CrisperCas9技术介绍-修正版.pdf
- 格式:pdf
- 大小:1.20 MB
- 文档页数:10


基因编辑技术CRISPRCas9的使用教程与最佳实践分享在现代生物学研究中,基因编辑技术的出现为研究人员提供了一种高效、精确、低成本的方式来研究基因功能和调控机制。
CRISPR-Cas9系统作为一种革命性的基因编辑工具被广泛应用于基因组编辑、疾病治疗和农业改良等领域。
本文将为您介绍CRISPR-Cas9基因编辑技术的使用教程,并分享一些最佳实践。
CRISPR-Cas9基因编辑技术概述CRISPR-Cas9是一种依靠细菌天然的免疫机制发展而来的基因编辑技术。
CRISPR是一种特殊的DNA序列,可与Cas9酶一起,通过识别和切割DNA序列来精确编辑基因组。
CRISPR-Cas9系统的主要组成部分包括CRISPR RNA (crRNA)、转录单元结构化RNA(tracrRNA)和Cas9酶。
crRNA负责识别目标DNA序列,而tracrRNA将crRNA与Cas9酶结合起来形成活跃的CRISPR-Cas9复合物。
CRISPR-Cas9基因编辑技术的使用教程1. 设计并合成RNA引导序列在使用CRISPR-Cas9进行基因编辑之前,首先需要设计并合成RNA引导序列。
该序列用于指导Cas9酶精确识别和切割目标基因组DNA。
合成的RNA引导序列通常由crRNA和tracrRNA合成而成,也可以合成一个融合的single-guide RNA (sgRNA)。
2. 构建CRISPR-Cas9载体CRISPR-Cas9基因编辑需要将Cas9酶和RNA引导序列导入目标细胞内。
可使用载体如质粒或病毒进行基因编辑构建。
选择合适的载体需考虑目标细胞类型、转染效率和所需编辑范围等因素。
将Cas9基因和RNA引导序列克隆至载体后,可通过转染或病毒介导转染等方法将其导入目标细胞。
3. 确定编辑效果在导入CRISPR-Cas9系统后,使用分子生物学方法来验证编辑效果。
例如,PCR、测序、Western blot或免疫组化等技术可以用于检测目标基因的突变、修复或敲除效果。



CRISPR-Cas9基因编辑技术简介中学生物科学登录CRISPR-Cas9基因编辑技术简介一林黄叶731 人赞同了该文章来源|公众号:中学生物科学CRISPR-Cas9基因编辑技术简介/s?__biz=MzUzMjE2ODkxOA==&mid=224749 0541&idx=1&sn=1db981f771aab1bf6ff57f070d35f468&chksm =fab63254cdc1bb4243bd7e781027a571dd3a76f2355e38eff36b 5bd2635ad5636e8ea9fe914c&token=1724268749&lang=zh_CN #rdCRISPR-Cas9是继ZFN、TALENs等基因编辑技术推出后的第三代基因编辑技术,短短几年内,CRISPR-Cas9技术风靡全球, 成为现有基因编辑和基因修饰里面效率最高、最简便、成本最低、最容易上手的技术之一,成为当今最主流的基因编辑系统。
一、什么是CRISPR-Cas系统CRISPR-Cas系统是原核生物的一种天然免疫系统。
某些细菌在遭到病毒入侵后,能够把病毒基因的一小段存储到自身的 DNA 里一个称为CRISPR 的存储空间。
当再次遇到病毒入侵时,细菌能够根据存写的片段识别病毒,将病毒的DNA切断而使之失效。
C RISPR-Cas系统包含CRISPR基因座和Cas基因(CRISPR关联基因)两部分。
1、CRISPR(/'krɪspər/)是原核生物基因组内的一段重复序列。
CRISPR全称Clustered Regularly Interspersed Short Palindromic Repeats(成簇的规律性间隔的短回文重复序列)。
分布在40%的已测序细菌和90%的已测序古细菌当中。
(注:生活在深海的火山口、陆地的热泉以及盐碱湖等极端环境中,有一些独特结构的细菌,称为古细菌)CRISPR基因序列主要由前导序列(leader)、重复序列(repeat)和间隔序列(spacer)构成。

CRISPR-Cas9文库技术原理及应用CRISPR-Cas9技术原理CRISPR-Cas9技术凭借着成本低廉,操作方便,效率高等优点,CRISPR-Cas9技术迅速风靡全球的实验室,成为了生物科研的有力帮手,是继“锌指核酸内切酶(ZFN)”、“类转录激活因子效应物核酸酶(TALEN)”之后出现的第三代“基因组定点编辑技术”。
CRISPR-Cas9系统最初在大肠杆菌基因组中被发现,是细菌中抵抗外源病毒的免疫系统。
CRISPR-Cas9系统由两部分组成,一部分是用来识别靶基因组的,长度为20bp左右的sgRNA 序列,另外一部分是存在于CRISPR位点附近的双链DNA核酸酶——Cas9,能在sgRNA的引导下对靶位点进行切割,最终通过细胞内的非同源性末端连接机制(NHEJ)和同源重组修复机制(HDR)对形成断裂的DNA进行修复,从而形成基因的敲除和插入,最终实现基因的(定向)编辑。
与前两代技术相比,CRISPR-Cas9技术最大的突破是不仅可以对单个基因进行编辑,更重要的是可以同时对多个基因进行编辑,这也为全基因组筛选提供了有效的方法。
目前比较常见的文库类型包括:●CRISPR-Cas9 knock out文库●CRISPR panal文库●CRISPRa/i文库●psgRNA文库CRISPR-Cas9文库建库流程●靶位点确认及sgRNA文库设计●sgRNA文库芯片合成●sgRNA文库构建●QC验证文库质量●sgRNA文库慢病毒包装●感染稳定细胞株●药物筛选实验●细胞表型筛选●NGS测序验证功能基因CRISPR-Cas9文库应用方向1、药物靶点确定与验证CRISPR-Cas9筛选技术可以应用于药物靶点筛选中,通过大规模筛选技术,可以系统的分析、验证一些与抗药性相关的基因,从而为疾病治疗提供相关数据。
SCIENCE发表文章[1],研究人员利用CRISPR-Cas9文库筛选人类黑色素瘤A375细胞中的18,080个基因进行筛选,最终发现NF2、CUL3等4个基因参与了黑色素瘤A375细胞中的耐药调节过程。
CRISPRCas9基因敲除技术是什么?
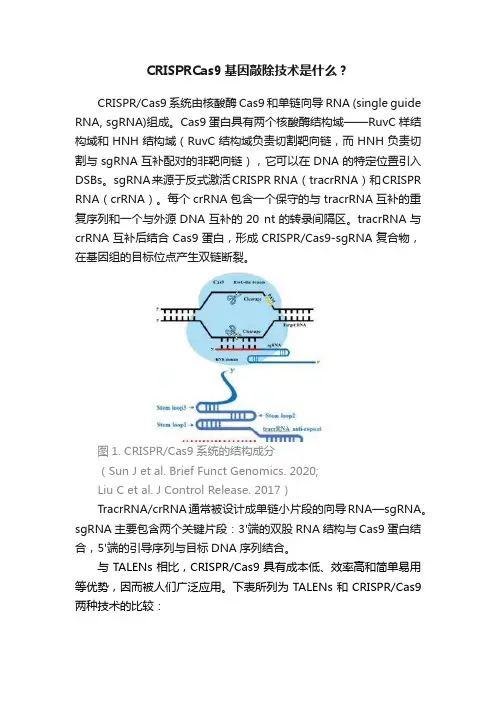
CRISPR/Cas9系统由核酸酶Cas9和单链向导RNA (single guide RNA, sgRNA)组成。
Cas9蛋白具有两个核酸酶结构域——RuvC样结构域和HNH结构域(RuvC结构域负责切割靶向链,而HNH负责切割与sgRNA互补配对的非靶向链),它可以在DNA的特定位置引入DSBs。
sgRNA来源于反式激活CRISPR RNA(tracrRNA)和CRISPR RNA(crRNA)。
每个crRNA包含一个保守的与tracrRNA互补的重复序列和一个与外源DNA互补的20 nt的转录间隔区。
tracrRNA与crRNA互补后结合Cas9蛋白,形成CRISPR/Cas9-sgRNA复合物,在基因组的目标位点产生双链断裂。
图1. CRISPR/Cas9系统的结构成分
(Sun J et al. Brief Funct Genomics. 2020;
Liu C et al. J Control Release. 2017)
TracrRNA/crRNA通常被设计成单链小片段的向导RNA—sgRNA。
sgRNA主要包含两个关键片段:3'端的双股RNA结构与Cas9蛋白结合,5'端的引导序列与目标DNA序列结合。
与TALENs相比,CRISPR/Cas9具有成本低、效率高和简单易用等优势,因而被人们广泛应用。
下表所列为TALENs和CRISPR/Cas9两种技术的比较:。

crispercas9基因编辑原理
CRISPR/Cas9基因编辑技术是一种基于RNA-蛋白质复合物的精准基因编辑技术,可以改变某一特定遗传物质的形状,从而产生新的基因表达。
CRISPR/Cas9是一种可以实现精准基因编辑的有效工具,它可以被用来修改基因的突变,改变基因的表达,插入新的基因,以及删除不需要的基因等。
CRISPR/Cas9基因编辑技术是基于一种叫做CRISPR的自然现象,它能够识别并切割特定的DNA序列。
这一技术的基本原理是利用一种叫做CRISPR-Cas9的RNA-蛋白质复合物,它能够精确地结合到特定的DNA序列,然后将DNA切割成两段。
随后,破裂的DNA段会被新的DNA段所替换,从而达到基因编辑的目的。
由于CRISPR/Cas9基因编辑技术的精确性高且成本低,它已经成为生物技术领域里最流行的研究工具之一。
在近几年,CRISPR/Cas9基因编辑技术已经被用于各种生物学研究,包括药物发现、疾病的诊断和治疗以及农业的育种等,取得了令人惊叹的成果。
此外,CRISPR/Cas9基因编辑技术也被用于临床试验,以研究疾病的病理机制以及治疗方法,从而为病人提供更好的治疗方案。
CRISPR/Cas9基因编辑技术是一种先进的生物技术,具有精确性高、成本低和操作简单等特点。
它已经被广泛应用于生物学研究,也可以用于治疗疾病。
因此,CRISPR/Cas9基因编辑技术有望在未来发
挥更大的作用,为人们的健康带来福音。

What is CRISPR/Cas9?Melody Redman,1Andrew King,2Caroline Watson,3David King41HYMS Centre for Education Development(CED),Hull York Medical School,University of York,York,UK2Molecular Haematology Unit, MRC Weatherall Institute of Molecular Medicine,University of Oxford,John Radcliffe Hospital,Oxford,UK3Department of Haematology, Oxford University NHS Foundation Trust,Churchill Hospital,Oxford,UK4Academic Unit of Child Health, Sheffield Children’s Hospital, Sheffield,UK Correspondence toDr David King,Academic Unit of Child Health,Sheffield Children’s Hospital,Western Bank, Sheffield S102TH,UK;*********************.uk Received5January2016 Revised18February2016 Accepted19February2016INTRODUCTIONClustered regularly interspaced palin-dromic repeats(CRISPR)/Cas9is agene-editing technology causing a majorupheaval in biomedical research.It makesit possible to correct errors in the genomeand turn on or off genes in cells and organ-isms quickly,cheaply and with relativeease.It has a number of laboratory applica-tions including rapid generation of cellularand animal models,functional genomicscreens and live imaging of the cellulargenome.1It has already been demonstratedthat it can be used to repair defective DNAin mice curing them of genetic disorders,2and it has been reported that humanembryos can be similarly modified.3Otherpotential clinical applications include genetherapy,treating infectious diseases such asHIV and engineering autologous patientmaterial to treat cancer and other diseases.In this review we will give an overview ofCRISPR/Cas9with an emphasis on how itmay impact on the specialty of paediatrics.Although it is likely to have a significanteffect on paediatrics through its impact inthe laboratory,here we will concentrate onits potential clinical applications.W e willalso describe some of the difficulties andethical controversies associated with thisnovel technology.OVERVIEW OF CRISPR/CAS9CRISPR/Cas9is a gene-editing technologywhich involves two essential components:a guide RNA to match a desired targetgene,and Cas9(CRISPR-associatedprotein9)—an endonuclease which causesa double-stranded DNA break,allowingmodifications to the genome(seefigure1).POTENTIAL CLINICAL USESCorrection of genetic disordersOne of the most exciting applications ofCRISPR/Cas9is its potential use to treatgenetic disorders caused by single genemutations.Examples of such diseasesinclude cystic fibrosis(CF),Duchenne’smuscular dystrophy(DMD)and haemo-globinopathies.The approach so far hascurrently only been validated in preclin-ical models,but there is hope it can soonbe translated to clinical practice.Schwank et al used CRISPR/Cas9toinvestigate the treatment of ingadult intestinal stem cells obtained fromtwo patients with CF,they successfullycorrected the most common mutationcausing CF in intestinal organoids.Theydemonstrated that once the mutation hadbeen corrected,the function of the CFtransmembrane conductor receptor(CFTR)was restored.4Another disease in which CRISPR/Cas9has been investigated is DMD.T abebordbaret al recently used adeno-associated virus(AA V)delivery of CRISPR/Cas9endonu-cleases to recover dystrophin expression ina mouse model of DMD,by deletion of theexon containing the original mutation.Thisproduces a truncated,but still functionalprotein.T reated mice were shown to par-tially recover muscle functional deficien-cies.5Significantly,it was demonstrated thatthe dystrophin gene was edited in musclestem cells which replenish mature muscletissue.This is important to ensure anytherapeutic effects of CRISPR/Cas9do notfade over time.T wo similar studies havedescribed using the CRISPR/Cas9system invivo to increase expression of the dys-trophin gene and improve muscle functionin mouse models of DMD.67Other studieshave used CRISPR/Cas9to target duplica-tion of exons in the human dystrophin genein vitro and have shown that this approachcan lead to production of full-length dys-trophin in the myotubules of an individualwith DMD.8CRISPR/Cas9could also be used totreat haemoglobinopathies.Canver et al9recently showed BCL11A enhancer dis-ruption by CRISPR/Cas9could inducefetal haemoglobin in both mice andprimary human erythroblast cells.In thefuture such an approach could allow fetalhaemoglobin to be expressed in patientswith abnormal adult haemoglobin.Thiswould represent a novel therapeutic strat-egy in patients with diseases such assickle cell disease orthalassaemias.RESEARCH IN PRACTICE Redman M,et al.Arch Dis Child Educ Pract Ed2016;0:1–3.doi:10.1136/archdischild-2016-3104591 Education & Practice Online First, published on April 8, 2016 as 10.1136/archdischild-2016-310459 Copyright Article author (or their employer) 2016. Produced by BMJ Publishing Group Ltd under licence. copyright. on December 25, 2023 by guest. Protected by / Arch Dis Child Educ Pract Ed: first published as 10.1136/archdischild-2016-310459 on 8 April 2016. Downloaded fromKnock-in of a fully functional β-globin gene is much more challenging,which is the reason for this some-what unusual approach.Treatment of HIVAnother potential clinical application of CRISPR/Cas9is to treat infectious diseases,such as HIV .Although antiretroviral therapy provides an effective treatment for HIV ,no cure currently exists due to permanent integration of the virus into the host genome.Hu et al showed the CRISPR/Cas9system could be used to target HIV-1genome activity .This inactivated HIV gene expression and replication in a variety of cells which can be latently infected with HIV ,without any toxic effects.Furthermore,cells could also be immu-nised against HIV-1infection.This is a potential thera-peutic advance in overcoming the current problem of how to eliminate HIV from infected individuals.After further refinement,the authors suggest their findings may enable gene therapies or transplantation of genet-ically altered bone marrow stem cells or inducible pluripotent stem cells to eradicate HIV infection.10Engineering somatic cells ex vivo to treat malignancy or other diseasesThere has been increasing interest in the possibility of using CRISPR/Cas9to modify patient-derived T-cells and stem/progenitor cells which can then be reintro-duced into patients to treat disease.This approach may overcome some of the issues associated with how to efficiently deliver gene editing to the right cells.T-cell genome engineering has already shown success in treating haematological malignancies and has the potential to treat solid cancers,primary immune deficiencies and autoimmune diseases.Genetic manipulation of T-cells has previously been inefficient.However,Schumann et al recently reported a more effective approach in human CD4+T-cells based on the CRISPR/Cas9system.Their tech-nique allowed experimental and therapeutic knock-out and knock-in editing of the genome in primary human T-cells.They demonstrated T-cells could be manipulated to prevent expression of the protein PD-1,which other work has shown may allow T-cells to target solid cancers.11There is also interest in using CRISPR/Cas9-mediated genome editing in pluripotent stem cells or primary somatic stem cells to treat disease.For example Xie et al 12showed the mutation causing β-thalassaemia could be corrected in human induced pluripotent stem cells ex vivo.They suggest that in the future such an approach could provide a source of cells for bone marrow transplantation to treat β-thalassaemia and other similar monogenic diseases.LIMITATIONSA number of challenges remain before the potential of CRISPR/Cas9can be translated to effective treatments at the bedside.A particular issue is how to deliver gene editing to the right cells,especially if the treat-ment is to be delivered in vivo.T o safely deliver Cas9-nuclease encoding genes and guide RNAs in vivo without any associated toxicity ,a suitable vector is needed.AA V has previously been a favoured option for gene delivery .1However,this delivery system may be too small to allow efficient transduction of the Cas9gene.1A smaller Cas9gene could be used,but this has additional implications on efficacy .1A number of other non-viral delivery systems are under investi-gation and this process requires further optimisation.Another significant concern is the possibility of off-target effects on parts of the genome separate from the region being targeted.Unintentional edits of the genome could have profound long-term complications for patients,including malignancy .The concentration of the Cas9nuclease enzyme and the length of time Cas9is expressed are both important when limiting off-target activity .1Although recent modifications in the nuclease have increased specificity ,further work is required to minimise off-target effects and to establish the long-term safety of any treatment.The therapeutic applications of CRISPR/Cas9con-sidered in this article have predominantly been direc-ted at somatic cells.A particularly controversial issue surrounding CRISPR/Cas9is that of gene editinginFigure 1The CRISPR/Cas9system.1Clustered regularlyinterspaced palindromic repeats (CRISPR)refers to sequences in the bacterial genome.They afford protection against invading viruses,when combined with a series of CRISPR-associated (Cas)proteins.Cas9,one of the associated proteins,is anendonuclease that cuts both strands of DNA.Cas9is directed to its target by a section of RNA.This can be synthesised as a single strand called a synthetic single guide RNA (sgRNA);the section of RNA which binds to the genomic DNA is 18–20nucleotides.In order to cut,a specific sequence of DNA of between 2and 5nucleotides (the exact sequence depends upon the bacteria which produces the Cas9)must lie at the 3’end of the guide RNA:this is called the protospacer adjacent motif (PAM).Repair after the DNA cut may occur via twopathways:non-homologous end joining,typically leading to a random insertion/deletion of DNA,or homology directed repair where a homologous piece of DNA is used as a repair template.It is the latter which allows precise genome editing:thehomologous section of DNA with the required sequence change may be delivered with the Cas9nuclease and sgRNA,theoretically allowing changes as precise as a single base-pair.Research in practice2Redman M,et al .Arch Dis Child Educ Pract Ed 2016;0:1–3.doi:10.1136/archdischild-2016-310459copyright.on December 25, 2023 by guest. Protected by /Arch Dis Child Educ Pract Ed: first published as 10.1136/archdischild-2016-310459 on 8 April 2016. Downloaded fromembryos.It has already been shown that CRISPR/ Cas9technology can alter the genome of human embryos3which theoretically could prove useful in the preimplantation treatment of genetic diseases. However,any genetic modification of the germline would be permanent and the long-term consequences are unclear.Many oppose germline modification under any circumstances,reasoning that an eventual consequence could be non-therapeutic genetic enhancement.13It is clear that the ethical boundaries, within which CRISPR/Cas9can be used,remain to be fully determined.▸CRISPR/Cas9technology has the potential to revolu-tionise the treatment of many paediatric conditions.▸A number of practical and ethical challenges must be overcome before this potential can be realised at thebedside.Contributors DK conceived the idea for this article.All authors were involved in writing and reviewing the final manuscript. Funding AK is supported by a W ellcome T rust Fellowship (108785/Z/15/Z).Competing interests None declared.Provenance and peer review Not commissioned;externally peer reviewed.Open Access This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution(CC BY4.0)license,which permits others to distribute,remix,adapt and build upon this work,for commercial use,provided the original work is properly cited. See:/licenses/by/4.0/REFERENCES1Hsu PD,Lander ES,Zhang F.Development and Applications of CRISPR-Cas9for Genome Engineering.Cell2014;157:1262–78.2Yin H,Xue W,Chen S,et al.Genome editing with Cas9in adult mice corrects a disease mutation and phenotype.NatBiotech2014;32:551–3.3Liang P,Xu Y,Zhang X,et al.CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes.Protein Cell2015;6:363–72.4Schwank G,Koo BK,Sasselli V,et al.Functional repair of CFTR by CRISPR/Cas9in intestinal stem cell organoids ofcystic fibrosis patients.Cell Stem Cell2013;13:653–8.5T abebordbar M,Zhu K,Cheng JK,et al.In vivo gene editing in dystrophic mouse muscle and muscle stem cells.Science2016;351:407–11.6Nelson CE,Hakim CH,Ousterout DG,et al.In vivo genome editing improves muscle function in a mouse model ofDuchenne muscular dystrophy.Science2016;351:403–7.7Long C,McAnally JR,Shelton JM,et al.Prevention of muscular dystrophy in mice by CRISPR/Cas9–mediated editing of germline DNA.Science2014;345:1184–8.8W ojtal D,Kemaladewi Dwi U,Malam Z,et al.Spell checking nature:versatility of CRISPR/Cas9for developing treatmentsfor inherited disorders.Am J Hum Genet2016;98:90–101.9Canver MC,Smith EC,Sher F,et al.BCL11A enhancer dissection by Cas9-mediated in situ saturating mutagenesis.Nature2015;527:192–7.10Hu W,Kaminski R,Y ang F,et al.RNA-directed gene editing specifically eradicates latent and prevents new HIV-1infection.Proc Natl Acad Sci USA2014;111:11461–6.11Schumann K,Lin S,Boyer E,et al.Generation of knock-in primary human T cells using Cas9ribonucleoproteins.ProcNatl Acad Sci USA2015;112:10437–42.12Xie F,Y e L,Chang JC,et al.Seamless gene correction of β-thalassemia mutations in patient-specific iPSCs usingCRISPR/Cas9and piggyBac.Genome Res2014;24:1526–33. 13Lanphier E,Urnov F,Haecker SE,et al.Don’t edit the human germ line.Nature2015;519:410–1.Research in practiceRedman M,et al.Arch Dis Child Educ Pract Ed2016;0:1–3.doi:10.1136/archdischild-2016-3104593copyright. on December 25, 2023 by guest. Protected by / Arch Dis Child Educ Pract Ed: first published as 10.1136/archdischild-2016-310459 on 8 April 2016. Downloaded from。

crispr-cas9CRISPR Cas9系统最初在细菌和古细菌中被发现,是一种细菌应对外来病毒或质粒不断攻击而演化来的获得性免疫防御机制。
当外源DNA首次侵入细菌时,外源DNA被Cas核酸酶切割,并以间隔序列形式整合到CRISPR基因座中。
当外源DNA再次入侵时,细菌会以间隔序列作为模板形成短的CRISPRRNA(crRNA),crRNA与反式激活crRNA (tracrRNA)形成复合RNA:该复合RNA作为引导链指导Cas9核酸酶结合到互补的外源DNA。
一旦结合,Cas9蛋白通过其NHN和RuvC1样核酸酶结构域分别切割外源DNA的两条链,使外源DNA降解。
CRISPRCas9靶向基因组编辑系统有两个组成部分:内切核酸酶-Cas9和指导RNA-gRNA。
内切核酸酶是来自酿脓链球菌的Cas9核酸酶蛋白。
Cas9核酸酶具有切割双链DNA的活性结构域(RuvC1和HNH样核酸酶结构域),使得双链DNA断裂。
gRNA是由起支架功能的tracrRNA与特异性的crRNA结合形成的嵌合RNA。
后来,人们将这两段RNA拼接成一条短RNA,称为sgRNA。
gRNA5'末端的20bp是特异性的结合序列-寻靶装置,通过RNA-DNA碱基配对将Cas9/gRNA复合物募集到特异的DNA靶点。
特异性结合序列相邻的是PAM (ProtospacerAdjacentMotif)基序。
Cas9核酸酶在PAM基序上游第3个碱基处切割双链DNA。
CRISPR Cas9系统产生双链DNA缺口后,生物体内一般通过非同源末端连接(NHEJ)或同源定向修复(HDR)这两种修复方式对断裂的双链DNA进行修复。
NHEJ修复途径是一种错误倾向修复机制,用于在缺少修复模板的情况下修复断裂的双链DNA。
该途径主要用于CRISPR Cas9系统介导的基因沉默。
主要用于当DNA发生断裂时,NHEJ修复途径被开启,DNA断裂处插入或缺失几个碱基,然后双链DNA的切割末端连接在一起,这个过程极容易导致切割位点发生移码突变,从而沉默该基因。
CRISPR/Cas9基因编辑技术具体步骤及方法CRISPR/Cas9 是一种能够对基因组的特定位点进行精确编辑的技术。
其原理是核酸内切酶Cas9蛋白通过导向性RNA(guide RNA, gRNA)识别特定基因组位点并对双链DNA进行切割,细胞随之利用非同源末端连接(Non homologous End Joining,NHEJ)或者同源重组(Homologous Recombination, HR)方式对切割位点进行修复,实现DNA水平基因敲除或精确编辑。
CRISPR基因敲除利用CRISPR / Cas9 进行单基因敲除目前研究最透彻、应用最广泛的II 型-CRISPR/Cas9 系统由两部分组成:1. 单链的guide RNA(single-guide RNA,sgRNA)2. 有核酸内切酶活性的Cas9 蛋白CRISPR/Cas9 系统利用sgRNA 来识别靶基因DNA,并引导Cas9 核酸内切酶剪切DNA(图1)。
当基因组发生双链DNA 断裂后,细胞通过非同源性末端接合(Non-homologous end joining, NHEJ) 将断裂接合,在此过程中,将随机引入N 个碱基的缺失或增加,若N 非3 的倍数,则目的基因发生移码突变,实表1 CRISPR/Cas9 基因敲除与RNAi 比较CRISPR过表达利用CRISPR / Cas9 进行单基因过表达通过修饰CRISPR/Cas9 系统中的一些元件,形成一种蛋白复合物-协同激活介质(SAM),可实现对多数细胞内源基因的特异性激活。
该系统灵活方便,为研究基因功能提供了极为便利的工具。
CRISPR-SAM 系统由三部分组成:1. 失去核酸酶活性的dCas9(deactivated Cas9)-VP64 融合蛋白2. 含2 个MS2 RNA adapter 的sgRNA3. MS2-P65-HSF1 激活辅助蛋白CRISPR-SAM 系统中的MS2-P65-HSF1 激活辅助蛋白就是SAM,全称为SynergisticActivation Mediator( 协同激活调节器),这也就是CRISPR-SAM 的命名由来。
基因敲除cas9【原创版】目录1.基因敲除技术简介2.CAS9 的作用原理3.CAS9 的应用领域4.CAS9 的安全性和伦理问题5.我国在基因敲除 CAS9 研究方面的进展正文1.基因敲除技术简介基因敲除技术是一种能够精准地删除目标基因的方法,对于研究生物系统的基因功能以及疾病治疗有着重要的意义。
近年来,随着基因编辑技术的不断发展,CRISPR-Cas9 系统成为了其中最为流行的一种基因敲除手段。
2.CAS9 的作用原理CRISPR-Cas9 系统中的 CAS9 蛋白,是一种来源于细菌的核酸酶,它能够识别并切割特定的 DNA 序列。
当 CAS9 与 RNA 结合后,可以精确地定位到目标 DNA 序列,然后通过切割使其失活。
这一过程可以看作是基因的“敲除”。
3.CAS9 的应用领域基因敲除 CAS9 技术在多个领域都有着广泛的应用。
在生物学研究中,科学家们可以利用 CAS9 技术来研究基因的功能,探究生物过程的机制。
在医学领域,CAS9 技术被用于治疗一些遗传性疾病,例如癌症、艾滋病等。
此外,CAS9 还被应用于农业领域,通过敲除某些基因来提高作物的抗病性和耐旱性等。
4.CAS9 的安全性和伦理问题虽然 CAS9 技术有着广泛的应用前景,但是其安全性和伦理问题也引起了人们的关注。
首先,CAS9 可能会引发意外的基因突变,导致细胞功能失调。
其次,基因编辑技术可能会被用于生物武器研发,造成严重的安全隐患。
另外,基因编辑技术涉及到生物个体的基因改变,可能引发伦理争议。
5.我国在基因敲除 CAS9 研究方面的进展我国在基因敲除 CAS9 研究方面取得了显著的进展。
我国科学家们在CAS9 蛋白的结构、功能研究方面做出了重要贡献,并且已经成功地利用CAS9 技术治疗了一些遗传性疾病。
同时,我国政府也积极推动基因编辑技术的规范化和标准化,以确保其在安全、可控的范围内发展。
总的来说,基因敲除 CAS9 技术为人类带来了巨大的科研价值和应用前景,同时也伴随着一些安全性和伦理问题。