医疗器械生产主流程图
- 格式:doc
- 大小:132.50 KB
- 文档页数:7

医疗器械工艺流程图编写要求英文回答:Medical Device Process Flow Diagram Writing Requirements.Purpose:To establish guidelines for the creation of process flow diagrams for medical devices.Scope:This document applies to all medical device processes, including design, development, manufacture, and distribution.Requirements:Accuracy: Process flow diagrams must accuratelyreflect the actual flow of activities and materials.Clarity: Diagrams should be easy to understand and interpret.Consistency: Diagrams should use standard symbols and terminology throughout.Completeness: Diagrams should include all relevant steps and activities.Validation: Diagrams must be validated to ensure that they accurately represent the intended process.Procedure:1. Identify the process to be diagrammed.2. Gather data on the process.3. Create a draft diagram.4. Review and validate the diagram.5. Implement the diagram.Specific Requirements:Symbols: Use standard symbols to represent activities, materials, and decisions.Terminology: Use clear and concise terminology that is consistent throughout the diagram.Flow lines: Use arrows to indicate the flow of materials and activities.Decision points: Use diamonds to represent decision points.Validation: Validate diagrams by comparing them to actual observations of the process.Additional Considerations:Software tools: Consider using software tools to create and manage process flow diagrams.Collaboration: Involve all relevant stakeholders in the creation and validation of diagrams.Continuous improvement: Regularly review and update diagrams to ensure accuracy and relevance.Benefits of Using Process Flow Diagrams:Improved communication: Diagrams provide a clear and concise way to communicate complex processes.Increased efficiency: Diagrams help identify bottlenecks and areas for improvement.Reduced errors: Diagrams help ensure that all steps in a process are followed correctly.Enhanced compliance: Diagrams help demonstratecompliance with regulatory requirements.中文回答:医疗器械工艺流程图编写要求。
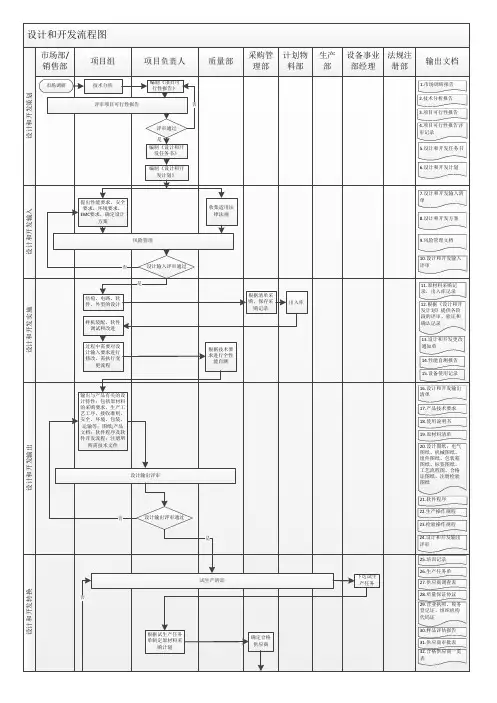
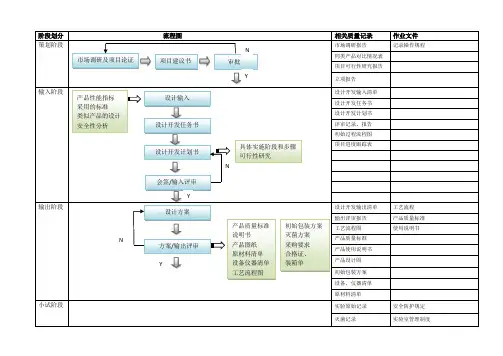
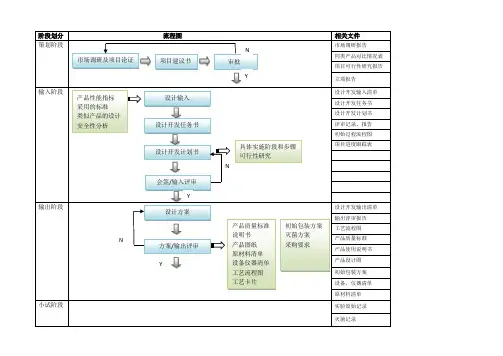
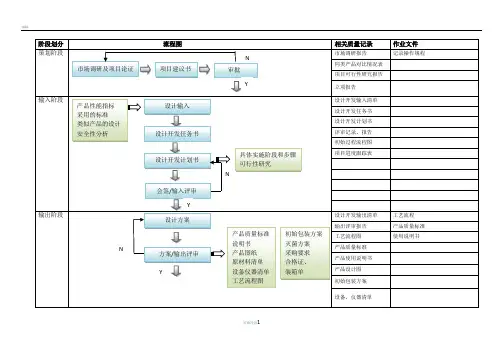
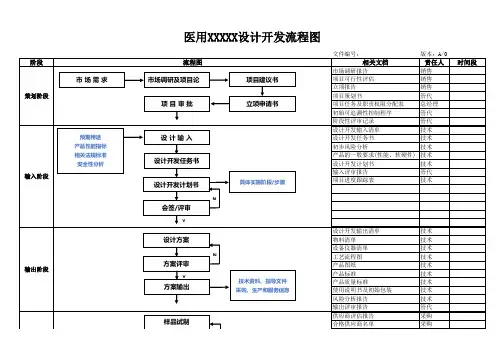

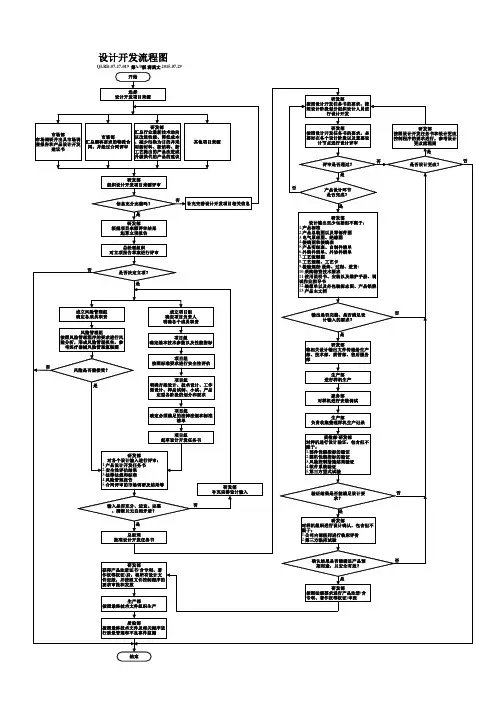
医疗器械项目开发设计流程图详图阶段划分:策划阶段:在这个阶段,我们需要进行市场调研和项目论证,确定产品性能指标和采用的标准,并进行类似产品的设计和安全性分析。
同时,我们需要绘制流程图,并进行项目建议书审批。
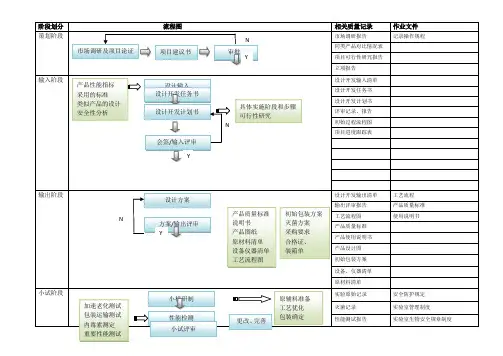
输入阶段:在这个阶段,我们需要制定设计开发任务书和计划书,列出设计开发输入清单,并记录操作规程、工艺流程、产品质量标准和使用说明书等相关质量记录。
输出阶段:在这个阶段,我们需要进行方案和输出评审,完成设计方案、产品质量标准、说明书、产品图纸、原材料清单、设备仪器清单、工艺流程图、初始包装方案、灭菌方案、采购要求、合格证、装箱单等输出。
小试阶段:在这个阶段,我们需要进行小样研制、加速老化测试、包装运输测试、性能检测、内毒素测定和重要性能测试,并进行小试评审。
中试阶段:在这个阶段,我们需要制定试产方案和操作规程,确定包装、灭菌工艺,选择生产、检验设备,并制定实验室管理制度、配液等实验标准操作规程、不合格品管理规程、采购与供方评估管理制度等相关规章制度。
同时,我们需要进行工艺优化、包装确定和更改完善,并撰写性能测试报告和评审记录、报告。
设计验证阶段:在这个阶段,我们需要进行试生产和型式检验,并进行自测报告、型式检验报告、验证记录、报告、批生产记录、批检验记录、留样记录、进货验证记录、报告、车间温、湿度监测记录和生产人员、产品清洁度的管理办法等相关工作。
定型阶段:在这个阶段,我们需要进行设计确认和临床试验,确定临床医院和入组病人选择,并进行实验过程跟踪和结果统计。
同时,我们需要完成注册资料和临床试验方案、合同和报告等相关工作。
准备阶段:在这个阶段,我们需要撰写产品技术报告。
标识和可追溯性流程图项研发部生产管理部质量管理部仓库管理员目开始原确定可追溯产品目录验证材料BOM表产品铭牌产品名称规格型号标追溯批号编号识区域、货架、标签零部件自带铭牌追溯批号编号库存标识标识保护无追溯编号时自作编号并加贴保护领用件标识生产铭牌、标签、领料单过程自制零部件标识产品标签、领料单标识组织调试产品标识流程单发货单上标准追溯批号、编号验证区域、货架、标签验证零部件自带铭牌追溯批号编号生产领用发放标识保护库存标识加贴成品铭牌验证成品标成品检验报告识流程单保存追溯记录库存标识交付时标识包装标识发货单产品铭牌结束状态标识分类检合格验不合格待检验状态标识分区颜色标牌、标签状态标识方法不合格品处理流程图各部门质量管理部总经理开始采购品不合格标识隔离问题分类半成品不合格一般不合格严重不合格成品不合格经理决策共同评审总经理组织各部门退回不合格检验记录评审记录流程单返工/返修退货让步接收报废返工返修单检验反馈问题责任部门报废否合格是入库纠正预防措施流程图记录回收存档完成采购流程图总经理采购主管质量管理部财务主管开始组织生产计划会议参考生产计划参考库存否制定采购计划否审批用款采购计划审批是是开始采购合格供方选择开发新供应商合格供方名录供应商选择签订合同流程图A 类质量协议合同通知供应商供货收货检验流程图验收记录是入库合格入库单否是供应商管理不合格处置流程图审批否按评审表处置定期评价供方业绩评定表质量统计表结束供应商选择流程图否是采购主管质量管理部总经理开始开发/更换供应商收集供应商基本资料供方调查表必要时实地考察否审批是物资分类ABC样品检验检验记录合格否是审批列为合格供应商合格供方名录正常供货结束取消供应商管理评审流程图是是总经理管理者代表质量管理部各部门开始制定管理评审计划管理评审计划否批准审核是三个工作日完成发放计划资料准备部门体系运行报告对体系运行报告内容进行讨论并作出结论主持会议汇总评价企业体系运行报告收集管理评审输入资料整理会议资料会议记录体系运行报告内容:1.内外审核结果;2.顾客反馈投诉;否批准审核是管理评审报告报告编制发放报告3.自查报告;4.产品监视测量;5.纠正预防措施;6.以往管评追踪;7.过程监视测量;8.体系变更;9.法律法规变更;10.改进建议;11.方针目标适宜性;12.目标统计;13.资源配置。
目录1.大容量注射剂生产区概况2.需要验证的关键工序及工艺验证3.工艺流程的实施4.操作过程及工艺条件5.技术安全、工艺卫生及劳动保护6.物料平衡及技经指标7.设备一览表8.岗位定员9.附件(含设备操作、清洁规程)10.变更记录1.大容量注射剂生产区概况本生产区面积904㎡,其中1万级净化区域167㎡,10万级净化区域174㎡。
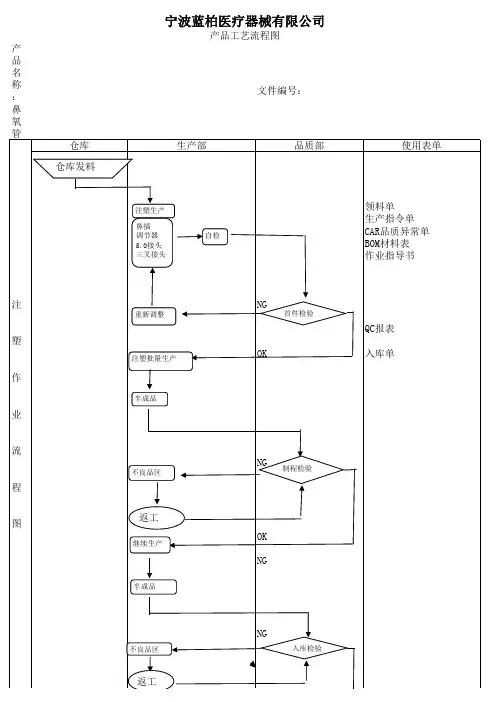
大容量注射剂车间采用10万级和1万级(局部百级)空气净化洁净级别,10万级区域设置缓冲、更衣、洗涤、洁具、称量、配碳、浓配、洗瓶等,1万级区域设置缓冲、更衣更鞋、稀配、化验、灌装、加塞、轧盖、洗涤、存放、洁具等功能间,所有隔断采用无粉尘产生的静电喷涂彩钢板,结合处采用圆角处理,不易产生积灰;人员经过三次更衣和二次更鞋后进入洁净区操作,物料进入洁净区均采用传递窗或气闸进行传递,人流、物流的进入相对分开,保证了洁净区空气洁净度要求;生产设备均采用优质不锈钢材料制造,采用洗瓶、灌装、压塞、轧盖联动线生产,其中洗瓶出口、灌装、加塞采用百级层流保护,灭菌器采用水浴式灭菌器。
1.1 大容量注射剂生产工艺流程图(见后页)1.2 大容量注射剂生产区工艺布局布置图(见后页)1.3 大容量注射剂生产区工艺设备布置图(见后页)1.4 大容量注射剂生产区送回风口平面布置图(见后页)以上项目按验证文件规定,均已在规定周期内进行相关的验证,验证方案及报告见相应文件。
大容量注射剂生产工艺流程图3.工艺流程的实施3.1 批生产指令的签发3.1.1 批生产指令由车间技术负责人根据生产计划表起草,并依据产品工艺规程于生产前一个工作日制定。
3.1.2 批生产指令应经QA质监员审核并签字,由车间主任签字批准后生效。
3.2 生产批记录的发放3.2.1 除配制工序和包装工序外,工序相应的生产批记录于生产当日由车间工艺质监员发放给各工序负责人,并于工序结束当日填写完整返回车间工艺质监员处汇总。
3.2.2 配制工序和包装工序的生产批记录于生产前一天由车间工艺质监员随同批生产指令或批包装指令一同发放,并于工序结束当日填写完整返回车间工艺质监员处汇总。