
First principles investigation of the structure and electronic properties of Cu 2
Te
Yinggan Zhang a ,Baisheng Sa a ,b ,Jian Zhou a ,Zhimei Sun a ,b ,?
a Department of Materials Science and Engineering,College of Materials,Xiamen University,361005Xiamen,China b
Collaborative Innovation Center of Chemistry for Energy Materials,Xiamen University,361005Xiamen,China
a r t i c l e i n f o Article history:
Received 7May 2013
Received in revised form 28June 2013Accepted 5August 2013
Available online 17September 2013Keywords:
Transition-metal telluride Density functional theory Chemical bonding Electronic structure
a b s t r a c t
By means of ab initio random structure search,we have revealed the crystal structure of Cu 2Te,which is in agreement with the experimentally proposed Nowotny’s model.We have then performed extensive cal-culations based on density functional theories (DFT)on this Cu 2Te structure.We have shown that the strong on-site Coulomb repulsion among the localized Cu 3d electrons has to be included via the addition of a proper U in order to describe the crystal structure precisely.Furthermore,the Te–Te bond in Cu 2Te shows the feature of van der Waals bonding,while the Cu–Te and Cu–Cu bonding are mainly strong cova-lent.By analyzing the density of states and electronic band structure,we have shown that Cu 2Te is a metallic conductor.Finally,the existence of a special Dirac-like cone at the K point in the electronic band structure of Cu 2Te reminisces that observed in graphene and topological insulators.
ó2013Elsevier B.V.All rights reserved.
1.Introduction
Chalcogenide alloys are widely investigated for the applications in various areas,for example,binary Sb 2Te 3,Bi 2Te 3were reported to be topological insulators (TIS)with a conducting surface state which consisting of a single Dirac cone at the C point [1,2],which have promising applications in quantum computer or spintronic devices.For ternary chalcogenides or multi-component chalcoge-nides,the fast reversible phase transition between amorphous and crystalline phases of the chalcogenides as well as the induced great contrast in physical properties between the two states make the optical and electrical data storage devices commercially avail-able [3,4].As far as we know,the stable phases of all the ternary and binary chalcogenides mentioned above are layered compound.The interaction between the adjacent Te atoms in these layered materials has been argued to be van der Waals-like weak bonding,which plays an important role in their physical and chemical prop-erties [5].
Among the layered chalcogenides,Cu 2Te has been widely used as a back contact material for CdTe solar cell,which is important cost-effective thin-?lm solar cells with a high cell ef?ciency of 16.4%[6].Moreover,Cu 2Te is an attractive material for its thermo-electric applications since tellurides not only have the ability to yield both p and n type materials by doping but also have very high thermopower [7,8].Pseudo binary Cu 2Te–Tl 2Te shows a higher ZT value than pure Tl 2Te [9].The superionic conductivity of Cu 2Te shows large ionic conductivities comparable to its electrolyte solu-tions well below its melting temperature which makes it an attrac-tive material [10].
However,the crystal structure of Cu 2Te is not yet clear,which could have at least ?ve phase transformations from room temper-ature to 850K.Vouroutzis et al.argued that the ‘‘average’’struc-ture of these phases could be represented by the Nowotny’s model [11–15].A clear picture of the crystal structure will be help-ful for the further understanding of Cu 2Te.Moreover,a compre-hensive understanding of the electronic structure and chemical bonding of Cu 2Te will provide guidances for its applications in var-ious areas.In this work,the equilibrium crystal structure and atom positions of Cu 2Te were obtained by ab initio random structure search.We found that the Nowotny’s structure shows the lowest total energy among all the possible structures.Furthermore,we have extensively investigated the electronic strong correlated ef-fect,electronic structure and chemical bonding of the Nowotny’s structured Cu 2Te.
https://www.doczj.com/doc/764275746.html,putational methods and details
The calculations are based on the density functional theory (DFT)in conjunction with the projector-augmented-wave (PAW)[16]potentials which is implemented in the Vienna ab initio Sim-ulation Package (VASP)[17,18].The generalized gradient approxi-mations (GGA)of Perdew–Burke–Ernzerhof (PBE)[19]and the local density approximation (LDA)[20]were used for the ex-
0927-0256/$-see front matter ó2013Elsevier B.V.All rights reserved.https://www.doczj.com/doc/764275746.html,/10.1016/https://www.doczj.com/doc/764275746.html,matsci.2013.08.009
?Corresponding author at:Department of Materials Science and Engineering,College of Materials,Xiamen University,361005Xiamen,China.Tel.:+865922186664.
E-mail addresses:zmsun@https://www.doczj.com/doc/764275746.html, ,zhmsun2@https://www.doczj.com/doc/764275746.html, (Z.Sun).
change–correlation functional.The k-points of9?9?5were automatically generated with Gamma symmetry.The valence elec-tron con?gurations for Cu and Te were3p63d104s1and5s25p4.Both the relaxation convergence for ions and electrons were 1?10à6eV.The tetrahedron method with Bl?chl corrections[21] was used.The crystal structure and electron charge density calcu-lated for the equilibrium structures were analyzed by the VESTA [22]code.
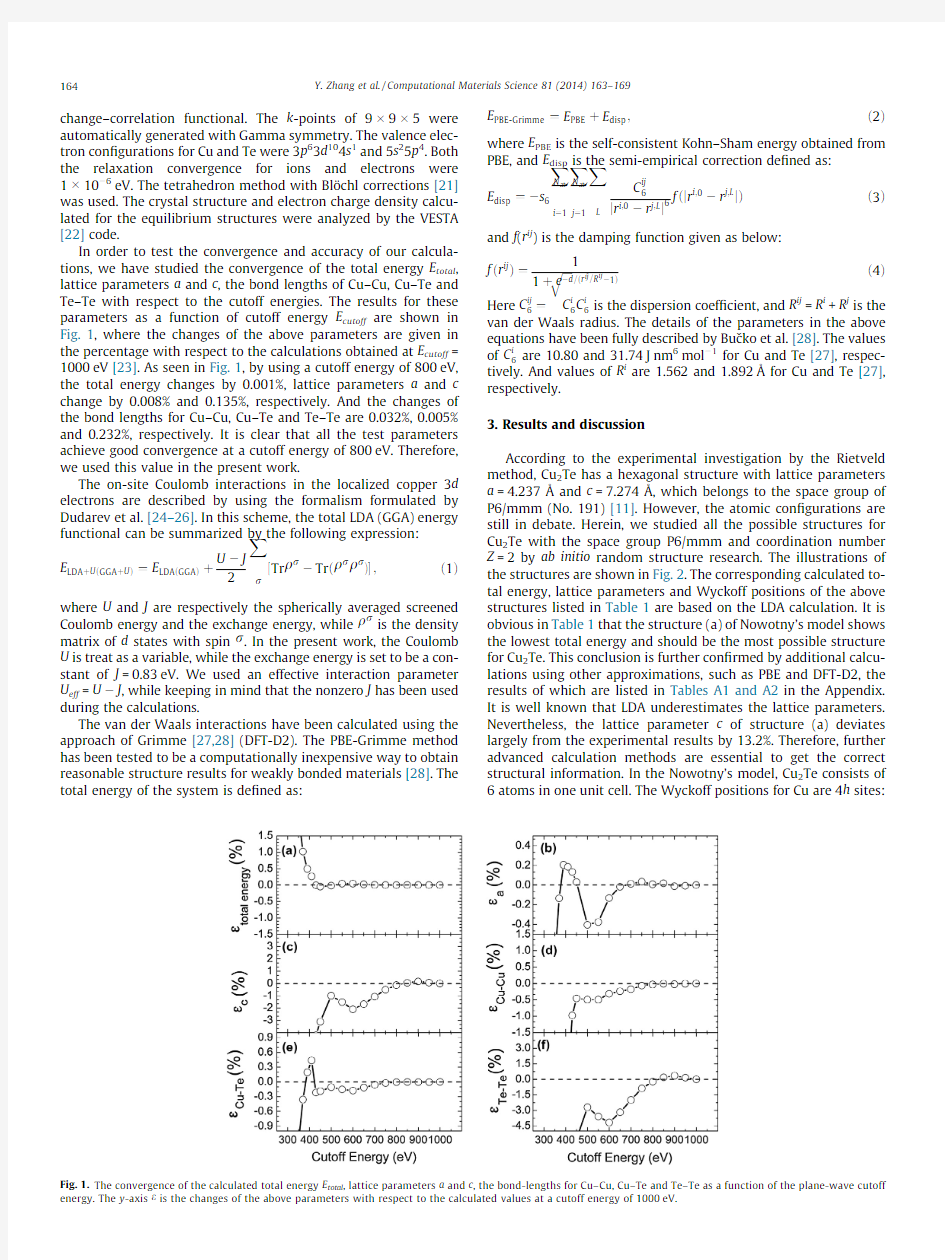
In order to test the convergence and accuracy of our calcula-tions,we have studied the convergence of the total energy E total, lattice parameters a and c,the bond lengths of Cu–Cu,Cu–Te and Te–Te with respect to the cutoff energies.The results for these parameters as a function of cutoff energy E cutoff are shown in Fig.1,where the changes of the above parameters are given in the percentage with respect to the calculations obtained at E cutoff= 1000eV[23].As seen in Fig.1,by using a cutoff energy of800eV, the total energy changes by0.001%,lattice parameters a and c change by0.008%and0.135%,respectively.And the changes of the bond lengths for Cu–Cu,Cu–Te and Te–Te are0.032%,0.005% and0.232%,respectively.It is clear that all the test parameters achieve good convergence at a cutoff energy of800eV.Therefore, we used this value in the present work.
The on-site Coulomb interactions in the localized copper3d electrons are described by using the formalism formulated by Dudarev et al.[24–26].In this scheme,the total LDA(GGA)energy functional can be summarized by the following expression:
E LDAtUeGGAtUT?E LDAeGGATtUàJ
2
X
r
?Tr q ràTreq r q rT ;e1T
where U and J are respectively the spherically averaged screened E PBE-Grimme?E PBEtE disp;e2Twhere E PBE is the self-consistent Kohn–Sham energy obtained from PBE,and E disp is the semi-empirical correction de?ned as:
E disp?às6
X N at
i?1
X N at
j?1
X
L
C ij
6
j r i;0àr j;L j
fej r i;0àr j;L jTe3Tand f(r ij)is the damping function given as below:
fer ijT?
1
1teàd=er ij=R ijà1T
e4T
Here C ij
6
?
???????????
C i
6
C i
6
q
is the dispersion coef?cient,and R ij=R i+R j is the van der Waals radius.The details of the parameters in the above equations have been fully described by Bucˇko et al.[28].The values
of C i
6
are10.80and31.74J nm6molà1for Cu and Te[27],respec-tively.And values of R i are1.562and1.892?for Cu and Te[27], respectively.
3.Results and discussion
According to the experimental investigation by the Rietveld method,Cu2Te has a hexagonal structure with lattice parameters a=4.237?and c=7.274?,which belongs to the space group of P6/mmm(No.191)[11].However,the atomic con?gurations are still in debate.Herein,we studied all the possible structures for Cu2Te with the space group P6/mmm and coordination number Z=2by ab initio random structure research.The illustrations of the structures are shown in Fig.2.The corresponding calculated to-tal energy,lattice parameters and Wyckoff positions of the above structures listed in Table1are based on the LDA calculation.It is
calculated total energy E total,lattice parameters a and c,the bond-lengths for Cu–Cu,Cu–Te and Te–Te as a changes of the above parameters with respect to the calculated values at a cutoff energy of1000eV.
164Y.Zhang et al./Computational Materials Science81(2014)163–169
e1323z T,e2313z T,e2313 z T,e1323
z Tand the Wyckoff positions for Te are 2e sites:e00 z T,(00z ).
To overcome the overestimating lattice parameter c by LDA and to get the correct structural information of Cu 2Te,we further opti-mized the structure by PBE and DFT-D2approaches.Table 2lists the calculated lattice parameters,bond lengths and atomic posi-tions of Cu 2Te using the LDA,PBE and DFT-D2approaches as well as the experimental results from Nowotny et al.[11].As seen in Table 2,the LDA and DFT-D2approximations predicted smaller
lattice parameters than PBE.Furthermore,the results of LDA and DFT-D2agree much better with the experimental results than PBE.It is because that the PBE approximation does not include the long range weak interaction force which dominates the Te–Te weak bonding in Cu 2Te.Therefore,in this paper below we mainly discuss the results based on the LDA and DFT-D2ap-proaches rather than PBE.As estimated from Table 1,the lattice parameter a deviates from experimental results by à1.6%and 0.2%using the LDA and DFT-D2,respectively.Hence,the calculated lattice parameter a is in good agreement with the experimental re-sult.However,the lattice parameter c is quite large in both LDA and DFT-D2.It is also noted in Table 2that the Cu–Cu and Te–Te bond lengths,especially the Te–Te bond length,are much larger than the experimental results.This indicates that the lattice parameter c is determined by the Te–Te bond since the bond of Te–Te is along the c direction.Therefore,more accurate descrip-tions of the Te–Te bond length are essential to get the correct structural information.
The results in Table 2show that standard DFT method fails to give the accurate lattice parameters for Cu 2Te.The description of strongly localized orbital such as d -orbital is an issue when using the density functional approximations [29].Very recently,R?san-der et al.investigated the electronic structure of ?uorite Cu 2Se using the LDA +U approach [30].They pointed out that the results obtained by LDA +U provide a good description for Cu 2Se.Hence we introduced DFT +U to further understand Cu 2Te.Table 3lists the calculated lattice parameters,bond lengths and atomic posi-tions of Cu 2Te with various U eff values.As seen in Table 3,the lat-tice parameter a increases with the increase of U values.Meanwhile,the lattice parameter c ?rstly decreases and then in-creases with the increase of U values.The same is true for the Te–Te bond length,and both correlate to the U values.It is clear that the lattice parameter c is closely correlated to the Te–Te bond length.At U =7.5eV,the Te–Te bond length is 3.173?.In this case,the bond length of Cu–Te and Cu–Cu as well as the positions of Cu and Te agree well with the experimental values [11].Even though the calculated lattice parameter c is still larger than that of exper-iments,it is within an acceptable error range.Based on the above analysis,we used a valid U =7.5eV for further calculations.On the other hand,to further investigate whether this U value will af-fect our previous conclusion of the lowest energy structure for Cu 2-Te,we have performed ab initio calculations for all the structures shown in 2using the LDA +U ,PBE +U and DFT-D2+U approxima-tions,where U =7.5eV.The calculated results are listed in Tables A3–A5in the Appendix,respectively.It is clearly that all the results support our previous conclusion that the Nowotny phase is the most stable
structure.
Fig.2.The possible crystal structures by ab initio random structure research.The blue balls are Cu atoms,the brown balls are Te atoms.The crystal structure (a)is the Nowotny’s model.(For interpretation of the references to color in this ?gure legend,the reader is referred to the web version of this article.)
Table 1
The calculated total energy,lattice parameters and Wyckoff positions for different structures of Cu 2Te in Fig.2using the LDA approach.
Total energy (eV)
Lattice parameters (?)Wyckoff position a
c Cu
Te
a à25.626 4.1698.2344h :e13233
20T
e231317
20T
2e :e0034
125Te0091
125T
e2313320Te13231720T
b à20.696 4.9087.1924h :e132369200Te2313131
200T2c :e13230Te2313
0T
e231369200Te1323131200T
c à22.855 4.920 4.9722e :e002371000Te00763
1000T
2d :e132312T
e23
1312T
2c :e13230Te231
30T
d à24.839 4.0267.0192d :e132312Te23131
2T
2e :e0014Te0034T
2c :e13230Te231
30T
e à21.752 4.4599.3562e :e00123500Te00377
500T
1b :e0012T2d :e132312Te23131
2T
1a :(000)f
à24.363
4.362
8.615
2d :e132312Te23131
2T
1b :e0012T2c :e13230Te231
30T
1a :e000T
Science 81(2014)163–169165
tron charge density of Cu 2Te in the (110)plane by the LDA +U ,PBE +U and DFT-D2+U approaches,where the value of U is 7.5eV.The bond points for the bonds are also presented in the ?g-ure.The bond point is the saddle point in the electron charge with two negative eigenvalues and one positive eigenvalue of the Hes-sian matrix of the charge density [31].It is seen in Fig.3that LDA +U and DFT-D2+U calculations give very similar chemical bonding picture for Cu 2Te.However,PBE +U does not present any effective chemical bonding between the adjacent Te layers.These results agree with our above analysis that PBE does not in-clude the long range weak interaction force which plays a key role in Cu 2Te.As seen in Fig.3(a and c),the bond point for Te–Te is 0.034e/au 3by LDA +U and is 0.036e/au 3by DFT-D2+U in Cu 2Te.The bond point of typical van der Waals type bond in tellurides is 0.016e/au 3for Sb 2Te 3(LDA)and is 0.011e/au 3for the [(GeTe)m
6.0 4.200
7.5980.1580.290 2.624 2.407 3.1866.5 4.2037.5920.1590.290 2.624 2.408 3.1867.0 4.2057.5690.1590.290 2.624 2.405 3.1737.5 4.2097.5590.1590.290 2.624 2.404 3.173
8.0 4.2188.2320.1530.264 2.599 2.526 3.8898.5 4.2218.2420.1530.263 2.599 2.530 3.905
9.0 4.2248.2340.1540.263 2.598 2.536 3.9049.5 4.2278.2330.1540.263 2.599 2.540 3.90710.0
4.230
8.229
0.155
0.263
2.598
2.550
3.909
Fig.3.The electron charge density in the (110)plane for Cu 2Te crystallized in the Nowotny’s model using (a)LDA +U ,(b)PBE +U ,(c)DFT-D2+U .The electron charge density using (d)LDA,(e)PBE,(f)DFT-D2are also illustrated for comparison.The graphs are under the same saturation levels.The interval between the contour lines 0.015e/au 3.The color scale is given at the left of the ?gure.(For interpretation of the references to color in this ?gure legend,the reader is referred to the web version of this article.)
Fig.4.The total and partial density of states for Cu 2Te crystallized in the Nowotny’s model based on LDA +U (left of graph)and DFT-D2+U (right of graph),where the value of U is 7.5eV.The black,red and blue lines represent s ,p and d states,respectively.The Fermi level is set to 0eV.(For interpretation of the references to color in this ?gure legend,the reader is referred to the web version of this article.)
(Sb 2Te 3)n ]supperlattice (DFT-D2)[32,33].Obviously,in Cu 2Te the
bond strength distributions of the charge density using LDA +U and DFT-D2+U predict a stronger Te–Te interaction rather than a pure van der Waals bonding.It is also noted in Fig.3that the electron charge densities around Te atoms show strong anisotropic property.Finally,the Te–Te bond is much weaker that the Cu–Te covalent bond (0.051e/au 3by LDA +U and 0.047e/au 3by DFT-D2+U ).The Cu–Cu bond is also shown as a strong covalent bond (0.045e/au 3by LDA +U and 0.041e/au 3by DFT-D2+U ).In addi-tion,the electron charge density using LDA,PBE and DFT-D2ap-proaches are also illustrated in Fig.3(d–f)for comparison.It is seen that the standard DFT calculations overestimate the Te–Te bond length in Cu 2Te by underestimating the Te–Te bonding strength.These results agree well with our previous analysis.
In order to further understand the properties of Cu 2Te,we stud-ied the density of states (DOS)based on the LDA +U and DFT-D2+U (U =7.5eV)approaches (given in Fig.4).According to the electrical measurements,Cu 2Te shows metallic conduction [34].While from optical studies,Cu 2Te was reported to be a semicon-ductor with an optical band gap 1.04eV [35].As seen in Fig.4
,
structure of Cu 2Te crystallized in the Nowotny’s model based on (a)LDA +U and (b)DFT-D2+U ,where the lines indicate the Cu s ,p and Te p states.(For interpretation of the references to color in this ?gure legend,Table A1
The calculated total energy,lattice parameters and Wyckoff positions for different structures in Fig.2of Cu 2Te using the PBE approach.
Total energy (eV)
Lattice parameters (?)Wyckoff position a
c Cu
Te
a à20.715 4.3228.6454h :e1323151
1000Te2313849
1000T
2e :e00131500T
e00369500Te23131511000Te132********T
b à16.569 5.0747.4124h :e132369200T
e23
13131
200T
2c :e132
30Te231
3
0T
e231369200Te1323131200T
c à18.103 5.071 5.1212e :e002371000Te00763
1000
T2d :e132312T
e23
131
2T
2c :e13230Te231
30T
d à19.697 4.1517.2082d :e132312Te23131
2T
2e :e0014Te0034T
2c :e13230Te231
30T
e à17.548 4.6169.6062e :e00123
500Te00377500T
1b :e0012T2d :e132312Te23131
2T
1a :(000)f
à19.842
4.485
9.200
2d :e132312Te23131
2T
1b :e001T2c :e13230Te231
30T
1a :(000)
Table A2
The calculated total energy,lattice parameters and Wyckoff positions for different structures in Fig.2of Cu 2Te using the DFT-D2approach.
Total energy (eV)
Lattice parameters (?)Wyckoff position a
c Cu
Te
a à22.465 4.2448.3024h :e132
31491000Te2313851
1000T2e :e0027
100Te0073
100Te2313
1491000Te132********Tb à18.108 4.9937.3334h :
e132369200Te2313131200T2c :e13230Te23130Te231
3
69200Te1323131200T
c à20.584 5.076 5.0552e :e0059
250Te00191250T2d :e132312T
e231312T
2c :e13230Te23130Td à21.766 4.1067.1162d :e132312Te231312T2e :e0014Te0034T
2c :e13230Te23130T
e à18.941 4.5219.5002e :e002471000Te00753
1000T1b :e0012T
2d :e132312Te231312T1a :(000)f
à21.717
4.591
5.950
2d :e121Te211T1b :e001T2c :
e13230Te23130T
1a :(000)
the total DOS of Cu 2Te obtained by LDA +U and DFT-D2+U show
similar characters,and there are ?nite values at the Fermi Levels,indicating the metallic conductivity of Cu 2Te.For the Nowotny’s model Cu 2Te,the Fermi Level locate at a valley of DOS,suggesting that this structure is stable [36].We also calculated the electronic structures of Cu 2Te using the Heyd–Scuseria–Ernzerhof (HSE06)hybrid functional [37,38]to double-check if the system is really metallic.It turns out that the HSE06calculations give a very similar metallic type electronic structure for Cu 2Te (data not shown).Therefore,Cu 2Te should be a metallic conductor.This metallic conductivity results from Te 5p electrons.This is clearly seen in Fig.4that the states at the Fermi level are from the Te 5p electrons.At around à5eV below the Fermi level the Te 5p electrons,Cu 3d electrons and Cu 4s electrons are strongly hybridized.These states can be divided into two parts:the Cu 3d –Cu 3d interaction above à5eV corresponding to the Cu–Cu covalent bond and the Te 5p –Cu 3d –Cu 4s interaction below à5eV corresponding to the Cu–Te bond.
Table A3
The calculated total energy,lattice parameters and Wyckoff positions for different structures in Fig.2of Cu 2Te using the LDA +U approach,where U =7.5eV.
Total energy (eV)
Lattice parameters (?)Wyckoff position a
c Cu Te
a à13.039 4.2097.5594h :e13
231591000Te2313841
1000T2e :e0029
100T
e0071
100Te2313
1591000Te132********Tb à8.527 4.9627.2174h :
e1323173500Te2313327500T2c :e132
3
0T
e23130Te231
3
173500Te1323327500T
c à10.524 4.981 4.9762e :e00119500Te00381
500T2d :e13
2312T
e231312T
2c :e120Te210Td à12.572 4.028 6.9832d :e121Te211T2e :e001Te003T
2c :e120Te210T
e à9.762 4.4809.4272e :e0049
Te00151T1b :e001T2d :e132312Te231312
T1a :e000Tf
à12.440
4.369
8.711
2d :e132312Te231312T1b :e0012T2c :
e13230Te23130T
1a :(000)
Table A4
The calculated total energy,lattice parameters and Wyckoff positions for different structures in Fig.2of Cu 2Te using the PBE +U approach,where U =7.5eV.
Total energy (eV)
Lattice parameters (?)Wyckoff position a
c Cu Te
a à8.364 4.3988.7724h :e13
2341250Te2313209
250T2e :e00129500T
e00371500T
e231341250Te1323209250T
b
à4.568
5.148
7.477
4h :e132369200Te2313131
200T2c :e132
30T
e231
30T
e231369200Te1323131200Te231369200Te1323131200T
c à5.929 5.149 5.1652e :e002371000Te00763
1000T
2d :e13231
2Te23131
2T
2c :e13230Te231
30T
d à7.464 4.1897.1862d :e132312Te23131
2T
2e :e0014Te0034T
2c :e13230Te231
30T
e à5.711 4.6679.7272e :e0061
250Te00189250T1b :e0012T
2d :e132312Te23131
2T
1a :(000)f
à7.965
4.515
9.173
2d :e132312Te23131
2T
1b :e0012T2c :e13230Te231
30T
1a :e000T
Table A5
The calculated total energy,lattice parameters and Wyckoff positions for different structures in Fig.2of Cu 2Te using the DFT-D2+U approach,where U =7.5eV.
Total energy (eV)
Lattice parameters (?)Wyckoff position a
c Cu
Te
a à9.885 4.3047.5404h :e132
3
425Te231321
25T2e :e0029
100T
e0071
100Te2313425Te13232125T
b à6.050 5.0647.3874h :e132369200Te2313131
200T2c :e132
3
0T
e23130Te231369200Te1323131200T
c à8.390 5.144 5.1002e :e002331000Te00767
1000T
2d :e13
2312T
e231312T
2c :e13230Te231
30T
d à9.507 4.1337.1092d :e132312Te23131
2T
2e :e0014Te0034T
2c :e13230Te231
30T
e à7.042 4.5579.5722e :e0031125Te0094
125T
1b :e0012T2d :e132312Te23131
2T
1a :e000Tf
à9.181
4.475
8.153
2d :e132312Te23131
2T
1b :e0012T2c :e13230Te231
30T
1a :e000T
168Y.Zhang et al./Computational Materials Science 81(2014)163–169
The electronic band structures in Fig.5give us a vivid descrip-tion of the electronic structure for Cu2Te.Similar to the charge den-sity and DOS,LDA+U and DFT-D2+U(U=7.5eV)calculations reveal similar band structure.Firstly,the band structure plotting unravels why Cu2Te shows metallic conductivity.There is a very ?at band cross the Fermi level which is occupied by the Te p states. Moreover,there are two more very light bands characterized as Cu s,p and Te p states cross the Fermi level.These bands reveal the metallic conductivity nature of Cu2Te.Hence we believe that the metallic conductivity of Cu2Te is intrinsic and is not due to the band gap problem by DFT calculations[39,40].Finally,it is inter-esting to note that the two Cu s,p and Te p bands at the K(coordi-nates)point in the Brillouin zone form a special cone which is very similar to the Dirac cone at the K and K0point in graphene and the surface states in topological insulators[41,42].Such a Dirac-like cone type band structure indicates that Cu2Te might exhibit very special electronic behaviors which need further investigations. 4.Conclusions
In summary,we have studied the possible structure of Cu2Te by ab initio random structure research.We found that the Nowotny’s model with the lowest total energy shows the highest stability. Based on DFT+U calculations,the equilibrium crystal structure and atomic positions of Cu2Te in agreement with experiments were obtained.We have also studied the electronic charge density, density of states and band structures to further understand the electronic properties of Cu2Te.The results show that both strongly localized Cu3d orbitals and van der Waals-like bonding play important roles in the chemical bonding of Cu2Te.There exists van der Waals-like type weak bonding in the adjacent Te–Te layers, while the Cu–Te and Cu–Cu bonding are mainly strong covalent in Cu2Te.Both the density of states and electronic band structures clearly show that Cu2Te is a metallic conductor.It is also worth to note that a special Dirac-like cone type band structure was ob-served in Cu2Te.Further study in deeper understanding the phys-ical original and electronic nature of this special Dirac-like cone is on-going.This work provides a comprehensive understanding of Cu2Te and should also be helpful for the understanding of Cu2Te related layered tellurides.
Acknowledgements
This work is supported by National Science Foundation for Distinguished Young Scientists of China(51225205),the National Natural Science Foundation of China(60976005,61274005)and the Outstanding Young Scientists Foundation of Fujian Province of China(2010J06018).Appendix
See Tables A1–A5.
References
[1]H.J.Zhang,C.X.Liu,X.L.Qi,X.Dai,Z.Fang,S.C.Zhang,Nature Phys.5(2009)
438.
[2]J.Zhang,C.-Z.Chang,P.Tang,Z.Zhang,X.Feng,K.Li,L.-L.Wang,X.Chen,C.Liu,
W.Duan,Science339(2013)1582.
[3]A.Kolobov,M.Krbal,P.Fons,J.Tominaga,T.Uruga,Nature Chem.3(2011)
311.
[4]Z.Sun,J.Zhou,A.Blomqvist,B.Johansson,R.Ahuja,Phys.Rev.Lett.102(2009)
075504.
[5]B.Sa,N.Miao,J.Zhou,Z.Sun,R.Ahuja,Phys.Chem.Chem.Phys.12(2010)
1585.
[6]J.H.Yun,K.H.Kim,D.Y.Lee,B.T.Ahn,Sol.Energy Mater.Sol.Cells75(2003)
203.
[7]K.Sridhar,K.Chattopadhyay,J.Alloys Compd.264(1998)293.
[8]V.M.Sklyarchuk,Y.O.Plevachuk,Semiconductors36(2002)1123.
[9]K.Kurosaki,K.Goto,A.Kosuga,H.Muta,S.Yamanaka,Mater.Trans.47(2006)
1432.
[10]H.Kikuchi,H.Iyetomi, A.Hasegawa,J.Phys.:Condens.Mater.10(1998)
11439.
[11]H.Nowotny,Z.Metallkd.37(1946)40.
[12]N.Vouroutzis,C.Manolikas,Phys.Status Solidi A111(1989)491.
[13]N.Vouroutzis,C.Manolikas,Phys.Status Solidi A115(1989)399.
[14]J.L.Da Silva,S.-H.Wei,J.Zhou,X.Wu,Appl.Phys.Lett.91(2007)091902.
[15]N.Vouroutzis,N.Frangis, C.Manolikas,Phys.Status Solidi A202(2005)
271.
[16]P.E.Bl?chl,Phys.Rev.B50(1994)17953.
[17]G.Kresse,J.Furthmüller,Phys.Rev.B54(1996)11169.
[18]G.Kresse,D.Joubert,Phys.Rev.B59(1999)1758.
[19]J.P.Perdew,K.Burke,M.Ernzerhof,Phys.Rev.Lett.77(1996)3865.
[20]W.Kohn,L.J.Sham,Phys.Rev.140(1965)A1133.
[21]P.E.Bl?chl,O.Jepsen,O.K.Andersen,Phys.Rev.B49(1994)16223.
[22]K.Momma,F.Izumi,J.Appl.Cryst.41(2008)653.
[23]J.L.F.Da Silva,A.Walsh,H.Lee,Phys.Rev.B78(2008)224111.
[24]S.Dudarev,D.N.Manh,A.Sutton,Philos.Mag.B75(1997)613.
[25]S.L.Dudarev,G.A.Botton,S.Y.Savrasov,C.J.Humphreys,A.P.Sutton,Phys.Rev.
B57(1998)1505.
[26]S.Dudarev,M.Castell,G.Botton,S.Savrasov,C.Muggelberg,G.Briggs,A.
Sutton,D.Goddard,Micron31(2000)363.
[27]S.Grimme,https://www.doczj.com/doc/764275746.html,put.Chem.27(2006)1787.
[28]T.Bucko,J.Hafner,S.Lebegue,J.G.Angyán,J.Phys.Chem.A114(2010)
11814.
[29]Y.Yang,W.Yang,P.Zhang,J.Chem.Phys.137(2012)214703.
[30]M.R?sander,L.Bergqvist,A.Delin,J.Phys.:Condens.Mater.25(2013)125503.
[31]R.F.Bader,J.Phys.Chem.A102(1998)7314.
[32]B.-T.Wang,P.Zhang,Appl.Phys.Lett.100(2012)082109.
[33]B.Sa,J.Zhou,Z.Sun,J.Tominaga,R.Ahuja,Phys.Rev.Lett.109(2012)096802.
[34]S.-Y.Miyatani,S.Mori,M.Yanagihara,J.Phys.Soc.Jpn.47(1979)1152.
[35]G.Sorokin,Y.M.Papshev,P.Oush,Sov.Phys.Solid State7(1966)1810.
[36]N.Miao,B.Sa,J.Zhou,Z.Sun,Comput.Mater.Sci.50(2011)1559.
[37]J.Heyd,G.E.Scuseria,M.Ernzerhof,J.Chem.Phys.118(2003)8207.
[38]J.Paier,M.Marsman,K.Hummer,G.Kresse,I.C.Gerber,J.G.ángyán,J.Chem.
Phys.124(2006)154709.
[39]A.Seidl,A.G?rling,P.Vogl,J.Majewski,M.Levy,Phys.Rev.B53(1996)
3764.
[40]https://www.doczj.com/doc/764275746.html,ny,A.Zunger,Phys.Rev.B78(2008)235104.
[41]K.S.Novoselov,Rev.Mod.Phys.83(2011)837.
[42]B.Sa,J.Zhou,Z.Song,Z.Sun,R.Ahuja,Phys.Rev.B84(2011)085130.
Y.Zhang et al./Computational Materials Science81(2014)163–169169
The prerequisite for vigorously developing our productivity is that we must be responsible for the safety of our company and our own lives. (安全管理) 单位:___________________ 姓名:___________________ 日期:___________________ 挖土安全操作规程(新编版)
挖土安全操作规程(新编版)导语:建立和健全我们的现代企业制度,是指引我们生产劳动的方向。而大力发展我们生产力的前提,是我们必须对我们企业和我们自己的生命安全负责。可用于实体印刷或电子存档(使用前请详细阅读条款)。 1挖土前根据安全技术交底了解地下管线、人防及其他构筑物情况和具体位置。地下构筑物外露时,必须进行加固保护。作业过程中应避开管线和构筑物。在现场电力、通信电缆2m范围内和现场燃气、热力、给排水等管道1m范围内挖土时,必须在主管单位人员监护下采取人工开挖。 2开挖槽、坑、沟深度超过1.5m,必须根据土质和深度情况按安全技术交底放坡或加可靠支撑,遇边坡不稳、有坍塌危险征兆时,必须立即撤离现场。并及时报告施工负责人,采取安全可靠排险措施后,方可继续挖土。 3槽、坑、沟必须设置人员上下坡道或安全梯。严禁攀登固壁支撑上下,或直接从沟;坑边壁上挖洞攀登爬上或跳下。间歇时,不得在槽、坑坡脚下休息。 4挖土过程中遇有古墓、地下管道、电缆或其他不能辨认的异物和液体、气体时,应立即停止作业,并报告施工负责人,待查明处理后,
合同编号:WU-PO-556-46 杂志刊登广告合同(二)标准样本 In Order T o Protect The Legitimate Rights And Interests Of Each Party, The Cooperative Parties Reach An Agreement Through Common Consultation And Fix The Responsibilities Of Each Party, So As T o Achieve The Effect Of Restricting All Parties 甲方:_________________________ 乙方:_________________________ 时间:________年_____月_____日 A4打印/ 新修订/ 完整/ 内容可编辑
杂志刊登广告合同(二)标准样本 使用说明:本合同资料适用于协作的当事人为保障各自的合法权益,经过共同协商达成一致意见并把各方所承担的责任固定下来,从而实现制约各方的效果。资料内容可按真实状况进行条款调整,套用时请仔细阅读。 广告刊出单位:《___________》杂志社(简称甲方) 广告客户单位:______________(简称乙方) 1.乙方须持营业执照,凡标明质量标准、获奖、优质产品称号、专利权、注册商标的产品应提交有关证明,实施生产许可证的产品应提交生产许可证。 2.乙方所刊登的广告内容要符合《广告法》,要实事求是,不得欺骗用户、弄虚作假。如因广告内容不当而造成不良后果的,应由乙方负全部责任。 3.乙方可对广告刊登版面和日期等提出要求
The way 的用法 Ⅰ常见用法: 1)the way+ that 2)the way + in which(最为正式的用法) 3)the way + 省略(最为自然的用法) 举例:I like the way in which he talks. I like the way that he talks. I like the way he talks. Ⅱ习惯用法: 在当代美国英语中,the way用作为副词的对格,“the way+ 从句”实际上相当于一个状语从句来修饰整个句子。 1)The way =as I am talking to you just the way I’d talk to my own child. He did not do it the way his friends did. Most fruits are naturally sweet and we can eat them just the way they are—all we have to do is to clean and peel them. 2)The way= according to the way/ judging from the way The way you answer the question, you are an excellent student. The way most people look at you, you’d think trash man is a monster. 3)The way =how/ how much No one can imagine the way he missed her. 4)The way =because
实验室岗位安全操作规程 1、在实验室工作当中存在化学灼伤、气体中毒、火灾触电、机械伤害等风险。 2、实验人员在实验室内必须穿戴工作服装,取样人员必须佩戴好相应的劳保用品与安全防护用具。 3、所有药品、标样、溶液都应有标签,绝对不要在容器内装入与标签不相符的物品。 4、不准穿拖鞋进入实验室,注意保持实验室的清洁卫生。 5、有毒、腐蚀、易燃、易爆的物品应妥善保管。贮存和使用应遵守?化学危险物品安全管理条例?。 6、实验室内严禁吸烟、饮水、进食。 7、开启易挥发液体试剂之前,先将试剂瓶放在自来水流中冷却几分钟,开启时,瓶口不要对人,最好在通风厨中进行。 8、易燃溶剂加热时,必须在水浴或沙浴中进行。 9、装过强腐蚀性、可燃性、有毒或易燃物品的器皿必须由使用者亲自洗净。 10、实验时若发现仪器设备出现故障或异常情况(如:有异味、冒烟等)时,应立即关闭电源开关,拨掉电源插头,并及时向实验室管理人员报告。 11、取下正在沸腾的溶液时,应用瓶夹摇动后取下以免溅出伤人。 12、玻璃棒、玻璃管、温度计等插入或拔出胶管时,均应垫有棉布且不可强行插入或拔出,以免折断刺伤人。
13、实验完毕,要关闭设备的电源、关好通风橱、整理好仪器设备,并打扫卫生。 14、配制药品或试验中能放出HCN、NO2、H2S、SO2、NH3及其它有毒和腐蚀性气体时,应在通风厨中进行,并带好必要的劳保用品。 15、实验室内应备有急救药品,消防器材和劳保用品。 16、化验室内应保持空气流通,环境清洁、安静。 17、易燃性气体不可与有助燃性的气体放到一个气瓶间,气瓶间内一定要有相应的防爆防倾设施。 18、样品的取样、接受、贮存和处置等要符合国家和公司的相关规定。 19、对实验产生的废液、废油、废物要分类存放并定期处置,禁止随意倾倒和储存。 20、实验室使用及存储的化学药剂或化学危险品都应备有相对应的化学品安全技术说明书(即MSDS),包括电子版和纸质版,并存放于实验室工作人员易于查找阅读的地方。同时实验室工作人员在使用化学药剂(特别是危险化学品)之前要对MSDS进行阅读学习,了解其危险特性及应急措施。 21、化学烧伤事故应急措施:当浓酸溅到眼睛或皮肤上时,应立即用大量清水冲洗,再用0.5%的碳酸氢钠溶液清洗;当强碱溅到眼睛或皮服上时,应迅速用大量清水冲洗再用2%的稀硼酸溶液清洗眼睛或用1%的醋酸溶液清洗皮肤。 当酸和碱滴溅到眼睛或皮肤上时,除经过上述处理外,还应马上送往医院进行救护。
( 操作规程 ) 单位:_________________________ 姓名:_________________________ 日期:_________________________ 精品文档 / Word文档 / 文字可改 挖土安全操作规程(新版) Safety operating procedures refer to documents describing all aspects of work steps and operating procedures that comply with production safety laws and regulations.
挖土安全操作规程(新版) 1挖土前根据安全技术交底了解地下管线、人防及其他构筑物情况和具体位置。地下构筑物外露时,必须进行加固保护。作业过程中应避开管线和构筑物。在现场电力、通信电缆2m范围内和现场燃气、热力、给排水等管道1m范围内挖土时,必须在主管单位人员监护下采取人工开挖。 2开挖槽、坑、沟深度超过1.5m,必须根据土质和深度情况按安全技术交底放坡或加可靠支撑,遇边坡不稳、有坍塌危险征兆时,必须立即撤离现场。并及时报告施工负责人,采取安全可靠排险措施后,方可继续挖土。 3槽、坑、沟必须设置人员上下坡道或安全梯。严禁攀登固壁支撑上下,或直接从沟;坑边壁上挖洞攀登爬上或跳下。间歇时,不得在槽、坑坡脚下休息。
4挖土过程中遇有古墓、地下管道、电缆或其他不能辨认的异物和液体、气体时,应立即停止作业,并报告施工负责人,待查明处理后,再继续挖土。 5槽、坑、沟边1m以内不得堆土、堆料、停置机具。堆土高度不得超过1.5m。槽、坑、沟与建筑物、构筑物的距离不得小于1.5m。开挖深度超过2m时,必须在周边设两道牢固护身栏杆,并立挂密目安全网。 6人工开挖土方,两人横向间距不得小于2m,纵向间距不得小于3m。严禁掏洞挖土,搜底挖槽。 7钢钎破冻土、坚硬土时,扶钎人应站在打锤人侧面用长把夹具扶钎,打锤范围内不得有其他人停留。锤顶应平整,锤头应安装牢固。钎子应直且不得有飞刺。打锤人不得戴手套。 8从槽、坑、沟中吊运送土至地面时,绳索、滑轮、钩子、箩筐等垂直运输设备、工具应完好牢固。起吊、垂直运送时,下方不得站人。 9配合机械挖土清理槽底作业时,严禁进入铲斗回转半径范围。
杂志刊登广告合同 甲方: 法定代表人: 乙方: 法定代表人: 上述各方经平等自愿协商,签订本合同以共同遵守。 第1条乙方须持营业执照,凡标明质量标准、获奖、优质产品称号、专利权、注册商标的产品应提交有关证明,实施生产许可证的产品应提交生产许可证。第2条乙方所刊登的广告内容要符合《广告法》,要实事求是,不得欺骗用户、弄虚作假。如因广告内容不当而造成不良后果的,应由乙方负全部责任。 第3条乙方可对广告刊登版面和日期等提出要求,由甲方参考乙方意见,根据来稿先后、版面情况及预付款等统一安排。 第4条乙方在上刊登广告的要求如下: 刊出时间版面版面费制版费设计费等合计 ¥元年/期 ¥元年/期 ¥元年/期 ¥元年/期 ¥元年/期 总计人民币(大写)(¥元)。 第5条广告刊出后,如发生影响效果的差错系原稿的错误,由乙方负责;系甲方差错,甲方负责用文字更正一次,但不予重登广告,原已刊出的广告,费用照收。
第6条广告设计、摄影、发排制版后,如乙方要求变更合同,则须付相应的设计费、摄影费、制版费以及赔偿有关损失。 第7条乙方在签订合同后,应及时付广告费。甲方将优先安排已有合同单、刊登资料和已付清广告费的客户。 第8条本合同一式两份,各方各执一份,具有同等法律效力。 第9条因本合同引起的或与本合同有关的任何争议,由合同各方协商解决,也可由有关部门调解。协商或调解不成的,按下列第种方式解决: (1)提交位于(地点)的仲裁委员会仲裁。仲裁裁决是终局的,对各方均有约束力; (2)依法向所在地有管辖权的人民法院起诉。 签约地点:市区 签约时间:年月日 甲方单位: 联系人: 电话: 传真: 开户行: 帐号: 乙方单位: 联系人: 电话:
The way的用法及其含义(二) 二、the way在句中的语法作用 the way在句中可以作主语、宾语或表语: 1.作主语 The way you are doing it is completely crazy.你这个干法简直发疯。 The way she puts on that accent really irritates me. 她故意操那种口音的样子实在令我恼火。The way she behaved towards him was utterly ruthless. 她对待他真是无情至极。 Words are important, but the way a person stands, folds his or her arms or moves his or her hands can also give us information about his or her feelings. 言语固然重要,但人的站姿,抱臂的方式和手势也回告诉我们他(她)的情感。 2.作宾语 I hate the way she stared at me.我讨厌她盯我看的样子。 We like the way that her hair hangs down.我们喜欢她的头发笔直地垂下来。 You could tell she was foreign by the way she was dressed. 从她的穿著就可以看出她是外国人。 She could not hide her amusement at the way he was dancing. 她见他跳舞的姿势,忍俊不禁。 3.作表语 This is the way the accident happened.这就是事故如何发生的。 Believe it or not, that's the way it is. 信不信由你, 反正事情就是这样。 That's the way I look at it, too. 我也是这么想。 That was the way minority nationalities were treated in old China. 那就是少数民族在旧中
实验室安全操作规范 一、穿着规定 1、进入实验室,必须穿戴工作服。 2、进行危害物质、挥发性有机溶机、特定化学物质或其它毒性化学物质等化学药品及生物样品操作,必须要穿戴防护具(例如防护手套、口罩、防毒面具等)。 3、进行实验中,严禁戴隐形眼镜(防止化学药剂溅入眼内而腐蚀灼伤眼睛)。 4、需将长发及松散衣服妥善固定,且实验室内不得穿拖鞋。 5、操作高温(低温)实验,必须戴防高温(低温)手套。 6、实验服严禁穿回办公区。 二、饮食规定 1、严禁在实验室内吃东西。 2、食物禁止储藏在实验室的冰箱或储藏柜内。 三、试剂存储及操作相关规定 1、未进检测室时对实验过程进行预习,掌握操作过程及原理,弄清所有检测仪器及试剂的特性。估计可能发生危险的实验,操作时注意防范。 2、实验开始前检查仪器是否完好,装置是否正确稳妥。 3、实验进行时应经常注意仪器是否漏液、漏气、碎裂,反应是否正常等。 4、操作危险性试剂时,必须严格遵守操作守则,严禁自行更改实验流程。 5、使用试剂时,要首先确认容器上标示的名称是否为需要的实验试剂。确认药品是否为危害品,有无警告标识。 6、使用挥发性有机溶剂、强酸强碱、腐蚀性试剂、有毒试剂等必须在通风橱内进行操作。 7、有机溶剂、固体化学药品、酸性和碱性化合物均需分开存放,挥发性的化学药品必需放于通风良好的试剂柜内保存。 8、避免独自一人在实验室做危险实验。 9、做危险性实验时必须经领导批准,有两人以上在场方可进行,节假日和夜间严禁做危险性实验。 10、做有危害性气体的实验时,必须在通风橱里进行。 11、进行实验操作时(蜗旋、挥干、吹干等),严禁容器口对着人。 12、绝对不允许任意混合各种试剂以免发生危险。不能用手接触药品,不要用鼻子去闻试剂的气味,不得品尝任何试剂的味道。 13、检测剩余的实验试剂不得随意丢弃或带出实验室,放回指定容器或按规定处理。 14、实验操作完毕必须仔细认真清洗或擦拭可重复使用的实验器材,放置于规定位置。 四、用电相关安全规定 1、实验室内电气设备的安装和使用管理,必须符合安全用电要求,大功率实验设备用电必须使用专线,严禁与照明线共用,谨防因超负荷用电着火。 2、实验室内不准乱拉乱接电线。 3、实验室内的用电线路和配电盘、板、箱、柜等装置及线路系统中的各种开关、插座、插头等均应经常保持完好可用状态,空气开关功率必须与线路允许的容量相匹配。室内照明器具都要经常保持稳固可用状态。 4、实验室内仪器设备凡本身要求安全接地的,必须接地;要定期检查线路。 5、实验室内不得使用明火取暖,严禁抽烟。 6、手上有水或潮湿的,禁止接触电器用品或电器设备。 7、实验室内的鉴定人员必须掌握本室仪器、设备的性能和操作方法,严格按照规程操作。
人工挖土安全操作规程示 范文本 In The Actual Work Production Management, In Order To Ensure The Smooth Progress Of The Process, And Consider The Relationship Between Each Link, The Specific Requirements Of Each Link To Achieve Risk Control And Planning 某某管理中心 XX年XX月
人工挖土安全操作规程示范文本 使用指引:此操作规程资料应用在实际工作生产管理中为了保障过程顺利推进,同时考虑各个环节之间的关系,每个环节实现的具体要求而进行的风险控制与规划,并将危害降低到最小,文档经过下载可进行自定义修改,请根据实际需求进行调整与使用。 1.人工挖孔桩施工适用于桩径800~2000mm、桩深 不超过25m的桩。 2.从事挖孔桩的作业人员必须视力、嗅觉、听觉、心 脏、血压正常,必须经过安全技术培训,考核合格方可上 岗。 3.人工挖孔桩施工前,应根据桩的直径、桩深、土质、 现场环境等状况进行混凝土护壁结构的设计,编制施工方 案和相应的安全技术措施,并经企业负责人和技术负责人 签字批准。 4.施工前,总承包的施工企业应和具有资质的分包施工 企业签订专业分包合同,合同中必须规定双方的安全责 任。
5.人工挖孔桩施工前应对现场环境进行调查,掌握以下情况: ⑴地下管线位置、埋深和现况; ⑵地下构筑物(人防、化粪池、渗水池、坟墓等)的位置、埋深和现况; ⑶施工现场周围建(构)筑物、交通、地表排水、振动源等情况; ⑷高压电气影响范围。 6.人工挖孔桩施工前,工程项目经理部的主管施工技术人员必须向承担施工的专业分包负责人进行安全技术交底并形成文件。交底内容应包括施工程序、安全技术要求、现况地下管线和设施情况、周围环境和现场防护要求等。 7.人工挖孔作业前,专业分包负责人必须向全体作业人员进行详细的安全技术交底,并形成文件。 8.施工前应检查施工物资准备情况,确认符合要求,并
杂志广告合同样本 :)甲方(广告刊户地址: 邮编: 电话: 代表人: 职务: 开户行: 帐户名称: 帐号: 乙方: 地址: 邮编: 电话: 代表人: 职务: 开户行: 帐户名称: 帐号: 甲乙双方根据国务院《中华人民共和国广告法》及有关规定,签订本合同,并共同遵守。. 1. 甲方委托乙方于____年____月____日至____年____月____日期间在《应用科技》杂志发布标题为__________广告。广告规格为__________.广告单价____ 元,加急费____ 元,其他费用____ 元,扣除优惠____ 元,刊登次数____.总计 ________元,大写:__________. 2. 甲方须向乙方的证明材料: 1)盖有本单位公章的营业执照复印件、产品生产许可证及产品经营许可证; 2)专利或技术成果转让须提供专利证书或成果鉴定证书; 3. 广告采用样稿,样稿由__________提供,并经__________同意,发布后如需 改动应经__________同意。 4. 乙方有权审查广告内容和表现形式,对不符合法律、法规的广告内容和表现 形式,乙方可要求甲方作出修改,甲方作出修改前,乙方有权拒绝发布。 5. 广告样稿为合同附件,为本合同不可分割的部分,与本合同一并保存。 6. 甲方应在____ 年____月____日前将广告发布费付给乙方,付款方式为甲方将款项汇至乙方帐户。 7. 违约责任 1)任何一方未履行本协议项下的任何一项条款均视为违约。违约方应承担因自己的违约行为而给守约方造成的经济损失。 2)甲方应按期支付费用,如延期付款,甲方应向乙方支付相当于迟延金额百分之
定冠词the的用法: 定冠词the与指示代词this ,that同源,有“那(这)个”的意思,但较弱,可以和一个名词连用,来表示某个或某些特定的人或东西. (1)特指双方都明白的人或物 Take the medicine.把药吃了. (2)上文提到过的人或事 He bought a house.他买了幢房子. I've been to the house.我去过那幢房子. (3)指世界上独一无二的事物 the sun ,the sky ,the moon, the earth (4)单数名词连用表示一类事物 the dollar 美元 the fox 狐狸 或与形容词或分词连用,表示一类人 the rich 富人 the living 生者 (5)用在序数词和形容词最高级,及形容词等前面 Where do you live?你住在哪? I live on the second floor.我住在二楼. That's the very thing I've been looking for.那正是我要找的东西. (6)与复数名词连用,指整个群体 They are the teachers of this school.(指全体教师) They are teachers of this school.(指部分教师) (7)表示所有,相当于物主代词,用在表示身体部位的名词前 She caught me by the arm.她抓住了我的手臂. (8)用在某些有普通名词构成的国家名称,机关团体,阶级等专有名词前 the People's Republic of China 中华人民共和国 the United States 美国 (9)用在表示乐器的名词前 She plays the piano.她会弹钢琴. (10)用在姓氏的复数名词之前,表示一家人 the Greens 格林一家人(或格林夫妇) (11)用在惯用语中 in the day, in the morning... the day before yesterday, the next morning... in the sky... in the dark... in the end... on the whole, by the way...
编号:SM-ZD-85177 实验室安全标准操作规程Through the process agreement to achieve a unified action policy for different people, so as to coordinate action, reduce blindness, and make the work orderly. 编制:____________________ 审核:____________________ 批准:____________________ 本文档下载后可任意修改
实验室安全标准操作规程 简介:该规程资料适用于公司或组织通过合理化地制定计划,达成上下级或不同的人员之间形成统一的行动方针,明确执行目标,工作内容,执行方式,执行进度,从而使整体计划目标统一,行动协调,过程有条不紊。文档可直接下载或修改,使用时请详细阅读内容。 1职责 1.1实验室主任对安全全面负责。经常进行安全督察,组织安全检查,负责处理安全事故。 1.2实验员负责水、电线路、消防器材的配置和设施安全检查。 1.3各科实验老师负责本科的化学药品、水电气、门窗的安全。 1.4实验员负责试剂、药品,特别是有毒有害,易燃、易爆物质的管理。 2. 工作程序 2.1 安全操作规范 2.1.1检测人员在工作中要严格按照操作规程,杜绝一切违章操作,发现异常情况立即停止工作,并及时登记报告。 2.1.2禁止用嘴、鼻直接接触试剂。使用易挥发、腐蚀性
强、有毒物质必须带防护手套,并在通风橱内进行,中途不许离岗。 2.1.3在进行加热、加压、蒸馏等操作时,操作人员不得随意离开现场,若因故须暂时离开,必须委托他人照看或关闭电源。 2.1.4各种安全设施不许随意拆卸搬动、挪作他用,保证其完好及功能正常。 2.1.5操作人员要熟悉所使用的仪器设备性能和维护知识,熟悉水、电、燃气、气压钢瓶的使用常识及性能,遵守安全使用规则,精心操作。 2.2 有毒有害物质的管理 2.2.1化学试剂、药品中凡属易燃易爆,有毒(特别是剧毒物品)、易挥发产生有害气体的均应列为危险物品,严格分类,加强管理,专人负责。 2.2.2建立详细帐目,帐、物、卡相符,专人限量采购,入库检查。 2.2.3危险物品、易燃易爆物品单独存放,有毒物品放入专用加锁铁柜内,注意通风。
工作行为规范系列 人工挖土安全要求规程(标准、完整、实用、可修改)
编号:FS-QG-78734人工挖土安全要求规程 Manual excavation safety requirements 说明:为规范化、制度化和统一化作业行为,使人员管理工作有章可循,提高工作效率和责任感、归属感,特此编写。 1.新工人必须参加入场安全教育,考试合格后方可上岗。 2.在从事挖土作业前必须熟悉作业内容、作业环境,对使用的工具要进行检修,不牢固者不得使用;作业时必须执行安全技术交底,服从带班人员指挥。 3.配合其他专业工种人员作业时,必须服从该专业工种人员的指挥。 4.作业时必须根据作业要求,佩戴防护用品,施工现场不得穿拖鞋。 5.作业时必须遵守劳动纪律。不得擅自动用各种机电设备。 6.挖土前应按安全技术交底要求了解地下管线、人防及其他构筑物情况,按要求坑探,掌握构筑物的具体位置。地下构筑物外露时,应按交底要求进行加固保护。作业中应避
开管线和构筑物。在现场电力、通讯电缆2m范围内和现场燃气、热力、给排水等管道1m范围内挖土时,必须在主管单位人员的监护下采取人工开挖。 7.开挖槽(坑、沟)深度超过1.5m时必须根据土质和开挖深度情况按技术交底要求放坡、支撑或护壁。遇边坡不稳、有坍塌危险征兆时,必须立即撤离现场。并报告有关负责人,经过安全处理后,方可继续施工。 8.槽、坑、沟边上口边1m以内,不得来回上人走动,不得堆放任何物、料、机具或杂物,堆±高度不得超过1.5m。槽、坑、沟与建筑物、构筑物之间距离不得小于1.5m。堆土不得遮压检查井、消防井等设施。 9.开挖深度超过2m时,必须在周边设置牢固的安全防护栏,并立挂密目安全网。槽深大于2.5m时,应分层挖土,层高不得超过2m,层间应设平台,平台宽度不得小于0.5m。作业时两人横向间距不得小于2m,纵向间距不得小于3m。严禁掏洞挖土、搜底扩槽,严禁在槽内或坑坡脚下休息。 10.槽、坑、沟根据情况必须设置上下坡道或安全梯。进出上、下沟槽必须走马道、安全梯。马道、安全梯间距不宜
“theway+从句”结构的意义及用法 首先让我们来看下面这个句子: Read the followingpassageand talkabout it wi th your classmates.Try totell whatyou think of Tom and ofthe way the childrentreated him. 在这个句子中,the way是先行词,后面是省略了关系副词that或in which的定语从句。 下面我们将叙述“the way+从句”结构的用法。 1.the way之后,引导定语从句的关系词是that而不是how,因此,<<现代英语惯用法词典>>中所给出的下面两个句子是错误的:This is thewayhowithappened. This is the way how he always treats me. 2.在正式语体中,that可被in which所代替;在非正式语体中,that则往往省略。由此我们得到theway后接定语从句时的三种模式:1) the way+that-从句2)the way +in which-从句3) the way +从句 例如:The way(in which ,that) thesecomrade slookatproblems is wrong.这些同志看问题的方法
不对。 Theway(that ,in which)you’re doingit is comple tely crazy.你这么个干法,简直发疯。 Weadmired him for theway inwhich he facesdifficulties. Wallace and Darwingreed on the way inwhi ch different forms of life had begun.华莱士和达尔文对不同类型的生物是如何起源的持相同的观点。 This is the way(that) hedid it. I likedthe way(that) sheorganized the meeting. 3.theway(that)有时可以与how(作“如何”解)通用。例如: That’s the way(that) shespoke. = That’s how shespoke.
编订:__________________ 单位:__________________ 时间:__________________ 实验室安全标准操作规程 (正式) Standardize The Management Mechanism To Make The Personnel In The Organization Operate According To The Established Standards And Reach The Expected Level. Word格式 / 完整 / 可编辑
文件编号:KG-AO-5509-66 实验室安全标准操作规程(正式) 使用备注:本文档可用在日常工作场景,通过对管理机制、管理原则、管理方法以及管理机构进行设置固定的规范,从而使得组织内人员按照既定标准、规范的要求进行操作,使日常工作或活动达到预期的水平。下载后就可自由编辑。 1职责 1.1实验室主任对安全全面负责。经常进行安全督察,组织安全检查,负责处理安全事故。 1.2实验员负责水、电线路、消防器材的配置和设施安全检查。 1.3各科实验老师负责本科的化学药品、水电气、门窗的安全。 1.4实验员负责试剂、药品,特别是有毒有害,易燃、易爆物质的管理。 2. 工作程序 2.1 安全操作规范 2.1.1检测人员在工作中要严格按照操作规程,杜绝一切违章操作,发现异常情况立即停止工作,并及时登记报告。
2.1.2禁止用嘴、鼻直接接触试剂。使用易挥发、腐蚀性强、有毒物质必须带防护手套,并在通风橱内进行,中途不许离岗。 2.1.3在进行加热、加压、蒸馏等操作时,操作人员不得随意离开现场,若因故须暂时离开,必须委托他人照看或关闭电源。 2.1.4各种安全设施不许随意拆卸搬动、挪作他用,保证其完好及功能正常。 2.1.5操作人员要熟悉所使用的仪器设备性能和维护知识,熟悉水、电、燃气、气压钢瓶的使用常识及性能,遵守安全使用规则,精心操作。 2.2 有毒有害物质的管理 2.2.1化学试剂、药品中凡属易燃易爆,有毒(特别是剧毒物品)、易挥发产生有害气体的均应列为危险物品,严格分类,加强管理,专人负责。 2.2.2建立详细帐目,帐、物、卡相符,专人限量采购,入库检查。 2.2.3危险物品、易燃易爆物品单独存放,有毒
杂志刊登广告合同四示范 文本 In Order To Protect Their Legitimate Rights And Interests, The Cooperative Parties Reach A Consensus Through Consultation And Sign Into Documents, So As To Solve And Prevent Disputes And Achieve The Effect Of Common Interests 某某管理中心 XX年XX月
杂志刊登广告合同四示范文本 使用指引:此合同资料应用在协作多方为保障各自的合法权益,经过共同商量最终得出一致意见,特意签订成为文书材料,从而达到解决和预防纠纷实现共同利益的效果,文档经过下载可进行自定义修改,请根据实际需求进行调整与使用。 合同编号:___________ 甲方:_________ 乙方:_________杂志社有限公司 签订日期: 甲、乙双方严格遵守中华人民共和国《广告法》及 《合同法》,就广告刊登具体事项签订以下条款: 一、广告刊登方式 二、广告发布内容 _________________ 三、广告采用由甲方提供的(□菲林□光盘□电子文档□照 片、文字□印刷品□样品□其它)。 四、甲方在提供广告资料的同时,提供相应的企业营
业执照、专利证书等证件的复印件,对广告内容自负法律责任。乙方有权审查广告内容和表现形式,对不符合法律、法规的广告内容和表现形式,乙方应要求甲方作出修改,甲方未作出修改前,乙方有权拒绝发布。 五、付款方式 _________________ 六、双方发生经济合同纠纷协商解决不成功时,提请经济仲裁委员会仲裁。 七、本合同一式贰份,甲、乙双方各执一份,自合同签订之日起合同即时生效。 甲方名称:(章)_________ 乙方名称:(章)_________ 法定代表:_______________代表:____________ 委托代理人:_____________地址:____________ 单位地址:_______________邮编:____________ 邮编:____________
表示“方式”、“方法”,注意以下用法: 1.表示用某种方法或按某种方式,通常用介词in(此介词有时可省略)。如: Do it (in) your own way. 按你自己的方法做吧。 Please do not talk (in) that way. 请不要那样说。 2.表示做某事的方式或方法,其后可接不定式或of doing sth。 如: It’s the best way of studying [to study] English. 这是学习英语的最好方法。 There are different ways to do [of doing] it. 做这事有不同的办法。 3.其后通常可直接跟一个定语从句(不用任何引导词),也可跟由that 或in which 引导的定语从句,但是其后的从句不能由how 来引导。如: 我不喜欢他说话的态度。 正:I don’t like the way he spoke. 正:I don’t like the way that he spoke. 正:I don’t like the way in which he spoke. 误:I don’t like the way how he spoke. 4.注意以下各句the way 的用法: That’s the way (=how) he spoke. 那就是他说话的方式。 Nobody else loves you the way(=as) I do. 没有人像我这样爱你。 The way (=According as) you are studying now, you won’tmake much progress. 根据你现在学习情况来看,你不会有多大的进步。 2007年陕西省高考英语中有这样一道单项填空题: ——I think he is taking an active part insocial work. ——I agree with you_____. A、in a way B、on the way C、by the way D、in the way 此题答案选A。要想弄清为什么选A,而不选其他几项,则要弄清选项中含way的四个短语的不同意义和用法,下面我们就对此作一归纳和小结。 一、in a way的用法 表示:在一定程度上,从某方面说。如: In a way he was right.在某种程度上他是对的。注:in a way也可说成in one way。 二、on the way的用法 1、表示:即将来(去),就要来(去)。如: Spring is on the way.春天快到了。 I'd better be on my way soon.我最好还是快点儿走。 Radio forecasts said a sixth-grade wind was on the way.无线电预报说将有六级大风。 2、表示:在路上,在行进中。如: He stopped for breakfast on the way.他中途停下吃早点。 We had some good laughs on the way.我们在路上好好笑了一阵子。 3、表示:(婴儿)尚未出生。如: She has two children with another one on the way.她有两个孩子,现在还怀着一个。 She's got five children,and another one is on the way.她已经有5个孩子了,另一个又快生了。 三、by the way的用法
实验室安全操作规程 1、化验室安全操作规程 (1)实验前应做好准备,必须对所用药品与设备性能有充分的了解,熟悉每个具体操作中的安全注意事项。 (2)实验前必须熟悉实验室及其周围的环境和水龙头、电闸门的位置 (3)实验时应保持安静,思想要集中,遵守操作规程,切勿粗心大意,马马虎虎,更不准在实验室内开玩笑。 (4)严禁在实验室内饮食或煮食,或者把食具带劲实验室。 (5)每次实验完毕后应把手洗净,并检查水、电、气等安全措施完善后才能离开实验室(6)每个实验室都必须备有灭火器或砂土,并尽可能放在显眼的地方,能同事备有消防用的消防栓或水缸则更好 (7)实验室应保持空气流通,并设有专用的卫生箱,以供及时治疗的需要。常备药品有:红汞药水:供一般破伤使用 酒精:轻微的灼烧伤可用进过酒精的脱脂棉擦拭 5%硼酸氢钠溶液:受酸性物灼伤可用作冲洗 3%硼酸溶液:受碱性物灼伤可用作冲洗 还需要备有碘酒:紫药水及绷带和药棉 2、火和电的安全预防 (1)在使用中电气动力时,必须事先检查电开关,马达以及机械设备各部门是否安置妥善(2)开始工作时和停止工作时,必须将开关彻底扣严和拉下 (3)在更换保险丝时,要按负荷量,不得加大或以铜丝代替使用。 (4)严禁用湿手、湿布或铁柄毛刷等去清扫或擦拭电闸刀、点插销等,防止触电 (5)凡电气动力设备过热时,应立即停止运转 (6)定碳、定硫电炉或其他高温炉,其硅碳棒露出部分应设有安全罩,严禁将安置妥善的安全罩随意撤掉,以免发生触电事故。 (7)禁止洒水在电气设备和电线路上,以免漏电 (8)凡使用110伏以上电源装置,仪器的金属部分必须安装地线 (9)电热设备,例如马弗炉、烘箱、电炉和电热板等,所用电源的导线应经常注意检查其各接触处是否妥当,导线有无损坏和被腐蚀等 (10)马弗炉、烘箱等用电设备,使用时必须要有人负责照管,以防发生事故 (11)马弗炉需放在水泥等不燃物砌成的坚固台子上,不要靠近木板墙或木质门窗。(12)使用易燃物时,必须在距离火源较远的地方进行,绝不可靠近火源,尤其是乙醚着火的危险性极大,用时必须小心,用完后的剩余部分也应及时的存放到专门的安全地方。(13)绝不可以将氧气钢瓶存放在靠近电源的地方,并需防止强烈震动,气体出口活门处绝不可涂油和与有机物接触,以免发生爆炸的危险。 3、化学药品的安全预防 (1)距度星的药品,例如KCN、AS2O3等等,必须制定保管使用规则,并严格遵守,及时工作人员很少,也不可例外的有所忽视。这类药品不能与一般药品同样的存放和任意使用,即使用过后的余量已经很少也应及时送保管员及时查收,不应任意放在工作台上。 (2)内服有毒药品,如氰化物、铅化物、汞及汞化物、络酸盐、氧化砷钡盐等,应装在坚固的瓶中保管,禁止入口,与手接触用后要洗手。 (3)接触皮肤有毒的药品,例如氰化物、氟氢酸、溴水、过氯酸等,要装在严禁坚固的瓶中保管,使用时要特别小心,不得与皮肤接触。 (4)呼吸有毒药品(及有毒气体和蒸气)如氰化氢、氮的氧化物、氯化氢、硫化氢、溴、
杂志刊登广告合同协议 书 公司内部档案编码:[OPPTR-OPPT28-OPPTL98-OPPNN08]
甲方(广告客户)填写内容单位名称、地址:______________________ 广告内容:____________________________ 广告类别、版面、刊期:_________________ 广告费合计:___________________________ 汇款时间:_____________________________ 经办人(签名、联系电话):_____________ 甲方(盖章)、填写合同日期:__________乙方(杂志社)填写内容 要求汇入广告费时间:___________________ 拟安排刊期:___________________________ 经办人(签名、联系电话):_____________ 开户银行:_____________________________ 户名:_________________________________ 帐号:_________________________________ 乙方(盖章)、填写合同日期:__________ 单期广告价目表 类别 版面
位置 收费(元/次) 彩色 全版 封面 6000 ? ? 封底 5000 ? ? 封二 4000 ? ? 封三 封三 3500 ? ? 插页 3500 ? 2个全版 插页 7000 黑白 全版 插页 1500 ? 1/2版 正文内页 800 ? 1/2版以内 正文内页 600 说明:? 1.客户需至少提前________天预定版面,可通过电话、传真、E-mail等方式与我们联系。