Supplier Audit Checklist(包材供应商稽核清单)
- 格式:xls
- 大小:112.50 KB
- 文档页数:1


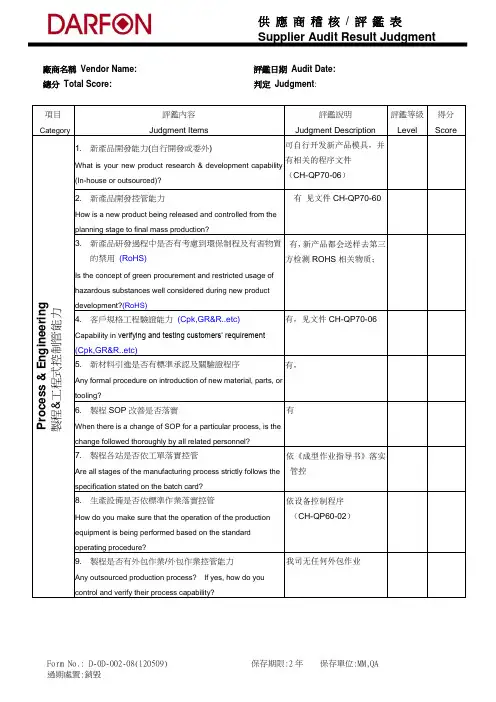
廠商名稱 Vendor Name: 評鑑日期 Audit Date: 總分 Total Score: 判定 Judgment : 項目Category評鑑內容 Judgment Items評鑑說明 Judgment Description 評鑑等級 Level得分 ScoreP r o c e s s & E n g i n e e r i n g 製程&工程式控制管能力1. 新產品開發能力(自行開發或委外)What is your new product research & development capability (In-house or outsourced)?可自行开发新产品模具,并有相关的程序文件 (CH-QP70-06)2. 新產品開發控管能力How is a new product being released and controlled from the planning stage to final mass production?有 见文件CH-QP70-603. 新產品研發過程中是否有考慮到環保制程及有害物質的禁用 (RoHS)Is the concept of green procurement and restricted usage of hazardous substances well considered during new productdevelopment?(RoHS)有,新产品都会送样去第三方检测ROHS 相关物质;4. 客戶規格工程驗證能力 (Cpk,GR&R..etc)Capability in verifying and testing customers’ requirement(Cpk,GR&R..etc)有,见文件CH-QP70-065. 新材料引進是否有標準承認及關驗證程序Any formal procedure on introduction of new material, parts, or tooling?有,6. 製程SOP 改善是否落實 When there is a change of SOP for a particular process, is the change followed thoroughly by all related personnel?有7. 製程各站是否依工單落實控管 Are all stages of the manufacturing process strictly follows the specification stated on the batch card?依《成型作业指导书》落实管控8. 生產設備是否依標準作業落實控管How do you make sure that the operation of the production equipment is being performed based on the standard operating procedure?依设备控制程序(CH-QP60-02)9. 製程是否有外包作業/外包作業控管能力Any outsourced production process? If yes, how do you control and verify their process capability?我司无任何外包作业項目Category評鑑內容Judgment Items評鑑說明 Judgment Description 評鑑等級 Level得分 ScoreP r o c e s s & E n g i n e e r i n g 製程&工程式控制管能力10. 製程良品、不良品是否明顯區隔Any distinct segregation between OK items and non-conforming items during the production process?有,见现场均有标识区分11. 製程上是否有引用SPC 方法來管控?Are SPC methods implemented to monitor the production process?有,SPC 作业规范12. 相關組織架構分工是否明確Is there an organization chart that clearly specifies the tasks and responsibilities?有,见质量手册(CH-QM-001)Q u a l i t y S y s t e m s品保系統控管能力13. 品保體系是否經過第三者驗證Has the quality system being certified by a third party?每年一次北京泰瑞特认证公司到我司审查验证;14. IQC 是否訂有檢驗計畫&檢驗規範(是否包含RoHS 之檢驗規範) Does IQC have an inspection plan and judgment specification that including of RoHS inspection spec .?有,进料检验作业规范(CH-WI-007),且要求供应商每年提供一次原材料的第三方检测的ROHS 报告;15. IQC 檢驗&表單作業是否落實How to make sure the integrity of IQC inspection?有,见原物料检验单16. IQC 良品、不良品區隔及異常追蹤是否落實Any distinct segregation between IQC inspected ok items and non-conforming items? How are non-conforming items being treated?有,见现场标识17. 抽樣計畫是否有明確定義?Are the sampling plans for inspections clearly defined?有,见文件CH-WI-01518. 是否有IPQC 來稽查製程作業及追蹤改善Is there IPQC to audit the process operations and to follow up on continuous process improvements?有IPQC 巡回检验,每小时一次并记录于《巡检日报表》上若有异常并开立制程异常分析改善报告追踪改善;19. IPQC 檢驗&表單作業是否落實How to make sure that the IPQC inspection is thoroughly carried out?有,如《巡检日报表》有按规定执行20. 成品檢驗是否訂有規範&檢驗規範Any inspection procedure and specification for acceptance criteria for finished product?有,如塑胶卷轴检验作业规范項目Category評鑑內容Judgment Items評鑑說明 Judgment Description 評鑑等級 Level得分 ScoreQ u a l i t y S y s t e m s 品保系統控管能力21. 出貨檢驗是否訂有規範&檢驗規範Any inspection procedure and specification for out-goinginspections?有,出货检验作业规范22. 信賴性檢驗作業及規範是否完整Any procedure and testing criteria on reliability testing?有,如塑胶卷轴检验作业规范23. 供应商是否有RMA 的管控流程?Do you have a RMA procedure?有24. 客訴處理時效及有效性是否良好How are customer complaints being handled?有,详见客诉处理单S t o r a g e a n d L o g i s t i c s 物流系統控管能力25. 倉儲管理良品、不良品是否區隔完整(RoHS 如何區別?)Any distinct segregation between ok and non-conforming items in storage or warehouse?(How to differentiate RoHS?)有相关区域标识,详见现场26. 出貨物流是否建立Barcode 系統管制,此系統能否與客戶Barcode 系統相容?Is there a barcode scanning system to control and confirmoutgoing shipment of customer orders? If so, is the barcode system corresponds to customers’ s ystem?我司出货物流是以生产批号和出货日期管制的27. 出貨管制作業能否確保符合客戶所需Does the procedure ensure that the final shipment conform to all customer’s requirement?能符合所需28. 倉儲存放作業是否依規範執行Are there procedures for handling, storing, packaging and delivery of product? How do you check the execution of the procedure?是,见文件<仓储管理办法》 CH-WI-022;29. 供應商是否使用正式的,檔化的全面經營計劃,包括短期和長期目標和計劃Does the company have a short term and long term operational goal or target that is recognizable in the official documentation?是項目 Category評鑑內容Judgment Items評鑑說明Judgment Description評鑑等級 Level得分 ScoreE n v i r o n m e n t a l (H SF ) M a n a g e m e n t 綠色環境(有害物質)系統控管能力30. 供應商送樣承認時,如何保證不含有環境管理物質。
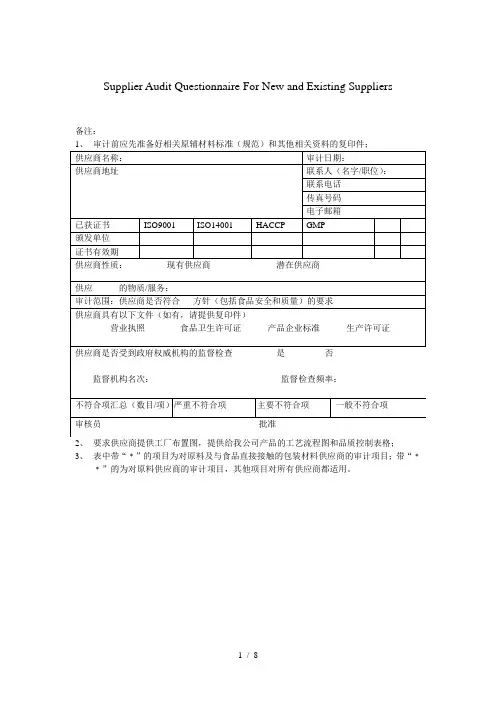

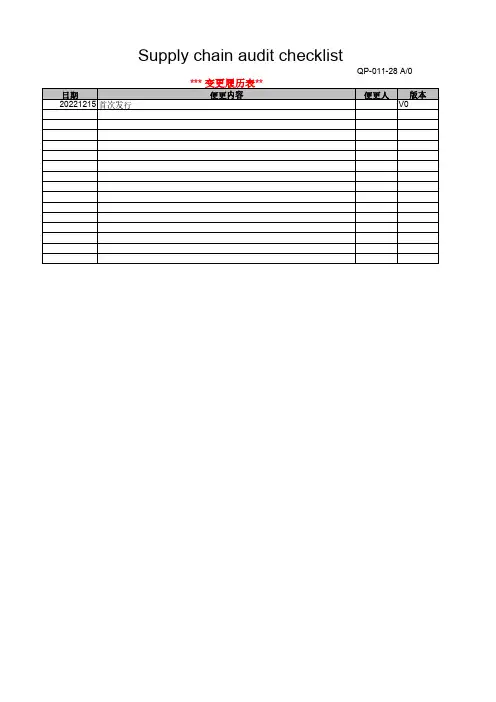

1. Supplier information (供应商) :Name (名称):Status (状态):Location (供应商厂址):□ Initial (首次)Telephone (电话号码):□ Follow-up (跟踪)Fax #: (传真号码)□ On-going (持续)Capital configuration(资本结构)Degree in industry(行业地位)Major customer (主要客户)Major equipment (主要测量设备)□ Yes □ No □ Yes□ No3. Supplier Remarks (供应商备注):4. Supplier Representative-Signature/Date (供应商代表-署名/日期):Signature (署名):Date (日期):5. Audit Status (稽核模式):"A1" - Approved by site visit 参观工厂式评估□ Yes "AS" - Approved by self assessment 自我评估□ Yes "CT" - Conditionally approved for Time 有时间条件地评估□ Yes "CL" - Conditionally approved for Limitatious 有限制条件地评估□ Yes "AW" - Approval Withheld 拒绝评估□ Yes "NR" - Not Rate 不用评估□ Yes6. Summary and evaluation of Audit (稽核总体概述及评价):7. Conclusion (结论,参照第9项)Total Score (稽核得分):Assess Grade (评定等级):Audit Result (稽核结果):8. Assessment -Signature/Date (评估-署名/日期):Assessor (评审员):Title (职务):Date (日期):Checked by (审核):Title (职务):Date (日期):Approved by (审批):Title (职务):Date (日期):Notes(注): No. 1-4 items should be filled by supplier.(第1-4项由供应商填写)Supplier Audit ChecklistLeviton Electronic (Dongguan) Co., Ltd.立维腾电子(东莞)有限公司2. Pre-Audit Mandatory Requirements (for initial audits only) 稽核的前提条件 :A. Does supplier conduct the QMS like as ISO 9001? 供应商是否导入质量管理体系(例如:ISO 9001)?B. Does supplier agree to upgrade the system to meet the audit requirements? 供应商是否同意提升自身系统以配合稽核的要求?请注明贵司获得的QMS 质量管理体系证书编号和有效期,认证机构名称等.。

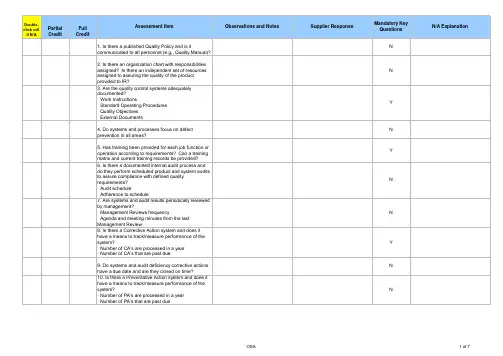
0-Jan-00RequirementsTypical Objective Evidence AUDIT FINDINGS & OBSERVATIONSN o t A p p l i c a b l eN o t V e r i f i e dSupplier Self-Audit SCORE SupplierCA-PA Req'd?(Y / N)On-Site Audit SCORE After CAPA Verif. SCORECompletionDate (mm/dd/yy)Status1.0 Q U A L I T Y M A N A G E M E N T1.1The quality system is documented,controlled, and maintained to clearly describe current practice.Documented procedures required.Records required.Quality manual and all QSprocedures show revision control (sign-offs & dates), history of changes, quality organization's responsibilities1.0 Q U A L I T Y M A N A G E M E N T1.2Quality reports, trend charts and data analysis identify areas of opportunity and are used bymanagement on a routine basis.Records required.Product quality yield data, top problems and corresponding improvement actions, status of preventive/corrective actions taken, internal audit results1.0 Q U A L I T Y M A N A G E M E N T1.3Quality performance targets are clearly defined, included in the business plan and monitored for improvements.Strategic and tactical objectives,goals, action plans, etc.1.0 Q U A L I T Y M A N A G E M E N T1.4Executive management participates in periodic quality system reviews that address quality related feedback from customers and internal quality metrics. Records required.Analysis of field failures,inspection yields, resource needs, internal audit results,corrective action status, etc.2.0 C O N T I N U O U S I M P R O V E M E N T2.1Preventive actions are taken based on the analysis of significant business trends, design reviews,customer satisfaction surveys or other meaningful inputs.Documented procedures required.Records required.Management review meetings,goal setting, performance measurement, internal audits,action plans, customer surveys2.0 C O N T I N U O U S I M P R O V E M E N T2.2A formal approach is used to actively pursue cost containment and other continual improvement activities throughout the organization. Documented procedures required. Records required.Employee involvement /recognition program, Lean, Six Sigma, kaizen, SPC, 5-S, cost reduction program, preventive actions0-Jan-00RequirementsTypical Objective Evidence AUDIT FINDINGS & OBSERVATIONSN o t A p p l i c a b l eN o t V e r i f i e dSupplier Self-Audit SCORE SupplierCA-PA Req'd?(Y / N)On-Site Audit SCORE After CAPA Verif. SCORECompletionDate (mm/dd/yy)Status0-Jan-00RequirementsTypical Objective Evidence AUDIT FINDINGS & OBSERVATIONSN o t A p p l i c a b l eN o t V e r i f i e dSupplier Self-Audit SCORE SupplierCA-PA Req'd?(Y / N)On-Site Audit SCORE After CAPA Verif. SCORECompletionDate (mm/dd/yy)Status0-Jan-00RequirementsTypical Objective Evidence AUDIT FINDINGS & OBSERVATIONSN o t A p p l i c a b l eN o t V e r i f i e dSupplier Self-Audit SCORE SupplierCA-PA Req'd?(Y / N)On-Site Audit SCORE After CAPA Verif. SCORECompletionDate (mm/dd/yy)Status0-Jan-00RequirementsTypical Objective Evidence AUDIT FINDINGS & OBSERVATIONSN o t A p p l i c a b l eN o t V e r i f i e dSupplier Self-Audit SCORE SupplierCA-PA Req'd?(Y / N)On-Site Audit SCORE After CAPA Verif. SCORECompletionDate (mm/dd/yy)Status0-Jan-00RequirementsTypical Objective Evidence AUDIT FINDINGS & OBSERVATIONSN o t A p p l i c a b l eN o t V e r i f i e dSupplier Self-Audit SCORE SupplierCA-PA Req'd?(Y / N)On-Site Audit SCORE After CAPA Verif. SCORECompletionDate (mm/dd/yy)Status0-Jan-00RequirementsTypical Objective Evidence AUDIT FINDINGS & OBSERVATIONSN o t A p p l i c a b l eN o t V e r i f i e dSupplier Self-Audit SCORE SupplierCA-PA Req'd?(Y / N)On-Site Audit SCORE After CAPA Verif. SCORECompletionDate (mm/dd/yy)Status0-Jan-00RequirementsTypical Objective Evidence AUDIT FINDINGS & OBSERVATIONSN o t A p p l i c a b l eN o t V e r i f i e dSupplier Self-Audit SCORE SupplierCA-PA Req'd?(Y / N)On-Site Audit SCORE After CAPA Verif. SCORECompletionDate (mm/dd/yy)Status0-Jan-00RequirementsTypical Objective Evidence AUDIT FINDINGS & OBSERVATIONSN o t A p p l i c a b l eN o t V e r i f i e dSupplier Self-Audit SCORE SupplierCA-PA Req'd?(Y / N)On-Site Audit SCORE After CAPA Verif. SCORECompletionDate (mm/dd/yy)Status0-Jan-00RequirementsTypical Objective Evidence AUDIT FINDINGS & OBSERVATIONSN o t A p p l i c a b l eN o t V e r i f i e dSupplier Self-Audit SCORE SupplierCA-PA Req'd?(Y / N)On-Site Audit SCORE After CAPA Verif. SCORECompletionDate (mm/dd/yy)Status0-Jan-00RequirementsTypical Objective Evidence AUDIT FINDINGS & OBSERVATIONSN o t A p p l i c a b l eN o t V e r i f i e dSupplier Self-Audit SCORE SupplierCA-PA Req'd?(Y / N)On-Site Audit SCORE After CAPA Verif. SCORECompletionDate (mm/dd/yy)Status0-Jan-00RequirementsTypical Objective Evidence AUDIT FINDINGS & OBSERVATIONSN o t A p p l i c a b l eN o t V e r i f i e dSupplier Self-Audit SCORE SupplierCA-PA Req'd?(Y / N)On-Site Audit SCORE After CAPA Verif. SCORECompletionDate (mm/dd/yy)Status0-Jan-00RequirementsTypical Objective Evidence AUDIT FINDINGS & OBSERVATIONSN o t A p p l i c a b l eN o t V e r i f i e dSupplier Self-Audit SCORE SupplierCA-PA Req'd?(Y / N)On-Site Audit SCORE After CAPA Verif. SCORECompletionDate (mm/dd/yy)StatusS0-Jan-00RequirementsTypical Objective Evidence AUDIT FINDINGS & OBSERVATIONSN o t A p p l i c a b l eN o t V e r i f i e dSupplier Self-Audit SCORE SupplierCA-PA Req'd?(Y / N)On-Site Audit SCORE After CAPA Verif. SCORECompletionDate (mm/dd/yy)Status0-Jan-00RequirementsTypical Objective Evidence AUDIT FINDINGS & OBSERVATIONSN o t A p p l i c a b l eN o t V e r i f i e dSupplier Self-Audit SCORE SupplierCA-PA Req'd?(Y / N)On-Site Audit SCORE After CAPA Verif. SCORECompletionDate (mm/dd/yy)Status0-Jan-00RequirementsTypical Objective Evidence 1.0 Q U A L I T Y M A N A G E M E N T1.1The quality system is documented,controlled, and maintained to clearly describe current practice.Documented procedures required.Records required.Quality manual and all QSprocedures show revision control (sign-offs & dates), history of changes, quality organization's responsibilities1.0 Q U A L I T Y M A N A G E M E N T1.2Quality reports, trend charts and data analysis identify areas of opportunity and are used bymanagement on a routine basis.Records required.Product quality yield data, top problems and corresponding improvement actions, status of preventive/corrective actions taken, internal audit results1.0 Q U A L I T Y M A N A G E M E N T1.3Quality performance targets are clearly defined, included in the business plan and monitored for improvements.Strategic and tactical objectives,goals, action plans, etc.1.0 Q U A L I T Y M A N A G E M E N T1.4Executive management participates in periodic quality system reviews that address quality related feedback from customers and internal quality metrics. Records required.Analysis of field failures,inspection yields, resource needs, internal audit results,corrective action status, etc.2.0 C O N T I N U O U S I M P R O V E M E N T2.1Preventive actions are taken based on the analysis of significant business trends, design reviews,customer satisfaction surveys or other meaningful inputs.Documented procedures required.Records required.Management review meetings,goal setting, performance measurement, internal audits,action plans, customer surveys2.0 C O N T I N U O U S I M P R O V E M E N T2.2A formal approach is used to actively pursue cost containment and other continual improvement activities throughout the organization. Documented procedures required. Records required.Employee involvement /recognition program, Lean, Six Sigma, kaizen, SPC, 5-S, cost reduction program, preventive actions0-Jan-00Requirements Typical Objective Evidence0-Jan-00Requirements Typical Objective Evidence0-Jan-00Requirements Typical Objective Evidence0-Jan-00Requirements Typical Objective Evidence0-Jan-00Requirements Typical Objective Evidence0-Jan-00Requirements Typical Objective Evidence0-Jan-00Requirements Typical Objective Evidence0-Jan-00Requirements Typical Objective Evidence0-Jan-00Requirements Typical Objective Evidence0-Jan-00Requirements Typical Objective Evidence0-Jan-00Requirements Typical Objective Evidence0-Jan-00Requirements Typical Objective Evidence S0-Jan-00Requirements Typical Objective Evidence0-Jan-00Requirements Typical Objective Evidence。

1. Supplier information (供应商) :Name (名称):
Status (状态):Location (供应商厂址):□ Initial (首次)Telephone (电话号码):□ Follow-up (跟踪)Fax #: (传真号码)
□ On-going (持续)Capital configuration(资本结构)Degree in industry(行业地位)Major customer (主要客户)Major equipment (主要测量设备)
□ Yes □ No □ Yes
□ No
3. Supplier Remarks (供应商备注):
4. Supplier Representative-Signature/Date (供应商代表-署名/日期):
Signature (署名):Date (日期):
5. Audit Status (稽核模式):
"A1" - Approved by site visit 参观工厂式评估□ Yes "AS" - Approved by self assessment 自我评估
□ Yes "CT" - Conditionally approved for Time 有时间条件地评估□ Yes "CL" - Conditionally approved for Limitatious 有限制条件地评估□ Yes "AW" - Approval Withheld 拒绝评估□ Yes "NR" - Not Rate 不用评估
□ Yes
6. Summary and evaluation of Audit (稽核总体概述及评价):
7. Conclusion (结论,参照第9项)Total Score (稽核得分):
Assess Grade (评定等级):
Audit Result (稽核结果):
8. Assessment -Signature/Date (评估-署名/日期):Assessor (评审员):Title (职务):Date (日期):Checked by (审核):Title (职务):Date (日期):Approved by (审批):
Title (职务):
Date (日期):
Notes(注): No. 1-4 items should be filled by supplier.(第1-4项由供应商填写)
Leviton Electronic (Dongguan) Co., Ltd.
立维腾电子(东莞)有限公司
2. Pre-Audit Mandatory Requirements (for initial audits only) 稽核的前提条件 :A. Does supplier conduct the QMS like as ISO 9001? 供应商是否导入质量管理体系(例如:ISO 9001)?
Supplier Audit Checklist
B. Does supplier agree to upgrade the system to meet the audit requirements? 供应商是否同意提升自身系统以配合稽核的要求?。