
Colloids and Surfaces A:Physicochem.Eng.Aspects
280 (2006) 232–236
Short communication
A novel method for the preparation of Bi4Ti3O12
nanoparticles in w/o microemulsion
Lijin Xie a,Junfeng Ma a,b,?,Zhongqiang Zhao a,Hua Tian a,Jun Zhou a,
Yonggang Wang b,Jiantao Tao b,Xiaoyi Zhu b
a College of Chemistry and Chemical Engineering,Ocean University of China,Qingdao266003,China
b Institute of Materials Science and Engineering,Ocean University of China,Qingdao266003,China
Received8August2005;received in revised form8February2006;accepted13February2006
Available online 6 March 2006
Abstract
Nanometer-sized Bi4Ti3O12particles have been prepared by chemical reaction of bismuth nitrate pentahydrate,titanium sulfate and ammonia solution in a reverse microemulsion system consisting of water,OP(P-octyl polyethylene glycol phenylether,non-ionic surfactant),n-butanol (co-surfactant),and cyclohexane(oil).Precursor hydroxides precipitated in the droplets of water-in-oil(w/o)microemulsion were calcined at 800?C for4h to form Bi4Ti3O12nanoparticles.The samples were investigated with X-ray diffraction(XRD),transmission electron microscopy (TEM),fourier transform infrared spectrophotometer(FT-IR)and ultraviolet visible spectrophotometer(UV–vis).It was found that the as-prepared Bi4Ti3O12nanoparticles had small particle sizes(35nm),high crystallinity,narrow size distributions and strong light absorption properties not only in the ultraviolet light but also in the visible light region.
? 2006 Elsevier B.V. All rights reserved.
Keywords:Bi4Ti3O12nanoparticles;Water-in-oil microemulsions;Synthesis
1.Introduction
As a member of the Aurivillius family compounds,which are well known as ferroelectric materials,bismuth titanate Bi4Ti3O12is of particular interest due to its unique electro-optic switching behavior.Generally,it is useful for various appli-cations such as memory storage,optical display,piezoelectric converters or pyroelectric devices over a wide range of tem-peratures[1–6].Bi4Ti3O12is also an important photocatalyst material[7].It is well known that the photocatalytic properties can be improved by decreasing the particle size of the pho-tocatalyst material in order to increase the number of active sites on its surfaces.Thus,for the application of Bi4Ti3O12as photocatalyst material,nanosized particles are desirable.Up to now,several methods,including solid state reaction[8,9], coprecipitation method[10],and hydrothermal synthesis[11], molten salt process[12],citrate method[13],laser sintering [14],were developed for the synthesis of Bi4Ti3O12powders. However,these methods still have some drawbacks.
?Corresponding author.Tel.:+8653282031623;fax:+8653282031623.
E-mail address:majf@https://www.doczj.com/doc/ac11734174.html,(J.Ma).
Reverse microemulsions are colloidal nanodispersions of water in oil stabilized by a surfactant?lm.These thermo-dynamically stable dispersions can be considered as true nanoreactors,which can be used to carry out chemical reactions and,in particular,to synthesize nanomaterials.The synthesis of nanoparticles in microemulsions,the formation mechanisms and growth control in recent years have been reviewed by MP. Pileni[15]and L′o pez-Quintela et al.[16,17].Now,water-in-oil (w/o)microemulsions have been successfully used to synthesize colloidal metals[18,19],superconducting materials[20,21], and magnetic materials[22,23].Unfortunately,no descriptions concerning the preparation of Bi4Ti3O12powders by the use of water-in-oil microemulsions have appeared.In this paper, for the?rst time,some results on the synthesis of Bi4Ti3O12 nanoparticles in water-in-oil microemulsions are presented. 2.Experimental
2.1.Chemicals
Bismuth nitrate pentahydrate(analytic reagent),titanium sul-fate(analytic reagent)were supplied by Shanghai Chemical
0927-7757/$–see front matter? 2006 Elsevier B.V. All rights reserved. doi:10.1016/j.colsurfa.2006.02.015
L.Xie et al./Colloids and Surfaces A:Physicochem.Eng.Aspects 280 (2006) 232–236233
Table1
Composition of the microemulsion system used for the synthesis reactions
M I M II wt.% Aqueous phase0.l M Bi(NO3)3and Ti(SO4)2 1.0M NH4OH20 Surfactant OP OP17 Co-surfactant n-Butanol n-Butanol10 Oil phase Cyclohexane Cyclohexane55
Reagent Corporation.Ammonia water(28wt.%),OP(analytic reagent),n-butanol(Analytic reagent)and cyclohexane(analytic reagent)were obtained from Laiyang Chemical Reagent Corpo-ration.All reagents were used without further puri?cation.The water used throughout this work was distilled water.
2.2.Synthesis of Bi4Ti3O12nanoparticles
Bi4Ti3O12powders were prepared via two processing meth-ods:(i)the conventional co-precipitation method and(ii)the microemulsion method.In the co-precipitation method,ammo-nia water(28wt.%)was added to aqueous solution including Bismuth and Titanium cations(molar ratio of Bi/Ti=4/3)until the pH of the mixture reached8,where a white precipitate was produced.The precipitate was?ltered,washed with distilled water and dried in a drying oven at100?C for24h.Then the dried precipitate was calcined for4h at800?C in an electric furnace in air.
For the microemulsion method,cyclohexane was used as the oil phase,OP was used as the surfactant and n-butanol was used as the co-surfactant.Two kinds of microemulsions(M I and M II)with different aqueous phase were obtained(see Table1). The aqueous phase in the M I was a solution of Bismuth and Titanium cations(molar ratio of Bi/Ti=4/3),while the aqueous phase in the M II containing the precipitating agent,NH4OH (1.0M).These two microemulsions were mixed for3h under constant stirring speed.The opaque appearance of the mixture upon vigorous stirring indicated the formation of the particles. Powder samples were obtained by?occulating the colloids with acetone and followed by separation in a centrifuge.The pre-cipitates were washed with acetone and ethanol to eliminate excess OP,and then dried at100?C for24h.The dried pre-cipitates were calcined at800?C for4h in an electric furnace in air.
2.3.Characterization
X-ray diffraction(XRD,D/max,Rigaku,Japan)employing Cu K?radiation was used to identify the crystalline phase of the prepared powders.JEM-1200EX transmission electron micro-scope(Tokyo,Japan)was employed to examine the morphology and size of the nanoparticles,operated at60.0kV.Samples were prepared by adding ethanol to a fraction of the powders synthesized,ultrasonic dispersing for10min and droplet of it was dropped on a carbon-coated copper grid.The fourier trans-form infrared(FT-IR)spectra were recorded on samples in KBr tablets using an A V ATAR360FT-IR spectrophotometer
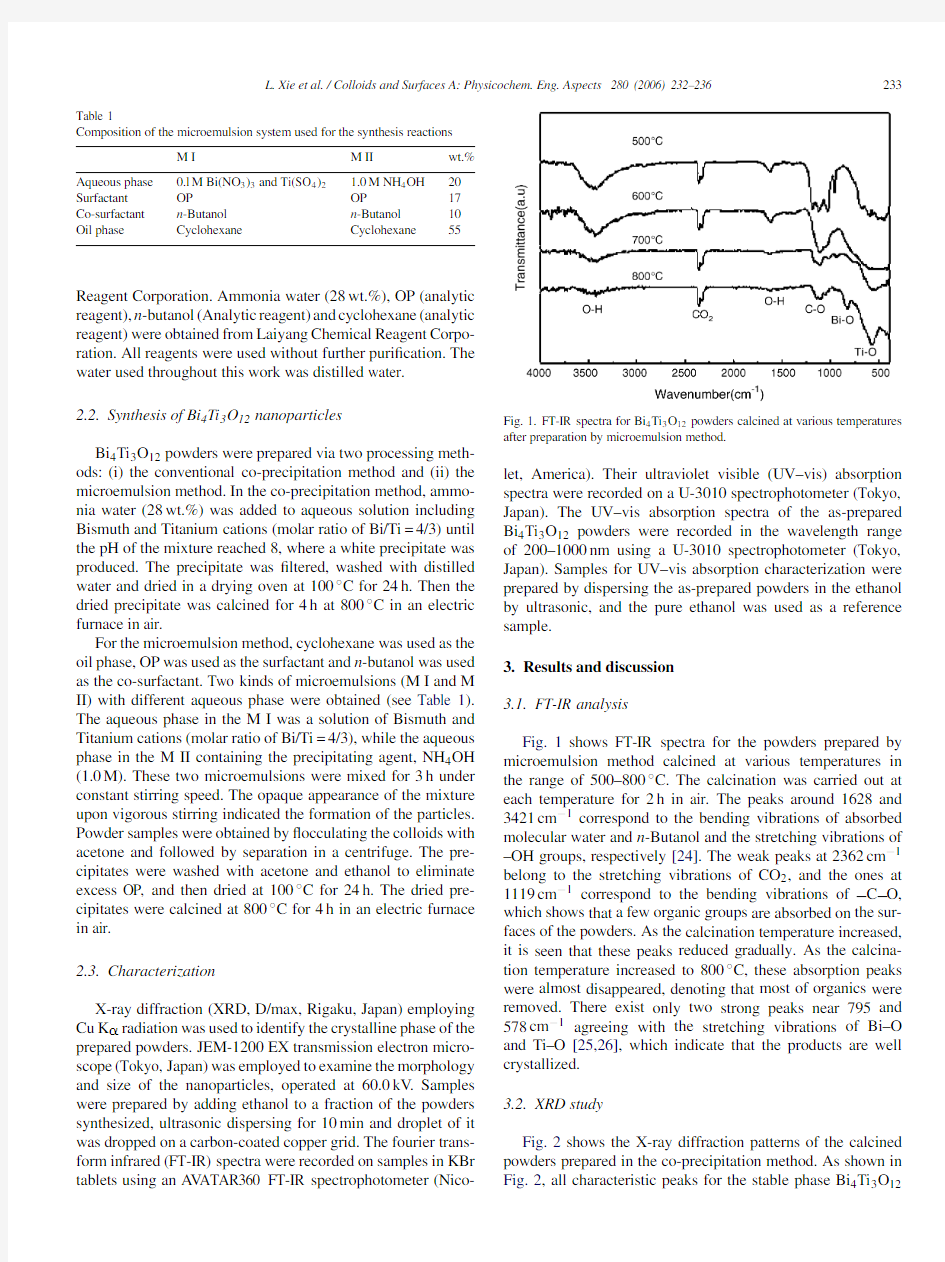
(Nico-Fig.1.FT-IR spectra for Bi4Ti3O12powders calcined at various temperatures after preparation by microemulsion method.
let,America).Their ultraviolet visible(UV–vis)absorption spectra were recorded on a U-3010spectrophotometer(Tokyo, Japan).The UV–vis absorption spectra of the as-prepared Bi4Ti3O12powders were recorded in the wavelength range of200–1000nm using a U-3010spectrophotometer(Tokyo, Japan).Samples for UV–vis absorption characterization were prepared by dispersing the as-prepared powders in the ethanol by ultrasonic,and the pure ethanol was used as a reference sample.
3.Results and discussion
3.1.FT-IR analysis
Fig.1shows FT-IR spectra for the powders prepared by microemulsion method calcined at various temperatures in the range of500–800?C.The calcination was carried out at each temperature for2h in air.The peaks around1628and 3421cm?1correspond to the bending vibrations of absorbed molecular water and n-Butanol and the stretching vibrations of –OH groups,respectively[24].The weak peaks at2362cm?1 belong to the stretching vibrations of CO2,and the ones at 1119cm?1correspond to the bending vibrations of C O, which shows that a few organic groups are absorbed on the sur-faces of the powders.As the calcination temperature increased, it is seen that these peaks reduced gradually.As the calcina-tion temperature increased to800?C,these absorption peaks were almost disappeared,denoting that most of organics were removed.There exist only two strong peaks near795and 578cm?1agreeing with the stretching vibrations of Bi–O and Ti–O[25,26],which indicate that the products are well crystallized.
3.2.XRD study
Fig.2shows the X-ray diffraction patterns of the calcined powders prepared in the co-precipitation method.As shown in Fig.2,all characteristic peaks for the stable phase Bi4Ti3O12
234L.Xie et al./Colloids and Surfaces A:Physicochem.Eng.
Aspects 280 (2006) 232–236
Fig.2.X-ray diffraction patterns of the powders calcined at800?C for4h after preparation by co-precipitation method.
with layered perovskite structure were marked by their indices. No other phases are detected.Therefore,from the X-ray diffrac-tion,it was con?rmed that pure phase Bi4Ti3O12was formed. Fig.3presents the XRD patterns for the Bi4Ti3O12powders prepared by the microemulsion method with calcining at800?C for1,2and4h,respectively.When the calcined time was1h,as shown in Fig.3c,the product contained Bi2Ti2O7and Bi20TiO32 phase as the impurities.The impurities were decreased gradually with an increase in the calcined times,and a puri?ed Bi4Ti3O12 phase with layered perovskite structure could be readily obtained in the calcined time of4h(Fig.3a).Moreover,as the
calcined Fig.3.X-ray diffraction patterns of the powders calcined at800?C for different calcined times:(a)4h;(b)2h;and(c)1h after preparation by microemulsion method.
times was prolonged,the XRD peaks were sharper and the sta-ble phase Bi4Ti3O12powders with higher crystallinity could be obtained.
3.3.Morphology of the particles
Fig.4a and b show the transmission electron microscopy (TEM)micrographs of the powders prepared by the co-precipitation and microemulsion method,respectively.It is apparent that the microemulsion method resulted in the forma-tion of nanoscale Bi4Ti3O12powders in the range of25–50
nm Fig.4.TEM micrographs of the powders prepared by different methods:(a)the co-precipitation method and(b)the microemulsion method.
L.Xie et al./Colloids and Surfaces A:Physicochem.Eng.Aspects 280 (2006) 232–236
235
Fig.5.Histogram of the Bi 4Ti 3O 12particles prepared by the microemulsion method.
in diameter.The particle size distribution,obtained from the TEM micrograph,is shown in Fig.5.The Gaussian ?tted result shows that the mean diameter of the particles is 35nm and the standard deviation (S.D.)is 8.8nm.On the other hand,the co-precipitation method led to the formation of Bi 4Ti 3O 12powders not only of larger particles but also of wider particle size distri-bution than those formed by the microemulsion method.These results seem to be caused by the fact that the powders prepared by the microemulsion method were formed in a controlled man-ner in nanosized domains,which kept the composition partition to a nanosized level.In contrast,the powders prepared by the co-precipitation method were formed in an uncontrolled manner,leading to an extensive growth and aggregation of precipitated particles.
3.4.UV–vis analysis
Fig.5a and b show the light absorption properties of the powders prepared by the microemulsion and co-precipitation method,respectively.As shown in Fig.6,there is a less differ-ence in intensity in UV absorption region between the
samples
Fig.6.UV–vis absorption spectra of the prepared Bi 4Ti 3O 12powders by differ-ent methods:(a)the microemulsion method and (b)the co-precipitation method.
prepared by the two methods,but the absorption edge and peak of the as-prepared Bi 4Ti 3O 12powders from the microemulsion method are red-shifted obviously with respect to those from the co-precipitation method.It indicates that the powders pre-pared by the microemulsion have stronger photocatalytic poten-tial than those prepared by the co-precipitation method in the visible light region,which is in a good agreement with TEM observations that the powders prepared by the microemulsion method have much smaller particle size than that prepared by the co-precipitation method.We can also ?nd that the peak at 350nm appears broader and non symmetric in Fig.5a.The result might be caused by the fact that the particles are so small and do not have anymore organic groups on their sur-face that they are less stable in solution and aggregate easily.Therefore,there may be the addition of the contribution of the scattering.4.Conclusion
To summarize,Bi 4Ti 3O 12nanoparticles were successfully prepared by the microemulsion method,and their histogram exhibits a narrow size distribution and average particle size of about 35nm.However,the powders from the co-precipitation method are not only of larger particles size with wider particle size distribution but also of irregular particle shape.In addition,the UV–vis absorption measurement initially indicated that the powders prepared by the microemulsion have stronger photo-catalytic potential than those prepared by the co-precipitation method in the visible light region.References
[1]A.M.Umabala,M.Suresh,A.V .Prasadarao,Mater.Lett.44(2000)175.[2]Y .Du,J.Fang,M.Zhang,J.Hong,Z.Yin,Q.Zhang,Mater.Lett.57
(2002)802.
[3]Y .Shi,C.Cao,S.Feng,Mater.Lett.46(2000)270.[4]E.C.Subbarao,J.Phys.Chem.Solids 23(1962)665.[5]A.Fouskova,L.E.Cross,J.Appl.Phys.41(1970)2834.
[6]A.Q.Jiang,G.H.Li,L.D.Zhang,J.Appl.Phys.83(1998)4878.
[7]Wei F.Yao,Hong Wang,Xiao H.Xu,Shu X.Shang,Yun Hou,Yin
Zhang,Min Wang,Mater.Lett.57(2003)1899.
[8]C.H.Lu,C.H.Wu,J.Eur.Ceram.Soc.22(2002)707.
[9]L.B.Kong,J.Ma,W.Zhu,O.K.Tan,Mater.Lett.51(2001)108.[10]Titipun Thongtem,Somchai Thongtem,Ceram.Int.30(2004)1463.[11]Pusit Pookmanee,Phunthanee Uriwilast,Sukon Phanichpant,Ceram.Int.
30(2004)1913.
[12]Yanmei Kan,Xihai Jin,Peiling Wang,Yongxiang Li,Yi-Bing Cheng,
Dongsheng Yan,Mater.Res.Bull.38(2003)567.
[13]S.R.Dhage,Y .B.Khollam,S.B.Dhespande,H.S.Potdar,V .Rav,Mater.
Res.Bull.39(2004)1993.
[14]Ze’lia Soares Macedo,Antonio Carlos Hernandes,Mater.Lett.55(2002)
217.
[15]M.P.Pileni,Reverse Micelles,Elsevier,1990.[16]M.A.L′o pez-Quintela,J.Rivas,M.C.Blanco,C.Tojo,Nanoscale Mate-rials,Kluwer,Academic/Plenum,2003,p.135.[17]M.A.L′o pez-Quintela,Curr.Opin.Colloid Interface Sci.8(2003)137.[18]M.Arturo,L.Quintela,J.Rivas,J.Colloid Interface Sci.158(1993)
446.
[19]P.Barnickel,A.Wokaun,W.Sager,H.F.Eicke,J.Colloid Interface Sci.
80(1992)148.
[20]D.Burgard,C.Kroph,R.Nass,H.Schmidt,Mater.Res.Soc.Symp.
Proc.101(1994)346.
236L.Xie et al./Colloids and Surfaces A:Physicochem.Eng.Aspects 280 (2006) 232–236
[21]S.Hingorani, D.O.Shah,M.S.Multani,J.Mater.Res.461(1995)
10.
[22]A.Koˇs ak,D.Makovec,M.Drofenik,A.ˇZnidarˇs iˇc,J.Magn.Magn.
Mater.272(2004)1542.
[23]Vuk Uskokovi′c,Miha Drofenik,Irena Ban,J.Magn.Magn.Mater.284
(2004)294.[24]C.P.Sbu,S.R.Kumar,P.Mukundan,K.G.K.Warrier,Chem.Mater.14
(2002)2876.
[25]H.S.Gu,A.X.Kuan,X.J.Li,Ferroelectrics211(1998)271.
[26]R.A.Nyquist,R.O.Kagel,Infrared Spectra of Inorganic Compound,
Academic Press,New York,1971,Fig.105.