大学化工专业英语Lesson-3
- 格式:doc
- 大小:45.00 KB
- 文档页数:7



前几课翻译链接:/s/1o6qiyuQLesson 10 ThermodynamicsThermodynamics is the physics of energy, heat, work, entropy and the spontaneity of processes. Thermodynamics is closely related to statistical mechanics from which many thermodynamic relationships can be derived.热力学是物理能量,热,工作过程,熵和自发性。
热力学是密切相关的统计力学,热力学关系可以推导出。
While dealing with processes in which systems exchange matter or energy, classical thermodynamics is not concerned with the rate at which such processes take place, termed kinetics. For this reason, the use of the term “thermodynamics”usually refers to equilibrium thermodynamics. In this connection, a central concept in thermodynamics is that of quasistatic processes, which are idealized, “infinitely slow”processes. Time-dependent thermodynamic processes are studied by non-equilibrium thermodynamics.在处理中,系统交换物质或能量的过程,经典热力学不关心这些过程发生的速率,称为动力学。



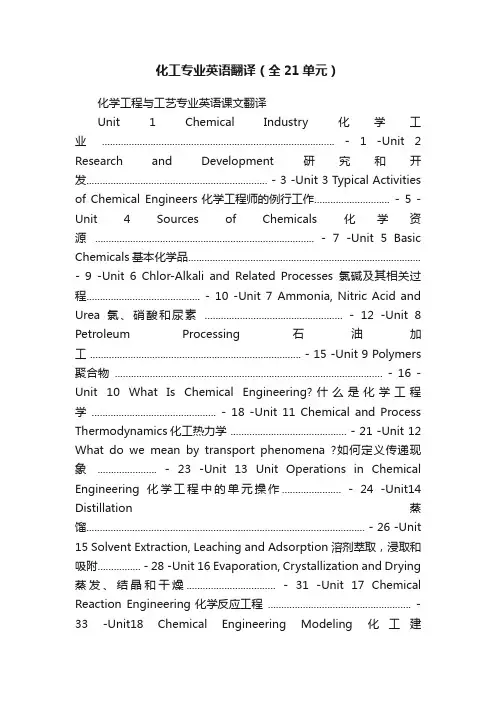
化工专业英语翻译(全21单元)化学工程与工艺专业英语课文翻译Unit 1 Chemical Industry化学工业...................................................................................... - 1 -Unit 2 Research and Development研究和开发................................................................... - 3 -Unit 3 Typical Activities of Chemical Engineers化学工程师的例行工作............................ - 5 -Unit 4 Sources of Chemicals化学资源................................................................................. - 7 -Unit 5 Basic Chemicals基本化学品...................................................................................... - 9 -Unit 6 Chlor-Alkali and Related Processes氯碱及其相关过程.......................................... - 10 -Unit 7 Ammonia, Nitric Acid and Urea氯、硝酸和尿素................................................... - 12 -Unit 8 Petroleum Processing石油加工 .............................................................................. - 15 -Unit 9 Polymers 聚合物 ................................................................................................... - 16 -Unit 10 What Is Chemical Engineering?什么是化学工程学 .............................................. - 18 -Unit 11 Chemical and Process Thermodynamics化工热力学 ........................................... - 21 -Unit 12 What do we mean by transport phenomena ?如何定义传递现象...................... - 23 -Unit 13 Unit Operations in Chemical Engineering化学工程中的单元操作...................... - 24 -Unit14 Distillation蒸馏....................................................................................................... - 26 -Unit 15 Solvent Extraction, Leaching and Adsorption溶剂萃取,浸取和吸附................ - 28 -Unit 16 Evaporation, Crystallization and Drying 蒸发、结晶和干燥................................. - 31 -Unit 17 Chemical Reaction Engineering化学反应工程 ..................................................... - 33 -Unit18 Chemical Engineering Modeling化工建模 ............................................................. - 36 -Unit 19 Introduction to Process Design过程设计简介...................................................... - 37 -Unit 20 Material Science and Chemical Engineer材料科学和化学工程........................... - 39 -Unit 21 Chemical Industry and Environment化学工业与环境 ......................................... - 42 - Unit 1 Chemical Industry化学工业1.化学工业的起源尽管化学品的使用可以追溯到古代文明时代,我们所谓的现代化学工业的发展却是非常近代(才开始的)。

第二部分化学化工专业基础知识第二节Nomenclature of Organic Chemistry一.The Need for Classification of Organic Compounds The field of organic chemistry is vast, for it includes not only the composition of all living organisms but also of a great many other materials that we use daily.It is physically impossible for one to study the properties of each of the hundreds of thousands of known organic compounds. Hence organic compounds with similar structural features are grouped into series or classes. Some of the classes of organic compounds are hydrocarbons, alcohols,aldehydes,ketones,ethers, carboxylic acids, esters, carbohydrates, and proteins. Each of these classes of compounds is identified by certain characteristic structural features.⏹hydrocarbons, 烃类,碳氢化合物⏹alcohols, 醇类⏹aldehydes, 醛类⏹ketones, 酮类⏹ethers, 醚类⏹carboxylic acids, 羧酸⏹esters, 酯类⏹carbohydrates,糖类,碳水化合物1. HydrocarbonsHydrocarbons are composed entirely of carbon and hydrogen atoms bonded to each other by covalent bonds.Several series of hydrocarbons are known. These include the alkanes, alkenes, alkynes, and aromatic hydrocarbons.alkanes, [‘ælkeinz]烷烃alkenes, [‘ælki:nz]烯烃alkynes, [‘ælkainz]炔烃aromatic hydrocarbons,芳香烃Classification of Hydrocarbons:1.1 Aliphatic compounds (脂肪烃)Rule A-1. Saturated Unbranched-chain Compounds and Univalent RadicalsRule A-2. Saturated Branched-chain Compounds and Univalent RadicalsRule A-3. Unsaturated Compounds and Univalent Radicals1.1.1 Alkanes (C n H2n+2)1.1.1.1 Straight-Chain Alkanes一.“元素”和“单质”的英文意思都是―element‖,有时为了区别,在强调―单质‖时可用―free element‖。

《化学工程与工艺专业英语》课文翻译-Unit3 Typical Activities of ChemicalEngineers本文将介绍化学工程师在日常工作中的一些典型活动。
化学工程师旨在将化学与工程学的原理应用于产业中,解决生产过程中的各种问题并开发新产品。
化学工程师随着技术的发展和市场的变化而发展和变化。
但以下是其典型活动:第一,设计新的工艺流程。
化学工程师需要了解化学反应原理、物质转化和热量传递方程式,以及流体力学和系统分析等方面的知识,创造出新的工艺流程。
在此基础上,他们可以设计出化学反应器、分离装置和加工设备等。
而在设计之前,化学工程师会根据产品需求和工厂生产线等因素,制定经济可行性和技术可行性分析,制定整个流程方案。
第二,工厂的设计和规划。
这些安装要求使用化学工程师的技能和知识。
工程师需要考虑由工厂所需的供电、供水和废物处理等各个方面。
他们需要选择合适的材料和设备,也需要在设计中采用节能和环境保护技术。
他们也必须为紧密的安全要求和法律法规做好准备。
第三,处理产品的生产和质量控制。
生产线上的任何错误都有可能导致生产过程劣化和生产失败。
化学工程师通过控制生产过程的不同参数和调整生产中的机器设备、材料流程和成品质量保障等,确保产品的质量和产品的性能。
如果有任何生产上的问题,他们都需要快速响应,并及时寻找解决方法。
第四,进行研究和开发。
在工业生产中,研究和开发是至关重要的。
化学工程师必须熟悉当前和未来的技术发展。
他们需要收集和分析大量的数据和材料,以探索和开发新的技术和产品,面对多种工艺流程,为生产线输入新的元素。
第五,进行销售和市场分析。
销售和市场分析也是化学工程师需要了解的另一个方面。
他们需要熟悉市场需求和市场潜力。
工程师必须在市场竞争激烈的环境中发挥创新、争取同行的业务和合作伙伴,为他们的产品寻找最佳销售渠道。
第六,维护和管理设备。
对于工厂设备的维护和管理是保证生产线平稳运转的必要条件。


Lesson 1Chemical Engineering1、What is chemical engineering and its content?The Institution of Chemical Engineers defines chemical engineering as “that branch of engineering which is concerned with processes in which materials undergo a required change in composition, energy content or physical state: with the means of processing; with the resulting products, and with their application to useful ends”.2、What concept is the landmark in the development of chemical engineering?Unit operations3、What are the basic laws of chemical engineering science?The principles of chemistry, physics, and mathematics.The laws of physical chemical and physics govern the practicability and efficiency of chemical engineering operations. Energy changes, deriving from thermodynamics consideration, are particularly important. Mathematics is a basic tool in optimization and modeling.4、Name the functions and branches of chemical engineering you know.Chemical Engineering Functions: The design and development of both processes and plant items.Branches of Chemical Engineering: Plastics, polymers, synthetic fibers, dyeing, pulp and paper manufactures, pharmaceutical industry, and separation of rare metals.Lesson 2 Chemical Equilibrium and Kinetics1、Which factors influence the reaction rates?a)Temperatureb)concentrations of reactants (or partial pressure of gaseous reactants)c)presence of a catalyst.2、How to determine the reaction equilibrium constants?For a reversible reaction:aA + bB = cC +dDthe equilibrium constant expression is written as follows:K = C c D d / A a B bLesson 3 The Second Law of Thermodynamics1、What are the applications of chemical thermodynamics?There is two major applications of thermodynamics:( i ) The calculation of heat and work effect associated with processes as well as the calculation of the maximum work obtainable from a process or the minimum work required to drive a process.( ii ) The establishment of relationship among the variables describing systems at equilibrium.Lesson 4 Chemical Reaction Engineering1、Homogeneous Reactions vs. Heterogeneous ReactionsHomogeneous reactions are those in which the reactants, products, and any catalyst used form one continuous phase; gaseous and liquid.Heterogeneous reactions are those in which two or more phases exist, and the overriding problem in the reactor design is to promote mass transfer between the phases. The possible combinations of phase are:( i ) Liquid-liquid ( ii ) Liquid-solid ( iii ) Liquid-solid-gas( iv ) Gas-solid ( v ) Gas-liquid2、Reactor Geometry(type)Stirred Tank ReactorsTubular ReactorsPacked Bed ReactorsFluidized Bed ReactorsLesson 5 Chlor-Alkali and Related Processes1、What are the mechanisms of chlor-alkali process?The reaction are based on the idea of using electrons as a reagent in chemical reactions. The basic reactions of brine electrolysis can be written as follows:Anode 2Cl- - 2e-—> Cl2Cathode 2H2O + 2e-—> H2 + 2OH-The overall reaction is: 2Na+ + 2Cl- + 2H2O —> NaOH + Cl2 + H2Lesson 7 Momentum, Heat, and Mass Transfer1、In some cases, momentum , heat and mass transfer all occurs simultaneously, explain with examples.In a water-cooling tower, where transfer of sensible heat (heat transfer) and evaporation (mass transfer) both take place from the surface of the water droplets. Momentum transfer take place between the water droplets and air.2、What will happen for two adjacent layers of fluid with different moving velocities?There will be a tendency for the faster moving layer to be retarded and the slower moving layer to be accelerated by virtue of the continuous passage of molecules in each direction. There will be a net transfer of momentum from the fast to the slow moving stream.Lesson 10 Gas Absorption1、There are three ways in which a large contact area can be established:1. The liquid is brought in contact with the gas in the form of thin films ( film scrubbers).2. The liquid is dispersed in the gas in the form of minute drops (spray scrubbers).3. The gas is dispersed in the liquid in the form of small bubbles (bubble scrubbers). All apparatus applied in gas absorption practice is based on one of these three principles or on a combination of them.Lesson 13 Filtration1、Which factors should be considered in the operation of filtration?a)The properties of the liquid , particularly its viscosity, density and corrosive properties.b)The nature of the solid - its particle size and shape, size distribution, and packing characteristics.c)The concentration of solids in suspension.d)The quality of material to be handled, and its value.e)Whether the valuable product is the solid, the fluid, or both.f)Whether it is necessary to wash the filtered solids.g)Whether very slight contamination caused by contact of the suspension or filtrate with the various components of the equipment is detrimental to the product.h)Whether the feed liquor may be heated.i)Whether any form of pretreatment would be helpful.2、Which factors will have a close relation with the rate of filtration?a) The drop in pressure from the feed to the far side of the filter medium.b) The area of the filtering surface.c) The viscosity of the filtrate,d) The resistance of the filter cake.e) The resistance of the filter medium and initial layers of cake.Lesson 15 Computer-Assisted Design of New Process1、Design for new processed proceed through at least three stagesConceptual Design: the generation of ideas for new processes (processed synthesis) and their translation into an initial design. This stage includes preliminary cost estimates to asses the potential profitability of the process, as well as analyses of process safety andenvironmental considerations.Final Design: a rigorous set of design calculations to specify all the significance details of a process,Detailed Design: preparation of engineering drawing and equipment lists needed for construction.TBC ...Lesson 16 Catalysis1、Catalytic reactions can be classified into three types:The most common is heterogeneous catalysis, in which the catalyst is a solid and the reactants and products are either gases or liquids. The second type is homogeneous catalysis, in which the reactants, products and catalyst are molecularly dispersed in a single phase, usually the liquid phase. The third type is enzyme catalysis.Lesson 18 Polymers and Polymerization Techniques1、There are five general methods of polymerization:( i ) Bulk (or mass) ( ii ) Solution ( iii ) Slurry (or precipitation)( iv ) Suspension(or dispersion) ( v) EmulsionFurther lesser-used methods include:( vi ) Interfacial ( vii ) Reaction injection moulding (RIM)( viii ) Reactive processing of molten polymers2、A polymerization process consists of three stages:( i ) Monomer preparation ( ii ) Polymerization ( iii ) Polymer recover。
Part 2 Unit 1. 元素、化合物和物质元素是用一般的化学方法无法使之分解为更简单物质的纯物质。
总的来说, 到2008年, 已经发现的元素有117种, 其中94种是地球上天然存在的元素。
人们熟悉的一些常见元素有碳、氢、氧、氮、磷、钾、硫、铝、铁、铜和金。
这些元素是物质组成的基本单元, 就像数字但是由0 - 9组成一样。
众所周知, 整个宇宙也同样是由这些已经在地球上发现的元素所构成。
这些自然界中存在的元素和其他元素结合在一起形成了矿物质、植物或水和二氧化碳等物质。
铜、银、金和其它20多种元素可以以高纯度形式存在于自然界中。
还有16种元素是自然界中没有发现的,这些元素是在核爆炸和核研究中所产生的少量物质。
因而,他们都是人造元素。
由两个或两个以上元素组成的纯物质称为化合物。
因为化合物含有两种或更多的元素, 因而其与元素的性质不同, 他们可以通过化学变化分解成更简单的物质。
化合物经化学分解最终产生的物质正是组成化合物的元素。
组成化合物的元素的原子以整数比例,而非以分数比,结合在一起。
(组成化合物的元素各原子间以整数比例而非分数比结合在一起)原子间相互结合形成的化合物以分子或离子状态存在。
一个化合物的分子是由两个或两个以上的原子组成的,化合物的分子体积很小,而且不带电(呈电中性).如果我们把一滴水细分成越来越小的微粒, 我们最终可以得到一个水的单体,即一个水分子。
这个水分子是由两个氢原子和一个氧原子键合在一起。
人们通常无法进一步分解分子单元, 只有破坏分子结构才能将其分解成所组成的元素。
因此, 水分子是水这种化合物的最小单元。
离子是带正电的或负电的原子或元素簇。
一个化合物中的离子依靠它们正电和负电基团间的相互引力结合在一起, 形成晶体结构。
由离子组成的化合物中不存在分子。
氯化钠是一种典型的非分子结构的化合物。
这类化合物包含大量的正、负离子, 其分子式如分子结构的化合物那样,通常采用组成化合物的原子间以简单比例式方式来表达。
The Second Law of Thermodynamics热力学第二定律Thermodynamics is concerned with transformation of energy, and the laws of thermodynamics describe the bounds within which these transformation are observed to occur.热力学讨论的是能量的转换,热力学定律描述了这些转变能够发生的边界条件。
The first law, stating that energy must be conserved in all ordinary processes, has been the underlying principle of the preceding chapters.第一定律指出了在一般过程中,能量是守恒的,它是前面章节遵循的根本原则。
The first law imposes no restriction on the direction of energy transformation.第一定律对能量转换的方向性没有限制。
Yet, all our experience indicates the existence of such a restriction.然而,我们的经验说明这种限制的存在。
To complete the foundation for the science of thermodynamics, it is necessary to formulate this second limitation. Its concise statement constitutes the second law.为了完善热力学的科学基础,有必要规定第二个限制条件,它简洁的容构成了第二定律。
The differences between the two forms of energy, heat and work, provide some insight into the second law.两种形式的能量:热和功之间的不同,为第二定律提供了一些见解。
大学化工专业英语Lesson-3The Second Law of Thermodynamics热力学第二定律Thermodynamics is concerned with transformation of energy, and the laws of thermodynamics describe the bounds within which these transformation are observed to occur.热力学讨论的是能量的转换,热力学定律描述了这些转变能够发生的边界条件。
The first law, stating that energy must be conserved in all ordinary processes, has been the underlying principle of the preceding chapters.第一定律指出了在一般过程中,能量是守恒的,它是前面章节遵循的根本原则。
The first law imposes no restriction on the direction of energy transformation.第一定律对能量转换的方向性没有限制。
Yet, all our experience indicates the existence of such a restriction.然而,我们的经验表明这种限制的存在。
To complete the foundation for the science of thermodynamics, it is necessary to formulate this second limitation. Its concise statement constitutes the second law. 为了完善热力学的科学基础,有必要规定第二个限制条件,它简洁的内容构成了第二定律。
The differences between the two forms of energy, heat and work, provide some insight into the second law.两种形式的能量:热和功之间的不同,为第二定律提供了一些见解。
These differences are not implied by the first law.这些不同是第一定律中没有提到的。
In an energy balance both work and heat are included as simple additive terms, implying that one unit of heat, such as a joule or BTU, is equivalent to the same unit of work.在能量平衡中,功和热作为简单的加和项,表明了,一个单位的热,如1J或1BTU,等于相同单位的功。
Although this is true with respect to an energy balance.虽然这是真正的能量平衡。
Experience teaches that there is a difference in quality between heat and work.经验告诉我们,热和功之间有一个质量的差异。
This experience can be summarized by the following facts.这方面的经验可以从以下事实中总结出来。
First, the efficiency of the transformation from one form of work to another such as electrical to mechanical as accomplished in an electric motor, can be made to approach 100 percent as closely as is desired.首先,使得从一种形式的功向另一种形式的功的转换效率尽可能的接近于我们所向往的100%,比如,从电能到机械能的转换能够通过电动机来实现。
One needs merely to exert more and more care in eliminating irreversibilities in the apparatus.我们仅仅需要越来越关心装置设备中不可逆性的消除。
On the other hand, efforts to convert energy transferred to a system as heat into any of the forms of work show this regardless of improvements in the machines employed.另一方面,将转移到系统中的热量转换成任何一种形式的功表明,这与所使用机器的改进无关。
The conversion is limited to low values(40 percent is an approximate maximum).这种转换率是被限制在一个较小的值内(40%大约是最大值)。
These efficiencies are so low, in comparison with these obtained for the transformation of work from form to another, that there can be no escape from the conclusion that there is an intrinsic difference between heat and work, in the reverse direction, the conversion of work into heat with 100 percent efficiency is very common.与不同形式的功之间转换的效率相比,这些效率是太低了,以至于得出一个不可逃避的结论:热与功之间有一个本质的不同,在相反的方向上,功向热转换的效率为100%,这是非常普通的。
Indeed, efforts are made in nearly every machine to eliminate this conversion, which decrease efficiency of operation.事实上,为了消除这种转变,我们在努力改进几乎每台机器,这降低了操作效率。
These facts lead to the conclusion that heat is a less versatile or more degraded form of energy than work.从这些事实中可以得出一个结论,热与功相比,没有那么多变,没有更多能量的退化形式。
Work might be termed energy of a higher quality than heat.功可以被称为是比热具有更高质量的能量。
To draw further upon our experience, we know that heat always flows from a higher temperature level to a lower one, and never in the reverse direction.为了进一步的总结我们的经验,我们知道从高温到低温将会产生热量,然而在相反的方向上不会有热量产生。
This suggests that heat itself may be assigned a characteristic quality as well as quantity, and that this quality depends upon temperature, the relation of temperature to the quality of heat is evident from the increased in efficiency with which heat may be converted into work as the temperature of the source is raised.这表明,热本身可能被分配了一定的质量和数量,这个质量取决于温度,从增加的效率来看,温度和热的质量的关系是明显的:在这样的效率下,随着原始材料温度的升高,热能够转化成功。
For example, the efficiency, or work output per unit of fuel burned, of a stationary power plant increases as the temperature of the steam in the boiler and superheater rises.例如,一个固定式发电站的效率,或者燃烧每单位燃料的输出功,随着锅炉或过热器中蒸汽温度的升高而增加。
Statements of the Second Law第二定律的陈述The observations just described are results of the restriction imposed by the second law on the direction of actual processes. Many general statements may be made which described this restriction and hence, serve as statements of the second law. Two of the most common are:刚刚描述的观察结果是第二定律对实际过程在方向上的限制的结果。
许多通用的语句能够描述这个限制,因此,这些描述可以作为第二定律的陈述。
最常见的两个是:1. No apparatus can operate in such a way that its only effect(in system and surroundings)is to convert heat absorbed by a system completely into work.1. 没有设备能够仅仅产生这样一种影响:实现将系统吸收的热量全部转化成功。
2. Any process which consists solely in the transfer of heat from one temperature to a higher one is impossible.任何一个从低温到高温的过程仅仅包含热量的转移是不可能的。