Cardiovascular Risk
- 格式:pdf
- 大小:817.55 KB
- 文档页数:10

Ann.N.Y.Acad.Sci.ISSN0077-8923 ANNALS OF THE NEW YORK ACADEMY OF SCIENCESIssue:The Year in Diabetes and ObesityCardiovascular disease and glycemic control in type2 diabetes:now that the dust is settling from largeclinical trialsFrancesco Giorgino,Anna Leonardini,and Luigi LaviolaDepartment of Emergency and Organ Transplantation,Section of Internal Medicine,Endocrinology,Andrology and Metabolic Diseases,University of Bari Aldo Moro,Bari,ItalyAddress for correspondence:Francesco Giorgino,M.D.,Ph.D.,Department of Emergency and Organ Transplantation, Section of Internal Medicine,Endocrinology,Andrology and Metabolic Diseases,University of Bari Aldo Moro,Piazza Giulio Cesare,11,I-70124Bari,Italy.francesco.giorgino@uniba.itThe relationship between glucose control and cardiovascular outcomes in type2diabetes has been a matter of controversy over the years.Although epidemiological evidence exists in favor of an adverse role of poor glucose control on cardiovascular events,intervention trials have been less conclusive.The Action to Control Cardiovascular Risk in Diabetes(ACCORD)study,the Action in Diabetes and Vascular Disease(ADV ANCE)study,and the Veterans Affairs Diabetes Trial(V ADT)have shown no beneficial effect of intensive glucose control on primary cardiovascular endpoints in type2diabetes.However,subgroup analysis has provided evidence suggesting that the potential beneficial effect largely depends on patients’characteristics,including age,diabetes duration,previous glucose control,presence of cardiovascular disease,and risk of hypoglycemia.The benefit of strict glucose control on cardiovascular outcomes and mortality may be indeed hampered by the extent and frequency of hypoglycemic events and could be enhanced if glucose-lowering medications,capable of exerting favorable effects on the cardiovascular system,were used.This review examines the relationship between intensive glucose control and cardiovascular outcomes in type2diabetes,addressing the need for individualization of glucose targets and careful consideration of the benefit/risk profile of antidiabetes medications.Keywords:type2diabetes;HbA1c;macrovascular disease;blood pressure;lipids;hypoglycemia;glucagon-like peptide-1;ADV ANCE;ACCORD;V ADTIntroductionCardiovascular disease(CVD)is the major cause of death in patients with type2diabetes(T2D), as more than60%of T2D patients die of my-ocardial infarction(MI)or stroke,and an even greater proportion of patients have serious burden-some complications.1The impact of glucose low-ering on cardiovascular complications is a hotly debated issue.The United Kingdom Prospective Di-abetes Study(UKPDS)was thefirst clinical trial to provide key evidence of the importance of using in-tensive therapy for diabetes control in individuals with newly diagnosed T2D.However,although the insulin or sulphonylurea-based intensified glucose-control treatment was effective in reducing the risk of major microvascular endpoints,the effects on CVD risk were modest and did not reach statisti-cal significance.2Recent large clinical trials(often referred to as“megatrials”),the Action in Diabetes and Vascular Disease(ADV ANCE),3Action to Con-trol Cardiovascular Risk in Diabetes(ACCORD),4 and the Veterans Affairs Diabetes Trial(V ADT),5 reported no significant decrease in primary cardio-vascular endpoints with intensive glucose control. In the ADV ANCE study,11,140type2diabet-ics were randomly assigned to receive either stan-dard or intensive glucose control,defined as the use of gliclazide plus any other drug required to achieve a glycosylated hemoglobin(HbA1c)level of 6.5%or less(Table1).3After a median follow-up of 5.0years,the mean HbA1c was lower in thedoi:10.1111/nyas.12044Giorgino et al.Intensive glucose control and cardiovascular risk Table1.Age,diabetes duration,median follow-up,HbA1c values,and outcomes in the ACCORD and ADV ANCE studies and the V ADTDiabetes HbA1c(%):duration(year):Median intensive Primary All-cause Age intensive follow-up History versus endpoint HR mortality HR Study(year)versus standard(year)of CVD standard Primary endpoint(95%CI)(95%CI)ACCORD(n=10,251)62.2±6.810vs.10 3.435% 6.4vs.7.5Nonfatal MI,nonfatalstroke,or death fromCVD0.90(0.78–1.04) 1.22(1.01–1.46)ADV ANCE (n=11,140)66±6.08.0±6.4vs.7.9±6.35.032%6.53±0.91vs.7.30±1.26Death fromcardiovascular causes,nonfatal MI,ornonfatal stroke0.94(0.84–1.06)0.93(0.83–1.06)V ADT(n=1,791)60±9.011.5±8vs.11.5±75.640%6.9vs.8.4MI,stroke,death fromCVD,CHF,surgery forvascular disease,inoperable CAD,oramputation forischemic gangrene0.88(0.74–1.05) 1.07(0.81–1.42)intensive control group(6.5%vs.7.3%),with a reduction in the incidence of combined major macrovascular and microvascular events primarily because of a reduction in the incidence of nephropa-thy.There were no significant effects of the inten-sive glucose control on major macrovascular events, death from cardiovascular causes,or death from any cause.Similarly,in the V ADT,1,791suboptimally controlled type2diabetics,40%with established CVD,were randomized to receive either intensive glucose control,targeting an absolute reduction of 1.5%in HbA1c levels,or standard glucose control (Table1).5After a median follow-up of5.6years, HbA1c was lower in the intensive-therapy group (6.9%vs.8.4%).Nevertheless,there was no sig-nificant difference between the two groups in the incidence of major cardiovascular events,or in the rate of death from any cause.ACCORD was another study designed to determine whether intensive glu-cose control would reduce the rate of cardiovas-cular events(Table1).4In this study,10,251type 2diabetics with median baseline HbA1c of8.1% were randomly assigned to receive either intensive therapy targeting an HbA1c level within the normal range,that is,below6.0%,or standard therapy tar-geting HbA1c between7.0%and7.9%.The primary outcome was a composite of nonfatal MI,nonfatal stroke,or death from cardiovascular causes.Even though the rate of nonfatal MI was significantly lower in the intensive therapy arm,thefinding of higher all-cause and cardiovascular cause mortal-ity in this group led to discontinuation of the in-tensive therapy after a mean follow-up of3.5years (Fig.1).Notably,hypoglycemia requiring assistance and weight gain of more than10kg were more fre-quent in the intensive therapy group.The results of the ACCORD study raised concern about not only the effectiveness but also the safety of inten-sive glycemic control in type2diabetics.Prespec-ified subgroup analysis of the participants in this trial suggested that patients in the intensive group without history of cardiovascular event before ran-domization or whose baseline HbA1c level was8.0% or less may have had fewer fatal or nonfatal car-diovascular events than did patients in the standard therapy group.Several recent meta-analyses of randomized con-trolled trials have also investigated the effects of intensive glucose lowering on all-cause mortal-ity,cardiovascular death,and vascular events in T2D.6–10In the largest and most recent meta-analysis by Boussageon et al.,13studies were in-cluded.6Of the34,533patients evaluated,18,315 received intensive glucose-lowering treatment and 16,218standard treatment.Intensive treatment did not significantly affect all-cause mortality or cardiovascular death.The results of this meta-analysis showed limited benefit of intensive glucose-lowering therapy on all-cause mortality and deaths from cardiovascular causes,and a10%reduction in the risk of microalbuminuria.6Results from other meta-analyses have also shown no effects ofIntensive glucose control and cardiovascular risk Giorgino etal.Figure 1.Effects of intensive glucose control on all-cause and cardiovascular mortality and myocardial infarction in the ACCORD study.CV,cardiovascular.∗P<0.05.Adapted from Ref.4.intensive glucose control on all-cause or cardiovas-cular mortality,while indicating a modest15–17% reduction in the incidence of nonfatal MI in these cohorts.7–10Several potential factors could have contributed to limit the potential benefit of intensive glucose-lowering therapies on CVD prevention in T2D in-dividuals in studies such as the ACCORD and AD-V ANCE and the V ADT:(1)Concomitant targeting of other potentiallymore potent cardiovascular risk factors,suchas blood pressure and lipids,might havedampened the favorable effects of controllinghyperglycemia.(2)Intensive control of hyperglycemia could havebeen directed to patients unable to exhibit theexpected benefit due to their specific clinicalcharacteristics(“wrong”patients).(3)Limited benefit might have derived from usingglucose-lowering drugs with no favorable im-pact on the global cardiovascular risk profile.(4)Glucose-lowering drugs might have producedadverse effects on the cardiovascular systemby inducing weight gain and hypoglycemicevents,resulting in somewhat increased riskfor CVD and mortality(“imperfect”drugs).(5)Excess mortality might have potentially re-sulted from using too many drugs and/or toocomplex drug regimens,leading to undesir-able drug–drug interactions with a potentiallyharmful impact on patients’health.These diverse factors and their potential role in the relationship between intensive glucose control and CVD/mortality are outlined in Figure2,and will be discussed individually below.Limited benefit due to other therapies Although several studies have focused on intensive glycemic control to decrease the risks of macrovas-cular and microvascular diseases in T2D,glucose control is only one of the factors to be considered. Comprehensive risk factor management,including blood pressure control,lipid management,weight reduction in overweight or obese individuals,and smoking cessation,are also needed.The results of the ACCORD and ADV ANCE studies and the V ADT should be interpreted in the context of comprehen-sive care of patients with diabetes.Interventions for simultaneous optimal control of comorbidities of-ten present in type2diabetics,such as hyperten-sion and hyperlipidemia,have been shown to be a more effective strategy in reducing cardiovascular risk than targeting only blood glucose levels per se.11 Evidence for an aggressive approach to lipid and blood pressure control was supported by the re-sults from the Steno-2study.11,12In Steno-2,inves-tigators used intensified multifactorial intervention with improved glycemia,renin–angiotensin system blockers,aspirin,and lipid-lowering agents and evaluated whether this approach would have an ef-fect on the rates of death from cardiovascular causes and from any cause.11,12The primary endpoint at 13.3years of follow-up was the time to death from any cause.Intensive therapy was also associated with a significantly lower risk of death from cardiovascu-lar causes and of cardiovascular events.The Steno-2study did demonstrate a difference in levels of glycemia achieved when compared with the AC-CORD study:4,11HbA1c was a mean of8.4%at study entry and7.9%at end of study intervention for the intensively treated group,whereas it was8.8%at baseline and9.0%at study end for conventional treatment.In addition,only a limited proportion of subjects in the intensively treated group reached an HbA1c level of less than6.5%(i.e.,∼15%),and this proportion was not statistically different than in the conventionally treated group,indicating poor success in achieving the prespecified glucose target and somewhat reducing the relevance of the spe-cific intervention on the hyperglycemia component for CVD and microvascular disease prevention.The observational study has continued,and the differ-ences observed in glycemia between intensive andGiorgino et al.Intensive glucose control and cardiovascularriskFigure2.Relationship between intensive glucose control and cardiovascular outcomes and mortality in the ACCORD study and other megatrials.The potential mechanisms affecting this relationship and limiting the clinical benefit are outlined in the box on the left.CV,cardiovascular;CVD,cardiovascular disease.conventional treatments are much less than at endof intervention.Nevertheless,over the long-termperiod of follow-up,intensive intervention with avaried drug regimen and lifestyle modification hadsustained beneficial effects with respect to vascularcomplications and rates of death from any cause andfrom cardiovascular causes.12Blood pressureThe ACCORD study also had an embedded bloodpressure trial that examined whether blood pressurelowering to systolic blood pressure(SBP)less than120mm Hg provided greater cardiovascular protec-tion than a SBP of130–140mm Hg in T2D patientsat high risk for CVD.13A total of4,733participantswere randomly assigned to intensive therapy(SBP <120mm Hg)or standard therapy(SBP<140mm Hg),with the mean follow-up being4.7years.Theblood pressure levels achieved in the intensive andstandard groups were119/64mm Hg and133/70mm Hg,respectively;this difference was attainedwith an average of3.4medications per participantin the intensive group and2.1in the standard ther-apy group.The intensive antihypertensive therapyin the ACCORD blood pressure trial did not consid-erably reduce the primary cardiovascular outcomeor the rate of death from any cause.However,theintensive arm of blood pressure control reduced therate of total stroke and nonfatal stroke,with the estimated number needed to treat with intensive blood pressure therapy to prevent one stroke over five years being89.There were indicators of possi-ble harm associated with intensive blood pressure lowering(SBP<120mm Hg),including a rate of serious adverse events in the intensive arm.It should be noted that of the subjects investigated in the ACCORD glucose control trial∼85%were on antihypertensive medications,and their blood pres-sure levels were126.4/66.9and127.4/67.7in the intensive and standard groups,respectively,a differ-ence that was statistically significant(P<0.001) (Table2).4Thus,the effects of intensive glucose lowering on the CVD and other outcomes were ex-amined in a context in which blood pressure was being actively targeted,with possible differences between the intensively and conventionally treated cohorts.The ADV ANCE study also included a blood pres-sure intervention trial.In this study,treatment with an angiotensin converting enzyme(ACE)inhibitor and a thiazide-type diuretic reduced the rate of death but not the composite macrovascular out-come.However,this trial had no specified targets for the randomized comparison,and the mean SBP in the intensive group(135mm Hg)was not as low as the mean SBP in the ACCORD standard-therapy group.14However,a post hoc analysis of blood pres-sure control in6,400patients with diabetes andIntensive glucose control and cardiovascular risk Giorgino et al. Table2.Blood pressure and lipid levels and use of statin,antihypertensive medications,and aspirin in the ACCORD and ADV ANCE studies and the V ADT participants at study end(adapted from Refs.3–5)ACCORD(n=10,251)aADV ANCE(n=11,140)b V ADT(n=1,791)cStandard Intensive Standard Intensive Standard Intensive Blood pressure(mm Hg)Systolic128±16126±17137±18135±17125±15127±16 Diastolic68±1067±1074±1073±1069±1068±10 Cholesterol(mg/dL)LDL87±3387±33103±41102±3880±3180±33 HDL49±13(♂)49±13(♂)48±1448±1441±1240±1140±11(♀)40±10(♀)Total164±42163±42153±40150±40 Triglycerides(mg/dL)166±114160±125161±102151±94159±104151±173 On statin(%)888848468385 On antihypertensivemedications(%)858389887576 On aspirin(%)767655578586a Intensive(target HbA1c<6%)vs.standard(HbA1c7–7.9%).b Intensive(target HbA1c<6.5%)vs.standard(HbA1c>6.5%).c Intensive(target HbA1c4.8–6.0%)vs.standard(HbA1c8–9.0%).Abbreviations:HDL,high-density lipoprotein;LDL,low-density lipoprotein;♂,men;♀,women.coronary artery disease enrolled in the International Verapamil/Trandolapril Study(INVEST)demon-strated that tight control(<130mm Hg)was not associated with improved cardiovascular outcomes compared with usual care(130–140mm Hg).15In the V ADT,blood pressure,lipids,diet,and lifestyle were treated identically in both arms.By improving blood pressure control in an identical manner in both glucose arms,the V ADT excluded the effect of blood pressure differences on cardiovas-cular events between treatment arms and reduced the overall risk of macrovascular complications dur-ing the trial.5Participants in the V ADT(n=1,791) with hypertension(72.1%of total)received stepped treatment to maintain blood pressure below the tar-get of130/80mm Hg in standard and intensive glycemic treatment groups.Blood pressure levels of all subjects at baseline and on-study were analyzed to detect associations with cardiovascular risk.The primary outcome was the time from randomiza-tion to thefirst occurrence of MI,stroke,conges-tive heart failure,surgery for vascular disease,in-operable coronary disease,amputation for ischemic gangrene,or cardiovascular death.From data analy-sis,increased risk of cardiovascular events with SBP ≥140mm Hg emphasizes the need for treatment of systolic hypertension.Also,this study for the first time demonstrated that diastolic blood pressure (DBP)<70mm Hg in T2D patients was indepen-dently associated with elevated cardiovascular risk, even when SBP was on target.16Lipid profileIt has been widely demonstrated that intensive targeting of low-density lipoprotein(LDL)choles-terol contributes to CVD prevention in T2D.As a consequence,most guidelines suggest a target LDL-cholesterol level below100mg/dL as the primary goal in T2D individuals,with the option of achieving an LDL-cholesterol level below70mg/dL in those with overt CVD.Regarding the overall lipid-lowering approach in the ACCORD glucose control study,it should be observed that mean LDL cholesterol was below90mg/dL in both the intensive and standard glucose control arms,and that88%of subjects were on statin therapy.4Thus, the results from this study do not clarify whether lipid control and glycemic control,respectively,areGiorgino et al.Intensive glucose control and cardiovascularriskFigure3.All-cause mortality in intensive versus standard glycemia groups according to use of antihypertensive medications, statins,and aspirin in the ACCORD and ADV ANCE studies.∗P=0.0305for subjects on aspirin versus subjects not on aspirin. Adapted from Refs.3and18.related or synergistic,since the large majority of enrolled subjects were already receiving a lipid control regimen.Data from the Steno-2trial on combined control of glucose,lipids,and blood pressure levels demonstrated significant short-and long-term benefits from this multifactorial approach.11In the study,the effect seemed to be cumulative rather than synergistic.Recent results from the ACCORD-LIPID study indicate that intensive lipid control(i.e.,addition of afibrate to statin therapy)does not reduce car-diovascular events.17Specifically,the lipid-lowering arm of ACCORD failed to demonstrate any ben-efit of add-on therapy with fenofibrate to LDL-lowering treatment with HMG-CoA reductase in-hibitors(statins)on vascular outcomes in patients with diabetes.However,data from earlier studies and from a subgroup analysis of ACCORD indi-cate a probable benefit of adding treatment with fibric acid derivatives to individuals with persis-tently elevated triglyceride levels and low high-density lipoprotein(HDL)cholesterol despite statin therapy.17In the ACCORD and ADV ANCE studies and the V ADT,a large proportion of subjects,ranging from55%to85%,were also treated with aspirin (Table2).Thus,results from ADV ANCE,ACCORD, and V ADT suggest that a large proportion of par-ticipants in these trials,which were being treated intensively or less intensively for glucose targets, received extensive antihypertensive,lipid-lowering, and antiplatelet medications.Median levels of SBP, SDP,and LDL cholesterol in these cohorts were also indicative of a significant proportion of them be-ing adequately controlled for blood pressure and lipid targets(Table2).Therefore,the possibility ex-ists that active interventions for simultaneous con-trol of hypertension and hyperlipidemia and use of aspirin may have affected the impact of intensive glucose control on cardiovascular outcomes in the megatrials.Subgroups analyses,however,do not ap-parently support this conclusion(Fig.3).Whether patients were on antihypertensive or lipid-lowering medications was not associated with a different out-come of the intensive glucose control on mortality in ACCORD and ADV ANCE patients,even thoughIntensive glucose control and cardiovascular risk Giorgino et al.the different groups were largely unbalanced in size. Only aspirin use in the ACCORD study seemed to modulate the effects of intensive versus standard glucose control on mortality.18The“wrong patient”concept and the need for individualization of glucose targets The ADV ANCE and ACCORD studies and the V ADT provide conflicting evidence of mortality risk with intensive glycemic control.These trials showed an approximate15%reduction in nonfatal MI with no benefit or harm in all-cause or cardiovascular mortality.Potential explanations for the lack of im-pact of intensive glycemic control on CVD can be found in the patients’characteristics.Indeed,these studies were of shorter duration and enrolled gener-ally older patients than previous studies,including the DCCT and UKPDS in which the intensive con-trol had shown better outcomes.In addition,mean diabetes duration was longer and a greater portion of patients had established CV disease in the mega-trials(approximately32–40%)than in earlier trials (Table1).It is also possible that the follow-up of these studies was too short to detect a clinical ben-efit.Consistent with this hypothesis,in the UKPDS no macrovascular benefit was noted in the inten-sive control arm in thefirst10years of follow-up. Nevertheless,posttrial monitoring for an additional 10years(UKPDS80)revealed a15%risk reduction in MI and13%reduction in all-cause mortality in the intensive treatment group.19A possible explanation is that the wrong patients were investigated in the megatrials(Fig.2).Indeed, the population of the ACCORD study may not rep-resent the average patient with T2D in clinical prac-tice.Participants in this trial had T2D on average for10years at the time of enrollment,had higher HbA1c levels than most type2diabetic patients in the United States and most Western countries to-day(average of8.2%at baseline),and had known heart disease or at least two risk factors in addi-tion to diabetes,such as high blood pressure,high cholesterol levels,obesity,and smoking.4In the UKPDS,the benefits of intensive glycemic control on CVD were observed only after a long duration of intervention in newly diagnosed younger patients.2 In older patients with T2D with longer disease dura-tion,atherosclerotic disease may already have been established and thus intensive glucose control may have had little benefit.Conversely,patients with shorter disease duration,lower HbA1c,and/or lack of established CVD might have benefited signifi-cantly from more intensive glycemic control.20,21 Relevant to this concept,the V ADT showed that ad-vanced CVD,as demonstrated by computed tomog-raphy(CT)-detectable coronary artery calcium,was associated with negative outcomes.In a substudy co-hort of301T2D participants in V ADT,the ability of intensive glucose therapy compared with standard therapy to reduce cardiovascular events was exam-ined based on the extent of coronary atherosclerosis as measured by a CT-detectable coronary artery cal-cium score(CAC).The data showed that there was a progressive diminution of the benefit of intensive glucose control with increasing CAC.In patients with CAC≤100,1of52individuals experienced an event(HR for intensive therapy=0.08;range, 0.008–0.770;P=0.03),whereas11of62patients with a CAC>100had an event(HR=0.74;range, 0.46–1.20;P=0.21).Thus,this subgroup analy-sis indicates that intensive glycemic therapy may be most effective in those with less extensive coronary atherosclerosis.22Why does intensive treatment of hyperglycemia appear to be ineffective in reducing cardiovascular events in T2D with advanced atherosclerosis?Two potential mechanisms may be involved.First,once the atherosclerotic plaque has developed,modified lipoproteins,activated vascular cells,and altered im-mune cell signaling may generate a self-propagating process that maintains atherogenesis,even in the face of improved glucose control.Second,advanced glycosylation end-product formation,which may be involved in CVD,is not readily reversible and may require more than three tofive years of in-tensive glucose control to be reverted.Thus,the presence of multiple cardiovascular risk factors or established CVD may have reduced the benefits of intensive glycemic control in the high-risk cohort of ACCORD,ADV ANCE,and V ADT compared with the low-risk population of the UKPDS cohort,of whom only a minority had prior CVD.23The pres-ence of long-standing disease and prolonged prior poor glycemic control may be additional factors ac-celerating the progression of atherosclerotic lesions in T2D.The goal of individualizing HbA1c targets has gained more attraction after these recent clinical trials in older patients with established T2D failed to show a benefit from intensive glucose-loweringGiorgino et al.Intensive glucose control and cardiovascular riskTable3.Potential criteria for individualization of glucose targets in type2diabetes2–5,25,29HbA1c HbA1c Criterion<6.5–7.0%7.0–8.0% Age(years)<55>60 Diabetes duration(years)<10>10Life expectancy(years)>5<5 Possibility to performIGC for>5yearsYes No Usual HbA1c level(%)<8.0>8.0 CVD No Yes Prone to hypoglycemia No No Reduction of HbA1clevel upon IGCYes NoAbbreviations:CVD,cardiovascular disease;IGC,in-tensive glucose control.therapies on CVD outcomes.Recommendations suggest that the goals should be individualized,such that(1)certain populations(children,pregnant women,and elderly patients)require special con-siderations and(2)more stringent glycemic goals (i.e.,a normal HbA1c<6.0%)may further re-duce complications at the cost of increased risk of hypoglycemia.24For the latter,the recommen-dations also suggest that less intensive glycemic goals may be indicated in patients with severe or frequent hypoglycemia.With regard to the less intensive glycemic goals,perhaps consideration should be given to the high-risk patient with mul-tiple risk factors and CVD,as evaluated in the ACCORD study.4Thus,aiming for a HbA1c of 7.0–8.0%may be a reasonable goal in patients with very long duration of diabetes,history of se-vere hypoglycemia,advanced atherosclerosis,sig-nificant comorbidities,and advanced age/frailty (Table3),even though with what priority each one of these criteria should be considered is not clear at present(current recommendations from scientific societies also do not provide this spe-cific information).In younger patients without documented macrovascular disease or the above-mentioned conditions,the goal of attaining an HbA1c<6.5–7.0%may provide long-lasting bene-fits.In patients with limited life expectancy,more liberal HbA1c values may be pursued.In deter-mining the HbA1c target for CVD prevention,one should consider that at least three tofive years are usually required before possible differences in the incidence of nonfatal MI in T2D are observed.2 Thus,a clinical setting that allows tight glucose control to be implemented for this period of time should be available.Finally,an excess mortality was observed in the ACCORD study in those T2D in-dividuals who showed an unexpected increase in HbA1c levels upon institution of intensive glucose control.25Accordingly,patients exhibiting the pat-tern of worsening glycemic control when exposed to intensive treatment should be set at higher glucose targets(Table3).The above guidelines are apparently incorpo-rated into the updated version of the American Diabetes Association and the European Associa-tion for the Study of Diabetes recommendations on the management of hyperglycemia in nonpreg-nant adults with T2D.The new recommendations are less prescriptive and more patient centered.In-dividualized treatment is explicitly defined as the cornerstone of success.Treatment strategies should be tailored to individual patient needs,preferences, and tolerances and based on differences in age and disease course.Other factors affecting individual-ized treatment plans include specific symptoms,co-morbid conditions,weight,race/ethnicity,sex,and lifestyle.26Current“imperfect”glucose-lowering drugsThe inability of ACCORD,ADV ANCE,and V ADT to demonstrate significant reductions in CVD out-comes with intensive glycemic control also suggests that current pharmacological tools for treating hy-perglycemia in patients with more advanced T2D may have counterbalancing consequences for the cardiovascular system.The available agents used to treat diabetes have not been conclusively shown to reduce macrovascular disease and,in some in-stances,their chronic use may promote negative cardiovascular effects in diabetic subjects despite improvement of hyperglycemia.Importantly,these adverse cardiovascular side effects appear in several instances to be directly due to the mode of drug ac-tion.Selection of a treatment regimen for patients with T2D includes evaluation of the effects of med-ications on overall cardiovascular risk.27 Sulfonylureas have the benefit of acting rapidly to lower glucose levels but,unfortunately,on a。


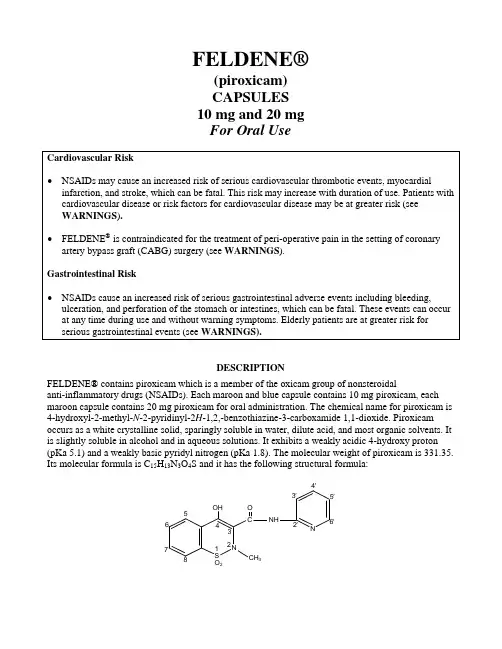
4′3′5′ C NH 6′ 2′N 3O 2 FELDENE®(piroxicam)CAPSULES 10 mg and 20 mgFor Oral Use Cardiovascular Risk• NSAIDs may cause an increased risk of serious cardiovascular thrombotic events, myocardialinfarction, and stroke, which can be fatal. This risk may increase with duration of use. Patients withcardiovascular disease or risk factors for cardiovascular disease may be at greater risk (seeWARNINGS ).• FELDENE ® is contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS ).Gastrointestinal Risk• NSAIDs cause an increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events (see WARNINGS).DESCRIPTION FELDENE® contains piroxicam which is a member of the oxicam group of nonsteroidalanti-inflammatory drugs (NSAIDs). Each maroon and blue capsule contains 10 mg piroxicam, each maroon capsule contains 20 mg piroxicam for oral administration. The chemical name for piroxicam is 4-hydroxyl-2-methyl-N -2-pyridinyl-2H -1,2,-benzothiazine-3-carboxamide 1,1-dioxide. Piroxicam occurs as a white crystalline solid, sparingly soluble in water, dilute acid, and most organic solvents. It is slightly soluble in alcohol and in aqueous solutions. It exhibits a weakly acidic 4-hydroxy proton (pKa 5.1) and a weakly basic pyridyl nitrogen (pKa 1.8). The molecular weight of piroxicam is 331.35. Its molecular formula is C 15H 13N 3O 4S and it has the following structural formula:The inactive ingredients in FELDENE capsules include: Blue 1, Red 3, lactose, magnesium stearate, sodium lauryl sulfate, starch.CLINICAL PHARMACOLOGYPharmacodynamicsFELDENE is a nonsteroidal anti-inflammatory drug (NSAID) that exhibits anti-inflammatory, analgesic, and antipyretic activities in animal models. The mechanism of action of FELDENE, like that of other NSAIDs, is not completely understood but may be related to prostaglandin synthetase inhibition.PharmacokineticsAbsorption: FELDENE is well absorbed following oral administration. Drug plasma concentrations are proportional for 10 and 20 mg doses and generally peak within three to five hours after medication. The prolonged half-life (50 hours) results in the maintenance of relatively stable plasma concentrations throughout the day on once daily doses and to significant accumulation upon multiple dosing. A single 20 mg dose generally produces peak piroxicam plasma levels of 1.5 to 2 mcg/mL, while maximum drug plasma concentrations, after repeated daily ingestion of 20 mg FELDENE, usually stabilize at 3– 8 mcg/mL. Most patients approximate steady state plasma levels within 7–12 days. Higher levels, which approximate steady state at two to three weeks, have been observed in patients in whom longer plasma half-lives of piroxicam occurred.With food there is a slight delay in the rate but not the extent of absorption following oral administration. The concomitant administration of antacids (aluminum hydroxide or aluminum hydroxide with magnesium hydroxide) have been shown to have no effect on the plasma levels of orally administered piroxicam.Distribution: The apparent volume of distribution of piroxicam is approximately 0.14 L/kg.Ninety-nine percent of plasma piroxicam is bound to plasma proteins. Piroxicam is excreted into human milk. The presence in breast milk has been determined during initial and long-term conditions (52 days). Piroxicam appeared in breast milk at about 1% to 3% of the maternal concentration. No accumulation of piroxicam occurred in milk relative to that in plasma during treatment. Metabolism: Metabolism of piroxicam occurs by hydroxylation at the 5 position of the pyridyl side chain and conjugation of this product; by cyclodehydration; and by a sequence of reactions involving hydrolysis of the amide linkage, decarboxylation, ring contraction, and N-demethylation. In vitro studies indicate cytochrome P4502C9 (CYP2C9) as the main enzyme involved in the formation to the 5′-hydroxy-piroxicam, the major metabolite (see Pharmacogenetics, and Special Populations, Poor Metabolizers of CYP2C9 Substrates). The biotransformation products of piroxicam metabolism are reported to not have any anti-inflammatory activity.Higher systemic exposure of piroxicam has been noted in subjects with CYP2C9 polymorphisms compared to normal metabolizer type subjects (see Pharmacogenetics, and Special Populations, Poor Metabolizers of CYP2C9 Substrates).Excretion: FELDENE and its biotransformation products are excreted in urine and feces, with about twice as much appearing in the urine as in the feces. Approximately 5% of a FELDENE dose is excreted unchanged. The plasma half-life (T½) for piroxicam is approximately 50 hours. Pharmacogenetics: CYP2C9 activity is reduced in individuals with genetic polymorphisms, such as the CYP2C9*2 and CYP2C9*3 polymorphisms. Limited data from one published report that included nine subjects each with heterozygous CYP2C9*1/*2 and CYP2C9*1/*3 genotypes and one subject with the homozygous CYP2C9*3/*3 genotype showed piroxicam systemic levels that were 1.7-, 1.7- and 5.3-fold, respectively, higher compared to the 17 subjects with CYP2C9*1/*1 or normal metabolizer genotype. The pharmacokinetics of piroxicam have not been evaluated in subjects with other CYP2C9 polymorphisms, such as *5, *6, *9 and *11. It is estimated that the frequency of the homozygous *3/*3 genotype is 0.3% to 1.0% in various ethnic groups.Special PopulationsPediatric: FELDENE has not been investigated in pediatric patients.Race: Pharmacokinetic differences due to race have not been identified.Hepatic Insufficiency: The effects of hepatic disease on FELDENE pharmacokinetics have not been established. However, a substantial portion of FELDENE elimination occurs by hepatic metabolism. Consequently, patients with hepatic disease may require reduced doses of FELDENE as compared to patients with normal hepatic function.Poor Metabolizers of CYP2C9 Substrates: Patients who are known or suspected to be poor CYP2C9 metabolizers based on genotype or previous history/experience with other CYP2C9 substrates (such as warfarin and phenytoin) should be administered piroxicam with caution as they may have abnormally high plasma levels due to reduced metabolic clearance.Renal Insufficiency: FELDENE pharmacokinetics have been investigated in patients with renal insufficiency. Studies indicate patients with mild to moderate renal impairment may not require dosing adjustments. However, the pharmacokinetic properties of FELDENE in patients with severe renal insufficiency or those receiving hemodialysis are not known.Other InformationIn controlled clinical trials, the effectiveness of FELDENE has been established for both acute exacerbations and long-term management of rheumatoid arthritis and osteoarthritis.The therapeutic effects of FELDENE are evident early in the treatment of both diseases with a progressive increase in response over several (8–12) weeks. Efficacy is seen in terms of pain relief and, when present, subsidence of inflammation.Doses of 20 mg/day FELDENE display a therapeutic effect comparable to therapeutic doses of aspirin, with a lower incidence of minor gastrointestinal effects and tinnitus.FELDENE has been administered concomitantly with fixed doses of gold and corticosteroids. TheINDICATIONS AND USAGECarefully consider the potential benefits and risks of FELDENE and other treatment options before deciding to use FELDENE. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS).FELDENE is indicated:•For relief of the signs and symptoms of osteoarthritis.•For relief of the signs and symptoms of rheumatoid arthritis.CONTRAINDICATIONSFELDENE is contraindicated in patients with known hypersensitivity to piroxicam.FELDENE should not be given to patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs. Severe, rarely fatal, anaphylactic-like reactions to NSAIDs have been reported in such patients (see WARNINGS: Anaphylactoid Reactions and PRECAUTIONS: Preexisting Asthma).FELDENE is contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS).WARNINGSCARDIOVASCULAR EFFECTSCardiovascular Thrombotic EventsClinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious cardiovascular (CV) thrombotic events, myocardial infarction, and stroke, which can be fatal. All NSAIDs, both COX-2 selective and nonselective, may have a similar risk. Patients with known CV disease or risk factors for CV disease may be at greater risk. To minimize the potential risk for an adverse CV event in patients treated with an NSAID, the lowest effective dose should be used for the shortest duration possible. Physicians and patients should remain alert for the development of such events, even in the absence of previous CV symptoms. Patients should be informed about the signs and/or symptoms of serious CV events and the steps to take if they occur.There is no consistent evidence that concurrent use of aspirin mitigates the increased risk of serious CV thrombotic events associated with NSAID use. The concurrent use of aspirin and an NSAID does increase the risk of serious GI events (see WARNINGS, Gastrointestinal Effects - Risk of Ulceration, Bleeding, and Perforation).Two large, controlled clinical trials of a COX-2 selective NSAID for the treatment of pain in the first 10–14 days following CABG surgery found an increased incidence of myocardial infarction and stroke (see CONTRAINDICATIONS).Hypertensionthiazides or loop diuretics may have impaired response to these therapies when taking NSAIDs. NSAIDs, including FELDENE, should be used with caution in patients with hypertension. Blood pressure (BP) should be monitored closely during the initiation of NSAID treatment and throughout the course of therapy.Congestive Heart Failure and EdemaFluid retention and edema have been observed in some patients taking NSAIDs. FELDENE should be used with caution in patients with fluid retention or heart failure.Gastrointestinal Effects - Risk of Ulceration, Bleeding, and PerforationNSAIDs, including FELDENE, can cause serious gastrointestinal (GI) adverse events including inflammation, bleeding, ulceration, and perforation of the stomach, small intestine, or large intestine, which can be fatal. These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. Only one in five patients who develop a serious upper GI adverse event on NSAID therapy is symptomatic. Upper GI ulcers, gross bleeding, or perforation caused by NSAIDs occur in approximately 1% of patients treated for 3–6 months, and in about 2–4% of patients treated for one year. These trends continue with longer duration of use, increasing the likelihood of developing a serious GI event at some time during the course of therapy. However, even short-term therapy is not without risk.NSAIDs should be prescribed with extreme caution in those with a prior history of ulcer disease or gastrointestinal bleeding. Patients with a prior history of peptic ulcer disease and/or gastrointestinal bleeding who use NSAIDs have a greater than 10-fold increased risk for developing a GI bleed compared to patients with neither of these risk factors. Other factors that increase the risk of GI bleeding in patients treated with NSAIDs include concomitant use of oral corticosteroids or anticoagulants, longer duration of NSAID therapy, smoking, use of alcohol, older age, and poor general health status. Most spontaneous reports of fatal GI events are in elderly or debilitated patients and, therefore, special care should be taken in treating this population.To minimize the potential risk for an adverse GI event in patients treated with an NSAID, the lowest effective dose should be used for the shortest possible duration. Patients and physicians should remain alert for signs and symptoms of GI ulcerations and bleeding during NSAID therapy and promptly initiate additional evaluation and treatment if a serious GI event is suspected. This should include discontinuation of the NSAID until a serious GI adverse event is ruled out. For high-risk patients, alternate therapies that do not involve NSAIDs should be considered.Renal EffectsLong-term administration of NSAIDs has resulted in renal papillary necrosis and other renal injury. Renal toxicity has also been seen in patients in whom renal prostaglandins have a compensatory role in the maintenance of renal perfusion. In these patients, administration of a nonsteroidal antiinflammatory drug may cause a dose-dependent reduction in prostaglandin formation and, secondarily, in renal blood flow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, heart failure, liver dysfunction, those taking diuretics and ACE-inhibitors, and the elderly. Discontinuation of NSAID therapy is usually followed by recovery to the pretreatment state.Advanced Renal DiseaseNo information is available from controlled clinical studies regarding the use of FELDENE in patientspatients with advanced renal disease. If FELDENE therapy must be initiated, close monitoring of the patient’s renal function is advisable.Anaphylactoid ReactionsAs with other NSAIDs, anaphylactoid reactions may occur in patients without known prior exposure to FELDENE. FELDENE should not be given to patients with the aspirin triad. This symptom complex typically occurs in asthmatic patients who experience rhinitis with or without nasal polyps, or who exhibit severe, potentially fatal bronchospasm after taking aspirin or other NSAIDs (see CONTRAINDICATIONS and PRECAUTIONS: Preexisting Asthma). Emergency help should be sought in cases where an anaphylactoid reaction occurs.Skin ReactionsNSAIDs, including FELDENE, can cause serious skin adverse events such as exfoliative dermatitis, Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. These serious events may occur without warning. Patients should be informed about the signs and symptoms of serious skin manifestations and use of the drug should be discontinued at the first appearance of skin rash or any other sign of hypersensitivity.Other Hypersensitivity ReactionsA combination of dermatological and/or allergic signs and symptoms suggestive of serum sickness have occasionally occurred in conjunction with the use of FELDENE. These include arthralgias, pruritus, fever, fatigue, and rash including vesiculobullous reactions and exfoliative dermatitis. PregnancyIn late pregnancy, as with other NSAIDs, FELDENE should be avoided because it may cause premature closure of the ductus arteriosus.PRECAUTIONSGeneralFELDENE cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroids may lead to disease exacerbation. Patients on prolonged corticosteroid therapy should have their therapy tapered slowly if a decision is made to discontinue corticosteroids.The pharmacological activity of FELDENE in reducing fever and inflammation may diminish the utility of these diagnostic signs in detecting complications of presumed noninfectious, painful conditions.Hepatic EffectsBorderline elevations of one or more liver tests may occur in up to 15% of patients taking NSAIDs, including FELDENE. These laboratory abnormalities may progress, may remain unchanged, or may be transient with continuing therapy. Notable elevations of ALT or AST (approximately three or more times the upper limit of normal) have been reported in approximately 1% of patients in clinical trials with NSAIDs. In addition, rare cases of severe hepatic reactions, including jaundice and fatal fulminant hepatitis, liver necrosis and hepatic failure, some of them with fatal outcomes have been reported.while on therapy with FELDENE. If clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, etc.), FELDENE should be discontinued (see ADVERSE REACTIONS).Hematological EffectsAnemia is sometimes seen in patients receiving NSAIDs, including FELDENE. This may be due to fluid retention, occult or gross GI blood loss, or an incompletely described effect upon erythropoiesis. Patients on long-term treatment with NSAIDs, including FELDENE, should have their hemoglobin or hematocrit checked if they exhibit any signs or symptoms of anemia.NSAIDs inhibit platelet aggregation and have been shown to prolong bleeding time in some patients. Unlike aspirin, their effect on platelet function is quantitatively less, of shorter duration, and reversible. Patients receiving FELDENE who may be adversely affected by alterations in platelet function, such as those with coagulation disorders or patients receiving anticoagulants, should be carefully monitored.Ophthalmologic EffectsBecause of reports of adverse eye findings with nonsteroidal anti-inflammatory agents, it is recommended that patients who develop visual complaints during treatment with FELDENE have ophthalmic evaluations.Preexisting AsthmaPatients with asthma may have aspirin-sensitive asthma. The use of aspirin in patients withaspirin-sensitive asthma has been associated with severe bronchospasm which can be fatal. Since cross-reactivity, including bronchospasm, between aspirin and other nonsteroidal anti-inflammatory drugs has been reported in such aspirin-sensitive patients, FELDENE should not be administered to patients with this form of aspirin sensitivity and should be used with caution in patients with preexisting asthma.Information for PatientsPatients should be informed of the following information before initiating therapy with an NSAID and periodically during the course of ongoing therapy. Patients should also be encouraged to read the NSAID Medication Guide that accompanies each prescription dispensed.• FELDENE, like other NSAIDs, may cause CV side effects, such as MI or stroke, which may result in hospitalization and even death. Although serious CV events can occur withoutwarning symptoms, patients should be alert for the signs and symptoms of chest pain, shortness of breath, weakness, slurring of speech, and should ask for medical advice when observing any indicative signs or symptoms. Patients should be apprised of the importance of this follow-up (see WARNINGS, CARDIOVASCULAR EFFECTS).• FELDENE, like other NSAIDs, can cause GI discomfort and, rarely, serious GI side effects, such as ulcers and bleeding, which may result in hospitalization and even death. Althoughserious GI tract ulcerations and bleeding can occur without warning symptoms, patients should be alert for the signs and symptoms of ulcerations and bleeding, and should ask for medicaladvice when observing any indicative signs or symptoms including epigastric pain, dyspepsia, melena, and hematemesis. Patients should be apprised of the importance of this follow-up (see WARNINGS, Gastrointestinal Effects - Risk of Ulceration, Bleeding, and Perforation).• FELDENE, like other NSAIDs, can cause serious skin side effects such as exfoliative dermatitis, SJS and TEN, which may result in hospitalization and even death. Although serious skin reactions may occur without warning, patients should be alert for the signs and symptoms of skin rash and blisters, fever, or other signs of hypersensitivity such as itching, and shouldask for medical advice when observing any indicative signs or symptoms. Patients should beadvised to stop the drug immediately if they develop any type of rash and contact theirphysicians as soon as possible.• Patients should promptly report signs or symptoms of unexplained weight gain or edema to their physicians.• Patients should be informed of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, pruritus, jaundice, right upper quadrant tenderness, and “flu-like” symptoms).If these occur, patients should be instructed to stop therapy and seek immediate medicaltherapy.• Patients should be informed of the signs of an anaphylactoid reaction (e.g., difficulty breathing, swelling of the face or throat). If these occur, patients should be instructed to seek immediate emergency help (see WARNINGS).• In late pregnancy, as with other NSAIDs, FELDENE should be avoided because it may cause premature closure of the ductus arteriosus.Laboratory TestsBecause serious GI tract ulcerations and bleeding can occur without warning symptoms, physicians should monitor for signs and symptoms of GI bleeding. Patients on long-term treatment with NSAIDs should have their CBC and a chemistry profile checked periodically. If clinical signs and symptoms consistent with liver or renal disease develop, systemic manifestations occur (e.g., eosinophilia, rash, etc.), or if abnormal liver tests persist or worsen, FELDENE should be discontinued.Drug InteractionsHighly Protein Bound Drugs: FELDENE is highly protein bound and, therefore, might be expected to displace other protein bound drugs. Physicians should closely monitor patients for a change in dosage requirements when administering FELDENE to patients on other highly protein bound drugs. Aspirin: When FELDENE is administered with aspirin, its protein binding is reduced, although the clearance of free FELDENE is not altered. Plasma levels of piroxicam are depressed to approximately 80% of their normal values when FELDENE is administered (20 mg/day) in conjunction with aspirin (3900 mg/day). The clinical significance of this interaction is not known; however, as with other NSAIDs, concomitant administration of piroxicam and aspirin is not generally recommended because of the potential for increased adverse effects.Methotrexate: NSAIDs have been reported to competitively inhibit methotrexate accumulation in rabbit kidney slices. This may indicate that they could enhance the toxicity of methotrexate. Caution should be used when NSAIDs are administered concomitantly with methotrexate.ACE-Inhibitors: Reports suggest that NSAIDs may diminish the antihypertensive effect of ACE- inhibitors. This interaction should be given consideration in patients taking NSAIDs concomitantly with ACE-inhibitors.Diuretics: Clinical studies, as well as postmarketing observations, have shown that FELDENE can reduce the natriuretic effect of furosemide and thiazides in some patients. This response has been attributed to inhibition of renal prostaglandin synthesis. During concomitant therapy with NSAIDs, the patient should be observed closely for signs of renal failure (see WARNINGS: Renal Effects), as well as to assure diuretic efficacy.Lithium: NSAIDs have produced an elevation of plasma lithium levels and a reduction in renal lithium clearance. The mean minimum lithium concentration increased 15% and the renal clearance was decreased by approximately 20%. These effects have been attributed to inhibition of renal prostaglandin synthesis by the NSAID. Thus, when NSAIDs and lithium are administered concurrently, subjects should be observed carefully for signs of lithium toxicity.Warfarin: The effects of warfarin and NSAIDs on GI bleeding are synergistic, such that users of both drugs together have a risk of serious GI bleeding higher than users of either drug alone. Carcinogenesis, Mutagenesis, Impairment of FertilitySubacute, acute, and chronic toxicity studies have been carried out in rats, mice, dogs, and monkeys. The pathology most often seen was that characteristically associated with the animal toxicology of anti-inflammatory agents: renal papillary necrosis (see PRECAUTIONS) and gastrointestinal lesions. Reproductive studies revealed no impairment of fertility in animals.PregnancyTeratogenic Effects: Pregnancy Category CReproductive studies conducted in rats and rabbits have not demonstrated evidence of developmental abnormalities. However, animal reproduction studies are not always predictive of human response. There are no adequate and well-controlled studies in pregnant women. FELDENE is not recommended for use in pregnant women since safety has not been established in humans. FELDENE should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus.Nonteratogenic Effects:Because of the known effects of nonsteroidal anti-inflammatory drugs on the fetal cardiovascular system (closure of ductus arteriosus), use during pregnancy (particularly late pregnancy) should be avoided. In animal studies of FELDENE, gastrointestinal tract toxicity was increased in pregnant females in the last trimester of pregnancy compared to nonpregnant females or females in earlier trimesters of pregnancy.Labor and DeliveryIn rat studies with NSAIDs, as with other drugs known to inhibit prostaglandin synthesis, an increased incidence of dystocia, delayed parturition, and decreased pup survival occurred. The effects of FELDENE on labor and delivery in pregnant women are unknown.Nursing MothersPiroxicam is excreted into human milk. The presence in breast milk has been determined during initial and long-term conditions (52 days). Piroxicam appeared in breast milk at about 1% to 3% of the maternal concentration. No accumulation of piroxicam occurred in milk relative to that in plasma during treatment. FELDENE is not recommended for use in nursing mothers.Pediatric UseSafety and effectiveness in pediatric patients have not been established.Geriatric UseAs with any NSAID, caution should be exercised in treating the elderly (65 years and older).Most spontaneous reports of fatal GI events with NSAIDs are in the elderly or debilitated patients and, therefore, care should be taken in treating this population. In addition to a past history of ulcer disease, older age and poor general health status (among other factors) may increase the risk for GI bleeding. To minimize the potential risk of an adverse GI event, the lowest effective dose should be used for the shortest possible duration (see WARNINGS, Gastrointestinal Effects – Risk of Ulceration, Bleeding, and Perforation).As with all other NSAIDs, there is a risk of developing renal toxicity in patients in which renal prostaglandins have a compensatory role in maintenance of renal perfusion. Discontinuation of nonsteroidal anti-inflammatory drug therapy is usually followed by recovery to the pretreatment state (see Warnings: Renal Effects).In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting a greater frequency of impaired drug elimination and of concomitant disease or other drug therapy.ADVERSE REACTIONSIn patients taking FELDENE or other NSAIDs, the most frequently reported adverse experiences occurring in approximately 1–10% of patients are:Cardiovascular System: Edema.Digestive System: Anorexia, abdominal pain, constipation, diarrhea, dyspepsia, elevated liver enzymes, flatulence, gross bleeding/perforation, heartburn, nausea, ulcers (gastric/duodenal), vomiting.Hemic and Lymphatic System: Anemia, increased bleeding time.Nervous System: Dizziness, headache.Skin and Appendages: Pruritus, rash.Special Senses: Tinnitus.Urogenital System: Abnormal renal function.Additional adverse experiences reported occasionally include:Body As a Whole: Fever, infection, sepsis.Cardiovascular System: Congestive heart failure, hypertension, tachycardia, syncope.Digestive System: Dry mouth, esophagitis, gastritis, glossitis, hematemesis, hepatitis, jaundice, melena, rectal bleeding, stomatitis.Hemic and Lymphatic System: Ecchymosis, eosinophilia, epistaxis, leukopenia, purpura, petechial rash, thrombocytopenia.Metabolic and Nutritional: Weight changes.Nervous System: Anxiety, asthenia, confusion, depression, dream abnormalities, drowsiness, insomnia, malaise, nervousness, paresthesia, somnolence, tremors, vertigo.Respiratory System: Asthma, dyspnea.Skin and Appendages: Alopecia, bruising, desquamation, erythema, photosensitivity, sweat.Special Senses: Blurred vision.Urogenital System: Cystitis, dysuria, hematuria, hyperkalemia, interstitial nephritis, nephrotic syndrome, oliguria/polyuria, proteinuria, renal failure.Other adverse reactions which occur rarely are:。

risk的用法总结大全risk有冒险的意思,关于它的知识点是比较丰富的,下面小编分享risk的用法给大家,希望对你们有所帮助!释义risk英 [r?sk] 美 [r?sk] n. 风险;危险;冒险vt. 冒…的危险n. (Risk)人名;(英、阿拉伯)里斯克[ 复数 risks 过去式 risked 过去分词 risked 现在分词 risking 第三人称单数 risks ]Risk premium 风险溢价 ; 风险贴水 ; 风险保费systematic risk 系统性风险 ; 系统风险 ; 体系性风险liquidity risk 流动性风险 ; 流通风险 ; 流动风险risk evaluation 风险评估 ; [经] 风险评价 ; 风险衡量all risk 全险 ; 一切险 ; 平安险 ; 战争险risk investment [金融] 风险投资 ; [经] 危险投资 ; 险投资 ; 危害投资Settlement risk 清算风险 ; 结算风险 ; 交收风险Risk Society 风险社会学说risk exposure 风险暴露 ; 风险敞口 ; 风险承担词语辨析risk, hazard, venture, chance, dare这组词都有“敢于冒险”的意思,其区别是:risk 指不顾个人安危去干某事,侧重主动承担风险。
hazard 主要指冒险作出某个选择,隐含碰运气意味。
venture 指冒风险试一试,或指有礼貌的反抗或反对。
chance 指碰运气、冒风险试试。
dare 可与venture换用,但语气较强,着重挑战或违抗。
risk的用法1. 用作动词时,为及物动词,表示“冒……的危险”“使遭受危险”。
如:They risk their lives in order that we may live more safely. 他们冒了生死危险使我们生活得更安全。
后接动词作宾语时,要用动名词,不能用不定式。

肝酶与代谢综合征关系的研究进展摘要】代谢综合征是以多种心血管危险因素集合为特点的一种疾病,包括高血糖、肥胖症、高血压、高甘油三酯血症和低高密度脂蛋白胆固醇血症等,已报道代谢综合征可增加2型糖尿病及心血管疾病的风险。
代谢综合征现在不仅成为一个临床问题,而且成为全球重要的公共卫生挑战,其在世界范围内的患病率日益增加,因此,早期发现和干预代谢综合征对预防心血管相关疾病的进展和减少公共卫生负担具有重要意义。
肝脏是脂质代谢、糖代谢的主要场所,肝酶浓度升高,且即使在正常范围内的肝脏转氨酶浓度升高,均可增加代谢综合征的发生风险。
故可根据肝酶浓度,有助于发现早期糖尿病、血脂异常、高血压、肥胖症等,并对其进行干预。
本文综述了肝酶与代谢综合征的相关性,以此可获得预测代谢综合征的方法。
【关键词】丙氨酸转氨酶;谷氨酰转肽酶;天冬氨酸转氨酶;代谢综合征【中图分类号】R589 【文献标识码】A 【文章编号】2095-1752(2018)25-0011-02【Abstract】Metabolic syndrome is aconstellation?of?several?cardiovascularrisk?factors,including?hyperglycemia,Obesity,h ypertension,hypertriglyceridemia,and?low?HDL-C,It is well?reportedthat?metabolic?syndrome?escalates?the?risk?of?type?2?diabetes?and?cardiovascular ?disease.Metabolic?syndrome?is?not?only?a?clinical?problem?but?also?an?importan t?public?health?challenge?worldwide,and?its?prevalence?in?the?world?is?increasing. Therefore,early?detection?and?intervention?of?metabolic?syndrome?is?of?great?sig nificance?in?preventing?the?progress?of?cardiovascular?related?diseases?and?reduci ng?the?burden?of?public?health.The?liver?is?the?main?place?for?lipid?metabolism? and?glucose?metabolism.The?concentration?of?liver?enzymes?is?elevated,and?even ?in?the?normal?range?of?liver?transaminase?concentration,the?risk?of?metabolic?sy ndrome?can?be?increased.Therefore,according?to?the?concentration?of?liver?enzym es,it?is?helpful?to?detect?early?diabetes,dyslipidemia,hypertension,obesity?and?so? on.This?article?reviews?the?correlation?between?liver?enzymes?and?metabolic?syn drome,so?as?to?obtain?a?method?for?predicting?metabolic?syndrome.【Key words】Alanine?aminotransferase; Glutamyl?transferase;Aspartate?aminotransferase; Metabolic?syndrome既往研究报道[9],随着时代的发展,发展中国家的MS的患病率逐渐增高,由于MS的发病率升高,心血管疾病及糖尿病的发生率随着升高,这些疾病都将对人类产生极大危害,导致生活及工作质量下降,甚至威胁人类的生命。


risk的同义词risk表危险,冒险; 保险额的意思,那么你知道risk的同义词有哪些吗?接下来小编为大家整理了risk的同义词,希望对你有帮助哦!risk的同义词辨析1:danger, risk, hazard, menace, peril, threat这些名词均含有"危险,威胁"之意。
danger :含义广,普通用词,指能够造成伤害、损害或不利的任何情况。
risk :指有可能发生的危险,尤指主动进行某种活动或去碰运气而冒的危险。
hazard比risk正式,多指偶然发生的或无法控制的危险,常含较严重或有一定风险的意味。
menace :所指的危险性最严重,表示使用暴力或造成破坏性的可能。
peril :指迫在眉睫很有可能发生的严重危险。
threat :普通用词,语气弱于menace,指任何公开侵犯对方的言行,给对方构成危险或威胁。
risk的同义词辨析2:venture, chance, dare, hazard, risk这些动词均含有"敢于冒险"之意。
venture :指冒风险试一试,或指有礼貌的反抗或反对。
chance :指碰运气、冒风险试试。
dare :可与venture换用,但语气较强,着重挑战或违抗。
hazard :主要指冒险作出某个选择,隐含碰运气意味。
risk :指不顾个人安危去干某事,侧重主动承担风险。
词组习语:at risk1. 处境危险;遭受危险非洲有2,300万人正处于饥饿的危险中。
23 million people in Africa are &B{at risk from} starvation.at one's (own) risk1. 自负责任,自负后果他们从事冒险活动,后果自负。
they undertook the adventure at their own risk.at the risk of doing something1. 冒(做某事)的危险冒着使人厌烦得要死的风险,我重复了最重要的绘画规则。



_______________________________________________________________________________________________ HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to useCaldolor safely and effectively.See full prescribing information for Caldolor.CALDOLOR (ibuprofen) Injection, for intravenous use Initial U.S. Approval: 1974WARNING: RISK OF SERIOUS CARDIOVASCULAR ANDGASTROINTESTINAL EVENTSSee full prescribing information for complete boxed warningCardiovascular Risk • Non-steroidal anti-inflammatory drugs (NSAIDs) may increase the risk of serious cardiovascular (CV) thrombotic events, myocardial infarction, and stroke, which can be fatal. Risk may increase with duration of use. (5.1) • Caldolor is contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery. (4.3, 5.1) Gastrointestinal Risk • NSAIDs increase the risk of serious gastrointestinal (GI) adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. Events can occur at any time without warning symptoms. Elderly patients are at greater risk. (5.2) -----------------------------INDICATIONS AND USAGE-------------------------- Caldolor is an NSAID indicated in adults for the: • Management of mild to moderate pain (1.1) • Management of moderate to severe pain as an adjunct to opioid analgesics (1.1) • Reduction of fever (1.2) -------------------------DOSAGE AND ADMINISTRATION--------------------- • Pain: 400 mg to 800 mg intravenously over 30 minutes every 6 hours as necessary. (2.1) • Fever: 400 mg intravenously over 30 minutes, followed by 400 mg every 4 to 6 hours or 100-200 mg every 4 hours as necessary. (2.2) • Patients must be well hydrated before Caldolor administration. • Caldolor must be diluted before administration. (2.3) ------------------------DOSAGE FORMS AND STRENGTHS------------------- Vials: 400 mg/4 mL or 800 mg/8 mL (3) -------------------------------CONTRAINDICATIONS------------------------------ • Known hypersensitivity to ibuprofen or other NSAIDS (4.1) • Asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs (4.2) • Use during the peri-operative period in the setting of coronary artery bypass graft (CABG) surgery (4.3, 5.1) -------------------------WARNINGS AND PRECAUTIONS----------------------• Serious and potentially fatal CV thrombotic events: Use lowest effective dose of Caldolor for shortest possible duration. (5.1)• Serious and potentially fatal GI reactions: Use lowest effective dose of Caldolor for shortest possible duration. Use with caution in patients with prior history of ulcer disease or GI bleeding. (5.2) • Hepatic effects: Range from transaminase elevations to liver failure. Discontinue Caldolor immediately if abnormal liver tests persist or worsen. (5.3, 5.15) • Hypertension: Can occur with NSAID treatment. Monitor blood pressure closely during treatment with Caldolor. (5.4) • Congestive heart failure and edema: Fluid retention and edema can occur with NSAID treatment. Use Caldolor with caution in patients with fluid retention or heart failure. (5.5) • Renal effects: Long-term administration of NSAIDs can result in renal papillary necrosis and other renal injury. Use Caldolor with caution in patients at risk (e.g., the elderly, those with renal impairment, heart failure, liver impairment, and those taking diuretics or ACE inhibitors. (5.6) • Anaphylactoid reactions: May occur in patients with the aspirin triad or in patients without prior exposure to Caldolor. Discontinue Caldolor immediately if an anaphylactoid reaction occurs. (5.7, 5.12) • Serious skin reactions: Include exfoliative dermatitis, Stevens-Johnson Syndrome, and toxic epidermal necrolysis, which can be fatal.Discontinue Caldolor if rash or other signs of local skin reaction occur. (5.8) -------------------------------ADVERSE REACTIONS------------------------------ The most common adverse reactions are nausea, flatulence, vomiting, headache, hemorrhage and dizziness (>5%). (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Cumberland Pharmaceuticals Inc. at 1-877-484-2700 or FDA at 1-800-FDA-1088 or/medwatch.---------------------------------DRUG INTERACTIONS----------------------------• ACE-inhibitors: NSAIDs may diminish the antihypertensive effect ofACE-inhibitors. (7.3) • Aspirin: Concomitant administration of ibuprofen and aspirin is notgenerally recommended because of the potential for increased adverseeffects. (7.1) --------------------------USE IN SPECIFIC POPULATIONS--------------------- • Pregnancy: Avoid use after 30 weeks gestation because premature closure of the ductus arteriosus in the fetus may occur. (8.1) • Nursing Mothers: Use with caution as it is not known if ibuprofen is excreted in human milk. (8.3) • Pediatric Use: Safety and effectiveness not established in patients less than 17 years of age. (8.4) See 17 for PATIENT COUNSELING INFORMATION Revised: 06/2009FULL PRESCRIBING INFORMATION: CONTENTS*WARNING: RISK OF SERIOUS CARDIOVASCULAR ANDGASTROINTESTINAL EVENTS 1 INDICATIONS AND USAGE 5.8 Serious Skin Reactions1.1 Analgesia (Pain) 5.9 Pregnancy 1.2 Antipyretic (Fever)5.10 Masking Inflammation and Fever 2 DOSAGE AND ADMINISTRATION5.11 Hematological Effects 2.1 Analgesia (Pain) 5.12 Preexisting Asthma 2.2 Antipyretic (Fever) 5.13 Ophthalmological Effects 3 DOSAGE FORMS AND STRENGTHS 5.14 Aseptic Meningitis 4 CONTRAINDICATIONS 5.15 Monitoring 4.1 Hypersensitivity 6 ADVERSE REACTIONS 4.2 Asthma and Allergic Reactions6.1 Clinical Studies Experience4.3 Coronary Artery Bypass Graft (CABG) 7 DRUG INTERACTIONS5 WARNINGS AND PRECAUTIONS 7.1 Aspirin 5.1 Cardiovascular Thrombotic Events 7.2 Anticoagulants 5.2 Gastrointestinal Effects: Risk of Ulceration, Bleeding and7.3 ACE Inhibitors Perforation7.4 Diuretics 5.3 Hepatic Effects 7.5 Lithium 5.4 Hypertension 7.6 Methotrexate 5.5 Congestive Heart Failure and Edema 7.7 H-2 Antagonists 5.6 Renal Effects 8 USE IN SPECIFIC POPULATIONS 5.7 Anaphylactoid Reactions8.1 Pregnancy8.2 Labor and Delivery8.3Nursingmothers8.4 Pediatric use 1412.1 Mechanism of action12.3Pharmacokinetics CLINICAL STUDIES101112 8.5 Geriatric useOVERDOSAGEDESCRIPTIONCLINICAL PHARMACOLOGY161714.1 Analgesia (Pain)14.2Antipyretic(Fever)HOW SUPPLIED/STORAGE AND HANDLINGPATIENT COUNSELING INFORMATION*Sections or subsections omitted from the Full Prescribing Information are not listed.FULL PRESCRIBING INFORMATIONWARNING: RISK OF SERIOUS CARDIOVASCULAR AND GASTROINTESTINAL EVENTSCardiovascular Risk• Non-steroidal anti-inflammatory drugs (NSAIDs) may increase therisk of serious cardiovascular (CV) thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increasewith duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at greater risk [see Warnings and Precautions (5.1)].• Caldolor is contraindicated for the treatment of peri-operative painin the setting of coronary artery bypass graft (CABG) surgery [see Contraindications (4.3) and Warnings and Precautions (5.1)]. Gastrointestinal Risk• NSAIDs increase the risk of serious gastrointestinal (GI) adverse events including bleeding, ulceration, and perforation of the stomachor intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events [see Warnings and Precautions (5.2)].1 INDICATIONS AND USAGE1.1 Analgesia (Pain)Caldolor is indicated in adults for the management of mild to moderate pain and the management of moderate to severe pain as an adjunct to opioid analgesics.1.2 Antipyretic (Fever)Caldolor is indicated for the reduction of fever in adults.2 DOSAGE AND ADMINISTRATIONUse the lowest effective dose for the shortest duration consistent with individual patient treatment goals [see Warnings and Precautions (5)]. After observing the response to initial therapy with Caldolor, the dose and frequency should be adjusted to suit an individual patient's needs. Do not exceed 3200 mg total daily dose.To reduce the risk of renal adverse reactions, patients must be well hydrated prior to administration of Caldolor.2.1 Analgesia (Pain)Administer 400 mg to 800 mg intravenously every 6 hours as necessary. Infusion time must be no less than 30 minutes.2.2 Antipyretic (Fever)Administer 400 mg intravenously, followed by 400 mg every 4 to 6 hours or 100-200 mg every 4 hours as necessary. Infusion time must be no less than 30 minutes.2.3 Preparation and AdministrationCaldolor must be diluted prior to intravenous infusion. Dilute to a final concentration of 4 mg/mL or less. Appropriate diluents include 0.9% Sodium Chloride Injection USP (normal saline), 5% Dextrose Injection USP (D5W), or Lactated Ringers Solution.• 800 mg dose: Dilute 8 mL of Caldolor in no less than 200 mL of diluent.• 400 mg dose: Dilute 4 mL of Caldolor in no less than 100 mL of diluent.Visually inspect parenteral drug products for particulate matter and discoloration prior to administration, whenever solution and container permit. If visibly opaque particles, discoloration or other foreign particulates are observed, the solution should not be used.Diluted solutions are stable for up to 24 hours at ambient temperature (approximately 20 to 25° C) and room lighting.Infusion time must be no less than 30 minutes.3 DOSAGE FORMS AND STRENGTHSCaldolor is available as a 400 mg/4 mL single-dose vial (100 mg/mL) and 800 mg/8 mL single-dose vial (100 mg/mL).4 CONTRAINDICATIONS4.1 HypersensitivityCaldolor is contraindicated in patients with known hypersensitivity (e.g., anaphylactoid reactions and serious skin reactions) to ibuprofen. [see Warnings and Precautions (5.7, 5.8)]4.2 Asthma and Allergic ReactionsCaldolor is contraindicated in patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs. Severe, rarely fatal anaphylactic-like reactions to NSAIDs have been reported in such patients [see Warnings and Precautions (5.7, 5.12)].4.3 Coronary Artery Bypass Graft (CABG)Caldolor is contraindicated for the treatment of perioperative pain in the setting of coronary artery bypass graft (CABG) surgery [see Warnings and Precautions (5.1)].5 WARNINGS AND PRECAUTIONS5.1 Cardiovascular Thrombotic EventsClinical trials of several COX-2 selective and nonselective NSAIDs of up to three years’ duration have shown an increased risk of serious cardiovascular (CV) thrombotic events, myocardial infarction and stroke, which can be fatal. All NSAIDs, both COX-2 selective and nonselective, may have a similar risk. Patients with known CV disease or risk factors for CV disease may be at greater risk. To minimize the potential risk for an adverse CV event in patients treated with an NSAID, use the lowest effective dose for the shortest duration possible. Physicians and patients should remain alert for the development of such events, even in the absence of previous CV symptoms. Patients should be informed about the signs and/or symptoms of serious CV events and the steps to take if they occur.Two large, controlled clinical trials of a COX-2 selective NSAID for the treatment of pain in the first 10-14 days following CABG surgery found an increased incidence of myocardial infarction and stroke [see Contraindications (4.3)].There is no consistent evidence that concurrent use of aspirin mitigates the increased risk of serious CV thrombotic events associated with NSAID use. The concurrent use of aspirin and an NSAID does increase the risk of serious gastrointestinal (GI) events [see Warnings and Precautions (5.2)].5.2 Gastrointestinal Effects: Risk of Ulceration, Bleeding, and PerforationNSAIDs, including ibuprofen, can cause serious GI adverse events including inflammation, bleeding, ulceration, and perforation of the stomach, small intestine, or large intestine, which can be fatal. These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. Only one in five patients who develop a serious upper GI adverse event on NSAID therapy is symptomatic. Upper GI ulcers, gross bleeding, or perforation caused by NSAIDs occur in approximately 1% of patients treated for 3-6 months and in about 2-4% of patients treated for one year. These trends continue with longer duration of use, increasing the likelihood of developing a serious GI event at some timeduring the course of therapy. However, even short-term therapy is not without risk.Prescribe NSAIDs, including Caldolor, with extreme caution in those with a prior history of ulcer disease or GI bleeding. Patients with a prior history of peptic ulcer disease and/or GI bleeding who use NSAIDs have a greater than 10fold increased risk for developing a GI bleed compared to treated patients with neither of these risk factors. Other factors that increase the risk of GI bleeding in patients treated with NSAIDs include concomitant use of oral corticosteroids or anticoagulants, longer duration of NSAID therapy, smoking, use of alcohol, older age, and poor general health status. Most reports of spontaneous fatal GI events are in elderly or debilitated patients, and therefore special care should be taken in treating this population.To minimize the potential risk for an adverse GI event in patients treated with an NSAID, use the lowest effective dose for the shortest possible duration. Patients and physicians should remain alert for signs and symptoms of GI ulcerations and bleeding during NSAID therapy and promptly initiate additional evaluation and treatment if a serious GI event is suspected. This should include discontinuation of the NSAID until a serious GI adverse event is ruled out. For high-risk patients, alternate therapies that do not involve NSAIDs should be considered.5.3 Hepatic EffectsBorderline elevations of one or more liver tests may occur in up to 15% of patients taking NSAIDs, including ibuprofen. These laboratory abnormalities may progress, may remain unchanged, or may be transient with continuing therapy. Notable elevations of ALT or AST (approximately three or more times the upper limit of normal) have been reported in approximately 1% of patients in clinical trials with NSAIDs. In addition, rare cases of severe hepatic reactions have been reported, including jaundice, fulminant hepatitis, liver necrosis and hepatic failure, some with fatal outcomes. A patient with symptoms and/or signs suggesting liver dysfunction, or with abnormal liver test values, should be evaluated for evidence of the development of a more severe hepatic reaction while on therapy with ibuprofen. If clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, etc.), ibuprofen should be discontinued.5.4 HypertensionNSAIDs, including ibuprofen, can lead to onset of new hypertension or worsening of preexisting hypertension, either of which may contribute to the increased incidence of CV events. Use NSAIDs, including ibuprofen, with caution in patients with hypertension. Monitor blood pressure closely during the initiation of NSAID treatment and throughout the course of therapy.Patients taking ACE inhibitors, thiazides, or loop diuretics may have impaired response to these therapies when taking NSAIDs.5.5 Congestive Heart Failure and EdemaFluid retention and edema have been observed in some patients taking NSAIDs. Use Caldolor with caution in patients with fluid retention or heart failure.5.6 Renal EffectsUse caution when initiating treatment with Caldolor in patients with considerable dehydration.Long-term administration of NSAIDs has resulted in renal papillary necrosis and other renal injury. Renal toxicity has also been seen in patients in whom renal prostaglandins have a compensatory role in the maintenance of renal perfusion. In these patients, administration of an NSAID may cause a dose dependent reduction in prostaglandin formation and, secondarily, in renal blood flow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, heart failure, liver dysfunction, those taking diuretics or ACE inhibitors, and the elderly. Discontinuation of NSAID therapy is usually followed by recovery to the pretreatment state.No information is available from controlled clinical studies regarding the use of Caldolor in patients with advanced renal disease. If Caldolor therapy must be initiated in patients with advanced renal disease, closely monitor the patient’s renal function.5.7 Anaphylactoid ReactionsAs with other NSAIDs, anaphylactoid reactions may occur in patients without known prior exposure to ibuprofen. CALDOLOR is contraindicated in patients with the aspirin triad. This symptom complex typically occurs in asthmatic patients who experience rhinitis with or without nasal polyps, or who exhibit severe, potentially fatal bronchospasm after taking aspirin or other NSAIDs [see Contraindications (4.2)].5.8 Serious Skin ReactionsNSAIDs, including ibuprofen, can cause serious skin adverse reactions such as exfoliative dermatitis, Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. These serious events may occur without warning. Inform patients about the signs and symptoms of serious skin manifestations, and to discontinue Caldolor at the first appearance of skin rash or any other sign of hypersensitivity.5.9 PregnancyStarting at 30 weeks gestation, Caldolor, and other NSAIDs, should be avoided by pregnant women as premature closure of the ductus arteriosus in the fetus may occur [see Use in Specific Populations (8.1)].5.10 Masking Inflammation and FeverThe pharmacological activity of ibuprofen in reducing fever and inflammation may diminish the utility of these diagnostic signs in detecting complications of presumed noninfectious, painful conditions.5.11 Hematological EffectsCaldolor must be diluted prior to use. Infusion of the drug product without dilution can cause hemolysis [see Dosage and Administration (2.3)].Anemia may occur in patients receiving NSAIDs, including ibuprofen. This may be due to fluid retention, occult or gross GI blood loss, or an incompletely described effect on erythropoiesis. In patients on long-term treatment with NSAIDs, including ibuprofen, check hemoglobin or hematocrit if they exhibit any signs or symptoms of anemia or blood loss.NSAIDs inhibit platelet aggregation and have been shown to prolong bleeding time in some patients. Unlike aspirin, their effects on platelet function are less severe quantitatively, of shorter duration, and reversible. Carefully monitor patients who may be adversely affected by alterations in platelet function, such as those with coagulation disorders or patients receiving anticoagulants.5.12 Preexisting Asthma6 Patients with asthma may have aspirin-sensitive asthma.The use of aspirin in patients with aspirin-sensitive asthma has been associated with severe bronchospasm, which can be fatal. Since cross-reactivity between aspirin and NSAIDs has been reported in such aspirin-sensitive patients, including bronchospasm, Caldolor is contraindicated in patients with this form of aspirin sensitivity and should be used with caution in all patients with preexisting asthma.5.13 Ophthalmological EffectsBlurred or diminished vision, scotomata, and changes in color vision have been reported with oral ibuprofen. Discontinue ibuprofen if a patient develops such complaints, and refer the patient for an ophthalmologic examination that includes central visual fields and color vision testing.5.14 Aseptic MeningitisAseptic meningitis with fever and coma has been observedin patients on oral ibuprofen therapy. Although it is probably more likely to occur in patients with systemic lupus erythematosus and related connective tissue diseases, it has been reported in patients who do not have underlying chronic disease. If signs or symptoms of meningitis develop in a patient on ibuprofen, give consideration to whether or not the signs or symptoms are related to ibuprofen therapy.5.15 MonitoringBecause serious GI tract ulcerations and bleeding can occur without warning symptoms, physicians should monitor for signs or symptoms of GI bleeding.Patients on long-term treatment with NSAIDs should have CBC and chemistry profiles checked periodically. If clinical signs and symptoms consistent with liver or renal disease develop, systemic manifestations occur (e.g., eosinophilia, rash), or abnormal liver tests persist or worsen, discontinue Caldolor.ADVERSE REACTIONSThe following serious adverse reactions are discussed elsewhere in the labeling:• Cardiovascular thrombotic events [see Boxed Warning and Warnings and Precautions (5.1)]• G astrointestinal effects [see Boxed Warning and Warnings and Precautions (5.2)]• H epaticeffects[see Warnings and Precautions (5.3)] • Hypertension [see Warnings and Precautions (5.4)]• Congestive heart failure and edema [see Warnings and Precautions (5.5)]• Renal effects [see Warnings and Precautions (5.6)]• Anaphylactoid reactions [see Warnings and Precautions (5.7)]• Serious skin reactions [see Warnings and Precautions(5.8)]The most common adverse reactions reported in clinical studies are nausea, flatulence, vomiting, and headache.The most common reason for discontinuation due to adverse events in controlled trials of Caldolor is pruritus (<1%).6.1 Clinical Studies ExperienceBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be compared directly to rates in the clinical trials of another drug and may not reflect the rates observed in practice.During clinical development, 560 patients were exposed to Caldolor, 438 in pain and 122 with fever. In the pain studies, Caldolor was started intra-operatively and administered at a dose of 400 mg or 800 mg every six hours for up to three days. In the fever studies, Caldolor was administered at doses of 100 mg, 200 mg, or 400 mg every four or six hours for up to 3 days.The most frequent type of adverse reaction occurring with oral ibuprofen is gastrointestinal.Pain StudiesThe incidence rates of adverse reactions listed in the following table were derived from multicenter, controlled clinical studies in post-operative patients comparing Caldolor to placebo in patients also receiving morphine as needed for postoperative pain.Table 1: Post-operative Patients with Adverse Reactions Observed in ≥ 3% of Patients in any Caldolor Treatment Group in Pain Studies*EventCaldolorPlacebo(N=287)400 mg(N=134)800 mg(N=304)Any Reaction 118 (88%) 260 (86%) 258 (90%)Nausea 77 (57%) 161 (53%) 179 (62%) Vomiting 30 (22%) 46 (15%) 50 (17%) Flatulence 10 (7%) 49 (16%) 44 (15%) Headache 12 (9%) 35 (12%) 31 (11%) Hemorrhage 13 (10%) 13 (4%) 16 (6%) Dizziness 8 (6%) 13 (4%) 5 (2%)Edema peripheral 1 (<1%) 9 (3%) 4 (1%)Urinary retention 7 (5%) 10 (3%) 10 (3%) Anemia 5 (4%) 7 (2%) 6 (2%)Decreased hemoglobin 4 (3%) 6 (2%) 3 (1%)Dyspepsia 6 (4%)+ 4 (1%) 2 (<1%) Wound hemorrhage 4 (3%) 4 (1%) 4 (1%)Abdominal discomfort 4 (3%)+ 2(<1%) 0 Cough 4 (3%)+ 2 (<1%) 1 (<1%)Hypokalemia 5 (4%) 3 (<1%) 8 (3%)* All patients received concomitant morphine during these studies.Fever StudiesFever studies were conducted in febrile hospitalized patients with malaria and febrile hospitalized patients with varying causes of fever. In hospitalized febrile patients with malaria. The adverse reactions observed in at least two Caldolor-treated patients included abdominal pain and nasal congestion.In hospitalized febrile patients (all causes), adverse reactions observed in more than two patients in any given treatment group are presented in the table below.Table 2: Patients with Adverse Reactions Observed in≥ 3% of Patients in any Caldolor Treatment Group in All-Cause Fever StudyCaldolorPlaceboN=28 Event100 mgN=30200 mgN=30400 mgN=31Any Reaction 27 (87%) 25 (83%) 23 (74%) 25 (89%)Anemia 5 (17%) 6 (20%) 11 (36%) 4 (14%) Eosinophilia 7 (23%) 7 (23%) 8 (26%) 7 (25%)Hypokalemia 4 (13%) 4 (13%) 6 (19%) 5 (18%)Hypoproteinemia 3 (10%) 0 4 (13%) 2 (7%)Neutropenia 2 (7%) 2 (7%) 4 (13%) 2 (7%)Blood urea increased 0 0 3 (10%) 0Hypernatremia 2 (7%) 0 3 (10%) 0Table 2: Patients with Adverse Reactions Observed in ≥ 3% of Patients in any Caldolor Treatment Group in Al Cause Fever Studyl-CaldolorPlacebo N=28Event 100 mg N=30200 mg N=30 400 mg N=31 Hypertension0 0 3 (10%) 0 Hypoalbuminemia 3 (10%) 1 (3%) 3 (10%) 1 (4%) Hypotension 0 2 (7%) 3 (10%) 1 (4%) Diarrhea 3 (10%) 3 (10%) 2 (7%) 2 (7%) Pneumonia bacterial 3 (10%) 1 (3%) 2 (7%) 0 Blood LDH increased 3 (10%) 2 (7%) 1 (3%) 1 (4%) Thromocythemia 3 (10%) 2 (7%) 1 (3%) 0 Bacteremia 4 (13%)7 DRUG INTERACTIONS 7.1 AspirinWhen ibuprofen is administered with aspirin, ibuprofen‘s protein binding is reduced, although the clearance of free ibuprofen is not altered. The clinical significance of this interaction is not known; however, as with other NSAIDs, concomitant administration of Caldolor and aspirin is not generally recommended because of the potential for increased adverse effects. 7.2 Anticoagulants The effects of warfarin and NSAIDs on GI bleeding are synergistic, such that the users of both drugs together have a higher risk of serious GI bleeding than users of either drug alone [see Warnings and Precautions (5.2)]. 7.3 ACE InhibitorsNSAIDs may diminish the antihypertensive effect of ACE inhibitors. This interaction should be given consideration in patients taking NSAIDs concomitantly with ACE inhibitors. 7.4 DiureticsClinical studies and postmarketing observations have shown that ibuprofen can reduce the natriuretic effects of furosemide and thiazides in some patients. This response has been attributed to inhibition of renal prostaglandin synthesis. During concomitant therapy with NSAIDs, observe patients closely for signs of renal failure, as well as to assure diuretic efficacy [see Warnings and Precautions (5.6)].7.5 LithiumNSAIDS have produced elevations of plasma lithium levels and a reduction in renal lithium clearance.. The mean minimum lithium concentration increased 15%, and the renal clearance of lithium decreased by 20%. This effect has been attributed to inhibition of renal prostaglandin synthesis by the NSAID. Thus, when NSAIDS and lithium are administered concurrently, observe patients carefully for signs of lithium toxicity. 7.6 MethotrexateNSAIDs have been reported to competitively inhibit methotrexate accumulation in rabbit kidney slices. This indicates that NSAIDs may enhance the toxicity of methotrexate. Use caution when NSAIDs are administered concomitantly with methotrexate. 7.7 H-2 AntagonistsIn studies of human volunteers, co-administration of cimetidine or ranitidine with ibuprofen had no substantive effect on ibuprofen serum concentrations. 8 USE IN SPECIFIC POPULATIONS 8.1 PregnancyTeratogenic effects - Pregnancy Category C prior to 30 weeks gestation; Category D starting at 30 weeks gestation. Starting at 30 weeks gestation, Caldolor, and other NSAIDs, should be avoided by pregnant women as premature closure of the ductus arteriosus in the fetus may occur. Caldolor can cause fetal harm when administered to a pregnant woman starting at 30 weeks gestation. There are no adequate, well-controlled studies in pregnant women. Prior to 30 weeks gestation, Caldolor should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.Reproductive studies conducted in rats and rabbits have not demonstrated evidence of developmental abnormalities.8.2 Labor and DeliveryThe effects of Caldolor on labor and delivery in pregnant women are unknown. In rat studies, maternal exposure to NSAIDs, as with other drugs known to inhibit prostaglandin synthesis, increased the incidence of dystocia and delayed parturition, and decreased pup survival. 8.3 Nursing MothersIt is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Caldolor, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother. 8.4 Pediatric Use Safety and effectiveness of Caldolor for management of pain and reduction of fever has not been established in pediatric patients below the age of 17 years.8.5 Geriatric Use Clinical studies of Caldolor did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Dose selectionfor an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Elderly patients are at increased risk for serious GI adverse events. 10 OVERDOSAGEThe following signs and symptoms have occurred in individuals following an overdose of oral ibuprofen: abdominal pain, nausea, vomiting, drowsiness, and dizziness. There are no specific measures to treat acute overdosage with Caldolor. There is no known antidote to ibuprofen. In case of an overdosage, discontinue Caldolor therapy and consider contacting a regional poison control center at 1-800-222-1222.11 DESCRIPTIONCaldolor contains the active ingredient ibuprofen, which is (±)-2-(p -isobutylphenyl) propionic acid. Ibuprofen is a white powder with a melting point of 74-77°C. It has a molecular weight of 206.28. It is very slightly soluble in water (<1 mg/mL) and readily soluble in organic solvents such as ethanol and acetone. The structural formula of ibuprofen is represented below:。
risk的用法和短语例句risk有危险;风险;投资报酬的风险等意思,那么你知道risk的用法吗?下面是店铺为大家整理的risk的用法和相关短语例句,欢迎大家学习!risk的用法:risk的用法1:risk的基本意思是“冒…的危险”,含有“以…孤注一掷并愿意承担后果”的意味。
risk的用法2:可指冒由于做某事而发生另一不幸之事的风险。
risk的用法3:可指虽然明知做某事会产生不幸后果,但仍不顾一切地进行。
risk的用法4:risk用作及物动词,接名词、代词、动名词(不接动词不定式)作宾语。
可用于被动结构。
risk的用法5:risk也可表示(玩纸牌、赛马等)赌钱,后接具体的钱数或money。
risk的常用短语:用作名词 (n.)at one's own riskat riskat risk toat the risk ofrun the risk oftake risks用作动词 (v.)risk itrisk的用法例句:1. I'm a blood donor; I can't risk any contagion.我是献血者,我不能冒任何接触传染的危险。
2. You could never eliminate risk, but preparation and training could attenuate it.风险不可能完全消除,但可以通过防范和培训来降低。
3. Smoking places you at serious risk of cardiovascular and respiratory disease.吸烟会大大增加罹患心血管和呼吸道疾病的风险。
4. You're taking a big risk showing this to Kravis.你把这个给克拉维斯看会有很大的风险。
5. Concerned people want to minimize the risk of developing cancer.相关人员希望尽可能降低罹癌风险。
2023血脂指南标准英文回答:The 2023 blood lipid guidelines have been updated to provide healthcare professionals with the latest recommendations for managing blood lipid levels in patients. These guidelines aim to address the increasing prevalenceof dyslipidemia and its associated cardiovascular risks.One of the key recommendations in the 2023 guidelinesis the use of statin therapy for the primary prevention of cardiovascular events in individuals with elevated low-density lipoprotein cholesterol (LDL-C) levels. Statins are a class of medications that help lower LDL-C levels and reduce the risk of heart disease. This recommendation is based on strong evidence from clinical trials showing the efficacy of statins in reducing cardiovascular events.In addition to statin therapy, the guidelines also emphasize the importance of lifestyle modifications inmanaging blood lipid levels. These include adopting a healthy diet, engaging in regular physical activity, maintaining a healthy weight, and avoiding smoking. Lifestyle modifications are crucial in reducing LDL-Clevels and improving overall cardiovascular health.Furthermore, the guidelines recommend the use of non-statin medications, such as ezetimibe and PCSK9 inhibitors, in individuals who require additional LDL-C lowering despite maximum tolerated statin therapy. These medications can be used as adjuncts to statin therapy to further reduce LDL-C levels and lower the risk of cardiovascular events.It is important for healthcare professionals to assess individual patient risk and personalize treatmentstrategies based on the guidelines. This involves considering factors such as age, gender, family history, presence of comorbidities, and overall cardiovascular risk profile. By tailoring treatment plans to the specific needs of each patient, healthcare professionals can optimize the management of blood lipid levels and reduce the risk of cardiovascular events.中文回答:2023年的血脂指南标准已经更新,旨在为医疗专业人员提供管理患者血脂水平的最新建议。
糖尿病和高密度脂蛋白(HDL)水平之间的关系和机制摘要:糖尿病是一种代谢紊乱性疾病,而高密度脂蛋白(HDL)是一种血脂的组成部分。
过去的研究表明,糖尿病患者通常具有较低的HDL水平,这可能与心血管疾病的风险增加相关。
本文旨在探讨糖尿病和HDL水平之间的关系以及可能的机制,以便更好地理解和管理糖尿病患者的心血管风险。
第一章:引言糖尿病是一种常见的慢性疾病,全球范围内的患病率不断增加。
糖尿病患者通常伴有高胆固醇和高甘油三酯血症,同时具有较低的HDL水平。
高密度脂蛋白是一种具有反动脉粥样硬化作用的脂蛋白。
第二章:糖尿病与HDL水平的相关性第二章将介绍糖尿病和HDL水平之间的相关性研究。
之前的研究表明,糖尿病患者通常具有较低的HDL水平,而这可能与心血管疾病风险的增加有关。
本章将综述这些研究结果,以便更好地理解糖尿病和HDL水平的关系。
第三章:HDL水平与心血管疾病的关联第三章将介绍HDL水平与心血管疾病的关联。
HDL被认为是一种具有反动脉粥样硬化作用的脂蛋白。
较高的HDL水平与较低的心血管疾病风险相关。
本章将详细探讨HDL水平与心血管疾病的关联,以及其在糖尿病患者中的影响。
第四章:可能的机制第四章将探讨糖尿病和HDL水平之间的可能机制。
其中包括胰岛素抵抗、炎症反应、脂代谢异常等方面。
胰岛素抵抗是糖尿病发展的主要生理机制之一,也与HDL水平降低有关。
炎症反应在糖尿病中普遍存在,可能对HDL水平产生负面影响。
脂代谢异常可能干扰HDL的合成和代谢过程。
本章将详细探讨这些可能的机制,以提供更深入的理解。
第五章:糖尿病患者的管理策略第五章将介绍糖尿病患者的管理策略,重点关注提高HDL水平以减少心血管风险。
糖尿病患者通常需要药物治疗以控制其血糖水平,但同时应注意HDL水平的管理。
本章将介绍药物治疗和生活方式干预对HDL 水平的影响,并提供一些建议,以帮助糖尿病患者管理其心血管风险。
第六章:结论本文总结了糖尿病和HDL水平之间的关系和可能的机制,以及管理策略。
n engl j med 368;17 nejm.org april 25, 20131575The new england journal of medicine
established in 1812 april 25, 2013 vol. 368 no. 17Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk
W.H. Wilson Tang, M.D., Zeneng Wang, Ph.D., Bruce S. Levison, Ph.D., Robert A. Koeth, B.S., Earl B. Britt, M.D., Xiaoming Fu, M.S., Yuping Wu, Ph.D., and Stanley L. Hazen, M.D., Ph.D.
ABSTRACT
From the Department of Cellular and Molecular Medicine, Lerner Research In-stitute, Cleveland Clinic, Cleveland. Ad-dress reprint requests to Dr. Hazen at the Cleveland Clinic, 9500 Euclid Ave. NC-10, Cleveland, OH 44195, or at hazens@ ccf.org.
N Engl J Med 2013;368:1575-84.DOI: 10.1056/NEJMoa1109400Copyright © 2013 Massachusetts Medical Society.
BackgroundRecent studies in animals have shown a mechanistic link between intestinal micro-bial metabolism of the choline moiety in dietary phosphatidylcholine (lecithin) and coronary artery disease through the production of a proatherosclerotic metabolite, trimethylamine-N-oxide (TMAO). We investigated the relationship among intestinal microbiota-dependent metabolism of dietary phosphatidylcholine, TMAO levels, and adverse cardiovascular events in humans.
MethodsWe quantified plasma and urinary levels of TMAO and plasma choline and betaine levels by means of liquid chromatography and online tandem mass spectrometry after a phosphatidylcholine challenge (ingestion of two hard-boiled eggs and deu-terium [d9]-labeled phosphatidylcholine) in healthy participants before and after the suppression of intestinal microbiota with oral broad-spectrum antibiotics. We further examined the relationship between fasting plasma levels of TMAO and in-cident major adverse cardiovascular events (death, myocardial infarction, or stroke) during 3 years of follow-up in 4007 patients undergoing elective coronary angiog-raphy.
ResultsTime-dependent increases in levels of both TMAO and its d9 isotopologue, as well as other choline metabolites, were detected after the phosphatidylcholine challenge. Plasma levels of TMAO were markedly suppressed after the administration of anti-biotics and then reappeared after withdrawal of antibiotics. Increased plasma levels of TMAO were associated with an increased risk of a major adverse cardiovascular event (hazard ratio for highest vs. lowest TMAO quartile, 2.54; 95% confidence in-terval, 1.96 to 3.28; P<0.001). An elevated TMAO level predicted an increased risk of major adverse cardiovascular events after adjustment for traditional risk factors (P<0.001), as well as in lower-risk subgroups.
ConclusionsThe production of TMAO from dietary phosphatidylcholine is dependent on me-tabolism by the intestinal microbiota. Increased TMAO levels are associated with an increased risk of incident major adverse cardiovascular events. (Funded by the National Institutes of Health and others.)
The New England Journal of Medicine Downloaded from nejm.org at Harvard Library on December 24, 2014. For personal use only. No other uses without permission. Copyright © 2013 Massachusetts Medical Society. All rights reserved. The new england journal of medicinen engl j med 368;17 nejm.org april 25, 20131576T he phospholipid phosphatidylcho-line (lecithin) is the major dietary source of choline, a semiessential nutrient that is part of the B-complex vitamin family.1,2 Choline has various metabolic roles, ranging from its es-sential involvement in lipid metabolism and cell-membrane structure to its role as a precursor for the synthesis of the neurotransmitter acetylcho-line. Choline and some of its metabolites, such as betaine, can also serve as a source of methyl groups that are required for proper metabolism of certain amino acids, such as homocysteine and methionine.3There is a growing awareness that intestinal microbial organisms, collectively termed micro-biota, participate in the global metabolism of their host.4-6 We recently described the potential role of a complex phosphatidylcholine–choline metabolic pathway involving gut microbiota in contributing to the pathogenesis of atheroscle-rotic coronary artery disease in animal models.7 We also reported an association between a history of cardiovascular disease and elevated fasting plasma levels of trimethylamine-N-oxide (TMAO), an intestinal microbiota-dependent metabolite of the choline head group of phosphatidylcholine that is excreted in the urine.7-12Here, we examine the relationship between oral intake of phosphatidylcholine and the involve-ment of the intestinal microbiota in the forma-tion of TMAO in humans. We also examine the relationship between fasting plasma levels of TMAO and the long-term risk of incident major adverse cardiovascular events.