PUBMED课后作业讲解和演示
- 格式:ppt
- 大小:1.48 MB
- 文档页数:16

PubMed Emba 入门教程章节一:PubMed 简介1.1 课程目标:了解PubMed 的基本概念、功能和用途。
掌握PubMed 的访问方式和基本操作。
1.2 教学内容:PubMed 的定义和背景PubMed 的功能和特点PubMed 的访问方式PubMed 的基本操作(搜索、浏览、筛选、排序等)1.3 教学活动:讲解PubMed 的基本概念和功能演示PubMed 的访问方式和基本操作学生实操:搜索并筛选文献章节二:PubMed 搜索技巧2.1 课程目标:学习PubMed 的搜索技巧,提高搜索效率和准确性。
2.2 教学内容:PubMed 的搜索方式(关键词搜索、高级搜索等)常用搜索策略和技巧(同义词、布尔运算、位置运算等)检索式的构建和优化2.3 教学活动:讲解PubMed 的搜索方式和常用搜索策略演示检索式的构建和优化方法学生实操:构建检索式并进行搜索章节三:PubMed 文献浏览和筛选3.1 课程目标:学习PubMed 文献的浏览和筛选方法,快速找到所需信息。
3.2 教学内容:PubMed 文献的浏览方式(、摘要、全文等)常用筛选条件(时间、语言、发表期刊等)排序和分组功能3.3 教学活动:讲解PubMed 文献的浏览和筛选方法演示常用筛选条件和排序分组功能学生实操:根据需求进行文献浏览和筛选章节四:PubMed 文献获取和引用4.1 课程目标:学习PubMed 文献的获取和引用方法,方便进行文献阅读和写作。
4.2 教学内容:PubMed 文献的获取方式(全文、引用信息等)文献引用格式(APA、MLA等)文献管理工具的使用(如EndNote、Zotero等)4.3 教学活动:讲解PubMed 文献的获取和引用方法演示文献引用格式的编写和文献管理工具的使用学生实操:全文、编写引用信息并使用文献管理工具章节五:PubMed 高级应用5.1 课程目标:学习PubMed 的高级应用,进行更深入的文献研究和分析。




一、用Pubmed检索下列各题,记录你的检索操作过程及命中文献数1.了解有关疯牛病(bovine spongiform encephalopathy ,BSE)起源(origin)的情况,最早的报道在哪年,记录出处。
#1 bovine spongiform encephalopathy#2 BSE#3 #1 OR #2#4 origin#5 #3 AND #4点击Display settings在sort by 下方勾选Pub Date后点击Apply进行按出版日期排序,选择最后一项的最后一条记录。
2.2005年度诺贝尔医学奖授予了幽门螺杆菌的发现者Barry J.Marshall(1951-)和J. Robin W arren(1937-),请检索两人合作发表的论文,记录最早的一篇文献题录。
#1 Marshall BJ[au]#2 W arren JR[au]#3 #1 AND #2点击Display settings在sort by 下方勾选Pub Date后点击Apply进行按出版日期排序,选择最后一项的最后一条记录。
3.请检索有关流感病毒H5N1人疫苗(vaccine)的临床试验(clinical trial)文献。
#1 H5N1#2 vaccine#3 #1 AND #2选择限定:Species √human(人类) Article types√clinical trial(临床试验)4.RNA干扰(RNA INTERFERENCE )用于肝癌治疗的综述文献。
#1 RNA INTERFERENCE#2 liver cancer#3 #1 AND #2选择限定:Article types√Review(综述)二、用Pubmed主题途径检索下列各题,记录你的检索操作过程及命中文献数1. 有关儿童哮喘(asthma)的药物疗法方面的文献。
在左上角数据库选择下拉菜单中选择进入MeSH,在词语输入框中输入asthma,查找asthma的主题词为asthma,点击有下划线的asthma进入主题检索页面,勾选副主题词drug therapy,在右上角选AND,然后点击Add to search builder将主题词及副主题词送入上面的检索构造框中,点击Search Pubmed按钮即进行主题检索:asthma/drug therapy在该题主题检索中,儿童不作为检索进行检索,而是选择限定中的年龄限定进行限制检索。




实验2 PubMed 的使用一、用PubMed检索作业1perform a search for the human disease list below: Creutzfeldt-Jakob, Gaucher, Leeukaemia, Breast cancer; Write down how many articles there are.Creutzfeldt-Jakob 6484Gaucher 4671Leeukaemia 240634Breast cancer 2376382 Perfom the same search, only for articles which appeared exactly during the past year. Print the Abstracts of the first 5 search results , sorted by Author .①ⅰCreutzfeldt-Jakobⅰ、Creutzfeldt-Jakob disease affecting the maxillofacial region: a case report.Abdel-Galil K, Williams C, Chambers P.SourceBradford Teaching Hospitals NHS Foundation Trust, St. Luke's Hospital, Bradford, United Kingdom. khalidabdelgalil@AbstractCreutzfeldt-Jakob disease (CJD) is a rare disorder caused by prions that can affect any part of the central nervous system. It is characterised by an initial non-specific illness of varying duration, followed by progressive neurological decline. We report a patient with sporadic CJD who presented with neurologicalsymptoms and bilateral dislocation of the temporomandibular joints (TMJs). To our knowledge this is the first report of sporadic CJD that involved the maxillofacial region.2009 The British Association of Oral and Maxillofacial Surgeons. Published by Elsevier Ltd. All rights reserved.ⅱ、Understanding amyloid aggregation by statistical analysis of atomic force microscopy images.Adamcik J, Jung JM, Flakowski J, De Los Rios P, Dietler G, Mezzenga R.SourceLaboratoire de Physique de la Matière Vivante, Ecole Polytechnique Fédérale de Lausanne (EPFL),CH-1015 Lausanne, Switzerland.AbstractThe aggregation of proteins is central to many aspects of daily life, including food processing, blood coagulation, eye cataract formation disease and prion-related neurodegenerative infections. However, the physical mechanisms responsible for amyloidosis-the irreversible fibril formation of various proteins that is linked to disorders such as Alzheimer's, Creutzfeldt-Jakob and Huntington's diseases-have not yet been fully elucidated. Here, we show that different stages of amyloid aggregation can be examined by performing a statistical polymer physics analysis of single-molecule atomic force microscopy images of heat-denatured beta-lactoglobulin fibrils. The atomic force microscopy analysis, supported by theoretical arguments, reveals that the fibrils have a multistranded helical shape with twisted ribbon-like structures. Our results also indicate a possible general model for amyloid fibril assembly and illustrate the potential of this approach for investigating fibrillar systems.ⅲ、Diagnostic pitfalls in a patient with posttraumatic exaggerated startle response and mutistic stupor.Agorastos A, Muhtz C, Kellner M.ⅳ、Refractory nonconvulsive status epilepticus inCreutzfeldt-Jakob disease.Aiguabella M, Falip M, Veciana M, Bruna J, Palasí A, Corral L, Herrero JI, Boluda S, Mora J, Iranzo A, Serrano C.SourceNeurology Department, Hospital de Bellvitge, Hospitalet de Llobregat, Barcelona, Spain.37785mam@comb.catAbstractCreutzfeldt-Jakob disease (CJD) is a rare human transmissible spongiform subacute encephalopathy. The most common clinical manifestations of CJD include rapidly progressive dementia, behavioural changes, cerebellar dysfunction and myoclonus. Other seizure types are rare and nonconvulsive status epilepticus (SE) is exceptional. We report a case of a 44-year-old man who presented a psychotic episode followed by akinetic mutism and refractory nonconvulsive SE. The final diagnosis was CJD. Continuous video-EEG monitoring revealed the ictal pattern of nonconvulsive SE to be periodic sharp wave complexes characteristic of CJD. [Published with video sequences].ⅴ、[Rare form of Creutzfeldt-Jakob disease].Al-Hamdany S, Holm IE.SourcePatologisk Institut, Århus Universitetshospital, Aalborg Sygehus, 9000 Aalborg, Denmark.AbstractCreutzfeldt-Jakob disease (CJD) is the most common type of prion disease. A considerable variation in disease phenotype is seen, primarily influenced by a naturally occurring polymorphism in the prion protein gene. We present a case of sporadic CJD of atactic type. Molecular genetic analysis showed a VV polymorphism at codon 129 in the prion protein gene. The various polymorphisms at codon 129 and their influence on the clinical picture and pathology are briefly discussed.②Gaucherⅰ、Velaglucerase (Vpriv) for Gaucher's disease.[No authors listed]ⅱ、In brief: Velaglucerase (Vpriv) for Gaucher's disease.[No authors listed]ⅲ、Infectious crystalline keratopathy caused by Streptococcus Abiotrophia defectiva.Abry F, Sauer A, Riegel P, Saleh M, Gaucher D, Speeg-Schatz C, Bourcier T.SourceDepartment of Ophthalmology, University Hospital of Strasbourg, Strasbourg, France.florence.abry@chru-strasbourg.frAbstractPURPOSE:To report the first case of Streptococcus Abiotrophia defectiva-associated crystalline keratopathy. METHODS:An 83-year-old woman underwent penetrating keratoplasty for pseudophakic bullous keratopathy in theOD. Ten months after surgery, the patient presented with decreased visual acuity in the OD. Slit-lampexamination showed crystalline keratopathy. Corneal scrapings were negative, and the patient wastreated empirically with 2 fortified antibiotics (ciprofloxacin and vancomycin). Despite these treatments,the surface of the infiltrate increased and corneal regrafting was performed 6 weeks later. The excisedprior graft was evaluated microbiologically [culture and polymerase chain reaction (PCR)] andhistopathologically.RESULTS:In the explanted corneal graft, cultures grew Streptococcus A. defectiva, and its DNA was demonstratedby broad-range PCR (16S ribosomal DNA).CONCLUSIONS:A. defectiva can be a causative organism of infectious crystalline keratopathy. Risk factors may include long-term corticosteroid use and prior corneal transplantation. The present case confirms that PCR canⅳ、Eye movement impairment recovery in a Gaucher patient treated with miglustat.Accardo A, Pensiero S, Ciana G, Parentin F, Bembi B.SourceDepartment of Electronics (DEEI), University of Trieste, 34127 Trieste, Italy.AbstractIn Gaucher Disease (GD) the enzyme (imiglucerase) replacement therapy (ERT) is not able to stop the progression of the neurological involvement, while the substrate reduction therapy (SRT), performed by N-Butyldeoxynojirimycin (miglustat), is an alternative that should be evaluated. Two sisters, presenting the same genotype (R353G/R353G), were diagnosed as suffering from GD; one of them later developed neurological alterations identified by quantitative saccadic eye movements analysis. The aim of the study was to quantitatively measure the miglustat effects in this GD neurological patient. Eye movement analysis during subsequent controls was performed by estimating the characteristic parameters of saccadic main sequence. The study demonstrates that the SRT alone can be effective in GD3. Moreover, it confirms that quantitative eye movement analysis is able to precociously identify also slight neurological alterations, permitting more accurate GD classification.ⅴ、Velaglucerase alfa.Aerts JM, Yasothan U, Kirkpatrick P.③Leeukaemiaⅰ、Chronic fatigue syndrome: going viral?[No authors listed]ⅱ、Toxicology and carcinogenesis studies of androstenedione (CAS No. 63-05-8) in F344/N rats and B6C3F1 mice (gavage studies).[No authors listed]AbstractAndrostenedione is an androgen steroid that is normally synthesized within men and women and may be metabolized to a more potent androgen or estrogen hormone. It was nominated to the National Toxicology Program for study due to concern for adverse health effects associated with its chronic use as a dietary supplement by athletes (prior to the banning of its over the counter sales). In order to evaluate its subchronic and chronic toxicity, male and female F344/N rats and B6C3F1 mice were administered androstenedione (98% pure) by gavage for 2 weeks, 3 months, or 2 years. Genetic toxicology studies were conducted in Salmonella typhimurium, Escherichia coli, rat bone marrow cells, and mouse peripheral blood erythrocytes. 2-WEEK STUDY IN RATS: groups of five male and five female rats were administered 0, 1, 5, 10, 20, or 50 mg androstenedione/kg body weight in a 0.5% aqueous methylcellulose solution by gavage, 5 days per week for 12 days. All rats survived to the end of the study, and the mean body weights of dosed groups were similar to those of the vehicle control groups. The development of cytoplasmic vacuoles within centrilobular hepatocytes in male rats was the only treatment-related effect observed. 2-WEEK STUDY IN MICE: groups of five male and five female mice were administered 0, 1, 5, 10, 20, or 50 mg androstenedione/kg body weight in a 0.5% aqueous methylcellulose solution by gavage, 5 days per week for 12 days. One vehicle control female, one 20 mg/kg female, and one 50 mg/kg female died early due to gavage accidents. There were no significant chemical-related histopathological or mean body weight changes. 3-MONTH STUDY IN RATS: groups of 10 male and 10 female core study rats were administered 0, 1, 5, 10, 20, or 50 mg androstenedione/kg body weight in a 0.5% aqueous methylcellulose solution by gavage, 5 days per week for 14 weeks; additional groups of 10 male and 10 female clinical pathology study rats received the same doses for 23 days. All rats survived to the end of the study. The mean body weights of the 20 mg/kg female group was significantly greater than those of the vehicle control group and there was significant increased weight gain in the 1, 20, and 50 mg/kg female groups. Female thymus weights were significantly increased in the 20 and 50 mg/kg groups, which may be related to the increase in mean body weight. The numbers of sperm per mg cauda epididymis in the 10, 20, and 50 mg/kg male groups and the total number of sperm per cauda epididymis in 50 mg/kg males were significantly less than those of the vehicle controls. No treatment-related histological lesions were observed in males or females. 3-MONTH STUDY IN MICE: groups of 10 male and 10 female mice were administered 0, 1, 5, 10, 20, or 50 mg androstenedione/kg body weight in a 0.5% aqueous methylcellulose solution by gavage, 5 days per week for 14 weeks.Except for one 10 mg/kg female that died early due to a dosing accident, all mice survived to the end of the study. The mean body weights of dosed groups were similar to those of the vehicle control groups. The number of spermatids per mg testis and the total number of spermatids per testis in 20 mg/kg males were significantly greater than those of the vehicle controls. Sperm motility in 50 mg/kg males was significantly lower than that in the vehicle controls. The incidences of x-zone atrophy of the adrenal cortex, an androgen-sensitive endpoint, were significantly increased in females administered 5 mg/kg or greater. There were also significant decreases in the incidences of x-zone cytoplasmic vacuolization in 20 and 50 mg/kg females. The incidences of bone marrow hyperplasia were significantly increased in 5 and 50mg/kg males. 2-YEAR STUDY IN RATS: groups of 50 male and 50 female rats were administered 0, 10, 20, or 50 mg androstenedione/kg body weight in a 0.5% aqueous methylcellulose solution by gavage, 5 days per week for at least 104 weeks. Survival of 10 mg/kg males was significantly greater than that of the vehicle controls. The mean body weights of 20 and 50 mg/kg females were greater than those of the vehicle controls after weeks 17 and 9, respectively. The incidences of mononuclear cell leukemia were significantly increased in 20 and 50 mg/kg females and significantly decreased in 20 and 50 mg/kg males. Incidences of alveolar/bronchiolar adenoma and alveolar/bronchiolar adenoma or carcinoma (combined) were significantly increased in 20 mg/kg males. The incidence of testicular interstitial cell adenoma (including bilateral) was significantly decreased in 50 mg/kg males. In females, the incidences of mammary gland fibroadenoma were significantly decreased in the 20 and 50 mg/kg groups, the incidences of mammary gland hyperplasia were significantly decreased in all dosed groups, and the incidences of mammary gland cyst were significantly decreased in the 10 and 50 mg/kg groups. In the liver of males, the incidences of basophilic focus in all dosed groups, the incidence of clear cell focus in the 20 mg/kg group, and the incidence of eosinophilic focus in the 50 mg/kg group were significantly increased. The incidences of pancreatic islet hyperplasia and atrophy of the exocrine pancreas were significantly increased in 50 mg/kg females. 2-YEAR STUDY IN MICE: groups of 50 male and 50 female mice were administered 0, 2 (females only), 10, 20 (males only), or 50 mg androstenedione/kg body weight in a 0.5% aqueous methylcellulose solution by gavage, 5 days per week for at least 104 weeks. Survival of dosed groups was similar to that of the vehicle control groups. Mean body weights of 10 and 50 mg/kg females were generally less than those of the vehicle controls after weeks 81 and 17, respectively. The incidences of hepatocellular adenoma in males and females were significantly increased in the 50 mg/kg groups. In females, the incidences of hepatocellular carcinoma were significantly increased in all dosed groups. Incidences of hepatocellular adenoma or carcinoma (combined) in males and females were significantly increased in the 50 mg/kg groups. Incidences ofhepatoblastoma were marginally increased in dosed males. Incidences of multiple hepatocellular adenomas and carcinomas were significantly increased in 10 and 50 mg/kg males, and there was an increased incidence of multiple hepatocellular adenomas in 50 mg/kg females. The incidence of eosinophilic focus was significantly increased in 50 mg/kg males, and the incidences of mixed cell focus and cytoplasmic vacuolization were significantly increased in 50 mg/kg females. There was a marginally increased incidence of pancreatic islet adenoma in 50 mg/kg males and in 10 and 50 mg/kg females, with an earlier day of first incidence in males. The incidences of clitoral gland hyperplasia and clitoral gland duct dilatation were significantly increased in 10 and 50 mg/kg females. The incidence of glomerular metaplasia of the kidney was significantly increased in 50 mg/kg females, and the incidences of cytoplasmic alteration of the submandibular salivary gland were significantly increased in all dosed female groups. The increased incidences of cytoplasmic alteration of the submandibular salivary gland and glomerular metaplasia of the kidney in female mice indicated a masculinizing effect from androstenedione treatment. In 50 mg/kg females, the incidence of malignant lymphoma was significantly decreased. GENETIC TOXICOLOGY: androstenedione was not mutagenic in either of two independent bacterial mutation assays conducted with and without exogenous metabolic activation. No significant increases in the frequencies of micronucleated polychromatic erythrocytes, indicators of chromosomal damage, were observed in bone marrow of male rats administered androstenedione by gavage once daily for 3 consecutive days. Results of a peripheral blood erythrocyte micronucleus test in mice, in which androstenedione was administered by gavage for 3 months, were negative in males but judged to be equivocal in females due to a small increase (twofold over background) in micronucleated normochromatic erythrocytes observed at the highest dose administered (50 mg/kg). CONCLUSIONS: under the conditions of these 2-year gavage studies, there was equivocal evidence of carcinogenic activity of androstenedione in male F344/N rats based on increased incidences of alveolar/bronchiolar adenoma and alveolar/bronchiolar adenoma or carcinoma (combined). There was equivocal evidence of carcinogenic activity of androstenedione in female F344/N rats based on increased incidences of mononuclear cell leukemia. There was clear evidence of carcinogenic activity of androstenedione in male B6C3F1 mice based on increased incidences of multiple hepatocellular adenoma and hepatocellular carcinoma and increased incidence of hepatoblastoma. There was clear evidence of carcinogenic activity of androstenedione in female B6C3F1 mice based on increased incidences of hepatocellular adenoma and hepatocellular carcinoma. Increased incidences of pancreatic islet adenoma in male and female mice were also considered chemical related. Androstenedione administration caused increased incidences in nonneoplastic lesions of the liver in male and female rats and mice; pancreatic islets andexocrine pancreas of female rats; and clitoral gland, kidney, and submandibular salivary gland of female mice. Decreases in the incidences of testicular interstitial cell adenoma in male rats, mammary gland fibroadenoma, cysts, and hyperplasia in female rats, and malignant lymphoma in female mice were considered related to androstenedione administration. Synonyms: Andro; androst-4-ene-3,17-dione;4-androstene-3,17-dione; delta-4-androstene-3,17-dione; delta-4-androstenedione;3,17-dioxoandrost-4-ene; 17-ketotestosterone; SKF 2170 Trade names: Androtex, Fecundin.ⅲ、Retraction: "Expanded and highly active proliferation centers identify a histological subtype of chronic lymphocytic leukemia ("accelerated" chronic lymphocytic leukemia) with aggressive clinical behavior" by Eva Gine', Antonio Martinez, Neus Villamor, Armando Lopez-Guillermo, Mireia Camos, Daniel Martinez, Jordi Esteve, Xavier Calvo, Ana Muntañola, Pau Abrisqueta, Maria Rozman, Ciril Rozman, Francesc Bosch, Elias Campo, and Emili Montserrat. Haematologica. 2010 Apr 7. [Epub ahead of print] with doi:10.3324/haematol. 2009.022277.[No authors listed]ⅳ、Cancer: the revolution has begun.[No authors listed]ⅴ、Ofatumumab: chronic lymphocytic leukaemia: a last resort. [No authors listed]AbstractAbout 50% of patients with symptomatic chronic lymphocytic leukaemia in whom chlorambucil and fludarabine have failed die within 6 to 9 months. In addition to appropriate palliative care, alemtuzumab may offer patients a few extra months of life, but at a cost of several serious adverse effects. Ofatumumab, a monoclonal antibody similar to rituximab, has been authorised in the United States for the treatment of patients with chronic lymphocytic leukaemia refractory to fludarabine and alemtuzumab. The European Medicines Agency has issued a favourable opinion on marketing authorisation of ofatumumab in this setting. Clinical assessment of ofatumumab is based on an interim subgroup analysisof a non-comparative trial in 154 patients. Fludarabine and alemtuzumab therapy had failed in 59patients. The median overall survival time in this subgroup of 59 patients was 13.7 months, and the timeto progression was 5.7 months. Thirty-one of these 59 patients had non-specific symptoms of leukaemia,which disappeared for at least 2 and 6 months in respectively 48% and 23% of cases. The adverse effectprofile of ofatumumab appears similar to that of rituximab, and includes hypersensitivity reactions,infections, cardiac disorders and neutropenia. In practice, despite the scarcity of data, the use ofofatumumab seems to be justified for patients who have no other valid therapeutic options, but more dataare needed.④Breast cancerⅰ、Breast Cancer Incidence in Canada Declines as Hormone Therapy Drops.[No authors listed]ⅱ、New technologies may reduce breast biopsies.[No authors listed]ⅲ、Breast disease increases with adolescent drinking.[No authors listed]ⅳ、Iodine. Monograph.[No authors listed]ⅴ、Oncologic drugs advisory committee recommends withdrawing approval for bevacizumab use in breast cancer treatment.[No authors listed]3Perform a new search for the same human disease , for articles authored by either Smith or Jones . Prient th Summary descriptions of the first 10 search results, sorted by Journal .①Creutzfeldt-Jakob1.ⅰ、RNA integrity in post mortem human variant Creutzfeldt-Jakob disease (vCJD) and control brain tissue.Sherwood KR, Head MW, Walker R, Smith C, Ironside JW, Fazakerley JK.Neuropathol Appl Neurobiol. 2011 Oct;37(6):633-42. doi: 10.1111/j.1365-2990.2011.01162.x.ⅱ、Generalized cerebral atrophy seen on MRI in a naturally exposed animal model for Creutzfeldt-Jakob disease.McKnight AL, Minkoff LA, Sutton DL, Thomsen BV, Habecker PL, Sweeney RW, Smith G, Dasanu CA, Ichim TE, Alexandrescu DT, Stutman JM.J Transl Med. 2010 Nov 26;8:125.ⅲ、A highly sensitive immunoassay for the detection of prion-infected material in whole human blood without the use of proteinase K.Tattum MH, Jones S, Pal S, Khalili-Shirazi A, Collinge J, Jackson GS.Transfusion. 2010 Dec;50(12):2619-27. doi: 10.1111/j.1537-2995.2010.02731.x.ⅳ、The application of in vitro cell-free conversion systems to human prion diseases. Jones M, Peden AH, Head MW, Ironside JW.Acta Neuropathol. 2011 Jan;121(1):135-43. Epub 2010 Jun 10. Review.ⅴ、Discrimination between prion-infected and normal blood samples by protein misfolding cyclic amplification.Tattum MH, Jones S, Pal S, Collinge J, Jackson GS.Transfusion. 2010 May;50(5):996-1002. Epub 2010 Feb 18.ⅵ、Evaluation of an animal product-free variant of MegaCell MEM as a storage medium for corneas destined for transplantation.Smith VA, Johnson T.Ophthalmic Res. 2010;43(1):33-42. Epub 2009 Oct 14.ⅶ、Evaluation of Megacell MEM as a storage medium for corneas destined for transplantation.Smith VA, Johnson T.Ophthalmic Res. 2010;43(1):18-25. Epub 2009 Oct 14.ⅷ、Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease.Zerr I, Kallenberg K, Summers DM, Romero C, Taratuto A, Heinemann U, Breithaupt M, Varges D, Meissner B, Ladogana A, Schuur M, Haik S, Collins SJ, Jansen GH, Stokin GB, Pimentel J, Hewer E, Collie D, Smith P, Roberts H, Brandel JP, van Duijn C, Pocchiari M, Begue C, Cras P, Will RG, Sanchez-Juan P.Brain. 2009 Oct;132(Pt 10):2659-68. Epub 2009 Sep 22.ⅸ、Ethical analysis of communicating the risk of iatrogenic Creutzfeldt-Jakob disease.Shalowitz DI, Barnosky A, Smith LB.Infect Control Hosp Epidemiol. 2009 Aug;30(8):805-6. No abstract available.ⅹ、MRI lesion profiles in sporadic Creutzfeldt-Jakob disease.Meissner B, Kallenberg K, Sanchez-Juan P, Collie D, Summers DM, Almonti S, Collins SJ, Smith P, Cras P, Jansen GH, Brandel JP, Coulthart MB, Roberts H, Van Everbroeck B, Galanaud D, Mellina V, Will RG, Zerr I.Neurology. 2009 Jun 9;72(23):1994-2001.②Gaucherⅰ、Two-dimensional nanosheets produced by liquid exfoliation of layered materials.Coleman JN, Lotya M, O'Neill A, Bergin SD, King PJ, Khan U, Young K, Gaucher A, De S, Smith RJ, Shvets IV, Arora SK, Stanton G, Kim HY, Lee K, Kim GT, Duesberg GS, Hallam T, Boland JJ, Wang JJ, Donegan JF, Grunlan JC, Moriarty G, Shmeliov A, Nicholls RJ, Perkins JM, Grieveson EM, Theuwissen K, McComb DW, Nellist PD, Nicolosi V.Science. 2011 Feb 4;331(6017):568-71.ⅱ、Safety, tolerability, and pharmacokinetics of eliglustat tartrate (Genz-112638) after single doses, multiple doses, and food in healthy volunteers.Peterschmitt MJ, Burke A, Blankstein L, Smith SE, Puga AC, Kramer WG, Harris JA, Mathews D, Bonate PL.J Clin Pharmacol. 2011 May;51(5):695-705. Epub 2010 Sep 23.ⅲ、Polymeric micelles for oral drug delivery.Gaucher G, Satturwar P, Jones MC, Furtos A, Leroux JC.Eur J Pharm Biopharm. 2010 Oct;76(2):147-58. Epub 2010 Jun 19. Review.ⅳ、Osteonecrosis is not a predictor of poor outcomes in primary total hip arthroplasty: a systematic literature review.Johannson HR, Zywiel MG, Marker DR, Jones LC, McGrath MS, Mont MA.Int Orthop. 2011 Apr;35(4):465-73. Epub 2010 Feb 25. Review.ⅴ、A randomized trial comparing the efficacy and safety of imiglucerase (Cerezyme) infusions every 4 weeks versus every 2 weeks in the maintenance therapy of adult patients with Gaucher disease type 1.Kishnani PS, DiRocco M, Kaplan P, Mehta A, Pastores GM, Smith SE, Puga AC, Lemay RM, Weinreb NJ.Mol Genet Metab. 2009 Apr;96(4):164-70. Epub 2009 Feb 4.ⅵ、Antistaphylococcal activity of dihydrophthalazine antifolates, a family of novel antibacterial drugs.Clark C, Ednie LM, Lin G, Smith K, Kosowska-Shick K, McGhee P, Dewasse B, Beachel L, Caspers P, Gaucher B, Mert G, Shapiro S, Appelbaum PC.Antimicrob Agents Chemother. 2009 Apr;53(4):1353-61. Epub 2009 Feb 2.ⅶ、The long-term international safety experience of imiglucerase therapy for Gaucher disease.Starzyk K, Richards S, Yee J, Smith SE, Kingma W.Mol Genet Metab. 2007 Feb;90(2):157-63. Epub 2006 Oct 31.ⅷ、Pseudo-Gaucher cells in myelodysplasia.Stewart AJ, Jones RD.J Clin Pathol. 1999 Dec;52(12):917-8.ⅸ、Regulation of expression of the gene encoding human acid beta-glucosidase in different cell types.Doll RF, Smith FI.Gene. 1993 May 30;127(2):255-60.ⅹ、Genetic diagnosis of Gaucher's disease.Mistry PK, Smith SJ, Ali M, Hatton CS, McIntyre N, Cox TM.Lancet. 1992 Apr 11;339(8798):889-92.③Leeukaemiaⅰ、Effectiveness of chemical agents in removing platinum from DNA isolated from cisplatin-treated HL-60 cells.Oliński R, Briggs RC, Basinger M, Jones MM.Acta Biochim Pol. 1992;39(4):327-34.ⅱ、Differentiating large cell lymphoma from indolent small B-cell lymphoma in fine needle aspirates using p53, PCNA and transformed lymphocyte count.Young NA, Ehya H, Klein-Szanto A, Litwin S, Smith MR, al-Saleem T.Acta Cytol. 2000 Jul-Aug;44(4):592-603.ⅲ、Human leukemic T and B lymphoblasts produce insulin-like growth factor binding proteins 2 and 4.Neely EK, Smith SD, Rosenfeld RG.Acta Endocrinol (Copenh). 1991 Jun;124(6):707-14.ⅳ、A fourth case of 8p11 myeloproliferative disorder transforming to B-lineage acute lymphoblastic leukaemia. A case report.JabbarAl-Obaidi M, Rymes N, White P, Pomfret M, Smith H, Starczynski J, Johnson R. Acta Haematol. 2002;107(2):98-100. Review.ⅴ、Chlorambucil lung toxicity.Giles FJ, Smith MP, Goldstone AH.Acta Haematol. 1990;83(3):156-8. Review.ⅵ、Acquired Pelger-Huet anomaly in a case of non-Hodgkin's lymphoma.Liesveld J, Smith BD.Acta Haematol. 1988;79(1):46-9.ⅶ、Allogeneic, syngeneic and autologous marrow transplantation in the acute leukemias and lymphomas--Baltimore experiences.Santos GW, Saral R, Burns WH, Braine HG, Sensenbrenner LL, Wingard JR, Yeager AM, Jones R, Ambinder RF, Rowley SD, et al.Acta Haematol. 1987;78 Suppl 1:175-80.ⅷ、Comparison of results of salvage therapy in adult acute myelogenous leukemia. Keating MJ, Estey E, Kantarjian HM, Walters R, Smith T, McCredie KB, Freireich EJ. Acta Haematol. 1987;78 Suppl 1:120-6.ⅸ、Lymphadenopathy complicating hairy cell leukaemia. A case of disseminated Mycobacterium kansasii infection.Mead GM, Dance DA, Smith AG.Acta Haematol. 1983;70(5):335-6.ⅹ、Unusual lymphocyte morphology in a case of chronic lymphatic leukaemia: apparent nuclear extrusion.Newell DG, Jayaswal U, Smith J, Roath S.Acta Haematol. 1978;59(1):25-30.④Breast cancerⅰ、Comparative Effectiveness of Medications To Reduce Risk of Primary Breast Cancer in Women [Internet].Nelson HD, Fu R, Humphrey L, Smith MEB, Griffin JC, Nygren P.Rockville (MD): Agency for Healthcare Research and Quality (US); 2009 Sep.ⅱ、Alexa Fluor 680-glycylglycylglycine-bombesin[7-14]NH2 peptide.Cheng KT, Smith CJ, Ma L, Yu P, Hoffman TJ.Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2010.2007 Jul 20 [updated 2007 Aug 25].ⅲ、Investigation of optimal use of computer-aided detection systems: the role of the "machine" in decision making process.Paquerault S, Hardy PT, Wersto N, Chen J, Smith RC.Acad Radiol. 2010 Sep;17(9):1112-21. Epub 2010 Jun 3.ⅳ、Investigation of reading mode and relative sensitivity as factors that influence reader performance when using computer-aided detection software.Paquerault S, Samuelson FW, Petrick N, Myers KJ, Smith RC.Acad Radiol. 2009 Sep;16(9):1095-107. Epub 2009 Jun 11.ⅴ、Detection and localization of occult lesions using breast magnetic resonance imaging: initial experience in a community hospital.Friedman P, Sanders L, Russo J, Sharo R, Swaminathan S, Smith R.Acad Radiol. 2005 Jun;12(6):728-38.ⅵ、Comparison of core needle breast biopsy techniques: freehand versusthree-dimensional US guidance.Smith WL, Surry KJ, Kumar A, McCurdy L, Downey DB, Fenster A.Acad Radiol. 2002 May;9(5):541-50.。
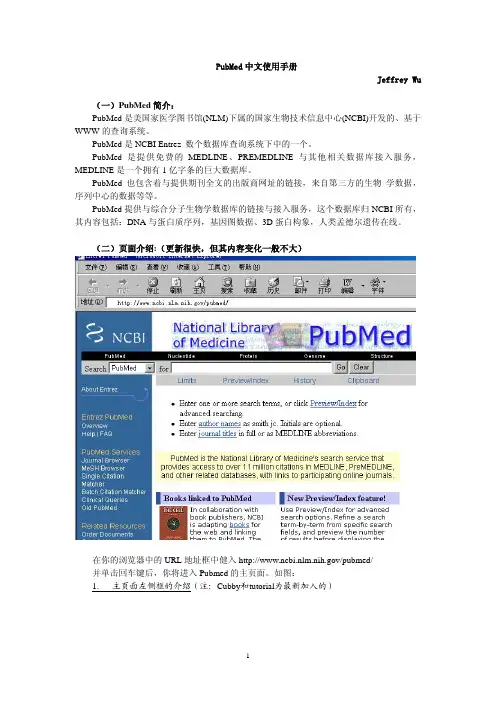
PubMed中文使用手册Jeffrey Wu(一)PubMed简介:PubMed是美国家医学图书馆(NLM)下属的国家生物技术信息中心(NCBI)开发的、基于WWW的查询系统。
PubMed是NCBI Entrez 数个数据库查询系统下中的一个。
PubMed 是提供免费的MEDLINE、PREMEDLINE与其他相关数据库接入服务,MEDLINE是一个拥有1亿字条的巨大数据库。
PubMed也包含着与提供期刊全文的出版商网址的链接,来自第三方的生物学数据,序列中心的数据等等。
PubMed提供与综合分子生物学数据库的链接与接入服务,这个数据库归NCBI所有,其内容包括:DNA与蛋白质序列,基因图数据、3D蛋白构象,人类孟德尔遗传在线。
(二)页面介绍:(更新很快,但其内容变化一般不大)在你的浏览器中的URL地址框中健入/pubmed/并单击回车键后,你将进入Pubmed的主页面。
如图:1. 主页面左侧框的介绍(注:Cubby和tutorial为最新加入的)MeSh Browser你可以用它来分层浏览MesH表Single Citation Matcher通过填表的形式输入期刊的信息可以找到某单篇的文献或整个期刊的内容。
Batch Citation Matcher用一种特定的形式输入期刊的信息一次搜索多篇文献。
Clinical Queries这一部分为临床医生设置,通过过滤的方式将搜索的文献固定在4个范围:治疗、诊断、病原学与预后。
Old PubMed (使用以前的PubMed查询方式)关于每一项的具体使用方法, 后面将会有详细介绍。
Related ResourcesOrder Documents提供一种收费性质服务,可以使用户在当地得到文献的全文拷贝(费用与发送方式各不相同)。
Grateful Med是对另一个NLM基于网络的查询系统的链接。
Grateful Med也提供MEDLINE的接入,并且还有一些其他的数据库如AIDSLINE、HISTLINE等等。

pubmed教程PubMed 检索系统PubMed 检索系统PubMed系统是由美国国立生物技术信息中心(NCBI)开发的用于检索MEDLINE、PreMED-LINE数据库的网上检索系统。
MEDLINE 是美国国立医学图书馆(U.S.NationalLibrary ofMedicine)最重要的书目文摘数据库,内容涉及医学、护理学、牙科学、兽医学、卫生保健和基础医学。
收录了全世界70多个国家和地区的4000余种生物医学期刊,现有书目文摘条目1000万余条,时间起自1966年。
PreMEDLINE是一个临时性医学文献数据库。
它每天都在不断地接受新数据,可为用户提供基本的文献条目和文摘,其文献条目在标引和加工后每周向MEDLINE移加一次。
PubMed还通过电子通讯方式接受出版商提供的文献条目数据,这种条目带有[MEDLINE record inprocess]的说明,并标有[Record as supplied bypublisher]的标识。
这种条目每天都在不停地向PreMEDLINE数据库中传送,但其中有些条目由于超出了MEDLARS数据库的收录范围,将永远不会被PreMEDLINE或MEDLINE条目所取代,例如在综合性的科学杂志(Science或Nature)上发表的地理学文章等。
PubMed 系统的主要特点PubMed 检索Details 键的用法存储检索策略--URL 键的用法特征栏(Feature Bar)介绍检索结果的显示、存盘、打印PubMed 详解PubMed 系统的主要特点1.词汇自动转换功能(Automatic TermMapping)在PubMed主页的检索提问框中键入检索词,系统将按顺序使用如下4种表或索引,对检索词进行转换后再检索。
(1)MeSH转换表(MeSH TranslationTable),包括MeSH词、参见词、副主题词等。
如果系统在该表中发现了与检索词相匹配的词,就会自动将其转换为相应的MeSH词和TextWord词(题名词和文摘词)进行检索。