Stille-Carbonylative cross-coupling
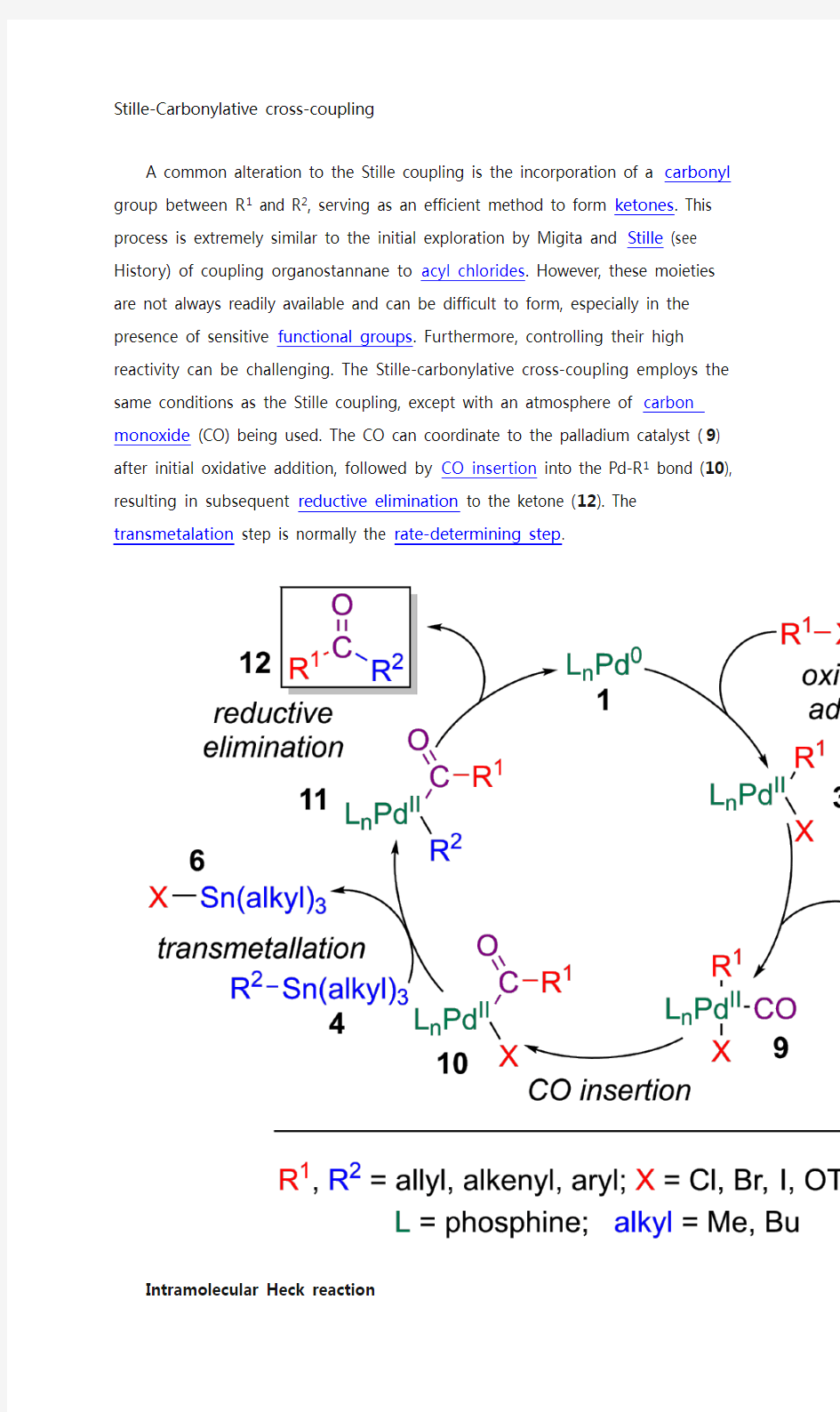
A common alteration to the Stille coupling is the incorporation of a carbonyl group between R1 and R2, serving as an efficient method to form ketones. This process is extremely similar to the initial exploration by Migita and Stille (see History) of coupling organostannane to acyl chlorides. However, these moieties are not always readily available and can be difficult to form, especially in the presence of sensitive functional groups. Furthermore, controlling their high reactivity can be challenging. The Stille-carbonylative cross-coupling employs the same conditions as the Stille coupling, except with an atmosphere of carbon monoxide (CO) being used. The CO can coordinate to the palladium catalyst (9) after initial oxidative addition, followed by CO insertion into the Pd-R1 bond (10), resulting in subsequent reductive elimination to the ketone (12). The transmetalation step is normally the rate-determining step.
Intramolecular Heck reaction
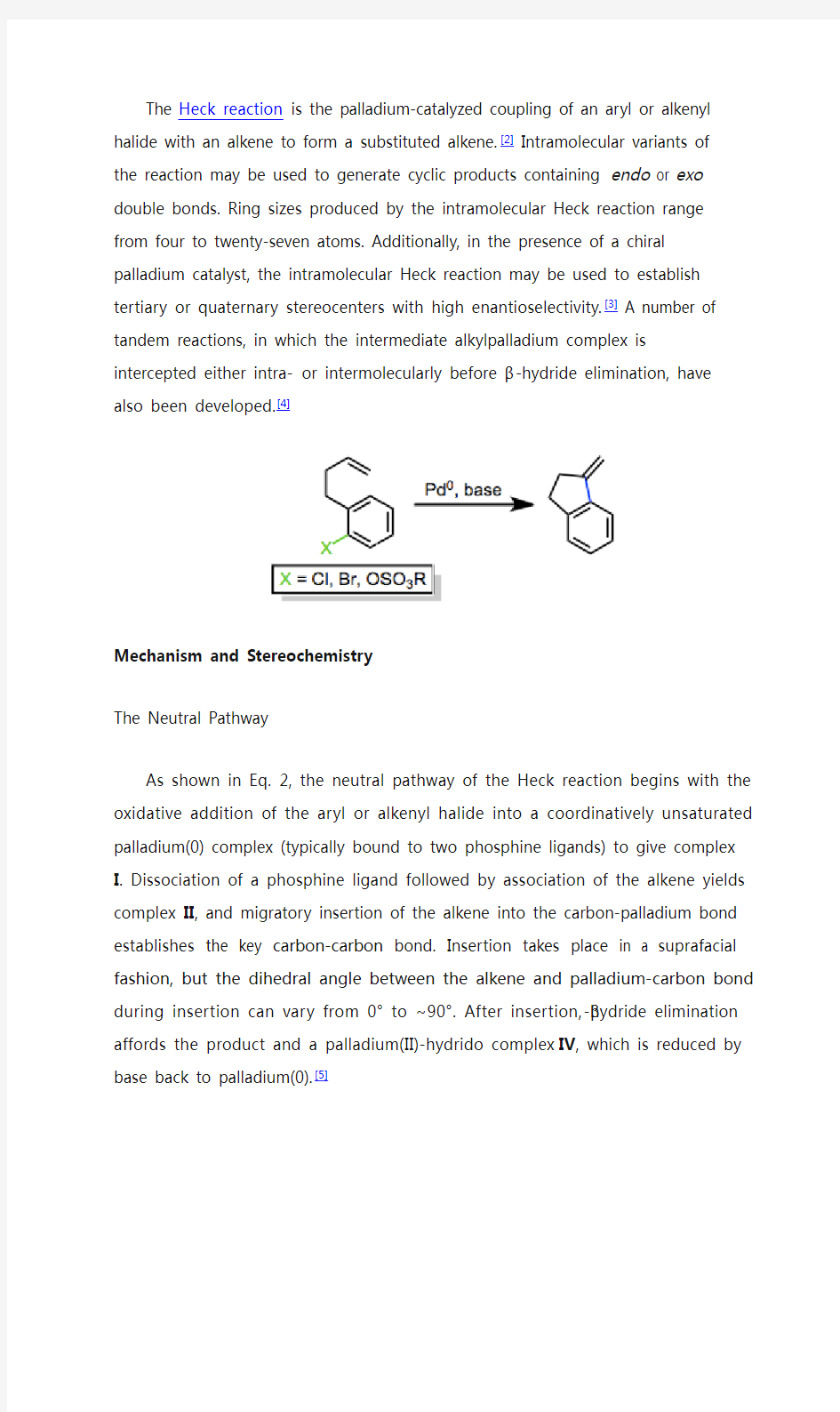
The Heck reaction is the palladium-catalyzed coupling of an aryl or alkenyl halide with an alkene to form a substituted alkene.[2]Intramolecular variants of the reaction may be used to generate cyclic products containing endo
or exo double bonds. Ring sizes produced by the intramolecular Heck reaction range from four to twenty-seven atoms. Additionally, in the presence of a chiral palladium catalyst, the intramolecular Heck reaction may be used to establish tertiary or quaternary stereocenters with high enantioselectivity.[3] A number of tandem reactions, in which the intermediate alkylpalladium complex is intercepted either intra- or intermolecul arly before β-hydride elimination, have also been developed.[4]
Mechanism and Stereochemistry
The Neutral Pathway
As shown in Eq. 2, the neutral pathway of the Heck reaction begins with the oxidative addition of the aryl or alkenyl halide into a coordinatively unsaturated palladium(0) complex (typically bound to two phosphine ligands) to give complex I. Dissociation of a phosphine ligand followed by association of the alkene yields complex II, and migratory insertion of the alkene into the carbon-palladium bond establishes the key carbon-carbon bond. Insertion takes place in a suprafacial fashion, but the dihedral angle between the alkene and palladium-carbon bond during insertion can vary from 0° to ~90°. After insertion, β-hydride elimination affords the product and a palladium(II)-hydrido complex IV, which is reduced by base back to palladium(0).[5]
The Cationic Pathway
Most asymmetric Heck reactions employing chiral phosphines proceed by the cationic pathway, which does not require the dissociation of a phosphine ligand. Oxidative addition of an aryl perfluorosulfonate generates a cationic palladium aryl complex V. The mechanism then proceeds as in the neutral case, with the difference that an extra site of coordinative unsaturation exists on palladium throughout the process. Thus, coordination of the alkene does not require ligand dissociation. Stoichiometric amounts of base are still required to reduce the palladium(II)-hydrido complex VIII back to palladium(0).[6] Silver salts may be used to initiate the cationic pathway in reactions of aryl halides.[7]
The Anionic Pathway
Reactions involving palladium(II) acetate and phosphine ligands proceed by a third mechanism, the anionic pathway.[8]Base mediates the oxidation of a phosphine ligand by palladium(II) to a phosphine oxide. Oxidative addition then generates the anionic palladium complex IX. Loss of halide leads to neutral complex X, which undergoes steps analogous to the neutral pathway to regenerate anionic complex IX. A similar anionic pathway is also likely operative in reactions of bulky palladium tri(tert-butyl)phosphine complexes.[9]
Establishing Tertiary or Quaternary Stereocenters
Asymmetric Heck reactions establish quaternary or tertiary stereocenters. If migratory insertion generates a quaternary center adjacent to the palladium-carbon bond (as in reactions of trisubstituted or 1,1-disubstituted alkenes), β-hydride elimination toward that center is not possible and it is retained in the product.[3]Similarly, β-hydride elimination is not possible if a hydrogen syn to the palladium-carbon bond is not available. Thus, tertiary stereocenters can be established in conformationally restricted systems.[10]
常用硅烷偶联剂——K H550、KH560、KH570、KH792、DL602 1.KH550 KH550硅烷偶联剂CAS号:919-30-2 一、国外对应牌号 A-1100(美国联碳),Z-6011(美国道康宁),KBM-903(日本信越)。本品有碱性,通用性强,适用于环氧、PBT、酚醛树脂、聚酰胺、聚碳酸酯等多种热塑性和热固性树脂。 二、化学名称分子式: 名称:γ-氨丙基三乙氧基硅烷 别名:3-三乙氧基甲硅烷基-1-丙胺 【3-TriethoxysilylpropylamineAPTES】, γ-氨丙基三乙氧基硅烷或3-氨基丙基三乙氧基硅烷【3-AminpropyltriethoxysilaneAMEO】 分子式:NH2(CH2)3Si(OC2H5)3 分子量:221.37 分子结构: 三、物理性质:
外观:无色透明液体 密度(ρ25℃):0.946 沸点:217℃ 折光率nD25:1.420 溶解性:可溶于有机溶剂,但丙酮、四氯化碳不适宜作释剂;可溶于水。在水中水解,呈碱性。 本品应严格密封,存放于干燥、阴凉、避光的室内。 四、KH550主要用途: 本品应用于矿物填充的酚醛、聚酯、环氧、PBT、聚酰胺、聚碳酸酯等热塑性和热固体树脂,能大幅度提高增强塑料的干湿态抗弯强度、抗压强度、剪切强度等物理力学性能和湿态电气性能,并改善填料在聚合物中的润湿性和分散性。 本品是优异的粘结促进剂,可用于聚氨酯、环氧、腈类、酚醛胶粘剂和密封材料,可改善颜料的分散性并提高对玻璃、铝、铁金属的粘合性,也适用于聚氨酯、环氧和丙烯酸乳胶涂料。 在树脂砂铸造中,本品增强树脂硅砂的粘合性,提高型砂强度抗湿性。 在玻纤棉和矿物棉生产中,将其加入到酚醛粘结剂中,可提高防潮性及增加压缩回弹性。 在砂轮制造中它有助于改进耐磨自硬砂的酚醛粘合剂的粘结性及耐水性。 2.KH560
硅烷偶联剂的使用说 明
硅烷偶联剂使用说明 一、选用硅烷偶联剂的一般原则 已知,硅烷偶联剂的水解速度取于硅能团Si-X,而与有机聚合物的反应活性则取于碳官能团C-Y。因此,对于不同基材或处理对象,选择适用的硅烷偶联剂至关重要。选择的方法主要通过试验预选,并应在既有经验或规律的基础上进行。例如,在一般情况下,不饱和聚酯多选用含CH2=CMeCOO、Vi及CH2-CHOCH2O-的硅烷偶联剂;环氧树脂多选用含CH2-CHCH2O及H2N-硅烷偶联剂;酚醛树脂多选用含H2N-及H2NCONH-硅烷偶联剂;聚烯烃多选用乙烯基硅烷;使用硫黄硫化的橡胶则多选用烃基硅烷等。由于异种材料间的黏接可度受到一系列因素的影响,诸如润湿、表面能、界面层及极性吸附、酸碱的作用、互穿网络及共价键反应等。因而,光靠试验预选有时还不够精确,还需综合考虑材料的组成及其对硅烷偶联剂反应的敏感度等。为了提高水解稳定性及降低改性成本,硅烷偶联剂中可掺入三烃基硅烷使用;对于难黏材料,还可将硅烷偶联剂交联的聚合物共用。 硅烷偶联剂用作增黏剂时,主要是通过与聚合物生成化学键、氢键;润湿及表面能效应;改善聚合物结晶性、酸碱反应以及互穿聚合物网络的生成等而实现的。增黏主要围绕3种体系:即(1)无机材料对有机材料;(2)无机材料对无机材料;(3)有机材料对有机材料。对于第一种黏接,通常要求将无机材料黏接到聚合物上,故需优先考虑硅烷偶联剂中Y与聚合物所含官能团的反应活性;后两种属于同类型材料间的黏接,故硅烷偶联剂自身的反亲水型聚合物以及无机材料要求增黏时所选用的硅烷偶联剂。 二、使用方法 如同前述,硅烷偶联剂的主要应用领域之一是处理有机聚合物使用的无机填料。后者经硅烷偶联剂处理,即可将其亲水性表面转变成亲有机表面,既可避免体系中粒子集结及聚合物急剧稠化,还可提高有机聚合物对补强填料的润湿性,通过碳官能硅烷还可使补强填料与聚合物实现牢固键合。但是,硅烷偶联剂的使用效果,还与硅烷偶联剂的种类及用量、基材的特征、树脂或聚合物的性质以及应用的场合、方法及条件等有关。本节侧重介绍硅烷偶联剂的两种使用方法,即表面处理法及整体掺混法。前法是用硅烷偶联剂稀溶液处理基体表面;后法是将硅烷偶联剂原液或溶液,直接加入由聚合物及填料配成的混合物中,因而特别适用于需要搅拌混合的物料体系。 1、硅烷偶联剂用量计算 被处理物(基体)单位比表面积所占的反应活性点数目以及硅烷偶联剂覆盖表面的厚度是决定基体表面硅基化所需偶联剂用量的关键因素。为获得单分子层覆盖,需先测定基体的Si-OH含量。已知,多数硅质基体的Si-OH含是来4-12个 /μ㎡,因而均匀分布时,1mol硅烷偶联剂可覆盖约7500m2的基体。具有多个可水解基团的硅烷偶联剂,由于自身缩合反应,多少要影响计算的准确性。若使用 Y3SiX处理基体,则可得到与计算值一致的单分子层覆盖。但因Y3SiX价昂,且覆
硅烷偶联剂kh570 一、概述: 偶联剂kh570是一类具有两不同性质官能团的物质,它们分子中的一部分官能团可与有机分子反应,另一部分官能团可与无机物表面的吸附水反应,形成牢固的粘合。偶联剂在复合材料中的作用在于它既能与增强材料表面的某些基团反应,又能与基体树脂反应,在增强材料与树脂基体之间形成一个界面层,界面层能传递应力,从而增强了增强材料与树脂之间粘合强度,提高了复合材料的性能,同时还可以防止不与其它介质向界面渗透,改善了界面状态,有利于制品的耐老化、耐应力及电绝缘性能。 化学名称:γ―甲基丙烯酰氧基丙基三甲氧基硅烷 化学结构式:CH3CCH2COO(CH2)3Si(OCH3)3 对应牌号:中科院KH-570、美国联碳公司A-174、美国道康宁公司Z-603、日本信越公司KBM-503 典型特征:偶联剂570为甲基丙烯酰氧基官能团硅烷,外观为无色或微黄透明液体,溶于丙酮、苯、乙醚、四氯化碳,与水反应。沸点为255℃,密度P25'g/m1:1.040,折光率ND:1.429,闪点:88℃,含量为≥97% 二、应用领域: 1、用于玻璃纤维的表面处理,能改善玻璃纤维和树脂的粘合性能,大大提高玻璃纤维增强复合材料的强度、电气、抗水、抗气候等性能,即使在湿态时,它对复合材料机械性能的提高,效果也十分显着。目前,
在玻璃纤维中使用硅烷偶联剂已相当普遍,用于这一方面的硅烷偶联剂约占其消耗总量的50%,其中用得较多的品种是乙烯基硅烷、氨基硅烷、甲基丙烯酰氧基硅烷等。 2、用于无机填料填充塑料。可预先对填料进行表面处理,也可直接加入树脂中。能改善填料在树脂中的分散性及粘合力,改善工艺性能和提高填充塑料(包括橡胶)的机械、电学和耐气候等性能。 3、用作密封剂、粘接剂和涂料的增粘剂,能提高它们的粘接强度、耐水、耐气候等性能。硅烷偶联剂往往可以解决某些材料长期以来无法粘接的难题。硅烷偶联剂作为增粘剂的作用原理在于它本身有两种基团;一种基团可以和被粘的骨架材料结合;而另一种基团则可以与高分子材料或粘接剂结合,从而在粘接界面形成强力较高的化学键,大大改善了粘接强度。硅烷偶联剂的应用一般有三种方法:一是作为骨架材料的表面处理剂;二是加入到粘接剂中,三是直接加入到高分子材料中。从充分发挥其效能和降低成本的角度出发,前两种方法较好。 三、使用方法 1、表面预处理法:将硅烷偶联剂配成0.5~1%浓度的稀溶液,使用时只需在清洁的被粘表面涂上薄薄的一层,干燥后即可上胶。所用溶剂多为水、醇、或水醇混合物,并以不含氟离子的水及价廉无毒的乙醇、异丙醇为宜。除氨烃基硅烷外,由其它硅烷偶联剂配制的溶液均需加入醋酸作水解催化剂,并将pH值调至3.5~5.5。长链烷基及苯基硅烷由于稳定性较差,不宜配成水溶液使用。氯硅烷及乙氧基硅烷水解过程中伴随有严重的缩合反应,也不宜配成水溶液或水醇溶液使用,而多配成醇
常用硅烷偶联剂介绍 1. KH550 KH550硅烷偶联剂CAS号:919-30-2 一、国外对应牌号 A-1100(美国联碳),Z-6011(美国道康宁),KBM-903(日本信越)。本品有碱性,通用性强,适用于环氧、PBT、酚醛树脂、聚酰胺、聚碳酸酯等多种热塑性和热固性树脂。 二、化学名称分子式: 名称:γ-氨丙基三乙氧基硅烷 别名:3-三乙氧基甲硅烷基-1-丙胺 【3-Triethoxysilylpropylamine APTES】, γ-氨丙基三乙氧基硅烷或3-氨基丙基三乙氧基硅烷 【3-Aminpropyltriethoxysilane AMEO】分子式:NH2(CH2)3Si(OC2H5)3 分子量:221.37 分子结构: 三、物理性质: 外观:无色透明液体 密度(ρ25℃):0.946 沸点:217℃
折光率nD25: 1.420 溶解性:可溶于有机溶剂,但丙酮、四氯化碳不适宜作释剂;可溶于水。在水中水解,呈碱性。 本品应严格密封,存放于干燥、阴凉、避光的室内。 四、KH550主要用途: 本品应用于矿物填充的酚醛、聚酯、环氧、PBT、聚酰胺、聚碳酸酯等热塑性和热固体树脂,能大幅度提高增强塑料的干湿态抗弯强度、抗压强度、剪切强度等物理力学性能和湿态电气性能,并改善填料在聚合物中的润湿性和分散性。 本品是优异的粘结促进剂,可用于聚氨酯、环氧、腈类、酚醛胶粘剂和密封材料,可改善颜料的分散性并提高对玻璃、铝、铁金属的粘合性,也适用于聚氨酯、环氧和丙烯酸乳胶涂料。 在树脂砂铸造中,本品增强树脂硅砂的粘合性,提高型砂强度抗湿性。 在玻纤棉和矿物棉生产中,将其加入到酚醛粘结剂中,可提高防潮性及增加压缩回弹性。 在砂轮制造中它有助于改进耐磨自硬砂的酚醛粘合剂的粘结性及耐水性。 2. KH560 一、国外对应牌号:
硅烷偶联剂的使用(完整篇) 一、选用硅烷偶联剂的一般原则 已知,硅烷偶联剂的水解速度取于硅能团Si-X,而与有机聚合物的反应活性则取于碳官能团C-Y。因此,对于不同基材或处理对象,选择适用的硅烷偶联剂至关重要。选择的方法主要通过试验预选,并应在既有经验或规律的基础上进行。例如,在一般情况下,不饱和聚酯多选用含CH2=CMeCOO、Vi及 CH2-CHOCH2O-的硅烷偶联剂;环氧树脂多选用含CH2-CHCH2O及H2N-硅烷偶联剂;酚醛树脂多选用含H2N-及H2NCONH-硅烷偶联剂;聚烯烃选用乙烯基硅烷;使用硫黄硫化的橡胶则多选用烃基硅烷等。由于异种材料间的黏接可度受到一系列因素的影响,诸如润湿、表面能、界面层及极性吸附、酸碱的作用、互穿网络及共价键反应等。因而,光靠试验预选有时还不够精确,还需综合考虑材料的组成及其对硅烷偶联剂反应的敏感度等。为了提高水解稳定性及降低改性成本,硅烷偶联剂中可掺入三烃基硅烷使用;对于难黏材料,还可将硅烷偶联剂交联的聚合物共用。硅烷偶联剂用作增黏剂时,主要是通过与聚合物生成化学键、氢键;润湿及表面能效应;改善聚合物结晶性、酸碱反应以及互穿聚合物网络的生成等而实现的。增黏主要围绕3种体系:即(1)无机材料对有机材料;(2)无机材料对无机材料;(3)有机材料对有机材料。对于第一种黏接,通常要求将无机材料黏接到聚合物上,故需优先考虑硅烷偶联剂中Y与聚合物所含官能团的反应活性;后两种属于同类型材料间的黏接,故硅烷偶联剂自身的反亲水型聚合物以及无机材料要求增黏时所选用的硅烷偶联剂。 二、使用方法 如同前述,硅烷偶联剂的主要应用领域之一是处理有机聚合物使用的无机填料。后者经硅烷偶联剂处理,即可将其亲水性表面转变成亲有机表面,既可避免体系中粒子集结及聚合物急剧稠化,还可提高有机聚合物对补强填料的润湿性,通过碳官能硅烷还可使补强填料与聚合物实现牢固键合。但是,硅烷偶联剂的使用效果,还与硅烷偶联剂的种类及用量、基材的特征、树脂或聚合物的性质以及应用的场合、方法及条件等有关。本节侧重介绍硅烷偶联剂的两种使用方法,即表面处理法及整体掺混法。前法是用硅烷偶联剂稀溶液处理基体表面;后法是将硅烷偶联剂原液或溶液,直接加入由聚合物及填料配成的混合物中,因而特别适用于需要搅拌混合的物料体系。 1、硅烷偶联剂用量计算 被处理物(基体)单位比表面积所占的反应活性点数目以及硅烷偶联剂覆盖表面的厚度是决定基体表面硅基化所需偶联剂用量的关键因素。为获得单分子层覆盖,需先测定基体的Si-OH含量。已知,多数硅质基体的Si-OH含是来4-12个/μ㎡,因而均匀分布时,1mol硅烷偶联剂可覆盖约7500m2的基体。具有多个可水解基团的硅烷偶联剂,由于自身缩合反应,多少要影响计算的准确性。若使用Y3SiX处理基体,则可得到与计算值一致的单分子层覆盖。但因Y3SiX价昂,且覆盖耐水解性差,故无实用价值。此外,基体表面的Si-OH数,也随加热条件而变化。例如,常态下Si-OH数为5.3个/μ㎡硅质基体,经在400℃或800℃下加热处理后,则Si-OH值可相应降为2.6个/μ㎡或<1个/μ㎡。反之,使用湿热盐酸处理基体,则可得到高Si-OH含量;使用碱性洗涤剂处理基体表面,则可形成硅醇阴离子。硅烷偶联剂的可润湿面积(WS),是指1g硅烷偶联剂的溶液所能覆盖基体的面积(㎡/g)。若将其与含硅基体的表面积值(㎡/g)关连,即可计算出单分子层覆盖所需的硅烷偶联剂用量。以处理填料为例,填料表面形成单分子
常用硅烷偶联剂介绍标准化管理部编码-[99968T-6889628-J68568-1689N]
常用硅烷偶联剂介绍 1.KH550 KH550硅烷偶联剂CAS号:919-30-2 一、国外对应牌号 A-1100(美国联碳),Z-6011(美国道康宁),KBM-903(日本信越)。本品有碱性,通用性强,适用于环氧、PBT、酚醛树脂、聚酰胺、聚碳酸酯等多种热塑性和热固性树脂。 二、化学名称分子式: 名称:γ-氨丙基三乙氧基硅烷 别名:3-三乙氧基甲硅烷基-1-丙胺 【3-TriethoxysilylpropylamineAPTES】, γ-氨丙基三乙氧基硅烷或3-氨基丙基三乙氧基硅烷【3-AminpropyltriethoxysilaneAMEO】 分子式:NH 2(CH 2 ) 3 Si(OC 2 H 5 ) 3 分子量:221.37 分子结构: 三、物理性质: 外观:无色透明液体 密度(ρ25℃):0.946
沸点:217℃ 折光率nD25:1.420 溶解性:可溶于有机溶剂,但丙酮、四氯化碳不适宜作释剂;可溶于水。在水中水解,呈碱性。 本品应严格密封,存放于干燥、阴凉、避光的室内。 四、KH550主要用途: 本品应用于矿物填充的酚醛、聚酯、环氧、PBT、聚酰胺、聚碳酸酯等热塑性和热固体树脂,能大幅度提高增强塑料的干湿态抗弯强度、抗压强度、剪切强度等物理力学性能和湿态电气性能,并改善填料在聚合物中的润湿性和分散性。 本品是优异的粘结促进剂,可用于聚氨酯、环氧、腈类、酚醛胶粘剂和密封材料,可改善颜料的分散性并提高对玻璃、铝、铁金属的粘合性,也适用于聚氨酯、环氧和丙烯酸乳胶涂料。 在树脂砂铸造中,本品增强树脂硅砂的粘合性,提高型砂强度抗湿性。 在玻纤棉和矿物棉生产中,将其加入到酚醛粘结剂中,可提高防潮性及增加压缩回弹性。 在砂轮制造中它有助于改进耐磨自硬砂的酚醛粘合剂的粘结性及耐水性。 2.KH560 一、国外对应牌号: A-187(美国联碳公司)。
一、选用硅烷偶联剂的一般原则 已知,硅烷偶联剂的水解速度取于硅能团Si-X ,而与有机聚合物的反应活性则取于碳官能团C-丫。因此,对于不同基材或处理对象,选择适用的硅烷偶联剂至关重要。选择的方法主要通过试验预选,并应在既有经验或规律的基础上进行。例如,在一般情况下,不饱和聚酯多选用含CH2=CMeC、OOVi 及CH2-CHOCH-2O 的硅烷偶联剂;环氧树脂多选用含CH2- CHCH2及H2N-硅烷偶联剂;酚醛树脂多选用含H2N-及H2NC0NH硅烷偶联剂;聚烯烃选用乙烯基硅烷;使用硫黄硫化的橡胶则多选用烃基硅烷等。由于异种材料间的黏接可度受到一系列因素的影响,诸如润湿、表面能、界面层及极性吸附、酸碱的作用、互穿网络及共价键反应等。因而, 光靠试验预选有时还不够精确,还需综合考虑材料的组成及其对硅烷偶联剂反应的敏感度等。为了提高水解稳定性及降低改性成本,硅烷偶联剂中可掺入三烃基硅烷使用;对于难黏材料,还可将硅烷偶联剂交联的聚合物共用。硅烷偶联剂用作增黏剂时,主要是通过与聚合物生成化学键、氢键;润湿及表面能效应;改善聚合物结晶性、酸碱反应以及互穿聚合物网络的生成等而实现的。增黏主要围绕 3 种体系:即(1)无机材料对有机材料;(2)无机材料对无机材料;(3)有机材料对有机材料。对于第一种黏接,通常要求将无机材料黏接到聚合物上,故需优先考虑硅烷偶联剂中丫与聚合物所含官能团的反应活性;后两种属于同类型材料间的黏接,故硅烷偶联剂自身的反亲水型聚合物以及无机材料要求增黏时所选用的硅烷偶联剂。 二、使用方法 如同前述,硅烷偶联剂的主要应用领域之一是处理有机聚合物使用的无机填料。后者经硅烷偶联剂处理,即可将其亲水性表面转变成亲有机表面,既可避免体系中粒子集结及聚合物急剧稠化,还可提高有机聚合物对补强填料的润湿性,通过碳官能硅烷还可使补强填料与聚合物实现牢固键合。但是,硅烷偶联剂的使用效果,还与硅烷偶联剂的种类及用量、基材的特征、树脂或聚合物的性质以及应用的场合、方法及条件等有关。本节侧重介绍硅烷偶联剂的两种使用方法,即表面处理法及整体掺混法。前法是用硅烷偶联剂稀溶液处理基体表面;后法是将硅烷偶联剂原液或溶液,直接加入由聚合物及填料配成的混合物中,因而特别适用于需要搅拌混合的物料体系。 1、硅烷偶联剂用量计算 被处理物(基体)单位比表面积所占的反应活性点数目以及硅烷偶联剂覆盖表面的厚度是决定基体表面硅基化所需偶联剂用量的关键因素。为获得单分子层覆盖,需先测定基体的Si—OH含量。已知,多数硅质基体的Si —OH含是来4-12 个/卩叭因而均匀分布时,1mol硅烷偶联剂可覆盖约7500m2的基体。具有多个可水解基团的硅烷偶联剂,由于自身缩合反应,多少要影响计算的准确性。若使用丫3SiX处理基体,则可得到与计算值一致的单分子层覆盖。但因丫3SiX价昂,且覆盖耐水解性差,故无实用价值。此外,基体表面的Si-OH数,也随加热条件而变化。例如,常态下Si —OH数为5.3个/卩川硅质基体,经在400C或800C 下加热处理后,则Si —OH值可相应降为2.6个/卩卅或V 1个/卩讥反之,使用湿热盐酸处理基体,则可得到高Si —OH含量;使用碱性洗涤剂处理基体表面,则可形成硅醇阴离子。硅烷偶联剂的可润湿面积(WS,是指ig硅烷偶联剂的溶液所能覆
常用硅烷偶联剂介绍 1.KH550 KH550硅烷偶联剂CAS号:919-30-2 一、国外对应牌号 A-1100(美国联碳),Z-6011(美国道康宁),KBM-903(日本信越)。本品有碱性,通用性强,适用于环氧、PBT、酚醛树脂、聚酰胺、聚碳酸酯等多种热塑性和热固性树脂。 二、化学名称分子式: 名称:γ-氨丙基三乙氧基硅烷 别名:3-三乙氧基甲硅烷基-1-丙胺 【3-TriethoxysilylpropylamineAPTES】, γ-氨丙基三乙氧基硅烷或3-氨基丙基三乙氧基硅烷【3-AminpropyltriethoxysilaneAMEO】 分子式:NH 2(CH 2 ) 3 Si(OC 2 H 5 ) 3 分子量:221.37 分子结构: 三、物理性质: 外观:无色透明液体 密度(ρ25℃):0.946
沸点:217℃ 折光率nD25:1.420 溶解性:可溶于有机溶剂,但丙酮、四氯化碳不适宜作释剂;可溶于水。在水中水解,呈碱性。 本品应严格密封,存放于干燥、阴凉、避光的室内。 四、KH550主要用途: 本品应用于矿物填充的酚醛、聚酯、环氧、PBT、聚酰胺、聚碳酸酯等热塑性和热固体树脂,能大幅度提高增强塑料的干湿态抗弯强度、抗压强度、剪切强度等物理力学性能和湿态电气性能,并改善填料在聚合物中的润湿性和分散性。 本品是优异的粘结促进剂,可用于聚氨酯、环氧、腈类、酚醛胶粘剂和密封材料,可改善颜料的分散性并提高对玻璃、铝、铁金属的粘合性,也适用于聚氨酯、环氧和丙烯酸乳胶涂料。 在树脂砂铸造中,本品增强树脂硅砂的粘合性,提高型砂强度抗湿性。 在玻纤棉和矿物棉生产中,将其加入到酚醛粘结剂中,可提高防潮性及增加压缩回弹性。 在砂轮制造中它有助于改进耐磨自硬砂的酚醛粘合剂的粘结性及耐水性。 2.KH560 一、国外对应牌号: A-187(美国联碳公司)。
碱性磷酸酶染色液(偶氮偶联法) 简介: 碱性磷酸酶(Alkaline phosphatase ,简称ALP 或AKP)为一类磷酸酯酶,广泛分布于哺乳动物组织内,其活性所需最适pH 9.2~9.8。碱性磷酸酶染色液(偶氮偶联法)是采用偶氮偶联法(又称同时偶联法),细胞内碱性磷酸酶可使AB-BI 磷酸盐水解,释放出磷酸与萘酚,后者与偶联重氮盐生成有色产物,定位于细胞质中, 碱性磷酸酶活性部位呈红色,位于胞桨。 组成: 操作步骤(仅供参考): 1、 血液、骨髓或细胞涂片、冰冻切片、石蜡切片入ALP 固定液固定,水洗。 2、滴加配制好的ALP 孵育液,放入湿盒中,避光孵育,水洗。 3、Lea 苏木素染色液复染或甲基绿染色液复染。 4、水洗、镜检或甘油明胶封固后镜检。 染色结果: 临床意义: 1、 类白血病反应积分明显增高,未经治疗的慢性粒细胞白血病积分明显减低。 2、 急性细菌性感染积分明显增高,病毒性感染积分多正常或减低。 3、 再生障碍性贫血积分常增高,PNH 、MDS 积分常减低。 编号 名称 DE0004 4×2ml DE0004 4×10ml DE0004 4×20ml Storage 试剂(A): ALP 固定液 2ml 10ml 20ml RT 避光 试剂(B): ALP 孵育液 B1: AS-BI 染色液 1ml 5ml 10ml -20℃ 避光 B2: FBB 染色液 1ml 5ml 10ml -20℃ 避光 临用前,按B1:B2=1:1比例混合,即为ALP 孵育液,即配即用。 试剂(C): Lea 苏木素染色液 2ml 10ml 20ml 4℃ 避光 试剂(D): 甲基绿染色液 2ml 10ml 20ml RT 避光 使用说明书 1份 ALP 活性部位 红色 细胞核 蓝色(苏木素)或绿色(甲基绿)
常用硅烷偶联剂 Document serial number【NL89WT-NY98YT-NC8CB-NNUUT-NUT108】
常用硅烷偶联剂——KH550、KH560、KH570、KH792、DL602 1.KH550 KH550硅烷偶联剂CAS号:919-30-2 一、国外对应牌号 A-1100(美国联碳),Z-6011(美国道康宁),KBM-903(日本信越)。本品有碱性,通用性强,适用于环氧、PBT、酚醛树脂、聚酰胺、聚碳酸酯等多种热塑性和热固性树脂。 二、化学名称分子式: 名称:γ-氨丙基三乙氧基硅烷 别名:3-三乙氧基甲硅烷基-1-丙胺 【3-TriethoxysilylpropylamineAPTES】, γ-氨丙基三乙氧基硅烷或3-氨基丙基三乙氧基硅烷 【3-AminpropyltriethoxysilaneAMEO】 分子式:NH 2(CH 2 ) 3 Si(OC 2 H 5 ) 3 分子量:221.37 分子结构: 三、物理性质: 外观:无色透明液体
密度(ρ25℃):0.946 沸点:217℃ 折光率nD25:1.420 溶解性:可溶于有机溶剂,但丙酮、四氯化碳不适宜作释剂;可溶于水。在水中水解,呈碱性。 本品应严格密封,存放于干燥、阴凉、避光的室内。 四、KH550主要用途: 本品应用于矿物填充的酚醛、聚酯、环氧、PBT、聚酰胺、聚碳酸酯等热塑性和热固体树脂,能大幅度提高增强塑料的干湿态抗弯强度、抗压强度、剪切强度等物理力学性能和湿态电气性能,并改善填料在聚合物中的润湿性和分散性。 本品是优异的粘结促进剂,可用于聚氨酯、环氧、腈类、酚醛胶粘剂和密封材料,可改善颜料的分散性并提高对玻璃、铝、铁金属的粘合性,也适用于聚氨酯、环氧和丙烯酸乳胶涂料。 在树脂砂铸造中,本品增强树脂硅砂的粘合性,提高型砂强度抗湿性。 在玻纤棉和矿物棉生产中,将其加入到酚醛粘结剂中,可提高防潮性及增加压缩回弹性。 在砂轮制造中它有助于改进耐磨自硬砂的酚醛粘合剂的粘结性及耐水性。 2.KH560 一、国外对应牌号: A-187(美国联碳公司)。 KBM-403(日本信越化学工业株式会社) 二、化学名称及分子式 化学名称:γ-缩水甘油醚氧丙基三甲氧基硅烷
硅烷偶联剂使用说明 一、选用硅烷偶联剂的一般原则 已知,硅烷偶联剂的水解速度取于硅能团Si-X,而与有机聚合物的反应活性则取于碳官能团C-Y。因此,对于不同基材或处理对象,选择适用的硅烷偶联剂至关重要。选择的方法主要通过试验预选,并应在既有经验或规律的基础上进行。例如,在一般情况下,不饱和聚酯多选用含CH2=CMeCOO、Vi及CH2-CHOCH2O-的硅烷偶联剂;环氧树脂多选用含CH2-CHCH2O及H2N-硅烷偶联剂;酚醛树脂多选用含H2N-及H2NCONH-硅烷偶联剂;聚烯烃多选用乙烯基硅烷;使用硫黄硫化的橡胶则多选用烃基硅烷等。由于异种材料间的黏接可度受到一系列因素的影响,诸如润湿、表面能、界面层及极性吸附、酸碱的作用、互穿网络及共价键反应等。因而,光靠试验预选有时还不够精确,还需综合考虑材料的组成及其对硅烷偶联剂反应的敏感度等。为了提高水解稳定性及降低改性成本,硅烷偶联剂中可掺入三烃基硅烷使用;对于难黏材料,还可将硅烷偶联剂交联的聚合物共用。 硅烷偶联剂用作增黏剂时,主要是通过与聚合物生成化学键、氢键;润湿及表面能效应;改善聚合物结晶性、酸碱反应以及互穿聚合物网络的生成等而实现的。增黏主要围绕3种体系:即(1)无机材料对有机材料;(2)无机材料对无机材料;(3)有机材料对有机材料。对于第一种黏接,通常要求将无机材料黏接到聚合物上,故需优先考虑硅烷偶联剂中Y与聚合物所含官能团的反应活性;后两种属于同类型材料间的黏接,故硅烷偶联剂自身的反亲水型聚合物以及无机材料要求增黏时所选用的硅烷偶联剂。 二、使用方法 如同前述,硅烷偶联剂的主要应用领域之一是处理有机聚合物使用的无机填料。后者经硅烷偶联剂处理,即可将其亲水性表面转变成亲有机表面,既可避免体系中粒子集结及聚合物急剧稠化,还可提高有机聚合物对补强填料的润湿性,通过碳官能硅烷还可使补强填料与聚合物实现牢固键合。但是,硅烷偶联剂的使用效果,还与硅烷偶联剂的种类及用量、基材的特征、树脂或聚合物的性质以及应用的场合、方法及条件等有关。本节侧重介绍硅烷偶联剂的两种使用方法,即表面处理法及整体掺混法。前法是用硅烷偶联剂稀溶液处理基体表面;后法是将硅烷偶联剂原液或溶液,直接加入由聚合物及填料配成的混合物中,因而特别适用于需要搅拌混合的物料体系。 1、硅烷偶联剂用量计算 被处理物(基体)单位比表面积所占的反应活性点数目以及硅烷偶联剂覆盖表面的厚度是决定基体表面硅基化所需偶联剂用量的关键因素。为获得单分子层覆盖,需先测定基体的
硅烷偶联剂 一项目建设的目的: 为减少单一产品的经营风险,改进有机硅主要产品的结构,考虑发展有机硅下游产品——硅烷偶联剂,降低经营风险,在市场占据有利形势。 近几年,由于我国玻纤行业和子午线轮胎生产的快速发展,使得市场对硅烷偶联剂的需求量增长很快。 我国的玻璃纤维产业属于朝阳产业,而随着建筑、机械、电子等玻璃纤维增强复合材料等应用领域的发展,使得我国的玻璃纤维产业正在进入新一轮高速发展期。预计“十一五”期间,玻纤生产量的发展速度将接近10%,2010年我国玻璃纤维量有望达到130万吨,对硅烷偶联剂的需求量将达到18000吨左右;加上橡胶行业及其他行业发展的需求,预计2010年国内硅烷偶联剂总需求量将达到25000吨以上。 目前国内虽有多家硅烷偶联剂生产企业,但绝大多数企业生产规模小,而且产品档次较低,品种规格较少。因此,有条件的地区或企业建设较大型的多功能硅烷偶联剂生产线,提高我国硅烷偶联剂的生产水平是必要的。 二概述 1 基本情况: 硅烷偶联剂是一类具有特殊结构的低分子有机硅化合物,其通式为RSiX3,式中R代表氨基、巯基乙烯基、环氧基、氯丙基、氰基及甲基丙烯酰氧基等基团,这些基团和不同的基体树脂均具有较强的反应能力,x代表能够水解的基团,如卤素、烷氧基、酰氧基等。 硅烷偶联剂是由三氯氢硅(HSiCl3)和带有反应性基团的不饱和烯烃在铂氨酸催化下加成,再经醇解而得。硅烷偶联剂既能与无机物中的羟基又能与有机聚合物中的长分子链相互作用,使两种不同性质的材料偶联起来,从而改善生物材料的各种性能。 2 用途:
硅烷偶联剂的应用大致可归纳为三个方面; (1) 用于玻璃纤维的表面处理。硅烷偶联剂能改善玻璃纤维和树脂的粘合性能,提高玻璃纤维增强复合材料的强度、抗水、抗气候等性能。2004年玻璃纤维使用的硅烷偶联剂约占其消耗总量的50%以上,其中用得较多的品种有乙烯基硅烷、氨基硅烷、甲基丙烯酰氧基硅烷等。 (2) 用于无机填料的表面处理。硅烷偶联剂在对无机填料及树脂进行偶联时可预先对填料进行表面处理,也可直接加入树脂中,以改善填料在树脂中的分散性及粘合力,提高工艺性能和填充塑料(包括橡胶)的机械、电学和耐气候等性能。 (3) 用作密封剂、粘接剂和涂料的增粘剂。硅烷偶联剂能提高它们的粘接强度、耐水、耐气候等性能。硅烷偶联剂往往可以解决某些材料长期以来无法粘接的难题。 3 硅烷偶联剂的品种: 硅烷偶联剂品种很多(常用硅烷偶联剂品种见下表),其中产量最大的是双-[3-(三乙氧基)硅丙基]四硫化物(Si-69或KH-846),它是由三氯氢硅、氯丙烯为原料催化合成γ-氯丙基三氯硅烷(它是生产多种硅烷偶联剂的中间产品),然后进行醇解得到γ-氯丙基三乙氧基硅烷,再与硫化物在一定条件下反应而制得。它是橡胶料行业中得到成功使用的多功能硅烷偶联剂,广泛应用在子线午轮胎及其它橡胶制品中。 目前常用的硅烷偶联剂品种
常用硅烷偶联剂介绍
常用硅烷偶联剂介绍 1. KH550 KH550硅烷偶联剂 CAS号:919-30-2 一、国外对应牌号 A-1100(美国联碳),Z-6011(美国道康宁),KBM-903(日本信越)。本品有碱性,通用性强,适用于环氧、PBT、酚醛树脂、聚酰胺、聚碳酸酯等多种热塑性和热固性树脂。 二、化学名称分子式: 名称:γ-氨丙基三乙氧基硅烷 别名:3-三乙氧基甲硅烷基-1-丙胺 【3-Triethoxysilylpropylamine APTES】, γ-氨丙基三乙氧基硅烷或3-氨基丙基三乙氧基硅烷 【3-Aminpropyltriethoxysilane AMEO】 分子式:NH2(CH2)3Si(OC2H5)3 分子量:221.37 分子结构: 三、物理性质: 外观:无色透明液体 密度(ρ25℃):0.946 沸点:217℃
折光率nD25: 1.420 溶解性:可溶于有机溶剂,但丙酮、四氯化碳不适宜作释剂;可溶于水。在水中水解,呈碱性。 本品应严格密封,存放于干燥、阴凉、避光的室内。 四、KH550主要用途: 本品应用于矿物填充的酚醛、聚酯、环氧、PBT、聚酰胺、聚碳酸酯等热塑性和热固体树脂,能大幅度提高增强塑料的干湿态抗弯强度、抗压强度、剪切强度等物理力学性能和湿态电气性能,并改善填料在聚合物中的润湿性和分散性。 本品是优异的粘结促进剂,可用于聚氨酯、环氧、腈类、酚醛胶粘剂和密封材料,可改善颜料的分散性并提高对玻璃、铝、铁金属的粘合性,也适用于聚氨酯、环氧和丙烯酸乳胶涂料。 在树脂砂铸造中,本品增强树脂硅砂的粘合性,提高型砂强度抗湿性。 在玻纤棉和矿物棉生产中,将其加入到酚醛粘结剂中,可提高防潮性及增加压缩回弹性。 在砂轮制造中它有助于改进耐磨自硬砂的酚醛粘合剂的粘结性及耐水性。 2. KH560 一、国外对应牌号:
碱性磷酸酶染色液(偶氮偶联法)使用说明 自备材料: 1、载玻片、湿盒 2、普通光学显微镜 产品简介: 碱性磷酸酶(Alkaline phosphatase,简称ALP或AKP)为一类磷酸酯酶,广泛分布于哺乳动物组织内,其活性所需最适pH9.2~9.8。此酶主要存在于物质交换活跃之处(细胞膜),如肠上皮和肾近曲小管的刷状缘、附睾上皮之静纤毛、肝的毛细胆管膜以及微动脉和毛细血管动脉部之内皮,还见于内质网、高尔基复合体、吞饮小泡、肠上皮之溶酶体、中性粒细胞之中性颗粒以及平滑肌之细胞膜。
碱性磷酸酶染色液(偶氮偶联法)不是采用金属沉淀法来显示碱性磷酸酶活性,而是采用偶氮偶联法(又称同时偶联法),其原理是在pH9.2~9.8的碱性条件下,细胞内碱性磷酸酶可使AB-BI磷酸盐水解,释放出磷酸不萘酚,后者不偶联重氮盐生成有色产物,定位于细胞质中。该染液可用于血液、骨髓或细胞涂片、冰冻切片、梯度入水后的石蜡切片等的碱性磷酸酶染色,碱性磷酸酶活性部位呈蓝色,位于胞桨,结果较金属盐沉淀法可靠。 操作步骤(仅供参考): 一、涂片或切片 1、血液、骨髓或细胞涂片、冰冻切片、石蜡切片入ALP固定液固定3min(梯度入水后的 石蜡切片无需固定),水洗。 2、滴加配制好的ALP孵育液,放入湿盒中,避光孵育15~20min,水洗。 3、入核固红染色液或甲基绿染色液复染3~5min。 4、水洗、镜检或甘油明胶封固后镜检。 二、贴壁培养细胞 1、取6孔板或其他容器培养的细胞,弃液,PBS清洗干净。 2、加入ALP固定液固定3min或4%多聚甲醛固定10~15min,PBS清洗。 3、滴加配制好的ALP孵育液,放入湿盒中,避光孵育15~20min,PBS清洗。 4、入核固红染色液或甲基绿染色液复染3~5min。 5、PBS清洗、镜检。
硅烷偶联剂介绍
目录 1 硅烷偶联剂 (1) 有机硅烷偶联剂的选择原则 (3) 偶联剂用量 (4) 硅烷偶联剂作用机理 (5) 硅烷偶联剂使用方法 (6) 硅烷偶联剂分类与用途 (7) 硅烷偶联剂A-151 (7) 硅烷偶联剂A-171 (8) 硅烷偶联剂A-172 (9) 硅烷偶联剂KH-540 (9) 硅烷偶联剂KH-550 (10) 硅烷偶联剂KH-551 (10) 硅烷偶联剂KH-560 (11) 硅烷偶联剂KH-570 (12) 硅烷偶联剂KH-580 (13) 硅烷偶联剂KH-602 (13) 硅烷偶联剂KH-791 (14) 硅烷偶联剂KH-792 (15) 硅烷偶联剂KH-901 (16) 硅烷偶联剂KH-902 (16) 硅烷偶联剂nd-22 (17) 硅烷偶联剂ND-42(南大42) (17) 硅烷偶联剂ND-43 (17) 硅烷偶联剂SI-69 (18) 苯基三甲氧基硅烷 (18) 苯基三乙氧基硅烷 (19) 甲基三乙氧基硅烷 (20)
钛酸酯偶联剂 (20) 钛酸酯偶联剂101(钛酸酯TTS) (20) 钛酸酯偶联剂102 (21) 钛酸酯偶联剂105 (21) 有机硅烷偶联剂的选择原则 有机硅烷偶联剂的选择一般凭借对有机硅烷偶联剂侧试数据进行经脸总结,准确.地预测有机硅烷偶联剂是非常困难的。使用有机硅烷偶联剂后增大的键强度是一系列复杂因素的综合,如浸润、表面能、边界层的吸附、极性吸附,酸碱相互作用等. 预选有机硅烷偶联剂可遵循以下规津:不饱和聚醋可选用乙烯纂、环氧基及甲基丙烯陈氧基型有机硅烷偶联剂;环氧树脂宜选用环氧基或氨基型有机硅烷偶联剂;酚醛树脂宜选用氨基或服基型有机硅烷偶联剂;烯烃聚合物宜选用乙烯基型右机硅烷偶联剂;硫磺硫化的橡胶宜选用疏基型有机硅烷偶联剂等, 一、选用硅烷偶联剂的一般原则已知,硅烷偶联剂的水解速度取于硅能团Si-X,而与有机聚合物的反应活性则取于碳官能团C-Y。因此,对于不同基材或处理对象,选择适用的硅烷偶联剂至关重要。选择的方法主要通过试验,预选并应在既有经验或规律的基础上进行。例如,在一般情况下,不饱和聚酯多选用含CH2=CMeCOOVi及CH2-CHOCH2O的硅烷偶联剂:环氧树脂多选用含CH2CHCH2O及H2N硅烷偶联剂:酚醛树脂多选用含H2N及H2NCONH硅烷偶联剂:聚烯烃多选用乙烯基硅烷:使用硫黄硫化的橡胶则多选用烃基硅烷等。由于异种材料间的黏接强度受到一系列因素的影响,诸如润湿、表面能、界面层及极性吸附、酸碱的作用、互穿网络及共价键反应等。因而,光靠试验预选有时还不够精确,还需综合考虑材料的组成及其对硅烷偶联剂反应的敏感度等。为了提高水解稳定性及降低改性成本,硅烷偶联剂中可掺入三烃基硅烷使用;对于难黏材料,还可将硅烷偶联剂交联的聚合物共用。 硅烷偶联剂用作增黏剂时,主要是通过与聚合物生成化学键、氢键;润湿及表面能效应:改善聚合物结晶性、酸碱反应以及互穿聚合物网络的生成等而实现的。增黏主要围绕3种体系:即(1)无机材料对有机材料;(2)无机材料对无机材料;(3)有机材料对有机材料。对于第一种黏接,通常要求将无机材料黏接到聚合物上,故需优先考虑硅烷偶联剂中Y与聚合物所含官能团的反应活性:后两种属于同类型材料间的黏接,故硅烷偶联剂自身的反亲水型聚合物以及无机材料要求增黏时所选用的硅烷偶联剂。 硅烷偶联剂牌号偶联剂应用领 域 偶联剂作用 KH-540 KH-550 胶黏剂行业●提高粘接力及粘接寿命 ●在潮湿和干燥的条件下仍具有良好的粘结效果●更佳的耐溶剂性、提高储存寿命 KH-560 KH-570 KH-792 Si-602 Si-563 KH-540 KH-550 涂料行业●有机聚合物和无机表面之间的附着力促进剂●粘合体系的交联剂和固化剂,共聚单体 ●填料和颜料的分散剂 ●在抗刮和抗腐蚀涂料中充当粘结组分及涂层 KH-560 KH-570 KH-792 Si-602 Si-563 A-151
常用硅烷偶联剂—— KH550 、 KH560 、KH570 、KH792 、DL602 1.KH550 KH550 硅烷偶联剂CAS 号:919-30-2 二、化学名称分子式: 名称:γ-氨丙基三乙氧基硅烷 别名:3-三乙氧基甲硅烷基-1- 丙胺 【3-Triethoxysilylpropylamine APTES 】, γ-氨丙基三乙氧基硅烷或3- 氨基丙基三乙氧基硅烷 【3-Aminpropyltriethoxysilane AMEO 】 分子NH 2(CH 2)3Si(OC 分子量:221.37 分子结构: 三、物理性质 外观:无色透明液体 密度(ρ25℃ ):0.946
沸点:217℃ 溶解性:可溶于有机溶剂,但丙酮、四氯化碳不适宜作释剂;可溶于水。在水中水解,呈碱性
本品应严格密封,存放于干燥、阴凉、避光的室内 2.KH560 、化学名称及分子式 化学名γ-缩水甘油醚氧丙基三甲氧基硅烷 CH2CH(O)CH 2O(CH2)3Si(OCH 3)3 分子式: 结构式: 分子量:236.3376 三、物理性质: 物理形态:液体。颜色:无色透明。沸点:290℃。折光率: (nD25) 1.4260-1.4280 ,密度( ρ 25℃ )1.065-1.072 。溶解性:溶于水,同时发生 水解反应,水解反应释放甲醇。溶于醇、丙酮和在5%以下的正常使 用水平溶于大多数脂肪族酯。 四、应用范围: KH-560 是一种含环氧基的偶联剂,用于多硫化物和聚氨酯的嵌 缝胶和密封胶,用于环氧树脂的胶粘剂、填充型或增强型热固性树脂、玻璃纤维胶粘剂和用于无机物填充或玻璃增强的热塑料性树脂等。
医院检验中心中性粒细胞碱性磷酸酶(NAP) 染色偶氮偶联法[ 原理] 中性粒细胞胞浆中的碱性磷酸酶在碱性环境中,能水解磷酸萘酚钠,释放的萘酚与重氮盐作用生成不溶性有色的偶氮染料,定位于胞浆中。 [ 试剂] (1)固定剂,为40 %甲醛25m1,加无水乙醇至100m1。 (2)丙二醇缓冲液。①贮备液(0 . 2mol/L 2—氨基-2-甲基 -1 ,3- 丙二醇液) 为2- 氨基-2- 甲基-1 ,3- 丙二醇10 .5g,蒸馏水加至500ml。②应用液(0.05mol/L pH9.75),为0.2mol/L 贮备液25m1,o.1mol/L 盐酸5m1 ,蒸馏水加 至100m1。 (3)基质孵育液pH9.5-9 . 6(用前临时配制),a—磷酸萘 酚钠20mg溶于0.05mol/L丙二醇缓冲液20m1,再加坚 牢蓝RR(或坚牢紫酱)20mg,混合后用滤纸过滤,立即应用
(4)10g/l 亮绿 [ 操作] (1)新鲜血片或骨髓片用4- 10C的固定剂固定30秒 (2)在涂片上滴加新配过滤的孵育液,在室温下作用 10-15 分钟。 (3)水洗,10g/L 亮绿复染 2 分钟。 (4)水洗,晾干镜检。 [ 结果观察] (-) :阴性反应。 (+) :胞浆中含少量紫黑色,无紫黑色颗粒或红色物质。 (++) :胞浆中含有中等量紫黑色颗粒或呈弥漫红色。 (+++) :胞浆中有丰富的紫黑色颗粒或弥漫较深红色。 (++++) :有极丰富的大紫黑色颗粒或弥漫深红色。 [ 参考值] 中性粒细胞碱性磷酸酶活性积分值为10—50 分。
[ 临床意义] 基本同改良Gomo;氏钙钻法。 [ 附注] (1)若涂片不能及时测定,可固定干燥后放冰箱4C保存。 (2)在甘油磷酸钠和巴比妥钠应临用前称取,分别为0.38 和 0. 28,再加蒸馏水20m1即配成。配制后的B -甘油磷酸钠 不宜久放。 (3)涂片从孵育液拿出可不用水洗,直接加硝酸钻,但与硝酸 钴作用后,应反复水洗,以免残留的硝酸钻与硫化胺作用后使整个涂片变黑,造成假阳性。 (4)每次染色时,应同时做 1 份感染思者的血片为阳性对照, 以增加结果的可靠性。对照片可 1 次涂几十片,固定干燥 后,放冰箱可保存几个月。 (5)硫化胺试剂本身黄色很谈,若变深黄色,应及时更换。 试剂放置时间越长黄色越深。这说明硫大量析出,硫 离子浓度大大减少。易引起假阴性。这是试验成功与
硅烷偶联剂的使用方法 硅烷偶联剂的使用方法主要有表面预处理法和直接加入法,前者是用稀释的偶联剂处理填料表面,后者是在树脂和填料预混时,加入偶联剂的原液。 (1)表面预处理法 将硅烷偶联剂配成0.5~1%浓度的稀溶液,使用时只需在清洁的被粘表面涂上薄薄的一层,干燥后即可上胶。所用溶剂多为水、醇(甲氧基硅烷选择甲醇,乙氧基硅烷选择乙醇)、或水醇混合物,并以不含氟离子的水及价廉无毒的乙醇、异丙醇为宜。除氨烃基硅烷外,由其它硅烷偶联剂配制的溶液均需加入醋酸作水解催化剂,并将pH值调至3.5~5.5。长链烷基及苯基硅烷由于稳定性较差,不宜配成水溶液使用。氯硅烷及乙氧基硅烷水解过程中伴随有严重的缩合反应,也不宜配成水溶液或水醇溶液使用,而多配成醇溶液使用。水溶性较差的硅烷偶联剂,可先加入0.1~0.2%(质量分数)的非离子型表面活性剂,然后再加水加工成水乳液使用。硅烷偶联剂配成溶液,有利于硅烷偶联剂在材料表面的分散,溶剂是水和醇配制成的溶液,溶液一般为硅烷(20%)、醇(72%)、水(8%),醇一般为乙醇(对乙氧基硅烷)甲醇(对甲氧基硅烷)及异丙醇(对不易溶于乙醇、甲醇的硅烷)因硅烷水解速度与PH值有关,中性最慢,偏酸、偏碱都较快,因此一般需调节溶液的PH值,除氨基硅烷外,其他硅烷可加入少量醋酸,调节PH值至4—5,氨基硅烷因具碱性,不必调节。因硅烷水解后,不能久存,最好现配现用,最好在一小时内用完。 (2)直接添加方法 将硅烷偶联剂直接加入到胶粘剂组分中,一般加入量为基体树脂量的1~5%。涂胶后依靠分子的扩散作用,偶联剂分子迁移到粘接界面处产生偶联作用。对于需要固化的胶粘剂,涂胶后需放置一段时间再进行固化,以使偶联剂完成迁移过程,方能获得较好的效果。实际使用时,偶联剂常常在表面形成一个沉积层,但真正起作用的只是单分子层,因此,偶联剂用量不必过多。 硅烷偶联剂具体使用方法 (1)预处理填料法 将填料放入固体搅拌机(高速固体搅拌机HENSHEL(亨舍尔)或V型固体搅拌机等),并将上述硅烷溶液直接喷洒在填料上并搅拌,转速越高,分散效果越好。