Puri?cation of antibody Fab and F(ab 0
)2fragments using
Gradi?ow technology
Gregory L.M.Cheung,*Theresa M.Thomas,and Dennis B.Rylatt
Gradipore Ltd.,22Rodborough Rd,Frenchs Forest NSW 2086,Australia
Received 22May 2003,and in revised form 1July 2003
Abstract
The Gradi?ow,a preparative electrophoresis instrument designed to separate molecules on the basis of their size and charge,was
used to purify antibody Fab and F(ab 0
)2fragments.The method described is charge based,utilizing the di?erence in the p I between
the antibody Fab/F(ab 0
)2fragments and antibody Fc fragments that occur after enzyme digestion of whole antibody molecules.This
method of puri?cation was successful across a range of monoclonal and polyclonal antibodies.In particular,F(ab 0
)2fragments were puri?ed from a number of mouse monoclonal antibodies (both IgG1and IgG2a isotypes)and Fab fragments were puri?ed from egg yolk IgY polyclonal antibodies.This is a rapid puri?cation method which has advantages over alternative methods that usually comprise ion exchange and gel ?ltration chromatography.This method may be applicable to most antibody digest preparations.ó2003Elsevier Inc.All rights reserved.
Antibody Fab fragments are commonly used in bi-ology for both in vivo and in vitro experiments and di-agnostic tests [1,2].Fab and F(ab 0
)2antibody fragments are preferable in assay systems where the presence of the Fc region may cause problems.For instance,when staining for cells,background staining can occur with cells containing Fc receptors such as macrophages or neutrophils.The Fc fragment is also the most antigenic portion of the molecule [3]and cleavage of the Fc fragment may be bene?cial when using antibodies in vivo to reduce allergenicity without adversely a?ecting antibody function.Conjugation of di?erent Fab frag-ments also provides the possibility of creating antibodies with multiple speci?cities [4].
Antibody Fab/F(ab 0
)2fragments are produced by digestion of whole intact antibodies with enzymes such as papain or pepsin,or by recombinant expression techniques.Puri?cation of digested fragments is usually carried out by a combination of ion exchange chroma-tography and gel ?ltration [5].
The Gradi?ow is a preparative electrophoresis in-strument designed to separate macromolecules,espe-cially proteins,on the basis of their size and charge
(Fig.1).Separation occurs in the separation unit where three polyacrylamide membranes of various nominal molecular weight cuto?s are sandwiched to form two separate streams.Proteins can transfer from one stream to another depending on the choice of mem-branes used,the bu?er composition and pH,and the voltage applied to perform the separation.The Gra-di?ow instrument and separation principles have been described in detail previously [6].Many di?erent types of proteins have been successfully puri?ed using this technology,including whole antibodies [6],re-combinant proteins [7],egg white proteins [8],milk whey proteins [7],and human plasma proteins [9].The Gradi?ow apparatus has also been successfully applied to fractionation of complex protein extracts prior to analysis in proteomics [10].
Here we describe the puri?cation of multiple anti-body Fab/F(ab 0
)2preparations using Gradi?ow tech-nology.All examples were monoclonal antibodies except one,which was a polyclonal antibody prepara-tion from chicken egg yolk (IgY).The methodology
used for the puri?cation of the Fab/F(ab 0
)2fragments is dependent upon the di?erence in the isoelectric point
(p I )of the Fab/F(ab 0
)2fragments and the Fc fragment.
The Fab/F(ab 0
)2fragments usually possess an observed p I of above 6.5and the Fc fragment usually possesses a
*
Corresponding author.Fax:+61-2-8977-9099.
E-mail address:gcheung@https://www.doczj.com/doc/6f13800320.html, (G.L.M.Cheung).1046-5928/$-see front matter ó2003Elsevier Inc.All rights reserved.
doi:10.1016/S1046-5928(03)00219-5
Protein Expression and Puri?cation 32(2003)
135–140
https://www.doczj.com/doc/6f13800320.html,/locate/yprep
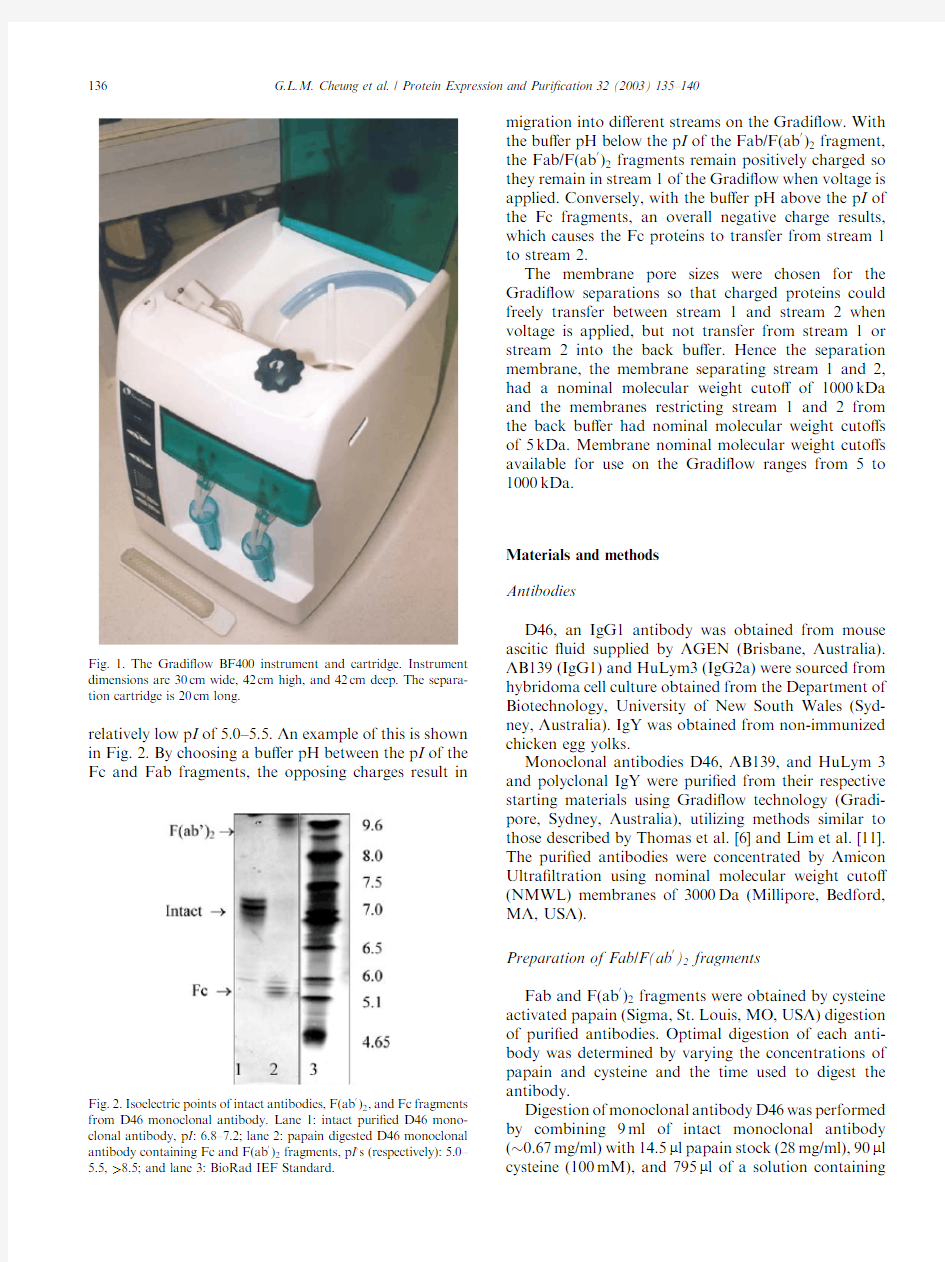
relatively low p I of 5.0–5.5.An example of this is shown in Fig.2.By choosing a bu?er pH between the p I of the Fc and Fab fragments,the opposing charges result in
migration into di?erent streams on the Gradi?ow.With
the bu?er pH below the p I of the Fab/F(ab 0
)2fragment,
the Fab/F(ab 0
)2fragments remain positively charged so they remain in stream 1of the Gradi?ow when voltage is applied.Conversely,with the bu?er pH above the p I of the Fc fragments,an overall negative charge results,which causes the Fc proteins to transfer from stream 1to stream 2.
The membrane pore sizes were chosen for the Gradi?ow separations so that charged proteins could freely transfer between stream 1and stream 2when voltage is applied,but not transfer from stream 1or stream 2into the back bu?er.Hence the separation membrane,the membrane separating stream 1and 2,had a nominal molecular weight cuto?of 1000kDa and the membranes restricting stream 1and 2from the back bu?er had nominal molecular weight cuto?s of 5kDa.Membrane nominal molecular weight cuto?s available for use on the Gradi?ow ranges from 5to 1000kDa.
Materials and methods Antibodies
D46,an IgG1antibody was obtained from mouse ascitic ?uid supplied by AGEN (Brisbane,Australia).AB139(IgG1)and HuLym3(IgG2a)were sourced from hybridoma cell culture obtained from the Department of Biotechnology,University of New South Wales (Syd-ney,Australia).IgY was obtained from non-immunized chicken egg yolks.
Monoclonal antibodies D46,AB139,and HuLym 3and polyclonal IgY were puri?ed from their respective starting materials using Gradi?ow technology (Gradi-pore,Sydney,Australia),utilizing methods similar to those described by Thomas et al.[6]and Lim et al.[11].The puri?ed antibodies were concentrated by Amicon Ultra?ltration using nominal molecular weight cuto?(NMWL)membranes of 3000Da (Millipore,Bedford,MA,USA).
Preparation of Fab/F(ab 0
)2fragments
Fab and F(ab 0
)2fragments were obtained by cysteine activated papain (Sigma,St.Louis,MO,USA)digestion of puri?ed antibodies.Optimal digestion of each anti-body was determined by varying the concentrations of papain and cysteine and the time used to digest the antibody.
Digestion of monoclonal antibody D46was performed by combining 9ml of intact monoclonal antibody ( 0.67mg/ml)with 14.5l l papain stock (28mg/ml),90l l cysteine (100mM),and 795l l of a solution
containing
Fig.2.Isoelectric points of intact antibodies,F(ab 0
)2,and Fc fragments from D46monoclonal https://www.doczj.com/doc/6f13800320.html,ne 1:intact puri?ed D46mono-clonal antibody,p I :6.8–7.2;lane 2:papain digested D46monoclonal
antibody containing Fc and F(ab 0
)2fragments,p I ?s (respectively):5.0–5.5,>8.5;and lane 3:BioRad IEF
Standard.
Fig.1.The Gradi?ow BF400instrument and cartridge.Instrument dimensions are 30cm wide,42cm high,and 42cm deep.The separa-tion cartridge is 20cm long.
136G.L.M.Cheung et al./Protein Expression and Puri?cation 32(2003)135–140
1mM dithiothreitol (DTT),1100mM Tris base,and 2mM disodium ethylene-diaminetetraacetate dihydrate (EDTA).This was incubated at 37°C for 1h.The reac-tion was stopped with 900l l iodoacetamide (500mM)and placed on ice for 30min.For digestion of intact IgY,8ml of puri?ed IgY at approximately 0.89mg/ml was combined with 400l l cysteine (100mM)and 400l l of 0.5mg/ml papain,and mixed thoroughly.The papain was previously prepared from a 28mg/ml stock and diluted into a solution containing 1mM dithiothreitol (DTT),100mM Tris base,and 2mM disodium ethylene-diam-inetetraacetate dihydrate (EDTA).The digestion mixture was incubated at 37°C for 24h.The reaction was stopped with 800l l iodoacetamide (500mM)and placed on ice for 30min.
Puri?cation of monoclonal antibody fragments using Gradi?ow
Table 1shows the isoelectric points and the condi-tions used to purify the F(ab 0
)2fragments from D46,AB139,and HuLym3monoclonal antibodies.Brie?y,8ml of freshly digested antibody (approximately 4.9mg)was mixed with 2ml of 10times concentrated bu?er and loaded into stream 1of the Gradi?ow.About 10ml of normal concentration bu?er was loaded in stream 2,and 1.8liters of bu?er was loaded into the back bu?er res-
ervoir.A Gradi?ow separation cartridge,referred to as ?pre-fractionation cartridge,p I cuts ?(Gradipore)was inserted and tightened in the cartridge slot.Prior to the separation,all streams were circulated for 5min.Protein separation was performed by applying voltage for 1h.During separation,the pH of stream 1and stream 2was checked every 15min.If the pH of the streams di?ered from the pH of the back bu?er by more than 0.3of a pH unit,then the pH was adjusted to the pH of the back bu?er using 2M Tris base or glacial acetic acid.At the completion of the separation,voltage was applied in reverse for 5s to release any protein attached to the membranes.Streams were circulated for 5min without voltage,then harvested.
Puri?cation of IgY polyclonal Fab fragments
The conditions used for separation of the Fab frag-ments from IgY polyclonal antibodies are listed in Table 2.The bu?er was made up using 10g Hepes and 12.5g EACA and diluted to 2liters using reverse os-mosis water.A 6ml volume of the digested antibody sample (approximately 4.4mg)was loaded in stream 2on the Gradi?ow along with 2ml of 10times concen-trated bu?er.A 10ml volume of normal concentration bu?er was placed in stream 1and 1.8liters of this bu?er was added to the back bu?er reservoir.A Gradi?ow separation cartridge,referred to as the ?Fab fragment transfer cartridge ?(Gradipore)was inserted and tight-ened into the cartridge slot.All streams were circulated for 5min,then voltage was applied for 3h.During the 3h,the pH was checked every half an hour and adjusted if necessary as mentioned above.After the 3h separa-tion,the voltage was reversed for 5s.The streams were circulated without voltage for 5min and the contents of streams 1and 2were collected.A 5ml volume of phosphate-bu?ered saline (PBS)was added to each stream and circulated without applied voltage for 10min to collect any residual protein.The PBS fractions were harvested.The contents of stream 1were combined with the stream 1PBS fraction to combine fractions con-taining puri?ed antibody.
1
Abbreviations used:Bis-Tris,bis(2-hydroxyethyl)imino-tris(hy-droxymethyl)methane;DTT,dithiothreitol;EACA,6-amino-n -hexa-noic acid;EDTA,disodium ethylene-diaminetetraacetate dihydrate;
Fab,fragment for antigen binding;F(ab 0
)2,dimeric fragment for antigen binding;Fc,fragment crystalizable;Hepes,[2-hydroxyethyl]piperazine N 0-[2-ethanesulfonic acid];HRP,horseradish peroxidase;IEF,isoelectric focusing;IgG,immunoglobulin G;IgY,immunoglob-ulin Y;imidazole,1,2-diaza-2,4-cyclopentadiene;kDa,kilodaltons;mg,milligram;ml,milliliter;Mopso,3-(N -morpholino)-2-hydroxypro-panesulfonic acid;p I ,isoelectric point;PBS,phosphate-bu?ered saline;PBS/T,phosphate bu?er saline +0.05%Tween 20;PBS/T/1%SM,phosphate-bu?ered saline +0.05%Tween 20+1%skim milk powder;PBS/T/5%SM,phosphate-bu?ered saline +0.05%Tween 20+5%skim milk powder;SDS,sodium dodecyl sulfate;Tris,2-amino-2-hydrox-ymethyl-1,3-propanediol.
Table 1
Experimental conditions and isoelectric points for monoclonal F(ab 0
)2separations Antibody
D46(IgG1)Ab139(IgG1)
HuLym3(IgG2a)
Starting material
Mouse ascitic ?uid Hybridoma cell culture Hybridoma cell culture p I :F(ab 0
)2fragment 8.5–9.2 6.5–7.57.5–9.5p I :Fc fragment 5.0–5.5 5.0–5.5<7.0Bu?er pH 7.8
6.1
7.0
Bu?er type
Tris/Hepes (34mM)Mopso/Bis-Tris (37mM)Hepes/Imidazole (39mM)Separation duration 60min 60min 60min Voltage
250V 250V 250V Membrane con?guration (upper–middle–lower)(nominal molecular weight cuto?s in kDa)5–1000–55–1000–55–1000–5Load Stream
Stream 1
Stream 1
Stream 1
G.L.M.Cheung et al./Protein Expression and Puri?cation 32(2003)135–140
137
Gel electrophoresis
Samples were analyzed on4–20%non-reduced SDS–PAGE iGels(Gradipore)and by Western blotting.Gels were stained in100ml of the Coomassie stain Gradipure (Gradipore)overnight.The gels were destained in6% acetic acid until a clear background was reached.Silver stained gels were stained according to the standard di-amine staining procedure,according to the protocol provided with the gels.
Isoelectric focusing was performed using Novex3–10 IEF gels(Invitrogen,Carlsbad,CA,USA)according to the instructions.Staining of isoelectric focusing gels was carried out using BioRad(Hercules,CA)IEF gel staining solution.
Western blotting
Freshly run gels were blotted onto BioRad Trans-Blot pure nitrocellulose using methods provided by the manufacturer.A BioRad Trans-Blot Semi-Dry transfer cell was used.The membrane was soaked in PBS/T/1%SM for10min before application of the primary antibody for60min at room temperature.The anti-bodies used were anti-mouse IgG Fab-speci?c HRP conjugated(Sigma)diluted1:2000in PBS/T/1%SM, anti-mouse IgG Fc-speci?c(Sigma)diluted1:500in PBS/T/1%SM,and anti-chicken IgY Fab-speci?c (Jackson Immunoresearch,West Grove,PA,USA)di-luted1:4000in PBS/T/5%SM.Development of the Western blots was performed using ECL chemilumi-nescent reagents from Amersham Biosciences(Piscata-way,NJ,USA).
Protein concentration determination
Protein concentration was determined using the BCA Protein Assay Reagent Kit(Pierce,Rockford,IL,USA) against a standard curve created using bovine serum albumin.
Results and discussion
Puri?cation of monoclonal F(ab0)2fragments The digestion and separation of monoclonal F(ab0)2 fragments is illustrated by non-reduced SDS–PAGE in Fig.3.The undigested antibody has a molecular weight of approximately150kDa and can be detected on both the anti-Fc and anti-Fab blots.Unlike the results on the IEF gel,the antibody migrates predominantly as a single molecular weight species.After digestion(lane2)there was little remaining intact antibody and the antibody existed as F(ab0)2( 100kDa)and Fc fragments ( 40kDa).Following Gradi?ow puri?cation of the di-gested sample the F(ab0)2was retained in stream1(lane
Table2
Experimental conditions for puri?cation of polyclonal Fab fragments Antibody IgY
Antibody origin Chicken egg yolk
p I:Fab fragment7.0–9.5
p I:Fc fragment 5.5–6.0
Bu?er pH 6.2
Bu?er type Hepes/EACA(69mM) Separation voltage250V
Membrane con?guration (nominal molecular weight cuto?s in kDa)25(upper)–1000(middle)–5 (lower)
Load stream Stream
2
Fig.3.Non-reduced SDS–PAGE gels and blots demonstrating separation of F(ab0)2and Fc fragments.Separation of D46F(ab0)https://www.doczj.com/doc/6f13800320.html,ne1: intact puri?ed antibody;lane2:papain digested antibody,(unpuri?ed F(ab0)2loaded onto the Gradi?ow);lane3:puri?ed F(ab0)2,stream1?nal sample;lane4:stream2?nal sample(Fc removed from F(ab0)2);and lane5:Benchmark protein ladder,Invitrogen.Importantly,there is no de-tectable Fc in the puri?ed F(ab0)2sample(lane3)on the anti-Fc blot or silver stain.The intact IgG and the F(ab0)2bands on the silver stain are negatively stained due to the high amounts of protein.The Fc fragments appear as two bands at25and40kDa.There is what appears to be antibody light chain at25kDa in undigested,unpuri?ed,and puri?ed samples(lanes1,2,and3,respectively)and heavy chain(50kDa)with the intact antibody sample(lane1).
138G.L.M.Cheung et al./Protein Expression and Puri?cation32(2003)135–140
3)and the Fc transferred fully to stream 2(lane 4).The silver stain indicates that there was only a small amount of lower molecular weight contaminants with the puri-?ed F(ab 0
)2.An equivalent Coomassie blue stained gel
showed a strongly stained F(ab 0
)2band with no visible contaminants (results not shown).The anti-Fab blot
shows reasonable retention of the F(ab 0
)2fragment from start to ?nish with little visible loss (lanes 2and 3).The anti-Fc blot shows the Fc fragment in the start sample (digested antibody,lane 2)but no detectable Fc present
in the puri?ed F(ab 0
)2sample (lane 3).There was also
little intact antibody in the puri?ed F(ab 0
)2sample as some of the remaining intact antibody transferred to stream 2during the separation.This was due to the in-tact antibody being slightly negatively charged at the
separation pH.Similar results were obtained for F(ab 0
)2/Fc fragment separation for all the other monoclonal antibodies.In each case no detectable Fc remained in
the puri?ed F(ab 0
)2sample (stream 1?nal)and little intact antibody was present.Puri?cation of a polyclonal Fab
The digestion of polyclonal IgY occurred with similar results to those obtained by Loeken and Roth [12],and similar isoelectric points of the intact antibody (6.0–8.5),Fab (7.0–9.5),and Fc (5.5–6.0)fragments were ob-served.Polyclonal IgY Fab fragments were successfully puri?ed using the Gradi?ow as demonstrated in Fig.4.
Unlike puri?cation of monoclonal F(ab 0
)2fragments where the sample was loaded in stream 1,digested polyclonal Fab was loaded in stream 2on the Gradi?ow (lane 5)and the Fab fragment transferred away from the Fc fragment into stream 1(lanes 1–3).Most of the Fab transferred from stream 2to stream 1as demonstrated by the disappearing Fab in lane 6and the appearance of Fab in lane 1during the ?rst hour.Also,unlike the
puri?cation of monoclonal F(ab 0
)2fragments as noted in Table 2,a 25kDa upper restriction was used instead of a
5kDa upper restriction.This was used to allow any low molecular weight contaminants transferring from stream 2to stream 1to potentially transfer from stream 1into the back bu?er.
Though the separation was performed for 3h,no Fab was visible in stream 2after 2h (lane 7)and the sepa-ration appears completed by this time.The puri?ed Fab in stream 1(lane 3)was a single major band on the Coomassie blue stained gel with one minor contami-nant.As shown in lane 9,a large amount of the puri?ed Fab in stream 1was collected in the PBS fraction.This sample (lane 9)would be pooled with the ?nal stream 1sample (lane 3)to obtain the ?nal puri?ed Fab pool.The ?nal Fab sample would contain reasonably pure Fab with two minor contaminants which could possibly be antibody light chains.It should be noted that samples taken during the separation may not have been an ac-curate indication of separation progress.With this sep-aration,signi?cant amounts of protein interacted with the membranes and were only collected in the PBS wash after completion of separation.This 3h procedure re-sulted in satisfactory separation of the Fab fragments from the Fc fragments,but shorter separation times between 2and 3h may also have been su?cient.
The Western blots (Fig.5)further demonstrate the puri?cation of polyclonal Fab.On the anti-IgY blot,intact antibody along with Fc and Fab fragments were detected in the digested sample (lane 2).The anti-Fab (IgY)blot detected only the intact antibody and Fab fragment.This indicated that the protein band just above 45kDa was the Fab fragment.The puri?ed Fab in lane 3appeared as a single band in the anti-IgY blot signifying the absence of any Fc and the near complete absence of intact antibody.Similar results were obtained for the residual Fab collected in the stream 1PBS fraction (lane 5),although a little more intact antibody was detected.The gel and blot results suggest that
there
Fig.4.A Coomassie blue stained non-reduced SDS–PAGE showing the puri?cation of IgY Fab polyclonal https://www.doczj.com/doc/6f13800320.html,ne 1:stream 1,after 1h;lane 2:stream 1,2h;lane 3:stream 1,3h (?nal puri?ed Fab sample);lane 4:marker;lane 5:stream 2(0h,start sample,digested antibody);lane 6:stream 2,1h;lane 7:stream 2,2h;lane 8:stream 2,3h;lane 9:stream 1,PBS wash;and lane 10:stream 2,PBS
wash.
Fig.5.Western blots demonstrating the puri?cation of Fab from https://www.doczj.com/doc/6f13800320.html,ne 1:intact undigested puri?ed IgY;lane 2:stream 2,start sample (digested IgY);lane 3:stream 1,?nal sample (puri?ed Fab);lane 4:stream 2,?nal sample (Fab removed);lane 5:stream 1,PBS wash (residual puri?ed Fab);and lane 6:stream 2,PBS wash.
G.L.M.Cheung et al./Protein Expression and Puri?cation 32(2003)135–140139
was no signi?cant loss of the Fab fragment during pu-ri?cation.
During the puri?cation of monoclonal antibody F(ab0)2fragments4.9mg of protein was loaded onto the Gradi?ow and during the puri?cation of polyclonal antibody Fab fragments4.4mg of polyclonal antibody was loaded onto the Gradi?ow.The maximum amount of Fab/F(ab0)2protein that can be separated from the Fc in a single separation was not investigated.In other applications using di?erent proteins on the Gradi?ow, up to100mg of protein was puri?ed during a single separation.As the separations described in this publi-cation are relatively straightforward,using fairly de?ned starting samples,separation of similarly large amounts of Fab/F(ab0)2protein may be possible.
In conclusion,a method to purify antibody Fab/ F(ab0)2fragments using Gradi?ow technology has been described.This charge based separation method was successful across di?erent Fab/F(ab0)2antibodies from di?erent starting materials.Though separation of monoclonal F(ab0)2was achieved with the sample loa-ded in stream1on the Gradi?ow,similar puri?cation should also be successful with sample loaded in stream 2,as in the puri?cation of the polyclonal Fab fragments. Acknowledgments
The author thanks the following people for their as-sistance and supplying puri?ed antibody for Fab/F(ab0)2 digestions and puri?cations.Sarah Gee,Irene Bate, Josie Quindere,Denise Thomas,Sharon Leong,Niza Hurst,Joannah Taylor,Lucy Ryan,Grace Li,and Andrew Gilbert.References
[1]P.Hudson,Recombinant antibody constructs in cancer therapy,
Curr.Opin.Immunol.11(1999)548–557.
[2]K.Wilson,M.Gerometta,D.Rylatt,P.Bundesen,D.McPhee,C.
Hillyard,B.Kemp,Rapid whole blood assay for HIV-1seropo-sivity using a Fab-peptide conjugate,J.Immunol.Methods138 (1991)111–119.
[3]R.Porter,The hydrolysis of rabbit c-globulin and antibodies with
crystalline papain,Biochem.J.73(1959)119–126.
[4]S.Martin,B.Hartyl,P.Martin,I.Bate,D.Rylatt,J.Young,P.
Bundesen,G.Cooksley,C.Hillyard,Rapid detection of circulat-ing hepatitis B surface antigen in whole blood,Asia Pac.J.Mol.
Biol.Biotechnol.5(1997)31–40.
[5]E.Harlow,https://www.doczj.com/doc/6f13800320.html,ne,Antibodies,A Laboratory Manual Harbor
Laboratory,Cold Spring,New York,1988,pp.626–629.
[6]T.M.Thomas,E.E.Shave,I.M.Bate,S.C.Gee,S.Franklin,D.B.
Rylatt,Preparative electrophoresis:a general method for the puri?cation of polyclonal antibodies,J.Chromatogr.A944(2002) 161–168.
[7]G.L.Corthals,B.M.Collins,B.C.Mabbut,K.L.Willams,A.A.
Gooley,Puri?cation by re?ux electrophoresis of whey proteins and of recombinant protein expressed in Dictyostelium discoideum, J.Chromatogr.A773(1997)299–309.
[8]D.L.Rothemund,T.M.Thomas,D.B.Rylatt,Puri?cation of the
basic avidin protein using Gradi?ow technology,Protein Expr.
Purif.26(2002)149–152.
[9]G.Li,R.Stewart,B.Conlan,A.Gilbert,P.Roeth,H.Nair,
Puri?cation of human immunoglobulin G:a new approach to plasma fractionation,Vox Sang.83(2002)332–338.
[10]V.L.Locke,T.S.Gibson,T.M.Thomas,G.L.Corthals,D.B.
Rylatt,Gradi?ow as a prefractionation tool for two-dimensional electrophoresis,Proteomics2(2002)1254–1260.
[11]S.Lim,H.P.Manusu,A.A.Gooley,K.L.Williams,D.B.Rylatt,
Puri?cation of monoclonal antibodies from ascitic?uid using preparative electrophoresis,J.Chromatogr.A827(1998)329–335.
[12]M.Loeken,T.Roth,Analysis of maternal IgG subpopulations
which are transported into the chicken oocyte,Immunology49 (1982)21–28.
140G.L.M.Cheung et al./Protein Expression and Puri?cation32(2003)135–140