
Capacity fade of LiNi (1àx ày )Co x Al y O 2cathode for lithium-ion batteries during accelerated calendar and cycle life https://www.doczj.com/doc/8e2631474.html,parison analysis between LiNi (1àx ày )Co x Al y O 2and LiCoO 2cathodes in cylindrical lithium-ion cells during long term storage test
Shoichiro Watanabe *,Masahiro Kinoshita,Kensuke Nakura
Portable Rechargeable Battery Business Division,SANYO Electric Co.,Ltd,Automotive &Industrial Systems Company of Panasonic Group,139-32,Toyohisa,Matsushige-Cho,Itano-gun,Tokushima 771-0213,Japan
h i g h l i g h t s
We investigate the degradation of Li-ion battery during long-term storage test. The degradation of Ni based cathode is compared with that of Co based cathode.
The storage performance of Ni based cathode is superior to that of Co based cathode. Change of both cathode surface and bulk affects the storage performance.
a r t i c l e i n f o
Article history:
Received 1May 2013Received in revised form 17August 2013
Accepted 20August 2013
Available online 6September 2013
Keywords:
Scanning transmission electron energy-loss spectroscopy Deterioration
Storage performance Cycle performance
Lithium nickel cobalt aluminum oxide Lithium-ion batteries
a b s t r a c t
Ni-based LiNi (1àx ày )Co x Al y O 2(NCA)and LiCoO 2(LCO)cathode materials taken out of lithium-ion cells after storage for 2years at 45 C were analyzed by various spectroscopic techniques.X-ray photoelectron spectroscopy exhibited that there was no difference between NCA and LCO.On the other hand,scanning transmission electron microscopy e electron energy-loss spectroscopy demonstrated there was a remarkably large difference between the two cathode materials.Ni-L 2,3energy-loss near-edge structure (ELNES)spectra of the NCA showed a peak at about 856.5eV,which was assigned to trivalent nickel,was maintained even after storage,indicating that the NCA had no signi ?cant change in its surface structure during storage.On the other hand,in the Co-L 2,3ELNES spectra of the LCO a peak at about 782.5eV,which was assigned to trivalent cobalt,signi ?cantly shifted to the lower energies after storage.These results suggest that crystal structure change of the active material surface is a predominant reason of deterioration during the storage test.
ó2013Elsevier B.V.All rights reserved.
1.Introduction
Lithium-ion batteries are widely used in portable devices such as notebook personal computers (PCs),cellular phones and digital still camera because they have high energy density.With a rise in awareness of environmental issues,lithium-ion batteries are expected to be applied to high-power applications and electric energy storage use.For EV and household electric energy storage applications,longer calendar life than conventional usage is
required.Batteries are often charged to high states of charge (SOC),and used in wide ranges of SOC during charge/discharge cycles.Ni-based oxides,LiNi (1àx ày )Co x Al y O 2(NCA),have been consid-ered as one of the most promising cathode materials because of their high capacity and low cost.It has been reported that partial substitution of Ni by Co and Al improved both thermal stability and cyclability [1e 3].However,lithium-ion batteries with the NCA cathode material were remarkably degraded during charge/discharge cycling and storage at high temperatures.The US Department of Energy ’s Advanced Technology Development (ATD)program suggested that high-power lithium ion batteries (0.8e 1.0A h,93W h kg à1)for hybrid electric vehicle applications demonstrated the increased rates of area-speci ?c impedance (ASI)
*Corresponding author.Tel.:t81886999395;fax:t81886999046.E-mail address:watanabe.sho-ichiro@https://www.doczj.com/doc/8e2631474.html, (S.
Watanabe).Contents lists available at ScienceDirect
Journal of Power Sources
journal ho mep age:www.elsevi https://www.doczj.com/doc/8e2631474.html,/locate/jpo
wsour
0378-7753/$e see front matter ó2013Elsevier B.V.All rights reserved.https://www.doczj.com/doc/8e2631474.html,/10.1016/j.jpowsour.2013.08.079
Journal of Power Sources 247(2014)412e 422
and power fade was strongly affected by temperature and time[4]. The SAFT team reported that the storage characteristics of a cylin-drical lithium-ion cell(85e145W h kgà1)were affected by SOC, which could be ascribed to an imperfect solid electrolyte interface (SEI)formed on the cathode[5].The mechanism of degradation upon aging and charge/discharge cycles was clari?ed by electro-chemical impedance analysis and several surface analyses.The AC-impedance studies with symmetrical cells demonstrated that the interfacial resistance at the cathode was a more predominant factor in a rise of impedance[6].
Various spectroscopic methods such as X-ray photoelectron spectroscopy(XPS),FT-IR,attenuated total re?ection-Fourier transform IR spectroscopy(ATR-FTIR)and high resolution hard XPS are often used for characterizing the cathode surface.Ostrovskii et al.and Anderson et al.clari?ed in XPS analysis that a cathode surface?lm consisted of a mixture of polycarbonates,LiF,Li x PF y and Li x PF y O z compounds[7].Song et al.found in ex-situ ATR-FTIR analysis that dicarbonyl anhydride and carbonyl ester(RCOOR0) were formed on the surface of the cathode which was charged to 4.2V[8].Shikano et al.found that Li2CO3,hydrocarbons,ROCO2Li, polycarbonate-type compounds and LiF were detected at the cathode surface by XPS and high-resolution hard XPS,and the amount of carbonates was increased during charge/discharge cycling[9].Recently,Saito et al.have reported that lithium car-bonate was mainly detected by ATR-FTIR spectroscopy and its amount decreased with decreasing SOC[10].On the other hand, Abraham et al.proposed that an-oxygen-de?cient surface layer was formed on an Li(Ni,Co)O2cathode due to oxygen-transfer reactions with the electrolyte[11].
The other spectroscopic analyses were also introduced to anal-ysis a surface layer formed during charge/discharge https://www.doczj.com/doc/8e2631474.html,ing in-situ X-ray absorption?ne structure(XAFS),in situ micro XAFS and surface sensitive conversion electron yield(CEY)mode X-ray absorption spectroscopy(XAS),Ukyo et al.found that the valence of Ni in LiNi0.8Co0.15Al0.05O2was converted from Ni3tto Ni4tduring charging,whereas that of Co was hardly changed.Inactive Ni2tatoms existed on the surface and the average valence of Ni was increased by charge/discharge cycling and aging[12,13].The elec-tron energy loss spectroscopy(EELS)and high resolution TEM revealed the an Ni e O layer with a rock salt(NaCl)-type crystal structure existed on the surface of cathode particle,and the appearance of an electrochemically inactive surface layer
Table1
Cell materials,cell capacity and test conditions of cylindrical18650cells.
Test condition NCR18650CGR18650E
Cathode material LiNi0.8Co0.15Al0.05O2LiCoO2
Anode material Graphite Graphite
Cell capacity 2.9A h 2.6A h
Cycle test Charge CCCV;charged at0.3C rate up to4.2V50mA-cutoff CCCV;charged at0.7C rate up to4.2V50mA-cutoff Charge rest20min20min
Discharge 1.0C rate,2.5V-cutoff 1.0C rate,3.0V-cutoff
Discharge rest20min20min
Temperature45 C45 C
Interval storage e cycle test Charge CCCV;charged at0.3C rate up to4.2V50mA-cutoff CCCV;charged at0.7C rate up to4.2V50mA-cutoff Charge rest24h24h
Discharge 1.0C rate,2.5V-cutoff 1.0C rate,2.5V-cutoff
Discharge rest20min20min
Temperature45 C45 C
Storage test Storage temperature45 C45 C
Charge before storage CCCV;charged at0.3C rate up to4.1V50mA-cutoff CCCV;charged at0.7C rate up to4.1V50mA-cutoff
Charge for checking capacity CCCV;charged at0.3C rate up to4.2V50mA-cutoff CCCV;charged at0.7C rate up to4.2V50mA-cutoff
Discharge for checking capacity0.2C rate,2.5V-cutoff0.2C rate,3.0V-cutoff
Capacity checking period90days90
days
Fig.1.Cycle performance of NCR18650(NCA cathode)and CGR18650(LCO cathode)at 45
C.
Fig.2.Interval storage and cycle test performance of NCR18650(NCA cathode)and
CGR18650E(LCO cathode)at45 C.
S.Watanabe et al./Journal of Power Sources247(2014)412e422413
contributed to the rise of cathode impedance during charge/discharge cycling and aging [15,16].Tatsumi et al.proposed a schematic degradation model of LiNi (1àx ày )Co x Al y O 2with combining these spectroscopic methods [9,14].
Panasonic https://www.doczj.com/doc/8e2631474.html,unched the highest capacity and light weight lithium-ion battery series with the nickel-based NCA cathode in 2006.The energy density of a cylindrical 18650-type battery was
620W h dm à3and 230W h kg à1or more.Battery packs inside notebook PCs were used under severe conditions like full charge voltages and high temperatures of 40 C or more because many customers used PCs with connecting the AC power supply.We noticed that the long term battery life of the NCA-cathode batteries (NCR18650)was several times as long as that of the LCO-cathode batteries (CGR18650E)[17].The difference in long-term reliability between these two commercial lithium-ion batteries must be caused by the difference of cathode material.In this study,the change in anode and cathode surface in the two 18650-type cells was investigated by XPS,ICP and TEM-EELS during the interval storage and cycle tests imitating actual usage conditions by PC users in the market (discharge/charge and 24h charge state storage at 45 C),and normal cycle and storage tests and the capacity fade was determined by the reconstructed model cell method.More-over,the mechanism of degradation was proposed on the basis of these results.
2.Experimental
2.1.Accelerated calendar and cycle life tests for cylindrical 18650cells
Two cylindrical battery cells made by Panasonic Co.were used in this study.The NCA and LCO cathode cells are NCR18650(2900mA h)and CGR18650E (2600mA h)whose cathode and anode materials,cell capacity and test conditions are shown in Table 1,
respectively.
Fig.4.Rate performances of the (a)NCR18650and (b)CGR18650.The cells were charged up to 4.2V by CVCC until the current reduced to 0.05C and discharged at (a)0.2C,(b)1C and (c)2C rate at 25 C.Straight line and closed circles indicate (e )fresh cell and (C )cell stored for 2years at 45 C after being charged to 4.1V,
respectively.
Fig.3.Storage performance at 45 C of NCR18650(NCA cathode)and CGR18650(LCO cathode)charged to 4.1
V.
Fig.5.Nyquist plots of (a)NCR18650and (b)CGR18650E which were (B )fresh or (C )stored for 2years at 45 C after being charged to 4.1V.Each impedance measurement was performed at 100%SOC and a temperature of 25 C.
S.Watanabe et al./Journal of Power Sources 247(2014)412e 422
414
The nickel-based oxide cathode/graphite cell was composed of nickel-based oxide cathode,graphite anode,electrolyte and micro-porous polyethylene/heat-resisting polymer layer-coated sepa-rator.The nickel-based oxide cathode was composed of a mixture of LiNi0.8Co0.15Al0.05O2,carbon black and poly(vinylidene?uoride), and aluminum foil.The cobalt-based oxide cathode/graphite cell was the same as the nickel-based oxide cathode/graphite cell except for the cathode active material.Electrolyte was a mixture of ethylene carbonate(EC),ethylmethyl carbonate(EMC)and dimethyl carbonate(DMC)containing1mol Là1(?M)lithium hexa?uorophosphate(LiPF6).
The cycle tests,the interval storage e cycle tests and the storage tests were performed according to protocols in Table1.The pro-tocols for the interval storage and cycle tests imagine user’s usage in Note-type PC.
For cycle test of NCR18650cell,the cell was charged at870mA (0.3C rate)up to4.2V followed by constant voltage until the cur-rent reduced to50mA.The cell was then discharged to2.5V at 2900mA(1C rate).For cycle test of CGR18650cell,the cell was charged at1820mA(0.7C rate)up to4.2V followed by constant voltage until the current reduced to50mA.The cell was then dis-charged to3.0V at2600mA(1C rate).Twenty minutes of relax-ation were used after charging and discharging.For interval storage e cycle test,24h of relaxation was used after charging,and the other conditions were the same as cycle test.Before storage test of NCA18650cell and CGR18650cell,the cells were charged at870mA
(0.3C rate)and1820mA(0.7C rate)until voltage reached4.1V at which constant-voltage charging was applied until current reduced to50mA.The charged cells were stored for720days together with capacity examined at580mA or520mA(0.2C rate)every90days. When checking capacity during storage,the cells were charged to 4.2V.
Cycle tests,interval storage-cycle tests and storage tests were done in a temperature of45 C.2.2.The model cell and impedance analysis
In order to determine which electrode was degraded,both the cathode and anode were taken out of each18650cell and used for assembling a2016coin cell with the lithium metal foil as a counter electrode under Ar atmosphere.An electrolyte and a separator of the coin cell were the same as those of the18650cell.Impedance measurements were performed using a Solartron
1260/1286
Fig.6.Discharge curves of(a)NCA cathode of NCR18650,(b)LCO cathode of CGR18650E,(c)graphite anode of NCR18650,(d)graphite anode of CGR18650E;the data of cathode obtained from the(B)fresh cell and(C)cell stored for2years at45 C after being charged to4.1V,respectively.Percentage of the initial value of discharge capacity was shown. Storage condition;the cells were stored for2years at45 C after being charged to4.1
V.
Fig.7.X-ray diffraction patterns of NCA cathode taken out from the charged
NCR18650;(a)fresh cathode material and(b)stored cathode https://www.doczj.com/doc/8e2631474.html,ler indexes
and lattice parameters were given by assuming a hexagonal lattice.The lattice pa-
rameters were obtained by a least-squares method using nine diffraction lines
depending on the number of well-de?ned diffraction lines.Storage condition;the cells
were stored for2years at45 C after being charged to4.1V.
S.Watanabe et al./Journal of Power Sources247(2014)412e422415
frequency response analyzer system.The amplitude of the ac signal was 10mV over the frequency range between 1MHz and 1mHz in this study.All measurements were carried out at 25 C.The capacity fade and impedance change of the cathode and anode were compared with their initial conditions.2.3.Surface and bulk analysis
XRD patterns were collected with a Panalytical X ’Celerator de-tector equipped with a Cu target X-ray tube and a diffracted beam
monochromator.Each XRD measurement was performed every 0.05 over a scattering angle range between 2q ?10 and 90 and the counting time was 10s.SEM images were obtained with a Hitachi S-4500scanning electron microscope equipped with an energy-dispersive X-ray (EDX)analyzer.
Surface structure of each cathode material was characterized by XPS and scanning transmission electron microscopy (STEM)-selected area electron diffraction (SAED)/EELS.XPS analysis was performed with a Perkin e Elmer PHI 560/ESCA-SAM system.XPS spectra were obtained after several times of Ar t-sputtering with 4keV energy ions and a current beam of 0.36m A cm à2.A sample for the XPS analysis was excited with 1486.6eV energy Al K a X-rays.Inductively coupled plasma atomic emission spectrometry (ICP-AES)measurement was performed using ICPS-7500in order to quantify the amount of metal deposited on the anode surface.STEM-SAED/EELS analysis was conducted with a 200kV JEOL JEM-2100F equipped with a parallel electron energy loss spectrometer (Gatan 863).XRD and SEM were used to study the phase change and microscopic morphology for each active material after storage.For surface and bulk analyses,each cell was charged to a voltage of 4.1V at 0.2C rate and then the voltage was kept until the current was reduced to 0.05C rate.After charging,the cell was dis-assembled.The samples taken out of the disassembled cells were washed with dry DMC and evaporated at room temperature.
3.Results and discussion 3.1.Performance of 18650cells
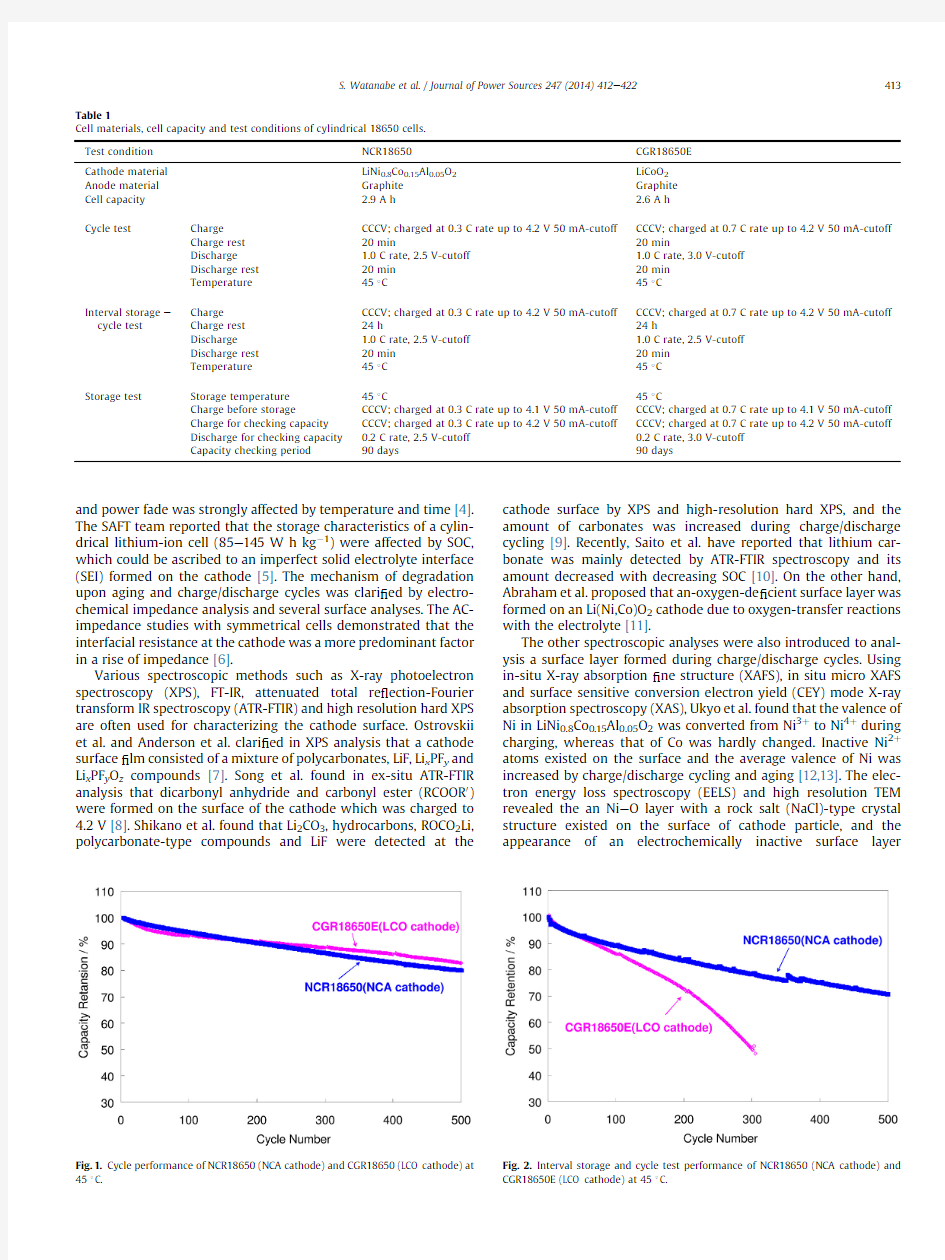
Fig.1shows cycle performance for an NCR18650(NCA cathode)and a CGR18650E (LCO cathode)at 45 C.This ?gure indicates there are only a few differences in cycle performance between both cells.However,as for the interval storage and cycle test performance,the NCR18650was much superior to the CGR18650E as shown in Fig.2
.
Fig.8.X-ray diffraction patterns of LCO cathode taken out of the charged CGR18650E;(a)fresh cathode material and (b)stored cathode https://www.doczj.com/doc/8e2631474.html,ler indexes and lattice parameters were given by assuming a hexagonal lattice.The lattice parameters were obtained by a least-squares method using nine diffraction lines depending on the number of well-de ?ned diffraction lines.Storage condition;the cells were stored for 2years at 45 C after being charged to 4.1
V.
Fig.9.SEM images of cathode material particles;(a)fresh NCR18650cathode,(b)stored NCR18650cathode,(c)fresh CGR18650cathode and (d)stored CGR18650cathode.Storage condition;the cells were stored for 2years at 45 C after being charged to 4.1V.
S.Watanabe et al./Journal of Power Sources 247(2014)412e 422
416
The life time of the70%capacity retention for the NCR18650was twice longer than that for the CGR18650E.
In order to verify the difference,the capacity fade analysis during a long term storage test was performed.Fig.3shows storage performance at45 C for the NCR18650and CGR18650E after they were charged to4.1V.The NCR18650showed better storage per-formance than the CGR18650E.The NCR18650maintained about 90%of the initial capacity even after storage for2years at45 C.
Fig.4shows rate performance for the NCR18650and CGR18650E which were fresh or stored for2years at45 C after being charged to4.1V.As be seen from Fig.4,the capacity fade and polarization of the CGR18650E was remarkably increased during the storage for2years.
Fig.5shows ac impedance spectra of the NCR18650and CGR18650E which were fresh or stored for2years at45 C after being charged to4.1V.Each impedance measurement was per-formed at100%SOC and a temperature of25 C.The charge transfer resistance(R ct)was evaluated from the lower frequency semicircle in each Nyquist plot.The R ct values for the fresh and stored NCR18650were0.0262and0.0356U,respectively,while those for the fresh and stored CGR18650E were0.0231and0.0407U, respectively.The impedance of both cells was increased during storage,but its increment for the CGR18650E was much larger than that for the NCR18650.
3.2.Analysis of disassembled battery
To reassemble a2016coin cell,the cathode or anode active materials taken out of the fresh and stored cells were used with a lithium metal counter electrode.
Fig.6shows discharge curves of each reassembled coin cell operated in the voltage range of2.5e4.3V or0.01e1.5V at a con-stant current of0.1C at25 C.As be seen from Fig.6(a)and(b),in the discharge process no signi?cant capacity fade was observed
for
Fig.10.P2p core level XPS spectra for cathode surface;(a)fresh NCA cathode,(b)fresh LCO cathode,(c)stored NCA cathode and(d)stored LCO cathode.Storage condition;the cells were stored for2years at45 C after being charged to4.1V.
S.Watanabe et al./Journal of Power Sources247(2014)412e422417
the NCA cathode after storage,while30%capacity fade was observed for the LCO cathode after storage.Moreover,as shown in Fig.6(c)and(d),in the charge process the notable capacity fade was not observed for the graphite anode in both NCR18650and CGR18650E.These results suggest that the difference in capacity fade of lithium ion cells during high temperature storage shown in Figs.2and3is ascribed to the degradation of cathode.
ICP analysis con?rmed that the lithium composition of both cathode active materials,NCA and LCO,before and after the storage tests were equal when each cell was charged to a voltage of4.1V before every analysis below.
Figs.7and8show XRD patterns of the fresh and stored NCA and LCO cathode active materials,respectively.The samples for XRD were prepared by clipping from4.1V charged cathode and then mixing with the standard reference material(NIST640c Si).All diffraction lines for fresh and stored cathode active materials were indexed by assuming a hexagonal lattice.All XRD patterns of the fresh and stored NCA and LCO cathode active materials were identical as a-NaFeO2having a space group of R-3m.Ramadass et al. pointed out that peak intensities of(003),(006),and(104)planes for the LCO changed during the cycle tests at high temperatures [18].However,as can be seen from Figs.7and8,additional diffraction lines and line broadening of each diffraction line were not speci?cally observed,indicating that the core structure of the cathode materials was not damaged during2year storage at45 C. The integrated intensity ratio of(003)to(104)lines in each XRD pattern indicates the amount of cobalt or nickel ion mixing into Li layer(cation mixing).There was no signi?cant difference in the intensity ratio between fresh and stored NCA cathode active ma-terials.On the other hand,the intensity ratio between fresh and stored LCO cathode active materials showed a signi?cant change.
SEM images of cathode active materials taken out of the dis-assembled cells are shown in Fig.9.No notable difference in cathode particles morphology between the fresh and
stored
Fig.11.F1s core level XPS spectra for anode surface(a)fresh anode of NCR18650,(b)fresh anode of CGR18650,(c)stored anode of NCR18650and(d)stored anode of CGR18650. Storage condition;the cells were stored for2years at45 C after being charged to4.1V.
S.Watanabe et al./Journal of Power Sources247(2014)412e422
418
samples was observed.In addition,we analyzed the electrolyte composition and separator SEM morphology and separator Gurley number change after storage,but the substantial change was not recognized.
These results show that the difference in degradation of LCO cathode and NCA cathode at high temperature long term storage is due to ease of forming cation mixing.
Besides,the in ?uence of cathode surface for battery degradation as has been previously pointed out [19]is discussed below.3.3.Analysis of electrode/electrolyte interface
In order to obtain more information on the cathode/electrolyte interface,the surface of the NCA and LCO cathodes was analyzed by XPS.Fig.10shows P2p core level spectra for the NCA and LCO cathodes before and after 2years storage at 45 C.P2p core level was used because of eliminating inappropriate in ?uence of PVdF as a cathode binder.In the P2p core level spectra,peaks at 135.5and 133.5eV are assigned to a P e F bond and a P e O bond,respectively.Anderson et al.clari ?ed these two peaks were ascribed to Li x PF y and Li x PF y O z compounds which were formed on the cathode sur-face [20].The thickness of the surface layer for the NCA cathode scarcely increased during the long-term storage,while that for the LCO cathode increased by only several nanometers.So the forma-tion of surface layer does not seem to be a predominant factor for the deterioration of cathode
performance.
Fig.13.Cross section TEM images and Ni-L 2,3ELNES spectra from NCA cathode material taken out from the charged NCR18650;(a)fresh cathode material and (b)stored cathode material.Dash lines show the peak positions of Ni-L 2,3ELNES spectrum from the surface of NiO particle.Storage condition;the cells were stored for 2years at 45 C after being charged to 4.1
V.
Fig.12.Amount of metal deposition on the anode surface of fresh cell and cell stored for 2years at 45 C;deposited (-)Ni and ()Co.
S.Watanabe et al./Journal of Power Sources 247(2014)412e 422419
Fig.11shows F1s core level spectra for the anode surface of NCR18650and CGR18650E before and after 2years storage at 45 C.In the F1s core level spectra,peaks at 688and 686eV are assigned to P e F and Li e F,respectively.In each spectrum these two peaks were observed,suggesting the formation of the anode surface layer or SEI with LiF and hydrolysis products such as LiPOF 3and Li x PO y F z compounds.LiPF 6as an electrolyte often decomposes to PF 5and LiF,and PF 5readily reacts with trace amount of moisture to form HF as
a
Fig.14.Cross section TEM images and Co-L 2,3ELNES spectra from the LCO cathode material taken out from the charged CGR18650E;(a)fresh cathode material and (b)stored cathode material.Dash lines show the peak positions of Co-L 2,3ELNES spectrum from the surface of CoO particle.Storage condition;the cells were stored for 2years at 45 C after being charged to 4.1
V.
Fig.15.The Ni-and Co-L 2,3ELNES peak position changes from surface of charged cathode particle;(B )Ni-L 2,3of NCA before storage,(C )Ni-L 2,3of NCA after storage,(>)Co-L 2,3of LCO before storage,(A )Co-L 2,3of LCO after storage.Storage condition;the cells were stored for 2years at 45 C after being charged to 4.1V.
S.Watanabe et al./Journal of Power Sources 247(2014)412e 422
420
hydrolysis product.Consequently,HF reacts with Li-carbonate compounds to form LiF [21].The anode surface layer after the long-term storage for the NCR18650had thickness close to that for the CGR18650E strongly suggesting that the formation of SEI on the anode surface also did not seem to be a predominant factor for the deterioration of cathode performance.
Fig.12shows the amount of Ni and Co components in deposits on the anode surface of NCR18650and CGR18650E before and after 2years storage at 45 C.The amount of the Ni and Co components was quanti ?ed by ICP-AES.Fig.12clearly indicated that in both cases Co and Ni dissolved from the cathode were deposited on the graphite anode surface.Amatucci et al.reported that there was a linear correlation between the capacity loss of the LiCoO 2cathode during high voltage cycling tests and the amount of Co deposited on the anode [22].In the present study,the amount of Ni dissolved from the NCA cathode during the long-term storage was less than half that of Co dissolved from the LCO cathode.This implies that the amount of Co and Ni dissolved from cathode material can be related to the change in surface crystal and electronic structures of the nickel-based and the cobalt-based oxide cathodes.
STEM-EELS was used in order to investigate the changes in the local structure and the electronic structure of active material before and after storage at 45 C for 2years.Figs.13and 14show the Ni-L 2,3energy-loss and the Co-L 2,3energy-loss near-edge structure (ELNES)spectra for the NCA and LCO cathode materials in the 18650cells before and after storage at 45 C for 2years.Fig.15summarized the Ni-and Co-L 2,3ELNES peak depth pro ?les of cathode particle before and after storage.
The Ni-L 2,3ELNES spectra were acquired in a depth range from the surface to 100nm for a selected NCA cathode material particle.The NiO powder as the standard has a peak at 854.5eV,indicating that it is assigned to divalent nickel.In contrast,the peak at 856.5eV is assigned to trivalent nickel.The Ni-L 2,3ELNES spectra after storage exhibited that the peak at 856.5eV in a depth range of 6e 10nm was shifted to 855.5eV during the storage test,suggesting that the sur-face layer composed of oxygen de ?cient NiO became thick.It was reported that the Ni 4tions which existed in the charged state could be transformed into Ni 2twith the oxidation of the electrolyte and solvents at the cathode/electrolyte interface,and the structure change into rock-salt type and oxygen loss also occurred simulta-neously [23].In this study,the charged NCA oxidized the electrolyte with oxygen loss to form the nano-order surface layer of lithium/oxygen-de ?cient Li(Ni,Co)O 2with the rock-salt structure at the cathode/electrolyte interface as follows [11]
:
Fig.16.The high resolution image and convergent beam electron diffraction patterns of NCA cathode material taken out from stored NCR18650.Storage condition;the cells were stored for 2years at 45 C after being charged to 4.1
V.
Fig.17.Experimental electron diffraction patterns exhibiting only fundamental re-?ections collected from stored NCA cathode material taken out from the charged NCR18650,(a)surface area which is indexed to the (1e 10)zone axis ((111)d ?0.24nm),(b)bulk area which is indexed to the (100)zone axis ((003)d ?0.47
nm).Fig.18.The high resolution image and convergent beam electron diffraction patterns of LCO cathode material taken out from stored CGR18650E.Storage condition;the cells were stored for 2years at 45 C after being charged to 4.1
V.
Fig.19.Experimental electron diffraction patterns exhibiting only fundamental re-?ections collected from stored LCO cathode material taken out from the charged NCGR18650E,(a)surface area which is indexed to the (1e 10)zone axis ((111)d ?0.24nm),(b)bulk area which is indexed to the (100)zone axis ((003)d ?0.47nm).
S.Watanabe et al./Journal of Power Sources 247(2014)412e 422421
The Co-L 2,3ELNES spectra for the LCO cathode material in the CGR18650E before and after storage are shown in Fig.14.The peak positions of CoO powder for the standard of divalent cobalt are shown in Figs.14and 15.Before storage the peak at 782.5eV assigned to trivalent cobalt was observed underneath the surface layer with thickness of around 10nm,but after storage it shifted to 781eV as shown in Fig.15.The crystal structure of the surface layer can be identi ?ed as a CoO-like rock-salt structure by selected area electron diffraction (SAED)analysis,as discussed by some groups [13,19].
Figs.16e 19show high resolution TEM images and experimental electron diffraction patterns for surface and bulk of active materials after storage at 45 C for 2years.Figs.16and 17show the image of stored NCA bulk-surface with two micro diffraction patterns layer.Figs.16and 18exhibited that the surface layer of NCA and LCO had about several nanometers in thickness and a cubic rock salt-type NiO and CoO structures.In the case of stored LCO particle,three types of micro diffraction layers were observed in Fig.18.The thickness of the surface cubic rock-salt layer was about 9nm which was thicker than that of the NCA particle.Moreover,a mixed layer which was composed of the a -NaFeO 2-type R -3m and cubic rock-salt structures was formed underneath the LCO surface layer,and its thickness was about 20nm.The lithium nickel/cobalt oxide layer with rock-salt structure formed on the NCA/LCO surface can bring low lithium ion conductivity and low electric conductivity,which caused an increase in cathode impedance.The growth of the rock-salt type surface layer for LCO was much faster than that for NCA.The difference of growth rate of the rock-salt type surface layer may be due to the difference in amount of Ni and Co dissolution shown in Fig.12.This is responsible for a big difference in storage char-acteristics between LCO and NCA.4.Conclusion
In order to verify good long life reliability of NCR18650(NCA cathode),its capacity fade analysis during the long-term storage test was investigated and compared with CGR18650E (LCO cathode).The ?ndings obtained in this study are summarized as follows,
(1)NCR18650was greatly superior to CGR18650E in the storage
characteristics.
(2)The increase in impedance and capacity fade was mainly
attributed to the degradation of the cathode,and the deterio-ration of LCO cathode was larger than that of NCA cathode.(3)SEI on NCA and LCO cathode surface analyzed by XPS was
composed of almost same elements and had same thickness level,suggesting that SEI was not the main factor of the dif-ference in degradation behavior between NCA and LCO cathodes.
(4)Change in the surface crystal/electronic structures and the
cation mixing of NCA cathode material during the long-term storage at high temperature was much smaller than that of
LCO cathode material,indicating that NCA had excellent stor-age characteristics.
In this paper,the difference between the degradation of LCO/graphite cell and that of NCA/graphite cell was particularly discussed.The results of detailed analysis on the in ?uence of cycling for degradation of the performance of these cells will be discussed in next paper.
Acknowledgments
This work was supported by the “LEAD ”program of the New Energy and Industrial Technology Development Organization (NEDO).The authors express their thanks to members of Lithium ion Battery Business Unit (A.Nagasaki,N.Yamamoto and H.Kaiya)and Prof.Hiroshi Inoue in Osaka Prefecture University.
References
[1]T.Ohzuku,A.Ueda,M.Kouguchi,J.Electrochem.Soc.142(1995)4033.[2]H.Arai,M.Tsuda,Y.Sakurai,J.Power Sources 90(2000)76.
[3] A.Kinoshita,K.Yanagida,A.Yanai,Y.Kida,A.Funahashi,T.Nohma,I.Yonezu,
J.Power Sources 102(2001)283.
[4]I.Bloom,S.A.Jones,V.S.Battaglia,G.L.Henriksen,J.P.Christophersen,
R.B.Wright,C.D.Ho,J.R.Belt,C.G.Motloch,J.Power Sources 124(2003)538.[5]M.Broussely,Ph.Biensan, F.Bonhomme,Ph.Blanchard,S.Herreyre,
K.Nechev,R.J.Staniewicz,J.Power Sources 146(2005)90.
[6] C.H.Chen,J.Liu,M.E.Stoll,G.Henriksen,D.R.Vissers,K.Amine,J.Power
Sources 128(2004)278.
[7] A.M.Andersson, D.P.Abraham,R.Haasch,S.MacLaren,J.Liu,K.Amine,
J.Electrochem.Soc.149(2002)A1358.
[8]S.W.Song,G.V.Zhuang,P.N.Ross Jr.,J.Electrochem.Soc.151(2004)A1162.[9]M.Shikano,H.Kobayashi,S.Koike,H.Sakaebe, E.Ikenaga,K.Kobayashi,
K.Tatsumi,J.Power Sources 174(2007)795.
[10]Y.Saito,M.Shikano,H.Kobayashi,J.Power Sources 196(2011)6889.
[11] D.P.Abraham,R.D.Twesten,M.Balasubramanian,I.Petrov,J.McBreen,
K.Amine,https://www.doczj.com/doc/8e2631474.html,mun.4(2002)620.
[12]T.Nonaka,C.Okuda,Y.Seno,K.Koumoto,https://www.doczj.com/doc/8e2631474.html,yo,Ceram.Int.34(2008)859.[13]T.Sasaki,T.Nonaka,H.Oka,C.Okuda,Y.Itou,Y.Kondo,Y.Takeuchi,https://www.doczj.com/doc/8e2631474.html,yo,
K.Tatsumi,S.Muto,J.Electrochem.Soc.156(2009)A289.
[14]H.Kobayashi,M.Shikano,S.Koike,H.Sakaebe,K.Tatsumi,J.Power Sources
174(2007)380.
[15] D.Mori,H.Kobayashi,M.Shikano,H.Nitani,H.Kageyama,S.Koike,
H.Sakaebe,K.Tatsumi,J.Power Sources 189(2009)676.
[16]S.Muto,Y.Sasano,K.Tatsumi,T.Sasaki,K.Horibuchi,Y.Takeuchi,https://www.doczj.com/doc/8e2631474.html,yo,
J.Electrochem.Soc.156(2009)A371.
[17]S.Watanabe,M.Kinoshita,K.Nakura,in:Abstract of the IMLB Meeting,
Montreal,2010,Abstract.
[18]P.Ramadass,B.Haran,R.White,B.N.Popov,J.Power Sources 112(2002)614.[19] D.P.Abraham,J.Liu,C.H.Chen,Y.E.Hyung,M.Stoll,N.Elsen,S.MacLaren,
R.Twesten,R.Haasch,E.Sammann,I.Petrov,K.Amine,J.Power Sources 119(2003)511.
[20] D.Ostrovskii,F.Ronci,B.Scrosati,P.Jacobsson,J.Power Sources 94(2001)183.[21] A.M.Andersson,M.Herstedt,A.G.Bishop,K.Edstrom,Electrochim.Acta 47
(2002)1885.
[22]G.G.Amatucci,J.M.Tarascon,L.C.Klein,Solid State Ionics 83(1996)167.
[23]J.Vetter,P.Novak,M.R.Wagner, C.Veit,K.-C.Moller,J.O.Besenhard,
M.Winter,M.Wohlfahrt-Mehrens,C.Vogler,A.Hammouche,J.Power Sources 147(2005)269
.
S.Watanabe et al./Journal of Power Sources 247(2014)412e 422
422
人民警察录用考试法律基础知识与公安业务知识分类模拟13 (总分:100.00,做题时间:90分钟) 一、单项选择题(总题数:40,分数:100.00) 1.人民警察的______,不仅指人民警察必须具有相应的文化程度(学历),而且要求人民警察具有良好的文化修养。 (分数:2.50) A.业务素质 B.法律素质 C.文化素质√ D.政治素质 解析:[解析] 文化素质不仅指人民警察必须具有相应的文化程度,而且要求人民警察具有良好的文化修养。文化程度为学习和掌握现代科学技术提供了知识基础,文化修养则使人讲文明、懂礼貌、注重礼仪。故选C。 2.______的规范化是实现各项工作规范化的前提和保证。 (分数:2.50) A.队伍管理 B.编制管理 C.人事管理√ D.机构管理 解析:[解析] 人事管理的规范化是实现各项工作规范化的前提和保证。故选C。 3.人民警察必须把______作为自己全部工作的出发点和归宿。 (分数:2.50) A.为人民谋利益√ B.维护治安秩序 C.维护国家利益 D.维护法律尊严 解析:[解析] 人民警察必须把为人民谋利益作为自己全部工作的出发点和归宿。故选A。 4.督察机构认为需要对公安机关的人民警察给予行政处分或者降低警衔、取消警衔的,可由______提出建议,移送有关部门按照国家有关规定办理。 (分数:2.50) A.督察机构√ B.行政首长 C.上级主管部门 D.督察长 解析:[解析] 公安机关的人民警察需要给予行政处分或者降低警衔、取消警衔的,可由督察机构提出建议,移送有关部门按照国家有关规定办理。故选A。 5.个人一等功奖励不属于公安部机关的,由______审批。 (分数:2.50) A.公安部 B.公安部政治部 C.省级公安机关√ D.地市级公安局 解析:[解析] 个人一等功奖励不属于公安部机关的,由省级公安机关审批。故选C。 6.______公安机关均设立督察机构。 (分数:2.50) A.省级人民政府 B.地级市以上地方各级人民政府
警察分为哪几种_警察分类 很多对警察不是很了解的人可能不知道警察分类的情况,今天乔布简历的小编就给大家来普及一下警察分为哪几种: 我国的警察包括人民警察和武警两个大类。 一、人民警察包括以下几个部分: (1)地方公安:刑事警察(包括缉毒、国保、经侦),治安警察,户籍警察(片警),交警,督察警察(警中警),监管警察(看守所,戒毒所)、巡警(包括防暴警察、特巡警--特警);(2)铁路公安:机构设置跟地方公安差不多,没有了交警,多了乘警和直属消防队,以前接受铁道部和公安部双重领导,估计现在划归公安部,“十局”;(3)民航公安:分为机场警察和空中警察(空保)两类,机场警察属地方公安,空中警察直属民航总局;(4)交通公安:数量很少,鲜为人知。听名字不要误以为是交警。此警种直属国家交通运输部,负责我国以长江、黄河为主的内河航运安全;(5)司法公安:如狱警,法警(检察,法院机关的执行警察);(6)林业公安:通常说的“森林警察”,受林业部、公安部双重领导;(7)国安警察:国家安全局的警察,类似FBI;(8)缉私公安:海关警察,受公安部、海关总署双重领导;(9)消防、边防、警卫,武警序列的警察。 二、武装警察部队,简称武警: (1)武警内卫部队,包括机动部队和特勤部队;(2)武警森林部队,受武警总部和国家林业局双重领导的武警;(3)武警黄金部队,受武警总部和国家黄金工业总公司-国家黄金工业管理局(原冶金工业部)双重领导的武警;(4)武警水电部队,受武警总部和水利部双重领导的武警;(5)边防、消防、警卫虽属武警序列,但是其由公安部管辖。 以上就是乔布简历的小编给大家整理的警察分类,希望大家能够很好的认识警察分为哪几种。 本文来源找工作 https://www.doczj.com/doc/8e2631474.html,/
中华人民共和国人民警察的警衔分为5等13级: 中华人民共和国人民警察警衔 ?总警监?副总警监 一等 ?一级警监?二级警监?三级警监二等 ?一级警督?二级警督?三级警督三等 ?一级警司?二级警司?三级警司四等 五等?一级警员?二级警员 人民警察实行警察职务等级编制警衔。担任行政职务的人民警察实行下列职务等级编制衔: 1、部级正职:总警监; 2、部级副职:副总警监; 3、厅(局)级正职:一级警监至二级警监; 4、厅(局)级副职:二级警监至三级警监; 5、处(局)级正职:三级警监至二级警督; 6、处(局)级副职:一级警督至三级警督; 7、科(局)级正职:一级警督至一级警司; 8、科(局)级副职:二级警督至二级警司; 9、科员(警长)职:三级警督至三级警司; 10、办事员(警员)职:一级警司至二级警员。 担任专业技术职务的人民警察实行下列职务等级编制警衔: 1、高级专业技术职务:一级警监至二级警督; 2、中级专业技术职务:一级警督至二级警司; 3、初级专业技术职务:三级警督至一级警员。
地方公安局: 领导层就是正副局长还有正副政委。还有办公室,政治处,内保队,经侦队,治安队,刑警队,网监队,交警队,110指挥中心等等。派出所除办公室,治安队,刑警队还有社区队。领导是正副所长和教导员 重案组应该属于刑警队伍,技术鉴定等等也应该是属于刑警内部的技术科 一、局领导职责 (一)局长职责 1、主持市公安局全面工作。 2、调查、分析、预测全市敌情和社会治安情况,并研究制定对策,维护全市政治稳定和治安秩序稳定。 3、负责指挥严厉打击刑事犯罪的重大行动,处置重大案件和严重危害社会治安的聚众闹事、骚乱事件及重大治安灾害事故。 4、负责全市公安队伍的教育管理,用长效机制规范队伍建设。 5、负责市公安局的党风廉政建设与思想政治工作,督促领导班子成员勤政、廉政、优政。 (二)政委职责 1、协助局长主持全市公安工作,着重抓全市公安队伍建设。 2、制定全局政治工作计划,加强党组织建设,抓公安民警思想、纪律作风建设和政治业务学习。 3、在局党委的集体领导下,抓好主管部门工作。 4、完成局党委和上级交办的其它工作事项。 (三)副局长职责
监狱人民警察职务分类框架的构想 监狱人民警察职务分类框架的构想 [摘要] 监狱人民警察职务分类问题研究已成为当前监狱学界及监狱管理高层的一个热门话题。目前在理论界也形成一些基本学说或流派,但总起来说与监狱体制改革的方向衔接不够紧密;与监狱主体职能发挥的衔接不够紧密,因此从理论层面来探讨监狱人民警察职务分类更具现实意义。笔者提出将监狱人民警察分为授予警衔的人民警察和不授 警衔的文职警察(也称文职干部),并从理性层面作一剖析,提出监狱人民警察职务分类的基本框架。 [关键词] 职务分类;授予警衔的人民警察;文职警察 目前就我国监狱系统来看,对监狱人民警察的职务分类还是处于比较粗放的状况,基本上按照国家公务员的基本分类方式分为领导职务和非领导职务,从其岗位性质来分大致可分为政治工作类、监管改造类、生产劳动管理类、行政后勤类四大类。监狱学术界与理论界对监狱人民警察的分类提出了“三分类”、“四分类”“五分类”等几种基本分类方法,这些分类应当说有其合理性、适应性的一面,但也不可避免地否
认其不适应的一面。主要表现为两个方面:一是与监狱的主体职能不相协调;二是监狱体制改革的改革方向不协调。本人在研究国外监狱工作人员分类以及总结我国监狱人民警 察现实分类不足基础上,着眼于监狱人民警察队伍的长远发展,以监狱职能纯化为视角来审视和提出监狱人民警察职务分类的基本构想,与各位专家学者共同探讨。 一、构建监狱人民警察职务分类框架的基本原则 监狱机关人民警察实施科学的分类和职务序列,要有利于监狱职能的法治化,有利于监狱行刑工作的科学化,有利于提高监狱行刑的效能,与公务员法、人民警察法以及相关条例相呼应为总体要求,具体要遵循以下若干原则。 (一)坚持职务分类的合法性原则。与《中华人民共和国公务员法》、《中华人民共和国人民警察法》、《中华人民共和国监狱法》以及与公安机关人民警察分类制度紧密衔接。但这种合法性不是机械的照抄照搬,而是要在理论层面上要有所创新、有所突破,以此来推动监狱立法进程。当前,《公安机关组织管理条例》正式颁布,也给监狱人民警察的职务分类提供了新的分类思路。 (二)坚持继承与借鉴的原则。既要符合中国国情,有社会主义中国的特色,继承中国历史监狱警察(古称狱吏等)分类的有益养份,又应借鉴并吸收世界各国的有益经验,与国际行刑趋势相衔接。我们要不断审视监狱人民警察职务分
资档次,按原人事部、财政部《关于印发<公务员工资制度改革实施办法>的通知》(国人部发[2006]58号)规定执行。 五、其他政策 (一)、公安机关执法勤务机构套改警员职务后,对按职务确定标准的津贴补贴,按所任警员职务执行;对其他津贴补贴,仍按国家现行规定执行。列入艰苦边远地区津贴实施范围的人民警察,一级警长和二级警长执行县处级的标准,三级警长、四级警长和一级警员执行乡科级的标准,二级警员和三级警员执行科级以下的标准。规范后津贴补贴的执行办法,由各地区根据实际情况制定。 (二)分配到公安机关执法勤务机构的军队转业干部,比照地方同等条件人员确定基本工资。如所任科员职务低于和其原军队职务等级相应的综合管理类职务对应最低级别相同的警员职务,职务工资和级别工资按和其原军队职务等级相应的综合管理类职务对应最低级别相同的警员职务确定。 (三)公安机关执法勤务机构人民警察在警员职务序列与公安机关综合管理机构和公安机关之外相互交流的,按照在接收单位所任职务,比照本单位同等条件人员确定工资。
(四)公安机关执法勤务机构新录用的警员的试用期工资,仍按国人部发[2006]58号文件规定执行。试用期满合格后的工资待遇,另行规定。 六、执行时间 公安机关执法勤务机构人民警察警员职务的工资政策,从2010年1月1日起执行。 七、组织实施 公安机关执法勤务机构人民警察实行警员职务序列及相应的工资政策,充分体现了党中央、国务院对广大基层一线人民警察的关怀和重视。各地区要加强领导,周密部署,切实做好组织实施工作。各级人力资源社会保障、财政部门要在当地人民政府统一领导下,密切配合,精心组织,会同公安部门认真抓好工资兑现工作。要加强指导和监督,严明政策和纪律,确保各项工作平稳顺利进行。 本通知由人力资源社会保障部负责解释。 附件:警员职务与级别对应关系表 级别警员职务 十二一级警长 十三
【转】根据几年的工作经验和跟同行的交流情况,对警察做一个分类比较(厅级的不考虑),大家都看看: 机关民警:主要是市局、分局局机关民警,待遇较基层民警差一些,前途比基层民警都好一些,有如法制办之类的部门,绝对的实权加好待遇部门; 经警:即经济犯罪侦查警察,跟经济打交道,绝对的好部门,津贴福利都不错,收礼有人请吃饭是经常的事情,工作也不累; 治安警:主要是治安支队、治安大队等,工作较闲,权利较大,接触违法乱纪的事情以及宾馆酒店等特种行业,待遇津贴很好; 刑警(侦查):做侦查刑警的一般都是年轻人,工作强度比较大,经常加班加点,待遇补贴较差,经常出差(差旅费多少看单位),但前途比较好,容易提拔; 刑警(技术):做技术的民警需要现场勘查,工作强度比侦查小一些,跟物证打交道不跟人打交道,关系网不容易形成,提拔比较难,而且是公安里面待遇最差警种,油水八杆子打不着,有关系的都想跳槽,公务员招录的时候,限制化学、法医等专业的基层公安职位,十有八九就是刑侦大队技术中队,慎重; 交警:工作比较累,年轻人要经常上街,皮肤都不好,但待遇津贴都不错,收入较可观; 特警:工作很无聊,属于武职,学不到什么东西,一般都是选拔小伙子当特警,里面之后再调到派出所等一线部门工作,另外有个特岗津贴,公安里面待遇属于中等; 巡警:有的地方叫防暴警察,工作更无聊,跟协警差不多,到处巡逻,待遇不详,一般情况吧; 派出所刑侦民警:业务上跟刑警紧密相连,地位比刑警低一些,事情比刑警多一些,工作较忙,但强度比刑警小一些,待遇比刑警好一点点;
派出所治安民警:业务上跟治安警相联系,地位比治安警低一些,事情忙一些,接触基层老百姓违法乱纪事情,收礼收卡会有,待遇不错,但不如治安警; 派出所片警:福利多少与工作强度大小与管辖片的人群的富裕程度有很大关系,除了基数工资,其他的收入或者油水情况不一定; 警校人员:包括警院(厅级)和各市警校(局级),也算机关,工作强度不大,待遇一般,管理模式和制度跟公安一样,主要是服从和守纪,跟普通大学两回事,不适合有想法的人,据说警院行政人员比教师好,主要是工作压力小,又能管着教师; 法警:属于司法系统,不归公安管理,分检察院法警和法院法警两类,检察院有贪污案件侦查任务,因而有油水,法院没有,待遇一般,负责保安和执行枪决等,没啥仕途,另外,以前法警过司考可以转检察官法官,现在编制紧,除非关系超级硬,一般要重新省考; 狱警:属于司法系统,也不归公安管理,工作地点一般较偏远,比较无聊,每天接触犯人,由于犯人劳动所得归监狱资金管理,因而待遇非常好,但没什么前途,工作较压抑; 缉毒警:工作较闲(涉毒案件少),其余不详; 国保警:工作很闲,其余也不详; 网警:网警是负责互联网安全的,主要工作是上网,删除有害帖子,qq24小时挂着也可以。这个工作,估计很多警察都羡慕; 技侦警:即技术侦查类警察,一般比较全能,搞技术开锁手机定位什么的,工作比较神秘,很少跟人交流工作的事情; 乘警:又称铁警,以前归铁道管,属于事业编制或企业编制,地位很低;现在归公安管了,地位提高了,直接变成国家公务员了,招考也是国考,但工作内容不
中国人民警察警衔级别 1、中国警察警衔级别简史 一般来说有八大警种:治安、刑警、交警、户籍、森警、武警(包括武警消防、武警黄金、武警水电、武警内卫、武警边防、武警特警、武警警卫等部队)、外事民警、乘警,新增有:网警、维和民警。 2、《警衔条例》规定人民警察的警衔设五等十三级 一等:总警监、副总警监; 二等:警监:一级、二级、三级; 三等:警督:一级、二级、三级; 四等:警司:一级、二级、三级; 五等:警员:一级、二级。 对担任专业技术职务的人民警察的警衔,在警衔前冠以“专业技术”。 二级警监以上是高级警官,三级警监、警督是中级警官,警司、警员是初级警官。 3、编制警衔 《警衔条例》第四条规定:“人民警察衽警察职务等级编制警衔。”编制警衔以人民警察的现任职务为主,兼顾担任现职时间和参加工作年限等条件,综合考核,评定警衔。 1)行政职务与警衔。《警衔条例》第八条规定:担任行政职务的人民警察实下列职务等级编制警衔:部级正职为总警监; 部级副职为副总警监; 厅(局)级正职为一级警监至二级警监; 厅(局)级副职为二级警监至三级警监; 处(局)级正职为三级警监至二级警督; 处(局)级副职为一级警督至三级警督; 科(局)级正职为一级警督至一级警司; 科(局)级副职为二级警督至二级警司; 科员(警长)职为三级督至三级警司; 办事员(警员)职为一级警司至二级警员。 2)技术职务与警衔。《警衔条例》第九条明确规定,担任专业技术职务的人民警察实行下列职务等级编制警衔: 高级专业技术职务:一级警监至二级警督; 中级专业技术职务:一级警督至二级警司; 初级专业技术职务:三级警督至一级警员。 此外:中国警察系列警衔的标志分别是: 总警监:部级正职。总警监警衔标志缀钉一枚橄榄枝环绕一周的国徽。 副总警监:部级副职。副总警监警衔标志缀钉一枚橄榄枝环绕半周的国徽。 警监警衔标志由一枚银色橄榄枝和银色四角星花组成。 一级警监:缀钉三枚四角星花; 二级警监:缀钉二枚四角星花; 三级警监:缀钉一枚四角星花。 警督警衔标志由二道银色横杠和银色四角星花组成。 一级警督:缀钉三枚四角星花; 二级警督:缀钉二枚四角星花; 三级警督:缀钉一枚四角星花。 警司警衔标志由一道银色横杠和银色四角星花组成。 一级警司:缀钉三枚四角星花; 二级警司:缀钉二枚四角星花; 三级警司:缀钉一枚四角星花。 一级警员:缀钉二枚四角星花; 二级警员:缀钉一枚四角星花。
人民警察录用考试法律基础知识与公安业务知识分类模拟5 (总分:100.00,做题时间:90分钟) 一、判断题(总题数:40,分数:100.00) 1.公安部不需要接受中央政法委员会的领导,但是各级地方公安机关要接受各级党委的政法委员会的领导。 (分数:2.50) A.正确 B.错误√ 解析:[解析] 公安部要接受中央政法委员会的领导,各级地方公安机关要接受各级党委的政法委员会的领导。 2.公安秘书工作,主要是指公安秘书行政工作和公安对策制定工作。 (分数:2.50) A.正确 B.错误√ 解析:[解析] 公安秘书工作,主要是指公安秘书行政工作和公安对策研究工作。 3.1996年召开的第十几次全国公安会议明确提出,建立强有力的公安工作,必须首先建设强大的公安队伍,走质量强警之路。 (分数:2.50) A.正确 B.错误√ 解析:[解析] 1991年召开的第十八次全国公安会议明确提出,建立强有力的公安工作,必须首先建设强大的公安队伍,走质量强警之路。 4.英国在19世纪建立了治安法官制度。 (分数:2.50) A.正确 B.错误√ 解析:[解析] 英国在中世纪(约476年~1640年)就建有治安法官制度。 5.公安政策实际上是一种公安原则,它与公安法制、公安专业对策、社会治安综合治理方针等,构成一个完整的公安原则体系。 (分数:2.50) A.正确 B.错误√ 解析:[解析] 公安政策实际上是一种公安对策,它与公安法制、公安专业对策、社会治安综合治理方针等,构成一个完整的公安对策体系。 6.依法从重从快惩处的对象是严重危害国家安全的犯罪分子。 (分数:2.50) A.正确 B.错误√ 解析:[解析] 依法从重从快惩处的对象是严重危害社会治安的犯罪分子。 7.在警察的萌芽时期,同时伴生的有监禁行为、审判行为的萌芽。 (分数:2.50)
人民警察录用考试法律基础知识与公安业务知识分类模拟8 (总分:100.00,做题时间:90分钟) 一、判断题(总题数:40,分数:100.00) 1.我国公安机关的专政职能是专门用以对付敌对势力、敌对分子和严重刑事犯罪分子的。 (分数:2.50) A.正确√ B.错误 解析: 2.为了保证准确有效地执行法律,公安机关在刑事诉讼活动中,必须坚持同人民检察院、人民法院分工负责,互相配合,必要时可互相代替,同时又要互相制约。 (分数:2.50) A.正确 B.错误√ 解析:[解析] 为了保证准确有效地执行法律,公安机关在刑事诉讼活动中,必须坚持同人民检察院、人民法院分工负责,互相配合,同时又要互相制约。 3.在中央集权制警政管理体制中,警察由政府统一领导。 (分数:2.50) A.正确 B.错误√ 解析:[解析] 在中央集权制警政管理体制中,警察由中央政府统一领导。 4.谨慎,就是重证据,重调查研究,不得草率,防止偏差,实行严格审批制度、监督制度,坚持有错必纠。 (分数:2.50) A.正确√ B.错误 解析: 5.专门机关与广大群众相结合,是在党委的领导下,把公安机关的职能作用与人民群众的积极主动精神结合起来。 (分数:2.50) A.正确√ B.错误 解析: 6.1950年10月15日召开第一次全国公安会议,研究解决了统一全国公安组织机构和公安机关的工作任务问题。 (分数:2.50) A.正确 B.错误√ 解析:[解析] 1949年10月15日召开第一次全国公安会议,研究解决了统一全国公安组织机构和公安机关的工作任务问题。 7.公安机关在办理刑事案件中,对任何人的犯罪行为都应一律平等地适应法律予以追究,不因其社会地位、经济状况、职业、受教育程度等方面的不同而有所不同。
人民警察录用考试法律基础知识与公安业务知识分类模拟12 (总分:100.00,做题时间:90分钟) 一、单项选择题(总题数:50,分数:100.00) 1.公安机关是______意志的忠实执行者。 (分数:2.00) A.政党 B.团体 C.国家√ D.阶级 解析:[解析] 公安机关是国家意志的忠实执行者。在人民当家作主的人民民主专政国家,公安机关是依据广大人民群众的意志建立的,忠实地执行人民的意志、国家的意志,以国家赋予公安机关的任务为公安工作的总根据、总目标,以国家政策和法律为全部活动的依据,因而是国家意志的忠实执行者。故选C。 2.限期出境和驱逐出境属于______的一种权力行使。 (分数:2.00) A.“警察命令” B.治安行政处罚权√ C.治安行政强制权 D.刑罚 解析:[解析] 治安行政处罚是我国行政处罚的一种。根据《治安管理处罚法》的规定,它的种类有警告、罚款、行政拘留、吊销公安机关发放的许可证。对违反治安管理的外国人,可以附加适用限期出境或者驱逐出境。故选B。 3.公安机关的性质,是指公安机关与其他国家机关相区别的______。 (分数:2.00) A.根本特点 B.根本性质 C.根本属性√ D.根本区别 解析:[解析] 公安机关的性质是指公安机关与其他国家机关相区别的根本属性。认识公安机关的性质是确定公安机关职能、任务、职权等问题的重要根据。公安机关的性质体现在公安机关的一切公安实践之中,体现在全体公安民警执行公安任务、行使公安职权的行为之中。故选C。 4.人民警察必须善于宣传群众、动员和组织群众,依靠人民群众的力量打击犯罪活动,维护社会治安,开展综合治理。这种能力属于______。 (分数:2.00) A.岗位专业能力 B.分析综合能力 C.群众工作能力√ D.管理沟通能力 解析:[解析] 群众工作能力,即人民警察必须善于宣传群众、动员和组织群众,依靠人民群众的力量打击犯罪活动,维护社会治安,开展综合治理。故选C。 5.热诚服务的要点是:情系民生,______,热情周到。 (分数:2.00) A.服务社会√ B.勇于担当 C.诚信友善 D.献身使命 解析:[解析] 公安机关人民警察职业道德规范要求之热诚服务:情系民生,服务社会,热情周到。故选A。
公安机关执法勤务机构人民警察警员职务套改方案为贯彻实施《国务院办公厅关于规范公安机关人民警察职务序列的意见》(国办发[2008]9号,以下简称《意见》),结合公安工作实际,制定本实施方案。 一、指导思想和目标 实施《意见》工作,要以科学发展观为统领,坚持依法治警、从优待警、以人为本,体现公安特色,倾斜基层一线,立足现实,着眼长远,统筹规划,突出重点,稳步推进,力争在2008年底基本完成全国公安民警职务序列分类、称谓规范和相关人员职务套改工作,并以此为契机,进一步规范公安机关内设机构设置,完善公安民警分类管理制度和选人用人机制,深化公安人事制度改革,推动公安队伍正规化建设,为建设一支政治坚定、业务精通.作风优良、执法公正的公安队伍,圆满完成各项公安保卫任务提供强有力的组织保证。 二、实施范围 (一)下列单位在编在职、已评授警衔的公安民警列入《意见》实施范围: 1.各级公安行政机关及其内设机构、派出机构 2.各级公安行政机关直属事业单位中,列入参照公务员法管理并列入评授人民警察警衔范围的机构; 3.已完成司法体制改革、纳入公务员法管理的铁路交通、民航、林业公安机构,海关缉私部门及其内设机构、派出机构; 4.其他属于公安机关人民警察建制单位的机构。
面试,工资,福利,薪水 上述机构中因涉嫌违法违纪被立案审查尚未作出结论的人员,以及尚未完成司法体制改革、未纳入公务员法管理的铁路、交通、民航、林业公安机构人员,暂不列入《意见》实施范围。 (二)下列人员不列入《意见》实施范围: 1.不担任人民警察职务的; 2.已批准离休、退休或者在2008年9月30日前已达到退休年龄的; 3.正在办理调离公安机关和辞职、辞退手续的; 4.被开除公职、劳动教养、判处刑罚、免予刑事处罚的; 5.因其他原因不宜列入实施范围的。 三、实施方法和步骤 各地公安机关要积极主动地向地方党委、政府汇报,与公务员主管部门加强沟通协调,从2008年10月1日起,按照自上而下、分期分批、积极稳妥的原则,对列入《意见》实施范围的单位,组织实施机构、人员分类、称谓规范和相关人员职务套改工作。列入《意见》实施范围的单位及其人员,应于2008年12月31日前完成规范机构设置和职务序列工作。具体实施步骤如下: (一)确定实施范围:县级以上地方公安机关会同公务员主管部门,梳理确定列入《意见》实施范围的单位及其人员,并报上一级公安机关备案。
人民警察录用考试法律基础知识与公安业务知识分类模拟4 (总分:100.00,做题时间:90分钟) 一、判断题(总题数:50,分数:100.00) 1.维护国家安全,就是保卫我国人民民主专政政权和社会主义制度不受侵犯,保卫我国的国家主权和领土完整不受侵犯。 (分数:2.00) A.正确√ B.错误 解析: 2.人民警察良好的身体素质是完成各项公安保卫任务、保存自己、克敌制胜的基本保证,而对文化程度的要求则不必太严格。 (分数:2.00) A.正确 B.错误√ 解析:[解析] 人民警察良好的身体素质是完成各项公安保卫任务、保存自己、克敌制胜的基本保证。同时,为了更好地学习和掌握现代科学技术,全面提高人民警察的业务素质,也应要求人民警察具有相应的文化程度。 3.任何违反治安管理的行为都具有社会危害性。 (分数:2.00) A.正确√ B.错误 解析: 4.公安机关人民警察内务,是指公安机关人民警察的内部事务。 (分数:2.00) A.正确√ B.错误 解析: 5.处理好公安机关与人民群众的关系是调整各种社会关系的基础。 (分数:2.00) A.正确√ B.错误 解析: 6.对于上级公安机关的重要部署及执行有重大社会影响的任务,要在党委的密切领导下进行。 (分数:2.00) A.正确√ B.错误 解析: 7.政治性、法律性和公正性,是人民警察职业道德三个最显著的特征。 (分数:2.00) A.正确
解析:[解析] 鲜明的阶级性、广泛的人民性和行为的表率性是人民警察职业道德三个最显著的特征。 8.当宽则宽,当严则严。有从宽情节的,一定要从宽;有从严情节的,一定要从严,否则就会失去政策的威力。 (分数:2.00) A.正确√ B.错误 解析: 9.1991年,公安部对人民警察的职业道德规范进行了全面系统的概括和阐述,并在全国公安系统颁布实施《人民警察职业道德规范》。 (分数:2.00) A.正确 B.错误√ 解析:[解析] 1994年,公安部对人民警察的职业道德规范进行了全面系统的概括和阐述,并在全国公安系统颁布实施《人民警察职业道德规范》。 10.对有违反治安管理行为者,我们坚持教育少数,处罚多数,并且要寓教育于处罚的全过程。 (分数:2.00) A.正确 B.错误√ 解析:[解析] 对有违反治安管理行为者,我们坚持教育多数,处罚少数,并且要寓教育于处罚的全过程。 11.公安机关的任务就是公安机关在国家法律所确定的管辖范围内为实现一定目标所承担的工作内容。 (分数:2.00) A.正确√ B.错误 解析:[解析] 公安机关的任务,是指公安机关在国家法律所确定的管辖范围内,为实现一定的目标所承担的工作内容。 12.公安机关在办理刑事案件中,对包括犯罪嫌疑人在内的诉讼参与人依法享有的诉讼权利和合法权益应给予平等的重视和保护。 (分数:2.00) A.正确√ B.错误 解析: 13.违反治安管理行为的构成要件,是指《治安管理处罚法》所规定的,构成违反治安管理行为所必须具备的一切主体要件与客体要件的总和。 (分数:2.00) A.正确 B.错误√ 解析:[解析] 违反治安管理行为的构成要件,是指《治安管理处罚法》所规定的,构成违反治安管理行为所必须具备的一切主观要件与客观要件的总和。 14.对公安工作的功过是非,要依靠各级党委和政府的检验和评价。 (分数:2.00) A.正确
人民警察赞歌(2006-12-03 10:47:18) 分类:警察人生人民警察,和平年代最具危险的神圣职业; 人民警察,新时期最具奉献精神的英雄群体。 你属于形形色色没完没了的现场,你属于厚厚薄薄没头没尾的案卷;你属于呼啸而来又呼啸而去的警笛,你属于由天安门、长城和橄榄枝构成的庄严国徽。 一颗火热的心总是深藏于冷峻的外表下.你挟带着一柄扬善惩恶的利剑,奔走在一条与众不同的路上。 你选择的是一种维护稳定秩序的生活,需要一种与挑战与生俱来的耐心,这耐心与职责熔合,锻造出一种叫平安的氛围,献给群众。 二 寻访、摸排、思考;伏击、审讯、追踪…… 品味着那上岗时不期而至的冷雨,呼吸着冬夜无孔不入的寒风,你的脸上,总是流露着倦意的憔悴;你的眼中,总是那带着血丝的失眠。 你总是在一个又一个无月的夜晚,将身体与大地融为一体,将心和耳贴紧夜幕,你听到了父老乡亲那酣畅的鼾声和香甜的梦呓,但你警觉的双耳如雷达,捕捉的却是那一串流窜了很久的罪恶足音是否在向善良和无辜逼近…… 三 也许就在今晚,罪犯又将导演一场惨剧,等待着你的又是一场短兵相接的肉搏,抑或一串较量的枪声。 曾几回,你的背后是尖刀,胸前是枪口。黑暗在你夜归的路上阴险地设置了包围。你无畏的笑声却让黑暗中的阴影胆战心惊,那慌乱的脚步和夜色一起逃遁…… 铐住罪恶的黑手,你披着微熹的曙光回到警营,虽然今晚的电视荧屏,会有一条关于你的“警方行动”现场播放,但你委实顾不上瞄上一眼,因为你累得只想钻进久违的梦乡…… 四 你总是那么天真、那么执着,期望一场酣畅的讯问,讯问时那子弹般射出的语言,揭开了一道道丑恶的黑幕;期望一腔谆谆的告诫,告诫里那烈火般燃烧的真情,能灼热一颗颗冷酷的灵魂。 你朝着人间幸福的方向,发出一声声情与法的警示;你捧出太阳般的心,托住永不倾斜
文件编号:2020年4月 公安机关执法勤务机构人民警察警员职务套 改方案版本号: A 修改号: 1 页次: 1.0 编制: 会签: 审核: 批准: 发布日期: 实施日期:
公安机关执法勤务机构人民警察警员职务套改方案为贯彻实施《国务院办公厅关于规范公安机关人民警察职务序列的意见》(国办发[2008]9号,以下简称《意见》),结合公安工作实际,制定本实施方案。 一、指导思想和目标 实施《意见》工作,要以科学发展观为统领,坚持依法治警、从优待警、以人为本,体现公安特色,倾斜基层一线,立足现实,着眼长远,统筹规划,突出重点,稳步推进,力争在2008年底基本完成全国公安民警职务序列分类、称谓规范和相关人员职务套改工作,并以此为契机,进一步规范公安机关内设机构设置,完善公安民警分类管理制度和选人用人机制,深化公安人事制度改革,推动公安队伍正规化建设,为建设一支政治坚定、业务精通.作风优良、执法公正的公安队伍,圆满完成各项公安保卫任务提供强有力的组织保证。 二、实施范围 (一)下列单位在编在职、已评授警衔的公安民警列入《意见》实施范围: 1.各级公安行政机关及其内设机构、派出机构 2.各级公安行政机关直属事业单位中,列入参照公务员法管理并列入评授人民警察警衔范围的机构; 3.已完成司法体制改革、纳入公务员法管理的铁路交通、民航、林业公安机构,海关缉私部门及其内设机构、派出机构;
4.其他属于公安机关人民警察建制单位的机构。 面试,工资,福利,薪水 上述机构中因涉嫌违法违纪被立案审查尚未作出结论的人员,以及尚未完成司法体制改革、未纳入公务员法管理的铁路、交通、民航、林业公安机构人员,暂不列入《意见》实施范围。 (二)下列人员不列入《意见》实施范围: 1.不担任人民警察职务的; 2.已批准离休、退休或者在2008年9月30日前已达到退休年龄的; 3.正在办理调离公安机关和辞职、辞退手续的; 4.被开除公职、劳动教养、判处刑罚、免予刑事处罚的; 5.因其他原因不宜列入实施范围的。 三、实施方法和步骤 各地公安机关要积极主动地向地方党委、政府汇报,与公务员主管部门加强沟通协调,从2008年10月1日起,按照自上而下、分期分批、积极稳妥的原则,对列入《意见》实施范围的单位,组织实施机构、人员分类、称谓规范和相关人员职务套改工作。列入《意见》实施范围的单位及其人员,应于2008年12月31日前完成规范机构设置和职务序列工作。具体实施步骤如下: (一)确定实施范围:县级以上地方公安机关会同公务员主管部门,梳理确定列入《意见》实施范围的单位及其人员,并报上一级公安机关备案。
人力资源和社会保障部、财政部关于公安机关执法勤务机构人民警察警员职务套改后工资问题的通知 各省、自治区、直辖市人力资源社会保障厅(局)、财政厅(局)、新疆生产建设兵团人事局、财务局:为贯彻落实《国务院办公厅关于规范公安机关人民警察职务序列的意见》(国办发[2008]9号)和人力资源社会保障部、公安部、国家公务员局《关于印发公安机关执法勤务机构人民警察警员职务套改方案的通知》(人社部发[2009]178号)规定,经报国务院批准,现就公安机关执法勤务机构人民警察套改为警员职务后的工资问题通知如下: 一、实施范围 执行警员工资政策的人员,仅限于列入人社部发[2009]178号文件规定实施范围的公安机关执法勤务机构中列入国家政法专项编制、执行公务员工资制度的人员。 二、基本工资标准 各警员职务的职务工资标准每人每月分别为,一级警长760元,二级警长590元,三级警长480元,四级警长440元,一级警员410元,二级警员380元,三级警员340元。 警员的级别工资标准,与综合管理类公务员相同。 三、套改工资的办法 职务工资。执法勤务机构人民警察按所任警员职务执行相应的职务工资标准。 级别和级别工资。执法勤务机构人民警察套改警员职务后,由办事员套为二级警员、科员套为一级警员、副主任科员套为三级警长,现级别未达到拟任警员职务对应最低级别的晋升到最低级别,已达到对应最低级别的晋升一个级别;由副主任科员套为四级警长,现级别未达到四级警长对应最低级别的晋升到最低级别,已达到对应最低级别的级别和级别工资不变;其他的级别和级别工资不变。晋升级别时,级别工资就近就高套入新任级别对应的工资标准。 四、正常晋升工资的办法 执法勤务机构人民警察晋升警员职务后,从晋升职务的次月起执行新任警员职务的职务工资和相应的级别工资。原级别低于新任职务对应最低级别的,晋升到新任职务的最低级别;原级别在新任职务对应级别以内的,由一级警员晋升为四级警长的级别和级别工资不变,其他的晋升一个级别。级别工资逐级就近就高套入晋升后级别对应的工资标准。 按年度考核结果晋升级别增加工资和晋升级别工资档次,按原人事部、财政部《关于印发<公务员工资制度改革实施办法>的通知》(国人部发[2006]58号)规定执行。 五、其他政策 (一)、公安机关执法勤务机构套改警员职务后,对按职务确定标准的津贴补贴,按所任警员职务执行;对其他津贴补贴,仍按国家现行规定执行。列入艰苦边远地区津贴实施范围的人民警察,一级警长和二级警长执行县处级的标准,三级警长、四级警长和一级警员执行乡科级的标准,二级警员和三级警员执行科级以下的标准。规范后津贴补贴的执行办法,由各地区根据实际情况制定。 (二)分配到公安机关执法勤务机构的军队转业干部,比照地方同等条件人员确定基本工资。如所任科员职务低于和其原军队职务等级相应的综合管理类职务对应最低级别相同的警员职务,职务工资和级别工资按和其原军队职务等级相应的综合管理类职务对应最低级别相同的警员职务确定。 (三)公安机关执法勤务机构人民警察在警员职务序列与公安机关综合管理机构和公安机关之外相互交流的,按照在接收单位所任职务,比照本单位同等条件人员确定工资。 (四)公安机关执法勤务机构新录用的警员的试用期工资,仍按国人部发[2006]58号文件规定执行。试用期满合格后的工资待遇,另行规定。 六、执行时间 公安机关执法勤务机构人民警察警员职务的工资政策,从2010年1月1日起执行。 七、组织实施 公安机关执法勤务机构人民警察实行警员职务序列及相应的工资政策,充分体现了党中央、国务院