzle has the function of extin-guishing theflame near the outer jacket and isolating it from the environment.A detailed description of the burner is given by Beltrame et al.[22].Technical purity methane(98%,AGA Gas Inc.)was seeded with atomic absorption purity acetylene(99.8%,AGA Gas Inc.).The experiments were conducted with constant fuel and oxidizer velocities and a strain rate equal to20sÀ1.A40mm long catalytic probe was introduced through theflame-protecting shield to the yellow soot-containing region of theflame.The central part oftheFig.1.Schematic of the experimental setup.600W.Merchan-Merchan et al./Carbon42(2004)599–608probe($25mm)was used to study the structure of deposited materials.The0.64mm diameter probe is an alloy with compositions of73%Ni+17%Cu+10%Fe. The axial position of the probeðZÞwas controlled by the positioning system.Reported experiments were con-ducted with residence time of10min.To generate radial electricfields on the probe surface, the probe was supported on Teflonâisolators while the burner nozzles were kept at ground potential(Fig.1).In general this configuration allows to generate a variety of electricfields controlling the probe potential with the external electric source.In the present work,experi-ments were conducted at two characteristic probe potentials:grounded probe and probe atfloating po-tential.When nodes one and two are connected no electric field is generated;this mode is further referred as the grounding probe mode(GPM).On the other hand, when node one and two are disconnected,electricfield is generated between the surface of the catalytic probe and the edges of the fuel and oxidizer nozzles;this configu-ration is further referred as thefloating potential mode (FPM).Due to the small size of the probe relative to size of the burner nozzles the electricfield around the probe can be well approximated as radial.The source of the floating potential is the transport of ions and electrons formed in high-temperature oxygenflame to the probe surface;a potential difference close to300mV was typically measured when the probe was in FPM.The initial surface scans of the catalyst probe were performed by a scanning electron microscope(JEOL Inc.,Model JSM-6320F)with a coldfield emission source.The specimens for TEM examinations were prepared by ultrasonic dispersion of carbon deposits collected from a specific probe location in acetone;a drop of the suspension was placed on the electron microscope grid.The detailed structural characteristics of the deposited material were obtained from high-res-olution electron microscopy studies performed in a JEOL JEM-3010electron microscope with the magnifi-cation range from50to1,500,000times.Images were collected on a Gatan digital imaging system and pro-cessed by Digital Micrograph software.3.Results and discussionWhen the catalytic probe was inserted in GPM a variety of carbon nanostructures were observed depending on position of catalytic probe in the fuel zone of the opposedflowflame.Those carbon structures in-clude MWNTs and MWNT bundles,nanofibers with varying degree of crystallinity,helical regularly coiled tubular carbon nanotubes,ribbon-like coiled nanofibers with rectangular cross section,and,finally,long($0.2 mm)uniform-diameter($100nm)tubular nanofibers with regular internal structure of carbon layers.Some of the observed structures,such as coiled tubular and rib-bon-like nanofibers,have been reported[23,24]to grow only under special conditions in CVD.Fig.2(a)shows a typical regularly coiled carbon nanofiber formed in ourflames.The diameters of these coils ranged from20to100nm with lengths of several microns.These structures were typically observed in the zone close to Z¼9mm,in the vicinity of theflame front.Qualitatively,the formation of helical nanotubes can be explained by variation of deposition rates and, hence,extrusion velocities along the contact curve be-tween the active catalytic particle and thealready Fig.2.SEM images of carbon nanomaterials grown in various loca-tion of the opposedflowflame on a single catalytic support:(a)heli-cally coiled spiral carbon nanotube,(b)multi-walled carbon nanotubes,(c)coiled carbon nanofiber with rectangular cross-section.W.Merchan-Merchan et al./Carbon42(2004)599–608601formed tube[23].The sharp gradients of temperature and chemical species in the vicinity of theflame zone induce sensible variations of carbon deposition rates providing the condition for the growth of helical struc-tures.Irregular carbon nanotubes are widely observed in theflame region close to Z¼8:5mm.They form a coating layer that typically contains entangled web of randomly directed tubes,carbon nanofibers and soot particulates,as shown in Fig.2(b).The tube diameters vary significantly,typically from10to45nm.The internal tube structure is regular,although carbon layers often form an angle with the tube axis.As a result bamboo-like structures are widely observed.Irregularly oriented nanotubes frequently change direction of growth;kinks and bends are common for these struc-tures.The other distinctive coiled carbon structure observed in ourflames possesses a nontubular form,Fig.2(c). These structures are typically found to be present in the low temperature zone Z¼8mm.The nanofiber has a distinctive ribbon-like appearance with unwound ribbon rolls.The rectangular cross section has a height of300 nm and a thickness of100nm.It was reported previously [25],that growth of these rarely observed ribbon-like filaments could be catalyzed by small iron-containing particles in CO containing atmosphere at700°C,which correspond to theflame environment at the vicinity of Z¼8mm.The variation of structure and morphology of formed carbon nanomaterials is directly attributed to the strong variation of temperature and chemical composition in the studiedflame region.The distributions of several major hydrocarbons(CH4,C2H2,CO,and C6H6)that can contribute to the growth of carbon deposits are shown in Fig.3along with the temperature profile[22]. It is easy to conclude that all above components vary significantly in theflame region of interest.Thus,con-centration of CH4and C2H2diminishes below100ppm for Z>10:5mm;the concentration of CO grows with Z reaching its maximum at this point;C6H6is present in essential quantities only from8to10mm,maximizing at 9.5mm.The essential variations of temperature and chemical composition are inevitable in a variety offlame config-urations.The effective control method is necessary to stabilize growth of carbon nanomaterials with specified structure and morphology.The electricfield control method was implemented in this study electrically insulating the probe from the grounded support and operating it at afloating potential.The catalytic probe of the same metallic composition was inserted at the axialflame position of Z¼8:5mm in FPM(approximate probe potential)300mV).The produced carbon deposits were analyzed with SEM (Figs.4and5).Fig.4shows that controlled electricfield growth generates a coat of VACNTs.Low magnifica-tion image shows that VACNT layer uniformly coat the catalytic substrate.The generated nanotubes are char-Fig.4.SEM image of orderly VACNT layer covering the probe sur-face;floating potential mode,Z¼8:5mm.The layer is partially re-moved revealing the bare catalytic surface.Fig.5.High-resolution SEM of the wall edge of the layer shown in Fig.4displays nanotube purity and alignment.602W.Merchan-Merchan et al./Carbon42(2004)599–608acterized by high purity and alignment,as shown in Fig.5.For this axial position,hundreds of nanotube diam-eters were measured.The results show a very narrow diameter distribution with a mean diameter close to 38nm.Fig.2(b)and Figs.4,5depict the carbon nanomate-rials grown on identical probes in the same flame loca-tion respectively in GPM and FPM conditions.Analysis of SEM images on the synthesized carbon materials on both catalytic substrates suggests that nanotube growth is greatly enhanced when the electric field is present (FPM)if compared with the case when the electric field is absent (GPM).Other interesting aspects,displayed in Figs.4and 5,include not only the presence of VACNTs but also the absence of contaminants such as soot or other nontubular carbon structures that are often pres-ent in the flame synthesis of carbon nanotubes.It should be noted that the samples analyzed in this study were never purified by any kind of chemical and/or physical treatment.With electric field stabilization,flame position re-mains the important factor in controlling the thickness of the coat layer of nanotubes formed under FPM.Figs.6and 7show the variation of nanotube formation along the burner axis.Fig.6is a low resolution SEM image of the catalytic substrate inserted in the FPM at the axial distance of Z ¼9:5mm.The well-defined layer formed here shows highly ordered carbon nanotube arrays,similar to those found in the previous position.The application of high-resolution imaging in Fig.6reveals a highly dense bundle of nanotubes attached to the tips of the undisturbed nanotube layer,as shown in Fig.7.High-resolution imaging shows that these nanotube bundles are free of contaminants as well.It is evident by comparing micrographs obtained at the axial position of Z ¼8:5mm to those obtained at Z ¼9:5mm that the thickness of the coating layer decreased when the sub-strate is inserted further down from the edge of the fuel nozzle.Several SEM images were collected at variouslocations of the probe surface;an average layer thick-ness of 9l m was measured for this flame location.Another interesting aspect of these nanotube layers is the strong van der Waals body attraction force existing between the tubes in the self-formed macro bundles.This aspect is unique since it greatly simplifies their harvesting.That is,micron size arrays of VACNTs can be easily removed from its roots.A catalytic substrate inserted at the axial flame po-sition of Z ¼10:5mm shows no apparent sign of arrays of VACNTs (Fig.8).But high-resolution SEM reveals that thinner and less uniform layers of VACNTs are still being grown as shown in Fig.9.The nanotube layer at this position is still aligned and defined but not as well as in the two previous positions.It can be speculated that at this flame position the conditions of CNT growth are far from optimal and the electric field control has only limited effect.A thickness of VACNT arrays of 3l m is characteristic for this flame position.Additionally,high and low resolution SEM analysis was performed on the surface of the support catalytic substrate at different locations in order to confirm the uniformity of the nanotube layer.In this particular experiment,the probe was inserted into the flame ataFig.6.Low resolution SEM image shows the catalyst substrate coated with a layer of carbon nanotubes (FPM),Z ¼9:5mm.Fig.7.Higher resolution SEM of a micro area in Fig.6.Fig.8.Low resolution SEM image on the catalyst substrate coated with a layer of carbon material (FPM),Z ¼10:5mm.W.Merchan-Merchan et al./Carbon 42(2004)599–608603position where the most thick layer of nanotubes were observed,Z¼8:5mm.After the exposure to theflame, the probe was placed on a stainless steel stud for microscope studies.Fig.10represents SEM images of arrays of orderly and high purity nanotubes scanned at top,center,and bottom of the probe surface,respectively.For all probe locations considered for microscopic analysis,arrays of nanotubes covered the probe with orientation perpen-dicular to the probe surface.By inspection of Fig.10it is evident that most of the formed material remains at-tached to the surface.Higher resolution SEM on the walls of this material showed that the bulk material is composed of arrays of nanotubes.The separated mate-rial always tends to remains packed like bundles of hay and always maintains its original length.In the upper right corner of Fig.10(b)a bundle of highly dense na-notubes is observed.This bundle has a cylindrical shape and a length of approximately40l m which coincide with the length of nanotubes in the attached VACNT layer.The material remains packed due to the strong van der Waals forces that exist between the tubes in the bundles.The same sample probe was then rotated180°for further SEM scanning and again,layers of nanotu-bes were present covering the catalytic substrate surface.High-resolution TEM was employed to study the inner morphology of the aligned nanotubes.For TEM studies,nanotubes formed on the catalytic support were carefully peeled offand transferred to acetone.After sonication,the drop of the suspension was placed ona Fig.9.Higher resolution SEM of a micro area in Fig.8.Fig.10.Low and high resolution(inserts)SEM images of scanned probe surface showing uniformity of VACNT coating layer.604W.Merchan-Merchan et al./Carbon42(2004)599–608copper-substrate/carbon-film of microscope specimen grid and dried.TEM images of nanotubes synthesized at Z ¼8:5mm are shown in Figs.11and 12.Very low resolution TEM imaging on the transferred material shows the presence of high-density closely packed nanotube bundles (Fig.11).It appears that even after sonication,closely packed bundles of nanotubes remain intact.Fig.11shows typical bundles of nanotubes ob-served by TEM in a sample grid,here the bundle pos-sesses a length and width of 31and 10l m,respectively.In a closer zoom on the nanotube bundle,no material other than tubular structures are present,Fig.12.From a number of micrographs the average diameter of the cylindrical multi-shells was measured,a monodisperse diameter distribution averaging 38nm was obtained.The application of high-resolution TEM imaging on these carbon nanotubes reveals a texture of well-aligned and highly graphitized concentric graphitic cylinders.The average interplanar distance of the concentric cylindrical graphene sheets was measured to be 0.34nm,as indicated in Fig.13.The layer planes appear to be perfectly parallel to the central tube axis.The experimental results show the strong influence of the electric field on alignment,size distribution,internal structure,and growth rate of carbon nanotubes.The mechanism of alignment is widely discussed in the lit-erature [26–28].As an example,employing a plasma discharge Merkulov and coworkers [27]successfully demonstrated,that the direction of the electric field lines determines the orientation of the carbon nanotubes and nanofibers;electric field alignment of single-walled na-notubes were considered by Zhang et al.[28].Consider the alignment mechanism of a single CNT growing on a metal catalytic support at the presence of the electric field E (Fig.14a).At the synthesis tempera-ture,both metallic and semiconducting CNT can be treated as good conductors.Consequently,the nanotube can be represented as a conductive cylinder of length L and radius R in contact with a conducting plane with a surface charge density r .For a cylinder of finite length,an electric field generated by the plate in the normal direction will induce a bound charge at the nanotube end to screen the external field over the nanotube vol-ume.The field-screening area of the single nanotube can be estimated as L 2.Accordingly,the total charge of the nanotube is q %r L 2¼e 0L 2E .The total electrostatic force acting on the nanotube is F %qE ¼e 0L 2E 2.The nanotube inclined at an angle h with respect to the normal direction will have axial F a and tangential F t components of the force F inducing respectively axial stress and alignment force.For a small deviation of the nanotube from the normal direction d ,the alignment force F t ¼F sin h ¼e 0LE 2d is proportional to the nano-tube length.The more rigorous solution of the electro-static problem [29]gives an expression for the total electrostatic force acting on the cylinder as F ¼pe 0E 2L 2=ðln ð2R =L ÞÀ3=2Þ,showing that the acting force is a weak function of R .The solution also shows the pres-ence of finite surface charges over the lower part of the nanotube with the charge density distribution close tolinear.Fig.11.Low resolution TEM image of material removed from the catalytic support.The material is produced using FPM at Z ¼8:5mm.Fig.12.TEM image of metal catalyzed carbon nanotubes from a micro area shown in Fig.11.Fig.13.High-resolution TEM image of the wall of a tubule produced in floating potential mode.W.Merchan-Merchan et al./Carbon 42(2004)599–608605For CNTs growing on a conductive substrate as a vertically aligned array with a tube-to-tube separation distance of D,the externalfield is reduced by mutual screening(Fig.14b).The depth of thefield penetration can be also estimated as D.Similar consideration for the charge induced on the nanotube tip provides estimation of the charge value q%e0D2E and the electrostatic force acting on the single tube F%qE¼e0D2E2.Conse-quently,the aligning force F t¼e0DE2d is linearly pro-portional to the nanotube separation D and the deviation from the vertical position d.The surface charges of the same polarity induced near the nanotube ends ensure their separation and alignment.These repulsive forces overcome van der Waals attraction forces.It is worth mentioning that the repulsive align-ment is quiet different from the alignment induced by van der Waals interactions.The latter mechanism leads to the formation of nanotube bundles which in turn reduces the thermal randomization of the CNT growth. However,a direction of the bundle growth remains arbitrary.Overall,the electricfields near the tips of growing nanotubes can be extremely high.Even applied poten-tials as small as few tens of millivolts can develop an electricfield exceeding1000V/cm at the characteristic nanotube diameter.The experimentally measuredfield enhancement factors are reaching800for multi-walled nanotubes[30]and3000for single-walled nanotubes [31].The enhancement of the electricfield at the tip of closed conducting nanotube was calculated by Maiti et al.[32];the resulting force estimated from the axial stress is in a good agreement with Taylor solution[29].The important aspect of the aligned growth is that the constant orientation of catalytic particles at the tips of the growing nanotubes is preserved by the electricfield. The non-symmetric catalytic particle is polarized in the electricfield,and the induced dipole moment tends to be aligned along the electricfield lines.The formation of helical and spiral nanotubes requires variation of the particle orientation.As a result the constant orientation of the catalytic particle stabilizes the linear CNT struc-tures.The high concentration of the electric charges near the nanotube end also induces axial stress between the growing nanotube body and conductive catalyst parti-cle.This can lead to the increase of the CNT growth rate.For clarity,we further consider a nanotube with a catalytic particle at the tip.The tip-growth proceeds via several important stages,including decomposition of flame hydrocarbons on catalytic surface,diffusion of carbon through and over metal particulate,formation of ordered CNT structure on the opposite side of the catalytic particle.The solution of electrostatic problem suggests strong concentration of charge and electricfield near the tip of the growing nanotube,namely on the catalytic particle attached to the nanotube end.The stress introduced between the particle and the CNT body may be estimated as r s¼F=p R2.While the force F has only weak dependence on R,the stress shows dra-matic increase with the reduction of contact area and can reach a critical value rÃscorresponding to the pos-sible particle separation.This can discriminate the growth of nanotubes with R<RÃ.The moderate value of stress,however,can increase the transformation of carbon to the CNT structure.As a result the concen-tration of free carbon on the CNT side will decrease leading to the faster carbon diffusion and the CNT growth will proceed at the increased rate.The sensitiv-ity of the stress to the nanotube diameter is a possi-ble explanation for the narrow diameter distribution which is characteristic for the synthesized VACNT arrays.Finally,the electricfield can influence the transport of charged particles inflames that include ions and charged soot particles.In this way,soot entrapment in the growing layer can be controlled by the electricfield.The effect of the electricfield control using various electricfield amplitudes and geometries is an interesting subject for the future studies.The selectedflame con-figuration allows ease of application of internal(probe generated)and external(generated by external elec-trodes)electricfields,as well as time-dependent elec-tromagneticfields.4.ConclusionsThe electricfield controlled synthesis of carbon nanomaterials on a Ni-based catalytic support posi-tioned at the fuel side of the opposedflow oxy-flame is studied experimentally.Theflame environment is formed by fuel and oxidizer with compositions of CH4+4%C2H2and50%O2+50%N2,respectively.Car-bon nanomaterials formed on the probe surface are comparatively analyzed for two characteristic opera-tional modes:a grounded probe mode and afloating probe mode.In a grounded mode a number of various carbon nanostructures are formed depending on the probe location inflame.Observed nanoforms include multi-walled carbon nanotubes(MWNTs),MWNT bundles, helically coiled tubular nanofibers,and ribbon-like coiled nanofibers with rectangular cross-section.The presence of various carbon nanoforms is attributed to the space variation offlame parameters,namelyflame temperature and concentration of chemical species.It is found that a presence of an electric potential (floating mode operation)provides an ability to control nanostructure morphology and synthesis rate.A thick layer(35–40l m)of vertically aligned carbon nanotubes (VACNTs)is found to be formed on the probe surface in thefloating potential mode.This layer is character-ized by high uniformity and narrow distribution of nanotube diameters.Electricfield control method demonstrates stabilization of the linear nanotube structure in a wideflame region.The growth rate re-mains dependent onflame location but the strong enhancement effect is observed with application of electricfield.AcknowledgementsThis work was supported by the National Science Foundation grant CTS-0304528.This work was par-tially supported by the Air Liquide Corp.under an Unrestricted Laboratory Development Grant.The au-thors extend special thank to Dr.Alan Nicholls,Mr. John Roth,Ms.Linda J.Juarez,and Ms.Kristina Jarosius from the UIC Research Resource Center for day-to-day assistance in SEM and TEM studies, encouragement and helpful discussions.The authors would also like to thank Mr.Attilio Milanese for his input and helpful discussions.References[1]Iijima S.Helical microtubules of graphitic carbon.Nature1991;354:56–8.[2]Kiang CH,Goddard WA,Beyers R,Salem JR,Bethune DS.Catalytic synthesis of single-layer carbon nanotubes with wide range of diameters.J Phys Chem1994;98:6612–8.[3]Shi Z,Lian Y,Zhou X,Gu Z,Zhang V,Iijima S,et al.Massproduction of single-wall carbon nanotubes by arc discharge method.Carbon1999;37:1449–53.[4]Guo T,Nikolaev P,Thess A,Colbert DT,Smalley RE.Catalyticgrowth of single-walled nanotubes by laser vaporization.Chem Phys Lett1995;243:49–54.[5]Andrews R,Jacques D,Rao AM,Derbyshire F,Qian D,Fan X,et al.Continuous production of aligned carbon nanotubes:a step closer to commercial realization.Chem Phys Lett1999;303:467–74.[6]Yuan L,Saito K,Pan C,Williams FA,Gordon AS.Nanotubesfrom methaneflames.Chem Phys Lett2001;340:237–41.[7]Yuan L,Saito K,Hu W,Chen Z.Ethyleneflame synthesis of well-aligned multi-walled carbon nanotubes.Chem Phys Lett2001;346: 23–5.[8]Vander Wal RL.Flame synthesis of substrate-supported metal-catalyzed carbon nanotubes.Chem Phys Lett2000;324:217–23.[9]Vander Wal RL,Lee JH.Ferrocene as a precursor reagent formetal-catalyzed carbon nanotubes:competing effbust Flame2002;130:27–36.[10]Vander Wal RL.Fe-catalyzed single-walled carbon nanotubesynthesis within aflame bust Flame2002;130: 37–47.[11]Vander Wal RL,Hall LJ,Berger GM.Optimization offlamesynthesis for carbon nanotubes using supported catalyst.J Phys Chem2002;B106:13122–32.[12]Height MJ,Howard JB,Tester JW.Synthesis of single-walledcarbon nanotubes in a premixed acetylene/oxygen/argonflame.Proceedings of the Third Joint Meeting of the U.S.Section of the Combustion Institute(2003).[13]Merchan-Merchan W,Saveliev A,Kennedy LA,Fridman A.Formation of carbon nanotubes in counter-flow,oxy-methane diffusionflames without catalyst.Chem Phys Lett2002;354:20–4.[14]Saveliev A,Merchan-Merchan W,Kennedy LA.Metal catalyzedsynthesis of carbon nanotubes in an opposedflow methane oxygenflbust Flame2003;135:27–33.[15]Kuzuya C,Kohda M,Hishikawa Y,Motojima S.Preparation ofcarbon micro-coils with the application of outer and inner electromagneticfields and bias voltage.Carbon2002;40:1991–2001.[16]Avigal Y,Kalish R.Growth of aligned carbon nanotubes bybiasing during growth.Appl Phys Lett2001;78(16):2291–3. [17]Srivastava AK,Srivastava ON.Curious aligned of carbon nano-tubes under applied electricfield.Carbon2001;39:201–6. [18]Lee KH,Cho JM,Sigmund W.Control of growth orientation forcarbon nanotubes.Appl Phys Lett2003;82(3):448–50.[19]Ant U,Yiming L,Hongjie D.Electric-field-aligned growth ofsingle-walled carbon nanotubes on surfaces.Appl Phys Lett 2002;81(18):3464–6.[20]Colbert DT,Smalley RE.Electric effects in nanotube growth.Carbon1995;33(7):921–4.[21]Srivastava A,Srivastava AK,Srivastava ON.Effect of externalelectricfield on the growth of nanotubules.Appl Phys Lett1998;72(14):1685–7.W.Merchan-Merchan et al./Carbon42(2004)599–608607。