Selective adsorption of phosphate from seawater and wastewater by amorphoUS zirconium hydroxide
- 格式:pdf
- 大小:216.53 KB
- 文档页数:8

化学及化工专业词汇英语翻译(P-Z)3reversibility 可逆性reversible cell 可逆电池reversible change 可逆变化reversible colloid 可逆胶质reversible electrode 可逆电极reversible engine 可逆机reversible gel 可逆凝胶reversible process 可逆过程reversible reaction 可逆反应reversible wave 可逆波reversion 硫化还原revivification 复活revolution counter 转数表revolvig tubular kiln 管式转炉revolving furnace 旋转炉revolving screen 旋转筛reynolds number 雷诺数rhamnetin 鼠李亭rhamnose 鼠李糖rhenate 铼酸盐rhenium 铼rhenium compound 铼化合物rhenium dioxide 二氧化铼rheology 龄学rheometer 龄仪rheopexy 震凝rheostat 变阻器rhodamine 若丹明rhodan 硫氰酸氨苯酯rhodan value 硫氰值rhodanate 硫氰酸盐rhodanine 绕丹宁rhodanizing 镀铑rhodanometry 硫氰酸盐滴定法rhodate 铑酸盐rhodinol 玫红醇rhodium 铑rhodium chloride 氯化铑rhodium dioxide 二氧化铑rhodium hydroxide 氢氧化铑rhodium nitrate 硝酸铑rhodochrosite 菱锰矿rhodonite 蔷薇辉石rhodopsin 视玫红质rhombic sulfur 斜方硫rhombic system 正交晶系rhombohedron 菱形体ribbed funnel 线沟漏斗ribitol 阿东醇riboflavin 核黄素ribonuclease 核糖核酸酶ribonucleic acid 核糖核酸ribose 核糖rice oil 米糠油rice starch 米淀粉rich gas 富煤气ricin 蓖麻毒ricinic acid 蓖麻醇酸ricinoleate 蓖麻酸盐rider 游码rigid body 刚体rigid foam 硬质泡沫塑料rigid plastics 硬质塑料rigid polyvinyl chloride 硬聚氯乙烯rigidity 刚性ring 环ring analysis 环分析ring burner 环焰灯ring cleavage reaction 开环反应ring closure 环化ring compound 环状化合物ring current 环形电流ring formation 成环ring isomerism 环异构ring kiln 环形窑ring opening 开环ring opening polymerization 开环聚合rinmann's green 林曼绿ripening 成熟ripening degree 成熟度rivanol 利凡诺river gold 砂金road octane number 路线辛烷值road oil 铺路沥青roaster 焙烧炉roasting 焙烧roasting furnace 焙烧炉roasting kiln 燃烧炉robot 机扑rochelle salt 罗谢尔盐rock candy 冰糖rock crystall 石英rock phosphate 磷酸岩rock salt 岩盐rock wool 石棉rocket propulsion 火箭推进rockwell hardness 罗氏硬度rockwell hardness tester 洛氏硬度试验机rod mill 棒磨机rod sulfur 棒状硫磺rodenticide 杀鼠剂roentgen 伦琴roentgen equivalent man 人体伦琴当量roll 辊子roll crusher 滚碎机roll mill 滚碎机roller mill 滚碎机rolling 轧制roman cement 罗马水泥rongalite 雕白粉roofing tile 屋瓦room temperature 室温room temperature curing method 室温固化法room temperature setting adhesive 室温固化粘合剂root's blower 罗茨式鼓风机rosaniline 蔷薇苯胺rosaniline chlorhydrate 蔷薇苯胺化盐酸rosaniline sulfate 蔷薇苯胺化硫酸rose crucible 罗斯坩埚rose oil 蔷薇油rose water 玫瑰香水rosemary oil 迷迭香油rosenmund reduction 罗森蒙得还原rosin 松香rosin ester 酯屎rosin oil 松香油rosin pitch 松香沥青rosin sizing 上松香胶rosin soap 松香皂rosin spirit 松香精rosin varnish 松香清漆rosolic acid 玫红酸rotamer 旋转异构体rotameter 转子临计rotary crusher 旋转压碎机rotary dryer 旋转干燥器rotary filter 旋转滤器rotary kiln 旋转窑rotary pump 旋转泵rotated dropping mercury electrode 旋转滴汞电极rotating crystal method 旋转晶体法rotating system of coordinates 转动坐标系rotation axis 旋转轴rotation group 旋转群rotation spectrum 转动光谱rotation vibration spectrum 转动振动谱rotational energy 转动能rotational isomer 旋转异构体rotational isomerism 旋光异构rotational level 转动能级rotational quantum number 转动量子数rotational viscometer 旋转粘度计rotenone 鱼藤酮roughness 粗糙度round flask 圆底烧瓶rubber 橡胶rubber cement 橡胶胶水rubber composition 橡胶配合物rubber compound 橡胶配合物rubber dispersion 橡胶分散液rubber dough 橡胶胶水rubber fabric 胶布rubber gum 屎脂rubber industry 橡胶工业rubber insulation 橡胶绝缘rubber isomer 橡胶异构体rubber latex 胶乳rubber like elasticity 似橡胶弹性rubber lining 橡皮衬里rubber resin 橡胶尸rubber solvent 橡胶溶剂rubber sponge 海绵橡胶rubber stopper 橡皮塞rubber substitute 油膏rubber thread 橡胶线rubber tube 橡皮管rubbing 磨损rubbing oil 摩擦用油rubeanic acid 红氨酸rubidium 铷rubine number 红玉数ruby 红玉ruff degradation 鲁弗降解rufol 绛醇rupture 破裂rust 锈rust inhibitor 抗腐蚀添加剂rust proof paint 防锈涂料rut preventives 抗腐蚀添加剂ruthenic chloride 四氯化钌ruthenium 钌ruthenium chloride 氯化钌ruthenium compound 钌化合物ruthenium dichloride 二氯化钌ruthenium tetrachloride 四氯化钌rutile 金红石rutin 芸香苷s acid s 酸sabadilla 赛藜芦sabinane 桧烷sabinene 桧萜saccharase 转化酶saccharate 蔗糖盐saccharic acid 蔗质酸saccharides 糖类saccharification 糖化saccharimeter 糖量计saccharimetry 糖量测定saccharin 糖精saccharometry 糖量测定saccharomyces 酵母属saccharose 蔗糖saddening 煤染固着处理safe handling 安全操作safe operating area 安全运转区域safety 安全safety device 保护设备safety engineering 安全工程safety explosive 安全炸药safety factor 安全率safety film 安全软片safety fuel 安全燃料safety glass 安全玻璃safety lamp 安全灯safety match 安全火柴safety technique 安全工程safety valve 安全阀safflower oil 红花油saffron 擦粉safranal 藏花醛safranine 藏红safrole 黄樟素saggar 烧箱sagger 烧箱sal soda 苏打晶体salad oil 生菜油salicin 水杨甙salicyl alcohol 水杨醇salicylaldehyde 水杨醛salicylamide 水杨酰胺salicylate 水杨酸盐salicylic acid 水杨酸saligenin 水杨苷saligenol 水杨醇salimeter 盐液密度计salimetry 盐度测量法salina 制盐所salinity 盐浓度salipyrine 萨利比林salivary gland hormone 唾液腺激素salmine 鲑精朊salol 萨罗salsoline 萨苏林salt 盐salt bath 盐浴salt bath furnace 盐浴炉salt bridge 盐桥salt effect 盐效应salt error 盐误差salt glaze 盐釉salt grainer 成盐蒸发器salt mine 盐矿salt of sorrel 草酸氢钾salt shrinking 盐缩salt spray cabinet 盐雾室salt spray test 盐雾试验salt water 盐水salt works 制盐所saltern 制盐所salting 盐腌salting out effect 盐析效应saltpeter 硝石salvage 废弃物salvaged material 废弃物salvarsan 塞佛散samarium 钐samarium poisoning 钐中毒samarskite 铌钇矿sample 样品sample bottle 样品瓶sample solution 样品溶液sampler 采样器sampling 取样sampling method 取样方法sand blast 砂喷sand cracking 砂子炉裂解sand culture 沙基培养sand filter 砂滤器sand lime brick 石灰砂粒碳sand paper 砂纸sandalwood oil 檀木油sandarac 山达尸sandbath 砂浴sandmeyer reaction 散得麦尔反应sandpaper 砂纸sandstone 砂岩sandwich moulding 夹层模塑sandwich structure 夹层结构sanforizing 防缩处理sanitary chemistry 卫生化学sanitary ware 卫生陶器sanitizer 消毒剂santalol 檀香醇santalyl acetate 乙酸檀香酯santonin 山道年santoninic acid 山道年酸saponarin 皂草苷saponated cresol solution 煤酚皂溶液saponid 合成洗涤剂saponifiability 皂化性saponification 皂化saponification equivalent 皂化当量saponification number 皂化值saponification value 皂化值saponifier 皂化剂saponifying agent 皂化剂saponin 皂荚苷sapphire 蓝宝石sapphire whisker 氧化铝纤维saran 偏氧纶sarcolysine 溶肉瘤素sarcosine 肌氨酸sardine oil 沙丁鱼油sarkomycin 肉瘤霉素sassafras oil 黄樟油sassoline 天然硼酸satellite 伴线satin spar 纤维石膏satin white 缎白saturated acid 饱和酸saturated activity 饱和活性saturated calomel electrode 饱和甘汞电极saturated compound 饱和化合物saturated fatty acid 饱和脂肪酸saturated hydrocarbon 饱和烃saturated solution 饱和溶液saturated vapor pressure 饱和蒸汽压saturation 饱和saturation curve 饱和曲线saturation point 饱和点saturation temperature 饱和温度saturator 饱和器sawdust 锯屑saxin 糖精saybolt chromometer 赛波特比色计saybolt viscometer 赛氏粘度计scale 标度scale beam 秤杆scale coefficient 生垢因数scale pan 天平盘scale up 尺度放大scaling 剥落scaling factor 污垢因子;缩尺因数scaling law 相似律scaly structure 鳞状构造scandium 钪scanning acoustic microscope 扫描声学显微镜scarlet 深红色scattered spectrum 散射光谱scattering 散射scattering coefficient 散射系数scattering cross section 散射截面scattering of light 光散射scattering plane 散射面scavenger 净化剂scent 气味scheele's green 许雷绿scheelite 白钨矿schist 片岩schmidt number 施密特数schradan 八甲磷schulze hardy's rule 舒尔茨哈代规则schungite 半石墨schweinfurth green 巴黎绿scintillation 闪炼scintillation counter 闪炼计数管sclerometer 硬度计scleroprotein 硬朊scopolamine 莨若胺scopoline 莨若林scorching 早期硫化scorification 熔融法scorifier 试金坩埚scouring 精练scouring agent 精练剂scouring soap 擦洗皂scrap paper 废纸scrapped rubber 废橡皮screen 筛子screen analysis 筛析screen printing 筛网印花screening 筛分screening agent 遮蔽剂screening constant 屏蔽常数screening effect 屏蔽效应screening machine 筛选机screw compressor 螺旋式压缩机screw conveyor 螺旋运输器screw feeder 螺旋进料机screw mixer 螺旋式混合机scrubber 煤气洗涤器scrubbing 涤气scrubbing bottle 洗气瓶scrubbing tower 涤气塔scum 浮渣scummer 消泡剂sea floor 海底扩张说sea salt 海盐sea water 海水sea water magnesia 海水镁sealant 嵌缝填料sealed tube 封闭管sealing compound 嵌缝填料sealing wax 密封蜡seaweed 海藻sebacate 癸二酸盐sebacic acid 癸二酸sebaconitrile 癸二腈second class ore 进厂矿石secondary accelerator 辅助促进剂secondary air 次级空气secondary alcohol 仲醇secondary amine 仲胺secondary battery 次级电池secondary carbon atom 仲碳原子secondary cell 次级电池secondary electron 二次电子secondary plasticizer 次级增塑剂secondary reaction 副反应secondary valence 副价secondhand energy 二次能源secretin 肠促胰液素secretion 分泌sectional titration 分区滴定sectrometer 真空管滴定计sediment 沱殿物sedimentary rock 沉积岩sedimentation 沉积酌sedimentation analysis 沉淀分析sedimentation balance 沉降天平sedimentation coefficient 沉降系数sedimentation constant 沉降常数sedimentation equilibrium 沉降平衡sedimentation velocity 沉降速度sedimentation volume 沉降容积sedoheptulose 景天庚酮糖seed crystal 晶种seed disinfectant 种子消毒剂seed fiber 种子纤维seed grain 晶种seepage 渗透segregation 偏析seignette salt 罗谢尔盐selection rule 选择定则selective absorption 选择吸收selective adsorbent 选择性吸附剂selective adsorption 选择吸附selective corrosion 选择腐蚀selective extraction 选择提取selective fermentation 选择发酵selective flotation 选择浮选selective permeability 选择渗透性selective polymerization 选择聚合selective reaction 选择反应selective reagents 选择试剂selectivity 选择性selectivity coefficient 选择系数selectivity factor 选择系数selenate 硒酸盐selenic acid 硒酸selenic anhydride 硒酐selenide 硒化物selenite 亚硒酸盐selenium 硒selenium cell 硒光电池selenium oxide 氧化硒selenium rectifier 硒整流selenochemistry 月球化学selenone 硒砜selenonic acid 硒代磺酸selenous acid 亚硒酸self absorption 自吸收self baking electrode 自动烧结电极self catalysis 自催化酌self cleaning 自净self coagulation 自凝聚self decomposition 自分解self discharge 自动放电self filtering 自滤selfenergy 自能selfextinguishing effect 自动灭火效应selfignition 自燃selfignition temperature 自燃温度selfluminous pigment 自发光颜料semi coking tar 低温焦油semiacetal 半缩醛semiautomatic electroplating 半自动电镀semiautomatic machine 半自动机器semibatch chemical reactor 半连续化学反应器semibituminous coal 半烟煤semicarbazide 氨基脲semicarbazide hydrochloride 氨基脲化氢氯semicarbazone 半卡胞腙semicellulose 半纤维素semichemical pulp 半化学纸浆semicoke 半焦炭semicolloid 半胶体semiconductor 半导体semiconductor rectifier 半导体整流semidine 半联胺semidry process 半干法semidrying oil 半干性油semiebonite 半硬质胶semifinished product 半成品semihard rubber 半硬橡胶semihydrate 半水合物semimetal 半金属semimicro analysis 半微分析semimicro balance 半微量天平seminose 甘露糖semioxamazide 氨基草酰阱semipermeability 半透性semipermeable membrane 半透膜semiplastic explosive 半塑性炸药semipolar bond 半极性键semipolar linkage 半极性键semiporcelain 半瓷器semiquinone 半醌semirigid plastics 半刚性塑料semisilica brick 半硅砖semisolid 半固体semisynthetic fiber 半合成纤维semitransparency 半透明semiwater gas 半水煤气senarmontite 方锑矿sensibility 感度sensible heat factor 显热因子sensible heat flow 可感热流显热流sensitive emulsion 感光乳剂sensitive layer 感光层sensitive paper 感光纸sensitivity curve 灵敏度曲线sensitivity limit 灵敏度限度sensitivity speck 敏化中心sensitization 敏化sensitized fluorescence 增感发光sensitizer 敏化剂sensitizing dye 敏化染料sensitometer 感光计sensitometry 感光度测定sensor 感受器separating funnel 分液漏斗separating power 分离能力separation 分离separation coefficient 分离系数separation of isotope 同位素分离separator 分离器sepia 乌贼墨颜料septic matter 腐败物septic poison 腐败毒sequestering agent 掩蔽剂sericin 丝胶朊sericite 绢云母series connection 串联series of spectral line 光谱系serine 丝氨酸seromycin 环丝氨酸serotonin 血清素serpentine 蛇纹石serum 血清serum albumin 血清蛋白serum globulin 血清球蛋白servomechanism 伺服机构sesame oil 芝麻油sesquiterpene 倍半萜烯setting 凝结setting agent 硬化剂setting point 凝固点setting temperature 加硫setting time 凝结时间settlement 沱殿物settler 沉淀箱settling 沉淀settling chamber 沉淀室settling curve 沉降曲线settling ratio 沉降比settling vat 沉降桶settling velocity 沉降速度sewage 污水sewage disposal 污水处理sewer gas 下水道气体sex hormone 性激素shaft furnace 竖式炉shaking 摇动shaking apperatus 摇动机shaking machine 摇动机shaking screen 振动筛shaking sieve 振动筛shale 页岩shale oil 页岩油shape factor 形状系数shape memory alloy 形状记忆合金shaping 成形shaving cream 刮脸膏shaving powder 刮脸粉shaving soap 刮脸皂shear modulus 剪切模量shear rate 剪切速率shear resistance 剪切阻力shear strength 剪切强度shearing 剪shearing stress 剪应力sheet 薄板sheet glass 板玻璃sheet rubber 生胶片shelf burner 搁板炉shelf life 贮藏寿命shell and tube condenser 列管式冷凝器shell and tube heat exchanger 列管式换热器shell model 壳模型shell structure 壳结构shellac 虫胶shellac varnish 虫胶清漆sherardizing 粉镀锌法sherwood number 舍伍德数sherwood oil 石油醚shift reagent 变位试剂shikimic acid 莽草酸shikonin 紫草醌ship bottom paint 船底涂料shock 碰撞shock absorber 冲稽收器shore hardness 肖氏硬度short fiber 短纤维short mash process 短时间糖化法short oil varnish 短油清漆short period 短周期shot capacity 注塑容量shrinkage 收缩shrinkage allowance 收缩容许量shrinkage crack 缩裂shrinkage limit 收缩限度shrinkage water 收缩水shrinking percentage 收缩率shutter test 快门试验sialic acid 唾液酸sialomucin 唾液酸粘蛋白siccative 干燥剂side chain 侧链side chain isomerism 侧链异构side fermentation 副发酵side reaction 副反应siderite 菱铁矿siderophile element 亲铁元素sienna 富铁黄土sieve 筛子sieve analysis 筛析sieve residue 筛渣量sieve shaker 振动筛sieve tray 筛盘sieve tray column 筛盘塔sieving 筛分sieving machine 筛选机sigma bond 结合signal 信号signal lamp 信号灯signal oil 信号油silane 硅烷silane coupling 硅烷耦合silanol 硅烷醇silent discharge 无声放电silica 硅石silica brick 硅砖silica gel 硅胶silica glass 石英玻璃silica modulus 硅酸率silica sand 石英砂silican 甲硅烷silicate 硅酸盐silicate paint 硅胶漆silication 硅化酌siliceous slag 硅土矿渣silicic acid 硅酸silicic acid anhydride 二氧化硅silicide 硅化物silicification 硅化酌silicochloroform 硅氯仿silicoethane 乙硅烷silicofluoride 氟硅化物silicomethane 甲硅烷silicon 硅silicon bromide 溴化硅silicon carbide 碳化硅silicon chloride 氯化硅silicon dioxide 二氧化硅silicon fluoride 氟化硅silicon hydride 硅氢化合物silicon nitride 氮化硅silicone 硅酮silicone grease 硅润脂silicone oil 硅酮油silicone resin 硅酮尸silicone rubber 硅酮橡胶silicosis 硅肺silicotungstic acid 硅钨酸silk 绸silk glue 丝胶朊sillimanite 硅线石siloxane 硅氧烷siloxene 硅氧烯silver 银silver acetate 醋酸银silver acetylide 乙炔银silver amalgam 银汞剂silver arsenite 亚砷酸银silver azide 叠氮化银silver bromide 溴化银silver carbide 碳化银silver carbonate 碳酸银silver chloride 氯化银silver chloride cell 氯化银电池silver chromate 铬酸银silver compound 银化合物silver cyanide 氰化银silver fluoride 氟化银silver foil 银箔silver fulminate 雷酸银silver halide 卤化银silver iodide 碘化银silver leaf 银箔silver mirror test 银镜试验silver nitrate 硝酸银silver oxide 氧化银silver plating 镀银silver protein 蛋白银silver salt 银盐silver selenide 硒化银silver telluride 碲化银silvering 镀银simazin 侮三嗪similar transformation 相似变换similarity 类似性simple cell 初基胞simple distillation 粗蒸馏simple elongation 简单伸长simple lattice 初基点阵simple protein 简单蛋白质simple substance 单质simulation 模拟simultaneous reaction 联立反应single bath process 一浴法single bond 单键single crystal 单晶single electrode 单电极single electrode potential 单电极电位single fiber 单根纤维single fluid cell 单液电池single phase system 单相系single screw extruder 单螺杆压出机singular equilibrium 独平衡singular point 奇点sintered diamond 烧结金刚石sintering 烧结sintering furnace 烧结炉sintering machine 矿石烧结机siphon 虹吸管sisal 剑麻sitosterol 谷甾醇six membered ring 六元环size 胶水sizing 上胶sizing agent 上浆剂skatole 粪臭素skein dyeing 绞纱染色skeletal vibration 骨架振动skid defied tire 防滑轮胎skim milk 脱脂乳skimmer 消泡剂skin effect 囚效应skin friction 表面摩擦slack coal 粉煤slack wax 疏松石蜡slag 炉渣slag brick 矿渣砖slag cement 矿渣水泥slag wool 渣棉slaked lime 消石灰slant culture 斜面培养slate 石板slime 稀矿泥slime bacteria 粘液细菌sling psychrometer 摇动湿度计slip 泥浆slit 缝隙slit ultramicroscope 缝隙超显微镜slow accelerator 缓动促进剂slow combustion 缓慢燃烧slow match 导火线slow oxidation 缓慢氧化slowing down 减速sludge 泥渣sludge acid 淤渣酸slurry 泥浆slushing oil 防锈油smalt 大青玻璃粉smaltite 砷钴矿smectic phase 碟状液晶分子相smelt 熔融smelting 冶炼smelting furnace 熔炉smithsonite 菱锌矿smoke agent 烟雾剂smoke point 烟点smoke screen 烟幕smokeless combustion 无烟燃烧smokeless fuel 无烟燃料smokeless powder 无烟火药smokeless propellant 无烟推进剂snapshot 快摄照片sni mechanism sni机构soakage 浸透soaking 浸透soaking drum 浸水转鼓soap 皂soap builder 肥皂配合剂soap flakes 皂片soap kettle 皂煮锅soap powder 皂粉soap spent lye 皂液soda 苏打soda ash 苏打灰soda feldspar 钠长石soda lime 碱石灰soda lime glass 钠钙玻璃soda lye 苛性钠溶液soda microcline 钠斜微长石soda nitre 天然硝石soda pulp 碱纸浆soda soap 钠皂sodalite 方钠石sodation 碳酸钠去垢sodium 钠sodium acetate 醋酸钠sodium alum 钠矾sodium aluminate 铝酸钠sodium amalgam 钠汞齐sodium amide 氨基钠sodium arsenate 砷酸钠sodium arsenite 亚砷酸钠sodium azide 叠氮化钠sodium bicarbonate 碳酸氢钠sodium bichromate 重铬酸钠sodium bismuthate 铋酸钠sodium bisulfate 硫酸氢钠sodium bisulfite 亚硫酸氢钠sodium borohydride 氢化硼钠sodium bromate 溴酸钠sodium bromide 溴化钠sodium carbonate 碳酸钠sodium chlorate 氯酸钠sodium chloride 氯化钠sodium chloroplatinate 氯铂酸钠sodium chromate 铬酸钠sodium citrate 柠檬酸钠sodium cobaltic nitrite 亚硝酸钴钠sodium cyanide 氰化钠sodium dichromate 重铬酸钠sodium dihydrogenphosphate 磷酸二氢钠sodium dithionite 亚硫酸氢钠sodium ethoxide 乙醇钠sodium ethylate 乙醇钠sodium ferricyanide 铁氰化钠sodium fluoride 氟化钠sodium fluoroacetate 氟醋酸钠sodium fluoroethanoate 氟醋酸钠sodium formate 甲酸钠sodium glutamate 谷氨酸钠sodium hydride 氢化钠sodium hydrogencarbonate 碳酸氢钠sodium hydrogensulfate 硫酸氢钠sodium hydrosulfite 亚硫酸氢钠sodium hydroxide 氢氧化钠sodium hypochlorite 次氯酸钠sodium hypophosphite 次磷酸钠sodium iodide 碘化钠sodium metaphosphate 偏磷酸钠sodium metasilicate 偏硅酸钠sodium molybdate 钼酸钠sodium nitrate 硝酸钠sodium nitride 一氮化三钠sodium nitrite 亚硝酸钠sodium nitroprusside 硝普钠sodium oxalate 乙二酸钠sodium oxide 氧化钠sodium perborate 过硼酸钠sodium perchlorate 高氢酸钠sodium peroxide 过氧化钠sodium phosphite 亚磷酸钠sodium polysulfide 多硫化钠sodium propionate 丙酸钠sodium pyrophosphate 焦磷酸钠sodium pyrosulfite 焦亚硫酸钠sodium salicylate 水杨酸钠sodium selenite 亚硒酸钠sodium silicate 硅酸钠sodium silicofluoride 氟硅化钠sodium stearate硬脂酸钠sodium sulfate 硫酸钠sodium sulfide 硫化钠sodium sulfite 亚硫化酸钠sodium thiocyanate 硫氰酸钠sodium thiosulfate 硫代硫酸钠soft air 湿空气soft charcoal 软炭soft coal 烟煤soft coke 软焦炭soft glass 软玻璃soft lead 软铅soft pitch 软焦油沥青soft porcelain 软瓷器soft rubber 软橡皮soft soap 钾皂soft water 软水softener 软化剂softening 软化softening agent 软化剂softening degree 软化度softening point 软化点softening temperature 软化点soil 土壤soil analysis 土壤分析soil chemistry 土壤化学soil conditioner 土壤改良剂soil culture 土壤培养soil disinfectant 土壤消毒剂soil stabilizer 土壤稳定剂sol 溶胶solanidine 茄啶solanine 茄碱solanorubin 茄玉红solar battery 太阳电池solar distillation 曝晒蒸发solar drying 日晒干燥solar oil 太阳油solar radiation 太阳辐射solar ray 太阳光线solar salt 太阳盐solar spectrum 太阳光谱solar still 太阳能蒸馏器solation 溶胶化solder 焊剂soldering acid 钎焊用酸solid 固体solid bitumen 固体沥青solid carbon dioxide 固体二氧化碳solid electrolyte 固体电解质solid fuel 固体燃料solid liquid equilibrium 固液平衡solid lubricant 固体润滑剂solid matter 固体物质solid paraffin 固态石蜡solid phase 固相solid phase polymerization 固相聚合solid phase reaction 固相反应solid solution 固溶体solid state 固态solid state chemistry 固体化学solid state physics 固态物理学solid state plasma 固态等离子体solidification 凝固solidifying point 凝固点solidus 固相线solubility 溶解度solubility curve 溶解度曲线solubility limit 溶解限度solubility parameter 溶解度参数solubility product 溶解度积solubilization 加溶solubilized vat dye 可溶性还原染料solubilizer 增溶剂solubilizing agent 增溶剂soluble ribonucleic acid 可溶性核糖核酸soluble saccharin 可溶性糖精soluble silica 可溶性硅石soluble starch 可溶性淀粉solute 溶质solution 溶液solution polymerization 溶液聚合solution pressure 溶解压力solutrope 共溶混合物solvate 溶剂化物solvated electron 溶剂化电子solvation 溶剂化solvay soda process 氨碱法solvent 溶剂solvent effect 溶剂效应solvent extraction 溶剂提取solvent molding 溶剂成型solvent naphtha 溶剂石脑油solvent parameter 溶剂参数solvent recovery 溶剂回收solvent refining 溶剂精制solvent resistance 耐溶剂性solventless varnish 无溶剂清漆solvolysis 溶剂分解sonic nucleation 声波核晶过程sonochemiluminescence 声化学发光sonochemistry 超声化学soot 烟灰sorbate 吸着物sorbic acid 山梨酸sorbin 山梨糖sorbite 山梨醇sorbitol 山梨醇sorbose 山梨糖sorel cement 损耳水泥sorption 吸着sorting machiner 分选机sosoloid 固溶体sound wave 声波source of energy 能源source of heat 热源soy oil 豆油soybean oil 豆油soybean plastic 大豆蛋白塑胶space charge layer 空间电荷层space chemistry 立体化学space formula 立体式space group 空间群space isomerism 空间异构space lattice 空间晶格space model 空间模型space polymer 立体聚合物space reflection 空间反射space time yield 时空产率space velocity 空间速度spacing 晶面间距spanish white 梧牙覆白spar varnish 桅杆清漆sparger 喷淋器多孔管分布器spark 火花spark discharge 火花放电spark explosion method 火花爆炸法spark spectrum 火花光谱sparteine 司巴丁spathic iron ore 菱铁矿spatial formula 立体式spatial frequency 空间频率spatula 刮勺special polymer 特种聚合体specific activity 比活性specific charge 比电荷specific cohesion 比凝聚力specific conductivity 比电导率specific energy 比能specific gravity。

石墨烯基材料应用于水污染物治理领域的研究进展孟亮;孙阳;公晗;王平;乔维川;甘露;徐立杰【摘要】石墨烯由于其独特的性能在水污染治理领域成为一种极具潜力的环境功能材料.本文综述了近几年石墨烯、氧化石墨烯及其复合材料在水污染物治理中的四方面典型应用,即作为吸附剂、光催化剂、电催化氧化剂和其它催化氧化剂(活化H2 O2 、过一硫酸盐).从不同类型的水污染物角度出发,分类概述了针对重金属离子、染料类污染物、新兴环境污染物和一些无机营养元素的处理过程中石墨烯的添加对材料功能及体系作用机理的影响,还综合分析了一些重要水环境因子(如pH值,污染物浓度等)、催化剂自身的性质和石墨烯掺杂量等因素对污染物去除效率的影响.最后,针对目前石墨烯基材料在水污染治理领域的研究应用存在的问题做了总结并展望了今后研究的方向.%Graphene-based nanomaterials have attracted increasing attention in different areas, such as material science, chemical engineering and environmental science. In recent years, it and its derivatives (e. g. graphene oxide, reduced graphene oxide) have been considered promising functional materials in water pollution control because of their many unique properties. Recent intense re-search on the development of graphene-based composite materials has expanded their application to water treatment. Progress in the use of graphene, graphene oxide and graphene-based composite materials in water pollutant treatment is reviewed, including their use as adsorbents, photocatalysts, and the oxidant and oxidant (e. g. H2 O2 , peroxymonosulfate) activators in electrocatalysis. Wa-ter pollutants are heavy metal ions, dyestuffs, some inorganic nutrients and the emerging environmental pollutants. Not onlyare the mechanisms of the use of graphene and its derivatives in different treatment processes considered, but the effects of important factors on the removal efficiency of pollutants are analyzed, such as environmental factors (e. g. pH, pollutant concentration), the proper-ties of the materials (e. g. particle size, surface charge) and the concentration and morphology of the material. Current problems of using graphene-based composite materials in water treatment are summarized and future research directions are proposed.【期刊名称】《新型炭材料》【年(卷),期】2019(034)003【总页数】18页(P220-237)【关键词】石墨烯;吸附;重金属离子;有机污染物;新兴环境污染物【作者】孟亮;孙阳;公晗;王平;乔维川;甘露;徐立杰【作者单位】南京林业大学生物与环境学院,江苏南京 210037;南京林业大学生物与环境学院,江苏南京 210037;华南农业大学海洋学院,广东广州 510642;南京林业大学生物与环境学院,江苏南京 210037;南京林业大学生物与环境学院,江苏南京 210037;南京林业大学材料科学与工程学院,江苏南京 210037;南京林业大学生物与环境学院,江苏南京 210037【正文语种】中文【中图分类】X521 前言近些年,随着现代化工的发展,越来越多的新型水环境污染物(重金属离子、各类顽固有机污染物)引起关注,严重威胁着人们的健康[1]。

第36卷 第3期 无 机 材 料 学 报Vol. 36No. 32021年3月Journal of Inorganic Materials Mar., 2021收稿日期: 2020-06-20; 收到修改稿日期: 2020-08-27; 网络出版日期: 2020-09-09基金项目: 国家自然科学基金(21776191, 21706181); 上饶师范学院校级自选课题(202028); 山西省重点研发计划(201803D421094); 国家重点研发计划政府间国际科技创新合作重点专项项目(2017YFE0129200)National Natural Science Foundation of China (21776191, 21706181); Project of Shangrao Normal University (202028); Key R&D project of Shanxi Province (201803D421094); National Key R&D Program of China (2017YFE0129200)作者简介: 杨言言(1983–), 女, 博士, 讲师.E-mail:******************YANGYanyan(1983–),female,PhD,lecturer.E-mail:******************通信作者: 郝晓刚, 教授.E-mail:**************.cn;*********************文章编号: 1000-324X(2021)03-0292-07 DOI: 10.15541/jim20200340电活性镍钴双金属氧化物高选择性去除/回收水中磷酸盐离子杨言言1,2, 李永国3, 祝小雯1, 杜 晓2, 马旭莉2, 郝晓刚2(1. 上饶师范学院 化学与环境科学学院, 上饶 334001; 2. 太原理工大学 化学化工学院, 太原 030024; 3. 中国辐射防护研究院, 太原 030006)摘 要: 磷是植物体生长的重要营养素, 也是引发水体富营养化的重要因素, 因此废水中磷酸盐的去除与回收均至关重要。

2017年第36卷第11期 CHEMICAL INDUSTRY AND ENGINEERING PROGRESS·4279·化 工 进展五种改性纳米纤维素吸附剂的制备及除磷性能比较王婷庭,刘敏,崔桂榕,陈滢(四川大学建筑与环境学院,四川 成都 610065)摘要:含磷废水的排放是造成水体富营养化的重要原因,吸附法可以有效去除废水中的磷。
开发环境友好的高效吸附剂是该法进一步推广的关键因素之一。
采用TEMPO 氧化+机械剪切结合的方法制备纳米纤维素(CNFs ),分别用Fe(OH)3、Al(OH)3、Mg(OH)2、La 2O 3和MnO 2对CNFs 进行改性。
将改性前后的CNFs 用于吸附去除废水中磷,并比较了不同pH 条件下的除磷效果。
结果表明,Fe(OH)3、Al(OH)3、Mg(OH)2、La 2O 3和MnO 2均能成功负载于CNFs 上。
经改性后的CNFs 对磷的吸附去除效果有明显提高,pH 越低吸附容量越高。
同一pH 条件下,吸附容量依次为Fe(OH)3@CNFs >Al(OH)3@CNFs >Mg(OH)2@CNFs >La 2O 3@CNFs >MnO 2@CNFs 。
Fe(OH)3@CNFs 对磷的吸附效果最好,且受pH 变化的影响不大。
在磷初始浓度为10mg/L 、pH 为4时,Fe(OH)3@CNFs 对磷的吸附容量为7.58mg/g ,为未负载CNFs 的94.75倍;当pH 升高至7时,其吸附容量仍可达到7.09mg/g 。
将其用于实际废水除磷时无需调节pH ,可节约药剂,降低处理成本。
关键词:纳米纤维素;TEMPO 氧化;改性;吸附;除磷中图分类号:X703.1 文献标志码:A 文章编号:1000–6613(2017)11–4279–07 DOI :10.16085/j.issn.1000-6613.2017-0376Preparation of several modified cellulose nanofiber hybrid adsorbents andperformance comparison of phosphate removalsWANG Tingting ,LIU Min ,CUI Guirong ,CHEN Ying(College of Architecture & Environment ,Sichuan University ,Chengdu 610065,Sichuan ,China )Abstract: The discharge of phosphorus wastewater is considered as a dominant factor for water eutrophication. Adsorption is an effective method for phosphorus removal in the wastewater treatment. The development of environment friendly adsorbents with good adsorption capacity is one of the key factors for the further a adsorption application. The TEMPO oxidation combined physical treatmentswas used to prepare the cellulose nanofiber hybrid (CNFs ). Then, Fe(OH)3, Al(OH)3,Mg(OH)2,La 2O 3 and MnO 2 were used to modify CNFs. CNFs and modified CNFs were applied in the phosphate removal from wastewater. The performance of phosphate removal at different pH was investigated. Fe(OH)3,Al(OH)3,Mg(OH)2,La 2O 3 and MnO 2 could be successfully loaded onto CNFs. While modified CNFs showed higher adsorption capacity than CNFs. The phosphate adsorption capacities of modified CNFs were as follows: Fe(OH)3@CNFs >Al(OH)3@CNFs >Mg(OH)2@CNFs >La 2O 3@CNFs >MnO 2@CNFs ,and they all had better phosphate adsorption performance in lower pH. It could be concluded that Fe(OH)3@CNFs had a superior adsorption performance. When the initial concentration of phosphate is 10mg/L ,the adsorption capacity of Fe(OH)3@CNFs was 7.58mg/g at pH 4,which is 95.75 times that of CNFs ,7.09mg/g at pH 7 for practical applications ,Fe(OH)3@ CNFs is*************。

氢氧化铝佐剂对Nogo-66免疫原性的影响岳红云;贺翔鸽;燕振国;曹虹;马岩梅【摘要】目的通过不同浓度氢氧化铝吸附Nogo-66后对SD大鼠免疫特征的影响,探索氢氧化铝佐剂对Nogo-66免疫原性的影响. 方法氢氧化铝混悬液1 ml与4%Nogo-66蛋白溶液以体积比例1:1,1:2,1:3配制,充分微量震荡混匀30 min.微板考马斯亮蓝蛋白定量法计算Nogo-66蛋白质的氢氧化铝吸附率.吸附氢氧化铝佐剂的Nogo-66蛋白免疫接种sD大鼠,免疫频次为1次/7 d,共免疫4次.流式细胞术检测处于活跃分裂期细胞数目. 结果单纯蛋白质组在所有实验组中,免疫原性最高,与其余实验组对比均具有统计学差异(P<0.05),中剂量佐剂组与小剂量佐剂组无统计学差异,二者与高剂量佐剂组对比均有显著性差异(P<0.05). 结论氢氧化铝佐剂对Nogo-66的影响表现为对免疫原性的抑制作用,其原因可能由于其刺激吞噬细胞活性,抑制抗原表位释放.【期刊名称】《山西医科大学学报》【年(卷),期】2010(041)001【总页数】3页(P24-26)【关键词】氢氧化铝佐剂;Nogo-66蛋白;免疫原性【作者】岳红云;贺翔鸽;燕振国;曹虹;马岩梅【作者单位】兰州军区兰州总医院眼科,兰州,730050;第三军医大学大坪医院眼科;兰州军区兰州总医院眼科,兰州,730050;兰州军区兰州总医院眼科,兰州,730050;兰州军区兰州总医院眼科,兰州,730050【正文语种】中文【中图分类】R392为了加强机体对一定免疫原的免疫应答,常应用各种免疫促进剂,称为免疫佐剂(immunological adjuvant,IA),佐剂与免疫原混合,可以加强对免疫原的免疫应答能力。
佐剂增强对免疫原的免疫应答,但并不赋予半抗原免疫原性。
Nogo-66蛋白的等电点为6.09;当 pH>pI的溶液中,蛋白质为阴离子,根据 Nogo-66蛋白的等电点,当 pH=7时,Nogo-66在电场中向阳极移动;而中性 pH值时,硫酸铝带负电荷,氢氧化铝带正电荷。

2021年第3期矿物选别化 工矿物与加工INDUSTRIAL MINERALS & PROCESSING文章编号:1008-7524(2021 )03-0013-04 DOI :10.16283/j. cnki. hgkwyjg. 2021. 03. 004云南某反浮选脱镁磷精矿再反浮选脱硅降MER 值试验研究"刘文彪",马航2,傅英2,饶峰3,黄文萱彳(1.昆明理工大学国土资源工程学院,云南 昆明650093;2.云南云天化股份有限公司研发中心,云南昆明650228;3•福州大学紫金矿业学院,福建福州350108)摘要:随着钙质胶磷矿资源的不断消耗,入选的胶磷矿中P 2O 5和MgO 含量持续降低,Si 。
?含量持续升高,使用 目前成熟的单一反浮选脱除MgO 工艺生产的磷精矿存在P 2O 5含量降低,Si 。
?杂质和MER 值升高的问题。
为了解 决此问题,以反浮选脱镁磷精矿为原料对研发的酸性条件下再反浮选脱硅捕收剂开展了药剂用量、pH 适应性及浮选低温耐受性试验,结果表明,该脱硅捕收剂具有较好的pH 适应性和低温耐受性,对降低磷精矿Si 。
?含量和MER 值以及提高最终磷精矿品质有明显作用。
关键词:磷精矿;捕收剂;反浮选;脱镁;脱硅;MER中图分类号:TD971 文献标志码:AExperimental study on SiO 2 removal and MER reduction for a phosphate concentrate after MgO removal byreverse flotation in Yunnan ProvinceLiu Wenbiao 1> 2, Ma Hang 2 ?Fu Ying 2, Rao Feng 3,Huang Wenxuan 3(1. Faculty of Land Resource Engineering , Kunming University of Science and Technology?Kunming Yunnan 650093,China ; 2. R & D Center Yunnan Yuntianhua Co. , Ltd. ,Kunming Yunnan 650228,China; 3・ School of Zijin Mining , Fuzhou University, Fuzhou Fujian 350108, China)Abstract : With the lasting consumption of calcareous phosphate resources , the content of P 2 O 5 and MgO in the feedto flotation of phosphate rock is ever decreasing , while SiO 2 content in the feed is increasing. As a result , the phos phate concentrate obtained from the reverse flotation circuit only for removal of MgO is assaying lower and lower P 2O 5, higher and higher SiO 2 and MER. The experiment using the phosphate concentrate obtained from the reverseflotation of the phosphate rock for removal of MgO as the feed to the reverse flotation for removal o£ SiO 2 was carriedout with optimization of operating conditions including dosage of the collector. The results shows that the collectorused for removal of SiO 2 by reverse flotation is of high adaptability to pH in pulp and o£ high efficiency at low tempera ture, which facilitates the reduction of SiO 2 content in the phosphate concentrate and MER value , eventually improve the quality of the final product.Keywords : phosphate concentrate ; collector 5 reverse flotation ; removal o£ MgO ; removal o£ SiO 2 jMER“+•引用格式:刘文彪,马航,傅英,等.云南某反浮选脱镁磷精矿再反浮选脱硅降MER 值试验研究[J].化工矿物与加工,2021,50(3):13-15 + 42. •F-0引言磷矿是磷肥生产不可或缺的重要原料,对粮 食作物的丰产丰收起着至关重要的作用。

2020年 第24期 广 东 化 工 第47卷 总第434期 · 95 ·丰乐河徽州区段水质分析与评价谈一鸣1,马明海1*,卢欢2,苏梦轩1,常祝芳1,丁荣艳1(1.黄山学院 生命与环境科学学院单位,安徽 黄山 245041;2.北京科技大学 能源与环境工程学院,北京 100083)[摘 要]以丰乐河(徽州区段)为研究对象,选择总磷、总氮、氨氮、化学需氧量、溶解氧、pH 、透明度等七个指标,利用污染指数法对10个代表性段面进行水质评价。
结果表明,丰乐河(徽州区段)10个断面均达到了地表水Ⅲ类水标准,水质较好,水体整体处于尚清洁水平。
下游的两个检测点位存在潜在的有机物超标风险,应加强管控。
[关键词]丰乐河;综合污染指数;水环境质量评价;徽州区段[中图分类号]X82 [文献标识码]A [文章编号]1007-1865(2020)24-0095-02Analysis and Evaluation of Water Quality in Huizhou Section of Fengle RiverTan Yiming 1, Ma Minghai 1*, Lu Huan 2, Su Mengxuan 1, Chang Zhufang 1, Ding Rongyan 1 (1. School of Life and Environmental Science, Huangshan University, Huangshan 245041;2. School of Energy and Environmental Engineering, Beijing University of Science and Technology, Beijing 100083, China)Abstract: Taking Fengle River (Huizhou Section) as the research object, seven indicators including total phosphorus, total nitrogen, ammonia nitrogen, chemical oxygen demand, dissolved oxygen, pH and transparency were selected to evaluate the water quality of 10 representative sections by pollution index method. The results show that the 10 sections of Fengle River (Huizhou section) meet the class Ⅲ surface water standard, the water quality is good, and the water body is still in a clean level. There is a potential risk of exceeding the standard of organic matter in the two detection points of downstream, so the control should be strengthened in the future.Keywords: fengle river ;comprehensive pollution index ;water quality assessment ;huizhou section1 引言河流作为城市地表水的主要来源,是城市生态环境的重要组成部分,具有疏浚、排涝、供水及景观作用,对当地城市建设具有积极作用[1]。

第34卷第6期2020年12月 江苏科技大学学报(自然科学版)JournalofJiangsuUniversityofScienceandTechnology(NaturalScienceEdition) Vol 34No 6Dec.2020 DOI:10.11917/j.issn.1673-4807.2020.06.018碳点修饰磷酸钴微米花对水中四环素的吸附施伟龙1,李铭洋2,郭 峰3,王 贝1,韩妹娜1,晏 超1(1.江苏科技大学材料科学与工程学院,镇江212100)(2.江苏科技大学环境与化学学院,镇江212018)(3.江苏科技大学能源与动力工程学院,镇江212100)摘 要:抗生素废水的肆意排放对人类健康和生态环境产生潜在的威胁.吸附技术被认为是处理此类污染物最有效的方法之一.本研究通过简单的共沉淀法制备碳点修饰的磷酸钴微米花复合材料(CDs-CoPi),并用其吸附去除水溶液中四环素(TC).采用XRD、XPS、FT-IR、SEM等手段对CDs-CoPi的晶相、结构、形貌和元素组成等进行表征分析.结果表明,CDs均匀地负载在CoPi微米花的表面,CDs-CoPi的比表面积显著提高,对TC的吸附性能显著增强,CDs16-CoPi(CDs质量分数为16%)显示出最优异的吸附性能.CDs16-CoPi对TC的吸附过程符合伪二阶动力学模型.等温吸附规律可用Langmuir模型很好地描述,最大单分子层吸附容量可达34 7mg/g.CDs-CoPi对TC的吸附性能提升主要归因于CDs表面含氧官能团的贡献.关键词:磷酸钴;碳点;吸附;复合材料;四环素中图分类号:X506 文献标志码:A 文章编号:1673-4807(2020)06-116-07收稿日期:2019-04-27 修回日期:2019-10-29基金项目:江苏科技大学博士科研启动基金资助项目(1062931806,1142931803)作者简介:施伟龙(1988—),男,博士,讲师,研究方向为碳纳米材料基复合材料的制备与应用.E mail:shiwl@just.edu.cn引文格式:施伟龙,李铭洋,郭峰,等.碳点修饰磷酸钴微米花对水中四环素的吸附[J].江苏科技大学学报(自然科学版),2020,34(6):116-122.DOI:10.11917/j.issn.1673-4807.2020.06.018.Adsorptionoftetracyclineinwaterontocarbon dotsmodifiedcobaltphosphatemicro flowerSHIWeilong1,LIMingyang2,GUOFeng3,WANGBei,1HANMeina,1YANChao1(1.SchoolofMaterialsScienceandEngineering,JiangsuUniversityofScienceandTechnology,Zhenjiang212100,China)(2.SchoolofEnvironmentalandChemicalEngineering,JiangsuUniversityofScienceandTechnology,Zhenjiang212018,China)(3.SchoolofEnergyandPowerEngineering,JiangsuUniversityofScienceandTechnology,Zhenjiang212100,China)Abstract:Theindiscriminatedischargeofantibioticwastewaterposesahugethreattohumanhealthandtheeco logicalenvironment.Adsorptiontechnologyisconsideredtobeoneoftheeffectivewaystodealwithsuchcontaminants.Here,carbondotmodifiedcobaltphosphatemicro flowercomposites(CDs/CoPi)werepreparedthroughafacileco precipitationmethodforadsorptionremovaloftetracycline(TC)fromaqueoussolution.Thecrystalphase,structure,morphologyandelementcompositionoftheCDs/CoPicompositewereanalyzedbyase riesofcharacterizationmethodssuchasXRD,XPS,FT IRandSEM.TheresultsshowthattheCDswereuni formlydepositedonthesurfacesofCoPimicroflowersinthecompositewhichcanimprovethespecificsurfacear eaofCoPiandeffectivelyimproveitsadsorptionperformanceofTC.TheCDs/CoPicompositesasadsorbentsex hibitenhancedadsorptionbehaviorforTCincomparisonofpureCoPi,inwhichtheCDs16 CoPi(withtheCDsmasscontentof16%)showsthehighestadsorptionactivity.Thecorrespondingadsorptionkineticswasfittedwellwiththepseudo second orderkineticmodel.Meanwhile,theadsorptionisothermresearchshowedthatthemeasuredexperimentaldataareinaccordwiththeLangmuirmodel,andthemaximumsingle layeradsorptionca pacityoftheCDs16 CoPicompositewascalculatedtobe(34 7mg/g).TheincreasedadsorptionperformanceofCDs/CoPicanattributetotherichoxygen containinggroupsonthesurfaceofCDs.Keywords:CoPi,carbondots,adsorption,compositematerial,tetracycline 抗生素是一类天然或合成的化学物质,可抑制或杀死微生物,广泛地应用于人类医学、水产养殖和畜牧业[1].自从青霉素问世以来,人们已发现数百种抗生素.近年来,抗生素的滥用及其废水的肆意排放现象频发,在许多水体中,如地表水、地下水、海水甚至饮用水中都可检出抗生素[2].由于其具有较高的生物毒性、可诱导细菌产生耐药基因、在环境中难降解等特点,对人类健康和生态环境构成了极大的威胁[3].到目前为止,去除水溶液中抗生素的方法有很多,如化学氧化法、膜过滤法、光催化降解法和吸附法等[4-8].其中,吸附法是去除水中各种有机污染物的一种最为有效、经济的方法[9-10].最近,磷酸钴(CoPi)由于成本低、地球储量丰富、无毒等优点而倍受学者们的关注[11].特别是三维多级结构的CoPi,具有较高的比表面积,可用于有机污染物的吸附.然而,CoPi的吸附能力仍远未达到实际应用的要求,仍需进一步优化,以提升其吸附性能.碳点(CDs),作为碳纳米家族的新成员,具有优异的光学性能、低毒性、高化学稳定性,被广泛应用于荧光探针、光伏器件、生物成像等领域[12-13].此外,CDs的表面具有丰富的含氧官能团,因此,具有良好的水溶液分散性.最近已经有报道证明,官能团(OH、COOH等)可有助于提高反应物的吸附性能[14].基于此,尝试将CDs与CoPi结合,构建一种高吸附容量的复合材料,以期有效地去除水环境中的抗生素.本研究采用简单的一步共沉淀法,制备出不同CDs含量的CDs-CoPi复合材料.利用该复合材料作为吸附剂(CDs-CoPi),以盐酸四环素(TC,分子式见图1)作为目标抗生素进行吸附性能评价.图1 TC抗生素的分子结构Fig.1 MolecularstructureforTCantibiotic1 实验1 1 催化剂的制备(1)CDs的制备首先利用电化学刻蚀石墨棒的方法制备CDs溶液.步骤如下:以超纯水为电解质,两根石墨棒作为两个电极.通过直流电源将30V的电压施加到两个电极上,持续搅拌120h后,阳极石墨棒收到电化学刻蚀,反应器中逐渐出现暗黄色溶液,经低速定量滤纸过滤后,经高速离心30min后,除去沉淀后的氧化石墨和石墨颗粒,进而得到CDs溶液.然后,将CDs溶液经过冻干,得到CDs粉末.(2)CDs-CoPi复合材料的制备在50mL去离子水中加入Co(NO3)62H2O(3mmol),超声15min,持续搅拌30min,同时,加入一定量的碳点粉末,再超声20min后,将含有2mmol的(NH4)2HPO4水溶液加入到前面的混合溶液中,继续磁力搅拌2h,混合均匀,静置沉淀10h,用去离子水和乙醇洗涤沉淀物,在90℃烘箱中,干燥8h,得到不同CDs含量的CDs-Co-Pi复合材料,其中质量比分别为1%、2%、4%、8%、16%和32%,所合成样品标记为CDs1-CoPi、CDs2-CoPi、CDs4-CoPi、CDs8-CoPi、CDs16-CoPi和CDs32-CoPi.1 2 吸附性能测试吸附性能测试的步骤与文献[24]报道类似,具体步骤如下:取50mgCDs-CoPi复合材料投加到100mL浓度为10mg/L的TC的水溶液中,在持续搅拌的条件下,进行吸附性能评价.经过一定时间间隔,当吸附剂与TC达到吸附平衡后,提取出3mL的样液,并离心与吸附剂分离.用紫外分光光度法测定上清液中TC的浓度,测定波长为357nm.达到吸附平衡时样品对TC的吸附量qe(mg·g-1)用式(1)计算:qe=(C0-Ce)Vm(1)式中:C0为TC的初始浓度,mg·L-1;Ce为达到吸附平衡时TC的浓度,mg·L-1,V为溶液的体积,L;m为吸附剂的质量,g.动力学参数测试步骤与达到吸附平衡的实验过程相同.每隔一定的时间间隔,从正在反应的溶液中抽取3mL样液,并离心与吸附剂分离.用紫外分光光度法测定上清液中TC的浓度,测定波长为357nm.时间为t时,样品对TC的吸附量qt(mg·g-1)可用式(2)计算:qt=(C0-Ct)Vm(2)式中:Ct为在任意时间t时TC的浓度,mg·L-1.711第6期 施伟龙,等:碳点修饰磷酸钴微米花对水中四环素的吸附1 3 实验与表征仪器Empyrean型X射线粉末衍射仪,荷兰Panalyt iclal公司;Axisultra-DLD型X射线光电子能谱仪,英国Kratos公司;激光共聚焦拉曼光谱仪,法国HoribaJobinYvon公司;UV-2550型紫外可见分光光度计,日本岛津公司;FT-IR650型红外光谱仪,日本岛津公司;S-4800冷场发射扫描电子显微镜,日本日立公司;ASAP-2050型比表面积分析仪,美国Micromeritics公司.2 结果与讨论2 1 XRD分析图2为纯相CoPi和CDs16-CoPi复合物的X射线(XRD)图谱.从图2可以看出,纯相CoPi样品的衍射峰与标准卡(JCPDSNO.41-0375)相吻合,并且没有出现杂峰,证明所合成样品为Co Pi[15].与纯相CoPi相比,CDs16-CoPi的衍射峰并没有发生变化,表明CDs的引入没有对CoPi的晶相结构产生破坏性影响.同时,在复合物CDs16-CoPi中,并没有观察到CDs在26°的特征衍射峰,这可能是由于CDs的负载量过少或者被CoPi较强的衍射峰覆盖所致[16].图2 CoPi和CDs16-CoPi的XRD图谱Fig.2 XRDpatternsofCoPiandCDs16-CoPi2 2 FT-IR和Raman分析图3为CoPi、CDs16-CoPi和CDs的傅里叶红外(FT-IR)光谱.从图3可以看出,在CoPi和CDs16-CoPi样品中,900~1100cm-1范围的振动峰主要归因于磷酸盐的非对称拉伸所引起的[17],而在660cm-1处的特征峰则是由金属与氧之间的金属-氧键振动所致[18].利用拉曼光谱(Raman)进一步分析所制备样品的成分组成.图4为CoPi和CDs16-CoPi的Raman光谱,从中可以观察到,除了碳材料D带和G带的特征峰外,CoPi呈现出与CoPi-CDs相似的图谱,这可以说明在CDs16-CoPi复合材料中确实有CDs的存在.此外,970cm-1和1120cm-1处的特征峰可归因于磷酸根基团[19].众所周知,在1348cm-1处的D带是由无序诱导模式或有缺陷的石墨结构所引起的,而1570cm-1处的G带是由碳原子的sp2平面振动导致[20].通常采用D带与G带的强度比(ID/IG)来确定碳材料的缺陷.较高的ID/IG比值表明碳材料的石墨化程度较低.通过观察,可以得知,所制备的CDs-CoPi复合材料中ID/IG的值大于1,意味着CDs-CoPi复合材料中的CDs含有缺陷,而一定程度的缺陷可有利于对有机污染物的捕获和吸附.图3 CoPi、CDs16-CoPi和CDs的FT-IR图谱Fig.3 FI-IRspectraofCoPi、CDs16-CoPiandCDs图4 CoPi和CoPi-CDs的Raman光谱图Fig.4 RamanspectraofCoPiandCDs16-CoPi2 3 SEM分析通过扫描电镜(SEM)和能谱(EDX)测试对CoPi和CDs16-CoPi样品进行形貌和组成进行分析.从图5(a)的SEM图片可以观察到,所合成的CoPi材料呈现出三维花状结构.进一步放大观察(图5(b)),CoPi纳米花结构是由若干个纳米薄片组装而成,且纳米薄片的厚度约为70~80nm.图5(c)为CDs16-CoPi的SEM图片,其形貌与纯相CoPi类似,但片层的厚度有所增加,大概增至150~160nm.此外,对CDs16-CoPi复合材料进行了EDX能谱分析,从图5(d)可以看出,CDs16-CoPi主要是由C、Co、P和O4种元素组成,这进一步证实了复合材料中存在CDs和CoPi.811江苏科技大学学报(自然科学版)2020年图5 CoPi和CDs16-CoPi的SEM图以及EDX能谱分析图Fig.5 SEMimagesandEDXspectumofCoPiandCDs16-CoPi2 4 XPS分析为进一步检测CDs16-CoPi的元素组成和化学价态,对CDs16-CoPi进行了X光电子能谱(XPS)分析.图6(a)为C1s高分辨谱图,在284 6、286 3和288 6eV处的3个结合能峰分别归属与来自CDs的C-C,C-O和C=O的结合能[15],说明CDs具有丰富的含氧官能团,有助于对有机污染物的吸附.由图6(b)可见,Co2p的2个特征峰分别对应于Co2p3/2(781 3eV)和Co2p1/2(797 2eV)的内层电子,此外伴随有2个卫星峰,可以说明Co2+存在CoPi中[20].图6 CDs16-CoPi的XPS图谱Fig.6 XPSspectraofCDs16-CoPi图6(c)可见,P2p3/2和P2p1/2的能谱峰分别对应于结合能133 0eV和134 1eV,表明CoPi-CDs复合物中磷酸基团的磷原子的存在[15].图6(d)为CoPi-CDs复合物的O1s谱图,位于530 7、531 2和532 4eV的这些峰可归属于CoPi-CDs复合物中的Co-O-C,C=O和C-O键的结合能,表明CoPi和CDs之间的强耦合作用[21-22].通过XPS分析表明,复合材料中CoPi和CDs间强烈的化学作用,证明成功制备了CDs-CoPi异质结复合材料.2 5 吸附性能研究在室温和黑暗条件下,对TC(10mg/L)进行吸附实验以评价所制备材料的吸附性能(图7),结果表明:所有合成的样品均对TC产生吸附效果,且随着时间的增加而增强,在50min后达到吸附平衡.几乎所有样品在最初5min内的吸附速度最快,然后变得缓慢,这可能是因为在吸附的初始阶段,材料上大量空置的表面吸附位点被填充.经过初始阶段后,由于吸附剂与TC分子间的斥力作用,剩下的空置吸附位点则难以被占据.值得注意的是,随着CDs在复合材料中的质量分数从1%增加到16%,CoPi在平衡状态下的吸附容量从3 98mg·g-1增加到18 1mg·g-1,说明CoPi中引入CDs可以提供更多的吸附空位,从而提高CoPi的吸附活性.当进一步增加CDs的含量(质量分数超过16%)时,复合材料的吸附容量明显下降.吸附活性下降的原因可归结为过量的CDs容易覆盖或占据了CoPi表面上的吸附活性位点和通道.基于以上数据,利用伪一级动力学模型、伪二级吸附动力学模型和Elovich模型来模拟CDs16-CoPi复合材料对TC吸附的动力学行为.以上3种动力学模型分别按式(3)、(4)和(5)的形式表示:ln(qe-qt)=lnqe-k1t2 303(3)tqt=1k2q2e-tqe(4)qt=b+alnt(5)式中:k1和k2分别是伪一级和伪二级方程的吸附速率常数,单位分别为min-1和g·mg-1·min-1;a和b则是Elovich常数.基于上述模型分析,图8(a~c)给出了不同动力学模型的拟合数据.其中,伪二级动力学方程(R2>0 99)展现出良好的线性相关.与其他的动力学模型相比,所计算出来的理论平衡吸附量qe(qe,cal)与实验所得到的平衡吸附量(qe,exp)相接近.因此,可以得出CDs16-CoPi复合材料对TC吸附过程符合伪二级动力学模型(图8).911第6期 施伟龙,等:碳点修饰磷酸钴微米花对水中四环素的吸附图7 TC(10mg·L-1)在制备的样品上的吸附性能测试Fig.7 AdsorptionofTC(10mg·L-1)overaspreparedsamples图8 CDs16-CoPi复合材料的TC吸附动力学模型Fig.8 AdsorptionkineticsofTCovertheCDs16-CoPi图9为不同初始TC浓度对CDs16-CoPi的吸附性能影响关系,可以看出复合材料对TC吸附容量随初始浓度的增加而上升.图9 TC初始浓度对CDs16-CoPi复合材料的影响Fig.9 EffetofinitialcocentiationofTCsolutiononadsorptionperformanceoverCDs16-CoPi为了进一步研究吸附平衡,利用Langmuir等温模型对数据进行拟合.众所周知,Langmuir等温理论是基于在均匀表面吸附的假设,用式(6)表示[23]:Ceqe=1Q0b+CeQ0(6)式中:Q0为每单位吸附剂在饱和条件下吸附的最大质量,mg·g-1;b为Langmuir等温线常数,L·mg-1.根据线性图Ce/qe对Ce的斜率和截距可以计算出Q0和b的值,因此可通过此拟合方程(图10)计算出CDs16-CoPi的最大单层吸附容量Q0为34 7mg/g.图10 CDs16-CoPi复合材料在吸附温度为20℃的Langmuir吸附等温线Fig.10 LangmuiradsorptionisothermofTCantoCDs16-CoPiat20℃2 6 BET分析图11为CoPi和CDs16-CoPi的N2吸附-脱附等温曲线,表面积以及所计算出的平均孔体积大小.CoPi和CDs16-CoPi的N2吸附-脱附等温曲线类型属于IUPAC分类中典型的Ⅳ型等温线.其中,图中所展现出的滞后环为H3型,说明CoPi基底材料具有典型的介孔材料特征.通过根据Brunauer Emmett Teller(BET)计算结果分析,与纯相CoPi(16 53m2·g-1)相比,CDs16-CoPi具有更大的比表面积(24 19m2·g-1)和孔容量(0 054cm3·g-1).通常,吸附剂的吸附性能的优劣与比表面积的大小密切相关,比表面积越大,所空置的表面吸附位点越多,更加有利于样品对有机污染物的吸附活性.纯相CoPi和CDs16-CoPi复合材料相应的孔径分布图(图12),可以看出CDs的引入对CoPi的孔径分布影响不明显.以上结果显示,CDs的引入可以提高花状CoPi的表面吸附位点和比表面积,进而有效提高其对TC的吸附性能.图11 纯相CoPi和CDs16-CoPi复合材料的N2吸附-脱附等温曲线及相应的测定参数Fig.11 NitrogenadsorptionisothermsandmeasuredparametersofCoPiandCDs16-CoPi021江苏科技大学学报(自然科学版)2020年图12 纯相CoPi和CDs16-CoPi复合材料的孔径分布图线Fig.12 DistributionsofporesizeoverCoPiandCDs16-CoPi3 结论(1)通过简单的一步共沉淀法制备了CDs-CoPi复合材料,并用作吸附剂去除水溶液中TC.与纯CoPi相比,CDs-CoPi复合物展现出更加优异的吸附性能,其中CDs16-CoPi显示出最优的吸附活性,单层的最大吸附容量为34 7mg/g.(2)CDs具有丰富的含氧官能团,有助于提高CoPi对有机污染物的吸附;CDs可以与CoPi之间形成强的耦合作用,增加CoPi的吸附位点,提高其比表面积.本研究为有效提升材料的吸附性能提供了一种有益的设计思路.参考文献(References)[1] OZGEH,BURINYLZ,SibelA,etal.Removaloftetracyclineandoxytetracyclinebymicroscalezerov alentironandformationoftransformationproducts[J].EnvironmentalScience&PollutionResearchInterna tional,2014,21(5):3774-82.DOI:10.1007/s11356-013-2342-1.[2] ZHANGQianqian,YINGGuangguo,PANChanggui,etal.ComprehensiveevaluationofantibioticsemissionandfateintheriverbasinsofChina:sourceanalysis,multimediamodeling,andlinkagetobacterialresist ance[J].EnvironmentalScience&Technology,2015,49(11):6772-6782.DOI:10.1021/acs.est.5b00729.[3] YANC,YANGY,ZHOUJ,etal.AntibioticsinthesurfacewateroftheYangtzeEstuary:Occurrence,dis tributionandriskassessment[J].EnvironmentalPol lution,2013,175(8):22-29.DOI:10.1016/j.envpol.2012.12.008.[4] NAVALONS,ALVAROM,GARCIAH.Reactionofchlorinedioxidewithemergentwaterpollutants:Prod uctstudyofthereactionofthreeβ lactamantibioticswithClO2[J].WaterResearch,2008,42(8):1935-1942.DOI:10.1016/j.watres.2007.11.023.[5] HOMAYOONFALM,MEHRNIAMR.AmoxicillinseparationfrompharmaceuticalsolutionbypHsensitivenanofiltrationmembranes[J].Separation&Purifica tionTechnology,2014,130:74-83.DOI:10.1016/j.seppur.2014.04.009.[6] HUANGH,JIEZ,LIANJ,etal.PreparationofcubicCu2OnanoparticleswrappedbyreducedgrapheneoxidefortheefficientremovalofrhodamineB[J].JournalofAlloys&Compounds,2017,718:112-115.DOI:10.1016/j.jallcom.2017.05.132.[7] BAHAMOND,CARROL,GURIS,etal.Computationalstudyofibuprofenremovalfromwaterbyadsorp tioninrealisticactivatedcarbons[J].JournalofColloid&InterfaceScience,2017,498:323-324.DOI:10.1016/j.jcis.2017.03.068.[8] CHENH,KOOPALLK,XIONGJ,etal.Mechanismsofsoilhumicacidadsorptionontomontmorillon iteandkaolinite[J].JournalofColloid&InterfaceScience,2017,504:457-467.DOI:10.1016/j.jcis.2017.05.078.[9] KIMSH,SHONHK,NGOHH.Adsorptioncharacteristicsofantibioticstrimethoprimonpowderedandgranularactivatedcarbon[J].JournalofIndustrial&EngineeringChemistry,2010,16(3):344-349.DOI:10.1016/j.jiec.2009.09.061.[10] KO ODYSKAD,KRUKOWSKAJ,THOMASP.Comparisonofsorptionanddesorptionstudiesofheavymetalionsfrombiocharandcommercialactivecarbon[J].ChemicalEngineeringJournal,2017,307:353-363.DOI:10.1016/j.cej.2016.08.088.[11] KANANMW,JUNKOY,YOGESHS,etal.Structureandvalencyofacobalt phosphatewateroxidationcatalystdeterminedbyinsituX rayspectroscopy[J].JournaloftheAmericanChemicalSociety,2010,132(39):13692.DOI:10.1021/ja1023767.[12] LIH,HEX,LIUY,etal.One stepultrasonicsynthesisofwater solublecarbonnanoparticleswithexcel lentphotoluminescentproperties[J].Carbon,2011,49(2):605-609.DOI:10.1016/j.carbon.2010.10.004.[13] LIH,KANGZ,LIUY,etal.Carbonnanodots:synthesis,propertiesandapplications[J].JournalofMa terialsChemistry,2012,22(46):24230-24253.DOI:10.1039/C2JM34690G.[14] LIUR,LIUJ,KONGW,etal.Adsorptiondominantcatalyticactivityofacarbondotsstabilizedgoldnanop articlessystem[J].DaltonTrans,2014,43(28):10920-10929.DOI:10.1039/C4DT00630E.121第6期 施伟龙,等:碳点修饰磷酸钴微米花对水中四环素的吸附[15] ZHUM,HANM,CHENGZ,etal.Strongcouplingeffectattheinterfaceofcobaltphosphate carbondotsboostphotocatalyticwatersplitting[J].JournalofColloid&InterfaceScience,2018,530:256-263.DOI:10.1016/j.jcis.2018.06.078.[16] WUX,ZHAOJ,GUOS,etal.CarbondotandBiVO4quantumdotcompositesforoverallwatersplittingviaatwo electronpathway[J].Nanoscale,2016,8(39):17314.DOI:10.1039/C6NR05864G.[17] SHAOH,PADMANATHANN,MCNULTYD,etal.Supercapatterybasedonbinder freeCo3(PO4)2·8H2Omultilayernano/microflakesonnickelfoam[J].AcsAppliedMaterials&Interfaces,2016,8(42):28592-28598.DOI:10.1021/acsami.6b08354.[18] AHMEDIM,GASSERMS.AdsorptionstudyofanionicreactivedyefromaqueoussolutiontoMg-Fe-CO3layereddoublehydroxide(LDH)[J].AppliedSurfaceScience,2012,259:650-656.DOI:10.1016/j.apsusc.2012.07.092.[19] ZHAIS,XUEW,YAMAZAKID,etal.CompressibilityofstrontiumorthophosphateSr3(PO4)2athighpressure[J].Physics&ChemistryofMinerals,2011,38(5):357-361.DOI:10.1007/s00269-010-0409-9.[20] YUANCZ,JIANGYF,WANGZ,etal.Cobaltphosphatenanoparticlesdecoratedwithnitrogen dopedcarbonlayersashighlyactiveandstableelectrocata lystsfortheoxygenevolutionreaction[J].JournalofMaterialsChemistryA,2016,4(21):8155-8160.DOI:10.1039/C6TA01929C.[21] THEERTHAGIRIJ,THIAGARAJANK,SENTHILKUMARB,etal.Synthesisofhierarchicalcobaltphosphatenanoflakesandtheirenhancedelectrochemi calperformancesforsupercapacitorapplications[J].Chemistryselect,2017,2(1):201-210.DOI:10.1002/slct.201601628.[22] HUG,DENGX,PENGZ,etal.Morphologyandluminescenceof(Y,Gd)BO3:Euphosphorparticlespre paredbyurea assistedspraypyrolysis[J].JournalofAlloys&Compounds,2008,452(2):462-466.DOI:10.1016/j.jallcom.2007.05.041.[23] SHIW,FENGG,WANGH,etal.CarbondotsdecoratedmagneticZnFe2O4nanoparticleswithenhancedadsorptioncapacityfortheremovalofdyefromaqueoussolution[J].AppliedSurfaceScience,2018,433:790-797.DOI:10.1016/j.apsusc.2017.10.099.[24] 曹煜,陈芳艳,高承娟,等.磺胺甲唑分子印迹聚合物的合成及其吸附性能研究[J].江苏科技大学学报(自然科学版),2018,32(5):723-728.DOI:10.11917/j.issn.1673-4807.2018.05.020.CAOYu,CHENFangyan,GAOChengjuan,etal.Researchonthesynthesisandasdsorptionperformanceofsulfamethoxazolemolecularlyimprintedpolymer[J].JournalofJiangsuUnversityofScienceandTechnology(NaturalScienceEdition),2018,32(5):723-728.DOI:10.11917/j.issn.1673-4807.2018.05.020.(inChinese)(责任编辑:顾琳)221江苏科技大学学报(自然科学版)2020年。
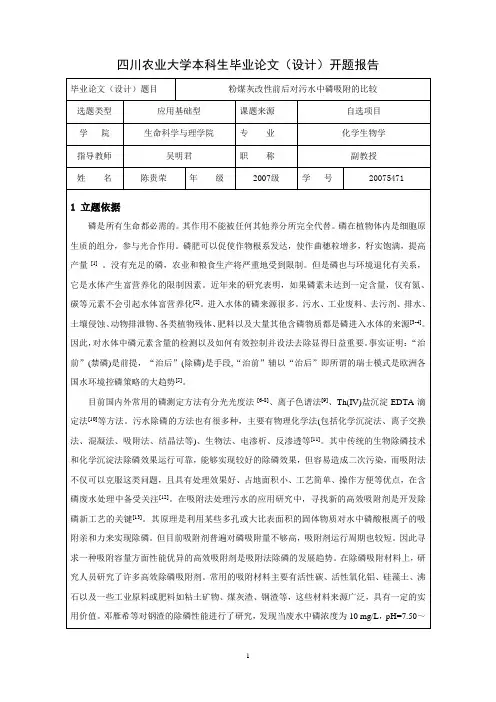

氧化锆-磁性壳聚糖材料对磷酸盐的吸附研究安风霞;刘景亮【摘要】制备了氧化锆负载的磁性壳聚糖复合材料,并研究了其对水体中磷酸盐的吸附行为.XRD表征结果表明负载在磁性壳聚糖上的氧化锆纳米颗粒并未影响材料的晶型结构.吸附实验结果表明负载氧化锆后磁性材料对磷酸盐表现出了良好的吸附性能,其吸附机理包括离子交换和静电作用.该磁性材料对磷酸盐的吸附量随溶液pH值的增大而减小;随温度的升高而升高;阴离子对水中磷酸盐吸附效果影响的次序为:Cl->NO-3>SO2-4.【期刊名称】《广州化工》【年(卷),期】2017(045)020【总页数】4页(P29-32)【关键词】磁性壳聚糖;氧化锆;吸附;磷【作者】安风霞;刘景亮【作者单位】国电环境保护研究院, 江苏南京 210031;南京晓庄学院, 江苏南京211171【正文语种】中文【中图分类】O647.3磷是水体富营养化的限制性元素之一,过量磷的引入能引起水体的富营养化,因此除磷对控制和防治水体富营养化有重要的意义[1]。
除磷的方法主要有生物法、化学沉淀法和吸附法。
吸附法除磷由于成本低、吸附效率高、可再生循环等优点,受到广泛关注[2-3]。
常用的吸附除磷材料如工业废渣[4]、天然材料[5-6]等对磷的吸附容量有限。
近年来,将金属氧化物或氢氧化物作为除磷吸附剂取得了显著效果,特别是铝[7-8]、钙[9]、铁[10-11]、锆[12-13]的氧化物对磷酸盐均有良好的吸附性能。
研究表明氧化锆化学性质稳定,与其他阴离子相比对磷酸盐具有较高的吸附选择性[14-15]。
Chitrakar[12]合成了无定形的氢氧化锆,对磷酸盐的最大吸附量为10 mg·g-1;Chubar 等[16]用溶胶-凝胶法合成的ZrO2·xH2O 对磷酸盐的最大吸附量约40 m g·g-1,结果表明,ZrO2 对磷酸盐的吸附机制在于 ZrOH 吸附位点与磷酸根离子的配位作用,吸附过程中-OH 被置换到水体中。

猱艺科枚Journal of Green Science and Technology第23卷第2期2021年1月牡蛎壳改性花生壳生物炭吸附去除水体中的磷李浩,夏港,关昊为,易群伟,李嘉融,刘妥淇,张静(武汉轻工大学土木工程与建筑学院,湖北武汉430023)摘要:基于牡蛎壳富含碳酸钙成分,采用800°C高温改性方法制备牡蛎壳改性花生壳生物炭,考察了其对溶液中稀的吸附性能。
实验结果表明:牡蛎壳改性后的花生壳生物炭对磷的吸附量显著高于未改性的花生壳生物炭。
溶液初始磷浓度为200mg/L,添加0.02g的生物炭.,在25°C下反应48h后,牡蛎壳及性花生壳生物炭磷吸附容量为197.3mg/g,约为未改性花生壳生物炭的17倍。
关键词:牡蛎壳改性;吸附性能;花生壳;生物炭;磷中图分类号:X172文献标识码:A文章编号:1674-9944(2021)02-0072-021引言磷是水生生物新陈代谢中不可或缺的一种元素口O 但当水体中磷浓度超标时,会刺激水体中藻类的生长,减少水体中的溶解氧浓度,使水生生物窒息而死,造成水体富营养化,对水体生态系统产生负面影响凤旳。
在我国,根据《城镇污水处理厂污染物排放标准》GB18918 -2002规定,排入自然水体的污废水的磷限值为0.5 mg/L,美国对排入湖泊的污废水的磷限量规定为0.05 mg/L"】。
工业、农业和生活污废水是磷进入自然水体环境的主要来源,含磷浓度为4〜15mg/L,远远超过规定限制旳。
因此,开发有效去除废水中磷的方法是亟待解决的一个重要的全球性问题。
目前,控制水体磷污染的有效方法主要有物理处理法(微滤、反渗透、电渗析、磁选)、化学处理法(沉淀、结晶、阴离子交换和吸附)和生物处理(同化、强化生物除磷、人工湿地、废水稳定池)[6~9\在众多的处理方法中,吸附处理法具有成本低、效率高、选择性好、操作简单,以及对废水pH值无明显影响等优点,而备受欢迎与传统炭质吸附剂相比,生物炭不仅具有传统炭质类材料的吸附特点,而且原材料易获得、制备方法简单、成本低廉,因而,成为一种备受关注的新型吸附剂[10>11\然而,由于生物炭的金属阳离子含量低以及其热解过程中金属离子的过度损耗,生物炭作为吸附剂一般对阳离子和有机污染物有很强的吸附能力,但它对阴离子污染物的吸附能力有限卫扛因此,需要对生物炭进行改性以提高其对阴离子污染物的吸附性。
Infrared spectroscopic and X-ray diffraction characterizationof the nature of adsorbed arsenate on ferrihydriteYongfeng Jiaa,*,Liying Xu a ,Xin Wang a ,George P.Demopoulosb,*aInstitute of Applied Ecology,Key Laboratory of Terrestrial Ecological Process,Chinese Academy of Sciences,Shenyang 110016,ChinabDepartment of Mining,Metals and Materials Engineering,McGill University,Montreal,QC,Canada H3A 2B2Received 30March 2006;accepted in revised form 14December 2006;available online 30January 2007AbstractFourier transformed infrared (FTIR)spectroscopy was used to characterize arsenate–ferrihydrite sorption solids synthe-sized at pH 3–8.The speciation of sorbed arsenate was determined based on the As–O stretching vibration bands located at 650–950cm À1and O–H stretching vibration bands at 3000–3500cm À1.The positions of the As–O and O–H stretching vibra-tion bands changed with pH indicating that the nature of surface arsenate species on ferrihydrite was strongly pH dependent.Sorption density and synthesis media (sulfate vs.nitrate)had no appreciable effect.At acidic pH (3,4),ferric arsenate surface precipitate formed on ferrihydrite and constituted the predominant surface arsenate species.X-ray diffraction (XRD)analyses of he sorption solids synthesized at elevated temperature (75°C),pH 3clearly showed the development of crystalline ferric arsenate (i.e.scorodite).In neutral and alkaline media (pH 7,8),arsenate sorbed as a bidentate surface complex (in both pro-tonated B FeO 2As ðO ÞðOH ÞÀand unprotonated B FeO 2As ðO Þ22Àforms).For the sorption systems in slightly acidic media (pH 5,6),both ferric arsenate and surface complex were probably present on ferrihydrite.It was further determined that the incor-porated sulfate in ferrihydrite during synthesis was substituted by arsenate and was more easily exchangeable with increasing pH.Ó2007Elsevier Ltd.All rights reserved.1.INTRODUCTIONFerrihydrite is a poorly ordered hydrous iron oxide commonly present in low-temperature geochemical pro-cesses.It is widely occurring in surface environments,e.g.in soils,lake and river sediments and water columns (Way-chunas et al.,1993;Cornell and Schwertmann,1996;Jam-bor and Dutrizac,1998).Arsenate is an important form of arsenic in both natural water systems and mineral pro-cessing tailings.Ferrihydrite shows strong affinity for arse-nate at mineral–water interfaces.Adsorption on ferrihydrite is an important factor controlling transport,fate and bioavailability of arsenic in soils,groundwaterand surface water systems (Smedley and Kinniburgh,2002).Ferrihydrite is also a common product in many hydrometallurgical operations such as the coprecipitation process of iron with arsenate as well as in acid mine drain-age (Jambor and Dutrizac,1998;Carlson et al.,2002).Hence the adsorption of arsenate on ferrihydrite plays an important role in the removal and immobilization of ar-senic from industrial effluents as well as the fate of arsenate in tailings impoundment.Adsorption of arsenate on ferrihydrite involves ligand exchange with H 2O and/or OH Àon the substrate surface (Jain et al.,1999).Factors influencing adsorption of arse-nate on ferrihydrite include medium pH,type and con-centration of co-ions,initial Fe/As molar ratios etc.pH is one of the most important factors that control aqueous arsenate speciation and surface functional groups of hy-drous oxides,consequently influencing the macroscopic characteristics of adsorption process and the microscopic characteristics such as bonding modes and the nature of0016-7037/$-see front matter Ó2007Elsevier Ltd.All rights reserved.doi:10.1016/j.gca.2006.12.021*Corresponding authors.Fax:+862483970436(Y.Jia),+15143984492(G.P.Demopoulos).E-mail addresses:yongfeng.jia@ (Y.Jia),george.demopoulos@mcgill.ca (G.P.Demopoulos)./locate/gcaGeochimica et Cosmochimica Acta 71(2007)1643–1654adsorbed arsenate on the surface of ferrihydrite(Mas-scheleyn et al.,1991;Hsia et al.,1992;Fuller et al., 1993;Bowell,1994;Wilkie and Hering,1996;Raven et al.,1998;Jain et al.,1999;Meng et al.,2000;Grafe et al.,2002;Dixit and Hering,2003).The degree of pro-tonation of arsenate anion in aqueous solution is a func-tion of pH with p K a1=2.3,p K a2=6.8and p K a3=11.6 (Goldberg and Johnston,2001),resulting in arsenate spe-cies varying from H3AsO4,H2AsO4À,HAsO42À,toAsO43Àwhen pH increases from acidic region to alkalineregion(Myneni et al.,1998;Raven et al.,1998;Goldberg and Johnston,2001).On the other hand,the presence and the density of surface groups of ferrihydrite,i.e. H2O,OHÀ,are also strongly pH dependent.The point of zero charge(PZC)is approximately8.5(Jain et al., 1999;Goldberg and Johnston,2001).Hence,the modes of complexation of arsenate anions with ferrihydrite by replacing surface hydroxyl groups and/or waters are lar-gely controlled by the pH of reaction medium.Ligand exchange in the mode of bidentate binuclear in-ner-sphere complexation is the widely accepted mechanism of the adsorption of arsenate on iron oxides.It has been proposed based on infrared(Harrison and Berkheiser, 1982)and extended X-ray absorptionfine structure(EX-AFS)(Waychunas et al.,1993)analyses and confirmed to be the dominant interaction mode of arsenate–ferrihydrite and arsenate–goethite systems(Lumsdon et al.,1984;Man-ceau,1995;Sun and Doner,1996;Waychunas et al.,1996; Fendorf et al.,1997;Foster et al.,1998;Myneni et al.,1998; O’Reilly et al.,2001;Roddick-Lanzilotta et al.,2002;Sher-man and Randall,2003;Arai et al.,2004;Cance`s et al., 2005;Waychunas et al.,2005).However,most of the stud-ies were conducted at neutral to alkaline pH.The applica-bility of the conclusions to the acidic arsenate–ferrihydrite adsorption system may be questionable.Moreover,most of the studies have dealt mainly with characterizing the bonding mechanism between arsenate anions and surface iron polyhedra without identifying arsenate species on the surface of iron oxide.Based on macroscopic measurements of the adsorption process,arsenate was adsorbedas B FeO2AsðOHÞ2;B FeO2AsðOÞðOHÞÀ;B FeO2AsðOÞ22Àon the surface of ferrihydrite at mildly acidic and alkaline pH(Jain et al.,1999).In a recent work on direct character-ization of arsenate coordination on mineral(portlandite, gibbsite,ettringite,Fe-oxyhydroxides)surfaces using FTIR, both protonated and unprotonated arsenate species(i.e.HAsO42Àand AsO43Àwere present on the goethite surfaceat alkaline pH(Myneni et al.,1998).When arsenate was ad-sorbed on schwertmannite and ferrihydrite at acidic pH(i.e. pH3),surface precipitates were proposed to form and were termed as ferric hydroxyarsenate(FeOHAs)(Carlson et al., 2002).Evidence for surface precipitation of phosphate on goethite has been observed(Ler and Stanforth,2003).Sim-ilarly,surface precipitation of ferric arsenate on ferrihydrite is likely to occur in addition to bidentate binuclear com-plexation according to the XRD and Raman spectroscopic evidence we reported recently(Jia et al.,2006).The objective of this paper was to provide further evi-dence of surface precipitation of ferric arsenate on ferrihy-drite.This was done via characterization of the interactions between arsenate and ferrihydrite in terms of bonding modes and surface arsenate species as a function of pH and coverage density by Fourier transformed infrared spec-troscopy(FTIR)and evolution of crystallinity at elevated temperature(75°C)by X-ray diffraction(XRD)analysis. The effect of ferrihydrite synthesis media(NO3Àvs.SO42À)was also evaluated since it significantly influenced arsenate adsorption capacity(Jia and Demopoulos,2005). Poorly crystalline ferric arsenate was used as reference material in the study.Moreover,relatively high arsenic con-centration solutions were used in this study since this is the case in important hydrometallurgical operations where arsenic removal is practiced.2.MATERIALS AND METHODS2.1.Synthesis of poorly crystalline ferric arsenatePoorly crystalline ferric arsenate was synthesized at 21°C by adjusting a0.02M As(V)/0.02M Fe(III)solution (as sodium arsenate and ferric sulfate)from initial pH1.3to 1.8with NaOH solution and maintained at that pH for1h (Jia et al.,2006).The resultant solid was separated byfiltra-tion,washed with de-ionized water(pH2)and vacuum-dried at60°C.The chemical formula(Fe1.02AsO4Æ2.4H2O) was determined by digestion with hydrochloric acid fol-lowed by ICP-AES analysis.2.2.Synthesis of arsenate–ferrihydrite sorption samplesTwo-line ferrihydrite samples were synthesized at21°C using a slightly modified procedure from that reported in the literature(Schwertmann and Cornell,1991).Both ni-trate(Fe(NO3)3Æ9H2O)and sulfate(Fe2(SO4)3Æ5H2O)salts were used as the sources of ferric iron[Fe(III)].Briefly, the Fe(III)solution was prepared by dissolving ferric ni-trate or ferric sulfate in de-ionized water.The pH of the solution was raised to$7.5in about5min using1M NaOH solution and maintained at that pH for1h with the slurry mechanically agitated vigorously.The ferrihy-drite samples synthesized from sulfate and nitrate media were termed as‘‘sulfate–ferrihydrite’’and‘‘nitrate–ferrihy-drite’’,respectively,for simplicity.The prepared ferrihydrite slurry was adjusted to differ-ent pH between3and8with NaOH and HNO3and al-lowed to equilibrate for1h.Arsenate solution was introduced into the ferrihydrite slurry from a burette over a10-min period with the slurry mechanically stirred moder-ately.The pH was controlled by addition of NaOH and/or HNO3solution and allowed to equilibrate at21°C for 2weeks.The volume of adsorption slurry was500mL for all experiments and the concentration of Fe(III)in the slur-ry system was4g/L.At each pH,three initial Fe/As molar ratios(i.e.Fe/As=2,4and8)were applied.Arsenate–fer-rihydrite sorption samples were also synthesized at75°C, Fe/As molar ratio of2and4.The pH of the slurry was con-trolled constant at pH3throughout the sorption reaction. Samples were taken at1day,3day,1week,2weeks and 2months of reaction time.The synthesized arsenate–fer-rihydrite sorption products werefiltered,DI water-rinsed1644Y.Jia et al./Geochimica et Cosmochimica Acta71(2007)1643–1654and vacuum-dried at60°C.The equilibrium concentration of arsenic was determined by ICP-AES analysis.2.3.FTIR analysisThe infrared spectra of the samples were obtained on a Bio-Rad FTS60Fourier Transformed Infrared Spectrome-ter with a MCT liquid nitrogen cooled detector.The KBr/ sample discs were prepared by mixing0.5%offinely ground samples in KBr.The sample chamber was purged by N2gas for10min before scans were started.The measurement res-olution was set at4cmÀ1and the spectra were collected in the range of400–4000cmÀ1with200co-added scans.2.4.X-ray diffraction(XRD)analysisThe powder XRD patterns were collected on a Rigaku D/Max2500PC X-ray diffractometer with graphite mono-chromated CuK a1radiation.The powder samples were scanned from10to90°2h with increments of0.02°2h.3.RESULTS AND DISCUSSION3.1.Effect of pH on the nature of adsorbed arsenateAqueous arsenate species have no direct bearing on the surface arsenate species adsorbed on mineral surfaces (Myneni et al.,1998).However,the effect of complexation of arsenate ions on oxide surfaces is similar to that of pro-tonation of aqueous arsenate species.A brief discussion on infrared absorption of aqueous arsenate can assist with understanding the infrared characteristics of surface species (Myneni et al.,1998;Goldberg and Johnston,2001;Rod-dick-Lanzilotta et al.,2002).The free arsenate anion,AsO43À,is present in highly alkaline(p K a3=11.6)aqueoussolution and belongs to T d symmetry.Only m3and m4funda-mental bands are infrared active in this form.The infrared spectrum of an AsO43Àdominated solution exhibits a major band at792cmÀ1(Roddick-Lanzilotta et al.,2002).Uponprotonation or complexation with metal cations,the sym-metry decreases and splitting of the m3band occurs(Harri-son and Berkheiser,1982;Myneni et al.,1998).AqueousHAsO42Àspecies belong to the C3v symmetry.Its infrared spectrum shows two broad bands at859and689cmÀ1, the latter was assigned to stretching vibration of As–OH (Myneni et al.,1998).Curvefitting of the former band gave two bands at865and846cmÀ1,which were assigned to asymmetric and symmetric stretching vibration of uncom-plexed As–O,respectively(Myneni et al.,1998).Poorly crystalline ferric arsenate was used as reference material in this study to identify the possible occurrence of a surface precipitate of arsenate on ferrihydrite.This compound is not well defined and often termed loosely as amorphous ferric arsenate(Krause and Ettel,1989)or amorphous scorodite(Langmuir et al.,1999),because it possesses similar bonding structures to crystalline ferric arsenate,i.e.scorodite(FeAsO4Æ2H2O).It is an unstable arsenate phase with increasing pH and tends to convert to ferrihydrite(Krause and Ettel,1989).The poorly crystalline ferric arsenate synthesized in this work was determined to have the formula Fe1.02AsO4Æ2.4H2O.Fig.1shows the effect of pH on the infrared spectra of the sorption samples of arsenate on sulfate–ferrihydrite(initial molar ratio of Fe/As=2).Both detailed display of the As–O stretching vibration region(500–1000cmÀ1)and the whole range of the scanning(400–4000cmÀ1)are shown in thefigure.Poorly crystalline ferric arsenate shows a strong well-resolved band at838cmÀ1.Within the crystalline ferric arsenate(i.e.scorodite)structure,AsO4tetrahedra and FeO4(OH2)2octahedra connect alternately at vertices(Kita-hama et al.,1975).The arsenate is coordinated with four iron octahedra with an average As–O bond length of1.68A˚.The band at838cmÀ1was attributed to the stretching vibration of As–O coordinating to iron atom,i.e.As–O–Fe.The weakThe nature of adsorbed arsenate on ferrihydrite1645shoulder at$750cmÀ1was probably caused by the hydrogen bonding between H2O and AsO4since the H-bonding re-sulted in increased bond length and a red shift of the wave number(Myneni et al.,1998).The1625cmÀ1band was due to water O–H bending mode whereas the stretching vibration bands of O–H were located at$3194and$3373cmÀ1.The bands between950and1250cmÀ1were assigned to struc-tural SO42Àions,which were incorporated into the poorlycrystalline ferric arsenate by substitution of AsO43Àions dur-ing synthesis from sulfate medium.Sulfate ions were incorpo-rated into crystalline scorodite synthesized from sulfate solution(Singhania et al.,2005).The infrared spectra of pH3and4sorption samples also exhibited a strong,well-resolved band in the As–O stretching vibration region at similar position($833cmÀ1)to that of poorly crystalline ferric arsenate,indicating similarities of the arsenate bonding structures between sorption samples and poorly crystalline ferric arsenate.This suggested the for-mation of a ferric arsenate surface precipitate in the arsenate–ferrihydrite sorption samples synthesized in acidic media. However,the weak shoulder at$750cmÀ1on the FTIR spec-trum of the poorly crystalline ferric arsenate was missing for the sorption solids for some unknown reasons.The band at$833cmÀ1for the pH3and4sorption samples was assigned to As–O stretching vibration of the As–O–Fe coordination of ferric arsenate precipitate on fer-rihydrite.The formation of ferric arsenate phase in pH3 and4sorption samples was also supported by the O–H stretching vibration band at$3190cmÀ1.All samples showed a strong broad O–H band at$3370,but only theacidic sorption samples displayed the$3190cmÀ1O–H stretching vibration band like the case of poorly crystalline ferric arsenate.This characteristic O–H band of poorly crystalline ferric arsenate at$3190cmÀ1was fading out with increasing pH,indicating the disappearance of ferric arsenate surface precipitate at neutral and alkaline pH.As pH increased from3to8,the As–O stretching vibra-tion band shifted gradually from$833cmÀ1down to $806cmÀ1.At the same time,a new band emerged at high-er frequency(870–880cmÀ1)and its intensity was more pronounced with increasing pH.At pH8,we could clearly see the splitting of the single band into two bands.Peak deconvolution and curvefitting of the band produced two peaks at$806–810and$878cmÀ1(Fig.2).It is well estab-lished that at mildly alkaline pH,arsenate is adsorbed on ferrihydrite via bidentate binuclear complexation with surface iron polyhedra(Harrison and Berkheiser,1982; Waychunas et al.,1993).The band at$878cmÀ1of the pH6–8sorption samples was assigned to uncomplexed/ unprotonated As–O,whereas the$806–808cmÀ1arose from the two As–O–Fe complexed to ferrihydrite surface. Two infrared bands at824/861and817/854cmÀ1were ob-served for the arsenate adsorbed on amorphous iron oxide at pH5and9,respectively(Goldberg and Johnston,2001). The lower frequency bands at817and824cmÀ1were as-signed to the stretching vibration of As–O–Fe and the high-er frequency bands at854and861cmÀ1were attributed to ‘‘non-surface-complexed’’As–O bonds of the adsorbed arsenate species(Goldberg and Johnston,2001).It is inter-esting to note that the lower frequency band increased from 817and824cmÀ1as pH increased from5to9.This obser-vation and the band assignments are in good agreement with present work.Roddick-Lanzilotta et al.(2002)also reported that the As–O stretching vibration band of the ad-sorbed arsenate on ferrihydrite shifted from$825to $800cmÀ1as pH increased from2.6to8.In the case of bidentate binuclear complexation,two of the four As–O bonding structures are complexed to iron atoms(i.e.As–O–Fe)and the remaining two are present either both as unprotonated As–O or one as unprotonated As–O and the other one as protonated As–O–H.In com-parison,arsenate ions are coordinated to four iron atoms in ferric arsenate.According to Myneni et al.(1998),the force constant of the As–OM bond increases with coordina-tion number and decreases compared to uncomplexed As–O.Hence,for the bidentate adsorbed arsenate ion,the force constant of the two coordinated As–O–Fe is lower than that of the As–O–Fe in ferric arsenate,whereas the uncomplexed/unprotonated As–O bond has larger force constant compared to ferric arsenate.Consequently,the stretching vibration frequency of the uncomplexed/unprot-onated As–O is located at higher position while the fre-quency of the complexed As–O–Fe band is located at lower position.The increase and decrease of the As–O force constant for the bidentate binuclear complexed arsenate compared to ferric arsenate is supported by the As–O bond length(two at1.62,1.67A˚and the other two at1.71A˚, compared to1.68A˚of ferric arsenate)(Sherman and Ran-dall,2003).The shorter bond distance results in a stronger force constant and consequently higher infrared frequency.1646Y.Jia et al./Geochimica et Cosmochimica Acta71(2007)1643–1654A very weak band was observed at$700cmÀ1on the infrared spectrum of pH8arsenate–ferrihydrite sorption sample(see Fig.1).This band was reasonably assigned to protonated As–O–H bond of the adsorbed arsenate species, which was located at similar position to that of aqueous protonated arsenate species(Myneni et al.,1998).Complex-ation with metals cannot give such a low As–O stretching vibration frequency.The presence of protonated arsenate species was also proposed for the adsorption of arsenate on freshly prepared hydrous iron oxide and goethite(Myn-eni et al.,1998).It was noted that the weak As–O–H band at$700cmÀ1was absent at acidic pH indicating the ab-sence of protonated adsorbed arsenate species on ferrihy-drite at acidic pH.In a previous study using a dispersion infrared instrument(Harrison and Berkheiser,1982),three bands at875,805and700cmÀ1were observed for the ad-sorbed arsenate on hydrous ferric oxide(HFO)at pH6.5. They are very similar to the infrared bands of the pH8 sorption samples of this work(878,806and700cmÀ1).pH3and8are the extreme cases for the adsorption of arsenate on ferrihydrite in this work.At pH3,a sur-face precipitate developed and the adsorbed arsenate spe-cies were present mainly as poorly crystalline ferric arsenate.The possibility of surface precipitation of arse-nate on ferrihydrite was also suggested previously by Stanforth(1999)and Carlson et al.(2002).According to the latter research,a poorly crystalline ferric hydroxy-arsenate(FeOHAs)surface precipitate was found to form during adsorption of arsenate on schwertmannite and fer-rihydrite at pH3(Carlson et al.,2002).Similarly,surface precipitation of phosphate on goethite has been proposed in recent studies(Zhao and Stanforth,2001;Ler and Stanforth,2003).It was suggested that the adsorption reaction consisted of two phases:thefirst phase of rapid surface complexation followed by the second phase of slow buildup of a surface precipitate(Zhao and Stan-forth,2001).At the other extreme(i.e.pH8)arsenate was adsorbed via bidentate binuclear complexation with surface iron atoms in the form of unprotonated and probably protonated arsenate species as well(i.e.B FeO2AsðOÞ22Àand B FeO2AsðOÞðOHÞÀ, where B Fe represents the surface of ferrihydrite).This is in good agreement with the model proposed previously by Myneni et al.(1998),Jain et al.(1999)and Goldberg and Johnston(2001).Myneni et al.(1998)suggested that both protonated and unprotonated arsenate species were present as surface arsenate species adsorbed on ferrihydrite at alkaline pH.The surface arsenate specieswere proposed to be XH2AsO4,XHAsO4À,XAsO42À(X was Al or Fe)(Jain et al.,1999;Goldberg and Johnston, 2001).As pH increased from3to8,surface arsenate species shifted from poorly crystalline ferric arsenate precipitates to bidentate surface complexes.This is reasonable since poorly crystalline ferric arsenate is stable only at acidic pH and decomposes with increasing pH(Krause and Ettel, 1989).For the sorption systems whose media pHs lay be-tween3and8,both types of arsenate species were probably present on the surface of ferrihydrite,with the feature of poorly crystalline ferric arsenate being more pronounced at lower pH and the feature of bidentate complexes being more pronounced at higher pH.Similar to the Fe/As=2systems,the single As–O stretching vibration band shifted down gradually and split into two bands as pH increased from3to8for the Fe/As= 4sorption samples(Fig.3).The single band at acidic pH was assigned to As–O–Fe of ferric arsenate surface pre-cipitate.The presence of$3190cmÀ1O–H stretching vibra-tion band also indicated the development of ferric arsenate in the sorption solids at acidic pH.This band was absent onThe nature of adsorbed arsenate on ferrihydrite1647the infrared spectra of sorption samples at alkaline pH.The two bands(808and878cmÀ1)at alkaline pH are attributed to As–O–Fe bidentate–binuclear coordinating to ferrihy-drite and the uncomplexed/unprotonated As–O bond, respectively.The presence of protonated As–O–H bond could not be ruled out,since there appeared to be a weak feature at$700cmÀ1.It was interesting to note that all sorption samples from pH3to8had very similar sorption density(i.e.As/Fe$0.25,see Table1),but the surface arse-nate species varied from poorly crystalline ferric arsenate to bidentate–binuclear surface complexes as pH increased. This was indicative that the nature of surface arsenate spe-cies strongly depended on the pH of the sorption media.The spectra of pH3and4sorption samples showed strong sulfate bands between950and1250cmÀ1.Sulfate ions were apparently adsorbed onto the ferrihydrite during synthesis from sulfate medium and displaced by arsenate during adsorption as discussed elsewhere(Jia and Demopo-ulos,2005).At higher pH(i.e.5–8),the sulfate ions were substituted by arsenate ions as indicated by the absence of sulfate bands on the infrared spectra.For the adsorption systems of Fe/As=2,no sulfate band was observed for all pH samples(see Fig.1).Ferrihydrite synthesized from sulfate media was found to adsorb significantly more arsenate than that from nitrate media(Jia and Demopoulos,2005),which could not be ex-plained by the difference in BET surface areas.Therefore,it was of interest to compare the surface arsenate species of the two types of arsenate–ferrihydrite sorption samples. Fig.4shows the effect of pH on the infrared spectra of arsenate adsorbed on nitrate–ferrihydrite.Apparently the spectra are similar to those of arsenate adsorbed on sul-fate–ferrihydrite(Fig.1).Both are dominated by the strong As–O stretching vibration bands at700–950cmÀ1and strong O–H stretching vibration bands at3000–3500cmÀ1.In the As–O stretching vibration region(700–950cmÀ1),the infrared spectra of acidic sorption samples (pH3,4)exhibited a strong,well-resolved single band at similar position to that of poorly crystalline ferric arse-nate.This was indicative that a ferric arsenate surface precipitate formed when arsenate adsorbed on nitrate–fer-rihydrite at acidic pH,similar to As(V)adsorption onTable1Arsenic equilibrium concentration(mg/L)and sorption density(mol-As/mol-Fe)for the adsorption of arsenate on ferrihydrite synthesized from sulfate and nitrate medium at pH3–8and initial Fe/As molar ratio of2,4and8pH Fe/As=2Fe/As=4Fe/As=8Sulfate Nitrate Sulfate Nitrate Sulfate3198(0.49)318(0.46) 1.0(0.25) 1.9(0.25)<0.02(0.125) 4321(0.46)471(0.43) 1.9(0.25) 3.4(0.25)0.2(0.125) 5440(0.44)615(0.41) 3.6(0.25)14.8(0.25)0.2(0.125) 6498(0.43)788(0.38) 5.0(0.25)29.6(0.25)0.2(0.125) 7702(0.40)1070(0.32)15.8(0.25)100(0.24)0.6(0.125) 81178(0.30)1439(0.25)141(0.24)178(0.23) 2.1(0.125)The numbers in bracket are sorption densities,i.e.As/Fe molar ratio of the solids.1648Y.Jia et al./Geochimica et Cosmochimica Acta71(2007)1643–1654sulfate–ferrihydrite.As pH increased,the single As–O stretching vibration band split into two strong bands at $806–810cmÀ1and$878cmÀ1as observed for the sul-fate–ferrihydrite samples(see Fig.1)and were attributed to the As–O stretching vibration of bidentate binuclear complexed arsenate ions.The formation of a ferric arse-nate surface precipitate at acidic pH was also evidenced by the change of the$3190cmÀ1O–H band with pH.The spectrum of ferrihydrite displayed strong NO3Àbands at1250–1500cmÀ1.These bands disappeared after adsorption of arsenate indicating that nitrate ions previ-ously adsorbed during ferrihydrite synthesis were dis-placed by arsenate ions.3.2.Effect of coverage density on the nature of adsorbed arsenateThe effect of coverage density on the nature of adsorbed arsenate on ferrihydrite was evaluated.At acidic pH(i.e. pH3,4),the As–O stretching vibration region(700–950cmÀ1)was dominated by a strong well-resolved band (Fig.5).As the Fe/As molar ratio increased from2to8(i.e.the adsorption density As/Fe decreasing from0.49to0.125)for the pH3sorption systems(see Table1),the intensity of the As–O stretching vibration band decreased markedly.The As–O stretching vibration peak was located at similar position.The pH4arsenate–ferrihydrite sorptionThe nature of adsorbed arsenate on ferrihydrite1649samples with various arsenate sorption densities exhibited very similar infrared spectra to that of pH3samples.Poor-ly crystalline ferric arsenate was the major surface arsenate species.The nature of surface arsenate species was not sig-nificantly influenced by arsenate sorption density and equi-librium concentration.At pH6,the major As–O stretching vibration peak was located at$822cmÀ1for all the initial Fe/As molar ratios used,i.e.2,4and8.A visible shoulder was also emerging at $878cmÀ1on the infrared spectra.A bidentate complex of arsenate was becoming detectable in addition to the major surface species of ferric arsenate.When pH increased to8,all infrared spectra exhib-ited two bands at808and878cmÀ1irrespective of the initial Fe/As molar ratios indicating that bidentate com-plexes were the dominant surface arsenate species.The equilibrium concentration of arsenic ranged from0.028 to15.8mM for the pH8sorption systems and the sorp-tion density(As/Fe)ranged from0.125to0.31.Waych-unas et al.(1993)conducted EXAFS analysis on samples with sorption density of0.001–0.1and concluded that arsenate was sorbed via bidentate surface complexation. Goldberg and Johnston(2001)used FTIR to character-ize the arsenate–iron oxide sorption solids synthesized at pH5and9with equilibrium concentration of0.1–1.0mM and suspension density of4g/L($8g/L for the present work).The obtained results showed similar As–O band to the present study,i.e.two bands located at$817–824and$854–861cmÀ1.The infrared spectrum obtained by Roddick-Lanzilotta et al.(2002)for the sorption system pH2.6and arsenic equilibrium concen-tration of$0.5mM displayed a well-resolved band $825,which is similar to the single As–O band of the acidic samples in this work.Carlson et al.(2002)pro-posed the formation of ferric hydroxyarsenate in Fe/As=2.6–3.2sorption solids prepared from solutions of pH3.Therefore,by comparing the present study with other works,it is proposed that the pH plays the most impor-tant role in controlling the nature of the surface arsenate species sorbed on ferrihydrite,whereas other conditions (e.g.coverage density,suspension density etc.)play less important factors.The fate of sulfate in synthetic ferrihydrite before and after adsorption of arsenate can also be monitored on the infrared spectra.As indicated by the bands between950 and1250cmÀ1,more sulfate was incorporated into the fer-rihydrite at acidic pH(3,4)than neutral to alkaline pH (6,8)(Fig.5).After adsorption of arsenate at pH3,4 and Fe/As=4,8,there was still measurable amount of sul-fate remaining in the ferrihydrite,while at Fe/As=2,all sulfate has been desorbed.It was proposed that sulfate ions were adsorbed on goethite as both inner-sphere and outer-sphere surface complexes at acidic pH(Peak et al.,1999). Adsorption of arsenate on the ferrihydrite involved ligand exchange with previously adsorbed sulfate ions.With increasing pH,the adsorbed sulfate ions on ferrihydrite were more easily displaceable by arsenate.At pH6,sulfate bands were visible only on the Fe/As=8infrared spectrum while no sulfate was detectable by FTIR in the pH8sam-ples.This is in good agreement with a previous study that reported the quantitative analysis of the sorption solids (Jia and Demopoulos,2005).It was suggested that surface precipitation of phosphate and arsenate on goethite occurred almost simultaneously (Zhao and Stanforth,2001;Ler and Stanforth,2003).The process of surface precipitation may involve slow dissolution of ferrihydrite,ternary complexation of Fe3+ and subsequent precipitating of arsenate(Ler and Stan-forth,2003).The process was depicted schematically in Fig.6.It was not surprising that arsenate surface precipitate formed at such a high initial Fe/As molar ratio of8where arsenic equilibrium concentration is below the detection limit(<0.02mg/L)in this work.There possibly also existed tridentate complex structure that formed initially upon contacting of the arsenate ions with ferrihy-drite given the amorphous nature of the latter.The saturation state with respect to poorly crystalline ferric arsenate in acidic media was estimated in a previous study(Jia et al.,2006).When the p K sp(ferrihydrite)=39 (Langmuir,1997)and p K sp(ferric arsenate)=22.89 (Mahoney,2002)were taken in the calculation,the log IAPð½Fe3þ ½AsO43À was obviously lower than log K sp (ferric arsenate)for the pH3,Fe/As=4and Fe/As=8 sorption systems,indicating that the systems were undersat-urated with respect to poorly crystalline ferric arsenate.For。
膜吸附磷酸根膜吸附磷酸根是一种常见的水处理技术,用于去除水体中的磷酸根离子。
膜吸附是一种通过物质与膜表面之间的吸附作用,将目标污染物从水中分离的过程。
在膜吸附磷酸根的过程中,往往会使用一些特定的吸附剂或吸附材料,下面是关于膜吸附磷酸根的相关参考内容。
1. Zhang L, Li J, Liu L, et al. Adsorptive removal of phosphate from water using graphene oxide-incorporated nanofiltration membranes[J]. Journal of Membrane Science, 2016, 501: 110-118. 这篇文章介绍了一种利用石墨烯氧化物(GO)改性的纳滤膜来吸附去除水中磷酸根的方法。
实验结果表明,GO纳滤膜具有良好的吸附去除磷酸根的性能。
2. Bastami T R, Bueken B, Denayer J F M, et al. Microporous coordination polymers as adsorbents for phosphate removal from water[J]. Chemical Engineering Journal, 2019, 360: 1124-1133.这篇文章研究了一种基于微孔配位聚合物(MCPs)的磷酸根吸附剂,通过调节MCPs的孔径和表面化学性质,实现了高效去除水中磷酸根的目的。
3. Khan M I, Lee J, Ilyas H, et al. Ultrathin graphene oxide membranes for effectively removing phosphate from water[J]. Nanotechnology, 2018, 29(41): 415701.该研究报道了一种利用超薄氧化石墨烯膜高效去除水中磷酸根的方法。
第51卷第8期 辽 宁 化 工 Vol.51,No. 8 2022年8月 Liaoning Chemical Industry August,2022收稿日期: 2022-03-07铈改性吸附材料去除水体中磷酸盐的研究进展韩雨桐,唐婧(沈阳建筑大学市政与工程学院, 辽宁 沈阳 110168)摘 要: 铈被证明具有去除磷酸盐的巨大潜力,将铈应用于常规吸附材料改性可提高其吸附磷酸盐的性能,可用于缓解日益加剧的水体富营养化。
归纳了国内外有关铈改性吸附材料除磷的相关研究,总结了铈改性吸附材料的吸附机理,介绍了铈改性的不同种类吸附材料,并指出了当前研究的不足和对未来的展望,为铈改性吸附材料去除水体中磷酸盐的发展提供了理论基础。
关 键 词:铈改性;吸附材料;除磷;吸附机理中图分类号:X703 文献标识码: A 文章编号: 1004-0935(2022)08-1137-04磷的过度排放会导致藻类和浮游生物的大量繁殖,使得水体能见度和水中溶解氧浓度的降低,造成水体富营养化。
目前已采用了生物法、化学沉淀法、电化学法、吸附法[1]和生态法等多种方法去除水体中的磷酸盐。
其中生物法可去除水体中90%的磷元素,但它的处理效果不够稳定,在实际应用中存在局限[2];化学法的投资相对较高,难以避免大量化学污泥所造成的二次污染;生态法更是难以在源头进行治理,存在见效时间过长等一系列问题;而吸附法具有性能稳定、吸附效果优良和污染少等优良特点,被认为是一种可行的高效低耗去除水体中磷酸盐的方法。
常用的除磷吸附剂有固体废弃物类吸附剂、天然黏土类吸附剂和金属及其氧化物类吸附剂等。
金属改性吸附材料具有比表面积大[3]、对磷酸盐的强亲和力和富含羟基团等特点,目前有很多用铝、铁、镁、钛、镧、铈等金属改性常规吸附材料制备除磷吸附剂的研究,其中铈对磷酸盐的吸附效果和选择性较好,是制备高效除磷吸附剂的重要选择对象。
1 铈对磷酸盐的吸附机制铈是一种发现较早、丰度最高、毒性较低的稀土元素,具有独特的4f 电子层,也是唯一具有双价态(Ce(III)和Ce(Ⅳ))的镧系元素。
淀粉质吸附剂选择性分离醇/水共沸物实验研究的开题报告一、选题背景及研究意义在化工生产中,醇/水共沸物是一类具有广泛应用前景和巨大经济价值的混合物。
但是,醇/水共沸物在分离过程中难以完全分离,从而造成生产成本的增加和产品质量的下降。
因此,开发一种简单、高效、经济、环保的醇/水共沸物分离技术至关重要。
本研究将探索一种新的淀粉质吸附剂选择性分离醇/水共沸物的方法,并进一步探究其分离机理,旨在为醇/水共沸物的高效分离提供理论支持,并为化工工业的推广应用提供技术储备。
二、研究内容1.淀粉质吸附剂制备及性质表征;2.淀粉质吸附剂选择性吸附不同醇/水共沸物实验研究;3.淀粉质吸附剂选择性冲洗醇/水共沸物实验研究;4.分析分离机理。
三、研究方法1.淀粉质吸附剂制备:采用化学合成法制备淀粉质吸附剂,并进行表征;2.选择性吸附实验:利用床层吸附器,采用不同体积比的DMSO/H2O、甲醇/H2O、乙醇/H2O醇/水共沸物进行选择性吸附实验;3.选择性冲洗实验:采用不同的冲洗方案进行选择性冲洗实验;4.分析分离机理:利用红外光谱、CTAB等方法对吸附前后吸附剂的形貌和表面化学特性进行分析,研究分离机理。
四、预期结果通过淀粉质吸附剂选择性吸附和分离实验,分析分离机理,预计能够获得以下结果:1.制备出性能优异的淀粉质吸附剂,表征其吸附性能和物化特性;2.确定淀粉质吸附剂对醇/水共沸物的选择性吸附性能,并优化操作条件;3.探究淀粉质吸附剂选择性冲洗方案,分析分离机理;4.建立醇/水共沸物淀粉质吸附剂分离机制的理论模型。
五、参考文献[1] Issa, R.M.; Haider, A.J.S; Khashman, B. (2017). The Effect of the Solvents on the Physical and Structural Properties of PVA/AA Blend Membranes, Acta Chromatographica, 29(1): 65-76.[2] Nghiem, L.D.; Huynh, L.H.; Nguyen, D.D.; et al. (2015). Development of Novel cationic cellulose-silica co-extruded adsorbent for the removal of anionic dyes from aqueous solution. Chemical Engineering Research and Design, 95 :43–54.[3] Bhattacharyya, D.; Gupta, S.S. (2008). Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: a review, Advances in Colloid and Interface Science, 140(2): 114–131.[4] He, L.N.; Zhang, X.Q.; Wu, X.Z. (2016). Investigation of the selective adsorption and separation of benzene, toluene, and xylene on the coal based activated carbon fibers. Microporous and Mesoporous Materials, 224:350–359.。
Journal of Colloid and Interface Science297(2006)426–433/locate/jcisSelective adsorption of phosphate from seawater and wastewater byamorphous zirconium hydroxideRamesh Chitrakar,Satoko Tezuka,Akinari Sonoda∗,Kohji Sakane,Kenta Ooi,Takahiro Hirotsu Health Technology Research Center,National Institute of Advanced Industrial Science and Technology(AIST),2217-14Hayashi-cho,Takamatsu761-0395,JapanReceived5September2005;accepted8November2005Available online7December2005AbstractPhosphate adsorption from single electrolyte(NaH2PO4),phosphate-enriched seawater,and model wastewater was studied using amorphous zirconium hydroxide,ZrO(OH)2·(Na2O)0.05·1.5H2O,as an adsorbent.Batch experiments were carried out to investigate the adsorption of phos-phate.The effect of pH on phosphate adsorption from seawater showed that the uptake of phosphate increased with an increase in pH up to6,and then decreased sharply with a further increase in pH of the solution.The equilibrium data of phosphate adsorption were followed with a Freundlich isotherm.The uptake of phosphate at the adsorbent/solution ratio0.05g/2L was10and17mg-P/g for the phosphate-enriched seawater and the model wastewater,respectively.A much higher adsorptivity toward phosphate ions in seawater was observed on ZrO(OH)2·(Na2O)0.05·1.5H2O than on other representative adsorbents based on layered double hydroxides of Mg(II)–Al(III),Mg(II)–Fe(III),and Ni(II)–Fe(III).The effective desorption of phosphate ions on ZrO(OH)2·(Na2O)0.05·1.5H2O could be achieved using a0.1M NaOH solution.The usefulness of experimental data for practical applications in removing phosphate in seawater and wastewater is discussed.©2005Elsevier Inc.All rights reserved.Keywords:Zirconium hydroxide;Adsorption;Selectivity;Phosphate;Seawater1.IntroductionPhosphorus occurs mainly as phosphate in aquasystems. Phosphate is involved in a wide variety of biological and chem-ical processes in natural waters,wastewater,and water treat-ment.The release of phosphate to surface water is of environ-mental concern,because it enhances the growth of organisms in most ecosystems and is therefore the cause of eutrophica-tion,resulting in deterioration of water quality.The need for selective adsorption of phosphate at trace concentration levels has been well recognized.The anion-exchange reaction has at-tracted much interest for the removal of anions.A number of studies have been carried out on the interaction of phosphate with natural or synthetic minerals:goethite(α-FeOOH)[1,2],manganese nodules(mixed oxides ofδ-MnO2,α-SiO2,FeOOH,and Al2O3)[3],manganese oxides[4],and aluminum-impregnated mesoporous silicates[5].X-ray absorp-*Corresponding author.E-mail address:a.sonoda@aist.go.jp(A.Sonoda).tion near edge structure spectroscopy(XANES)has recently been used to study phosphate adsorption on mineral oxides[6].Hydrous oxides of iron(III),aluminum,zirconium,and man-ganese(IV)have been investigated for phosphate adsorption from a single electrolyte[7–10].These adsorbents are gener-ally amphoteric,giving them both cationic and anionic ion-exchange behavior depending on the pH of a contacting so-lution.Adsorption of phosphate from seawater with hydrous oxides of Fe(III),Al,and Mn(IV)has also been studied in de-tail[11–13].However,these adsorbents showed low uptake of phosphate(1–3mg-P/g)from seawater due to the presence of competing Cl−,HCO−3,and SO2−4ions.We have found that calcined Mg(II)–Mn(III)layered double hydroxide shows high selectivity toward phosphate ion in seawater[14].Studies on the adsorption of phosphate from wastewater be-fore discharge into the environment have been carried out using industrial waste materials and by-products as adsorbents,in-cludingfly ash[15],blast furnace slag(an industrial by-product derived from iron ore processing)[16],and iron oxide tail-ings(iron oxide with sulfate and silicates)[17].A new class0021-9797/$–see front matter©2005Elsevier Inc.All rights reserved. doi:10.1016/j.jcis.2005.11.011R.Chitrakar et al./Journal of Colloid and Interface Science297(2006)426–433427of polymeric ion exchangers(chelating resins)was found to be effective for the selective adsorption of phosphate from waste-water[18].Three types of zirconium oxides are known to exist:cu-bic,tetragonal,and monoclinic.They can be synthesized with different chemical compositions depending on the method of preparation[19].We are interested in amorphous zirconium hydroxide,because of its simplicity of preparation,its large surface area,and its strong affinity for certain anions such as phosphate orfluoride from a single electrolyte[20].Many stud-ies have been done on the adsorptive properties of cations on zirconium hydroxides(amorphous)[21]and monoclinic types [22]in single or dilute electrolyte solutions.To our knowledge, no study has been done on phosphate adsorption by zirconium hydroxide from seawater or wastewater.This paper describes original work on the effectiveness of amorphous zirconium hy-droxide for the selective adsorption of phosphate from a sodium phosphate solution,seawater and model wastewater.2.Experimental2.1.Preparation of amorphous zirconium hydroxideA0.1M(M=mol/L)NaOH solution was slowly added dropwise to200ml of a0.1M ZrOCl2solution.The latter solution was stirred magnetically during the addition of the sodium hydroxide solution till the pH reached10.The precip-itate was aged overnight,separated by centrifugation,washed with deionized water until free of chloride ions,andfinally dried in air at room temperature.The air-dried sample was ground and sieved to100–200mesh size(150–230µm).2.2.Preparation of other adsorbents for comparison studyThree types of layered double hydroxides,[Mg0.71Al0.29 (OH)2][Cl0.29·0.59H2O],[Mg0.84Fe0.16(OH)2][Cl0.16·0.68 H2O],and[Ni0.79Fe0.21(OH)2][Cl0.21·0.63H2O],were pre-pared in chloride forms and were designated as MgAl,MgFe, and NiFe,respectively[23].2.3.Physical propertiesPowder X-ray diffraction analysis was carried out using a Rigaku X-ray diffractometer(RINT1200)equipped with Ni-filtered Cu Kαradiation(λ=1.5404Å)and a graphite mono-chromator.TG-DTA experiments were conducted on a MAC Science thermal analyzer(200TG-DTA)at a heating rate of 10◦C/min in air.Surface area was determined by nitrogen gas adsorption using Quantachrome Type1-C apparatus after sam-ple was degassed at150◦C for4h.2.4.Chemical analysisA known amount of each powder sample was dissolved in a0.5M HCl solution.The dissolved Zr4+ion was analyzed bya Seiko ICP atomic emission plasma spectrometer(SPS7800), and the Na+,K+,Mg2+,and Ca2+ions were analyzed by a Shimadzu atomic absorption spectrometer(AA-670).The Cl−and SO2−4ions were determined by a Shimadzu liquid chro-matograph(LC-10Ai).Phosphate was determined with a portable colorimeter, Model DR/700,HACH Company,USA.All the reagents and chemicals were supplied by the HACH Company.The analy-sis was performed according to the procedures described in the manual.2.5.Preparation of phosphate-enriched seawater and model wastewater solutionsPhosphate-enriched seawater was prepared by the addition of a NaH2PO4solution to fresh seawater up to an appropriate phosphate concentration(0.30mg-P/L)with the pH of the so-lution at7.8.Model wastewater was prepared as described in the literature[24].The following concentrations werefixed in the model wastewater with a pH value of9:phosphate=2mg-P/L,alkalinity=100mg/L(NaHCO3),and Ca=25mg/L (CaCl2).2.6.Adsorption of phosphate from different solutionsAll the adsorption studies were carried out by a batch method at room temperature.The rates of phosphate removal from a NaH2PO4solution,phosphate-enriched seawater,and a model wastewater were determined by stirring a known amount of ZrO(OH)2·(Na2O)0.05·1.5H2O in a known volume of solu-tion.A small amount of supernatant solution was sampled at different intervals of time,and the phosphate concentrations were determined.Adsorption isotherms were obtained by stir-ring known amounts of the sample with2L of solution for 7d to attain satisfactory equilibrium.The phosphate concentra-tions in the supernatant solutions were determined.The uptakes of phosphate were calculated from the decreases of phosphate concentrations relative to those of initial concentration.The effect of pH on phosphate adsorption from a NaH2PO4 solution and phosphate-enriched seawater were studied at dif-ferent pH values.The pH of the solution was adjusted by the addition of a1M HCl or a1M NaOH solution.2.7.Uptake of cations and anions from seawaterA quantity of0.3g of ZrO(OH)2·(Na2O)0.05·1.5H2O was stirred in50L of seawater for3d.After the supernatant solution was discarded,the experiment was repeated three times by re-placement with fresh seawater.The solid sample was collected and washed till free of chloride ions.After drying,the solid sample was dissolved in an appropriate acid to elute cations and anions adsorbed in the sample.2.8.Desorption of phosphate and repetition of the adsorption/desorption cyclePhosphate desorption was studied using a phosphate-loaded sample,which was prepared by treatment of0.50g of ZrO(OH)2·(Na2O)0.05·1.5H2O with a Na2HPO4solution(2L,phosphate428R.Chitrakar et al./Journal of Colloid and Interface Science297(2006)426–433concentration50mg-P/L)for3d.The phosphate-loaded ad-sorbent was immersed in200ml of a0.1M NaOH solution for1d.The amount of phosphate desorbed was determined by the analysis of the phosphate concentration in the solution.The adsorption/desorption cycles were repeatedfive times.The desorption rate(R des)was calculated as R des=(A des/ Q ad)×100,where A des is the amount of phosphate released after desorption and Q ad is the initial phosphate uptake.The regeneration rate(R reg)was evaluated as R reg=(R ad/Q ad)×100,where R ad is the phosphate uptake by the regenerated ad-sorbent.Q ad,A des,and R ad are expressed in mg-P/g.3.Results and discussion3.1.Characterization of ZrO(OH)2·(Na2O)0.05·1.5H2OChemical analysis data for the solid sample showed the com-position ZrO2(71.3%),Na2O(2.0%),and H2O(26.2%)on a wt%basis.The water content was obtained from ignition of the solid sample at400◦C in air.This corresponds to the for-mulation ZrO2·(Na2O)0.05·2.5H2O or ZrO(OH)2·(Na2O)0.05·1.5H2O.The hydroxide content was formulated on the basis of electrical neutrality.The theoretical ion exchange capacity was evaluated as1.5mmol/g,consistent with the molar content of OH groups in the unit weight of ZrO(OH)2·(Na2O)0.05·1.5H2O. The surface area was228m2/g as determined from the nitrogen adsorption isotherm.XRD patterns of ZrO(OH)2·(Na2O)0.05·1.5H2O,and its cal-cined product at500◦C are shown in Fig.1.This sample could be regarded as an amorphous material.After calcina-tion at500◦C for3h in air,the crystallization of the sam-ple occurred,and the diffraction pattern showed a tetragonal phase[25].The TG-DTA results of decomposition of ZrO (OH)2·(Na2O)0.05·1.5H2O are shown in Fig.2.There was weight loss up to400◦C showing dehydration.The DTA curve showed one endothermic peak at around70◦C,which was at-tributed to the physically adsorbed water.There was a broad exothermic peak around470◦C,which was attributed to crys-tallization of ZrO(OH)2·(Na2O)0.05·1.5H2O to a tetragonal phase,while the broad exothermic peak might reflect a lack of homogeneity in the sample[25].3.2.Rate of phosphate removalThe removal of phosphate from a NaH2PO4solution, phosphate-enriched seawater,and a model wastewater as a function of time were examined.Fig.3a shows the effect of the initial phosphate concentration of the NaH2PO4solu-tion on phosphate removal at an adsorbent/solution ratio of 0.10g/1L.The phosphate removal from seawater and the model wastewater with initial concentrations of0.3and2mg-P/L were performed at adsorbent/solution ratios of0.05g/2L and0.20g/1L,respectively(Figs.3b and3c).The phosphate removal from the0.3and1.0mg-P/L NaH2PO4solution and the2mg-P/L model wastewater were almost100%in4–6d. To know the exact equilibrium time,phosphate removals were performed at higher concentrations of Na2HPO4solution.The Fig.1.XRD patterns of ZrO(OH)2·(Na2O)0.05·1.5H2O and its calcined prod-uct at500◦C.Fig.2.TG-DTA curves of ZrO(OH)2·(Na2O)0.05·1.5H2O.rate of phosphate removal was found to be slow for all solutions studied,requiring7d to attain equilibrium.The slow phosphate adsorption on ZrO(OH)2·(Na2O)0.05·1.5H2O could be explained on the basis of the random struc-ture of amorphous zirconium hydroxide proposed by Clearfield et al.[20],whofirst pointed out that when zirconium hydroxide is precipitated from a solution of ZrOCl2·8H2O by the addi-tion of a base,a polymeric oxohydroxide of the general for-mula[ZrO b(OH)4−2b·x H2O]is formed,most probably based on a tetramer of Zr(IV).The random structure has two types of hydroxyl groups,which are responsible for ion-exchange reac-tions:(i)terminal–OH groups exist on the surface of the solid,R.Chitrakar et al./Journal of Colloid and Interface Science297(2006)426–433429Fig.3.Rate of phosphate adsorption by ZrO(OH)2·(Na2O)0.05·1.5H2O in NaH2PO4solution(a),phosphate-enriched seawater(b),and model wastewa-ter(c).For(a):adsorbent=0.10g,volume=1L,pH5.0;For(b):adsorbent =0.05g,volume=2L,pH7.7;For(c):adsorbent=0.20g,volume=1L, pH8.8.and(ii)bridging–OH groups lie in the interior part of the solid and are connected by two metal atoms.In the present study, we assume that ZrO(OH)2·(Na2O)0.05·1.5H2O has both types of hydroxyl groups,as shown in Fig.4.Thus,the phosphate adsorption at the surface hydroxyl groups is fast,but the diffu-sion of phosphate ions into the interior is expected to be slow. Accordingly,the slow diffusion may hinder the access of phos-phate ions to the bridging hydroxyl groups,resulting in the slow adsorption rate.In general,adsorption over a period of minutes to hours occurs by anion adsorption on the surface hydroxyl groups of the adsorbent[26,27].Diffusion of anions into the lattice or adsorbent matrix requires a longer time to attain equi-librium[1].Fig.4.Structure of amorphous zirconium hydroxide formed by addition of base to aqueous zirconyl solution.Table1Ion uptake by ZrO(OH)2·(Na2O)0.05·1.5H2O from seawaterIons Ion uptake(mg/g)Ion concentration inseawater(mg/ml)K d(ml/g) Na+<0.110.8<0.1K+<0.10.4<0.2Ca2+<0.10.4<0.2Cl−9.019.40.4SO2−4192.77P8.06×10−51.3×105 Note.K d:distribution coefficient.K d=ion uptake(mg/g)/ion concentration (mg/ml).3.3.Uptake of phosphate and other ions from seawaterSeawater contains considerable amounts of Na+,K+,Ca2+, Cl−,and SO2−4ions.The uptake of these ions by ZrO(OH)2·(Na2O)0.05·1.5H2O was determined.The uptakes and the dis-tribution coefficients of these ions are summarized in Table1. The distribution coefficient(K d)can be calculated as K d(ml/g) =ion uptake(mg/g)/ion concentration in seawater(mg/ml). The ion uptakes were<0.1mg/g(Na+),<0.1mg/g(K+),<0.1mg/g(Ca2+),9.0mg/g(Cl−),19mg/g(SO2−4),and 8mg/g(P).A higher K d value was observed for phosphate than for the other ions.The selectivity order from the K d val-ues was Na+,K+,Ca2+<Cl−<SO2−4 P.These results show that ZrO(OH)2·(Na2O)0.05·1.5H2O has no selectivity to-ward the metal ions,but high selectivity toward phosphate ions in seawater.3.4.Effect of pH on phosphate adsorptionIt is known that hydrous trivalent and tetravalent metal hy-droxides exhibit amphoteric behavior,exchanging anions in an acidic solution and cations in a basic one.The anion-exchange capacity is strongly governed by the pH of the so-lution,ionic species of phosphate,and surface chemistry of the solids.The effect of pH on phosphate adsorption was demonstrated by measuring the uptake of phosphate by ZrO (OH)2·(Na2O)0.05·1.5H2O as a function of pH in a NaH2PO4430R.Chitrakar et al./Journal of Colloid and Interface Science297(2006)426–433Fig.5.The effect of pH on the uptake of phosphate by ZrO(OH)2·(Na2O)0.05·1.5H2O from NaH2PO4solution(a)and phosphate-enriched seawater(b). For(a):adsorbent=0.10g,conc.=50mg-P/L,volume=100ml.For(b): adsorbent=0.10g,conc.=0.33mg-P/L,volume=2L.Contact time=7d.solution,and in phosphate-enriched seawater.The results in Fig.5a for the NaH2PO4solution show that the uptake of phos-phate tends to decrease with an increase in pH,from50mg-P/g at pH2to13mg-P/g at pH10.The dissolution of zirconium ions at different pH was about1%.Phosphate can exist in dif-ferent ionic species as monovalent H2PO−4,divalent HPO2−4, and trivalent PO3−4ions,depending on the pH of the solution (p K1=2.15,p K2=7.20,and p K3=12.33)[28].The pH de-pendence of the uptake is likely attributable to the fact that a lower pH causes the ZrO(OH)2·(Na2O)0.05·1.5H2O surface to carry a more positive charge,and thus would more significantly attract the negatively charged monovalent H2PO−4ions in so-lution.The similar pH dependence of the anion adsorption is generally observed in trivalent and tetravalent metal hydrox-ides[29].In the case of seawater,the uptake of phosphate increases with an increase in pH,reaching a maximum around pH6and then decreasing progressively with an increase in pH up to a value of9(Fig.5b).In pH range2–4,sulfate anions in sea-water may affect the phosphate adsorption greatly,because the adsorbent prefers divalent SO2−4to monovalent HPO−4.The up-take of phosphate was maximal at pH around6.A decrease in the uptake of phosphate in the region pH>7may be due to the fact that the surface charge of the adsorbent becomes more negative at higher pH and causes greater electrostatic repulsion toward the more negatively charged phosphate anions HPO2−4 and PO3−4.Fig.6.Isotherm of phosphate adsorption by ZrO(OH)2·(Na2O)0.05·1.5H2O in NaH2PO4(a),phosphate-enriched seawater(b),and model wastewater(c). For(a):adsorbent=0.10g,conc.=1–10mg-P/L,volume=1L,pH5.0. For(b):adsorbent=0.012–0.400g,conc.=0.30mg-P/L,volume=2L, pH7.7.For(c):adsorbent=0.04–0.20g,conc.=2mg-P/L,volume=2L, pH8.8.Contact time=7d.3.5.Adsorption isothermIsotherm studies were carried out to determine the con-ditions for maximum uptake of phosphate on ZrO(OH)2·(Na2O)0.05·1.5H2O from a NaH2PO4solution,phosphate-enriched seawater,and a model wastewater.Fig.6shows that the uptake of phosphate increases with the phosphate equilib-rium concentration in all three solutions.The phosphate ad-sorption data werefitted to the Freundlich equation,shown as q e=K F C1/n e,where q e is the amount of phosphate ad-R.Chitrakar et al./Journal of Colloid and Interface Science297(2006)426–433431Table2Freundlich isotherm constants for adsorption of phosphate by ZrO(OH)2·(Na2O)0.05·1.5H2OSolution Freundlich isothermK F n R2 NaH2PO417.430.998 Seawater43.510.956 Wastewater14.9110.981sorbed on the solid phase(mg-P/g),C e is the equilibrium phosphate concentration in solution phase(mg-P/L),and K F (mg/g)and n(dimensionless)are Freundlich constants.The Freundlich isotherm constants and R2values for different so-lutions are given in Table2.The error bars were±10%for seawater and±3%for NaHPO4solution and wastewater.The constant n refers to the interaction between exchange sites at the adsorbent and phosphate ions.A high value for n>1indicates favorable adsorption.The maximum uptake of phosphate from seawater on ZrO(OH)2·(Na2O)0.05·1.5H2O was10mg-P/g at a phosphate equilibrium concentration of0.2mg-P/yered double hydroxide is known to have a high phosphate exchange capacity of60mg-P/g at pH8[30],but the maximum uptake of phosphate from seawater was only1mg-P/g.To summarize the adsorption isotherm data,the uptakes of phosphate from NaH2PO4solution,seawater,and wastewater on ZrO(OH)2·(Na2O)0.05·1.5H2O are30,10,and15mg-P/g at equilibrium pH5,7.7,and8.8,respectively.The uptakes of phosphate are comparable with their respective pH titration data (Figs.5a,5b).But the uptake of phosphate from seawater is too low as compared to that from single electrolyte(NaH2PO4)at pH7.8(Fig.5a).The decrease in uptake may be related to low phosphate concentration and the presence of major and minor seawater components,which strongly reduce the phosphate ad-sorption.These adsorption isotherms demonstrate that ZrO(OH)2·(Na2O)0.05·1.5H2O may be used as an adsorbent for phosphate from wastewater and seawater,because of its high phosphate uptake.It is noted that the values presented here are high as compared to the reported data in the literature;the maximum uptake is1–3mg-P/g by hydrous oxides of Fe(III),Al,and Mn(IV)in seawater[11–13],and1and7mg-P/g by blast fur-nace slags[16]and alkalinefly ash[15],respectively,in waste-water.3.6.Desorption of phosphate and repetition of the adsorption/desorption cycleFor reusability,the adsorbed phosphate should be easily desorbed.In the present study,phosphate-loaded ZrO(OH)2·(Na2O)0.05·1.5H2O was desorbed with a0.1M NaOH solu-tion.The desorption and regeneration experimental results are shown in Fig.7.The amount of phosphate desorption was 82%.The phosphate adsorption decreased slightly after the first cycle,but remained almost constant till thefifth repeti-tions of the adsorption/desorption cycle.It is concluded that the adsorption of phosphate on ZrO(OH)2·(Na2O)0.05·1.5H2O is reversible,and the bonding between exchange sitesand Fig.7.Change of regeneration(R reg)and desorption(R des)rates with recycle times.the adsorbed phosphate is not so strong.This result suggests that ZrO(OH)2·(Na2O)0.05·1.5H2O has practical potential to be used as an adsorbent for phosphate removal.parison of phosphate adsorption fromphosphate-enriched seawater with other adsorbentsPhosphate adsorption from phosphate-enriched seawater were studied on layered double hydroxides of MgAl,MgFe, NiFe,and ZrO(OH)2·(Na2O)0.05·1.5H2O at an adsorbent/ seawater ratio of0.05g/2L after stirring for7d.The uptakes of phosphate are summarized as follows;the phosphate adsorptiv-ity increases in the order,MgAl(1mg-P/g)<MgFe(1.5mg-P/g)<NiFe(1.8mg-P/g)<ZrO(OH)2·(Na2O)0.05·1.5H2O (10.0mg/g).The uptake of phosphate by the present adsorbent is higher than those for the other adsorbents.Although MgAl and MgFe are known to have large phosphate exchange capaci-ties in NaH2PO4solution[30–32],they also show high selectiv-ity for sulfate ions.Therefore,these layered double hydroxide adsorbents are not useful for the adsorption of phosphate from the seawater environment,where the sulfate concentration is markedly higher than the phosphate concentration,as shown in Table1.3.8.Mechanism of adsorption of phosphate onZrO(OH)2·(Na2O)0.05·1.5H2OCation or anion adsorption on amphoteric metal hydroxides has been extensively studied[20].Functional groups are mostly represented by hydrolyzed species,and acid–base reactions can be represented for ZrO(OH)2·(Na2O)0.05·1.5H2O as follows:≡Zr–OH+H+ ≡ZrOH+2protonation in an acidicmedium(anion exchange),(1)≡Zr–OH ≡ZrO−+H+deprotonation in an alkalinesolution(cation exchange).(2) It is known that certain anions(phosphate,arsenate,selenate, etc.)that are strongly adsorbed do undergo ligand exchange (inner sphere or outer sphere complex),but simple anions(chlo-ride,nitrate,and sulfate)do not.An inner sphere complex is formed when the adsorbed ligand is directly linked to the metal ion by covalent bonding,and an outer complex,which involves432R.Chitrakar et al./Journal of Colloid and Interface Science297(2006)426–433 electrostatic bonding,is formed when a water molecule is re-tained between the exchange site and the adsorbed ligand[4].For a single electrolyte,an increase in pH should cause a de-crease in the amount of phosphate adsorption.If the adsorptionis only a simple ion-exchange mechanism between the nega-tively charged phosphate ion and the oxide surface,there shouldbe no adsorption of phosphate ions at pH above5,because thevalue of zero point charge of zirconium hydroxide reported inthe literature was close to4[33].However,a significant amountof phosphate adsorption occurs at pH>4,as shown in Fig.5.The formation of an outer-sphere complex is more likely tooccur during phosphate adsorption,because the adsorbed phos-phate ions are easily desorbed with a0.1M NaOH solution.Forthe inner-sphere complex formation,phosphate ions are bounddirectly to the metal ion of the adsorbent by the formation ofa covalent bond,and desorption involves only a portion of thetotal phosphate[1].Accordingly,we assume that the phosphate adsorption onZrO(OH)2·(Na2O)0.05·1.5H2O is due to the formation of theouter sphere complex.The reactions are given as≡Zr–OH+H++H2PO−4 ≡Zr–OH+2–H2PO−4(at pH2–4),(3)≡Zr–OH+2H++HPO2−4 ≡Zr–OH+2–HPO2−4+H+(at pH6–10),(4)≡Zr–OH+3H++PO3−4 ≡Zr–OH+2–PO3−4+2H+(at pH>10),(5)where–OH is a hydroxyl group,and–H2PO−4,HPO2−4,andPO3−4are the adsorbed ligands at corresponding pH values.In seawater,the competition of anions for exchange sites de-pends on the relative affinity of the anions for the exchangesites,the relative concentration of the anions,and the pH ofthe solution.At pH<4,phosphate adsorption is low due to thepresence of a high concentration of chloride and sulfate ions inseawater.Although chloride and sulfate ions may compete forthe available exchange sites on ZrO(OH)2·(Na2O)0.05·1.5H2O with electrostatic interactions,the adsorption of phosphate isprobably followed by outer sphere complex formation,as dis-cussed above.The uptake of phosphate increases with an in-crease of the pH value up to7.A decrease in uptake of phos-phate in the region pH>7is explained by the fact that thesurface charge of the adsorbent changes to be more negative athigher pH,resulting in electrostatic repulsion of the more nega-tively charged phosphate anion.A similar outer sphere complexformation has been reported for the adsorption of phosphatefrom seawater on tetravalent manganese oxide[13].AlthoughZrO(OH)2·(Na2O)0.05·1.5H2O is regarded as a weak cation ex-changer at high pH[20],the uptake of cations from seawater is negligible at neutral pH,as shown in Table1.4.ConclusionsThe results of Fig.6suggest that the adsorption of phos-phate is high at low equilibrium concentration of phosphate(0.2mg-P/L).The maximum uptake of phosphate ions fromseawater and wastewater is10and17mg-P/g,respectively,on ZrO(OH)2·(Na2O)0.05·1.5H2O,much greater than the1–2mg-P/g observed on the layered double hydroxide adsorbents stud-ied so far and reported literature data for seawater[11–13],and 1–7mg-P/g reported for wastewater on blast slag andfly ash [15,16].ZrO(OH)2·(Na2O)0.05·1.5H2O can be effectively used for the removal of phosphate from effluents containing phos-phate at wider pH ranges.The significance of the experimental data in seawater is to demonstrate that ZrO(OH)2·(Na2O)0.05·1.5H2O is highly se-lective for phosphate anions even at extremely low phosphate concentration,and its practical application may be applied to natural waters.The adsorbent may also be used to collect phos-phate ions from seawater,because of increasing industrial de-mand of phosphate.Adsorption/desorption studies show that phosphate is easily desorbed with a0.1M NaOH solution,indicating that the ad-sorbent can be reused.These results suggest that ZrO(OH)2·(Na2O)0.05·1.5H2O can act as a suitable adsorbent for the safe and economical removal of harmful phosphate ions from sea-water and contaminated ecosystems.References[1]K.C.Makris,W.G.Harris,G.A.O’Connor,H.El-Shall,J.Colloid Inter-face Sci.287(2005)552.[2]A.Genz,A.Kornmüller,M.Kekel,Water Res.38(2004)3523.[3]K.M.Parida,S.Mohanty,J.Colloid Interface Sci.199(1998)22.[4]S.Ouvrard,M.O.Simonnot,M.Sardin,Ind.Eng.Chem.Res.41(2002)2785.[5]E.W.Shin,J.S.Han,M.Jang,S.H.Min,J.K.Park,R.M.Rowell,Environ.Sci.Technol.38(2004)912.[6]N.Khare,D.Hesterberg,J.D.Martin,Environ.Sci.Technol.39(2005)2152.[7]R.S.Juang,J.Y.Chung,J.Colloid Interface Sci.275(2004)53.[8]N.I.Chubar,V.A.Kanibolotskyy,V.V.Strelko,G.G.Gallios,V.F.Samanidou,T.O.Shaposhnikova,V.G.Mailgrandt,I.Z.Zhuravlev,Col-loids Surf.A Physicochem.Eng.Aspects225(2005)55.[9]W.P.Tang,O.Shima,A.Okubo,K.Ooi,J.Pharm.Sci.86(1997)230.[10]M.Kawashima,Y.Tainaka,T.Hori,M.Koyama,T.Takamatsu,WaterRes.20(1986)471.[11]Y.Gao,A.Mucci,Chem.Geol.199(2003)91.[12]S.Tanada,M.Kabeyama,N.Kawasaki,T.Sakiyama,T.Nakamura,M.Araki,T.Tamura,J.Colloid Interface Sci.257(2003)135.[13]W.Yao,lero,Environ.Sci.Technol.30(1996)536.[14]R.Chitrakar,S.Tezuka,A.Sonoda,K.Sakane,K.Ooi,T.Hirotsu,J.Col-loid Interface Sci.290(2005)45.[15]K.C.Cheung,T.H.Venkitachalam,Chemosphere41(2000)243.[16]L.Johansson,J.P.Gustafsson,Water Res.34(2000)259.[17]L.Zeng,X.Li,J.Liu,Water Res.38(2004)1318.[18]D.Zhao,A.K.SenGupta,Water Res.32(1998)1613.[19]A.Clearfield,Inorg.Chem.3(1964)146.[20]A.Clearfield,G.H.Nancollas,R.H.Blessing,in:J.A.Marinsky(Ed.),IonExchange and Solvent Extraction,vol.5,Dekker,New York,1973,pp.1–120.[21]Y.Inoue,H.Yamazaki,Bull.Chem.Soc.Jpn.60(1987)891.[22]R.Paterson,H.Rahman,J.Colloid Interface Sci.103(1985)106.[23]S.Tezuka,R.Chitrakar,A.Sonoda,K.Ooi,Chem.Lett.32(2003)722.[24]S.Nagamine,T.Ueda,I.Masuda,T.Mori,E.Sasaoka,I.Joko,Ind.Eng.Chem.Res.42(2003)4748.[25]C.J.Norman,P.A.Goulding,I.McAlpine,Catal.Today20(1994)313.[26]L.N.Ho,T.Ishihara,S.Ueshima,H.Nishiguchi,Y.Takita,J.Colloid In-terface Sci.272(2004)399.。