
Available online at https://www.doczj.com/doc/598168913.html, International Journal of Hydrogen Energy29(2004)173–
185
https://www.doczj.com/doc/598168913.html,/locate/ijhydene
Biohydrogen production:pros pectsand limitationsto practical
application
David B.Levin a;b;?,Lawrence Pitt b,Murray Love b
a Department of Biology,University of Victoria,Victoria,BC,Canada V8W3P6
b Institute for Integrated Energy Systems,University of Victoria,Victoria,BC,Canada V8W3P6
Accepted24March2003
Abstract
Hydrogen may be produced by a number of processes,including electrolysis of water,thermocatalytic reformation of hydrogen-rich organic compounds,and biological processes.Currently,hydrogen is produced,almost exclusively,by elec-trolysis of water or by steam reformation of methane.Biological production of hydrogen(Biohydrogen)technologies provide a wide range of approaches to generate hydrogen,including direct biophotolysis,indirect biophotolysis,photo-fermentations, and dark-fermentation.The practical application of these technologies to every day energy problems,however,is unclear.In thispaper,hydrogen production ratesof variousbiohydrogen s ys temsare compared by?rs t s tandardizing the unitsof hydro-gen production and then by calculating the size of biohydrogen systems that would be required to power proton exchange membrane(PEM)fuel cellsof variouss izes.
?2003International Association for Hydrogen Energy.Published by Elsevier Ltd.All rights reserved.
Keywords:Biological hydrogen production;Dark-fermentation;Fuel cells
1.Introduction
Energy isvital to global pros perity,yet dependence on fossil fuels as our primary energy source contributes to global climate change,environmental degradation,and health problems[1].Hydrogen(H2)o erstremendouspo-tential asa clean,renewable energy currency.Hydrogen has the highest gravimetric energy density of any known fuel and iscompatible with electrochemical and combus-tion processes for energy conversion without producing carbon-based emissions that contribute to environmental pollution and climate change.Hydrogen fuel cellsand related hydrogen technologies provide the essential link between renewable energy sources and sustainable energy services.The transition from a fossil fuel-based economy to a hydrogen energy-based economy,however,is fraught
?Corresponding author.Tel.:+1-250-472-4069;fax:+1-250-472-4075.
E-mail address:dlevin@uvic.ca(D.B.Levin).with many technical challenges,from the production of suf-?cient quantities of hydrogen to its storage,transmission, and distribution[2].
Hydrogen may be produced by a number of processes, including electrolysis of water,thermocatalytic reformation of hydrogen-rich organic compounds,and biological pro-cesses.Currently,hydrogen is produced,almost exclusively, by electrolysis of water or by steam reformation of methane. Biological production of hydrogen(biohydrogen),using (micro)organisms,is an exciting new area of technology development that o ersthe potential production of us able hydrogen from a variety of renewable resources.Biological systems provide a wide range of approaches to generate hydrogen,and include direct biophotolysis,indirect biopho-tolysis,photo-fermentations,and dark-fermentation[3–5]. While biohydrogen systems can produce H2,no commer-cial systems are yet available,and questions concerning the practical application of biohydrogen loom large.Can bio-hydrogen systems be developed as practical and commer-cial applications?For example,can biohydrogen systems be
0360-3199/03/$30.00?2003International Association for Hydrogen Energy.Published by Elsevier Ltd.All rights reserved. doi:10.1016/S0360-3199(03)00094-6
174 D.B.Levin et al./International Journal of Hydrogen Energy29(2004)173–185
integrated with hydrogen fuel cell technologiesto generate electricity at a practical scale?
One of the major limitationsto the practical application of biohydrogen systems is that scientists who study biohydro-gen systems do not talk to engineers who develop hydrogen fuel cell technologies(and vice versa).Thus,the rates of hy-drogen produced by biological systems are unknown to fuel cell engineersand the amountsof H2required for practical applications,such as fuel cells,are unknown to biohydrogen researchers.Moreover,the rates of hydrogen produced by the various biohydrogen systems are expressed in di erent units,making it di cult to assess and compare the rates and amounts of hydrogen synthesized by di erent biohydrogen technologies.
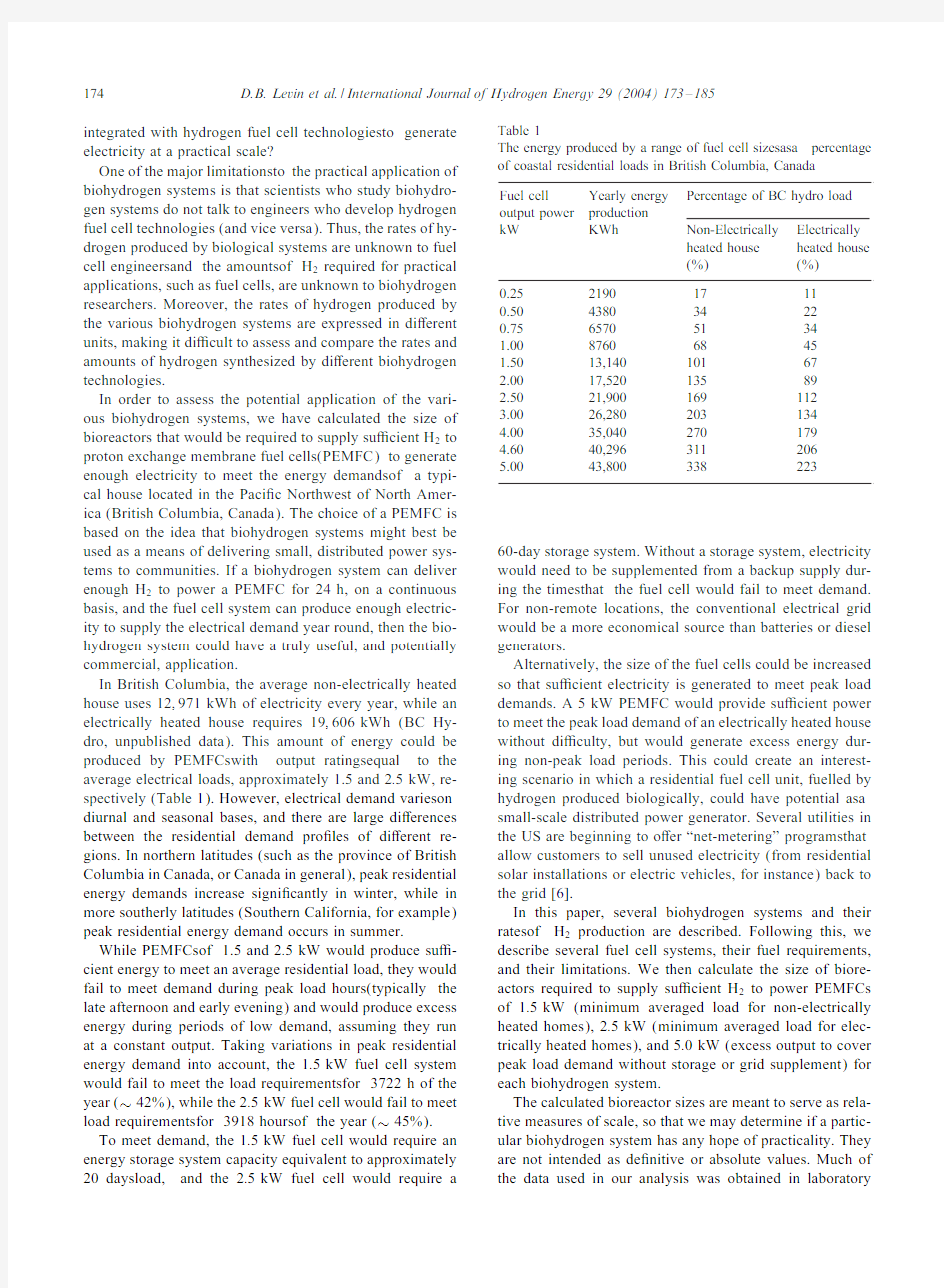
In order to assess the potential application of the vari-ous biohydrogen systems,we have calculated the size of bioreactors that would be required to supply su cient H2to proton exchange membrane fuel cells(PEMFC)to generate enough electricity to meet the energy demandsof a typi-cal house located in the Paci?c Northwest of North Amer-ica(British Columbia,Canada).The choice of a PEMFC is based on the idea that biohydrogen systems might best be used as a means of delivering small,distributed power sys-tems to communities.If a biohydrogen system can deliver enough H2to power a PEMFC for24h,on a continuous basis,and the fuel cell system can produce enough electric-ity to supply the electrical demand year round,then the bio-hydrogen system could have a truly useful,and potentially commercial,application.
In British Columbia,the average non-electrically heated house uses12;971kWh of electricity every year,while an electrically heated house requires19;606kWh(BC Hy-dro,unpublished data).This amount of energy could be produced by PEMFCswith output ratingsequal to the average electrical loads,approximately1.5and2:5kW,re-spectively(Table1).However,electrical demand varieson diurnal and seasonal bases,and there are large di erences between the residential demand pro?les of di erent re-gions.In northern latitudes(such as the province of British Columbia in Canada,or Canada in general),peak residential energy demands increase signi?cantly in winter,while in more southerly latitudes(Southern California,for example) peak residential energy demand occurs in summer. While PEMFCsof1.5and2:5kW would produce su -cient energy to meet an average residential load,they would fail to meet demand during peak load hours(typically the late afternoon and early evening)and would produce excess energy during periods of low demand,assuming they run at a constant output.Taking variations in peak residential energy demand into account,the1:5kW fuel cell system would fail to meet the load requirementsfor3722h of the year(~42%),while the2:5kW fuel cell would fail to meet load requirementsfor3918hoursof the year(~45%). To meet demand,the1:5kW fuel cell would require an energy storage system capacity equivalent to approximately 20daysload,and the2:5kW fuel cell would require a Table1
The energy produced by a range of fuel cell s izesasa percentage of coastal residential loads in British Columbia,Canada
Fuel cell Yearly energy Percentage of BC hydro load output power production
kW KWh Non-Electrically Electrically
heated house heated house
(%)(%)
0.2521901711
0.5043803422
0.7565705134
1.0087606845
1.5013,14010167
2.0017,52013589
2.5021,900169112
3.0026,280203134
4.0035,040270179
4.6040,296311206
5.0043,800338223
60-day storage system.Without a storage system,electricity would need to be supplemented from a backup supply dur-ing the timesthat the fuel cell would fail to meet demand. For non-remote locations,the conventional electrical grid would be a more economical source than batteries or diesel generators.
Alternatively,the size of the fuel cells could be increased so that su cient electricity is generated to meet peak load demands.A5kW PEMFC would provide su cient power to meet the peak load demand of an electrically heated house without di culty,but would generate excess energy dur-ing non-peak load periods.This could create an interest-ing scenario in which a residential fuel cell unit,fuelled by hydrogen produced biologically,could have potential asa small-scale distributed power generator.Several utilities in the US are beginning to o er“net-metering”programsthat allow customers to sell unused electricity(from residential solar installations or electric vehicles,for instance)back to the grid[6].
In this paper,several biohydrogen systems and their ratesof H2production are described.Following this,we describe several fuel cell systems,their fuel requirements, and their limitations.We then calculate the size of biore-actors required to supply su cient H2to power PEMFCs of1:5kW(minimum averaged load for non-electrically heated homes),2:5kW(minimum averaged load for elec-trically heated homes),and5:0kW(excess output to cover peak load demand without storage or grid supplement)for each biohydrogen system.
The calculated bioreactor sizes are meant to serve as rela-tive measures of scale,so that we may determine if a partic-ular biohydrogen system has any hope of practicality.They are not intended as de?nitive or absolute values.Much of the data used in our analysis was obtained in laboratory
D.B.Levin et al./International Journal of Hydrogen Energy 29(2004)173–185175
bench-scale experiments with pure,de?ned substrates,and in some cases with pure cultures of known bacteria.We ac-knowledge that scaling up biohydrogen systems to 1000l or more will be a great challenge with respect to substrate com-position and supply,culture impurities,and removal of gas productsfrom the aqueousphas e,aswell asH 2separation,puri?cation,and storage.The intent of this paper is,there-fore,to provide a benchmark by which we may determine which biohydrogen system(s)o er the greatest potential for practical applications.2.Methods
Descriptions of the biohydrogen systems and their rates of H 2production were gathered from the literature and from direct,personal communications with scientists.One major
obstacle
to comparison of di erent biohydrogen systems is that di erent unitsof H 2synthesis rate are used for di er-ent biohydrogen technologies.Rates of H 2production are reported variously as ml of H 2=ml of culture/h,l of H 2=l of culture/h, mol of H 2=l of culture/h,nmol of H 2= g of pro-tein in the culture/h,or mol of H 2=mg of chlorophyll (chl)a=h.In the following analysis,rates of H 2production by the various biohydrogen systems were converted to a standard format ;mmolesof H 2=l of culture/h,and is expressed as mmol H 2=(l ×h).
For systems that reported the rate of H 2production as ml of H 2=ml =h or l of H 2=l culture/h,the rateswere con-verted to the standard format by dividing the number of l (or the fraction of a l in the ca e of ml of H 2=ml of cul-ture/h)by 24.8to derive the number of moles(1mole H 2occupies24:8l at 303K(=30?C),a common temperature at which mesophilic bacteria are cultured)and standard pres-sure (1atm =760mm =Hg =101:3kPa).For systems that reported the rate of H 2production asnmolesof H 2= g of protein in the culture/h,the rateswere converted to the s tan-dard format by multiplying the concentration of protein in the culture (usually given as mg protein/ml of culture)by the nmolesof H 2=mg of protein in the culture times1000,giving the molesof H 2=l of culture.For systems that re-ported the rate of H 2production as mol of H 2=h =mg of chl a ,the rates were converted to the standard format using the number of mg of chl a=l of culture.Because the protein and chlorophyll content can vary for each type of culture,con-centrations used in each calculation were based on values reported by authors.The rate of H 2cons umption by PEMFCswascalculated as described below based on well established equations [7,8]The anodic,cathodic,and overall reactionsin a PEMFC are H 2(g)→2H +(aq)+2e ?;
(1)1
2O 2
(g)+2H +(aq)+2e ?→H 2O(l);
(2)H 2(2)+12O 2
(g)→H 2O(l):(3)
The anode reaction (1)generatestwo electronsfor every
molecule of H 2reacted.The charge transfer for the reaction isgiven by
charge =zF ×amount of reactant ;
(4)
where z isthe number of electronstrans ferred per molecule of fuel (z =2for H 2)and F isthe Faraday cons tant,96;485Coulombs(C).The maximum thermodynamic e ciency (ámax )of a fuel cell isde?ned asthe ratio of the electrical energy produced in the cell ( g f =change in Gibbsfree energy)to the energy that would be produced by
burning the fuel ( h
f =change in enthalpy of formation):ámax =
g f
h
f :(5)
The maximum cell voltage (E max )isthe voltage that would result if all the fuel’s energy were transformed into electrical energy:
E max =? h
f zF
:(6)
For H 2fuel, h
f is ?285:85kJ =mol (HHV)and z =2.E max is,therefore,1:48V.The actual cell e ciency can be related to the cell voltage V c asfollows :
cell e ciency =
V c
E max
:(7)Since part of the fuel entering the fuel cell always passes through unused,a fuel utilization coe cient ( f )isde?ned as
f =mass of fuel reacted in cell mass of fuel input to cell :
Hydrogen fuel cellsoften have a fuel utilization coe cient ( f )approaching 0.95[7].The overall cell e ciency (á)is therefore
á= f V c
E max
:(8)
The cell voltage ,if not known,can be calculated by rear-ranging Eq.(8):
V c =áE max
f :(9)
To calculate the rate of H 2consumption (rate of fuel use)of a PEMFC,the charge isdivided by time yielding the current (I ),in amperes.The rate of fuel use can be calculated for a single cell,where z =2:
fuel usage =I
2F
(mole =s):
For a stack of n cells:
fuel usage =In
2F
(mole =s):(10)
The output power (P e )of the stack with cell voltage V c (calculated using Eq.(9),above)isgiven by P e =V c In;therefore,
I =P e
V c n
:
176 D.B.Levin et al./International Journal of Hydrogen Energy29(2004)173–185 Table2
Flow ratesof H2required to power PEM fuel cells
Size of PEMFC H2 ow rate required
(kW)(g/h)(mol/h)(SL/h)
1.04923.9577.7
1.57335.9866.6
2.512159.91444.3
5.0243119.72888.5
50%e ciency,95%H2utilization rate,average cell voltage
0:779V.
Substituting this into Eq.(10)givesthe fuel usage asa
function of output power and cell voltage:
fuel usage=P e
2V c F
(mole=s):(11)
To express fuel usage as a mass ow rate(kg=s),it is nec-
essary to multiply Eq.(11)by the molar mass of the fuel
(2:02×10?3kg=mol for H2).Similarly,to express fuel us-
age asa volumetric ow rate(SL/s),the density of the fuel
isrequired(8:4×10?5kg=SL at NTP for H2).Substituting
the above?guresinto Eq.(11)yieldsthe following relations:
H2usage=2:02×10?3kg=mol P e
2V c F
=1:05×10?8×P e
V c
(kg=s)
=1:25×10?4×P e
V c
(SL=s):(12) For hydrogen fuel cells,the storage volume(V s)required at a temperature T s of25?C(298K)and a given pressure P s is estimated by assuming hydrogen to be an ideal gas, following the relation:P s V s=nRT s. Thiswasrearranged to s olve for volume and convert to standard units(litres):
V s=1000nRT s
P s
;(13) where P s is storage pressure,in kPa,n isthe number of molesof H2required,and R isthe univers al gascons tant 8:314×10?3kJ mol=K.The ow ratesof H2required to power PEMFCsof variouss izeswere calculated us ing the above equationsand are pres ented in Table2.
3.Biohydrogen systems
3.1.Direct biophotolysis
Photosynthetic production of hydrogen from water is a bi-ological process that can convert sunlight into useful,stored chemical energy by the following general reaction:
2H2O Light energy
?????→2H2+O2:(14)
Green algae,under anaerobic conditions,can either use H2 asan electron donor in the CO2-?xation process or evolve H2.Hydrogen production by green microalgae requiress ev-eral minutesto a few hoursof anaerobic incubation in the dark to induce the synthesis and/or activation of enzymes involved in H2metabolism,including a reversible hydroge-nase enzyme.The hydrogenase combines protons(H+)in the medium with electrons(donated by reduced ferredoxin) to form and release H2.Thus,green microalgae possess the genetic,enzymatic,metabolic,and electron-transport ma-chinery to photoproduce H2gas.The synthesis of H2per-mits sustained electron ow through the electron-transport chain,which supports synthesis of ATP[9].
The process of algal photosynthesis oxidizes H2O and evolvesO2.Light energy absorbed by photosystem II(PSII) generateselectronswhich are trans ferred to ferredoxin, using light energy absorbed by photosystem I(PSI).A reversible hydrogenase accepts electrons directly from the reduced ferredoxin to generate H2[10].Because the hy-drogenase enzyme responsible for evolution of molecular H2is highly sensitive to O2,photosynthetic production of H2and O2must be temporally and/or spatially separated. In a two-phase process,CO2is?rs t?xed into H2-rich sub-strates during normal photosynthesis(Phase1),followed by light-mediated generation of molecular H2when the mi-croalgae are incubated under anaerobic conditions(Phas e 2).Phase2of the two-stage process can be achieved by incubating the microalgae in medium that doesnot contain sulfur-containing nutrients[9].
When culture of the green alga Chlamydomonas rein-hardtii are deprived of inorganic S,the ratesof O2synthesis and CO2?xation decline signi?cantly within24h(in the light).The reas on for thislos sof activity isdue to the need for frequent replacement of the H2O-oxidizing protein D1in the PSII reaction centre[11].Depletion of sulfur blocks the synthesis of the D1polypeptide chain(32kDa),which con-tains many sulfur containing amino acids,such as cysteine and methionine.While photosynthetic capability declines greatly,respiration continues,and after approximately22h of sulfur deprivation,C.reinhardtii culturesmaintained in the light become anaerobic,and begin to synthesize H2[9]. Based on this phenomenon,systems for sustained H2 gasproduction in C.reinhardtii have been developed [12,13].The rate of H2production by C.reinhardtii re-ported by Kosourov et al.[12]was7:95mmol H2=l of culture after100h,which corresponds to approximately 0:07mmole H2=(l×h).Meliset al.[13]reported similar values.
3.2.Indirect biophotolysis
Cyanobacteria can also synthesize and evolve H2through photosynthesis via the following processes:
12H2O+6CO2Light energy
?????→C6H12O6+6O2;(15) C6H12O6+12H2O Light energy
?????→12H2+6CO2:(16)
D.B.Levin et al./International Journal of Hydrogen Energy 29(2004)173–185177
Cyanobacteria (also know as blue-green algae,cyano-phyceae,or cyanophytes)are a large and diverse group of photoautotrophic microorganisms,which evolved and di-versi?ed early in Earth’s history [14].Cyanobacteria con-tain photosynthetic pigments,such as chl a ,carotenoids,and phycobiliproteinsand can perform oxygenic photos ynthe-sis.They are a morphologically diverse group that includes unicellular,?lamentous,and colonial species.Within the ?l-amentouscyanobacteria,vegetative cellsmay develop into structurally modi?ed and functionally specialized cells,such as the akinetes (resting cells)or heterocysts (specialized cellsthat perform nitrogen-?xation ;
[15]).The nutritional requirementsof cyanobacteria are s imple:air (N 2and O 2),water,mineral salts,and light [16].
Species of cyanobacteria may possess several enzymes directly involved in hydrogen metabolism and synthesis of molecular H 2.These include nitrogenases which catalyze the production of H 2asa by-product of nitrogen reduction to ammonia,uptake hydrogenases which catalyze the oxida-tion of H 2synthesized by the nitrogenase,and bi-directional hydrogenases which have the ability to both oxidize and synthesize H 2(reviewed by Tamagrini et al.[15]).
Hydrogen production by cyanobacteria hasbeen s tud-ied for over three decadesand hasrevealed that e cient photoconversion of H 2O to H 2isin uenced by many fac-tors[17].Hydrogen production has been assessed in a very wide variety of species and strains,within at least 14genera,under a vast range of culture conditions [17].The ratesof H 2evolution are aswide-ranging asthe s pecies and conditions used in these studies.Rates of H 2pro-duction by non-nitrogen-?xing cyanobacteria range from 0:02 mol H 2=mg chl a=h (Synechococcus PCC 6307)to 0:40 mol H 2=mg chl a=h (Aphanocapsa montana )[18].These rates are very low compared with those of hetero-cystous cyanobacteria,which range from 0:17 mol H 2=mg chl a=h (Nostoc linckia IAM M-14)to 4:2 mol H 2=mg chl a=h (Anabaena variablilis IAM M-58)[19].
Because of the higher rates of H 2production by Anabaena species and strains,these have been subject to intense study for the past several years.Mutant strains of A.variabilis have demonstrated signi?cantly higher rates of H 2produc-tion compared with wild-type strains.A.variabilis PK84,for example,produced H 2at a rate of 6:91nmol = g of pro-tein/h (in 350ml cultures).When A.variablis PK84was cultured under conditionsof nitrogen s tarvation,the rate of H 2synthesis was 12:6mol = g of protein/h (in 350ml cultures).The concentration of total protein in the culture was28:2 g =ml of culture [20].Assuming no change as the culture volume iss caled up,a 1l culture would contain 28;200 g protein and would produce 355;320nmol H 2=h or approximately 0:355mmol H 2=(l ×h).3.3.Photo-fermentation
Purple non-sulfur bacteria evolve molecular H 2catalyzed by nitrogenase under nitrogen-de?cient conditions using
light energy and reduced compounds(organic acids ).C 6H 12O 6+12H 2O Light energy
?????→12H 2+6CO 2:
(17)
These photoheterotrophic bacteria have been investigated for their potential to convert light energy into H 2using waste organic compoundsass ubs trate [21–25]in batch processes [26],continuouscultures[27,28],or culturesof bacteria im-mobilized in carrageenan [29],in agar gel [30],on porous glass [24],on activated glass [31],or on polyurethane foam [23].
In general,ratesof hydrogen production by photo-heterotrophic bacteria are higher when the cellsare im-mobilized in or on a solid matrix,than when the cell are free-living.Continuousculturesof Rhodopseudomonas capsulata and Rhodobacter spheroides were reported to produce H 2at ratesthat range from 40to 50ml H 2=l of culture/h [21,32,33],80ml to 100ml H 2=l of culture/h [25].Continuousculturesof Rhodospirillum rubrum were reported to produce H 2at a rate of 180ml H 2=l of culture/h [28].Culturesof Rb.spheroides immobilized on porous glass,on the other hand,were reported to produce H 2at a rate of 1:3ml H 2=ml =h (1:3l H 2=l of immobilized cul-ture/h)[24].Ratesof H 2production by Rb.spheroides GL1immobilized on activated glass were 3.6–4:0ml H 2=ml =h [31,34].If the system of culturing Rb.spheroides on porous glass could be scaled-up without compromising the rate of H 2synthesis,this would result in rates of 3.6–4:0l H 2=l of immobilized culture/h,which would correspond to 0:145mmol H 2=(l ×h)to 0:161mmol H 2=(l ×h).3.4.Hydrogen synthesis via the water–gas shift reaction of photoheterotrophic bacteria
Certain photoheterotrophic bacteria within the superfam-ily Rhodospirillaceae can grow in the dark using CO as the sole carbon source to generate ATP with the concomitant release of H 2and CO 2[35–37].The oxidation of CO to CO 2with the release of H 2occursvia a water–gass hift reaction:CO(g)+H 2O(l)→CO 2(g)+H 2(g); G ?
=?20(kJ =mol):
(18)
In these organisms,however,the reaction is mediated by proteinscoordinated in an enzymatic pathway.The reaction takes place at low (ambient)temperature and pressure.Thermodynamicsof the reaction are very favorable to CO-oxidation and H 2synthesis since the equilibrium is strongly to the right of this reaction.
Stoichiometric amountsof CO 2and H 2are produced dur-ing CO-oxidation [37].The enzyme that bindsand oxidizes CO,carbon monoxide:acceptor oxidoreductase (carbon monoxide dehydrogenase =CODH)is part of a membrane bound enzyme complex [37,38].Rubrivivax gelatinosus CBS isa purple non-s ulfur bacterium that not only performs
178 D.B.Levin et al./International Journal of Hydrogen Energy29(2004)173–185
the CO–water–gas shift reaction in darkness,convert-ing100%CO in the atmosphere into near stoichiometric amountsof H2,it also assimilates CO into new cell mass in the light(via CO2?xation)when CO is the sole source of carbon[39,40].Even when an organic substrate is available with CO,R.gelatinosus CBS will utilize both substrates simultaneously,indicating that the CO-oxidation pathway is fully functional even when a more favorable substrate is included[41].
R.gelatinosus CBS exhibitsa doubling time of7h in light when CO s ervesasthe only carbon s ource[42,41]. The mass transfer of CO may be enhanced by a high ratio of gas phase to liquid bacterial suspension,and by stirring the culture vigorously.The hydrogenase from this organism istolerant to O2,exhibiting a half-life of21h when whole cellswere s tirred in full air[43].
A speci?c rate of CO oxidation to H2production of 0:8mmol=min=g of cells,dry weight(cdw)[41]wasmea-sured using a low-density culture(?nal OD660?0:2), stirred at a high rate(250rpm),and supplemented with 20%CO in the gas phase.Because the conversion of CO to H2iss toichiometric,thiscorres pondsto a rate of CO uptake and conversion of approximately1:34g CO=h=g cdw,or48mmol CO=h=g dcw.An OD660of2.0yields 2:0g R.gelatinosus CBS cdw/l.Thiscorres pondsto a H2production rate of96mmol H2=2g cdw/h or 96mmol H2=(l×h).
3.5.Dark-fermentation
Hydrogen can be produced by anaerobic bacteria, grown in the dark on carbohydrate-rich substrates.Fer-mentation reactionscan be operated at mes ophilic(25–40?C),thermophilic(40–65?C),extreme thermophilic (65–80?C),or hyperthermophilic(?80?C)temperatures. While direct and indirect photolysis systems produce pure H2,dark-fermentation processes produce a mixed biogas containing primarily H2and carbon dioxide(CO2),but which may also contain lesser amounts of methane(CH4), CO,and/or hydrogen sul?de(H2S).The gascompos ition presents technical challenges with respect to using the biogasin fuel cells(s ee below).
Bacteria known to produce hydrogen include species of Enterobacter,Bacillus,and Clostridium.Carbohydratesare the preferred substrate for hydrogen-producing fermenta-tions.Glucose,isomers of hexoses,or polymers in the form of starch or cellulose,yield di erent amounts of H2per mole of glucose,depending on the fermentation pathway and end-product(s).When acetic acid is the end-product,a theoretical maximum of4mole H2per mole of glucose is obtained:
C6H12O6+2H2O
→2CH3COOH+4H2+2CO2:(19)
When butyrate isthe end-product,a theoretical maximum of2molesH2per mole of glucose is obtained:
C6H12O6+2H2O
→CH2CH2CH2COOH+2H2+2CO2:(20)
Thus,the highest theoretical yields of H2are asso-ciated with acetate asthe fermentation end-product.In practice,however,high H2yields are associated with a mixture of acetate and butyrate fermentation products, and low H2yields are associated with propionate and reduced end-products(alcohols,lactic acid).Clostridium pasteurianum,C.butyricum,and C.beijerinkii are high H2producers,while C.propionicum isa poor H2producer [44].
Hydrogen production by these bacteria is highly de-pendent on the proces sconditionss uch aspH,hydraulic retention time(HRT),and gas partial pressure,which a ect metabolic balance.Thus,fermentation end-products pro-duced by a bacterium depend on the environmental condi-tionsin which it grows.Reduced fermentation end-products like ethanol,butanol,and lactate,contain hydrogen that has not been liberated asgas.To maximize the yield of H2,the metabolism of the bacterium must be directed away from alcohols(ethanol,butanol)and reduced acids(lactate) towardsvolatile fatty acids(VFA).C.pasteurianum isa classic H2and VFA producer,but itsmetabolis m can be directed away from H2production and towardss olvent production by high glucose concentrations(12.5%w/v),by CO(which inhibitsFe-hydrogenas e),and by limiting Fe concentrations[45].
The partial pressure of H2(pH2)isan extremely impor-tant factor for continuousH2synthesis.Hydrogen synthesis pathways are sensitive to H2concentrationsand are s ubject to end-product inhibition.AsH2concentrationsincreas e, H2synthesis decreases and metabolic pathways shift to pro-duction of more reduced substrates such as lactate,ethanol, acetone,butanol,or alanine.As the temperature increases, however,conditionsthat favor reaction(15),shown above, are less a ected by H2concentration.ContinuousH2syn-thes isrequirespH2of?50kPa at60?C[46],?20kPa at 70?C[47],and?2kPa at98?C[48].
Some pure culturesof Clostridium species can degrade insoluble starch without pre-treatment,while some pure cul-turesof Enterobacter species can degrade soluble starch [49].Speciesof Clostridium generally yield high ratesof H2 synthesis using xylose as a substrate.For example,Taguchi et al.[50]found that a pure strain of Clostridium(species No.2)could produce H2in a continuousculture,at pH6.0, using xylose(a major product of hemicellulose hydrolysis) as substrate.In other studies with a variety of natural sub-strates,the rate of H2production varied with the speci?c substrate used[51–53].The best rate obtained with a pure culture of Clostridium(s peciesNo.2)was21:03mmol H2=
D.B.Levin et al./International Journal of Hydrogen Energy29(2004)173–185179
(l×h)using3%xylose as substrate[53].The yield, after5h HRT,was2.36mol of H2per mole of xylose.
Stable fermentation processes capable of using non-sterile feedstocks,however,require mixed cultures of bacteria en-riched from natural sources.Such mixed cultures are re-ported to contain mostly species of https://www.doczj.com/doc/598168913.html,y et al.
[54]observed a rate of1600l H2=m3=day(66:7ml H2=l of culture/h),and a yield of2:14mol of H2per mol of hex-ose using a0.75%solution of soluble starch.H2synthesis wasobtained with a HRT of17h,at an organic loading of6g of total organic sludge per litre of culture,at37?C, and a pH of5.2.This system produced H2at a rate of 64:5mmol H2=(l×h).
Using?xed-bed bioreactors containing mesophilic bacte-ria(predominantly Clostridium spp.)derived from domes-tic sewage sludge,Chang et al.[55]compared the use of expanded clay matricesor activated carbon ass upport ma-trixesfor the bacteria within the bioreactors.The HRT was varied from0.5to5h.At an HRT of1h,with sucrose as substrate(20g=l of culture),the activated carbon ma-trix bioreactor produced1:3l H2=l of culture/h.The use of a hollow-?ber micro?ltration membrane increased the rate of H2production to3:0l H2=l of culture/h.H2production began2–3dayspos t-inoculation and wascontinuousover a 2-week period.Thiscorres pondsto a rate of approximately 121mmol H2=(l×h).
Ueno et al.[56]observed rates of H2production of up to198mmol H2=l of culture after24h,using an unde-?ned population of bacteria,enriched for spore-forming Clostridium species,at thermophilic temperatures(60?C). With sugar factory wastewater as the substrate,a yield of 2:52molesof H2per mole of glucose was observed.Max-imum H2synthesis was obtained with a HRT of12h,at an organic loading of5g of total organic sludge per litre of culture,at a pH of6.8.The bioreactor wasmixed at a rate of200rpm and argon gaswasus ed to replace the gasphas e.H2synthesis did not begin until the26th day of operation,but once H2production began,gasevolution was observed for at least20days for each HRT cycle,and wascontinuousfor a total of212days[56].This system produced H2at a rate of8:2mmol H2=(l×h). Hydrogen production by the extreme thermophiles Caldicellulosiruptor saccharolyticus and Thermotoga el?i iscurrently under inves tigation[57].The maximum rate of H2production by C.saccharolyticus on sucrose(10g=l of culture)was8:4mmol H2=(l×h).The maximum rate of H2production by T.el?i on glucose(10g=l of culture)was 2.7–4:5mmol H2=(l×h).Maximal H2production rates were observed during exponential growth phase,which wasin the order of8–12h,within a total growth cycle (lag phase to stationary phase)of approximately2days. The total amount of H2produced during these batches were110mmol H2for C.saccharolyticus cultured on su-crose(at10g=l),and76mmol H2for T.el?i cultured on glucose.3.6.Fuel cell technologies
Fuel cellsare electrochemical devicesthat create an elec-tron ow using charged ions.A variety of di erent fuel cells systems have been developed.They di er in the type of electrolyte used,in the operating conditions,in their power dens ity range,in their application,and each hasitsadvan-tagesand dis advantages(reviewed by Larminie et al.[7]; Table3).
Alkaline fuel cells(AFC)utilize hydroxyl ions(OH?)as the mobile ion(derived from potassium hydroxide,KOH), operate in the50to200?C range,and are extremely sensitive to the presence of CO2.Phosphoric acid fuel cells(PAFC) utilize protons(H+)asthe mobile ion and operate at ap-proximately200?C.PAFC systems were the?rst fuel cells produced commercially and are used as stationary power sources,generating up to200kW of electricity.Many are in use in the USA and in Europe.The high operating tem-perature and corrosive nature of the electrolyte makes them unsuitable for use in mobile and transportation applications. Molten carbonate fuel cells(MCFC)utilize carbonate ions (CO2?3)asthe mobile ion,operate at approximately650?C, and can take H2,CO2,CO,and/or CH4asfuel,which means they can use natural gas,coal gas,or biogas as fuel sources. Like PAFC,MCFC are used as stationary power sources, generating electricity in the MW range.Solid oxide fuel cells(SOFC)utilize oxygen radicals(O2?)asthe mobile ion and operate between500?C and1000?C.Like MCFC, SOFC can utilize H2,CO,and/or CH4asfuel,which means they can use methane,coal gas,or biogas as fuel sources. Carbon dioxide isnot utilized asa fuel and isdis charged as a waste gas.Like other high-temperature fuel cell systems (PAFC and MCFC)SOFC systems are used as stationary power sources,generating electricity from the low kW to the MW range.
PEMFC utilize hydrogen protons(H+)asthe mobile ion, operate in the50–100?C range,require pure H2and are extremely sensitive to the presence of CO.Of all the fuel cell systems that are available,PEMFC systems are espe-cially suitable for mobile and transportation applications, and PEMFC engines have been demonstrated successfully in both cars and buses.Small PEMFCs,in the1–10kW range, are also under commercial development as small stationary power unitsto provide electricity to homesand s mall bus i-nesses.Because of their imminent commercial applications, PEMFC technologies are under intense research and devel-opment.The rate of H2cons umption by PEMFCsisus ually expressed in kg of H2=sor in mol of H2=s.We have cal-culated the ratesof H2consumption by PEMFCs of several sizes and expressed them as g H2=h or mol H2=h(Table2).
3.7.Rates of biohydrogen synthesis
A comparison of H2production ratesreported for s everal biohydrogen systems is presented in Table4.Conversion of reported unitsof H2production to the standardized unit
180 D.B.Levin et al./International Journal of Hydrogen Energy29(2004)173–185
Table3
The fuel cons traintsfor di erent fuel cell types(adapted from[30])
Fuel cell type PEMFC a AFC b PAFC c MCFC d SOFC e (operating tempt.)(80?C)(200?C)(220?C)(600–700?C)(800–1100?C) Component in fuel gas
H2Fuel Fuel Fuel Fuel Fuel
CH4Diluent Diluent Diluent Diluent Diluent
CO Poison if?10ppm Poison Poison if?0:5%Fuel Fuel
CO2Diluent Poison Diluent Diluent f Diluent
S(H2S and COS)Poison if?10ppm Poison Poison?50ppm Poison?1ppm
a PEMFC—proton exchange membrane fuel cell.
b AFC—alkaline fuel cell.
c PAPF—phosphoric aci
d fuel cell.
d MCFC—molten carbonat
e fuel cell.
e SOFC—solid oxide fuel cell.
f CO2must be supplied to cathode of MCFC.
Table4
Comparison of the rates of H2synthesis by di erent technologies
BioH2System H2synthesis rate H2synthesis rate References
(reported units)(converted units)
Direct photolysis4:67mmol H2=l=80h0:07mmol H2=(l×h)[29] Indirect photolysis12:6nmol H2= g protein/h0:355mmol H2=(l×h)[51] Photo-fermentation4:0ml H2=ml=h0:16mmol H2=(l×h)[60,61] CO-oxidation by R.gelatinosus0:8mmol H2=g cdw/min96:0mmol H2=(l×h)[71]
Dark-fermentations
Mesophilic,pure strain a21:0mmol H2=1l=h21:0mmol H2=(l×h)[56] Mesophilic,unde?ned b1;600:0l H2=m3=h64:5mmol H2=(l×h)[32] Mesophilic,unde?ned3:0l H2=l=h121:0mmol H2=(l×h)[8] Thermophilic,unde?ned198:0mmol H2=l=24h8:2mmol H2=(l×h)[66] Extreme thermophilic,pure strain c8:4mmol H2=l=h8:4mmol H2=(l×h)[67,68]
a Clostridium species#2.
b A consortium of unknown microorganisms cultured from a natural substrate and selected by the bioreactor culture conditions.
c Caldicellulosiruptor saccharolyticus.
(mmol H2=(l×h))revealsthe wide range of H2synthesis by di erent biohydrogen systems.Light-dependent biohy-drogen systems(direct photolysis,indirect photolysis,and photo-fermentation)all have ratesof H2synthesis well be-low1mmol H2=(l×h).dark-fermentation systems,all pro-duce H2at ratesthat are well above1mmol H2=(l×h).The ratesof H2synthesis by an unde?ned consortium of ther-mophilic Clostridium[56]and by the extreme thermophilic Caldicellulosiruptor saccharolyticus[47,57]are very sim-ilar(8.2and8:4mmol H2=(l×h),respectively).A pure strain of mesophilic Clostridium[53]demonstrated very good ratesof H2synthesis with xylose as a substrate(21.0 mmol H2=(l×h),and two dark-fermentation systems that uti-lized unde?ned consortia of mesophilic bacteria[55,54,58] had impressively higher rates of H2synthesis(64.5and 121:0mmol=H2=(l×h),respectively).3.8.Biohydrogen:prospects for practical application Our analyses indicate that photosynthesis-based systems do not produce H2at ratesthat are s u cient to meet the goal of providing enough H2to power even a1kW PEMFC on a continuousbas is(Table5).Thisdoesnot mean that thes e systems should be abandoned.There may be applications other than our hypothetical objective to which they may be more suited.Moreover,continued research will no doubt result in signi?cant improvements in their respective tech-nologies,and thusin the ratesof H2production(see below). Thermophilic and extreme thermophilic biohydrogen sys-temswould require bioreactorsin the range of approximately 2900–14;600l to provide su cient H2to power PEMFCs of1.5–5:0kW,and a bioreactor of approximately5700l would be required to power the5:0kW fuel cell using the
D.B.Levin et al./International Journal of Hydrogen Energy29(2004)173–185181 Table5
IsBiohydrogen practical?The s ize of bioreactor required to power PEM fuel cellsof di erent output
BioH2system H2synthesis rate Size of bioreactor required b to power a:
(mmol H2(l×h))a1:0kW FC(l)1:5kW FC(l)2:5kW FC(l)5:0kW FC(l) Direct photolysis0.073:41×1055:12×1058:56×1051:71×106 Indirect photolysis0.3556:73×1041:01×1051:69×1053:37×105 Photo-fermentation0.161:49×1052:24×1053:74×1057:58×105 CO-oxidation by R.gelatinosus96.02:49×1023:74×1026:24×1021:25×103 Dark-fermentations
Mesophilic,pure strain c21.01:14×1031:71×1032:85×1035:70×103 Mesophilic,unde?ned d64.53:71×1025:57×1029:29×1021:86×103 Mesophilic,unde?ned121.01:98×1022:97×1024:95×1029:89×102 Thermophilic,unde?ned8.22:91×1034:38×1037:31×1031:46×104 Extreme thermophilic,unde?ned e8.42:85×1034:28×1037:13×1031:43×104
a Conrested units.
b Approximate volumes.Calculated volumes were rounded up to nearest whole value.
c Clostridium species#2.
d A consortium of unknown microorganisms cultured from a natural substrat
e and selected by the bioreactor culture conditions.
e Caldicellulosiruptor saccharolyticus.
pure culture of mesophilic Clostridium sp.(strain No.2). The size of bioreactors required for these systems are very large,and thus these systems may be considered impractical for our hypothetical application at thistime.
Some dark-fermentation systems and the CO–water shift reaction of R.gelatinosus CBS,however,appear promis-ing.Bioreactors of reasonable size would be su cient to power the5.0kW fuel cell using unde?ned consortia of mesophilic bacteria,enriched for Clostridium species.The system reported by Chang et al.[55]in particular appears most promising.A bioreactor of approximately500l(495l, in Table5)would provide enough H2to power a2:5kW PEMFC,while a bioreactor of approximately1000l(989l, Table5)would provide su cient H2to power a5:0kW PEMFC.The CO–water shift reaction of R.gelatinosus CBS isintriguing asit o ersthe potential to capture and reform CO,and produce H2.A bioreactor of approximately624l would be required provide enough H2to power the2:5kW PEMFC,while a bioreactor of approximately1250l(1247l, Table5)would provide su cient H2to power a5:0kW PEMFC.
By way of comparison,the current state-of-the-art for distributed,on-site production of hydrogen is via station-ary electrolyzerswhich can generate H2from H2O at a rate of1000l=h,or40:3mol=h(see https://www.doczj.com/doc/598168913.html,). Equivalent ratesof H2synthesis could be achieved by a bioreactor of approximately334l using a dark-fermentation system,or by a bioreactor of approximately420l contain-ing R.gelatinosus CBS.
The CO-water shift reaction of R.gelatinosus CBS of-fersan additional advantage.The CO–water s hift reaction als o yieldsequi-molar amountsof CO2,which R.gelati-nosus CBS can assimilate into new cell biomass using organic compounds,such as butyrate or propionate,as a carbon source and ATP as an energy source[59].Thus,in addition to reformation of CO and H2synthesis,this pro-cess could be incorporated into an integrated energy system to sequester CO2in light,after H2isrecovered from the system.Bioreactors of1000–1500l in the basement of a home isnot unthinkable.Before electrical and natural gas heating,it wasnormal for North American homeslocated in northern latitudes to have,in their basements,tanks of heating oil that were approximately thiss ize.
While dark-fermentation systems and the CO-water shift reaction may have practical applications,there are a number of technical challenges that must be considered and over-come before these systems can be used to produce H2to power a PEMFC.The most signi?cant of these problems is whether the systems can be scaled up to volumes large enough to generate the required ow rate(22:1mol H2=h for the5:0kW fuel cell).The working volume of the biore-actor described that produced121mmol H2=(l×h)was3l, and used sucrose(at20g=l of culture)asthe carbon s ource for bacterial growth[55].The biogasproduced was25–35% H2and65–75%CO2.Further research is required to deter-mine if the rate of H2production will remain at high levels if these systems are scaled up to much larger volumes(183l or more),and if carbon sources other than pure sucrose can be used.The major challenge for the CO–water shift reac-tion isthe problem of mas strans fer:the gasmus t be avail-able to the bacteria,in solution,at a su cient concentra-tion that the bacteria can absorb and metabolize e ciently. Thismay require radically new technologiesand bioreactor designs.
182 D.B.Levin et al./International Journal of Hydrogen Energy29(2004)173–185
3.9.Biohydrogen:future directions
Both light-dependent(direct photolysis,indirect photol-ysis,and photo-fermentation)and dark-fermenation biohy-drogen systems are under intense investigation to?nd ways to improve both the ratesof H2production and the ultimate yield of H2.Hydrogen production by direct photolysis using green algae iscurrently limited by three parameters[60]:(i) solar conversion e ciency of the photosynthetic apparatus; (ii)H2synthesis processes(i.e.the need to separate the pro-cesses of H2O oxidation from H2synthesis);and(iii)biore-actor design and cost.A number of approaches to improve H2production by green algae are currently under investi-gation.These include genetic engineering of light gathering antennae[61],optimization of light input into photobioreac-tors[62],and improvementsto the two-phas e H2production systems used with green algae[63,64].
Hydrogen production via indirect photolysis using cyanobacteria can be improved by screening for wild-type strains possessing highly active hydrogen evolving enzymes (nitogenases and/or hydrogenases),in combination with high heterocyst formation[17].Genetic modi?cation of strains to eliminate uptake hydrogenases and increase levels of bidirectional hydrogenase activity may yield signi?cant increases in H2production.For example,a mutant strain of Anabaena(AMC414),in which the large subunit of the uptake hydrogenase(hup L)wasinactivated by a deletion event[65],produced H2at a rate that wasmore than twice that of the parent wild-type strain,Anabaena PCC7120 [66].Finally,optimization of cultivation conditionss uch aslight intens ity,pH,temperature,and nutrient content,as well as maintaining low partial pressures of H2and CO2 (see below)will contribute to increased H2production. Many of the parametersthat limit H2production by green algae and cyanobacteria also apply to photo-heterotrophic bacteria used in photo-fermentation systems.
E ortsto improve H2production in these bacteria also include elimination of competing microorganisms,such asmicroalgae,us ing light?lters[67],co-culturesof pho-toheterotrophic bacteria with di erent light utilization characteristics[68],two-phase fermentation systems in which photoheterotrophic bacteria utilize substrates pro-duced by anaerobic bacteria during dark-fermentation pro-cesses[69],novel photobioreactor designs[70,68],and use of speci?c waste streams as substrate for photo-fermentation [71].
Dark-fermentation systems also appear to have the great potential to be developed as practical biohydrogen systems. Substantial improvements,however,can be made through rapid gas removal and separation,bioreactor design,and genetic modi?cations in the microorganisms. Improvementsin gass eparation will contribute to s igni?-cant increases in H2production.As discussed above,the pH2 isan extremely important factor for continuousH2synthesis. AsH2concentrationsincreas e,H2synthesis decreases and metabolic activity shifts to pathways that synthesize more reduced substrates.The concentration of CO2also a ects the rate of synthesis and?nal yield of H2.Cells synthesize succinate and formate using CO2,pyruvate,and reduced nicotinamide adenine dinucleotide(NADH)via the hexose monophosphate pathway[3].Thispathway competeswith reactionsin which H2is synthesized by NADH-dependent hydrogenases(which oxidize NADH to NAD+).E cient removal of CO2from the fermentation system would reduce competition for NADH and thus result in increased H2syn-thesis.
In dark-fermentation processes,this problem is com-pounded by the fact that the gasproduced isa mixture of primarily H2and CO2,but may also contain other gases such as CH4,H2S,or ammonia(NH4).Moreover,the H2 content of the gasmixture may be low(?50%).PEMFCs require high-purity H2(?99%)and cannot tolerate CO at concentrations?10ppm[7].To both maintain continu-ousH2synthesis and remove diluting(CO2;CH4)and/or contaminating(CO)gases,rapid removal of the gases and puri?cation of the H2are essential.
Methodsto enhance H2production by removal of H2and CO2include sparging with N2or argon(Ar)gasand ap-plying a vacuum to the head space to reduce the H2partial pressure[72].An increase in H2production of over50% wasobtained after periodic s parging with N2[73].Too much sparging,however,dilutes the H2and createsa s eriousprob-lem with respect to separation of the H2from the sparging gas.
Removal and selective puri?cation of H2hasbeen demonstrated using membrane technologies.A hollow ?ber/silicone rubber membrane e ectively reduced biogas partial pressure in a dark-fermentation system,resulting in a10%improvement in the rate of H2production and a 15%increase in H2yield[74],and a non-porous,synthetic polyvinyltrimethylsilane(PVTMS)membrane was used for production of high-purity H2from three di erent H2 producing bioreactor systems[75].
Substantial gains in H2production can also be achieved through optimization of bioreactor https://www.doczj.com/doc/598168913.html,ing ?xed-bed bioreactorscontaining an unde?ned cons or-tium of mesophilic bacteria,Chang et al.[8]observed ratesof H2synthesis far greater than other studies (121mmol H2=(l×h)).Thisremarkable rate of H2pro-duction wasachieved us ing activated carbon asa s upport matrix that allowed retention of the H2producing bacteria within the bioreactor,plus a membrane?lter system to remove the biogas and maintain low gas partial pressures.
A theoretical study of H2production by(hyper)thermo-philic bacteria in a high rate bioreactor wasconducted by van Groenesttijn et al.[76].In this system,(hyper)thermophilic bacteria would be growing asa bio?lm within an anaerobic trickling?lter containing packing material with a very high surface area.The liquid-suspended biomass substrate would be passed continuously through the?lter,so that the biomass substrate,the H2producing bacteria,and the resulting gas phase would be in close proximity.Low H2and CO2partial
D.B.Levin et al./International Journal of Hydrogen Energy29(2004)173–185183
pressures would be maintained by stripping the H2gasfrom the bioreactor using steam within the?lter.
Finally,gainsin H2production may be achieved through genetic modi?cation of H2producing bacteria.Hydrogen producing strains of bacteria can be genetically modi?ed in several ways to increase H2synthesis,including(1) over-expression of cellulases,hemi-cellulases,and lignases that can maximize substrate(glucose)avaliability;(2) elimination of uptake hydrogenases;(3)over-expression of H2evolving hydrogenases that have themselves been modi-?ed to be oxygen tolerant;and(4)elimination of metabolic pathwaysthat compete for reducing equivalentsrequired for H2synthesis.
4.Concluding remarks
Biohydrogen technologiesare s till in their infancy.Ex-isting technologies o er potential for practical application, but if biohydrogen systems are to become commercially competitive they must be able to synthesize H2at ratesthat are su cient to power fuel cells of su cient size to do practical work.Further research and development aimed at increasing rates of synthesis and?nal yields of H2are essen-tial.Optimization of bioreactor designs,rapid removal and puri?cation of gases,and genetic modi?cation of enzyme pathwaysthat compete with hydrogen producing enzyme systems o er exciting prospects for biohydrogen systems. Even a10-fold increase in the rate of H2synthesis by some dark-fermentation systems would reduce bioreactor size dramatically.This would greatly facilitate overcom-ing the engineering challengesof s cale up,and create new opportunitiesfor practical applications.
Acknowledgements
We wish to thank the following people for their encour-agement and helpful commentsduring the preparation of this manuscript:Maria Ghirardi,Freda Hawkes,Pin-Ching Maness,and Ed van Niel.
References
[1]BockrisJO’M.The origin of ideason a hydrogen economy and
itss olution to the decay of the environment.Int J Hydrogen Energy2002;27:731–40.
[2]Dunn S.Hydrogen futures:toward a sustainable energy
system.Int J Hydrogen Energy2002;27:235–64.
[3]DasD,Nejat Veziroglu T.Hydrogen production by biological
processes:a survey of literature.Int J Hydrogen Energy 2001;26:13–28.
[4]Hallenbeck P,Benemann JR.Biological hydrogen production:
fundamentals and limiting processes.Int J Hydrogen Energy 2002;27:1185–94.
[5]Nandi R,Sengupta S.Microbial production of hydrogen:an
overview.Crit Rev Microbiol1998;24:61–84.
[6]Kempton W,Tomic J,Letendre S,BrooksA,Lipman
T.Vehicle-to-grid power:battery,hybrid,and fuel cell vehiclesasres ourcesfor dis tributed electric power in California.Working Paper UCD-ITS-RR-01-03,Institute of Transportation Studies,2001.
[7]Larminie J,Dicks A.Fuel cell systems explained.New York:
Wiley,2000.
[8]Moran M,Shapiro H.Fundamentalsof engineering
thermodynamics,3rd ed.New York:Wiley,1996.
[9]Ghirardi ML,Zhang L,Lee JW,Flynn T,Seibert M,
Greenbaum E,MelisA.Microalgae:a green s ource of renewable H2.TrendsBiotechnol200018:506–11. [10]Adams MWW.The structure and mechanism of iron-
hydrogenases.Biochem Biophys Acta1990;1020:115–45.
[11]Wyko DD,DaviesJP,MelisA,Gros s man AR.The
regulation of photosynthetic electron-transport during nutrient deprivation in Chlamydomonas reinhardtii.Plant Physiol 1998;117:129–39.
[12]Kosourov S,Tsygankov A,Seibert M,Ghirardi ML.Sustained
hydrogen photoproduction by Chlamydomonas reinhardtii:
e ectso
f culture parameters.Biotechnol Bioeng2002
;
78: 731–40.
[13]MelisA,Zhang L,Fores tier M,Ghirardi ML,Seibert M.
Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii.Plant Physiol2000;122:127–35.
[14]Schopf JW.The fossil record.Tracing the roots of the
cyanobacterial lineage.In:Whitton BA,PottsM,editors.
The ecology of cyanobacteria.Dordrecht,The Netherlands: Kluwer Academic Publishers,2000.
[15]Tamagnini P,Axelsson R,Lindberg P,Oxelfelt F,Wunschiers
R,Lindblad P.Hydrogenases and hydrogen metabolism in cyanobacteria.Microbiol Mol Biol Rev2002;66:1–20. [16]Hansel A,Lindblad P.Mini-review:toward optimization
of cyanobacteria asbiotechnologically relevant producers of molecular hydrogen,a clean energy source.Appl Environ Microbiol1998;50:153–60.
[17]Pinto FAL,Troshina O,Lindblad P.A brief look at three
decadesof res earch on cyanobacterial hydrogen evolution.Int J Hydrogen Energy2002;27:1209–15.
[18]Howarth DC,Codd GA.The uptake and production of
molecular hydrogen by unicellular cyanobacteria.J Gen Microbiol1985;131:1561–9.
[19]Masukawa H,Nakamura K,Mochimaru M,Sakurai H.
Photohydrogen production and nitrogenase activity in some heterocystous cyanobacteria.BioHydrogen2001;II:63–6. [20]Sveshnikov DA,Sveshnikov NV,Rao KK,Hall DO.
Hydrogen metabolism of Anabaena variabilis in continuous cultures and under nutritional stress.FEBS Lett1997;147: 297–301.
[21]Arik T,Gunduz U,Yucel M,Turker L,Sediroglu V,Eroglu
I.Photoproduction of hydrogen by Rhodobacter sphaeroides
OU001.In:Viroglu TN,Winter CJ,Baselt JP,Kreysa G, editors.Proceedings of the11th World Hydrogen Energy Conference,Stuttgart,Germany.Frankfurt:Scon&Wetzel GmbH,1996.p.2417–26.
[22]Bolton JR.Solar photoproduction of hydrogen.Sol Energy
1996;57:37–50.
[23]Fedorov AS,Tsygankov AA,Rao KK,Hall DO.Hydrogen
photoproduction by Rhodobacter sphaeroides immobilised on polyurethane foam.Biotechnol Lett1998;20:1007–9.
184 D.B.Levin et al./International Journal of Hydrogen Energy29(2004)173–185
[24]Tsygankov AA,Hirata Y,Miyake M,Asada Y,Miyake J.
Photobioreactor with photosynthetic bacteria immobilized on porousglas sfor hydrogen photoproduction.J Ferment Bioeng 1994;77:575–8.
[25]Tsygankov AA,Fedorov AS,Laurinavichene TV,Gogotov
IN,Rao KK,Hall DO.Actual and potential ratesof hydrogen photoproduction by continuousculture of the purple non-sulphur bacteria Rhodobacter capsulatus.Appl Microbiol Biotechnol1998;49:102–7.
[26]Zurrer H,Bachofen R.Hydrogen production by the
photosynthetic bacterium,Rhodospirillum rubrum.Appl Environ Microbiol1979;37:789–93.
[27]Fascetti E,Todini O.Rhodobacter sphaeroides RV cultivation
and hydrogen production in a one-and two-stage chemostat.
Appl Microbiol Biotechnol1995;22:300–5.
[28]Zurrer H,Bachofen R.Aspects of growth and hydrogen
production of the photosynthetic bacterium Rhodospirillum rubrum in continuousculture.Biomas s19822:165–74. [29]Francou N,VignaisPM.Hydrogen production by
Rhodopseudomonas capsulata cellsentrapped in carrageenan beads.Biotechnol Lett1984;6:639–44.
[30]Vincenzini M,Materassi R,Sili C,Florenzano G.Hydrogen
production by immobilized cellsIII.Prolonged and s table H2photoevolution by Rhodopseudomonas palaustris in light-dark-cycles.Int J Hydrogen Energy1986;11:623–6. [31]Tsygankov AA,Fedorov AS,Talipova IV,Laurinavichene
TV,Miyake J,Gogotov IN,Rao KK,Hall DO.Application of immobilized phototrophic bacteria for simultaneous waste water treatment and hydrogen photoproduction.Appl Biochem Microbiol1998;34:1–5(in Russian).
[32]Jouanneau Y,Lebecque S,VignaisPM.Ammonia and light
e ect on nitrogenase activity in nitrogen-limited continuous
culturesof Rhodopseudomonas capsulata:role of glutamate synthetase.Arch Microbiol1984;119:326–31.
[33]Willison JC,Jouanneau Y,Michalski WP,Colbeau A,
VignaisPM.Nitrogen?xation and H2metabolism in the photosynthetic bacterium Rhodopseudomonas capsulata.
In:Papageorgiou GC,Packer L,editors.Photosynthetic prokaryotes:cell di erentiation and function.New York: Elsevier,1983.p.333–52.
[34]Tsygankov AA,Fedorov AS,Talipova IV,Laurinavichene
TV,Miyake J,Gogotov https://www.doczj.com/doc/598168913.html,e of immobilized phototrophic microorganisms for waste water treatment and simultaneous production of hydrogen.Appl Biochem Microbiol1998;34:362–6(in Russian).
[35]Champine JE,U en RL.Membrane topography of anaerobic
carbon monoxide oxidation in Rhodocyclus gelatinosus.
J Bacteriol1987;169:4784–9.
[36]Kerby RL,Ludden PW,Robert GP.Carbon monoxide-
dependent growth of Rhodospirillum rubrum.J Bacteriol 1995;177:2241–4.
[37]U en RL.Metabolism of carbon monoxide by
Rhodopseudomonas gelatinosa:cell growth and propertiesof the oxidation system.J Bacteriol1983;155:956–65. [38]Wakim BT,U en RL.Membrane association of the
carbon monoxide oxidation system in Rhodopseudomonas gelatinosa.J Bacteriol1982;153:571–3.
[39]Maness PC,Weaver PF.A potential bioremediation role for
photosynthetic bacteria.In:Sikdal SK,Irvine RL,editors.
Bioremediation:principlesand practice,vol II.Technomic Publishing Co,Inc.,Lancaster PA,1997.[40]U en RL.Anaerobic growth of a Rhodopseudomonas species
in the dark with carbon monoxide ass ole carbon and energy substrate.Proc Natl Acad Sci USA1976;73:3298–302. [41]Watt AS,Huang J,Smolinski S,Maness PC,Wolfrum EJ.
The water–gass hift pathway In:Rubrivivax gelatinosus.In preparation.
[42]Maness PC,Weaver PF.Hydrogen production from
a carbon-monoxide oxidation pathway in Rubrivivax
gelatinosus.Int J Hydrogen Energy2002;27:1407–11. [43]Maness PC,Smolinski S,Dillon AC,Heben MJ,Weaver PF.
Characterization of the oxygen tolerance of a hydrogenase linked to a carbon monoxide oxidation pathway in Rubrivivax gelatinosus.Appl Environ Microbiol2002;68:2633–6. [44]Hawkes FR,Dinsdale R,Hawkes DL,Hussy I.
Sustainable fermentative biohydrogen:challenges for process optimization.Int J Hydrogen Energy2002;27:1339–47. [45]Dabrock B,Bahl H,Gottschalk G.Paramenters a ecting
solvent production by Clostridium pasteurianum.Appl Environ Microbiol1992;58:1233–9.
[46]Lee MJ,Zinder SH.Hydrogen partial pressures in a
thermophilic acetate-oxidizing methanogenic co-culture.Appl Environ Microbiol1988;54:1457–61.
[47]van Niel EWJ,Claassen PAM,Stams AJM.Substrate and
product inhibition of hydrogen production by the extreme thermophile Caldicellulosiruptor saccharolyticus.Biotechnol Bioeng2002;81:255–62.
[48]AdamsMWW.The metabolis m of hydrogen by extremely
thermophilic sulphur-dependent bacteria.FEMS Microbiol Rev1990;75:219–38.
[49]Taguchi F,Yamada K,Hasegawa K,Saito-Taki T,Hara K.
Continuoushydrogen production by Clostridium sp.No.2 from cellulose hydrolysate in an aqueous two-phase system.
J Ferment Bioeng1996;82:80–3.
[50]Taguchi F,Mizukami N,Yamada K,Hasegawa K,Saito-Taki
T.Direct conversion of cellulosic materials to hydrogen by Clostridium sp.strain No.2.Enzyme Microbiol Biotechnol 1995;17:147–50.
[51]Taguchi F,Mizukami N,Hasegawa K,Saito-Taki T.Microbial
conversion of arabinose and xylose to hydrogen by a newly isolated Clostridium sp.No.2.Can J Microbiol1994;40:228–33.
[52]Taguchi F,Mizukami N,Saito-Taki T,Hasegawa K.Hydrogen
production from continuousfermentation of xylos e during growth of Clostridium sp.strain No.2.Can J Microbiol 1995;41:536–40.
[53]Taguchi F,Hasegawa K,Saito-Taki T,Hara K.Simultaneous
production of xylanase and hydrogen using xylan in batch culturesof Clostridium sp.strain X53.J Ferment Bioeng 1996;18:178–80.
[54]Lay JJ.Modeling and optimization of anaerobic digestion
sludge converting starch to hydrogen.Biotechnol Bioeng 2000;68:269–78.
[55]Chang J-S,Lee K-S,Lin P-J.BioHydrogen Production
with?xed-bed bioreactors.Int J Hydrogen Energy2002;27: 1167–74.
[56]Ueno Y,Otauka S,Morimoto M.Hydrogen production from
industrial wastewater by anaerobic micro ora in chemostat culture.J Ferment Bioeng1996;82:194–7.
[57]van Niel EWJ,Budde MAW,de HaasGG,van
der Wal FJ,Claassen PAM,Stams AJM.Distinctive propertiesof high hydrogen producing extreme thermophiles,
D.B.Levin et al./International Journal of Hydrogen Energy29(2004)173–185185
Caldicellulosiruptor saccharolyticus and Thermotoga el?i.
Int J Hydrogen Energy2002;27:1391–8.
[58]Lay JJ.Biohydrogen generation by mesophilic anaerobic
fermentation of microcrystalline cellulose.Biotechnol Bioeng 2001;74:280–7.
[59]Madigan MT,Martinko JM,Parker J.Biology of
microorganisms.Upper Saddle River,NJ:Prentice-Hall,1996.
p.649.
[60]Melis T.Green alga production:process,challenges,and
prospects.Int J Hydrogen Energy2002;27:1217–28. [61]Polle JEW,Kanakagiri S,Jin E,Masuda T,Melis A.
Truncated chlorophyll antenna size of the photosystems—
a practical method to improve microalgal productivity and
hydrogen production in mass culture.Int J Hydrogen Energy 2002;27:1257–64.
[62]Gordon J.Tailoring optical systems to optimized
photobioreactors.Int J Hydrogen Energy2002;27:1175–84.
[63]Laurinavichene TV,Tolstygina IV,Galiulina RR,Ghirardi
ML,Seibert M,Tsygankov AA.Dilution methods to deprive Chlamydomonas reinhardtii culturesof s ulphur for subsequent hydrogen photoproduction.Int J Hydrogen Energy 2002;27:1245–50.
[64]Tsygankov AA,Kosourov S,Seibert M,Ghirardi ML.
Hydrogen photoproduction under continuousillumination by sulphur-deprived,synchronous Chlamydomonas reinhardtii cultures.Int J Hydrogen Energy2002;27:1139–44. [65]Carrasco CD,Buettner JA,Golden JW.Programmed DNA
rearrangement of a cyanobacterial hup L gene in heterocysts.
Proc Natl Acad Sci USA1995;92:791–5.
[66]Lindblad P,Christensson K,Lindberg P,Federov A,Pinto F,
Tsygankov A.Photoproduction of H2by wildtype Anabaena PCC1720and a hydrogen uptake de?cient mutant:from laboratory to outdoor culture.Int J Hydrogen Energy 2002;27:1271–81.
[67]Ko I-B,Noike https://www.doczj.com/doc/598168913.html,e of blue optical?lters for suppression
of growth of algae in hydrogen producing non-axenic cultures of Rhodobacter sphaeroides RV.Int J Hydrogen Energy 2002;27:1297–302.[68]Kondo T,Arawaka M,Wakayama T,Miyake J.Hydrogen
production by combining two typesof photos ynthetic bacteria with di erent characteristics.Int J Hydrogen Energy 2002;27:1303–8.
[69]Lee C-M,Chen P-C,Wang C-C,Tung Y-C.Photohydrogen
production using purple non-sulfur bacteria with hydrogen fermentation reactor e uent.Int J Hydrogen Energy 2002;27:1308–14.
[70]Hoekema S,Bijmans M,Janssen M,Tramper J,Wij els
RH.A pneumatically agitated at-panel photobioreactor with gasrecirculation:anaerobic photoheterotrophic cultivation of a purple non-sulfur bacterium.Int J Hydrogen Energy 2002;27:1331–8.
[71]Zhu H,Ueda S,Asada Y,Miyake J.Hydrogen production
as a novel process of wastewater treatment—studies on tofu wastewater with entrapped R.sphaetoides and mutagenesis.
Int J Hydrogen Energy2002;27:1349–58.
[72]Kataoka N,Miya A,Kiriyama K.Studieson hydrogen
production by continuousculture of hydrogen producing anaerobic bacteria.Proceedingsof the Eighth International Conference on Anaerobic Digestion,vol2.Sendai,Japan, 1997.p.383–90.
[73]Mizuno O,Dins dale R,HawkesFR,HawkesDL,Noike
T.Enhancement of hydrogen production from glucose by nitrogen gas sparging.Bioresource Technol2000;73:59–65.
[74]Liang T-M,Cheng S-S,Wu K-L.Behavioral study on
hydrogen fermentation reactor installed with silicone rubber membrane.Int J Hydrogen Energy2002;27:1157–65. [75]Teplyakov VV,Gassanova LG,Sostina EG,Slepova
EV,Modigell M,Netrusov https://www.doczj.com/doc/598168913.html,b-scale bioreactor integration with active membrane system for hydrogen production:experience and prospects.Int J Hydrogen Energy 2002;27:1149–55.
[76]van Groenestijn JW,Hazewinkel JHO,Nienord M,Bussmann
PJT.2002.Energy aspects of biological hydrogen production in high rate bioreactorsoperated in the thermophilic temperature range.Int J Hydrogen Energy2002;27:1141–7.