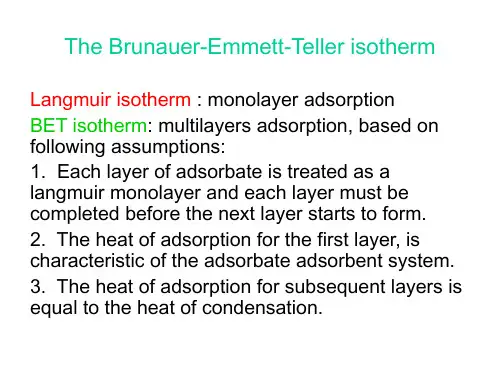
Kinetic modelling of the adsorption of nitrates
- 格式:pdf
- 大小:437.16 KB
- 文档页数:7

化工进展Chemical Industry and Engineering Progress2024 年第 43 卷第 3 期基于分子模拟的多孔炭材料结构模型构建方法研究进展周逸寰,解强,周红阳,梁鼎成,刘金昌(中国矿业大学(北京)化学与环境工程学院,北京 100083)摘要:结构模型的构建是多孔炭材料结构表征、“构效”关系探究、吸附模拟研究等的前提和基础。
本文对基于分子模拟的多孔炭材料结构模型构建方法、应用及特点进行了综述性评介,以挥发性有机物(volatile organic compounds ,VOCs )吸附净化用活性炭的选型需求为导向,分析各种模型构建方法的适用性。
结果表明,由片段单元组装构成多孔炭结构的早期模型,能展现多孔炭材料的部分表观性质,但对多孔炭吸附性能的解析与机理阐释尚缺乏指导意义。
多孔炭结构模型构建方法可归为仿真过程法和结构重建法,前者适于炭材料微观结构演变的研究,但所需算力高;后者通过拟合多孔炭的实验、表征数据、在一定约束条件下重建模型,其中的随机填充法可以针对性地调控模型的孔结构和官能团,应用于吸附模拟研究时有助于确定吸附特定VOCs 的最优孔结构、筛选合适的活性炭,进而指导多孔炭材料的制备。
然而,对包括随机填充法在内的结构重建法,尚需掌握量化调控结构模型孔结构、表面官能团的方法与关键参数,发展能够进行多参数、多指标“构效”关系研究的多尺度化模型,才能对多孔炭材料的实际应用提供指导。
关键词:分子模拟;活性炭;结构模型;动力学模型;随机填充法中图分类号:X51;O647 文献标志码:A 文章编号:1000-6613(2024)03-1535-17Modeling of porous carbon materials based on molecular simulation:State-of-the artZHOU Yihuan ,XIE Qiang ,ZHOU Hongyang ,LIANG Dingcheng ,LIU Jinchang(School of Chemical and Environmental Engineering, China University of Mining and Technology (Beijing ), Beijing100083, China)Abstract: Modelling of porous carbon materials serves as prerequisite and foundation for the characterization, structure-performance relationship investigation and adsorption simulation study. In this article, a critical literature survey was conducted on the strategy, application and merits/demerits of approaches to modelling of porous carbon materials based on molecular simulation, and the applicability of various modelling methods was analyzed in demand oriented for screening activated carbon for the purification of volatile organic compounds (VOCs). The results showed that early models constructed by either fragment, basic structural units (BSUs) or basic buildings elements (BBEs) can exhibit some apparent properties of porous carbon materials. Meanwhile, they were incapable of providing guidance for the elucidation of adsorption performance and mechanism of porous carbons. Various modelling methods of porous carbon material can be classified into two groups according to their construction strategy, theDOI :10.16085/j.issn.1000-6613.2023-0485收稿日期:2023-03-29;修改稿日期:2023-05-21。

Journal of Hazardous Materials 181 (2010) 535–542Contents lists available at ScienceDirectJournal of HazardousMaterialsj o u r n a l h o m e p a g e :w w w.e l s e v i e r.c o m /l o c a t e /j h a z m atAdsorptive removal of methyl orange from aqueous solution with metal-organic frameworks,porous chromium-benzenedicarboxylatesEnamul Haque a ,Ji Eun Lee a ,In Tae Jang b ,Young Kyu Hwang b ,Jong-San Chang b ,Jonggeon Jegal c ,Sung Hwa Jhung a ,∗aDepartment of Chemistry,Kyungpook National University,Daegu 702-701,Republic of KoreabResearch Center for Nanocatalysts,Korea Research Institute of Chemical Technology,P.O.Box,107,Yusung,Daejeon 305-600,Republic of Korea cMembrane and Separation Research Center,Korea Research Institute of Chemical Technology,P.O.Box 107,Yusung,Daejeon 305-600,Republic of Koreaa r t i c l e i n f o Article history:Received 8February 2010Received in revised form 8May 2010Accepted 10May 2010Available online 16 May 2010Keywords:Porous chromium-benzenedicarboxylates MOFsMethyl orange DyeAdsorption Removala b s t r a c tTwo typical highly porous metal-organic framework (MOF)materials based on chromium-benzenedicarboxylates (Cr-BDC)obtained from Material of Institute Lavoisier with special structure of MIL-101and MIL-53have been used for the adsorptive removal of methyl orange (MO),a harmful anionic dye,from aqueous solutions.The adsorption capacity and adsorption kinetic constant of MIL-101are greater than those of MIL-53,showing the importance of porosity and pore size for the adsorption.The performance of MIL-101improves with modification:the adsorption capacity and kinetic constant are in the order of MIL-101<ethylenediamine-grafted MIL-101<protonated ethylenediamine-grafted MIL-101(even though the porosity and pore size are slightly decreased with grafting and further protonation).The adsorption capacity of protonated ethylenediamine-grafted MIL-101decreases with increasing the pH of an aqueous MO solution.These results suggest that the adsorption of MO on the MOF is at least partly due to the electrostatic interaction between anionic MO and a cationic adsorbent.Adsorption of MO at various temperatures shows that the adsorption is a spontaneous and endothermic process and that the entropy increases (the driving force of the adsorption)with MO adsorption.The adsorbent MIL-101s are re-usable after sonification in water.Based on this study,MOFs can be suggested as potential re-usable adsorbents to remove anionic dyes because of their high porosity,facile modification and ready re-activation.© 2010 Elsevier B.V. All rights reserved.1.IntroductionRecently,considerable amount of waste water having color has been generated from many industries including textile,leather,paper,printing,dyestuff,plastic and so on [1].Removal of dye mate-rials from water is very important because water quality is greatly influenced by color [1]and even small amount of dyes is highly visible and undesirable.Moreover,many dyes are considered to be toxic and even carcinogenic [1–3].It is difficult to degrade dye materials because they are very sta-ble to light and oxidation [3].For the removal of dye materials from contaminated water,several methods such as physical,chemical and biological methods have been investigated [1,3].Among the proposed methods,removal of dyes by adsorption technologies is regarded as one of the competitive methods because adsorption does not need a high operation temperature and several color-ing materials can be removed simultaneously [1].The versatility∗Corresponding author.Fax:+82539506330.E-mail address:sung@knu.ac.kr (S.H.Jhung).of adsorption,especially with activated carbons,is due to its high efficiency,economic feasibility and simplicity of design [3].Methyl orange (MO)is one of the well-known acidic/anionic dyes,and has been widely used in textile,printing,paper,food and pharmaceutical industries and research laboratories [2].The struc-ture of MO is shown in Scheme 1and the removal of MO from water is very important due to its toxicity [2,3].Harmful MO is selected in this study as a representative acidic dye.Several adsorbents such as ammonium-functionalized MCM-41and layered double hydroxides (LDH)have been studied for the removal of MO dye [4,5].Adsorbents,including activated carbon,made from wastes attract attention to decrease the cost of adsorp-tion [2,6].Agricultural wastes such as lemon [7],banana and orange peel [8]and biopolymers [9]like alginate have also been used.Functionalized adsorbents such as hyper-crosslinked polymers [10]and diaminoethane sporollenin [11]have also been studied.For efficient separation of adsorbent from liquid after adsorption,magnetic particles have also been dispersed/incorporated in the adsorbent [9,12].The thermodynamics parameters of MO adsorption have been studied over various adsorbents [2,5–7,10].In every case,the G0304-3894/$–see front matter © 2010 Elsevier B.V. All rights reserved.doi:10.1016/j.jhazmat.2010.05.047536 E.Haque et al./Journal of Hazardous Materials181 (2010) 535–542Scheme1.is negative for spontaneous adsorption;however H depends on adsorbents[2,5–7,10]and S is generally positive[2,5–7]. The adsorption kinetics has been interpreted with various models [2–10]and the adsorption of MO over MCM-41illustrates that the adsorption process is composed of complex processes like diffusion in surface or mesopore and adsorption on mesopore[4].Moreover, the kinetic constants vary widely depending on the adsorbents [2–10].Metal-organic frameworks(MOFs)are crystalline porous mate-rials which are well known for their various applications[13–20]. The particular interest in MOF materials is due to the easy tunability of their pore size and shape from a microporous to a mesoporous scale by changing the connectivity of the inorganic moiety and the nature of organic linkers[13–15].MOFs are especially inter-esting in thefield of adsorption,separation and storage of gases and vapors[16,19].For example,the storage of hydrogen[16]and carbon dioxide/methane[21]using MOFs has been extensively investigated.The removal of hazardous materials such as sulfur-containing materials has also been studied[22,23].Among the numerous MOFs reported so far,two of the most topical solids are the porous chromium-benzenedicarboxylates (Cr-BDCs)namely MIL-53[24](MIL stands for Material of Insti-tute Lavoisier)and MIL-101[25,26],which are largely studied for their potential -53with a chemical formula of Cr(OH)[C6H4(CO2)2]·n H2O,has an orthorhombic structure and pore volume of0.6cc/g[24].MIL-101,Cr3O(F/OH)(H2O)2[C6H4(CO2)2], has a cubic structure and huge pore volume of1.9cc/g[25,26].The pore sizes of MIL-53and MIL-101are around0.85and2.9–3.4nm, respectively[24–26].MIL-53is very interesting due to the breath-ing effect[27],and has been widely studied for adsorption[23]and drug delivery[20,28].MIL-101is a very important material due to its mesoporous structure and huge porosity,and is widely studied for adsorption[29],catalysis[30]and drug delivery[20,28].However,so far,there has been no report of the use of MOFs including Cr-BDCs in the removal of dye materials.In this work,we report,for thefirst time,the results of the adsorption of MO over MOFs,especially well-studied Cr-BDCs,to understand the charac-teristics of adsorption and possibility of using MOFs as adsorbents for the removal of dye materials from waste water.2.ExperimentalThe Cr-BDCs such as MIL-53and MIL-101were prepared follow-ing the reported methods[24–26,31,32].Ethylenediamine-grafted MIL-101(ED-MIL-101)was obtained by grafting ethylenediamine according to the method reported earlier[26,30].The protonated ED-MIL-101(PED-MIL-101)was obtained by acidification of ED-MIL-101with0.1M HCl solution at room temperature for6h. Activated carbon was purchased from Duksan chemical company (granule,size:2–3mm).The textural properties of the adsor-bents were analyzed with a surface area and porosity analyzer (Micromeritics,Tristar II3020)after evacuation at150◦C for12h. The surface area,pore volume and average pore size were calcu-lated using the nitrogen adsorption isotherms.An aqueous stock solution of MO(1000ppm)was prepared by dissolving MO(molecular formula:C14H14N3NaO3S,molecu-lar weight:327.34,Daejung Chemicals co.Ltd.,Korea)in deionized water.Aqueous MO solutions with different concentrations of MO (5–200ppm)were prepared by successive dilution of the stock solution with water.The MO concentrations were determined using the absorbance(at464nm)of the solutions after getting the UV spectra of the solution with a spectrophotometer(Shimadzu UV spectrophotometer,UV-1800).The calibration curve was obtained from the spectra of the standard solutions(5–50ppm)at a specific pH5.6.Before adsorption,the adsorbents were dried overnight under vacuum at100◦C and were kept in a desiccator.An exact amount of the adsorbents(∼10mg)was put in the aqueous dye solutions (50mL)havingfixed dye concentrations from20ppm to200ppm. The MO solutions(pH:5.6)containing the adsorbents were mixed well with magnetic stirring and maintained for afixed time(10min to12h)at25◦C.After adsorption for a pre-determined time,the solution was separated from the adsorbents with a syringefil-ter(PTFE,hydrophobic,0.5m),and the dye concentration was calculated,after dilution(if necessary),with absorbance obtained using UV spectra.The adsorption rate constant was calculated using pseudo-second or pseudo-first order reaction kinetics[33–35]and the maximum adsorption capacity was calculated using the Lang-muir adsorption isotherm[33,36]after adsorption for12h.To get the thermodynamic parameters of adsorption such as G(free energy change), H(enthalpy change)and S(entropy change) the adsorption was further carried out at35and45◦C.To determine the adsorption capacity at various pHs,the pH of the MO solution was adjusted with0.1M HCl or0.1M NaOH aqueous solution.The used adsorbent was activated with deion-ized water(0.05g adsorbent in25ml water)under ultrasound (Sonics and Materials,USA;Model:VC X750,power:750W,ampli-tude;35%)at room temperature for60min(temperature raised to 65◦C–70◦C after sonification).After separation,the adsorbent was dried and reused for the next adsorption.3.Result and discussion3.1.Adsorption kineticsTo understand the adsorption kinetics,MO was adsorbed at var-ious times up to12h,and the quantity of adsorbed MO is displayed in Fig.1when the initial MO concentration is30-50ppm.As shown in Fig.1,the adsorbed quantity of MO is in the order of acti-vated carbon<MIL-53<MIL-101<ED-MIL-101<PED-MIL-101for the whole adsorption time from any initial MO concentration.The adsorbed MO slightly increases with the initial MO concentration, showing the favorable adsorption at high MO concentration.The adsorption time needed for saturation of the adsorbed amount is in the order of activated carbon>MIL-53>MIL-101>ED-MIL-101>PED-MIL-101(Supplementary data Fig.S1).The adsorption over modified MIL-101s is practically completed in2h,showing the rapid adsorption of MO over the modified MIL-101s.However, the adsorbed amount increases steadily with time over the acti-vated carbon.To compare the adsorption kinetics precisely,the changes of adsorption amount with time are treated with the versa-tile pseudo-second-order kinetic model[33–35]because the whole data during adsorption time can be treated successfully:dq tdt=k2(q e−q t)2(1) or,tq t=1k2q2e+1q et(2)where q e:amount adsorbed at equilibrium(mg/g);q t:amount adsorbed at time t(mg/g);t:adsorption time(h).E.Haque et al./Journal of Hazardous Materials181 (2010) 535–542537Fig.1.Effect of contact time and initial MO concentration on the adsorption of MO over thefive adsorbents:(a)C i=30ppm;(b)C i=40ppm;(a)C i=50ppm.Therefore,the second-order kinetic constant(k2)can be calcu-lated byk2=slope2int erceptwhen the t/q t is plotted against t.Fig.2shows the plots of the pseudo-second-order kinetics of the MO adsorption over thefive adsorbents at threeinitial Fig.2.Plots of pseudo-second-order kinetics of MO adsorption over thefive adsor-bents:(a)C i=30ppm;(b)C i=40ppm;(a)C i=50ppm.dye concentrations.The calculated kinetic constants(k2)and cor-relation coefficients(R2)are shown in Table1.The adsorption kinetic constants for MO adsorption are in the order of activated carbon<MIL-53<MIL-101<ED-MIL-101<PED-MIL-101,similar to the adsorbed quantity(Fig.1).Therefore,PED-MIL-101is the most effective adsorbent for MO removal in the viewpoint of adsorption amount and rate.538 E.Haque et al./Journal of Hazardous Materials181 (2010) 535–542 The adsorption kinetic constants over activated carbon and Cr-BDCs are larger than those over crosslinked polymer[10]andactivated carbon(obtained from agricultural product,Phragmitesaustralis)[3].On the contrary the constants are smaller than theconstants over NH3+-MCM-41,activated carbon(entrapping mag-netic cellulose bead)[12],lemon peel[7]and alginate bead[9].The kinetic constants over activated carbon and Cr-BDCs increaseslightly with increasing the initial MO concentration,showingrapid adsorption in the presence of MO in high concentration.Theincrease of the kinetic constant with increasing initial adsorbateconcentration has been reported too[33,37,38].The kinetic con-stants of adsorption over Cr-BDCs show that the adsorption overPED-MIL-101is the fastest and the adsorption kinetics increaseswith modification of virgin MIL-101.The kinetic constant over PED-MIL-101is around10times greater than that of the activated carbonunder the experimental conditions.The adsorption data were also analyzed using the pseudo-first-order kinetic model[33]:ln(q e−q t)=ln q e−k1t(3)Therefore,thefirst order kinetic constant(k1)can be calculatedbyk1=−slope when the ln(q e−q t)is plotted against t.The plots of the pseudo-first-order kinetics of the dye adsorp-tions over thefive adsorbents at the initial MO concentrations of30–50ppm are shown in Fig.S2(adsorption time is only0–1hfor good linearity[33])and the kinetic constants are displayed inTable S1.Similar to the second-order kinetic constants,the rateconstant generally increases in the order of activated carbon<MIL-53<MIL-101<ED-MIL-101<PED-MIL-101,confirming once againthe most rapid adsorption over PED-MIL-101.However,the lin-earity of thefirst order kinetics is relatively poor(low correlationcoefficients R2).The fast adsorption of MO over MIL-101,compared with theadsorption over MIL-53,is probably due to the large pore size ofMIL-101as the kinetic constant of adsorption generally increaseswith the increasing pore size of a porous material not only inliquid-phase adsorption[39,40]but also in gas phase adsorption[41].However,the kinetic constants of the MIL-101s show that thepore size of the MIL-101s(Table1)does not have any effect on thekinetics of adsorption,suggesting the presence of a specific inter-action between PED-MIL-101or ED-MIL-101and MO because theadsorption kinetic constant increases with increasing pore size ifthere is no specific interaction between adsorbate and adsorbent[39–41].3.2.Adsorption thermodynamicsThe adsorption isotherms were obtained after adsorption forsufficient time of12h,and the results are compared in Fig.3a.Theamount of adsorbed dye is in the order of PED-MIL-101>ED-MIL-101>MIL-101>MIL-53>activated carbon for the experimentalconditions,suggesting the efficiency of the Cr-BDCs,especially PED-MIL-101.As shown in Fig.3b,the adsorption isotherms have beenplotted to follow the Langmuir equation[33,36]:C e q e =C eQ0+1Q0b(4)where C e:equilibrium concentration of adsorbate(mg/L) q e:the amount of adsorbate adsorbed(mg/g)Q0:Langmuir constant(maximum adsorption capacity)(mg/g) b:Langmuir constant(L/mg or L/mol)So,the Q0can be obtained from the reciprocal of the slope of a plot of C e/q e against C e.The Q0for all of the samples is determined from Fig.3b and the values are summarized in Table1.Generally the Q0increases inthe Fig.3.(a)Adsorption isotherms for MO adsorption over thefive adsorbents and(b) Langmuir plots of the isotherms(a).The arrows in(b)show that the y-axis scale of activated carbon is different to that of MOFs.order of activated carbon<MIL-53<MIL-101<ED-MIL-101<PED-MIL-101,and the adsorption capacity of PED-MIL-101is194mg/g. The large adsorption capacity of MIL-101for MO,compared to that of MIL-53,is probably due to the large porosity of MIL-101as the adsorption capacity generally increases with increasing the poros-ity of adsorbents[42,43].So far many adsorbents have been evaluated as candidates for the removal of MO from water and their adsorption capacities have varied widely from2.1to about366mg/g depending on the adsor-bent[1–12].Even though the adsorption capacity of Cr-BDCs is less than that of NH3+-MCM-41[4],LDH[5]and activated carbon(made from an agricultural product,Phragmites australis)[3],its capacity is relatively greater than that of the most adsorbents[1,2,6–12].To shed light on the MO adsorption over Cr-BDCs,the adsorp-tion free energy,enthalpy and entropy change were calculated from the adsorption of MO over PED-MIL-101at various temperatures. Fig.4a shows the adsorption isotherms at the temperature of25, 35and45◦C,and the Langmuir plots are displayed in Fig.4b.The adsorption capacity increases with increasing adsorption temper-ature,suggesting endothermic adsorption.The Gibbs free energy change G can be calculated by the fol-lowing Eq.(5)[5,6,10,35]:G=−RT ln b(5)(where R is the gas constant)The Langmuir constant b(dimension:L/mol)can be obtained from the slope/intercept of the Langmuir plot of Fig.4b.The negative free energy change( G)shown in Table2suggests spontaneous adsorption under the adsorption conditions.E.Haque et al./Journal of Hazardous Materials181 (2010) 535–542539Fig.4.(a)Adsorption isotherms for MO adsorption over PED-MIL-101at25,35and45◦C;and(b)Langmuir plots of the isotherms(a);(c)Adsorption isotherms for MO adsorption over MIL-101at25,35and45◦C;and(d)Langmuir plots of the isotherms(c).The enthalpy change H can be obtained by using the van’t Hoffequation[5,44]:ln b= SR− HRT(6)The calculated H,obtained from the(−slope×R)of the van’tHoff plot(Fig.5),is29.5kJ/mol,confirming endothermic adsorp-tion in accord with the increasing adsorption capacity associatedwith increasing adsorption temperature(Fig.4a).The endothermicTable1Textural properties,the pseudo-second-order kinetic constants(k2)with correlation constants(R2)at various MO concentrations and the maximum adsorption capacities (Q0)of thefive adsorbents.Adsorbent(pore size,nm)BET surfacearea(m2/g)Total porevolume(cm3/g)Pseudo-second-order kinetics constants k2(g/(mg min))Maximum adsorptioncapacity,Q o(mg/g) 30ppm40ppm50ppmk2R2k2R2k2R2Activated carbon(<1.0)10680.50 2.17×10−40.987 2.34×10−40.981 2.59×10−40.97811.2MIL-53(<1.0)14380.557.23×10−40.9997.70×10−40.9997.84×10−40.99957.9MIL-101(1.6,2.1)3873 1.709.01×10−40.9999.19×10−40.9969.29×10−40.998114ED-MIL-101(1.4,1.8)3491 1.37 1.06×10−30.998 1.19×10−30.999 1.27×10−30.999160PED-MIL-101(1.4,1.8)3296 1.18 2.75×10−3 1.00 2.95×10−3 1.00 3.04×10−30.999194Table2The maximum adsorption capacity and thermodynamic parameters of MO adsorption over PED-MIL-101and MIL-101at different temperatures.Adsorbent Temp.(◦C)Q o(mg/g) G(kJ/mol) H(kJ/mol) S(J/mol/K)PED-MIL-10125194−32.535219−34.529.520945241−36.6MIL-10125114−31.935121−33.1 4.0012045140−34.3540 E.Haque et al./Journal of Hazardous Materials 181 (2010) 535–542Table 3The equilibrium adsorbed amount (q e )and pseudo-second-order kinetic constant (k 2)of fresh and reused PED-MIL-101s and ED-MIL-101s (Temperature:25◦C,C i :50ppm).Adsorbent Items Fresh 1st reuse 2nd reuse PED-MIL-101q e (mg/g)187186181k 2(g/mg min) 3.04×10−3 1.87×10−3 1.18×10−3ED-MIL-101q e (mg/g)130127123k 2(g/mg min)1.27×10−31.07×10−37.62×10−4Fig.5.van’t Hoff plots to get the H and S of the MO adsorption over the MIL-101and PED-MIL-101.adsorption may be due to a stronger interaction between pre-adsorbed water and the adsorbent than the interaction between MO and the adsorbent.However,further work is necessary to clarify this suggestion.The entropy change S can be obtained from the (intercept ×R )of the vant’t Hoff plot (Fig.5),and the obtained entropy change S is 208J/(mol K).The positive S means the increased randomness with adsorption of MO probably because the number of desorbed water molecule islarger than that of the adsorbed MO molecule (MO is very bulky compared with water;therefore several water may be desorbed by adsorption of a MO molecule).Therefore,the driving force of MO adsorption (negative G )on PED-MIL-101is due to an entropy effect (large positive S )rather than an enthalpy change ( H is positive).Fig.6.Effect of pH of MO solution on the adsorbed amount of MO over activated carbon and PED-MIL-101(C i :50ppm,adsorption time:4h).The thermodynamic properties were also determined for the case of MIL-101using the adsorption isotherms (Fig.4c)and the Langmuir plots at different temperatures (Fig.4d).Generally,the isotherms and Langmuir plots are similar to those of PED-MIL-101(Fig.4a and b).However,it should be mentioned that the adsorbed amount of MO over PED-MIL-101is high at very low concentra-tion (especially at 45◦C),which is very helpful to remove MO even at a low concentration.The adsorption free energy,enthalpy and entropy change over MIL-101,obtained from Fig.4d and Fig.5,are displayed in Table 2.Similar to PED-MIL-101,the adsorption of MO over MIL-101is a spontaneous process (negative G ).However,the absolute value of H is very small for the case of MIL-101.This may be due to a physical adsorption of MO and little desorption of pre-adsorbed water.The small free energy change ( S )sup-ports this assumption (small number of desorbed water molecules).However,a more detailed study will be necessary to understand the difference between MIL-101and PED-MIL-101in terms of the adsorption enthalpy and entropy changes.3.3.Effect of pH and regeneration of MOFsThe adsorption of a dye usually highly depends on the pH of the dye solution [2,6].MO adsorption at various pH values was mea-sured after equilibration withPED-MIL-101and activated carbon.As shown in Fig.6,the adsorbed amounts decrease with increas-ing the pH of the MO solution,which is quite similar to previously reported results [2,6].The decreasing of adsorbed MO with increas-ing pH might be due to the fact that the concentration of positive charge of adsorbents is decreased with increasing pH.Scheme 2.E.Haque et al./Journal of Hazardous Materials181 (2010) 535–542541Fig.7.(a)Effect of contact time on the MO adsorption and(b)Pseudo-second-order plots to show the re-usability of the spent PED-MIL-101.Even though more detailed work is necessary to clearly under-stand the mechanism of MO adsorption on Cr-BDCs,the mechanism may be explained with electrostatic interaction between MO and adsorbents(as proposed in Scheme2).MO usually exists in the sulfate form;therefore,there will be a strong electrostatic inter-action with an adsorbent having a positive charge.The positive charge distribution increases in the order of MIL-101<ED-MIL-101<PED-MIL-101as suggested in Scheme2.On the other hand the positive charge of PED-MIL-101will be decreased with increasing the pH of the solution due to the deprotonation of the proto-nated adsorbent.Therefore,the increase of adsorption capacity and kinetic constant with modification(MIL-101<ED-MIL-101<PED-MIL-101)or increasing acidity may be explained with the increased positive charge of the adsorbent,especially PED-MIL-101in a low pH condition.A similar adsorption mechanism of anionic dyes via electrostatic interaction has been reported in the case of chitosan [44]and NH3+-MCM-41[4].Facile regeneration of an adsorbent is very important for commercial feasibility.The re-usability of spent adsorbent(PED-MIL-101)is shown in Fig.7a.Even though the adsorption kinetic constants(shown in Table3which are derived from Fig.7b) decrease noticeably with the cycle of adsorption,the amount of adsorbed MO does not decrease much,suggesting the applicabil-ity of Cr-BDCs in the adsorptive removal of anionic dye materials from waste water.Similar re-usability of ED-MIL-101has also been observed(Table3,Supporting Fig.3).4.ConclusionThe liquid-phase adsorption of MO over MOF-type materials has been studied to understand the characteristics of dye adsorption on these materials.The adsorption capacity and adsorption kinetic constant of MIL-101are greater than those of MIL-53,showing the importance of porosity and pore size for adsorption,which is sim-ilar to reported -101,after being modified to have a positive charge,can be efficiently used to remove MO via liquid-phase adsorption.Based on the rate constant(pseudo-second or pseudo-first-order kinetics for adsorption)and adsorption capac-ity,it can be suggested that there is a specific interaction like electrostatic interaction between MO and the adsorbent for rapid and high uptake of the dye.The adsorption of MO over PED-MIL-101 at various temperatures shows that the adsorption is spontaneous and endothermic and the randomness increases with the adsorp-tion of MO.The driving force of MO adsorption over PED-MIL-101 is mainly due to an entropy effect rather than an enthalpy change. From this study,it can be suggested that the MOF-type materials may be applied in the regenerable adsorptive removal of anionic dye materials from contaminated water.AcknowledgementsThis work was supported by the National Research Founda-tion of Korea(NRF)grant funded by the Korea Government(MEST) (2008-0055718,2009-0083696).Appendix A.Supplementary dataSupplementary data associated with this article can be found,in the online version,at doi:10.1016/j.jhazmat.2010.05.047. References[1]G.Crini,Non-conventional low-cost adsorbents for dye removal:a review,Bioresour.Technol.97(2006)1061–1085.[2]A.Mittal,A.Malviya,D.Kaur,J.Mittal,L.Kurup,Studies on the adsorptionkinetics and isotherms for the removal and recovery of Methyl Orange from wastewaters using waste materials,J.Hazard.Mater.148(2007)229–240. [3]S.Chen,J.Zhang,C.Zhang,Q.Yue,Y.Li,C.Li,Equilibrium and kinetic studies ofmethyl orange and methyl violet adsorption on activated carbon derived from Phragmites australis,Desalination252(2010)149–156.[4]Q.Qin,J.Ma,K.Liu,Adsorption of anionic dyes on ammonium-functionalizedMCM-41,J.Hazard.Mater.162(2009)133–139.[5]Z.-M.Ni,S.-J.Xia,L.-G.Wang,F.-F.Xing,G.-X.Pan,Treatment of methyl orangeby calcined layered double hydroxides in aqueous solution:adsorption prop-erty and kinetic studies,J.Colloid Interface Sci.316(2007)284–291.[6]K.P.Singh,D.Mohan,S.Sinha,G.S.Tondon,D.Gosh,Color Removal fromWastewater Using Low-Cost Activated Carbon Derived from Agricultural Waste Material,Ind.Eng.Chem.Res.42(2003)1965–1976.[7]A.Bhatnagar,E.Kumar,A.K.Minocha,B.-H.Jeon,H.Song,Y.-C.Seo,Removalof anionic dyes from water using Citrus limonum(lemon)peel:equilibrium studies and kinetic modeling,Sep.Sci.Technol.44(2009)316–334.[8]G.Annadurai,R.-S.Juang,D.-J.Lee,Use of cellulose-based wastes for adsorptionof dyes from aqueous solutions,J.Hazard.Mater.92(2002)263–274.[9]V.Rocher,J.-M.Siaugue,V.Cabuil,A.Bee,Removal of organic dyes by magneticalginate beads,Water Res.42(2008)1290–1298.[10]J.-H.Huang,K.-L.Huang,S.-Q.Liu,A.-T.Wang,C.Yan,Adsorption of rhodamineB and methyl orange on a hypercrosslinked polymeric adsorbent in aqueoussolution,Colloid Surf.A:Physicochem.Eng.Aspects330(2008)55–61. [11]A.Ayar,O.Gezici,M.Küc¸ükosmano˘g lu,Adsorptive removal of methyleneblue and methyl orange from aqueous media by carboxylated diaminoethane sporopollenin:on the usability of an aminocarboxilic acid functionality-bearing solid-stationary phase in column techniques,J.Hazard.Mater.146(2007) 186–193.[12]X.Luo,L.Jhang,High effective adsorption of organic dyes on magnetic cellulosebeads entrapping activated carbon,J.Hazard.Mater.171(2009)340–347. [13]G.Férey,Hybrid porous solids:past,present,future,Chem.Soc.Rev.37(2008)191–214.[14]S.Kitagawa,R.Kitaura,S.-I.Noro,Functional porous coordination polymers,Angew.Chem.Int.Ed.43(2004)2334–2375.[15]O.M.Yaghi,M.O’Keeffe,N.W.Ockwig,H.K.Chae,M.Eddaoudi,J.Kim,Reticularsynthesis and the design of new materials,Nature423(2003)705–714.。


Adsorption of uranium(VI)from aqueous solutionby diethylenetriamine-functionalized magnetic chitosanJinsheng Xu •Mansheng Chen •Chunhua Zhang •Zhengji YiReceived:16February 2013/Published online:12June 2013ÓAkade´miai Kiado ´,Budapest,Hungary 2013Abstract In this paper,the modified magnetic chitosan resin containing diethylenetriamine functional groups (DETA-MCS)was used for the adsorption of uranium ions from aqueous solutions.The influence of experimental conditions such as contact time,pH value and initial ura-nium(VI)concentration was studied.The Langmuir,Fre-undlich,Sips and Dubinin–Radushkevich equations were used to check the fitting of adsorption data to the equilib-rium isotherm.The best fit for U(VI)was obtained with the Sips model.Adsorption kinetics data were tested using pseudo-first-order and pseudo-second-order models.Kinetic studies showed that the adsorption followed the pseudo-second-order kinetic model,indicating that the chemical adsorption was the rate-limiting step.The present results suggest that DETA-MCS is an adsorbent for the efficient removal of uranium(VI)from aqueous solution.Keywords Uranium ÁAdsorption ÁDiethylenetriamine-functionalized magnetic chitosan (DETA-MCS)ÁIsothermIntroductionUranium is not only a main raw material for nuclear industry,but also a radioactivity element,which is one of metal ions such as cesium and strontium are highly toxic,causes progressive or irreversible renal injury and in acutecases may lead to kidney failure and death [1–5].For this reason,the recovery,accumulation,and removal of ura-nium are of great importance.Nowadays,recovery of uranium(VI)from dilute aqueous solution commonly includes coagulation,chromatographic extraction,chemi-cal precipitation,ion exchange,membrane dialysis,etc.[3,4],but they have several disadvantages,like clogging,high cost and ineffectiveness when uranium(VI)ions are present in the wastewater at low concentrations,especially in the range of 1–100mg/L [6,7].Therefore,the above methods have limitation in application.In recent years,much attention has been focused on various adsorbents with metal-binding capacities and low cost,such as chitosan,zeolites,clay or certain waste products [8].As well known,chitosan and its derivatives have great potential application in the areas of biotech-nology,biomedicine,food ingredients,and cosmetics.Furthermore,chitosans are the most important materials examined for the removal of toxic metal ions due to their inexpensive and effective in natures [9,10].Magnetic chitosan resins (MCR)have been widely used in various applications such as enzyme purification,cell separation,and waste treatment [11,12].On the other hand,as we know,one of the promising methods is the use of chelating resins that have suitable functional groups capable of interaction with metal ions.And the number of chelate rings can increase the stability of complex formed by poly amine,furthermore,the diethylenetriamine can exhibit different kinds of coordination modes of interaction of the U(VI)ions by five-memberd chelating rings in order to increase the adsorption capacity.Based on the above dis-cussion,in this study,the diethylenetriamine-modified magnetic chitosan resins (DETA-MCS)were synthesized.It was expected that DETA-MCS should be efficient for the removal of U(VI)ions,owing to the strong adsorptionJ.Xu (&)ÁM.Chen ÁC.Zhang ÁZ.YiDepartment of Chemistry and Material Sciences,KeyLaboratory of Functional Organometallic Materials of Hengyang Normal University,College of Hunan Province,Huangbai Road No.165,Hengyang 421008,Hunan,China e-mail:hynuxujs@J Radioanal Nucl Chem (2013)298:1375–1383DOI 10.1007/s10967-013-2571-2capacity for the target ions and quick separation from aqueous by the magnetism.The different factors affecting the uptake behavior such as pH,initial concentration of the U(VI)ions,and contact time were investigated.Moreover, the adsorption isotherms,kinetics and regeneration studies were also identified.Materials and methodsChitosan with40mesh,90%degree of deacetylation(DD) and molecular weight of1.39105,and diethylenetriamine were purchased from Shanghai Medicine Company.0.45l m polypropylene membrane(PPM)filter was pur-chased from Sinopharm Chemical Reagent Co.Ltd.(ori-ginal from Kenker Company,US).A stock solution of U(VI)(1,000mg/L)was prepared by dissolving U3O8in a mixture of HCl,H2O2and HNO3.The U3O8was provided by School of Nuclear Resources and Nuclear Fuel Engi-neering,University of South China.All working solutions of different U(VI)concentrations were obtained by diluting the stock solution with distilled and deionized water at room temperature.All other reagents and solvents used in this study were of analytical grade.Uranium adsorption experimentsBatch sorption experiments of U(VI)were conducted in a series of250mL conicalflasks.Generally speaking, 100mL U(VI)solution was mixed with a known amount of DETA-MCS powder.The pH of the U(VI)solution was adjusted as desired using1.0mol/L NaOH and1.0mol/L HCl before mixing with the adsorbent(20mg DETA-MCS powder).A sample of solution was collected at suitable time intervals andfiltered through a0.45l m PPMfilter which does not adsorb uranyl cations.Then thefiltrates were analyzed for U(VI)concentration in the supernatants using a standard method given by Xie et al.[13].The U(VI)removal efficiency and adsorption capacity of U(VI) onto the DETA-MCS were calculated using the following equations:removal efficiencyð%Þ¼C0ÀC fC0Â100ð1Þq e¼ðC0ÀC fÞVWð2Þwhere q e denotes the adsorption capacity of U(VI)onto the DETA-MCS(mg/g);C0and C f the concentrations of the U(VI)in the solution before and after adsorption(mg/L), respectively;V the volume of the aqueous solution(L);and W is the mass of dry adsorbent used(g).Results and discussionPreparation and characterizationThe magnetic chitosan microspheres(MCS)were prepared according to the literature[14].The MCS(10.0g)were suspended in120mL isopropyl alcohol to which10mL epichlorohydrine(125mmol)dissolved in200mL ace-tone/water mixture(1:1v/v)was added.The solid which is modified MCS with epichlorohydrine(MCS-ECH)was filtered and washed by ethanol followed by water for three times.Then the MCS-ECH obtained were suspended in 200mL ethanol/water mixture(1:1v/v),then diethylene-triamine(10mL)was added.The reaction mixture was stirred at60°C for12h,and the solid products DETA-MCS werefiltered and successively washed with acetone, demineralized water,and methanol,and dried in a vacuum oven at60°C.The resins studied were synthesized as shown in Fig.1.Figure2shows the FTIR spectra of DETA-MCS and MCS,The peaks at560–660cm-1were assigned to Fe–O bond vibration of Fe3O4.The absorption band around 3,380cm-1,revealing the stretching vibration of N–H group and–OH group in magnetic chitosan,and at 1,588cm-1confirms the N–H scissoring from the primary amine,due to the free amino groups in the cross-linked chitosan.But in DETA-MCS,the peak becomes broad because of the existence of–NH2.The increasing intensity at1,667and1,082cm-1in the spectrum of DETA-MCS indicates that DETA-MCS has more amine groups than the unmodified magnetic chitosan(MCS).Effect of contact timeSince the contact time between the adsorbate and adsorbent is a key parameter for the adsorption process,the contact time required for the sorption equilibrium experiments was first determined.Under the conditions of50mL solution contain20mg adsorbent amount,pH 3.5,298K and 50mg/L U(VI),the adsorption experiments were carried out for contact times ranging from20to180min.The results are shown in Fig.3.The sorption capacity increased with increasing contact time and a larger amount of ura-nium was removed by DETA-MCS in thefirst60min of contact time.Then the U(VI)sorption process proceeded slowly and reached saturation levels gradually at about 120min.After120min,the change of adsorption capaci-ties for U(VI)did not show notable effects.In our study,a contact time of120min was selected to guarantee an optimum U(VI)uptake.Effect of initial pH valuesIt is known that the medium pH has an influence upon the uranium sorption process because it controls the solubility of metals as well as the dissociation state of some func-tional groups,such as carboxyl,hydroxyl and amino on the adsorbent surface [15–17].In order to search for the opti-mum pH for the adsorption process as well as to find out whether the DETA-MCS was able to show a good U(VI)uptake at extreme pH values,metal uptake was studied at pH ranging from 1.0to 6.0.The dependence of adsorption percentage of U(VI)ions on the pH of solution was given in Fig.4.The adsorption percentage of the U(VI)ions adsorbed on the DETA-MCS indicated a marked influencewith increasing pH of solution from 1.0to 3.5then started to decrease slightly with further increase in the pH of solution after reaching a maximum of 96.1%at pH 3.5.In strong acidic solutions (pH \3.5),more protons will be available to protonate amine groups to form groups –NH 3?,reducing the number of binding sites for the adsorption of UO 22?,therefore,the removal efficiency of uranium is lower in strong acidic solutions (pH \3.5).However,the availability of free U(VI)ions is maximum at pH 3.5and hence maximum adsorption,when pH value increase beyond 3.5,hydrolysis precipitation starts because of the formation of complexes in aqueous solution [18].The hydrolysis of uranyl ions play significant role in deter-mining the equilibrium between U(VI)in solution and on adsorbent.Hydrolysis products occur,includingUO 2(OH)?,(UO 2)2(OH)22?,(UO 2)3(OH)53?,which results in decline of adsorption removal efficiency of U(VI),similar results were also observed [19].The hydrolysis equilibria are as follows:UO 2þ2þ2H 2O UO 2ðOH ÞþþH 3O þpK 1¼5:82UO 2þ2þ4H 2O ðUO 2Þ2ðOH Þ2þ2þ2H 3O þpK 2¼5:623UO 2þ2þ10H 2O ðUO 2Þ3ðOH Þþ5þ5H 3OþpK 3¼15:63where pKs are the logarithms of the equilibrium constants.When the pH becomes low enough,the divalent free UO 22?becomes the dominant ion form in the solution.Along with increasing pH,the percentage of UO 22?in the solution decreases,whereas the percentage oftheFig.1Scheme for the synthesis ofDETA-MCSFig.2FT-IR spectra MCS andDETA-MCSFig.3Effect of contact time on the adsorption of uranium (VI)([UO 22?]=50mg/L,DETA-MCS =20mg,pH =3.5,and T =298K)Fig.4Effect of initial pH on the adsorption of uranium (VI)([UO 22?]=50mg/L,DETA-MCS =20mg,and T =298K)monovalent hydrolyzed species,UO 2(OH)?,(UO 2)3(OH)5?,increases.At higher pH [5.5,dissolved solid schoepite (4UO 3Á9H 2O)exist in the solution.In view of the above result,all subsequent experiments were performed at pH 3.5.Effect of initial uranium(VI)concentrationThe percentage removal and adsorption capacity of U(VI)by contacting a fixed mass of DETA-MCS (20mg)at the temperature (298K)and initial pH (3.5)using a range of initial U(VI)concentrations were shown in Fig.5.It was found that the adsorption removal efficiency of U(VI)decreased with increasing the initial U(VI)concentration in the aqueous solution.On one hand,this is because more mass of uranium is put into the system with increasing the initial U(VI)concentration in the aqueous solution.On the other hand,because of the higher mobility of uranyl ions (UO 2)2?in the diluted solutions,the interaction of this ion with the adsorbent also increases slowly.All in all,the adsorption capacity of DETA-MCS for uranium increased with increase in the initial uranium concentration.Similar results on the influence of the U(VI)biosorption has beenreported by Ku¨tahyal ıet al.[20]in their study using acti-vated carbon prepared from charcoal by chemical activation.Adsorption isothermThe adsorption isotherm is the most important information,which indicates how the adsorbent molecules distribute between the liquid and the solid phase when the adsorption process reaches an equilibrium state [21].The parameters of Langmuir,Freundlich,Sips and Dubinin–Radushkevich(D–R)models obtained are given in Table 1.In the research,the sorption data have been subjected to different sorption isotherms,namely the Langmuir,Freundlich,Sips and D–R isotherm models.Figure 6shows the adsorption isotherm of uranium(VI)on the DETA-modified MCR from the non-linear models.The Langmuir equation assumes that:(i)the solid surface presents a finite number of identical sites which are energetically uniform;(ii)there is no interactions between adsorbed species,meaning that the amount adsorbed has no influence on the rate of adsorption and (iii)a monolayer is formed when the solid surface reaches saturation.The Langmuir isotherm con-siders the adsorbent surface as homogeneous with identical sites in terms of energy.Equation (3)represents the Langmuir isotherm:q e ¼q m K L C e 1þK L C eð3Þwhere C e is the concentration of the adsorbate in solution at equilibrium (mg/g),q e is the adsorption capacity at equilibrium (mg/g),q m is the maximum adsorption capacity of the adsorbent (mg/g),and K L is the Langmuir adsorption constant related to the energy of adsorption (L/mg).The empirical Freundlich equation based on adsorption on a heterogeneous surface is given as follows:q e ¼K F C 1=neð4Þwhere q e denotes the equilibrium adsorption capacity (mg/g);C e the residual U(VI)concentration in the solution at equilibrium (mg/g);K F the Freundlich constant related to the adsorption capacity of sorbent (mg/g);n the Freundlich exponent related to adsorption intensity.To resolve the problem of continuing increase in the adsorbed amount with a rising concentration as observed for Freundlich model (Fig.6),an expression was proposed as Sips isotherm model [22,23],which is a combined form of Langmuir and Freundlich expressions deduced for predict-ing the heterogeneous adsorption systems.It is given as:q e ¼q s K s C 1=me1þK s C 1=með5Þwhere q s (mg/g)is the Sips maximum uptake of U(VI)per unit mass of DETA-MCS,K S (L/mg)is Sips constant related to energy of adsorption,and parameter m could be regarded as the Sips parameter characterizing the system heterogeneity.Figure 6shows the equilibrium adsorption of U(VI)ions onto the DETA-MCS and the fitting plot of the three iso-therm models.For the studied system,the Sips isotherm correlates best (R =0.998)with the experimental data from adsorption equilibrium of U(VI)ions by DETA-MCS in these models.The phenomenon also suggeststheFig.5Effect of initial concentration on the adsorption of ura-nium(VI)(DETA-MCS =20mg,pH =3.5,and T =298K)heterogeneity of the adsorption,which may be attributed to the complicated form of U(VI)ions at the acid pH regions and the heterogeneous distribution of the active sites on DETA-MCS surface.The maximum adsorption capacity of DETA-MCS for U(VI)ions obtained by Sips isotherm model is 69.68mg/g.Dubinin–Radushkevich isotherm is more general than the Langmuir isotherm because it does not assume a homogeneous surface or constant sorption potential [24].Therefore,in this paper,the D–R isotherm is also used to analyze the experimental isotherm data.The linearized form of the D–R isotherm may be written as:ln C ads ¼ln q m ÀKE 2ð6Þwhere C ads is the amount of metal ions adsorbed on per unit weight of adsorbent,q m is the maximum sorption capacity and K is the activity coefficient related to the mean adsorption energy and E is the Polanyi potential which is equal to:E ¼RT ln ð1þ1=C e Þð7ÞThe values of q m and K deduced by plotting ln C ads versus E 2(Fig.7),and the mean energy of adsorption (E )was calculated from the equation,according to the D–R isotherm,as:E ¼1=ðÀ2K Þ1=2ð8ÞThe plot of ln C ads versus E 2as shown in Fig.7is a straight line.From the slope and intercept of this plot the values of K =-5.9983910-9mol 2/kJ 2and q m =70.52mg U(VI)/g have been estimated.As we know,the adsorption value of the mean sorption energy is in the range of 1–8kJ/mol and in that of 9–16kJ/mol predicted the physical adsorption and the chemical adsorption,respectively [25].The value of E is calculated to be E =9.13kJ/mol and evaluated in the range of 9–16kJ/mol for composite adsorbent.The value of E is expected for chemical adsorption.It is assumed to be heterogeneous in the structure of composite.The results of linearized equations are shown in Table 1,the Langmuir model effectively described the sorption data with all R values [0.99.The adsorption isotherms of U(VI)ions exhibit Langmuir behavior which indicates a mono-layer paring the four isotherm models described above,Sips isotherm is most suitable to char-acterize the uranium-sorption behavior of DETA-MCS according to the values of R .Adsorption kineticsIn order to investigate the kinetic mechanism,which con-trols the adsorption process,the pseudo-first-order andTable 1Isotherm constants and values of R for DETA-MCS ParameterValue R Langmuir isotherm q m (mg/g)65.160.991K L (L/mg)1.24Freundlich isotherm K F (mg/g)36.350.898n3.36Sips isotherm q s (mg/g)69.680.998Ks (L/mg) 1.27m2.74Dubinin–Radushkevich (D–R)isotherm K (mol 2/kJ 2)-5.998391090.955q m (mg/g)70.52Eads (kJ/mol)9.13Fig.6Plots of q e versus C e for the adsorption of uranium(VI)on DETA-MCS (DETA-MCS =20mg,pH =3.5,and T =298K)Fig.7Dubinin–Radushkevich isotherm of sorption U(VI)onto DETA-MCS adsorbentpseudo-second-order models were used to test the experi-ment data [26,27].The pseudo-first-order kinetic model is given as:ln ðq e Àq t Þ¼ln q e Àk 1tð9Þwhere q e is the amounts of adsorbed metal per unit mass (mg/g)at equilibrium and k 1is the rate constant of pseudo first-order sorption (min -1).The value of the rate constant k 1and q e for the pseudo-first-order sorption reaction can be obtained by plotting ln (q e -q t )versus t as well as further linear regression analysis (Fig.8).A series of parameters,including kinetic constants,correlation coefficients and q e values,were obtained via linear regression analysis and shown in Table 2.The calculated q e value of first-order kinetic model (54.35mg/L)cannot give reasonable values,which was lower greater than experimental value Q exp (62.75mg/L).Hence,this equation cannot provide an accurate fit of the experimental data.The pseudo-second order kinetic model defines that the rate controlling mechanism formed by chemical reaction for the sorption of metal ions on adsorbents [28–31].In order to describe U(VI)sorption on the DETA-MCS resin for the initial U(VI)concentrations at constant temperature (298K),the kinetic data obtained from batch adsorption experiments have been analyzed using the pseudo-second order kinetic equation given below.t q t ¼t q e þ1k 2q eð10Þwhere k 2is the rate constant of pseudo-second-order adsorption (g/mg min).The pseudo-second-order plot (Fig.9)is also linear with correlation coefficient of 0.994(Table 2),however the calculated value of adsorption capacity,q e ,cal (61.33mg/g)is close to the value of experimental adsorption capacity,q e,exp (62.75mg/g).Therefore,the pseudo-second-order rate kinetic model best described the experimental data.In other words,U(VI)sorption by DETA-MCS followed the pseudo-second-order kinetic reaction.The goodness of the fit to the pseudo-second-order kinetic model indicates that U(VI)adsorption on the DETA-MCS resin occurred by chemical adsorption [32].Comparison of U(VI)sorption capacity with other adsorbentsTo evaluate the potential application prospect of DETA-MCS,the prepared magnetic adsorbent can be well dis-persed in the water and can be easily separated magneti-cally from the medium after adsorption.These unique features present this adsorbent as a novel,promising and feasible alternative for uranium removal as compared with other adsorbents [33–45](Table 3).This paper emphasizes the material of DETA-MCS as a novel adsorbent in envi-ronmental remediation.Although a direct comparison of DETA-MCS with other adsorbents is very difficultowingFig.8Pseudo-first-order kinetics of uranium(VI)adsorption on DETA-MCS ([UO 22?]=50mg/L,DETA-MCS =20mg,pH =3.5,and T =298K)Table 2Kinetic parameters of uranium(VI)adsorbed onto DETA-MCSPseudo-first-order Pseudo-second-orderExperimental Q e valuek 1(min -1)Q e 1(mg/g)R k 2(g/(mg min))Q e 2(mg/g)R Q exp (mg/g)0.0326554.350.9490.004461.330.99462.75Fig.9Pseudo-second-order kinetics of uranium(VI)adsorption on DETA-MCS ([UO 22?]=50mg/L,DETA-MCS =20mg,pH =3.5,and T =298K)to different experimental conditions adopted,it is con-cluded that U(VI)sorption capacity of DETA-MCS is higher than that of chitosan grafted MWCNTs,magnetic Fe3O4@SiO2and Fe3O4/GO,but lower than that of ion-imprinted MCR.The lower uptake values observed may be attributed to the increased extent of protonation of amino groups in the acidic solution.All in all,it is noteworthy that DETA-MCS-bound U(VI)can be favorably and quickly separated from a solution by using the external magnetic field and it is a prospective adsorbent for application in the field of U(VI)removal.Resins regenerationTo make the process more effective and economically feasible,sorbent regeneration and U(VI)recovery must be evaluated.Regeneration of the DETA-MCS resins was carried out using0.5M HNO3.Adsorption/desorption cycles were carried out repeatedly.The regeneration effi-ciency was calculated using equation[46,47].Regeneration efficiencyð%)¼Uptake of UðVIÞin the second cycleUptake of UðVIÞin the first cycleÂ100ð11ÞRegeneration efficiency of94.4%was achieved for DETA-MCS over three cycles with a standard deviation of ±1.1%.On the other hand,the DETA-MCS after being used for three cycles could still be aggregated very fast from the solution by a3,000G magnet.This higher regeneration efficiency along with easy separation from adsorption medium using external magneticfield indicate promising application in thefield of U(VI)removal.ConclusionsThe U(VI)adsorption capacity by DETA-MCS was strongly dependent on contact time,pH,and initial ura-nium(VI)concentration.The adsorption capacity of U(VI) onto DETA-MCS increases with an increase of contact time and reaches adsorption equilibrium within120min, the adsorption removal efficiency increased with increasing pH to a maximum value(pH3.5)and then declines slowly with further increase in pH.Four adsorption models were used for the mathematical description of the adsorption equilibrium.It is found that the equilibrium isotherm data werefitted well by Sips isotherm and pseudo-second-order equation.In summary,DETA-MCS could be used as an effective adsorbent for U(VI)removal from aqueous solution.The resins loaded by U(VI)are easily regenerated for repeated use using HNO3.Acknowledgments This study was supported by the Key Project of Science and Technology Plan of Hunan Province(No.2012FJ2002), the Innovation Platform Open fund of Hunan Provincial Education Department(No.11K009),the Hunan Provincial Natural Science Foundation of China(No.13JJ6069)and the Hunan Provincial Key Discipline Construction.References1.Mellah A,Chegrouche S,Barkat M(2006)The removal of ura-nium(VI)from aqueous solutions onto activated carbon:kinetic and thermodynamic investigations.J Colloid Interface Sci 296:434–4412.Anirudhan TS,Bringle CD,Rijith S(2010)Removal of ura-nium(VI)from aqueous solutions and nuclear industry effluents using humic acid-immobilized zirconium-pillared clay.J Environ Radioact101:267–2763.Donia AM,Atia AA,Moussa EMM,El-Sherif AM,Abd El-Magied MO(2009)Removal of uranium(VI)from aqueous solutions using glycidyl methacrylate chelating resins.Hydro-metallurgy95:183–1894.Kulkarni PS,Mukhopadhyay S,Bellary MP,Ghosh SK(2002)Studies on membrane stability and recovery of uranium(VI)fromTable3Reported values of adsorption capacities of U(VI)by dif-ferent adsorbentsAdsorbents Adsorptioncapacity(mg/g)ReferencesChitosan22.3[33] Cross-linked chitosan with citric acid191[33] Cross-linked chitosan withepichlorohydrin49.05[32] Chitosan/amine resin493.2[12] Chitosan-tripolyphosphate236.9[34] Chitosan grafted MWCNTs39.2[35]Ion-imprinted magnetic chitosanresins(IMCR)187.26[36]Non-imprinted magnetic chitosanresins160.77[36]Magnetic chitosan grafted withSaccharomyces Cerevisiae72.4[37] Magnetic chitosan42[38] Glycidyl methacrylate(GMA)withdivinylbenzene/magnetite460.3[3]Ion-imprinted cross-linked chitosanHQ-type(IIC-HQ-CTS)218[39]Attapulgite/iron oxide magnetic(ATP/IOM)8.31[40] Magnetic Schiff base94.30[41] Magnetic Fe3O4@SiO252.36[42] Ethylenediamine-modified magneticchitosan82.83[43]Fe3O4/GO69.49[44] Magnetite nanoparticles5[45]aqueous solutions using a liquid emulsion membrane process.Hydrometallurgy64:49–585.Wang JS,Hu XJ,Liu YG,Xie SB,Bao ZL(2010)Biosorption ofuranium(VI)by immobilized Aspergillus fumigatus beads.J Environ Radioact101:504–5086.Sangi MR,Shahmoradi A,Zolgharnein J,Azimi GH,Ghorban-doost M(2008)Removal and recovery of heavy metals from aqueous solution using Ulmus carpinifolia and Fraxinus excelsior tree leaves.J Hazard Mater155:513–5227.Xie SB,Yang J,Chen C,Zhang XJ,Wang QL,Zhang C(2008)Study on biosorption kinetics and thermodynamics of uranium by Citrobacter freudii.J Environ Radioact99:126–1338.Babel S,Kurniawan TA(2003)Low-cost adsorbents for heavymetals uptake from contaminated water:a review.J Hazard Mater 97:219–2439.Merrifield JD,Davids WG,MacRae JD,Amirbahman A(2004)Uptake of mercury by thiol-grafted chitosan gel beads.Water Res 38:3132–313810.Wan Ngah WS,Kamari A,Koay YJ(2004)Equilibrium andkinetics studies of adsorption of copper(II)on chitosan and chitosan/PVA beads.Int J Biol Macromol34:155–16111.Justi KC,Favere VT,Laranjeira MC,Neves A,Peralta RA(2005)Kinetics and equilibrium adsorption of Cu(II),Cd(II),and Ni(II) ions by chitosan functionalized with2[bis-(pyridylmethyl)ami-nomethyl]-4-methyl-6-formylphenol.J Colloid Interface Sci 291:369–37412.Atia AA(2005)Studies on the interaction of mercury(II)and ura-nyl(II)with modified chitosan resins.Hydrometallurgy80:13–22 13.Xie SB,Zhang C,Zhou XH,Yang J,Zhang XJ,Wang JS(2009)Removal of uranium(VI)from aqueous solution by adsorption of hematite.J Environ Radioact100:162–16614.Zhou LM,Wang YP,Liu ZR,Huang QW(2009)Characteristicsof equilibrium,kinetics studies for adsorption of Hg(II),Cu(II), and Ni(II)ions by thiourea-modified magnetic chitosan micro-spheres.J Hazard Mater161:995–100215.O¨zerog˘lu C,Kece˛li G(2011)Investigation of Cs(I)adsorption ondensely crosslinked poly(sodium methacrylate)from aqueous solutions.J Radioanal Nucl Chem289:577–58616.O¨zerog˘lu C,Kece˛li G(2009)Kinetic and thermodynamic studieson the adsorption of U(VI)ions on densely crosslinked poly(methacrylic acid)from aqueous solutions.Radiochim Acta 97:709–71717.Venkatesan KA,Shymala KV,Antony MP,Srinivasan TG,RaoPRV(2008)Batch and dynamic extraction of uranium(VI)from nitric acid medium by commercial phosphinic acid resin,Tulsion CH-96.J Radioanal Nucl Chem275:563–57018.Li WC,Victor DM,Chakrabarti CL(1980)Effect of pH anduranium concentration on interaction of uranium(VI)and ura-nium(IV)with organic ligands in aqueous solutions.Anal Chem 52:520–53419.Parab H,Joshi S,Shenoy N,Verma R,Lali A,Sudersanan M(2005)Uranium removal from aqueous solution by coir pith: equilibrium and kinetic studies.Bioresour Technol96:1241–1248 20.Ku¨tahyalıC,Eral M(2004)Selective adsorption of uranium fromaqueous solutions using activated carbon prepared from charcoal by chemical activation.Sep Purif Technol40:109–11421.Hasan M,Ahmad AL,Hameed BH(2008)Adsorption of reactivedye onto crosslinked chitosan/oil palm ash composite beads.Chem Eng J136:164–17222.Ncibi MC(2008)Applicability of some statistical tools to predictoptimum adsorption isotherm after linear and non-linear regres-sion analysis.J Hazard Mater153:207–21223.Foo KY,Hameed BH(2010)Insights into the modeling ofadsorption isotherm systems.Chem Eng J156:2–1024.Oguz E(2005)Thermodynamic and kinetic investigations ofPO43-adsorption on blast furnace slag.J Colloid Interface Sci 28:62–6725.Saeed MM(2003)Adsorption profile and thermodynamicparameters of the preconcentration of Eu(III)on2-thenoyltri-fluoroacetone loaded polyurethane(PUR)foam.J Radioanal Nucl Chem256:73–8026.Chiou MS,Li HY(2002)Equilibrium and kinetic modeling ofadsorption of reactive dye on cross-linked chitosan beads.J Hazard Mater93:233–24827.Ho S,Mckay G(1999)Pseudo-second order model for sorptionprocess.Process Biochem34:451–46528.Ho YS,McKay G(2002)Application of kinetic models on thesorption of copper(II)on to peat.Adsorpt Sci Technol20: 797–81529.Liu Y,Liu Y,Cao X,Hua R,Wang Y,Pang C,Hua M,Li X(2011)Biosorption study of uranium(VI)on crosslinked chitosan: isotherm,kinetic and thermodynamic aspects.J Radioanal Nucl Chem290:231–23930.Wu FC,Tseng RL,Juang RS(2001)Enhanced abilities of highlyswollen chitosan beads for color removal and tyrosinase immo-bilization.J Hazard Mater81:166–17731.Ho YS(2004)Citation review of Lagergren kinetic rate equationon adsorption reactions.Scientometrics59:171–17732.Wang GH,Liu JS,Wang XG,Xie ZY,Deng NS(2009)Adsorption of uranium(VI)from aqueous solution onto cross-linked chitosan.J Hazard Mater168:1053–105833.Suc NV,Yeu Ly HT(2011)Adsorption of U(VI)from aqueoussolution onto modified chitosan.Int J ChemTech Res3: 1993–200234.Sureshkumara MK,Dasb D,Malliac MB,Gupta PC(2009)Adsorption of uranium from aqueous solution using chitosan-tripolyphosphate(CTPP)beads.J Hazard Mater184:65–72 35.Shao DD,Hu J,Wang XK(2010)Plasma induced graftingmultiwalled carbon nanotube with chitosan and its application for removal of UO22?,Cu2?,and Pb2?from aqueous solutions.Plasma Process Polym7:977–98536.Zhou LM,Shang C,Liu ZR,Huang GL,Adesina AA(2012)Selective adsorption of uranium(VI)from aqueous solutions using the ion-imprinted magnetic chitosan resins.J Colloid Interface Sci366:165–17237.Saifuddin N,Sultanbayeva D(2012)Immobilization of Saccha-romyces cerevisiae onto cross-linked chitosan coated with mag-netic nanoparticles for adsorption of uranium(VI)ions.Adv Nat Appl Sci6:249–26738.Stopa LCB,Yamaura M(2010)Uranium removal by chitosanimpregnated with magnetite nanoparticles:adsorption and desorption.Int J Nucl Energy Sci Technol5:283–28939.Liu YH,Cao XH,Le ZG,Luo MB,Xu WY,Huang GL(2010)Pre-concentration and determination of trace uranium(VI)in environments using ion-imprinted chitosan resin via solid phase extraction.J Braz Chem Soc21:533–54040.Fan QH,Li P,Chen YF,Wu WS(2011)Preparation and appli-cation of attapulgite/iron oxide magnetic composites for the removal of U(VI)from aqueous solution.J Hazard Mater 192:1851–185941.Zhang XF,Jiao CS,Wang J,Liu Q,Li RM,Yang PP,Zhang ML(2012)Removal of uranium(VI)from aqueous solutions by magnetic Schiff base:kinetic and thermodynamic investigation.Chem Eng J198–199:412–41942.Fan FL,Qin Z,Bai J,Rong WD,Fan FY,Tian W,Wu XL,ZhaoL(2012)Rapid removal of uranium from aqueous solutions using magnetic Fe3O4@SiO2composite particles.J Environ Radioact 106:40–46。


第22卷第1期化 学 研 究中国科技核心期刊2011年1月CH EMICAL RESEARCH hxyj@表面活性剂改性有机沸石对废水中亚甲基蓝的吸附行为张秀兰,栗印环,张灵芝,张至杰(信阳师范学院化学化工系,河南信阳464000)摘 要:用表面活性剂十六烷基三甲基溴化铵(CTMAB)和十二烷基硫酸钠(SDS)复合改性沸石,制备了阴阳离子表面活性剂改性沸石,利用X射线衍射和红外吸收光谱表征了改性前后沸石的结构,并研究了改性沸石对亚甲基蓝的吸附行为.结果表明,改性后沸石的吸附性能明显增强;在溶液p H8、常温、吸附时间100min、改性沸石用量20g/L条件下,160mg/L亚甲基蓝溶液的脱色率达99.32%.其吸附动力学行为遵循准二级吸附速率方程;平衡吸附量Q e与平衡浓度c e之间的关系符合Langmuir等温吸附方程.关键词:表面活性剂;改性沸石;废水;亚甲基蓝;吸附性能中图分类号:O647.32文献标志码:A文章编号:1008-1011(2011)01-0080-04Adsorption of methylene blue in w aste w aterby surfactant2modif ied zeoliteZHAN G Xiu2lan,L I Y in2huan,ZHAN G Ling2zhi,ZHAN G Zhi2jie(Depart ment of Chemist ry and Chemical Engineering,X iny ang Teachers College,X iny ang464000,Henan,China)Abstract:Zeolite modified wit h anion2cation surfactant s was prepared by incorporating bot h cat2ionic surfactant cetylt rimet hylammonium bromide(CTMAB)and anionic surfactant sodium do2decyl sulfate(SDS).The st ruct ure of t he zeolites before and after modification was analyzed bymeans of inf rared spect romet ry and X2ray diffraction.The adsorption behavior of t he modifiedzeolite to met hylene blue in wastewater was evaluated.Result s show t hat modification wit h t hesurfactant s cont ributes to greatly increase t he adsorption capacity of zeolite to met hylene blue.At a solution p H of8,room temperat ure,absorption time of100min,and modified zeolitedosage of20g/L,t he met hylene blue in wastewater(160mg/L)was removed at a rate of upto99.32%.The adsorption kinetics followed t he laws of p seudo2second order kinetic model,and t he relationship between adsorption capacity(Q e)and equilibrium mass concent ration(c e)was in accordance wit h Langmuir isot hermal adsorption equations.K eyw ords:surfactant;modified zeolite;wastewater;met hylene blue;adsorption behavior 我国印染工业排放的废水由于其成分复杂,色度深,排放量大,高COD和BOD,成为难处理的工业废水,而对其脱色则成为治理过程的关键.目前,新型廉价的吸附材料的开发是印染废水处理方面研究的热点之一[1-4].沸石是一种具有骨架结构的水合铝硅酸盐矿物,储量丰富,价廉易得,具有良好的吸附性能和阳离子交换性能[5].通过有机改性,增加沸石的有机含量,从而增强其对有机污染物的吸附能力.国内外研究中尚未有利用阴阳离子表面活性剂复合改性沸石的脱色报道.作者采用十六烷基三甲基溴化铵(CTMAB)和十二烷基硫酸钠(SDS)为复合改性剂,对信阳上天梯非金属矿斜发沸石进行改性,通过X射线衍射、红外吸收光谱对改性前后沸石的结构进行表征,探讨阴阳离子表面活性剂改性沸石对亚甲基蓝的吸附性能,为染收稿日期:2010-09-11.基金项目:河南省科技攻关项目(0524440058).作者简介:张秀兰(1968-),女,副教授,学士,主要从事无机非金属材料和水处理方面的研究.E2mail:zxlxynu2005@.第1期张秀兰等:表面活性剂改性有机沸石对废水中亚甲基蓝的吸附行为81 料废水的处理寻找廉价的原料.1 实验部分1.1 实验原料、试剂及仪器河南信阳上天梯斜发沸石,200~300目;亚甲基蓝、十六烷基三甲基溴化铵、十二烷基硫酸钠均为分析纯;D8/Advance 型X 射线粉末衍射仪,德国Bruker 公司;PE 2680型红外光谱仪,美国Perkin 2Elmer 公司;723型分光光度计,四川仪器九厂;SHA 2C 水浴恒温振荡器,北京仪器有限公司;ZK 282B 型真空干燥箱,上海市实验仪器总厂;p HS 23C 型精密p H 计,上海雷磁仪器厂;分析天平,湘仪天平制造有限公司.1.2 阴阳离子表面活性剂改性沸石的制备在一定体积的十六烷基三甲基溴化铵饱和溶液中加入一定量的十二烷基硫酸钠(C TMAB 与SDS 的质量比为13∶1),混合均匀,制得阴阳离子表面活性复配液.将一定量的沸石(质量为SDS 的36倍)配成一定浓度的悬浮液,分散均匀后加入上述复配液,在常温下搅拌1.5h ,产品经抽滤,充分洗涤后,在90℃下烘干2.0h ,获得阴阳离子表面活性剂改性沸石.产品经研磨,过200目筛,密封备用.1.3 改性沸石对亚甲基蓝的吸附取160mg/L 的亚甲基蓝溶液50mL 置于具塞锥形瓶中,分别用一定量的天然沸石和改性沸石作为吸附剂,在一定的p H 值、振荡时间和吸附温度下进行吸附实验,用分光光度法于波长665nm 处测定亚甲基蓝的残余质量浓度.平衡吸附量根据公式Q e =V (c 0-c e )/m 计算.式中:Q e 是平衡吸附量(mg/g ),V 是染料溶液的体积(L ),m 是沸石的质量(g ),c 0、c e 分别为染料溶液的初始质量浓度及吸附平衡时的质量浓度(mg/L ).吸附率以η=[(c 0-c e )/c 0]×100%计算.2 结果与讨论2.1 沸石结构的表征2.1.1 X 射线衍射分析分别将过200目筛的天然沸石和改性沸石,用D8/Advance 型X 射线衍射仪进行分析,结果见图1.由图1可知,天然沸石经有机改性后,首峰的位置发生了变化,与天然沸石相比改性沸石首峰的2θ明显减小.由Bragg 方程:2d sinθ=n λ(其中d 为层面间距,θ为入射角,λ为入射光的波长,n 为衍射级数)可知:有机改性沸石的层面间距d 明显增大,说明改性后阴阳离子表面活性剂已经进入沸石晶格间.图1 沸石的XRD 谱图Fig.1 XRD of the zeolite 图2 沸石的IR 谱图Fig.2 IR spectra of the zeolite2.1.2 红外吸收光谱分析将天然沸石和改性沸石用溴化钾压片,在4000~400cm -1范围内摄谱,结果见图2.由图2可知,与天然沸石相比,改性沸石的红外光谱发生了如下变化:在2918cm -1及2850cm -1处出现了两个尖锐的强吸收峰,这归属于十六烷基三甲基溴化铵和十二烷基硫酸钠改性剂中-CH 3和-CH 2产生的对称和反对称伸缩振动;在1470cm -1附近的吸收峰归属于有机改性剂分子中-CH 2的弯曲振动;在3429cm -1位置出现一宽峰,是N -H 与O -H 伸缩振动发生重叠的吸收峰;沸石晶格内和晶格外羟基群的吸收峰(3632cm -1、3437cm -1、1639cm -1)的强度显著减弱;沸石骨架中Si -O 或Al -O 吸收振动峰(1043cm -1、720cm -1、82 化 学 研 究2011年468cm -1)的强度和面积也发生了变化,说明两种物质骨架相似,但不属于同一种物质.表明一定量的十六烷基三甲基溴化铵和十二烷基硫酸钠已复合进入了沸石中.2.2 吸附条件对吸附效果的影响2.2.1 吸附时间对吸附率的影响取50mL 160mg/L 亚甲基蓝溶液,加入0.5g 的改性沸石,常温下振荡吸附不同时间.实验结果表明:改性沸石的吸附率随吸附时间的延长而升高,在吸附开始的几十分钟内吸附率迅速上升,随着时间的延长吸附率上升缓慢.100min 时基本达到吸附平衡.以下实验中选定吸附时间为100min.2.2.2 沸石用量对吸附率的影响在160mg/L 亚甲基蓝溶液中,随改性沸石投加量的增加,改性沸石对亚甲基蓝的吸附率逐渐升高;当投加量增加到20g/L 后,吸附率趋于稳定,再增加沸石用量,吸附率变化不大,因此以下实验确定沸石用量为20g/L.2.2.3 温度对吸附率的影响改性沸石对亚甲基蓝的吸附率随温度升高而降低,说明吸附过程为物理吸附,升高温度不利于吸附作用的发生.当温度为25℃时,吸附率可达98.62%,考虑到实际应用,吸附温度选择常温为宜.2.2.4 p H 值对吸附率的影响溶液p H 值对改性沸石吸附作用的影响较为明显,在p H 2~10的范围内,随着p H 值的增大吸附率逐渐升高,当p H 值达到8附近时吸附率最高,然后吸附率下降.这可能是因为在酸性条件下亚甲基蓝在水溶液中形成一价有机“阳离子”的季胺盐离子基团,溶液中H +与亚甲基蓝形成较强的竞争吸附,从而导致吸附率较低;随着p H 值增大,沸石表面负电性显著提高,与水溶液中一价有机亚甲基蓝阳离子靠静电引力在表面结合,吸附作用增强;p H 值过大,沸石溶解出一定量的SiO (O H )-3和Al (O H )-4,而与亚甲基蓝阳离子在溶液中结合,与表面吸附的亚甲基蓝阳离子产生竞争效应[6],从而减少其在沸石表面的吸附.以下实验选择吸附液的p H 值为8.2.3 吸附等温线分别取初始浓度为200mg/L 、400mg/L 、600mg/L 、800mg/L 、1000mg/L 的亚甲基蓝溶液进行吸附实验,测得一系列吸附平衡数据,可得亚甲基蓝在改性沸石上的吸附等温线如图3所示.分别采用Lang 2muir 等温方程和Freundlich 等温方程对图3中的等温吸附数据进行线性拟合,结果如图4、图5所示.图中Q e 为平衡吸附量(mg ・g -1),c e 为平衡质量浓度(mg ・L -1),r 2为相关系数.由图4、图5可以看出:采用Langmuir 方程能够较好地描述亚甲基蓝在有机改性沸石上的吸附等温线.图3 改性沸石对亚甲基蓝的吸附等温线Fig.3 The adsorption isotherm of the modified zeolite 图4 Langmuir 吸附等温线Fig.4 The adsorption isotherm of the Langmuir2.4 阴阳离子有机沸石对亚甲基蓝的吸附动力学曲线分别取0.5g 沸石置于11个100mL 具塞锥形瓶中,加入160mg/L 的亚甲基蓝溶液50mL ,在常温条件下进行振荡吸附不同时间实验,测得亚甲基蓝在改性沸石上的吸附量随吸附时间的变化曲线如图6所示.吸附开始时亚甲基蓝主要被吸附在改性沸石的外表面,吸附较快;随着吸附过程的进行,亚甲基蓝的质量浓度逐渐减小,同时吸附质沿改性沸石向内部扩散,扩散阻力渐增,吸附速率主要受扩散控制,导致吸附速率变慢,到吸附后期,主要在吸附剂内表面吸附,且浓度推动力越来越小,吸附已基本达到平衡.关于吸附的动力第1期张秀兰等:表面活性剂改性有机沸石对废水中亚甲基蓝的吸附行为83 学模型已有不少研究报道,准二级动力学方程是常用的动力学方程之一[7].其线性表达式为:t/Q t =[1/(k 2Q 2e )]+(1/Q e )t .式中:t 为吸附时间(min );Q e 和Q t 分别为吸附平衡时和吸附过程中任意时间t 的吸附量(mg/g );k 2为二级吸附反应的速率常数[g /(mg ・min )].用上式对图6中的数据进行线性拟合,得动力学方程为:t/Q t =0.0625t +0.1261,r 2=0.9999,可以看出准二级反应速率模型拟合的直线相关性很好,由上式可算出平衡吸附量Q e =16.03mg ・L -1,称为模型计算平衡吸附量.与实验值的相对误差在3%以内,所以用准二级吸附速率方程可较准确地描述亚甲基蓝在改性沸石上的吸附行为.图5 Freundlich 吸附等温线Fig.5 The adsorption isotherm of Freundlic 图6 改性沸石对亚甲基蓝的吸附动力学曲线Fig.6 The kinetic curve ofmodified zeolite on methylene blue2.5 模拟实验分别取1L 浓度为160mg/L 的亚甲基蓝模拟染色废水两份,在吸附剂用量为20g 、p H 值为8、吸附时间为100min 、常温的条件下,分别用天然沸石和有机改性沸石对其进行吸附实验,经吸附处理后,废水中残余亚甲基蓝的质量浓度分别为54.19mg/L 和1.09mg/L ,脱色率分别达66.13%和99.32.%,可见有机改性沸石对亚甲基蓝的脱色率明显增高.说明沸石的有机改性效果很好,适用于处理高浓度染色废水,出水几乎无色度.3 结论1)阴阳离子表面活性剂改性沸石对亚甲基蓝的吸附效果较好.当废水p H 值为8、常温、吸附时间100min 、沸石用量为20g/L 时,改性沸石对质量浓度为160mg/L 的亚甲基蓝的去除率达99.32%,残余质量浓度远远低于国家排放标准[8].2)阴阳离子表面活性剂改性沸石对亚甲基蓝的吸附规律较好地符合Langmuir 吸附等温式,吸附过程符合准二级动力学方程.参考文献:[1]HAN Run Ping ,WAN G Y i ,ZOU Wei Hua ,et parison of linear and nonlinear analysis in estimating the Thomasmodel parameters for methylene blue adsorption onto natural zeolite in fixed 2bed column [J ].J Hazard Mater ,2007,145(1/2):341-345.[2]张秀兰,栗印环,胡福欣.膨润土微波改性及对废水中甲基橙吸附的研究[J ].信阳师范学院学版:自然科学版,2008,21(2):266-269.[3]王亚玲,习霞,邵健.壳聚糖2二氧化硅凝胶复合物对甲基橙的吸附研究[J ].水处理技术,2009,35(8):69-71.[4]栗印环,李鸿雁,张秀兰.阴2阳离子有机膨润土对酸性靛蓝的吸附及动力学研究[J ].信阳师范学院学版:自然科学版,2010,23(2):284-287.[5]张秀兰,栗印环,马万山.改性沸石对水中硫化物的吸附及再生研究[J ].非金属矿,2007,30(5):57-59.[6]路英杭,冯翰林,孙中溪.铝硅酸盐对亚甲基蓝的吸附行为[J ].济南大学学报:自然科学版,2009,23(1):18-21.[7]HO Yuh Shan.Second 2order kinetic model for the sorption of cadmium onto tree fern :a comparison of linear and nonlinearmethods[J ].Water Res ,2006,40(1):119-125.[8]奚旦立.环境工程手册环境监测卷[M ].北京:高等教育出版社,1998.。

Reduction of NO x by H 2on Pt/WO 3/ZrO 2catalysts in oxygen-rich exhaustF.J.P.Schott,P.Balle,J.Adler,S.Kureti *Institut fu¨r Technische Chemie und Polymerchemie,Universita ¨t Karlsruhe,Kaiserstrasse 12,D-76128Karlsruhe,Germany 1.IntroductionNitrogen oxides (NO x )emitted by lean-burn engines con-tribute to various environmental problems,for instance forma-tion of acid rain as well as ozone.As a consequence,the emission limits have been worldwide tightened in the past.For the removal of NO x from oxygen-rich exhaust the selective catalytic reduction (SCR)using NH 3and NO x storage reduction catalyst (NSR)are currently the most favoured technologies.However,a serious constraint of these techniques is the minor deNO x performance below 2008C.Contrary,the catalytic reduction of NO x by H 2(H 2-deNO x )reveals an interesting potential for the low-temperature NO x abatement being particularly crucial for diesel passenger cars.In the driving cycle of the European Union the exhaust temperature is below 1508C for about 60%of cycle time.Thus,SCR and NSR do not cover the most part of the certification cycle and might therefore come under pressure when the exhaust limits will be markedly tightened in the future.This clearly indicates the need for a deNO x technique operating at low temperatures.Furthermore,low-temperature NO x reduction exhibits a potential for industrial applications as well, e.g.for fossil power plants,waste combustion plants,nitric acid production and air separation.First published in 1971Jones et al.show the effective NO x reduction by H 2in slight excess of O 2using a Pt/Al 2O 3catalyst [1].High NO x conversions are observed between 65and 2008C while indicating a high yield of nitrous oxide as well;at maximum deNO x the molar ratio of N 2/N 2O is shown to be unity.The mechanism of the reaction of NO with H 2on Pt/Al 2O 3involves the reduction of the active Pt sites by H 2followed by adsorption and dissociation of NO [2].The recombination of two N atoms leads to the formation of N 2,whereas the oxygen is retained onto the Pt surface.Contrary,N 2O is produced by combination of a N atom and NO being adsorbed on neighbouring Pt sites.In the last years some Pt H 2-deNO x catalysts are presented revealing considerable low-temperature activity even under strongly oxidising conditions [3–6].Wildermann reports on a very active Pt/Al 2O 3catalyst that shows maximum performance already at 708C [3].However,this material produces a huge proportion of N 2O being in line with the results from Jones et al.[1].For example,at peak NO x conversion the N 2O selectivity amounts to 80%.Moreover,Wildermann indicates the enhancement of activity and N 2production of Pt/Al 2O 3by using the promoter Mo (3.4wt.%)resulting in a N 2selectivity of 40%.The performance of this Pt/Mo/Al 2O 3catalyst is additionally enhanced by Co,whereas the N 2selectivity is slightly increased only.However,the activityApplied Catalysis B:Environmental 87(2009)18–29A R T I C L E I N F O Article history:Received 30June 2008Received in revised form 19August 2008Accepted 26August 2008Available online 31August 2008Keywords:NO x reduction H 2Pt WO 3ZrO 2Diesel exhaust Mechanism DRIFTSA B S T R A C TThis work addresses the low-temperature NO x abatement under oxygen-rich conditions using H 2as reductant.For this purpose Pt/ZrO 2and Pt/WO 3/ZrO 2catalysts are developed and characterised by temperature-programmed desorption of H 2(H 2-TPD),N 2physisorption (BET)and powder X-ray diffraction (PXRD).The most active catalyst is a Pt/WO 3/ZrO 2pattern with a Pt load of 0.3wt.%and a W content of 11wt.%.This material reveals high deNO x activity below 2008C and high overall N 2selectivity of about 90%.Additionally,the catalyst exhibits outstanding hydrothermal stability as well as resistance against SO x .Furthermore,the transfer from the powder level to real honeycomb systems leads to promising performance as well.Diffuse reflectance Fourier transform infrared spectroscopic studies,kinetic modelling of tempera-ture-programmed desorption of O 2(O 2-TPD)and NO x -TPD examinations indicate that the pronounced H 2-deNO x performance of the Pt/WO 3/ZrO 2catalyst is related to the electronic interaction of WO 3with the precious metal.The tungsten promoter increases the electron density on the Pt thus activating the sample for H 2-deNO x and N 2formation,respectively.Contrary,NO x surface species formed on the WO 3/ZrO 2support are not supposed to be involved in the H 2-deNO x reaction.ß2008Elsevier B.V.All rights reserved.*Corresponding author.Tel.:+497216088090;fax:+497216082816.E-mail address:kureti@ict.uni-karlsruhe.de (S.Kureti).Contents lists available at ScienceDirectApplied Catalysis B:Environmentalj o ur n a l h o m e p a g e :w w w.e l se v i e r.c om /l oc a t e /a p c a t b0926-3373/$–see front matter ß2008Elsevier B.V.All rights reserved.doi:10.1016/j.apcatb.2008.08.021declines when CO exceeds0.15vol.%[7,8]being in accordance with Lambert and Macleod[9,10].Costa et al.report a Pt/La0.7Sr0.2Ce0.1FeO3catalyst with pronounced low-temperature activity as well as substantially increased N2selectivity up to80–90%[11,12].Detailed examina-tions performed with a related Pt/La0.5Sr0.2Ce0.51MnO3sample[12] indicate a different reaction mechanism as compared to that elucidated for Pt/Al2O3[2].Costa et al.postulate chemisorption of NO x on the support resulting in nitro and nitrato surface species, while H2adsorbs dissociatively on the Pt component.Then,the atomic hydrogen spills over to the support reducing the NO x surface complexes to release N2and H2O.Following Costa et al.this mechanism suppresses the formation of N2O.A very similar mechanism is postulated for a Pt/MgO–CeO2catalyst being very active as well[13,14].In contrast to platinum,Ru,Ir,Rh,Pd and Ag [3,15]as well as perovskite catalysts[11,16–18]reveal no or at least low performance in excess of O2.An exception is Pd/LaCoO3 showing considerable activity[19].Furthermore,it is worth mentioning that the H2-deNO x reaction is an important feature of the TWC technology as well as in regeneration of NSR catalystsreducing NO x under stoichiometric and rich conditions,respec-tively.The aim of this paper is the development of a H2-deNO x catalyst showing both pronounced low-temperature activity and minimum N2O production,whereas the present study mainly focuses on diesel exhaust.For this purpose a series of Pt/ZrO2and Pt/WO3/ ZrO2samples is systematically prepared,characterised andfinally tested for H2-deNO x.These systems are selected as a result of pre-studies in which different support materials have been screened [20,21],e.g.Al2O3,SiO2,ZrO2,TiO2and MgO carriers all modified with alkaline and alkaline earth metals,elements of the1st period of transition metals,Ce,La and Mo.For the evaluation of the technical potential of the most promising catalyst relevant conditions are varied,while a coated honeycomb is employed as well.Additionally,mechanistic examinations are performed to gain insight into the effectiveness of the best catalyst.2.Experimental2.1.Development of the ZrO2support and catalyst preparationPreliminary catalytic investigations show that the synthesis route,crystalline phase and BET surface area of the ZrO2substrate strongly affect the performance of the H2-deNO x catalysts[22].The best result is obtained with a self-prepared zirconia existing in the tetragonal phase.This material is synthesised by advancing the so-called hydrazine route[23,24]providing reliable product quality and sufficient mass to coat several full size honeycombs for automotive applications.For the synthesis a solution of234g ZrO(NO3)2(Fluka)in 1.6l distilled H2O is added to a boiling mixture of400ml N2H4ÁH2O(Fluka)and1.2l distilled H2O.The resulting blend is digested for12h under reflux,whereas shorter reaction time leads to unwanted monoclinic ZrO2as well(Fig.1). Afterfiltration and washing with H2O the solid is dried overnight at 1008C and calcined in air at7508C for6h.The yield of ZrO2is almost100%(85g).The introduction of Pt is carried out by incipient wetness method.In this impregnation a defined volume of Pt(NO3)2 (Chempur)solution is taken such that it is completely absorbed by the substrate.The adjusted Pt loads referring to the support range from0.1to2.0wt.%.After impregnation,the samples are dried overnight at1008C and are then activated by dosing a gas mixture of9vol.%H2and91vol.%N2.In the activation step the temperature is increased from20to3008C at the rate of1.0K minÀ1;the end temperature is held for30min.Finally,the samples are condi-tioned by heating in air at5008C for5h.For reference purposes a classical Pt/Al2O3catalyst is also prepared taking commercially available g-Al2O3balls(d=0.6mm,Sasol).The modification of ZrO2with tungsten is performed by incipient wetness method as well using a solution of (NH4)6H2W12O41(Fluka).Different loads of W(up to22wt.%) relating to the mass of ZrO2are established by varying the concentration of(NH4)6H2W12O41.After impregnation,the sample is dried overnight at1008C and is then impregnated by Pt as mentioned above.It is worth noting that in the H2activation tungsten oxide is not reduced[24].2.2.Characterisation of the catalystsCrystalline phase of the pure supports as well as catalysts is examined by powder X-ray diffraction(PXRD).The PXRD patterns are recorded at room temperature on a Siemens D501using Ni filtered Cu K a radiation.A2u step size of0.028is used with an integration time of4s.The diffractogram of the commercial Al2O3 carrier confirms the g-modification,while the prepared ZrO2is in the tetragonal phase as already demonstrated in Fig.1.Regardless of the load of W no reflexes of crystalline tungsten oxide are observed suggesting amorphous WO x domains,at least at W contents above6wt.%when tungsten oxide exists in sufficient abundance to be monitored.Additionally,signals of Pt are not found as well being associated with its low contents.The dispersion of Pt is studied by temperature-programmed desorption of H2(H2-TPD).In these analyses,same laboratory bench is used as in the catalytic investigations(Section2.4).Respective sample(1.5g)is charged into the quartz glass tube reactor(i.d. 8mm)and pre-treated in Arflow at6008C for15min.Subsequently, the catalyst is cooled to3008C and exposed to a gas mixture of 5vol.%H2and95vol.%Ar for30min to reduce the active Pt surface. The pattern is then rapidly cooled to308C in the H2/Arflow.After saturation,it isflushed with Ar and H2-TPD is started using Ar as carrier gas(100ml minÀ1,STP).In TPD the catalyst is heated to 6008C at the rate of20K minÀ1,whereupon temperature is recorded by a K type thermocouple(TC)fitted directly in front of the sample. Desorbing H2is continuously monitored by thermal conductivity detection(TCD,Shimadzu).For specific analysis of H2the reactor effluents pass a cold trap(À508C)removing H2O.The Pt dispersion (d Pt)is determined by supposing that one H adsorbs per active Pt site (Eq.(1))[25].The molar amount of desorbing H2ðn H2Þis obtained by integrating the corresponding TCD signal,whereas the total proportion of Pt(n Pt)is known from the impregnationprocedure. Fig.1.PXRD patterns of the prepared ZrO2support depending on the digestion time (a)5min,(b)1h,(c)4h,(d)7h,(e)12h and(f)17h;*monoclinic phase;+ tetragonal phase);final calcination is performed in air at7508C for6h;analytical parameters of PXRD are described in next section.F.J.P.Schott et al./Applied Catalysis B:Environmental87(2009)18–2919As derived from blank experiments,e.g.Pt free 11W/ZrO 2,the H 2desorption is specific for Pt.d Pt ¼0:5n H 2n Pt(1)Furthermore,the ZrO 2-based catalyst with a Pt load of 0.3wt.%and a W content of 11wt.%is exemplarily characterised by X-ray photoelectron spectroscopy.The spectrometer is a Phoibos 150MCD from Specs being equipped with a Mg anode (E (Mg K a )=1253eV).The spectrum shows a clear absorption at 36eV (W4f 7/2)being related to W 6+species [26].The BET surface area of the catalysts is investigated by multi-point Sorptomatic 1990using N 2as adsorbate.The BET data are listed in Table 1along with the loading,Pt dispersion and sample codes.It is worth mentioning that the declining BET surface area of the 0.3Pt/W/ZrO 2samples is mainly related to the increasing proportion of WO 3exhibiting negligible surface area and it is not due to the blocking of pores of the ZrO 2carrier.2.3.O 2-and NO x -TPD studiesO 2-TPD studies are performed to investigate the kinetics of the adsorption and desorption of O 2on selected catalysts.Oxygen is used as probe molecule since it represents a dominating species on the Pt surface under lean burn conditions [27].The procedure of O 2-TPD is similar to that described above for H 2-TPD.The catalyst (5.00g)is heated in Ar at 7508C for 15min,cooled to 508C and is then exposed to a mixture of 2vol.%O 2and 98vol.%Ar (99.996%,<6ppm O 2)until the sample is saturated.After flushing with Ar (500ml min À1,STP)TPD is started with a rate of 20K min À1.O 2is detected by CIMS (Airsense 500,V &F).As the axial and radial temperature gradients along the catalyst bed are below 10K heat transfer effects are to be neglected.Furthermore,Mears and Weisz Prater criteria [28],that amount to 10À5and 10À2,respectively,exclude transport limitation by film and pore diffusion.NO x -TPD examinations are conducted similar to H 2-TPD as well using a catalyst mass of 1.50g.After the pre-treatment performed at 5008C the sample is cooled to 1258C in Ar flow and is then exposed to a mixture of 2800ppm NO and 6vol.%O 2in Ar.In this treatment NO is partially converted into NO 2(15%)due to catalytic oxidation.After saturation,the dosage of NO and O 2is stopped and the reactor is flushed by Ar followed by starting the TPD with a rateof 20K min À1.Additionally,before beginning the TPD a mixture of (a)1500ppm H 2,6vol.%O 2,Ar (balance)or (b)1500ppm H 2in Ar is added for 10min to study the reaction of NO x surface species with H 2.Subsequently,it is purged again and TPD is finally started employing exclusively the CLD analyzer mentioned in the following section.2.4.H 2-deNO x studiesThe catalytic investigations are performed on a laboratory bench using a diesel model exhaust.Before the measurements,the samples are pressed to pellet with 40MPa,granulated and sieved in a mesh size of 125–250m m;an exception is the Pt/Al 2O 3pattern which is kept in form of balls.The samples (1.50g)are charged into the quartz glass tube reactor (i.d.8mm),fixed with quartz wool and pre-treated in Ar flow at 5008C for 15min to remove possible impurities and to provide reproducible conditions.Subsequently,the model exhaust is added and the temperature is decreased to 408C with a rate (b )of 1.0K min À1.Furthermore,some experi-ments are carried out under stationary conditions at selected temperatures.The standard feed (500ml min À1,STP)is composed of 500ppm NO,2000ppm H 2,6.0vol.%O 2and Ar as balance.To evaluate the best catalyst the concentration of H 2and O 2is varied and other relevant exhaust gas species,i.e.CO,H 2O and CO 2are dosed additionally.The feed is obtained by blending a special mixture of 2000ppm H 2and 6.0vol.%O 2in Ar with the other components (Air Liquide).The flow of each component is controlled by independent mass flow controllers (MKS Instru-ments),whereas water is supplied by a liquid pump (Kronlab).Temperature is measured by a K type TC located directly in front of and behind the catalyst ing the standard feed the temperature difference between inlet and outlet is below 10K and therefore only the inlet temperature is presented.The analysis of NO x is conducted by means of CLD (EL-ht,Eco Physics),while N 2O,CO and CO 2are monitored by NDIR spectroscopy (Uras 10E,Hartmann &Braun).N 2is detected by GC/TCD (RGC 202withpacked columns Haye Sep Q 60and mol sieve 5A˚,Siemens)resulting in a time resolution of 9min that corresponds to a temperature interval of 15K.Oxygen is analysed by using magnetomechanics (Magnos 6G,Hartmann &Braun).The NO x conversion (X (NO x ))refers to the formation of N 2and N 2O as defined by Eq.(2),whereas the temperature programmed H 2-deNO x data are found to be equal to stationary results.For the major part of the reaction the mass of N is balanced being associated with the production of N 2and N 2O thus excluding the genesis of NH 3.Nevertheless,below 808C H 2-deNO x is slightly interfered by NO x adsorption corresponding to less than 10%conversion.X ðNO x Þ¼2c ðN 2Þþ2c ðN 2O Þc ðNO x Þin(2)The selectivity of N 2(S (N 2))is defined by Eq.(3),whereupon a corresponding expression is used for the N 2O selectivity (S (N 2O)).Moreover,for the comparison of different catalysts the overall selectivity of N 2(S (N 2)overall )is taken as well (Eq.(4));T 1and T 2are the temperatures with NO x conversion of 20%.Selectivity data are exclusively presented for deNO x above 20%to minimise error propagation.S ðN 2Þ¼c ðN 2Þ22(3)S ðN 2Þoverall ¼ZT 2T 1S ðN 2Þd T21(4)Table 1Load of Pt and W,sample code,BET surface area and Pt dispersion of the H 2-deNO x catalysts Catalyst system m(Pt)(%)m(W)(%)Sample codeS BET(m 2g À1)d Pt (%)Pt/Al 2O 30.50.5Pt/Al 2O 3150522Pt/Al 2O 31489Pt/ZrO 20.10.1Pt/ZrO 299300.30.3Pt/ZrO 2100250.50.5Pt/ZrO 21003622Pt/ZrO 29811Pt/WO 3/ZrO 2a0.330.3Pt/3W/ZrO 297360.360.3Pt/6W/ZrO 2106500.3110.3Pt/11W/ZrO 268900.3220.3Pt/22W/ZrO 26295b 2112Pt/11W/ZrO 27011aThe loads of Pt and W refer to the ZrO 2support.bWhile the Pt dispersion of 0.3Pt/11W/ZrO 2is checked by HRTEM (particles <2nm),double H 2desorption is observed for 0.3Pt/22W/ZrO 2.Hence,different H/Pt stoichiometry is assumed being speculated to be 2.This change might be associated with Pt–W interactions dominating at high tungsten oxide coverages amounting to ca.45%for 22%W.F.J.P.Schott et al./Applied Catalysis B:Environmental 87(2009)18–29202.5.DRIFTS studiesThe DRIFT spectroscopic studies are performed with a Nicolet 5700FTIR spectrometer(Thermo Electron)being equipped with a MCT detector and DRIFTS optics(Thermo Mattson).The sample compartment is continuously purged with N2to avoid diffusion of air.The IR cell made of stainless steel contains a ZnSe window and is connected to a gas-handling system.The spectra are recorded in the range from1000to4000cmÀ1with an instrument resolution of 4cmÀ1.100scans are accumulated to a spectrum resulting in a time resolution of1min.Before the analysis,the catalyst powder is charged into the sample holder of the cell and is heated for30min at 5008C in N2or Arflow(500ml minÀ1,STP).In the studies using CO as probe molecule the sample is then exposed at2508C for30min to a mixture of2000ppm H2and6vol.%O2(N2balance);this is done to establish similar conditions as in catalytic studies(Section2.4). Subsequently,the catalyst is cooled in N2flow to258C and then a background spectrum is recorded.After this,the sample is treated for5min with a mixture of500ppm CO in N2followed by purging with N2.Finally,the sample spectrum is collected.The DRIFTS investigation of H2-deNO x is performed sequen-tially.Firstly,the catalyst is cooled from500to1258C under flowing Ar and then the background spectrum is taken.Subse-quently,the sample is exposed for10min to a mixture of 1000ppm NO and6vol.%O2(Ar balance)followed by purging with Ar and taking the spectrum.After this,the blend of40or2000ppm H2and6vol.%O2(Ar balance)is added while continuously collecting data.The H2concentration of40ppm is adjusted to definitely avoid hot spots on the catalyst.The DRIFT spectra are presented in terms of Kubelka Munk transformation defined as F(R)=(1ÀR)2/(2R)with R=R s/R r, whereas R s is the reflectance of the sample under reaction conditions and R r that under the Arflow.3.Results and discussion3.1.Performance of the Pt/Al2O3reference and the Pt/ZrO2catalystsA preliminary investigation performed in the absence of a catalyst shows no conversion of NO x excluding H2-deNO x by gas-phase reactions.In contrast to that,the0.5Pt/Al2O3referencereveals pronounced low-temperature activity,whereas the opera-tion window is rather narrow(50–1508C)and N2O forms as the major product;S(N2)overall amounts to20%(Fig.2).The perfor-mance of Pt/Al2O3is considered to be in fair agreement with literature addressing the same catalytic system[1,3,4].Fig.3illustrates the performance of0.3Pt/ZrO2indicating NO x conversion in the entire temperature range with two deNO x maxima at140and1708C.Furthermore,the peak NO x removal is higher as compared to0.5Pt/Al2O3.Another interesting feature is the markedly improved N2selectivity of0.3Pt/ZrO2,i.e.N2is formed as the major product showing an overall selectivity of55%.The Pt/ZrO2samples with Pt contents of0.1and0.5wt.%show similar peak NO x conversions of78and82%,respectively,whereas their operation range is restricted covering a range of ca.140K only.Furthermore,S(N2)overall is very similar to0.3Pt/ZrO2.Hence, the latter material is considered to be superior and is therefore adopted to the Pt/WO3/ZrO2system.The superiority of0.3Pt/ZrO2 is difficult to explain as Table1shows very similar physical–chemical properties for the Pt/ZrO2samples.3.2.Performance of the0.3Pt/W/ZrO2catalystsFor comparison of the0.3Pt/W/ZrO2samples revealing different loads of W the maximum NO x conversion(X(NO x)max)is used in addition to S(N2)overall.Fig.4shows that a little amount of tungsten is sufficient to increase deNO x as well as N2selectivity.The optimum content of W is11wt.%resulting in an overall N2 selectivity of85%and a peak NO x conversion of95%;assuming planar tungsten oxide the ZrO2coverage by WO3is estimated to be 0.22being significantly less than a monolayer.Furthermore,the results of XPS and PXRD suggest that the tungsten component exists in the form of amorphous WO3.For clarity the performance of the0.3Pt/11W/ZrO2catalyst is presented in Fig.5.The data point to a broad range of deNO x with two conversion peaks at90and2508C.As a consequence,0.3Pt/ 11W/ZrO2covers the low-as well as high-temperature regime thus representing a promising catalytic material.Nevertheless,it should be stated that the low-temperature activity is much more pronounced,while above2508C deNO x declines.The latter feature is attributed to increasing conversion of H2with O2being present in excess;a more detailed discussion of the educt selectivity is presented in Section3.4.The two NO x conversion maximums are ascribed to different active Pt sites as stated in Section 3.5.3, whereas no evidence for active NO x species located on the support is found as discussed in Section3.5.3as well.Contrary,the bare 11W/ZrO2support does not directly participate in deNO x as deduced from a measurement without Pt.However,0.3Pt/11W/ ZrO2still shows significant formation of N2O below1508C,e.g.at Fig.2.H2-deNO x performance of0.5Pt/Al2O3(X(NO x)—,S(N2)--,S(N2O)). Conditions:m=1.50g,c(NO)=500ppm,c(H2)=2000ppm,c(O2)=6.0vol.%,Ar balance,F=500ml minÀ1(STP),S.V.=22.000hÀ1,b=1.0K minÀ1.Fig.3.H2-deNO x performance of0.3Pt/ZrO2(X(NO x)—,S(N2)--,S(N2O)). Conditions:m=1.50g,c(NO)=500ppm,c(H2)=2000ppm,c(O2)=6.0vol.%,Ar balance,F=500ml minÀ1(STP),S.V.=22,000hÀ1,b=1.0K minÀ1.F.J.P.Schott et al./Applied Catalysis B:Environmental87(2009)18–2921the low-temperature deNO x peak S (N 2O)is about 45%.In contrast to that,N 2O is substantially suppressed above 2008C correspond-ing to a N 2selectivity of approximately 90%.Furthermore,it should be mentioned that no NH 3forms in H 2-deNO x on the 0.3Pt/11W/ZrO 2catalyst as referred from a NDIR analysis (Binos 1.1,Leybold-Heraeus).The suppression of NH 3formation is reported to be typical for NO x reduction by H 2on Pt catalysts under oxygen-rich conditions [1].The turnover frequency (TOF)being defined as the number of converted NO x molecules per Pt atom and time is 8.0Â10À3s À1for the deNO x peak at 90%and 5.0Â10À3s À1for the 2508C maximum.These specific H 2-deNO x data are very close to that of 0.1Pt/MgO–CeO 2showing a TOF of ca.8Â10À3s À1(908C)in a similar NO/H 2/O 2feed [14].A direct comparison of the activity range of both catalysts is problematic as data recorded under analogue condi-tions are not available in the study of 0.1Pt/MgO–CeO 2[14].3.3.Evaluation of the 0.3Pt/11W/ZrO 2catalyst3.3.1.Effect of O 2and CO on H 2-deNO x performanceThe previous section provides evidence that the content of O 2is a crucial parameter for the H 2-deNO x reaction.Hence,theeffect of O 2is systematically investigated,whereas for simplicity the performance of 0.3Pt/11W/ZrO 2is exemplarily illustrated at 1258C being representative for the low-temperature range (Fig.6).It is apparent that the O 2concentration does not drastically affect the low-temperature activity, e.g.deNO x is about 90%for 1.5vol.%O 2and ca.75%for 18vol.%.Moreover,the N 2selectivity does not change at all.On the contrary,the high-temperature activity declines markedly with growing O 2content being completely suppressed above 12vol.%O 2(Fig.7).This effect is referred to the increasing reaction of H 2with O 2(Section 3.4).However,diesel engines provide low temperatures in connection with rather high O 2concentrations and vice versa,e.g.at 1508C the O 2content is in the range of 10–15vol.%.Therefore,the decline in high-temperature deNO x observed for relatively high O 2contents is not a substantial issue for practical application.Furthermore,the effect of CO on H 2-deNO x is investigated as well,since this component is known to block active Pt sites at low temperatures which might affect the performance of 0.3Pt/11W/ZrO 2[27].For this examination two representative CO concentra-tions are supplied,i.e.a rather low (40ppm)and a rather high one (400ppm).Fig.8shows that the latter CO concentrationcausesFig.4.Effect of W load on the H 2-deNO x performance of the 0.3Pt/W/ZrO 2samples (X (NO x )max *,S (N 2)overall *).Conditions:m =1.50g,c (NO)=500ppm,c (H 2)=2000ppm,c (O 2)=6.0vol.%,Ar balance,F =500ml min À1(STP),S.V.=22.000h À1,b =1.0K min À1.Fig.5.H 2-deNO x performance of 0.3Pt/11W/ZrO 2(X (NO x )—,S (N 2)--,S (N 2O)).Conditions:m =1.50g,c (NO)=500ppm,c (H 2)=2000ppm,c (O 2)=6.0vol.%,Ar balance,F =500ml min À1(STP),S.V.=22.000h À1,b =1.0K min À1.Fig.6.Effect of O 2on the H 2-deNO x performance of 0.3Pt/11W/ZrO 2at 1258C (X (NO x )*,S (N 2)*).Conditions:m =1.50g,c (NO)=500ppm,c (H 2)=2000ppm,c (O 2)=1.5–18vol.%,Ar balance,F =500ml min À1(STP),S.V.=22.000h À1,b =1.0K min À1.Fig.7.H 2-deNO x performance of 0.3Pt/11W/ZrO 2with a O 2content of 12vol.%(X (NO x )—,S (N 2)--,S (N 2O)).Conditions:m =1.50g,c(NO)=500ppm,c (H 2)=2000ppm,c (O 2)=12vol.%,Ar balance,F =500ml min À1(STP),S.V.=22.000h À1,b =1.0K min À1.F.J.P.Schott et al./Applied Catalysis B:Environmental 87(2009)18–2922a drastic decline in catalytic activity,whereupon significant deNO x begins in parallel to the CO light-off (ca.1108C).This effect is in line with the literature which shows the appearance of free Pt sites being capable of reducing NO only when CO removal starts [29].Consequently,as deNO x is inhibited below 1108C no substantial quantity of N 2O is formed resulting in an overall N 2selectivity of about 90%.Additionally,it is worth mentioning that four maxima of NO x conversion appear pointing to a broad variety of active Pt sites.Furthermore,the CO concentration of 40ppm does not affect the performance of 0.3Pt/11W/ZrO 2at all.These results clearly show that high CO concentrations have to be avoided in practice to maintain H 2-deNO x .Indeed,this can be simply achieved by using a diesel oxidation catalyst (DOC)located in front the H 2-deNO x catalyst.DOC systems are a state-of-the-art technology and are applied in every diesel vehicle released in the industry countries oxidising CO and HC close to the engine outlet.3.3.2.Hydrothermal stability and resistance against SO xFor the use of 0.3Pt/11W/ZrO 2in diesel exhaust its hydrothermal resistance as well as chemical stability towards SO x is of particular concern.To pursue these requirements the catalyst (1.50g)is hydrothermally aged at 7808C for 15h adjusting a gas mixture of 2.5vol.%H 2O and 97.5vol.%Ar (500ml/min,STP),whereas exposure to SO x is carried out at 3508C for 24h while supplying a blend of 40ppm SO 2and synthetic air (500ml min À1,STP).The latter conditions are considered to be appropriate to form SO 3on the catalyst being a strong catalyst poison [30].Nevertheless,both aging procedures do not affect the catalytic performance at all evidencing high resistance of 0.3Pt/11W/ZrO 2against hydrothermal and sulphur exposure.parison of the reducing efficiency of H 2with C 3H 6and COTo assess the efficiency of H 2in the deNO x reaction on 0.3Pt/11W/ZrO 2additional reductants are used.For this purpose C 3H 6and CO are taken as they are potentially formed as major or side product in the on-board production of H 2from diesel,e.g.by catalytic cracking,catalytic partial oxidation or catalytic steam reforming [31];C 3H 6is taken as a model hydrocarbon.For an accurate comparison 10,000ppm H 2,1000ppm C 3H 6or 9000ppm CO are dosed to the standard feed.These concentra-tions demand very similar amount of oxygen for complete conversion.Fig.9shows that the presence of 10,000ppm H 2causes outstanding NO x conversion.Contrary,in the study withC 3H 6significant deNO x appears between 160and 3208C only with a peak conversion of ca.80%corresponding to a N 2selectivity of 45%.A minor deNO x activity is obtained with CO being in line with the results demonstrated in Section 3.3.1.As discussed there,the inhibition of deNO x is related to the blocking of active Pt sites by C 3H 6and CO suppressing substantial dissociation of NO [29].This effect is obviously stronger for CO,whereas it has to be taken into account that much more CO molecules are dosed as compared to C 3H 6.Additionally,Fig.9demonstrates that C 3H 6and CO mainly react with O 2,particularly above 3008C.Finally,from the present experiments it is concluded that among the tested reductants only H 2shows efficient NO x reduction on 0.3Pt/11W/ZrO 2.Fig.9.Effect of H 2,C 3H 6and CO on the deNO x performance of 0.3Pt/11W/ZrO 2(X (NO x )—,S (N 2)--,S (N 2O),X (C 3H 6)—,X (CO)—),The concentration of reducing agent 10,000ppm H 2,1000ppm C 3H 6or 9000ppm CO;remaining conditions are the same as described in Fig.5.Fig.8.Effect of CO on the H 2-deNO x performance of 0.3Pt/11W/ZrO 2(X (NO x )—,S (N 2)--,S (N 2O),X (CO)—).Conditions:m =1.50g,c (NO)=500ppm,c (CO)=400ppm,c (H 2)=2000ppm,c (O 2)=6.0vol.%,Ar balance,F =500ml min À1(STP),S.V.=22.000h À1,b =1.0K min À1.F.J.P.Schott et al./Applied Catalysis B:Environmental 87(2009)18–2923。
Adsorption of CO 2,CH 4,and N 2on Gas Diameter Grade Ion-Exchange Small Pore ZeolitesJiangfeng Yang,Qiang Zhao,Hong Xu,Libo Li,Jinxiang Dong,and Jinping Li *Research Institute of Special Chemicals,Taiyuan University of Technology,Taiyuan 030024,Shanxi,P.R.Chinastructure like CO 2.From the viewpoint of the equilibrium selectivity for CO 2and N 2or CO 2andof CO 2;K-zeolites with high S CO 2/N 2and S CH 4/N 2,adsorption potential order was K-zeolites >Na-zeolites >The removal of CO 2from gaseous mixtures is important for CO 2capture from flue gas,biogas,or land fill gases.These sources of natural gas mainly contain CH 4,CO 2,and N 2.Therefore,the separation of CO 2,CH 4and N 2mixtures canupgrade low quality natural energy gas and also mitigate the problem of excess CO 2emissions.1Of the available adsorption-based separation processes,energy (CH 4)and CO2capture isconsidered to be an energy-and cost-e fficient alternative.Thus,adsorption is an important solution for separation,CO 2capture,CH 4storage,and transportation.2−4This approach uses various types of sorbents or adsorption materials such as carbon materials (activated carbon and carbon molecular sieves),5,6molecular sieves (zeolites),7−10and the popular new metal −organic frameworks (MOFs).11−14Sorbents are considered to be the most important factors a ffecting adsorption techniques.A comparison of di fferent adsorption materials showed that carbon materials are di fficult to have a balance of small pores and large pores,for optimum balance between capacity and dynamics,and MOFs had lower thermal stability,but zeolites produced very homogeneous structures with high surface area and good thermal stability,and the size of the pore volume could be modulated.15,16Thus,zeolites are the most commonly used adsorbents in the field,such as LTA structures (4A,5A,and 13X).17The question is how do we choose appropriate zeolites for a speci fic application such as CO 2,CH 4,and N 2adsorption and First,an analysis of gases showed that nonpolar gases have very similar diameters and gas dynamics:CO 2=0.33nm,CH 4=0.38nm,and N 2=0.36nm,18while the adsorptionstrength of the sorbents was CO2>CH4>N2.19,20Therefore,the adsorption and separation of most gases by zeolites isreliant on their surface potential or the balance of ions in surface channels,particularly the low silicon Li-X zeolite usedfor O 2and N 2separation.21The pore diameters of zeolites areusually bigger than the gas molecules,so gases can di ffusethrough these pores.But what would happen if the pores had a similar size to or smaller than the molecular diameter of the three gases during zeolite adsorption?Titanium silicalite ETS-4had a pore size that is similar to the molecular diameter,and it can be modulated by temperature changes for the aperture separation of various gases,such as CO 2and CH 4,N 2,and CH 4,although its lower adsorptioncapacity limits theapplication of this approach.22−24The ori fice diameters of the zeolites,KFI,CHA,and LEV,are very close to the kinetic diameters of CO 2,CH 4,and N2(Figure 1).These three small-pore zeolites have cage-like structures,and the larger cavity in the hole is ideal for gas uptake.This type of small-pore size microporous zeolite is a hot research area in the field of catalysis and adsorption.25−27Received:August 28,2012Accepted:October 31,2012Published:November 7,2012Krishna and van Baten found frequent correlation e ffects with cage-type zeolites,such as LTA,CHA,and DDR,where narrow windows separated the cages.26Webley et al.studied the gas adsorption selectivity of M(Ca,K)-CHA and concluded that the high O 2/Ar selectivity was possibly due to partial pore blockage by a large K +located near the 8-member ring,producing a 20-hedron cage.28The small pore size and the metal cation balance probably plays an important role in the gas diameter grade structure of zeolites where it determines the adsorption capacity and shape-selective catalysis.It is less common to focus on these types of small pore size cage-like structures and the adsorption of gas molecules close to the aperture.In this study,we synthesized three types of zeolites,Na-LEV,K-CHA,and K-KFI,using the hydrothermal method,whereas Na-KFI,Li-KFI,Ca-KFI,Na-CHA,Li-CHA,and Ca-CHA were obtained by ion exchange.The aims of this study were to evaluate the pore size e ffect and the metal cation e ffect on the adsorption of CO 2,CH 4,and N 2.We also discuss the most suitable method for the separation of CO 2,CH 4,and N 2based on calculations of the adsorption equilibrium.■MATERIALS AND METHODSThe chemicals used in this study are described in more detail in Table 1.Partial Aluminum Potassium.[50.00g of water +29.76g of potassium hydroxide +15.80g of alumina]were heated to boiling until clear,cooled to room temperature,and corrected for any weight loss due to boiling.30,31K-KFI.The synthesis following the procedure reported by Johannes et al.30−32The batch composition was:7.2partial aluminum potassium/0.1strontium nitrate/7.5silicon dioxide/130water,and the typical procedure involved mixing the required amounts of partial aluminum potassium and water,followed by the addition of silica sol,and mixing until smooth (approximately 10min),before strontium nitrate was added to the mixture and stirred for 10min.The resulting mixture was transferred into a 23mL Te flon-lined autoclave and heated in an oven for 5days at 423K.After cooling to ambient temperature,the product was filtered,washed with water,and dried at 373K.The crystal structure of KFI and the degree of crystallinity were con firmed by powder X-ray di ffraction(XRD).Figure 1.Structures of zeolites KFI (a),CHA (b),and LEV (c).29Table 1.Purity of Chemicals Used in This Study and Their Detailschemical name source initial mass fraction purityalumina Aladdin,China >0.99silica sol QingdaoHaiyang Chemical Co.,Ltd.0.401,1-dimethylpiperidinium chloride Shanghai Bangcheng Chemical Co.,Ltd.0.98strontium nitrate Aladdin,China >0.99potassium hydroxide TianjinKemiou Chemical Reagent Co.,Ltd.0.82sodium hydroxide TianjinKemiou Chemical Reagent Co.,Ltd.0.96sodium chloride TianjinKemiou Chemical Reagent Co.,Ltd.0.99lithium hydroxide Tianjin Kemiou Chemical Reagent Co.,Ltd.0.98lithium chloride TianjinKemiou Chemical Reagent Co.,Ltd.0.97calcium hydroxide TianjinKemiou Chemical Reagent Co.,Ltd.0.95calcium chloride Tianjin Kemiou Chemical Reagent Co.,Ltd.0.96K-CHA.The synthesis method was similar to that for K-KFI,but the batch composition was:7.2partial aluminum potassium:0.1strontium nitrate:6silicon dioxide/130water,so there were lower levels of Si/Al.The crystal structure of CHA and the degree of crystallinity were con firmed by powder XRD.Na-LEV.This was prepared from:6partial aluminum sodium/10silicon dioxide/31,1-dimethylpiperidinium chlor-ide/200H 2O,which was made according to our previously reported method.33Ion Exchange.The Na-KFI described above was converted from its potassium form (K-KFI)via three consecutive ion exchanges.In general,300mL of 1M sodium chloride at pH =9(adjusted with 0.01M sodium hydroxide)was added to 5g of zeolite,and the solution was heated to 363K and stirred for 12h.The solution was decanted,and fresh solution was added.After successive washes with the requisite solution,the resulting zeolite was vacuum-filtered and washed with 500mL of deionized water.The zeolite was dried at 373K for 24h.Li-KFI was prepared from Na-KFI by five consecutive ion exchanges of Na-KFI with 2M lithium chloride (5g of zeolite:300mL of lithium chloride)at pH =9(adjusted with 0.01M lithium hydroxide).Ca-KFI was prepared from Na-KFI by five consecutive ion exchanges of Na-KFI with 1M calcium chloride (1g of zeolite:300mL of calcium chloride).The solution was heated at 353K for 12h and decanted,and fresh solution was added.This procedure was repeated five times.Finally,the Ca-KFI was filtered and washed with copious amounts of deionized water and dried at 373K overnight.Na-CHA,Li-CHA,and Ca-CHA,were prepared using the same procedure.34Characterization.The crystallinity and phase purity of the molecular sieves were measured by powder XRD using a Rigaku Mini Flex II X-ray di ffractometer with Cu K αradiation operated at 30kV and 15mA.The scanning range was from 5to 40°(2theta)at 1°/min.Morphological data were acquired by scanning electron microscopy (SEM)using a JEOL JSM-6700F scanning electron microscope operated at 15.0kV.The samples were coated with gold to increase their conductivity before scanning.The Si/Al ratio and the metal ion content of the zeolites were determined by elemental analysis using a spectrophotometer-723P.High-Pressure Gas Adsorption Measurements.The purity of the carbon dioxide was 99.999%,methane was 99.95%,and nitrogen was 99.99%.The adsorption isotherms were measured under high pressure using an Intelligent Gravimetric Analyzer (IGA 001,Hiden,UK).Before measuring the isotherm,a 50mg sample was predried under reduced pressure and then outgassed overnight at 673K under a high vacuum until no further weight loss was observed.Each adsorption/desorption step was allowed to approach equilibrium over a period of 20−30min,and all of the isotherms for each gas were measuredusing a single sample.■RESULTS AND DISCUSSION Synthesis,Ion Exchange,and Characterization.The zeolite K-KFI was synthesized according to a published method.32Figure 2a shows the XRD pattern of the synthesized molecular sieve K-KFI compared with the standard XRD data for the molecular sieve KFI.The peak positions and relative di ffraction intensity were similar to the reported values,which demonstrated that the molecular sieve K-KFI had been synthesized.Figure 2a also shows the XRD pattern of the molecular sieve K-KFI,which was calcined from (573to 1073)K.Its peak positions and relative di ffraction intensity were the same as a sample synthesized below 973K,while the skeleton collapse temperature was 1073K.The method used for the synthesis of K-CHA was similar to that used for K-KFI.Figure 2b shows the XRD pattern of the synthesized molecular sieve K-CHA compared with the standard XRD data for the molecular sieve CHA.The peak positions and relative di ffraction intensity were similar to the reported values,which demonstrated that the molecular sieve K-CHA had been synthesized.The samples had similar behavior at below 973K,while the skeleton collapse temperature was 1073K.The data showed that the structures of KFI and CHA had good thermal stability.The Si/Al values in K-KFI and K-CHA were 4.59and 2.63,respectively.The low Si/Al value indicated that K +occupied more channels in the zeolites,where the large space allowed access to small metal ions or divalent ion exchange.In addition,K-CHA with a lower Si/Al value indicated that more ions could be exchanged,while the size of the pore space could change greatly.However,the higher Si/Al value of K-KFI indicated lower ion exchange,so the size of the pore space could be regulated at a finer level.Table 2shows the experimental K-zeolites,which were changed to Na-zeolites via ion exchange,and the uncertainty is within ±0.005.This shows that the ion exchange degree was increased with temperature and frequency.K-CHA with a lower Si/Al value was produced more completely and easier than K-KFI with a higher Si/Al value from Na +exchanged with K +.The ion exchange degree of Na-CHA from K-CHA wasveryFigure 2.XRD patterns of KFI (a)and CHA (b).high in the first cycle,because the higher temperature is helpful to ion exchange in low Si/Al zeolites.35We selected the highest exchange degree for K +(Na-KFI with 0.91and Na-CHA with 0.99)and the Na-zeolites were used to obtain Li-zeolites and Ca-zeolites.Table 3shows that Li-KFI and Ca-KFI were 0.96and 0.83,respectively,based on Li +and Ca 2+exchange for Na +,while Li-CHA and Ca-CHA were 0.92and 0.96,respectively.The XRD patterns of the Na-,Li-,and Ca-zeolites showed that the zeolite structures were highly stable throughout ion exchange (Figure 3).The samples that changed in appearance are shown in Figure 4.The morphologies of the zeolites with KFI and CHA structures were observed by SEM.For K-KFI to Na-KFI,Li-KFI,and Ca-KFI,the morphologies of the crystals changed from regular cubic accumulations until they completely lost their regular structures.We also showed that there was a slight change in their appearance after a single exchange of materials,followed by a more obvious change after the secondary exchange of materials,particularly M 2+exchange with M +based on the morphologies of M-CHA (M-metal).The pore diameter of some of the samples was too small to be measured or analyzed using a liquid nitrogen adsorption isotherm,for LEV with the ori fice very close to the nitrogen-diameter,so the surfaces of all samples were measured andanalyzed using a CO 2adsorption isotherm at 273K (Figure 5).The microporous surface area and microporous volumes werecalculated using the Dubinin −Radushkevitch (D-R)equation(Table 4):β=−··⎡⎣⎢⎤⎦⎥V V B T PP log()log()log0202(1)where V was volume adsorbed at equilibrium pressure;V 0was the micropore capacity;P 0was saturation vapor pressure of gas attemperature T ;P was equilibrium pressure;B was a constant,βwasthe a ffinity coe fficient of analysis gas relative to P 0gas (for this application βis taken to be 1);T was the analysis bath temperature.36−38All of the samples with a high surface area could be used foradsorption;the surfaces and the pore volumes of the zeoliteswere changed very obvious by ion exchange.Table 4shows thesurface of the samples where the greatest changes were in theappearance of the M-CHAs with the lowest surface areas (K-CHA with 278.5m 2·g −1)and the highest (Li-CHA with 638m 2·g −1,the same situation also appear in the pore volumechange (K-CHA with 0.07cm 3·g −1and Li-KFI with 0.17cm 3·g −1).because they had lower Si/Al values and morebalanced metal ions could be exchanged,so with the smallersize of Li +instead of K +(Table 3),more space can be obtained.However,M-KFI had a higher Si/Al value than M-CHA,so the area of its surface and microporous volume that could be regulated by ion exchange was smaller;that is,the surface area ranges from 333.5m 2·g −1(Ca-KFI)to 566.2m 2·g −1(Na-KFI),and the pore volume ranges from 0.07cm 3·g −1(Ca-KFI)to 0.15cm 3·g −1(Na-KFI).We also found that the surfaces and pore volumes of Ca-zeolites were smaller than Li-or Na-zeolites,which showed that the introduction of Ca 2+reduced the number of balanced ions,while the plug was very strong because the size of Ca 2+larger than Li +and Na +.Li-KFI and Na-KFI had similar surfaces,so it was inferred from Li +that there was no hole in Na-KFI to produce major changes.The systematic errors of surface areas and pore volume have been estimated to be less than 5m 2·g −1and 0.01cm 3·g −1.Table 2.Ion Exchange Degree from K-Zeolite to Na-Zeolite at Di fferent Exchange Times and Temperatures of (323and 363)K a 0.5870.7570.8800.910363Na-CHA 0.7500.7640.9240.9343230.9240.9710.9740.991363a Uncertainties are:U (exchange degree)=0.005;U (T )=0.1K.Table 3.Ion Exchange Degree of Li and Ca-Zeolite from Na-Zeolite a zeolites cation ionic radius (nm)Na +per unit cell (wt %)exchange degree for Na +K-KFI 0.133Na-KFI 0.095 5.29Li-KFI 0.0680.200.96Ca-KFI 0.0990.900.83K-CHA 0.133Na-CHA 0.0957.94Li-CHA 0.0680.640.92Ca-CHA 0.0990.290.96a Uncertainties are:U (Na +contents)=0.02wt %;U (exchange degree)=0.005.Figure 3.XRD patterns of Na,Li,and Ca-KFI (a)and Na,Li,and Ca-CHA (b).Gas Sorption Isotherm Measurements.The CO 2,CH 4,and N 2adsorption and desorption isotherms of the samples are shown in Figure 6,where all of the isotherms have a Langmuir I form and the relative uncertainties of adsorption volumes are estimated to be 0.05V .Most of the samples exhibited rapid desorption,and this correlated with their adsorption curve.Only sample K-CHA exhibited desorption hysteresis during CH 4adsorption.We inferred that the pore size of K-CHA was too close to the kinetics of the di ffusion diameter of CH 4,so it had a very strong CH 4adsorption potential,whereas desorption was hindered by the steric e ffect of the micropores.Table 5also shows the orders of the volumes for CO 2,CH 4,and N 2adsorption of the samples at 0.1MPa.The order of CO 2adsorption at 298K corresponded to the surfaces of the samples.Based on the CO2adsorption,we can also determine the zeolites with Li +and Na +exchange with bigger surfaces and greater adsorption volumes.Based on the high levels (>100cm 3·g −1)of CO 2adsorption with Li,Na-KFI,and Li,Na-CHA at high pressurea,we conclude that micropore Li-zeolites and Na-zeolites could be used for CO 2capture and storage (CCS).The smallest CH 4adsorption volume was found with Na-LEV,so we inferred that CH4adsorption would not occur in itsmicropores because the levels were far less than with othersamples.We conclude that CH4could not di ffuse through theholes in Na-LEV because the ori fice diameter (0.36×0.48nm)was too small.The CH 4adsorption results showed thatM-Figure 4.SEM of the samples:(a)K-KFI,(b)Na-KFI,(c)Li-KFI,(d)Ca-KFI,(e)K-CHA,(f)Na-CHA,(g)Li-CHA,(h)Ca-CHA.CHA was better than M-KFI,so CH 4adsorption or storage was based on the surface features and the ori fice diameter (CHA with 0.38×0.38nm,KFI with 0.39×0.39nm),which indicated a higher adsorption potential.39The low N 2adsorption with Na-LEV shows that molecules could not di ffuse through its pores.N 2adsorption was not a ffected by the metal ion or the structure,so the pore size or surfaces of porous materials were always su fficient for liquid nitrogen adsorption.In the other samples,the N 2adsorption results showed that the pores were expanded by small metal ions or divalent ion exchange,that is,twice then once,because the order was Li,Ca-zeolite >Na-zeolite >K-zeolite (Table 5).Adsorption Equilibrium Selectivity.To evaluate the adsorption equilibrium selectivity and predict the adsorption of the gas mixture from the pure component isotherms,the adsorbent selection parameter S i /j is de fined in the following equation:=ΔΔS qq a i j i j /12/(2)where Δq 1and Δq 2are the adsorption equilibrium capacity di fferences at the adsorption pressure and desorption pressure for components 1and 2.The adsorption equilibrium selectivity a i /j between components i and j is de fined as follows:==a K K q b q b i j i j mi imj j /(3)Henry ’s law:=q Kp (4)Langmuir isotherm model:=+q q bpbp 1m (5)where q m and b are Langmuir isotherm equation parameters,which can be determined from the slope and intercept of a linear Langmuir plot of (1/q )versus (1/p )where q m i and q m jand b i and b j are the Langmuir equation constants for components i and j ,respectively.The equilibrium selectivity de fined in the above equation is basically the ratio of Henry ’s constants for the two components.40Based on the results for S CO 2/CH 4and S CO 2/N 2(in Table 6andthe relative uncertainties of S i /j are estimated to be 0.05S ),all ofthe micropore zeolites produced excellent results in the evaluations,because of the high adsorption of CO 2in the gas diameter grade micropore structures.Na-LEV was the bestmicroporous sieve for gas materials with the highest S CO 2/CH 4=137and S CO 2/N 2=934due to the almost total nonadsorption ofCH 4and N 2but high adsorption of CO 2,which is rare among sorbents.41The second best was Na-KFI (S CO 2/CH 4=92andS CO 2/N 2=374),which had produced better results than otherM-KFIs.In addition,Na-CHA had a higher SCO 2/CH 4than K andLi-CHA,and at the same time Na-CHA also had a higher S CO 2/N 2than Li,Ca-CHA,so we can conclude that Na-zeoliteshad higher CO 2adsorption.A reanalysis of the data showed that Li-zeolites were followed by Na-zeolite,so the e ffects of the smaller Li +were lower than the e ffects of the bigger Na +,and we inferred that the size of the metal ions was an important impact in zeolites.The aerodynamic diameter and physicochemical propertiesof CH 4and N 2were very similar,so separating CH4and N 2wasmuch more di fficult than CO 2and CH4or CO 2and N 2separation which used an adsorption technique.The exper-imental data for S CH 4/N 2in Table 6also con firmed this point.K-CHA had the highest S CH 4/N 2=14.5,while the second was K-KFI (S CH 4/N 2=8.5),which indicated that the introduction of alarge K +increased the potential adsorption of CH4.The Na-zeolites and Li-zeolites have very low data for S CH 4/N 2.Based on the relatively good K-zeolite data for S CO 2/N 2and the smallersurface pores,we can conclude that K +had a signi ficant role in the adsorption of CH4and CO 2,although a smaller hole did not allow greater nitrogen di ffusion.From the perspective of the adsorption potential,we conclude that the large K +was better than Na +and the small Li +;the order was K-zeolites >Na-zeolites >Li-zeolites,indicating that bigger ions had a stronger a ffinity.While the introduction of divalent ions could halve the total number of ions,thus,Ca 2+formed fewer of these small pore type zeolites,and it did not produce very good resultsfor adsorption separation.■CONCLUSION We prepared nine di fferent surfaces of gas diameter grade small pore zeolites,which were characterized by XRD,SEM,and elemental analysis.We synthesized K-CHA with a lower Si/Al value,and more balanced metal ions could be exchanged,so the surfaces and microporous volumes were changedgreatly.Figure 5.CO 2adsorption of the samples on 273K.Table 4.Microporous Surface (MS)and Microporous Volume (MV)of the Samples Obtained from CO 2Adsorption Isotherms at 273K a K-KFI 430.40.10Na-KFI 566.20.15Li-KFI 550.60.14Ca-KFI 333.50.07K-CHA 278.50.07Na-CHA 594.80.16Li-CHA 638.00.17Ca-CHA 487.20.12a Uncertainties are:U (MS)=5m 2·g −1;U (MV)=0.01cm 3·g −1.However,K-KFI had a higher Si/Al value,and the area of its surface and microporous volumes could be modulated to make it smaller.We focused on the CO 2,CH 4,and N 2adsorption isotherms of the samples under high pressure (1MPa)at room temperature (298K),and we found that the smaller Li +and Na +exchanged more with the surface and they had higher adsorption volumes,whereas the larger K +led to severe channel congestion.We calculated and evaluated the adsorption equilibrium selectivity of CO 2/CH 4/N 2,which showed that the ori fice diameter had a very important role in the sieving of CO 2and N 2or CO 2and CH 4,where Na-LEV produced the best sieving e ffect.From the viewpoint of the adsorption equilibria,the Na-zeolites produced the best results for adsorption equilibrium selectivity with CO 2and N 2or CO 2and CH 4,followed by Li-zeolites,whereasK-zeolites withhigh Figure 6.CO 2(▲),CH 4(■),and N 2(●)adsorption (solid)and desorption (hollow)isotherm of the samples at 298K and 1MPa:(a)K-KFI,(b)K-CHA,(C)Na-LEV,(d)Na-KFI,(e)Li-KFI,(f)Ca-KFI,(g)Na-CHA,(h)Li-CHA,(i)Ca-CHA.Table 5.Volumes of CO 2,CH 4,and N 2Adsorption on the Samples at 0.1MPa a K-KFI 67.317.57.1Na-KFI 93.617.79.5Li-KFI 88.315.99.3Ca-KFI 54.816.29.5K-CHA 47.119.1 5.5Na-CHA 104.330.316.7Li-CHA 106.633.016.9Ca-CHA 83.221.418.7a The relative uncertainties of adsorption volumes are estimated to be 0.05V .Table 6.Separation Factor of CH 4/N 2,CO 2/CH 4,and CO 2/N 2Calculated from Pure Component Adsorption Isothermsof the Samplesa zeolite S CO 2/CH 4S CO 2/N 2S CH 4/N 2Na-LEV 137934 6.8K-KFI 353038.5Na-KFI 92374 4.1Li-KFI 80237 3.0Ca-KFI 1959 3.0K-CHA 2435214.5Na-CHA 42187 4.3Li-CHA 32154 4.7Ca-CHA 51127 1.6a The relative uncertainties of Si /j are estimated to be 0.05S .S CH 4/N 2and S CO 2/N 2.Based on the adsorption equilibrium selectivity results,we can conclude that the adsorption potential order was K-zeolites >Na-zeolites >Li-zeolites,so the bigger ions had a stronger a ffinity.Divalent ions were less likely to be captured in the structures than univalent ions,so their separation was somewhat poorer.■AUTHOR INFORMATION Corresponding Author *E-mail:Jpli211@.Tel.:86-3516010908.Fax:86-3516010908.Funding We gratefully acknowledge financial support from the National Natural Science Foundation of China (Nos.21136007,51002103)and Research Fund for the Doctoral Program of Higher Education of China (No.20091402110006).This work was financially supported by the program for the Top Science and Technology Innovation Teams of Higher Learning Institutions of Shanxi.Notes The authors declare no competing financial interest.■REFERENCES (1)Kikkinides,E.S.;Yang,R.T.;Cho,S.H.Concentration and recovery of CO 2from flue gas by pressure swing adsorption.Ind.Eng.Chem.Res.1993,32,2714−2720.(2)Hao,G.P.;Li,W.C.;Qian,D.;Wang,G.H.;Zhang,W.P.;Zhang,T.;Wang,A.Q.;Schu t h,F.;Bongard,H.J.;Lu,A.H.Structurally Designed Synthesis of Mechanically Stable Poly-(benzoxazine-co-resol)-Based Porous Carbon Monoliths and TheirApplication as High-Performance CO 2Capture Sorbents.J.Am.Chem.Soc.2011,133,11378−11388.(3)Matranga,K.R.;Myers,A.I.;Glandt,E.D.Storage of natural gas by adsorption on activated carbon.Chem.Eng.Sci.1992,47,1569−1579.(4)Matranga,K.R.;Stella,A.;Myers,A.L.;Glandt,E.D.Molecular simulation of adsorbed natural gas.Sep.Sci.Technol.1992,27,1825−1836.(5)Liu,Y.;Wilcox,J.Effects of Surface Heterogeneity on the Adsorption ofCO 2in Microporous Carbons.Environ.Sci.Technol.2012,46,1940−1947.(6)Chen,J.;Loo,L.S.;Wang,K.An Ideal Absorbed Solution Theory (IAST)Study of Adsorption Equilibria of Binary Mixtures ofMethane and Ethane on a Templated Carbon.J.Chem.Eng.Data 2011,56,1209−1212.(7)Ducrot-Boisgontier,C.;Parmentier,J.;Faour,A.;Patarin,J.;Pirngruber,G.D.FAU-Type Zeolite Nanocasted Carbon Replicas for CO 2Adsorption and Hydrogen Purification.Energy Fuels 2010,24,3595−3602.(8)Jayaraman,A.;Hernandez-Maldonado,A.J.;Yang,R.T.;Chinn,D.;Munson,C.L.;Mohr,D.H.;Donald,H.Clinoptilolites for nitrogen/methane separation.Chem.Eng.Sci.2004,59,2407−2417.(9)Wang,Y.;LeVan,M.D.Adsorption Equilibrium of BinaryMixtures of Carbon Dioxide and Water Vapor on Zeolites 5A and 13X.J.Chem.Eng.Data 2010,55,3189−3195.(10)Bao,Z.;Yu,L.;Dou,T.;Gong,Y.;Zhang,Q.;Ren,Q.;Lu,X.;Deng,S.Adsorption Equilibria of CO 2,CH 4,N 2,O 2,and Ar on High Silica Zeolites.J.Chem.Eng.Data 2011,56,4017−4023.(11)Coudert,F.O.-X.;Mellot-Draznieks,C.;Fuchs,A.H.;Boutin,A.Prediction of Breathing and Gate Opening Transitions Upon Binary Mixture Adsorptionin Metal −Organic Frameworks.J.Am.Chem.Soc.2009,131,11329−11331.(12)Zheng,S.T.;Bu,J.T.;Li,Y.;Wu,T.;Zuo,F.;Feng,P.;Bu,X.Pore Space Partition and Charge Separation in Cage-within-Cage Indium −Organic Frameworks with High CO 2Uptake.J.Am.Chem.Soc.2010,132,17062−17064.(13)He,Y.;Xiang,S.;Chen,B.A Microporous Hydrogen-BondedOrganic Framework for Highly Selective C 2H 2/C 2H 4Separation at Ambient Temperature.J.Am.Chem.Soc.2011,133,14570−14573.(14)Sumida,K.;Rogow,D.L.;Mason,J.A.;McDonald,T.M.;Bloch,E.D.;Herm,Z.R.;Bae,T.H.;Long,J.R.Carbon Dioxide Capture in Metal −Organic Frameworks.Chem.Rev.2012,112,724−781.(15)Gemma,L.H.;Edward,B.;Martin,C.S.;Neil,C.H.;Ewan,M.M.;Paul,F.M.;Joseph,A.H.High-Pressure and Temperature IonExchange of Aluminosilicate and Gallosilicate Natrolite.J.Am.Chem.Soc.2011,133,13883−13885.(16)Aguilar-Armenta,G.;Hernandez-Ramirez,G.;Flores-Loyola,E.;Ugarte-Castaneda, A.;Silva-Gonzalez,R.;Tabares-Munoz, C.;Jimenez-Lopez,A.;Rodriguez-Castellon,E.Adsorption Kinetics of CO 2,O 2,N 2,and CH 4in Cation-Exchanged Clinoptilolite.J.Phys.Chem.B 2001,105,1313−1319.(17)Walton,K.S.;Abney,M.B.;LeVan,D.M.CO 2adsorption in Yand X zeolites modified by alkali metal cation exchange.Microporous Mesoporous Mater.2006,91,78−84.(18)Yang,R.T.Adsorbents:Fundamentals and Applications ;Wiley-Interscience:New York,2003.(19)Van den Bergh,J.;Mittelmeijer-Hazeleger,M.;Kapteijn,F.Modeling Permeation of CO 2/CH 4,N 2/CH 4,and CO 2/Air Mixturesacross a DD3R Zeolite Membrane.J.Phys.Chem.C 2010,114,9379−9389.(20)Dunne,J.A.;Mariwala,R.;Rao,M.;Sircar,S.;Gorte,R.J.;Myers, A.L.Calorimetric Heats of Adsorption and Adsorption Isotherms.1.O 2,N 2,Ar,CO 2,CH 4,C 2H 6,and SF6on Silicalite.Langmuir 1996,12,5888−5895.(21)Baksh,M.S.A.;Kikkinde,E.S.;Yang,R.T.Lithium Type XZeolite as a Superior for Air Separation.Sep.Sci.Technol.1992,27,277−294.(22)Kuznicki,S.M.;Bell,V.A.;Nair,S.;Hillhouse,H.W.;Jacubinas,R.M.;Braunbarth,C.M.;Toby,B.H.;Tsapatsis,M.A titanosilicatemolecular sieve with adjustable pores for size-selective adsorption ofmolecules.Nature 2001,412,720−724.(23)Marathe,R.P.;Farooq,S.;Srinivasan,M.P.Modeling GasAdsorption and Transport in Small-Pore Titanium ngmuir2005,21,4532−4546.(24)Pillai,R.S.;Peter,S.A.;Jasra,R.V.Adsorption of carbondioxide,methane,nitrogen,oxygen and argon in NaETS-4.Micro-porous Mesoporous Mater.2008,113,268−276.(25)Altwasser,S.;Welker,C.;Traa,Y.;Weitkamp,J.Catalyticcracking of n-octane on small-pore zeolites.Microporous MesoporousMater.2005,83,345−356.(26)Krishna,R.;van Baten,J.M.Onsager coefficients for binary mixture diffusion in nanopores.Chem.Eng.Sci.2008,63,3120−3140.(27)Saxton,C.G.;Kruth,A.;Castro,M.;Wright,P.A.;Howe,R.F.Xenon adsorption in synthetic chabazite zeolites.Microporous Mesoporous Mater.2010,129,68−73.(28)Singh,R.K.Adsorption of N 2,O 2,and Ar in PotassiumChabazite.Adsorption 2005,11,173−177.(29)Baerlocher,Ch.;McCusker,L.B.;Olson,D.H.Atlas of Zeolite Framework Types ,6th revised ed.;Elsevier:New York,2007.(30)Johannes,V.Zeolite ZK-5.U.S.Patent US4994249,1991(31)Robson,H.How to read a patent.Microporous Mesoporous Mater.1998,22,551−662.(32)Schwarz,S.;Corbin,D.R.;Sonnichsen,G.C.The effect ofcrystal size on the methylamines synthesis performance of ZK-5zeolites.Microporous Mesoporous Mater.1998,22,409−418.(33)Dong,J.;Wang,X.;Xu,H.;Zhao,Q.;Li,J.Hydrogen storage inseveral microporous zeolites.Int.J.Hydrogen Energy 2007,32,4998−5004.(34)Zhang,J.;Singh,R.;Webley,P.A.Alkali and alkaline-earthcation exchanged chabazite zeolites for adsorption based CO2capture.Microporous Mesoporous Mater.2008,111,478−487.(35)Xu,R.;Pang,W.Chemistry-Zeolites and Porous Materials(Chinese);Science Press:Beijing,2004.。

2,4-二氯苯酚降解研究进展李碧;陈轶;孔殿超【摘要】2,4-二氯苯酚(2,4-DCP)常用于农药生产,在水体和土壤中分布广泛,是一种难降解高毒性有机物,文章围绕近年来对2,4-DCP的各种降解方法,分析了2,4-DCP的降解机理,探讨了不同降解方法的适用范围及影响因素,并提出了进一步研究的方向.【期刊名称】《广州化工》【年(卷),期】2013(041)005【总页数】3页(P40-42)【关键词】2,4-二氯苯酚;降解;物理化学;生物法【作者】李碧;陈轶;孔殿超【作者单位】合肥工业大学资源与环境工程学院,合肥230009【正文语种】中文【中图分类】X592氯酚类化合物 (CPs)是一类使用广泛、毒性较大且污染严重的异型生物质,被广泛用作木材防腐剂、杀菌剂和除草剂等,其中2,4-二氯酚(DCP)主要用以合成农药除草醚和二氯苯氧酸及其酯类,2,4-二氯酚的大量使用给自然环境造成很大的危害,被美国环保局和我国政府列入优先污染物行列[1]。
2,4-DCP是水环境中分布最广的氯酚之一,在中国,较多河流土壤中均能检测到2,4-DCP的存在[2],部分水体中2,4-DCP浓度甚至达到0.1~1 mg/L[3]。
已有证实2,4-DCP能引起病理学症状,改变人体内分泌系统[4],因此2,4-DCP的降解得到了国内外重点关注。
本文探讨了2,4-DCP的各种降解方法,并分析降解机理及各种方法的影响因素。
1 物理化学法由于高浓度氯酚对微生物的抑制,在处理高浓度氯酚废水时,生物法往往不能取得较高的处理效率,因此往往使用物理化学降解高浓度2,4-DCP,常见的方法主要有光降解、氧化还原、吸附和离子交换。
1.1 光降解关于2,4-DCP的光降解一直是研究热点,且已有不同光催化系统对2,4-DCP降解效果的对比研究,紫外光将氯酚提升至激发态和三重态,通过产生的羟自由基与2,4-DCP及中间产物不断反应,最终生成CO2和H2O。
总结“五种等温线”的研究和应用情况,每一种等温线至少列举2例说明。
吸附等温曲线是指在一定温度下溶质分子在两相界面上进行的吸附过程达到平衡时它们在两相中浓度之间的关系曲线。
在一定温度下,分离物质在液相和固相中的浓度关系可用吸附方程式来表示。
作为吸附现象方面的特性有吸附量、吸附强度、吸附状态等,而宏观地总括这些特性的是吸附等温线。
吸附等温曲线用途广泛,在许多行业都有应用[1]。
Ⅰ型—①I-A型—称为兰缪尔型吸附等温线,可用单分子层吸附来解释。
在2.5nm以下微孔吸附剂上的吸附等温线属于这种类型。
Ⅰ型—②I-B型固体吸附剂具有超微孔(0.5~2.0nm)和极微孔(<1.5nm) ,外表面积比孔表面积小很多。
例1:半胱氨酸在TiO2上的吸附在不同的PH值下,通过红外光谱仪和兰缪尔吸附等温线分析表面复合结构。
兰缪尔吸附等温线被应用分析结合物常量,这是一种与静电吸附物质一致,在PH为8.0,TiO2膜很难改变,氨分子接触TiO2的表面通过氨的质子组,这种新的排列生成很大的浓度在饱和浓度围[2]。
在TiO2上吸附半胱氨酸的吸附等温线,吸附等温线的半胱氨酸在二氧化钛pH值5.0和15℃吸附等温线的半胱氨酸在二氧化钛pH值2.0和15℃吸附等温线的半胱氨酸在二氧化钛pH值8.0和15℃在pH值为2.0,半胱氨酸主要吸附在完全质子化了的和两性离子形式。
从吸附剂获得的配位,吸附等温线实验表明在二氧化钛表面吸附物种存在竞争效应,但两性离子形式表现出更多的亲和力。
这是由一个主要的积累物种在二氧化钛表面上,反映在各自的吸光度比值。
吸附的半胱氨酸生产减少pKa1(羧基)值从1.96到1.37。
在pH值为5.0,氨基酸的两性离子形式。
这个事实与红外光谱谱和吸附等温线是一致的。
在pH值为8.0,有良好的静电相互作用在高度带负电荷的二氧化钛表面和质子化了的胺半胱氨酸的部分地区,而羧酸盐和硫醇盐组从表面的静电排斥。
因此,氨基酸集团接触到表面的时候,这导致了另外一个基团扩散到溶液中,由于半谷氨酸在TiO2表面的空间布局导致这里有一个大的饱和吸附量。
中间体的吸附、偶联等反应英文回答:The adsorption and coupling reactions of intermediates play crucial roles in various chemical processes. These reactions are often involved in the formation of new chemical bonds and the transformation of reactants into products. Understanding the mechanisms and kinetics of these reactions is essential for designing efficient catalytic systems and optimizing reaction conditions.Adsorption is the process by which molecules or atoms bind to the surface of a solid or liquid. It can occur through various interactions, such as van der Waals forces, hydrogen bonding, or covalent bonding. The adsorption of intermediates onto a catalyst surface is often the first step in a catalytic reaction. It provides a platform for further reactions to take place and influences the overall reaction rate.Coupling reactions involve the formation of new chemical bonds between two or more reactant molecules. These reactions are commonly used in organic synthesis to construct complex molecules. In many cases, the intermediates formed during the reaction play a crucialrole in the coupling process. They can undergo various transformations, such as oxidative addition, reductive elimination, or nucleophilic attack, to form the desired products.The study of adsorption and coupling reactions of intermediates is often carried out using various experimental techniques, such as surface science methods, spectroscopy, and kinetic measurements. These techniques provide valuable information about the nature of the intermediates, their binding sites on the catalyst surface, and their reactivity. Computational methods, such as density functional theory (DFT) calculations, are also widely used to investigate the reaction mechanisms and energetics of these processes.Understanding the factors that influence the adsorptionand coupling reactions of intermediates is crucial for catalyst design and optimization. The nature of thecatalyst surface, including its composition, morphology, and crystal structure, can significantly affect the adsorption and reactivity of intermediates. The presence of co-adsorbates or additives can also influence these reactions by modifying the electronic properties of the catalyst surface or providing additional reaction pathways.In conclusion, the adsorption and coupling reactions of intermediates are important processes in various chemical reactions. Studying these reactions can provide valuable insights into the mechanisms and kinetics of catalytic processes. By understanding the factors that influence these reactions, researchers can design more efficient catalysts and optimize reaction conditions for various applications.中文回答:中间体的吸附和偶联反应在各种化学过程中起着关键作用。
Kinetic modeling of methanol synthesis over commercial catalysts based on three-site adsorptionNonam Park a ,b ,Myung-June Park a ,b ,⁎,Yun-Jo Lee c ,Kyoung-Su Ha c ,1,Ki-Won Jun c ,⁎⁎a Department of Chemical Engineering,Ajou University,Suwon 443-749,Republic of Koreab Department of Energy Systems Research,Ajou University,Suwon 443-749,Republic of KoreacResearch Center for Green Catalysis,Korea Research Institute of Chemical Technology (KRICT),Daejeon 305-600,Republic of Koreaa b s t r a c ta r t i c l e i n f o Article history:Received 6December 2013Received in revised form 26March 2014Accepted 31March 2014Available online 25April 2014Keywords:Methanol synthesis Kinetic modelThree-site adsorption Parameter estimation Effectiveness factorA kinetic model for the synthesis of methanol over commercial catalysts was developed based on the adsorption of CO and CO 2onto various active sites of Cu,while the kinetic parameters were estimated by fitting 118exper-imental sets of data under a variety of conditions.When both CO and CO 2hydrogenations take place,CO conver-sion was in fluenced by the change in temperature as well as in space velocity and pressure,while CO 2conversion was minimally in fluenced as a result of its correlation to the water –gas shift reaction.However,the CO and H 2fractions signi ficantly in fluenced both conversions.The analysis based on the model developed in this investiga-tion clearly con firmed that the contribution of each reaction and the maximum methanol concentration was expressed as a function of temperature and the CO fraction.Additionally,catalysts with varying particle sizes were used to evaluate the effect of the internal diffusion limitation on catalytic performance and the effectiveness factors were subsequently calculated and regressed with respect to the particle size.©2014Elsevier B.V.All rights reserved.1.IntroductionMethanol (MeOH)is a key industrial chemical that can be used ei-ther as a solvent or a fuel,in addition to being able to be readily convert-ed into other useful products such as formaldehyde,amines,acetic acid,and esters [1].MeOH is also used to produce ethylene or propylene in the methanol-to-ole fins process and is capable of conveniently storing and transporting CO and H 2[2,3].While fossil fuels have contributed substantially to atmospheric pollution by emitting signi ficant amounts of greenhouse gases,particularly CO 2[4],recycling excess CO 2from in-dustrial waste and the atmosphere and reacting it with H 2to produce MeOH could potentially mitigate the global warming problem.Addi-tionally,MeOH is directly oxidized in air (without H 2)to CO 2and H 2O to produce electricity in direct-MeOH fuel cells [5].Since the reaction employed in the synthesis of MeOH is highly exothermic,the heat released from the reaction needs to be ef ficiently removed to keep the reactor isothermal.For this reason,much research is devoted to kinetic and reactor modeling when designing commercial processes for mass production [3].Previously reported kinetic models include mechanistic kinetic models based on detailed reactionmechanisms [6–9]and the power law model [10,11].Grabow and Mavrikakis [12]developed a microkinetic model for the synthesis of MeOH,including the water –gas shift (WGS)reaction using density functional theory calculations,while Ovesen et al.[13]described a static and dynamic (changes in particle shape and active surface area)microkinetic model for the synthesis of MeOH over a Cu/ZnO catalyst.Both CO and CO 2hydrogenations can be used in the synthesis of MeOH to achieve high productivity,and Klier et al.[14]proposed three models based on different assumptions concerning how CO,CO 2,and H 2compete for the active sites.McNeil et al.[15]assumed that the adsorption of hydrogen occurs on ZnO in contrast to the adsorption of CO and CO 2on Cu 1+and Cu 0,respectively,and it was also determined that the catalyst has both reduced and oxidized (such as Cu 2+which may be formed by the oxidation of Cu metal)sites for CO and CO 2hy-drogenations,respectively,in the presence of an optimal amount of CO 2or H 2O [16].When CO and CO 2hydrogenations are occurring simul-taneously,the MeOH production rate reaches a maximum and subse-quently decreases as the CO 2concentration increases,indicating that CO 2inhibits CO hydrogenation [17],while the synergetic effects of CO 2had been suggested in our previous work [8].However,despite many useful kinetic models for MeOH synthesis (as discussed above,and as shown in the list of models in the literature [18,19]),very few investigations have addressed the development of ki-netic models based on three-site adsorption (Cu 1+,Cu 0,and ZnO).Therefore,detailed elementary steps for both CO and CO 2hydrogena-tions based on two-site adsorption [19]were modi fied by considering additional adsorption site for CO 2in our previous work [8],and theFuel Processing Technology 125(2014)139–147⁎Correspondence to:M.-J.Park,Department of Chemical Engineering,Ajou University,Suwon 443-749,Republic of Korea.Tel.:+82312192383;fax:+82312191612.⁎⁎Corresponding author.Tel.:+82428607671;fax:+82428607388.E-mail addresses:mjpark@ajou.ac.kr (M.-J.Park),kwjun@krict.re.kr (K.-W.Jun).1Present address:Department of Chemical and Biomolecular Engineering,Sogang University,Seoul 121-742,Republic ofKorea./10.1016/j.fuproc.2014.03.0410378-3820/©2014Elsevier B.V.All rightsreserved.Contents lists available at ScienceDirectFuel Processing Technologyj o u r n a l h o m e p a g e :w w w.e l s e v i e r.c o m/l o c a t e /f u p r o cmodel for the three-site adsorption was developed for the purpose of its application to the catalysts developed in the Korea Research Institute of Chemical Technology(KRICT).It is worth noting that the KRICT catalysts exhibited different rate determining step(RDS)(the surface reaction of a methoxy species,the hydrogenation of the formate intermediate HCO2,and the formation of a formate intermediate for CO and CO2hy-drogenations and WGS reaction),relative to the reported model for commercial catalysts[19].Therefore,since commercial catalysts were used in the present study because of their use in large scale commercial reactors,the elementary steps in our previous work[8]were considered while the RDS assumption for commercial catalyst[19]was applied.In such a way,the model for the three-site adsorption was developed in the present study.2.ExperimentalA commercial catalyst(Cu/ZnO/Al2O3,Süd-Chemie,MegaMax700) is used for the kinetic experiment regarding the synthesis of MeOH. The pelletized catalyst(5mm diameter;3mm height)was broken and sieved to produce three samples of uniform size:0.15mm–0.25mm,0.75mm–0.85mm,and1.5mm–2.5mm;which were denot-ed as S1,S2,and S3,respectively.Additionally,the original catalyst pel-let,denoted as S4,was also used without breaking.The S1catalyst was used for the kinetic investigation,while the others were used to evalu-ate the effects of the particle sizes on the catalytic performance.The kinetic data was collected using a continuous tubularflowfixed-bed microreactor(see Fig.1for the schematic of the experimental sys-tem).The temperature within the reactor was controlled by adjusting the furnace temperature and theflow rate was controlled by a mass flow controller.The pressure was precisely controlled by a back pres-sure regulator and monitored by a digital pressure sensor.The catalyst samples(0.4g)were diluted with similar-sized and inertα-Al2O3parti-cles(1.2g)and packed together into a stainless steel reactor(internal diameter=7mm).The catalyst was reduced under aflow of H2 (100mL/min)and temperature was increased from room temperature to a predetermined point at a rate of2K/min and held for3h.After reduction,the pressure was increased according to the operating condi-tions,and the H2gas was then replaced by the gas used in the synthesis of MeOH.Detailed conditions regarding temperature,pressure,and feed gas composition are listed in Table1.The reaction products were ana-lyzed by an on-line gas chromatograph(Young Lin),where a Carboxen 1000column was installed to separate the CO,CO2,H2,and Ar that were detected with the thermal conductivity detector and a HP-PLOT Q cap-illary column coupled toflame ionization detector was used to separate and analyze all hydrocarbons,including MeOH.3.Results and discussion3.1.Reaction ratesThe synthesis of MeOH was comprised of three main reactions:(1) CO hydrogenation,(2)the WGS reaction,and(3)CO2hydrogenation. Additionally,the dehydration of MeOH to produce dimethyl ether (DME)was considered in this study.COþ2H2⇄CH3OHð1ÞCO2þH2⇄COþH2Oð2ÞCO2þ3H2⇄CH3OHþH2Oð3Þ2CH3OH⇄CH3OCH3þH2O:ð4ÞAll reactions occurred simultaneously on the surface of the catalyst and CO and CO2were assumed to adsorb on the various sites of catalyst during the synthesis of MeOH[8,14].Table2displays the elementary re-actions for CO and CO2hydrogenations and the WGS reaction,where the adsorption site of CO2in the present study was denoted as“s3”(“s1”was used to denote both CO and CO2hydrogenations in the literature[19]).It is worth noting that the dehydration of methanol to dimethyl ether was included since experimental data showed the pro-duction of DME,while other byproducts such as higher alcohol,meth-ane and hydrocarbons were detected with trace(negligible)amount. Therefore,although it is well-known that methane and other hydrocar-bons could appear as byproducts under certain reaction conditions and catalysts,those reactions were not considered in the present study.The elementary steps of(A3),(B2),and(C3)were chosen to be the RDSs for CO hydrogenation,WGS reaction,and CO2hydrogenation, respectively,as determined in the literature[19],and all the other steps were assumed to be at equilibrium.The adsorption of MeOH was assumed to be negligible,while the fractions of surface COregulator (BPR)Fig.1.Schematic of the experimental system used in this study. 140N.Park et al./Fuel Processing Technology125(2014)139–147(θCO(s1)),CO 2(θCO2(s3)),hydrogen atoms (θH(s2)),and water (θH2O(s2))were determined from the adsorption steps as follows:θCO s1ðÞ¼K CO f CO θs1ð5ÞθCO2s3ðÞ¼K CO2f CO2θs3ð6ÞθH s2ðÞ¼K 0:5H2f 0:5H2θs2ð7ÞθH2O s2ðÞ¼K H2O f H2O θs2:ð8ÞIn the step (A3)of Table 2,the fractions of H 2CO(s1)and H 3CO(s1)were calculated by inserting the above equations as follows:θH2CO s1ðÞ¼K A1K A2θCO s1ðÞθ2H s2ðÞ=θ2s2¼K CO K H2K A1K A2ðÞθs1f CO f H2ð9ÞθH3CO s1ðÞ¼θs1f CH3OH =K A 4K 0:5H2f 0:5H2:ð10ÞEqs.(9)and (10)were inserted into the rate of the (A3)step to obtain:r CO¼k A3θH2CO s1ðÞθH s2ðÞ−θH3CO s1ðÞθs2=K A3¼k 0A K CO f CO f 1:5H2−f CH3OH K P ;A f 0:5H2!θs1θs2:ð11ÞSite balance equations for site 1,2and 3were calculated by assum-ing that the total number of sites per weight of catalyst was constant in addition to neglecting terms originating from intermediate products [19],as follows:θs1¼11þK CO f CO ;θs2¼11þK 0:5H2f 0:5H2þK H2O f H2O;θs3¼11þK CO2f CO2:ð12ÞBy inserting Eqs.(12)to (11),the following CO hydrogenation reac-tion rate was obtained:r CO ¼k 0A K CO f CO f 1:5H2−f CH3OH =K P ;A f 0:5H2h i1þK CO f CO ðÞ1þK 0:5H2f 0:5H2þK H2O f H2OÀÁ:ð13ÞIn the same manner,the CO 2hydrogenation and the WGS reactionrates were determined as follows:r WGS ¼−k B2θHCO2s3ðÞθH s2ðÞ−θCO s3ðÞθH2O s2ðÞK B2¼−k B2K CO2K 0:5H2K B1f CO2f 0:5H2θs3 K 0:5H2f 0:5H2θs2 −K CO f CO θs3ðÞKH2O f H2O θs2ðÞK B2!¼−k 0B K CO2f CO2f H2−f CO f H2O =K P ;Bh i 1þK CO2f CO2H2f H2H2O f H2O ÀÁð14Þr CO2¼k C3θH2CO2s3ðÞθH s2ðÞ−θH3CO2s3ðÞθs2K C3¼k C3K CO2K H2K C1K C2f CO2f H2θs3ðÞK 0:5H2f 0:5H2θs2 −K H2O f H2O f CH3OH θs3K H2K C4K C5K C6f H2!θs2C3"#¼k 0C K CO2f CO2f 1:5H2−f H2O f CH3OH =K P ;C f 1:5H2h i 1þK CO2f CO2ðÞ1þK 0:5H2f 0:5H2þK H2O f H2OÀÁð15Þwhere f denotes the fugacity,which was calculated using the general-ized correlations for the fugacity coef ficient in the literature [20],and K P,i denotes the reaction equilibrium constant,which was obtainedfrom the literature [21].Since only two of the four reaction rates are independent from a thermodynamic standpoint,the K P,C can be established as linear combinations of the other values.ln K P ;Abar−2h i ¼1:183Â104T K ½−29:061ð16Þln K P ;B −½ ¼−4:773Â103T K ½þ4:672ð17ÞK P ;C ¼K P ;A K P ;B :ð18ÞFor the DME production,a reaction rate equation was available in the literature [22]and used without alteration:r DME ¼k DME K 2CH3OH C 2CH3OH −C H2O C DME ðÞ=K P ;DME h i 1þ2ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiK CH3OH C CH3OH p þK H2O ;DME C H2Oð19Þwhere C i was the concentration of component i in mol/m 3,and thereaction equilibrium constant was:K P ;DME¼0:106exp2:1858Â104RT!:ð20Þ3.2.Reactor model and parameter estimationThe dimensionless Mears parameter [23,24]was calculated using experimental data and physical properties to evaluate the contribution of external mass diffusion.The values ranged from 2.34×10−2to 3.17×10−2,which were below the threshold value of 0.15,indicating negligible external mass diffusion.The occurrence of internal pore diffu-sion limitation was determined based on the Weisz –Prater criterion [23,25,26],where the existence of the concentration gradient was deter-mined if the value of the dimensionless Weisz –Prater parameter (C WP )was much greater than one (C WP ≫1).Since the values of C WP in this study were close to one during each experiment,no internal diffusion limitations were detected.Therefore,a plug flow model without disper-sion was used to simulate the reactor as follows [8]:Mass balance :−u sdC iþρB X NR j ¼1r i ;j ¼0ð21ÞEnergy balance :ρg u s C PdT dz ¼ρBX NR j ¼1−ΔH ðÞj r j þ4UD t T w −T ðÞð22ÞBoundary conditions :at z ¼0;C i ¼C i ;in ;T ¼T in :ð23ÞThe estimation of the parameters was based on minimizing the ob-jective function (F obj )and the residuals of square errors of the objectiveelements (CO and CO 2conversions)were expressed as follows:F obj ¼XNE nX iw iX i ;calc −X i ;expi ;exp!2"#nð24Þwhere NE and w i represent the number of experimental conditions and the weighting factor,respectively.It is important to note that prior to the estimation,the experimental values of the CO and CO 2con-versions were compared to the equilibrium conversions under the141N.Park et al./Fuel Processing Technology 125(2014)139–147Table1Experimental conditions.Case Pressure[bar]Temp.[K]Space velocity[mL/(g cat·h)]Feed composition CO/(CO+CO2)H2/(2CO+3CO2)Conversion[%]Close to the equil.Remarks CO CO2H2Ar CO CO215052320,0000.190.110.7000.64 1.0041.057.84Temperature 25054320,0000.190.110.7000.64 1.0033.78 6.0535057320,0000.190.110.7000.64 1.0018.06 6.18●45059320,0000.190.110.7000.64 1.009.14 6.8●55061320,0000.190.110.7000.64 1.00 3.278.38●65052380000.190.110.7000.64 1.0054.289.46●Space velocity 75052320,0000.190.110.7000.64 1.0041.21 6.185052330,0000.190.110.7000.64 1.0034.13 4.8295052340,0000.190.110.7000.64 1.0028.93 4.62105052380000.190.110.7000.64 1.0052.2910.68Pressure 117052380000.190.110.7000.64 1.0066.7810.05●129052380000.190.110.7000.64 1.0074.7810.98●135052380000.000.240.720.040.00 1.00−9.3623.32CO/(CO+CO2) 145052380000.110.160.690.050.40 1.0052.5410.73●155052380000.170.110.670.050.60 1.0052.68.09165052380000.220.070.660.050.75 1.0049.90.37175052380000.260.050.650.050.85 1.0045.620.05185052380000.270.030.640.050.90 1.0044.60195052380000.280.020.640.050.93 1.0042.210205052380000.290.020.640.050.95 1.0036.50215052380000.320.000.630.05 1.00 1.0020−0.66225052380000.180.100.670.050.64 1.0057.557.82●H2/(2CO+3CO2) 235052380000.160.090.700.050.64 1.1757.968●245052380000.130.070.770.040.64 1.6562.319.38●255052380000.090.050.830.030.64 2.5069.7715.11●265052380000.190.110.7000.64 1.0057.16 5.8●Inert gas 275052380000.140.080.500.290.64 1.0040.9 4.32287052380000.140.080.500.290.64 1.0057.4 5.32297052380000.190.110.7000.64 1.0073.0311.01●307052380000.000.240.720.040.00 1.00−7.428.7CO/(CO+CO2) 317052380000.170.110.670.050.60 1.0064.4610.02●327052380000.220.070.660.050.75 1.0060.85 4.21337052380000.270.030.640.050.90 1.0057.41−6.43347052380000.290.020.640.050.95 1.0054.19−23.02357052380000.320.000.630.05 1.00 1.0031.58−1.24367054380000.000.240.720.040.00 1.00−10.2226.46●CO/(CO+CO2) 377054380000.170.110.670.050.60 1.0049.93 4.78●387054380000.220.070.660.050.75 1.0048.44−1.27397054380000.270.030.640.050.90 1.0046.38−35.65●407054380000.290.020.640.050.95 1.0045.66−47.62●417054380000.320.000.630.05 1.00 1.0041.31−4.34425052380000.000.240.720.040.00 1.00−8.4424.61●CO/(CO+CO2)=0 435052380000.000.220.750.040.00 1.16−9.1325.39●445052380000.000.170.800.030.00 1.60−9.8831.47●455052380000.000.120.860.020.00 2.39−10.5140.94●466052380000.000.240.720.040.00 1.00−7.8926.21●CO/(CO+CO2)=0 476052380000.000.220.750.040.00 1.16−8.3528486052380000.000.170.800.030.00 1.60−8.9634.23496052380000.000.120.860.020.00 2.39−8.5845.15●507052380000.000.240.720.040.00 1.00−7.328.36CO/(CO+CO2)=0 517052380000.000.220.750.040.00 1.16−7.830.37527052380000.000.170.800.030.00 1.60−8.3335.95537052380000.000.120.860.020.00 2.39−8.0248.44548052380000.070.050.620.260.58 2.0475.9316.75●Close to ICI plantoperating conditions 558053380000.070.050.620.260.58 2.0467.3618.94568054380000.070.050.620.260.58 2.0459.0520.96578054310,0000.070.050.620.260.58 2.0457.9117.01585050320,0000.190.110.7000.64 1.0037.128.29Temperature 595051320,0000.190.110.7000.64 1.0043.798.34605052320,0000.190.110.7000.64 1.0042.668.32615053320,0000.190.110.7000.64 1.0038.427.86625054320,0000.190.110.7000.64 1.0032.217.14635055320,0000.190.110.7000.64 1.0027.94 6.85645056320,0000.190.110.7000.64 1.0023.75 6.94●655057320,0000.190.110.7000.64 1.0017.56 5.88●665058320,0000.190.110.7000.64 1.0011.61 5.77●675059320,0000.190.110.7000.64 1.008.42 4.63●685061320,0000.190.110.7000.64 1.00 6.31 5.75●695050320,0000.110.060.8300.64 2.0055.4315.62Temperature 705051320,0000.110.060.8300.64 2.0052.8230.12715052320,0000.110.060.8300.64 2.0055.815.29725053320,0000.110.060.8300.64 2.0051.814.04735054320,0000.110.060.8300.64 2.0045.9512.12●745055320,0000.110.060.8300.64 2.0039.149.29●142N.Park et al./Fuel Processing Technology125(2014)139–147corresponding experimental conditions,and the values that deviated from the equilibrium conversion by more than 15%were subsequently used for the kinetic data.Thus,only 74conditions (out of 118total conditions)were selected for the estimation (the conditions marked with solid circles in Table 1were determined to be close to the equilib-rium conversion and were excluded from the estimation).Additionally,Table 1(continued )CasePressure [bar]Temp.[K]Space velocity [mL/(g cat ·h)]Feed composition CO/(CO +CO 2)H 2/(2CO +3CO 2)Conversion[%]Close to the equil.RemarksCO CO 2H 2Ar CO CO 2755056320,0000.110.060.8300.64 2.0032.77.42●765057320,0000.110.060.8300.64 2.0026.75 3.88●775050380000.190.110.7000.64 1.0056.4212.15Temperature785051380000.190.110.7000.64 1.0055.6511.28795052380000.190.110.7000.64 1.0051.3710.09805054380000.190.110.7000.64 1.0036.579.22815050380000.110.060.8300.64 2.0076.1818.88●Temperature825051380000.110.060.8300.64 2.0072.2817.86●835052380000.110.060.8300.64 2.0065.9317.07●845053380000.110.060.8300.64 2.0059.2715.9●855054380000.110.060.8300.64 2.0050.3211.59●865055380000.110.060.8300.64 2.0044.1410.24●875056380000.110.060.8300.64 2.0039.76 6.94885057380000.110.060.8300.64 2.0036.69 2.51897052320,0000.180.100.670.050.64 1.0054.23 5.45Temperature907049320,0000.180.100.670.050.64 1.0031.72 4.0991*******,0000.180.100.670.050.64 1.0055.62 5.61927051320,0000.180.100.670.050.64 1.0057.92 5.61937052320,0000.180.100.670.050.64 1.0053.21 3.98947053320,0000.180.100.670.050.64 1.0049.76 3.72957054320,0000.180.100.670.050.64 1.0044.3 3.35967055320,0000.180.100.670.050.64 1.0037.45 1.58977057320,0000.180.100.670.050.64 1.0027.950.31●987052320,0000.180.100.670.050.64 1.0053.18 4.23997057320,0000.180.100.670.050.64 1.0026.22 2.891007052320,0000.180.100.670.050.64 1.0053.73 5.181015052320,0000.180.100.670.050.64 1.0041.26 6.37Pressure1027052320,0000.180.100.670.050.64 1.0048.59 4.281039052320,0000.180.100.670.050.64 1.0053.21 3.981045052320,0000.000.240.720.040.00 1.00−15.1325.64CO/(CO +CO 2)1055052320,0000.110.160.690.050.40 1.0033.558.641065052320,0000.170.110.670.050.60 1.0035.57 4.231075052320,0000.220.070.660.050.75 1.0038.76 6.291085052320,0000.270.030.640.050.90 1.0030.45−11.951095052320,0000.290.020.640.050.95 1.0028.93−15.011105052320,0000.320.000.630.06 1.00 1.0013.76−0.821115052380000.180.100.670.050.64 1.0053.927.47●H 2/(2CO +3CO 2)1125052380000.130.070.770.040.64 1.6564.8417.21●1135052380000.090.050.830.030.64 2.5071.9324.26●1145052320,0000.180.100.670.050.64 1.0030.367.16H 2/(2CO +3CO 2)1155052320,0000.160.090.700.050.64 1.1731.598.851165052320,0000.130.070.770.040.64 1.6536.8314.021*********,0000.110.060.800.030.64 2.0038.7117.641185052320,0000.090.050.830.030.642.5040.120.91Table 2Elementary reactions for synthesis of MeOH.Elementary stepEquilibrium constantAdsorptionCO +s1⇆CO(s1)K CO ¼θCO s1ðÞf CO θs1CO 2+s3⇆CO 2(s3)K CO2¼θCO2s3ðÞf CO2θs3H 2+2s2⇆2H(s2)K H2¼θ2H s2ðÞf H2θ2s2H 2O +s2⇆H 2O(s2)K H2O ¼θH2O s2ðÞf H2O θs2Surface reactionElementary stepsEquilibrium constant (A)CO hydrogenation reaction(A1):CO(s1)+H(s2)⇆HCO(s1)+s2K A1(A2):HCO(s1)+H(s2)⇆H 2CO(s1)+s2K A2(A3):H 2CO(s1)+H(S2)⇆H 3CO(s1)+s2K A3(A4):H 3CO(S1)+H(s2)⇆CH 3OH +s1+s K A4(B)Water –gas shift reaction (B1):CO 2(S3)+H(s2)⇆HCO 2(s3)+s2K B1(B2):HCO 2(S3)+H(s2)⇆CO(s3)+H 2O(s2)K B2(C)CO 2hydrogenation reaction(C1):CO 2(s3)+H(s2)⇆HCO 2(s3)+s2K C1(C2):HCO 2(s3)+H(s2)⇆H 2CO 2(s3)+s2K C2(C3):H 2CO 2(s3)+H(s2)⇆H 3CO 2(s3)+s2K C3(C4):H 3CO 2(s3)+H(s2)⇆H 2CO(s3)+H 2O(s2)K C4(C5):H 2CO(s3)+H(s2)⇆H 3CO(s3)+s2K C5(C6):H 3CO(s3)+H(s2)⇆CH 3OH +s3+s2K C6143N.Park et al./Fuel Processing Technology 125(2014)139–147the sensitivity analysis con firmed that the adsorption equilibrium con-stants do not signi ficantly impact the overall reaction rates;therefore,the reported values [18,19,22]were used without modi fication,but the adsorption coef ficient of H 2O (K H2O )was estimated because it was not reported in the literature.The estimation was conducted using the “lsqcurve fit ”subroutine in MATLAB (MathWorks,Inc.),where the Levenberg –Marquardt method was applied and the estimated parameters are listed in Table 3.Fig.2displays the comparison between the experimental data and the simu-lated results with the corresponding statistics included and the effec-tiveness of the developed kinetic model was also shown.The mean of absolute relative residuals (MARR)value for the CO 2conversion was higher than that of CO conversion.This result was attributed to the fact that the experimental values of the CO 2conversion under some conditions were close to zero and the relative errors were thus calculat-ed to be very high due to very small denominator values.Additionally,when water and methanol in the reactor ef fluent were trapped to prevent it from entering the GC column,a small amount of CO 2might have remained dissolved in the liquid phase,resulting in increased mea-surement errors [27].It was hard to directly measure dissolved CO 2in a blank test because a minimal amount of CO 2was dissolved in the liquid phase compared to that in the gas phase (but large enough to affect the measurement errors of the CO 2conversions).We instead calculated the weight fractions of CO 2in the liquid phase using a process simulator to determine the amount of CO 2that can be dissolved in the liquid phase as a result of condensation.The values ranged from 0.14wt.%to 6.74wt.%under all operating conditions,indicating that the dissolved CO 2varies signi ficantly with respect to the experimental conditions which results in high individual errors in some cases.The activation energies of the estimated kinetic parameters were nearly the same as the values reported in the literature [19],with the ex-ception of the DME production rate constant being approximately 17%higher than the value reported in the literature [22].Meanwhile,thepre-exponential factor of k C′was determined to be approximately 30times higher than the previously reported value,indicating that the CO 2hydrogenation rates were relatively high.This result was con firmed by the conditions in groups (9)–(11)of Fig.2(experimental conditions 42–53in Table 1),and the CO 2conversion was determined to be suf ficiently high.It is also important to note that the CO conversion exhibited slightly negative values as a result of the weak reverse WGS reaction rate and the CO 2was almost entirely consumed by the CO 2hydrogenation.Although much effort was devoted towards maintaining the isother-mal condition within the reactor in this study,there was still the possi-bility of a temperature gradient existing due to the high exothermicity of the reaction;therefore,the energy balance equation (Eq.(22))was taken into consideration.It has been previously reported that the overall heat transfer coef ficient in the catalytic bed typically falls in the range ofC O c o n v e r s i o n [%]Case010********60708090100110120C O 2 c o n v e r s i o n [%](a)(b)parison of the (a)CO and (b)CO 2conversions between the experimental and simulated data.The mean of the absolute relative residuals was 9.40%and 72.12%for the CO andCO 2conversions,respectively.Numbers in parentheses above the diagram correspond to the effects of various operating conditions (detailed conditions are listed in Table 1):effects of (1)temperature;(2)space velocity;(3)pressure;(4)CO fraction at 50bar and 250°C;(5)H 2fraction;(6)inert gas fraction;CO fraction at 70bar,(7)250°C and (8)270°C;H 2/CO 2ratio with no CO for (9)50bar,(10)60bar and (11)70bar;(12)temperature and space velocity (close to ICI plant operating conditions);temperature with H 2/(2CO +3CO 2)and SV [mL/(g cat ·h)](13)1and 20000;(14)2and 20000;(15)1and 8000;and (16)2and 8000;(17)temperature at 70bar;(18)pressure;(19)CO fraction;H 2fraction with SV of (20)8000and (21)20,000.144N.Park et al./Fuel Processing Technology 125(2014)139–147100–300W/(m 2·K)[28–30],and the heat transfer coef ficient that was also estimated was determined to be 143W/(m 2·K).It should be noticed that,although the model developed in the presented study is based on the intrinsic kinetics,its application is limited to the catalyst with similar composition.This is attributable to the fact that different composition may have different RDS,as discussed in the introduction section (RDS's of KRICT and commercial catalysts were different).3.3.Effects of operating conditionsThe effects of temperature,space velocity,and pressure on the CO conversion are provided in groups (1),(2),and (3),respectively,in Fig.2.The negative effects of temperature were attributed to the prox-imity of the conversion to equilibrium,which was negatively in fluenced by the elevated temperature in exothermic reactions [20].However,as shown in groups (13)–(15),when the conversion was relatively far from equilibrium,the positive effects of the temperature were observed as a result of the reaction rates increasing as the temperature increased.The negative effects of space velocity were attributed to the reduced residence time,and the positive effects of the pressure were attributed to the increased concentration of the reactants.Alternatively,the CO 2conversions in groups (1)–(3)exhibited little variation with respect to the operating conditions,since the WGS reaction that produces CO 2may compensate for the CO 2consumption through hydrogenation when CO and CO 2hydrogenations occur simultaneously (as discussed in Section 3.2,the contribution of theE f f e c t i v e n e s s f a c t o rParticle radius [mm]C O c o n v e r s i o n [%](a)(b)Fig.4.(a)Effectiveness factor calculated using experimental data and regressed lines:y =1.019exp(−262.2x )for 523K (R 2=0.9552)and y =1.034exp(−250.4x )for 533K (R 2=0.9692);and (b)the CO conversion for various particle sizes and temperatures (Catalyst weight 0.4g,inert weight =1.2g,P =50bar,SV =20,000mL/(g cat h),CO/CO 2/H 2/Ar =18/10/67/5).The MARR value was calculated to be 7.58%.0.05.0e-51.0e-41.5e-42.0e-42.5e-43.0e-43.5e-45005105205305400.20.40.60.81.0A c c u m u l a t e d r C O [m o l /k g c a t /s ]T e m pe r a t u r e [K ]CO f r a c t i o n -5.0e-50.05.0e-51.0e-41.5e-42.0e-42.5e-45005105205305400.20.40.60.81.0A c c u m u l a t e d r W G S [m o l /k g c a t /s ]T em p e r a t u re [K ]CO f r a c t i o n0.05.0e-51.0e-41.5e-42.0e-42.5e-43.0e-45005105205305400.20.40.60.81.0A c c u m u l a t e d r C O 2 [m o l /k g c a t /s ]T e m pe r a t u r e [K ]CO f r a c t i o n (a)(c)(b)(d)204060801001201401605005105205305400.20.40.60.81.0M e O H c o n c e n t r a t i o n [m o l /m 3]T em pe r a t u re [K ]C O fr a c t i o nFig.3.Effects of temperature and CO fraction (=CO/(CO +CO 2))on (a)the MeOH concentration upon exiting the reactor,and the accumulated rates of (b)the CO hydrogenation,(c)theWGS reaction,and (d)the CO 2hydrogenation.The pressure,space velocity,and H 2fraction (=H 2/(2CO +3CO 2))were speci fied as 50bar,8000mL/(g cat h)and 1.0,respectively.145N.Park et al./Fuel Processing Technology 125(2014)139–147。
1.Unpolarized light: Most sources of electromagnetic radiation contain a large number of atomsor molecules that emit light. The orientation of the electric fields produced by these emitters may not be correlated, in which case the light is said to be unpolarized.2.Polarized light:The polarization of light is described by specifying the orientation of thewave's electric field at a point in space over one period of the oscillation. When light travels in free space, in most cases it propagates as a transverse wave—the polarization is perpendicular to the wave's direction of travel.3.Optical isotropic:Substance in which light has the same velocity (refractive index) in alldirections is called optical isotropic.4.Optical indicatrix:The optical indicatrix is constructed by plotting indices of refraction asradii parallel to the vibration direction of the light.5.Birefringence: Birefringence is the optical property of a material having a refractive index thatdepends on the polarization and propagation direction of light. These optically anisotropic materials are said to be birefringent (or birefractive). The birefringence is often quantified as the maximum difference between refractive indices exhibited by the material.6.Pleochroism: Pleochroism results when lights of different wavelength are absorbed differentlyby different crystallographic directions.7.Relief:Relief is a measure of the relative difference in n between a mineral grain and itssurroundings.8.X-rays: X-rays are produced when electrically charged particles of sufficient kinetic energy arerapidly decelerated.9.Continuous radiation:Continuous radiation is produced as the impinging high-energyelectrons, decelerated by the atomic electrons of the target element. Its wavelength depends on accelerating voltage.10.Characteristic X-ray: Characteristic X-ray is indirectly produced when an electron isdisplaced through a collision with a primary beam electron, and is replaced by another electron.The resultant loss of energy is given off in the form of an X-ray. Characteristic lines are dependent on target electronic structure, energies are independent of accelerating voltage.11. Secondary electrons: Secondary electrons are usually the result of an inelastic collision, inwhich the transferred energy of the primary beam is transferred to an electron that is then emitted from the atom. Secondary electrons typically have energy of 50 eV or less.12. Backscattered electrons: Backscattered electrons are the result of elastic collisions with atomsof the specimen. They result in emitted electrons that have energy of 80% or more of the original energy of the primary beam electron.13. Depth of field: The height over which a sample can be clearly focused is called the depth offield. The SEM has a large depth of field which produces the images that appear 3-dimensional in nature.14. Topographic contrast: Topographic contrast has a complex origin with strong number andtrajectory components of both backscattered and secondary electron signals. Normally the yield of secondary electron signals is more sensitive to the topography of the sample surface. Yet the secondary electron signals give more contribution to topographic contrast.15. Atomic number contrast: Atomic number contrast (compositional contrast) arises fromdifferences in the local chemical composition within an object under study. Compositional contrast is principally a backscattered electrons number contrast mechanism, with a component of energy dependence.16. Energy-dispersive X-ray spectroscopy (EDS or EDX or EDAX): EDS is an analyticaltechnique used for the elemental analysis or chemical characterization of a sample.17. The Wavelength dispersive X-ray spectroscopy (WDS): WDS is a method used to count thenumber of X-rays of a specific wavelength diffracted by a crystal. The x-ray is diffracted according to Bragg Law, and detected by a gas-filled proportional counter.18. Mass-thickness contrast: mass contrast is due to the difference of density and thickness,which is called mass-thickness constrast.19. Diffraction contrast: Diffraction contrast results from scattering of the incident electron waveby structural defects in the case of crystalline materials; Diffraction contrast is attributed to the difference of diffraction of different parts of the sample.()θπθcos N N =。
1.With increasing demand for good quality water around the world, people today pay more attention than ever to the importance of water treatment, water reclamation and desalination processes.磁场has been widely used in the water industry since the年s。
Accord-ing to the latest edition of the WHO guideline for drinking water quality, boron concentration is set at 0.5 mg/L as the limit2.The ζ -potential is an important and reliable indicator of the…3. It should be noted that calcite is not easily distinguishable from the lepidocrocite trace using XRD analysis4. To this a10 ml aliquot of Fe(II) stock was added to initiate the oxidation run and a series of samples were withdrawn at time intervals of 0, 10, 30, 60 and 120 min.摘要:In recent years,…has shown a grea t potential as a low-cost, environment al friendly and sustainable treatment technology to align with the“zero ” wa ste scheme in the water /wastewater industry. The ability of this advanced……technology has been widely demonstrated to remove…… in water . At present, the main technical….. that….water treatment.For the first time, we describe how to utilize a mu lti-variables optimization approach to det ermine the optimum operat ion parameters so as to enhance process……and photooxidation efficiency.Both …and…associated with …. water treatment process are detailed. A brief discussion on the life cycle assessment for …. technology as an alternative waste treatment process is presented. This paper will deliver a scientific and technical overview and useful information to scientists and engineers who work in this field. Introduction:Increasing demand and shortage of clean water sources due to the rapid development of industrialisation, population growth and long-term droughts have become an issue worldwide. With this growing demand, various practical strategies and solutions have been adopted to yield more viable water resources. The storage of rainwater for daily activities andincreasing the catchment capacity for stormwater are just a few examples that could resolve the problems in short-term.Water industries and governments in some arid areas with abundant of sunlight, less rainfall and long-term drought have a challenge to seek viable water resources. It is estimated that around 4 billion people worldwide experience to have no or little access to clean and sanitised water supply, and millions of people died of severe waterborne diseases annually。
A PCBEE Proce dia 5( 2013 )349 – 3572212-6708 © 2013 The Authors. Published by Elsevier B.V.Selection and peer review under responsibility of Asia-Pacific Chemical, Biological & Environmental Engineering Societydoi: 10.1016/j.apcbe e.2013.05.060Available online at 350M. Abdelkreem / A PCBEE Procedia 5( 2013 )349 – 357during chlorination treatment of water for domestic supply.The toxic and hazardous nature of phenols and their derivatives have been well documented [1,2 ,3 ,4]and can cause several health problems (Slein and Sansone,[5]). Phenolic compounds have been classified as high-priority pollutants by the USA EPA (Environmental Protection Agency)[6]. Phenolic compounds are usually present in wastewater generated from the paint, solvent, petrochemical, coal conversion, pharmaceutical, plastic, iron-steel and paper and pulp industries.Several methods are currently used for the removal of phenol and its derivatives from wastewater, e.g. microbial degradation, chemical oxidation, incineration, solvent extraction and irradiation[7,8,9,4]. However, by far the most frequently used technology is adsorption by a solid phase. Several different adsorbent solids such as activated carbon[10 ]silica[11], glass powder[12], polymeric resins[13,14], fly ash[15,16,17,18,19,20 ] , peat[20,21], kaolinite[22] and zeolites[23,24] have all been proposed to remove phenolic pollutants from wastewater.2.Experimental procedure2.1.AdsorbentStarting material olive mill waste was provided by a factory from Al Wahhat (Egypt). The preparation of the adsorbent was as followed, olive mill wasteovernight, crushed, sieved. The resulting material was used without any further treatment in the adsorption process.2.2.AdsorbateAnalytical grade phenol from J.T. Baker was used in the experiments. Stock solutions of phenol were prepared using de-ionized water.2.3.Preliminary kinetic studyA preliminary kinetic investigation was made at certain operating conditions in order to determine the time neededpractically to reach equilibrium. A large number of conical flasks containing the same adsorption mixture were well shaken at temperature 25 C and samples were separately withdrawn at different time intervals. The withdrawn samples were filtered then analyzed. Samples are repeatedly withdrawn until equilibrium is reached.2.4.Adsorption studiesSorption studies were conducted in a routine manner by both batch and continuous techniques. For batch experiments a fixed amount (0.25 gm) of olive mill waste were placed into 250-mL flasks containing 20 mL solution with different initial concentrations (50 - 700 mg/L) of phenol solution. The flasks were shacked on a shaker at 150 rpm with a constant shaking rate for the time needed to reach equilibrium from the preliminary kinetic investigation then the samples was filtered and the phenol concentration was determined. All the experiments were carried out in duplicates and the average value were used for further calculations. The studies were performed at a constant temperature of 25ºC to be representative of environmentally relevant conditions.Kinetic experiments were conducted using a known weight of adsorbent dosage and employing phenol concentration.The effects of the dosage of adsorbent, the initial phenol concentration, and contact time will be studied. The amount of phenol adsorbed on olive mill waste will be determined by the difference between the initial concentration (C o) and the equilibrium concentration (C e).The amount of phenol adsorbed at equilibrium q e (mg/g) was calculated from the following equation: q e = (C o - C e)V /m (1)351 M. Abdelkreem / A PCBEE Procedia 5( 2013 )349 – 357352M. Abdelkreem / A PCBEE Procedia 5( 2013 )349 – 357(2) that the amount adsorbed increased with the increase in the concentration of solution. When the initial phenolconcentration was increased from 100 to 600 mg/L, the adsorption uptake of olive mill waste increased.A higher initial concentration provides an important driving force to overcome all resistances for the phenol betweenthe aqueous and solid phases, thus increasing the uptake. The effect of contact time on the removal of phenol by the olive mill waste at initial concentrations 100-600 mg/L showed rapid adsorption of phenol in the first 30 min.The adsorption rate decreased gradually and the adsorption reached equilibrium in about one hour for all the initial concentrations used.The time required to attain this state of equilibrium was termed the equilibrium time and the amount of phenol adsorbed at the equilibrium time reflected the maximum phenol adsorption capacity of the adsorbent under these particular conditions.These results were also obtained by Reza Ansari[25].Fig.2.Effect of initial phenol concentration on adsorption of phenol ,contact time 2h, temperature 293 K, amount of phenol 0.2 gm/20 ml solution, 150 r.p.m.3.3.Effect of olive mill waste dosage on adsorptionFigure (3) shows the phenol removal from aqueous solutions as a function of added olive mill waste. Olive mill waste dosage ranged from 0.25 to 1 g for the 20 mL of phenol solutions. It can be seen that the maximum removal expressed asa percentage removal was about85% from initial phenol at dosages 1 g of olive mill waste. Phenol removal increasedquickly from 52% to 85% when the olive mill waste dosage increased from 0.25 g to 1 g and reached a maximum for 1 g olive mill waste.This trend is expected because as the adsorbent dose increases, the number of active sites for binding phenol molecules on the adsorbent increases and thus more phenol was attached to their surface. Thus it results in the increment of adsorption efficiency until saturation. These results were agreed with those abstained by M. Ghaedi[26], and T. Smithaa[27].353M. Abdelkreem / A PCBEE Procedia 5( 2013 )349 – 357Fig. 3. Effect of adsorbent dose on adsorption of phenol , contact time 2h, temperature 293 K, initial phenol concentration 250 ppm,150r.p.m.3.4.Adsorption isotherms modelsAdsorption isotherms are mathematical models that describe the distribution of the adsorbate species among liquid andadsorbent, based on a set of assumptions that are mainly related to the heterogeneity/homogeneity of adsorbents, the typeof coverage, and possibility of interaction between the adsorbate species. It also provides a panorama of the course takenby the system under study in a concise form, indicating how efficiently an adsorbent will adsorb and allows an estimate ofthe economic viability of the adsorbent commercial applications for the specified solute 5. Four isotherms equations havebeen tested in the present study to analyze the equilibrium data of the adsorbent, Langmuir[28], Freundlich[1] ,Temkin[10] and Dubinin-Radushkevich21. Langmuir model is the most widely used isotherm and its equation written as:C e/q e = (1/q L K L) + (1/q L) C e (3)where q e is the mono-layer adsorption capacity of adsorbent (mg/g), K L is the Langmuir adsorption constant (L/mg) andq L is the mono-layer adsorption capacity of adsorbent (mg/g). Therefore, a plot of C e/q e versus C e gives a straight line ofslope 1/q L and intercepts 1/(q L K L).The Freundlish model can be expressed as:Log q e = log K F + (1/n) log C e(4) Where K F and n are the Freundlich adsorption constants which can be determined by the linear plot of log q e versus logC e.The Temkin isotherm equation is given as:q e = B ln A + B ln C e(5) where A and B are Temkin constants. A plot of q e versus ln C e can be used to determine the constants A and B.The Dubinin-Radushkevich (D-R) isotherm was used to fit with the experimental data, and it can be represented as: ln q e =ln q m ß2 (6)where ß is a coefficient related to the mean free energy of adsorption (mmol2/J2), q m is the maximum adsorption capacityand is the Polanyi potential (J/mmol) that can be written as:= RT (1+1/C e) (7) Where R is the gas constant and T is the absolute temperature.The equilibrium adsorption isotherms were performed by plotting phenol adsorbed (q e) against the equilibriumconcentration of phenol (C e) in solution.Correlation coefficients and parameter values for the four isotherms were presented in table (1). Based on thecorrelation coefficients, the applicability of the isotherms was compared. The experimental results indicate that thesorption of phenol onto olive mill waste followed Dubinin-Radushkevich (D-R) isotherm model.To determine if adsorption process was favorable or unfavorable for Langmuir type adsorption process, Langmuirisotherm is then classified using a dimensionless constant separation factor (R L), which can be defined as:R L = 1/(1+K L C max) (8) where C max is the highest (initial) metal concentration in solution (mg/L). If the value of R L < 1, it indicates a favorableadsorption and if R L > 1 then, an unfavorable adsorption. The R L values for the adsorption of phenol onto olive mill wastewas in the range 0 < R L <1, indicating that Langmuir adsorption is favorable.354 M . Abdelkreem / A PCBEE Procedia 5 ( 2013 ) 349 – 357 3.5.Kinetic ModelingThe kinetics of phenol adsorption can be modeled by various equations. The pseudo-first-order Lagergren equation is given as [16],Log (q e -q ) = log q t e (k 1k /2.303) t (9)Where q t and q e are the amounts of ion adsorbed at time t and at equilibrium (mg/g), respectively, and k 1is the rate constant of pseudo-first-order adsorption process (h 1). The slope and intercept of plots of log (q e q t ) versus t were used to determine the first-order rate constant k 1and equilibrium adsorption capacity q e .The pseudo-second-order kinetic model by Ho and McKay 22, with the linear form:t/q t =1/(k 2k q e )+(1/2q /) t e (10)Where k 2is the equilibrium rate constant of pseudo-second-order adsorption (g/mg.h). If the pseudo-second-order equation is applicable, the plot of t/q t versus t gives a linear relationship, and then k 2and q e can be calculated from the slope and intercept of the line.Fig.4. Plot of time vs. t/q e of pseudo-second order graph.The adsorption data may also be analyzed using the Elovich equation [23], which has the linear form:qt=1/ß/ln (() + (1/)/) ln t ß(11)Where ßis the initial sorption rate constant (mg/g h), and the parameter ßis related to the extent of surface coverage and activation energy for chemisorptions (g/mg). The constant can be obtained from the slope and intercept of the plot of q t versus ln t.The intra-particle diffusion model was also tested. The initial rate constant for intra-particle diffusion is obtained using the equation [7]:q e =C + k int k t 1/2(12)Where k int is the intra-particle rate constant (mg/g.h 1/2).Table 2 represents the constants of kinetics models for phenol adsorption on olive mill waste. The obtained data revealed that pseudo-second order equation provides the best correlation coefficient (Figure 4) with extreme high values (>0.99). In addition, the calculated q e values also agree with the experimental data in the case of pseudo-second-order kinetics. These suggest that the adsorption data are well represented by pseudo-second-order kinetics and it supports the assumption that the chemical adsorption is the rate-limiting. The reaction mechanism may be partly a result of the ion355M . Abdelkreem / A PCBEE Procedia 5( 2013 )349 – 357 exchange between phenol and the hydroxyl and carboxylic groups on the olive mill waste surface [23].Table 1.Values of the used isotherm parameters.Isotherm ModelParameter R 2Langmuirq L =62.5L K =0.010.968FreundlichF K = 10.351n= 3.460.904TemkinA=1.244B= 2.530.949Dubinin-Radushkevich q m =24.4*105ß=1E-070.999Table 2 Values of parameters of each kinetic modelkinetic ModelParameter R 2Pseudo-first-orderq e =10k 1=0.0440.987Pseudo-second-orderq e =22.22k 2=0.01310.999Elovich=1033.9ß=0.5020.876Intra-particle-diffusion k int =0.5710.7423.6.Fixed Bed Studies3.6.1.Effect of flow rateThe results for different phenol solution flow rates are plotted for a bed height of 0.2m and inlet phenol concentration of 250 ppm,Fig. 5shows that as the flow rate increases from 0.2to 0.6cm3/sec, the breakthrough curve becomes steeper.The break point time decreases without considering the velocity variation along the bed. This is because of the residence time of the solute in the column, which is not long enough for adsorption equilibrium to be reached at high flow rate. So at high flow rate the phenol solution leaves the column before equilibrium occurs. Furthermore, a fixed saturation capacity of bed based on the same driving force gives rise to a shorter time for saturation at higher flow rate.Fig. 5.Effect of Volumetric Flow Rate on Break Time.3.6.2.Effect of initial concentrationThe effect of initial phenol concentration on effluent concentration is shown in Fig. 6.During these, simulations other parameters such as bed height and flow rate are kept constant. It is observed that as the inlet phenol concentration increases,the break point time decreases without considering the velocity variation. For larger feed concentration, steeper356M. Abdelkreem / A PCBEE Procedia 5( 2013 )349 – 357breakthrough curves are found, because of the lower mass-transfer flux from the bulk solution to the particle surface due to the weaker driving force. In addition, at high concentration, the isotherm gradient is lower, yielding a higher driving force along the pores. Thus the equilibrium is attained faster for values of higher phenol concentration.Fig. 6.Effect of Initial Phenol Concentration on Break Time.4.ConclusionThe adsorption of phenol from aqueous solution using olive mill waste as an adsorbent was investigated under different experimental conditions in batch process and column process. The Dubinin-Radushkevich (D-R) adsorption isotherm was found to have the best fit to the experimental data. The adsorption kinetics can be predicted by pseudo-second-order kinetic.From the fixed bed investigations, as the flow rate is increased, the breakthrough curve becomes steeper. The break point time is obtained earlier and effluent phenol concentration ratio increases more rapidly. For smaller bed height, the effluent phenol concentration ratio increases more rapidly than for a higher bed height. For larger initial phenol concentration, steeper breakthrough curves are obtained and break point time is achieved sooner.The results indicate that olive mill waste can be used as adsorbent material for adsorption of phenol from aqueous solutions.References[1]Greminger DC, Burns GP, Lynn S, Hanson DJ and King CJ (1982) Solvent-extraction of phenols from water.Ind. Eng. Chem. 2151-54.[2]Brandt S, Zeng A and Deckwer DW (1997) Adsorption and desorption of pentachlorophenol on cells of Mycobacteriumchlorophenolicum PCP-1. Biotechnol. Bioeng.55480-489.[3]Bülbül G, Aksu Z and Turkish J (1997) Investigation of wastewater treatment containing phenol using free and Ca-alginate gelimmobilized P. putida in a batch stirred reactor.Turkish J. Eng. Environ. Sci. 21175-183.[4]Denizli A, Cihanger N, Taner A, Taner M and Alsacak G (2004) Removal of chlorophenols from syn thetic solutions usingPhanerochaete chrysosporium.Process Biochem.39 2025-2030[5]Slein MW and Sansone EB (1980)Degradation of Chemical Carcinogens. Van Nostrand Reinhold, New York, USA.[6]Environmental Protection Agency (1984) Methods 604, Phenols in Federal Register. October 26, Part VIII, 40, CFR, 58, USA.[7]Munaf E, Zein R, Kurniadi R and Kurniadi I I (1997) The use of rice husk for removal of phenol from waste water as studiedusing 4-aminoantipyrine spectrophotometric method. Environ. Technol. 18 (3) 355-358357M. Abdelkreem / A PCBEE Procedia 5( 2013 )349 – 357[8] Bertoncini C, Raffaelli J, Fassino L, Odetti HS and Botani EJ (2003) Phenol adsorption on porous and non-porous carbons.Carbon 41 (6) 1101-1111[9] Khalid M, Joly G, Renaud A and Mangnoux P (2004) Removal of phenol from water by adsorption using zeolites. Ind. Eng. Chem. Res. 43 5275-5280.[10] Nouri S, Hagaseresht F and Max LU GQ (2002) Comparison of adsorption capacity of p-Cresol and p-Nitrophenol by activated carbon in single and double solute. Adsorption 8 (3) 215-223.[11] Hanna K, Beurroies I, Denoyerl R, Desplantiergiscard D, Galarneu A and Di Renzo F (2002) Sorption of hydrophobic molecules by organic/inorganic mesostructures. J. Colloid Interface Sci. 252 276-283.[12] Atun G (1992) The adsorption of nitrophenols on a special adsorbent prepared from glass powder. Spectrosc. Lett.1992 25 (5) 741-756.[13] Wagner K and Schultz S (2001) Adsorption of phenol, chlorophenols, and dihydroxybenzens onto unfunctionalized polymericresins at temperatures from 294.15 K to 318.15 K. J. Chem. Eng. Data. 46 322-330.[14] Abburi K (2003) Adsorption of phenol and p-chlorophenol from their single and bisolute aqueous solutions on Amberlite XAD-16 resin. J. Hazard. Mater. 105 143-156.[15] Akgrman A and Zardkoohi M (1996) Adsorption of phenolic compounds on fly ash. J. Chem. Eng. Data. 41 185-187.[16] Kao PN, Tzeng JH and Huang TL (2000) Removal of chlorophenols from aqueous solution by fly ash. J. Hazard. Mater. 76 237-249[17] Sarkar M and Acharya KP (2006) Use of fly ash for the removal of phenol and its analogues from contaminated water. Waste Manage. 26 559-570.[18] Sarkar M, Acharya KP and Bhattacharya B (2005) Removal characteristics of some priority organic pollutants from water in a fixed bed fly ash column. J. Chem. Technol. Biotechnol. 80 1349-1355.[19] Srivastava VC, Swamy MM, Mall ID, Prasad B and Mishra IM (2006) Adsorptive removal of phenol by bagasse fly ash and activated carbon: Equilibrium, kinetics and t hermodynamics. Colloids Surf. A. 272 89-104.[20] Viraraghavan T and Alfaro F (1998) Adsorption of phenol from wastewater by peat, fly ash and bentonite. J. Hazard. Mater. 5759-70.[21] Allen SJ (1987) Equilibrium adsorption isotherms for peat. Fuel 66 1169-1175.[22] Barhoumi M, Beurroies I, Denoyel R, Said H and Hanna K (2003) Co-adsorption of alkylphenols and nonionic surfactants onto kaolinite. Colloids Surf A. 219 25-33.[23]Koh S and Dixon JB (2001) Preparation and application of organominerals as adsorbents of phenol, benzene and toluene. Appl.Clay Sci. 18 111-122[24] Sismanoglu T and Pura S, (2001) Adsorption of aqueous nitrophenols on clinoptilolite. Colloids Surf. A. 180 1-2[25] Reza Ansari, Samaneh Alaie and Ali Khah, (Sept.2011), Application of polyaniline for removal of acid green 25 from aqueous solutions, Journal of scientific and industrial research, vol. 70, 804-809.[26]M. Ghaedi, S. Ramazani and M. Roosta (2011), Gold nanoparticle loaded activated carbon as novel adsorbent for the removal of Congo red, Indian Journal of Science and Technology Vol. 4 No. 10 (Oct 2011) ISSN: 0974- 6846[27] T. Smithaa, S. Thirumalisamy A*, and S. Manonani, (2012), Equilibrium and Kinetics Study of Adsorption of Crystal Violet onto the Peel of Cucumis sativa Fruit from Aqueous Solution, ISSN: 0973-4945; CODEN ECJHAO E-Journal of Chemistry 2012, 9(3), 1091-1101.[28] Garcia-Araya JF, Beltran FJ, Alvarez P and Masa FJ (2003) Activated carbon adsorption of some phenolic compounds present in agro industrial wastewater. Adsorption 9 (1) 107-115.。
Chemical Engineering Journal125(2006)111–117Kinetic modelling of the adsorption of nitratesby ion exchange resinM.Chabani a,A.Amrane b,∗,A.Bensmaili aa Facult´e de G´e nie des Proc´e d´e s et G´e nie M´e canique,U.S.T.H.B.BP32,El Allia,Bab ezzouar,Algeriab Equipe Chimie et Ing´e nierie des Proc´e d´e s-ENSCR/Universit´e de rennes1,UMR CNRS6226“Sciences chimiques de Rennes”,ENSCR,Campus de Beaulieu,av.du G´e n´e ral Leclerc,35700Rennes,FranceReceived21April2006;received in revised form17July2006;accepted6August2006AbstractThe capacity of ion exchange resins,Amberlite IRA400,for removal of nitrates from aqueous solution was investigated under different initial concentrations.The suitability of the Freundlich,Langmuir and Dubinin-Radushkevich adsorption models to the equilibrium data was investigated.The equilibrium data obtained in this study were found to follow Freundlich adsorption isotherm.The maximum sorption capacity was769.2mg/g at25◦C.Reversible-first-order,intraparticle diffusion,film diffusion and Bangham models were used tofit the experimental data. The adsorption of nitrates on Amberlite IRA400resin follows reversible-first-order kinetics.The overall rate constants were estimated for different initial concentrations.Results of the intra-particle diffusion and thefilm diffusion models show that thefilm diffusion was the main rate-limiting step.The low correlation of data to the Bangham’s equation also confirms that diffusion of nitrates into pores of the resin was not the only rate-controlling step.The thermodynamic constants of adsorption phenomena, H◦and S◦were found to be−26.122kJ/mol and−68.76J/mol in the range298–318K and+19.205kJ/mol and+68.76J/mol in the range318–343K,respectively.The negative values of the Gibbs free energy ( G)demonstrate the spontaneous nature of adsorption of nitrates onto Amberlite IRA400.©2006Elsevier B.V.All rights reserved.Keywords:Adsorption;Resin;Nitrates;Kinetics1.IntroductionSeveral nitrogenous compounds,including ammonia,nitrite and nitrate are frequently present in drinking water[1].Nitrates can cause several environmental problems.Nitrates and phos-phates can stimule eutrophication where pollution is caused in waterways by heavy algal growth,as they are both rate-limiting nutrients for process.A high concentration of nitrate-nitrogen in drinking water leads to the production of nitrosamine,which is related to cancer,and increases the risks of diseases such as methemoglobinemia in new-born infants[2,3].Several methods that serve to reduce nitrates in drinking water have been developed.The use of biological reactor seems to be the most promising technique in the treatment of high nitrate concentrations.However,maintaining biological processes at their optimum conditions is difficult,and the problems of con-∗Corresponding author.Tel.:+33223235755;fax:+33223238120.E-mail address:abdeltif.amrane@univ-rennes1.fr(A.Amrane).tamination by dead-bacteria have to be solved to make such processes satisfactory for a safety use in drinking water treat-ment.Adsorption is a useful process for in situ treatment of underground and surface water,primarily due to it’s easy of use [3].Adsorbent resins are considered the most promising owing to their chemical stability and ability to control surface chemistry [4].The characteristics of adsorption behaviour are generally inferred in terms of both adsorption kinetics and equilibrium isotherms.They are also important tools to understand the adsorption mechanism,viz.the theoretical evaluation and inter-pretation of thermodynamic parameters[5,6].The objective of this study was to investigate equilibrium and kinetic parameters for the removal of nitrates from aqueous solutions by adsorption onto an ionized adsorbent,Amberlite IRA400.The Langmuir,Freundlich and Dubinin-Radushkevich (D-R)equations were used tofit the equilibrium isotherms.Ther-modynamic parameters were also evaluated through adsorption measurements.1385-8947/$–see front matter©2006Elsevier B.V.All rights reserved. doi:10.1016/j.cej.2006.08.014112M.Chabani et al./Chemical Engineering Journal125(2006)111–117Nomenclatureb Langmuir constant(cm3/mg)C0initial concentration in solution(mg/l)C e equilibrium concentration(mg/l)C t concentration at time t(mg/l)D p pore diffusion coefficient(cm2/s)D ffilm diffusion coefficient(cm2/s)E free energy of adsorption(kJ/mol)G Gibbs free energy(kJ/mol)H◦enthalpy of adsorption(kJ/mol)k overall rate constant of adsorption(min−1)k1forward rate constant(min−1)k2backward rate constant(min−1)k d distribution constant(l/g)k f Freundlich’s constant related to the sorptioncapacityk i intraparticle diffusion parameter(mg/g min0.5)m mass of adsorbent(g)n Freundlich’s constant related to the sorptionintensity of a sorbentq the amount of nitrates adsorbed or transferred(mg/l)q o maximum adsorption capacity(mg/g)q e adsorption capacity in equilibrium(mg/g)q t amount of adsorption at time t(mg/g)R ideal gas constant(8.314J/mol K)R p radius of the adsorbent(cm)S◦entropy of adsorption(J/mol K)t time(min)t1/2time for half adsorption(min)T temperature(K)v volume of solution(l)Greek letterεfilm thickness(cm)2.Materials2.1.Pre-treatment of resinBefore use,the resin was washed in distilled water to remove the adhering dirt and then dried at50◦C.After drying,the resin was screened to obtain a particle size range of0.3–0.8mm.2.2.The resin characteristicsThe main characteristics of the Amberlite IRA400(Merck, Darmstadt,Germany),a macroporous anion exchange resin,are given in Table1.2.3.Nitrate solutionsThe stock solution of NO3−used in this study was prepared by dissolving an accurate quantity of KNO3in distilled water.Table1Characteristics of Amberlite IRA400ion exchange resinPolymer matrix Polystyrene DVBFunctional group–N+R3Ionic form Cl−Exchange capacity 2.6–3eq kg−1of dry massAppearance Yellow to golden spherical beads,translucent Effective size0.3–0.8mmWater retention42–48%Visual density in wet state0.66–0.73g/mlTrue density in wet state 1.07–1.10g/mlA range of dilutions,1–18mg/l,was prepared from the stock solution.2.4.Sorption experimentsThe pH of the aqueous solutions of NO3−was approximately 6.8and did not varied significantly with the dilution.The effect of temperature on the sorption rate of nitrates on Amberlite IRA400was investigated by equilibrating the sorption mix-ture(800ml)containing dried resin(2g)and nitrates(15mg/l) in a temperature range298–343K.The solutions were placed in flasks and stirred for3h.Experiments were mainly carried out without initial adjustment of the pH.Preliminary tests showed that the adsorption was complete after1h.The concentration of residual nitrate ions was determined spectrophotometrically according to Rodier protocol[7].The sorption capacity at time t,q t(mg/g)was obtained as follows: q t=(C0−C t)vm(1) where C0and C t(mg/l)were the liquid-phase concentrations of solutes at the initial and a given time t,respectively,v(l)the volume of solution and m the mass resin(g).The amount of adsorption at equilibrium,q e was given by: q e=(C0−C e)vm(2) C e(mg/l)was the concentration of nitrates at equilibrium.The distribution constant k d was calculated using the follow-ing equation:k d=Amount of nitrates in adsorbentAmount of nitrates in solution×vm.(3) 3.Results and discussion3.1.Adsorption isothermsSorption equilibrium is usually described by an isotherm equation whose parameters express the surface properties and affinity of the sorbent,at afixed temperature and pH[8].An adsorption isotherm describes the relationship between the amount of adsorbate adsorbed on the adsorbent and the con-centration of dissolved adsorbate in the liquid at equilibrium. Equations often used to describe the experimental isotherm data are those developed by Freundlich[9],by Langmuir[10]and by Dubinin-Radushkevich[11].The Freundlich and LangmuirM.Chabani et al./Chemical Engineering Journal 125(2006)111–117113isotherms are the most commonly used to describe the adsorp-tion characteristics of adsorbent used in water and wastewater.3.1.1.Freundlich isotherm (1906)The empirical model can be applied to non-ideal sorption on heterogeneous surfaces as well as multilayer sorption and is expresses by the following equation:q e =k f C 1/ne(4)n and k f are the Freundlich constants.The fit of data to Freundlich isotherm indicates the hetero-geneity of the sorbent surface.The magnitude of the exponent 1/n gives an indication of the adequacy and capacity of the adsor-bent/adsorbate system [12].In most cases,an exponent between 1and 10shows beneficial adsorption.The linear plot of ln q e versus ln C e (Fig.1)shows that the adsorption obeys to the Fre-undlich model.k f and n were determined from the Freundlich plot and found to be 0.982and 1.074(Fig.1),respectively.n values above 1indicate favourable adsorption.ngmuir isotherm (1916)The Langmuir model is probably the best known and most widely applied sorption isotherm.It may be represented as fol-lows:q e =q o bC e1+bC e (5)The Langmuir constants q o and b are related to the adsorption capacity and the energy of adsorption,respectively.The linear plot of 1/q e versus 1/C e shows that the adsorption obeys to the Langmuir model (Fig.2),and gave the following values for q o and b ,769.2mg/g and 1.02cm 3/mg,respectively.3.1.3.Dubinin-Radushkevich (D-R)isothermRadushkevich [13]and Dubinin [14]have reported that the characteristic sorption curve is related to the porous structure of the sorbent.The sorption data were applied to the D-R model in order to distinguish between physical and chemical adsorption [15].The D-R equation was given by:ln q e =ln q o −βε2(6)Fig.1.Linearized Freundlich isotherm (Eq.(4))for nitrates adsorption by Amberlite IRA 400;T =25◦C,pH 6.8,adsorbent dose 1.25g/l.Fig.2.Linearized Langmuir isotherm (Eq.(5))for nitrates adsorption by Amber-lite IRA 400;T =25◦C,pH 6.8,adsorbent dose 1.25g/l.where βwas the activity coefficient related to mean sorption energy and εthe Polanyi potential given by:ε=RT ln1+1e (7)where R was the gas constant (kJ/mol K)and T the temperature (K).The slope of the plot ln q e versus εgives β(mol 2/J 2)and the ordinate intercept yields the sorption capacity q o (mg/g).The mean sorption energy (E )is given by E =1/√−2β;for a magnitude of E between 8and 16kJ/mol,the adsorption process follows chemical ion-exchange [6,16],while values of E below 8kJ/mol characterize a physical adsorption process [17].The plot of ln q e against ε2for nitrate ions sorption on resin is shown in Fig.3.The E value was 40.8J/mol,which corresponds to a physical adsorption.The linear correlation coefficients for Freundlich,Langmuir and D-R are shown in Table 2,and are always greater than 0.980.The Freundlich equation represents a better fit of experimental data than Langmuir and D-Requations.Fig.3.Radushkevich-Dubinin isotherm (Eq.(6))for nitrates adsorption by Amberlite IRA 400;T =25◦C,pH 6.8,adsorbent dose 1.25g/l.114M.Chabani et al./Chemical Engineering Journal 125(2006)111–117Table 2Isotherm parameters collected for removal of nitrates by Amberlite IRA 400Freundlich constants k f 0.982n 1.074R 20.9951Langmuir constants q o (mg/g)769.2b (cm 3/mg) 1.02R 20.9933D-R constants q o (mg/g)76.5E (J/mol)40.8R 20.9873.2.Adsorption kinetics3.2.1.Effect of initial concentrationsPredicting the adsorption rate,in addition to the adsorbate residence time and the reactor dimensions,controlled by the system’s kinetics,are probably the most important factors in adsorption system design [18].Preliminary experiments showed high initial rates of adsorp-tion of nitrates followed by lower rates near equilibrium.Kinet-ics of nitrates removal at 298K showed high rates during the initial 15min,and decreased thereafter until nearly constant val-ues after 40min of adsorption (Fig.4).In batch adsorption processes the adsorbate molecules diffuse in porous adsorbent and the rate process usually depends upon t 1/2rather than the contact time,t [19,20]:q t =k i t 0.5(8)The plot of q t ,the amount of adsorbate adsorbed per unit weight of adsorbent versus square root of time has been com-monly used to describe an adsorption process controlled by diffusion in the adsorbent particle and consecutive diffusion in the bulk of the solution [21].Therefore,as can be observed in Fig.5,adsorption was linear and charaterized by extremelyfastFig.4.Time-courses of nitrates adsorption for different initial concentrations of nitricnitrogen.Fig.5.Effect of t 1/2on adsorption of nitrates by Amberlite IRA 400(Eq.(8)),adsorbent dose 1.25g/l,pH 6.8.uptake.During this phase,nitrates were adsorbed within a t 1/2value of about 10min;this behaviour can be attributed to the rapid use of the most readily available adsorbing sites on the adsorbent surface.After this phase,adsorption became negligi-ble.It may be attributed to a very slow diffusion of adsorbed nitrates from the film surface into the micropores,which are the less accessible sites of adsorption [20,22,23].Fig.5shows two consecutive linear steps during the adsorption of nitrates on Amberlite IRA 400,and the deviation from the straight line indicates that the pore diffusion is not the only rate-limiting step.The slope of the linear portion from Fig.5was used to derive values for the rate parameter,k i ,for the intra-particle diffusion (Eq.(8)).The results show that intraparticle diffusion model was valid for the considered system.Kinetic parameters and correlation coefficients obtained by the intra-particle model are given in Table 3.The values of k i increased for increasing initial concentrations.Kinetic data can further be used to check whether pore-diffusion was the only rate-controlling step or not in the adsorp-tion system using Bangham’s equation [24–26]:log log C 0C 0−qm =log k B m2.303v+a log t (9)As observed,the model did not match experimental data(Fig.6),showing that the diffusion in the pores of the sorbent wasTable 3Intraparticle diffusion (Eq.(8))kinetic parameters for adsorption of nitrates on Amberlite IRA 400resin at different concentrations Concentration of nitric nitrogen (mg/l)Intraparticle diffusion Correlation coefficient R 2Kinetic parameters,k i (mg/g min 0.5)10.8740.5120.990 1.23.50.987 2.9150.990 3.93100.9038.75140.98211.7180.96615.5M.Chabani et al./Chemical Engineering Journal125(2006)111–117115Fig.6.Fit of adsorption kinetics at different initial concentrations of nitric nitro-gen by means of the Bangham’s model(Eq.(9)).not the only rate-controlling step.The correlation coefficients given by the Bangham’s equation(Fig.6)confirmed that the model did notfit satisfactory experimental data.Similar results were previously reported for4-CP adsorption by macroreticular resins[27]and for adsorption of4-chlorophenol by XAD-4[12].3.2.2.First-order reversible modelWhen a single species is considered to adsorb on a hetero-geneous surface,the adsorption of a solute from an aqueous solution follows reversible-first-order kinetics[28,29].The het-erogeneous equilibrium between the solute in solution and the ion exchange resin may be expressed as:A k1k2B,(10)With k1the forward reaction rate and k2the backward reaction rate constant.If C0(mg/l)was the initial concentration of nitrates and q(mg/l)the amount transferred from the liquid phase to the solid phase at a given time t,then the rate was:d q d t =−d(C0−q)d t=k(C0−q)(11)where k(min−1)is the overall reaction rate constant.Since k1 (min−1)and k2(min−1)are the rate constants for the forward and the reverse processes,the rate can be expressed as:d qd t=k1(C0−q)−k2q(12)If X e(mg/l)represents the concentration of nitrates adsorbed at equilibrium,then at equilibrium:k1(C0−X e)−k2X e=0,d qd t=(k1+k2)(X e−q)(13) after integration,The above equation can be rearranged:ln(1−U t)=−(k1+k2)t=−kt(14) where U t=q/X e and k was the overall rate constant;U t,called the fractional attainment of equilibrium of nitrates,is givenby Fig.7.Fit of adsorption kinetics at different initial concentrations of nitric nitro-gen by means of the reversible-first-order model(Eq.(14)).the following expression:U t=C0−C tC0−C e(15) where C0,C t and C e were the initial nitrates concentration,its concentration at a given time t and at equilibrium,respectively. The overall constant rate k for a given concentration corre-sponded to the slope of the straight line of the plot ln(1−U t) versus t(Fig.7).The equilibrium constant and the forward and backward constant rates k1and k2were calculated using Eq.(14)and are collected in Table4.The high correlation coefficient values,if compared to that given in the available literature[30],confirm the applicability of the model.It could be seen that the forward rate constants for the removal of nitrates were much higher than the backward rate constants for the des-orption process(Table4).The constant rates depend on the adsorption capacity,the diffusion coefficient,the effective mass transfer area,the hydrodynamic of the system and other physico-chemical parameters.Ourfinding agreed with previous results [12].3.2.3.Diffusion processIn order to assess the nature of the diffusion process respon-sible for adsorption of nitrates on Amberlite IRA400,attempts were made to calculate the coefficients of the process.Iffilm dif-fusion was to be the rate-determining step in the adsorption of nitrates on the surface of the resin,the value of thefilm diffusion coefficient(D f)should be in the range10−6to10−8cm2/s.If pore diffusion was to be rate limiting,the pore diffusion coeffi-cient(D p)should be in the range10−11to10−13cm2/s[31,32]. Assuming spherical geometry for the sorbent,the overall rate constant of the process can be correlated with the pore diffu-sion coefficient and thefilm diffusion coefficient independently according to[29]:Pore diffusion coefficient:D p=0.03R2pt1/2,(16)andfilm diffusion coefficient:D f=0.23R pε1/2q(17)116M.Chabani et al./Chemical Engineering Journal125(2006)111–117 Table4Constant rates for the removal of nitrates with Amberlite IRA400resin using reversible-first-order model(Eq.(14))C0(mg/l)Correlationcoefficients,R2Overall rate constant,k=k1+k2(min−1)Forward rate constant,k1(min−1)Backward rate constant,k2(min−1)10.9540.0860.0680.018 20.9940.0890.0770.012 3.50.9780.1160.1070.009 50.9850.1410.1290.011 100.9800.1370.1280.008 140.9750.1650.1510.013 180.9920.1630.1460.017 where R p was the radius of the adsorbent(R p=0.045cm),εthefilm thickness(10−3cm)[19],q(mg/l)the amount of nitratesadsorbed and C0the initial concentration.By considering theappropriate data and the respective overall rate constants,poreandfilm diffusion coefficients for various concentrations ofnitrates were determined(Table5).It clearly appeared thatnitrates removal on Amberlite IRA400resin was controlledbyfilm diffusion since coefficient values were in the range10−6to10−8cm2/s.3.3.Thermodynamic studiesThe amounts of sorption of nitrates ions by Amberlite IRA400were measured in the range temperature298–343K.Ther-modynamic parameters were determined using the followingrelation[15,33–36]:ln k d= S◦R−H◦RT(18)where k d was the distribution coefficient, H, S,and T the enthalpy,entropy and temperature(in Kelvin),respectively,and R was the gas constant.The values of enthalpy( H)and entropy ( S)were obtained from the slope and intercept of ln(k d)versus 1/T plot.Fig.8shows two linear parts in the temperature range explored.Afirst decreasing part for a temperature range from 318to343K,and a second and increasing linear part for a tem-perature range from298to318K.Gibbs free energy( G)was calculated using the well-known equation:G= H−T S(19) The slope was positive in the temperature range298–318K and negative in a second step,from318to343K(Fig.8).The thermodynamic parameters are collected in Table6.Table5Diffusion coefficients for the removal of nitrates by Amberlite IRA400resinC0(mg/l)Pore diffusion coefficient,Eq.(16)(×10−7cm2/s)Film diffusion coefficient, Eq.(17)(×10−8cm2/s)10.670.73 20.780.92 3.50.84 1.06 50.92 1.15 10 1.01 1.29 14 1.26 1.58 18 1.692.06Fig.8.Estimation of thermodynamic parameters(Eq.(18))for the adsorption of nitrates onto Amberlite IRA400.Table6Thermodynamic parameters for the adsorption of nitrates on Amberlite IRA400 298K308K318K318K333K343K G(kJ/mol)−5.631−4.944−4.256−2.66−3.692−4.38 H◦(kJ/mol)−26.122+19.205S◦(J/mol K)−68.76+68.76For thefirst range from298to318K,the negative value of enthalpy showed that the adsorption of nitrates was exother-mic.The negative values of adsorption entropy indicated that the adsorption process was reversible and demonstrated the sponta-neous nature of adsorption(Table6).In the range318–343K,Gibbs free energy( G)decreased for increasing temperatures,indicating that the reaction was spontaneous and favoured by higher temperatures.The positive value of enthalpy indicated that the adsorption was endothermic. The positive value of adsorption entropy showed the irreversibil-ity of the adsorption process and favoured complexation and stability of sorption.The resultant effect of complex bonding and steric hindrance of the sorbed species probably increased the enthalpy and the entropy of the system.4.ConclusionsThese results show that Amberlite IRA400is an effective adsorbent for the removal of nitrates from aqueous solution.TheM.Chabani et al./Chemical Engineering Journal125(2006)111–117117equilibrium between nitrates and resin was achieved in approx-imately60min,leading to96%removal of nitrates.The corre-lation coefficient showed that Freundlich model gave a betterfit of experimental data than Langmuir and Dubinin-Radushkevich models.Adsorption kinetics was found to followfirst order reversible model expression.Thefilm diffusion was the main rate-limiting step.Temperature variations were used to evaluate enthalpy H, entropy S and Gibbs free energy G values.The negative value of G showed the spontaneous nature of adsorption.In the tem-perature range298–318K, H and S were negative,and the reaction was exothermic and reversible.From318to343K, H and S were positive,and the reaction was endothermic and irre-versible.In addition,positive S values favoured complexation and stability of sorption.References[1]N.¨Ozt¨u rk,T.Ennil Bektas,Nitrate removal from aqueous solution byadsorption onto various materials,J.Hazard.Mater.B112(2004)155–162.[2]H.Bouwer,Agricultural contamination:problems and solutions,WaterEnviron.Technol.(1989)292–297.[3]K.Mizuta,T.Matsumoto,Y.Hatate,K.Nishihara,T.Nakanishi,Removalof nitrate-nitrogen from drinking water using bamboo powder charcoal, Bioresour.Technol.95(2004)255–257.[4]N.I.Chubar,V.F.Samanidou,V.S.Kouts,G.G.Gallios,V.A.Kanibolotsky,V.V.Strelko,I.Z.Zhuravlev,Adsorption offluoride,chloride,bromide,and bromate ions on a novel ion exchanger,J.Colloid Interface Sci.291(2005) 67–74.[5]S.J.Allen,G.Mckay,J.F.Porter,Adsorption isotherm models for basicdye adsorption by peat in single and binary component systems,J.Colloid Interface Sci.280(2004)322–333.[6]A.¨Ozcan,A.S.¨Ozcan,S.Tunali,T.Akar,I.Kiran,Determination of theequilibrium,kinetic and thermodynamic parameters of adsorption of cop-per(II)ions onto seeds of Capsicum annuum,J.Hazard.Mater.B124(2005) 200–208.[7]J.Rodier,Water Analysis,seventh ed.,Dunod,Paris,1996(in French).[8]S.Sohn,D.Kim,Modelisation of Langmuir isotherm in solution systems-definition and utilization of concentration dependent factor,Chemosphere 58(2005)115–123.[9]H.M.F.Freundlich,¨Uber die adsorption in l¨o sungen,Zeitschrift f¨u rPhysikalische Chemie(Leipzig)57A(1906)385–470.[10]ngmuir,The constitution and fundamental properties of solids andliquids,J.Am.Chem.Soc.38(11)(1916)2221–2295.[11]M.M.Dubinin,E.D.Zaverina,L.V.Radushkevich,Sorbtsiyai strukturaaktivnykh uglei.I.Issledovanie adsorbtsii organicheskikh parov,Zhurnal Fizicheskoi Khimii21(11)(1947)1351–1363.[12]M.S.Bilgili,Adsorption of4-chlorophenol from aqueous solutions byXAD-4resin:isotherm,kinetic,and thermodynamic analysis,J.Hazard.Mater.137(2006)157–164.[13]L.V.Radushkevich,Potential theory of sorption and structure of carbons,Zhurnal Fizicheskoi Khimii23(1949)1410–1420.[14]M.M.Dubinin,Modern state of the theory of volumefilling of microporeadsorbents during adsorption of gases and steams on carbon adsorbents, Zhurnal Fizicheskoi Khimii39(1965)1305–1317.[15]R.Donat,A.Akdogan,E.Erdem,H.Cetisli,Themodynamics of Pb2+andNi2+adsorption onto natural bentonite from aqueous solutions,J.Colloid Interface Sci.286(2005)43–52.[16]F.Helffrich,Ion Exchange,McGraw Hill,New York,1962.[17]M.S.Onyango,Y.Kojima,O.Aoyi,E.C.Bernardo,H.Matsuda,Adsorp-tion equilibrium modelling and solution chemistry dependence offluoride removal from water by trivalent-cation exchange zeolite F-9,J.Colloid Interface Sci.279(2004)341–350.[18]Y.S.Ho,Review of second-order models for adsorption systems,J.Hazard.Mater.136(2006)681–689.[19]W.J.Weber,J.C.Morris,Adsorption Processes for Water Treatment,But-terworth,London,1987.[20]Q.Feng,Q.Lin,F.Gong,S.Sugita,M.Shoya,Adsorption of lead andmercury by rice husk ash,J.Colloid Interface Sci.278(2004)1–8. [21]M.Ahmaruzzaman,D.K.Sharma,Adsorption of phenols from wastewater,J.Colloid Interface Sci.287(2005)14–24.[22]O.Keskinkan,M.Z.L.Goksu,M.Basibuyuk,C.F.Forster,Heavy metaladsorption properties of a submerged aquatic plant(Ceratophyllum demer-sum),Bioresour.Technol.92(2004)197–200.[23]zaridis, D.D.Asouhidou,Kinetics of sorptive removal ofchromium(VI)from aqueous solutions by calcined Mg-Al-CO3hydrotal-cite,Water Res.37(2003)2875–2882.[24]D.H.Bangham,F.P.Burt,The behaviour of gases in contact with glasssurfaces,Proc.R.Soc.Lond.Ser.A–Containing Pap.A Mathemat.Phys.Character105(1924)481–488.[25]I.D.Mall,V.C.Srivastava,N.K.Agarwal,Removal of orange-G and methylviolet dyes by adsorption onto bagassefly ash kinetic study and equilibrium isotherm analyses,Dyes Pigments69(2006)210–223.[26]W.A.House,F.H.Denise,P.D.Armitage,Comparison of the uptake ofinorganic phosphorus to suspended and stream bed sediment,Water Res.29(1995)767–779.[27]R.-S.Juang,J.-Y.Shiau,Adsorption isotherms of phenols from water ontomacroreticular resins,J.Hazard.Mater.B70(1999)171–183.[28]Y.S.Ho,G.Mckay,Pseudo-second order model for sorption processes,Process Biochem.34(1999)451–465.[29]A.K.Battacharya,C.Venkobachar,Removal of cadmium(II)by low costadsorbents,J.Environ.Eng.Div.ASCE Proc.(1984)110–122.[30]K.V.Kumar,S.S.Ramamurthi,Adsorption of malachite green ontPithophora sp.a fresh water algae:Equilibrium and kinetic modelling, Process Biochem.40(2005)2865–2872.[31]S.Rengaraj,S.H.Moon,Kinetics of adsorption of Co(II)removal fromwater and wastewater by ion exchange resins,Water Res.36(2002) 1783–1793.[32]L.D.Michelson,P.G.Gideon,E.G.Pace,L.H.Kutal,Removal of Sol-uble Mercury from Wastewater by Complexing Techniques,Bull No.74,US.Dept.Industry,Office of Water Research and Technology, 1975.[33]J.Berrueta,J.M.Freije,G.Adrio,J.Coca,Synergistic effect in the mixturesof extraction of phenol from aqueous-solutions with n-butylacetate and acetophenone.Solvent extract,Ion Exchange8(1990)817–825.[34]M.K.Purkait,S.DasGupta,S.De,Adsorption of eosin dye on activatedcarbon and its surfactant based desorption,J.Environ.Manage.76(2005) 135–142.[35]M.Ajmal,R.A.Khan Rao,M.Ali Khan,Adsorption of copper from aque-ous solution on Brassica cumpestris(mustard oil cake),J.Hazard.Mater.B122(2005)177–183.[36]R.Naseem,S.S.Tahir,Removal of Pb(II)from aqueous/acidic solutionsby using bentonite as an adsorbent,Water Res.35(2001)3982–3986.。