
REVIEW
Wangxia Wang?Basia Vinocur?Arie Altman
Plant responses to drought,salinity and extreme temperatures: towards genetic engineering for stress tolerance
Received:18June2003/Accepted:12August2003/Published online:26September2003
óSpringer-Verlag2003
Abstract Abiotic stresses,such as drought,salinity, extreme temperatures,chemical toxicity and oxidative stress are serious threats to agriculture and the natural status of the environment.Increased salinization of arable land is expected to have devastating global e?ects, resulting in30%land loss within the next25years,and up to50%by the year2050.Therefore,breeding for drought and salinity stress tolerance in crop plants(for food supply)and in forest trees(a central component of the global ecosystem)should be given high research priority in plant biotechnology programs.Molecular control mechanisms for abiotic stress tolerance are based on the activation and regulation of speci?c stress-related genes.These genes are involved in the whole sequence of stress responses,such as signaling,tran-scriptional control,protection of membranes and pro-teins,and free-radical and toxic-compound scavenging. Recently,research into the molecular mechanisms of stress responses has started to bear fruit and,in parallel, genetic modi?cation of stress tolerance has also shown promising results that may ultimately apply to agricul-turally and ecologically important plants.The present review summarizes the recent advances in elucidating stress-response mechanisms and their biotechnological applications.Emphasis is placed on transgenic plants that have been engineered based on di?erent stress-response mechanisms.The review examines the follow-ing aspects:regulatory controls,metabolite engineering, ion transport,antioxidants and detoxi?cation,late embryogenesis abundant(LEA)and heat-shock pro-teins.Keywords Abiotic stress?Antioxidant?Hsp?
Ion transport?LEA protein?Osmolyte?Transcription factor
Abbreviations HSF:heat-shock factor?Hsp:heat-shock protein?LEA protein:late embryogenesis abundant protein?ROS:reactive oxygen species?SP1:stable protein1?TF:transcription factor
Introduction
Abiotic stresses,such as drought,salinity,extreme temperatures,chemical toxicity and oxidative stress are serious threats to agriculture and result in the deterio-ration of the environment.Abiotic stress is the primary cause of crop loss worldwide,reducing average yields for most major crop plants by more than50%(Boyer1982; Bray et al.2000).Drought and salinity are becoming particularly widespread in many regions,and may cause serious salinization of more than50%of all arable lands by the year2050.Abiotic stress leads to a series of morphological,physiological,biochemical and molecu-lar changes that adversely a?ect plant growth and pro-ductivity(Wang et al.2001a).Drought,salinity,extreme temperatures and oxidative stress are often intercon-nected,and may induce similar cellular damage.For example,drought and/or salinization are manifested primarily as osmotic stress,resulting in the disruption of homeostasis and ion distribution in the cell(Serrano et al.1999;Zhu2001a).Oxidative stress,which fre-quently accompanies high temperature,salinity,or drought stress,may cause denaturation of functional and structural proteins(Smirno?1998).As a conse-quence,these diverse environmental stresses often acti-vate similar cell signaling pathways(Shinozaki and Yamaguchi-Shinozaki2000;Knight and Knight2001; Zhu2001b,2002)and cellular responses,such as the production of stress proteins,up-regulation of anti-oxi-dants and accumulation of compatible solutes(Vierling
Planta(2003)218:1–14
DOI10.1007/s00425-003-1105-5
W.Wang?B.Vinocur?A.Altman(&)
The Robert H.Smith Institute of Plant Sciences and Genetics in Agriculture,and the Otto Warburg Center for Agricultural Biotechnology,Faculty of Agricultural,Food and Environmental Quality Sciences,The Hebrew University of Jerusalem,P.O.Box 12,76100Rehovot,Israel
E-mail:altman@agri.huji.ac.il
Fax:+972-8-9489899
and Kimpel 1992;Zhu et al.1997;Cushman and Bohnert 2000).
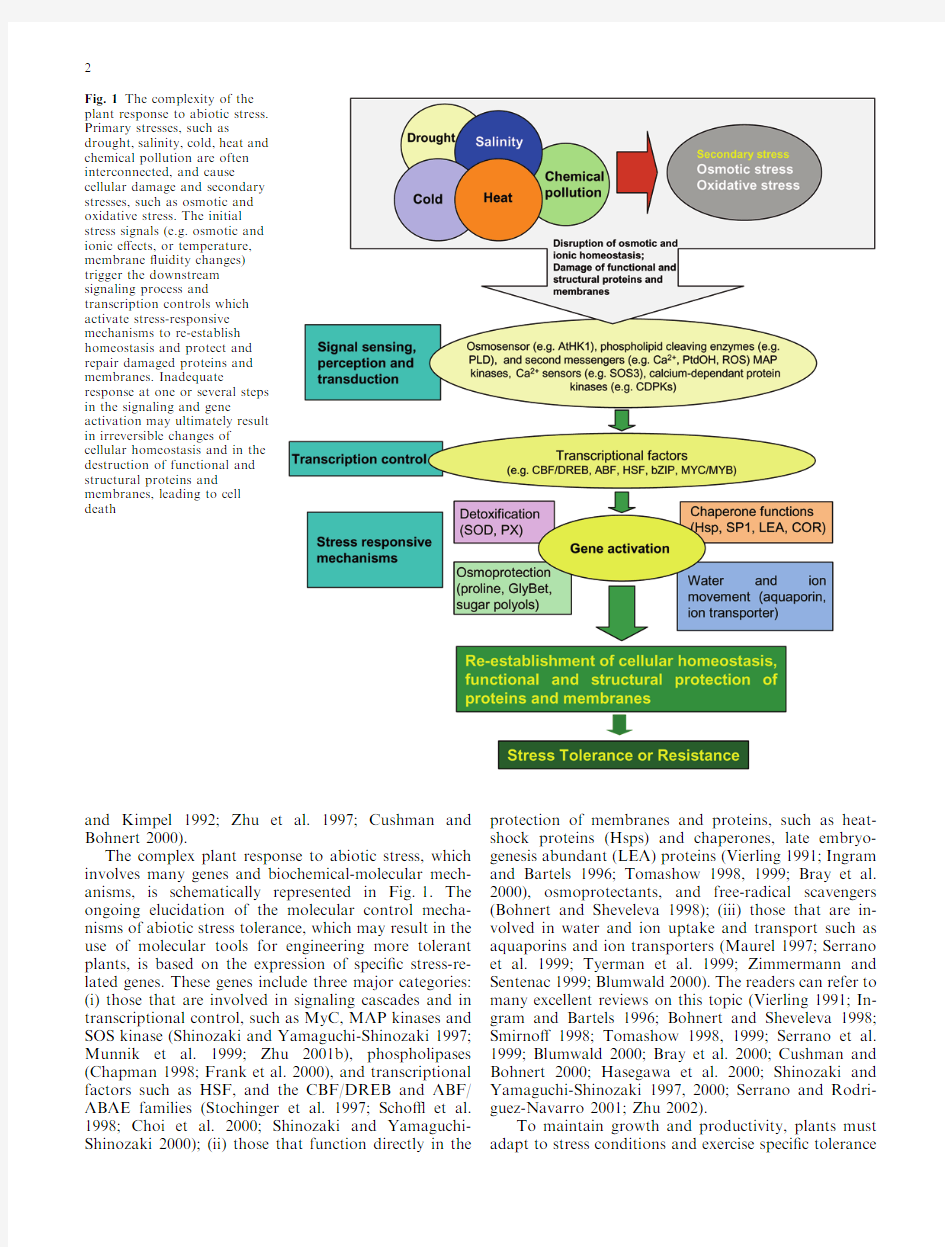
The complex plant response to abiotic stress,which involves many genes and biochemical-molecular mech-anisms,is schematically represented in Fig.1.The ongoing elucidation of the molecular control mecha-nisms of abiotic stress tolerance,which may result in the use of molecular tools for engineering more tolerant plants,is based on the expression of speci?c stress-re-lated genes.These genes include three major categories:(i)those that are involved in signaling cascades and in transcriptional control,such as MyC,MAP kinases and SOS kinase (Shinozaki and Yamaguchi-Shinozaki 1997;Munnik et al.1999;Zhu 2001b),phospholipases (Chapman 1998;Frank et al.2000),and transcriptional factors such as HSF,and the CBF/DREB and ABF/ABAE families (Stochinger et al.1997;Scho ?et al.1998;Choi et al.2000;Shinozaki and Yamaguchi-Shinozaki 2000);(ii)those that function directly in the protection of membranes and proteins,such as heat-shock proteins (Hsps)and chaperones,late embryo-genesis abundant (LEA)proteins (Vierling 1991;Ingram and Bartels 1996;Tomashow 1998,1999;Bray et al.2000),osmoprotectants,and free-radical scavengers (Bohnert and Sheveleva 1998);(iii)those that are in-volved in water and ion uptake and transport such as aquaporins and ion transporters (Maurel 1997;Serrano et al.1999;Tyerman et al.1999;Zimmermann and Sentenac 1999;Blumwald 2000).The readers can refer to many excellent reviews on this topic (Vierling 1991;In-gram and Bartels 1996;Bohnert and Sheveleva 1998;Smirno?1998;Tomashow 1998,1999;Serrano et al.1999;Blumwald 2000;Bray et al.2000;Cushman and Bohnert 2000;Hasegawa et al.2000;Shinozaki and Yamaguchi-Shinozaki 1997,2000;Serrano and Rodri-guez-Navarro 2001;Zhu 2002).
To maintain growth and productivity,plants must adapt to stress conditions and exercise speci?c
tolerance
Fig.1The complexity of the plant response to abiotic stress.Primary stresses,such as
drought,salinity,cold,heat and chemical pollution are often interconnected,and cause
cellular damage and secondary stresses,such as osmotic and oxidative stress.The initial stress signals (e.g.osmotic and ionic e?ects,or temperature,membrane ?uidity changes)trigger the downstream signaling process and
transcription controls which activate stress-responsive mechanisms to re-establish homeostasis and protect and repair damaged proteins and membranes.Inadequate
response at one or several steps in the signaling and gene
activation may ultimately result in irreversible changes of
cellular homeostasis and in the destruction of functional and structural proteins and membranes,leading to cell death
2
mechanisms.Plant modi?cation for enhanced tolerance is mostly based on the manipulation of genes that pro-tect and maintain the function and structure of cellular components.In contrast to most monogenic traits of engineered resistance to pests and herbicides,the genetically complex responses to abiotic stress condi-tions are more di?cult to control and engineer.Present engineering strategies rely on the transfer of one or several genes that are either involved in signaling and regulatory pathways,or that encode enzymes present in pathways leading to the synthesis of functional and structural protectants,such as osmolytes and antioxi-dants,or that encode stress-tolerance-conferring pro-teins.The current e?orts to improve plant stress tolerance by gene transformation have resulted in important achievements;however,the nature of the genetically complex mechanisms of abiotic stress toler-ance,and the potential detrimental side e?ects,make this task extremely di?cult.
The primary objective of this review is to report re-cent advances in the stress-response mechanisms and their biotechnological applications in plants.Emphasis is given to transgenic plants that were engineered for stress tolerance,based on di?erent mechanisms of stress re-sponse.The following major mechanisms and aspects are discussed:regulatory controls,osmolyte engineering,ion transport,detoxi?cation mechanisms and the role of LEA and Hsps.The di?erent transgenic plants that are discussed in this review are summarized in Table1. Transcription factors and their signi?cance
in plant stress tolerance
Plant stress responses are regulated by multiple signaling pathways that activate gene transcription and its downstream machinery.Plant genomes contain a large number of transcription factors(TFs);for example,
Table1Mechanisms,genes and genetically modi?ed plant species implicated in plant responses to abiotic stress Mechanism Genes Species Reference
Transcription control CBF1Arabidopsis thaliana Jaglo-Ottosen et al.1998
DREB1A A.thaliana Kasuga et al.1999
CBF3 A.thaliana Gilmour et al.2000
CBFs Brassica napus Jaglo et al.2001
CBF1Lycopersicon
esculentum
Hsieh et al.2002
CBF4 A.thaliana Haake et al.2002
AtMYC2and AtMYB2 A.thaliana Abe et al.2003
ABF3or ABF4 A.thaliana Kang et al.2002
HSF1and HSF3 A.thaliana JH Lee et al.1995;Pra ndl et al.1998
HsfA1L.esculentum Mishra et al.2002
spl7Oryza sativa Yamanouchi et al.2002
Compatible solute
Proline P5CS Nicotiana tabacum Kishor et al.1995;Konstantinova et al.2002;
Hong et al.2000
ProDH A.thaliana Nanjo et al.1999
Myo-inositol IMT1N.tabacum Sheveleva et al.1997
Sorbitol stpd1N.tabacum Sheveleva et al.1998
Antioxidants and detoxi?cation CuZn-SOD N.tabacum Gupta et al.1993a,1993b;Pitcher and Zilinskas1996 Mn-SOD or Fe-SOD Medicago sativa,
N.tabacum
McKersie et al.1996,1999,2000;Van Camp et al.1996
GST and GPX N.tabacum Roxas et al.1997
chy B A.thaliana Davison et al.2002
Aldose-aldehyde reductase N.tabacum Oberschall et al.2000
Ion transport AtNHX1 A.thaliana Apse et al.1999
B.napus Zhang et al.2001
L.esculentum Zhang and Blumwald2001 SOS1 A.thaliana Shi et al.(2003)
HAL1Cucurbita melo Bordas et al.1997
Rus et al.2001
AVP1 A.thaliana Gaxiola et al.2001
Hsps and molecular chaperones Hsp17.7Daucus carota Malik et al.1999 Hsp21 A.thaliana Ha rndahl et al.1999 AtHSP17.6A A.thaliana Sun et al.2001 DnaK1N.tabacum Sugino et al.1999 SP1Populus tremula Wang et al.2003
LEA-type proteins COR15a A.thaliana Artus et al.1996;Steponkus et al.1998;
Jaglo-Ottosen et al.1998
HVA1O.sativa Xu et al.1996
Triticum aestivum Sivamani et al.2000
WCS19 A.thaliana Ndong et al.2002
3
Arabidopsis dedicates about5.9%of its genome coding for more than1,500TFs(Riechmann et al.2000).Most of these TFs belong to a few large multigene families, e.g.MYB,AP2/EREBP,bZIP and WRKY.Individual members of the same family often respond di?erently to various stress stimuli;on the other hand,some stress-responsive genes may share the same TFs,as indicated by the signi?cant overlap of the gene-expression pro?les that are induced in response to di?erent stresses(Bo-hnert et al.2001;Seki et al.2001;Chen et al.2002; Fowler and Thomashow2002;Kreps et al.2002).Some key examples are discussed below.
The dehydration-responsive transcription factors (DREB)and C-repeat binding factors(CBF)bind to DRE and CRT cis-acting elements that contain the same motif(CCGAC).Members of the CBF/DREB1family, such as CBF1,CBF2,and CBF3(or DREB1B, DREB1C,and DREB1A,respectively)are themselves stress-inducible.DREB/CBF proteins are encoded by AP2/EREBP multigene families and mediate the tran-scription of several genes such as rd29A,rd17,cor6.6, cor15a,erd10,kin1,kin2and others in response to cold and water stress(Ingram and Bartels1996;Stockinger et al.1997;Gilmour et al.1998;Liu et al.1998;Seki et al.2001;Thomashow et al.2001).
Signi?cant improvement of stress tolerance was found upon overexpression of a single TF in engineered Arabidopsis thaliana plants.Arabidopsis cold acclimation is associated with the induction of COR(cold-regulated) genes by the CRT/DRE cis-regulatory elements (Thomashow1998).Jaglo-Ottosen et al.(1998)showed that increased expression of Arabidopsis CBF1induces the expression of the cold-regulated genes cor6.6,cor15a, cor47,and cor78,and increased the freezing tolerance of non-acclimated Arabidopsis plants.Arabidopsis trans-formation with the DREB1A gene(Kasuga et al.1999), driven by either the strong constitutive promoter of the cauli?ower mosaic virus(35SCaMV)or by a DRE-containing promoter from the dehydration-induced gene (rd29A),resulted in a marked increase in tolerance to freezing,water and salinity stress.Similar to the CBF1 transgene,constitutive expression of DREB1A tran-scription factor resulted in increased expression of its downstream targeted genes,such as rd29A,rd17,cor6.6, cor15a,erd10,and kin1.Overexpression of CBF3in Arabidopsis also increased freezing tolerance and,more interestingly,resulted in multiple biochemical changes associated with cold acclimation:elevated levels of proline and total soluble sugars,including sucrose,raf-?nose,glucose,and fructose(Gilmour et al.2000). Plants overexpressing CBF3also had elevated D1-pyrr-oline-5-carboxylate synthetase(P5CS)transcript levels, suggesting that the increase in proline levels had resul-ted,in part,from increased expression of the key proline biosynthetic enzyme P5CS.
Components of the Arabidopsis CBF/DREB cold-response pathway were also found in Brassica napus and other plant species(Jaglo et al.2001).Constitutive overexpression of the Arabidopsis CBF genes in transgenic B.napus plants induced expression of or-thologs of Arabidopsis CBF-targeted genes and in-creased the freezing tolerance of both non-acclimated and cold-acclimated plants.Recently,expression of Arabidopsis CBF1in tomato plants has been shown to confer elevated tolerance to chilling and oxidative stress (Hsieh et al.2002).However,the expression of cor genes was not induced,while reactive oxygen species (ROS)scavenger genes, e.g.CAT1,were activated. Recently,a close CBF/DREB1homolog in Arabidopsis, CBF4,was isolated(Haake et al.2002).The expression of CBF4is rapidly induced during drought stress and by abscisic aid(ABA)treatment,but not by cold, thereby distinguishing it from CBF/DREB1transcrip-tion factors.Overexpression of CBF4under the con-stitutive CaMV35S promoter resulted in the expression of cold-and drought-induced genes under non-stress conditions,and the transgenic Arabidopsis plants showed more tolerance to freezing and drought con-ditions(Haake et al.2002).
ABA signaling plays a vital role in plant stress res-ponses as evidenced by the fact that many of the drought-inducible genes studied to date are also induced by ABA.Two TF families bZIP and MYB,are involved in ABA signaling and its gene activation.Many ABA-inducible genes share the(C/T)ACGTGGC consensus, cis-acting ABA-responsive element(ABRE)in their promoter regions(Guiltinan et al.1990;Mundy et al. 1990).Several ABRE-binding proteins,including rice TRAB and Arabidopsis AREB/ABF and ABI5,which interact with ABRE and regulate gene expression,have been isolated(Hobo et al.1999;Choi et al.2000;Fin-kelstein and Lynch2000;Lopez-Molina and Chua2000; Uno et al.2000;Kang et al.2002).Recently,Abe et al. (2003)showed that the Arabidopsis MYB transcription factor proteins AtMYC2and AtMYB2function as transcriptional activators in ABA-inducible gene expression,suggesting a novel regulatory system for gene expression in response to ABA,other than the ABRE-bZIP regulatory system.
Constitutive expression of ABF3or ABF4demon-strated enhanced drought tolerance in Arabidopsis plants,with altered expression of ABA/stress-responsive genes,e.g.rd29B,rab18,ABI1and ABI2(Kang et al. 2002).Several ABA-associated phenotypes,such as ABA hypersensitivity and sugar hypersensitivity,were observed in transgenic plants.Moreover,salt hypersen-sitivity was observed in ABF3-and ABF4-overexpress-ing plants at the germination and young seedling stage, indicating the possible participation of ABF3and ABF4 in the salt response at these particular developmental stages.Improved osmotic-stress tolerance in35S:At-MYC2/AtMYB2transgenic plants,as judged by an electrolyte-leakage test(Abe et al.2003),is yet another example of how plant engineering with transcriptional activators of ABA signaling can provide a means of improving plant stress tolerance.
Similar to osmotic stress,the heat-shock response is primarily regulated at the transcriptional level.
4
Thermo-inducibility is attributed to conserved cis-reg-ulatory promoter elements(HSEs)that are the binding sites for the trans-active heat-shock factors(HSFs; Scho?et al.1998).The HSEs share a common con-sensus sequence‘‘nGAAnnTTCnnGAAn’’.Plant HSFs,which are further categorized into three classes, A,B and C,appear to be a unique family containing a number of members:21from Arabidopsis,more than 16from tomato,and15from soybean(Nover et al. 2001).Hsps are chaperones,which function during both normal cell growth and stress conditions;there-fore it is not surprising that HSFs provide diverse functions that di?erentially control the activation of heat-shock genes(Morimoto1998;Scho?et al.1998; Mishra et al.2002).It has been shown that overex-pression of HSF1and HSF3(class A)leads to the expression of several hsp genes conferring thermo-tol-erance in transgenic plants(JH Lee et al.1995;Pra ndl et al.1998).In tomato plants,overexpression of HsfA1 resulted in heat-stress tolerance,while HsfA1antisense plants and fruits were extremely sensitive to elevated temperatures(Mishra et al.2002).Analysis of the transgenic plants disclosed that HsfA1has a unique role as a master regulator for the synthesis of other HSFs such as HSFs A2and B1as well as Hsps.HSFs may also play a role in controlling cell death.The rice spl(spotted leaf)gene spl7encodes a class-A HSF,and the spl7transgenic rice showed no lesions(spotted leaf) or delay in development of lesions(Yamanouchi et al. 2002).The experiment suggested that spl7might par-ticipate in controlling cell death that is caused by environmental stresses such as high temperature.
These studies demonstrate the important role of TFs in the acquisition of stress tolerance,which may ulti-mately contribute to agricultural and environmental practices.Although plant transformation with stress-responsive TFs permits overexpression of downstream stress-associated multiple genes,it may also activate additional non-stress genes that adversely a?ect the normal agronomic characteristics of a crop.One com-mon negative e?ect of TF-modi?ed plants is the growth retardation in transgenic plants that constitutively ex-press TFs(Kasuga et al.1999;Hsieh et al.2002;Kang et al.2002;Abe et al.2003).For example,a positive correlation was found between the levels of DREB1A expression,the level of expression of the target gene RD29A,and the degree to which plants growth is stunted(Liu et al.1998).These negative e?ects can be partially prevented by the use of stress-inducible pro-moters that control the expression of the TF(Kasuga et al.1999).
Applications of compatible-solute engineering Compatible solutes,or osmolytes,accumulate in organisms in response to osmotic stress.The primary function of compatible solutes is to maintain cell turgor and thus the driving gradient for water uptake.Recent studies indicate that compatible solutes can also act as free-radical scavengers or chemical chaperones by di-rectly stabilizing membranes and/or proteins(Lee et al. 1997;Hare et al.1998;Bohnert and Shen1999;McNeil et al.1999;Diamant et al.2001).Compatible solutes fall into three major groups:amino acids(e.g.proline), quaternary amines(e.g.glycine betaine,dimethylsulfo-niopropionate)and polyol/sugars(e.g.mannitol,treha-lose).Overexpression of compatible solutes in transgenic plants can result in improved stress tolerance.
Proline is synthesized from glutamate via glutamic-c-semialdehyde(GSA)and D1-pyrroline-5-carboxylate (P5C).P5C synthase(P5CS)catalyzes the conversion of glutamate to P5C,followed by P5C reductase(P5CR), which reduces P5C to proline.In the reverse reaction, proline is metabolized to glutamate in a feed-back manner,via P5C and GSA with the aid of proline dehydrogenase(ProDH)followed by P5C dehydroge-nase(P5CDH).Transgenic tobacco(Nicotiana tabacum) overexpressing the p5cs gene that encodes P5CS pro-duced10-to18-fold more proline and exhibited better performance under salt stress(Kishor et al.1995). Freezing tolerance was achieved by transforming to-bacco with the same gene(p5cs;Konstantinova et al. 2002).Because P5CS is the rate-limiting enzyme in proline biosynthesis in plants,and is subject to feedback inhibition by proline,removal of the feedback inhibition can result in high level of proline accumulation(Hong et al.2000).Transgenic tobacco overexpressing the mutated form of Vigna aconitifolia P5CS(P5CSF129A) accumulated about2-fold more proline than plants expressing wild-type P5CS when the feedback inhibition induced by proline was eliminated and this di?erence was further increased in plants under salt stress.The elevated proline also reduced free-radical levels in re-sponse to osmotic stress and signi?cantly improved the ability of the transgenic seedlings to grow in a medium containing up to200mM NaCl(Hong et al.2000).
An alternative strategy for sustaining a high level of proline during stress is to down-regulate proline catab-olism.Proline dehydrogenase(ProDH)catalyzes the?rst step of proline degradation.Repression of Arabidopsis proline dehydrogenase(AtProDH)mRNA in antisense transgenic plants resulted in the constitutive accumula-tion of proline(Nanjo et al.1999).AtProDH-antisense-transgenic plants showed more tolerance to freezing ()7°C)and to NaCl(600mM)than wild-type and vector-transformed plants.
Betaines are quaternary ammonium compounds,i.e. amino acid derivatives in which the nitrogen atom is fully methylated.In plants,glycine betaine,a represen-tative member of this group of osmolytes,is synthesized in the chloroplast from choline by a two-step process. The?rst step(choline to betaine aldehyde)is mediated by choline monooxygenase(CMO),which can be in-duced by drought and salinity(Russell et al.1998).The second step(betaine aldehyde to glycine betaine)is catalyzed by betaine aldehyde dehydrogenase(BADH),
5
an NAD-dependent dehydrogenase.Many important crops,such as rice,potato and tomato,do not accu-mulate glycine betaine and are therefore potential can-didates for the engineering of betaine biosynthesis (McCue and Hanson1990).Genetic engineering for glycine betaine biosynthesis in non-accumulating plants has been extensively reported(Lilius et al.1996;Hayashi et al.1997;Alia et al.1998a,1998b;Sakamoto et al. 1998,2000;also see review of Sakamoto and Murata 2002,and references therein).Transgenic plants expressing bacterial choline-oxidizing enzymes displayed increased tolerance to various stresses such as high salt concentrations and extreme temperatures(reviewed in Sakamoto and Murata2001).The main constraint to glycine betaine accumulation in transgenic plants ap-pears to be the endogenous choline supply(Nuccio et al. 1998).Therefore,up-regulation of the de novo synthesis of choline to increase glycine betaine synthesis is also imported in non-accumulators,which express foreign choline-oxidizing enzymes(Nuccio et al.1998;Huang et al.2000).In addition,correct subcellular targeting of the inserted gene also has a signi?cant impact on its expression and expected functions(Sakamoto et al. 1998;Konstantinova et al.2002).
A number of‘‘sugar alcohols’’(mannitol,trehalose, myo-inositol and sorbitol)have been targeted for the engineering of compatible-solute overproduction.Tar-czynski et al.(1993)introduced a bacterial gene that encodes mannitol1-phosphate dehydrogenase into to-bacco plants,resulting in mannitol accumulation and enhanced tolerance to salinity.In addition,transgenic tobacco plants carrying a cDNA encoding myo-inositol O-methyltransferase(IMT1)accumulated D-ononitol and,as a result,acquired enhanced photosynthesis protection and increased recovery under drought and salt stress(Sheveleva et al.1997).When engineering these’sugar polyols’in plants,pleiotropic e?ects some-times occurred.Abnormal phenotypes associated with sorbitol accumulation were also found in transgenic tobacco transformed with stpd1,a cDNA encoding sorbitol-6-phosphate dehydrogenase from apple(Shev-eleva et al.1998).Plants with low levels of sorbitol(less than2–3l mol g)1fresh weight)developed normally, but necrotic lesions and reduced shoot and root growth, as well as low fertility,were associated with an excessive sorbitol accumulation.The adverse e?ects observed in these sorbitol overproducers may have resulted from a disturbance in carbohydrate transport and allocation (Sheveleva et al.1998).
Recently,Garg et al.(2002)showed rice tolerance to multiple abiotic stresses by engineering trehalose over-expression without the negative pleiotropic e?ects ob-served in some other studies.The modest increase in trehalose levels in transgenic lines,using either the tissue-speci?c or stress-dependent promoters,resulted in a higher capacity for photosynthesis and a con-comitant decrease in the photo-oxidative damage dur-ing stress.Although the elevated trehalose levels do not seem to account for osmoprotection activity,the ?ndings demonstrate the feasibility of engineering rice for increased tolerance and enhanced productivity through tissue-speci?c or stress-dependent overproduc-tion of trehalose.
Genetic manipulations of compatible solutes do not always lead to a signi?cant accumulation of the com-pound(except some cases of proline over-production; Chen and Murata2002),suggesting that the function of compatible solutes is not restricted to osmotic adjust-ment,only.Accumulation of compatible solutes may also protect plants against damage by scavenging of reactive oxygen species,and by their chaperone-like activities in maintaining protein structures and func-tions.Engineered overproduction of these compatible solutes provides an opportunity to generate more tol-erant plants.Incorrect gene expression of compatible solutes often causes pleiotropic e?ects(e.g.necrosis and growth retardation)due to disturbance in endogenous pathways of primary metabolisms.For agricultural practices,over-synthesis of compatible solutes should not account for the primary metabolic costs.Moreover, to minimize the pleiotropic e?ects,the over-production of compatible solutes should be stress-inducible and/or tissue speci?c(Garg et al.2002).
Antioxidants and detoxi?cation genes
Salt,drought,heat and oxidative stress are accompanied by the formation of ROS such as O2,H2O2,and OH) (Price et al.1989;Moran et al.1994,Mittler2002), which damage membranes and macromolecules.Plants have developed several antioxidation strategies to scav-enge these toxic compounds.Enhancement of antioxi-dant defense in plants can thus increase tolerance to di?erent stress factors.Antioxidants(ROS scavengers) include enzymes such as catalase,superoxide dismutase (SOD),ascorbate peroxidase(APX)and glutathione reductase,as well as non-enzyme molecules such as ascorbate,glutathione,carotenoids,and anthocyanins. Additional compounds,such as osmolytes,proteins(e.g. peroxiredoxin)and amphiphilic molecules(e.g.tocoph-erol),can also function as ROS scavengers(Bowler et al. 1992;Noctor and Foyer1998).
Transgenic tobacco plants overexpressing chloro-plastic Cu/Zn-SOD showed increased resistance to oxi-dative stress caused by high light and low temperatures (Gupta et al.1993a,1993b).Transgenic alfalfa plants (Medicago sativa)expressing Mn-SOD evinced reduced injury from water-de?cit stress,as determined by chlo-rophyll?uorescence,electrolyte leakage and re-growth (McKersie et al.1996).In another study,transgenic alfalfa plants expressing either Mn-SOD or Fe-SOD had increased winter survival rates and yields(McKersie et al.1999,2000).However,this was not associated with protection against primary injury caused by freezing, and the Fe-SOD transgenic alfalfa did not show greater tolerance to oxidative stress(McKersie et al.2000).As
6
proposed by the authors,Fe-SOD overexpression may reduce secondary injury symptoms and thereby enhance recovery from the stress experienced during the winter. Mn-SOD-expressing transgenic tobacco showed a2-to 3-fold reduction in leaf injury compared to wild-type plants following exposure to ozone(Van Camp et al. 1996).In addition,overexpression of Cu/Zn-SOD in the cytosol of transgenic tobacco plants was found to confer partial resistance to ozone-induced foliar necrosis (Pitcher and Zilinskas1996).Transgenic tobacco seed-lings,overexpressing cDNA which encodes an enzyme with both glutathione S-transferase(GST)and gluta-thione peroxidase(GPX)activity,grew signi?cantly faster than control seedlings following exposure to chilling or salt stress(Roxas et al.1997).
In Arabidopsis,overexpression of the chy B gene that encodes b-carotene hydroxylase(an enzyme active in the zeaxanthin biosynthetic pathway)resulted in a2-fold increase in the pool of the xanthophyll cycle(Davison et al.2002).These transgenic plants showed greater tolerance to high light and increased temperatures,and it was suggested that the stress protection was most likely due to the action of zeaxanthin in preventing oxidative damage to membranes.
Transgenic tobacco plants expressing alfalfa aldose-aldehyde reductase,a stress-activated enzyme,showed reduced damage when exposed to oxidative stress and increased tolerance to heavy metals,salt and dehydra-tion stress(Oberschall et al.2000).This novel enzyme is an NADPH-dependent aldose/aldehyde reductase, which is believed to be involved in detoxi?cation by reducing the level of reactive aldehydes.
Targeting detoxi?cation pathways is an appropriate approach for obtaining plants with multiple stress-tol-erance traits(Bartels2001).It is expected that with our increasing understanding of those pathways,it will be-come possible to produce transgenic plants that can be sustained under true?eld conditions with multiple environmental stresses.
Engineering for ion transport
Osmotic stress,ion toxicity and high salt content in the soil and the irrigation water,especially Na+and Cl), signi?cantly impair plant growth.Ion transporters selectively transport ions and maintain them at physio-logically relevant concentrations while Na+/H+anti-porters also play a crucial role in maintaining cellular ion homeostasis,thus permitting plant survival and growth under saline conditions.The Na+/H+anti-porters catalyze the exchange of Na+for H+across membranes and have a variety of functions,such as regulating cytoplasmic pH,sodium levels and cell turgor (Serrano et al.1999).Plant Na+/H+antiporters have been isolated from Arabidopsis(AtNHX1,SOS1;Gaxi-ola et al.1999;Shi et al.2000)and rice plants(Fukuda et al.1999)and from the halophytic plants Atriplex gmelini(Hamada et al.2001)and Mesembryanthemum crystallinum(Chauhan et al.2000).Overexpression of the vacuolar Na+/H+antiporter AtNHX1in Arabid-opsis plants(Apse et al.1999)promoted growth and development in potting medium irrigated with up to 200mM sodium chloride.This salinity tolerance was positively correlated with elevated levels of AtNHX1 transcript,and with protein and vacuolar Na+/H+an-tiporter activity.Transgenic Brassica napus plants overexpressing AtNHX1were able to grow,?ower and produce seeds,in the presence of200mM sodium chloride,even though they accumulated sodium at a rate of up to6%dry weight.Moreover,their seed yields and seed oil quality were not altered by the high soil salinity (Zhang et al.2001).Similarly,transgenic tomato plants overexpressing this gene were able to grow,?ower and produce fruit in the presence of200mM sodium chlo-ride(Zhang and Blumwald2001).Although the tomato leaves accumulated high sodium concentrations,the fruits displayed very low sodium content,demonstrating the potential to maintain fruit yield and quality at high salt levels.
The A.thaliana plasma membrane Na+/H+anti-porter,encoded by the SOS1gene,was suggested to be essential for salt tolerance(Shi et al.2002),and recently Shi et al.(2003)reported that overexpression of SOS1 improves salt tolerance in transgenic Arabidopsis.The authors also showed that the increased salt tolerance was correlated with reduced Na+accumulation.
Saccharomyces cerevisiae cation transport systems, such as HAL1and HAL3,are involved in the regulation of K+and Na+transport,respectively.Shoots from transgenic melon plants expressing the HAL1gene showed some level of salt tolerance in vitro(Bordas et al. 1997),and transgenic tomato lines expressing the HAL1 gene were found to be more salt-tolerant than the wild-type plants,as judged by both callus and plant growth in short-term experiments(Gisbert et al.2000).Such transgenic lines also demonstrated better fruit yield un-der salt stress(Rus et al.2001).
In plants,protons are used as coupling ions for ion transport systems,and the proton gradient,generated by proton pumps found in the cell membrane,is the driving force for nutrient uptake(Serrano et al.1999).Three distinct proton pumps are responsible for the generation of the proton electrochemical gradients(Sze et al.1999): (i)the plasma membrane H-ATPase pump(PM H-AT-Pase)which extrudes H+from the cell and thus gener-ates a proton motive force;(ii)the vacuolar-type H-ATPase pump(V-ATPase);(iii)the vacuolar H-pumping pyrophosphatase pump(H-PPase).The latter two acidify the vacuolar lumen and other endo-membrane compartments.Arabidopsis plants were transformed with a vacuolar H+-PPase pump that is encoded by a single gene,AVP1(Gaxiola et al.2001), which can generate an H+gradient across the vacuolar membrane,similar in magnitude to that of the multi-subunit vacuolar H+-ATPase pump.These transgenic plants expressed higher levels of AVP1and were more resistant to salt and drought than wild-type plants.It
7
was also found that the resistant phenotypes had an increased vacuolar proton gradient,resulting in increased solute accumulation and water retention.
Maintenance and re-establishment of cellular ion homeostasis during stress and/or following stress is extremely important for plant survival and growth, especially for prevention,compartmentation or exclu-sion of sodium ions under salinity stress.Overexpres-sion of the genes involved in cellular ion homeostasis have already resulted in signi?cant improvement; however more careful utilization of speci?c genes, including targeting to di?erent types of cells and organelles,should result in even greater salt-stress tol-erance under true?eld conditions.
The role of Hsps and LEA-type proteins
To cope with environmental stress,plants activate a large set of genes leading to the accumulation of speci?c stress-associated proteins(Vierling1991;Ingram and Bartels1996;Bohnert and Sheveleva1998;Thomashow 1999;Hoekstra et al.2001).Heat-shock proteins(Hsps) and late embryogenesis abundant(LEA)-type proteins are two major types of stress-induced proteins that accumulate upon water,salinity,and extreme tempera-ture stress.They have been shown to play a role in cel-lular protection during the stress(Vierling and Kimpel 1992;Boston et al.1996;Close1996;Ingram and Bartels 1996;Waters et al.1996;Thomashow1998).
Hsps and molecular chaperones
Dysfunction of enzymes and proteins usually accompa-nies abiotic stress.Therefore,maintaining proteins in their functional conformations and preventing aggrega-tion of non-native proteins are particularly important for cell survival under stress.Many stress-responsive proteins,especially Hsps,have been shown to act as molecular chaperones,which are responsible for protein synthesis,targeting,maturation and degradation in a broad array of normal cellular processes.Furthermore, molecular chaperones function in the stabilization of proteins and membranes,and in assisting protein refolding under stress conditions(Vierling1991;Hend-rick and Hartl1993;Boston et al.1996;Hartl1996; Waters et al.1996;To ro k et al.2001).
Among?ve conserved families of Hsps(Hsp100, Hsp90,Hsp70,Hsp60and sHsp),the small heat-shock proteins(sHsps)are found to be most prevalent in plants.sHsps are Hsps that vary in size from12to 40kDa(Vierling1991).Various studies have shown that plant sHsps are not only expressed in response to heat shock but also under water,salt,and oxidative stress, and at low temperature(Almoguera et al.1993;Alamillo et al.1995;Sabehat et al.1998;Ha rndahl et al.1999; Hamilton and Heckathorn2001).In the resurrection plant Craterostigma plantagineum,sHsp-like proteins are expressed constitutively in vegetative tissues(Ala-millo et al.1995).Two tomato sHsps,tom66and tom111,were induced by low temperature in pre-heated fruits(Sabehat et al.1998).In another study,Hsp21was found to be involved in oxidative stress(Ha rndahl et al. 1999).Recently,Hamilton and Heckathorn(2001)sug-gested that sHsps might act as antioxidants in protecting Complex-I electron transport in mitochondria during NaCl stress.Moreover,At-HSP17.6A expression was induced by heat and osmotic stress as well as during seed development(Sun et al.2001).In addition,sHsps are involved in many developmental processes,such as em-bryo development,seed germination,somatic embryo-genesis,pollen development,and fruit maturation (Waters et al.1996).
Plant sHsps show less sequence similarity than Hsps of other organisms.The sequence similarity spans approximately100amino acids proximal to the car-boxyl-terminal,and exhibits pronounced homology with the a-crystallin family(Waters et al.1996).Plant sHsps, like other sHsps and a-crystallins,tend to form large oligomeric complexes(Chen et al.1994;GJ Lee et al. 1995;Collada et al.1997;Suzuki et al.1998).The major chaperone activity of sHsps is to bind and hold dena-tured substrates in a folding-competent state for sub-sequent refolding by a chaperone network(Horwitz 1992;Ehrnsperger et al.1997;Lee et al.1997;Veinger et al.1998;Haslbeck et al.1999;Ding and Candido 2000;Studer and Narberhaus2000).However,some members of the plant sHsps can also stabilize or reac-tivate inactivated enzymes(GJ Lee et al.1995;Hook and Harding1998;Muchowski and Clark1998;Hasl-beck et al.1999;Smy kal et al.2000;Marini et al.2000; Sun et al.2001).
sHsps,as well as other Hsps,are believed to play an important role in plant stress tolerance.Carrot trans-genic cells and regenerated plants,which constitutively expressed the carrot Hsp17.7gene,showed more ther-motolerance than the vector controls(Malik et al.1999). In contrast,heat-inducible Hsp17.7antisense lines were less thermotolerant than the vector controls.Under high light conditions,transgenic Arabidopsis that constitu-tively expressed a chloroplast Hsp21were more resistant to heat stress than the wild-type plants(Ha rndahl et al. 1999).The results lead us to suggest that Hsps overpro-duction may also protect plants from oxidative stress. Recently,Sun et al.(2001)demonstrated that the expression of Arabidopsis AtHSP17.6A is regulated by heat shock and osmotic stress,and is induced during seed maturation.Overexpression of AtHSP17.6A in A.thali-ana conferred higher osmotolerance;however,the AtH-SP17.6A transgene failed to increase heat tolerance.
DnaK1,a member of Hsp70from the halotolerant Cyanobacterium aphanothece,was overexpressed in the cytosol of transgenic tobacco plants and was found to improve their salt tolerance(Sugino et al.1999).Under salt stress,the CO2?xation rate decreased to40%in the control plants while its activity in the transgenic plants was approximately85%.In addition,leaf sodium
8
concentrations were signi?cantly increased in control plants but those of the transgenic plants remained at levels similar to the non-stressed plants.
The LEA-type proteins
LEA-type proteins have been found in a wide range of plant species in response to water de?cit resulting from desiccation,cold and osmotic stress.LEA-type proteins fall into a number of families,with diverse structures and functions(Close1996;Ingram and Bartels1996; Thomashow1998).Predictions of secondary structures suggest that most LEA proteins exist as random coiled a-helices(Bray et al.2000).It was therefore proposed that most LEA and dehydrin proteins exist as largely unfolded structures in their native state,although a few members exist as dimers or tetramers(Ceccardi et al. 1994;Kazuoka and Oeda1994).
Hydrophilicity is a common characteristic of LEA-type and other osmotic stress-responsive proteins.LEA proteins have been grouped together with other osmotic stress-induced proteins from Saccharomyces cerevisiae and Escherichia coli into a class of proteins termed hy-drophilins,based on criteria of high hydrophilicity index (>1.0)and glycine content(>6%;Garay-Arroyo et al. 2000).Heat stability is another notable feature of LEA proteins,i.e.they do not coagulate upon boiling(Close et al.1989;Ceccardi et al.1994;Houde et al.1995; Thomashow1998,1999).Another common character-istic of LEA-type proteins is that,in most cases,their related gene expression is transcriptionally regulated and responsive to ABA(Mundy and Chua1988;Skriver and Mundy1990;Leung and Giraudat1998).
The functions of LEA-type proteins are largely un-known.Nevertheless,their considerable synthesis during the late stage of embryogenesis,their induction by stress and their structural characteristics(hydrophilicity,ran-dom coils and repeating motifs)permits the prediction of some of their functions.It has been suggested that LEA-type proteins act as water-binding molecules,in ion sequestration and in macromolecule and membrane stabilization(i.e.chaperone-like activity;Dure1993a, 1993b;Close1996;Ingram and Bartels1996;Thoma-show1998,1999).COR85,a group-II LEA protein,was shown to be involved in cryoprotection of freezing-sen-sitive enzymes(Kazuoka and Oeda1994).Moreover,it was found that COR15am,the mature COR15a poly-peptide,acts directly as a cryoprotective protein by inhibiting the formation of hexagonal II phase lipids,a major type of freeze-induced membrane lesion in non-acclimated plants(Steponkus et al.1998).
Overexpression of COR15a,which was targeted to the chloroplasts,increased freezing tolerance of chlo-roplasts in vivo,and of protoplasts in vitro(Artus et al. 1996).This increase most likely resulted from the membrane-stabilizing e?ect of COR15a(Artus et al. 1996;Steponkus et al.1998).However,the protective e?ect of COR15a was insigni?cant for the survival of whole plants during freezing(Jaglo-Ottosen et al.1998). Xu et al.(1996)reported that the expression of HVA1, an LEA III family protein in barley,confers tolerance to water de?ciency and salt stress in transgenic rice plants. Moreover,constitutive expression of the same protein in transgenic wheat plants improved biomass productivity and water-use e?ciency under water-de?cit conditions (Sivamani et al.2000).More recently,Ndong et al. (2002)reported that constitutive expression of a wheat chloroplast LEA-like protein(WCS19)in Arabidopsis resulted in a signi?cant increase in freezing tolerance.
Overexpression of a single LEA-type protein is not always su?cient to confer plant stress tolerance.Trans-genic tobacco plants that had been transformed with three Craterostigma plantagineum cDNAs,pcC6-19(homolo-gous with rice rab16),pcC3-06(homologous with lea D29)and pcC27-45(homologous with lea14), expressed high levels of the encoded proteins,but this increase did not result in drought tolerance(Iturriaga et al. 1992).In contrast,multi-expression of LEA-type proteins activated by their common transcription factors was found to correlate with stress tolerance in transgenic plants(Jaglo-Ottosen et al.1998;Kasuga et al.1999;Jaglo et al.2001).This suggested that the LEA-type proteins might function synergistically with other members. Perspective
Complex traits of abiotic stress phenomena in plants make genetic modi?cation for e?cient stress tolerance di?cult to achieve.However,the modi?cation of a sin-gle trait(e.g.TFs,antiporters,and others)resulted in several cases in signi?cant improvements in stress tolerance,as discussed earlier.
In addition to TFs,the modulation of upstream sig-naling regulators such as rice calcium-dependent protein kinase(OsCDPK)or Arabidopsis glycogen synthase kinase(AtGSK1),can also be a promising method for improving stress tolerance in plants(Piao et al.2001; Saijo et al.2000).However,little is known about the molecular mechanisms underlying these signaling com-ponents.Moreover,alteration of further upstream molecules in the pathway often activates a much wider network of genes,other than stress-speci?c ones.Such ’overactivation’may have deleterious e?ects on total plant performance,eventually becoming useless for agricultural practices.
The discovery and use of new stress-tolerance-asso-ciated genes,as well as heterologous genes,to confer plant stress tolerance(including those unique to extreme-growth-environment organisms e.g.halophytes, thermophilic organisms),has been the subject of ongo-ing e?orts to obtain tolerant plants.Recently,we re-ported on a stress-responsive gene,sp1,which is a representative member of a novel protein family.SP1is a homo-oligomeric protein that possesses exceptional stability under a variety of harsh conditions,such as boiling,proteolysis,detergents and high-salt
9
denaturation.Biochemical analysis demonstrated that SP1functions as a molecular chaperone in protecting and repairing di?erent heat-labile enzymes(Wang et al. 2001b,2002;Wang et al.unpublished data).Preliminary results showed that elevated expression of SP1is corre-lated to salt-stress tolerance(Wang et al.2003;Barak 2003).Like many newly discovered genes,SP1may serve as a candidate for modifying plant stress tolerance.A further understanding of the biochemical and molecular mechanisms underlying stress should be achieved with the advent of functional genomics,transcriptomics and proteomics.Many heterologous genes,such as the bac-terial mtlD gene producing mannitol,bacterial choline-oxidizing enzyme and yeast HAL genes(Tarczynski et al.1993;Lilius et al.1996;Bordas et al.1997)have been used successfully in genetically modi?ed plants. Recently,transgenic tobacco transformed with the ani-mal cell death suppressors Bcl-xL and Ced-9showed enhanced resistance to UV-B,paraquat,salt,cold and wounding stress(Mitsuhara et al.1999;Qiao et al. 2002).The utility of foreign genes in modifying plants opens a new avenue with a wide range of gene resources. Nevertheless,the availability of many diverse metabo-lites in plants,the di?erent post-transcriptional or translational modi?cations of selected foreign genes,as well as human health and environmental considerations, should be taken into account before foreign genes are designed for expression in plants.
While adaptation to stress under natural conditions has some ecological advantages,the metabolic and en-ergy costs may sometimes mask and limit its bene?t to agriculture and result in yield penalty.Therefore,the improvement of abiotic stress tolerance of agricultural plants can only be achieved,practically,by combining traditional and molecular breeding(Kasuga et al.1999; Dunwell2000;Wang et al.2001a).Thus,a comprehen-sive breeding strategy for abiotic stress tolerance should include the following steps and approaches:(i)conven-tional breeding and germplasm selection,especially of wild relevant species;(ii)elucidation of the speci?c molecular control mechanisms in tolerant and sensitive genotypes;(iii)biotechnology-oriented improvement of selection and breeding procedures through functional genomics analysis,use of molecular probes and markers for selection among natural and bred populations,and transformation with speci?c genes;and(iv)improvement and adaptation of current agricultural practices.
An ideal genetically modi?ed crop should possess a highly regulated stress-response capability that does not a?ect crop performance when stress is absent.In this respect,conventional breeding and selection techniques will continue to make a contribution(Wang et al. 2001a).While certain transgenic crops have already been moved from the laboratory,only a few stress-resistant transgenic crops have been evaluated in?eld trials under real stress conditions(Dunwell2000).In addition,most of the genetically modi?ed stress-tolerant plants gener-ated to date are non-agronomic plants.When facing the deleterious e?ects of drought and salinity,it is imperative that more crops,which are genetically resis-tant to abiotic stress,be designed,tested,and eventually released for application as new commercial varieties. Acknowledgements This work was supported by the European Union(grant no.QLK5-2000-01377-ESTABLISH),and by the Horowitz Fund,Yissum,Israel.
References
Abe H,Urao T,Ito T,Seki M,Shinozaki K,Yamaguchi-Shinozaki K(2003)Arabidopsis AtMYC2(bHLH)and AtMYB2(MYB) function as transcriptional activators in abscisic acid signaling.
Plant Cell15:63–78
Alamillo J,Almoguera C,Bartels D,Jordano J(1995)Constitutive expression of small heat shock proteins in vegetative tissues of the resurrection plant Craterostigma plantagineum.Plant Mol Biol29:1093–1099
Alia,Hayashi H,Sakamoto A,Murata N(1998a)Enhancement of the tolerance of Arabidopsis to high temperatures by genetic engineering of the synthesis of glycinebetaine.Plant J16:155–161
Alia,Hayashi H,Chen THH,Murata N(1998b)Transformation with a gene for choline oxidase enhances the cold tolerance of Arabidopsis during germination and early growth.Plant Cell Environ21:232–239
Almoguera C,Coca MA,Jordano J(1993)Tissue-speci?c expres-sion of sun?ower heat shock proteins in response to water stress.Plant J4:947–958
Apse MP,Aharon GS,Snedden WA,Blumwald E(1999)Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis.Science285:1256–1258
Artus NN,Uemura M,Steponkus PL,Gilmour SJ,Lin C, Thomashow MF(1996)Constitutive expression of the cold-regulated Arabidopsis thaliana COR15a gene a?ects both chloroplast and protoplast freezing tolerance.Proc Natl Acad Sci USA93:13404–13409
Barak T(2003)The possible function of SP1in sp1-transgenic aspen plants that express high or low levels of this protein.MSc thesis,Hebrew University of Jerusalem
Bartels D(2001)Targeting detoxi?cation pathways:an e?cient approach to obtain plants with multiple stress tolerance.Trends Plant Sci6:284–286
Blumwald E(2000)Sodium transport and salt tolerance in plants.
Curr Opin Cell Biol12:431–434
Bohnert HJ,Shen B(1999)Transformation and compatible solutes.Sci Hort78:237–260
Bohnert HJ,Sheveleva E(1998)Plant stress adaptations—making metabolism move.Curr Opin Plant Biol1:267–274
Bohnert HJ,Ayoubi P,Borchert C,Bressan RA,Burnap RL, Cushman JC,Cushman MA,Deyholos M,Fischer R, Galbraith DW(2001)A genomics approach towards salt stress tolerance.Plant Physiol Biochem39:295–311
Bordas M,Montesinos C,Debauza M,Salvador A,Roig LA, Serrano R,Moreno V(1997)Transfer of the yeast salt tolerance gene HAL1to Cucumis melo L.cultivars and in vitro evaluation of salt tolerance.Transgenic Res6:41–50
Boston RS,Viitanen PV,Vierling E(1996)Molecular chaperones and protein folding in plants.Plant Mol Biol32:191–222 Bowler C,Van Montagu M,Inze D(1992)Superoxide dismutases and stress tolerance.Annu Rev Plant Physiol Plant Mol Biol 43:83–116
Boyer JS(1982)Plant productivity and environment.Science 218:443–448
Bray EA,Bailey-Serres J,Weretilnyk E(2000)Responses to abiotic stresses.In:Gruissem W,Buchannan B,Jones R(eds) Biochemistry and molecular biology of plants.American Society of Plant Physiologists,Rockville,MD,pp1158–1249
10
Ceccardi TL,Meyer NC,Close TJ(1994)Puri?cation of a maize dehydrin.Protein Express Purif5:266–269
Chapman D(1998)Phospholipase activity during plant growth and development and in response to environmental stress.Trends Plant Sci.3:419–426
Chauhan S,Forsthoefel N,Ran Y,Quigley F,Nelson DE,Bohnert HJ(2000)Na+/myo-inositol symporters and Na+/H+-antiport in Mesembryanthemum crystallinum Plant J24:511–522
Chen Q,Osteryoung K,Vierling E(1994)A21-kDa chloroplast heat shock protein assembles into high molecular weight com-plexes in vitro and in organelle.J Biol Chem269:13216–13233 Chen THH,Murata N(2002)Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compat-ible solutes.Curr Opin Plant Biol5:250–257
Chen W,Provart NJ,Glazebrook J,Katagiri F,Chang HS,Eul-gem T,Mauch F,Luan Sh,Zou G,Whitham SA,Budworth PR,Tao Y,Xie Z,Chen X,Lam S,Kreps JA,Harper JF,Si-Ammour A,Mauch-Mani B,Heinlein M,Kobayashi K,Hohn T,Dangl JL,Wang X,Zhu T(2002)Expression pro?le matrix of arabidopsis transcription factor genes suggests their putative functions in response to environmental stresses.Plant Cell 14:559–574
Choi HI,Hong JH,Ha J,Kang JY,Kim SY(2000)ABFs,a family of ABA-responsive element binding factors.J Biol Chem 275:1723–1730
Close TJ(1996)Dehydrins:emergence of a biochemical role of a family of plant dehydration proteins.Physiol Plant97:795–803 Close TJ,Kortt AA,Chandler PM(1989)A cDNA-based com-parison of dehydration-induced proteins(dehydrins)in barley and corn.Plant Mol Biol13:95–108
Collada C,Gomez L,Casado R,Aragoncillo C(1997)Puri?cation and in vitro chaperone activity of a class I small heat-shock protein abundant in recalcitrant chestnut seeds.Plant Physiol 115:71–77
Cushman JC,Bohnert HJ(2000)Genomic approaches to plant stress tolerance.Curr Opin Plant Biol3:117–124
Davison PA,Hunter CN,Horton P(2002)Overexpression of b-carotene hydroxylase enhances stress tolerance in Arabidop-sis.Nature418:203–206
Diamant S,Eliahu N,Rosenthal D,Goloubino?P(2001)Chem-ical chaperones regulate molecular chaperones in vitro and in cells under combined salt and heat stresses.J Biol Chem 276:39586–39591
Ding L,Candido EPM(2000)HSP25,a small heat shock protein associated with dense bodies and M-lines of body wall muscle in Caenorhabditis elegans.J Biol Chem275:9510–9517
Dunwell JM(2000)Transgenic approaches to crop improvement.
J Exp Bot51:487–496
Dure III L(1993a)Structural motifs in Lea proteins.In:Close TJ, Bray EA(eds)Plant response to cellular dehydration during environmental stress.American Society of Plant Physiologists, Rockville,MD,pp91–103
Dure III L(1993b)A repeating11-mer amino acid motif and plant desiccation.Plant J3:363–369
Ehrnsperger M,Gra ber S,Gaestel M,Buchner J(1997)Binding of non-native protein to Hsp25during heat shock creates a res-ervoir of folding intermediates for reactivation.EMBO J16:221–229
Finkelstein RR,Lynch TJ(2000)The Arabidopsis abscisic acid response gene ABI5encodes a basic leucine zipper transcription factor.Plant Cell12:599–609
Fowler S,Thomashow MF(2002)Arabidopsis transcriptome pro-?ling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway.Plant Cell14:1675–1690
Frank W,Munnik T,Kerkmann K,Salamini F,Bartels D (2000)Water de?cit triggers phospholipase D activity in the resurrection plant Craterostigma plantagineum.Plant Cell 12:111–124
Fukuda A,Nakamura A,Tanaka Y(1999)Molecular cloning and expression of the Na+/H+exchanger gene in Oryza sativa.
Biochim Biophys Acta1446:149–155Garay-Arroyo A,Colmenero-Flores JM,Garciarrubio A,Cova-rrubias AA(2000)Highly hydrophilic proteins in prokaryotes and eukaryotes are common during conditions of water de?cit.
J Biol Chem275:5668–5674
Garg AK,Kim JK,Owens TG,Ranwala AP,Choi YD,Kochian LV,Wu RJ(2002)Trehalose accumulation in rice plants con-fers high tolerance levels to di?erent abiotic stresses.Proc Natl Acad Sci USA99:15898–15903
Gaxiola RA,Rao R,Sherman A,Grisa?P,Alper SL,Fink GR (1999)The Arabidopsis thaliana proton transporters,AtNhx1 and Avp1,can function in cation detoxi?cation in yeast.Proc Natl Acad Sci USA.96:1480–1485
Gaxiola RA,Li J,Undurraga S,Dang LM,Allen GJ,Alper SL, Fink GR(2001)Drought-and salt-tolerant plants result from overexpression of the AVP1H+-pump.Proc Natl Acad Sci USA98:11444–11449
Gilmour SJ,Zarka DG,Stockinger EJ,Salazar MP,Houghton JM,Thomashow MF(1998)Low temperature regulation of the Arabidopsis CBF family of AP2transcriptional activators as an early step in cold-induced COR gene expression.Plant J16:433–442
Gilmour SJ,Sebolt AM,Salazar MP,Everard JD,Thomashow MF(2000)Overexpression of the arabidopsis CBF3transcrip-tional activator mimics multiple biochemical changes associated with cold acclimation.Plant Physiol124:1854–1865
Gisbert C,Rus AM,Bolar?n MC,Lo pez-Coronado JM,Arrillaga I,Montesinos C,Caro M,Serrano R,Moreno V(2000)The yeast HAL1gene improves salt tolerance of transgenic tomato.
Plant Physiol123:393–402
Guiltinan MJ,Marcotte WR,Quatrano RS(1990)A plant leucine zipper protein that recognizes an abscisic acid response element.
Science250:267–271
Gupta AS,Heinen JL,Holaday AS,Burke JJ,Allen RD(1993a) Increased resistance to oxidative stress in transgenic plants that overexpress chloroplastic Cu/Zn superoxide dismutase.Proc Natl Acad Sci USA90:1629–1633
Gupta AS,Webb RP,Holaday AS,Allen RD(1993b)Overex-pression of superoxide dismutase protects plants from oxidative stress.Plant Physiol103:1067–1073
Haake V,Cook D,Riechmann JL,Pineda O,Thomashow MF, Zhang JZ(2002)Transcription factor CBF4is a regulator of drought adaptation in Arabidopsis.Plant Physiol130:639–648 Hamada A,Shono M,Xia T,Ohta M,Hayashi Y,Tanaka A, Hayakawa T(2001)Isolation and characterization of a Na+/ H+antiporter gene from the halophyte Atriplex gmelini.Plant Mol Biol46:35–42
Hamilton III EW,Heckathorn SA(2001)Mitochondrial adapta-tions to https://www.doczj.com/doc/6b12076314.html,plex I is protected by anti-oxidants and small heat shock proteins,whereas complex II is protected by proline and betaine.Plant Physiol126:1266–1274
Hare PD,Cress WA,Van Staden J(1998)Dissecting the roles of osmolyte accumulation during stress.Plant Cell Environ 21:535–553
Ha rndahl U,Hall RB,Osteryoung KW,Vierling E,Bornman JF, Sundby C(1999)The chloroplast small heat shock protein undergoes oxidation-dependent conformational changes and may protect plants from oxidative stress.Cell Stress Chaper-ones4:129–138
Hartl FU(1996)Molecular chaperones in cellular protein folding.
Nature381:571–579
Hasegawa PM,Bressan AB,Zhu JK,Bohnert HJ(2000)Plant cellular and molecular responses to high salinity.Annu Rev Plant Physiol Plant Mol Biol51:463–499
Haslbeck M,Walke S,Stromer T,Ehrnsperger M,White HE, Chen S,Saibil HR,Buchner J(1999)Hsp26:a temperature-regulated chaperone.EMBO J18:6744–6751
Hayashi H,Mustardy L,Deshnium P,Ida M,Murata N(1997) Transformation of Arabidopsis thaliana with the codA gene for choline oxidase:accumulation of glycinebetaine and enhanced tolerance to salt and cold stress.Plant J12:133–142 Hendrick JP,Hartl FU(1993)Molecular chaperone functions of heat-shock proteins.Annu Rev Biochem62:349–384
11
Hobo T,Kowyama Y,Hattori T(1999)A bZIP factor,TRAB1, interacts with VP1and mediates abscisic acid-induced tran-scription.Proc Natl Acad Sci USA96:15348–15353 Hoekstra FA,Golovina EA,Buitink J(2001)Mechanisms of plant desiccation tolerance.Trends Plant Sci6:431–438
Hong Z,Lakkineni K,Zhang K,Verma DPS(2000) Removal of feedback inhibition of D1-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress.Plant Physiol122: 1129–1136
Hook DWA,Harding JJ(1998)Protection of enzymes by a-crys-tallin acting as a molecular chaperone.Int J Biol Macromol 22:295–306
Horwitz J(1992)a-Crystallin can function as a molecular chaper-one.Proc Natl Acad Sci USA89:10449–10453
Houde M,Daniel C,Lachapelle M,Allard F,Laliberte S,Sarhan F (1995)Immunolocalization of freezing-tolerance-associated proteins in the cytoplasm and nucleoplasm of wheat crown tissue.Plant J8:583–593
Hsieh TH,Lee JT,Yang PT,Chiu LH,Charng YY,Wang YC, Chan MT(2002)Heterology expression of the Arabidopsis C-repeat/dehydration response element binding factor1gene confers elevated tolerance to chilling and oxidative stresses in transgenic tomato.Plant Physiol129:1086–1094
Huang J,Hirji R,Adam L,Rozwadowski KL,Hammer-lindl JK,Keller WA,Selvaraj G(2000)Genetic engineer-ing of glycinebetaine production toward enhancing stress tolerance in plants:metabolic limitations.Plant Physiol 122:747–756
Ingram J,Bartels D(1996)The molecular basis of dehydration tolerance in plants.Annu Rev Plant Biol47:377–403 Iturriaga G,Schneider K,Salamini F,Bartels D(1992)Expression of desiccation-related proteins from the resurrection plant in transgenic tobacco.Plant Mol Biol20:555–558
Jaglo KR,Kle?S,Amundsen KL,Zhang X,Haake V,Zhang JZ, Deits T,Thomashow MF(2001)Components of the Arabid-opsis C-repeat/dehydration-responsive element binding factor cold-response pathway are conserved in Brassica napus and other plant species.Plant Physiol127:910–917
Jaglo-Ottosen KR,Gilmour SJ,Zarka DG,Schabenberger O, Thomashow MF(1998)Arabidopsis CBF1overexpression in-duces COR genes and enhances freezing tolerance.Science 280:104–106
Kang JY,Choi HI,Im MY,Kim SY(2002)Arabidopsis basic leucine zipper proteins that mediate stress-responsive abscisic acid signaling.Plant Cell14:343–357
Kasuga M,Liu Q,Miura S,Yamaguchi-Shinozaki K,Shinozaki K (1999)Improving plant drought,salt,and freezing tolerance by gene transfer of a single stress-inducible transcription factor.
Nature Biotech17:287–291
Kazuoka T,Oeda K(1994)Puri?cation and characterization of COR85-oligomeric complex from cold-acclimated spinach.
Plant Cell Physiol35:601–611
Kishor KPB,Hong Z,Miao GH,Hu CAA,Verma DPS(1995) Overexpression of D1-pyrroline-5-carboxylate synthetase increase proline production and confers osmotolerance in transgenic plants.Plant Physiol108:1387–1394
Knight H,Knight MR(2001)Abiotic stress signalling pathways: speci?city and cross-talk.Trends Plant Sci6:262–267 Konstantinova T,Parvanova D,Atanassov A,Djilianov D(2002) Freezing tolerant tobacco,transformed to accumulate osmo-protectants.Plant Sci163:157–164
Kreps JA,Wu Y,Chang HS,Zhu T,Wang X,Harper JF(2002) Transcriptome changes for Arabidopsis in response to salt, osmotic,and cold stress.Plant Physiol130:2129–2141
Lee GJ,Pokala N,Vierling E(1995)Structure and in vitro molecular chaperone activity of cytosolic small heat shock proteins from pea.J Biol Chem270:10432–10438
Lee GJ,Roseman AM,Saibil HR,Vierling E(1997)A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state.EMBO J 3:659–671Lee JH,Hu bel A,Scho?F(1995)Derepression of the activity of genetically engineered heat shock factor causes constitutive synthesis of heat shock proteins and increased thermotolerance in transgenic Arabidopsis.Plant J8:603–612
Leung J,Giraudat J(1998)Abscisic acid signal transduction.Annu Rev Plant Biol49:199–222
Lilius G,Holmberg N,Bu low L(1996)Enhanced NaCl stress tolerance in transgenic tobacco expressing bacterial choline dehydrogenase.Bio-Technology14:177–180
Liu Q,Kasuga M,Sakuma Y,Abe H,Miura S,Yamaguchi-Shinozaki K,Shinozaki K(1998)Two transcription factors, DREB1and DREB2,with an EREBP/AP2DNA binding do-main separate two cellular signal transduction pathways in drought-and low-temperature-responsive gene expression, respectively,in Arabidopsis.Plant Cell10:1391–1406
Lopez-Molina L,Chua NH(2000)A null mutation in a bZIP factor confers ABA-insensitivity in Arabidopsis thaliana.Plant Cell Physiol41:541–547
Malik MK,Slovin JP,Hwang CH,Zimmerman JL(1999)Modi-?ed expression of a carrot small heat shock protein gene, Hsp17.7,results in increased or decreased thermotolerance.
Plant J20:89–99
Marini I,Moschini R,Corso AD,Mura U(2000)Complete pro-tection by a-crystallin of lens sorbitol dehydrogenase undergo-ing thermal stress.J Biol Chem275:32559–32565
Maurel C,(1997)Aquaporins and water permeability of plant membranes.Annu Rev Plant Biol48:399–429
McCue KF,Hanson AD(1990)Drought and salt tolerance:toward understanding and application.Trends Biotechnol8:358–362 McKersie BD,Bowley SR,Harjanto E,Leprince O(1996)Water-de?cit tolerance and?eld performance of transgenic alfalfa overexpressing superoxide dismutase.Plant Physiol111:1177–1181
McKersie BD,Bowley SR,Jones KS(1999)Winter survival of transgenic alfalfa overexpressing superoxide dismutase.Plant Physiol119:839–848
McKersie BD,Murnaghan J,Jones KS,Bowley SR(2000)Iron-superoxide dismutase expression in transgenic alfalfa increases winter survival without a detectable increase in photosynthetic oxidative stress tolerance.Plant Physiol122: 1427–1438
McNeil SD,Nuccio ML,Hanson AD(1999)Betaines and related osmoprotectants:targets for metabolic engineering of stress resistance.Plant Physiol120:945–949
Mishra SK,Tripp J,Winkelhaus S,Tschiersch B,Theres K,Nover L,Scharf KD(2002)In the complex family of heat stress transcription factors,HsfA1has a unique role as master regu-lator of thermotolerance in tomato.Gene Dev16:1555–1567 Mittler R(2002)Oxidative stress,antioxidants and stress tolerance.
Trends Plant Sci7:405–410
Mitsuhara I,Malik KA,Miura M,Ohashi Y(1999)Animal cell-death suppressors Bcl-xL and Ced-9inhibit cell death in tobacco plants.Curr Biol9:775–778
Moran JF,Becana M,Iturbe-Ormaetxe I,Frechilla S,Klucas RV, Aparicio-Tejo P(1994)Drought induces oxidative stress in pea plants.Planta194:346–352
Morimoto RI(1998)Regulation of the heat shock transcriptional response:cross talk between family of heat shock factors, molecular chaperones,and negative regulators.Genes Dev 12:3788–3796
Muchowski PJ,Clark JI(1998)ATP-enhance molecular chaperone functions of the small heat shock protein human a B crystallin.
Biochemistry95:1004–1009
Mundy J,Chua NH(1988)Abscisic acid and water stress induce the expression of a novel rice gene.EMBO J7:2279–2286 Mundy J,Yamaguchi-Shinozaki K,Chua,NH(1990)Nuclear pro-teins bind conserved elements in the abscisic acid-responsive promoter of a rice rab gene.Proc.Natl Acad Sci USA87:406–410 Munnik T,Ligterink W,Meskiene I,Calderini O,Beyerly J, Musgrave A,Hirt H(1999)Distinct osmo-sensing protein ki-nase pathways are involved in signaling moderate and severe hyper-osmotic stress.Plant J20:381–388
12
Nanjo T,Kobayashia M,Yoshibab Y,Kakubaric Y,Yamaguchi-Shinozaki K,Shinozaki K(1999)Antisense suppression of proline degradation improves tolerance to freezing and salinity in Arabidopsis thaliana.FEBS Lett461:205–210
Ndong C,Danyluk J,Wilson KE,Pocock T,Huner NPA,Sarhan F(2002)Cold-regulated cereal chloroplast late embryogenesis abundant-like proteins.Molecular characterization and func-tional analyses.Plant Physiol129:1368–1381
Noctor G,Foyer C(1998)Ascorbate and glutathione:keeping active oxygen under control.Annu Rev Plant Physiol Plant Mol Biol49:249–279
Nover L,Bharti K,Do ring P,Mishra SK,Ganguli A,Scharf KD (2001)Arabidopsis and the heat stress transcription factor world:How many heat stress transcription factors do we need?
Cell Stress Chaperones6:177–189
Nuccio ML,Russell BL,Nolte KD,Rathinasabapathi B,Gage DA,Hanson AD(1998)The endogenous choline supply limits glycine betaine synthesis in transgenic tobacco expressing cho-line monooxygenase.Plant J16:487–498
Oberschall A,Dea k M,To ro k K,Sass L,Vass I,Kova cs I,Fehe r A,Dudits D,Horva th GV(2000)A novel aldose/aldehyde reductase protects transgenic plants against lipid peroxidation under chemical and drought stresses.Plant J24:437–446
Piao HL,Lim JH,Kim SJ,Cheong GW,Hwang I(2001)Con-stitutive over-expression of AtGSK1induces NaCl stress re-sponses in the absence of NaCl stress and results in enhanced NaCl tolerance in Arabidopsis.Plant J27:305–314
Pitcher LH,Zilinskas BA(1996)Overexpression of copper/zinc superoxide dismutase in the cytosol of transgenic tobacco confers partial resistance to ozone-induced foliar necrosis.Plant Physiol110:583–588
Pra ndl R,Hinderhofer K,Eggers-Schumacher G,Scho?F(1998) HSF3,a new heat shock factor from Arabidopsis thaliana, derepresses the heat shock response and confers thermotoler-ance when overexpressed in transgenic plants.Mol Gen Genet 258:269–278
Price AM,Atherton NM,Hendry GAF(1989)Plants under drought-stress generate activated oxygen.Free Rad Res Com-mun8:61–66
Qiao J,Mitsuhara I,Yazaki Y,Sakano K,Gotoh Y,Miura M, Ohashi Y(2002)Enhanced resistance to salt,cold and wound stresses by overproduction of animal cell death suppressors Bcl-xL and Ced-9in tobacco cells—their possible contribution through improved function of organella.Plant Cell Physiol 43:992–1005
Riechmann JL,Heard J,Martin G,Reuber L,Jiang C,Keddie J, Adam L,Pineda O,Ratcli?e OJ,Samaha RR.(2000)Arabid-opsis transcription factors:genome-wide comparative analysis among eukaryotes.Science290:2105–2110
Roxas VP,Smith RK Jr,Allen ER,Allen RD(1997)Overexpres-sion of glutathione S-transferase/glutathione peroxidase en-hances the growth of transgenic tobacco seedlings during stress.
Nature Biotech15:988–991
Rus AM,Estan o MT,Gisbert C,Garcia-Sogo B,Serrano R,Caro M,Moreno V,Bolar?n MC(2001)Expressing the yeast HAL1 gene in tomato increases fruit yield and enhances K+/Na+ selectivity under salt stress.Plant Cell Environ24:875–880 Russell BL,Rathinasabapathi B,Hanson AD(1998)Osmotic stress induces expression of choline monooxygenase in sugar beet and amaranth.Plant Physiol116:859–865
Sabehat A,Lurie S,Weiss D(1998)Expression of small heat-shock proteins at low temperatures.Plant Physiol117:651–658
Saijo Y,Hata S,Kyozuka J,Shimamoto K,Izui K(2000)Over-expression of a single Ca2+-dependent protein kinase confers both cold and salt/drought tolerance on rice plants.Plant J 23:319–327
Sakamoto A,Murata N(2001)The use of bacterial choline oxi-dase,a glycinebetaine-synthesizing enzyme,to create stress-resistant transgenic plants.Plant Physiol125:180–188 Sakamoto A,Murata N(2002)The role of glycine betaine in the protection of plants from stress:clues from transgenic plants.
Plant Cell Environ25:163–171Sakamoto A,Alia,Murata N(1998)Metabolic engineering of rice leading to biosynthesis of glycinebetaine and tolerance to salt and cold.Plant Mol Biol38:1011–1019
Sakamoto A,Valverde R,Alia,Chen THH,Murata N(2000) Transformation of Arabidopsis with the codA gene for choline oxidase enhances freezing tolerance of plants.Plant J22:449–453
Scho?F,Pra ndl R,Reindl A(1998)Regulation of the heat-shock response.Plant Physiol117:1135–1141
Seki M,Narusaka M,Abe H,Kasuga M,Yamaguchi-Shinozaki K, Carninci P,Hayashizaki Y,Shinozaki K(2001)Monitoring the expression pattern of1,300Arabidopsis genes under drought and cold stresses by using a full-length cDNA microarray.Plant Cell13:61–72
Serrano R,Rodriguez-Navarro A(2001)Ion homeostasis during salt stress in plants.Curr Opin Cell Biol13:399–404
Serrano R,Mulet JM,Rios G,Marquez JA,de Larrinoa IF,Leube MP,Mendizabal I,Pascual-Ahuir A,Proft M,Ros R, Montesinos C(1999)A glimpse of the mechanisms of ion homeostasis during salt stress.J Exp Bot50:1023–1036 Sheveleva E,Chmara W,Bohnert HJ,Jensen RG(1997) Increased salt and drought tolerance by D-ononitol produc-tion in transgenic Nicotiana tabacum L.Plant Physiol115:1211–1219
Sheveleva EV,Marquez S,Chmara W,Zegeer A,Jensen RG, Bohnert HJ(1998)Sorbitol-6-phosphate dehydrogenase expression in transgenic tobacco.Plant Physiol117:831–839 Shi H,Ishitani M,Kim C,Zhu JK(2000)The Arabidopsis thaliana salt tolerance gene SOS1encodes a putative Na+/H+anti-porter.Proc Natl Acad Sci USA97:6896–6901
Shi H,Quintero FJ,Pardo JM,Zhu JK(2002)Role of SOS1as a plasma membrane Na+/H+antiporter that controls long dis-tance Na+transport in plant.Plant Cell14:465–477
Shi H,Lee BH,Wu SJ and Zhu JK(2003)Overexpression of a plasma membrane Na+/H+antiporter gene improves salt tol-erance in Arabidopsis thaliana.Nature Biotech21:81–85 Shinozaki K,Yamaguchi-Shinozaki K(1997)Gene expression and signal transduction in water-stress response.Plant Physiol 115:327–334
Shinozaki K,Yamaguchi-Shinozaki K(2000)Molecular responses to dehydration and low temperature:di?erences and cross-talk between two stress signalling pathways.Curr Opin Plant Biol 3:217–223
Sivamani E,Bahieldin A,Wraithc JM,Al-Niemia T,Dyera WE, Hod THD,Qu R(2000)Improved biomass productivity and water use e?ciency under water de?cit conditions in transgenic wheat constitutively expressing the barley HVA1gene.Plant Sci 155:1–9
Skriver K,Mundy J(1990)Gene expression in response to abscisic acid and osmotic stress.Plant Cell2:503–512
Smirno?N(1998)Plant resistance to environmental stress.Curr Opin Biotech9:214–219
Smy kal P,Mas?n J,Hrdy I,Konopa sek I,Z a rsky V(2000) Chaperone activity of tobacco HSP18,a small heat-shock protein,is inhibited by ATP.Plant J23:703–713
Steponkus PL,Uemura M,Joseph RA,Gilmour SJ,Thomashow MF(1998)Mode of action of the COR15a gene on the freezing tolerance of Arabidopsis thaliana.Proc Natl Acad Sci USA 95:14570–14575
Stockinger EJ,Gilmour SJ,Thomashow MF(1997)Arabidopsis thaliana CBF1encodes an AP2domain-containing transcrip-tional activator that binds to the C-repeat/DRE,a cis-acting DNA regulatory element that stimulates transcription in re-sponse to low temperature and water de?cit.Proc Natl Acad Sci USA94:1035–40
Studer S,Narberhaus F(2000)Chaperone activity and homo-and hetero-oligomer formation of bacterial small heat shock pro-teins.J Biol Chem275:37212–37218
Sugino M,Hibino T,Tanaka Y,Nii N,Takabe T(1999)Overex-pression of DnaK from a halotolerant cyanobacterium Apha-nothece halophytice acquires resistance to salt stress in transgenic tobacco plants.Plant Sci146:81–88
13
Sun W,Bernard C,van de Cotte B,Van Montagu M,Verbruggen, N(2001)At-HSP17.6A,encoding a small heat-shock protein in Arabidopsis,can enhance osmotolerance upon overexpression.
Plant J27:407–415
Suzuki TC,Denise C,Krawitz DC,Vierling E(1998)The chloro-plast small heat-shock protein oligomer is not phosphorylated and does not dissociate during heat stress in vivo.Plant Physiol 116:1151–1161
Sze H,Lia X,Palmgrenb MG(1999)Energization of plant cell membranes by H-pumping ATPases:regulation and biosyn-thesis.Plant Cell11:677–690
Tarczynski MC,Jensen RG,Bohnert HJ(1993)Stress protection of transgenic tobacco by production of the osmolyte mannitol.
Science259:508–510
Thomashow MF(1998)Role of cold-responsive genes in plant freezing tolerance.Plant Physiol118:1–7
Thomashow MF(1999)Plant cold acclimation:freezing tolerance genes and regulatory mechanisms.Annu Rev Plant Biol50:571–599
Thomashow MF,Gilmour SJ,Stockinger EJ,Jaglo-Ottosen KR, Zarka DG(2001)Role of the Arabidopsis CBF transcriptional activators in cold acclimation.Physiol Plant112:171–175
To ro k Z,Goloubino?P,Horva th I,Tsvetkova NM,Glatz A, Balogh G,Varvasovszki V,Los DA,Vierling E,Crowe JH, V?gh L(2001)Synechocystis HSP17is an amphitropic protein that stabilizes heat-stressed membranes and binds denatured proteins for subsequent chaperone-mediated refolding.Proc Natl Acad Sci USA98:3098–3103
Tyerman SD,Bohnert HJ,Maurel C,Steudle E,Smith JAC (1999)Plant aquaporins:their molecular biology,biophysics and signi?cance for plant water relations.J Exp Bot50:1055–1071
Uno Y,Furihata T,Abe H,Yoshida R,Shinozaki K,Yamaguchi-Shinozaki K(2000)Novel Arabidopsis bZIP transcription fac-tors involved in an abscisic-acid-dependent signal transduction pathway under drought and high salinity conditions.Proc Natl Acad Sci USA97:11632–11637
Van Camp W,Capiau K,Van Montagu M,Inze D,Slooten L.
(1996)Enhancement of oxidative stress tolerance in transgenic tobacco plants overproducing Fe-superoxide dismutase in chloroplasts.Plant Physiol112:1703–1714
Veinger L,Diamant S,Buchner J,Goloubino?P(1998)The small heat-shock protein IbpB from Escherichia coli stabilizes stress-denatured proteins for subsequent refolding by a multichaper-one network.J Biol Chem273:11032–11037
Vierling E(1991)The roles of heat-shock proteins in plants.Annu Rev Plant Biol42:579–620Vierling E,Kimpel JA(1992)Plant responses to environmental stress.Curr Opin Biotech3:164–170
Wang WX,Vinocur B,Shoseyov O,Altman A(2001a)Biotech-nology of plant osmotic stress tolerance:physiological and molecular considerations.Acta Hort560:285–292
Wang WX,Pelah D,Alergand T,Shoseyov O,Altman A (2001b)Denaturate stable and/or protease resistant,chaperone-like oligomeric proteins,polynucleotides encoding same and their uses.Provisional Patent Application No.60/272,771, USA
Wang WX,Pelah D,Alergand T,Shoseyov O,Altman A(2002) Characterization of SP1,a stress-responsive,boiling-soluble, homo-oligomeric protein from aspen(Populus tremula L.).
Plant Physiol130:865–875
Wang WX,Barak T,Vinocur B,Shoseyov O,Altman A(2003) Abiotic resistance and chaperones:possible physiological role of SP1,a stable and stabilizing protein from Populus.In:Vasil IK(ed)Plant biotechnology2000and beyond.Kluwer, Dordrecht,pp439–443
Waters ER,Lee GJ,Vierling E(1996)Evolution,structure and function of the small heat shock proteins in plants.J Exp Bot 47:325–338
Xu D,Duan X,Wang B,Hong B,Ho THD,Wu R(1996) Expression of a late embryogenesis abundant protein gene HVA1,from barley confers tolerance to water de?cit and salt stress in transgenic rice.Plant Physiol110:249–257 Yamanouchi U,Yano M,Lin H,Ashikari M,Yamada K(2002)A rice spotted leaf gene,Spl7,encodes a heat stress transcription factor protein.Proc Natl Acad Sci USA99:7530–7535
Zhang HX,Blumwald E(2001)Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit.Nature Biotech 19:765–768
Zhang HX,Hodson JN,Williams JP,Blumwald E(2001)Engi-neering salt-tolerant Brassica plants:characterization of yield and seed oil quality in transgenic plants with increased vacuolar sodium accumulation.Proc Natl Acad Sci USA98:12832–12836
Zhu JK(2001a)Plant salt tolerance.Trends Plant Sci6:66–71 Zhu JK(2001b)Cell signaling under salt,water and cold stresses.
Curr Opin Plant Biol4:401–406
Zhu JK(2002)Salt and drought stress signal transduction in plants.Annu Rev Plant Biol53:247–73
Zhu JK,Hasegawa PM,Bressan RA(1997)Molecular aspects of osmotic stress in plants.Crit Rev Plant Sci16:253–277 Zimmermann S,Sentenac H(1999)Plant ion channels:from molecular structures to physiological functions.Curr Opin Plant Biol2:477–482
14
交通事故三维模拟演示系统 产品简介 交通事故三维模拟演示系统集成了三维360度全景照相技术、三维虚拟现实动态仿真技术(增强现实技术)为一体,完全满足现在公安系统里现场全景照相、全景三维测量、三维重建、模拟、和分析的应用。是北京金视和科技有限公司集十几年来图形图像和三维仿真领域的尖端科研成果,并结合多年来对公安交通系统的调研数据进行定制化开发的解决方案。 交通事故三维模拟演示系统生成高度逼真的三维场景图片和动画片。把这些全景图片、三维场景、动画片和声音、文字结合,为侦查、技术、指挥人员生成各种三维虚拟案件现场场景的多媒体影音和影像材料。对这些数字化多媒体信息进行分析、演示,并可以在网络服务器上发布、保存、修改案例,其他用户可以通过网络服务器进行查询、观看案例。为案件的侦破、记录、汇报、存档查询,都提供了便利的直观方便。 交通事故三维模拟演示系统是由三维数字化图形软件和360°全自动机器 人拍摄系统组成。是基于图形图像和三维仿真领域的尖端科研成果,并结合多年来对交通事故处理部门的调研数据进行定制化开发的解决方案。 产品特点 交通事故三维模拟演示系统搭载的全景拍摄系统,由高端单反数码相机、精密鱼眼镜头和全自动拍摄云台组成,可以在一分钟内拍摄一组完整的现场全景图片,并以全自动方式进行拼接融合,无须人工干预拼接过程。达到交通事故现场快速全景重建的目的。 在传统的工作流程当中,由于人为、天气等外界因素干扰,事故现场很容易在短时间内遭到破坏和干扰。鉴于事故现场的特殊性,快速、完整、准确的保存事故现场,交通事故现场不可能像刑事案件现场那样长时间保留。交警在记录事故现场时,大多是通过昂贵的单反数码相机,直观的对事故现场进行大量的物证和场景拍摄,在后期分析事故现场时由于照片数量繁多,很难建立起事故现场的形象认知,甚至有可能会漏拍一些关键信息。借助360°全自动机器人拍摄系统将真实交通事故现场的完整的保存下来,可以在撤离交通事故现场后随时在交通事故现场全景图上进行截图、测量和分析。 现场采集物证图像及多场景热点添加在真实的交通事故现场中需要拍摄大量的现场细节图像(车辆痕迹、道路痕迹、现场环境、尸体、现场散落物和遗留物、血迹等)。但大量的现场图片容易让技术人员混淆物证,这对于日后的案情分析来说有重大影响。交通事故三维模拟演示系统的热点添加功能可以将所有采集到的现场细节图像以超级链接方式添加到现场全景图和三维重建现场中,并且可以用鼠标双击放大凸显细节图像,还可以创建文件夹对物证图像进行组织分类、可重命名以及删改细目照片资源,为事故过程分析带来极大的帮助。
模拟演练与3D培训系统方案 煤科集团沈阳研究院有限公司 2014/2/28
一、模拟演练技术资料 DMX-135A仿真模拟与演练评价系统说明 1.系统型号 DMX-135 A 通道长度 多功能模拟训练系 统 2.系统简介 “3D-VR煤矿事故仿真和安全培训 演练系统”是我院与澳大利亚新南威尔 士大学合作开发的一套应用于煤矿安全培训的大型沉浸式仿真演练系统。我院通过借助UNSW(新南威尔士大学)的世界领先技术及软硬件平台,开发出符合我国国情的煤矿安全培训模块。本系统可由危险识别、灾害模拟、自救逃生、救护救援等多个安全培训模块组成,本系统模块是按照国家煤矿安全规程和新编煤矿安全技术培训教材编制
而成。采用虚拟现实(VR)技术、360度环屏投影播放技术、12.1环绕立体声技术、3D电影技术、计算机网络协同处理技术为煤矿工人安全和技能培训提供一流的“沉浸式”培训环境,将安全培训装备和质量提升到一个崭新的水平。 通过使用本系统,创建一个逼真的虚拟现实世界,使学员在体验各种真实场景同时,学习各模块相关操作知识,认识灾害的发生、发展过程及危害,并通过问答式的交互学习,快速掌握每个模块的操作步骤及要点。从而提高矿工的整体技术水平、思想素质、及各种突发状况的应对能力,从而使煤矿整体操作能力及防范意识得到提高,真正进入规范化管理阶段,降低各种事故发生的概率。 DMX-135A多功能模拟训练系统通道总长135米,分三层,旨在通
过仿真救灾现场的严峻条件,人为设置测试科目,使训练人员背负呼吸器,在黑暗、噪音、浓烟、高温的模拟条件下,按照预先设定的工作程序,完成正确穿越各种障碍,并按规定动作完成抢险、伤员营救等工作。它可以测量出训练人员最大身体承受能力和心理承受能力,评定受训人员能否正确使用呼吸器及抢险救援任务等的完成情况。 最 额定电压:380V,允许偏差±10%; 谐波:不大于5%; 频率:50Hz,允许偏差±5%; 3.3 系统组成 系统由以下部分组成:
摘要 随着我国汽车保有量的大幅度上升,高新技术产品和装置在汽车上的不断引入和普及与汽车维修业高素质从业人员不足之间的矛盾显得日益突出。发动机电控技术是汽车专业的一门核心技术,理论性与实践性很强。开发发动机电控系统模拟教学实验台,能便于学生认识和学习发动机电控系统的工作原理。发动机电控系统模拟实验台可以广泛应用于科研、教学等方面,通过对发动机工作过程的模拟、显示,可以提高汽车从业人员的知识掌握水平及实际操作能力,增强对汽车电控系统的认识和掌握,为学生学习和掌握发动机电控系统的工作原理提供帮助。 本设计是以教学展示为前提,主要介绍了发动机电控系统的控制内容、发展简史与前景、组成与工作原理,并在此基础上设计电控系统模拟实验台的相关内容。主要包括实验台架的设计、电控系统的零部件布置、电动机转速控制等内容。这个教学实验台能够演示发动机的起动和正常工作过程,并且设置了测量点。通过完成对实验台的设计,可以深刻理解和掌握发动机电控系统的组成和工作原理、机械设计与机械制图的相关知识,最终设计出一款及机械、电控于一体化的产品。 关键词:发动机电控系统;电动机控制;实验台;单片机;PWM
ABSTRACT With significant increase of the number of automobile,the contradiction between high-tech products and devices, the constant introduction in the car and popularization of high-quality vehicle maintenance trade and lack of practitioners become increasingly prominent. Engine electronic control technology is the theoretical and practical of a core technology and theoretical and practical very strong. Electronic control system simulation teaching test can make students to understand and learn the engine electronic control system works easy. Engine electronic control simulation system can be widely used in scientific research, teaching, etc. By displaying of the course of the engine simulation process, it can be employed to improve motor vehicle mastery level of knowledge and practical ability, and enhance the automotive electronic control system of understanding and mastering, for the students to learn and master the engine control system works to help. The design is based on the premise of teaching show, this paper introduces control content system of the engine electronic control, brief history of and prospects for development, composition and working principle and design on the basis of electronic control system simulation test-bed of related content. It mainly includes the design of bench testing, electrical control system layout of components, such as motor speed control. The teaching experiment platform to demonstrate the engine start and normal work processes, and set the measurement points. The design of the test-bed by accomplish can help learns to understand and master the engine electronic control system components, a final design and mechanical and electrical control products in the integration. Key words: Engine Electrical Control System;Motor Control;Test-bed;microcontroller;PWM
第1章模拟演练系统 模拟训练系统软件应用上与其它终端台分离,形成一套能够独立完成模拟接处警过程和调度过程的软件,不影响系统正常的接处警工作。系统能够为操作人员、指挥员分别提供模拟演练功能以及在线考试功能,并能对训练和考试情况进行评估。模拟训练软件分为五大功能:典型案例查询、指挥员调度指挥训练、在线考试和训练质量评估。 典型案例查询 典型案例查询模块实现对典型案例进行查询,对查询结果进行浏览。根据查询信息可以演示的发生、发展及处臵的全过程。 指挥员训练试题维护 此功能用于添加、维护和删除指挥员训练的试题。其中又包括训练因素和训练试题维护。训练试题是由一个一个的训练因素组成,用户可以根据自己的需求来不断丰富训练因素库,从而达到生成情况复杂多样的指挥员训练试题的效果。 指挥员模拟训练 指挥员模拟训练模块则是训练、考核指挥员员的业务能力和调度水平。指挥员要为训练试题中的各种复杂因素制定出合理的调度方案,并给出详细的总体调度方案。 训练试题评审 接警员和指挥员的训练试题生成后还不能马上用来训练,必须经过有权限的领到审批发布后才会出现在可训练的试题库中。审批和发布是分开的,只有通过了审批的训练试题才可以发布。 训练评估 训练质量评估系统则是根据训练过程中系统记录下来的各项考核指标结果对训练质量人为的给出一个合理的评估。评估过程中系统将给出事先录入的标准答案。 虚拟现实(Virtual Reality)模拟训练 通过在训练中设定各种实战中的实际环境及情境,达到对实际情况的仿真,达到实战训练的效果。
在线考试 在线考试是基于WEB的一个功能模块,用户可以在线维护考试科目,考试题目,生成考卷,考试,查询成绩等。
智能用电综合模拟演示平台的建设 邵瑾,李铮,张晶 (中国电力科学研究院,北京市海淀区 100192) CONSTRUCTION OF COMPREHENSIVE SIMULATION AND DEMO PLATFORM FOR SMART ELECTRIC CONSUMPTION SHAO Jin, LI Zheng, ZHANG Jing (China Electric Power Research Institute, Haidian District, Beijing 100192, China) ABSTRACT: This paper analyzes need and practical significance of comprehensive simulation and demo platform of smart electric consumption, present with construction principle, system structure, function module, networking of communication, and application. The focus of discussion is put on design scheme for functional modules, such as load management, monitoring of distribution transformer, automatic meter reading and monitoring of unusual electricity consumption. Finally, summary and expectation is forwarded to application of the demo platform. KEY WORDS: smart grid, smart electric consumption, comprehensive simulation, demo platform 摘要:本文分析了智能用电综合模拟演示平台的需求和现实意义,介绍了演示平台的建设原则、系统结构、功能模块、通信组网、应用分析等内容。重点介绍了负荷管理、配变监测、集中抄表及异常用电监测等功能模块的设计方案,最后对演示平台的应用进行了总结和展望。 关键词:智能电网,智能用电,综合模拟,演示平台 1引言 利用现代通信技术、信息技术、营销技术,建设智能用电服务体系是智能电网的重要内容之一。智能用电,依托坚强电网和现代管理理念,利用高级量测、高效控制、高速通信、快速储能等技术,实现市场响应迅速、计量公正准确、数据实时采集、收费方式多样、服务高效便捷,构建智能电网与电力用户电力流、信息流、业务流实时互动的新型供用电关系。将供电端到用户端的所有设备,透过传感器连接,形成紧密完整的用电网络,并对信息加以整合分析,实现电力资源的最佳配置,达到降低用户用电成本、提升可靠性、提高用电效率的目的。 智能用电综合模拟演示平台(以下称演示平台),是智能用电各种专业系统和技术的集成实验环境。演示平台既是一个一体化的数据采集和监控系统,也是一个硬件、软件、业务、技术高度融合的模拟实验平台。演示平台是以软件为中枢指挥、设备为支撑骨架、信道为信息通道、功能为业务流程、展示为技术目标的综合模拟系统,该平台的应用对于引领智能电网科技创新,展示和验证智能用电和智能通信科技成果、宣传普及节能减排和清洁能源利用都具有重要意义。 2演示平台建设原则 演示平台建设原则是分阶段原则、分模块原则与可扩展原则。 分阶段原则:建设初期是已现有用电信息采集系统为基础,包括负荷管理、配变监测、集中抄表和异常用电监测等内容。建设后期演示平台将不断引入智能用电最新技术的研究和应用成果,对初期平台进行改进和完善。 分模块原则:智能用电涉及的内容很多,这就需要选择主要和典型的功能和业务,通过建立功能模块的方式对各项功能和业务进行综合展示。 可扩展原则:考虑到未来智能电网新标准、新规范和新技术的应用,对系统的软硬件设备都会造成一定的影响,因此在整个系统和设备的冗余度、开放性和接口设计上预留有足够的扩展空间,为未来的系统及设备升级和扩展做好技术准备。 3演示平台系统结构
系统演示方案 编写本方案目的在于,演示时有较好的步骤性和目的性,充分演示系统的各种功能及性能。 1、系统初始状态 系统的初始状态如下,各PLC均为停机状态,通信控制服务器设备状态表显示均为黄点显示。 2、系统的启动 1、首先启动各上位机服务器,然后通过查询分析器模拟工程师服务器启动MPLC,命令如下: SP_NewCommand 'Engineer_Server','Comunication_Server','SB08','Z000','KJ' 执行此命令后,通信控制服务器中设备状态表炉门PLC应为运行状态,绿点显示。指令表中正确显示各指令。 2、然后依次开启HPLC1~HPLC4 SP_NewCommand 'Engineer_Server','Comunication_Server','SB02','Z000','KJ' SP_NewCommand 'Engineer_Server','Comunication_Server','SB03','Z000','KJ' SP_NewCommand 'Engineer_Server','Comunication_Server','SB04','Z000','KJ' SP_NewCommand 'Engineer_Server','Comunication_Server','SB05','Z000','KJ' 通信控制服务器中各PLC应显示正确状态。 3、通过PLC模拟程序,模拟CPLC1和ZPLC1上传请求开机指令。 @XT03Z100SB06QQKJ 1196# @XT04Z100SB07QQKJ 1198# 通信控制服务器应做出正确应答,并开启相应PLC,设备列表显示