AD和diabetes
- 格式:pdf
- 大小:489.54 KB
- 文档页数:19
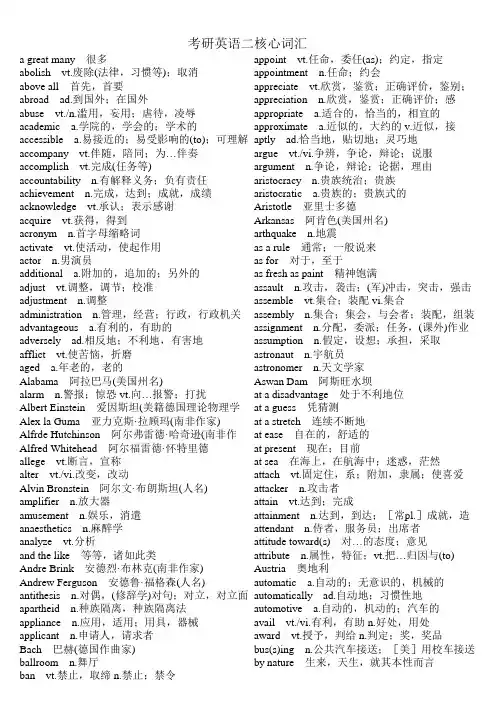
考研英语二核心词汇a great many 很多appoint vt.任命,委任(as);约定,指定abolish vt.废除(法律,习惯等);取消appointment n.任命;约会above all 首先,首要appreciate vt.欣赏,鉴赏;正确评价,鉴别;abroad ad.到国外;在国外appreciation n.欣赏,鉴赏;正确评价;感abuse vt./n.滥用,妄用;虐待,凌辱appropriate a.适合的,恰当的,相宜的academic a.学院的,学会的;学术的approximate a.近似的,大约的 v.近似,接accessible a.易接近的;易受影响的(to);可理解aptly ad.恰当地,贴切地;灵巧地accompany vt.伴随,陪同;为…伴奏argue vt./vi.争辨,争论,辩论;说服accomplish vt.完成(任务等)argument n.争论,辩论;论据,理由accountability n.有解释义务;负有责任aristocracy n.贵族统治;贵族achievement n.完成,达到;成就,成绩aristocratic a.贵族的;贵族式的acknowledge vt.承认;表示感谢Aristotle 亚里士多德acquire vt.获得,得到Arkansas 阿肯色(美国州名)acronym n.首字母缩略词arthquake n.地震activate vt.使活动,使起作用as a rule 通常;一般说来actor n.男演员as for 对于,至于additional a.附加的,追加的;另外的as fresh as paint 精神饱满adjust vt.调整,调节;校准assault n.攻击,袭击;(军)冲击,突击,强击adjustment n.调整assemble vt.集合;装配 vi.集合administration n.管理,经营;行政,行政机关assembly n.集合;集会,与会者;装配,组装advantageous a.有利的,有助的assignment n.分配,委派;任务,(课外)作业adversely ad.相反地;不利地,有害地assumption n.假定,设想;承担,采取afflict vt.使苦恼,折磨astronaut n.宇航员aged a.年老的,老的astronomer n.天文学家Alabama 阿拉巴马(美国州名)Aswan Dam 阿斯旺水坝alarm n.警报;惊恐 vt.向…报警;打扰at a disadvantage 处于不利地位Albert Einstein 爱因斯坦(美籍德国理论物理学at a guess 凭猜测Alex la Guma 亚力克斯·拉顾玛(南非作家)at a stretch 连续不断地Alfrde Hutchinson 阿尔弗雷德·哈奇逊(南非作at ease 自在的,舒适的Alfred Whitehead 阿尔福雷德·怀特里德at present 现在;目前allege vt.断言,宣称at sea 在海上,在航海中;迷惑,茫然alter vt./vi.改变,改动attach vt.固定住,系;附加,隶属;使喜爱Alvin Bronstein 阿尔文·布朗斯坦(人名)attacker n.攻击者amplifier n.放大器attain vt.达到;完成amusement n.娱乐,消遣attainment n.达到,到达;[常pl.]成就,造anaesthetics n.麻醉学attendant n.侍者,服务员;出席者analyze vt.分析attitude toward(s) 对…的态度;意见and the like 等等,诸如此类attribute n.属性,特征;vt.把…归因与(to) Andre Brink 安德烈·布林克(南非作家)Austria 奥地利Andrew Ferguson 安德鲁·福格森(人名)automatic a.自动的;无意识的,机械的antithesis n.对偶,(修辞学)对句;对立,对立面automatically ad.自动地;习惯性地apartheid n.种族隔离,种族隔离法automotive a.自动的,机动的;汽车的appliance n.应用,适用;用具,器械avail vt./vi.有利,有助 n.好处,用处applicant n.申请人,请求者award vt.授予,判给 n.判定;奖,奖品Bach 巴赫(德国作曲家)bus(s)ing n.公共汽车接送;[美]用校车接送ballroom n.舞厅by nature 生来,天生,就其本性而言ban vt.禁止,取缔 n.禁止;禁令barrier n.障碍;障碍物cadre n.干部;基础结构Basil D'Oliviera 贝兹尔·戴里维尔拉(南非板球运calculation n.计算,计算结果;仔细考虑basis n.基础,根据;主要成份;军事基地calculator n.计算者;计算器BBC 英国广播公司California 加利福尼亚(美国州名)be beneficial to 对…有益Cambridge 剑桥;剑桥大学be central to 对…极为重要的camera n.照相机,摄影机be concerned with 关于,涉及;忙于…;关心,campaign n.战役;运动 v.参加运动,参加竞be confronted with 面临,面对Camry 汽车牌名be contrary to 与…相反candidate n.候选人,候补者;应试者be deserving of 值得;应得capture vt.捕获;夺得,占领 n.捕获,捕获物be free from 没有…的;不受…的cargo n.船货,货物be incapable of 不会…,不能…cast vt.投,扔;投射;铸造 n.投,掷;模具be irrelevant to 与…不相干;不切题cease vt./vi./n.停止,结束be opposed to 反对Cees van Wendel de Joode 齐思·范·万德尔·德·be relevant to 与…有关cell n.细胞;小房间,单人牢房be subversive of 破坏…的certainty n.一定;必定be supposed to 应该Cesar Frank 弗兰克(法国比利时作曲家)be true of 符合于…,对…适用character n.性格,品质;特性;人物;符号,be unaware of 不知道…,没觉察到…Charles Richter 查尔斯·里克特be worth doing 值得做…chess n.国际象棋Beatle [the Beatles]披头士摇滚乐队Chile 智利Beethoven 贝多芬(德国作曲家)chip n.片屑;薄片;电子集成电路片,芯片belonging n.[常pl.]所有物;行李Cicely Saunders 茜西莉·桑德斯(人名) beloved a.为…所爱的;亲爱的 n.心爱的人,爱Cincinnati 辛辛那提(美国俄亥俄州西南部城Berkeley 伯克利;加利福尼亚大学伯克利分校circumstance n.[pl.]情况,环境;境遇Berlioz 柏辽兹(法国作曲家,指挥家及音乐评论circus n.马戏团,杂技团;马戏场,杂技场bias n.偏见 v.[常用被动语态]有偏见civil a.国民的,民用的;国内的,民间的binary a.二,双;二进制的 n.双(体);联星Clare Bolderson 克莱尔·博尔德森(人名) biomedical a.生物医学的clarification n.澄清,阐明Birmingham 伯明翰(英格兰中部城市)classify vt.把…分类,把…分等级;把…列为birthrate n.出生率classless a.无阶级的;不属于任何阶级的blindness n.无视,视而不见;盲目性clavichord n.(音)击弦古钢琴Bloke Modisane 布娄克·莫狄森(南非作家)clerk n.办事员,公务员;(美)店员blues n.布鲁斯;慢四步舞Clinton 比尔·克林顿(Bill Clinton)Bob Dylan 鲍伯·狄伦clipboard n.带弹簧夹子的书写板bodily a.身体的,肉体的cloudless a.无云的,晴朗的boring a.令人厌烦的clutch vt./vi.抓住,握紧boundary n.分界线,边界cobbler n.补鞋匠breadwinner n.养家糊口的人coin n.硬币,钱币 vt.创造(新词)brightness n.明亮,晴朗;聪敏,机灵coincide vi.一致,相符(with)Briton n.大不列颠人;英国人Colin Blackmore 科林·布莱克默(人名) budget n.预算 vt.把…编入预算;安排,预定collapse vt./vi./n.(使)倒塌,(使)崩溃;瓦解burden n.负担;责任,义务 vt.使负重担;麻烦collectively ad.总体地;集体地coloured a.有色的correctness n.正确,正确性coming a.正在到来的,即将来到的 n.来到,到correlation n.相互关系,关联communicator n.传播者,传播工作者correspondence n.符合,一致;通信community n.社区;共同体correspondent n.对应物;新闻通讯员,记者,companion n.同伴,同事;[天]伴星(=~star)cortisol n.[生]皮质(甾)醇compassion n.同情;怜悯(for)council n.理事会;委员会compel vt.强迫(to)county n.(英国)郡;县compensatory a.赔偿的,补偿的crash a.紧急的,速成的competition n.竞争;比赛creative a.创造性的competitive a.竞争的,比赛的creativity n.创造性competitor n.竞争者;对手crisis n.危机;决定性时刻completeness n.完整,圆满;完成,结束criterion n.标准,准则completion n.完成,结束;完满critical a.批评(性)的;紧要的,关键性的,危composer n.作曲家criticism n.批评;评论compulsion n.强制,强迫crowd n.群,人群 vi.聚集,群集computerize vt.电子计算机化,用电子计算机计curtail vt.截短,缩短;削减conception n.概念,观念concerned a.有关的;关切的,担心的Danie Carluccio 丹尼尔·卡鲁齐奥(人名) concession n.让步;特许权;租界,租界地datum n.([复]data)资料,材料;数据concrete a.具体的;混凝土的 n.混凝土 vt.使凝固David Morton 大卫·莫顿(人名)confront vt.面对,遭遇;正视,对抗day to day work 日常工作congress n.国会,议会;参议院,上院daydream vi./n.白日做梦congressman n.([复]congressmen) (美)国会议daytime n.白天,日间conquer vt.征服,战胜 vi.得胜,胜利debate vt./n.争论,辩论 vi.对…进行争论,辩Conrad Hilton 希尔顿(美国旅馆业巨头)Debussy 德彪西(法国作曲家) consciousness n.意识,知觉;觉悟decay vi./vt.(使)腐朽,腐烂;衰变 n.腐烂;衰conservative a.保存的,防腐的;保守的,守旧decline vi.下降;衰退vt.拒绝 n.下降;衰落consideration n.考虑;体谅,照顾deem vt.认为,相信conspiracy n.阴谋,密谋;阴谋集团,阴谋帮派defective a.有缺点的;有缺陷的constant a.永恒的,经久不变的;经常的 n.常数deficiency n.缺乏,不足constitutional a.宪法上规定的;组成的,构成的define vt.解释,给…下定义;限定,规定constraint n.强制;强制因素,制约条件definitive a.决定的,确定的;限定的,明确constructive a.建设的,建设性的degrade vt.降级,贬低;堕落;退化contemplate vt.注视,凝视;沉思delightful a.令人高兴的;讨人喜欢的content a.满足的,满意的 vt.使满意 n.满足,满demand for 对…的要求contented a.满足的,满意的democrat n.民主主义者,民主人士;民主党党contest n.竞争,比赛;争夺,竞争;争论,争辩democratic a.民主的,民主主义的continued a.继续的,连续的demographer n.人口学家contrary a.相反的,相对的,与…相反(to)demography n.人口统计学conventional a.惯例的,常规的;(艺术等)因袭density n.密集度,稠密度;[物][化]密度convict vt.证明…有罪(of);宣判 n.罪犯deny vt.否定,否认;拒绝接受,拒绝给予convince vt.使确认,使信服;使认识错误dependency n.从属;依赖(on)convincing a.有说服力的,使人信服的deport vt.驱逐出境cope vi.对付,妥善处理(with)deprive vt.夺去,剥夺;使失去(of)coronary a.冠的;冠状的 n.冠状动脉deserving a.应得的,值得的(of)correction n.改正,纠正;责备,惩罚desirable a.称心的,合意的,理想的despite prep.尽管,任凭earning n.警告;警报 a.警告的destination n.目的地,终点eastern a.东方的,东部的;向东方的,来自detail n.细节,详情 vt.详述,详细说明eclecticism n.折衷主义deteriorate vt./vi.(使)恶化economics n.[用作单或复]经济学;经济情developmental a.发展的,开发的;促使成长的economy n.经济;节约device n.装置,器械;方法,手段Edgar Varese 瓦雷兹(法裔美国作曲家) devotion n.献身,忠诚effect on 对…的作用diabetes n.糖尿病efficiency n.效率;功效,效能,实力differ vi.不同,相异(from);与…意见不同efficiently ad.效率高地;有能力地digital a.手指的,指状的;数字的,计数的effortless a.不作努力的;不费力的,容易的digression n.离题;偏离elderly a.较老的,人过中年的 n.近老年人diploma n.执照,特许证;毕业文凭,学位证书election n.选举;选举权diplomat a.外交家;外交官electoral a.选举的disabled a.伤残的;使失去战斗力的electorate n.全体选民;选区disaffection n.不满electronic a.电子的disapproval n.不赞成;不许可electronics n.[复][用作单]电子学discredit vt.使不可置信 n.丧失信义;不信,怀eliminate vt.排除,消灭discrepancy n.差异;不一致elite n.精英,杰出人物 a.杰出的,精英的disorder n.混乱;失调,紊乱 vt.使混乱;使失调elitist n.杰出人物 a.杰出人物(统治论)的distinct a.与其他不同的,独特的;明显的elsewhere ad.在别处;向别处distract vt.分散(注意,心思等);使人分心elude vt.(巧妙地)逃避,躲避distraction n.精神涣散,精神不集中;消遣,娱elusive a.躲避的;难以捉摸的,难以理解的distractor n.分散注意力的东西emergency n.紧急情况;突发事件district n.区,行政区;地区,区域employee n.雇员,雇工District of Columbia (美) (D.C.)哥伦比亚特区employment n.使用;雇佣;职业,工作ditch n.沟,沟渠 vt./vi.开渠;筑渠encounter vt./vi.遭遇,遇到n.遭遇,冲突;偶diversified a.多样化的enhance vt.提高;增强diversity n.多样性enjoyable a.愉快的;快乐的;有趣的division n.分开,分割;除(法);部门,科,处enrol(l)ment n.登记,接收,招生;招收人divisive a.造成不和的,制造分裂的ensure vt.保证,担保domestic adj.家庭的,家务的;国内的n.家仆,entity n.存在,实体;统一性dominance n.优势,控制,统治entrance n.进入;入口,门口;入场,入会,dominant a.占优势的;支配的entry n.进入;入口;登记,条目,账目Don Claxton 唐·克莱克斯顿(人名)equilibrium n.平衡,均衡,相称;均势;平静donation n.捐献;赠送equivalent a.相等的;等价的 n.等价(物);对dose n.(一次)剂量error n.谬误;错误dozen n.一打,十二个;十来个,十几个eruption n.(火山)喷发;(战争,感情等)爆发,dreamer n.做梦的人;空想家escalator n.自动扶梯dreamlike a.梦一般的,梦幻的escape vi./vt.逃跑;避免 n.逃跑;逃路,出口drift n.漂流;趋势,倾向 vi./vt.(使)漂流;漂泊establishment n.建立,创办;机构duel n./vi.决斗;(双方的)斗争esteem vt./n.尊敬,尊重duration n.持续;持续时间ethics n.伦理学;伦理观,道德标准Dutch a.荷兰人的;荷兰语的 n.荷兰人;荷兰语euthanasia n.无痛楚的死亡;安乐死Dutchman n.([复]Dutchmen)荷兰人evaluate vt.估价,评价dwarf n.矮子;[天]矮星eventual a.最后的,结局的dynamo n.[电]发电机;精力,精力充沛的人evident a.明显的,明白的evolve vt.使发展,推论 vi.进展;进化forecast vt.预测,预报;预示excellence n.优秀,杰出foreigner n.外国人exceptional a.例外的;异常的,特殊的formerly ad.以前,从前excerpt n.摘录,节录 vt.摘,引用foster vt.鼓励,促进;养育 a.收养的excessive a.过多的,过分的founder n.创始者;缔造者excitement n.刺激;兴奋frame n.构架,框架excretion n.排泄;分泌France 法兰西,法国execute vt.实行,执行,完成,贯彻;将…处死Francis Bacon 培根exert vt.尽(力);施加(压力等);行使(职权等)Franz Schubert 舒伯特(奥地利作曲家)expansion n.扩张;膨胀Frederick W.Taylor 弗雷德里克·泰勒expectation n.期待;估计寿命freshman n.新手,生手;大学一年级学生experiential a.经验的;凭经验的fruitful a.有成果的,有收获的experimental a.实验的;经验的functional a.功能的;职务上的;实用的explode vt.使爆炸 vi.爆炸;突发exploit vt.开发,开采;利用;剥削galaxy n.[天]星系,[G-]银河系expose vt.使暴露,使面临;揭露,揭发gamble vi./vt.赌博;打赌;投机 n.赌博;冒险extension n.伸张,伸展,扩大;电话分机gang n.一队,一族;一群,一帮extent n.广度,范围;程度gap n.裂口,裂缝external a.外在的,在外的Garm 加尔姆(俄国城市)eyeball n.眼球gathering n.聚集;集会eyewitness n.目击者;见证人George Bush 乔治·布什George McGovern 乔治·麦戈文fade vi.凋谢,枯萎;(颜色)褪去vt.使褪色George Wallace 乔治·华莱士faithfully ad.忠诚地;如实地Georgia 佐治亚(美国州名)far-fetched a.牵强的;未必会的,靠不住的Gesualdo 杰苏阿尔多(意大利作曲家)fault n.缺点,毛病;错误,过失;[地]断层giant n.巨人;巨物,巨大的动物 a.巨大的fearful a.可怕的,吓人的;害怕的,胆怯的Giuseppe Verdi 威尔弟(意大利作曲家,作歌feasible a.可行的,可能的glitter vi.闪闪发光,闪烁 n.闪光Ferris Greenlet 费里斯·格林里特(人名)goal n.目的,目标;得分进球,球门fiercely ad.凶猛地,凶残地;猛烈地going n.进行状况 a.进行中的;现行的Filipino n.菲律宾人(语) a.菲律宾人的;菲律宾goings and comings 来往;活动,发生的事finance n.财政,金融;经费,资金grab vt./n.攫取;抓取 vi.攫取;抓住(at) fireman n.消防队员graduate v.(使)(大学)毕业 n.大学毕业生,研flee vi./vt.(fled,fled)逃离;逃避grant vt.同意;准予 n.同意,授予;拨款flexible a.柔韧的,柔顺的;可变通的,灵活的gravity n.严肃,认真;重要性;[物]重力flight n.飞行,飞翔;航班,班机;逃跑,溃退grayscale 灰度Florence Nightingale 南丁格尔grip vt./n.紧握,紧夹;掌握,控制fluctuate vi.波动,起伏;动摇 vt.使波动,使起grouse n.松鸡fluctuation n.波动,起伏Guam 关岛(美国在西太平洋的重要海、空军focal n.焦点的Guatemala 危地马拉fold vt./vi.折叠;对折 n.褶(痕)guilt n.有罪;内疚folk n.人们;[口]家属,亲属 a.民间的guitar n.六弦琴,吉他foolishness n.愚蠢;可笑foolproof a.连傻子都懂的;不会出毛病的habitual a.习惯性的,习以为常的;惯常的for the sake of 为了…之好处;为了…的目的Haicheng 海城(中国辽宁省城市)fore ad.在前面 a.先前的;在前部的 n.前部handle n.柄,把手 vt.运用,操纵;经营,管Hannah Arendt 汉纳·阿伦特(人名)in other words 换句话说harmony n.协调,和谐;融洽,一致in part 部分地,在某种程度上Harry Emerson Fosdick 福斯迪克in power 掌权的,执政的Harry S. Truman 杜鲁门(美国第33任总统)in question 正被谈论的Harvard (美国)哈佛大学in reality 实际上,事实上headmaster n.(中学或小学的)校长in return 作为回报headquarters n.司令部,指挥部;总部,总店in series 串联电路的;成串联的healthcare n.保健in that 在于,原因是heartbreaking a.使人心碎的in the running 参赛,参加竞选hell n.地狱,阴间;用以咒骂或表示愤怒,不满in the strict sense 在严格的意义上Henry Kaiser 凯泽(美国实业家)in the way 挡路;碍事hiker n.徙步旅行者in time 及时;终于hinder vt.阻止;妨碍(from)in unison 完全一致地hormonal a.荷尔蒙的,激素的in vain 无效地;无结果地;徒然hospice n.(晚期病人)收容所inactive a.不活动的;不活跃的House n.[英]议院incapable a.无能力的;不能的(of) housekeeper n.管理家务的主妇;女管家incidence n.影响程度,影响范围;发生率How come...? 怎么会…?inconsiderate a.不替别人考虑的;不体谅人的Hugo Wolf 沃尔夫(奥地利作曲家)increasingly ad.不断增加地humanistic a.人文主义的,人本主义的,人道主indication n.指示,表示;象征,迹象humanity n.个性,博爱,仁慈;人类indifference n.冷漠;不感兴趣(to) Huntsville 亨茨维尔(城市名,位于阿拉巴马州individual n.个人,个体,独立单位 a.个人hysterical a.癔病的,歇斯底里的;患癔病的individualistic a.个人主义(者)的inefficiency n.无效;效能差identity n.同一,一致;身份,本体inefficient a.无效的;效率低的illegal a.非法的;违规的inevitable a.不可避免的,必然(发生)的illusion n.错觉;幻觉inevitably ad.不可避免地,必然地illustration n.说明;例证,插图infant n.婴儿,幼儿 a.婴儿的,幼儿的imitator n.模仿者infiltrate vt./vi.渗入,透过;浸润immigrant a.移民的,侨民的 n.移民,侨民infinitely ad.无限地,无穷地immigration n.移居;外来的移民inflexible a.不可弯曲的;不可改变的,固执impact n.冲击,碰撞;影响 vt.装紧,压紧inflict vt.使遭受(损伤,苦痛等),使承受impact on 对…之影响influence on 对…的影响impart vt.把…分给;给予(to)infrastructure n.基础;基础结构imperative a.绝对必要的;命令的,强制的;祈infrequent a.很少发生的implement vt.实现;完成;履行inhumane a.不人道的,残忍的implication n.含意,暗示;牵连,涉及,卷入initial a.最初的,开始的;词首的 n.首字母impoverished a.贫困的,赤贫的injection n.注射;注射剂,针剂improvise vt.即兴创作;临时准备,临时凑成inner a.内部的,里面的;思想的 n.内部;里in a sense 在某种意义上inspire vt.鼓舞;使产生灵感in advance 在前面;预先install vt.安装in between 在中间;每间隔;在…期间instantaneously ad.瞬间地;即刻地in favour of 赞成,支持;为…的利益,有利于instrument n.仪器;乐器in hand 手头上有integrate vt./vi.使结合,使并入;使成一体in large measure 很;大半;大部分intellectual n.知识分子 a.智力的,理智的in one's mind's eye 在脑海里intensity n.强烈,剧烈interchangeable a.可交换的;可互换的leading a.领导的,指引的;最重要的,主要internal a.内部的,内在的;国内的leaflet n.小叶,嫩叶;传单,活页interstate a.[主美]州际的leap v./n.(leapt或leaped)跳跃;飞跃interview vt./n.面谈,采访;面试,口试legal a.法律上的;合法的interviewer n.接见者;面谈者legislate vi.立法 vt.通过立法intimidate vt.恐吓,恫吓legislation n.立法;法律,法规invariably ad.不变地lengthen vt.使延长 vi.变长,延伸inventor n.发明者,创造者lesser a.较小的;更少的;次要的investment n.投资;(时间,精力的)投入lethal a.致死的irrelevant a.不相干的,离题的,与…不相干liability n.责任,义务;债务,负债;不利条irresponsible a.无责任感的,不负责任的liberty n.自由,自由权;冒昧,失礼;特许Isaac Newton 牛顿(英国物理学家,数学家和天lighting n.照明,照明设备It goes without saying that 不言而喻,理所当然light-sensitive a.光敏的ivy n.常青藤limitless a.无限制的,无限的link n.环节,联系 vt.用环连接;联系James Carville 詹姆斯·卡维尔Lionel Ngakane 莱昂内尔·恩卡纳(南非演员) jaw n.颔,颚literal a.精确的,如实的;逐字的,字面的jazz n.爵士乐litter n.干草;杂乱无章;一窝(仔畜) vt.铺草Jefferson Airplane 杰弗逊飞机(美国摇滚乐队名)Little Rock 小石城(美国阿肯色州首府) Jerome Singer 杰罗姆·辛格(人名)location n.定位,测位;位置,场所jet n.喷射;喷嘴;喷气式飞机,喷气发动机logic n.逻辑(学);逻辑性,条理性;理由,道Jimmy Carter 吉米·卡特longshoreman n.码头装卸工人Joan Freyberg 琼·弗雷伯格(人名)loom vi.隐隐呈现;逼近Johannes Brahms 勃拉姆斯(33-97年,德国作曲loose a.松的,宽的;宽松;放荡的John Anderson 约翰·安德森lovable a.可爱的,讨人喜欢的John Foster Dulles 杜勒斯(美国国务卿)loyalty n.忠诚;忠心John Major 约翰·梅杰(前英国首相)lvy League 常春藤联合会;名牌大学的John Rae 约翰·雷(人名)Lydia Garcia 莉迪亚·加西亚(人名)Johnson 约翰逊(美国第36任总统)jounalism n.新闻业;[总称]报刊;新闻学maid n.少女;侍女,女仆junction n.连接,结合;结合点,交叉点majority n.多数,大半;多数党,多数派juvenile a.青少年的 n.青少年maker n.制造者;制造商maladjustment n.失调;不适应环境Kathleen Weinstein 凯思琳·温斯坦(人名)manageable a.易管理的kid vt./vi./n.戏弄,开玩笑;欺骗,哄骗managerial a.经理的,管理人的;管理上的,kindergarten n.幼儿园Manitou Park 曼尼托公园kingdom n.王国;领域mankind n.[用作单或复]人类Kumari 库马里(人名)manual a.手的,用手(操作)的;体力的 n.手册kwashiorkor n.[医]恶性营养不良症marble n.弹子;大理石 a.大理石的,大理石般marvel(l)ous a.奇异的,惊人的;[口]了不La Traviata 茶花女(威尔第的歌剧名)materialism n.[哲]唯物主义,唯物论;物质laborer n.劳动者;工人measurement n.衡量,测量lag vi.走得慢,落后 n.落后,滞后mechanism n.[机]机构,机制;作用过程largely ad.大量地;主要地merchant n.商人launch vt.发射;使(船)下水 n.发射,(船)下水merit n.优点,长处;功绩,功劳leadership n.领导;[总称]领导人员merry a.欢乐的,愉快的Michael LaSane 麦克尔·拉森(人名)Northern 北爱尔兰microcassette n.微型卡式录音带northwestern a.在西北的,向西北的;来自西microscopic a.显微镜的;微观的;微小的,细not (never) for a moment 通常;多半mid-afternoon a.下午三点左右的not that... 并不是说Middletown 米道镇(镇名)notebook n.笔记本midst n.中间,当中 prep.(=amidst)在…当中notion n.概念;想法,看法Milky Way 银河;银河系(=Milky Way galaxy)nourishment n.滋补品,营养品Milton Kramer 弥尔顿·克莱默(人名)now that (连词)既然,由于miniature n.缩样,小型物 a.微型的,小型的minimum n.最小量;最低限度 a.最小的;最低objective n.目标,目的 a.客观的;无偏见的miniskirt n.超短裙obscure a.昏暗的;模糊的 vt.使暗,遮掩;使Miriam Makeba 末丽亚姆·马齐巴(南非演员)observatory n.天文台;了望台miserable a.悲惨的;可怜的observer n.遵守者,奉行者;观察者,监视者mislead vt.把…带错路,使…错或做错occupation n.占领;占有;职业misleading a.引入歧途的;使人误解的Ocean County 县名moderate a.中等的,适度的;温和的,有节制of...importance 有…重要性moderation n.温和,适度;缓和,减轻of...interest 有…兴趣modest a.谦虚的,谦恭的;适中的,不过分的of...value 有…价值mold n.(=mould)模子;模型 vt.用模子做,浇铸Ohio 俄亥俄州(美国州名)monopolize v.垄断;专卖old-boy n.老同学;(招呼用)老朋友,老弟,老monopoly n.垄断;专卖on and off 不时;断断续续地,间歇地motivate vt.作为…的动机,激发on average 平均motivation n.动机;动力on occasion 有时,间或Moussorgsky 穆索尔斯基(俄国作曲家)on one's head 归罪于某人,(责任)落到某人身moving a.活动的,移动的;动人的,令人感动on the alert 警戒,处于戒备状态multimedia a.多种手段的;多媒体的 n.多媒体on the contrary 相反地multiple a.多样的,复合的 n.倍数on the part of 就…而言muse vt./vi./n.沉思,冥想ongoing a.进行中的,前进的musically ad.在音乐方面;好听地;悦耳地operate v.运转,起作用;动手术;操作;经musician n.音乐家;作曲家oppose vt.反对,反抗;使相对,使对抗(to)opposition n.反对,反抗;对立,意见相反namely ad.即,也就是opt vi.抉择,选择(for),在…之间选择Nat Nakasa 纳特·纳卡萨(南非作家)optimal a.最适宜的;最理想的nationwide a.全国性的 ad.在全国范围内organizational a.组织(上)的neat a.整洁的;简洁的;整齐的originate vi./vt.发源;发生,发起need for 对…的需要out of power 丧失权力negative a.否定的;负的,阴性的 n.底片;负数out of step 步伐不一致;不协调neglect vt.忽视,忽略;疏忽 n.忽略;疏忽outcome n.结果,结局;出路,出口negotiation n.谈判,协商outer a.外部的Negro n.黑人 a.黑人的output n.产量;输出network n.[纺]网眼织物;网状物,网络outrage n.暴行;愤慨 vt.对…施暴;激怒New Jersey 新泽西州(美国州名)overcome vt.战胜;克服no other...than 除…外没有,只有;正是,就是overestimate vt.过高估计;过高评价nominate vt.提名;任命;命名oversupply vt./n.过多供应nomination n.提名;任命overturn vt./n.打翻;推翻,颠覆,毁灭 vi.翻nominee n.被提名者;被任命者overwhelming a.压倒之势的ownership n.拥有;所有权,所有制pledge v.发誓;保证 n.誓言,誓约;抵押品Oxbridge 牛津大学和剑桥大学;该校之学生plenty of 大量的;丰富的Oxford 牛津;牛津大学plus prep.加,加上 a.正的;附加的poet n.诗人p.a. system (=public address) system有线广播系point of view 观点painful a.痛苦的;费力的polio n.[医]脊髓灰质炎,小儿麻痹症painstaking a.苦干的;费力的poll n.选举;民意测验 vt.得到选票 vi.投票painter n.漆工;画家portray vt.描绘;描写;描述Palestrina 帕莱斯特里纳(意大利作曲家)positive a.确实的;积极的,肯定的;正的,palm n.手掌possess vt.具有,拥有panel n.专门小组possession n.有,拥有;[常pl.]占有物;财paradox n.似非而可能是的论点;自相矛盾的话postgraduate a.大学毕业后的,大学研究院的paralyse vt.使麻痹,使瘫痪;使无力,使气馁precede vt.先于…,比…优先 vi.在前面,领先parenting n.父母对孩子的养育precedent n.先例,前例parliament n.议会,国会;[P-]议会Precious Mackenzie 普莱舍斯·麦肯齐(南非举parliamentary a.议会的,国会的predict vt./vi.预言;预示partial a.偏袒的,偏心的,对…偏袒;部分的preliminary a.预备的;初步的 n.初试;预赛participant n.参加者 a.参与的prescribe vt.指示,规定;开处方,开药participation n.参加,参与presidency n.总统(或校长)职务(职权,任期);partly ad.部分地;在一定程度上presidential a.总统(校长)的;总统(校长)职务passive a.被动的;消极的prevail vi.胜过;流行,盛行passport n.护照prevalent a.流行的,普通的paternalistic a.家长式统治的;家长作风的primarily ad.首先,起初;首要地,主要地peaceful a.平静的,安宁的;和平的,和平方式prime a.最初的,基本的;主要的;最好的penetrating a.穿透的,贯穿的;深刻的,透彻的Princeton (美国)普林斯顿大学perchance ad.[古]偶然,意外地;可能,或许private a.私人的;私营的;秘密的,私下的performance n.执行;表现,工作性能;演出,privilege n.特权 vt.给予…特权periodicity n.周期性,间发性proceeding n.程序,进程;项目,活动,会议permissive a.容许的,许可的;随意的,开放的productivity n.生产率;丰饶,多产persist vi.坚持,固执(in);持续,存留profession n.职业(尤指脑力劳动或受过专业训persistently ad.坚持地;固执地profitability n.赚钱,获得personality n.个性;人格;品格profound a.深刻的,浑奥的personnel n.全体人员,全体职员;人事(部门)profusion n.丰富,大量;过分perspective n.透视,透视画法;远景,展望;观progressive a.进步的,先进的;渐次的,累进persuasion n.说服,劝服prohibition n.禁止;禁令pervasive a.弥漫的,渗透的;遍布的project n.设计,规划;项目 vt.方案,计划;pet n.宠物,爱畜 a.宠爱的,表示亲昵的prolong vt.延长;拉长pharmacological a.药物学的,药理学的promote vt.促进,发扬;提升,升级;发起,photocopy vt./n.复印,影印;照相复制本promotion n.促进;提升phrase n.短语,词语;习惯用语proof n.证据,证明;校样physiological a.生理的,生理学的proportion n.比率,比例 vt.使成比例,使相称physiology n.生理学prosecute vt.对…起诉,告发planet n.行星prosecutor n.起诉人;检察官,公诉人plantation n.种植园,大农场;植树造林prospect n.展望,景象;[常pl.]前景,前程plateau n.([复]plateaus或plateaux)高原prospective a.预期的;未来的plea n.请求,恳求;托词provision n.供应,供应品;条款,规定;给psychology n.心理学;心理replace vt.把…放回(原处);更换,以…替代publish vt.出版,刊印;公布,发表replacement n.复位,复职;替换,代替punishment n.处罚,罚,刑罚;折磨,损害replicate vt.重复;复制punk n.(俚)阿飞;朋克 a.颓废派的representation n.描写,表现;代表,代理pursue vt.追赶;追求,寻求;进行,从事representative n.代表,代表人 a.典型的,有代repressive a.镇压的;抑制的queue n.辫子;列队 vi.排队(for)reproduce vt.繁殖;再生产;复制;再现,重quicksand n.流沙reprogramme v.再次(重新)设定程序republican a.共和国的;共和党的 n.共和党党rabbit n.兔request vt./n.请求,要求racial a.种族的research n.研究,调查 vi.调查,研究racist n.种族主义者 a.种族主义的;种族歧视的reset vt./n.重新安排,重调radiation n.放射,发光;放射物,辐射线,辐射resettlement n.重新定居,重新安置radioactive a.[原]放射性的;放射引起的resistance to 对…的阻力radium n.镭resonance n.回声,反响;共振,共鸣radon n.氡respect for 对…的尊敬rating n.等级,规格; (电视)收视率respectively ad.各自地,分别地raw a.未煮过的,生的;未加工的response n.作答,回答;响应,反应readily ad.乐意地;很快地,容易地restriction n.限制;约束readjustment n.再整理,再调整resume n.摘要,梗概;个人简历realistic a.现实的,实际的;现实主义的retention n.保持;保留recognition n.认出;承认,公认revision n.修订,修改recorder n.记录者;录音机revolve vi.旋转;绕转recruit vt./vi.征募(新兵),吸收;补充 n.新成员rhythm n.韵律,格律;节奏reduction n.减少,减小;降级,降职;归纳,rhythmic a.有韵律的;有节奏的refine vt./vi.提纯,精制;使改进,变优雅rightly ad.公正地,正当地;合适地,恰当地refinement n.精炼,精制ritualize vt./vi.(行为模式)仪式化reflection n.反射,反映,映像;深思,考虑rivalry n.竞争;对抗regeneration n.新生,再生,复兴robot n.机器人;自动控制装置regrow vt.再生长,重新生长robotics n.机器人学,机器人技术regulate vt./n.管理;调节rock'n'roll n.摇滚乐,摇滚舞regulatory a.规章的;调节的Ronald Reagan 罗纳德·里根reinforce vt.增援,支援;加强,增加Ross Perot 罗斯·佩罗reinforcement n.增强,加固;强化rote n.死记硬背;机械的方法re-introduction n.重新采用,重新引入route n.路线;航线rejection n.拒绝,抵制;驳回routine n.日常工作 a.日常的;例行的;常规relaxation n.松弛,放松;缓和,减轻;休养running n.跑,赛跑;竞选relevant a.贴切的,中肯的;与…有关的(to)reliability n.可靠性safeguard vt.保护,捍卫 n.保护措施relief n.(痛苦,压迫等)减轻,宽慰;救济sake n.缘故religion n.宗教;宗教信仰salient a.突出的,凸起的;显著的remark vt./vi.说,评论,议论 n.评论,看法San Andreas fault (美)圣安德烈亚斯断层remedial a.治疗的,治疗上用的;补救的San Francisco 旧金山(或称三藩市)remedy n.治疗;补救(法) vt.治疗,补救satisfaction n.满意,满足renewal n.更新;重新开始Saudi n.沙特阿拉伯人 a.沙特阿拉伯(人或语) rephrase vt.重新措辞,改用别的话表示scale n.刻度,表度;规模;比例(尺);天平scarce a.缺乏的,不足的;稀有的,珍贵的speculation about 关于…猜测scheme n.计划;方案 vt./vi.计划,策划speechless a.不会说话的;不说话的schoolhouse n.(小学或乡村学校)校舍spokesman n.发言人;代言人secondary a.第二位的,次要的;中等的spontaneous a.自发的,本能的,自动的;出secretary n.秘书;书记;部长,大臣sportsman n.爱好运动的人;运动员Seine 塞纳河(法国北部河流,流经巴黎)spray n.水花;喷雾 vt.喷;喷涂 vi.喷;溅散seismic a.地震spur vt.激励,鞭策 n.踢马刺;刺激,激励,senior a.年长的;大学四年级的 n.年长者Sri Lanka 斯里兰卡sensible a.感觉得到的;明智的,明白事理的Stanford (美国)斯坦福大学sensitive a.敏感的;灵敏的,感光的Star of Bethlehem 圣诞星sensitivity n.敏感性;灵敏度starvation n.饥饿;饿死sentiment n.感情,情绪;感伤starve vi.饿死;挨饿;极需,渴望 vt.使挨饿separation n.分开,分类;分隔statistically ad.在统计方面serene a.安详的;宁静的statistics n.统计数字,统计资料;[用作单]series n.一系列,连续;丛书,套;[电]串联status n.情形,状况;地位,身份sexually ad.在性方面steadily ad.稳固地;稳定地shackle n.[常pl.]镣铐;[pl.]束缚,枷锁sterile a.不育的,贫瘠的;无菌的,消过毒的shade n.荫,阴影 vt.遮蔽,遮光sterility n.不生育,不结果,贫瘠;消毒,无shelf n.(壁橱,书橱内)搁板;架子Steve Makone 史蒂夫·马孔(南非足球运动员) shift vt./vi.替换;转移 n.转换,转移;轮班stir vt.搅拌;激起 vi.走动;活动 n.惊动shocking n.令人震惊的,骇人听闻的stopwatch n.(赛跑用的)跑表 vt.用秒表测定时。


Aacclimatization [əklaimətaizeiʃən] n环境适应性accommodation [ə,kɔmə'deiʃən] n 住处accumulation [əkju:mjʊ’leiʃ(ə)n] n 积聚,累积,积聚物acetic acid n 乙酸acid [æsid] adj酸味的,酸的尖刻的n〈化>酸酸味物质acidification [əsidifikeiʃən]n 使发酸,酸化,成酸性ad libitum [æd`libitəm] adv 随意,自由采食additive [’æditiv] n 添加剂algae ['ældӡi:] n 海藻alimentary canal n 食道Amino Acids,their salts and analogues: 氨基酸盐及其类似物ammonia [æməunjə]n氨,氨水ammonian n 氨水animal feed ingredient 动物饲料成分antibiotic[æntibai'ɔtik] a(adj) 抗菌的n 抗生素antigen ['æntidʒən]n 抗原antinutritional adj 抗营养的 appetite ['æpitait]n 食欲,胃applicable ['æplikəbəl] adj 合适的,适用的applicable feed category:适用饲喂类群appraisal [əpreizəl] n 估计,估量,评价approximate adj 大约的,近似的ash [æʃ] n. 灰分assess v 估定,评定attain v 达到,获得attenuate [ətenjueit] vt使变细减弱, 贬值aujeszky's disease 伪狂犬病Bbacteria[bæk’tiəriə] n 细菌betterment ['betəmənt] n改善,改进BHBA β-羟基丁酸bioavailability[͵baiəuə͵veilə’biliti] n 生物学效价biodiversity [baiəudaivə:siti] n 生物品种biotin [’baiətin] n 生物素,boar [bɔ:] n (未阉割)公猪bovine ['bəuvain] adj 牛的; n 牛bovine spongiform encephalopathy 疯牛病breeding ['bri:diŋ] n 生育;育种繁殖; 饲养教养; 熏陶; 训练breeding stock 种畜brucellosis[͵bru:sə'ləusis] n 布鲁氏菌病,地中海热,马耳他热buffer [bʌfə] n 起缓冲作用的人(或物)<机〉缓冲器,减震器vt 缓冲,减轻buildup 富集,积累bulk milk 散奶,原料奶bulk milk collection 收集牛奶butyric acid n 丁酸Ccalculate [kælkjuleit] vt & vi 计算,估计calf [kɑ:f]n呆子;犊 ;腓;小牛calving date 分娩日期:对本胎次而言.carbohydrate['kɑ:bəu'haidreit] n碳水化合物; 糖类淀粉质或糖类食物carbon dioxide 二氧化碳castrate vt 阉割catalyst [kætəlist] n〈化〉催化剂,触媒促进因素; 有感染力的人, 能激发对方的人catecholamine [kætikəuləmi:n] n[生化]儿茶酚胺category [kætiɡəri] n种类, 类别cellulose [’seljuləus] n纤维素cereal [siəriəl]n谷类植物, 谷物谷类食物, 麦片粥chloride ['klɔ:raid]n [化]氯化物choline [’kəuli:n]n胆碱chromium['krəumjəm]n〈化〉铬chromium picolinate ['krəumjəm]吡啶羧酸铬coenzyme [kəu’enzaim]n辅酶compromised [’kɔmprəmaiz] adj缺乏抵抗力的,缺乏免疫力的concentrate feed 浓缩料concentration [kɔnsəntreiʃən]n专心,专注;集中,集结conception [kənsepʃən]n怀孕confinement [kənfainmənt] n产期, 分娩conjugated [kɔndӡʊɡeɪtɪd] adj 共轭的,成对的consumption [kən’sʌmpʃən]n消费contaminant [kəntæminənt]n 致污物,污染物contaminate [kəntæmineit] vt 把…弄脏, 污染contamination [kən͵tæmi’neiʃən] n 玷污,污染,污染物contentious [kən’tenʃəs] adj 容易引起争论的,好争论的,有异议的copulation [͵kɔpju'lei∫ən] n 性交,交配coronary [’kɔrənəri] adj冠状动脉的counteract [kauntərækt]vt 对抗; 抵消cowpea [kaupi:] n 豇豆cremate [krɪ'meɪt] v 火葬;焚化crude protein 粗蛋白质crust [krʌst] n 外壳,坚硬的外壳,面包皮 v 盖以硬皮,结硬皮crustacean [krʌs’tei∫jən] adj,n 甲壳类的(动物)cystine [`sɪsti:n,-tɪn] n 胱氨酸Ddairy [dɛəri] n 牛奶场,乳品店dairy cow 奶牛dairy form 乳房质地dairy herd improvement (DHI) 奶牛群改良dam [dæm] n 母畜detergent [di’tə:dʒənt]adj 洗涤的detritus [di’traitəs] n 残屑,沉渣,腐质diabetes mellitus [’melitəs] n 糖尿病digestible [didʒestəbl] adj 可消化的digestive systems 消化系统dilute [dai’lju:t] v 冲淡,稀释DIM (days in milk) 干物质采食量(泌乳天数)direction [di'rekʃən,dai'rekʃən]n. 指导,用法说明disaccharide [dai'sækəraid]n 二糖,双糖dissolve [kɔndʌkt] vt & vi (使)溶解distributor’s name and address in EU:欧盟经销商的姓名和地址disturbance [distə:bəns] n 打扰,扰乱diversification [daivə:sifi’kei∫ən] n 变化,多样性diversity[dai'və:siti] n 多样化,多样性DL—Methionine DL-蛋氨酸domestic animals n 家畜domestication [dəʊ,mesti'keiʃən] n 驯服,教化dominance [dɔminəns]n 优势;支配地位;控制力dominate [dɔmineit] vt & vi 控制,支配,统治;在…中占首要地位dominated 占主导地位的due date—预产期:以上次的配种日期计算出的预产期.duration [djuəreiʃən] n 持续,持续的时间,期间Duroc [’djuərɔk] n 杜洛克猪enteric [enterik] adj 肠的enterocyte n 肠道细胞epinephrine [͵epi’nefrin—ri:n] n [生化]肾上腺素(o)estrus['i:strəs] n 发情oestrus cycle 发情周期estrous cycle manipulation 发情周期调控estrus synchronization 发情同期化euthanasia [juːθə’neɪzjə] n 安乐死eutrophication [ju:trɔfikeiʃən] n 超营养作用excrement [’ekskrimənt] n 粪便excreta [ekskri:tə] n排泄物(如汗、尿、粪等)excrete vt 排泄,排除,分泌excretion [ekskri:ʃən] n (动植物的)排泄,排泄物excretory [ekskri:təri] adj 排泄的,分泌的,有排泄功能的extract [ekstrækt] vt (费力地)拔出,抽出提取,榨出n 摘录, 引用提炼物,浓缩物F%—乳脂率:测定日乳样的乳脂肪百分比.F. Teat Place 前乳头位置F/P-乳脂与蛋白比:测定日乳样中乳脂肪与乳蛋白的比值。

眼科英文缩写对照表收集者:罗志杰abd┃abdominal 腹部的ABK┃aphakic bullous keratopathy无晶体性大泡性角膜病变abn┃abnormal异常ac┃before meals餐前AC┃anterior chamber前房ACC┃accommodation调节ACG┃angle-closure glaucoma闭角青光眼ACIOL┃anterior chamber intraocular lens前房型眼内晶体add┃adduction or reading additional power内收;阅读另加屈光力ad lib┃as desired当需要时adv┃advanced高度AIBSE┃acute idiopathic blind spot enlargement急性特发性盲点扩大综合征AIDS┃acquired immunodeficiency syndrome艾滋病AION┃arteritic ischemic optic neuropathy动脉缺血性视神经病变┃anterior ischemic optic neuropathy前部缺血性视神经病变AKS┃ankylosing spondylitis强直性脊椎炎AL┃argon laser氩激光ALPI┃argon laser peripheral iridoplasty氩激光周边虹膜成形术ALT┃argon laser trabeculoplasty氩激光小梁成形术AMA┃against medical advice违反医疗忠告ambl┃amblyopia弱视AMD┃age-related macular degeneration年龄相关性黄斑变性AMN┃acute macular neuroretinopathy急性黄斑神经视网膜病变AMPPE┃acute multifocal placoid pigment epitheliopathy 急性多病灶性盾鳞状色素上皮病变AMPPPE┃acute multifocal posterior placoid pigment epitheliopathy急性多病灶性后部盾鳞状色素上皮病变ANA┃antinuclear antibody抗核抗体ant┃anterior前APMPPE┃acute posterior multifocal placoid pigment epitheliopathy 急性后部多病灶性盾鳞状色素上皮病变AODM┃adult-onset diabetes mellitus成人(发病)型糖尿病AP┃anteroposterior前后APD┃afferent pupillary defect瞳孔传入缺陷approx┃approximately大约APTT┃activated partial thromboplastin time激活部分促凝血酶原激酶时间ARC┃anomalous retinal correspondence异常视网膜对应ARMD┃age-retinal pigment epitheliitis年龄相关性黄斑变性ARN┃acute retinal necrosis急性视网膜坏死ARPE┃acute retinal pegment epitheliitis急性视网膜色素上皮炎art┃artificial tears人工泪液AS┃anterior synechia前粘连ASA┃aspirin(acetylsalicylic acid)阿司匹林ASAP┃as soon as possible尽快地ASC┃anterior subcapsular cataract前囊下白内障ASCVD┃atherosclerotic cardiovascular disease动脉硬化性心血管病ASHD┃atherosclerotic heart disease动脉硬化性心脏病astig┃astigmatism散光ATR┃against-the-rule(astigmatism)逆规性(散光)AU┃anterior uveitis前葡萄膜炎AVM┃arteriovenous malformation动静脉畸形A/V┃arteriolar/venous(ratio,nicking)小动脉/静脉(比,压痕)AZOOR┃acute zonal occult outer retinopathy急性区域性隐匿性外层视网膜病变BBas┃basophils嗜碱细胞BC┃base curve基底曲线BCC┃basal cell carcinoma基底细胞癌BCP┃birth control pill避孕丸BCR┃base curve radius基底弯曲半径BCVA┃best corrected visual acuity最佳矫正视力BD┃base down底向下BDR┃background diabetic retinopathy背景型糖尿病性视网膜病变BI┃base in底向内bid┃twice a day一日两次bilat┃bilateral两侧性BIO┃binocular indirect ophthalmoscope双眼间接检眼镜BK┃bullous keratopathy大泡性角膜病变bleph┃blepharitis睑缘炎BM┃basement membrane基底膜BO┃base out 底向外BP┃blood pressure血压BRAO┃branch retinal artery occlusion视网膜分枝动脉阻塞BRVO┃branch retinal vein occlusion视网膜分枝静脉阻塞BU┃base up底向上BUT┃break-up time泪膜破裂时间BVA┃best visual acuity最佳视力bx┃biopsy活检Cc┃with与c┃cornea角膜CA┃carcinoma癌CABG┃coronary artery bypass graft冠状动脉旁路移植CAD┃coronary artery disease冠状动脉病CAI┃carbonic anhydrase inhibitor碳酸酐酶抑制剂Cap ┃capsule胶囊CAR┃cancer-associated retinopathy癌相关性视网膜病变cat┃cataract白内障CAT┃computed axial tomography计算机体层摄影术CB┃ciliary body睫状体CBC┃complete blood count完全血细胞计数cc┃with correction矫正(视力)cc┃chief complaint主诉CCA┃common carotid artery颈总动脉CCT┃computer-assisted corneal topography角膜地形图仪C/D┃cup-to-disc ratio杯/盘比CF┃count fingers数指CFZ┃capillary-free zone无毛细血管地带C&F┃cells and flare细胞和光带(闪光)CHF┃congestive heart failure充血性心力衰竭CHI┃closed head injury闭合性头颅外伤CHRPE┃congenital hypertrophy of retinal pigment epithelium先天性视网膜色素上皮肥厚CK┃conductive keratoplasty传导性角膜成形术CL┃contact lens,clear接触镜,透明CME┃cystoid macular edema囊样黄斑水肿cmv┃cytomegalovirus巨细胞病毒CN┃cranial nerve脑神经CNS┃central nervous system中枢神经系统CNV┃choroidal neovascularization脉络膜新生血管形成CNVM┃choroidal neovascular membrane脉络膜新生血管膜c/o┃complaining of主诉COAG┃chronic open-angle glaucoma慢开角青光眼cong┃congenital先天性conj┃conjunctiva结膜COP┃cicatricial ocular pemphigoid瘢痕性眼类天疱疮COPD┃chronic obstructive pulmonary disease慢性阻塞性肺病CP┃cerebral palsy大脑麻痹CPEO┃chronic progressive external ophthalmoplegia慢性进行性眼外肌麻痹CPR┃cardiopulmonary resuscitation心肺复苏术CR┃chorioretinal脉络膜视网膜CRAO┃central retinal artery occlusion视网膜中央动脉阻塞CRF┃chronic renal failure慢性肾衰竭CRT┃corneal refractive therapy角膜屈光矫正法CRVO┃central retinal vein occlusion视网膜中央静脉阻塞C&S┃culture and sensitivity培养和敏感性CSCR┃central serous chorioretinopathy中心性浆液性脉络膜视网膜病变CSF┃cerebrospinal fluid脑脊髓液CSME┃clinical significant macular edema临床意义性黄斑水肿CSR┃central serous retinopathy中心性浆液性视网膜病变csw┃corneoscleral wound角膜巩膜创伤CT┃cover test遮盖试验┃computed tomography计算机体层摄影CT scan┃computed tomographic scan计算机体层扫描CV┃color vision色觉CVA┃cerebrovascular accident脑中风CVD┃cardiovascular disease 心血管病CWS┃cotton-wool spot棉絮斑Cx┃culture(微生物)培养CXR┃chest x-ray film胸部X光片cyl┃cylinder柱镜d┃days天DD┃diopter or diameter屈光度或直径D/C┃discontinue停止D&C┃deep and clear(前房)深而透明DD┃disc diameter视盘直径DDx┃differential diagnosis鉴别诊断DFE┃dilated fundus examination扩瞳检查眼底D&I┃dilation and irrigation(泪道)扩张和灌洗DHD┃dissociated horizontal deviation分离性水平偏斜dia┃diameter直径disp┃dispense配药DM┃diabetes mellitus糖尿病DR┃diabetic retinopathy糖尿病视网膜病变DV┃distance vision远距离视力DVA┃distance vision acuity远距离视力DVD┃dissociated vertical deviation分离性垂直偏离DW┃daily wear每日戴(接触镜)Dx┃diagnosis诊断DX┃Diamox醋氮酰胺EE┃esophoria at far内隐斜(视远)E’ ┃esophoria at near内隐斜(视近)ECA┃external carotid artery颈外动脉ECCE┃extracapsular cataract extraction白内障囊外摘除术EDTA┃ethylene dianline tetraacctic acid乙烯dianline tetraacctic酸EEG┃electroencephalogram脑电图EF┃eccentric fixation离心注视EKC┃epidemic keratoconjunctivitis流行性角结膜炎EKGorECG┃electrocardiogram心电图ELISA┃enzyme-linked immunosorbent assay酶连结免疫吸收测定EMM┃epimacular membrane黄斑表面膜endo┃endothelium内皮细胞ENT┃ear,nose,throat耳鼻喉EOG┃electro-oculogram眼电图EOM┃extraocular muscles眼外肌eos┃eosinophil嗜酸型细胞epi┃epithelium上皮细胞ER┃emergency room急诊室ERG┃electroretinogram视网膜电图ERM┃epiretinal membrane视网膜表面膜ESR┃erythrocyte sedimentation rate血沉ET┃esotropia at far内斜视(视远)ET’ ┃esotropia at near内隐斜(视近)E(T)orIET┃intermittent esotropia at far间歇性内隐斜(视近)E(T)’orIET’┃intermittent esotropia at near间歇性内隐斜(视远)ETOH┃ethyl alcohol乙醇,酒精EUA ┃examination under anesthesia全麻下检查EW┃extended wear长期戴用(接触镜)EXT┃external ocular examination外眼检查exoph┃exophthalmos眼球突出FFA┃fluorescein angiography荧光素血管造影FAZ┃fluorescein angiography中心凹无血管地带FB┃foreign body异物FBS┃fasting blood sugar空腹血糖FC┃finger count数指FFA┃fundus fluorescein angiography荧光素眼底血管造影FHorFHx┃family history家族史fl┃fluorescein荧光素FMHx┃family medical history家族病史FML┃fluorometholone弗米隆FOHx┃family ocular history家族眼病史FR┃foveal reflex中心凹反光FROM┃full range of motion运动范围完整FTA-ABS┃fluorescent treponemal antibody absorption test荧光密螺旋体抗体吸收试验FTMH┃full-thickness macular hole黄斑全层裂孔f/u┃follow-up随访GGC┃gonorrhea or gonococcus淋病或淋菌GCA┃giant cell arteritis巨细胞性动脉炎gent┃gentamicin庆大霉素GI┃gastrointestine胃肠GLC┃glaucoma,glucose青光眼,葡萄糖fonio┃gonioscopy前房角镜检查GPC┃giant papillary conjunctivitis巨乳头性结膜炎gr┃grain 谷(=0.065克)gt or gtt┃drop(Plural,gtt)滴(复数,gtt)GTT┃glucose tolerance test葡萄糖耐量试验GVF┃Goldmann visual field Goldmann视野HH or hr(hrs) ┃hour小时HA┃headache头痛Hb,Hgb┃hemoglobin血红蛋白HCL┃hard contact lens硬性接触镜Hct┃hematocrit血细胞比容HCTZ┃hydrocholorothiazide呋塞米(双氢克尿塞)hem┃hemorrhage出血HIV┃human immunodeficiency virus人类免疫缺陷病毒HLA┃histocompatibility locus antigen or human leukocyte antigen组织相容性基因位点抗原或人类白细胞抗原h/o┃history of历史HM┃hand motion手动H&P┃history and physical病史和体格hs┃at bedtime在就寝时间HSK┃herpes simplex keratitis单纯疱疹性角膜炎HSV┃herpes simplex virus单纯疱疹病毒HT┃hypertropia at far上斜视(远距离)HT’ ┃hypertropia at near上斜视(近距离)HTN┃hypertension高血压HVF┃Humphrey visual field Humphrey视野Hx┃history历史HZO┃herpes zoster ophthalmicus眼带状疱疹II┃iris虹膜I&A┃irrigation and aspiration灌注和抽吸IBx┃incisional biopsy切口性活检IC┃iridocyclitis虹膜睫状体炎ICA┃internal carotid artery颈内动脉ICCE┃intracapsular cataract extraction白内障囊内摘除术ICE┃inidocorneal endothelial syndrome虹膜角膜内皮综合征ICG┃indocyanine green吲哚青绿ICP┃intracranial pressure颅内压ICU┃intensive care unit监护病房I&D┃incision and drainage切开引流术IDDM┃insulin-dependent diabetes mellitus胰岛素依赖糖尿病IDU┃idoxuridine疱疹净IFIOL┃iris-fixated intraocular lens虹膜固定眼内晶体Ig┃immunoglobulin免疫球蛋白IK┃interstitial keratitis基质性角膜炎Im┃intramuscular肌内Imp┃impression印象inf┃inferior下inj┃injection注射INO┃internuclear ophthalmoplegia核间眼肌麻痹INQ┃inferonasal quadrant鼻下象限int┃intermittent间歇性IOFB┃intraocular forergn body眼内异物IOH┃intraocular hemorrhage眼内出血IOL┃intraocular lens眼内晶状体,人工晶体ION┃ischemic optic neuropathy缺血性视神经病变IOP┃intraocular pressure眼压IR┃infrared红外线IRMA┃intraretinal microvascular abnormality视网膜内微血管异常ITQ┃inferotemporal quadrant颞下象限iu┃international unit or intermediate uveitis国际单位或中间葡萄膜炎IV┃intravenous静脉内JJCT┃Juxtacanalicular Tissue管旁组织JODM┃juvenile-onset diabetes mellitus青少年型糖尿病JRA┃juvenile rheumatoid arthritis少年类风湿性关节炎KK┃cornea or potassium角膜或钾KCS┃keratoconjunctivitis sicca干燥性角膜结膜炎kg┃kilogram公斤KP┃keratic precipiate角膜后沉着物LL┃left or lens左或晶状体lab┃laboratory试验室lac┃lacrimal泪LASEK┃laser subepithelial keratomileusis激光上皮下角膜磨削术LASIK┃laser in situ keratomileusis激光原位角膜磨削术LCG┃light contrast glare光对比炫耀LCTP┃lateral canthal tendon plication外眦腱折叠术LI┃laser iridotomy or laser interferometry激光虹膜切开术或激光干涉仪LK,LKP┃lamellar keratoplasty板层角膜移植术LIO┃left inferior oblique左眼下斜肌LIR┃left inferior rectus左眼下直肌LL┃lower lid or lids and lashes下睑或眼睑和睫毛LLR┃left lateral rectus左眼外直肌LMR┃left medial rectus左眼内直肌LN┃lymph node淋巴结LP┃light perception,light projection,or lumber puncture光感,光定位,或腰椎穿刺LPI┃laser peripheral iridoplasty激光周边虹膜成形术LS┃lid scrubs睑缘擦洗(治疗睑缘炎)LSO┃laser scanner ophthalmolscope激光扫描检眼镜LSO┃left superior oblique左眼上斜肌LSR┃left superior rectus左眼上直肌LTP┃laser trabeculoplasy激光小梁成形术LTQ┃lower temporal quadrant颞下象限LVA┃low vision aids低视力辅助lymph┃lymphocytes or lymphatic淋巴细胞或淋巴。
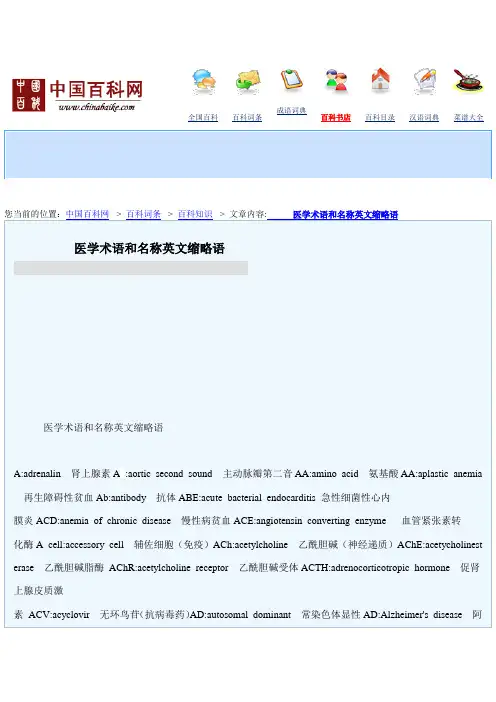
全国百科百科词条成语词典百科书店百科目录汉语词典菜谱大全您当前的位置:中国百科网-> 百科词条-> 百科知识-> 文章内容:医学术语和名称英文缩略语医学术语和名称英文缩略语医学术语和名称英文缩略语A:adrenalin肾上腺素A:aortic second sound主动脉瓣第二音AA:amino acid氨基酸AA:aplastic anemia 再生障碍性贫血Ab:antibody抗体ABE:acute bacterial endocarditis 急性细菌性心内膜炎ACD:anemia of chronic disease慢性病贫血ACE:angiotensin converting enzyme血管紧张素转化酶A cell:accessory cell辅佐细胞(免疫)ACh:acetylcholine乙酰胆碱(神经递质)AChE:acetycholinest erase乙酰胆碱脂酶AChR:acetylcholine receptor乙酰胆碱受体ACTH:adrenocorticotropic hormone促肾上腺皮质激素ACV:acyclovir无环鸟苷(抗病毒药)AD:autosomal dominant常染色体显性AD:Alzheimer's disease阿尔茨海默氏病ADA:adenosine deaminase腺苷脱氨酶ADCC:antibody-dependent cellular cytotoxicity抗体依赖性细胞介导细胞毒作用ADD:attention deficit disorder注意缺陷障碍(即多动综合征)ADH:antidiuretic hormone抗利尿激素ADP:adenosine diphosphate二磷酸腺苷ADR:adverse drug reaction药物不良反应AFB:acid-fast bacilli抗酸杆菌AFP:alpha fetoprotein甲胎蛋白AG:anion gap 阴离子间隙A/G:albumin/globulin ratio白/球蛋白比率Ag:antigen抗原AGL:acute granulocytic leukemi a 急性粒细胞白血病AGN:acute glomerulonephritis急性肾小球肾炎AHF:antihemophilic factor抗血友病因子AI:aortic insufficiency主动脉瓣关闭不全AI:artificial insemination人工授精AID:artificial inseminati on with doner's semen非配偶间人工授精AIDS:acquired immunodeficiency syndrome 获得性免疫缺陷综合征,艾滋病AIH:artificial insemination with husband's semen配偶间人工授精AIHA:autoimmune hemolytic anemia自身免疫性溶血性贫血AISN:acute interstitial nephritis 急性间质性肾炎AKP:alkaline phosphatase碱性磷酸酶Ala:alanine 丙氨酸ALA:aminolevulinic acid氨基酮戊酸(卟啉前体)ALD:aldolase醛缩酶ALG:antilymphocyte glo bulin抗淋巴细胞球蛋白ALL:acute lymphoblastic leukemia急性淋巴细胞白血病ALP:alkaline phosphatase碱性磷酸酶ALS:amyotrophic lateral sclerosis 肌萎缩性侧索硬化症ALS:antilymphocyte serum抗淋巴细胞血清ALT:alanine aminotransferase 丙氨酸转氨酶(即SGPT)AML:acute myeloblastic leukemia 急性原粒细胞性白血病AMMOL:acute myelomonoblastic leukemia 急性髓单核细胞性白血病AMOL:acute monoblastic leukemia 急性单核细胞性白血病AMS:acute mountain sickness急性高山病AMS:atypical measles syndrome非典型麻疹综合征AMY: amylase淀粉酶AN:analgesic nephritis止痛药肾炎ANA:antinuclear antibody抗细胞核抗体ANP:atrial n atriuretic peptide心房利钠肽(即心钠素)Anti-HBc:antibody to hepatitis B core antigen 抗乙型肝炎核心抗体Anti-HBe:antibody to hepatitis B e antigen抗乙型肝炎e抗体Anti-HBs:antibody to hepatitis B surface antigen抗乙型肝炎表面抗体(即AuAg)ANUG:acute necrotizing ulcerative gingivitis急性坏死性溃疡性龈炎AP:alternate pathway旁路途径(补体活化)APB:atrial premature beat房性期前收缩APC:antigen-presenting cell抗原呈递细胞APC:acute pharyngoconjunctival fever急性咽结合膜热APP:acute-phase protein急性期蛋白APRT:adenine phosphoribosyltransferase腺嘌呤磷酸核糖转移酶(嘌呤回收途径)APTT:activated partial thromboplastin time活化部分凝血活酶时间APUD:amine precursor uptake and decarboxylation胺前体摄取和脱羧(细胞)AR:aortic regurgitation主动脉反流AR:autosomal recessive常染色体隐性AR A-A:adenine arabinoside阿糖腺苷(抗病毒药)ARA-C:cytosine arabinoside阿糖胞苷(抗癌药)ARAS:as cending reticular activation system上行网状激活系统ARC:AIDS-related complex艾滋病相关复合征ARD:acute respiratory disease急性呼吸道病ARDS:adult respiratory distress syndrome成人呼吸窘迫综合征ARF:acute renal failure急性肾功能衰竭ARF:acute rheumatic fever急性风湿热AS:aortic s tenosis主动脉瓣狭窄AS:ankylosing spondylitis强直性脊柱炎Arg:arginine精氨酸ASA:acetylsalicylic ac id乙酰水杨酸ASD:Alzheimer's senile dementia 阿尔茨海默氏老年性痴呆ASD:atrial septal defect房间隔缺损Asn:asparagine天门冬酰胺ASO:antistreptolysin O抗链球菌(溶血)素OAsp:aspartic acid天门冬氨酸AST:aspartate aminotransferase天门冬氨酸转氨酶(即SGOT)AT:angiotensin血管紧张素ATL:adult T cell leukemia成人T细胞白血病ATN:acute tubular necrosis急性肾小管坏死ATP:adenosine triphosphate三磷酸腺苷AuAg:Australia antigen澳大利亚抗原(即Anti-HBs),澳抗AUC:area under concentration curve药-时曲线下面积AV:atrioventricalar房室AV:arteriovenous动静脉AVF:(augmented voltage,left leg) 加压单极左腿导联AVL:(augmented voltage,left arm) 加压单极左臂导联AVM:arteriovenous malformation动静脉畸形AV node:atrioventricular node房室结AVP:arginine vasopressin精氨酸加压素AVR:(augmented voltage,right arm)加压单极右臂导联AZT:azidothymidine叠氮脱氧胸腺嘧啶核苷BAEP:brain-stem auditory evoked potential 脑干听觉诱发电位BAL:British anti-lewisite(dimercaprol)英国抗路易士毒气剂(二巯基丙醇)BBB:bundle branch block束支传导阻滞BBB:blood-brain barrier血脑屏障BB P:bedside blood purification床边血液净化BBT:basal body temperature基础体温B cell:bone marrow-deri ved lymphocyte 骨髓源淋巴细胞BCG:bacillus Calmette-Guérin卡介苗BCNU:bis-chloroethyl-nitrosourea氯乙亚硝脲,卡氮芥(抗癌药)BE:base excess剩余碱BEAM:brain electrical activity mapping脑电位分布图BEE:basal energy expenditure基础能量消耗量BK:bradykinin缓激肽BME:biomedical engineering 生物医学工程BMF:bone marrow failure骨髓功能衰竭BMI:body mass index 体重指数(以体表面积为基数)BMP:bone morphogenic protein骨形态形成蛋白BMR:basal metabolic rate基础代谢率BMT:bone ma rrow transplantation骨髓移植BP:blood pressure血压BPH:benign prostatic hypertrophy良性前列腺肥大Bq:becquerel伯克(放射强度单位)BRM:biological response modulator 生物反应调节剂BSA:body surface area体表面积BSP:bromsulphalein酚四溴肽磺酸钠,磺溴肽钠(肝功能试验)BT:bleeding time出血时间BUN:blood urea nitrogen血尿素氮C:complement补体CABG:cor onary artery bypass grafting冠状动脉旁路移植术CAD:coronary artery disease冠状动脉病CAD:computer-aided diagnosis计算机辅助诊断CAH:c ongenital adrenal hyperplasia先天肾上腺增生cAMP:cyclic adenosine monophosphate 环一磷酸腺苷CAPD:continuous ambulatory peritnoeal dialysis持续性非卧床式腹膜透析CAT:calcium antagonist钙拮抗剂CAVH:continuous arteriovenous hemofiltration 连续动静脉血液滤过CAVHD:continuous arteriovenous hemodialysis 连续动静脉血液透析CAVHP:continuous arteriovenous hemoperfusion连续动静脉血液灌流CAVP:continuous arteriovenous plasmapheresis连续动静脉血浆换出CBC:complete blood count全部血细胞计数CBF:cerebral blood flow脑血流量CCK-P Z:cholecystokinin-pancreozymin 缩胆囊素-促胰酶素CCNU:cyclohexyl-chloroethyl-nitrosourea 氯乙环己亚硝脲(抗癌药)CCU:cardiac care unit心脏病监护中心CD:cluster of differentiation 免疫细胞表面分化抗原CDC:U.S.Centers for Disease Control 美国疾病监控中心C dyn:dynamic compliance动态顺应性CEA:carcinoembryonic antigen癌胚抗原CEI:converting enzy me inhibitor转化酶抑制剂CEP:chronic eosinophilic pneumonia慢性嗜酸细胞增多性肺炎CF:complement fixation补体结合CF:chemotactic factor趋化因子CF:cystic fibrosis囊性纤维化CFU:colony forming unit集落形成单位CGD:chronic granulomatous disease慢性肉芽肿病CGL:chr onic granulocytic leukemia 慢性粒细胞白血病CH:compromized host免疫力低下寄主C:constant domain of heavy chain重链稳定区(免疫球蛋白)CHD:coronary heart disease冠心病CHF:congestive heart failure充血性心力衰竭CHO:carbohyd rate碳水化合物,糖CI:cardiac index心脏指数Ci:curie居里(放射强度单位)CIC:circulating immune c omplex循环免疫复合物CIE:countercurrent immunoelectrophoresis对流免疫电泳CIS:carcinoma in situ原位癌CISN:chronic interstitial nephritis 慢性间质性肾炎CK:creatine kinase肌酸激酶Cl:clearance清除率C:constant domain of light chain轻链稳定区(免疫球蛋白)CLL:chronic lymphocytic leukemia慢性淋巴细胞白血症C(L+T):compliance of lungs and thorax 肺-胸廓顺应性CMG:cystometrogram膀胱压力容积曲线CMI:cell-mediated immunity细胞介导免疫CML:chronic my elogenons leukemia慢性髓细胞白血病CMV:cytomegalovirus巨细胞病毒CNS:central nervous system中枢神经系统CO:cardiac output心输出量CoA:coenzyme A辅酶ACO CP:CO combining power二氧化碳结合力COMT:catechol-O-methyltrans ferase 儿茶酚氧位甲基转移酶COPD:chronic obstructive pulmonary disease慢性阻塞性肺疾病CP:classic pathway经典途径(补体活化)CP:cor pulmonale肺原性心脏病CPAP:continuou s positive airway pressure呼吸道持续正压结氧CPCR:cardio-pulmonary-cerebral resuscitation心肺脑复苏CPDD:cis-platinum-diamino dichloride顺铂CPPV:continuous positive pressure ventilation连续正压通气结氧CPR:cardiopulmonary resuscitation心肺复CPZ:chlorpromazine氯丙嗪Cr:creatinine 肌酐CR:complement receptor补体受体CREST:calcinosis,Raynaud's phenomenon,esophageal dysfunction,sclerodactyly,telangiectasia 钙质沉着-雷诺氏现象-食管功能失调-硬皮病指(趾)-毛细管扩张(综合征)CRF:chronic renal failure慢性肾功能衰竭CRP:C-reactive p rotein C反应蛋白CSD:cat scratch disease猫抓病CSF:cerebrospinal fluid脑脊液CSF:colony stimulating factor集落刺激因子C stat:static compliance静态顺应性CT:calcitonin降钙素CT:computed tomography 电子计算机断层成像CT:clotting time凝血时间CTL:cytotoxic T lymphocyte细胞毒T淋巴细胞CTX:c ytoxan环磷酰胺CV:closing volume闭合容积CVA:cerebrovascular accident脑血管意外CVID:common variable immunoglobulin deficiency常见变异性免疫缺陷CVP:central venous pressure中央静脉压CWP:coal worker's pneumoconiosis煤矿工人尘肺Cys:cysteine半胱氨酸D:ergocalciferol麦角骨化醇(维生素D)D:cholecalciferol胆骨化醇(维生素D)1,25-(OH)D:1,25-dihydroxycholecalciferol 1,25-双羟胆骨化醇DAG:diacylglycerol甘油二脂D&C:dilatation &curettage刮宫DDS:diaminodiphenylsulfon e氨苯砜DDT:dichloro-diphenyl-trichloroethane滴滴涕DDVP:dichlorvos敌敌畏DEC:diethylcarbamazine 乙胺嗪(海群生,抗丝虫药)DES:diethylstilbestrol乙烯雌酚DF:differentiation factor分化因子DHEA:deh ydroepiandrosterone脱氢表雄甾酮DHT:dihydrotestosterone二氢睾丸酮DI:diabetes insipidus尿崩症DIC: disseminated intravascular coagulation弥漫性血管内凝血DIP:distal interphalangeal远指间(关节)DIT:diiodotyrosine二碘酪氨酸DJD:degenerative j oint disease退行性关节病(即骨性关节病)DKA:diabetic ketoacidosis糖尿病酮症酸中毒DLE:discoid lupus erythromatosus盘状红斑狼疮DM:diabetes mellitus糖尿病DM:dermatomyositis皮肌炎DNA:deoxyribonucleic acid脱氧核糖核酸D OC:11-deoxycorticosterone11-脱氧皮质酮Dopa:dihydroxyphenylalanine二羟苯丙氨酸,多巴DP:diastolic pressure舒张压DP:discharge precautions排出物隔离DPG:diphosphoglyceric acid二磷酸甘油酸DPN:dip hosphopyridine nucleotide二磷酸吡啶核苷酸(同NAD,即辅酶I)DSA:digital substraction angiography数字减影血管造影DSCG:disodium cromoglycate色甘酸钠(过敏反应介质阻释药)DSH:deliberate self harm蓄意自伤DSM:Di gnostic nd St tistic l M nu l of Ment l Disorders《精神障碍诊断统计手册》DST:dexamethasone suppression test地塞米松抑制试验DT:delirium tremens震颤谵妄DTH:delayed-type hypersensitivity迟发过敏DTIC:dimethyl imidazole carboxamide 氮烯咪胺(抗癌药)DTP:diphtheria tetanus pertussis白喉-破伤风-百日咳(三联疫苗)DUB:dysfunctional uterine bleeding功能失调性子宫出血DVT:deep vein thrombosis深静脉血栓形成D/W:dextrose in water葡萄糖液E:enzyme酶E:estr one雌酮E:estradiol雌二醇E:estriol雌三醇EABV:effective arterial blood volume有效动脉血容量EACA:epsilon-aminocaproic acid6-氨基己酸(纤溶酶激活剂抑制药)EAC-rosette:erythrocyte-antibody-complementrosette红细胞-抗体-补体玫瑰花结EAE:experimental allergic encephalomyelitis 实验性变应性脑脊髓炎EBA:epidermolysis bullosa acquisita 获得性大疱性表皮松解症EBP:eosinophilic basic protein嗜酸细胞碱性蛋白EBV:Epstein-Barr virus爱泼斯坦-巴尔二氏病毒ECF:extracellular fluid细胞外液ECF-A:eosinophil chemotactic factor ofanaphylaxis 过敏反应嗜酸细胞趋化因子ECG:electrocardiogram心电图ECHO:echocardiography超声心动图ECHOvirus:enteric cytopathogenic human orphanvirus人类肠道细胞病变孤儿病毒ECM:external cardiac massage胸外心脏按压ECM:erythema chronicummigrans 慢性游走性红斑(见LD)ECT:electroconvulsive therapy 电惊厥疗法,电抽搐疗法(即电休克疗法)ECT:emission computed tomography发射计算机断层成像EDD:expected date of delivery预产期EDTA:ethylene diamine tetraacetic acid 依地酸(金属解毒药)EEG:electroencephalogram脑电图EF:ejection fraction射血分数EFA:essential fatty acid必需脂肪酸EGD:esophagogastroduodenoscopy食管胃十二指肠镜检EGF:epidermal growth factor表皮生长因子EGRI:enterogastric reflux index肠胃反流指数EIA:enzyme immunoassay酶免疫测定EKC:epidemic keratoconjunctivitis 流行性角结膜炎EKG:electrocardiogram心电图ELISA:enzyme-linked immunosorbent assay 酶联免疫吸附测验EM:electron microscope电镜EMB:ethambutol乙胺丁醇(抗结核药)EMG:electromyogram肌电图EMSS:emergency medical service system 急诊医学勤务系统END:endorphin内啡肽ENL:erythema nodosum leprosum麻风结节性红斑(反应)ENT:ear,nose and throat耳鼻喉EOP:endogeneous opioid peptide内源性阿片肽EP:epinephrine肾上腺素EP:electrophoresis电泳EP:endogenous pyrogen内源致热源EP:endorphin内啡肽EP:enteric precauti ons肠道隔离EPEC:enteropathogenic E.coli肠致病性大肠杆菌EPO:erythropoietin红细胞生成素(即RE F)EPS:expressed prostatic secretion前列腺按摩液ERCP:endoscopic retrograde cholangiopancreatography内窥镜逆行胰胆管造影E-rosette:erythrocyte rosette红细胞玫瑰花结ERP:effective refractory period 有效不应期ERP:estrogen receptor protein雌激素受体蛋白ERPF:effective renal plasma flow有效肾血浆流量ERV:expiratory reserve volume补呼气容积ESR:erythrocyte sedimentation rate红细胞沉降率,血沉ESRD:end-stage renal disease终期肾疾病ETEC:enterotoxigenic E.coli产肠毒素大肠杆菌EXP:excret ion precautions排泄物隔离f:respiratory frequency呼吸频率F:folic acid叶酸Fab:antigen-binding fragme nt抗原结合片断(免疫球蛋白)FAD:flavin adenine dinucleotide 黄素腺嘌呤二核苷酸(黄酶辅基)FAS:fetal alcohol syndrome胎儿酒精综合征Fc:crystalizable fragment 结晶片断(免疫球蛋白)FDA:U.S.Food and Drug Administration美国食物药品局FDP:fibrinogen degradation products 纤维蛋白降解产物FEV:forced expiratory volume in 1 second 第一秒用力呼气量FFA:free fatty acid游离脂肪酸FFM:fat-free mass不含脂肪物质FH:tetrahydrofolate四氢叶酸FIA:fluoroimmunoassay荧光免疫测定Flu:influenza流行性感冒FMD:foot and mouth disease口蹄疫F MF:familial mediterranean fever家族性地中海热FMN:flavin mononucleotide 黄素单核苷酸(黄酶辅基)FM S:fibromyalgia syndrome纤维肌痛综合征FN:fibronectin纤维粘连蛋白FNA:fine needle aspiration biopsy 细针吸取活检FRC:functional residual capacity功能残气量FSH:follicle-stimulating hormone促滤泡激素FTA-ABS:fluorescent treponemal antibody-absorption 荧光螺旋体抗体吸收(试验)5-FU:5-fluorouracil5-氟尿嘧啶(抗癌药)FUO:fever of unkno wn origin无明热FVC:forced vital capacity用力肺活量G-:gram negative革兰氏阴性G+:gram positive 革兰氏阳性GABA:gamma-aminobutyric acidγ氨基丁酸GAG:glycosaminoglycan糖氨聚糖(即粘多糖)Gal:galactose半乳糖GALT:gut-associated lymphatic(lymphoid)tissue肠道相关淋巴组织GBS:Guillain-Barr syndrome 吉兰-巴雷二氏综合征GERD:gastroesophageal refux diseas e 胃食管反流病GF:growth factor生长因子GFR:glomerular filtration rate肾小球过滤率GGT:gamma-glutamyl transfera seγ-谷氨酰转移酶GH:growth hormone生长激素GI:gastrointestinal胃肠GIP:gastric inhibitory peptide抑胃肽Glc:glucose葡萄糖Gln:glutamine谷酰胺Glu:glutamic acid谷氨酸Gluc:glucuronic acid葡萄糖醛酸Gly:glycine甘氨酸GN:glomerulonephritis肾小球肾炎GN:glomerulonephropathy肾小球肾病GNB:gr am-negative bacilli革兰氏阴性杆菌GnRH:gonadotropin-releasing hormone 促性腺激素释放激素GP:glycoprotein糖蛋白G6PD:glucose 6-phosphate dehydrogenase6-磷酸葡萄糖脱氢酶GRH:GH-releasing hormone生长激素释放激素GSD:glycogen storage disease糖原贮积病GS H:glutathione(reduced)谷胱甘肽(还原型)GSSG:glutathione(oxidized)谷胱甘肽(氧化型)GTD:gestation al trophoblastic diseases妊娠性滋养细胞疾病GTT:glucose tolerance test葡萄糖耐量试验GU:genitourinary泌尿生殖GVH:graft-versus-host 移植物抗寄主(疾病)Gy:gray戈瑞(放射吸收剂量)HA:hemagglutination血细胞凝集HACE:high-altit ude cerebral edema高原脑水肿HACH:hypertensive atherosclerotic cerebralhemorrhage高血压性动脉硬化性脑出血HAI:hemagglutination-inhibition血凝抑制HA(N)E:hereditary angi oneurotic edema 遗传性血管神经性水肿HAPE:high-altitude pulmonary edema高原肺水肿HAT:hypoxanthine,aminopterin,thymidine 次黄嘌呤-氨基蝶呤-胸腺嘧啶核苷(培养基)HAV:hepatitis A virus甲型肝炎病毒HB:heart block心传导阻滞HbA:adult hemoglobin成人型血红蛋白HBcAg:hepatitis B core antigen乙型肝炎核心抗原HBE:His bundl e electrocardiogram希氏束心电图HBeAg:hepatitis B eantigen乙型肝炎e抗原HBIG:hepatitis B immune globulin乙型肝炎免疫球蛋白HbF:fetal hemoglobin胎儿型血红蛋白HbS:sickle hemoglobin 血红蛋白S,镰状细胞血红蛋白HBsAg:h epatitis B surface antigen 乙型肝炎表面抗原HBV:hepatitis B virus乙型肝炎病毒HCD:heavy chain disease重链病hCG:human chorionic gonadot ropin人类绒毛膜促性腺激素HCL:hairy cell leukemia毛细胞白血病HCT(Hct):hematatocrit血细胞比容(即PCV)HCV:hepatiti s C virus丙型肝炎病毒HD:Hodgkin's disease何杰金氏病,霍奇金氏病HD:hemodialysis血液透析HDL: high-density liporotein高密度脂蛋白HDN:hemolytic disease of newborn新生儿溶血病HDV:hepatitis D v irus丁型肝炎病毒HE:hematoxylin eosin苏木精伊红(染色剂)HE:hypertensive encephalopathy高血压性脑病HEV:hepatitis E virus戊型肝炎病毒HF:hepatic failure肝功能衰竭HF:Hageman factor哈格曼氏因子(凝血因子)HF:hemorrhagic fever出血热HFA:Health for All“人人享受健康服务”HFRS:hemorrhagi c fever with renal syndrome肾综合征出血热(即流行性出血热)HGG:human gamma globulin人丙种球蛋白HGPRT:hypoxanthine-guanine phosphoribosyltransferase次黄嘌呤-鸟嘌呤磷酸核糖转移酶(嘌呤回收途径)HHNK:hyperglycemic,hyperosmotic nonketotic coma高血糖高渗性非酮症性昏迷5-HIAA:5-hydroxyindoleacetic acid 5-羟基吲哚乙酸(5-羟色胺脱氨产物)His:histidine组氨酸HIV:human immunodeficiency virus人类免疫缺陷病毒(即HTLV-和LAV,为AIDS 病原体)HLA:human leukocyte antigen人白细胞抗原HLP:hyperlipopr oteinemia高脂蛋白血症HMG:human menopausal gonadotropin 人绝经期促性腺激素HMG-CoA:hydroxymethyl glutaryl CoA3-羟-3-甲基-戊二酰辅酶A(合成酮体和胆固醇的中间产物)HMM:hexamethymelamine六甲密胺(抗癌药)HMP:hexos e monophosphate磷酸己糖(支路)HMWK:high molecular weight kininogen高分子激肽原hnRNA:heterogeneous nuclear RNA核异质核糖核酸HPA axis:hypothalamic-pituitary-adrenal axis下丘脑-垂体-肾上腺轴HPETE:hydroperoxy-eicosatetraenoic acid 氢过氧二十碳四烯酸(合成白细胞三烯的中间产物)HPV:human papilloma virus人类乳头瘤病毒HR:heart rate 心率HS:heparin sulfate硫酸肝素HSE:herpes simplex encephalitis单纯疱疹脑炎HSP:Henoch-Sch nlein p urpura享舍二氏紫癜(即过敏性紫癜)5-HT:5-hydroxytrptamine(serotnin)5-羟色胺(血清素)HTLV:human T cell leukemia virus人类T细胞白血病病毒HTLV:human T lymphotropic virus嗜人T淋巴细胞病毒(同前,另名)HU:hydroxyurea羟基脲(抗癌药)HUS:hemolytic-uremic syndrome 溶血性尿毒症综合征HVA:homovanillic acid 高香草酸(多巴胺代谢产物)HX:histiocytosis X组织细胞增生症XH-Y:histoco mpatibility antigen组织相容性抗原Y(其结构基因位于Y染色体)HZ:herpes zoster带状疱疹IBD:inflammatory bowel disease炎症性肠道疾病IBS:irritable bowel syndrome肠道激惹综合征IC:inspiratory capacity深吸气量IC:immune complex免疫复合物ICD:intracranial pressure颅内压ICD:Intern tion l Cl ssific tion of Dise ses国际疾病分类ICF:intracellular fluid细胞内液ICG:indocyanine green 吲哚氰绿(试验)(肝排泌功能试验)ICS:immotile cilia syndrome纤毛不动综合症ICU:intensive care unit监护中心Id:idiotype独特型ID:intradermal皮内IDDM:insulin-dependent diabetes mellitus胰岛素依赖性糖尿病IDL:intermediate-density lipoprotein中等密度脂蛋白IDU:idoxuridine碘苷,疱疹净(抗病毒药)IE:infective endocarditis感染性心膜炎IEC:invasive E.col i侵袭性大肠杆菌IEP:immunoelectrophoresis免疫电泳IF:inhibiting factor抑制因子IFA:immune fluoresc ence antibody免疫荧光抗体IFA:indirect fluorescent antibody间接荧光抗体IGF-1:insulin-like growth fact or I胰岛素样生长因子I(即SMC)IgG:immunoglobin G G型免疫球蛋白IGT:impaired glucose tolerance糖耐量低减IHA:i ndirect hemagglatination间接血凝(试验)IL:interleukin白细胞介素ILD:interstitial lung disease肺间质病Ile:isoleucine异亮氨酸IM:infectious mononucleosis 传染性单核细胞增多症IM:intramuscular肌内IMD: immunologically mediated diseases 免疫机制介导疾病INH:isonicotinic acid hydrazide(isoniazid)异烟肼INQ:index of nutritional quality营养质量指标IPD:inflammatory pelvic disease盆腔炎IPF:idiopathic p ulmonary fibrosis 特发性肺间质纤维化IPH:idiopathic pulmonary hemosiderosis特发性肺含铁血黄素沉着症IPPV:intermittent positive pressure ventilation间歇正压通气给氧IQ:intelligence quotient智商Ir:immune response免疫应答(基因)IRD:immune renal disease免疫性肾病iRNA:informational RNA信息核糖核酸IRV:inspiratory reserve volume补吸气容积I s:immune suppressor免疫抑制(基因)ISG:immune serum globulin免疫血清球蛋白ITP:idiopathic thromb ocytopenic purpura 特发性血小板减少性紫癜ITP:inosine triphosphate三磷酸肌苷IU:international unit国际单位IUD:intra-uterine devic e宫内节育器IUI:intra-uterine insemination宫腔内人工授精IV:intravenous静脉内IVF:in vitro fertilizati on体外受精IVP:intravenous pyelogram静脉肾盂造影JCA:juvenile chronic arthritis青年慢性关节炎JE: Japanese encephalitis日本脑炎JEV:Japanese encephalitis vaccine日本脑炎疫苗JGA:juxtaglomerular apparatus近血管球复合体K cells:killer cells杀伤细胞Kb:kilobase纤碱基Km:Michaelis constant米氏常数1 7-KS:17-ketosteroid17-酮类固醇17-KGS:17-ketogenic steroid17-生酮类固醇KS:Kaposi's hemorrhagic sarc oma 卡波济氏出血性肉瘤KUB:kidney,ureter,bladder肾、输尿管及膀胱(平片)LABD:linear IgA bullous dermatosus线状免疫球蛋白A大疱性皮肤病LAH:left anterior hemiblock左前分支阻滞LAK cell:lymphokine activated killer cell 淋巴因子活化杀伤细胞LAT:latex agglutination test乳胶凝集试验LATS:long-acting thyroid stimulator 长效甲状腺刺激素LAV:lymphadenopathy-associated virus淋巴结病相关病毒(同HIV)LBM:lean body mass不计脂肪体重LBW:low birth weight低体重(儿)LC:Langerhan' s cell 郎格汉斯氏细胞(皮肤免疫细胞),郎格汉斯氏细胞(妊娠滋养细胞)LC:Langhans'cell郎汉斯氏细胞多核巨细胞LCAR:late cutaneous allergic reactions晚期变态反应性皮肤反应LCAT:lecithin cholesterol acyltransferase 卵磷脂胆固醇乙酰基转移酶(催化胆固醇脂化)LCM:lymphocytic choriomeningitis淋巴脉络丛脑膜炎LD:Lyme disease莱姆病LD body:Leishman-Donovan body 利士曼-多诺万二氏小体LD:median lethal dose半数致死量LDCF:lymphocyte-derived chemotactic factor淋巴细胞趋化因子LDH:lactate dehydrogenase乳酸脱氢酶LDL:low-density lipoprotein低密度脂蛋白LE:lupus erythematosus红斑狼疮Leu:leucine亮氨酸LGA:large-for-gestational-age大于胎龄(儿)LGV:lymphogra nuloma venerum性病性淋巴肉芽肿LH:leuteinizing hormone黄体生成素LL:lepromatous leprosy瘤型麻风LP:lumbar puncture腰椎穿刺LPAM:1-phenylalanine mustard左旋苯丙氨酸氮芥LPH:left posterior hem iblock左后分支阻滞β-LPH:β-lipotropic hormoneβ-促脂素LPL:lipoprotein lipase脂蛋白脂酶LPS:lipopol ysaccharide脂多糖LRI:lower respiratory illness下呼吸道病LS:life support生命支持LSD:lysergic aciddiethylamide赖瑟酸二乙胺(致幻剂)LT:leukotriene白细胞三烯LT:lymphotoxin淋巴毒素LUF syndrome:luteinized-unruptured follicle syndrome黄体化卵巢未破综合征LVSW:left ventricular stroke work左心室每搏功Lyb:lymphocyte antige n on B cells B淋巴细胞表面抗原(鼠)Lys:lysine赖氨酸Lyt:lymphocyte antigen on T cells T淋巴细胞表面抗原(鼠)βM:βmicroglobulinβ微球蛋白MAC:membrane attack complex 膜攻击复合物(补体活化产物)MAF:macrophage arming(activating)factor 巨噬细胞武装(活化)因子MAI:Mycob cterium vium-intr cellul re鸟-胞内分支杆菌MAMA:midarm muscle area中臂肌肉面积MAO:monoamine oxidase单胺氧化酶MAOI:monoa mine oxidase inhibitor单胺氧化酶抑制药MBC:minimal bactericidal concentration最低杀菌浓度MBC:maximal breathing capacity(MVV)最大换气量(同MVV)MBD:minimal brain damage轻微脑损伤(即多动综合征)MBF:myocardial blood flow心肌血流量MBP:myelin basic protein髓鞘碱性蛋白MIP:maximal inspir atory pressure最大吸气压力MCGN:mesangiocapillary glomerulonephritis 系膜毛细血管性肾小球肾炎(同MPGN)MCH:mean corpuscular hemoglobin 平均红细胞血红蛋白含量MCHC:mean corpuscular hemoglobin concentration平均红细胞血红蛋白浓度MCLS:mucocutaneons lymph node syndrome皮肤粘膜淋巴结综合征(即川崎氏病)MCNS:minimal change nephrotic syndrome微小病变性肾病综合征MCP:metacarpophalangeal掌指(关节)MCTD:mixed connective tissue disease混合结缔组织病MCV:mean corpuscular volume平均红细胞体积MD:muscular dystrophy肌肉营养不良MDS:myelod ysplastic syndrome骨髓异常增生综合征MEA:multiple endocrine adenomatosis 多发性内分泌腺瘤(同MEN)MEFV:maximal expiratory flow volume 最大呼气流量MEN:multiple endocrine neoplasia多发性内分泌腺瘤(同MEA)MET:metabolic equivalent梅脱(代谢当量)Met:methione甲硫氨酸,蛋氨酸MetHb:methe moglobin高铁血红蛋白MF:myelofibrosis骨髓纤维化MF:mycosis fungoides蕈样霉菌病(即蕈样肉芽肿)MG:myasthenia gravis重症肌无力MGN:membranous glomerulonephritis 膜性肾小球肾炎MHC:major histoc ompatibility complex主要组织相容性复合物MI:mitral insufficiency二尖瓣关闭不全MI:myooardial infarction心肌梗死MIC:minimum inhi bitory concentration最低抑制浓度MIF:migration inhibitory factor (单核-巨噬细胞)移动抑制因子MIF:M llerian inhibitory factor 米勒氏管抑制因子MIG:measles immune globulin麻疹免疫球蛋白MIT:monoiodotyrosine一碘酪氨酸MLD:minimum lethal dose最小致死量MLR:mixed lymphocyte response混合淋巴细胞反应MM:myeloid metaplasia骨髓外化生MMEF:maximum mid-expiratory flow最大呼气中期流量MMM:myelofibrosis with myeloid metaplasia 骨髓纤维化伴髓外化生MoAb:monoclonal antibody单克隆抗体6-MP:mercaptopurine6-巯基嘌呤(抗癌药)MPG N:membranoproliferative glomerulonephritis 膜增殖性肾炎(同MCGN)MPO:myeloperoxidase髓过氧化物酶MPS:mononuclear phagocyte system单核吞噬细胞系统MPS:mucopolysaccharidoses粘多糖病MR:mental retardation精神发育迟滞MR:mitral regurgitation二尖瓣反流MR:myelography脊髓造影MRI:magnetic resonance imaging磁共振成像MS:mitral stenosis二尖瓣狭窄MS:multiple sclerosis多发性硬化MSH:melanocyte-stimulating hormone促黑素细胞激素,促黑素MSOF:multiple system organ failure多发性系统器官衰竭MTP:metatarsophalangeal跖趾(关节)MTX:methotrexate氨甲蝶呤MVA:mevalonic acid甲羟戊酸(合成胆固醇的中间代谢物)MVV:maximal voluntary ventilation 最大通气量(同MBC)m:macrophage巨噬细胞N:neuraminidase神经氨酸酶NA:noradrenaline去甲肾上腺素NA:neutralizing antibody中和抗体NAD:nicotinamide adenine dinucleotide(oxidized)烟酰胺腺嘌呤二核苷酸(同DPN,即辅酶)NADP:nicotinamide adenine dinucleotide(oxidized)烟酰胺腺嘌呤二核苷酸磷酸(同TPN,即辅酶)NARES:non-allergic rhinitis with eosinophilia 嗜酸细胞增多性非变应性鼻炎NBT:nitroblue tetrazolium亚硝基蓝四氮唑NCF-A:neutrophil chemotactic factor ofanaphylaxis过敏反应嗜中性细胞趋化因子NDI:nephrogenic diabetes insipidus肾源性尿崩症NE:norepinep hrine去甲肾上腺素NEC:nerotizing enterocolitis坏死性小肠结肠炎NGU:nongonococcal urethritis非淋病性尿道炎NIDDM:noninsulin-dependent diabetes mellitus非胰岛素依赖性糖尿病NIH:U.S.National Institutes of Health 美国国立卫生研究所NK cell:natural killer cells天然杀伤细胞NMR:nuclear magnetic resonance 核磁共振(同MRI)NPN:nonprotein nitrogen非蛋白氮NPT:nocturnal penile tumescence夜间阴茎勃起NREM:nonrapid-eye-mo vement非快眼动(睡眠)NS:nephrotic syndrome肾变综合征NSAID:nonsteroidal anti-inflammatory drug 非甾体抗炎药OA:osteoarthritis骨性关节炎OA:orotic acid乳清酸(合成嘧啶的中间产物)OAF:osteoclast activat ing factor破骨细胞活化因子OC:oral contraceptive口服避孕药OCG:oral cholecystography口服造影剂胆囊造影OGTT:oral glucose tolerance test口服糖耐量试验17-OH CS:17-hydroxycorticosteroid17-羟皮质类固醇OLD:obstructive lung disease阻塞性肺疾病OPV:oral(live attenuated virus)polio vaccine(Sabin)口服脊髓灰质炎减毒活疫苗(萨宾氏)ORS:oral rehydration solution口服补水溶液OS:opening sn ap开瓣音OT:old tuberculin旧结核菌素OTC:over-the-counter非处方(药),柜台(药)P:atrial deplor ation(ECG wave)心房除极波(心电图)P:proline脯氨酸-p:short arm of chromosome染色体短臂P:properdin备解素(补体活化旁路途径)P :pulmonic second sound肺动脉瓣第二音PA:pernicous anemia恶性贫血P CO:arterial carbon dioxide p ressure动脉血三氧化碳分压PAF:platelet-activating factor血小板活化因子PAH:para-aminohippurate 对氨基马尿酸(肾血浆流量测定)PAM:pyridine aldoxime methiodide 解磷定(有机磷解毒药)PAM:primary amebic meningoencephalitis原发性阿米巴脑膜脑炎PAM:pulmonary alveolar macrophage肺泡巨噬细胞PAN:polyarteritis nodosa结节性多动脉炎P CO:arterial oxygen pressure动脉血氧分压P O:alveolar oxygen pressure肺泡氧分压PAP:prostatic acid phosphatase前列腺酸性磷酸酶Pap:Papanicolaou巴氏(染色)(找瘤细胞)PAS:para-aminosalicylic acid对位氨基水杨酸PAS:periodic acid-Schiff(reaction)过碘酸希夫氏(反应)PAWP:pulmonary arterial wedge pressure肺小动脉嵌顿压PBC:primary biliary cirrhosis 原发性胆汁性肝硬变PBG:porphobilinogen卟吩胆色素原PBI:protein-b ound iodine蛋白结合碘PC:phosphatidylcholine磷脂酰胆碱PCA:passive cutaneous anaphylaxis 被动皮肤过敏反应PCD:plasma cell dyscrasia浆细胞病PCG:phonocardiography心音图PCH:paroxysmal cold hemoglobinu ria阵发性寒冷性血红蛋白尿PCM:protein-calorie malnutrition蛋白质能量营养不良PCO syndrome:polycystic ovarian syndrome多囊卵巢综合征PCP:Pneumocystis c rinii pneumonia卡氏肺孢子虫肺炎,卡氏肺囊虫肺炎PCR:polymerase chain reaction 聚合酶链锁反应PCV:packed cell volume血细胞压积(同HCT)PD:peritoneal dialysis腹腔透析PDA:patent ductus arteriosus动脉导管未闭PDGF:platelet -derived growth factor 血小板源生长因子PDT:photodynamic therapy光动力学治疗PE:physical examination体格检查,体检PECT:positron emission computed tomography正电子发射计算机断层成像PEEP:positive end-expiratory pressure呼气终末正压给氧PEFR:peak expiratory flow rate呼气高峰流量PEG:pneumoencephalography气脑造影PET:positron emission tomography正电子发射断层成像PFNA:percutaneons fine needle aspiration经皮细针抽吸PG:prostaglandin前列腺素PGI:prostacyclin前列腺环素PGL:persistent generalized lymphadenopat hy持续性全身淋巴腺病(艾滋病)PGN:proliferative glomerulonephritis增殖性肾小球肾炎PH:portal hypertension门静脉高压PHA:passive hemagglutination被动血细胞凝集作用PHC:primar y health care初级卫生保健Phe:phenylalanine苯丙氨酸PI:phosphatidylinositol磷脂酰肌醇PI:protective i solation(reverse isolation)保护性隔离(反向隔离)PID:pelvic inflammatory disease盆腔炎PIE:pulmonary infiltration with eosinophilia嗜酸细胞增多性肺浸润PIF:prolactin inhibiting factor泌乳素抑制因子PIH:pregnancy-induced hypertension妊娠高血压PIP:proximal interphalangeal近端指(趾)间(关节)PIT:plasma iron transport rate血浆铁周转率PKD:polycystic kidney disease多囊性肾病PK reaction: Prausnitz-K stner reaction普库二氏反应(同PCA)PKU:phenylketonuria苯丙酮尿症PM:polymyositis多发性肌炎PMA:progressive mu cular atrophy进行性肌萎缩PML:progressive multifocal leukoencephalopathy进行性多灶性脑白质病PMN:polymorphonuclear neutrophilic leukocyte多形核嗜中性白细胞PMR:polymyalgia rheumatica多发性风湿性肌痛PMS:premenstrual syndrome经前综合征PMT:premenstrual tension经前紧张症PMV:prolapse of mitral valve二尖瓣脱垂PN:polyarteritis nodos a结节性多动脉炎PNH:paroxysmal nocturnal hemoglobinuria 阵发性夜间血红蛋白尿PNS:partial nonprogressing stroke 非进行性部分中风PNS:peripheral nervous system周围神经系统PNT:papulonecrotic tuberculid丘疹坏死性结核疹P.O.:per os经口POMC:pro-opiomelanocortin内啡肽-促黑素-促皮质素前体POMR:problem-oriented medical record以问题为中心的医案记录PP:pellagra preventive抗糙皮病(因子)(即烟酰胺和烟酸)PP:periodic paralyses周期性麻痹PP:pancreatic polypeptide胰多肽PPD:purified protein derivat ive 纯蛋白衍化物(精制结核菌素)PPLO:pleuropneumonia-like organism类胸膜肺炎微生物PR interval:PR间期(心电图)PRA:plasma renin activity血浆肾素活性(同PRC)PRC:plasma renin concentration血浆肾素浓度(同PRA)PRF:prolactin releasing factor泌乳素释放因子PRIH:prolactin release-inhibiting hormone 泌乳素释放抑制激素PRIST:paper radio-immuno-sorbent test 试纸放射免疫吸附试验PRL:prolactin泌乳素Pro:proline脯氨酸PROM:premature rupture of the membrane胎膜早破PRP:progesterone receptor protein孕酮受体蛋白PRPP:phosphoribosy1 pyrophosphate焦磷酸磷酸核糖(合成嘌呤、嘧啶和辅酶&的前体)PSA:psoriatic arthritis银屑病关节炎PSE:portal-systemic enceph alopathy门-体循环性脑病PSGN:poststreptococcal glomerulonephritis 链球菌感染后肾小球肾炎PSP:phenolsulfonphthalein酚磺肽,酚红PSS:progressive systemic sclerosis进行性系统性硬化症PST:phthalylsulfathiazole酞磺噻唑PS(V)T:paroxysmal supraventricular tachycardia阵发性室上性心动过速PT:prothrombin time凝血酶原时间PTA:plasma thromboplastin antecedent(factorⅪ)血浆凝血活酶前体(凝血因子Ⅺ)PTC:plasma thromboplastin component(factorⅨ)血浆凝血活酶组分(凝血因子Ⅸ)PTC:percutaneous transhepatic cholangiogram 经皮肝穿刺胆道造影PTCA:percutaneous transluminal coronaryangioplasty经皮穿刺冠状动脉管腔内成形术PTH:parathyroid hormone甲状旁腺素PTT:partial thromboplas tin time 部分凝血活酶时间PTU:propylthiouracil丙基硫氧嘧啶PV:plasma volume血浆容积PV:polycythe mia vera真性红细胞增多症PVR:peripheral vascular resistance周围血管阻力PWM:pokeweed mitogen美洲商陆有丝分裂原PXE:pseudoxanthoma elasticum弹性假黄瘤PZA:pyrazinamide吡嗪酰胺(抗结核药)PZI:protamine zine insulin鱼精蛋白锌胰岛素-q:long arm of chromosome 染色体长臂:perfusion rate肺血流灌注率Q fever:query fever疑向热,Q热QRS complex:ventricular depolarization(ECGwaves)心室除极波(心电图)QT interval: QT间期(心电图)R:roentgen伦琴(放射剂量单位):rate o f drug elimination药物排泄率RA:rheumatoid arthritis 类风湿性关节炎RA:refractory anemia难治性贫血rad:radiation absorbed dose 拉德(放射吸收剂量)RAI:radioactive iodine uptake test放射性碘摄取试验RANA:rheumatoid arthritis nuclear antigen 类风湿关节炎核抗原RAS:reticular activating system网状激活系统RAS:renin-angiotensin system肾素-血管紧张素系统RAS:renal artery stenosis肾动脉狭窄RAST:radio-allergo-sorbent test 放射-变应-吸附试验RAW:airway resistance气道阻力RBC:red blood cell红细胞RBF:renal blood flow肾血流量RC C:renal cell carcinoma肾细胞癌RDA:recommended daily allowance推荐膳食需要量RDS:respiratory distr ess syndrome 呼吸窘迫综合征RE system:reticulo-endothelial system 网状内皮系统REF:renal erythropoietic factor肾生血因子(即EPO)REM:rapid eye movement快眼动(睡眠)rem:roentgen equivalent of man雷姆,人体伦琴当量(放射剂量当量)RF:rheumatic fever风湿热RF:rheumatoid factor类风湿因子RF:releasing factor释放因子RFLP:restriction fragment length polymorphism 限制性内切片断长度多样性Rh:Rhesus group of red cell agglutinogen猕猴血型RH:releasing hormone释放激素RI:respiratory isolation呼吸道隔离RIA:radioimmunoassay放射免疫分析RIF:release inhibiting factor释放抑制因子RIH:release inhibiting hormone释放抑制因子RIND:reversibl e ischemic neurologic disability可逆性缺血性神经系统病RIP:radioimmunoprecipitation放射免疫沉淀RIST:radioimmunosorbent test放射免疫吸附试验RLD:restrictive lung disease限制性肺疾病RMP:rifampin利福平(抗结核及抗麻风药)RN A:ribonucleic acid核糖核酸RNP:ribonucleoprotein核蛋白RPGN:rapidly progressive glomerulonephripis 急进。

眼科名词缩写大全abd┃abdominal 腹部的ABK┃aphakic bullous keratopathy无晶体性大泡性角膜病变abn┃abnormal异常ac┃before meals餐前AC┃anterior chamber前房ACC┃accommodation调节ACG┃angle-closure glaucoma闭角青光眼ACIOL┃anterior chamber intraocular lens前房型眼内晶体add┃adduction or reading additional power内收;阅读另加屈光力ad lib┃as desired当需要时adv┃advanced高度AIBSE┃acute idiopathic blind spot enlargement急性特发性盲点扩大综合征AIDS┃acquired immunodeficiency syndrome艾滋病AION┃arteritic ischemic optic neuropathy动脉缺血性视神经病变┃anterior ischemic optic neuropathy前部缺血性视神经病变AKS┃ankylosing spondylitis强直性脊椎炎AL┃argon laser氩激光ALPI┃argon laser peripheral iridoplasty氩激光周边虹膜成形术ALT┃argon laser trabeculoplasty氩激光小梁成形术AMA┃against medical advice违反医疗忠告ambl┃amblyopia弱视AMD┃age-related macular degeneration年龄相关性黄斑变性AMN┃acute macular neuroretinopathy急性黄斑神经视网膜病变AMPPE┃acute multifocal placoid pigment epitheliopathy 急性多病灶性盾鳞状色素上皮病变AMPPPE┃acute multifocal posterior placoid pigment epitheliopathy急性多病灶性后部盾鳞状色素上皮病变ANA┃antinuclear antibody抗核抗体ant┃anterior前APMPPE┃acute posterior multifocal placoid pigment epitheliopathy 急性后部多病灶性盾鳞状色素上皮病变AODM┃adult-onset diabetes mellitus成人(发病)型糖尿病AP┃anteroposterior前后APD┃afferent pupillary defect瞳孔传入缺陷approx┃approximately大约APTT┃activated partial thromboplastin time激活部分促凝血酶原激酶时间ARC┃anomalous retinal correspondence异常视网膜对应ARMD┃age-retinal pigment epitheliitis年龄相关性黄斑变性ARN┃acute retinal necrosis急性视网膜坏死ARPE┃acute retinal pegment epitheliitis急性视网膜色素上皮炎art┃artificial tears人工泪液AS┃anterior synechia前粘连ASA┃aspirin(acetylsalicylic acid)阿司匹林ASAP┃as soon as possible尽快地ASC┃anterior subcapsular cataract前囊下白内障ASCVD┃atherosclerotic cardiovascular disease动脉硬化性心血管病ASHD┃atherosclerotic heart disease动脉硬化性心脏病astig┃astigmatism散光ATR┃against-the-rule(astigmatism)逆规性(散光)AU┃anterior uveitis前葡萄膜炎AVM┃arteriovenous malformation动静脉畸形A/V┃arteriolar/venous(ratio,nicking)小动脉/静脉(比,压痕)AZOOR┃acute zonal occult outer retinopathy急性区域性隐匿性外层视网膜病变Bas┃basophils嗜碱细胞BC┃base curve基底曲线BCC┃basal cell carcinoma基底细胞癌BCP┃birth control pill避孕丸BCR┃base curve radius基底弯曲半径BCVA┃best corrected visual acuity最佳矫正视力BD┃base down底向下BDR┃background diabetic retinopathy背景型糖尿病性视网膜病变BI┃base in底向内bid┃twice a day一日两次bilat┃bilateral两侧性BIO┃binocular indirect ophthalmoscope双眼间接检眼镜BK┃bullous keratopathy大泡性角膜病变bleph┃blepharitis睑缘炎BM┃basement membrane基底膜BO┃base out 底向外BP┃blood pressure血压BRAO┃branch retinal artery occlusion视网膜分枝动脉阻塞BRVO┃branch retinal vein occlusion视网膜分枝静脉阻塞BU┃base up底向上BUT┃break-up time泪膜破裂时间BVA┃best visual acuity最佳视力bx┃biopsy活检c┃with与c┃cornea角膜CA┃carcinoma癌CABG┃coronary artery bypass graft冠状动脉旁路移植CAD┃coronary artery disease冠状动脉病CAI┃carbonic anhydrase inhibitor碳酸酐酶抑制剂Cap ┃capsule胶囊CAR┃cancer-associated retinopathy癌相关性视网膜病变cat┃cataract白内障CAT┃computed axial tomography计算机体层摄影术CB┃ciliary body睫状体CBC┃complete blood count完全血细胞计数cc┃with correction矫正(视力)cc┃chief complaint主诉CCA┃common carotid artery颈总动脉CCT┃computer-assisted corneal topography角膜地形图仪C/D┃cup-to-disc ratio杯/盘比CF┃count fingers数指CFZ┃capillary-free zone无毛细血管地带C&F┃cells and flare细胞和光带(闪光)CHF┃congestive heart failure充血性心力衰竭CHI┃closed head injury闭合性头颅外伤CHRPE┃congenital hypertrophy of retinal pigment epithelium先天性视网膜色素上皮肥厚CK┃conductive keratoplasty传导性角膜成形术CL┃contact lens,clear接触镜,透明CME┃cystoid macular edema囊样黄斑水肿cmv┃cytomegalovirus巨细胞病毒CN┃cranial nerve脑神经CNS┃central nervous system中枢神经系统CNV┃choroidal neovascularization脉络膜新生血管形成CNVM┃choroidal neovascular membrane脉络膜新生血管膜c/o┃complaining of主诉COAG┃chronic open-angle glaucoma慢开角青光眼cong┃congenital先天性conj┃conjunctiva结膜COP┃cicatricial ocular pemphigoid瘢痕性眼类天疱疮COPD┃chronic obstructive pulmonary disease慢性阻塞性肺病CP┃cerebral palsy大脑麻痹CPEO┃chronic progressive external ophthalmoplegia慢性进行性眼外肌麻痹CPR┃cardiopulmonary resuscitation心肺复苏术CR┃chorioretinal脉络膜视网膜CRAO┃central retinal artery occlusion视网膜中央动脉阻塞CRF┃chronic renal failure慢性肾衰竭CRT┃corneal refractive therapy角膜屈光矫正法CRVO┃central retinal vein occlusion视网膜中央静脉阻塞C&S┃culture and sensitivity培养和敏感性CSCR┃central serous chorioretinopathy中心性浆液性脉络膜视网膜病变CSF┃cerebrospinal fluid脑脊髓液CSME┃clinical significant macular edema临床意义性黄斑水肿CSR┃central serous retinopathy中心性浆液性视网膜病变csw┃corneoscleral wound角膜巩膜创伤CT┃cover test遮盖试验┃computed tomography计算机体层摄影CT scan┃computed tomographic scan计算机体层扫描CV┃color vision色觉CVA┃cerebrovascular accident脑中风CVD┃cardiovascular disease 心血管病CWS┃cotton-wool spot棉絮斑Cx┃culture(微生物)培养CXR┃chest x-ray film胸部X光片cyl┃cylinder柱镜d┃days天D┃diopter or diameter屈光度或直径D/C┃discontinue停止D&C┃deep and clear(前房)深而透明DD┃disc diameter视盘直径DDx┃differential diagnosis鉴别诊断DFE┃dilated fundus examination扩瞳检查眼底D&I┃dilation and irrigation(泪道)扩张和灌洗DHD┃dissociated horizontal deviation分离性水平偏斜dia┃diameter直径disp┃dispense配药DM┃diabetes mellitus糖尿病DR┃diabetic retinopathy糖尿病视网膜病变DV┃distance vision远距离视力DVA┃distance vision acuity远距离视力DVD┃dissociated vertical deviation分离性垂直偏离DW┃daily wear每日戴(接触镜)Dx┃diagnosis诊断DX┃Diamox醋氮酰胺E┃esophoria at far内隐斜(视远)E’┃esophoria at near内隐斜(视近)ECA┃external carotid artery颈外动脉ECCE┃extracapsular cataract extraction白内障囊外摘除术EDTA┃ethylene dianline tetraacctic acid乙烯dianline tetraacctic酸EEG┃electroencephalogram脑电图EF┃eccentric fixation离心注视EKC┃epidemic keratoconjunctivitis流行性角结膜炎EKGorECG┃electrocardiogram心电图ELISA┃enzyme-linked immunosorbent assay酶连结免疫吸收测定EMM┃epimacular membrane黄斑表面膜endo┃endothelium内皮细胞ENT┃ear,nose,throat耳鼻喉EOG┃electro-oculogram眼电图EOM┃extraocular muscles眼外肌eos┃eosinophil嗜酸型细胞epi┃epithelium上皮细胞ER┃emergency room急诊室ERG┃electroretinogram视网膜电图ERM┃epiretinal membrane视网膜表面膜ESR┃erythrocyte sedimentation rate血沉ET┃esotropia at far内斜视(视远)ET’┃esotropia at near内隐斜(视近)E(T)orIET┃intermittent esotropia at far间歇性内隐斜(视近)E(T)’orIET’┃intermittent esotropia at near间歇性内隐斜(视远)ETOH┃ethyl alcohol乙醇,酒精EUA ┃examination under anesthesia全麻下检查EW┃extended wear长期戴用(接触镜)EXT┃external ocular examination外眼检查exoph┃exophthalmos眼球突出FA┃fluorescein angiography荧光素血管造影FAZ┃fluorescein angiography中心凹无血管地带FB┃foreign body异物FBS┃fasting blood sugar空腹血糖FC┃finger count数指FFA┃fundus fluorescein angiography荧光素眼底血管造影FHorFHx┃family history家族史fl┃fluorescein荧光素FMHx┃family medical history家族病史FML┃fluorometholone弗米隆FOHx┃family ocular history家族眼病史FR┃foveal reflex中心凹反光FROM┃full range of motion运动范围完整FTA-ABS┃fluorescent treponemal antibody absorption test荧光密螺旋体抗体吸收试验FTMH┃full-thickness macular hole黄斑全层裂孔f/u┃follow-up随访GC┃gonorrhea or gonococcus淋病或淋菌GCA┃giant cell arteritis巨细胞性动脉炎gent┃gentamicin庆大霉素GI┃gastrointestine胃肠GLC┃glaucoma,glucose青光眼,葡萄糖fonio┃gonioscopy前房角镜检查GPC┃giant papillary conjunctivitis巨乳头性结膜炎gr┃grain 谷(=0.065克)gt or gtt┃drop(Plural,gtt)滴(复数,gtt)GTT┃glucose tolerance test葡萄糖耐量试验GVF┃Goldmann visual field Goldmann视野H or hr(hrs) ┃hour小时HA┃headache头痛Hb,Hgb┃hemoglobin血红蛋白HCL┃hard contact lens硬性接触镜Hct┃hematocrit血细胞比容HCTZ┃hydrocholorothiazide呋塞米(双氢克尿塞)hem┃hemorrhage出血HIV┃human immunodeficiency virus人类免疫缺陷病毒HLA┃histocompatibility locus antigen or human leukocyte antigen组织相容性基因位点抗原或人类白细胞抗原h/o┃history of历史HM┃hand motion手动H&P┃history and physical病史和体格hs┃at bedtime在就寝时间HSK┃herpes simplex keratitis单纯疱疹性角膜炎HSV┃herpes simplex virus单纯疱疹病毒HT┃hypertropia at far上斜视(远距离)HT’┃hypertropia at near上斜视(近距离)HTN┃hypertension高血压HVF┃Humphrey visual field Humphrey视野Hx┃history历史HZO┃herpes zoster ophthalmicus眼带状疱疹I┃iris虹膜I&A┃irrigation and aspiration灌注和抽吸IBx┃incisional biopsy切口性活检IC┃iridocyclitis虹膜睫状体炎ICA┃internal carotid artery颈内动脉ICCE┃intracapsular cataract extraction白内障囊内摘除术ICE┃inidocorneal endothelial syndrome虹膜角膜内皮综合征ICG┃indocyanine green吲哚青绿ICP┃intracranial pressure颅内压ICU┃intensive care unit监护病房I&D┃incision and drainage切开引流术IDDM┃insulin-dependent diabetes mellitus胰岛素依赖糖尿病IDU┃idoxuridine疱疹净IFIOL┃iris-fixated intraocular lens虹膜固定眼内晶体Ig┃immunoglobulin免疫球蛋白IK┃interstitial keratitis基质性角膜炎Im┃intramuscular肌内Imp┃impression印象inf┃inferior下inj┃injection注射INO┃internuclear ophthalmoplegia核间眼肌麻痹INQ┃inferonasal quadrant鼻下象限int┃intermittent间歇性IOFB┃intraocular forergn body眼内异物IOH┃intraocular hemorrhage眼内出血IOL┃intraocular lens眼内晶状体,人工晶体ION┃ischemic optic neuropathy缺血性视神经病变IOP┃intraocular pressure眼压IR┃infrared红外线IRMA┃intraretinal microvascular abnormality视网膜内微血管异常ITQ┃inferotemporal quadrant颞下象限iu┃international unit or intermediate uveitis国际单位或中间葡萄膜炎IV┃intravenous静脉内JCT┃Juxtacanalicular Tissue管旁组织JODM┃juvenile-onset diabetes mellitus青少年型糖尿病JRA┃juvenile rheumatoid arthritis少年类风湿性关节炎K┃cornea or potassium角膜或钾KCS┃keratoconjunctivitis sicca干燥性角膜结膜炎kg┃kilogram公斤KP┃keratic precipiate角膜后沉着物L┃left or lens左或晶状体lab┃laboratory试验室lac┃lacrimal泪LASEK┃laser subepithelial keratomileusis激光上皮下角膜磨削术LASIK┃laser in situ keratomileusis激光原位角膜磨削术LCG┃light contrast glare光对比炫耀LCTP┃lateral canthal tendon plication外眦腱折叠术LI┃laser iridotomy or laser interferometry激光虹膜切开术或激光干涉仪LK,LKP┃lamellar keratoplasty板层角膜移植术LIO┃left inferior oblique左眼下斜肌LIR┃left inferior rectus左眼下直肌LL┃lower lid or lids and lashes下睑或眼睑和睫毛LLR┃left lateral rectus左眼外直肌LMR┃left medial rectus左眼内直肌LN┃lymph node淋巴结LP┃light perception,light projection,or lumber puncture光感,光定位,或腰椎穿刺LPI┃laser peripheral iridoplasty激光周边虹膜成形术LS┃lid scrubs睑缘擦洗(治疗睑缘炎)LSO┃laser scanner ophthalmolscope激光扫描检眼镜LSO┃left superior oblique左眼上斜肌LSR┃left superior rectus左眼上直肌LTP┃laser trabeculoplasy激光小梁成形术LTQ┃lower temporal quadrant颞下象限LVA┃low vision aids低视力辅助lymph┃lymphocytes or lymphatic淋巴细胞或淋巴mac┃macular黄斑mand┃mandibular下颌骨MAR┃melanoma-associated retinopathy黑色素相关的视网膜病变max┃maximum or maxillary最大值或上颌骨MCH┃mean corpuscular hemoglobin 平均血细胞血红蛋白MCHC┃mean corpuscular hemoglobin concentration平均血细胞血红蛋白浓度MCV┃mean corpuscular volume平均血细胞体积ME┃macular edema黄斑水肿MEDs┃medications药物治疗mERG┃multifocal ERG多区域(多焦)视网膜电图mfERG┃multifocal ERG多区域(多焦)视网膜电图MEWDS┃multiple evanescent white dot syndrome多数消散性白点综合征MFC┃multifocal choroiditis多病灶性脉络膜炎M.G. ┃Marcus Gunn瞳孔传入缺陷MHx┃medical history病史MI┃myocardial infarction心肌梗塞min┃minute or minimum分钟或最小量ml┃milliliter毫升mmHg┃millimeters of mercury毫米汞柱M&N┃Mydriacyl(tropicamide)and Neo-synephrine(phenylephrine) 两种最常用的扩瞳剂:托庇卡按及新福林mon┃monocytes单核细胞MR┃manifest refraction显性验光┃magnetic resonance磁共振MRA┃magnetic resonance angiography磁共振血管造影术MRD┃上睑缘至角膜中心反光点的距离MRI┃magnetic resonance imaging磁共振成像术MS┃multiple sclerosis多发性硬化MVA┃motor vehicle accident交通工具意外事故(车祸)N/A┃not applicable不适用NAG┃narrow-angle glaucoma窄角型青光眼NAION┃nonarteritic ischemic optic neuropathy非动脉缺血性视神经病变NAP┃no apparent pathology无明显病理学NCT┃noncontact tonometer非接触眼压计neg┃negative隐性NFL┃nerve fiber layer神经纤维层NFLD┃nerve fiber layer defect神经纤维层缺陷NG┃nongranulomatous非肉芽肿性NI┃no improvement无进步NIDDM┃non-insulin-dependent diabetes mellitus非胰岛素依赖型糖尿病NION┃Nonarteritic ischemic optic neuropathy非动脉缺血性视神经病变NKA┃no known allergies无已知的过敏反应nl┃normal正常NLP┃no light perception无光感Non repet┃not to be repeated(refilled)不重复(再次发药)NPA┃near point of accommodation调节近点NPC┃near point of convergence集合近点NPDR┃non-proliferative diabetic retinopathy非增生性糖尿病视网膜病变NR┃no refill不能再次发药NRC┃normal retinal correspondence正常视网膜对应NS┃nuclear sclerosis核性白内障(核硬化)NSA┃no significant abnormalities无重要异常NSAID┃no steroidal anti-inflammatory drug非类固醇消炎药NTG┃normal tension glaucoma正常眼压性青光眼NV┃near vision or neovascularization近视力或新生血管形成NV┃neovascularization新生血管形成N/V┃nausea and vomiting恶心和呕吐NVA┃Near visual acuity近视力NVD┃neovascularization of disc视盘新生血管形成NVE┃neovascularization of retina elsewhere视网膜新生血管形成NVG┃neovascular glaucoma新生血管性青光眼NVI┃neovascularization of the iris虹膜新生血管形成OA┃optic atrophy视神经萎缩OAG┃open-angle glaucoma开角型青光眼OCP┃ocular cicatricial pemphigoid眼瘢痕性类天疱疮OCT┃optic coherence tomography光学相干体层扫描术,光学相干层析术OD┃right eye右眼ODM┃ophthalmodynamometry眼动脉压测量OFHx┃ocular family history眼病家族史OGTT┃oral glucose tolerance test口服葡萄糖耐量试验OHTN┃ocular hypertension高眼压病OHx┃ocular history眼病史OKN┃optokinetic nystagmus视运动性眼球震颤ON┃optic nerve视神经ONAorOA┃optic nerve atrophy or optic atrophy视神经萎缩ONH┃optic nerve head视神经头ONSD┃optic nerve sheath decompression视神经鞘减压术OP┃outpatient门诊病人Op┃operation(Post Op)手术(术后)OR┃operating room手术室┃Over-refraction重叠验光Ortho┃orthophoria正视轴Ortho-K┃CRT,corneal refractive therapy角膜屈光矫正法OS┃left eye左眼OU┃both eyes双眼OZ┃optical zone光学地带p┃pupil瞳孔PA┃posteroanterior后前PACG┃primary angle-closure glaucoma原发性闭角型青光眼PACT┃prism alternating cover test棱镜交替遮盖试验PAM┃potential acuity meter潜在视力计PAN┃preauricular node耳前淋巴结PAS┃peripheral anterior synechia周边虹膜前粘连Pat or pt┃patient病人path┃pathology病理学P4,P2,etc. pilocarpine4%,2%,etc.drops毛果云香碱4%,2%等,眼药水PBK┃pseudophakic bullous keratopathy无晶状体性大泡性角膜病变PC┃after meals餐后PC┃posterior capsule后囊PCF┃pharyngoconjunctival fever咽结膜热PCIOL┃posterior chamber intraocular lens后房眼内晶体PCN┃penicillin青霉素PCO┃posterior capsular opacity后囊混浊PD┃prism diopter棱镜屈光度PD┃pupillary distance瞳孔距离PDR┃proliferative diabetic retinopathy增生性糖尿病视网膜病变PDS┃pigment dispersion syndrome色素播散综合征PDT┃photodynamic therapy光动力疗法PE┃physical examination体格检查PED┃pigment epithelial detachment色素上皮脱离PEE┃punctate epithelial erosions点状上皮侵蚀PEK┃punctate epithelial keratitis点状上皮角膜炎PERG┃pattern electroretinogram图形视网膜电图PERRL┃pupils equal,round,reactive to light瞳孔相等,圆,对光反射PERRLA┃pupils equal,round,reactive to light and accommodation瞳孔相等,圆,对光反应和调节PF┃Pred FortePg┃Prostaglandin前列腺素ph┃pinhole针孔phako┃phakoemulsification晶状体乳化术PHNIorNIph┃pinhole no improvement针孔无进步PHPV┃persistent hyperplastic primary vitreous永存原始玻璃体增生症PI┃present illness,peripheral iridotomy,or peripheral iridectomy现有疾病,周边虹膜切开术,或周边虹膜切除术PKPorPK┃penetrating keratoplasty穿透性角膜移植术PMMA┃polymethyl methacrylate聚甲基丙烯酸甲酯(有机玻璃)PMN┃polymorphonuclear neutrophil多形核中性白细胞po┃per os,by mouth口服POAG┃primary open-angle glaucose原发性开角型青光眼POHS┃presumed ocular histoplasmosis syndrome假定眼组织胞浆菌病综合征post┃posterior后PPA┃peripapillary atrophy视乳头周围萎缩PPD┃purified protein derivative or posterior polymorphous dystrophy纯蛋白质衍生物或后多形性营养不良PPDR┃pre-proliferative diabetic retinopathy增生前型糖尿病视网膜病变PPL┃pars plana lensectomy平部晶状体切除术PPV┃pars plana vitrectomy平部玻璃体切割术prep┃preparation准备PRK┃photorefractive keratectomy激光屈光角膜切除术prn┃take as needed当需要时服用PRP┃pan-retinal photocoagulation全视网膜光凝术PS┃posterior synechia虹膜后粘连PSC┃posterior subcapsular catarcat后囊下白内障PTC┃pseudotumor cerebri大脑假性肿瘤PTK┃phototherapeutic keratectomy光治疗性角膜切除术PVD┃posterior vitreous detachment玻璃体后脱离PVEP┃pattern visual evoked potential图形视觉诱发电位PVR┃proliferative vitreoretinopathy增生性玻璃体视网膜病变Px┃prognosis预后PXE┃psuedoxanthoma elasticum弹力性黄假瘤q┃every每qd┃every day每天q2h┃every 2 hours每2小时qid┃four times a day一天四次qod┃every other day每隔一日R┃right右RA┃rheumatoid arthritis类风湿性关节炎RB┃rose Bengal,retinoblastoma孟加拉玫瑰红,视网膜母细胞瘤RBC┃red blood cell红血细胞RBON┃retrobulbar optic neuritis球后视神经炎RCE┃recurrent corneal erosion复发性角膜侵蚀RD┃retinal ditachment视网膜脱离RF┃rheumatoid factor类风湿因子R-G┃red-green红-绿RGB┃Red green blue红绿蓝RGP┃rigid gas permeable刚质透气(接触镜)RI┃rubeosis iridis虹膜红变RIO┃right inferior oblique右眼下斜肌RIR┃right inferior rectus右眼下直肌RK┃radial keratotomy放射状角膜切开术RLR┃right lateral tectus右眼外直肌RMR┃right medial rectus右眼内直肌R/O┃rule Out摈除,排除ROP┃retinopathy of prematurity早产儿视网膜病变RP┃retinitis pigmentosa视网膜色素变性RPE┃retinal pigment epithelium视网膜色素上皮RRD┃rhegmatogenous retinal detachment孔原性视网膜脱离RSO┃right superior oblique右眼上斜肌RSR┃right superior rectus右眼上直肌RT┃return复诊RTC┃return to clinic复诊RTO┃return to office复诊RV┃return visit复诊Rx┃prescription处方s┃without没有SB┃scleral buckle巩膜扣带术sc┃without correction未戴眼镜矫正SCL┃soft contact lens软接触镜SEI┃subepithelial infiltrate上皮下侵润SI┃sector iridectomy扇形虹膜切除术sibs┃siblings同胞Sig┃write on label用法SJS┃Stevens-Johnson syndrome综合征SL┃Schwalbe’s line or serum lysozyme Schwalbe线或血清溶菌霉SLE┃slit lamp examination or systemic lupus erythematosus裂隙灯检查或系统性红斑狼疮SLK┃superior limbic keratoconjunctivitis上部角膜缘角结膜炎Sn┃sign体征SNQ┃superonasal quadrant鼻上象限SOB┃shortness of breath呼吸短促Sol┃solution溶液S/P┃status post后状态(例如XX手术后)Sph┃spherical球面镜SPK┃superficial punctuate keratitis浅层点状角膜炎SR┃subjective refraction主觉验光SRF┃subretinal fluid视网膜下液体SRNV┃subretinal neovascularization视网膜下新生血管形成SRNVM┃subretinal neovascular membrane视网膜下新生血管膜SRx┃spectacle prescription眼镜处方Ss┃scleral spur or sickle cell disease巩膜突或镰状细胞病stat┃immediately立即STD┃sexually transmitted disease性传播疾病STQ┃superotemporal quadrant颞上象限strab┃strabismus斜视subcu┃subcutaneous皮下sup┃superior下susp┃suspension悬浮液SVP┃spontaneous venous pulsation自发性静脉波动Sx┃symptom症状synech┃synechia虹膜粘连T1/2┃Timoptic drops0.5%0.5%赛马心安眼药水TA┃applanation tonometry or temporal arteritis压平式眼压测量法或颞动脉炎Tab ┃tablet 片剂Tap┃applanation tonometry压平式眼压测量法TB┃tuberculosis结核tbs┃tablespoon汤匙TBUT┃tear break-up time泪膜破裂时间TDx┃tentative diagnosis试验性诊断TIA┃transient ischemic attack暂时性缺血性发作tid┃three times a day一日三次TM┃trabecular meshwork小梁网状组织,小梁网络Tnct┃noncontact(air puff)tonometry非接触性(空气冲击)眼压测量法TPR┃temperature,pulse,respiration体温,脉搏,呼吸trab┃trabeculectomy小梁切除术TSH┃thyroid-stimulating hormone促甲状腺激素Tsp┃teaspoon茶匙Tx┃treatment治疗UA┃urinalysis尿分析UBM┃ultrasound biomicroscope超声活体显微镜UCT┃unilateral cover test单眼遮盖试验UL┃upper lid上睑ung┃ointment软膏UNQ┃upper nasal quadrant鼻上象限URI┃upper respiratory infection上呼吸道感染UTI┃urinary tract infection尿路感染UTT┃unable to test不能试验UV┃ultraviolet紫外线V┃vitreous玻璃体VA┃visual acuity视力Vcc┃vision with correction较正视力Vccl┃vision with contact lens接触镜矫正视力VD┃venereal disease性病VDRL┃Venereal disease research laboratory性病研究实验室(梅毒试验)VEP┃visual evoked potential视诱发电位VER┃visual evoked response视诱发反应VF┃visual fields视野VH┃vitreous hemorrhage玻璃体积血VKC┃vernal keratoconjunctivitis春季角膜结膜炎VKH┃Vogt-koyanagi-harada syndrome综合征VOR┃vestibuloocular reflex前庭眼反射Vsc┃vision without correction裸眼视力VZV┃varicella-zoster correction水痘-带状疱疹病毒W-4-D┃Worth-four-dot Worth四点灯WBC┃white blood cell白血细胞WC┃warm compresses热敷WC/LS┃warm compresses and lid scrubs热敷及擦洗睑缘Wk┃week星期WNL┃within normal limits在正常限度内WRA┃with-the-rule astigmatism顺规性(散光)wt┃weight重量X┃exophoria at far or axis外隐斜(视远)或轴X’┃exophoria at near外隐斜(视近)XT┃constant exotropia at far外斜视(视远)XT’┃contant exotropia at near外斜视(视近)X(T)orIXT┃intermittent exotropia at far间歇性外斜视(视远)X(T)’orIXT’┃intermittent exotropia at near间歇性外斜视(视近)YAG┃yttrium aluminum garnet亿铝石榴激光YLI┃YAG laser iridotomy YAG激光虹膜切开术Y/O┃years old岁(年龄)YPC┃YAG laser posterior capsulotomy YAG激光后囊切开术常见英文缩写PVD玻璃体后脱离CRVO中静阻BRVO分支静阻CRAO中动阻CME黄斑囊样水肿RD视网膜脱离RRD孔源性RD PVR增殖性视网膜病变CNV脉络膜新生血管NVI虹膜新生血管PCV息肉样脉络膜病变PPV玻璃体切除术Phaco+IOL+GSL白内障+晶体植入+房角分离术AION前部缺血性视网膜病变PDR增殖性DR BDR背景期DR RP视网膜色素变性PK全层角膜移植LK板层角膜移植。


糖化血红蛋白——提高糖尿病诊断效率的新手段上海交通大学附属第六人民医院内分泌代谢科贾伟平教授、包玉倩教授的课题组对中国人糖尿病诊断方法的研究又取得新进展,其研究成果最近被世界著名的四大综合性医学期刊之一——英国医学会官方月刊《British Medical Journal》在线发布,并配发了专家述评。
糖尿病是目前严重危害人类健康的三大慢性病之一。
近期在中国4.6万余人的调查显示,20岁以上人群糖尿病的患病率达9.7%,糖尿病前期的患病率达15.5%,目前估计中国糖尿病患者数已达9240万。
空腹血糖及口服葡萄糖耐量试验是诊断糖尿病的常用方法。
但是,无论进行口服葡萄糖耐量试验或检测空腹血糖均有时间及采样要求,需要空腹或多次取血,因此受试者依从性较差。
近年来有学者将糖化血红蛋白(HbA1c)这个评价糖尿病血糖控制水平的监测指标引入到糖尿病诊断领域。
HbA1c检测的优势在于方便、易行、不受进餐时间以及短期生活方式改变的影响,变异性小,反映出的血糖情况相对稳定。
2009年6月美国糖尿病学会(ADA)、国际糖尿病联盟(IDF)和欧洲糖尿病学会(EASD)组成的国际专家委员会建议HbA1c≥6.5%可作为诊断糖尿病的标准。
2010年ADA指南已将HbA1c≥6.5%列为糖尿病的诊断标准之一,将HbA1c≥6.0%作为糖尿病的筛查标准之一。
然而,应用HbA1c诊断糖尿病的切点存在种族差异。
贾伟平的团队在上海社区人群中进行空腹血糖、口服葡萄糖耐量试验以及HbA1c诊断糖尿病效率的研究。
研究结果提示,在社区普通人群中HbA1c≥6.3%诊断效率等同于空腹血糖≥7.0 mmol/l。
而在糖尿病的高危人群(包括年龄≥45岁、体重指数≥24 kg/m2)中HbA1c≥6.3%诊断效率明显优于空腹血糖≥7.0 mmol/l,也优于用HbA1c≥6.5%诊断糖尿病的效率。
目前中国的大部分综合性医院都有HbA1c检测仪,中国卫生部正在实施HbA1c检测的质量控制体系。

各种疾病的英文PART1:=各种疾病的英文=咳嗽coughing 肝炎hepatitis 癌症cancer 心脏病heart disease/attack Acidosis 酸中毒Adams-Stokes syndrome 亚—斯氏综合症alcoholism, alcoholic intoxication 酒精中毒alkalosis 碱中毒anaphylaxis 过敏症anemia 贫血iron deficie ncy an emia 缺铁性贫血megaloblastic an emia 巨幼红细胞性贫血aplastic an emia 再生障碍性贫血an giitis脉管炎angina pectoris 心绞痛arteriosclerosis 动脉硬化apoplexy 中风auricular fibrillati on 心房纤颤auriculo-ve ntricular block 房室传导阻滞bron chial asthma 支气管哮喘bron chitis 支气管炎bronchiectasis 支气管扩张bron chop neum onia 支气管肺炎carcinoma 癌cardiac arrhythmia 心律紊舌L cardiac failure 心力衰竭cardiomyopathy 心肌病cirrhosis 肝硬化coronary arteriosclerotic heart disease 冠状动脉硬化性心脏病Crohn disease 克罗恩病Cushing's syndrome 库欣综合症diabetes 糖尿病diffuse intravascular coagulation 弥散性血管凝血dyse ntery 痢疾enteritis 肠炎gastric ulcer 胃溃疡gastritis 胃炎gout 痛风hepatitis 肝炎Hodgkin's disease 霍奇金病hyperlipemia高脂血症,血脂过多hyperparathyroidism甲状旁腺功能亢进hypersple nism 脾功能亢进hypertension 高血压hyperthyroidism甲状腺功能亢进hypoglycemia 低血糖hypothyroidism 甲状腺功能减退in fectiveen docarditis 感染性心内膜炎in flue nza 流感leukemia 白血病lobar pneumonia 大叶性肺炎lymphadenitis 淋巴结炎lymphoma 淋巴瘤malaria 疟疾malnutrition 营养不良measles 麻疹myeloma 骨髓瘤myocardial infarction 心肌梗死myocarditis 心肌炎nephritis 肾炎nephritic syndrome 肾综合症obstructive pulm onary emphysema 阻塞性肺气肿pancreatitis 胰腺炎peptic ulcer 消化性溃疡peritonitis 腹膜炎pleuritis 胸膜炎pneumonia 肺炎pneumothorax 气胸purpura 紫癜allergic purpura 过敏性紫癜thrombocytolytic purpura血小板减少性紫癜pyelonephritis 肾盂肾炎renal failure肾功能衰竭rheumatic fever 风湿病rheumatoid arthritis 类风湿性关节炎scarlet fever 猩红热septicemia 败血症syphilis 梅毒tachycardia 心动过速tumour 肿瘤typhoid 伤寒ulcerativecolitis 溃疡性结肠炎upper gastr oin testi nal hemorrhage 上消化道血Neurology 神经科brain abscess 脑脓肿cerebral embolism 脑栓塞cerebral infarction 脑梗死cerebral thrombosis 脑血栓cerebral hemorrhage 脑出血con cussi on of brain脑震荡craniocerebral injury 颅脑损伤epilepsy癫痫intracranial tumour 颅内肿瘤in tracra nial hematoma 颅内血肿meningitis 脑膜炎migraine 偏头痛neurasthenia 神经衰弱neurosis 神经官能症paranoid psychosis 偏执性精神病Parki nso n's disease 帕金森综合症psychosis 精神病schizophrenia 精神分裂症Surgery 夕卜科abdominal externalhernia 腹外疝acute diffuse peritonitis 急性弥漫性腹膜炎acute mastitis 急性乳腺炎acute pan creatitis A急性胰腺炎acute perforati on of gastro-duode nal ulcer 急性胃十二指肠溃疡穿孑L acute pyel on ephritis急性肾盂肾炎anal fissure 肛裂analfistula 肛工痿anesthesia 麻醉angioma 血管瘤appendicitis 阑尾炎bleeding ofgastro-duode nal ulcer 胃十二指肠溃疡出血bone tumour 骨肿瘤breast adenoma 孚L 房腺瘤burn 烧伤cancer of breast 乳腺癌carbuncle 痈carcinoma of colon 结肠炎carc in oma of esophagus 食管癌carc in oma of gallbladder 胆囊癌carcinoma of rectum 直肠癌carcinoma of stomach 胃癌cholecystitis 胆囊炎cervical spondylosis颈椎病choledochitis胆管炎cholelithiasis 胆石症chondroma软骨瘤dislocation of joint 关节脱位erysipelas 丹毒fracture 骨折furuncle 疖hemorrhoid 痔hemothorax 血胸hypertrophy of prostate 前列腺肥大intestinal obstruction 肠梗阻intestinal tuberculosis 肠结核lipoma 脂肪瘤lithangiuria 尿路结石liver abscess 肝脓肿melanoma 黑色素瘤osseous tuberculosis 骨结核osteoclastoma 骨巨细胞瘤osteoporosis 骨质疏松症osteosarcoma骨质疏松症osteosarcoma 骨肉瘤Paget's disease 佩吉特病perianorecrtal abscess 肛管直肠周围脓肿phlegmon 蜂窝织炎portal hyperte nsion 门静脉高压prostatitis 前列腺炎protrusion of in tervertebral disc 椎间盘突出purule ntarthritis 化脓性关节炎pyoge nic ostcomyclitis 化脓性骨髓炎pyothorax 脓胸rectal polyp 直肠息肉rheumatoid arthritis 类风湿性关节炎rupture of splee n脾破裂scapulohumeral periarthritis 肩周炎tenosynovitis 腱鞘炎tetanus 破伤风thromboa ngiitis 血栓性脉管炎thyroidade no care inoma 甲状腺腺癌thyroid adenoma 甲状腺腺瘤trauma 创伤urinary infection 泌尿系感染varicose vein of lower limb 下肢静脉曲张Paediatrics 丿儿科acute militarytuberculosis of the lung 急性粟粒性肺结核acute n ecrotic en teritis 急性坏死性结肠炎an aphylactic purpura 过敏性紫癜ancylostomiasis 钩虫病ascariasis 蛔虫病asphyxia of the n ewbor n 新生儿窒息atrial septal defect 房间隔缺损birth injury 产伤cephalhematoma 头颅血肿cerebral palsy 脑性瘫痪congenital torticollis 先天性斜颈convulsion 惊厥Dow n's syn drome 唐氏综合症glomerul on ephritis 肾小球肾炎hemophilia 血友病infan tile diarrhea 婴儿腹泻intracranial hemorrhage of the n ewbor n 新生儿颅内出血in tussuscepti on肠套叠necrotic enterocolitis of newborn 新生儿坏死性小肠结膜炎neon atal jau ndice新生儿黄疸nu triti on al iron deficie ncyan emia 营养性缺铁性贫血n utritio nal megaloblastic an emia 营养性巨幼细胞性贫血pate nt ductus arteriosis 动脉导管未闭poliomyelitis 骨髓灰质炎prematureinfant 早产儿primary tuberculosis 原发性肺结核progressive muscular dystrophy 进行性肌肉营养不良pulm onary ste no sis肺动脉狭窄purulent meningitis 化脓性脑膜炎rickets 佝偻病sepsis of the n ewbor n 新生儿败血症teta nus of thenewborn 新生儿破伤风tetralogy of Fallot法洛四联症thrush 鹅口疮,真菌性口炎varicella 水痘ventricular septal defect 室间隔缺损viral encephalitis 病毒性脑炎viral myocarditis 病毒性心肌炎Gyn ecology and Obstetrics 妇,产科aborti on 流产ade no myosis 子宫内膜异位症amniotic fluid embolism 羊水栓塞Bartholin's cyst 巴氏腺囊肿carcinoma ofcervix 子宫颈癌carcinoma ofendometrium 子宫内膜癌carcinoma of ovary 卵巢癌cervicitis 宫颈炎chorio-epithelioma 绒毛膜上皮癌corpora luteum cyst 黄体囊肿dystocia 难产eclampsia 子痫edema-protei nu ria-hyperte nsion syndrome 水肿蛋白尿高血压综合征(妊娠高血压综合征)en dometriosis 子宫内膜异位症extrauteri ne preg nancy 子宫外孕hydatidiform mole 葡萄胎hyperemesis gravidarum 妊娠剧吐in fertility 不育症irregular menstruation 月经失调lochia 恶露monilial vaginitis 念珠菌性阴道炎multiple pregnancy 多胎妊娠myoma ofuterus 子宫肿瘤oligohydramnios 羊水过少ovarian tumour 卵巢肿瘤pelvic inflammatory disease 盆腔炎placentaprevia 前置胎盘placental abruption 胎盘早期剥离pregnancy-hypertension syn drome 妊娠高血压综合症prematurebirth 早产premature rupture of membrane 胎膜早破postpartumhemorrhage 产后出血puerperal infection产褥感染rupture of uterus 子宫破裂trichom onas vagi nitis 滴虫性阴道炎uteroplace ntal apoplexy 子宫胎盘卒中vulvitis 夕卜阴炎Ophthalmology and Otorhinolaryngology 五官科amblyopia弱视amygdalitis, tonsillitis 扁桃体炎astigmatism 散光carcinoma of nasopharynx 鼻咽癌carcinoma of larynx喉癌cataract 白内障tinnitus 耳鸣chalazion 霰粒肿,脸板腺囊肿colour blindness 色盲deflection of nasalseptum 鼻中隔偏曲deafness 聋furuncle of nasalvestibule 鼻前庭疖glaucoma 青光眼heterotropia 斜视hyperopia 远视injury of cornea 角膜损伤ceruminal impaction 耵聍嵌塞iritis 虹膜炎keratitis 角膜炎labyrinthitis 迷路炎,内耳炎laryn gitis 喉炎PART2:=用英语描述疾病=⑴一般病情:He feels headache, n ausea and vomiti ng.(他觉得头痛、恶心和想吐。

二级预防abcde原则如果某种疾病还没得,我们防治得病,我们就叫做这种疾病的一级预防,比如:预防得冠心病就叫冠心病的一级预防。
但是如果已经得了,防止冠心病发作或进一步加重,就叫做冠心病的二级预防。
冠心病根据病情的轻重,分为稳定的冠心病(慢性冠脉综合征CCS)和不稳定的冠心病(急性冠脉综合征,ACS)。
但不论哪一种,都要遵循以下的“ABCDE”方桉来进行二级预防。
所谓“ABCDE”均为英文缩写的字头。
A:抗血小板治疗(Anti-platelet therapy)、血管紧张素转化酶抑制剂(ACEI)B:β受体阻滞剂(Beta blocker)、血压控制(Blood pressure control)C:戒烟(Cigarette quitting)、血脂控制(Cholesterol lowering)D:饮食(Diet)、糖尿病控制(Diabetes control)E:运动(Exercise)、教育(Education)冠心病的二级预防abcde原则,指冠心病治疗的最基本原则,包括以下10个方面:A-抗血小板和抗心绞痛:第一个A是抗血小板,抗血小板包括吃阿司匹林或替格瑞洛片双联抗血小板;第二个A是抗心绞痛,当胸口闷痛的时候,服用中成药,或曲美他嗪,或尼可地尔抗心绞痛。
B-降压和服用β受体阻滞剂:B也有两个意思,一个是降压,一个是β受体阻滞剂。
C-戒烟和降血脂:C的意思一个是戒烟,第二个C是降血脂,吃他汀类的药物把胆固醇降下来。
D-降血糖和低盐低脂饮食:D的意思一个是糖尿病要降血糖,第二个D指低盐低脂。
E-锻炼和接受健康教育:得了冠心病,不能整天躺在床上,还是要活动的,要散步、游泳,什么活动都可以,但是要不引起心痛为前提。
第二个E即健康教育,建议患者按时吃药、运动、降血压,让患者接受一些卫生健康知识,了解自己、了解健康知识。

常用缩写AAA, abdominal aortic aneurysm 腹主动脉瘤ABFB, aortobifemoral bypass 主双股动脉旁路术ABI, ankle-brachial index 踝肱指数ACA, anterior cerebral artery 大脑前动脉ACE, angiotensin-converting enzyme 血管紧张素转化酶ACT, activated clotting time 活化凝血时间ADA, American Diabetes Association 美国糖尿病协会ADP, adenosine diphosphate 二磷酸腺苷AEF, aortoenteric fistula 主动脉肠道瘘AF, atrial fibrillation 房颤AFB, aortofemoral bypass 主股旁路术AGE, advanced glycosylation end product 高级糖基化终末产物AHA, American Heart Association 美国心脏病协会AHRQ, Agency for Healthcare Research and Quality 健康保健研究及质量控制委员会AI,aortoiliac 主髂AIDS, acquired immunodeficiency syndrome 获得性免疫缺陷综合征AKA, above-knee amputation 膝上截肢术AMP, adenosine monophosphate 单磷酸腺苷APC, activated protein C 活化蛋白CAPG, air plethysmography 空气体积描记术aPTT, activated partial thromboplastin time 活化部分凝血酶原时间ARB, angiotensin receptor blocker 血管紧张素受体阻滞剂ARDS, acute respiratory distress syndrome 急性呼吸窘迫综合征ARF, acute renal failure 急性肾衰ASA, acetylsalicylic acid 阿司匹林ATN, acute tubular necrosis 急性肾小管坏死ATP, adenosine triphosphate 三磷酸腺苷AVE, arteriovenous fistula 动静脉瘘AVG, arteriovenous graft 动静脉移植物AVM, arteriovenous malformation 动静脉畸形AVP, ambulatory venous pressure 非卧床静脉压bFGF, basic fibroblast growth factor碱性成纤维细胞生长因子BKA, below-knee amputation 膝下截肢BSA, body surface area 体表面积BUN, blood urea nitrogen血尿素氮CABG, coronary artery bypass grafting 冠状动脉旁路术CAD, coronary artery disease 冠状动脉疾病CAMP, cyclic adenosine monophosphate 环磷酸腺苷CAS, carotid artery stenting 颈动脉支架置入术CAVH, continuous arteriovenous hemofiltration连续动静脉血液滤过CAVHDF, continuous arteriovenous hemodiafiltration 连续动静脉血液透析滤过法CCA, common carotid artery 颈总动脉CCB, calcium channel blocker 钙通道阻滞剂CDC, Centers for Disease Control and Prevention疾病控制和预防中心CEA, carotid endarterectomy 颈动脉内膜切除术CEAP, clinical, etiologic, anatomic, pathologic [staging system] 临床的,病因学,解剖学,病理学(分级体系)CEA, common femoral artery 股总动脉CFV, common femoral vein 股总静脉cGMP, cyclic guanosine monophosphate 环磷酸鸟苷CI, confidence interval 置信区间CIA, common iliac artery 髂总动脉CK-MB, MB isozyme of creatine kinase 肌酸激酶同工酶CLI, critical limb ischemia 严重下肢缺血CMS, Centers for Medicare and Medicaid Services 医疗保险和补助中心CNS, central nervous system 中枢神经系统CO, carbon monoxide 一氧化碳CO2, carbon dioxide 二氧化碳COPD, chronic obstructive pulmonary disease 慢性阻塞性肺疾患COX, cyclooxygenase 环氧合酶CRI, chronic renal insufficiency 慢性肾功能不全CRP, C-reactive protein C反应蛋白CRPS, complex regional pain syndrome 复杂区域性疼痛CSF, cerebrospinal fluid 脑脊液CT, computed tomography 计算机断层扫描CTA, computed tomographic angiography 计算机断层扫描血管造影CTD, connective tissue disease 结缔组织病CTV, computed tomographic venography 计算机断层扫描静脉造影CVI, chronic venous insufficiency 慢性静脉功能不全CVP, central venous pressure 中心静脉压CWH, continuous venovenous hemofiltration 连续型静脉-静脉血液滤过CWHDF, continuous venovenous hemodiafiltration 连续型静脉-静脉血液透析法2D, two-dimensional 二维3D, three-dimensional 三维DBI, digital-brachial index 趾肱指数DBP, diastolic blood pressure 舒张压DDAVP, desmopressin去氨加压素DES, drug-eluting stent 药物洗脱支架DFU, diabetic foot ulcer 糖尿病足溃疡DIC, disseminated intravascular coagulation弥散性血管内凝血DM, diabetes mellitus 糖尿病DNA, deoxyribonucleic acid脱氧核糖核酸2,3-DPG, 2,3-diphosphoglvcerate 2,3二磷酸甘油酸酯DRIL, distal revascularization-interval ligation 远端血运重建间隔结扎DSA, digital subtraction angiography 数字减影血管造影DSE, dobutamine stress echocardiography 多巴酚丁胺负荷超声心动图DTAA, descending thoracic aortic aneurysm 降主动脉瘤DUS, duplex ultrasound 多普勒超声DVT, deep venous thrombosis 深静脉血栓EC,endothelial cell 内皮细胞ECA, external carotid artery 颈外动脉ECG, electrocardiogram 心电图EC-IC, extracranial-intracranial [bypass] 颅外-颅内旁路术ECM, extracellular matrix 细胞外基质ED,erectile dysfunction 勃起功能障碍EDS, Ehlers-Danlos syndrome 埃勒斯-当洛综合征EDV, end-diastolic velocity 舒张末期流速EEG, electroencephalography 脑电描记法EF, ejection fraction 射血分数EIA, external iliac artery 髂外动脉ELAM-1, endothelial leukocyte adhesion molecule-1 内皮细胞白细胞粘附分子-1 ELISA, enzyme-linked immunosorbent assay 酶联免疫吸附测定法ELT, euglobulin lysis time 优球蛋白溶解时间EMG, electromyography 肌电图描记术eNOS, endothelial nitric oxide synthase 内皮一氧化氮合酶ePTFE, expanded polytetrafluoroethylene 膨体聚四氟乙烯ESR, erythrocyte sedimentation rate 红细胞沉降率,血沉ESRD, end-stage renal disease 终末期肾病EVAR, endovascular aneurysm repair 腹主动脉瘤腔内修复术FDA, Food and Drug Administration 食品和药品管理局FDP, fibrin/fibrinogen degradation product 纤维蛋白/纤维蛋白原降解产物FEV, forced expiratory volume in 1 second 一秒用力呼气量FFP, fresh frozen plasma 新鲜冰冻血浆FGF, fibroblast growth factor 成纤维细胞生长因子FMD, fibromuscular dysplasia 纤维肌性发育不良FRC, functional residual capacity 功能残气量FVC, forced vital capacity 最大肺活量GA, general anesthesia 全麻GFR, glomerular filtration rate 肾小球滤过率GI, gastrointestinal 胃肠的GMP, guanosine monophosphate 单磷酸鸟(嘌呤)核苷G6PD, glucose-6-phosphate dehydrogenase 6-磷酸葡萄糖脱氢酶GP-IIb/IIIa, glycoprotein Ilb/IIIa 糖蛋白Ilb/IIIaGSM, gray-scale median 灰度中间值GSV, great saphenous vein 大隐静脉GSW, gunshot wound 枪弹伤GTP, guanosine triphosphate三磷酸鸟(嘌呤)核苷GUI, graphic-user interface 图形用户界面GW, guide wire 导丝HD, hemodialysis 血液透析HDL, high-density lipoprotein 高密度脂蛋白HIPPA, Health Insurance Portability and Accountability Act 健康保险流通与责任法案HIT, heparin-induced thrombocytopenia 肝素诱导的血小板减少症HIV, human immunodeficiency virus 人类免疫缺陷病毒HLA, human leukocyte antigen 人类白细胞抗原HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A羟甲基戊二酰辅酶AHR, hazard ratio 危害比HRQoL, health-related quality of life健康相关生活质量hsCRP, high-sensitivity C-reactive protein 高灵敏度C反应蛋白5-HT, serotonin 5-羟色胺HTN, hypertension 高血压ICA, internal carotid artery 颈内动脉ICAM-1, intercellular adhesion molecule-1 细胞间粘附分子-1ICAVL, Intersocietal Commission for the Accreditation of Vascular Laboratories 国家血管检查评估委员会ICH, intracerebral hemorrhage 颅内出血ICU, intensive care unit 重症监护病房IDL, intermediate-density lipoprotein 中密度脂蛋白IEL, internal elastic lamina 内弹力层IFN, interferon 干扰素IFU, instructions for use 使用说明书IGF, insulin-like growth factor 胰岛素样生长因子IH, intimal hyperplasia 内膜增生IL-6, interleukin-6 白介素-6IMA, inferior mesenteric artery 肠系膜下动脉iNOS, inducible nitric oxide synthase诱生型一氧化氮合酶IOM, Institute of Medicine 医学会IPC, intermittent pneumatic compression 间断性肺压缩IPG, impedance plethysmography 阻抗体积描记法IPPB, intermittent positive pressure breathing 间断性正压呼吸I/R, ischemia-reperfusion 缺血再灌注IVC,inferior vena cava 下腔静脉IVUS, intravascular ultrasound 血管内超声JAK-2, Janus kinase-2 蛋白酪氨酸激酶-2JNK, jun N-terminal kinase N-端氨基酸激酶K/DOQI, Kidney Disease Outcomes Quality Initiative 肾脏疾病预后质量测评KM, Kaplan-Meier卡普兰-迈耶曲线LAO, left anterior oblique 左前斜位LDL, low-density lipoprotein 低密度脂蛋白LMWH, low-molecular-weight heparin 低分子量肝素LOS, length of stay 住院时间Lp(a), lipoprotein (a) 脂蛋白LS, lumbosacral 腰骶的LV, left ventricular 左心室LVEDP, left ventricular end diastolic pressure 左心室舒张末期压LVEDV, left ventricular end diastolic volume 左室舒张末期容积LVH, left ventricular hypertrophy 左心室肥大MAP, mean arterial pressure 平均动脉压MCA, middle cerebral artery 大脑中动脉MI, myocardial infarction 心肌梗死MIP, maximum intensity projection 最大强度投影MMP, matrix metalloproteinase 基质金属蛋白酶MOF, multiple organ failure 多器官衰竭MRA, magnetic resonance angiography 核磁共振血管造影MR, magnetic resonance 核磁共振MRI, magnetic resonance imaging 核磁共振成像MRSA, methicillin-resistant Staphylococcus aureus 耐甲氧西林金葡菌MRV, magnetic resonance venography 核磁共振静脉成像MTHFR, 5,10-methylenetetrahydrofolate reductase 5,10 -亚甲基四氢叶酸还原酶NAC, N-acetylcysteine N-乙酰半胱氨酸NAD*, oxidized nicotinamide dinucleotide 氧化烟酰胺二核苷酸NADH, reduced nicotinamide adenine dinucleotide 还原型烟酰胺腺嘌呤二核苷酸,还原型辅酶INADPH, reduced nicotinamide adenine dinucleotide phosphate 还原型烟酰胺腺嘌呤二核苷酸磷酸,还原型辅酶ⅡNAIS, neo-aortoiliac system 新主髂动脉系统Nd:YAG, neodymium:yttrium-aluminum-garnet 钕钇铝石榴石NF-K B, nuclear factor K B核因子K BNIH, National Institutes of Health 国立卫生研究院NTS, National Inpatient Sample 全国住院样本NOS, nitric oxide synthase 一氧化氮合酶NPV, negative predictive value阴性预测值NSAID, nonsteroidal anti-inflammatory drug非甾体抗炎药NSQIP, National Surgical Quality Improvement Program 全国外科质量改进计划OR, odds ratio 优势比OTW, over-the-wire 导丝支撑的PA, pulmonary artery 腓动脉PAD, peripheral arterial disease 周围动脉疾病PAI, proximalization of arterial inflow 动脉流入道近端PAI-1, plasminogen activator inhibitor-1纤溶酶原激活物抑制剂-1 PAOD, peripheral arterial occlusive disease 周围动脉闭塞性疾病PBI, penile-brachial index 阴茎-肱(动脉收缩压)指数PBRCs, packed red blood cells 浓缩红细胞PCA, posterior cerebral artery 大脑后动脉PCI, percutaneous coronary intervention 经皮冠脉介入PCNA, proliferating cell nuclear antigen 增殖细胞核抗原PCWP, pulmonary artery wedge pressure 肺动脉楔压PD, peritoneal dialysis 腹膜透析PDE, phosphodiesterase磷酸二酯酶PDGF, platelet-derived growth factor血小板源生长因子PE, pulmonary embolism 肺栓塞PECAM-1, platelet-endothelial cell adhesion molecule-1血小板-内皮细胞粘附分子-1PEEP, positive end-expiratory pressure呼气末正压通气PEG, polyethylene glycol聚乙二醇PET, positron emission tomography正电子发射体层摄影PF4, platelet factor 4血小板因子ⅣPEA, profunda femoris artery 股深动脉PFT, pulmonary function test/testing 肺功能测试PGE2, prostaglandin E2 前列腺素E2PGI2, prostaglandin I, 前列腺素IPKC, protein kinase C蛋白激酶CPMN, polymorphonuclear neutrophil 多形核中性粒细胞PPG, photoplethysmography光学体积描记术PPV, positive predictive value阳性预测(价)值PRBCs, packed red blood cells 浓缩红细胞PSA, pseudoaneurysm 假性动脉瘤psi, pounds per square inch 磅/平方英寸PSV, peak systolic velocity最大收缩速度PT, prothrombin time凝血酶原时间PTA, percutaneous transluminal angioplasty 经皮腔内血管成形术PTFE, polytetrafluoroethylene聚四氟乙烯PTT, partial thromboplastin time部分凝血致活酶时间PVI, peripheral vascular intervention 周围血管介入PVR, pulse volume recording脉搏容积记录仪QALY, quality-adjusted life year质量调整生命年QoL, quality of life 生活质量RAAA, ruptured abdominal aortic aneurysm 破裂腹主动脉瘤RAGE, receptor for advanced glycosylation end products高级糖基化终末产物受体RAO, right anterior oblique 右前斜位RAS, renal artery stenosis 肾动脉狭窄RBC, red blood cell 红细胞RCT, randomized controlled trial 随机对照试验Re, Reynolds number 雷诺数RFA, radiofrequency ablation射频消蚀RGD, Arg-Gly-Asp 精氨酸-甘氨酸-天冬氨酸RI, resistive index 对抗指数RIND, reversible ischemic neurologic deficit 可逆性缺血性神经障碍RP, retroperitoneal 腹膜后的RR, relative risk 相对危险度RS, Raynaud's syndrome 雷诺氏综合征rt-PA, recombinant tissue plasminogen activator 重组组织型纤维蛋白酶原激活剂RUDI, revision using distal inflow 流入道远端的修复SBP, systolic blood pressure 收缩压SD, standard deviation 标准差SE, standard error 标准误SEPS, subfascial endoscopic perforator surgery 筋膜内镜下手术SF-36, Short Form (36) Health Survey 健康调查简表-36SFA, superficial femoral artery 股浅动脉SFJ, saphenofemoral junction 股隐交界处SK, streptokinase 链激酶SLE, systemic lupus erythematosus 系统性红斑狼疮SMA, superior mesenteric artery 肠系膜上动脉SMC, smooth muscle cell 平滑肌细胞SOD, superoxide dismutase超氧化物歧化酶SPECT, single-proton emission computed tomography 单光子发射计算体层摄影SPJ, saphenopopliteal junction 隐腘静脉交界处SSV, small saphenous vein 小隐静脉STEMI, ST-segment myocardial infarction ST段异常心肌梗死SVC, superior vena cava 上腔静脉SVS, Society for Vascular Surgery 血管外科协会TAA, thoracic aortic aneurysm 胸主动脉瘤TAAA, thoracoabdominal aortic aneurysm 胸腹主动脉瘤TAAD, thoracic aortic aneurysm and dissection 胸主动脉瘤和夹层TAO, thromboangiitis obliterans 血栓闭塞性脉管炎TASC, Trans-Atlantic Inter-Society Consensus for the Management of Peripheral Arterial Disease 周围动脉疾病治疗的泛大西洋跨协会建议TCD, transcranial Doppler 经颅多普勒TEE, transesophageal echocardiography 经食道超声TEVAR, thoracic endovascular aortic repair 胸主动脉修复术TF, tissue factor 组织因子TGF-β, transforming growth factor-β转化生长因子-βTIMP-1, tissue inhibitor of matrix metalloproteinase-1 基质金属蛋白酶抑制剂-1TIPS, transjugular intrahepatic portosystemic shunting 经颈静脉肝内门体分流术TLR, target lesion revascularization 靶病变血管重建TMA, transmetatarsal amputation 经跖骨截肢术TNF-α, tumor necrosis factor-α肿瘤坏死因子-αTOS, thoracic outlet syndrome 胸廓出口综合征t-PA, tissue plasminogen activator 组织纤溶酶原激活剂TT, thrombin time 凝血酶时间TTE, transthoracic echocardiography 经胸壁超声心动图TXA2, thromboxane A2 血栓素A2UFH, unfractionated heparin 普通肝素UK, urokinase 尿激酶u-PA, urinary plasminogen activator (urokinase) 尿纤溶酶原激活物USPSTF, U.S. Preventive Services Task Force美国预防服务工作队VATS, video-assisted thoracoscopic surgery电视辅助胸腔镜手术VCAM-1, vascular cell adhesion molecule-1血管细胞粘附分子-1VEGF, vascular endothelial growth factor 血管内皮生长因子VFI, venous filling index 静脉充盈指数VLDL, very-low-density lipoprotein 极低密度脂蛋白VSMC, vascular smooth muscle cell 血管平滑肌细胞VSS, Venous Severity Score 静脉严重性分级VTE, venous thromboembolism 静脉血栓栓塞性疾病vWF, von Willebrand factor 血管假性血友病因子WBC, white blood cell 白细胞WIQ, Walking Impairment Questionnaire 步行曲线调查表欢迎您的下载,资料仅供参考!致力为企业和个人提供合同协议,策划案计划书,学习资料等等打造全网一站式需求。
阿尔兹海默症与糖病的关系及干预措施阿尔兹海默症与糖尿病的关系及干预措施阿尔兹海默症(Alzheimer's disease,AD)是一种进行性神经退行性疾病,主要表现为认知和行为功能的丧失。
糖尿病(diabetes)是一种代谢紊乱疾病,由于胰岛素分泌或作用异常而导致血糖升高。
近年来,研究发现阿尔兹海默症与糖尿病之间存在密切的关系。
本文将探讨阿尔兹海默症与糖尿病的关系,并提出干预措施。
一、阿尔兹海默症与糖尿病的关系1. 共同风险因素:阿尔兹海默症和糖尿病的发病风险在一定程度上与年龄、遗传因素、心血管疾病等共同相关。
老年人容易同时患有阿尔兹海默症和糖尿病。
2. 炎症和氧化应激:炎症和氧化应激是阿尔兹海默症和糖尿病共同的病理生理特征。
炎症和氧化应激过程中产生的炎性细胞因子和自由基对大脑和胰岛有损害作用,促进了这两种疾病的发展。
3. 血糖控制:高血糖是糖尿病的主要特征,而长期高血糖有可能导致阿尔兹海默症的发生。
高血糖会损伤神经细胞,增加阿尔兹海默症的风险。
4. 胰岛素抵抗:糖尿病患者常伴随胰岛素抵抗的状况。
胰岛素抵抗可导致大脑内胰岛素受体的异常,进而影响细胞信号传导通路,加速阿尔兹海默症的发展。
二、干预措施1. 生活方式改变:良好的生活习惯对预防和控制阿尔兹海默症和糖尿病起到重要作用。
建立规律的运动习惯、科学的饮食结构、充足的睡眠等都有助于维持身体健康。
2. 药物治疗:针对糖尿病,胰岛素和药物治疗是常见的干预手段。
提前识别糖尿病风险,遵循医生的指导,合理用药,有助于减轻胰岛素抵抗、控制血糖水平。
对于阿尔兹海默症,药物干预可以通过改善认知功能和减轻症状带来一定帮助。
3. 心理和认知训练:通过心理和认知训练可以改善阿尔兹海默症患者的记忆力和思维能力。
同时,阿尔兹海默症患者的情绪管理也很重要,积极的心态和情绪对疾病的干预有积极的促进作用。
4. 科学监测和干预:定期检查血糖、胰岛素水平、认知能力等指标,以及进行相关的体格检查和影像学检查,有助于及早了解阿尔兹海默症和糖尿病的变化情况,及时采取干预措施。
“二型糖尿病”的英文表达有哪些1. Type 2 Diabetes Mellitus (T2DM)2. NonInsulinDependent Diabetes Mellitus (NIDDM)3. AdultOnset Diabetes4. MaturityOnset Diabetes5. Insulin Resistance Diabetes6. SecondType Diabetes7. Diabetes Mellitus Type 28. DM2这些术语都可以用来描述二型糖尿病,但在不同的医学文献和临床实践中,可能会使用不同的表达方式。
在与国际同行交流时,建议使用“Type 2 Diabetes Mellitus”这一最常用且被广泛认可的术语。
“二型糖尿病”在英文中有两个常用的表达方式,分别是“Type2 Diabetes”和“Diabetes Mellitus Type 2”。
其中,“Type 2 Diabetes”是最常用的表达方式,而被普遍接受和使用的缩写为“T2D”或“T2DM”。
“Diabetes Mellitus Type 2”这个表达方式在学术文献和专业报告中较为常见,它的缩写为“DM2”。
除此之外,还有一些其他的英文表达方式,如“NonInsulinDependent Diabetes Mellitus”,缩写为“NIDDM”。
这个表达方式虽然不如“Type 2 Diabetes”常用,但在某些文献中仍然可以见到。
另外,“AdultOnset Diabetes”也是“二型糖尿病”的一个英文表达方式,主要用来描述成年期发病的糖尿病。
还有一种表达方式是“ maturityonset diabetes”,它主要用来描述成年期发病的糖尿病,但与“Type 2 Diabetes”相比,使用较少。
“Impaired Glucose Tolerance”和“Impaired Insulin Sensitivity”这两个表达方式,分别用来描述血糖耐受受损和胰岛素敏感性降低的情况,它们通常与“二型糖尿病”相关联。
DIABETES NEW WORLD糖尿病新世界[基金项目]吉林省科技发展计划项目(182447SF010945097)。
[作者简介]杨天资(1995-),女,硕士,研究方向为全科医学及认知障碍。
[通信作者]白春艳(1974-),女,博士,主任医师,主要从事认知障碍及癫痫研究工作,E-mail:********************。
阿尔茨海默病(AD)是一组病因未明的神经退行性疾病,主要病理特征是老年斑(SP)和神经原纤维缠结(NFT)。
SP 是由β淀粉样蛋白(Aβ)形成[1],可造成神经细胞损伤。
NFT 是由tau 蛋白过度磷酸化形成[1],可产生神经毒性。
轻度认知障碍(MCI)是介于正常与痴呆之间的过渡状态,是AD 发展中的一个阶段。
2型糖尿病(T2DM)是以胰岛素分泌不足或抵抗导致高血糖为特征的慢性代谢性疾病,可引起多种组织器官结构和功能上的改变。
随着老龄化的加剧,年龄相关性疾病的发病率也在增长。
最新调查显示,中国60岁及以上人群中MCI 发病率为15.5%,AD 发病率3.93%[2]。
且我国糖尿病患病率已增至11.2%[3]。
研究表明,T2DM 是MCI 和AD 的独立危险因素[4]。
一项研究结果显示,糖尿病患者的认知表现较正常人有更显著的下降[5]。
因此,T2DM 可增加AD 发生的危险性,两种疾病之间存在相互作用、协同作用的重叠机制。
甚至有学者将AD 称为“3型糖尿病”[6]。
所以对T2DM 与MCI 关系的研究尤为必要,探索两病之间的作DOI:10.16658/ki.1672-4062.2021.18.195轻度认知障碍与2型糖尿病关系的研究进展杨天资1,白春艳21.长春中医药大学全科医学专业,吉林长春130117;2.吉林省人民医院神经内科,吉林长春130021[摘要]阿尔茨海默病(Alzheimer's disease,AD)是以记忆减退、认知障碍为主要表现的神经退行性疾病,是一个连续的病理生理过程。
病名英语缩写ARDS 成人呼吸窘迫综合症CAP 社区获得性肺炎COPD 慢性阻塞性肺气肿IPF 特发性肺纤维化HAP 医院获得性肺炎PAP 肺泡蛋白质沉积症PIE 间质肺气肿PTE 肺栓塞TB 肺结核PCP 卡式肺囊虫肺炎AVB 房室传导阻滞Af 房颤ASD 房缺AI 主闭AS 主狭ASO 闭塞性动脉硬化AT 房速AMI 急性心梗AP 心绞痛APB 房早BBB 束支传导阻滞CHD冠心病CHF 充血性心衰CF 心衰F3 法三F4 法四IHD 缺血性心脏病PDA 动脉导管未闭PS 肺狭PAT 阵发性房性心动过速MI 心梗MVP 二间瓣脱垂RBBB右束支传导阻滞SSS 病态窦房结综合症SBE 亚急性感染性心内膜炎UA 不稳定性心绞痛VSD 室缺VDH 心脏瓣膜病ALT 成人T细胞白血病AA 再障AIHA 自身免疫性溶血性贫血CLL 慢淋CGN 慢粒DIC 弥漫性血管内凝血ITP过敏性紫殿IDA 缺铁贫HD 霍奇金病PNH 阵发性睡眠性血红蛋白尿MM 多发性骨髓瘤MDS 骨髓增生异常综合症NHL 非霍奇金RAEB 难治性贫血伴原始细胞增多型TIP 血栓性血小板减少性紫殿AIH 自身免疫性肝炎AP 急性胰腺炎DU十二指肠溃疡ERCP内镜逆行胰胆管造影术FD 功能性消化不良GU 胃溃疡GERD胃食管反流病HE 肝性脑病IBS 肠易激综合症IBD 炎症性肠病SAP 急性重症胰腺炎UC 溃疡性结肠炎WD 肝豆状核变性DM 糖尿病T2DM 2型糖尿病DR 糖尿病视网膜病变DN 糖尿病肾病DKA 糖尿病酮症酸中毒DLE 盘状红斑狼疮GD 甲亢 GravesHNKHC 高渗性非酮症糖尿病昏迷IGT 糖耐量减低IDD 胰岛素依赖性糖尿病IIM 特发性炎症性肌病PKU 苯丙酮尿症MAS或POED 多发性骨纤维构造不良SSc 系统性硬化病SLE 系统性红斑狼疮AD 阿尔海默茨病ARF 急性肾功能不全AIN 急性间质性肾炎AGN 急性肾炎CGN 慢性肾炎CRF 慢性肾功能不全CIN 慢性间质性肾炎HIE 新生儿缺血缺氧性脑病PD 帕金森氏病PEM 蛋白质-热能营养不良PID 盆腔炎SCA 脊髓小脑共济失调MG 重症肌无力RA 类风湿关节炎TIA 短暂性脑缺血发作AIDS:艾滋病〔Acquired immune deficiency syndrome or acquired immunodeficiency syndrome)CRF:慢性肾衰竭〔Chronic renal failure〕ARF:急性肾功能衰竭(Acute renal failure)HMD:新生儿肺透明膜病〔hyaline membrane disease〕RDS:呼吸窘迫综合征〔Respiratory Distress Syndrome)ARDS:新生儿急性呼吸窘迫综合征(Acute Respiratory Distress Syndrome) NARDS:新生儿急性呼吸窘迫综合征〔Neonatal acute respiratory distress syndrome)RA:类风湿关节炎〔Rheumatoid arthritis〕OA:骨性关节炎〔Osteoarthritis〕DJD:退行性骨关节病〔Degenerative joint disease〕AS:强直性脊柱炎〔Ankylosing spondylitis〕SLE:系统性红斑狼疮(Systemic lupus erythematosus)SC:全身性硬皮病(Systemic scleroderma)ALL:急性淋巴细胞性白血病(Acute lymphocytic leukemia)ANLL:急性非淋巴细胞性白血病(acute nonlymphocytic leukemia )HHT:遗传性出血性毛细血管扩张症(Rendu-Osler-Weber病)(Hereditary hemorrhagic telangiectasia)PDA:动脉导管未闭(patent ductus arteriosus)VSD:室间隔缺损(ventricular septal defect)ASD:房间隔缺损(atrial septal defect)CASD:先天性房间隔缺损(congenital atrial septal defect)CML:慢性粒细胞性白血病(Chronic myelogenous leukemia)AML:急性粒细胞性白血病(Acute myelogenous leukemia)DM:糖尿病〔diabetes mellitus〕HBP:高血压(hypertension or high Blood pressure)HHD:高血压性心脏病(hypertensive heart disease)HRD:高血压性肾病(hypertensive renal disease)HE:高血压性脑病(hypertensive encephalopathy)CAHD:冠状动脉粥样硬化性心脏病(Coronary atherosclerotic heart disease) PTB:肺结核(pulmonary tuberculosis)MM:多发性骨髓瘤(multiple myeloma)COE:慢性阻塞性肺气肿(chronic obstructive emphysema)COPD:慢性阻塞性肺病〔chronic obstructive pulmonary diseases〕是一种慢性气道阻塞性疾病的统称,主要指具有不可逆性气道阻塞的慢性支气管炎和肺气肿两种疾病.ABC:动脉瘤样骨囊肿〔aneurysmal bone cyst〕CSO:慢性化脓性骨髓炎(chronic suppurative osteomyelisis)IPIF:特发性肺间质纤维化(idiopathic pulmonary interstitial fibrosis) FHB:骨纤维异常增生症(fibrous hyperplasia of bone)ANEFH:股骨头骨骺缺血坏死又称Legg-Perthes-Calve病、扁平髋、股骨头骨骺骨软骨炎等(Avascular necrosis of epiphysis of femoral head)INFH:股骨头缺血性坏死(ischemic necrosis of femoral head)(Avascular necrosis of femoral head)ARDS 成人呼吸窘迫综合症CAP 社区获得性肺炎COPD 慢性阻塞性肺气肿IPF 特发性肺纤维化HAP 医院获得性肺炎PAP 肺泡蛋白质沉积症PIE 间质肺气肿PTE 肺栓塞TB 肺结核PCP 卡式肺囊虫肺炎AVB 房室传导阻滞Af 房颤ASD 房缺AI 主闭AS 主狭ASO 闭塞性动脉硬化AT 房速AMI 急性心梗AP 心绞痛APB 房早BBB 束支传导阻滞CHD冠心病CHF 充血性心衰CF 心衰F3 法三F4 法四IHD 缺血性心脏病PDA 动脉导管未闭PS 肺狭PAT 阵发性房性心动过速MI 心梗MVP 二间瓣脱垂RBBB右束支传导阻滞SSS 病态窦房结综合症SBE 亚急性感染性心内膜炎UA 不稳定性心绞痛VSD 室缺VDH 心脏瓣膜病ALT 成人T细胞白血病AA 再障AIHA 自身免疫性溶血性贫血CLL 慢淋CGN 慢粒DIC 弥漫性血管内凝血ITP过敏性紫殿IDA 缺铁贫HD 霍奇金病PNH 阵发性睡眠性血红蛋白尿MM 多发性骨髓瘤MDS 骨髓增生异常综合症NHL 非霍奇金RAEB 难治性贫血伴原始细胞增多型TIP 血栓性血小板减少性紫殿AIH 自身免疫性肝炎AP 急性胰腺炎DU十二指肠溃疡ERCP内镜逆行胰胆管造影术FD 功能性消化不良GU 胃溃疡GERD胃食管反流病HE 肝性脑病IBS 肠易激综合症IBD 炎症性肠病SAP 急性重症胰腺炎UC 溃疡性结肠炎WD 肝豆状核变性DM 糖尿病T2DM 2型糖尿病DR 糖尿病视网膜病变DN 糖尿病肾病DKA 糖尿病酮症酸中毒DLE 盘状红斑狼疮GD 甲亢 GravesHNKHC 高渗性非酮症糖尿病昏迷IGT 糖耐量减低IDD 胰岛素依赖性糖尿病IIM 特发性炎症性肌病PKU 苯丙酮尿症MAS或POED 多发性骨纤维构造不良SSc 系统性硬化病SLE 系统性红斑狼疮AD 阿尔海默茨病ARF 急性肾功能不全AIN 急性间质性肾炎AGN 急性肾炎CGN 慢性肾炎CRF 慢性肾功能不全CIN 慢性间质性肾炎HIE 新生儿缺血缺氧性脑病PD 帕金森氏病PEM 蛋白质-热能营养不良PID 盆腔炎SCA 脊髓小脑共济失调MG 重症肌无力RA 类风湿关节炎TIA 短暂性脑缺血发作AAA 腹主动脉瘤AD 主动脉夹层TAO 脉管炎ARDS 成人呼吸窘迫综合症CAP 社区获得性肺炎COPD 慢性阻塞性肺气肿IPF 特发性肺纤维化HAP 医院获得性肺炎PAP 肺泡蛋白质沉积症PIE 间质肺气肿PTE 肺栓塞TB 肺结核PCP 卡式肺囊虫肺炎AVB 房室传导阻滞Af 房颤ASD 房缺AI 主闭AS 主狭ASO 闭塞性动脉硬化AT 房速AMI 急性心梗AP 心绞痛APB 房早BBB 束支传导阻滞CHD冠心病CHF 充血性心衰CF 心衰F3 法三F4 法四IHD 缺血性心脏病PDA 动脉导管未闭PS 肺狭PAT 阵发性房性心动过速MI 心梗MVP 二间瓣脱垂RBBB右束支传导阻滞SSS 病态窦房结综合症SBE 亚急性感染性心内膜炎UA 不稳定性心绞痛VSD 室缺VDH 心脏瓣膜病ALT 成人T细胞白血病AA 再障AIHA 自身免疫性溶血性贫血CLL 慢淋CGN 慢粒DIC 弥漫性血管内凝血ITP过敏性紫殿IDA 缺铁贫HD 霍奇金病PNH 阵发性睡眠性血红蛋白尿MM 多发性骨髓瘤MDS 骨髓增生异常综合症NHL 非霍奇金RAEB 难治性贫血伴原始细胞增多型TIP 血栓性血小板减少性紫殿AIH 自身免疫性肝炎AP 急性胰腺炎DU十二指肠溃疡ERCP内镜逆行胰胆管造影术FD 功能性消化不良GU 胃溃疡GERD胃食管反流病HE 肝性脑病IBS 肠易激综合症IBD 炎症性肠病SAP 急性重症胰腺炎UC 溃疡性结肠炎WD 肝豆状核变性DM 糖尿病T2DM 2型糖尿病DR 糖尿病视网膜病变DN 糖尿病肾病DKA 糖尿病酮症酸中毒DLE 盘状红斑狼疮GD 甲亢 GravesHNKHC 高渗性非酮症糖尿病昏迷IGT 糖耐量减低IDD 胰岛素依赖性糖尿病IIM 特发性炎症性肌病PKU 苯丙酮尿症MAS或POED 多发性骨纤维构造不良SSc 系统性硬化病SLE 系统性红斑狼疮AD 阿尔海默茨病ARF 急性肾功能不全AIN 急性间质性肾炎AGN 急性肾炎CGN 慢性肾炎CRF 慢性肾功能不全CIN 慢性间质性肾炎HIE 新生儿缺血缺氧性脑病PD 帕金森氏病PEM 蛋白质-热能营养不良PID 盆腔炎SCA 脊髓小脑共济失调MG 重症肌无力RA 类风湿关节炎TIA 短暂性脑缺血发作。
阿尔茨海默病和糖尿病的关联:揭示一种新的治疗策略王冰洁;张研【摘要】阿尔茨海默病(AD)是2型糖尿病(T2D)的危险因素,反之亦然.越来越多的证据表明这些疾病在流行病学、临床和分子水平上都有关联.最近的研究揭示了AD 和2型糖尿病具有共同的致病机制.在AD的动物模型中发现神经元胰岛素信号传导受损和内质网(ER)应激,类似于在T2D外周组织中的观察结果.这些研究揭示了一些新的糖尿病相关机制,这些机制能够引起AD脑功能障碍.在这里,我们回顾了这两种疾病之间共同出现的机制的文献,并讨论如何从这些机制中探索AD新的可能治疗靶点.%Alzheimer's disease(AD) is a risk factor for type 2 diabetes(T2D) and vice versa.A growing body of evidence indicates that these diseases are related both at epidemiological, clinical and molecular levels. Recent studies have begun to reveal common pathogenic mechanisms shared by AD and type 2 diabetes. Impaired neuronal insulin signaling and endoplasmic reticulum(ER) stress are found in animal models of AD,similar to observations in peripheral tissue in T2D. These findings shed light into mechanisms leading to brain dysfunction of AD in T2D patients. Here,we review the literatures on selected mechanisms shared between these diseases and discuss how the identification of such mechanisms may lead to novel therapeutic targets in AD.【期刊名称】《基础医学与临床》【年(卷),期】2018(038)003【总页数】5页(P294-298)【关键词】阿尔茨海默病;糖尿病;胰岛素抵抗;炎性反应;神经节苷脂;雷帕霉素靶蛋白(mTOR)【作者】王冰洁;张研【作者单位】北京大学生命科学学院膜生物学国家重点实验室;北京大学麦戈文脑科研究所,北京100871【正文语种】中文【中图分类】Q189代谢紊乱疾病——2型糖尿病(type 2 diabetes, T2D)和肥胖症是全球范围内的重要的健康问题,很大程度上是由于现代社会不健康的生活方式所引起。
ReviewCommon pathological processes in Alzheimer disease and type 2diabetes:A reviewLin Li a ,Christian Hölscher b,⁎a Gerontology Institute,Shanxi Medical University,#86South Xin Jian Road (030001),Taiyuan,Shanxi,China bSchool of Biomedical Sciences,Ulster University,Cromore Road,Coleraine BT521SA,UKA R T I C L E I N F O AB S T R AC TArticle history:Accepted 5September 2007Available online 11September 2007Alzheimer disease (AD)and type 2diabetes mellitus (T2DM)are conditions that affect a large number of people in the industrialized countries.Both conditions are on the increase,and finding novel treatments to cure or prevent them are a major aim in research.Somewhat surprisingly,AD and T2DM share several molecular processes that underlie the respective degenerative developments.This review describes and discusses several of these shared biochemical and physiological pathways.Disturbances in insulin signalling appears to be the main common impairment that affects cell growth and differentiation,cellular repair mechanisms,energy metabolism,and glucose utilization.Insulin not only regulates blood sugar levels but also acts as a growth factor on all cells including neurons in the CNS.Impairment of insulin signalling therefore not only affects blood glucose levels but also causes numerous degenerative processes.Other growth factor signalling systems such as insulin growth factors (IGFs)and transforming growth factors (TGFs)also are affected in both conditions.Also,the misfolding of proteins plays an important role in both diseases,as does the aggregation of amyloid peptides and of hyperphosphorylated proteins.Furthermore,more general physiological processes such as angiopathic and cytotoxic developments,the induction of apoptosis,or of non-apoptotic cell death via production of free radicals greatly influence the progression of AD and T2DM.The increase of detailed knowledge of these common physiological processes open up the opportunities for treatments that can prevent or reduce the onset of AD as well as T2DM.©2007Elsevier B.V.All rights reserved.Keywords:Ageing Alzheimer's DiabetesNeurodegeneration Insulin Growth factorContents1.Introduction ..........................................................3852.Similarities between physiological processes underlying T2DM and AD .........................3853.Epidemiological studies that link T2DM and AD ......................................3864.Similarities between T2DM and AD:1.Amyloid peptides .................................3865.Similarities between T2DM and AD:2.NFTs and hyperphosphorylated tau protein ...................3886.Similarities between T2DM and AD:3.Insulin and AD................ (388)B R A I N R E S E A RC H R E V I E W S 56(2007)384–402⁎Corresponding author.Fax:+442870324375.E-mail address:c.holscher@ (C.Hölscher).0165-0173/$–see front matter ©2007Elsevier B.V.All rights reserved.doi:10.1016/j.brainresrev.2007.09.001ava i l a b l e a t w w w.s c i e n c e d i r e c t.c o mw w w.e l s ev i e r.c o m /l o c a t e /b r a i n r e s r ev7.Similarities between T2DM and AD:4.Glyceraldehyde-derived advanced glycation end products(AGEs)andO-linked N-acetylglucosamine acylation (392)7.1.Glucose toxicity in diabetes and accelerating effects on AD development:the hexosamine biosyntheticpathway(HSP)and O-linked N-acetylglucosamine acylation(O-GlcNAc) (392)8.Similarities between T2DM and AD:5.TGF-β (393)8.1.Basic structure and biological activity of TGFs (393)8.1.1.Roles and functions of TGFs in T2D (394)8.1.2.Functions of TGF in the brain (394)9.Similarities between T2DM and AD:6.Evidence from animal models (394)10.Conclusion (395)References (395)1.IntroductionType2diabetes mellitus(T2DM)is one of the most common metabolic disorders,and its prevalence increases with age. Insulin resistance or T2DM is often associated with the most commonly occurring metabolic and physiologic problems,in-cluding elevated blood pressure,cardiovascular disease,dysli-pidemia(high triglyceride levels and low levels of high-density lipoproteins),and high cholesterol levels.Together with visce-ral obesity,this clustering of risk factors is known as the metabolic syndrome(Ahmed and Goldstein,2006;Levine, 2006).Recent evidence has identified T2DM as a risk factor of Alzheimer's disease(AD).AD,a progressive neurodegenera-tive disorder of hitherto unknown etiology leading progres-sively to severe incapacity and death,has been described as the pandemic of the21st century(Jellinger,2006).Familial AD is caused by mutations in the amyloid precursor protein(APP) and presenilin genes,both linked to Aβsynthesis.The etiology of the sporadic form of Alzheimer's disease(SAD)is complex, with an interaction of both genetic and environmental risk factors(Blennow et al.,2006).A key event in AD pathogenesis is the conversion of Aβfrom its soluble monomeric form into various aggregated forms in the brain.Preventing aggregation of Aβis being actively pursued as one therapeutic strategy for treating AD(Liu et al.,2005).2.Similarities between physiological processes underlying T2DM and ADSeveral studies have shown that there are many similarities between T2DM and AD,and that both conditions underlie common physiological processes.These include aging-related processes,degeneration,high cholesterol levels,metabolic disorders and degenerative processes,β-amyloid aggregation, and also second messenger system abnormalities such as glycogen synthase kinase3(GSK3)overactivity,and deregu-lated protein phosphorylation(Doble and Woodgett,2003; Ristow,2004),association with cardiovascular disease,blood vessel abnormalities(Brands et al.,2004),increased oxidative stress and increased inflammation response(de la Monte and Wands,2006;Haan,2006),correlation with the Apolipoprotein E(APOE)ε4allele(Qiu and Folstein,2006),and glyceraldehyde-derived advanced glycation end products(AGEs).These com-mon properties of both conditions will be described in detail in this review.One important aspect that links AD with T2DM is the fact that insulin receptors are not only expressed in the peripheral system but are also found on neurons in the CNS (Havrankova et al.,1981;Havrankova et al.,1978a,b;Havran-kova et al.,1978a,b).Insulin receptors not only regulate blood sugar levels but also have growth factor properties and re-gulate neuronal differentiation,stem cell and progenitor cell proliferation,dendritic sprouting,and cellular repair mechan-isms(Gispen and Biessels,2000;Hoyer,2004;Northam et al., 2006;Steen et al.,2005).AD is characterized by intracellular neurofibrillary tangles (NFTs),containing an abnormally hyperphosphorylated form of tau protein,and extracellular senile plaques(SPs),mainly composed of aggregatedβ-amyloid.Both of the pathologic hallmarks of AD are also found in T2DM(Churcher,2006;Glabe, 2006).Another property common to both conditions is the loss of cells and associated degenerative changes.AD is the most common neurodegenerative disease with extensive neuronal loss.T2DM is also a degenerative disease that results from the selective destruction of pancreaticβcells and associated neu-ropathies(Brands et al.,2004;Ristow,2004;Roche et al.,2005). Studies have shown that a higher serum insulin level in pre-diabetes and early T2DM has been associated with impaired cognitive function(Stolk et al.,1997).Mechanistically,this im-plies that elevation of Aβlevels is associated with elevated serum insulin content(Watson et al.,2003).The main physio-logical link between AD and T2DM is that both conditions are associated with peripheral and central insulin signalling ab-normalities(Hoyer,2004).These then lead to neurodegenera-tive processes and cognitive impairments(Sun and Alkon, 2006;Watson and Craft,2004).The complex relationship be-tween insulin,cholesterol,and AD has been investigated by many groups(Nelson and Alkon,2005).Insulin regulates cholesterol biosynthesis by stimulating activity of3-hydroxy-3-methylglutaryl-CoA reductase,a rate-limiting enzyme in cholesterol biosynthesis.Cholesterol levels also play a crucial role in AD and high levels are considered a risk factor.Hyper-cholesterolemia is also a known risk factors of T2DM(Kompoti et al.,2006).High levels of cholesterol affectβ-amyloid syn-thesis,APOEε4has been shown to affect cholesterol transport and Aβdeposition,amyloid precursor protein(APP)metabo-lism is affected since cholesterol levels modulate gamma secretase activity andβ-amyloid synthesis(Cole et al.,2005; Simons and Ehehalt,2002).Common biochemical pathways found in the pancreas and in the brain also include shared enzymes that are involved in neurotransmitter synthesis:glu-tamic acid decarboxylase,tyrosine hydroxylase,and dopa385B R A I N R E S E A RC H R E V I E W S56(2007)384–402decarboxylase.Numerous growth factor receptors and hor-mones are also found in bothβcells and in neurons;e.g., thyrotrophin-releasing hormone,P75receptors,neuronal growth factor receptors and glucagon-like peptide-1(Hoyer, 2004;Li,2007;Mohanty et al.,2005;Perry and Greig,2002).All those similarities support the assumption that common signalling mechanisms exist in response to similar physiolog-ical stimuli,and that the same signalling impairments can develop in bothβcells and neurons.One could also conclude from this thatβ-cells resemble neurons in several aspects:they are electrically excitable,express the same types of ion channels,and respond to hormonal stimuli and glucose by depolarization and exocytosis,in a process that resembles neurotransmitter release from synaptic vesicles.The insulin-releasing mechanism is under the control of cAMP and IP3 signalling(Green et al.,2004),as is the neurotransmitter release mechanism in neurons(Hölscher et al.,1999).3.Epidemiological studies that linkT2DM and ADNumerous epidemiological studies have linked T2DM with an increased risk of developing AD(Haan,2006).Recent evidence from a population-based study shows a link between T2DM and AD,with an incidence of AD as much as2to5times higher in population suffered from T2DM.Four risk factors(T2DM, hypertension,heart disease,and smoking)were attributed to a higher risk of AD.The risk of AD increased with accumulation of the associated risk factors(Luchsinger et al.,2005).An investigation in Sweden also showed that T2DM increased the risk of dementia.In this study,a dementia-free cohort of1301 residents75years or older was longitudinally examined to detect dementia cases.During the following6years,350sub-jects developed dementia,including260AD.T2DM was asso-ciated with hazard ratios of1.5for general dementia and1.3 for AD(Xu et al.,2004).In another survey of683subjects aged 65years and older,hyperinsulinemia is associated with a higher risk of AD and a faster decline in memory.In this investigation,149persons developed dementia,including137 cases of AD.The risk of AD doubled in the population that had hyperinsulinemia.The increased risk of developing AD when hyperinsulinemia or diabetes was present was39%(Luch-singer et al.,2004).In a prospective population-based cohort study among6370elderly subjects,an increased risk of AD in T2DM cases was also observed.In all6370study participants, 692(11%)were diagnosed with T2DM.During the follow-up period,126participants became demented and89(70%of dementia cases)were diagnosed with AD.27%patients with dementia were diagnosed with T2DM.Diabetes mellitus al-most doubled the risk of dementia and AD.Interestingly enough,patients treated with insulin were at highest risk of developing dementia(Ott et al.,1999).The authors suggest that diabetes has been contributing to the clinical syndrome in a substantial proportion of all dementia patients.A different study evaluated the risk factor of T2DM alone or combined with the APOEε4gene for developing dementia.A population-based cohort of2574Japanese-American men had been analysed.A regression analysis showed that T2DM was associated with AD development.Individuals with both T2DM and the APOEε4allele had a higher risk for developing AD than those with neither risk factor.Furthermore,the patients with both T2DM and the APOEε4allele had a higher number of plaques and NFTs in the cortex and hippocampus,and they also showed a higher risk of cerebral amyloid angiopathy.The association between T2DM and AD is particularly increased by the APOEε4allele(Peila et al.,2002).More epidemiologic inquiries have shown similar results.In a study of1455cases of T2DM followed for7years,101de-veloped dementia,including77AD cases.Persons with T2DM exhibited significantly increased risk of developing dementia (Leibson et al.,1997).In a study over for5.5years,127out of824 participants that were over55years old were diagnosed with T2DM,and151cases developed AD.After adjusting for age,sex, and educational level,those with T2DM had a65%increase in the risk of developing AD(Arvanitakis et al.,2004).In conclusion,the evidence presented by numerous epide-miological studies show a clear association between T2DM and an increased risk of developing AD.4.Similarities between T2DM and AD:1.Amyloid peptidesThere is considerable evidence to suggest that the accumula-tion of Aβis the cause of the neurodegeneration that occurs in AD(Hölscher,2005;Small and Cappai,2006).Similarly,loss of βcells in pancreas in T2DM is partly attributed to amyloid deposits in the islets(Clark et al.,1988).Aβis a product from the cleavage of its precursor protein,APP.Similarly,islet amy-loid is derived from islet amyloid polypeptide(IAPP)(Cooper et al.,1987).The90%structural similarity between APP and IAPP strengthen the link between AD and T2DM and suggest similar physiological roles(Janson et al.,2004).AD is charac-terized by formation of SPs which consist of Aβ(Glenner and Wong,1984).These plaques have toxic properties and are likely linked to the induction of inflammatory processes that cause neurotoxicity(Yan et al.,2003).IAPP aggregates are commonly observed in pancreatic islets of diabetic patients (Hoppener et al.,2000;Hoppener and Lips,2006;Mosselman et al.,1988).The islet of Langerhans in T2DM is characterized byβ-cell loss,and amyloid deposits contribute to this de-velopment(Westermark and Wilander,1978).It was shown transgenic mouse models that overexpress IAPP and develop aggregates of IAPP in the pancreas also developed diabetes (Janson et al.,1996;Verchere et al.,1996).Conversely,targeted disruption of IAPP expression lead to enhanced insulin sec-retion and improved glucose tolerance(Gebre-Medhin et al., 1998).Considering the pathogenetic similarities and the90% structural similarity between Aβprecursor protein and IAPP,it should not be surprising that AD seems to predispose for insulin resistance,insulin hypersecretion,and T2DM(Haan, 2006).As described above,individuals suffering from T2DM have a much higher likelihood of developing dementia(Bies-sels and Kappelle,2005;Ott et al.,1999).Great progress has been made in identifying IAPP and islet amyloid as potentially important contributors to the patho-genesis of theβ-cell dysfunction of T2DM(Hull et al.,2004). The physiological role of IAPP include the regulation of food intake and body weight.An effect of inhibition of food intake386B R A I N R E S E A R C H R E V I E W S56(2007)384–402was shown after central or peripheral administration of IAPP in rats(Arnelo et al.,2000;Lutz et al.,1998).Conversely,an increase of food intake and body weight was induced by administered IAPP antagonist intracerebroventricularly(i.c.v.) (Rushing et al.,2001).IAPP-receptors are localized in the nu-cleus accumbens,area postrema,nucleus of the solitary tract, and various hypothalamic regions in rodents that mediate food intake and body weight effectively(Sexton et al.,1994). Moreover,effects of IAPP also include the regulation of renal filtration(Harris et al.,1997),calcium homeostasis(Alam et al., 1993),and vasodilatation(Chin et al.,1994).IAPP is involved in the pathogenesis of the islet-cell dysfunction in T2DM.Under physiological conditions,IAPP is synthesized,processed,and secreted from theβ-cell along with insulin and does not accu-mulate as amyloid fibrils.However,misfolding of ICE occurs whenβ-cells are damaged(before the onset of T2DM)(Kahn, 2001).The misfolded and/or unprocessed(pro)IAPP in secre-tory granules is released from theβcell.The structure of this peptide is altered as it is exposed to an altered chemical envi-ronment(e.g.increased pH and decreased calcium concen-tration)as well as to other molecules(such as heparan sulfate and proteoglycans),and further amyloid fibril is formed.A different study showed an aggregation of pro-IAPP and IAPP in β-cell lysosomes in patients with T2DM and human IAPP expressing transgenic mice(de Koning et al.,1994).There is evidence that misfolded(pro)IAPP is more resistant to normal proteolytic processes by lysosomes.These amyloid fibrils faci-litate or seed a second stage of rapid amyloid fibril accumu-lation.T2DM is associated with chronically elevated glucose and free fatty acids,both of which have been demonstrated to enhance amyloid fibril formation.The toxicity of amyloid peptides associated with T2DM have been well documented (Lorenzo et al.,1994;Yankner et al.,1990).IAPP induces apop-tosis and cell death when incubated with isolated islets or islet cells(Lorenzo et al.,1994).In addition to inducing cell death, IAPP may also inhibitβcell replication(Lundmark et al.,2005). Formation and progression of islet amyloid may be concom-itant with the pathogenesis(a worsening ofβcell function and glucose homeostasis and a loss of islet-cell mass)of T2DM.It seems that aging is accompanied with islet amyloid forma-tion.The latter was found in older individuals who do not have diabetes,but show elevated postprandial glucose levels(Chen et al.,1985).Thus,islet amyloid formation may play a role in the impaired glucose metabolism of aging.In addition to IAPP amyloid deposits also contain other components:APOE,hepa-ran sulfate proteoglycans(HSPGs),and serum amyloid P com-ponent(Kahn et al.,1999).These peptides are found or play crucial roles both in T2DM and AD.As mentioned earlier, APOEε4is an important risk factor for AD.HSPGs are asso-ciated with the earliest stages of the formation of Aβin AD (Hamaguchi et al.,2006).Alois Alzheimer was the first to describe the special his-tological hallmarks in AD:“distributed all over the cortex,but especially numerous in the upper layers,there are minute military foci which are caused by the deposition of a special substance in the cortex.”(Alzheimer,1907;Alzheimer et al., 1907).It is now over two decades since component of this military foci was sequenced(Glenner and Wong,1984).and identified as a39-to43-amino-acid peptide,named asβ-amy-loid peptide(Wong et al.,1985).Aβis a cleavage product of the amyloid precursor protein(APP).Three secretases(α,β,andγ) are involved in the cleavage of APP.APP is cleaved by at least two pathways.In theαpathway,APP is cleaved by the enzyme α-secretase to produce the neuroprotective soluble APPαfragment.There is no Aβformation in this pathway,sinceα-secretase cleaves APP within the beta-amyloid sequence.Aβis formed in another pathway,in which APP is sequentially cleaved byβ-and thenγ-secretases.Aβcan evoke a series of pathophysiological processes and finally aggregate to produce senile plaques(SPs)(Hölscher,2005;Turner et al.,2004).The amyloid cascade hypothesis has received a lot of support in the recent years.Aβpeptides impair neuronal activity in different ways,including the impairment of synaptic function and the induction of cell death(Small et al.,2001;Walsh and Selkoe, 2004).Yankner and colleagues showed neuronal degeneration in cultured hippocampal cells after applying Aβ(Yankner et al., 1989).Similar toxic effects of Aβwere observed after i.c.v. injection in vivo(Hölscher,1998).The ability to memorize spatial tasks,synaptic plasticity,and neuronal survival was impaired after injection of Aβinto the hippocampus or cerebral cortex,even in the absence of neurotoxic effects(Gengler et al., 2007;Hölscher et al.,2007;Kowall et al.,1991).Interestingly enough,Aβcan be neurotrophic to undifferentiated hippo-campal neurons at low concentrations and neurotoxic to mature neurons at higher concentrations in cultural neurons (Yankner et al.,1990).Aβtoxicity was increased by oxidative stress(Miranda et al.,2000)and by additional challenge by glutamate(Koh et al.,1998;Koh et al.,1990).Further analysis showed that sections on theβ-amyloid peptide that facilitate the aggregation process are important for the cytotoxic effects. Fragments of the peptide that cannot aggregate were found to be non-toxic(Pike et al.,1995).The analysis of the tertiary configuration ofβ-amyloid further gave additional informa-tion on what crucial properties are linked with neurotoxicity. The monomeric alpha-helical conformation did not induce neuronal toxicity.Aβbecame neurotoxic afterβ-sheet trans-formation and aggregation(Talafous et al.,1994).Aβalso increases neurotoxicity by increasing calcium influx,in part by activating calcium channels(Freir and Herron,2003)and partly by activating neuronal metabotropic receptors,or even by forming ion channels in cell membranes(Hölscher,1998,2005). Recently,the focus has shifted from analysing the effects of amyloid plaques to investigating what effects small aggregates that are still soluble can have.It was found that such pre-aggregated oligomers(also called ADDLs)already have pro-nounced effects on neuronal transmission,memory forma-tion,and cell survival(Gong et al.,2003;Walsh and Selkoe, 2004).These effects can be reversible(Gong et al.,2003; Hölscher et al.,2007)and might be the first stage in cascade that eventually leads to irreversible damage and cell loss.The strongest support for the Aβcascade hypothesis originates from investigations of familial AD.Three different genes related to familial AD have been linked to abnormal Aβproduction or aggregation(Selkoe,2001).Specific point muta-tions in the APP gene shift the processing of APP towards the Aβpathway,e.g.by reducing the affinity to alpha-secretase,or increasing turnover byβ-secretase(Almkvist et al.,1997).The fact that such simple point mutations greatly accelerate the development of AD support the concept that Aβis the main cause of AD.Aβaccumulation on a large scale is seen only in387B R A I N R E S E A RC H R E V I E W S56(2007)384–402AD brains,and much less so in age matched control brains, which suggest that some abnormal factors facilitate the aggregation of Aβin AD.There is further evidence for a relationship betweenβ-amyloid,AD,and T2DM.To determine whether insulin affected plasma Aβlevels,insulin and Aβlevels of AD patients had been compared with those of normal older adults after infusions of insulin.Results showed that AD patients had higher plasma insulin and Aβlevels vs.normal adults.Insulin infusion reduced the plasma Aβlevels in normal adults.In contrast,insulin raised plasma Aβlevels in AD patients (Kulstad et al.,2006).The results suggest that AD patients show reduced insulin clearance and insulin-provoked plasma Aβelevation.Abnormal increase of peripheral Aβby insulin may well contribute to AD.Considering the importance of Aβin the pathogenesis of AD,a lot of research is focused on developing therapies for AD by targeting amyloid production,aggregation,clearance or toxicity(Golde,2006;Kennedy et al.,2007).5.Similarities between T2DM and AD:2.NFTs and hyperphosphorylated tau proteinAnother pathologic hallmark of AD is the accumulation of intracellular neurofibrillary tangles(NFTs),containing a hy-perphosphorylated form of tau protein.The activation of insulin receptors triggers tau phosphorylation:increasing the peripheral insulin level by insulin injection significantly increased tau phosphorylation at Ser202in the brain within 10min(Freude et al.,2005).Further,this study reported that insulin receptor signalling and tau phosphorylation was com-pletely abolished under hyperinsulinemic conditions in the brains of mice lacking the insulin receptor,indicating that the cerebral insulin receptors are a direct target of peripherally administered insulin.Tau phosphorylation in AD and T2DM involves glycogen synthase kinase-3(GSK-3)activation,a se-rine/threonine kinase that phosphorylates glycogen synthase in the rate-limiting step of glycogen biosynthesis(Doble and Woodgett,2007;Phiel et al.,2003;Sivaprakasam et al.,2006). GSK-3,in particular the GSK-3βisoform,therefore is a crucial step in the formation of NFTs.Consequently,developing inhi-bitors of GSK-3is an attractive research target in order to evolve new treatments for diseases such as T2DM and AD (Bhat et al.,2004;Cole et al.,2007)(Fig.1).A cell signaling pathway that has received increasing attention in the research of the dual pathogenicities underly-ing AD and T2DM is the Cdk5/P35/P25signaling axis.The analysis of genome data sets for genes associated with T2DM has recently reported findings that associate this pathway with both diseases.Studies undertaken in the USA,Finland, Iceland,and the UK uncovered a strong association of Cdkal1 with T2DM(Helgason et al.,2007;Saxena et al.,2007;Stein-thorsdottir et al.,2007;Zeggini et al.,2007).Cdkal1is a ho-molog of Cdk5rap1,the Cdk5regulatory subunit associated protein-1,an inhibitor of Cdk5activities.P35is an activating peptide of Cdk5,and is also involved in the impairment of insulin secretion by pancreatic beta cells in response to gluco-toxicity.Studies further showed that inhibition of Cdk5acti-vity protects pancreatic beta cells from glucotoxicity(Ubeda et al.,2006).In addition,Cdk5activity plays a role in AD de-velopment.Importantly,Cdk5is involved in the hyper-phos-phorylation of tau(Orellana et al.,2007;Wang et al.,2007).This might be due to abnormalities in the ratios of P25/P35found in AD patients that control the activation of Cdk5,and cause hyper-phosphorylation of tau(Ubeda et al.,2004;Ubeda et al., 2006).It appears that the hyper-phosphorylation of tau is not directly caused by Cdk5activity,but by the modulation of GSK-3βactivity by Cdk5(Plattner et al.,2006).Glucose-induced expression of the Cdk5activator p35involved in AD regulates insulin gene transcription,and therefore is involved in control of insulin signalling.The Cdk5activator p35can act as an activator of GSK-3since it maintains the active form of GSK-3 by promoting its N-terminal cleavage(Goni-Oliver et al.,2007). Therefore,novel drugs that reduce Cdk5activity are tested as potential treatments for AD and Parkinson's disease(Camins et al.,2006).6.Similarities between T2DM and AD:3.Insulin and ADThere is growing evidence that impairments in insulin sig-nalling is partly responsible for the cognitive decline in AD(de la Monte and Wands,2005;Gasparini and Xu,2003;Watson and Craft,2004).One impairment that has been described repeat-edly is the observation that in AD,insulin resistance in the CNS develops due to alterations of insulin receptor sensitivity.This affects the expression and metabolism of Aβand tau protein. Insulin and the insulin receptor(IR)are abundantly expressed in the rodent brain.They are highly expressed in the olfactory bulb,cerebral cortex,hippocampus,hypothalamus,amygdala, and septum(Watson and Craft,2004;Zhao et al.,2004).Insulin acts also as a‘neuromodulator’in the regulation of food intake and body weight.It influences the release and reuptake of neurotransmitters,and also appears to improve learning and memory(Biessels et al.,2004;Gispen and Biessels,2000).The insulin/IR distributed in the hypothalamus is involved in the regulation of the body energy homeostasis.The hippocampus-and cerebral cortex-distributed insulin/IR has also been shown to be involved in brain cognitive functions.Insulin can modu-late activities of excitatory and inhibitory receptors,and trigger signal transduction cascades leading to long-term memory consolidation.It has also been demonstrated that insulin sig-nalling plays a role in synaptic plasticity(Gispen and Biessels, 2000).Conversely,deterioration of insulin receptor signalling is involved in aging-related brain degeneration such as the AD and cognitive impairment in T2DM patients(Zhao et al.,2004). Furthermore,in the brains of AD and PD patients,a decrease of expression of IR has been described(Moroo et al.,1994;Steen et al.,2005).When comparing the expression of insulin and IR of AD patients in neocortical brain areas with aged matched controls,it was found that insulin and insulin receptor densi-ties decrease with aging(Frolich et al.,1998a,b).Brain IR den-sities in AD are also decreased compared to aged matched controls(Hoyer,1998).In addition to this,it has been dis-covered that IR receptors are not only desensitized in T2DM but also in the brains of patients with AD.This impairment in insulin signalling in the brain is considered unique in devel-opment and distribution and has been termed‘Type3388B R A I N R E S E A R C H R E V I E W S56(2007)384–402。