Preparation of polypyrrole-polyvinylalcohol (PPy-PVA)
- 格式:pdf
- 大小:335.78 KB
- 文档页数:4

第33卷第6期高校化学工程学报No.6 V ol.33 2019 年12月 Journal of Chemical Engineering of Chinese Universities Dec. 2019文章编号:1003-9015(2019)06-1509-07溶剂挥发法制备大孔聚合物微球冯子雄, 徐建昌, 章莉娟(华南理工大学化学与化工学院, 广东省绿色化学产品技术重点实验室, 广东广州 510640)Ⅳ为原料,通过溶剂挥发法制备大孔聚合物微球。
摘要:以乙基纤维素(EC)和具有pH敏感性的聚丙烯酸树脂(PAR)聚合物浓度和乳化剂聚乙烯醇(PVA)的浓度影响聚合物微球的粒径大小,聚合物浓度减小或乳化剂浓度增大有利于形成较小的微球。
研究了pH和PAR/EC质量比对微球孔结构、比表面积的影响,分析了微球多孔结构形成的机理。
溶液的pH可改变PAR的亲疏水性,影响微球的孔结构:酸性环境中得到的微球表面出现致密的大孔,内部是复杂的多孔网络结构,碱性环境中则得到表面无孔的微球。
因此,可通过调节溶液PAR/EC质量比和pH来调控微球的孔结构。
关键词:聚丙烯酸树脂IV;聚合物微球;溶剂挥发法;大孔;pH敏感性中图分类号:TQ031 文献标志码:A DOI:10.3969/j.issn.1003-9015.2019.06.028 Preparation of macroporous polymer microspheres by a solvent evaporation methodFENG Zi-xiong, XU Jian-chang, ZHANG Li-juan(School of Chemistry and Chemical Engineering, Guangdong Provincial Key Lab of Green Chemical Product Technology, South China University of Technology, Guangzhou 510640, China)Abstract: Macroporous polymer microspheres were prepared using ethyl cellulose (EC) and pH-sensitive polyacrylic resin Ⅳ(PAR) by a solvent evaporation method. The particle size of polymer microspheres was affected by the concentrations of polymers and emulsifier polyvinyl alcohol (PV A). The reduction of polymer concentration or the increase of emulsifier concentration was beneficial for the formation of microspheres with smaller size. The effects of pH and PAR/EC mass ratio on the pore structure and specific surface area of microspheres were studied. The results show that the change of solution pH affects the hydrophilicity of PAR, which results in different pore structures of microspheres. The microspheres synthesized in acidic solution have dense surface macropores and complex inner porous network structures, while nonporous microspheres can be obtained in alkaline environment. Therefore, the pore structure of microspheres can be regulated by adjusting PAR/EC mass ratios and solution pH.Key words: polyacrylic resin IV; polymer microspheres; solvent evaporation; macroporous;pH-sensitive1 前言大孔材料(孔径大于50 nm)相较于微孔与介孔具有许多独特的性能,例如良好的通透性、材质选择的广泛性、高效的吸附和分离性能,以及易于化学改性[1-2]。


ph敏感的聚丙烯酰胺水凝胶的制备方法英文、Preparation Method of pH-Sensitive Polyacrylamide HydrogelPolyacrylamide hydrogel is a versatile material that has many applications in biomedical engineering, drug delivery, and tissue engineering. In this study, we present a method for preparing pH-sensitive polyacrylamide hydrogel using a crosslinker and a pH-responsive monomer.Materials:- Acrylamide (AAm)- N,N'-methylenebisacrylamide (MBAA)- N,N,N',N'-tetramethylethylenediamine (TEMED)- Ammonium persulfate (APS)- N-(3-aminopropyl) methacrylamide hydrochloride (APMA) - Sodium hydroxide (NaOH)- Hydrochloric acid (HCl)Method:1. Preparation of monomer solution: Dissolve APMA in deionized water (DI water) at a concentration of 1 M.2. Preparation of crosslinker solution: Dissolve MBAA in DI water at a concentration of 1% (w/v).3. Preparation of initiator solution: Dissolve APS in DIwater at a concentration of 10% (w/v).4. Preparation of gelation buffer: Dissolve NaOH in DI water to obtain a 0.1 M solution. Adjust the pH to 7.4 using HCl.5. Preparation of hydrogel: Mix AAm, APMA, and MBAA at a ratio of 99:1:0.1 (w/w/w). Add TEMED and APS to the mixture to initiate polymerization. Pour the mixture into a mold and allow it to polymerize at room temperature for 2 hours.6. pH sensitivity test: Cut the hydrogel into small pieces and place them in different pH solutions (pH 2, 4, 6,7.4, 8, and 10). Observe the swelling behavior of the hydrogel at different pH values.Results:The resulting hydrogel exhibited pH sensitivity, as evidenced by its swelling behavior in different pH solutions. The hydrogel swelled more in alkaline solutions than in acidic solutions. This can be attributed to the presence of thepH-responsive monomer APMA, which undergoes protonation and deprotonation at different pH values.Conclusion:In conclusion, we have developed a simple method for preparing pH-sensitive polyacrylamide hydrogel using acrosslinker and a pH-responsive monomer. The resulting hydrogel exhibited pH sensitivity and may have potential applications in drug delivery and tissue engineering.。

印染废水排放量大、有机污染物含量高、水质变化大、有毒有害物质含量高,对水环境污染日益加剧。
太阳能光催化被认为是解决日益严重的水污染问题最有前途的方法[1-2]。
二氧化钛(TiO 2)因成本低、光化学稳定性好、无毒等优点,是最具吸引力的光催化剂[3-4]。
因此,利用TiO 2进行环境修复(包括对水、空气和土壤进行净化)得到广泛研究[5-8]。
然而,TiO 2半导体能隙为3.2eV ,只能被波长不超过387nm 的光波激发,吸收的太阳光不足5%,利用率低[9-10]。
为了将TiO 2的应用范围拓宽到可见光区域,对其进行适当的掺杂或表面改性。
常用方法有表面光敏化、复合半导体、贵金属沉积、离子掺杂修饰、导电高聚物掺杂等[11-12]。
导电共轭聚合物在可见光区吸收效率高、易于制备及掺杂,引起科研人员的广泛关注[13-16]。
本研究采用具有共轭环状结构、类似导电聚合物的预氧化PAN 对纳米TiO 2进行改性,减少光生电子-空穴张杰(衡水学院应用化学系,河北衡水053000)摘要:采用水相沉淀法合成聚丙烯腈(PAN ),并将其复合到纳米二氧化钛(TiO 2)表面,制备PAN/TiO 2复合微粒。
PAN/TiO 2经预氧化得到共轭结构的复合微粒,拓宽了纳米TiO 2的光响应范围。
采用FTIR 和DTA-TG 对PAN/TiO 2进行表征,结果表明,纳米TiO 2上附着有预氧化的PAN ,PAN/TiO 2热稳定性较好。
改变n (AN )∶n (TiO 2)制备不同的PAN/TiO 2,并用于太阳光下罗丹明B 的降解。
结果表明,n (AN )∶n (TiO 2)为1∶120时,PAN/TiO 2光催化性能较好。
关键词:二氧化钛;聚丙烯腈;复合微粒中图分类号:X791文献标志码:B文章编号:1004-0439(2021)04-0037-03Photocatalytic activity of preoxidized PAN/TiO 2composite particles under sunlightZHANG Jie(Department of Applied Chemistry,Hengshui University,Hengshui 053000,China)Abstract:Polyacrylonitrile (PAN)was prepared by precipitation method,and PAN/TiO 2composite particleswere prepared by compounding PAN onto the surface of nano-TiO 2.PAN/TiO 2composite particles were preox⁃idized to obtain composite particles with conjugated structure,so as to broaden the light response range of na⁃no-TiO 2.PAN/TiO 2were characterized by FTIR and DTA-TG.The results showed that the preoxidized polyacry⁃lonitrile attached to nano-titanium dioxide,and the thermal stability of PAN/TiO 2was good.PAN/TiO 2of differ⁃ent ratio were prepared by changing the molar ratio of AN and TiO 2,and it was applied to the degradation of Rhodamine B under sunlight.When the molar ratio of AN and TiO 2was 1∶120,the photocatalytic properties were better.Key words:TiO 2;PAN;composite particles收稿日期:2020-09-21基金项目:2021年河北省高等学校科学技术研究项目(ZC2021238);衡水市科技局项目(2020011008Z );衡水学院校级科研课题(2020ZR21)作者简介:张杰(1982—),女,河北人,讲师,硕士,E-mail :********************。

Preparation and characterization of Ag-TiO2 hybrid clusters powders[1](Ag-TiO2混合团簇粉末的制备和表征)Abstract:液相电弧放电法被用于制备纳米Ag-TiO2复合超细粉末。
XRD和TEM图表明颗粒呈葫芦状形态,分布狭窄。
我们讨论了实验条件对产品的影响,比较了这种方法制备的粉末和其他γ射线辐照法制备的粉末。
Introduction:材料合成技术,提高了研究特定电子和光学特性的能力。
这也导致了设备和不同效应的快速发展,如集成光学型偏振器[1]和量子霍耳效应。
所需的长度尺度对于这些结构的控制是在纳米级别的[ 2 ]。
科学家面临的一个新的挑战是半导体量子点的生长,它具有新的光学响应,引起了对其基础物理方面和三阶非线性光致发光的应用等的研究兴趣。
这方面的一个例子是Ag-TiO2复合材料通过胶体方法合成[ 3 ]或由γ射线辐照法合成[ 4 ]。
对比其他制备超细金属颗粒的方法,γ射线辐照法能在室温的环境压力下产生粉末。
在这封信中,我们开发了一种新的方法,即液相电弧放电法,用以制备纳米复合材料,当它经水热处理可以得到纳米级别的超细粉。
Preparation and photocatalytic activity of immobilized composite photocatalyst (titania nanoparticle/activated carbon)[2]固定化复合光催化剂(TiO2纳米颗粒/活性炭)的制备和光催化活性研究Abstract:制备了一种固定化复合光催化剂——TiO2纳米颗粒/活性炭(AC),并研究了它在降解纺织染料的光催化活性。
AC通过油菜籽壳制备。
碱性红18(BR18)和碱性红46(BR46)被用来作为模型染料。
并采用了傅里叶变换红外(FTIR),波长色散X射线光谱(WDX),扫描电子显微镜(SEM),紫外可见分光光度法,化学需氧量(COD)和离子色谱(IC)分析。

第三章Polymer StructureThis chapter is concerned with aspects of the structure of polymeric materials outside those of simple chemical composition. The main topics covered are polymer stereochemistry, crystallinity, and the character of amorphous polymers including the glass transition. These may be thought of as arising from the primary structure of the constituent molecules in ways that will become clearer as the chapter progresses.本章所关注的这些简单的化学成分之外的高分子材料的结构方面。
主要内容包括:聚合物立体化学,结晶,包括无定形聚合物的玻璃化转变的特征。
这些可能被认为是章进展变得更为清晰的方式,将组成分子的一级结构所产生的。
Before proceeding, a word on nomenclature is necessary. Polymer chemists, following the example of P.J. Flory, have tended to use the words configuration and conformation in a sense that differs from that conventionally employed within organic chemistry. In this book, by contrast, I intend to go along with F. W. Billmeyer, and use these words in the way that they apply more widely throughout chemistry. Thus configuration is the term given to an arrangement of atoms that cannot be altered except by breaking chemical bonds, while conformation is the term applied to the individual, recognisable arrangement of atoms that can be altered by simple rotation around a single bond. Configurations include head-to-tail arrangements, described in the previous chapter, conformations include trans versus gauche arrangements of successive carbon-carbon bonds along the backbone of an individual macromolecule.在继续之前,一个命名的话是必要的。

Preparation of Nonylphenyl polyethyleneglycol ether filmsPurposes1. To master the basic methods of water-soluble film preparation2. To understand the function of films-forming materials such as polyvinyl alcoholIntroductionFilms refer to the film-like preparations which are made by dissolving or dispersing the drug to the film-forming materials uniformly. Mass production of films is through flow casting method. The procedure of this method is as follows: First, preparing the slurry of the film-forming material. Then drugs, colorants etc. are added. After defoaming the slurry, painting and dying it, we can detach it and get the product which is ready to be packed. However, coating method is used when small amount production is needed. There are two concrete methods to achieve this.1. casting/pouring method pouring the slurry to the dish, dying it at proper temperature and at last detach it. The thickness of the film can be controlled by the amount of the slurry. Besides, aluminum alloy plate with a little square groove can be used in the process. For example, a aluminum alloy plate with the dimension of 165mm×135mm×15mm is manufactured by milling and adding 100 little square groove with the dimension of 15mm×12mm×0.3mm. After pouring the slurry, scraping the plate to the flat condition. Films prepared by this method have accurate drug content, regular shape, good appearance and can be easily split.2. scraping method choosing appropriate clean glass or stainless steel plate, washing and drying it, spreading some talc powder on it and then wiping it with a clean gauze. Pouring the slurry on it, scraping it to flat using a scraper with a definite distance and then dying it in the oven. By doing so, we can get the films.Besides the talc powder, we can use some release agent such as liquid paraffin to make the detachment easier. Also, polyethylene film can be used as a cushion material to get better effect. The process of this method is as follows: wiping the glass plate with 75% alcohol, putting a piece of plastic film on the glass when it is still wet.(the film should be wider than the glass on both sides), expelling the remaining bubble to make the film stick on the glass tightly and the process of film-making can be started. This method not only has the advantage of detaching the films easily but also can prevent the mutual adherence between the drug films and the package because the drug films can be cut together with the plastic films. It is convenient for us to split the film just before the use.Equipments and Materials1. EquipmentsSmooth and purity glass plate (30×45cm), circle copper stick (1×45cm), beaker(50ml, 100ml), volumetric flask (50ml), seconds-counter, little iron folder, haustorial tube (5ml), thickness tester, nylon sieve (100 mesh), analytic balance, 751 type spectrophotometer, etc.2. MaterialsPolyvinyl alcohol 0486, nona phenyl polyglycol ether, glycerol, distilled water, etc.Procedures1. Formulationnona phenyl polyglycol ether 5gpolyvinyl alcohol 0486 7.5gglycerol 1gdistilled water about 20g2. Preparationweigh 5g nonylphenyl polyethyleneglycol ether, 1g glycerol, 20g water, and put them into a beaker of 50 ml, heat for a while and stir until it dissolves. Add polyvinyl alcohol 0486 after it cools down and keep it still overnight. After polyvinyl alcohol 0486 is completely wetted and expanded, heat it under the water bath of 70℃until it totally dissolves. Filter when it is still hot if necessary with a nylon mesh of #100, keep its temperature under 50℃standing for a certain length of time or defoam it by ultrasonic wave. Then pour the film material to the lower edge of the glass plate with the same temperature, push the material forward with a push rod, put it in the oven of 70-80℃for 5-10 min and detach the film immediately. After cooling, cut it into small films of 5cm×5cm. The finished product can be got after package.3. Usagethe product can kill sperms as an external drug for contraception. It can dissolve within 50 seconds after it is administrated into the deep part of vagina. It has larger contact area comparing with suppository and has an apparent and instant effect.4. Quality Control of Films(1) dissolution timetake a piece of film, immerge it into a distilled water bath of 37±0.5℃with its upper side fixed by a little iron clamp. At the same time open the stopwatch. Record the time when the film falls off the clamp. The time should not exceed 30 seconds.(2) thicknessit is determined by thickness gage. Usually the thickness should be 0.065 ± 0.015 mm.(3) weight variation testtake 20 pieces of films (except for special regulations), weigh accurately the total weight and calculate the mean weight, then weigh accurately every piece, comparing the results with the mean weight. There should be less than 2 pieces of films whose weight are beyond the limitation of weight variation and at the same time none of them could exceed twice the limitation of weight variation.Weight variation requirement of films: a) ≤0.02g: ±15%; b) 0.02g-0.2g: ±10%; c) ≥0.2g: ±7.5%.(4) Content determinationput a piece of film into a beaker of 50ml, add some distilled water to dissolve it and transfer it to a volumetric flask of 50ml, add water to scale and shake evenly. Accurately measure 5ml to a volumetric flask of 50ml, add water to scale and determine its absorbance at wavelength of 273nm. The content can be got by calculationExperimental InstructionsRequirements for preview1. Review the quality requirements for film-forming material and the properties of PV A.2. Review the preparation process of films3. Write down the procedures of the experiment according to the notes and main points of operation.Procedures and Precautions1. polyvinyl alcohol is a kind of ideal and common film-forming material. It is a water-soluble multi-hydroxyl polymer which is got by the alcoholysis of polyvinyl acetate. The alcoholysis degree is affected by the solubility of the product(Table1).Table 1 The alcoholysis degree and the solubility of PVAAlcoholysis degree, % Residual acetate, % Solubility>99.5 <0.5 Only soluble in water with T 95℃95-99.5 0.5-5 Soluble in water with T 65-70℃88 12 Having the maximum solubility50 50 Can not dissolve in waterThe dissolving process of polyvinyl alcohol in water is similar to that of hydrophilic colloid. It must go through several stages which include:affinity with water , wetting, permeation, expansion and dissolving.The time for PVA to swell should be enough or it will not dissolve completely.2. During the process of arranging the ingredients, spreading the slurry and drying, the temperature should not be too high and the time should not be too long. If temperature is higher than 70℃ when preparing the slurry , the hydrogen bonds between the polyethenyl and water are easily broken which may lead to non-uniformity of the drug in the film. If temperature is too high when spreading the slurry, bubbles can be found in the film which makes the process of film-forming and detachment difficult and the product would be brittle and have a low drug load.3. When keeping the slurry still, try to wait until the bubbles completely disappear. In order to avoid the formation of the bubbles, do not stir before spreading the slurry.4. Try to control the temperature and time of drying after the film is formed. Less or over drying will both lead to detaching difficulty.5. The glass should be clean and smooth. Spread some liquid paraffin before heating to avoid detaching difficulty. Different film-forming materials show different degrees of affinity to the glass plate. Too little affinity would make the spreading process hard to perform and the film uneven. Too much affinity would make the detachment difficult. Usually it can be improved by changing the plate or cushion layer or lubricant.surfactant. Its structural formula is:The polymerization degree of polyethylene is about 9-10%, the clouding point is 65-70℃, hydroxyl value is 84, HLB value is 13.2, and ultraviolet absorption coefficient at wavelength of 275nm is 23.7±1.Questions1. What relationships between the characteristics and the alcoholysis degree or molecular weight of PVA?2. What is the meaning of the polymerization degree and the alcoholysis degree? What is the polymerization degree and the alcoholysis degree of PV A 05-88,17-88, respectively?3. Besides PV A, how many kinds of film-forming materials commonly used are there?。


注射用盐酸吡硫醇制备流程英文回答:Preparation of Pyrithione Hydrochloride for Injection.Introduction:Pyrithione hydrochloride is a broad-spectrum antifungal and antibacterial agent that is used to treat a variety of infections. It is a synthetic compound that is prepared by the reaction of pyrithione sodium with hydrochloric acid. The resulting product is a white or off-white powder that is soluble in water.Materials:Pyrithione sodium.Hydrochloric acid.Water.Activated carbon.Equipment:Reaction vessel.Stirrer.Thermometer.Vacuum filter.Drying oven.Procedure:1. Dissolve pyrithione sodium in water in the reaction vessel.2. Add hydrochloric acid to the solution and stir untilthe reaction is complete.3. Filter the solution through activated carbon to remove impurities.4. Vacuum filter the solution to remove the solvent.5. Dry the product in a drying oven.Quality Control:The product should meet the following specifications:Appearance: White or off-white powder.Assay: 98.0% to 102.0%。
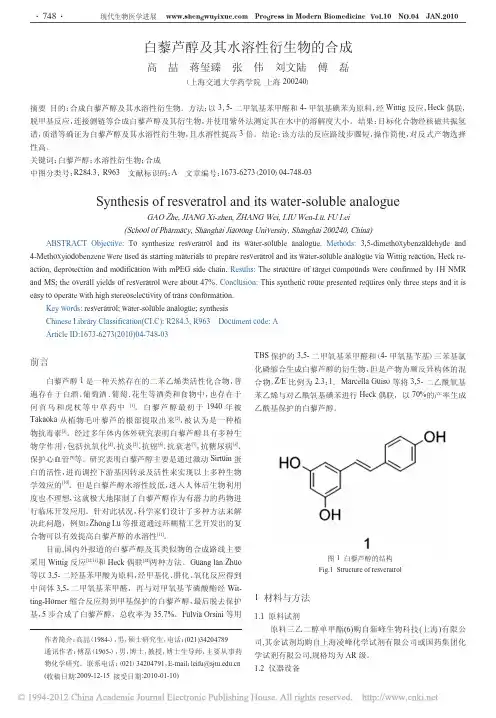
![高分子材料与工程专业英语词汇[1]](https://uimg.taocdn.com/9aa0e19cdd88d0d233d46a56.webp)
聚乙烯醇缩甲醛是利用聚乙烯醇缩与甲醛在盐酸催化的作用下而制得的,其反应如下CH 2O+H+C +H 2O HC H 2C HC H 2C H C H 2O HO HC +H 2OH +C H 2C HC H 2C H C H 2O O HC H 2+~~~~~~~~~~~~+H 2OC H 2C HC H 2C H C H 2O O HC H 2+~~~~~~C H 2C HC H 2C H C H 2OO~~~~~~C H 2+H+由于几率效应,聚乙烯醇中邻近羟基成环后,中间往往会夹着一些无法成环的孤立的羟基,因此缩醛化反应不能完全。
为了定量表示缩醛化的程度,定义已缩合的羟基量占原始羟基量的百分数为缩醛度。
聚乙烯醇是水溶性的高聚物,如果用甲醛将它进行部分缩甲醛化,随着缩醛度的增加,水溶性愈差。
作为维尼纶纤维的聚乙烯醇缩甲醛的缩醛度一般控制在35%左右。
它不溶于水,是性能优良的合成纤维。
本实验是合成水溶性聚乙烯醇缩甲醛胶水。
反应过程中须控制较低的缩醛度,使产物保持水溶性。
如反应过于猛烈,则会造成局部高缩醛度,导致不溶性物质存在于水中,影响胶水质量。
因此在反应过程中,特别要注意严格控制催化剂用量、反应温度、反应时间及反应物比例等因素。
聚乙烯醇缩甲醛随缩醛化程度的不同,性质和用途各有所不同。
它能溶于甲酸、乙酸、二氧六环、氯化烃(二氯乙烷、氯仿、二氯甲烷)、乙醇-苯混合物(30:70)、乙醇-甲苯混合物(40:60)以及60%的含水乙醇等。
本实验中,由于缩醛化反应的程度较低,胶水中尚含有未反应的甲醛,产物往往有甲醛的刺激性气味。
缩醛基团在碱性环境下较稳定,故要调整胶水的pH 值。
Polyvinylformal is the use of polyvinyl with formaldehyde obtained under the action of hydrochloric acid catalyzed reaction is as followsCH 2O+H+C +H 2O HC H 2C HC H 2C H C H 2O HO HC +H 2OH+C H 2C HC H 2C H C H 2O O HC H 2+~~~~~~~~~~~~+H 2OC H 2C HC H 2C H C H 2O O HC H 2+~~~~~~C H 2C HC H 2C H C H 2OO~~~~~~C H 2+H+Since the probability of effect, polyvinyl alcohol after the neighboring hydroxyl group to form a ring, the intermediate will often sandwiched between some of the isolated hydroxyl groups can not form a ring, the acetalization reaction is not completely. In order to quantify the degree of acetalization, the definitions have been condensed hydroxy accounts for a percentage of the original amount of hydroxyl groups for the acetalization degree.Polyvinyl alcohol is a water-soluble polymer, if it with formaldehyde partial formals, as the acetalization degree of the increase in the water-soluble worse. Vinylon fiber polyvinyl acetal of formaldehyde general control of about 35%. It does not dissolve in water, is the excellent performance of the synthetic fibers.This experiment is a synthetic water-soluble polyvinyl formaldehyde glue. A lower acetalization degree is required to control the course of the reaction, the product remains water soluble. If the reaction is too violent, and will result in the local high acetal degrees, lead to insoluble substances present in the water, affecting the glue quality. Therefore, during the reaction, in particular, to pay attention to strictly control the amount of catalyst, reaction temperature, reaction time, reactant ratios and other factors.Polyvinylformal with different degree of acetalization, the nature and purpose of each different. It was dissolved in formic acid, acetic acid, dioxane, chlorinated hydrocarbons (dichloroethane, chloroform, dichloromethane), ethanol - benzene (30:70) mixture of ethanol - toluene mixture (40:60), and 60 % aqueous ethanol and the like. This experiment, due to the lower degree of acetalization reaction, glue still contains unreacted formaldehyde, and the product tend to have a pungent odor of formaldehyde. The acetal group is more stable in an alkaline environment is necessary to readjust the pH value of the glue.。
第35卷第5期内蒙古民族大学学报(自然科学版)Vol.35No.5 2020年9月Journal of Inner Mongolia University for Nationalities Sept.2020DOI:10.14045/ki.15-1220.2020.05.008雷公藤甲素普朗尼克混合载药胶束的制备桂荣,曹瑞珍,金玲玲,尹美珍(内蒙古民族大学医学院,内蒙古通辽028000)[摘要]目的:采用薄膜水化法制备包载雷公藤甲素的普朗尼克F127/F68混合胶束.方法:对制备方法中的可变因素,以及水化温度与药物泄漏相关性进行考察,在考虑混合胶束稳定的前提下优化制备工艺,提高载药量和包封率.结果:载药胶束的载药量和包封率与投药量、加水量以及水化温度均有关,当加水量达到一定时与普朗尼克F127和F68的质量比无关,并且水化温度越高越易导致胶束提早释药.按照载体材料F127/F68(1:1)200mg,加水量20mL,水化温度50℃的制备工艺制备载药胶束,可得到载药量和包封率分别为3.47%和90.09%.结论:制备的雷公藤甲素普朗尼克混合载药胶束的制备方法简单,虽然载药量较低,但适合作为低剂量抗癌药物雷公藤甲素的载体.实验结果将为进一步研究雷公藤甲素的多功能载体打下基础.[关键词]雷公藤甲素;普朗尼克;聚合物胶束;载药量[中图分类号]R944.9[文献标识码]A[文章编号]1671-0185(2020)05-0408-05Preparation of Triptolide-loaded Pluronic Mixed MicellesGuirong,CAO Rui-zhen,JIN Ling-ling,YIN Mei-zhen(School of Medicine,Inner Mongolia University for Nationalities,Tongliao028000,China)Abstract:Objective:To prepare Pluronic F127/F68mixed micelles containing triptolide(TPL)by the film-hy-dration method.Methods:The variable factors in the preparation method and the correlation between hydrationtemperature and drug leakage were investigated.Under the premise of considering the stability of the mixed mi-celles,the preparation process was optimized to improve drug-loading amount and encapsulation rate.Results:The drug loading amount and encapsulation rate were related to the dosage of TPL,water quantity and hydrationtemperature,but not to the mass ratio of Pluronic F127and F68when water quantity reaches a certain level,and the higher the hydration temperature is,the more likely the micelle was to release early.According to theoptimized preparation process:carrier material F127/F68(1:1)was200mg,water volume was20mL,hydra-tion temperature was50℃,to prepare drug-loaded micelles,the drug-loaded capacity and encapsulation ratewere3.47%and90.09%respectively.Conclusion:The preparation method of triptolide-loaded Pluronic mixedmicelles is simple.Although the drug loading is low,it is suitable as a carrier for the low-dose anticancer drugtriptolide.The experimental results will lay the foundation for further research on the multifunctional carrier oftriptolide.Key words:Triptolide;Pluronic;Polymeric micelles;Drug loading amount聚合物胶束是由两亲性嵌段共聚物分子在水溶液中自组装成的具有亲水性外壳和疏水性内核的胶体分散系,不仅能增溶药物,还可以延长药物在体内的循环时间,提高生物利用度,并且可以依赖于肿瘤组织的高通透性和滞留效应(enhanced permeability and retention effect,EPR)[1-4],实现药物向肿瘤部位基金项目:内蒙古自治区自然科学基金项目(2020MS08026);内蒙古自治区布鲁氏菌病防治工程技术研究中心开放课题(MDK2018053);内蒙古民族大学科研项目(BS427);内蒙古民族大学研究生科研项目(NMDSS1946)作者简介:桂荣,内蒙古民族大学硕士研究生.通讯作者:尹美珍,内蒙古民族大学医学院教授,博士,硕士研究生导师.第5期桂荣等:雷公藤甲素普朗尼克混合载药胶束的制备409的被动靶向,从而降低药物的全身毒性.近年来,出现了大量有关聚合物胶束的研究报道.普朗尼克是一种结构简单、来源方便、成本低的两亲性三嵌段共聚物,由聚氧乙烯(PEO)、聚氧丙烯(PPO)组成,PPO和PEO嵌段比例不同构成多种不同型号的普朗尼克.普朗尼克在水溶液中自组装形成“核-壳”结构的纳米聚合物胶束.普朗尼克两端PEO嵌段的羟基,很容易对其进行化学改造[5,6],实现药物向肿瘤细胞的主动靶向.此外,普朗尼克还具有多种途径的逆转肿瘤耐药性作用[6].因而,普朗尼克作为载体材料得到了广泛关注.研究报道了多种单一普朗尼克F127、P85、P105载药胶束[7-9],以及双普朗尼克F127/P105、F127/ P1123、F127/L61载药胶束[10-12],用于靶向递送阿霉素、紫杉醇、甲氨蝶呤、多西他赛等多种化疗药物.研究发现两种不同普朗尼克的亲水和疏水嵌段之间相互作用,可增加递药系统的稳定性,不受血液稀释的影响[13],可以解决单一普朗尼克胶束因在血液大量稀释后药物提前释放的问题.雷公藤甲素(Triptolide,TPL)是从传统中药雷公藤中分离的一种双萜类化合物,具有抗风湿、抗老年痴呆、抗炎症、抗氧化、抗肿瘤,免疫调节、保护神经元和心血管等多种药理作用,其抗肿瘤作用倍受人们关注.研究证明TPL的抗肿瘤活性相比传统化疗药物顺铂、丝裂霉素和紫杉醇更强[14].但同时对消化、泌尿和血液系统等也表现出较大的毒副作用,因而限制了其在临床上的应用.普朗尼克F127和F68作为多功能药用辅料被收载于中国药典和美国药典中,具有良好的生物相容性和安全性.因此,本实验选择普朗尼克F127和F68作为载体材料,采用薄膜水化法制备双普朗尼克胶束,装载疏水性的雷公藤甲素,并研究其载药量和包封率,为雷公藤甲素的靶向递送提供合适的载体,并为进一步多功能载体研究打下基础.1材料与方法1.1材料和试剂普朗尼克F127、F68(BASF,Ludwigshsfen,Germany),雷公藤甲素(Triptolide,纯度98.5%,Aladdin),雷公藤甲素对照品(中国药品生物制品检定所).1.2F127/F68-TPL混合载药胶束的制备采用薄膜水化法制备聚合物载药胶束.称取一定量的载体材料,即普朗尼克F127和F68,以及适量的疏水性药物雷公藤甲素(TPL),无水乙醇使其充分溶解,氮气流蒸发将有机溶剂蒸干,真空干燥过夜,得干燥透明的一层薄膜(药膜);然后加一定量的去离子水,在一定的水浴温度下,在300rpm转速下恒速搅拌30min,进行水化,冷却至室温,10000rpm离心10min,用无菌0.22μm醋酸纤维素滤膜过滤,得到透明的普朗尼克-雷公藤甲素聚合物胶束溶液,冷冻干燥可得到冻干粉.1.3载药量和包封率测定采用RP-HPLC法测定F127/F68-TPL中TPL的含量.(1)色谱条件:反相色谱柱(Agilent,C18,150 mm×4.6mm,5μm),流动相为甲醇:水=30∶70;流速0.8mL/min,检测波长213nm;柱温30℃,进样量20μl.(2)采用TPL对照品,建立标准曲线y=34.23x-54.88(R2=0.9994).TPL在0.1~50μg/mL浓度范围内,色谱峰面积与浓度呈良好的线性关系.该方法专属性良好,普朗尼克对TPL检测无干扰.(3)样品溶液的制备及测定.精密量取一定量载药胶束溶液,加一定量甲醇稀释,混匀,漩涡l min,10000rpm离心10min,取上清液,0.45μm微孔有机滤膜过滤,取滤液20μL注入色谱仪,测定主成分的峰面积.(4)根据如下公式计算载药量(Drug Loading,DL)和包封率(Encapsulation rate,ER).DL%=胶束中药物的重量/所加聚合物和药物的总重量×100%,ER%=胶束中药物的重量/所加药物的重量×100%.1.4单因素考察载体材料质量为200mg,以ER%及DL%为指标,对薄膜水化法中的可变因素进行考察.1.4.1混合载体F127和F68的质量比考察将TPL投药量固定为5mg,水化温度50℃,加水量分别为5mL,10mL和20mL,考察F127的质量分数为33.3%、50%、66.7%和100%时,对DL%和ER%的影响.1.4.2投药量考察内蒙古民族大学学报2020年F127和F68各为100mg,水化温度50℃,加水量10mL,考察TPL 投药量为3mg、5mg、7mg、8mg 和9mg 时,对DL%和ER%的的影响.1.4.3加水量考察F127和F68各为100mg,TPL 投药量为5mg,水化温度50℃,考察水化时用水量分别为5、10、15、20、25mL 时DL%和ER%的变化.1.4.4水化温度考察F127和F68各为100mg,TPL 投药量为5mg,用水量为10mL,考察水化温度分别是30℃、50℃、70℃、80℃和90℃时,对DL%和ER%的影响.1.5水化温度对胶束泄漏药物的影响将1.4.4制备好的载药胶束溶液恒温水浴振荡(37℃,100rpm )48h 后,再取样RP-HPLC 法测定载药胶束中TPL 的含量,计算TPL 的泄漏量即TPL 的沉淀百分数.根据公式计算TPL 的泄漏量.泄漏量(%)=(原胶束中药物的含量-震荡放置后胶束中药物的含量)/原胶束中药物的含量×100%1.6优化工艺验证通过单因素考察,以及水化温度对泄漏量影响的考察,综合考虑ER%值和DL%值,优化薄膜水化法的制备工艺,当载体量200mg,F127与F68的质量比为1,TPL 投药量8mg,加水量20mL,水化温度50℃时,DL%和ER%的值均较高.2结果2.1载药量和包封率的影响因素2.1.1载体质量分数对包封率和载药量的影响见图1a,图1b.当TPL 的投药量为5mg,水化温度50℃,加水量分别为5mL 时,随F127质量比的增大,DL%和ER%的值均逐渐增大;而当水量分别为10mL 和20mL 时,随F127质量比增大,DL%和ER%的值均没有明显变化.但是,随水量增多,相应的DL%和ER%值均增大.此结果说明加水量不足时,混合载体中F127的质量分数不同对胶束的载药量和药物的包封率有影响,而当水量达到一定程度时,则没有明显的影响.2.521.510.50载药量(%)F127的质量(mg)包封率(%)1009080706050403020100F127的质量(mg )a F127与F68的质量比对载药量的影响b F127与F68的质量比对包封率的影响图1F127与F68的质量比对载药量和包封率的影响Fig.1Influence of mass ratio of F127to F68on drug loading amount and encapsulation rate2.1.2投药量对包封率和载药量的影响见图2.当水量为10mL 时,随投药量的增加,载药量呈现明显先上升趋势,而包封率则呈现明显下降趋势.结果表明投药量对胶束的载药量以及药物的包封率影响较大,增加投药量可提高载药量,但可能会降低包封率.4102.1.3加水量对包封率和载药量的影响见图3.当加水量为20mL 以下时,随加水量的增加,载药量和包封率均逐渐升高,当加水量达到20mL 以上时,水量增加,载药量和包封率均逐渐降低.表明加水量对载药量和包封率有较大的影响,提高加水量可提高胶束的载药量以及药物的包封率,最佳水量为20mL.载药量(%)2.421.61.20.80.4投药量(mg )包封率(%)投药量(mg )水量(mL )载药量(%)2.521.510.50120100806040200包封率(%)图2投药量对载药量和包封率的影响图3加水量对载药量和包封率的影响Fig.2Influence of dosage on drug loadingFig.3Influence of water addition amount onand encapsulation ratedrug loading and encapsulation rate2.1.4水化温度对包封率和载药量的影响见图4.水化温度在30~80℃的温度范围内,随温度升高载药量和包封均增大,而温度达到80℃~90℃时,ER%值和DL%值又均降低.因此,水化温度对ER%值和DL%值又较大影响,提高水化温度可提高胶束的载药量以及药物的包封率,但水化温度越高,静置于室温后,吸附于疏水内核的药物分子可能越易泄漏.2.2水化温度对泄漏量的影响见图5.水化温度越高,药物在胶束上48h 内的泄漏量越高,尤其是水化温度达80℃以上,药物泄漏的百分比更大.12010080604020包封率(%)载药量(%)2.521.510.50水化温度(℃)泄漏量(%)20151050水化温度(℃)图4水化温度对载药量和包封率的影响图5水化温度对载药胶束泄漏量的影响Fig.4Influence of hydration temperature on drugFig.5Influence of hydration temperature onloading and encapsulation rateleakage rate of drug-loaded micelle2.3优化制备工艺F127与F68各100mg 时,采用薄膜水化法的优化工艺,TPL 投药量8mg,加水量20mL,水化温度50℃,制备得载药胶束F127/F68-TPL,其载药量为3.47%,TPL 的包封率为90.09%.此优化的制备工艺获得较高的胶束载药量和药物包封率.3讨论胶束的制备方法主要有透析法、薄膜水化法和乳化溶剂蒸发法[7].薄膜水化法是比较简单而方便的制第5期桂荣等:雷公藤甲素普朗尼克混合载药胶束的制备411内蒙古民族大学学报2020年412备方法,且该方法的应用最为广泛.本实验采用薄膜水化法制备了F127/F68-TPL混合载药胶束,通过单因素考察发现,当加水适量时,F68和F127的质量比对胶束的载药量和药物的包封率没有显著影响.由于混合普朗尼克可以避免大量血液稀释导致药物早泄[13],因而本实验选择F68和F127的质量比为1:1制备载药胶束.单因素考察结果还表明投药量、加水量以及水化温度对胶束的载药量和药物的包封率均有较大的影响,不同的制备工艺条件得到不同的载药量和包封率,尤其水化温度的提高对载药量和包封率的提高更为明显.本实验中水化温度对泄漏量影响的实验结果表明:在50~90℃的水化温度中以50℃可以得到最稳定的载药胶束.因此,通过单因素分析,综合考虑载药量、包封率以及泄漏量,进行制备工艺的优化,提高载药胶束的载药量.在此基础上可以采用星点设计-效应面法最优化载药胶束的制备工艺,并通过验证进行确定.TPL的抗肿瘤活性较强,有研究证实其对多种肿瘤细胞具有较强的抑制作用,平均使用浓度为12nM[15].由于TPL的使用浓度极低,即使F127/F68-TPL的载药量最低为1%,也能远远满足制剂的使用要求.总之,本实验制备的F127/F68-TPL混合载药胶束,载药量虽低,在1.3%~3.5%之间,但对于使用剂量较低的药物,需要较低含量的制剂.此外,薄膜水化法制备方法简单易行,载体材料经济可得,制备的双普朗尼克载药胶束稳定.因此,普朗尼克F127/F68为载体材料的混合胶束可以作为TPL的靶向递送载体.参考文献[1]伍善广,杨帆,吴和谋,等.药物胶束嵌段共聚合物的研究进展[J].现代食品与药品杂志,2007,17(2):14-17.[2]曾昭武,肖人钟,王小丽,等.聚乙二醇-聚乳酸嵌段共聚物纳米粒的研究进展[J].现代生物医学进展,2012,12(1):179-182.[3]王香梅,张晓丽,陈韩根.两亲聚合物胶束自组装包药研究进展[J].高分子通报,2013,10(7):27-31.[4]Stapleton S,Jaffray D,Milosevic M.Radiation effects on the tumor microenvironment:Implications for nanomedicine delivery[J].Advanced drug delivery reviews,2017,109:119-130.[5]Song H,He R,Wang K,et al.Anti-HIF-1alpha antibody-conjugated pluronic triblock copolymers encapsulated with Pa-clitaxel for tumor targeting therapy[J].Biomaterials,2010,31(8):2302-2312.[6]李伟男,孙佳琳,管庆霞,等.逆转肿瘤多药耐药性的普朗尼克靶向药物传递系统[J].中国药学杂志,2016,51(15):1270-1273.[7]雷丸,唐鹏,王莹,等.九节龙皂苷Ⅰ聚合物载药胶束的制备及体外表征[J].西北药学杂志,2017,32(1):76-80.[8]王永中,方晓玲,李雅娟,等.紫杉醇Pluronic P105聚合物胶束的制备、表征与逆转肿瘤多药耐药性的体外研究[J].药学学报,2008,43(6):640-646.[9]Nguyen DH,Lee JS,Bae JW,et al.Targeted doxorubicin nanotherapy strongly suppressing growth of multidrug resis-tant tumor in mice[J].Int J Pharm,2015,495(1):329-335.[10]Chen Y,Zhang W,Huang Y,et al.Pluronic-based functional polymeric mixed micelles for co-delivery of doxorubi-cin and paclitaxel to multidrug resistant tumor[J].International Journal of Pharmaceutics,2015,488(1-2):44-58.[11]Zhang W,Shi Y,Chen YZ,et al.Multifunctional Pluronic P123/F127mixed polymeric micelles loaded with pacli-taxel for the treatment of multidrug resistant tumors[J].Biomaterials,2011,32(11):2894-2906.[12]Oerlemans C,Bult W,Bos M,et al.Polymeric Micelles in Anticancer Therapy:Targeting,Imaging and Triggered Re-lease[J].Pharm Res,2010,27(12):2569-2589.[13]Hong W,Chen D,Zhang X,et al.Reversing multidrug resistance by intracellular delivery of Pluronic P85unimers [J].Biomaterials,2013,34(37):9602-9614.[14]Yang S,Chen J,Guo Z,et al.Triptolide inhibits the growth and metastasis of solid tumors[J].Mol Cancer Ther,2003,2(1):65-72.[15]Alsaied OA,Sangwan V,Banerjee S,et al.Sorafenib and triptolide as combination therapy for hepatocellular carcino-ma[J].Surgery,2014,156(2):270-279.[责任编辑赵贤芳]。
Supporting InformationScope of the aminoethylation Suzuki-Miyaura reactionusing organotrifluoroboratesGary A. Molander* and Ludivine Jean-GérardRoy and Diana Vagelos Laboratories, Department of Chemistry, University of Pennsylvania,Philadelphia, Pennsylvania 19104-6323Table of ContentsGeneral Considerations........................................................................................................S2 General Method for the Preparation of Potassium β-Aminoethyltrifluoroborates................S2 Representative procedure for the cross-coupling reaction of Potassiumβ-Aminoethyltrifluoroborates and aryl electrophiles............................................................S3 Method A............................................................................................................................S3 Method B............................................................................................................................S4 References.........................................................................................................................S11NMR Spectra....................................................................................................................S12General considerations: N -Boc Vinyl carbamate and N -Cbz Vinyl carbamate were prepared according to procedures described in the literature.1 PdCl 2(dppf)•CH 2Cl 2, Pd(OAc)2, RuPhos (2-dicyclohexylphosphino-2’,6’-diisopropoxybiphenyl) were used as received. THF was distilled from Na/benzophenone ketyl and toluene from Na. H 2O and toluene were degassed prior to use. Standard benchtop techniques were employed for handling air-sensitive reagents. Melting points (ºC) were determined using a Thomas-Hoover melting point apparatus and are uncorrected. 1H, 13C, 11B and 19F NMR spectra were recorded at 500, 125.8, 128.4 and 470.8 MHz, respectively. Analytical thin-layer chromatography (TLC) was performed on silica gel aluminum plates precoated with a fluorescent indicator. Visualization was effected with ultraviolet light or vanillin. Standard flash chromatography procedures were performed using 32-63 µm silica gel.GeneralMethod for the Preparation of Potassium β-Aminoethyltrifluoroborates.Potassium 2-(benzyloxycarbonylamino)ethyltrifluoroborate (1a).A solution of 2,5-dimethyl-2,4-hexadiene (9.58 g, 86.9 mmol) in THF (12.4 mL) was treated with BH 3•THF (40 mL, 40 mmol) at 0 ºC. The mixture was stirred for 3.5 h at 0 ºC and treated with a solution of N -Cbz vinylcarbamate (2.8 g, 15.8 mmol) in THF (9.2 mL) while maintaining the temperature at 0 ºC. The reaction mixture was allowed to warm to rt, stirred for 2 h, cooled in an ice bath, and H 2O (5 mL) was carefully added. After an additional 1.5 h at rt, a 37% solution of CH 2O (2 mL) was added. The mixture was stirred overnight, quenched with brine, and the resulting mixture was extracted with EtOAc (80 mL). The organic layers were combined, dried (MgSO 4) and then filtered. The solvent was removed under vacuum, and to the residue was added KHF 2 (4.94 g, 63.2 mmol), acetone (53 mL) and H 2O (21 mL). The mixture was stirred for 4 h at rt, evaporated, and the resulting white solid was dried under high vacuum overnight. The crude product was then extracted with hot acetoneKF 3BNHCbz(150 mL). The insoluble salts were filtered off, and the filtrate was concentrated in vacuo . The crude compound was purified by dissolving in a minimum of hot acetone (~ 150 mL) and precipitating in Et 2O (~ 50mL) to obtain the title compound as a white solid in 71% yield (3.2 g, 11.2 mmol). mp = > 195 ºC; 1H-NMR (500 MHz, DMSO-d6) δ 7.34-7.25 (m, 5H), 6.36 (br s, 1H), 4.93 (s, 2H), 2.90-2.86 (m, 2H), 0.16-0.13 (m, 2H); 13C-NMR (125.8 MHz, DMSO) δ 156.1, 137.9, 128.4, 127.7 (2C), 65.3, 39.1; 11B-NMR (128.4 MHz, DMSO-d6) δ 4.17 (br s); 19F-NMR (470.8 MHz, DMSO -d6) δ -136.9 (m); IR (KBr) 3411, 2975, 2360, 1686, 1522, 1272 cm -1; HRMS (ES-) m/z calcd. for C 10H 12BF 3NO 2 (M-K +) 246.0913, found 246.0923.Potassium 2-(tert -butoxycarbonylamino)ethyltrifluoroborate (1b).This compound was obtained by the general method for the preparation of potassium β-aminoethyltrifluoroborates using N -Boc vinylcarbamate (1.14 g, 8.0 mmol) as the starting material. The title compound was obtained as a white solid in 60% yield. mp = > 185 ºC; 1H-NMR (500 MHz, DMSO-d6) δ 5.79 (s, 1H), 2.85-2.81 (m, 2H), 1.34 (s, 9H), 0.14-0.12 (m, 2H); 13C-NMR (125.8 MHz, DMSO-d6) δ 155.1, 76.6, 38.5, 28.3; 11B-NMR (128.4 MHz, DMSO-d6) δ 3.93 (br s); 19F-NMR (470.8 MHz, DMSO-d6) δ -136.6 (m); IR (KBr) 3411, 2975, 2360, 1676, 1522, 1364, 1034 cm -1; HRMS (ES -) m/z calcd. for C 7H 14BF 3NO 2 (M-K +) 212.1069, found 212.1078.Representative procedure for the Suzuki-Miyaura cross-coupling reaction of potassium β-aminoethyltrifluoroborates and aryl electrophiles:METHOD ABenzyl 4-cyanophenethylcarbamate (3a). To a mixture of potassium β-aminoethyltrifluoroborate (1a) (69.1 mg, 0.242 mmol), 4-bromobenzonitrileKF 3B NHBoc(43.7 mg, 0.240 mmol), Cs 2CO 3 (234.6 mg, 0.72 mmol), and PdCl 2(dppf)•CH 2Cl 2 (9.8 mg, 0.012 mmol) under nitrogen was added toluene/H 2O (3:1, 1.5 mL). The reaction was heated at 80 ºC with stirring under a nitrogen atmosphere in a sealed tube for 12 h and then cooled to rt. A saturated aqueous solution of NH 4Cl (4 mL) was added, and the resulting mixture was extracted with CH 2Cl 2 (3 × 5 mL). The organic layer was dried (MgSO 4) and then filtered. The solvent was removed in vacuo and the crude product was purified by silica gel chromatography (elution with EtOAc/hexane 3:7) to afford the product as a white solid in 75% yield (47.7 mg, 0.17 mmol). mp = 81-83 ºC; 1H-NMR (500 MHz, CDCl 3) δ 7.56 (d, 2H, J = 7.9 Hz), 7.37-7.31 (m, 5H), 7.27 (d, 2H, J = 7.7 Hz), 5.08 (s, 2H), 4.78 (br s, 1H), 3.48-3.44 (m, 2H), 2.88 (app t, 2H, J = 6.6 Hz); 13C-NMR (125.8 MHz, CDCl 3) δ 156.1, 144.3, 136.3, 132.3, 129.5, 128.5, 128.2, 128.1, 118.7, 110.4, 66.7, 41.6, 36.2; IR (KBr) 3341, 2938, 2359, 2227, 1703, 1529, 1248, 1136, 823 cm -1; HRMS (CI+) m/z calcd. for C 17H 17N 2O 2 (M+H +) 281.1290, found 281.1284.METHOD BBenzyl 2,4-dimethoxyphenethylcarbamate (5a). To a mixture of potassium β-aminoethyltrifluoroborate (1a ) (69.1 mg, 0.242 mmol), 1-bromo-2,4-dimethoxybenzene (46.0 mg, 0.24 mmol), Cs 2CO 3 (234.6 mg, 0.72 mmol), Pd(OAc)2 (2.7 mg, 0.012 mmol), and RuPhos (11.2 mg, 0.024 mmol) under nitrogen was added toluene/H 2O (3:1, 1.5 mL). The reaction was heated at 95 ºC with stirring under a nitrogen atmosphere in a sealed tube for 12 h, and then cooled to rt. A saturated aqueous solution of NH 4Cl (4 mL) was added and the resulting mixture was extracted with CH 2Cl 2 (3 × 5 mL). The organic layer was dried (MgSO 4) and then filtered. The solvent was removed in vacuo and the crude product was purified by silica gel chromatography (elution with EtOAc/hexane 3:7) to afford the product as a white solid in 79% yield (59.5 mg, 0.19 mmol). mp = 91-92 ºC; 1H-NMR (500 MHz, CDCl 3) δ 7.35-7.27 (m, 5H), 6.99 (d, 1H, J = 8.1 Hz), 6.42-6.39 (m, 2H), 5.07 (s, 2H), 4.88 (br s, 1H), 3.77 (s, 6H), 3.40-3.36 (m, 2H), 2.75 (app t, O O2H, J = 6.7 Hz); 13C-NMR (125.8 MHz, CDCl 3) δ 159.8, 158.5, 156.5, 136.9, 130.9, 128.5, 128.11, 128.07, 119.6, 104.1, 98.7, 66.5, 55.4, 55.3, 41.4, 30.1; IR (KBr) 3341, 1697, 1546, 1465, 1260, 1133 cm -1; HRMS (ES+) m/z calcd. for C 18H 21NO 4Na + (M+Na +) 338.1368, found 338.1369.Benzyl 4-formylphenethylcarbamate (3b). This product was prepared from 4-bromobenzaldehyde (44.4 mg, 0.24 mmol) according to Method A to afford the desired compound as a white solid in 87% yield (59.1 mg, 0.21 mmol). mp = 74-76 ºC; 1H-NMR (500 MHz, CDCl 3) δ 9.97 (s, 1H), 7.80 (d, 2H, J = 7.8 Hz), 7.37-7.33 (m, 7H), 5.09 (s, 2H), 4.81 (br s, 1H), 3.50-3.47 (m, 2H), 2,91 (app t, 2H, J = 6.6 Hz); 13C-NMR (125.8 MHz, CDCl 3) δ 192.0, 156.4, 146.2, 136.5, 135.1, 130.2, 129.6, 128.7, 128.3, 128.4, 66.9, 41.9, 36.5; IR (KBr) 3313, 2948, 1721, 1681, 1533, 1267, 1241 cm -1; HRMS (CI+) m/z calcd. for C 17H 18NO 3 (M+H +) 284.1287, found 284.1286.Benzyl 4-nitrophenethylcarbamate (3c). This product was prepared from 1-bromo-4-nitrobenzene (48.6 mg, 0.240 mmol) according to Method A to afford the desired compound as a white solid in 87% yield (59.1 mg, 0.21 mmol). The spectroscopic data corresponds to that reported in the literature.2Benzyl 3-acetylphenethylcarbamate (3d). This product was prepared from3-bromoacetophenone (47.8 mg, 0.240 mmol) according to Method A toafford the desired compound as a colorless oil in 91% yield (64.9 mg, 0.22 mmol). 1H-NMR (500 MHz, CDCl 3) δ 7.80-7.77 (m, 2H), 7.38-7.26 (m, 7H), 5.07 (s, 2H), 4.96 (br s, 1H), 3.46 (m, 2H), 2.87 (app t, 2H, J = 6.6 Hz), 2.56 (s, 3H); 13C-NMR (125.8 MHz, CDCl 3) δ 198.2, 156.4, 139.4, 137.4, 136.5, 133.6, 128.8, 128.5, 128.2, 128.1, 126.7, 66.6, 42.1, 36.0, 26.6; IR (neat) 3339, 2933, 1706, 1682, 1530,O 21248 cm -1; HRMS (CI+) m/z calcd. for C 18H 20NO 3 (M+H +) 298.1443, found 298.1434.Benzyl 2-cyanophenethylcarbamate (3e). This product was prepared from 2-bromobenzonitrile (43.7 mg, 0.240 mmol) according to Method A to afford the desired compound as a colorless oil in 70% yield (46.8 mg, 0.17 mmol). 1H-NMR (500 MHz, CDCl 3) δ7.60 (m, 1H), 7.50 (m, 1H), 7.36-7.25 (m, 7H), 5.08 (s, 2H), 4.94 (br s, 1H), 3.53-3.49 (m, 2H), 3.05 (app t, 2H, J = 6.8 Hz); 13C-NMR (125.8 MHz, CDCl 3) δ 156.3, 142.8, 136.6, 133.0, 132.98, 130.2, 128.6, 128.19, 128.17, 127.2, 118.0, 112.9, 66.8, 41.5, 34.9; IR (neat) 3340, 2937, 2224, 1705, 1528, 1248 cm -1; HRMS (ES+) m/z calcd. for C 17H 16N 2O 2Na + (M+Na +) 303.1109, found 303.1122.Benzyl 4-(methoxycarbonyl)phenethylcarbamate (3f). This product was prepared from methyl 4-bromobenzoate (51.6 mg, 0.240 mmol) according to Method A to afford the desired compound as a white solid in 86% yield (64.6 mg, 0.205 mmol). mp = 96-98 ºC; 1H-NMR (500 MHz, CDCl 3) δ 7.96 (d, 2H, J = 8.1 Hz), 7.36-7.23 (m, 7H), 5.08 (s, 2H), 4.86 (br s, 1H), 3.88 (s, 3H), 3.45 (m, 2H), 2.85 (app t, 2H, J = 6.5 Hz); 13C-NMR (125.8 MHz, CDCl 3) δ 167.0, 156.4, 144.3, 136.6, 130.0, 128.9, 128.6, 128.22, 128.18, 66.8, 52.1, 42.0, 36.2; IR (KBr) 3363, 2943, 1709,1719, 1534,1285, 1250 cm -1; HRMS (CI+) m/z calcd. for C 18H 20NO 4 (M+H +) 314.1392, found 314.1384.Benzyl 4-acetylphenethylcarbamate (3g). This product was prepared from 4-bromoacetophenone (47.8 mg, 0.240 mmol) according to Method A to afford the desired compound as a white solid in 89% yield (63.6 mg, 0.214 mmol). The spectroscopic data corresponds to that reported in the literature.1MeO 21-bromo-4-chlorobenzene (46.0 mg, 0.240 mmol) according to Method A to afford the desired compound as a white solid in 70% yield (48.7 mg, 0.168 mmol). mp = 76-78 ºC; 1H-NMR (500 MHz, CDCl 3) δ 7.36-7.30 (m, 5H), 7.24 (m, 2H), 7.08 (d, 2H, J = 7.8 Hz), 5.07 (s, 2H),4.81 (br s, 1H), 3.43-3.39 (m, 2H), 2.76 (app t, 2H, J = 6.7 Hz); 13C-NMR (125.8 MHz, CDCl 3) δ 156.4, 137.2, 136.6, 132.4, 130.2, 128.8, 128.6, 128.23, 128.18, 66.8, 42.1, 35.5; IR (KBr) 3443, 3053, 1720, 1513, 1264, 715 cm -1; HRMS (CI+) m/z calcd. for C 16H 16NO 2Cl (M+H +) 290.0948, found 290.0936.Benzyl 4-(trifluoromethyl)phenethylcarbamate (3i). This product was prepared from 4-bromobenzotrifluoride (54.0 mg, 0.240 mmol) according to Method A to afford the desired compound as a white solid in 88% yield (67.9 mg, 0.21 mmol). The spectroscopic data corresponds to that reported in the literature.1Benzyl 4-methylphenethylcarbamate (5b). This product was prepared from4-bromotoluene (41.1 mg, 0.240 mmol) according to Method B to afford thedesired compound as a white solid in 75% yield (48.2 mg, 0.18 mmol). The spectroscopic data corresponds to that reported in the literature.1Benzyl phenethylcarbamate (5c). This product was prepared frombromobenzene (37.7 mg, 0.240 mmol) according to Method B to afford the desiredcompound as a white solid in 86% yield (52.0 mg, 0.21 mmol). The spectroscopic data corresponds to that reported in the literature.1F 3from p -bromoanisole (44.9 mg, 0.240 mmol) according to Method B to afford the desired compound as a white solid in 80% yield (54.8 mg, 0.206 mmol). The spectroscopic data corresponds to that reported in the literature.3 mp = 85-87 ºC.Benzyl 2-methylphenethylcarbamate (5e). This product was prepared from 2-bromotoluene (41.1 mg, 0.240 mmol) according to Method B to afford the desiredcompound as a colorless oil in 82% yield (53.0 mg, 0.197 mmol). 1H-NMR (500 MHz, CDCl 3) δ 7.33-7.28 (m, 5H), 7.12-7.10 (m, 4 H), 5.09 (s, 2H), 4.86 (br s, 1H), 3.41-3.38 (m, 2H), 2.81(app t, 2H, J =7.1 Hz), 2.3 (s, 3H); 13C-NMR (125.8 MHz, CDCl 3) δ 156.4, 136.9, 136.7, 136.4, 130.5, 129.4, 128.6, 128.2, 126.7, 126.2, 66.7, 41.2, 33.6, 19.3; IR (neat) 3335, 2946, 1702, 1526, 1250 cm -1; HRMS (ES+) m/z calcd. for C 17H 19NO 2Na + (M+Na +) 292.1314, found 292.1326.Benzyl 3-methoxyphenethylcarbamate (5f). This product was preparedfrom m -bromoanisole (44.9 mg, 0.240 mmol) according to Method B to affordthe desired compound as a colorless oil in 86% yield (58.6 mg, 0.206 mmol). 1H-NMR (500 MHz, CDCl 3) δ 7.35-7.30 (m, 5H), 7.23-7.18 (m, 1H), 6.76-6.71 (m, 3H), 5.08 (s, 2H), 4.82 (br s, 1H), 3.76 (s, 3H), 3.46-3.42 (m, 2H), 2.77 (app t, 2H, J = 6.7 Hz); 13C-NMR (125.8 MHz, CDCl 3) δ 159.9, 156.4, 140.4, 136.6, 129.7, 128.62, 128.61, 128.2, 121.2, 114.6, 112.0, 66.7, 55.2, 42.2, 36.2; IR (neat) 3336, 2939, 1708, 1526, 1489, 1260 cm -1; HRMS (ES+) m/z calcd. for C 17H 19NO 3Na + (M+Na +) 308.1262, found 308.1274.Benzyl 4-acetamidophenethylcarbamate (5g). This product was prepared from 4-bromoacetanilide (51.4 mg, 0.240 mmol) according toOOMethod B to afford the desired compound as a white solid in 46% yield (34.5 mg, 0.11 mmol). mp = 148-150 ºC; 1H-NMR (500 MHz, CDCl 3) δ 7.67 (br s, 1H), 7.40 (d, 2H, J = 8.3 Hz), 7.34-7.30 (m, 5H), 7.08 (d, 2H, J = 8.1 Hz), 5.08 (s, 2H), 4.88 (br s, 1H), 3.42-3.38 (m, 2H), 2.75 (app t, 2H, J = 6.7 Hz), 2.12 (s, 3H); 13C-NMR (125.8 MHz, CDCl 3) δ 168.6, 156.5, 136.62, 136.58, 134.7, 129.3, 128.6, 128.2, 128.1, 120.4, 66.7, 42.3, 35.6, 24.5; IR (KBr) 3312, 3035, 2944, 2869, 1685, 1663, 1530, 1257 cm -1; HRMS (ES+) m/z calcd. for C 18H 20N 2O 3Na + (M+Na +) 335.1372, found 335.1385.Benzyl 2,4,6-trimethylphenethylcarbamate (5h). This product was preparedfrom 2-bromomesitylene (47.8 mg, 0.240 mmol) according to Method B tpafford the desired compound as a white solid in 86% yield (61.1 mg, 0.205 mmol). mp = 91-93 ºC; 1H-NMR (500 MHz, CDCl 3) δ 7.35-7.29 (m, 5H), 6.83 (s, 2H), 5.11 (s, 2H), 4.88 (bs, 1H), 3.30-3.26 (m, 2H), 2.84 (app t, 2H, J = 7.4 Hz), 2.30 (s, 6H), 2.24 (s, 3H); 13C-NMR (125.8 MHz, CDCl 3) δ 156.5, 136.7, 135.8, 132.2, 129.2, 128.6, 128.2, 66.7, 40.3, 30.0, 20.9, 19.9; IR (KBr) 3329, 2954, 1689, 1542, 1256 cm -1; HRMS (ES+) m/z calcd. for C 19H 23NO 2Na + (M+Na +) 320.1626, found 320.1634.Benzyl 2-(isoquinolin-4-yl)ethylcarbamate (7a). This product was preparedfrom 4-bromoisoquinoline (49.9 mg, 0.240 mmol) according to Method B to afford the desired compound as a white solid in 84% yield (61.8 mg, 0.202 mmol). mp = 105-107 ºC; 1H-NMR (500 MHz, CDCl 3) δ 9.03 (s, 1H), 8.31 (s, 1H), 8.07 (d, 1H, J = 8.4 Hz), 7.91 (d, 1H, J = 8.3 Hz), 7.71 (m, 1H), 7.58 (app t, 1H, J = 7.5 Hz), 7.33-7.30 (m, 5H), 5.36 (br s, 1H), 5.11 (s, 2H), 3.55-3.51 (m, 2H), 3.22 (app t, 2H, J = 7.0 Hz); 13C-NMR (125.8 MHz, CDCl 3) δ 156.6, 151.8, 143.2, 136.6, 134.8, 130.6, 128.6, 128.5, 128.4, 128.2, 128.1, 127.1, 122.8, 66.7, 41.7, 30.5; IR (KBr) 3201, 3022, 1714, 1544, 1260 cm -1; HRMS (CI+) m/z calcd. for C 19H 19N 2O 2 (M+H +) 307.1446, found 307.1433.Benzyl 2-(pyridin-3-yl)ethylcarbamate (7b). This product was prepared from 3-bromopyridine (37.9 mg, 0.240 mmol) according to Method B to afford the desired compound as a colorless oil in 81% yield (49.7 mg, 0.194 mmol). 1H-NMR (500 MHz, CDCl 3) δ 8.44-8.41 (m, 2H), 7.49 (br d, 1H, J = 7.2 Hz), 7.34-7.30 (m, 5H), 7.21-7.18 (m, 1H), 5.19 (br s, 1H), 5.08 (s, 2H), 3.44-3.42 (m, 2H), 2.81 (app t, 2H, J = 6.8 Hz); 13C-NMR (125.8 MHz, CDCl 3) δ 156.4, 150.1, 148.0, 136.5, 136.3, 134.3, 128.6, 128.2, 128.1, 123.5, 66.7, 41.9, 33.4; IR (neat) 3326, 3032, 2934, 1707, 1534, 1260 cm -1; HRMS (CI+) m/z calcd. for C 15H 17N 2O 2 (M+H +) 257.1290, found 257.1299.Benzyl 2-(pyrimidin-5-yl)ethylcarbamate (7c). This product was prepared from 5-bromopyrimidine (38.2 mg, 0.240 mmol) according to Method B to afford the desired compound as a colorless oil in 80% yield (49.4 mg, 0.192 mmol). 1H-NMR (500 MHz, CDCl 3) δ 9.05 (s, 1H), 8.56 (s, 2H), 7.35-7.31 (m, 5H), 5.31 (br s, 1H), 5.08 (s, 2H), 3.43 (m, 2H), 2.81 (m, 2H); 13C-NMR (125.8 MHz, CDCl 3) δ 157.2, 157.1, 156.4, 136.3, 132.2, 128.6, 128.3, 128.1, 66.9, 41.5, 30.9; IR (neat) 3322, 3033, 2937, 1713, 1562, 1410, 1255 cm -1; HRMS (CI+) m/z calcd. for C 14H 16N 3O 2 (M+H +) 258.1242, found 258.1231.Benzyl 2-(thiophen-2-yl)ethylcarbamate (7d). This product was prepared from2-bromothiophene (39.1 mg, 0.240 mmol) according to Method B to afford thedesired compound as a white solid in 79% yield (49.6 mg, 0.19 mmol). mp = 41-43 ºC; 1H-NMR (500 MHz, CDCl 3) δ 7.34-7.30 (m, 5H), 7.14 (dd, 1H, J = 5.1, 1.0 Hz), 6.92 (dd, 1H, J = 5.1, 3.4 Hz), 6.81 (br d, 1H, J = 2.5 Hz), 5.09 (s, 2H), 4.91 (br s, 1H), 3.46 (m, 2H), 3.02 (app t, 2H, J = 6.5 Hz); 13C-NMR (125.8 MHz, CDCl 3) δ 156.3, 141.2, 136.6, 128.6, 128.2, 127.1, 125.5, 124.0, 66.8, 42.5, 30.4; IR (KBr) 3411, 2927, 1711, 1531, 1256 cm -1; HRMS (ES+) m/z calcd. for C 14H 15NO 2SNa + (M+Na +)SNHCbz284.0721, found 284.0729.Benzyl 2-(1-(tert -butoxycarbonyl)-1H-indol-5-yl)ethylcarbamate (7e). This product was prepared from tert -butyl 5-bromo-1H -indole-1-carboxylate 4 (71.1 mg, 0.240 mmol) according to Method B to afford the desired compound as a yellow oil in 84% yield (79.4 mg, 0.202 mmol). 1H-NMR (500 MHz, CDCl 3) δ 8.05 (br d, 1H, J = 7.3 Hz),7.57 (d, 1H, J = 3.4 Hz), 7.34-7.29 (m, 6H), 7.12 (d, 1H, J = 8.3 Hz), 6.5 (d, 1H, J = 3.6 Hz), 5.08 (s, 2H), 4.79 (br s, 1H), 3.51-3.47 (m, 2H), 2.89 (app t, 2H, J = 6.7 Hz), 1.66 (s, 9H); 13C-NMR (125.8 MHz, CDCl 3) δ 156.4, 149.8, 136.7, 134.1, 133.0, 131.0, 128.6, 128.2, 126.3, 125.1, 120.9, 115.3, 107.1, 83.7, 66.7, 42.7, 36.0, 28.3; IR (neat) 3345, 2977, 2933, 1721, 1713, 1524, 1371, 1254 cm -1; HRMS (ES+) m/z calcd. for C 23H 26N 2O 4Na + (M+Na +) 417.1790, found 417.1808.tert-Butyl 4-formylphenethylcarbamate (9a). This product was prepared from 4-bromobenzaldehyde (44.4 mg, 0.240 mmol) and potassium 2-(tert -butoxycarbonylamino)ethyltrifluoroborate (61 mg, 0.242 mmol) according to Method A to afford the desired compound as a white solid in 79% yield (47.1 mg, 0.19 mmol). The spectroscopic data correspond to the commercially available compound.tert-Butyl 4-acetylphenethylcarbamate (9b). This product was preparedfrom 4-bromoacetophenone (47.8 mg, 0.240 mmol) and potassium 2-(tert -butoxycarbonylamino)ethyltrifluoroborate (1b ) (61 mg, 0.242 mmol) according to Method A to afford the desired compound as a white solid in 72% yield (45.3 mg, 0.172 mmol). mp = 70-72 ºC ; 1H-NMR (500 MHz, CDCl 3) δ 7.90 (d, 2H, J = 8.2 Hz), 7.29 (d, 2H, J = 8.1 Hz), 4.67 (br s, 1H), 3.40 (m, 2H),2.87 (app t, 2H, J = 6.9 Hz), 2.59 (s, 3H), 1.43 (s, 9H); 13C-NMR (125.8 MHz, CDCl 3) δ 197.8, 155.9,144.9, 135.6, 129.1, 128.7, 79.4, 41.5, 36.3, 28.4, 26.6; IR (KBr) 3364, 2977, 1704, 1682, 1509, 1268, 1169 cm -1; HRMS (ES+) m/z calcd. for C 15H 21NO 3Na + (M+Na +) 286.1419, found 286.1426.tert-Butyl 2,4-dimethoxyphenethylcarbamate (9c). This product was prepared from 1-bromo-2,4-dimethoxybenzene (46.0 mg, 0.240 mmol) and potassium 2-(tert -butoxycarbonylamino)ethyltrifluoroborate (1b ) (61 mg, 0.242 mmol) according to Method B to afford the desired compound as a white solid in 86% yield (57.7 mg, 0.205 mmol). mp = 67-69 ºC; 1H-NMR (500 MHz, CDCl 3) δ 7.02 (d, 1H, J = 8.0 Hz), 6.44-6.42 (m, 2H), 4.61 (br s, 1H), 3.80 (s, 6H), 3.31 (m, 2H), 2.73 (app t, 2H, J = 6.6 Hz), 1.43 (s, 9H); 13C-NMR (125.8 MHz, CDCl 3) δ 159.8, 158.6, 156.1, 130.9, 119.9, 104.1, 98.7, 79.0, 55.5, 55.4, 40.9, 30.2, 28.6; IR (KBr) 3366, 2979, 1690, 1533,1261, 1170 cm -1; HRMS (CI+) m/z calcd. for C 15H 23NO 4+ (M +) 281.1627, found 281.1616.tert-Butyl phenethylcarbamate (9d). This product was prepared fromphenyltrifluoromethanesulfonate (54.3 mg, 0.240 mmol) and potassium 2-(tert -butoxycarbonylamino)ethyltrifluoroborate (1b ) (61 mg, 0.242 mmol) according to Method B to afford the desired compound as a white solid in 78% yield (41.5 mg, 0.188 mmol). The spectroscopic data corresponds to that reported in the literature.5tert-Butyl 2-(pyrimidin-5-yl)ethylcarbamate (9e). This product was prepared from 5-bromopyrimidine (38.2 mg, 0.240 mmol) and potassium 2-(tert -butoxycarbonylamino)ethyltrifluoroborate (9e ) (61 mg, 0.242 mmol) according to Method B to afford the desired compound as a white solid in 83% yield (44.5 mg, 0.20 mmol). mp = 77-79 ºC; 1H-NMR (500 MHz, CDCl 3) δ 9.05 (s, 1H), 8.56 (s, 2H), 4.83 (br s, 1H), 3.37-3.33 (m, 2H), 2.79 (app t, 2H, J = O6.5 Hz), 1.37 (s, 9H); 13C-NMR (125.8 MHz, CDCl3) δ 157.3, 157.2, 155.9, 132.5, 79.8, 41.2, 31.1, 28.5; IR (KBr) 3365, 2980, 2937, 1692, 1530, 1273, 1172 cm-1; HRMS (CI+) m/z calcd. for C11H18N3O2 (M+H+) 224.1399, found 224.1389.References1 Kamatani, A.; Overman, L. E. J. Org. Chem. 1999, 64, 8743-8744.2 Hatori, K.; Sajiki, H.; Hirota, K. Tetrahedron2000, 56, 8433-8441.3 Salvatore, R. N.; Chu, F.; Nagle, A. S.; Kapxhiu, E. A.; Cross, R. M.; Jung, K. W. Tetrahedron2002, 58, 3329-3347.4 Witulski, B.; Buschmann, N.; Bergsträßer, U. Tetrahedron 2000, 56, 8473-8480.5 Caddick, S.; Haynes, A. K. de K.; Judd, D. B.; Williams, M. R. V. Tetrahedron Lett.2000, 41, 3513-3516.NHCbz K F3B 1aNHC bz K F3B 1aNHCbz K F3B 1aNHC bz K F3B 1aNHB o c K F3B 1bNHB o c K F3B 1bNHB o c K F3B 1bNHBo c K F3B 1bH C b zN3aH C b zN3azbCHb3HOzbCHb3HOHC b zO 23cHC b zO 23cHC b z A 3dHC b z A 3dH C b zC N 3eH C b zCN 3eH C bz M e O23fH C bz M e O23fH Cb zA3gH Cb zA3gHC b zC3hHC b zC3hHC b zF 33iH C b zF 33iH C b z OO5aH C b z OO5aHCbz 5bHCbz 5bzbCHc5zbCHc5HC b zO5dHC b zO5dH C b z5eH C b z5eH C b zO 5f。
化学试剂,2008,30(12),927~928;934(+)2邻氯苯甘氨酸甲酯盐酸盐的合成工艺研究熊维,申东升3,王国华(广东药学院药物合成研究所,广东广州 510006)摘要:以(+)2邻氯苯甘氨酸为原料,在氯化亚砜2甲醇溶液中直接反应成功的合成了标题化合物。
考察了滴加氯化亚砜时的温度、原料与氯化亚砜的物质的量比,滴加完氯化亚砜后的反应温度、反应时间等因素对酯化和成盐产率的影响,并对其进行了优化,确定了最佳反应条件:当滴加氯化亚砜的温度为-5~0℃,原料与氯化亚砜的物质的量比1∶2,滴加完氯化亚砜后的反应温度为50℃,反应时间为6h 时,总产率达到98%。
用IR 对产物的结构进行了表征。
关键词:(+)2邻氯苯甘氨酸;酯化;合成工艺中图分类号:O623.624 文献标识码:A 文章编号:025823283(2008)1220927202收稿日期:2008204202作者简介:熊维(19822),女,湖南常德人,硕士生,主要从事药物合成工艺改进的研究。
(+)2邻氯苯甘氨酸甲酯是一种非天然的具有生物活性的氨基酸酯,是合成氯吡格雷的一种重要中间体[1]。
由于氨基酸酯上的氨基易氧化变质、易聚合,稳定性较差,并且分子间生成氢键而具有较高的沸点,不易使用蒸馏等手段进行纯化。
(+)2邻氯苯甘氨酸甲酯的经典合成方法是用浓硫酸催化(+)2邻氯苯甘氨酸和甲醇酯化[2]。
硫酸催化氨基酸酯化时又分为氨基酸的直接酯化法,以及先保护氨基后酯化再去保护基的方法。
用硫酸作催化剂时,由于硫酸具有强氧化性和脱水性,常常三废量较多,甚至由于控温不好,局部温度较高而导致(+)2邻氯苯甘氨酸甲酯发生消旋,产物不易结晶,呈油状,对设备腐蚀严重。
考虑到(+)2邻氯苯甘氨酸甲酯的盐酸盐是固体并且容易结晶,干燥后可较长时间保存备用,所以我们设计用氯化亚砜作为成盐酸源和酯化催化剂[326],将(+)2邻氯苯甘氨酸与甲醇在酯化的同时又变为其甲酯盐酸盐,然后方便地进行重结晶提纯。
Preparation of polypyrrole/polyvinylalcohol (PPy/PV A)composite foam electrode materialXin Yi Wang a ,b ,Li Ping Heng b ,Nai Liang Yang b ,Qiang Xie a ,*,Jin Zhai b ,ca School of Chemical and Environmental Engineering,China University of Mining &Technology,Beijing 100083,Chinab Institute of Chemistry,Chinese Academy of Sciences,Beijing 100190,Chinac School of Chemistry and Environment,Beijing University of Aeronautics and Astronautics,Beijing 100081,ChinaReceived 23October 2009AbstractPolypyrrole/polyvinylalcohol (PPy/PV A)foam was prepared by direct foam polymerization in water and then it was coated on the indium-tin oxide transparent conductive glass (ITO)to form conventional three-electrode cell.FTIR and UV–vis spectra were adopted to characterize the molecular structure and the absorption spectra of foam material,respectively.The porous structure of PPy/PV A foams and their photoelectric conversion behaviors were studied.The dimension of the pores is bigger than 100m m in pared with the smooth film,the V oc and I sc of the foam film enhanced by 1.58-fold and 5.59-fold,respectively.#2010Qiang Xie.Published by Elsevier B.V .on behalf of Chinese Chemical Society.All rights reserved.Keywords:Conjugated polymers;Foams;Photoelectric conversion;PolymerizationThe conductive polymer (CP)performs not only the functions of metals,semiconductors and ferromagnets,but also has the activity of electrochemical oxidation–reduction and the flexibility of the mechanical properties and processability,which suggests that CP materials play an important role in the development of organic optoelectronic devices and electrochemical devices in the future [1–3].CP-based foams have many advantages,such as varied structure design,high chemical stability,and so on [4–7].Although the preparation of the foam polymer by conventional way needs a high-pressure vessel,a direct foam polymerization method was established and the foam materials utilizing conducting PANI were fabricated [8].So far,some representative conductive polymer electrode materials have been synthesized [9,10].PPy has been regarded as the material that takes on the prospect of industrial application,because it not only shares common characters with other conductive polymers,but also has good air stability and strong electrochemical reversibility [11–13].The composite membrane of PPy has attractive application prospects in wide fields [14–16].CP-based electrodes are well structured and have high surface area.Besides,its electrical conductivity can tune over a wide range [3,8,17–19].Up to present,PPy has been employed to PPy/TiO 2anode or solid electrolyte for dye-sensitized solar cells [20–22].Because of all those advantages mentioned above,PPy is promising in fabricating CP-based solar /locate/ccletAvailable online at Chinese Chemical Letters 21(2010)884–887*Corresponding author.E-mail address:dr-xieq@ (Q.Xie).1001-8417/$–see front matter #2010Qiang Xie.Published by Elsevier B.V .on behalf of Chinese Chemical Society.All rights reserved.doi:10.1016/let.2010.01.005Herein,we prepared conductive PPy/PV A composite foam film based on the method reported previously [8].Then the photoelectric conversion behavior was studied in details,finding that the improvement of photoelectric conversion efficiency is achieved.1.ExperimentalAt room temperature,3.5g PV A was dissolved into 45mL saturated ferric chloride (the oxidant)solution under mechanical stirring at 1000rpm for one hour [8].Then 600m L pyrrole was added slowly with microinjector and stirred for 8h.The smooth thin film electrode was prepared for comparison.The scanning electron microscopy (SEM)images were obtained using a JEOL JSM-6700F scanning electron microscope at 3.0kV .UV–vis spectrum was measured with a Hitachi U-3010UV–vis spectrometer.Fourier transform infrared spectrum (FTIR)was measured on a Bruker Tensor 27spectrophotometer.The photoelectric conversion behaviors of the films were measured by conventional three-electrode cell at AM 1.5simulated solar light (100mW/cm 2).2.Results and discussionsFrom Fig.1,the characteristic vibrations are the symmetry stretching vibration peak of pyrrole central at 1559cm À1and the asymmetric stretching vibration peak of pyrrole at 1426cm À1.PPy’s steady-state is the p-type doping conductive state at room temperature.The characteristic absorption peaks at 1207cm À1and 837cm À1are ascribed to the miscellaneous doped states of PPy [23,24].The UV–vis absorption spectrum of the foam film is shown in Fig.2.The band at 494nm is due to the p –p *absorption peak of long conjugated chain in polymer main chain,which also proves the presence of PPy [25].The morphology of foam film can be seen in Fig.3.The dimension of the pores is bigger than 100m m in diameter but the defects indicate the destruction of porous network structure.Fig.3(a)shows the surface of smooth film.Obviously,the foam wall forms a continuous network structure,which ensures the overall conductivity.In addition,the density and the porosity of the as-prepared PPy/PV A composite foam are 0.2847g/mL and 85.57%,respectively,being obtained with nitrogen adsorption experiments.This kind of foam will have the potential applications in the photovoltaic devices.Fig.4(a)shows the photocurrent generation at the foam film and the smooth film.It can be observed that the introduction of the foam will increase the photo-generated current density from 0.41m A/cm 2to 2.91m A/cm 2.As shown in Fig.4(b),a short-circuit current density (I sc )of 5.76m A/cm 2and an open-circuit voltage (V oc )0.054V for the foam film were obtained.As compared,an I sc and a V oc were 1.03m A/cm 2and 0.034V ,respectively for the smooth film.The V oc of the foam film was increased by 1.58-fold,and I sc by about 5.59-fold.This result indicates that the foam structures of PPy/PV A composite increase the photoelectric properties more efficiently than the PANI/PV A composite foam reported before [8].X.Y.Wang et al./Chinese Chemical Letters 21(2010)884–887885Fig.1.FTIR spectrum of the porous foam.The relationship between photocurrent and bias voltage of the foam is shown in Fig.4(c).It is 12.23m A/cm 2under a bias voltage of À180mV .Anodic photocurrent generation is achieved when the applied bias voltage is larger than 30mV;and it is À13.40m A/cm 2when the bias voltage is 180mV .This shows that when a negative bias voltage is applied,this electric field promotes the separation of the electron and hole and hence electron transfers in this pathway.On the contrary,when a positive bias voltage is applied,the electric field depresses the electron transfer from ITO electrode to electrolyte,and hence the cathodic photocurrent decreases.In this case,when the positive bias potential is higher than 30mV ,the electrons are transferred from electrolyte to ITO electrode through the film,which changes the photocurrent direction from cathodic to anodic.3.ConclusionsPPy can be foamed directly by means of polymerization of pyrrole in water using PV A foam as a template,which broadens the application scope of the foaming-polymerization method reported previously.Moreover,the porousX.Y.Wang et al./Chinese Chemical Letters 21(2010)884–887886Fig.2.UV/vis absorption spectra of the porousfoam.Fig.3.(a)and (b)top and section views of the prepared foam film,(c)top view of prepared smooth film,(d)optical photo of the preparedelectrode.Fig.4.Photoelectric conversion properties of PPy/PV A foams and smooth films:(a)Photocurrent generation of the films,(b)I–V characteristics of the foam and smooth films,(c)Photocurrent response of the foam film in applied electric fields.X.Y.Wang et al./Chinese Chemical Letters21(2010)884–887887 foamy PPy enhances the photoelectric conversion behavior of the porous materials by improving the light capture ability.It shows the evidence for using different conductive polymer to fabricating CP-based solar cells.AcknowledgmentsThis work was supported by the National Natural Science Foundation of China(No.20773142,50533030),the National Research Fund for Fundamental Key Project(No.2006CB806200,2006CB932100and2007CB936403)and 863project(No.2007AA032348and2008AA05Z308).References[1]A.Malinauskas,J.Power Sources126(2004)214.[2]P.Fernando,R.Jose,C.Cristina,Magn.Polym.232(1993)173.[3]Y.F.Li,Chin.Polym.Bull.4(2005)51.[4]Y.B.Wang,G.A.Sotzing,R.A.Weiss,Chem.Mater.15(2003)375.[5]M.C.Lonergan,E.J.Severin,B.J.Doleman,et al.Chem.Mater.8(1996)2298.[6]S.L.Shenoy,D.Cohen,C.Erkey,et al.Ind.Eng.Chem.Res.41(2002)1484.[7]X.B.Xu,Z.M.Li,L.Shi,et al.Small3(2007)408.[8]L.P.Heng,X.Y.Wang,J.Zhai,et al.Chem.Phys.Chem.9(2008)1559.[9]C.A.Furtado,P.P.Souza,Electrochem.Acta46(2001)1629.[10]T.Yamamoto,K.Sanechika,A.Yamamoto,J.Polym.Sci.Polym.Lett.Ed.18(1980)9.[11]A.G.MacDiarmid,Synth.Met.84(1997)27.[12]E.Kupila,J.Kankare,Synth.Met.74(1995)241.[13]S.Bereznev,J.Kois,I.Golovtsov,et al.Thin Solid Films511/512(2006)425.[14]R.Gangopadlhyay,A.De,Chem.Mater.12(2000)608.[15]A.Rarnanaviciene,A.Ramanavicius,Crit.Rev.Anal.Chem.32(2002)245.[16]Q.Ameer,S.B.Adeloju,Sens.Actuators B106(2005)541.[17]I.M.Arabatzis,P.Falaras,Nano Lett.3(2003)249.[18]F.Carn,A.Colin,et al.Adv.Mater.17(2005)62.[19]L.J.M.Jacobs,K.C.H.Danen,M.F.Kemmere,et p.Mater.Sci.38(2007)751.[20]G.K.R.Senadeera,T.Kitamura,Y.Wada,et al.J.Photochem.Photobiol.A184(2006)234.[21]H.Nagai,H.Segawa,mun.(2004)974.[22]T.Kitamura,M.Maitani,M.Matsuda,et al.Chem.Lett.(2001)1054.[23]X.T.Zhang,J.Zhang,W.H.Song,et al.J.Phys.Chem.B(2006)110.[24]W.Zhong,S.Liu,X.Chen,et al.Macromolecules39(2006)3224.[25]W.Yan,Z.X.Wei,L.L.Wang,J.Xi’an Jiaotong Univ.36(2002)94.。