
Diphenyl ethers from Aspergillus sp.and their anti-A β42aggregation activities
Huan Zhao a ,1,Gao-Qian Wang a ,1,Xu-Peng Tong a ,Guo-Dong Chen b ,?,Yuan-Fan Huang a ,Jia-Yu Cui a ,Ming-Zhu Kong c ,Liang-Dong Guo d ,Yi-Zhi Zheng c ,Xin-Sheng Yao a ,Hao Gao a ,?
a Institute of Traditional Chinese Medicine and Natural Products,College of Pharmacy,Jinan University,Guangzhou 510632,People's Republic of China
b Department of Pharmaceutical Engineering,College of Natural Resources and Environment,South China Agricultural University,Guangzhou 510642,People's Republi
c of China c Shenzhen Key Laboratory of Microbial Genetic Engineering,College of Life Sciences,Shenzhen University,Shenzhen 518060,People's Republic of China d
State Key Laboratory of Mycology,Institute of Microbiology,Chinese Academy of Sciences,Beijing 100190,People's Republic of China
a r t i c l e i n f o a
b s t r a
c t
Article history:
Received 16May 2014
Accepted in revised form 5July 2014Available online 16July 2014Two new compounds with the character of diphenyl ether structure,oxisterigmatocystin D (1)and 9-acetyldiorcinol B (6),were isolated from the endolichenic fungal strain Aspergillus sp.(No.16-20-8-1),along with six known compounds,oxisterigmatocystin A (2),oxisterigmatocystin C (3),sterigmatocystin (4),diorcinol B (5),violaceol-I (7),and violaceol-II (8).The structures of the new compounds were determined by extensive NMR spectroscopic data,and the absolute configuration of 1was established by single-crystal X-ray diffraction analysis.Moreover,the A β42aggregation inhibitory activities of 5–8were evaluated by the standard thioflavin T (ThT)fluorescence assay using epigallocatechin gallate (EGCG)as the positive https://www.doczj.com/doc/a49094641.html,pounds 7and 8displayed significant anti-A β42aggregation activity with IC 50values of 5.1and 2.3μM,respectively.Preliminary structure –activity relationship of these diphenyl ethers as anti-A β42aggregation inhibitors was proposed.
?2014Elsevier B.V.All rights reserved.
Keywords:Aspergillus sp.Diphenyl ethers
Anti-A β42aggregation activity
1.Introduction
The diphenyl ethers are reported from microorganisms,plantae and animalia,including Aspergillus sp.[1],Cordyceps sp.[2],Dendrospora sp.[3],Dysidea sp.[4–7],Kirschsteiniothelia sp.[8],Neoplaconema sp.[9],Penicillium sp.[10,11],Leathesia nana [12],Phyllanthus atropurpureus [13],Symphyocladia latiuscula [14],and so on.Diphenyl ethers exhibit a wide range of interesting biological activities,such as antimicrobial [1,3,6,8,10],cytotoxic [2,6,9],radical-scavenging [11],enzyme inhibitory [5],actin inhibitory [15],anti-HSV-1[2],and phytocidal activities [16,17].In early chemical investigation,we reported the isolation of two diphenyl ethers,2-isopentenyldiorcinol (9)and diorcinol (10),from a strain of
endolichenic fungus Aspergillus sp.(No.16-20-8-1)[18].In subsequent bioactive screening,diorcinol (10)showed signif-icant A β42aggregation inhibitory activity with IC 50value of 20.1μM,while 2-isopentenyldiorcinol (9)was inactive.It is firstly discovered that diorcinol (10)with the character of diphenyl ether structure has anti-A β42aggregation activity.However,not all of diphenyl ethers displayed anti-A β42aggregation activity.This phenomenon arouses our interest in the structure –activity relationship of these diphenyl ethers as anti-A β42aggregation inhibitors.
Alzheimer's disease (AD)is one of neurodegenerative diseases,which is characterized by the aggregation of amyloid βpeptides (A β)into fibrillar plaques [19].Therefore,blocking the neurotoxicity of A βis one of the strategies for AD treatment.In order to search more diphenyl ethers with A β42aggregation inhibitory activity and to reveal their structure –activity relationship as anti-A β42aggregation inhibitors,the solid culture of the strain Aspergillus sp.(No.16-20-8-1)was re-fermented at the same scale.Further chemical investigation
Fitoterapia 98(2014)77–83
?Corresponding authors.Tel./fax:+862085221559.
E-mail addresses:chgdtong@https://www.doczj.com/doc/a49094641.html, (G.-D.Chen),tghao@https://www.doczj.com/doc/a49094641.html, (H.Gao).1
These authors contributed equally to this
work.
https://www.doczj.com/doc/a49094641.html,/10.1016/j.?tote.2014.07.007
0367-326X/?2014Elsevier B.V.All rights reserved.
Contents lists available at ScienceDirect
Fitoterapia
j o u r na l h o me p a g e :w ww.e l s e v i e r.c o m /l oc a te /f i t o t
e
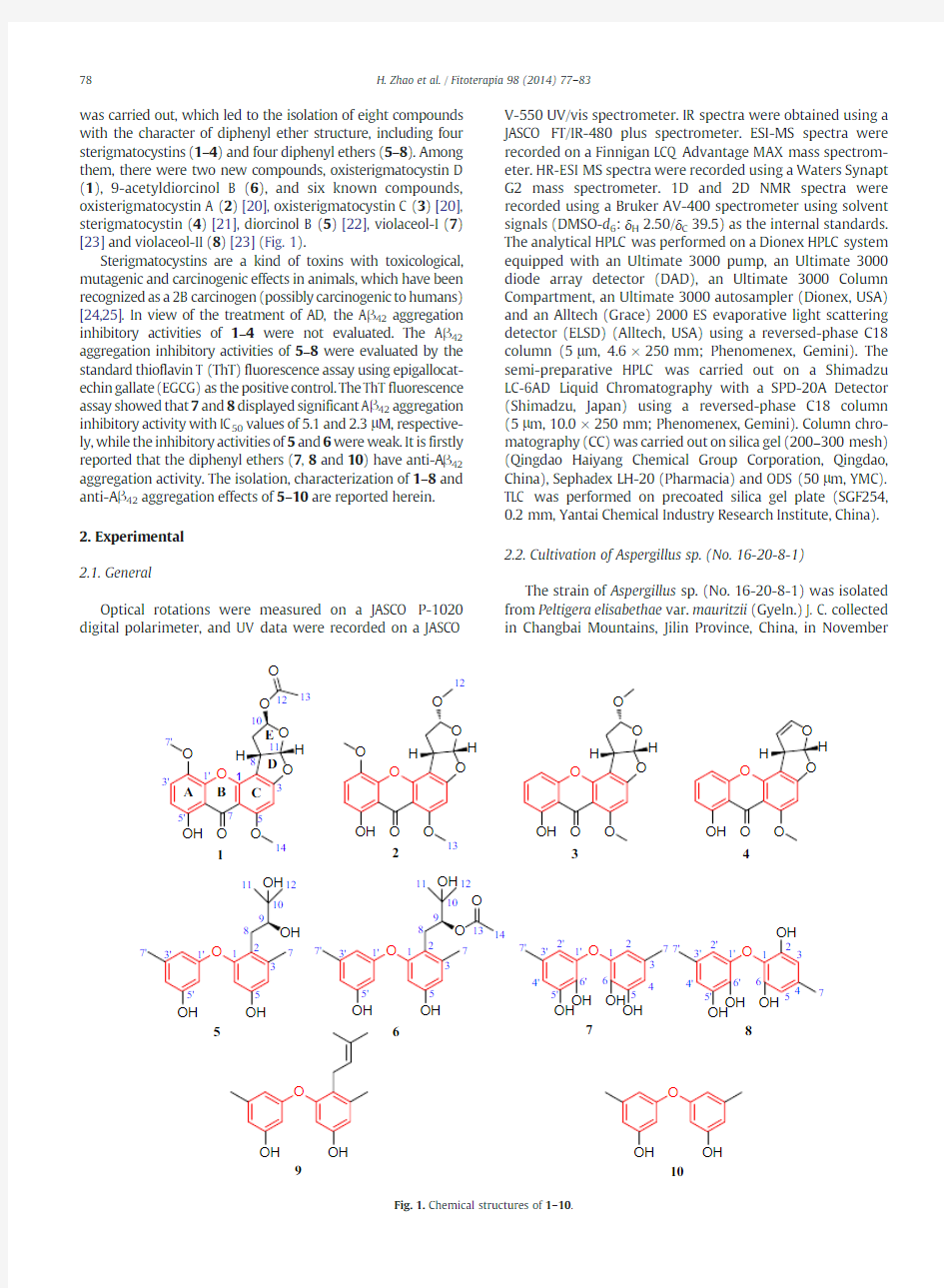
was carried out,which led to the isolation of eight compounds with the character of diphenyl ether structure,including four sterigmatocystins (1–4)and four diphenyl ethers (5–8).Among them,there were two new compounds,oxisterigmatocystin D (1),9-acetyldiorcinol B (6),and six known compounds,oxisterigmatocystin A (2)[20],oxisterigmatocystin C (3)[20],sterigmatocystin (4)[21],diorcinol B (5)[22],violaceol-I (7)[23]and violaceol-II (8)[23](Fig.1).
Sterigmatocystins are a kind of toxins with toxicological,mutagenic and carcinogenic effects in animals,which have been recognized as a 2B carcinogen (possibly carcinogenic to humans)[24,25].In view of the treatment of AD,the A β42aggregation inhibitory activities of 1–4were not evaluated.The A β42aggregation inhibitory activities of 5–8were evaluated by the standard thioflavin T (ThT)fluorescence assay using epigallocat-echin gallate (EGCG)as the positive control.The ThT fluorescence assay showed that 7and 8displayed significant A β42aggregation inhibitory activity with IC 50values of 5.1and 2.3μM,respective-ly,while the inhibitory activities of 5and 6were weak.It is firstly reported that the diphenyl ethers (7,8and 10)have anti-A β42aggregation activity.The isolation,characterization of 1–8and anti-A β42aggregation effects of 5–10are reported herein.2.Experimental
2.1.General
Optical rotations were measured on a JASCO P-1020digital polarimeter,and UV data were recorded on a JASCO
V-550UV/vis spectrometer.IR spectra were obtained using a JASCO FT/IR-480plus spectrometer.ESI-MS spectra were recorded on a Finnigan LCQ Advantage MAX mass spectrom-eter.HR-ESI MS spectra were recorded using a Waters Synapt G2mass spectrometer.1D and 2D NMR spectra were recorded using a Bruker AV-400spectrometer using solvent signals (DMSO-d 6:δH 2.50/δC 39.5)as the internal standards.The analytical HPLC was performed on a Dionex HPLC system equipped with an Ultimate 3000pump,an Ultimate 3000diode array detector (DAD),an Ultimate 3000Column Compartment,an Ultimate 3000autosampler (Dionex,USA)and an Alltech (Grace)2000ES evaporative light scattering detector (ELSD)(Alltech,USA)using a reversed-phase C18column (5μm,4.6×250mm;Phenomenex,Gemini).The semi-preparative HPLC was carried out on a Shimadzu LC-6AD Liquid Chromatography with a SPD-20A Detector (Shimadzu,Japan)using a reversed-phase C18column (5μm,10.0×250mm;Phenomenex,Gemini).Column chro-matography (CC)was carried out on silica gel (200–300mesh)(Qingdao Haiyang Chemical Group Corporation,Qingdao,China),Sephadex LH-20(Pharmacia)and ODS (50μm,YMC).TLC was performed on precoated silica gel plate (SGF254,0.2mm,Yantai Chemical Industry Research Institute,China).2.2.Cultivation of Aspergillus sp.(No.16-20-8-1)
The strain of Aspergillus sp.(No.16-20-8-1)was isolated from Peltigera elisabethae var.mauritzii (Gyeln.)J.C.collected in Changbai Mountains,Jilin Province,China,in November
1
2
3
4
6
5
O
O
O
O O
O O OH
12
13
14
O O
7
8
O OH OH
O
10
OH 9
1'
3'
5'7'
O
1'
3'
5'7'
O
1314
12
13
OH
OH 2
1
34
5
6
71'2'
3'4'
5'
6'
7'O
OH
OH 2
1
34
5
6
7
1'2'
3'
4'
5'
6'
7'
OH
Fig.1.Chemical structures of 1–10.
78H.Zhao et al./Fitoterapia 98(2014)77–83
2006.The isolate was identified by one of the authors (L.-D.Guo)and assigned the accession number 16-20-8-1in the culture collection at the Institute of Traditional Chinese Medicine and Natural Products,College of Pharmacy,Jinan University,Guangzhou.
The fungal strain was cultured on slants of potato dextrose agar (PDA)at 25°C for 5days.Agar plugs were used to inoculate four Erlenmeyer flasks (250mL),each containing 100mL of potato dextrose broth (PDB).Four flasks of the inoculated media were incubated at 25°C on a rotary shaker at 200rpm for 5days to prepare the seed culture.Fermentation was carried out in 30Erlenmeyer flasks (500mL),each containing 70g of rice.Distilled H 2O (105mL)was added to each flask,and the rice was soaked overnight before autoclav-ing at 120°C for 30min.After cooling to room temperature,each flask was inoculated with 10.0mL of the spore inoculum and incubated at 25°C for 40days.2.3.Extraction and isolation
The fermented rice substrate was extracted three times with EtOAc,and the organic solvent was removed under reduced pressure to yield crude extract (39.8g).The crude extract was suspended in 90%MeOH/H 2O and simultaneous-ly extracted with the same volume cyclohexane for three times.The residue of the 90%MeOH/H 2O layer (18.3g)was subjected to ODS Medium Performance Liquid Chromatogra-phy (MPLC)eluted with MeOH/H 2O (30:70,50:50,70:30and 100:0,v/v)to afford four fractions (W1–W4).Fraction W2(6.5g)was subjected to silica gel open column by eluting with CH 2Cl 2–MeOH (100:0to 0:100,v/v)to give six subfractions (W2-1–W2-6).Subfraction W2-3(2.4g)was subjected to semi-preparative HPLC (CH 3CN/H 2O,35:65;3.0mL/min,254nm)to give three sub-subfractions (W2-3-1–
W2-3-3).Sub-subfraction W2-3-1(457.4mg)was subjected to semi-preparative HPLC (CH 3CN/H 2O,30:70,0.1%formic acid; 3.0mL/min,254nm)to obtain 7(35.9mg)and 8(47.6mg).Sub-subfraction W2-3-2(163.0mg)was sub-jected to semi-preparative HPLC (CH 3CN/H 2O,25:75,0.1%formic acid;3.0mL/min,254nm)to obtain 5(6.0mg).Sub-subfraction W2-3-3(78.0mg)was separated by semi-preparative HPLC (CH 3CN/H 2O,25:75,0.1%formic acid;3.0mL/min,254nm)to obtain 6(3.9mg).Fraction W3(3.1g)was subjected to ODS column by eluting with MeOH/H 2O (35:65,45:55,65:35,75:25and 100:0v/v)to give five subfractions (W3-1–W3-5).Subfraction W3-4(1.2g)was subjected to silica gel open column by eluting with cyclohexane-ethylacetate (100:0to 0:100,v/v)to obtain 1(3.7mg),2(20.9mg),3(8.5mg)and 4(12.0mg).
2.3.1.Oxisterigmatocystin D (1)
Pale yellow needle-like crystals;[a]25D —169.0(c 0.2,CH 2Cl 2);UV (CH 3OH)λmax (log ε)231(4.39),247(4.49),253(4.47),277(4.06),325(3.57)nm;IR (KBr)νmax 3433(br),2926,2856,1655,1628,1588,1487,1245,1051,814cm ?1;ESI-MS (positive)m /z :415[M +H]+,437[M +Na]+,851[2M +Na]+;and HR-ESI-MS (positive)m /z :415.1032[M +H]+(calcd for C 21H 19O 9,414.1029).1H and 13C NMR data see Table 1.
2.3.2.9-Acetyldiorcinol B (6)
Colorless oil;[α]25D —19.7(c 1.0,CH 3OH);UV (CH 3OH)λmax (log ε)207(4.67),279(3.67)nm;IR (KBr)νmax 3295(br),2937,1706,1599,1546,1042cm ?1;ESI-MS (positive):m /z 397[M +Na]+;ESI-MS (negative):m /z 373[M —H]?;and HR-ESI-MS (positive):m /z 397.1622[M +Na]+(calcd.for C 21H 26O 6Na,397.1627).1H and 13C NMR data see Table 2.
Table 113
C NMR and 1H NMR data of 1and 2(400MHz for 1H;100MHz for 13
C;in DMSO-d 6).
No.
12δC,mult
δH (J in Hz)
δC,mult δH (J in Hz)
1153.8,C 153.0,C 2106.5,C 107.8,C 3164.4,C 164.9,C 491.1,CH 6.70,s 90.7,CH 6.60,s
5163.2,C 162.9,C 6105.3,C 104.6,C 7180.3,C 180.2,C 841.8,CH 4.38,ddd (9.5,5.8,4.0)
41.9,CH 4.20,dd (9.1,6.2)935.4,CH 2 2.60,ddd (13.6,4.8,4.0),H α36.2,CH 2 2.27,d (13.2),H α
2.45,ddd (1
3.6,9.5,
4.8),H β 2.40,ddd (13.2,9.1,4.9),H β1097.8,CH 6.25,t (4.8)106.4,CH
5.26,d (4.9)11112.3,CH
6.59,d (5.8)
113.7,CH 6.58,d (6.2)12169.3,C 54.5,CH 3 3.10,s 1320.8,CH 3 2.07,s 56.6,CH 3 3.88,s
1456.7,CH 3 3.90,s
1′144.0,C 143.9,C 2′139.1,C 139.0,C 3′120.8,CH 7.40,d (9.0)120.8,CH 7.38,d (8.9)4′109.0,CH 6.67,d (9.0)108.9,CH 6.65,d (8.9)
5′154.1,C 154.1,C 6′108.8,C 108.7,C 7′
57.5,CH 3
3.88,s 57.5,CH 3
3.87,s 5′\OH
12.63,s 12.71,br s
79
H.Zhao et al./Fitoterapia 98(2014)77–83
2.3.3.X-ray crystallographic analysis of oxisterigmatocystin D(1)
Upon crystallization from CHCl3using the vapor diffusion method,pale yellow needle-like crystals of1were obtained. Data were collected using a Sapphire CCD with a graphite monochromated Cu Kαradiation,λ=1.54178?at173.00 (10)K.Crystal data:C21H18O9?CHCl3,M=533.72,space group monoclinic,P21;unit cell dimensions were determined to be a=11.2742(3)?,b=6.7798(2)?,c=14.8812(3)?,α=90.00°,β=93.337°(2),γ=90.00°,V=1135.54 (5)?3,Z=2,Dx=1.561mg/m3,F(000)=548,andμ(Cu Kα)=4.130mm?1.18,596reflections were collected to θmax=62.80°,in which3189reflections were observed [F2N4σ(F2)].The structure was solved by direct methods using the SHELXS-97program,and refined by the program SHELXL-97and full-matrix least-squares calculations.In the structure refinements,nonhydrogen atoms were placed on the geometrically ideal positions by the“ride on”method. Hydrogen atoms bonded to oxygen were located by the structure factors with isotropic temperature factors.The final refinement gave R=0.0858,R W=0.2385,S=1.081,and Flack=?0.01(5).The paragraph crystallographic data for1 have been deposited at the Cambridge Crystallographic Data Centre(CCDC No.998240).
2.3.4.X-ray crystallographic analysis of oxisterigmatocystin A(2)
Upon crystallization from CHCl3using the vapor diffusion method,Colorless needle crystals of2were obtained. Data were collected using a Sapphire CCD with a graphite monochromated Cu Kαradiation,λ=1.54184?at173.01 (10)K.Crystal data:C20H18O8,M=386.34,space group monoclinic,P21;unit cell dimensions were determined to be a=11.9706(2)?,b=10.08898(13)?,c=14.7982(3)?,α=90.00°,β=106.3751°(19),γ=90.00°,V=1714.70 (5)?3,Z=4,Dx=1.497mg/m3,F(000)=808,andμ(Cu Kα)=0.990mm?1.16,414reflections were collected to θmax=62.76°,in which4982reflections were observed [F2N4σ(F2)].The structure was solved by direct methods using the SHELXS-97program,and refined by the program SHELXL-97and full-matrix least-squares calculations.In the structure refinements,nonhydrogen atoms were placed on the geometrically ideal positions by the“ride on”method. Hydrogen atoms bonded to oxygen were located by the structure factors with isotropic temperature factors.The final refinement gave R=0.0378,R W=0.1052,S=1.079,and Flack=0.05(14).The paragraph crystallographic data for2 have been deposited at the Cambridge Crystallographic Data Centre(CCDC No.998221).
2.3.5.X-ray crystallographic analysis of diorcinol B(5)
Upon crystallization from MeOH using the vapor diffusion method,colorless needle crystals of5were obtained.Data were collected using a Sapphire CCD with a graphite monochromated Cu Kαradiation,λ=1.54184?at173.0(3)K.Crystal data:C19 H24O5,M=332.38,space group orthorhombic,P212121; unit cell dimensions were determined to be a=5.3217(2)?, b=17.2707(7)?,c=19.0297(6)?,α=90.00°,β=90.00°,γ=90.00°,V=1749.01(11)?3,Z=4,Dx=1.262mg/m3, F(000)=712,andμ(Cu Kα)=0.742mm?1.13,682reflec-tions were collected toθmax=62.87°,in which2613reflec-tions were observed[F2N4σ(F2)].The structure was solved by direct methods using the SHELXS-97program,and refined by the program SHELXL-97and full-matrix least-squares calcula-tions.In the structure refinements,nonhydrogen atoms were placed on the geometrically ideal positions by the“ride on”method.Hydrogen atoms bonded to oxygen were located by the structure factors with isotropic temperature factors.The final refinement gave R=0.0325,R W=0.0834,S=1.026, and Flack=?0.07(18).The paragraph crystallographic data for5have been deposited at the Cambridge Crystallographic Data Centre(CCDC No.998222).
Table2
1D and2D NMR data of6(400MHz for1H;100MHz for13C;in DMSO-d
6
).
No.6
δC,multδH a(J in Hz)1H–1H COSY HMBC ROESY 1/5155.6/155.8,C
2118.1,C
3139.1,C
4112.2,CH 6.3262,6,7
6103.3,CH 6.02,d(2.0)41,2,4,5
719.6,CH3 2.23,s2,3,4
825.4,CH2 2.78,dd(13.7,10.6),Ha92,32′
2.68,dd(1
3.7,1.8),Hb91,2,3,92′,6′979.0,CH
4.92,dd(10.6,1.8)8a,8b132′,6′1070.5,C
1125.4,CH3 1.08,s9,10,12
1226.0,CH3 1.09,s9,10,11
13169.3,C
1420.6,CH3 1.80,s13
1′/5′158.0/158.4,C
2′109.5,CH 6.22,br s4',6'4′,6′,7′8a,8b,9 3′139.8,C
4′110.7,CH 6.322',6'2′,6′,7′
6′102.5,CH 6.15,br s2',4'1′,2′,4′,5′8b,9 7′21.1,CH3 2.17,s2′,3′,4′
5/5′\OH9.14/9.39,br s
a Overlapped signals are reported without designating multiplicity.
80H.Zhao et al./Fitoterapia98(2014)77–83
2.4.ThT?uorescence assay
The protocol for standard thioflavin T(ThT)fluorescence assay was described in references[26,27].Fluorescence reached at300min(Ex444nm;Em485nm)in a microplate reader(Fluoroskan Ascent,Thermo Scientific,USA).The bioactivities were indicated by relative inhibitory rate(Vi) according to the formula:Vi=100?[(Fi?Fb)/F0]?100, where Fi is the fluorescence value of the sample groups (containing sample,Aβ42and ThT),Fb is its blank value (containing sample and ThT),and F0is the fluorescence value for free aggregation of Aβ42incubated in the same buffer/HFIP/ DMSO system(containing Aβ42and ThT).Relative inhibitory activity(%)was from the mean of seven different independent experiments with standard errors.For active inhibitor(relative inhibitory activity N60%),the inhibitory constant(IC50)was determined by testing in triplicate5–7concentrations in three independent experiments.The statistics were calculated with SPSS Statistics19.0software.
3.Results and discussion
Compound1was pale yellow needle-like crystal.Its molecular formula was determined as C21H18O9based on HR-ESI-MS,indicating13degrees of unsaturation.The13C NMR spectrum showed21carbon signals,which was consistent
with the deduction of the https://www.doczj.com/doc/a49094641.html,bined with the DEPT experiment,these carbons can be categorized into two carbonyl carbons(δC180.3,169.3),nine olefinic or aromatic quaternary carbons(δC164.4,163.2,154.1,153.8,144.0,139.1,108.8,106.5, and105.3),six methine carbons(δC120.8,112.3,109.0,97.8, 91.1and41.8),one methylene carbon(δC35.4),and three methyl carbons(δC57.5,56.7,and20.8).The proton resonances were assigned to relevant carbon atoms through the HSQC experiment.Careful analysis of1D NMR data(Table1)revealed that compound1had a similar sterigmatocystin skeleton as that of oxisterigmatocystin A(2)[20],except that the signals of the methoxyl group(δH3.10;δC54.5)in oxisterigmatocystin A (2)were absent and the additional resonances for an O-acetyl group(δH2.07;δC20.8and169.3)were observed in the NMR spectra of1.The planar structure and the absolute configuration (8S,10R,11S)of1were unambiguously determined by the single crystal X-ray crystallographic analysis(Fig.2).And, compound1was named as oxisterigmatocystin D(Fig.1).
The structure of compound2was identified as oxisterig-matocystin A by the analyses of1D,and2D NMR data,which were consistent with the previous reported data[20].
Until now,more than40sterigmatocystins have been isolated from fungi,whose structural diversity of E ring mainly lies in(1)whether theΔ9(10)-double bond exists;and (2)whether the C-10is oxidated and the configuration of C-10.However,only the X-ray data of the acetate of5, 6-dimethoxysterigmatocystin was reported and demonstrat-ed that E ring withΔ9(10)-double bond adopted a planar conformation[28].The crystal of1and2was obtained.This is the first report of the X-ray data of sterigmatocystins oxidated at C-10with10β(1)or10α(2)configuration (Figs.2,3).The X-ray data revealed that the E ring in1 adopted a half-chair conformation(C-9was above the plane of O\C-11\C-8and C-10was under the plane of O\C-11\C-8),while the E ring in2adopted an envelope conformation(C-10was out of the plane of O\C-11\C-8\C-9).In conclusion,the solid conformation of E ring lies in whether theΔ9(10)-double bond exists and the configuration of C-10.Furthermore,the solution conformation of E ring lies on the configuration of C-10.For instance,J8–9αwas~0Hz in10α-oxisterigmatocystins(such as in2,3)while J8–9αwas~4.0Hz in10β-oxisterigmatocystins(such as in1, oxisterigmatocystin B[20]).
The structure of compound5was identified as diorcinol B by the analyses of1D,and2D NMR data which
were Fig.2.Perspective drawing of X-ray structure of1
.
Fig.3.Perspective drawing of X-ray structure of2.
81
H.Zhao et al./Fitoterapia98(2014)77–83
consistent with the previous reported data [22].The X-ray data of 5is firstly reported(Fig.4).
The molecular formula of compound 6(a colorless oil)was C 21H 26O 6(nine degrees of unsaturation)as determined by HR-ESI-MS (m /z 397.1622,[M +Na]+),which was C 2H 2O more than that of 5.The 13C NMR spectrum showed 21carbon signals,which was consistent with the deduction of the https://www.doczj.com/doc/a49094641.html,bined with the DEPT experiment,these carbons can be categorized into one ester carbonyl carbon (δC 169.3),seven olefinic or aromatic quaternary carbons (δC 158.4,158.0,155.8,155.6,139.8,139.1,and 118.1),five sp 2methine carbons (δC 112.2,110.7,109.5,103.3,and 102.5),one sp 3oxygenated quaternary carbon (δC 70.5),one sp 3oxygenated methine carbon (δC 79.0),one
sp 3methylene carbon (δC 25.4),and five methyl carbons (δC 26.0,25.4,21.1,20.6,and 19.6).The proton resonances were assigned to relevant carbon atoms through the HSQC experiment.The NMR data of 6revealed that the structural feature was similar to that of 5[22](Table 2),except that the additional acetyl group NMR resonances (δH 1.80;δC 20.6and 169.3)were observed in 6.The linkage site of the acetyl group was deduced at C-9by the downfield shift of H-9(δH 4.92),as well as the key HMBC correlation from H-9(δH 4.92)to C-13(δC 169.3)(Fig.5).Detailed analyses of the 1D and 2D NMR spectra data allowed for the establishment of the planar structure of 6(Fig.5).The crystal of 5was obtained (Fig.4),which established the absolute configuration of 5as 9S.Considering the co-occurrence of 5and 6in Aspergillus sp.(No.16-20-8-1)and the similar optical rotations of 5[([α]25D —28.2(c 1.0,CH 3OH)]and 6[([α]25D —19.7(c 1.0,CH 3OH)],the absolute configuration of C-9in 6should be same as that of 5.On the basis of the above data,6was determined as 9-acetyldiorcinol B (Fig.1).
The structures of 7and 8were identified as violaceol-I (7)and violaceol-II (8),by the analyses of 1D,and 2D NMR data.The NMR data of 7and 8in DMSO-d 6are firstly reported in this paper (Tables 3,4).The structures of 3and 4were identified as asoxisterigmatocystin C (3)[20],and sterigmatocystin (4)[21],based on the detailed comparison of their reported NMR
data.
Fig.4.Perspective drawing of X-ray structure of 5.
1H -1H
COS Y
HMB C (H
C )
Fig.5.Key 1H –1H COSY and HMBC correlations of 6.
Table 313
C NMR and 1H NMR data of 7(400MHz for 1H;100MHz for 13C;in DMSO-d 6).No.
7δC,mult
δH (J in Hz)
1/5145.3/146.5,C 2/4110.2/111.5,CH 6.04,d (1.5)/6.35,d (1.5)
3133.9,C 6127.3,C 7
20.6,C
2.06,s
5/6\OH 8.36,br s/8.89,br s 1′/5′145.3/146.5,C 2′/4′110.2/111.5,CH 6.04,d (1.5)/6.35,d (1.5)
3′133.9,C 6′127.3,C 7′
20.6,C
2.06,s
5′/6′\OH
8.36,br s/8.89,br s
Table 413
C NMR and 1H NMR data of 8(400MHz for 1H;100MHz for 13
C;in
DMSO-d 6).NO
8δC,mult
δH a (J in Hz)
1128.2,C 2,6150.6,C 3,5108.3,CH 6.224134.2,C 7
21.0,CH 3
2.14,s 2,6\OH 8.74
1′/5′146.1/147.2,C 2′/4′109.9/105.8,CH 6.22/5.79,br s
3′126.8,C 6′131.7,C 7′
20.8,CH 3
1.99,s 5′/6′\OH
8.74
a
Overlapped signals are reported without designating multiplicity.
82H.Zhao et al./Fitoterapia 98(2014)77–83
The inhibitory activities against Aβ42aggregation of the compounds5–8were tested by a ThT assay[26]with epigallocatechin gallate(EGCG)as the positive control (Table5).Among them,7and8showed significant Aβ42 aggregation inhibitory activity with IC50values of5.1and 2.3μM,while5and6had weak activity.The positive control EGCG(a polyphenol from green tea,prevented Aβ42aggrega-tion as a trial in a phase2–3RCT in patients with early Alzheimer's disease[29,30])showed the Aβ42aggregation inhibitor activity with IC50value of6.8μM.It is firstly reported that the diphenyl ethers(7,8and10)have anti-Aβ42 aggregation activity.Analysis of the structure–activity relation-ship suggests that the existence of the large groups at the ortho-position of ether bond may weaken the anti-Aβ42 aggregation activity,which could provide guide for further structural modification and optimization of diphenyl ethers for Aβ42aggregation inhibitor activity.
4.Con?ict of interest
There are no possible conflicts of interest. Acknowledgments
This project was supported financially by grants from the Ministry of Science and Technology of China (2012ZX0930*******),the Natural Science Foundation of China(81373306,81202441),the Guangdong Natural Science Funds for Distinguished Young Scholar(S2013050014287),the program for New Century Excellent Talents in University (NCET-10-0120)from the Ministry of Education of China, and the National Undergraduate Innovative Test Program (1210559047).
References
[1]Chen M,Shao CL,Fu XM,Xu RF,Zheng JJ,Zhao DL,et al.Bioactive indole
alkaloids and phenyl ether derivatives from a marine-derived Aspergillus sp.fungus.J Nat Prod2013;76:547–53.
[2]Bunyapaiboonsri T,Yoiprommarat S,Intereya K,Kocharin K.New
diphenyl ethers from the insect pathogenic fungus Cordyceps sp.BCC 1861.Chem Pharm Bull2007;55:304–7.
[3]Oh H,Kwon TO,Gloer JB,Marvanova L,Shearer CA.Tenellic acids A–D:
new bioactive diphenyl ether derivatives from the aquatic fungus Dendrospora tenella.J Nat Prod1999;62:580–3.
[4]Salvfi J,Faulkner DJ.A new brominated diphenyl ether from a
Philippine Dyszdea species.J Nat Prod1990;53:757–60.
[5]Fu X,Schmitz FJ,Govindan M,Abbas SA,Hanson KM,Horton PA,et al.
Enzyme inhibitors:new and known polybromena ted phenols and diphenyl ethers from four Indo-Pacific Dyszdea sponges.J Nat Prod 1995;58:1384–91.
[6]Zhang H,Skildum A,Stromquist E,Rose-Hellekant T,Chang LC.
Bioactive polybrominated diphenyl ethers from the marine sponge Dysidea sp J Nat Prod2008;71:262–4.
[7]Fu X,Schmitz FJ.New brominated diphenyl ether from an unidentified
species of Dysidea sponge.13C NMR data for some brominated diphenyl ethers.J Nat Prod1996;59:1102–3.
[8]Poch GK,Gloer JB,Shearer CA.New bioactive metabolites from a
freshwater isolate of the fungus Kirschsteiniothelia sp J Nat Prod 1992;55:1093–9.
[9]Wang FW,Ye YH,Chen JR,Wang XT,Zhu HL,Song YC,et al.Neoplaether,a
new cytotoxic and antifungal endophyte metabolite from Neoplaconema napellum IFB-E016.FEMS Microbiol Lett2006;261:218–23.
[10]Zhang Y,Li XM,Shang Z,Li CS,Ji NY,Wang BG.Meroterpenoid and
diphenyl ether derivatives from Penicillium sp.MA-37,a fungus isolated from marine mangrove rhizospheric soil.J Nat Prod2012;75:1888–95.
[11]Yang G,Yun K,Nenkep VN,Choi HD,Kang JS,Son BW.Induced
production of halogenated diphenyl ethers from the marine derived fungus Penicillium chrysogenum.Chem Biodivers2010;7:2766–70. [12]Xu XL,Song FH,Wang SJ,Li S,Xiao F,Zhao JL.Dibenzyl bromophenols
with diverse dimerization patterns from the brown alga Leathesia nana.
J Nat Prod2004;67:1661–6.
[13]Sarg T,Abdel-Ghani A,Zayed R,El-Sayed M.Bioactive compounds from
Phyllanthus atropurpureus.J Nat Prod2012;5:10–20.
[14]Duan XJ,Li XM,Wang BG.Highly brominated mono-and bis-phenols
from the marine red alga Symphyocladia latiuscula with radical-scavenging activity.J Nat Prod2007;70:1210–3.
[15]Asami Y,Jang JH,Oh H,Sohn JH,Jong WK,Dong OM,et al.Violaceols
function as actin inhibitors inducing cell shape elongation in fibroblast cells.Biosci Biotechnol Biochem2012;76:1431–7.
[16]Camilleri P,Weaver K,Bowyer JR,Hallahan BJ,Clark MT,Hallahan BJ.
Some novel diphenyl ether herbicides with peroxidizing activity.J Agric Food Chem1988;36:1061–3.
[17]Camilleri P,Gray A,Weaver K,Bowyer JR,Williams DJ.Herbicidal
diphenyl ethers:stereochemical studies using enantiomers of a novel diphenyl ether phthalide.J Agric Food Chem1989;37:519–23.
[18]Huang YF,Li XX,Chen GD,Hao Gao,Guo LD,Yao XS.A new diphenyl
ether from an endolichenic fungal strain,Aspergillus sp Mycosystema 2012;31:769–74.
[19]Rochet JC,Lansbury Jr PT.Amyloid fibrillogenesis:themes and
variations.Curr Opin Struct Biol2000;10:60–8.
[20]Cai SX,Zhu TJ,Du L,Zhao BY,Li DH,Gu QQ.Sterigmatocystins from the
deep-sea-derived fungus Aspergillus versicolor.J Antibiot2011;64:193–6.
[21]Zhu F,Lin YC.Three Xanthones from a marine-derived mangrove
endophytic fungus.Chem Nat Compd2007;43:132–5.
[22]Gao HQ,Zhou LN,Cai SX,Zhang GJ,Zhu TJ,Gu QQ,et al.Diorcinols B–E,
new prenylated diphenyl ethers from the marine-derived fungus Aspergillus versicolor ZLN-60.J Antibiot2013;66:539–42.
[23]Takenaka Y,Tanahashi T,Nagakura N,Hamada N.Phenyl ethers from
cultured lichen mycobionts of Graphis scriptavar.Serpentine and G.
rikuzensis.Chem Pharm Bull2003;51:794–7.
[24]Ver?ilovskis A,De SS.Sterigmatocystin:occurrence in foodstuffs and
analytical methods—an overview.Mol Nutr Food Res2010;54:136–47.
[25]Mori H,Kawai K,Ohbayashi F,Kuniyasu T,Yamazaki M,Hamasaki T,
et al.Genotoxicity of a variety of mycotoxins in the hepatocyte.Cancer Res1984;44:2918–23.
[26]Chakrabortee S,Liu Y,Zhang L,Matthews HR,Zhang H,Pan N,et al.
Macromolecular and small-molecule modulation of intracellular Aβ42 aggregation and associated toxicity.Biochem J2012;442:507–15. [27]Zheng QC,Chen GD,Kong MZ,Li GQ,Cui JY,Li XX,et al.
Nodulisporisteriods A and B,the first3,4-seco-4-methyl-progesteroids from Nodulisporium sp Steroids2013;78:896–901.
[28]Hamasaki T,Nakagomi T,Hatsuda Y,Fukuyama K,Katsube Y.5,6-
Dimethoxysterigmatocystin and related metabolites from Aspergillus multicolor.Agric Biol Chem1980;44:1149–55.
[29]Mangialasche F,Solomon A,Winblad B,Mecocci P,Kivipelto M.
Alzheimer's disease:clinical trials and drug https://www.doczj.com/doc/a49094641.html,ncet Neurol 2010;9:702–16.
[30]Mandel SA,Amit T,Kalfon L,Reznichenko L,Weinreb O,Youdim MB.
Cell signaling pathways and iron chelation in the neurorestorative activity of green tea polyphenols:special reference to epigallocatechin gallate(EGCG).J Alzheimers Dis2008;15:211–22.
Table5
The Aβ42aggregation inhibition of5–10.
Compounds ThT assay
Relative inhibitory activity(%)(100μM)IC50a(μM)
EGCG68.4±0.8 6.8
548.2±1.5
655.2±5.5
774.7±7.0 5.1
873.1±7.0 2.3
9?36.6±3.1
1069.2±2.220.1
a When the relative inhibitory activities of compounds are higher than 60%,the IC50values are evaluated.83
H.Zhao et al./Fitoterapia98(2014)77–83