
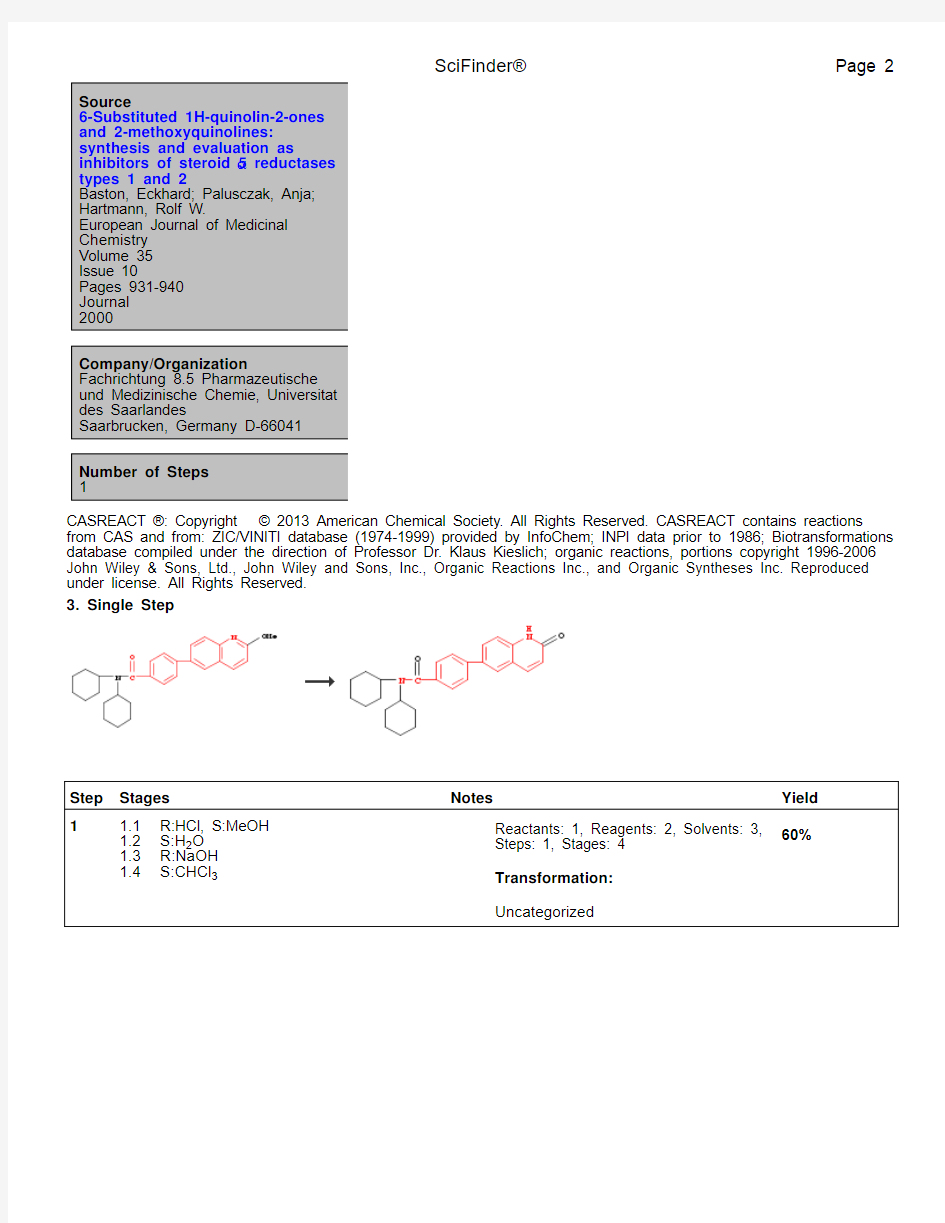
1. Single Step
Step Stages Notes Yield
1 1.1R:HCl, S:H2O
1.2S:H2O
1.3R:NaOH
1.4S:CHCl3
Reactants: 1, Reagents: 2, Solvents: 2,
Steps: 1, Stages: 4
Transformation:
Uncategorized
41%
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
2. Single Step
Step Stages Notes Yield
1 1.1R:HCl, S:H2O
1.2S:H2O
1.3R:NaOH
1.4S:CHCl3
Reactants: 1, Reagents: 2, Solvents: 2,
Steps: 1, Stages: 4
Transformation:
Uncategorized
65%
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
3. Single Step
Step Stages Notes Yield
1 1.1R:HCl, S:MeOH
1.2S:H2O
1.3R:NaOH
1.4S:CHCl3
Reactants: 1, Reagents: 2, Solvents: 3,
Steps: 1, Stages: 4
Transformation:
Uncategorized
60%
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
4. Single Step
Step Stages Notes Yield 1 1.1R:Pd(OAc)2, S:MeCN, overnight, reflux Reactants: 1, Reagents: 1, Solvents: 1,
Steps: 1, Stages: 1
Transformation:
Uncategorized
26% Experimental Procedure
Palladium(II) complex 8. Diazuliporphyrin hydrobromide salt 5a (20.0 mg; 0.028 mmol) and
palladium(II) acetate (9.4 mg; 0.042 mmol) were dissolved in acetonitrile (20 mL) and heated under refluxed while stirring overnight. The solvent was removed under reduced pressure and the residue eluted through a silica column with 1% methanol-chloroform. The first greenish fraction was collected and recrystallized from dichloromethane-petroleum ether (60-90°) to afford the palladium(II) complex (5.4 mg; 0.0074 mmol; 26%) as a dark powder, dark powder, mp >300 °C ;UV-vis (CHCl 3):λ max (log 10ε) 592 (4.50), 401 (4.84), 321 nm (4.45); UV-vis (MeOH): λ max (log 10ε) 567 (4.28), 393 nm (4.62); 1
H NMR (d
6
-DMSO): δ 1.51 (6H, t, J = 7.6 Hz), 1.57 (18H, s), 2.95 (6H, s), 3.25-3.32 (4H, q,obscured by solvent), 7.92 (1H, s), 8.06 (2H, dd, J = 2.0, 10.2 Hz), 8.13 (2H, dd, J = 2.0, 10.6 Hz),8.80 (2H, s), 9.36 (2H, d, J = 10.8 Hz), 9.47 (2H, d, J = 10.8 Hz), 9.98 (1H, s); 13C NMR (d 6-DMSO): δ10.3, 16.2, 18.5, 31.1, 38.7, 91.8, 105.2, 125.7, 130.2, 130.7, 130.8, 131.1, 134.6, 136.2, 136.3,
140.2, 145.5, 150.2, 153.8, 162.1, 174.3; HR MS (FAB): calcd for C 46H 46N 2Pd: m/z 732.2696. Found:732.2713.
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.5. Single Step
Step Stages Notes Yield
1
1.1R:HBTU, R:EtN(Pr-i )2, S:DMF, 30 min, rt 1.2
R:NH 3, S:Me 2CHOH, 1 h, rt
Reactants: 1, Reagents: 3, Solvents: 2,Steps: 1, Stages: 2Transformation:
1.Acylation of Nitrogen Nucleophiles
by Carboxylic Acids
98%
Experimental Procedure
Preparation of 3-Cyclohexyl-4-[2-(2,4-dimethyl-thiazol-5-yl)-quinolin-6-yl]-5-(2-morpholin-4-yl-2-oxo-ethyl)-benzamide (11.a). A mixture of 11.a (0.142 g, 0.249 mmol), HBTU (0.189 g, 0.498 mmol) and
diisopropylethylamine (0.14 mL, 0.8 mmol) in DMF (2 mL) was stirred at room temperature for 30 min
and 2 M ammonia in 2-propanol (1.5 mL) was then added. The mixture was stirred at room
temperature for further 1 h. After evaporation of the solvent, the residue was extracted with CHCl
3-MeOH (5:1, 100 mL 2×) and the organic phase was washed with water (50 ml×3), dried over
anhydrous Na 2SO 4 and evaporated. The residue was purified by chromatography (CH 2Cl 2-MeOH,6:1) to give 11.b(1.387 g, 98%). MS: 569.2 (M+H +).
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.6. 2 Steps
1
Step Stages Notes
Yield 1
1.1R:NaOH, S:H 2O, S:MeOH, S:THF, 2 h, 45°C 1.2
R:HCl, S:H 2O, pH 6
Reactants: 1, Reagents: 2, Solvents: 3,Steps: 1, Stages: 2
39%
Experimental Procedure
Preparation of 3-cyclohexyl-4-[2-(2,4-dimethyl-thiazol-5-yl)-quinolin-6-yl]-5-(2-morpholin-4-yl-2-oxo-ethyl)-benzoic acid (7). To a solution of 10.h (0.78 g, 1.34 mmol) in THF (8 mL) and MeOH (4 mL)was added 2N aq. NaOH (2.5 mL) and the mixture was stirred at 45°C. for 2 h. The mixture was
neutralized with 5 N HCl to pH 6. After evaporation of solvent, the residue was washed with water (25mL 2×) and dried to give compound 9 (30 mg, 39%). MS: 570.2 (M+H +). 1H-NMR (DMSO-d 6): δ (ppm)8.45 (d, 1H, J =8.7 Hz), 8.01 (d, 1H, J =8.4 Hz), 7.88 (d, 1H, J =8.7 Hz), 7.86 (d, 1H, J =1.2 Hz), 7.71-7.70 (m, 2H), 7.47 (dd, 1H, J =1.8, 8.7 Hz), 3.42 (d, 2H, J =4.5 Hz), 3.30-3.22 (m, 4H), 3.02-2.97 (m,4H), 2.72 (s, 3H), 2.68 (s, 3H), 2.22 (m, 1H), 1.64-0.82 (m, 10H).
2
Step Stages Notes
Yield 2
2.1R:HBTU, R:EtN(Pr-i )2, S:DMF, 30 min, rt 2.2
R:NH 3, S:Me 2CHOH, 1 h, rt
Reactants: 1, Reagents: 3, Solvents: 2,Steps: 1, Stages: 2
98%
Experimental Procedure
Preparation of 3-Cyclohexyl-4-[2-(2,4-dimethyl-thiazol-5-yl)-quinolin-6-yl]-5-(2-morpholin-4-yl-2-oxo-ethyl)-benzamide (11.a). A mixture of 11.a (0.142 g, 0.249 mmol), HBTU (0.189 g, 0.498 mmol) and diisopropylethylamine (0.14 mL, 0.8 mmol) in DMF (2 mL) was stirred at room temperature for 30 min and 2 M ammonia in 2-propanol (1.5 mL) was then added. The mixture was stirred at room
temperature for further 1 h. After evaporation of the solvent, the residue was extracted with CHCl 3-MeOH (5:1, 100 mL 2×) and the organic phase was washed with water (50 ml×3), dried over
anhydrous Na 2SO 4 and evaporated. The residue was purified by chromatography (CH 2Cl 2-MeOH,6:1) to give 11.b(1.387 g, 98%). MS: 569.2 (M+H +).
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.7. 3 Steps
1
Step Stages Notes Yield
1 1.1R:F3CCO2H, R:PhOMe, 1 h, rt
1.2 R:
R:EtN(Pr-i)2, S:DMF, 30 min, rt
1.3 2 h, rt
Reactants: 2, Reagents: 4, Solvents: 1,
Steps: 1, Stages: 3
94%
Experimental Procedure
Preparation of 3-cyclohexyl-4-[2-(2,4-dimethyl-thiazol-5-yl)-quinolin-6-Yl]-5-(2-morpholin-4-yl-2-oxo-
ethyl)-benzoic acid methyl ester (10.h). To 10.g (0.654 g, 1.27 mmol) was added TFA (4 mL) and
anisole (0.2 mL). The mixture was stirred at room temperature for 1 h. After evaporation of solvent, to
the residue was added DMF (5 ml), HATU (0.72 g, 1.91 mmol) and diisopropylethylamine (0.44 ml,
2.54 mmol). The mixture was stirred at room temperature for 30 min and morpholine (0.22 ml, 2.54
mmol) was then added. The mixture was stirred at room temperature for 2 h. After evaporation of
solvent, the product was purified by chromatography (from EtOAc-hexanes 1:1 to EtOAc). Yield 94%.
MS: 584.3 (M+H+).
2
Step Stages Notes Yield
2 2.1R:NaOH, S:H2O, S:MeOH, S:THF, 2 h, 45°C
2.2R:HCl, S:H2O, pH 6
Reactants: 1, Reagents: 2, Solvents: 3,
Steps: 1, Stages: 2
39% Experimental Procedure
Preparation of 3-cyclohexyl-4-[2-(2,4-dimethyl-thiazol-5-yl)-quinolin-6-yl]-5-(2-morpholin-4-yl-2-oxo-
ethyl)-benzoic acid (7). To a solution of 10.h (0.78 g, 1.34 mmol) in THF (8 mL) and MeOH (4 mL)
was added 2N aq. NaOH (2.5 mL) and the mixture was stirred at 45°C. for 2 h. The mixture was
neutralized with 5 N HCl to pH 6. After evaporation of solvent, the residue was washed with water (25
mL 2×) and dried to give compound 9 (30 mg, 39%). MS: 570.2 (M+H+). 1H-NMR (DMSO-d6): δ (ppm)
8.45 (d, 1H, J=8.7 Hz), 8.01 (d, 1H, J=8.4 Hz), 7.88 (d, 1H, J=8.7 Hz), 7.86 (d, 1H, J=1.2 Hz), 7.71-
7.70 (m, 2H), 7.47 (dd, 1H, J=1.8, 8.7 Hz), 3.42 (d, 2H, J=4.5 Hz), 3.30-3.22 (m, 4H), 3.02-2.97 (m,
4H), 2.72 (s, 3H), 2.68 (s, 3H), 2.22 (m, 1H), 1.64-0.82 (m, 10H).
3
Step Stages Notes
Yield 3
3.1R:HBTU, R:EtN(Pr-i )2, S:DMF, 30 min, rt 3.2
R:NH 3, S:Me 2
CHOH, 1 h, rt
Reactants: 1, Reagents: 3, Solvents: 2,
Steps: 1, Stages: 2
98%
Experimental Procedure
Preparation of 3-Cyclohexyl-4-[2-(2,4-dimethyl-thiazol-5-yl)-quinolin-6-yl]-5-(2-morpholin-4-yl-2-oxo-ethyl)-benzamide (11.a). A mixture of 11.a (0.142 g, 0.249 mmol), HBTU (0.189 g, 0.498 mmol) and diisopropylethylamine (0.14 mL, 0.8 mmol) in DMF (2 mL) was stirred at room temperature for 30 min and 2 M ammonia in 2-propanol (1.5 mL) was then added. The mixture was stirred at room
temperature for further 1 h. After evaporation of the solvent, the residue was extracted with CHCl 3-MeOH (5:1, 100 mL 2×) and the organic phase was washed with water (50 ml×3), dried over
anhydrous Na 2SO 4 and evaporated. The residue was purified by chromatography (CH 2Cl 2-MeOH,6:1) to give 11.b(1.387 g, 98%). MS: 569.2 (M+H +).
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.8. 4 Steps
1
Step Stages Notes Yield
1 1.1R:Zn, S:THF, 3 min, 50°C;
2 h, rt
1.2C:53199-31-8, S:THF, S:NMP, 16 h, 100°C
Reactants: 2, Reagents: 1, Catalysts: 1,
Solvents: 2, Steps: 1, Stages: 2
75% Experimental Procedure
Preparation of 3-tert-butoxycarbonylmethyl-5-cyclohexyl-4-[2-(2,4-dimethyl-thiazol-5-yl)-quinolin-6-yl]-
benzoic acid methyl ester (10.g). Zinc powder (0.5 g) was washed with 10% HCl, acetone and dried
under a high vacuum overnight. Anhydrous THF (30 mL) was added. To the suspension in the
presence of a catalytic amount of 12 was added tert-butyl bromoacetate (0.562 ml, 3.81 mmol) under
Ar. The mixture was heated at 50°C. for 3 min and then stirred at room temperature for 2 h. This
mixture was then transferred to a mixture of 10.f (0.68 g, 1.27 mmol), Pd(P(t-Bu)3)2 (33 mg) in NMP (5
mL) and anhydrous THF (60 mL) under Ar. The reaction mixture was stirred at 100° C. under Ar for 16
h. After filtration, the filtrate was evaporated to dryness and the residue was purified by
chromatography (EtOAc-hexanes, 2:3) to give 10.g (0.69 g, 75%). MS: 537.1 (M+H+).
2
[Reactant]
Step
Stages Notes
Yield
2 2.1R:F3CCO2H, R:PhOMe, 1 h, rt
2.2 R:
R:EtN(Pr-i)2, S:DMF, 30 min, rt
2.3 2 h, rt
Reactants: 2, Reagents: 4, Solvents: 1,
Steps: 1, Stages: 3
94%
Experimental Procedure
Preparation of 3-cyclohexyl-4-[2-(2,4-dimethyl-thiazol-5-yl)-quinolin-6-Yl]-5-(2-morpholin-4-yl-2-oxo-
ethyl)-benzoic acid methyl ester (10.h). To 10.g (0.654 g, 1.27 mmol) was added TFA (4 mL) and
anisole (0.2 mL). The mixture was stirred at room temperature for 1 h. After evaporation of solvent, to
the residue was added DMF (5 ml), HATU (0.72 g, 1.91 mmol) and diisopropylethylamine (0.44 ml,
2.54 mmol). The mixture was stirred at room temperature for 30 min and morpholine (0.22 ml, 2.54
mmol) was then added. The mixture was stirred at room temperature for 2 h. After evaporation of
solvent, the product was purified by chromatography (from EtOAc-hexanes 1:1 to EtOAc). Yield 94%.
MS: 584.3 (M+H+).
3
Step Stages Notes Yield
3
3.1R:NaOH, S:H 2O, S:MeOH, S:THF, 2 h, 45°C 3.2
R:HCl, S:H 2O, pH 6
Reactants: 1, Reagents: 2, Solvents: 3,Steps: 1, Stages: 2
39%
Experimental Procedure
Preparation of 3-cyclohexyl-4-[2-(2,4-dimethyl-thiazol-5-yl)-quinolin-6-yl]-5-(2-morpholin-4-yl-2-oxo-ethyl)-benzoic acid (7). To a solution of 10.h (0.78 g, 1.34 mmol) in THF (8 mL) and MeOH (4 mL)was added 2N aq. NaOH (2.5 mL) and the mixture was stirred at 45°C. for 2 h. The mixture was
neutralized with 5 N HCl to pH 6. After evaporation of solvent, the residue was washed with water (25mL 2×) and dried to give compound 9 (30 mg, 39%). MS: 570.2 (M+H +). 1H-NMR (DMSO-d 6): δ (ppm)8.45 (d, 1H, J =8.7 Hz), 8.01 (d, 1H, J =8.4 Hz), 7.88 (d, 1H, J =8.7 Hz), 7.86 (d, 1H, J =1.2 Hz), 7.71-7.70 (m, 2H), 7.47 (dd, 1H, J =1.8, 8.7 Hz), 3.42 (d, 2H, J =4.5 Hz), 3.30-3.22 (m, 4H), 3.02-2.97 (m,4H), 2.72 (s, 3H), 2.68 (s, 3H), 2.22 (m, 1H), 1.64-0.82 (m, 10H).
4
Step Stages Notes
Yield 4
4.1R:HBTU, R:EtN(Pr-i )2, S:DMF, 30 min, rt 4.2
R:NH 3, S:Me 2CHOH, 1 h, rt
Reactants: 1, Reagents: 3, Solvents: 2,Steps: 1, Stages: 2
98%
Experimental Procedure
Preparation of 3-Cyclohexyl-4-[2-(2,4-dimethyl-thiazol-5-yl)-quinolin-6-yl]-5-(2-morpholin-4-yl-2-oxo-ethyl)-benzamide (11.a). A mixture of 11.a (0.142 g, 0.249 mmol), HBTU (0.189 g, 0.498 mmol) and diisopropylethylamine (0.14 mL, 0.8 mmol) in DMF (2 mL) was stirred at room temperature for 30 min and 2 M ammonia in 2-propanol (1.5 mL) was then added. The mixture was stirred at room
temperature for further 1 h. After evaporation of the solvent, the residue was extracted with CHCl 3-MeOH (5:1, 100 mL 2×) and the organic phase was washed with water (50 ml×3), dried over
anhydrous Na 2SO 4 and evaporated. The residue was purified by chromatography (CH 2Cl 2-MeOH,6:1) to give 11.b(1.387 g, 98%). MS: 569.2 (M+H +).
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
9. 6 Steps
1
Step Stages Notes Yield
1 1.1R:H2, C:Pd, S:MeOH, S:AcOEt, 40 min, rt Reactants: 1, Reagents: 1, Catalysts: 1,
Solvents: 2, Steps: 1, Stages: 1
100% Experimental Procedure
Preparation of 3-amino-5-cyclohexyl-4-[2-(2,4-dimethyl-thiazol-5-yl)-quinolin-6-yl]-benzoic acid methyl
ester (10.e). Compound 10.d (1.0 g) was dissolved in EtOAc/MeOH(2:1, 40 mL) and hydrogenated
under reduced pressure of 40 psi of H2 over 5% Pd/C for 40 min. 10.e was obtained in a quantitative
yield. MS: 472.2 (M+H+).
2
Step Stages Notes Yield
2
2.1R:HBr, S:H
2
O, S:Me2CO, 0°C
2.2R:NaNO2, S:H2O, 8 min, 0°C; 10 min, 0°C
2.3R:CuBr, 15 min, 5°C
Reactants: 1, Reagents: 3, Solvents: 2,
Steps: 1, Stages: 3
75% Experimental Procedure
Preparation of 3-bromo-5-cyclohexyl-4-[2-(2,4-dimethyl-thiazol-5-yl)-quinolin-6-yl]-benzoic acid methyl
ester (10.f). To a fine suspension of 10.e (0.81 g, 1.72 mmol) in acetone (35 mL) at 0°C. was added
48% aq. HBr (0.58 mL). A solution of NaNO2 (0.148 g, 2.15 mmol) in water (1 mL) was added
dropwise over 8 min. The mixture was stirred at 0°C. in an ice bath for another 10 min, and CuBr (30
mg) was added in three portions. The mixture was stirred at 5°C. for 15 min. After evaporation of the
solvent, the residue was extracted with EtOAc (150 ml.), washed with H2O and dried over Na2SO4.
The product was purified by chromatography (EtOAc-hexanes; 2:3) to give 10.f (0.69 g, 75%). MS:
537.1 (M+H+).
3
[Reactant]
Step Stages Notes Yield
3 3.1R:Zn, S:THF, 3 min, 50°C; 2 h, rt
3.2C:53199-31-8, S:THF, S:NMP, 16 h, 100°C
Reactants: 2, Reagents: 1, Catalysts: 1,
Solvents: 2, Steps: 1, Stages: 2
75% Experimental Procedure
Preparation of 3-tert-butoxycarbonylmethyl-5-cyclohexyl-4-[2-(2,4-dimethyl-thiazol-5-yl)-quinolin-6-yl]-
benzoic acid methyl ester (10.g). Zinc powder (0.5 g) was washed with 10% HCl, acetone and dried
under a high vacuum overnight. Anhydrous THF (30 mL) was added. To the suspension in the
presence of a catalytic amount of 12 was added tert-butyl bromoacetate (0.562 ml, 3.81 mmol) under
Ar. The mixture was heated at 50°C. for 3 min and then stirred at room temperature for 2 h. This
mixture was then transferred to a mixture of 10.f (0.68 g, 1.27 mmol), Pd(P(t-Bu)3)2 (33 mg) in NMP (5
mL) and anhydrous THF (60 mL) under Ar. The reaction mixture was stirred at 100° C. under Ar for 16
h. After filtration, the filtrate was evaporated to dryness and the residue was purified by
chromatography (EtOAc-hexanes, 2:3) to give 10.g (0.69 g, 75%). MS: 537.1 (M+H
+).
4
[Reactant]
Step Stages Notes Yield
4 4.1R:F3CCO2
H, R:PhOMe, 1 h, rt
4.2
R:
R:EtN(Pr-i)2, S:DMF, 30 min, rt
4.3 2 h, rt
Reactants: 2, Reagents: 4, Solvents: 1,
Steps: 1, Stages: 3
94%
Experimental Procedure
Preparation of 3-cyclohexyl-4-[2-(2,4-dimethyl-thiazol-5-yl)-quinolin-6-Yl]-5-(2-morpholin-4-yl-2-oxo-
ethyl)-benzoic acid methyl ester (10.h). To 10.g (0.654 g, 1.27 mmol) was added TFA (4 mL) and
anisole (0.2 mL). The mixture was stirred at room temperature for 1 h. After evaporation of solvent, to
the residue was added DMF (5 ml), HATU (0.72 g, 1.91 mmol) and diisopropylethylamine (0.44 ml,
2.54 mmol). The mixture was stirred at room temperature for 30 min and morpholine (0.22 ml, 2.54
mmol) was then added. The mixture was stirred at room temperature for 2 h. After evaporation of
solvent, the product was purified by chromatography (from EtOAc-hexanes 1:1 to EtOAc). Yield 94%.
MS: 584.3 (M+H+).
5
Step Stages Notes Yield
5 5.1R:NaOH, S:H2O, S:MeOH, S:THF, 2 h, 45°C
5.2R:HCl, S:H2O, pH 6
Reactants: 1, Reagents: 2, Solvents: 3,
Steps: 1, Stages: 2
39% Experimental Procedure
Preparation of 3-cyclohexyl-4-[2-(2,4-dimethyl-thiazol-5-yl)-quinolin-6-yl]-5-(2-morpholin-4-yl-2-oxo-ethyl)-benzoic acid (7). To a solution of 10.h (0.78 g, 1.34 mmol) in THF (8 mL) and MeOH (4 mL)was added 2N aq. NaOH (2.5 mL) and the mixture was stirred at 45°C. for 2 h. The mixture was
neutralized with 5 N HCl to pH 6. After evaporation of solvent, the residue was washed with water (25mL 2×) and dried to give compound 9 (30 mg, 39%). MS: 570.2 (M+H +). 1H-NMR (DMSO-d 6): δ (ppm)8.45 (d, 1H, J =8.7 Hz), 8.01 (d, 1H, J =8.4 Hz), 7.88 (d, 1H, J =8.7 Hz), 7.86 (d, 1H, J =1.2 Hz), 7.71-7.70 (m, 2H), 7.47 (dd, 1H, J =1.8, 8.7 Hz), 3.42 (d, 2H, J =4.5 Hz), 3.30-3.22 (m, 4H), 3.02-2.97 (m,4H), 2.72 (s, 3H), 2.68 (s, 3H), 2.22 (m, 1H), 1.64-0.82 (m, 10H).
6
Step Stages Notes
Yield 6
6.1R:HBTU, R:EtN(Pr-i )2, S:DMF, 30 min, rt 6.2
R:NH 3, S:Me 2CHOH, 1 h, rt
Reactants: 1, Reagents: 3, Solvents: 2,Steps: 1, Stages: 2
98%
Experimental Procedure
Preparation of 3-Cyclohexyl-4-[2-(2,4-dimethyl-thiazol-5-yl)-quinolin-6-yl]-5-(2-morpholin-4-yl-2-oxo-ethyl)-benzamide (11.a). A mixture of 11.a (0.142 g, 0.249 mmol), HBTU (0.189 g, 0.498 mmol) and diisopropylethylamine (0.14 mL, 0.8 mmol) in DMF (2 mL) was stirred at room temperature for 30 min and 2 M ammonia in 2-propanol (1.5 mL) was then added. The mixture was stirred at room
temperature for further 1 h. After evaporation of the solvent, the residue was extracted with CHCl 3-MeOH (5:1, 100 mL 2×) and the organic phase was washed with water (50 ml×3), dried over
anhydrous Na 2SO 4 and evaporated. The residue was purified by chromatography (CH 2Cl 2-MeOH,6:1) to give 11.b(1.387 g, 98%). MS: 569.2 (M+H +).
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.10. 5 Steps
1
Step Stages Notes Yield
1 1.1R:HBr, S:H2O, S:Me2CO, 0°C
1.2R:NaNO2, S:H2O, 8 min, 0°C; 10 min, 0°C
1.3R:CuBr, 15 min, 5°C
Reactants: 1, Reagents: 3, Solvents: 2,
Steps: 1, Stages: 3
75% Experimental Procedure
Preparation of 3-bromo-5-cyclohexyl-4-[2-(2,4-dimethyl-thiazol-5-yl)-quinolin-6-yl]-benzoic acid methyl
ester (10.f). To a fine suspension of 10.e (0.81 g, 1.72 mmol) in acetone (35 mL) at 0°C. was added
48% aq. HBr (0.58 mL). A solution of NaNO2 (0.148 g, 2.15 mmol) in water (1 mL) was added
dropwise over 8 min. The mixture was stirred at 0°C. in an ice bath for another 10 min, and CuBr (30
mg) was added in three portions. The mixture was stirred at 5°C. for 15 min. After evaporation of the
solvent, the residue was extracted with EtOAc (150 ml.), washed with H2O and dried over Na2SO4.
The product was purified by chromatography (EtOAc-hexanes; 2:3) to give 10.f (0.69 g, 75%). MS:
537.1 (M+H+).
2
[Reactant]
Step Stages Notes Yield
2 2.1R:Zn, S:THF,
3 min, 50°C; 2 h, rt
2.2C:53199-31-8, S:THF, S:NMP, 16 h, 100°C
Reactants: 2, Reagents: 1, Catalysts: 1,
Solvents: 2, Steps: 1, Stages: 2
75% Experimental Procedure
Preparation of 3-tert-butoxycarbonylmethyl-5-cyclohexyl-4-[2-(2,4-dimethyl-thiazol-5-yl)-quinolin-6-yl]-
benzoic acid methyl ester (10.g). Zinc powder (0.5 g) was washed with 10% HCl, acetone and dried
under a high vacuum overnight. Anhydrous THF (30 mL) was added. To the suspension in the
presence of a catalytic amount of 12 was added tert-butyl bromoacetate (0.562 ml, 3.81 mmol) under
Ar. The mixture was heated at 50°C. for 3 min and then stirred at room temperature for 2 h. This
mixture was then transferred to a mixture of 10.f (0.68 g, 1.27 mmol), Pd(P(t-Bu)3)2 (33 mg) in NMP (5
mL) and anhydrous THF (60 mL) under Ar. The reaction mixture was stirred at 100° C. under Ar for 16
h. After filtration, the filtrate was evaporated to dryness and the residue was purified by
chromatography (EtOAc-hexanes, 2:3) to give 10.g (0.69 g, 75%). MS: 537.1 (M+H+).
3
[Reactant]
Step Stages Notes Yield
3 3.1R:F3CCO2H, R:PhOMe, 1 h, rt
3.2 R:
R:EtN(Pr-i)2, S:DMF, 30 min, rt
3.3 2 h, rt
Reactants: 2, Reagents: 4, Solvents: 1,
Steps: 1, Stages: 3
94%
Experimental Procedure
Preparation of 3-cyclohexyl-4-[2-(2,4-dimethyl-thiazol-5-yl)-quinolin-6-Yl]-5-(2-morpholin-4-yl-2-oxo-
ethyl)-benzoic acid methyl ester (10.h). To 10.g (0.654 g, 1.27 mmol) was added TFA (4 mL) and
anisole (0.2 mL). The mixture was stirred at room temperature for 1 h. After evaporation of solvent, to
the residue was added DMF (5 ml), HATU (0.72 g, 1.91 mmol) and diisopropylethylamine (0.44 ml,
2.54 mmol). The mixture was stirred at room temperature for 30 min and morpholine (0.22 ml, 2.54
mmol) was then added. The mixture was stirred at room temperature for 2 h. After evaporation of
solvent, the product was purified by chromatography (from EtOAc-hexanes 1:1 to EtOAc). Yield 94%.
MS: 584.3 (M+H+).
4
Step Stages Notes Yield
4 4.1R:NaOH, S:H
2
O, S:MeOH, S:THF, 2 h, 45°C
4.2R:HCl, S:H2O, pH 6
Reactants: 1, Reagents: 2, Solvents: 3,
Steps: 1, Stages: 2
39% Experimental Procedure
Preparation of 3-cyclohexyl-4-[2-(2,4-dimethyl-thiazol-5-yl)-quinolin-6-yl]-5-(2-morpholin-4-yl-2-oxo-
ethyl)-benzoic acid (7). To a solution of 10.h (0.78 g, 1.34 mmol) in THF (8 mL) and MeOH (4 mL)
was added 2N aq. NaOH (2.5 mL) and the mixture was stirred at 45°C. for 2 h. The mixture was
neutralized with 5 N HCl to pH 6. After evaporation of solvent, the residue was washed with water (25
mL 2×) and dried to give compound 9 (30 mg, 39%). MS: 570.2 (M+H+). 1H-NMR (DMSO-d6): δ (ppm)
8.45 (d, 1H, J=8.7 Hz), 8.01 (d, 1H, J=8.4 Hz), 7.88 (d, 1H, J=8.7 Hz), 7.86 (d, 1H, J=1.2 Hz), 7.71-
7.70 (m, 2H), 7.47 (dd, 1H, J=1.8, 8.7 Hz), 3.42 (d, 2H, J=4.5 Hz), 3.30-3.22 (m, 4H), 3.02-2.97 (m,
4H), 2.72 (s, 3H), 2.68 (s, 3H), 2.22 (m, 1H), 1.64-0.82 (m, 10H).
5
Step Stages Notes Yield
5
5.1R:HBTU, R:EtN(Pr-i )2, S:DMF, 30 min, rt 5.2
R:NH 3, S:Me 2CHOH, 1 h, rt
Reactants: 1, Reagents: 3, Solvents: 2,Steps: 1, Stages: 2
98%
Experimental Procedure
Preparation of 3-Cyclohexyl-4-[2-(2,4-dimethyl-thiazol-5-yl)-quinolin-6-yl]-5-(2-morpholin-4-yl-2-oxo-ethyl)-benzamide (11.a). A mixture of 11.a (0.142 g, 0.249 mmol), HBTU (0.189 g, 0.498 mmol) and diisopropylethylamine (0.14 mL, 0.8 mmol) in DMF (2 mL) was stirred at room temperature for 30 min and 2 M ammonia in 2-propanol (1.5 mL) was then added. The mixture was stirred at room
temperature for further 1 h. After evaporation of the solvent, the residue was extracted with CHCl
3-MeOH (5:1, 100 mL 2×) and the organic phase was washed with water (50 ml×3), dried over
anhydrous Na
2SO 4 and evaporated. The residue was purified by chromatography (CH 2Cl 2-MeOH,6:1) to give 11.b(1.387 g, 98%). MS: 569.2 (M+H +).
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.11. 2 Steps
1
Step Stages Notes Yield 1
1.1
R:C 5H 5N, R:O(C(=O)CF 3)2, S:DMF, 0°C; 6 h, rt Reactants: 1, Reagents: 2, Solvents: 1,
Steps: 1, Stages: 1
68%
Experimental Procedure
Preparation of 3-Cyclohexyl-4-[2-(2,4-dimethyl-thiazol-5-yl)-quinolin-6-yl]-5-(2-morpholin-4-yl-2-oxo-ethyl)-benzonitrile (11.c). To a solution of 11.b (0.102 g, 0.179 mmol) in anhydrous DMF (1.5 mL) was added trifluoroacetic anhydride (75 μL, 0.537 mmol) and pyridine (38 μL, 0.716 mmol) at 0°C. The mixture was then stirred at room temperature for 6 h. After evaporation of solvent, the residue was separated by chromatogaraphy eluting with CH 2Cl 2-MeOH (10:1) to give 11.c. Yield 68%. MS: 551.2(M+H +).
2
Step Stages Notes
Yield 2
2.1
R:
S:PhMe, 3 d, reflux
2.2
R:HCl, S:H 2O, S:MeOH, 30 min, rt
Reactants: 1, Reagents: 2, Solvents: 3,Steps: 1, Stages: 2
46%
Experimental Procedure
Preparation of 2-[3-cyclohexyl-2-[2-(2,4-dimethyl-thiazol-5-yl)-quinolin-6-yl]-5-(1H-tetrazol-5-yl)-phenyl]-1-morpholin-4-yl-ethanone (11). A mixture of 11.c (77 mg, 0.14 mmol) and azidotrimethyltin (43 mg, 0.207 mmol) in toluene (7 mL) was stirred under Ar at refluxing for 3 days. After evaporation of solvent, the residue was dissolved in MeOH (15 mL) and 5N HCl (1 mL) was added. The mixture was stirred at room temperature for 30 min and the solvent was evaporated. The residue was
separated by chromatography eluting with CH 2Cl 2-MeOH (8:1) to give compound 11 (38.1 mg, 46%).MS: 594.46 (M+H +). 1H-NMR (DMSO-d 6): δ (ppm) 8.47 (d, 1H, J =8.4 Hz), 8.07 (br s, 1H), 8.03 (d, 1H,J =8.4 Hz), 7.90 (d, 1H, J =8.4 Hz), 7.84 (br s, 1H), 7.75 (br s, 1H), 7.51 (d, 1H, J =8.7 Hz), 3.45 (d, 2H,J =4.8 Hz), 3.31-3.23 (m, 4H), 3.03-2.99 (m, 4H), 2.73 (s, 3H), 2.70 (s, 3H), 2.25 (m, 1H), 1.74-1.52(m, 6H), 1.22-1.16 (m, 2H), 0.94-0.85 (m, 2H).
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006 John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
12. 3 Steps
1
Step Stages Notes Yield
1 1.1R:HBTU, R:EtN(Pr-i)2, S:DMF, 30 min, rt
1.2R:NH3, S:Me2CHOH, 1 h, rt
Reactants: 1, Reagents: 3, Solvents: 2,
Steps: 1, Stages: 2
98% Experimental Procedure
Preparation of 3-Cyclohexyl-4-[2-(2,4-dimethyl-thiazol-5-yl)-quinolin-6-yl]-5-(2-morpholin-4-yl-2-oxo-
ethyl)-benzamide (11.a). A mixture of 11.a (0.142 g, 0.249 mmol), HBTU (0.189 g, 0.498 mmol) and
diisopropylethylamine (0.14 mL, 0.8 mmol) in DMF (2 mL) was stirred at room temperature for 30 min
and 2 M ammonia in 2-propanol (1.5 mL) was then added. The mixture was stirred at room
temperature for further 1 h. After evaporation of the solvent, the residue was extracted with CHCl3-
MeOH (5:1, 100 mL 2×) and the organic phase was washed with water (50 ml×3), dried over
anhydrous Na2SO4 and evaporated. The residue was purified by chromatography (CH2Cl2-MeOH,
6:1) to give 11.b(1.387 g, 98%). MS: 569.2 (M+H+).
2
Step Stages Notes Yield
2 2.1R:C5H5N, R:O(C(=O)CF3)2, S:DMF, 0°C; 6 h, rt Reactants: 1, Reagents: 2, Solvents: 1,
Steps: 1, Stages: 1
68% Experimental Procedure
Preparation of 3-Cyclohexyl-4-[2-(2,4-dimethyl-thiazol-5-yl)-quinolin-6-yl]-5-(2-morpholin-4-yl-2-oxo-
ethyl)-benzonitrile (11.c). To a solution of 11.b (0.102 g, 0.179 mmol) in anhydrous DMF (1.5 mL) was
added trifluoroacetic anhydride (75 μL, 0.537 mmol) and pyridine (38 μL, 0.716 mmol) at 0°C. The
mixture was then stirred at room temperature for 6 h. After evaporation of solvent, the residue was
separated by chromatogaraphy eluting with CH2Cl2-MeOH (10:1) to give 11.c. Yield 68%. MS: 551.2
(M+H+).
3
Step Stages Notes
Yield 3
3.1
R:
S:PhMe, 3 d, reflux
3.2
R:HCl, S:H 2O, S:MeOH, 30 min, rt
Reactants: 1, Reagents: 2, Solvents: 3,Steps: 1, Stages: 2
46%
Experimental Procedure
Preparation of 2-[3-cyclohexyl-2-[2-(2,4-dimethyl-thiazol-5-yl)-quinolin-6-yl]-5-(1H-tetrazol-5-yl)-phenyl]-1-morpholin-4-yl-ethanone (11). A mixture of 11.c (77 mg, 0.14 mmol) and azidotrimethyltin (43 mg, 0.207 mmol) in toluene (7 mL) was stirred under Ar at refluxing for 3 days. After evaporation of solvent, the residue was dissolved in MeOH (15 mL) and 5N HCl (1 mL) was added. The mixture was stirred at room temperature for 30 min and the solvent was evaporated. The residue was
separated by chromatography eluting with CH 2Cl 2-MeOH (8:1) to give compound 11 (38.1 mg, 46%).MS: 594.46 (M+H +). 1H-NMR (DMSO-d 6): δ (ppm) 8.47 (d, 1H, J =8.4 Hz), 8.07 (br s, 1H), 8.03 (d, 1H,J =8.4 Hz), 7.90 (d, 1H, J =8.4 Hz), 7.84 (br s, 1H), 7.75 (br s, 1H), 7.51 (d, 1H, J =8.7 Hz), 3.45 (d, 2H,J =4.8 Hz), 3.31-3.23 (m, 4H), 3.03-2.99 (m, 4H), 2.73 (s, 3H), 2.70 (s, 3H), 2.25 (m, 1H), 1.74-1.52(m, 6H), 1.22-1.16 (m, 2H), 0.94-0.85 (m, 2H).
CASREACT ?: Copyright ? 2013 American Chemical Society. All Rights Reserved. CASREACT contains reactions from CAS and from: ZIC/VINITI database (1974-1999) provided by InfoChem; INPI data prior to 1986; Biotransformations database compiled under the direction of Professor Dr. Klaus Kieslich; organic reactions, portions copyright 1996-2006John Wiley & Sons, Ltd., John Wiley and Sons, Inc., Organic Reactions Inc., and Organic Syntheses Inc. Reproduced under license. All Rights Reserved.
13. 4 Steps
1
Step Stages Notes Yield
1 1.1R:NaOH, S:H2O, S:MeOH, S:THF,
2 h, 45°C
1.2R:HCl, S:H2O, pH 6
Reactants: 1, Reagents: 2, Solvents: 3,
Steps: 1, Stages: 2
39% Experimental Procedure
Preparation of 3-cyclohexyl-4-[2-(2,4-dimethyl-thiazol-5-yl)-quinolin-6-yl]-5-(2-morpholin-4-yl-2-oxo-
ethyl)-benzoic acid (7). To a solution of 10.h (0.78 g, 1.34 mmol) in THF (8 mL) and MeOH (4 mL)
was added 2N aq. NaOH (2.5 mL) and the mixture was stirred at 45°C. for 2 h. The mixture was
neutralized with 5 N HCl to pH 6. After evaporation of solvent, the residue was washed with water (25
mL 2×) and dried to give compound 9 (30 mg, 39%). MS: 570.2 (M+H+). 1H-NMR (DMSO-d6): δ (ppm)
8.45 (d, 1H, J=8.7 Hz), 8.01 (d, 1H, J=8.4 Hz), 7.88 (d, 1H, J=8.7 Hz), 7.86 (d, 1H, J=1.2 Hz), 7.71-
7.70 (m, 2H), 7.47 (dd, 1H, J=1.8, 8.7 Hz), 3.42 (d, 2H, J=4.5 Hz), 3.30-3.22 (m, 4H), 3.02-2.97 (m,
4H), 2.72 (s, 3H), 2.68 (s, 3H), 2.22 (m, 1H), 1.64-0.82 (m, 10H).
2
Step Stages Notes Yield
2 2.1R:HBTU, R:EtN(Pr-i)2, S:DMF, 30 min, rt
2.2R:NH3, S:Me2CHOH, 1 h, rt
Reactants: 1, Reagents: 3, Solvents: 2,
Steps: 1, Stages: 2
98% Experimental Procedure
Preparation of 3-Cyclohexyl-4-[2-(2,4-dimethyl-thiazol-5-yl)-quinolin-6-yl]-5-(2-morpholin-4-yl-2-oxo-
ethyl)-benzamide (11.a). A mixture of 11.a (0.142 g, 0.249 mmol), HBTU (0.189 g, 0.498 mmol) and
diisopropylethylamine (0.14 mL, 0.8 mmol) in DMF (2 mL) was stirred at room temperature for 30 min
and 2 M ammonia in 2-propanol (1.5 mL) was then added. The mixture was stirred at room
temperature for further 1 h. After evaporation of the solvent, the residue was extracted with CHCl3-
MeOH (5:1, 100 mL 2×) and the organic phase was washed with water (50 ml×3), dried over
anhydrous Na2SO4 and evaporated. The residue was purified by chromatography (CH2Cl2-MeOH,
6:1) to give 11.b(1.387 g, 98%). MS: 569.2 (M+H+).
乙酰苯胺的制备 一.实验目的 1.学习实验室制备芳香族酰胺的原理和方法。 2.训练固体有机物的热过滤、脱色、洗涤、重结晶、干燥等纯化技术。 二.实验原理 NH 2+CH 3COOH 3+H 2O 芳香族酰胺通常用伯或仲芳胺与酸酐或羧酸反应制备,因为酸酐的价格较贵,所以一般选羧酸。本反应是可逆的,为提高平衡转化率,加入了过量的冰醋酸,同时不断地把生成的水移出反应体系,可以使反应接近完成。为了让生成的水蒸出,而又仅可能地让沸点接近的醋酸少蒸出来,本实验采用较长的分馏柱进行分馏。实验加入少量的锌粉,是为了防止反应过程中苯胺被氧化。 三.试剂及物理常数 四、实验流程 5ml 苯胺 7.4ml 冰醋酸0.1g 锌粉 称重计算产率
抽滤装置 干燥装置 布氏漏斗 抽滤瓶 反应装置 六、操作要点和说明 1.合成 (1).反应物量的确定: 本实验反应是可逆的,采用乙酸过量和从反应体系中分出水的方法来提高乙酰苯胺的产率,但随之会增加副产物二乙酰基苯胺的生成量。二乙酰苯胺很容易水解成乙酰苯胺和乙酸,在产物精制过程中通过水洗、重结晶等操作,二乙酰基苯胺水解成乙酰苯胺和乙酸,经过滤可除去乙酸,不影响乙酰苯胺的产率和纯度。 苯胺极易氧化,在空气中放置会变成红色,使用时必须重新蒸馏除去其中的杂质。反应过程中加入少许锌粉。锌粉在酸性介质中可使苯胺中有色物质还原,防止苯胺继续氧化。在实验中可以看到,锌粉加得适量,反应混合物呈淡黄色或接近无色。但锌粉不能加得太多,一方面消耗乙酸,另一方面在精制过程中乙酸锌水解成氢氧化锌,很难从乙酰苯胺中分离出来。 (2).合成反应装置的设计: 水沸点为100℃,乙酸沸点为117℃,两者仅差17℃,若要分离出水而不夹带更多的乙酸,必须使用分馏反应装置,而不能用蒸馏的反应装置。本实验用分馏柱。 一般有机反应用耐压、耐液体沸腾冲出的圆形瓶作反应器。由于乙酰苯胺的熔点为114℃,稍冷即固化,不易从圆形瓶中倒出,因此用锥形瓶作反应器更方便。 分出的水量很少,分馏柱可以不连接冷凝管,在分馏柱支口上直接连尾接管,兼作空气冷凝管即可,使装置更简单。 为控制反应温度,在分馏柱顶口插温度计。 (3).操作条件的控制 保持分馏柱顶温度低于105℃的稳定操作,开始缓慢加热,使反应进行一段时间,有水生成
11种过硫酸氢钾检测方法 实际上有些概念行业内可能一直没有清晰,比如单过硫酸氢钾和复合过硫酸氢钾 的差异到底有多大可能大多数人都没有细想过,这里面的差距大到你可能想像不到, 复合过硫酸氢钾盐的配方实际上是产品的生命线!当然这一点并没有让足够的经销商 和养殖户重视,因为他们没有条件研究到这一步,有的甚至对产品的真假都无法分辨!而这篇文章把当前的一些过硫酸氢钾检测方法进行汇总,为大家在判断真品时提供一 些帮助: 一、液—质联用法(LC-MS)或气-质联用(GC-MS)法 (一)方法简介 液—质联用法(LC-MS)是指利用液相色谱法与质谱法共同分析样品; 气-质联用(GC-MS)法是指利用气相色谱法与质谱法共同分析样品; 这两种检测方法都可以准确的分离产品,并且可以分析样品中的相关组成。 具体方法的选择还要考虑产品的配方组成。 (二)综合评价 项目检测准确率检测方便程度检测费用 评星★★★★★☆☆☆☆☆★★★★★ 原因定性、定量都非常准确极不方便:相关的仪器一般只在大专院校及科研院校才有,日常检测并不方便在一般的检测方法中,费用属于最高的 二、EDX等分析法 (一)方法简介 可以选择EDX(X射线能量光谱仪)分析法,当然也可以选择使用XRF(核磁共 振波谱分析仪)、FTIR(红外光谱分析)、XRD(X射线荧光光谱仪)进行钾离子的 定性分析。 通过仪器设备直接分析产品中是否含有钾离子从而辅助判断产品真假。 (二)综合评价 项目检测准确率检测方便程度检测费用
评星★★★☆☆☆☆☆☆☆★★★☆☆ 原因 1、只能判断是否含有钾离子,但不能判断其来源 2、当然也可以结合其实方法通过进一步断定是否含有硫酸根离子,来进一步确定产品真假。极不方便:相关的仪器一般只在大专院校及科研院校才有,日常检测并不方便在一般的检测方法中,费用属于较高的 三、碘化钾检测法 (一)方法简介 确认为真品过硫酸氢钾的情况下将将1g左右的过硫酸氢钾片/粉完全溶解于50ml 左右的净水中,摇匀后加入1g左右的KI固体,颜色越深则含量越高。 此方法是活性氧检测方法的简易版,为了方便操作,只能通过深浅判断高低,不 能确认具体含量。 (二)综合评价 项目检测准确率检测方便程度检测费用 评星★★★☆☆★★★☆☆★★☆☆☆ 原因 1、如果是真品,可以通过此方法判定产品含量高低; 2、具有高氧化性的化学物都可以对变色造成影响,比如亚氯酸钠、氯制剂;需 购买碘化钾。相对于一般的检测方法属于较高的 四、活性氧检测法 (一)方法简介 因真品是由复合过硫酸氢钾盐是由单过硫复配而成,而单过硫纯品一般含氧在%,那么相应含量的复合过硫酸氢钾应该对应含有一定量的活性氧。 此方法只能再确认真品的情况下,检测过硫酸氢钾含量的高低,并不能分辨产品 真假。 (二)综合评价 项目检测准确率检测方便程度检测费用 评星★★★☆☆★★☆☆☆★★★☆☆
有机合成课程设计 题目香兰素的合成工艺 系(院)化学与化工系 专业应用化学 班级11应化本2 学生姓名王春莲 学号1114100327 指导教师张圣燕 职称讲师 2013年 12月 20日
香兰素的合成工艺设计 1 产品简介 1.1 中英文名称,化学式,结构式 中文名称:香兰素 别名:香荚兰醛;香荚兰素;香兰醛 化学名称:3-甲氧基-4-羟基苯甲醛 英文名称:Vanillin 分子式:C8H8O3 结构式: CHO OH OCH3 1.2 物化性质 白色至微黄色鳞片状结晶或结晶性粉末,存在有不同熔点的四种结晶变型。呈甜克力香气及强烈的香兰素所独有的芳香气,香气比香兰素强3-4 倍。沸点285 ℃,点76.5 ℃。微溶于水,溶于乙醇、乙醚、甘油、丙二醇、氯仿和碱溶液。基本上无毒害,但其蒸气对皮肤及粘膜有局部刺激作用 1.3 用途 香兰素是重要的食用香料之一,是食用调香剂,具有香荚兰豆香气及浓郁的奶香,是食品添加剂行业中不可缺少的重要原料,广泛运用在各种需要增加奶香气息的调香食品中,如蛋糕、冷饮、巧克力、糖果、饼干、方便面、面包以及烟草、调香酒类、牙膏、肥皂、香水、化妆品、冰淇淋、饮料以及日用化妆品中起增香和定香作用。 香兰素在国外的应用领域很广,大量用于生产医药中间体,也用于植物生长促进剂、杀菌剂、润滑油消泡剂、电镀光亮剂、印制线路板生产导电剂等。国内香兰素主要用于食品添加剂,近几年在医药领域的应用不断拓宽,已成为香兰素应用最
有潜力的领域。 香兰素在国外的应用领域很广,大量用于生产医药中间体,也用于植物生长促进剂、杀菌剂、润滑油消泡剂、电镀光亮剂、印制线路板生产导电剂等。国内香兰素主要用于食品添加剂,近几年在医药领域的应用不断拓宽,已成为香兰素应用最有潜力的领域。目前国内香兰素消费:食品工业占55%,医药中间体占30%,饲料调味剂占10%,化妆品等占5%。 1.4 前景分析 国内外行业现状中国是世界香兰素出口大国,2002年国内需求量2350吨,占产量的30%,其余70%用于出口。而1988年仅出口273吨,1993年为1700吨,2002年为4653吨。1993~2002年,中国香兰素出口量年均增长率为12%。中国香兰素在北美、欧洲、东南亚等地市场享有良好信誉。 2 合成方法 2.1 第一种合成方法——愈创木酚法 (1)合成基本原理 愈创木酚在碱性条件下和乙醛缩合成3-甲氧基-4-羟基苯乙醇酸,3-甲氧基-4-羟基苯乙醇酸在碱性条件下被氧化成3-甲氧基-4-羟基苯乙酮酸(香草扁桃酸),然后在碱性条件下脱羧生成香兰素。其反应方程式如下: OCH 3 OH CHOCOOH CHOHCOOH OH OCH3 O2 OH OCH 3 CCOOH O CHO OH OCH3
2006年第26卷有机化学V ol. 26, 2006第11期, 1548~1552 Chinese Journal of Organic Chemistry No. 11, 1548~1552 ygzhou@https://www.doczj.com/doc/4d14721364.html, * E-mail: Received February 14, 2006; revised April 10, 2006; accepted May 23, 2006.
No. 11 陈国英等:1-取代异喹啉合成新方法的研究1549 Scheme 1 1.2 合成1-取代-2-苄氧羰基-1,2-二氢异喹啉 以合成1-正丁基-2-苄氧羰基-1,2-二氢异喹啉(1c)为例: 氮气保护下在一个50 mL反应瓶中, 加入镁(86 mg, 3.6 mmol), 几粒碘, 10 mL 干燥的乙醚. 滴加正丁基溴(493 mg, 0.38 mL, 3.6 mmol), 加毕, 室温搅拌30 min, 制备好格氏试剂备用. 氮气保护下的100 mL反应瓶中, 加入异喹啉(315 mg, 2.4 mmol), 再加入30 mL 干燥的乙醚. 冷却至-78 ℃后, 将制备好的格氏试剂滴加到异喹啉中, 继续搅拌10 min, 滴加氯甲酸苄酯(494 mg, 0.42 mL, 2.9 mmol). 加毕, 5 min后撤去冷浴. TLC跟踪反应, 原料消失后, 加入饱和氯化铵溶液40 mL, 乙醚(20 mL×2)萃取, 饱和食盐水洗涤, 无水硫酸钠干燥. 除去溶剂, 剩余物柱层析, 得到淡黄色油状物1c 724 mg (收率94%). 其它化合物的合成采用类似的操作进行. 化合物1a~1i的谱图数据如下: 1-甲基-2-苄氧羰基-1,2-二氢异喹啉(1a): 1H NMR (CDCl3, 400 MHz) δ: 1.30 (d, J=6.0 Hz, 3H), 5.23~5.28 (m, 2H), 5.32, 5.47 (q, J=6.4 Hz, 1H), 5.78, 5.88 (d, J=8.0 Hz, 1H), 6.80, 6.90 (d, J=8.0 Hz, 1H), 7.02~7.41 (m, 8H). 1-乙基-2-苄氧羰基-1,2-二氢异喹啉(1b): 1H NMR (CDCl3, 400 MHz) δ: 0.78~0.87 (m, 3H), 1.63~1.73 (m, 2H), 5.16~5.28 (m, 3H), 5.79, 5.91 (d, J=7.8 Hz, 1H), 6.83~7.40 (m, 10H); 13C NMR (CDCl3, 100 MHz) δ: 10.1, 28.2, 28.8, 57.1, 57.6, 68.0, 109.0, 109.3, 124.7, 124.8, 125.0, 125.5, 126.5, 126.7, 126.9, 127.7, 127.8, 128.3, 128.4, 128.5, 128.8, 130.3, 132.7, 136.3, 153.1, 153.9. HRMS calcd for C19H19NO2 (M++1) 294.1489, found 294.1465. 1-正丁基-2-苄氧羰基-1,2-二氢异喹啉(1c): 1H NMR (CDCl3, 400 MHz) δ: 0.77~0.85 (m, 3H), 1.18~1.25 (m, 4H), 1.61~1.66 (m, 2H), 5.20~5.35 (m, 3H), 5.81, 5.92 (d, J=7.6 Hz, 1H), 6.82~7.41 (m, 10H); 13C NMR (CDCl3, 100 MHz) δ: 14.2, 22.7, 22.8, 27.7, 34.9, 35.5, 55.9, 56.4, 68.0, 68.1, 69.9, 109.2, 109.4, 124.6, 124.8, 125.0, 125.5, 126.4, 126.6, 126.8, 127.0, 127.6, 127.7, 128.3, 128.4, 128.5, 128.8, 130.3, 133.1, 133.2, 136.3, 153.1, 153.8. HRMS calcd for C21H23NO2 (M++1) 322.1802, found 322.1774. 1-苄基-2-苄氧羰基-1,2-二氢异喹啉(1d): 1H NMR (CDCl3, 400 MHz) δ: 2.74~2.97 (m, 2H), 4.76~5.16 (m, 2H), 5.37~5.52 (m, 1H), 5.81, 5.97 (d, J=7.8 Hz, 1H), 6.59, 6.84 (d, J=7.8 Hz, 1H), 6.97~7.38 (m, 14H); 13C NMR (CDCl3, 100 MHz) δ: 41.0, 41.6, 57.4, 58.1, 68.0, 68.1, 108.9, 109.4, 124.4, 124.7, 125.0, 125.1, 126.6, 127.0, 127.8, 128.0, 128.2, 128.3, 128.5, 128.7, 129.9, 130.1, 131.7, 137.2, 152.8, 153.6. HRMS calcd for C24H21NO2 (M++1) 356.1645, found 356.1620. 1-苯基-2-苄氧羰基-1,2-二氢异喹啉(1e): 1H NMR (CDCl3, 400 MHz) δ: 5.16~5.29 (m, 2H), 5.86, 5.92 (d, J=7.4 Hz, 1H), 6.33, 6.52 (s, 1H), 6.89~7.34 (m, 15H). 1-(2-甲氧基苯基)-2-苄氧羰基-1,2-二氢异喹啉(1f): 1H NMR (CDCl 3 , 400 MHz) δ: 3.67 (s, 3H), 3.94 (s, 1H), 5.05~5.26 (m, 2H), 5.78, 5.86 (d, J=7.7 Hz, 1H), 6.78~7.51 (m, 14H); 13C NMR (CDCl3, 100 MHz) δ: 52.6, 55.4, 55.9, 68.0, 107.6, 107.8, 110.8, 111.3, 121.1, 121.3, 125.1, 125.2, 126.0, 126.6, 126.8, 127.3, 127.5, 127.6, 127.8, 128.1, 128.3, 128.5, 128.7, 129.2, 133.5, 136.1, 152.9, 154.0, 154.1, 154.9. HRMS calcd for C24H21NO3 (M++1) 372.1594, found 372.1573. 1-(3-甲氧基苯基)-2-苄氧羰基-1,2-二氢异喹啉(1g): 1H NMR (CDCl 3 , 400 MHz) δ: 3.68 (d, J=24.2 Hz, 3H), 5.17~5.29 (m, 2H), 5.85, 5.92 (d, J=7.7 Hz, 1H), 6.29, 6.49 (s, 1H), 6.73~ 7.35 (m, 14H); 13C NMR (CDCl3, 100 MHz) δ: 55.2, 5 8.2, 5 9.3, 68.3, 108.8, 109.1, 112.6, 112.9, 113.3, 119.0, 119.7, 125.0, 125.2, 125.4, 125.9, 127.3, 127.4, 127.5, 128.1, 128.3, 128.5, 128.7, 129.6, 130.3, 131.7, 136.0, 143.6, 144.3, 153.3, 159.7. HRMS calcd for C24H21NO3 (M++1) 372.1594, found 372.1572. 1-(4-甲氧基苯基)-2-苄氧羰基-1,2-二氢异喹啉(1h): 1H NMR (CDCl 3 , 400 MHz) δ: 3.74 (s, 3H), 5.16~5.29 (m, 2H), 5.85, 5.94 (d, J=7.6 Hz, 1H), 6.29, 6.49 (s, 1H), 6.72~6.87 (m, 2H), 7.05~7.35 (m, 12H); 13C NMR (CDCl3, 100 MHz) δ: 55.4, 57.7, 58.6, 68.3, 109.0, 113.9,
过硫酸氢钾复合盐的检测方法 <该方法为连云港新江环保材料有限公司过硫酸氢钾复合盐的检测标准> 一、活性氧和有效成分的检测 试剂: 10%硫酸溶液;25%碘化钾溶液;0.5%的淀粉指示剂;0.1N的硫代硫酸钠标准溶液。 1、10%硫酸溶液 量取60ml硫酸,缓缓注入约700ml水中,冷却,用水稀释到1000ml。 2、25%碘化钾溶液 称取250g碘化钾,溶于500ml水中,稀释到1000ml。 3、0.5%的淀粉指示剂 称取1.0g可溶性淀粉,加水10ml,在搅拌条件下注入200ml沸水中,再微沸2min,放置待冷,取上层清液使用,此溶液于使用前制备。 4、0.1N的硫代硫酸钠标准溶液
称取26g硫代硫酸钠(或16g无水硫代硫酸钠),溶于1000ml水中,缓缓煮沸10min,冷却,放置两周后过滤备用。 标定: 称取0.15g于120℃烘干至恒重的基准重铬酸钾,称准至0.0002g,置于碘量瓶中,溶于25ml水,加入2g碘化钾及20ml4N硫酸,摇匀,于暗处放置10min。加150ml水,用0.1N的硫代硫酸钠标准溶液滴定,接近终点加3ml0.5%淀粉指示剂,继续滴定至溶液由蓝色变为亮绿色。同时做空白实验。 硫代硫酸钠标准溶液的当量浓度N=m/(V1-V2)*0.04903 其中: V1-滴定重铬酸钾耗用的硫代硫酸钠标准溶液体积,ml; V2-空白实验耗用的硫代硫酸钠标准溶液体积,ml; m-重铬酸钾的质量,g; 0.04903-每毫克当量K2Cr2O7。 比较: a.测定方法:量取30.00~35.00ml0.1N碘标准溶液,置于碘量瓶中,加150ml水,用0.1N硫代硫酸钠标准溶液滴定,接近终点加3ml0.5%淀粉指示剂,继续滴定至溶液蓝色消失。 同时做水所消耗碘的空白实验,方法如下:取250ml水,加0.05ml0.1N碘标准溶液和3ml0.5%淀粉指示剂,用硫代硫酸钠标准溶液滴定至溶液蓝色消失。
香兰素生产工艺及其改进 始有溴蒸气从液面下逸出的瞬闻),就应及时 停止通溴,并分次少量地补加粉末状碳酸锂进 行中和调整至pH3.0~5.0范围内,直至通溴 操作结束为止. 加完碳酸镪后,将料绩由6O℃逐渐升温至 80℃,调节并控制料液的pH值为5.0(可先用 精密试纸粗测,再取样液用甲基红试液检查剐 呈黄色即可)无变化后,即达合成反应的终 点.停止通溴和搅拌,关上蒸汽. 取样液进行杂质检查.如果溶液中尚存有 过量的溴素(当用pH试纸测定时,其尾部呈 血红色条纹状),应补加尿素进行处理J如含有 溴酸盐成分(当往样液的试管中加入稀硫酸 时,样液呈黄色),则应加入少量硫脲进行还 原处理若料液中所含有的硫酸根超过标准, 就需将溶液升温歪沸,并调节溶液的pH值至 4.0左右,加入适量的氢氧化钡进行处理,并 搅拌半小时,静置4h后取样再复查硫酸根是否 合格如溶液中的硫酸根消失,然而钡盐出
现,就应再将溶液加入少许硫酸锂饱和溶渡并‘ 升温至沸,以赊尽钡离子.最后,还要复查该 溶液的pH值是否仍为5.0,否则应予以调整. 将上述已经净化合格的溴化镪溶液,在快 速搅拌下加入少量的粉状活性炭进行脱色处理,然后进至过滤工序.将所收集的滤液用泵 打入浓缩罐进行浓缩.在浓缩过程中,要随着 罐内液位的下降,补加滤液若干次.同时,在 浓缩过程中,会有一些混浊物析出,这是溶液 中含有的溶解度较小的碳酸锂在浓缩时析出的缘故此时,应将其除去(采用捞晶的方法). 当浓缩至溴化锂浓溶液的液温升至为190~ l9℃肘,即达到终点(在这以前1h停止补加 滤液)趁热放料进行过滤,以除尽”水不溶 物”杂质等.滤缓经冷却,搅拌,结晶,离心 分离,得一木合溴他锂.由于溴他锂(LiBr? HO)投易潮解,困此应立即密封包装,并置 故于干燥的库房内. 4.产品质量 外观,纯自色立方晶体或均匀状粉末 含量:>98.6(L2LiBr?H±O计)
乙酰苯胺的制备实验 一、实验原理 酰胺可以用酰氯、酸酐或酯同浓氨水、碳酸铵或(伯或仲)胺等作用制得。同冰醋酸共热来制备。这个反应是可逆的。在实际操作中,一般加入过量的冰醋酸,同时,用分馏柱把反应中生成的水(含少量的冰醋酸)蒸出,以提高乙酰苯胺的产率。 主反应: 二、反应试剂、产物、副产物的物理常数 三、药品 四、流程图
五、实验装置图 (1)分馏装置(2)抽滤装置(3)干燥装置六、实验内容 在60ml锥形瓶上装一个分馏柱,柱顶插一支200℃温度计,用一个小锥形瓶收集稀醋酸溶液。 在锥形瓶中放入5.0ml(0.055mol)新蒸馏过的苯胺、7.4ml(0.13mol)冰醋酸和0.1g锌粉,缓慢加热至沸腾,保持反应混合物微沸约10min,然后逐渐升温,控制温度,保持温度计读数在105℃左右。经过40~60min,反应所生
成的水(含少量醋酸)可完全蒸出。当温度计的读数发生上下波动或自行下降时(有时反应容器中出现白雾),表明反应达到终点。停止加热。这时,蒸出的水和醋酸大约有4ml。 在不断搅拌下把反应混合物趁热以细流慢慢倒入盛100ml冷水的烧杯中。继续剧烈搅拌,并冷却烧杯,使粗乙酰苯胺成细粒状完全析出。用布氏漏斗抽滤析出的固体,用玻璃瓶塞把固体压碎,再用5~10ml冷水洗涤以除去残留的酸液。把粗乙酰苯胺放入150ml热水中,加热至沸腾。如果仍有未溶解的油珠,需补加热水,直到油珠完全溶解为止。稍冷后加入约0.5g粉末状活性炭,用玻璃棒搅动并煮沸5-10min。趁热用保温漏斗过滤或用预先加热好的布氏漏斗减压过滤。冷却滤液,乙酰苯胺呈无色片状晶体析出。减压过滤,尽量挤压以除去晶体中的水分。产品放在表面皿上晾干后测定其熔点。产量:约5.0g。 纯乙酰苯胺为无色片状晶体。熔点mp=114.3℃。 (一)制备阶段 1.安装分馏装置:如图(1)所示,在100ml锥形瓶上装一个分馏柱,柱顶插一支200℃温度计,用一个100ml锥形瓶收集稀醋酸溶液。 2.加药品:在100ml锥形瓶中放入5ml新蒸馏过的苯胺、7.4ml冰醋酸和0.1g锌粉。
单过硫酸氢钾复合盐 基本信息 化学名称:过一硫酸氢钾复合盐 别名:单过硫酸钾盐,单过硫酸氢钾 分子式:2KHSO5·KHSO4·K2SO4 分子量: CAS号:70693-62-8; 产品规格:过硫酸氢钾粉 产品规格:过硫酸氢钾片 产品简介
过一硫酸氢钾复合盐(单过硫酸钾盐,单过硫酸氢钾)是一种稳定、方便、具有广泛用途的优良的酸性氧化剂,其应用领域涉及到口腔清洁、泳池及温泉水体消毒、线路板蚀刻剂、纸浆漂白、羊毛织物防缩处理、贵重金属提炼等;过一硫酸氢钾复合盐是有机合成中的一种重要助剂,能使有机物分子中的双键发生环氧化,是许多聚 合反应的自由基引发剂。 医药/化工合成是制备过氧化酮(Dioxirasnes)系列催化剂例 如DMD、TFD的基本原料,过氧化酮以其温和的反应条件、高 效的氧化活性、极好的选择性而开辟了不对称反应和天然药物合成的新路径。烯烃不对称反应催化剂设计中可以原位氧化手性胺、手性亚胺盐聚合引发剂,醋酸乙烯酯、丙烯酸乙酯及丙烯腈的聚合反应;乙烯基单体的聚合反应;粘合剂、调和剂。 印刷线路板PCB/金属表面处理铜板表面清洗剂、微蚀刻剂、黑化处理助剂。 消毒/动物养殖业动物环境消毒,水产养殖业水处理,能杀灭 几乎所有的人畜共患疾病的细菌和病毒,对口蹄疫、禽流感、SARS等具有优异的杀灭作用。 泳池/SPA水处理游泳池消毒与水冲击性处理(SHOCKS),快速清除尿素等有机物,净化水质,提高ORP。 羊毛服装行业优质的羊毛防缩剂。
油田、石化、金属电镀企业污水处理、废气处理絮凝剂、净 化剂,油田、石化、建材工业含聚合物污水处理、硫磺回收、 油层压裂助剂。 化妆品、日用化学品漂白配方、假牙清洗剂、抽水马桶清洗 剂、染发剂。 造纸行业废纸脱墨浆漂白、氧化淀粉制造。 运输信息 运输名称:腐蚀性固体,(含过一硫酸氢钾复合盐) 危险等级:8(腐蚀性) UN编号:3260 包装:PGII EMS:F-A,S-B 包装与储存 包装:内:25Kg/700kg塑料袋(Polyethylene film)外:编织袋储存:储存于干燥洁净、通风条件良好的仓间内。远离火种、热源和直接的阳光照射。注意防潮和雨淋,保持容器的密封,注意标签完好无遗漏。搬运时要轻装轻卸防止包装及容器损坏,注意保持容器压力排泄的正常。雨天不宜运输。应与易燃或可燃物、还原剂、硫、磷等分开存放,切忌混储混运。避免与其它易引起产品分解的物质接触。 新型消毒剂--单过硫酸氢钾复合盐消毒剂
单过硫酸氢钾复合盐
单过硫酸氢钾复合盐 基本信息 化学名称:过一硫酸氢钾复合盐 别名:单过硫酸钾盐,单过硫酸氢钾 分子式:2KHSO5·KHSO4·K2SO4 分子量:614.70 CAS号:70693-62-8; 产品规格:过硫酸氢钾粉 产品规格:过硫酸氢钾片 产品简介
过一硫酸氢钾复合盐(单过硫酸钾盐,单过硫酸氢钾)是一种稳定、方便、具有广泛用途的优良的酸性氧化剂,其应用领域涉及到口腔清洁、泳池及温泉水体消毒、线路板蚀刻剂、纸浆漂白、羊毛织物防缩处理、贵重金属提炼等;过一硫酸氢钾复合盐是有机合成中的一种重要助剂,能使有机物分子中的双键发生环氧化,是许多聚合反应 的自由基引发剂。 ?医药/化工合成是制备过氧化酮(Dioxirasnes)系列催化剂例如DMD、TFD的基本原料,过氧化酮以其温和的反应条件、高效的氧化活性、极好的选择性而开辟了不对称反应和天然药物合成的新路径。烯烃不对称反应催化剂设计中可以原位氧化手性胺、手性亚胺盐聚合引发剂,醋酸乙烯酯、丙烯酸乙酯及丙烯腈的聚合反应;乙烯基单体的聚合反应;粘合剂、调和剂。 ?印刷线路板PCB/金属表面处理铜板表面清洗剂、微蚀刻剂、黑化处理助剂。 ?消毒/动物养殖业动物环境消毒,水产养殖业水处理,能杀灭几乎所有的人畜共患疾病的细菌和病毒,对口蹄疫、禽流感、 SARS等具有优异的杀灭作用。 ?泳池/SPA水处理游泳池消毒与水冲击性处理(SHOCKS),快速清除尿素等有机物,净化水质,提高ORP。 ?羊毛服装行业优质的羊毛防缩剂。
?油田、石化、金属电镀企业污水处理、废气处理絮凝剂、净化剂,油田、石化、建材工业含聚合物污水处理、硫磺回收、 油层压裂助剂。 ?化妆品、日用化学品漂白配方、假牙清洗剂、抽水马桶清洗剂、染发剂。 ?造纸行业废纸脱墨浆漂白、氧化淀粉制造。 运输信息 运输名称:腐蚀性固体,N.O.S (含过一硫酸氢钾复合盐) 危险等级:8(腐蚀性) UN编号:3260 包装:PGII EMS:F-A,S-B 包装与储存 包装:内:25Kg/700kg塑料袋 (Polyethylene film)外:编织袋 储存:储存于干燥洁净、通风条件良好的仓间内。远离火种、热源和直接的阳光照射。注意防潮和雨淋,保持容器的密封,注意标签完好无遗漏。搬运时要轻装轻卸防止包装及容器损坏,注意保持容器压力排泄的正常。雨天不宜运输。应与易燃或可燃物、还原剂、硫、磷等分开存放,切忌混储混运。避免与其它易引起产品分解的物质接触。
实验五乙酰苯胺的制备及红外光谱鉴定 一、实验目的 1. 掌握苯胺乙酰化反应的原理和实验操作。 2. 学习固体有机物提纯的方法——重结晶。 3、了解红外光谱法鉴定有机化合物结构的方法。 二、实验原理 1、苯胺的乙酰化反应 胺的酰化在有机合成中有着重要的作用。作为一种保护措施,一级和二级芳胺在合成中通常被转化为它们的乙酰基衍生物以降低胺对氧化降解的敏感性,使其不被反应试剂破坏;同时氨基酰化后降低了氨基在亲电取代反应(特别是卤化)中的活化能力,使其由很强的第Ⅰ类定位基变为中等强度的第Ⅰ类定位基,使反应由多元取代变为有用的一元取代,由于乙酰基的空间位阻,往往选择性的生成对位取代物。 芳胺可用酰氯、酸酐或与冰醋酸加热来进行酰化,酸酐一般来说是比酰氯更好的酰化试剂,用游离胺与纯乙酸酐进行酰化时,常伴有二乙酰胺[ArN(COCH3)2]副产物的生成。但如果在醋酸-醋酸钠的缓冲溶液中进行酰化,由于酸酐的水解速度比酰化速度慢得多,可以得到高纯度的产物。但这一方法不适合于硝基苯和其它碱性很弱的芳胺的酰化。另外,酸酐的价格较贵,所以一般选羧酸。 本反应是可逆的,为提高平衡转化率,加入了过量的冰醋酸,同时不断地把生成的水移出反应体系,可以使反应接近完成。为了让生成的水蒸出,而又尽可能地让沸点接近的醋酸少蒸出来,本实验采用较长的分馏柱进行分馏。实验加入少量的锌粉,是为了防止反应过程中苯胺被氧化。 NH2 +CH3COOH HN C CH3 O +H2O 2、乙酰苯胺的重结晶 固体有机物在溶剂中的溶解度一般随温度的升高而增大。把固体有机物溶解在热的溶剂中使之饱和,冷却时由于溶解度降低,有机物又重新析出晶体。利用溶剂对被提纯物质及杂质的溶解度不同,使被提纯物质从过饱和溶液中析出。让杂质全部或大部分留在溶液中,从而达到提纯的目的。 重结晶只适宜杂质含量在5%以下的固体有机混合物的提纯。从反应粗产物直接重结晶是不适宜的,必须先采取其他方法初步提纯,然后再重结晶提纯。 重结晶提纯的一般过程为: (1)将不纯的固体有机物在溶剂的沸点或接近沸点的温度下溶解在溶剂中,制成接近饱和的浓溶液。若固体有机物的熔点较溶剂沸点低,则应制成在熔点温度以下的饱和溶液; (2)若溶液含有色杂质,可加入活性炭煮沸脱色; (3)过滤此热溶液以除去其中的不溶性物质及活性炭;
过硫酸氢钾简介 1、产品分子式、分子量、结构式 过硫酸氢钾复合物是一种无机酸性氧化剂,又名单过硫酸氢钾复合盐、过 一硫酸氢钾三合盐过氧化单硫酸钾盐,英文名称0x0ne, Potassium monopersulfate compound ,potassium ,monopersulfate triple salt or potassium peroxymonopersulfate。简称:PMPS或KMPS。是常用功能化学品Oxone、Caroat ZA200/100、Basolan2448的基本有效组分。 分子式:2KHSO5?KHSO4?K2SO4 分子量:614.7 CAS No. 70693-62-8 化学式: K+ 2、主要物理化学性质和技术数据表 物理性状:呈可以自由流动的白色粉状固体,易溶于水,在20C( 68OF)时, 水溶解度大于250g/L。堆积密度1.1-1.2。 化学特性:过硫酸氢钾复合盐的活性物质为过硫酸氢钾KHSO5 (或称之为过一
硫酸氢钾)。具有非常强大而有效的非氯氧化能力,使用和处理过程符合安全和环保要求,因而被广泛的应用于工业生产和消费领域。通常状态下比较稳定,当温度高于65r 时易发生分解反应。比较活泼,易于参与多种化学反应,可作为氧化剂、漂白剂、催化剂、消毒剂、蚀刻剂等。 主要质量技术数据如下表:
3、典型应用 应用行业 是制备过氧化酮(Dioxirasnes )系列催化剂例如 DMD 、TFD 的基本原料,过氧化酮以其温和的反 应条件、高效的氧化活性、极好的选择性而开辟 对称反应催化剂设计中可以原位氧化手性胺、手 性亚胺盐 聚合引发剂,醋酸乙烯酯、丙烯酸乙酯 及丙烯腈的聚合反应;乙烯基单体的聚合反应; 粘合剂、调和剂 使用说明 医药/化工合成 了不对称反应和天然药物合成的新路径。烯烃不
合成香兰素的工作任务 1.香兰素简介 香兰素(Vanillin )为白色或微黄色针状结晶,具有类似香荚兰豆的香气及浓郁的奶香,味微甜;熔点81~83℃,化学名为3-甲氧基-4-羟基苯甲醛。香兰素是重要的食用香料之一,为香料工业中最大的品种,是人们普遍喜爱的奶油香草香精的主要成份,广泛用于食品、巧克力、冰淇淋、饮料以及日用化妆品中起增香和定香作用。另外香兰素还可作饲料的添加剂、电镀行业的增亮剂、制药行业的中间体。 13.2 合成香兰素工作任务分析 13.2.1目标化合物分子结构的分析 ①香兰素的分子式:C 8H 8O 3 ②香兰素的分子结构式: CHO OCH 3 不难看出,目标化合物基本结构为取代苯酚结构,醛基和甲氧基分别处于酚羟基的对位和邻位。 13.2.2香兰素的合成路线分析 从苯环上基团引入的角度看,醛基可以直接引入,也可以采用氧化(或还原)的方法引入。 分析1: 相应合成路线1: CH 3 O NH 2 CH 3 O +HSO 4 N 23 分析2: 相应合成路线2: OH CH 3 O CH 3 O HO CHCOOH OH 分析3: OCH 3 OH FGI CH 3 O CHCOOH OH HO CH 3 O OH CH CH CH 3 TM FGR FGI OCH 3 OH + OHCCOOH 丁香酚 CHO OCH 3 OH 2 OCH 3 NaNO 2 H 2SO 4 CH 3Cl NaOH [O] OHCCOOH TM TM TM
相应合成路线3: CH 2CH=CH 2 OCH 3 ONa CH=CH-CH 3 OCH 3 ONa CHO OCH 3 OH 分析4: 相应合成路线4: 从基团变换的角度,也可以有下面的逆向推导。 分析5: CHO OH Br CHO OH 相应合成路线5: 分析6: CH 3 相应合成路线6 显然,路线5和路线6由于卤化时能发生多卤化及甲氧基化反应较难的问题,不是好的合成路线。路线1~4各有特点,只要条件具备,都可值得尝试。 13.2.3 文献中常见的香兰素的合成方法 目前香兰素的生产方法较多,典型的主要有: 路线一:以邻硝基氯苯为原料的多步合成的方法。 (1)甲氧基化反应 ++CH 3O NO 2 Cl KOH CH 3OH NO 2 ++KCl H 2 O (2)还原反应 OCH 3 OH CO 2 CHO OH TM CH 3 O OH COOH FGI FGR FGR [O] 异构化 TM TM OCH 3 OH CH 3 O OH COOH [H] NaOCH 3 TM TM CH3 OH TM Cl 2 CH 3 Cl NaOCH 3 CH 3 OCH 3OH
产能转移吡喹酮唯我独大 吡喹酮上市使用至今已有30多年历史,多年来,吡喹酮一直作为抗血吸虫病的主力药物和抗其他寄生虫的有效药物在临床一线应用,为人类健康起到了重要的作用。一线抗寄生虫药物吡喹酮最先由Seubere等人于1975年首先合成,德国默克(即现在的默克雪兰诺)和拜耳两药厂成功开发出药物制剂。1980年德国默克公司以商品名“Cesol”率先上市的吡喹酮,目前已在全世界范围内广泛应用。吡喹酮上市后,因其高效、低毒、抗寄生虫谱广、口服方便等特点,深受患者欢迎,销售额不断增加,市场占有率迅速扩大,很快成为世界上治疗血吸虫病和多种寄生虫病的主要药物。除用于人体外,它也广泛用作动物、家禽等的抗寄生虫治疗。吡喹酮又名环吡异喹酮、8440,为广谱抗寄生虫病用药,其抗蠕虫谱很广,对日本血吸虫、埃及血吸虫、曼氏血吸虫等均有杀灭作用。此外,它对并殖吸虫(肺吸虫)、华支睾吸虫、包虫、囊虫、孟氏裂头蚴、姜片虫、绦虫等也有杀灭作用。其作用特点是疗效高、剂量小、疗程短、代谢快、毒性小和口服方便。毋庸置疑,吡喹酮的问世是寄生虫病化疗史上的一项重大突破,现在,它仍是治疗多种寄生虫病的首选药物。 《中国药典》自1985年版以来,修订的历版均将吡喹酮收载其中,同时亦被收载于美国、英国等许多国家的药典以及《欧洲药典》、《国际药典》等。目前,吡喹酮已被列入我国《基本医疗保险及工伤保险药品目录》中抗吸虫病药物的甲类品种。多年来,每当我国长江流域以及其他南方广大地区遭受洪水袭击后,吡喹酮作为预防和治疗血吸虫的一线药物,均发挥了很大的作用。产能占全球近半. 目前,全世界有多家公司生产吡喹酮,如德国默克、拜耳、Miles、韩国大宇等,产量最大的是拜耳公司,年产量达50多吨。但是,近年来因环保等各种原因,这些公司的产量均有不同程度的减少甚至停产。前几年,吡喹酮全世界年总产量为200吨左右。 我国于1977年研发成功吡喹酮并开始临床试验,1982年正式投放市场。30余年来,我国吡喹酮年生产能力和产量均稳步上升。20世纪80年代,我国吡喹酮年生产能力不到20吨,年产量仅为10余吨,到20世纪90年代,年生产能力达到30多吨,年产量20余吨。20世纪末,我国吡喹酮的年生产能力已达到近50吨,年产量为35吨左右。多年来,南京制药厂、上海制药六厂为吡喹酮的主要生产企业,年产量和出口量占据全国80%以上的份额。21世纪以来,江苏、浙江、安徽等地有多家企业先后投入吡喹酮的生产,前些时候,浙江海正药业又与国外合作,兴建了年产40吨规模的吡喹酮生产线,这使我国的年生产能力增至150余吨,年产量80余吨,从而成为世界吡喹酮的主要生产国。现在全国共有吡喹酮生产批准文号25个,其中吡喹酮片剂生产批准文号有18个,吡喹酮原料药生产批准文号7个,原料药主要生产企业有:南京制药厂、浙江海正药业、常州亚邦制药、上海新华联制药、绍兴民生医药、江苏红豆杉药业、安徽省润康药业等。 此外,全国还有多家化工企业也生产吡喹酮原料药作为兽用药并出口,如石家庄嘉一药业和上海嘉一药业最近共同打造了国内规模最大的吡喹酮原料药产销集团。上海嘉一公司的吡喹酮原料药已于2011年4月通过农业部GMP认证,产品质量符合USP、EP、CVP等最新版
一.实验目的 1.学习实验室制备芳香族酰胺的原理和方法。 2.训练固体有机物的热过滤、脱色、洗涤、重结晶、干燥等纯化技术。 二.实验原理 NH 2+CH 3COOH 3+H 2O 芳香族酰胺通常用伯或仲芳胺与酸酐或羧酸反应制备,因为酸酐的价格较贵,所以一般选羧酸。本反应是可逆的,为提高平衡转化率,加入了过量的冰醋酸,同时不断地把生成的水移出反应体系,可以使反应接近完成。为了让生成的水蒸出,而又仅可能地让沸点接近的醋酸少蒸出来,本实验采用较长的分馏柱进行分馏。实验加入少量的锌粉,是为了防止反应过程中苯胺被氧化。 三.试剂及物理常数 四、实验流程 5ml 苯胺 7.4ml 冰醋酸0.1g 锌粉 称重计算产率 五、仪器装置
抽滤装置 干燥装置 布氏漏斗 抽滤瓶 反应装置 六、操作要点和说明 1.合成 (1).反应物量的确定: 本实验反应是可逆的,采用乙酸过量和从反应体系中分出水的方法来提高乙酰苯胺的产率,但随之会增加副产物二乙酰基苯胺的生成量。二乙酰苯胺很容易水解成乙酰苯胺和乙酸,在产物精制过程中通过水洗、重结晶等操作,二乙酰基苯胺水解成乙酰苯胺和乙酸,经过滤可除去乙酸,不影响乙酰苯胺的产率和纯度。 苯胺极易氧化,在空气中放置会变成红色,使用时必须重新蒸馏除去其中的杂质。反应过程中加入少许锌粉。锌粉在酸性介质中可使苯胺中有色物质还原,防止苯胺继续氧化。在实验中可以看到,锌粉加得适量,反应混合物呈淡黄色或接近无色。但锌粉不能加得太多,一方面消耗乙酸,另一方面在精制过程中乙酸锌水解成氢氧化锌,很难从乙酰苯胺中分离出来。 (2).合成反应装置的设计: 水沸点为100℃,乙酸沸点为117℃,两者仅差17℃,若要分离出水而不夹带更多的乙酸,必须使用分馏反应装置,而不能用蒸馏的反应装置。本实验用分馏柱。 一般有机反应用耐压、耐液体沸腾冲出的圆形瓶作反应器。由于乙酰苯胺的熔点为114℃,稍冷即固化,不易从圆形瓶中倒出,因此用锥形瓶作反应器更方便。 分出的水量很少,分馏柱可以不连接冷凝管,在分馏柱支口上直接连尾接管,兼作空气冷凝管即可,使装置更简单。 为控制反应温度,在分馏柱顶口插温度计。 (3).操作条件的控制 保持分馏柱顶温度低于105℃的稳定操作,开始缓慢加热,使反应进行一段时间,有水生成后,再调节反应温度使蒸汽缓慢进入分馏柱,只要生成水的速度大于或等于分出水的速度,即
.- 香兰素的合成方法及技术展望 吴志尚化工一班 3014207025 (天津大学化工学院,天津 300072) 摘要:香兰素是世界上最重要的香料之一,广泛应用在食品饮料、香精香料和医药工业等领域中, 全球每年的需求量超过16000t。鉴于人们对纯天然绿色食品的追求日益增长,天然香兰素高效的生产方法也成为研究的热点。本文综述了香兰素的多种不同的合成途径以及合成关键因素等方面的研究进展, 分析探讨了不同合成途径的优劣之处。并展望了利用微生物高产天然香兰素存在的瓶颈以及有潜力的发展方向。 关键词:香兰素;天然香料;合成途径 The synthesis methods of vanillin and technical outlook Wu Zhishang Class 1 3014207025 (School of chemical engineering institute,Tianjin University,Tianjin 300072) Abstract:Vanillin is one of the most important flavoring compounds, and it is widely used in the food industry, spice fragrance, and medicine industry, etc. The annual worldwide consumption is estimated over 16 000 tons. Due to people's increasing concern for natural food,the product of natural vanillin has become the major point of scientific research. By comparing different production methods of vanillin, we concluded that the microbial transformation to vanillin is the most promising method. Research developments on different biosynthetic pathways for vanillin, as well as the genes and enzymes involved, were discussed. In addition,the advantages and disadvantages of each pathway were compared and explained. Finally, the existing bottlenecks in biosynthesis of high-yield natural vanillin with the help of genetic and metabolic engineering, and the potential development direction in this field were elucidated. Natural spices ; Synthetic pathway Vanillin;Keywords: 香兰素(Vanillin, 4-羟基-3-甲氧基苯甲醛)主要存在于天然植物香荚兰中, 是世界上最重要的香料之一。香兰素的晶体为白色针状,呈香兰荚特有的香气,它微溶于冷水,易溶[1]于热水、乙醇、乙醚、氯仿和热挥发油中。其化学结构为: