Nitrogen dynamics at undisturbed and burned
- 格式:pdf
- 大小:363.49 KB
- 文档页数:11


第32卷第16期2012年8月生态学报ACTA ECOLOGICA SINICAVol.32,No.16Aug.,2012基金项目:国家自然科学基金项目(31271661);国家重点基础研究发展计划(973)课题(2009CB118602);公益性行业(农业)科研专项(201203100)收稿日期:2012-02-27;修订日期:2012-06-03*通讯作者Corresponding author.E-mail :zlwang@sdau.edu.cn ;jianggm@126.comDOI :10.5846/stxb201202270263吴光磊,郭立月,崔正勇,李勇,尹燕枰,王振林,蒋高明.氮肥运筹对晚播冬小麦氮素和干物质积累与转运的影响.生态学报,2012,32(16):5128-5137.Wu G L ,Guo L Y ,Cui Z Y ,Li Y ,Yin Y P ,Wang Z L ,Jiang G M.Differential effects of nitrogen managements on nitrogen ,dry matter accumulation and transportation in late-sowing winter wheat.Acta Ecologica Sinica ,2012,32(16):5128-5137.氮肥运筹对晚播冬小麦氮素和干物质积累与转运的影响吴光磊1,2,郭立月1,崔正勇1,李勇2,尹燕枰1,王振林1,*,蒋高明1,2(1.山东农业大学作物生物学国家重点试验室,泰安271018;2.中国科学院植物研究所植被与环境变化国家重点实验室,北京100093)摘要:氮素平衡对干物质积累与分配的影响是农业生态系统研究的重要内容,在保障产量前提下减少氮肥施用量可减少环境污染与温室气体排放。
以晚播冬小麦为研究对象,设置4个施氮量水平:0kg /hm 2(N0)、168.75kg /hm 2(N1)、225kg /hm 2(N2)、281.25kg /hm 2(N3),每个施氮量水平下设置2个追氮时期处理:拔节期(S1)、拔节期+开花期(S2),研究了氮肥运筹对晚播冬小麦氮素和干物质积累与转运及氮肥利用率的影响。

全国2018年4月高等教育自学考试英语科技文选试题课程代码:00836parta :vocabularyⅠ. Directions: Add the affix to each word according to the given Chinese, making changes when necessary.(10%)1. relevant 不相干的 1._____________2. hedron 多面体 2._____________3. recur 重新产生 3._____________4.topic 副主题 4._____________5.scan 扫描仪 5._____________6.plant 移植 6._____________7.ceptibility 敏感性7._____________press 压缩的8._____________9.smooth 平滑9._____________10.sell 吹嘘10._____________Ⅱ.Directions: Fill in the blanks, each using one of the given words or phrases below in its proper form.(10%)plug in run out of to the tune of look into a wide range of as to transform into adept at bring into play project oneself into11. She’s very______ making people feel at their ease.12. Even ______ all the resources and staff available would not be likely to help resolve the immediate shortfall in production.13. Before switching on the radio, make sure that the mains lead ______.14. The problem will ______.15. It is the failure of the architect to ______ the mind and spirit of the people who are to experience his designs that causes much of the staccato feeling to be noted in work today.16. Reactors can be used to ______ fertile material ______ fissionable material.17. In order to expand, they will need capital ______ six million dollars.18. When light passes through a prism, it spreads out into ______ colors.19. The aircraft will ______ fuel in another hour.20. ______ your second question, I am afraid I can give you no information at the moment.1Ⅲ. Directions: Fill in each blank with a suitable word given below.(10%)countless or creatures shelter produce through influenceits for relationshipsNo living creature, plant 21 animal, can exist in complete isolation. An animal is bound to depend on other living 22 , ultimately plants, for 23 food supply; it must also depend upon the activities of plants 24 a continued oxygen supply for its respiration. Apart from these two basic 25 it may be affected directly or indirectly in 26 different ways by other plants and animals around it. Other animals prey on it or compete with it for the same food; plants may provide 27 , concealment or nesting material, and so on. Similarly, the animal will 28 its own effects on the surrounding plants and animals and 29 its contribution of manure it may 30 the texture and fertility of the soil.21.______ 22.______ 23.______ 24.______ 25.______26.______ 27.______ 28.______ 29.______ 30.______PART B: TRANSLATIONⅣ. Directions: Translate the following sentences into English, each using one of the given words or phrases below.(10%)manipulate customary arbitrary save from pop into31.在上一世纪,奎宁(quinine)使千千万万人免于得疟疾(malaria)。
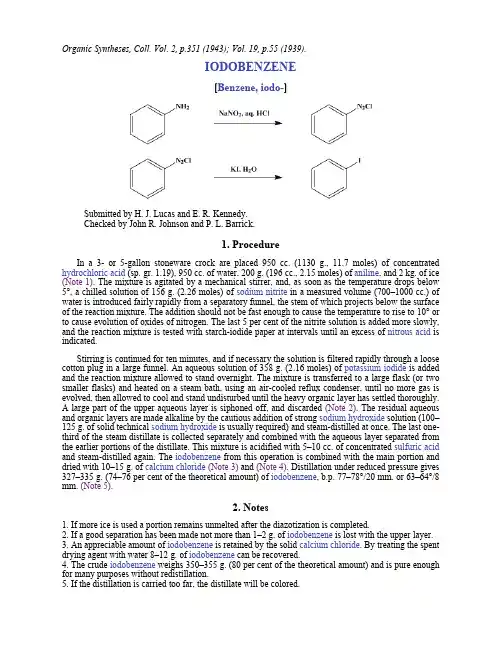
Organic Syntheses, Coll. Vol. 2, p.351 (1943); Vol. 19, p.55 (1939).IODOBENZENE[Benzene, iodo-]Submitted by H. J. Lucas and E. R. Kennedy.Checked by John R. Johnson and P. L. Barrick.1. ProcedureIn a 3- or 5-gallon stoneware crock are placed 950 cc. (1130 g., 11.7 moles) of concentrated hydrochloric acid (sp. gr. 1.19), 950 cc. of water, 200 g. (196 cc., 2.15 moles) of aniline, and 2 kg. of ice (Note 1). The mixture is agitated by a mechanical stirrer, and, as soon as the temperature drops below 5°, a chilled solution of 156 g. (2.26 moles) of sodium nitrite in a measured volume (700–1000 cc.) of water is introduced fairly rapidly from a separatory funnel, the stem of which projects below the surface of the reaction mixture. The addition should not be fast enough to cause the temperature to rise to 10° or to cause evolution of oxides of nitrogen. The last 5 per cent of the nitrite solution is added more slowly, and the reaction mixture is tested with starch-iodide paper at intervals until an excess of nitrous acid is indicated.Stirring is continued for ten minutes, and if necessary the solution is filtered rapidly through a loose cotton plug in a large funnel. An aqueous solution of 358 g. (2.16 moles) of potassium iodide is added and the reaction mixture allowed to stand overnight. The mixture is transferred to a large flask (or two smaller flasks) and heated on a steam bath, using an air-cooled reflux condenser, until no more gas is evolved, then allowed to cool and stand undisturbed until the heavy organic layer has settled thoroughly.A large part of the upper aqueous layer is siphoned off, and discarded (Note 2). The residual aqueous and organic layers are made alkaline by the cautious addition of strong sodium hydroxide solution (100–125 g. of solid technical sodium hydroxide is usually required) and steam-distilled at once. The last one-third of the steam distillate is collected separately and combined with the aqueous layer separated from the earlier portions of the distillate. This mixture is acidified with 5–10 cc. of concentrated sulfuric acid and steam-distilled again. The iodobenzene from this operation is combined with the main portion and dried with 10–15 g. of calcium chloride(Note 3) and (Note 4). Distillation under reduced pressure gives 327–335 g. (74–76 per cent of the theoretical amount) of iodobenzene, b.p. 77–78°/20 mm. or 63–64°/8 mm. (Note 5).2. Notes1. If more ice is used a portion remains unmelted after the diazotization is completed.2. If a good separation has been made not more than 1–2 g. of iodobenzene is lost with the upper layer.3. An appreciable amount of iodobenzene is retained by the solid calcium chloride. By treating the spent drying agent with water 8–12 g. of iodobenzene can be recovered.4. The crude iodobenzene weighs 350–355 g. (80 per cent of the theoretical amount) and is pure enough for many purposes without redistillation.5. If the distillation is carried too far, the distillate will be colored.3. DiscussionThe preparation of iodobenzene by iodination of benzene, with iodine and nitric acid, and a survey of preparative methods have been given in an earlier volume.1 The present procedure, based upon the method of Gattermann,2 gives a purer product.This preparation is referenced from:z Org. Syn. Coll. Vol. 5, 660z Org. Syn. Coll. Vol. 5, 665References and Notes. Syn. Coll. Vol. I, 1941, 323.2.Gattermann-Wieland, "Laboratory Methods of Organic Chemistry," p. 283. Translated from thetwenty-fourth German edition by W. McCartney, The Macmillan Company, New York, 1937.AppendixChemical Abstracts Nomenclature (Collective Index Number);(Registry Number)oxides of nitrogencalcium chloride (10043-52-4)sulfuric acid (7664-93-9)hydrochloric acid (7647-01-0)Benzene (71-43-2)aniline (62-53-3)sodium hydroxide (1310-73-2)nitric acid (7697-37-2)potassium iodide (7681-11-0)sodium nitrite (7632-00-0)nitrous acid (7782-77-6)iodine (7553-56-2)Iodobenzene,Benzene, iodo-(591-50-4)Copyright © 1921-2005, Organic Syntheses, Inc. All Rights Reserved。




氮气物理吸附英文Nitrogen Gas Physical AdsorptionNitrogen gas, with its chemical formula N2, is a colorless, odorless, and inert gas that makes up approximately 78% of the Earth's atmosphere. This ubiquitous gas has a wide range of applications, from industrial processes to medical and scientific research. One of the fundamental properties of nitrogen gas is its ability to undergo physical adsorption, a process that has significant implications in various fields.Physical adsorption, also known as physisorption, is a phenomenon where molecules or atoms of a substance (the adsorbate) accumulate on the surface of another substance (the adsorbent) without forming chemical bonds. This process is driven by the attractive forces between the adsorbate and the adsorbent, such as van der Waals forces and electrostatic interactions. In the case of nitrogen gas, the physical adsorption of N2 molecules onto various adsorbents has been extensively studied and has found numerous applications.One of the primary applications of nitrogen gas physical adsorption is in the field of gas separation and purification. Nitrogen gas can beselectively adsorbed onto specific adsorbents, such as activated carbon, zeolites, or metal-organic frameworks (MOFs), while other gases, such as oxygen or carbon dioxide, are not adsorbed as strongly. This selective adsorption allows for the efficient separation and purification of nitrogen gas from air or other gas mixtures. This process is particularly useful in industrial settings, where high-purity nitrogen gas is required for various applications, such as in the electronics industry, food packaging, or the production of chemicals.Another important application of nitrogen gas physical adsorption is in the area of gas storage and transportation. Nitrogen gas can be adsorbed onto porous adsorbents, such as activated carbon or metal-organic frameworks, to create high-density storage systems. These adsorbent-based storage systems can store a significantly larger amount of nitrogen gas compared to traditional compressed gas cylinders, making them more efficient and cost-effective for transportation and storage. This technology is particularly relevant in applications where large volumes of nitrogen gas are required, such as in the industrial or medical sectors.The physical adsorption of nitrogen gas is also crucial in the field of catalysis. Many catalytic processes involve the interaction of reactants with the surface of a catalyst, and the adsorption of nitrogen gas can provide valuable information about the catalyst's surface properties and accessibility. By studying the physicaladsorption of nitrogen gas on catalyst surfaces, researchers can gain insights into the catalyst's pore structure, surface area, and other characteristics that are essential for optimizing catalytic performance.In the field of material science, the physical adsorption of nitrogen gas is used to characterize the porous structure and surface properties of various materials, such as zeolites, activated carbon, and metal-organic frameworks. The analysis of nitrogen adsorption-desorption isotherms, which describe the relationship between the amount of nitrogen adsorbed and the pressure at a constant temperature, can provide information about the material's surface area, pore size distribution, and other structural features. This information is crucial for the development and optimization of materials with specific applications, such as in catalysis, adsorption, or energy storage.Furthermore, the physical adsorption of nitrogen gas is widely used in the field of environmental science and engineering. Nitrogen-based compounds, such as nitrates or nitrites, can be adsorbed onto various adsorbents, including activated carbon or clay minerals, for the removal of these pollutants from water or soil. This process is particularly important in the treatment of wastewater or the remediation of contaminated sites, where the removal of nitrogen-containing compounds is crucial for environmental protection.In conclusion, the physical adsorption of nitrogen gas is a fundamental phenomenon with a wide range of applications across various scientific and technological fields. From gas separation and purification to gas storage, catalysis, material characterization, and environmental remediation, the understanding and manipulation of nitrogen gas physical adsorption have been instrumental in advancing scientific knowledge and driving technological innovation. As research in this field continues to evolve, new and exciting applications of nitrogen gas physical adsorption are likely to emerge, further expanding its impact on our modern world.。


定额市鞍钢阳光实验学校2014高考英语阅读理解基础极品训练题(14)及答案阅读理解----------BAir pollution is damaging 60% of Europe’s prime wildlife sites in meadows, forests and bushes, according to a new report.A team of EU scientists said nitrogen emissions(氮排放) from cars, factories and farming were threatening biodiversity. It’s the second report this week warning of the on-going risks and threats linked to nitrogen pollution.Nitrogen in the atmosphere is harmless in its inert(惰性的) state, but the report says reactive forms of nitrogen, largely produced by human activity, can be a menace to the natural world.Emissions mostly come from vehicle exhausts(排气), factories, artificial fertilizers(肥料) and animal waste from intensive farming. The reactive nitrogen they emit to the air disrupts the environment in two ways: It can make acidic soils too acidic to support their previous mix of species. But primarily, because nitrogen is a fertilizer, it favors wild plants that can maximize the use of nitrogen to help them grow.In effect, some of the nitrogen spread to fertilize crops is carried in the atmosphere to fertilize weeds, possibly a great distance from where the chemicals were first applied.The effects of fertilization and acidification favor common aggressive species like grasses, brambles and nettles. They harm more delicate species like mosses(苔藓), and insect-eating sundew plants.The report said 60% of wildlife sites were now receiving a critical load of reactive nitrogen. The report’s lead author, Dr Kevin Hi cks from the University of York’s Stockholm Environment Institute (SEI), told BBC News that England’s Peak District had a de finitely low range of species as a result of the reactive nitrogen that fell on the area.“Nitrogen creates a rather big problem tha t seems to me to have been given too little attention,” he said. “Governments are responsible for protecting areas like this, but they are clearly failing.”He said more research was needed to understand the knock-on effects for creatures from the changes in vegetation accidentally caused by emissions from cars, industry and farms.At the conference, the representative s agreed “The Edinburgh Declaration on Reactive Nitrogen”. The document highlights the importance of reducing reactive nitrogen emissions to the environment, adding that the benefits of reducing nitrogen outweigh the costs of taking action.5. The underlined word “menace” is used to express that the reactivenitrogen, largely produced by human activity can be ___________.A. frighteningB. threateningC. uniqueD.unusual6. We can infer from the passage that _________.A.it’s harmless to have reactive nitrogen existing in the atmosphereB.reactive nitrogen emissions help aggressive species less thancropsC.the harm to those delicate species has a negative impact onbiodiversityD.reactive nitrogen can fertilize soils and keep their biodiversity7. The team of EU scientists released the second report of nitrogenemissions this week when __________.A.no action was taken to stop nitrogen emissionernments were willing to protect areas harmed by nitrogenC.“The Edinburgh Declaration on Reactive Nitrogen” was agreedD.nitrogen emissions were threatening wildlife sites’biodiversity8. Which of the following would be the best title for the passage?A. Keeping Away From Nitrogen EmissionsB. Stopping NitrogenEmissionsC. Air Pollution Damaging Europe’s WildlifeD. SavingEurope’s Wildlife【参考答案】5、B 6—8、CDC阅读理解-----------D“Mom, I have cancer.”These four words catapulted my son and me on a journey that lasted two years. On that dat I felt a wave of paralyzing fear.Scott was the oldest of my four children. He was 33 years old and a successful assistant principal at SamRayburn Hifht School in Pasadena, Texas. He and his wife Carolyn were busy raising four active children. Scott was 6’2’’, weighed 200 pounds and had ne ver been sick a day in his life.A few month earlier a mole(痣)on his neck had changed color. “Dr.Warner called,” Scott said that spring morning. “It’s melanoma.(黑素瘤)” I tried to comfort him, naming all the people I knew who had survived skin cancer. Yet, I felt small tentacles of fear begin to wrap around my chest.Our next stop was MDAnderson, the famous cancer hospital in Houston. Scott had surgery at the end of May and was scheduled for radiation treatments over the summmer recess. “There is an 80 percen t chance it won’t reoccur,” the doctors said. At the end of summer, all his tests came back negative and Scott was back at school in the fall. However, in December, Scott discovered a lump on his neck. It was examined and the result came back “malignant.(恶性的)” We now relized that Scott fell into the 20 percent category. I could feel the tentacles tightening around my chest. He entered the hospital for an aggressive treatment,a combination of interferon and interleukin.After five months of treatment, he had radical surgery on his neck. The test results were encourging, only three of the 33 lymph nodes(淋巴结) removed were malignant. We were very hopefull.For the next six months, Scott’s follow-up visits went well. Then in October, X-ray revealed a spot on his lung. The spot was removed during surgery and the doctors tried to be optimistic. It was a daily battle to control the fear and panic each setback brought.In January, he was diagnosed as having had a “disease explosion.” The cancer had spread to his lungs, spine and liver and he was given three to six months to live. There were times during this period when I felt like I was having a heart attack. The bands constricting my chest made breathing difficult.When you watch your child battle cancer, you experience a roller coaster of emotions. There are moments of hope and optimism but a bad test result or even an unusual pain can bring on dread and panic.Scott was readmitted to the hospital for one last try with chemotherapy. He died, quite suddenly, just six weeks after his last diagnosis. I was completely destroyed. I had counted on those last few months.The next morning I was busy notifying people and making funeral arrangements. I remember having this nagging feeling that something was physically wrong with me. It took a moment to realize that the crushing sensation in my chest was gone. The thing every parent fears the most had happened. My son was gone. Of course, the fear had been replaced by unbearable sorrow.After you lose a child, it is so difficult to go on. The most minimal tasks, combing your hair or taking a shower, becoming monumental. For months I just sat and stared into space. That spring, the trees began to bloom; flowers began to pop up in my garden. Friendswood was coming back to life but I was dead inside.During those last weeks, Scott and I often spoke about life and death. Fragments of those conversations kept playing over and over in my mind.“Don’t let this ruin your life, Mom.”“Make sure Dad re models his workshop.”“Please, take care of my family.”I remember wishing I could have just one more conversation with him.I knew what I would say, but what would Scott say? “I know how much you love me, Mom. So just sit on the couch and cry.” No, I knew him better than that. Scott loved life and knew how precious it is. I could almost hear his voice saying, “Get up Mom, Get on with your life. It’s too valuable to waste.”That was the day I began to move forward. I signed up for a cake decorating class. Soon I was making cakes for holidays and birthdays.My daughter-in-law told me about a writing class in Houston. I hadn’t written in years, but since I was retired I decided it be time to start again. The local college advertised a Life Story Writing class that I joined. There I met women who had also lost their children. The Poet Laureate of Texas was scheduled to speak at our local Barnes and Noble.I attended and joined our local poetry society. I never dreamed that writing essays and poems about Scott could be so therapeutic. Several of those poems have ever been published. In addition, each group brought more and more people into my life..I don’t believe you ever recover from the loss of a child. Scott is in my heart and mind every day. However, I do believe you can survive.Scott fought so bravery to live and he never gave up. He taught me that life is a gift that should be cherished, not wasted. It has taken years to become the person I am today. The journey has been a difficult , painful process but certainly worth the effort and I know that my son would be proud.55.What might be the best title of the passage ?A.Life is valuable B.Grieving and Recovery[来源:]C.Love and sorrow D.Alive or dead56.How old was Scott probably when he died?A.33 B.35 C.37 D.4057.What does the underlined sentence “ The bands constricting my chest made breathing difficult” probably imply?A.It implies that Scott’s mother was likely to have a heart attack. B.It implies that there was something wrong with Scott’s mother’s chest.C.It implies that Scott’s mother was very upset and panic because of Scott’s severe illness.D.It implies that the cancer had spread to her chest just like her son.58.Which of the followi ng statements best shows the author’s feeling about Scott’s dath?A.It was a daily battle to control the fear and panic each setback brought.B.She felt a wave of fear.C.She felt a feeling of fear begin to wrap around her chest.D.The fear had been replaced by unbearable sorrow.59.From Scott and his mother’s conversation, we can know that Scott is ________.A.considerable B.humorous C.determined D.sensitive60.The author intends to tell us that___________.A.it takes a long time to make a person recover from the shock of losing a childB.Scott is proud of his motherC.life is full of happiness and sorrow.D.We’d better make our life count instead of counting your days.【答案】阅读理解(共20小题;每小题2分,满分40分)阅读下列短文,从每题所给的A、B、C、D四个选项中,选出最佳选项。
高中英语真题:2015高考英语短文改错及阅读理解训练(6)文中共有10处语言错误,每句中最多有两处。
每处错误仅涉及一个单词的增加、删除或修改。
注意:1.每处错误及其修改均仅限一词;2.只允许修改10处,多者(从第11处起)不计分。
My friend Dick was seven years old, and her sister Katherine w as five. One day their mother take them to their aunt’s to play w hile she went to the city buy some new clothes. They played for a hour, and then their aunt took Dick into the kitchen. She gave him a nicely cake and a knife and said, “Dick, cut this cake in h alf, and give one of the piece to your sister. But remembering to do it like a gentleman.”“How do gentlemen do it?” Dick asked.“They always give the bigger one piece to the other person,” a nswered his aunt. Dick thought up this for a few seconds. Then he took the cake to his sister but said to her, “Cut this cake in h alf, Katherine.”【参考答案】4.My friend Dick was seven years old, and her (his) sister Katheri ne was five. One day their mother take (took) them to their aunt’s to play while she went to the city ∧ (加to ) buy some new clothes. They played for a (an) hour, and the n their aunt took Dick into the kitchen. She gave him a nicely (ni ce) cake and a knife and said, “Dick, cut this cake in half, and gi ve one of the piece (pieces) to your sister. But remembering (re member) to do it like a gentleman.”“How do gentlemen do it?”Dick asked. “They always give the bigger one piece to the other person,” answered his aunt. Dick thought up (about) this for a f ew seconds. Then he took the cake to his sister but (and) said t o her, “Cut this cake in half, Katherine.”5.短文改错下面短文中有10处语言错误。
余铭,梁钻好,陈海强,等. 低频电场冰温保鲜对虾的水分迁移规律及品质变化的影响[J]. 食品工业科技,2022,43(19):372−378.doi: 10.13386/j.issn1002-0306.2021100109YU Ming, LIANG Zuanhao, CHEN Haiqiang, et al. Water Migration and Quality Change of Prawn Preserved under Controlled Freezing-point Storage Combined with Low Frequency Electric Field Technology[J]. Science and Technology of Food Industry, 2022,43(19): 372−378. (in Chinese with English abstract). doi: 10.13386/j.issn1002-0306.2021100109· 贮运保鲜 ·低频电场冰温保鲜对虾的水分迁移规律及品质变化的影响余 铭1,2,梁钻好1,2,陈海强1,2,梁凤雪1,2,敖菲菲1,2,邓锦杰1,2(1.阳江职业技术学院食品与环境工程系,广东阳江 529566;2.广东省食品低温加工工程技术研究中心,广东阳江 529566)摘 要:为探明低频电场延长冰温保鲜对虾货架期的可行性。
在冰温基础上施加低频电场(LFEF+冰温)保鲜对虾,监测贮藏期间对虾的菌落总数和总挥发性盐基氮(TVB-N )含量变化,分析微观结构变化,通过低场核磁共振(LF-NMR )技术探究虾肉和虾头的水分迁移和变化规律,并与常规冰温保鲜作对照。
结果表明:贮藏第9 d ,LFEF+冰温组的菌落总数和TVB-N 含量开始表现出显著低于对照组的趋势(P <0.05);第12 d 后,LFEF+冰温组的菌落总数比对照组低一个数量级;其TVB-N 含量在第11 d 比对照组显著低36%(P <0.05),货架期可达13 d 以上。
工商管理专业学位外语考试模拟试题2010.10一、语音题(每空1分,共10分)01、notice A. stomachs B. houses C. mouths D. reasonable['nəutis] ['stʌməks] ['hauziz] [mauθ] ['ri:zənəbl]02、winkle A. windy B. drink C. footprint D. interesting['wiŋkl] ['windi] [driŋk] ['futprint] [ˈɪntrɪstɪŋ] 03、shook A. shoot B. food C. shoe D. wood[ʃuk] [ʃu:t] [fu:d] [ʃu:] [wud]04、occasionally A. population B. Russian C. questionD. television[ə'keiʒənəli] [pɔpju'leiʃən] ['rʌʃən] ['kwestʃən] ['teli,viʒən]05、pressure A. directly B. oxygen C. absence D. camera['preʃə] ['direkli] 'ɔksidʒən] ['æbsəns] ['kæmərə]06、float A. flower B. brown C. hometown D. bellows[fləut] ['flauə] [braun] ['həʊmtaʊn] ['beləuz] 07、bulletin A. bury B. Prussian C. bullet D. punishment['bulitin]['beri]['prʌʃən] ['bulit] ['pʌniʃmənt]08、breach A. break B. theatre C. meadow D. least[bri:tʃ] [breik] ['θiətə] ['medəu] [li:st] 09、opposite A. companion B. balloon C. stroll D. historic['ɔpəzit] [kəm'pænjən] [bə'lu:n] [strəul] [his'tɔrik]10、scatter A. laboratory B. separate C. gravity D. various['skætə] [lə'bɔrətəri] ['sepərit] ['ɡræviti] ['vεəriəs]11、essay A. holiday B. says C. away D. mayor[e'sei] ['hɔlədi] [sez] [ə'wei] ['mεə]12、singer A. anger B. tongue C. eager D. single['siŋə] ['æŋɡə] [tʌŋ]['i:ɡə] ['siŋɡl]13、splendid A. watched B. refused C. wretched D. impressed['splendid] [wɔtʃd] [ri'fju:z ] ['retʃid]['impres ]14、owner A. powerful B. brown C. narrow D. power['əunə] ['pauəful] [braun] ['nærəu] [pauə] 15、latent A. squirrel B. centigrade C. level D. mend['leitənt] ['skwə:rəl] ['sentiɡreid] ['levəl] [mend] 16、flood A. loose B. blood C. moon D. noon[flʌd] [lu:s] [blʌd] [mu:n] [nu:n]17、mud A. music B. human C. huge D. lung[mʌd] ['mju:zik] ['hju:mən] [hju:dʒ] [lʌŋ]18、creature A. effect B. energy C. reduce D. belief['kri:tʃə] [i'fekt] ['enədʒi] [ri'dju:s] [bi'li:f] 19、mountain A. explain B. remain C. campaign D. captain['mauntin] [ik'splein] [ri'mein] [kæm'pein]['kæptin]20、cookie A. frog B. oxygen C. wolf D. obvious['kuki] [frɔɡ] ['ɔksidʒən] [wulf] ['ɔbviəs]21、period A. request B. perseverance C. RecognizeD. require['piəriəd] [ri'kwest] [pə:si:'viərəns] ['rekəɡnaiz] [ri'kwaiə]22、geography A. regret B. envy C. remark D. deck[dʒi'ɔɡrəfi] [ri'ɡ ret] ['envi] [ri'mɑ:k] [dek] 23、replied A. entered B. asked C. stepped D. added[ri'plaid] ['entəd] [æskt] [stept]['ædid]24、counter A. country B. south C. tough D. enough['kauntə] ['kʌntri] [sauð] [tʌf] [i'nʌf] 25、eyebrow A. town B. follow C. slow D. fellow['aibrau] [taun] ['fɔləu] [sləu] ['feləu] 26、schoolyard A. coo B. cook C. poor D. childhood['sku:ljɑ:d] [ku:] [kuk] [pɔ:]['tʃaildhud]27、master A. Alsace B. tiresome C. impress D. unable['mæstə] ['a:əθas] ['taiəsəm] ['impres][ʌn'eibl]28、twinkle A. windy B. drink C. footprint D. interesting['twiŋkl] ['windi] [driŋk] ['futprint][ˈɪntrɪstɪŋ]二、单选题(每空1.5分,共30分)01、Since your supervisor has __ specified ___ the time for a talk, you must make sure that you will be there on time.因为你的上司有特定的时间谈谈,你必须保证你会准时到那儿的。
Generating nitrogen on-site provides many benefits to users, who are often unaware of hidden potential issues and additional requirements, when using gas cylinders.10-15% sent back unused - wasted money.PSA NITROGEN GENERATORSGAS CYLINDER DISADVANTAGESNITROSourceWASTED GAS COSTIMPURITIES SPOILT PROCESSES/PRODUCTSImpurities desorb from the cylinder wall below 30 barg, coating equipment and pipework.Ruining process or product – increasing downtime and cost.INSPECTION REQUIREMENTS Time consuming and potential for dangerous errors (safety).At least 15 checks every time a cylinder is changed – time consuming, liability issues.Monitoring deliveries and stock- labour intensive.Ruining process or product - increasing downtime and cost.Gas could run out or spoil process/product or need to repeat task.One in use, one empty, one full.Additional cost to certify employees to work with compressed gas cylinders.Personnel have to be trained in cylinder handling and regulator inspection every 3 years – Additional cost and liability.Heavy, manual handling, stored volume of asphyxiant.High pressure cylinders need specialist handling equipment – cost of handling equipment, possible injuries, additional risk.Additional cost, takes up valuable space. Requires maintenance and checking.ROGUE CYLINDERSNO INDICATION OF VOLUME REMAINING OR ACTUAL OXYGEN CONTENT 3 CYLINDERS POTENTIALLY RENTED FOR EVERY APPLICATION TRAINING SAFETY INSURANCE AND HSE CONCERNSREQUIRE SPECIALIST RACKS FOR STORAGE。
tpo53三篇托福阅读TOEFL原文译文题目答案译文背景知识阅读-1 (2)原文 (2)译文 (5)题目 (8)答案 (16)背景知识 (18)阅读-2 (21)原文 (21)译文 (24)题目 (27)答案 (34)背景知识 (36)阅读-1原文Evidence of the Earliest Writing①Although literacy appeared independently in several parts of the prehistoric world, the earliest evidence of writing is the cuneiform Sumerian script on the clay tablets of ancient Mesopotamia, which, archaeological detective work has revealed, had its origins in the accounting practices of commercial activity. Researchers demonstrated that preliterate people, to keep track of the goods they produced and exchanged, created a system of accounting using clay tokens as symbolic representations of their products. Over many thousands of years, the symbols evolved through several stages of abstraction until they became wedge-shaped (cuneiform) signs on clay tablets, recognizable as writing.②The original tokens (circa 8500 B.C.E.) were three-dimensional solid shapes—tiny spheres, cones, disks, and cylinders. A debt of six units of grain and eight head of livestock, for example might have been represented by six conical and eight cylindrical tokens. To keep batches of tokens together, an innovation was introduced (circa 3250 B. C. E.) whereby they were sealed inside clay envelopes that could be brokenopen and counted when it came time for a debt to be repaid. But because the contents of the envelopes could easily be forgotten, two-dimensional representations of the three-dimensional tokens were impressed into the surface of the envelopes before they were sealed. Eventually, having two sets of equivalent symbols—the internal tokens and external markings—came to seem redundant, so the tokens were eliminated (circa 3250-3100 B.C.E.), and only solid clay tablets with two-dimensional symbols were retained. Over time, the symbols became more numerous, varied, and abstract and came to represent more than trade commodities, evolving eventually into cuneiform writing.③The evolution of the symbolism is reflected in the archaeological record first of all by the increasing complexity of the tokens themselves. The earliest tokens, dating from about 10,000 to 6,000 years ago, were of only the simplest geometric shapes. But about 3500 B.C.E., more complex tokens came into common usage, including many naturalistic forms shaped like miniature tools, furniture, fruit, and humans. The earlier, plain tokens were counters for agricultural products, whereas the complex ones stood for finished products, such as bread, oil, perfume, wool, and rope, and for items produced in workshops, such as metal, bracelets, types of cloth, garments, mats, pieces of furniture, tools, and a variety of stone and pottery vessels. The signs marked onclay tablets likewise evolved from simple wedges, circles, ovals, and triangles based on the plain tokens to pictographs derived from the complex tokens.④Before this evidence came to light, the inventors of writing were assumed by researchers to have been an intellectual elite. Some, for example, hypothesized that writing emerged when members of the priestly caste agreed among themselves on written signs. But the association of the plain tokens with the first farmers and of the complex tokens with the first artisans—and the fact that the token-and-envelope accounting system invariably represented only small-scale transactions—testifies to the relatively modest social status of the creators of writing.⑤And not only of literacy, but numeracy (the representation of quantitative concepts) as well. The evidence of the tokens provides further confirmation that mathematics originated in people’s desire to keep records of flocks and other goods. Another immensely significant step occurred around 3100 B.C.E., when Sumerian accountants extended the token-based signs to include the first real numerals. Previously, units of grain had been represented by direct one-to-one correspondence―by repeating the token or symbol for a unit of grain the required number of times. The accountants, however, devisednumeral signs distinct from commodity signs, so that eighteen units of grain could be indicated by preceding a single grain symbol with a symbol denoting “18.”Their invention of abstract numerals and abstract counting was one of the most revolutionary advances in the history of mathematics.⑥What was the social status of the anonymous accountants who produced this breakthrough? The immense volume of clay tablets unearthed in the ruins of the Sumerian temples where the accounts were kept suggests a social differentiation within the scribal class, with a virtual army of lower-ranking tabulators performing the monotonous job of tallying commodities. We can only speculate as to how high or low the inventors of true numerals were in the scribal hierarchy, but it stands to reason that this laborsaving innovation would have been the brainchild of the lower-ranking types whose drudgery is eased.译文最早文字的证据①虽然读写能力是在史前世界的几个地方分别出现的,但书写的最早证据是古代美索不达米亚泥板上的苏美尔楔形文字,根据考古探查工作揭示,它起源于商业活动的会计实践。
分析测试新成果 (39 ~ 46)惰气熔融-红外吸收/热导法同时测定无烟煤中氮和氢王 琳1,王 楠1,沈峰满2(1. 东北大学 分析测试中心,辽宁 沈阳 110819;2. 东北大学 冶金学院,辽宁 沈阳 110819)摘要:首次使用惰气熔融-红外吸收/热导法实现无烟煤中氮、氢元素的同时、快速、准确测定. 探究分析条件,发现当称样量为0.030 0 g ,分析功率为5 500 W ,氮元素的积分延迟时间为15 s ,集成时间为55 s ,氢元素的积分延迟时间为5 s ,集成时间为85 s ,且使用石墨套埚时,氮氢元素的释放最完全、合理. 方法中氮、氢校准曲线的相关系数分别为0.994 9、0.994 0,检出限分别为0.321%、0.189%,定量限分别为0.326%、0.194%,精密度分别为3.60%、0.63%,满足线性关系及方法要求. 惰气熔融-红外吸收/热导法重复性好、高效便捷、操作和维护简单,可用于无烟煤中氮、氢元素的定量检测.关键词:惰气熔融;红外吸收/热导法;无烟煤;氮;氢中图分类号:O657. 3 文献标志码:B 文章编号:1006-3757(2024)01-0039-08DOI :10.16495/j.1006-3757.2024.01.007Simultaneous Determination of Nitrogen and Hydrogen in Anthracite by Inert Gas Melting-Infrared Absorption/Thermal Conductivity MethodWANG Lin 1, WANG Nan 1, SHEN Fengman2(1. Analysis and Measurement Centre , Northeastern University , Shenyang 110819, China ;2. School ofMetallurgy , Northeastern University , Shenyang 110819, China )Abstract :The contents of nitrogen and hydrogen in anthracite were simultaneously, rapidly and accurately determined by the inert gas melting-infrared absorption/thermal conductivity method. A series of experiments were studied. The results indicated that the most complete and reasonable release of nitrogen and hydrogen was achieved when the sample was 0.030 0 g, the analysis power was 5 500 W, the integration delay time of nitrogen was 15 s, the integration time of nitrogen was 55 s, the integration delay time of hydrogen was 5 s, the integration time of hydrogen was 85 s, and the graphite sleeve crucible was used. The correlation coefficients of calibration curves of nitrogen and hydrogen were 0.994 9and 0.994 0, respectively. The limits of detection were 0.321% and 0.189%, the limits of quantification were 0.326% and 0.194%, and the precision were 3.60% and 0.63%, respectively, which met the requirements of linearity and method. The inert gas melting-infrared absorption/thermal conductivity method is reproducible, efficient and convenient, easy to operate and maintain, and can be used for the quantitative determination of nitrogen and hydrogen in anthracite.Key words :inert gas melting ;infrared absorption/thermal conductivity method ;anthracite ;nitrogen ;hydrogen自2020年我国提出碳达峰、碳中和的发展目标以来[1],我国的能源、经济等发展始终围绕碳排放、绿色清洁等话题. 煤是工业原料之一,素来被称为“工业之母”,是世界工业、制造业、经济、民生等的重要支撑,其用途广泛,在新材料制备、化工生产、生活供暖、交通出行、发电等方面有着不可替代的作用. 我国属于煤矿矿产丰富的国家[2],煤、石油、天然气是重要的能源,特点是“富煤、贫油、少气”[3].收稿日期:2023−10−11; 修订日期:2023−12−18.基金项目:国家自然科学基金资助项目 (51974073) [National Natural Science Foundation of China (51974073)]作者简介:王琳(1990−),女,实验师,主要从事气体成分分析等化学分析,E-mail :****************.第 30 卷第 1 期分析测试技术与仪器Volume 30 Number 12024年1月ANALYSIS AND TESTING TECHNOLOGY AND INSTRUMENTS Jan. 2024煤根据品种及品质的不同,分为烟煤、无烟煤、焦炭等,并应用于不同行业,其中无烟煤因其燃烧无烟、煤化程度高、含碳量高、热值高、挥发分低等特点,普遍用于燃料及燃料电池、先进碳材料[4-7]、催化剂[8]、吸附剂[9-10]、滤料、民用煤等. 而据统计显示,我国空气污染源中的粉尘、PM2.5、SO2及NO x等大部分来自于民用煤燃烧的排放[11],因此加强对无烟煤的质量监测,是提升煤炭质量、发展低碳与绿色能源的重要环节.煤炭的检测标准溯源到上世纪60年代,检测指标一般包括工业分析[12](水分、灰分、挥发分、固定碳)、元素分析[13-15](C、S、O、N、H)、有价元素分析[16-17](As、Ga、Se、Ge等)、阴离子[18](氟等)等. 其中无烟煤中的氮元素在燃烧后会形成NO x,对人类及居住环境污染影响较大[11]. 无烟煤中氢元素含量的多少,代表了热值的大小. 因此准确快速测定无烟煤中氮、氢含量对煤炭质量控制,煤炭行业的检验检测、标准制定、能源开发及环境保护等均具有重要意义.对于无烟煤中氮、氢元素的检测,通常使用半微量开氏法和半微量蒸汽法[19]、高温燃烧-检测器测定法[14, 20]测定无烟煤中的氮含量,采用三节炉法、二节炉法[13]、电量-重量法[21]、高温燃烧-检测器测定法[14]测定无烟煤中的氢含量. 其中三节炉法、二节炉法、电量-重量法均存在硫、氯等元素的干扰,需使用铬酸铅、银丝、二氧化锰等试剂消除干扰,污染较大且成本高. 随着科技的进步,仪器法逐渐被用于测定无烟煤中的氮、氢元素含量,现有的仪器法[22]原理是将无烟煤在氧气下燃烧,对燃烧生成的H2O、N2气体进行检测. 但该法存在燃烧炉/管升降温时间长、分析时间长、维护复杂、耗材昂贵等缺点. 而以惰气熔融-红外吸收/热导法为分析原理设计的氧氮氢分析仪通常用于陶瓷、粉末[23]、钢铁[24]等无机材料中氧、氮、氢元素的测定,并以快速、精准的优势成为冶金、材料等领域以及检验检测机构在气体元素分析方面的常用仪器. 但目前为止,未见其应用于无烟煤类产品的检测工作中,其在使用中无需强酸、重金属等试剂,具有无需等待升降温、分析时间短、样品前处理简易、维护相对简单等优势,满足绿色、安全、快速、准确分析的要求,因此本文首次尝试将惰气熔融-红外吸收/热导法应用于无烟煤中氮、氢元素的检测.1 试验部分1.1 仪器与试剂氧氮氢分析仪:美国力可公司,ONH836;天平:赛多利斯,SQP;石墨套埚(内坩埚加外坩埚)、石墨标准坩埚、镍嚢,LECO公司;有机元素分析仪:德国元素公司,Vario MACRO cube.氦气(99.999%),氮气(99.5%),沈阳顺泰特种气体有限公司;无烟煤标准物质:ZBM093、ZBW112A、ZBM095A,济南众标科技有限公司生产;GBW11104j,国家煤炭质量监督检验中心;GBW11108o,山东省冶金科学研究院. 对氨基苯磺酰胺(C6H8N2O2S)、WO3,德国元素公司;未知样品为某学生客户日常送检的无烟煤样品.1.2 试验原理在惰性气体氦气保护下,样品置于上下电极间的石墨坩埚中,经过坩埚脱气、吹扫、脉冲炉通电,上、下电极及石墨坩埚形成电路并加热,使待测样品完全熔融,N、H元素分别以N2、H2分子形式释放,随载气氦气流经热的氧化铜催化剂,H2被完全氧化成H2O,N2、H2O一起进入红外检测池,根据H2O的特征红外吸收波长,检测得到氢元素的含量,之后H2O被高氯酸镁等过滤试剂吸收,N2进入热导检测池完成氮元素的测定,其原理图如图1所示.样品上电级红外检测池检测 H2O热导检测池检测 N2坩埚下电极脉冲熔融炉N2N2H2催化剂H2OH2O图1 氧氮氢分析仪测定氮、氢的工作原理图Fig. 1 Working principle diagram ofOxygen/Nitrogen/Hydrogen Analyzer determined nitrogenand hydrogen1.3 试验方法1.3.1 准备工作将标准物质、待测样品置于110 ℃洁净的烘箱中烘干2 h,保证粒度在0.074 mm以下,然后再置40分析测试技术与仪器第 30 卷于干燥器中冷却备用.对氧氮氢分析仪进行彻底维护,包括上电极、下电极、投样口的清扫清洁,催化剂、过滤试剂等试剂的更换,并通过漏气检查,保证仪器的气密性.1.3.2 试验步骤打开稳压电源、氧氮氢分析仪主机及软件,将下电极升高,在氦气保护模式下进行仪器预热至少1 h,预热完成后打开氦气至流速为450 mL/min,开通冷却水,使检测器保持在稳定的工作温度. 本方法以镍嚢及空白石墨套锅作为空白,分别称取0.010 0~0.100 0 g(精确到±0.000 3 g)的样品,小心倾倒于镍嚢内,等待投样,设置4 500~6 000 W的分析功率,对比石墨套埚与石墨标准坩埚的分析效果,分别设置0~15 s的分析延迟时间、50~85 s数据集成时间等仪器参数. 开始测试后进行投放样品、取下坩埚、更换新的内坩埚、脱气、吹扫等操作,依次进行空白、标准物质及未知样品的测试,建立标准曲线,并对方法进行检出限、定量限、精密度等试验验证.1.3.3 未知样品对比试验本文使用有机元素分析仪作为未知样品测试的对比方法,并命名为方法1. 对有机元素分析仪(CHNS模式)的燃烧管进行清理并更换试剂及灰分坩埚,还原管内铜及银丝重新装填,酒精擦拭干净后放回到炉子内,通高纯氦气,流速为600 mL/min,室温检漏通过后,分别升至1 150、850 ℃工作温度下吹扫4 h后进行试验. 使用仪器自带标准曲线,以75 mg的锡纸包裹,称取25 mg的对氨基苯磺酰胺作为“run”和漂移标准物质进行曲线校正,待测样品称样量为50 mg,加入WO3助熔,75 mg锡纸包裹,使用工具压除空气后置于自动进样器中进样,试样在1 150 ℃下通高纯氧气燃烧,850 ℃下催化还原,释放出N2和H2O,进入相应检测池分析检测,经过“吹扫-捕集”吸附解析的分离过程,得到氮、氢的分析数据,完成检测.2 结果与讨论2.1 进样方式的确定本试验采用直投法进样,对于粉末类样品以此方式进样时,会造成进样系统污染、进样量减少、分析数据偏低等问题,为避免因进样造成的分析误差,需采用镍嚢作为样品包裹体,保证进样量的准确性及释放完全性.2.2 进样量的确定样品的进样量会影响熔融效果,使用标准物质ZBM095A作为待测样品,对比0.010 0、0.020 0、0.030 0、0.040 0、0.050 0、0.060 0、0.080 0、0.100 0 g 进样量对氮、氢元素释放效果的影响. 由图2可见,随着进样量的增加,氮质量比在进样量为0.010 0~ 0.030 0 g时的测定结果变化不大,而在0.0300 g时出现拐点呈下降趋势,随着进样量的继续增加,由于释放条件不足,氮质量比下降,因此氮的最佳进样量为0.0300 g. 氢质量比随进样量增加,先呈明显上升趋势,在进样量为0.030 0 g时,氢质量比达到了最高点,而随着进样量的继续增大,氢质量比缓慢降低,在进样量大于0.060 0 g时,氢质量比迅速下降. 由此可见,0.0300 g是其最佳进样量. 产生该现象的原因可能是进样量较低时,样品分析浓度不够,导致氢元素质量比偏低,而进样量过高时,样品的分析条件不足以使氢完全释放,氢元素质量比降低,且就仪器本身的检测范围而言,氢的测量上限绝对质量为0.002 5 g,因此对于标准物质ZBM095A 的氢元素质量比的测定,当进样量超过0.050 0 g时,检测池处于饱和状态,无法正常检测. 因此,0.030 0 g 为该方法的最佳进样质量.4.54.03.53.02.52.01.51.00.500.020 00.040 00.060 0NH0.080 00.100 0m/g质量比/%图2 不同进样量下氮、氢的测试结果Fig. 2 Test results of nitrogen and hydrogen underdifferent sample masses2.3 分析功率的确定在氮、氢元素分析中,分析功率是决定样品释放的重要参数. 本试验依次设置4 500、5 000、5 500、6 000 W的功率梯度,观察功率对于无烟煤中氮、氢元素检测的影响. 图3为氮、氢的测试值随功率变化的关系图. 由图3可见,当功率较低,在4 500、5 000 W时,氮、氢元素质量比偏低,说明过低的功第 1 期王琳,等:惰气熔融-红外吸收/热导法同时测定无烟煤中氮和氢41率不足以使无烟煤完全熔融释放,这与无烟煤本身含碳量高、燃点高的特性一致. 但当功率为6 000 W 时,质量比再次下降,这是因为功率过高,导致氮、氢元素过早溢出,数据捕捉不及时,导致数据偏低.当分析功率为5 500 W 时,氮、氢元素的释放最完全,测定值最高. 由此可见,无烟煤的最佳分析功率为5 500 W.2.4 分析坩埚的对比氮、氢元素分析的样品载体一般分为石墨套埚(外坩埚加内坩埚)和标准坩埚. 本试验对比二者的分析效果,观察图4(a )的氮元素及图4(b )的氢元素在使用不同坩埚时的测定谱图,可发现氮、氢元素在使用石墨套埚得到的测定值明显高于标准坩埚,说明石墨套埚的分析效果优于标准坩埚. 究其原因,标准坩埚对比石墨套埚来说相对单薄,在5 500 W 的高功率下其承压能力小,甚至存在标准坩埚被烧漏或者断裂的情况,因而标准坩埚的使用会导致数据偏低,对于无烟煤这类燃点高、熔融产生热量大的样品来说,双层结构的套埚更适用. 因此,本试验选用石墨套埚作为分析坩埚.2.5 分析参数的设定(包括分析延迟时间、数据集成时间)本方法对仪器分析参数(分析延迟时间、数据集成时间)进行了探究. 对比了15、10、5、0 s 四种延迟时间,观察图5(a )可见,15 、10 s 时氢的出峰过早、不完整且峰形不佳,导致氢元素的数据捕集不完全,测试数据偏低. 当调整为5 s 时,氢峰的前端有平缓的基线,0 s 时出峰过缓. 因此,5 s 是合理的延迟时间. 由图5(b )可见,氮的测试值随延迟时间的增加而增大,其延迟时间设置为15 s 较合理.对于出峰不完全的问题,本试验采用将数据集成时间延长的方式,分别设置为55、65、75、80、85 s ,观察图6(a )发现,当集成时间为55、65、75 s 时,氢峰的末端均未回到基线的位置,数据偏低. 80 s 时谱线回到基线,85 s 时形成相对完整的正态分布峰,与图6(b )的数据趋势吻合. 同时观察图6(b )发现,氮的集成时间为55s 数据更合理. 因此本方法选择氮的延迟时间为15 s 、集成时间为55 s ,氢的延迟时间为5 s 、集成时间为85 s 为最佳分析参数.2.6 标准曲线建立及检出限测定无烟煤中的氮、氢元素含量范围较宽泛,单点校准的方式并不适用. 本文采用建立标准曲线的校准方式,在称样质量为0.030 0 g 、分析功率为5 500W ,氮、氢元素延迟时间分别为15、5 s ,捕集时间分别为55、85 s ,使用石墨套埚的试验条件下,选择有证标准物质ZBM093、GBW11104j 、GBW11108o 、2.754.34.24.14.03.92.702.652.602.554 5005 000N H5 5006 000P /W质量比/%质量比/%图3 分析功率的探究试验Fig. 3 Test results of nitrogen and hydrogen underdifferent analytical powers100(a)608040积分强度石墨套锅标准坩埚2000102030t /s405060100(b)608040积分强度石墨坩埚标准坩埚2000102030t /s405060图4 石墨套埚与标准坩埚的确定试验(a)不同坩埚对氮元素的测试谱图,(b)不同坩埚对氢元素的测试谱图Fig. 4 Comparison of test results between graphite sleeve pote and standard crucible (a) spectra of nitrogen in different crucibles, (b) spectra of hydrogen in different crucibles42分析测试技术与仪器第 30 卷ZBW112A 建立标准曲线,其认定值及测量值结果如表1所列. 氮、氢元素的线性方程分别为:Y =2.098 404 22X −0.000 200 66、Y =0.789 376 46X −0.000 044 57,相关系数分别为0.994 9、0.994 0,满足线性关系. 对空白坩埚连续测试11次,得到氮、氢元素的平均值分别为0.318 9%、0.186 9%,以该结果与3倍标准偏差之和作为检出限,分别为0.321%、0.189%,以平均值与10倍标准偏差之和作为定量限,分别为0.326%、0.194%,结果如表2所列,表明该方法检测范围较宽,适用于无烟煤中氮、氢元素的定量检测.2.7 方法的准确度、精密度测试精密度测试是验证方法可靠性的重要指标,本试验使用有证无烟煤标准物质ZBM095A 进行精密度测试,平行测定7次,并计算其精密度. 如表3所列,其氮、氢元素的测定平均值分别为1.30%、3.30%,由表1可知,其认证值分别为1.31%±0.07%、3.23%±0.10%,因此该方法准确度较好. 经计算,氮、氢的精密度分别为3.60%、0.63%,满足方法精密度要求. 由此可见该方法准确可靠.表 1 标准物质及其认证值、测量值Table 1 Certified and measured values of standardsubstances/%标准物质NH 认证值测量值认证值测量值ZBM0930.56±0.060.563 3.01±0.12 2.92GBW11104j 0.94±0.070.929 2.64±0.15 2.71GBW11108o 1.30±0.06 1.30 4.58±0.13 4.59ZBW112A 1.10±0.06 1.12 3.78±0.10 3.79ZBM095A1.31±0.071.303.23±0.103.3010015 s 10 s 5 s 0 s(a)8060积分强度402005101520253035t /s 404550556065702.655.04.03.02.01.00N H(b)2.602.552.50质量比/%质量比/%2.452.402.3551015t /s图5 氮、氢的分析延迟时间对比试验(a) 不同延迟时间下氢的测试谱图, (b)延迟时间对氮、氢的影响Fig. 5 Comparison test of analysis delay times of nitrogen and hydrogen(a) spectra of hydrogen in different delay times, (b) effect of delay times on nitrogen and hydrogen100 2.705.04.94.84.74.62.682.662.642.622.6055606570758085909585 s 80 s 75 s 65 s 55 s806040积分强度质量比/%质量比/%20002040t /st /s6080100(a)(b)图6 氮、氢的集成时间对比试验(a)不同集成时间下氢的测试谱图, (b)集成时间对氮、氢的影响Fig. 6 Comparison test of integration times of nitrogen and hydrogen(a) spectra of hydrogen in different integration times, (b) effect of integration times on nitrogen and hydrogen第 1 期王琳,等:惰气熔融-红外吸收/热导法同时测定无烟煤中氮和氢432.8 未知样品测试对日常送检的无烟煤样品进行抽检,并标号为样品1、样品2,使用方法1与本方法进行对比,随试验进行ZBM095A的测试. 分别平行测定7次,其测试结果如表4所列. 由表可见,方法1测得样品1、样品2、ZBM095A中氮的平均值分别为0.096 6%、1.086%、1.30%,相对标准偏差(RSD)分别为2.67%、1.75%、3.60%. 氢的平均值分别为2.899%、3.312%、3.30%,RSD分别为1.90%、1.50%、0.63%. 本方法测得样品1、样品2、ZBM095A中氮的平均值分别为0.094 6%、1.067%、1.25%,RSD分别为2.99%、1.69%、3.90%. 氢的平均值分别为2.927%、3.300%、3.20%,RSD分别为1.87%、1.56%、0.72%. 对比两种方法,准确度与精密度均能够满足试验要求,再次证实本文建立的方法适用于无烟煤中的氮、氢两种元素的定量测定.表 3 ZBM095A的精密度试验Table 3 Precision test of ZBM095A/%元素测定值平均值RSDN 1.28、1.26、1.34、1.35、1.36、1.30、1.24 1.30 3.60H 3.30、3.32、3.33、3.29、3.29、3.33、3.28 3.300.63表 4 两种方法测试未知样品的对比试验Table 4 Comparison of two methods for testing unknown samples/%样品方法1平均值方法1 RSD本方法平均值本方法RSD N H N H N H N H样品10.096 6 2.899 2.67 1.900.094 6 2.927 2.99 1.87样品 2 1.086 3.312 1.75 1.50 1.067 3.300 1.69 1.56 ZBM095A 1.30 3.30 3.600.63 1.25 3.20 3.900.723 结论(1)本文首次将惰性气体熔融-红外吸收/热导法应用于无烟煤类产品的检测中,该方法满足同时、快速、准确的特点,减少了强酸化学试剂的使用,体现了绿色化学宗旨.(2)建立了无烟煤中氮、氢元素定量测试的方法,为煤炭行业的检验检测、标准制定、贸易等提供参考.(3)拓展了氧氮氢分析仪的使用范围,在有色金属、高温合金、难熔金属、稀土、陶瓷、矿石等材料的使用范围之外,增加了无烟煤类产品的使用.参考文献:习近平. 在第七十五届联合国大会一般性辩论上的讲话[N]. 人民日报, 2020-09-23(3).[ 1 ]元雪芳, 任恒星, 郭鑫, 等. 不同物质对无烟煤生物转化的影响研究[J].煤化工,2022,50(5):79-82.[YUAN Xuefang, REN Hengxing, GUO Xin, et al.Study on impact of adding different substances on bio-transformation of anthracite[J]. Coal Chemical In-dustry,2022,50 (5):79-82.][ 2 ]吕俊复, 蒋苓, 柯希玮, 等. 碳中和背景下循环流化床燃烧技术在中国的发展前景[J]. 煤炭科学技术,2023,51(1):514-522. [LV Junfu, JIANG Ling, KEXiwei, et al. Future of circulating fluidized bed com-[ 3 ]表 2 方法的线性与检出限Table 2 Linearity and limits of detection元素线性方程线性相关系数(R2)平均值/(%,n=11)检出限/%定量限/% N Y=2.098 404 22X − 0.000 200 660.994 90.318 90.3210.326H Y=0.789 376 46X − 0.000 044 570.994 00.186 90.1890.19444分析测试技术与仪器第 30 卷bustion technology in China for carbon neutralization [J ]. Coal Science and Technology ,2023,51 (1):514-522.]于昭仪, 谢卫宁, 邱钿, 等. 添加剂对煤基石墨微观结构的影响[J ]. 煤炭科学技术,2023,51(5):302-308.[YU Zhaoyi, XIE Weining, QIU Tian, et al. Effect of additives on microstructure of coal-based graphite [J ].Coal Science and Technology ,2023,51 (5):302-308.][ 4 ]传秀云, 鲍莹. 煤制备新型先进炭材料的应用研究[J ]. 煤炭学报,2013,38(S1):187-194. [CHUAN Xiuyun, BAO Ying. Application of coal as raw materi-als in preparing new advanced carbon materials [J ].Journal of China Coal Society ,2013,38 (S1):187-194.][ 5 ]唐跃刚, 陈鹏翔, 李瑞青, 等. 京西煤制备氧化石墨烯分子结构模型的构建与优化[J ]. 煤炭科学技术,2021,49(6):126-134. [TANG Yuegang, CHEN Pengxiang, LI Ruiqing, et al. Model construction and optimization of molecule structure of coal-based grapheme oxide from Jingxi coal [J ]. Coal Science and Technology ,2021,49 (6):126-134.][ 6 ]Zhang C, Xie Y C, Zhang C, et al. Upgrading coal tomultifunctional graphene-based materials by direct laser scribing [J ]. Carbon ,2019,153 :585-591.[ 7 ]杨丽, 刘帅, 辛春梅, 等. 炭黑负载增加活性炭缺陷位点催化甲烷裂解制氢[J/OL ]. 煤炭科学技术, 2023:1-11[2023-12-08]. https:///kcms/detail/11.2402.td.20230706.1933.007.html. [YANG Li, LIU Shuai, XIN Chunmei, et al. The loading of carbon black increased the defect site of activated carbon Catalytic pyrolysis of methane for hydrogen produc-tion [J/OL ]. Coal science and technology, 2023: 1-11[2023-12-08]. https:///kcms/detail/11.2402.td.20230706.1933.007.html.][ 8 ]张昆, 孟召平, 金毅, 等. 不同煤体结构煤的孔隙结构分形特征及其研究意义[J ]. 煤炭科学技术,2023,51(10):198-206. [ZHANG Kun, MENG Zhaoping,JIN Yi, et al. Fractal characteristics of pore structures on different coal structures and its research signific-ance [J ]. Coal Science and Technology ,2023,51 (10):198-206.][ 9 ]狄军贞, 曹洋, 赵文琦. 巯基改性褐煤的制备及其对Fe 2+、Mn 2+的吸附特性研究[J ]. 煤炭科学技术,2023,51(3):261-270. [DI Junzhen, CAO Yang, ZHAO Wenqi. Preparation of mercapto modified lignite and its adsorption characteristics for Fe 2+、Mn 2+[J ]. Coal[ 10 ]Science and Technology ,2023,51 (3):261-270.]何绪文. 民用燃煤大气污染物控排技术对策[J ]. 洁净煤技术,2017,23(4):12-17. [HE Xuwen. Counter-measure of air pollutant controlled pollutation for ci-vilian coal [J ]. Clean Coal Technology ,2017,23 (4):12-17.][ 11 ]国家质量监督检验检疫总局, 中国国家标准化管理委员会. 煤的工业分析方法 仪器法: GB/T 30732—2014[S ]. 北京: 中国标准出版社, 2014. [General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China,Standardization Administration of the People's Re-public of China. Proximate analysis of coal-instru-mental method: GB/T 30732—2014[S ]. Beijing:Standards Press of China, 2014.][ 12 ]国家质量监督检验检疫总局. 煤的元素分析方法:GB/T 476—2001[S ]. 北京: 中国标准出版社, 2001.[General Administration of Quality Supervision, In-spection and Quarantine of the People's Republic of China. Ultimate analysis of coal: GB/T476—2001[S ]. Beijing: Standards Press of China,2001.][ 13 ]国家质量监督检验检疫总局, 中国国家标准化管理委员会. 煤中碳氢氮的测定 仪器法: GB/T 30733—2014[S ]. 北京: 中国标准出版社, 2014. [General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China,Standardization Administration of the People's Re-public of China. Determination of total carbon, hy-drogen and nitrogen content in coal-instrumental method: GB/T 30733—2014[S ]. Beijing: Standards Press of China, 2014.][ 14 ]杜强, 王喜武. 煤直接液化工艺硫氮元素分布及其影响研究[J ]. 中国煤炭,2022,48(8):104-108. [DU Qiang, WANG Xiwu. Research on the distribution of sulfur and nitrogen and its influence in direct coal li-quefaction process [J ]. China Coal ,2022,48 (8):104-108.][ 15 ]国家市场监督管理总局, 国家标准化管理委员会.煤中有价元素含量分级及应用导则: GB/T 41042—2021[S ]. 北京: 中国标准出版社, 2021.[State Administration for Market Regulation, Na-tional Standardization Administration. Guidance for utilization and classification of content of valuable elements in coal: GB/T 41042—2021[S ]. Beijing:Standards Press of China, 2021.][ 16 ]姬晓燕, 张志峰, 祁风华, 等. 任家庄井田晚古生代煤[ 17 ]第 1 期王琳,等:惰气熔融-红外吸收/热导法同时测定无烟煤中氮和氢45系战略性金属元素富集特征[J/OL ]. 煤炭科学技,2023: 1-15 [2023-12-08]. https://doi. org/10.13199/ki.cst.2023-0146. [JI Xiaoyan, ZHANG Zhifeng, QI Fenghua, et al. Characteristics of strategic metal ele-ment enrichment in late Paleozoic coal measures in Renjiazhuang Jingtian [J/OL ]. Coal Science and Technology, 2023: 1-15[2023-12-08]. https:///10.13199/ki.cst.2023-0146.]唐佳伟, 张锁, 刘兆峰, 等. 吸附法去除水中F -进展及其在矿井水处理中发展方向[J/OL ]. 煤炭科学技术,2023: 1-16[2023-12-08]. https: //doi. org/10.13199/ki. cst. 2022-1835. [TANG Jiawei, ZHANG Suo,LIU Zhaofeng, et al. Progress in removal of F -in wa-ter by adsorption method and its development direc-tion in mine water treatment [J/OL ]. Coal science and technology, 2023: 1-16[2023-12-08]. https:///10.13199/ki.cst.2022-1835.][ 18 ]国家质量监督检验检疫总局, 中国国家标准化管理委员会. 煤中氮的测定方法: GB/T 19227—2008[S ]. 北京: 中国标准出版社, 2009. [General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China,Standardization Administration of the People's Re-public of China. Determination of nitrogen in coal:GB/T 19227—2008[S ]. Beijing: Standards Press of China, 2009.][ 19 ]龚婉莉. 采用元素分析仪测定煤中碳氢氮含量的应用研究[J ]. 煤质技术,2018(1):38-41, 49. [GONG Wanli. The application study on determination of car-bon, hydrogen and nitrogen content in coal by ele-mental analyzer [J ]. Goal Quality Technology ,2018(1):38-41, 49.][ 20 ]国家质量监督检验检疫总局, 中国国家标准化管理委员会. 煤中碳和氢的测定方法: GB/T 476—2008[S ]. 北京: 中国标准出版社, 2009. [General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China,Standardization Administration of the People's Re-public of China. Determination of carbon and hydro-gen in coal: GB/T 476—2008[S ]. Beijing: Standards Press of China, 2009.][ 21 ]孙玉芳, 王曦. 元素分析仪测定土壤氮、碳含量的不确定度评定[J ]. 分析测试技术与仪器, 2016, 22(4):240-245. [SUN Yufang, WANG Xi. Uncertainty eval-uation of measurement results for determination of total carbon and nitrogen in soil samples using ele-mental analyzer [J ]. Analysis and testing technology and instruments. 2016, 22(4): 240-245.][ 22 ]朱春要, 秦建, 赵希文, 等. 脉冲熔融-红外/热导法测定钛合金粉末微注射成形脱脂坯中氧氮氢[J ]. 中国无机分析化学,2023,13(5):499-504. [ZHU Chunyao, QIN Jian, ZHAO Xiwen, et al. Determina-tion of oxygen, nitrogen and hydrogen in micro injec-tion molding of titanium alloy powder about degrease billets by impulse fusion-infrared absorption and thermal conductivity method [J ]. Chinese Jorunal of Inorganic Analytical Chemistry ,2023,13 (5):499-504.][ 23 ]侯桂臣, 张琳, 李一懿, 等. “直投法” 应用于金属粉末样品中氧氮氢分析[J ]. 冶金分析,2019,39(6):7-13. [HOU Guichen, ZHANG Lin, LI Yiyi, et al. Ap-plication of "direct-dropping" for analysis of oxygen,nitrogen and hydrogen in metal powder sample [J ].Metallurgical Analysis ,2019,39 (6):7-13.][ 24 ]46分析测试技术与仪器第 30 卷。
REGULAR ARTICLENitrogen dynamics at undisturbed and burned Mediterranean shrublands of Salento Peninsula, Southern ItalyMichael Dannenmann&Georg Willibald&Sebastian Sippel&Klaus Butterbach-BahlReceived:13May2010/Accepted:16August2010/Published online:10September2010#Springer Science+Business Media B.V.2010Abstract Fire is a major disturbance in shrubland ecosystems of the Mediterranean basin,with high potential to alter ecosystem nitrogen(N)stocks and N cycling.However,postfire effects on gross rates of soil N turnover(ammonification,nitrification,micro-bial immobilization,denitrification)have rarely been investigated.We determined gross rates of N turnover including nitrous oxide fluxes and dinitrogen emissions in the mineral soil of unburned and burned shrublands of Southern Italy6months after a natural fire.In soil of burned plots,both gross ammonification and gross nitrification were significantly higher than in soil of unburned plots(2.2±0.3versus0.6±0.1mg N kg−1sdw day−1for ammonification and1.1±0.1versus0.5±0.1mg N kg−1sdw day−1for nitrification).Microbial immobilization,in particular of nitrate,could not compensate for the increase in inorganic N production, therefore soil nitrate concentrations were considerably higher at the burned plots.Soil microbial biomass carbon and nitrogen concentrations were significantly lower in soils of burned plots than in soils of unburned plots.Dinitrogen was the dominant end product of denitrification and emitted at higher rates from the unburned plots than from the burned plots(0.094±0.003versus0.004±0.002mg N kg−1sdw day−1, while there was no net nitrous oxide flux(burned plots)or slight net nitrous oxide uptake(control plots). These results show that postfire patterns of gross N turnover in soil can exhibit a significant reduction of both microbial N retention and N gas losses via denitrification.Keywords Maquis.Fire.N cycling. Ammonification.Nitrification.Denitrification. Nitrous oxide.Dinitrogen.Microbial biomass.He flow soil core techniqueIntroductionFire is a major disturbance in Mediterranean ecosys-tems,with high potential to alter ecosystem N stocks and N cycling(Moreno and Oechel1995;Certini 2005;Castaldi and Aragosa2002;Knicker2007). Fire frequency may increase under future environ-mental conditions,since available regional predictions assume that air temperatures and drought event probability are significantly increasing due to climate change(Lavorel et al.1998;Piñol et al.1998).Plant Soil(2011)343:5–15DOI10.1007/s11104-010-0541-9Responsible Editor:Per Ambus.M.Dannenmann(*)Institute of Forest Botany and Tree Physiology, Chair of Tree Physiology,University of Freiburg, Georges-Koehler-Allee53/54,79110Freiburg,Germanye-mail:michael.dannenmann@M.Dannenmann:G.Willibald:S.Sippel:K.Butterbach-BahlKarlsruhe Institute of Technology(KIT),Institute for Meteorology and Climate Research(IMK-IFU), Kreuzeckbahnstrasse19,82467Garmisch-Partenkirchen,GermanyHowever,our understanding how fire may affect soil microbial N cycling in Mediterranean ecosystems is still limited.Soil microbial nitrogen(N)cycling in terrestrial ecosystems is of high ecological significance,as it regulates ecosystem N retention;N loss along gaseous and hydrological pathways which can affect atmospheric chemistry,climate change and water quality;and plant nutrient availability (Schimel and Bennett2004;Rennenberg et al. 2009).Gross N ammonification,i.e.the microbial production of ammonium(NH4+)from organic N compounds,is a key processes of soil N cycling, since free NH4+in plant-free soil is subject to two competing microbial processes and fates,i.e.nitrifi-cation to nitrate(NO3-)and immobilization into microbial biomass.After nitrification,NO3--N may also either be immobilized by soil microorganisms, undergo dissimilatory nitrate reduction to ammonium (Silver et al.2001),or may be denitrified.Via denitrification,NO3-is reduced stepwise to nitrite, the secondary greenhouse gas nitric oxide(NO),the potent primary greenhouse gas and most important destruent of stratospheric ozone(Ravishankara et al. 2009)nitrous oxide(N2O)as intermediates,and to molecular dinitrogen(N2)as the dominant end-product.Production of these N gases by denitrifica-tion leads to N loss from the ecosystem.The last step of denitrification,i.e.the reduction of N2O to N2 catalyzed by the enzyme nitrous oxide reductase,is converting reactive nitrogen back into its inert form, and hence,significantly contributes to closing the global nitrogen cycle(Galloway et al.2003). Furthermore it reduces soil N2O losses(Chapuis-Lardy et al.2007;Dannenmann et al.2008). However,the conversion of reactive N back to N2 by denitrification is thought to represent the largest uncertainty of the N cycle at all scales(Galloway et al.2004;Groffman et al.2006).Due to methodo-logical difficulties(Butterbach-Bahl et al.2002; Groffman et al.2006)reliable measurements of N2 emissions from terrestrial ecosystems are scarce which limits our understanding of the significance of the single permanent sink for reactive nitrogen, but also impedes the quantification and comprehen-sion of the denitrification process as a whole (Davidson and Seitzinger2006;Groffman et al. 2006).The latter also feedbacks on our understand-ing of microbial NO3-immobilization,as this is often calculated from the consumption of15NO3-, assuming that gaseous N losses via denitrification are not significant for the NO3-mass balance (Davidson et al.1992;Stark2000).Hence,underes-timation of denitrification probably lead to frequent overestimation of microbial NO3-immobilization in 15N pool dilution experiments.Our understanding of N ammonification,nitrifi-cation and microbial immobilization of inorganic N has significantly improved in the last decades for a wide range of ecosystems.In particular,the devel-opment and application of15N isotope pool dilution and—tracing techniques(Kirkham and Bartholomew 1954;Davidson et al.1991,1992,Stark2000; Murphy et al.2003;Booth et al.2005)facilitated a more holistic view of actual N turnover and its environmental controls compared to the more widely used determination of net rates of N turnover(Eno 1960),which confound simultaneously occurring production and consumption of inorganic N,as e.g. net nitrification is the balance of actual microbial nitrate production(gross nitrification)and microbial nitrate consumption via e.g.microbial nitrate immobilization and denitrification(Davidson et al. 1991).However,Mediterranean shrubland ecosystems are still being severely understudied with respect to gross rates of N turnover and denitrification activity and the importance of fire as a potential driver for soil N cycling has largely been ignored.It is well known that fire increases mineral N concentrations in the uppermost mineral soil(Marion et al.1991), but,as there is still extremely little knowledge on postfire effects on gross rates of ammonification, nitrification,microbial immobilization and denitrifi-cation,it remains unknown to what extent postfire increases of inorganic N concentrations are caused by direct ash input or altered inorganic N production rates.The goal of the present study was to investigate gross rates of soil N turnover(ammonification, nitrification,microbial immobilization of ammonium and nitrate as well as denitrification)at unburned and burned Mediterranean macchia shrublands. Furthermore,we aimed at the clarification of the importance of denitrification versus the other N turnover processes in the investigated ecosystem, i.e.if denitrification is insignificant as an N sink or not.Material and methodsSite characteristicsThe study site is located in Salento Penninsula, Southern Italy(18°23′17.34″E,40°18′5.70″N)at a distance of1km to the sea.The whole site area is completely flat and characterized by homogenous typical Mediterranean Macchia vegetation cover of 0.3–0.8m height.The dominating plant species are Erica australis,Rosmarinus officinalis,Pistacia lentis-cus and Myrtus communis.Mid of August2007, approximately half of the site was burned by a natural fire.The soil is a shallow Rendzic Leptosol on sandy carbonatic bedrock.The height of the densely rooted,organic matter-rich Ah layer was 4.6±0.8cm across the site.At the bottom of the Ah layer there was either a direct transition to the weathered bedrock or a scarcely rooted B layer above the bedrock.The weathered but still compact bedrock was always found at a depth of20cm.The gravel content of both the A and B horizons was moderate (ca.10%).Sampling designEnd of January2008,three unburned and three burned plots of100m2across an area of approx. 2ha were randomly selected and sampled.The distance between burned and unburned plots was 30–50m.The litter layer amounted to663±58g dry mass m−2while the ash layer at the burned plots amounted to72±6g dry mass m−2.At the sampling time,there was a herbal layer covering approximately 30%of the soil at the burned parts of the site. Furthermore,resprouting of burned shrubs had begun at the sampling date.Sampling took place at every plot at seven40*40cm spots randomly selected across the plot.First,the organic(unburned plots)or ash(burned plots)layer was quantitatively sampled and trans-ferred to plastic bags until weight determination and drying(24h at100°C)of subsamples.Subsequently, in the centre of every sampling spot,the Ah layer was sampled with three adjacent soil cores(4cm depth, 100cm3volume).Soil cores were sealed with pin-holed parafilm to facilitate gas exchange but to avoid water loss.Soil cores were stored in cooling boxes and transferred to the laboratories of IMK-IFU in Garmisch-Partenkirchen,Germany,within48h after sampling.After arrival at IMK-IFU they were stored at4°C until further processing and analysis.All samples were processed within two weeks after sampling.One intact soil core of every sampling spot was used for the simultaneous measurement of N2O and N2fluxes.The second soil core was used for the determination of gross rates of microbial N turnover after compositing and sieving samples for single plots.Also the third soil core was composited at the plot level and sieved for analysis of extractable concentrations of inorganic N,dis-solved organic nitrogen(DON),dissolved organic carbon(DOC),microbial biomass C and N,and pH values.Gross rates of ammonification,nitrificationand microbial N immobilizationGross rates of ammonification,nitrification and microbial immobilization were determined using a 15N pool dilution technique described in more detail by Dannenmann et al.(2009).We decided to use a sieved soil technique,as the stone content of the soil hampered sufficient homogenous15N injection into intact soil cores.Three days before the start of the experiment,the still intact soil samples were pre-incubated at the in situ during sampling deter-mined soil temperature(10°C).Immediately before 15N application,soil was removed out of the cores and roots,gravel and other coarse materials were removed by carefully breaking the intact soil sample portions by hand prior to sieving(5mm mesh width).Soil samples were composited for single plots.Mechanical disruption of the soil was mini-mized as far as possible.Two subsamples(230g sieved soil each)were labelled with7ml30%15N-enriched KNO3solution(for determination of gross nitrification rates)or7ml30%15N-enriched (NH4)2SO4solution(for determination of gross ammonification rates),respectively(time t0).The subsamples were spread in a thin layer and then the 15N label solution was sprayed homogenously on the samples.The amount of added N corresponded to1μg N g−1sdw.While aliquots of180g of the subsamples were transferred into six250ml plastic bottles(Carl Roth GmbH,Karlsruhe,Germany)(30g each),the residual soil was used for determi-nation of the gravimetric water content.The plastic bottles were incubated in the dark at10°C.At time t1(=t0+24h)and time t2(=t0+48h)soil in three of the bottles was extracted with1M KCl,respectively (Dannenmann et al.2006).Subsamples of the filtrate were passed through0.45μm syringe-filters and immediately frozen until colorimetrical measurement of NH4+and NO3-concentrations by a commercial laboratory(Dr.Janssen,Gillersheim,Germany).The diffusion method was used for trapping NH4+or NO3-as NH3on acid traps made of ashless paper filters(Brooks et al.1989).The14/15N-ratio of the N captured on the dried filter papers was analyzed using an elemental analyzer(EA1110, Carlo Erba Instruments,Milan,Italy)coupled to a mass spectrometer(MAT Delta Plus,Thermo Finnigan,Bremen,Germany).Gross ammonification and gross nitrification rates were calculated using the equations given by Kirkham and Bartholomew (1954).Microbial immobilization of NH4+was calculated by subtracting nitrification rates from NH4+consumption rates(Davidson et al.1992). This approach underestimates NH4+immobilization when there is heterotrophic nitrification(direct oxidation of organic substrate to NO3-).Overestima-tion of NH4+immobilization can occur by substrate-stimulation of NH4+consumption in the15NH4+ treatment,while the subtracted nitrification rate calculated from15NO3-pool dilution is not affected by substrate stimulation.Here,we tried to minimize experiment-inherent substrate stimulation by mini-mizing NH4+bel application increased ambient NH4+pools by only36%and56%in soils of burned and unburned plots,respectively.Nitrate immobilization was calculated by subtracting deni-trification rates(see below)from NO3-consumption rates.Physical and chemical soil parametersFor the determination of mineral N concentrations, 30g of unlabelled soil free of limestone and roots was extracted with1M KCl solution and analyzed for NH4+and NO3-concentrations as described above (see Dannenmann et al.2006).Soil pH values (0.01M CaCl)were measured with three subsamples of every plot by use of a combined electrode as described by Dannenmann et al.(2007).Denitrification:simultaneous measurement of N2 and N2O emissions from seven intact soil cores Dinitrogen and N2O emissions from intact soil cores were measured by use of the helium gas flow soil core method as described by Butterbach-Bahl et al. (2002)and Dannenmann et al.(2008).This method is based on the exchange of the soil and headspace atmospheres by a helium-oxygen atmosphere con-taining only25PPM N2and the subsequent simultaneous automated detection of N2O and N2 concentration changes in the headspace above the cores by use of an electron capture detector(ECD) for N2O and a pulse discharge helium ionization detector(PDHID)for N2(Fig.1).In order to facilitate the application of the method to shallow soils and in order to improve the spatial resolution of the measurements,we designed a new system for simultaneous measurement of N2and N2O from seven small soil cores.While the general setup of the system including the steering unit,automated flushing of soil cores and headspace,automated sampling and the detection technique and conditions for N2and N2O(see Fig.1)were the same as described by Butterbach-Bahl et al.(2002),two new incubation cuvettes were designed.The new incuba-tion cuvette facilitated the simultaneous flushing of seven soil cores(height4cm,100cm3volume each) via the porous porcellaine plates at the bottom of the soil cores,while the cuvette described by Butterbach-Bahl et al.(2002)contained only one soil core of20cm height and12.5cm diameter)(Fig.2). The soil cores are automatically pressed into the fittings sealed via O rings when closing the cuvette to ensure that He purge gas flow is taking place from bottom to top in the soil cores(Fig.2).Also with the new cuvette,the same huge constructive efforts were made to facilitate an extremely gastight system and hence avoid diffusion of atmospheric N2into the system(Butterbach-Bahl et al.2002), e.g.the cuvette had double sealings which were additionally purged with He(Fig.2),and He leakage tests were performed.The smaller size of the new cuvette compared to the version described by Butterbach-Bahl et al.(2002)allowed to further improve the gastightness of the system by placing the whole incubation cuvette including fittings of the gas tubings under water in a water bath.The water surrounding the cuvette is also used for the regula-tion of the incubation temperature.Based on this setup,no significant increase in N 2concentrations in the cuvettes was found during 8h when the system was run with an empty cuvette.Here,the soil cores (soil moisture 19or 21.4%sdw for burned or unburned soil samples;incuba-tion temperature:10°C for both treatments)were flushed for 72h to quantitatively remove N 2from the soil and headspace atmospheres.Subsequently,an artificial headspace atmosphere was created (80%He,20%O 2,25PPM N 2,400PPB N 2O)and the concentration change of N 2and N 2O in the two cuvettes was monitored automatically for 8h on hourly basis according to Butterbach-Bahl et al.(2002).Every sample air analysis was accompanied by 6automated calibration gas measurements at the gas chromatographs.For each treatment (burned/unburned),3measurements with 7soil cores were performed.Flux rates were calculated from the linear change in N 2and N 2O concentrations in theheadspace as described by Butterbach-Bahl et al.(2002).After every measurement,soil water content and soil dry weight of the incubated soil were deter-mined.Denitrification was calculated as the sum of N 2O plus N 2fluxes and related to a soil dry weight basis.Microbial biomass C and NMicrobial biomass C and N was determined by use of the chloroform fumigation-extraction technique (Brookes et al.1985).For this purpose,soil from seven soil cores was pooled at the plot level and sieved.Subsequently three subsamples of 30g were immediately extracted with 60ml 0.5M K 2SO 4,while three subsamples were fumigated with Chloroform vapour for 24h.Fumigated samples were extracted in a similar way like control samples.Total chemically bound nitrogen (TNb)andtotalFig.1Schematic representation of the measuring system used to simultaneously quantify N 2and N 2O emissions from seven intact soil cores.PDHID:Pulse Discharge Helium Ionization Detector;ECD:Electron Capture Detectororganic Carbon (TOC)were analyzed by use of a chemoluminescence detector for TNb analysis coupled to the TOC analyzer (Dannenmann et al.2006).Correction factors (0.54for microbial biomass N and 0.379for microbial biomass C,(Brookes et al.1985;Vance et al.1987)were applied to the difference in TNb and TOC between paired untreated and fumigated subsamples to estimate microbial biomass C and N.TOC values of the extracts of unfumigated control samples are referred to as extractable DOC concentrations.StatisticsTest for significant differences of the determined parameters between control and burning treatment were made by means of the Mann Whitney u-test using plots as statistical units (N =3).Also corre-lation analysis was performed using plot means from both burned and unburned treatments.All statistical analyses were performed with SPSS 10.0(SPSS Inc.,Chicago,USA)and Microcal Origin7.0.Fig.2Newly designed incubation vessel for simultaneous measurements of N 2and N 2O emissions from seven small intact soil cores (4cm height,100cm 3volume each)after purging with He/O.All soil cores are purged from bottom to top with He/O mixture.Double outside sealings are used which are additionally purged with He.Furthermore,the wholeincubation vessel is placed for purging and measuring under water in a water bath to finally reach gas tightness.After three days of purging,fully automated hourly measurements of N 2and N 2O concentrations in the headspace were conducted over 8h.The system contains two vesselsResults Soil parametersHalf a year after burning,the remaining ash and charcoal layer at the burned plots was one order magnitude smaller compared to the organic layer at the control plots (Table 1).Mineral soil pH was 7.5and slightly (7.53versus 7.45)but significantly higher at the burned plots (Table 1).Soil moisture content in the mineral soil was significantly lower at the burned plots as compared to the control plots.Both microbial biomass C (Table 1)and N (Fig.3)were significantly lower in the Ah horizon of the burned plots.However,no difference in the microbial C:N ratio was found between burned and unburned plots.In contrast to microbial biomass,extractable DOC was found to be significantly higher at the burned plots (Table 1).Gross rates of N turnoverGross ammonification was nearly four times higher at burned plots than at unburned plots (Fig.3).However,extractable soil NH 4+concentrations were approximately only 50%higher.Microbial NH 4+immobilization was threefold higher at the burned plots than at the control plots (Fig.3).Gross nitrification was more than twofold larger at burned plots than at control plots (Fig.3).Extractable soil NO 3-concentrations in the mineral soil of the burned plots were thirteen times the concentrations of soil NO 3-in the mineral soil horizon of the control plots (Fig.3).At burned plots,the amounts of extracted soil NO 3--N equaled the amounts of extracted soil NH 4+-N concentrations.In contrast,soil NO 3-con-centrations were considerably lower than soil NH 4+concentrations at unburned control plots.Microbial NO 3-immobilization was not significantlydifferentFig.3N turnover [mg N kg −1sdw day −1]and N pools [mg N kg −1sdw]in the Ah layer of unburned and burned plots.SON:soil organic nitrogen.A:gross ammonification;B:gross nitrification;C:microbial NH 4+immobilization;D:microbial NO 3-immobilization;E:N 2O flux;F:N 2flux.Different indices indicate significant differences between control and burned plots.Errors represent standard errors of the meanTable 1Soil parameters litter/ash mass parameters are given for the Ah layer.Errors represent standard errors of the mean calculated from N =3plots.DOC:dissolved organic carbon.MBC:microbial biomass carbon;MBN:microbial biomass nitrogen.Different indices indicate significant differences between burned and unburned plots litter/ash mass[g m −2]soil moisture [%sdw)pHDOC[mg C kg −1sdw]MBC[mg C kg −1sdw]MBC/MBN [ratio]control 617±86a 21.4±0.5a 7.45±0.02a 134±3a 1844±112a 15.3±0.2burned72±6b19.0±0.4b7.53±0.03b160±14b1380±143b15.9±1.31Soil parameters for control and burned plots.Except for litter/ash mass parameters are given for the Ah layer.Errors represent standard errors of the mean calculated from N =3plots.DOC:dissolved organic carbon.MBC:microbialbiomass carbon;MBN:microbial biomass nitrogen.Different indices indicate significant differences between burned and unburned plotsbetween control and burned plots and overall several fold lower than microbial NH 4+immobilization.Relative N retention,i. e.(microbial NH 4+immobilization +microbial NO 3-immobilization)/(gross ammonification +gross nitrification),was significantly lower at the burned plots than at the control plots (Table 2).This was caused in particular by an increase in gross nitrification which was not outbalanced by a concomitant increase in microbial NO 3-immobilization.Soil N 2O and N 2measurements revealed that dinitrogen was the dominant end product of denitri-fication both at control and burned plots (Fig.3).However,N 2emissions were more than one magni-tude larger at unburned control plots (94±2μg N kg -1sdw day −1)than at burned plots (4±2μg N kg −1sdw day −1).At the control plots,there was significant net uptake of N 2O of approx.1μg N kg −1sdw day −1,while at the burned plots N 2O fluxes were not significantly different from zero.At control plots,N 2O uptake was approximately two orders of magni-tude smaller than N 2emission.Due to the dominance of N 2as the end product,denitrification rates equalled N 2emissions.For burned plots,denitrification amounted to less than 1%compared to the other processes of N turnover.However,for the unburned plot denitrification was a significant sink process for microbial N cycling.Here,denitrification was on average 16%of ammonification,20%of nitrification and 98%of microbial NO 3-immobilization at the unburned control plots (Table 2).Correlation analysesSoil microbial biomass N was negatively correlated with gross ammonification (R =−0.91,p =0.01)and gross nitrification (R =−0.83,p =0.04)and net soil-atmosphere N 2O flux (R =−0.84,p =0.04)(i.e.positively correlated with net N 2O uptake rate)but positively correlated with soil water content (R =0.93,p =0.006),and N 2emission rate (R =0.94,p =0.005).Gross ammonification was positively correlated with independently determined soil NH 4+concentrations of unlabelled soil (R =0.885,p =0.02)as well as gross nitrification with soil NO 3-concentrations (R =0.98,p <0.001).DiscussionFire effects on gross rates of N turnoverFire has been shown to potentially alter a wide range of physical,biological and chemical soil parameters like soil organic matter quanity and quality (variable effects of fire across soil horizons),pH values (increase),nutrient availability (increase),and soil microbial biomass (decrease)(Marion et al.1991;Castaldi and Aragosa 2002;Certini 2005;Knicker 2007).The recovery of these effects is mainly depend-ing on plant recolonization (Certini 2005).However,little information is available on postfire effects on actual gross rates of N turnover in Mediterranean soils.In this study we show that 6months after burning,when vegetation re-growth already had started,gross ammonification and gross nitrification were considerably larger in the Ah horizon soil of the burned plots than in soil of unburned plots.As microbial immobilization,in particular of NO 3-,could not compensate for the increase in inorganic N production,inorganic N concentrations were higher and relative microbial nitrogen retention were smaller at burned plots.Since soil moisture values at burned plots were significantly lower as compared to un-burned plots (Table 1),the stimulation of microbial N turnover must have been due to the increased availability of organic substrates and mineral N due to burning of the vegetation (Andersson et al.2004a ,b ;Knicker 2007).This interpretation is supported by our measurements on higher DOC concentrations inretention:(microbial NH 4+immobilization +microbial NO 3-immobilization)/(gross ammonification +gross nitrification).Errors represent standard errors of the mean Relative N retentionDenitrification/ammonification Denitrification/nitrification Denitrification/NO 3-immobilization control 0.96±0.11a 0.16±0.02a 0.20±0.02a 0.98±0.65a burned0.66±0.12b0.002±0.001b0.003±0.002b0.002±0.001bTable 2Relative importance of microbial immobilization and denitrification versus inorganic N production.Relative Nretention:(microbial NH 4+immobilization +microbial NO 3-immobilization)/(gross ammonification +gross nitrification).Errors represent standard errors of the meanthe mineral soils at burned plots(Table1).Our findings on higher inorganic N concentrations in the uppermost mineral soil horizon are in agreement with earlier fire studies in Mediterranean shrublands (Marion et al.1991;Castaldi and Aragosa2002). Besides increased N turnover and reduced microbial immobilization also lower plant competition for mineral N at burned plots may have additionally contributed to the increase in mineral N.It has been assumed that net N ammonification and nitrification rates in Mediterranean shrublands are low because of the quality of the typical sclerophyllus leaf and because of leaching of allelopathic compounds from plants(Scalbert1991;Gallardo and Merino 1992;Castaldi et al.2009).As fire may destroy allelopathic compounds,the increased gross rates of N turnover at the burned plots observed here,could also be caused by a release of inhibiting plant effects on microbial N turnover(Castaldi and Aragosa2002).Only little studies investigated fire effects on gross rates of soil N turnover while,to our knowledge,there is no study conducted in a comparable ecosystem like investigated here.Bastias et al.(2006)reported minor but significant reduction of gross ammonification by 16%and gross nitrification by12%three weeks after a fire in the soil of a wet sclerophyll forest of Australia,subjected to high frequencies of prescribed burning.LeDuc and Rothstein(2007)did not observe significant effects of wildfire neither on gross pro-duction nor on immobilization of both ammonium and nitrate3–6years after burning of a jack pine forest in Michigan,USA.Anderson and Poth(1998) explained increased NH4+concentrations in Brazilian cerrado soils within the first2months after experi-mental fires by a stimulation of gross ammonification while gross nitrification was suppressed by burning. In contrast,addition of wildfire-produced charcoal to soil sampled in ponderosa pine forests of Montana, USA,strongly promoted gross nitrification,probably due to absorption of phenolic compounds which inhibited gross nitrification(DeLuca et al.2006).It remains unclear whether such variable responses of gross N turnover to fire are caused by variable responses of N cycling across ecosystems,different fire intensities or-frequencies and time elapsed between the burning and sampling events,or simply result from limited temporal resolution,which is characterizing almost all studies on gross rates of N turnover.In our study,the microbial biomass C and N pools were positively correlated with denitrification, i.e.smaller at burned plots but negatively correlated with gross rates of ammonification and nitrification. Microbial biomass is responsible for both production and consumption of inorganic N.Furthermore,it can serve as a substrate for N ammonification itself following microbial dieback due to drought events (Borken and Matzner2008).Therefore,the relation-ships between microbial biomass and rates of N turnover may be variable in time.The observed reduction in soil microbial biomass at the burned plots may be explained by generally more extreme environmental conditions at the burned plots,as the missing shadowing effect of vegetation as well as the dark ash may have lead to higher temperature fluctuations and quicker drying of the soil at the burned plots.However,lower microbial biomass may also be interpreted by retarded long-term recovery of soil microbes after fire(Castaldi and Aragosa2002),e.g. in association with a decreased rhizodeposition of labile C compounds by roots.However,larger DOC concentrations at the burned plots(Table1)do not support the latter hypothesis.Furthermore,the similar microbial C:N ratios in burned and unburned soil (Table1)do not indicate a fire-induced shift in microbial community composition,i.e.a promotion of fungi with a higher C:N ratio at the expense of bacteria with a lower C:N ratio.Still,there could have been an altered abundance of functional microbial groups which was not reflected in the microbial C:N ratio.Despite both gross rates of ammonification and nitrification as well as soil NO3-concentrations were higher in soil of burned plots,denitrification rates were considerably larger at unburned control plots(Fig.3).This may—analoguously like fire effects on microbial biomass—be explained by significantly decreased soil water content at burned plots(Table1),given that denitrification is a predominantly anaerobic process(Conrad1996). However,these differences in soil moisture were low,i.e.soil moisture was approximately10%lower at burned burned plots than at control plots only (Table1).Furthermore,soil moisture was at compa-rably low level both at control and burned plots (21.4and19.0%of soil dry mass).Lower denitrifi-cation at burned plots could be also a consequence of reduced rhizodeposition of labile C compounds,。