
Current Biology17,520–527,March20,2007a2007Elsevier Ltd All rights reserved DOI10.1016/j.cub.2007.01.052
Report Clathrin-Mediated Constitutive
Endocytosis of PIN
Auxin Ef?ux Carriers in Arabidopsis
Pankaj Dhonukshe,1Fernando Aniento,2
Inhwan Hwang,3David G.Robinson,4Jozef Mravec,1 York-Dieter Stierhof,1and Jirˇ?′Friml1,*
1Center for Plant Molecular Biology(ZMBP)
Auf der Morgenstelle3
University of Tu¨bingen
D-72076Tu¨bingen
Germany
2Departamento de Bioqu?′mica y Biolog?′a Molecular Facultad de Farmacia
Universidad de Valencia
Avda.Vicente Andre′s Estelle′s,s/n
E-46100Burjassot,Valencia
Spain
3Division of Molecular and Life Sciences
Pohang University of Science and Technology Pohang,790-784
Korea
4Department of Cell Biology
Heidelberg Institute for Plant Sciences
University of Heidelberg
69120Heidelberg
Germany
Summary
Endocytosis is an essential process by which eukary-otic cells internalize exogenous material or regulate signaling at the cell surface[1].Different endocytic pathways are well established in yeast and animals; prominent among them is clathrin-dependent endocy-tosis[2,3].In plants,endocytosis is poorly de?ned, and no molecular mechanism for cargo internalization has been demonstrated so far[4,5],although the inter-nalization of receptor-ligand complexes at the plant plasma membrane has recently been shown[6].Here we demonstrate by means of a green-to-red photo-convertible?uorescent reporter,EosFP[7],the consti-tutive endocytosis of PIN auxin ef?ux carriers[8]and their recycling to the plasma https://www.doczj.com/doc/406453837.html,ing a plant clathrin-speci?c antibody,we show the presence of clathrin at different stages of coated-vesicle formation at the plasma membrane in Arabidopsis.Genetic inter-ference with clathrin function inhibits PIN internaliza-tion and endocytosis in general.Furthermore,pharma-cological interference with cargo recruitment into the clathrin pathway blocks internalization of PINs and other plasma-membrane proteins.Our data demon-strate that clathrin-dependent endocytosis is opera-tional in plants and constitutes the predominant pathway for the internalization of numerous plasma-membrane-resident proteins including PIN auxin ef?ux carriers.Results and Discussion
Constitutive Endocytosis and Recycling of PIN Proteins In Vivo
Recent reports have suggested that the internalization of certain transporters,receptors,and extracellular-peptide ligands occurs at the plant plasma membrane (for detailed reviews,see[4,5]).The presumptive consti-tutive internalization and recycling of PIN auxin ef?ux carriers[9]is of particular interest because this process is considered to be important for regulation of the direc-tionality and throughput of intercellular auxin signaling [10,11]and thus plays a central role in many plant developmental processes including patterning and tropisms[12–14].
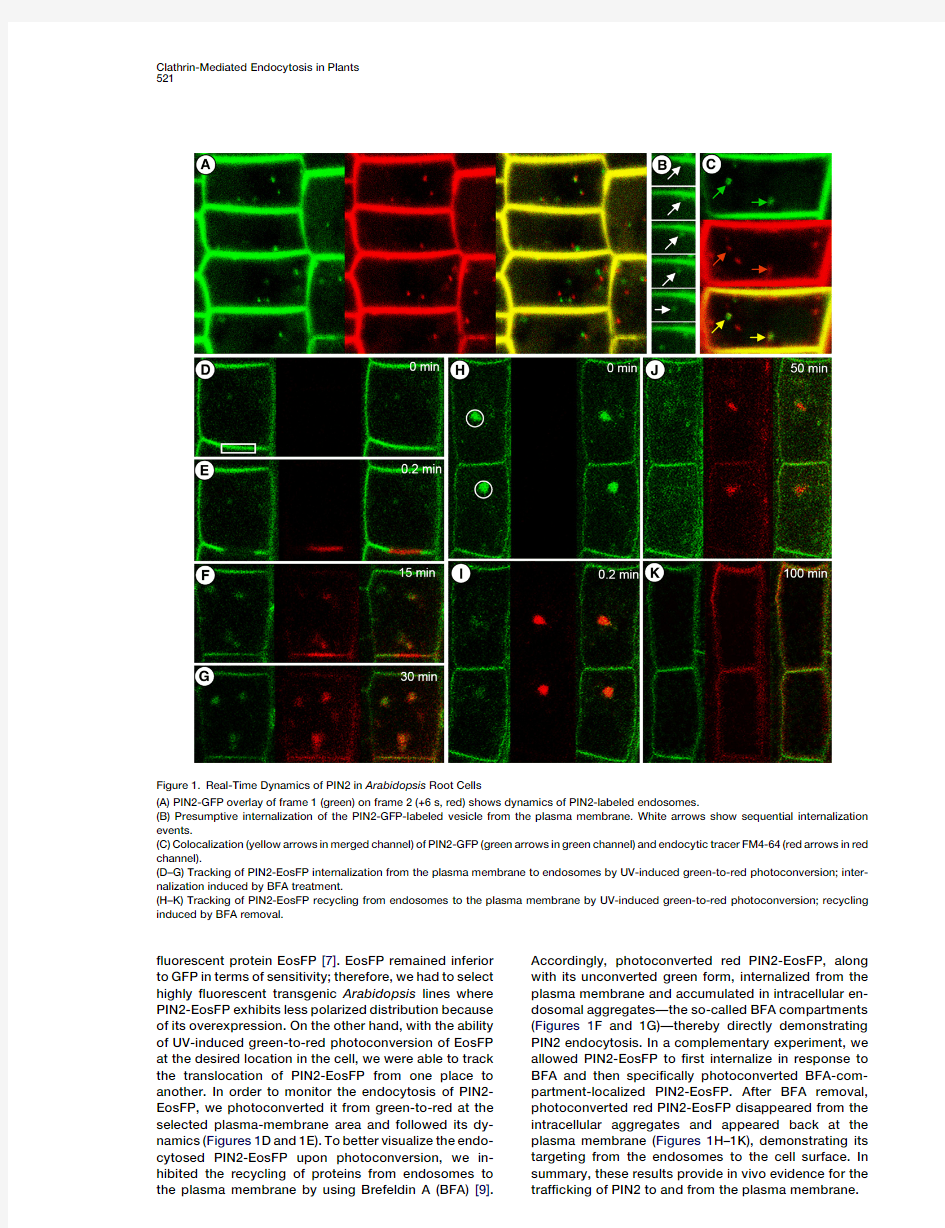
The?nding that PIN constitutively cycles is based on the pharmacological inhibition of de novo protein syn-thesis and protein traf?cking to the plasma membrane and is supported by the immunocytochemical visualiza-tion of PIN proteins[9].To gain further insights into PIN cycling,we followed the subcellular dynamics of PIN2 in root epidermis cells of Arabidopsis lines expressing a functional PIN2-Green Fluorescent Protein(PIN2-GFP) fusion[15,16].In these lines,because of the high signal intensity,PIN2-GFP exhibited less pronounced polarity of its localization.This strategy,however,allowed the detection of PIN2-GFP intracellularly,in addition to its known plasma-membrane localization,in control cells (Figure1A)as well as in cells treated with the protein-synthesis inhibitor cycloheximide(CHX)(see Figure S1A in the Supplemental Data available online).The intracel-lular PIN2-GFP punctate structures were dynamic and moved with a velocity of about8m m/min(see superim-position of two successive frames color-coded in red and green;6s interval between frames).To test whether the intracellular PIN2-GFP localizes to the endocytic pathway,we treated the PIN2-GFP-expressing Arabi-dopsis seedlings with the?uorescent endocytic tracer FM4-64[17–19].Quanti?cation of intracellular signals of FM4-64and PIN2-GFP showed that39%(64%)of the internalized FM4-64overlaps with the PIN2-GFP punctae,whereas93%(66%)of intracellular PIN2-GFP colocalizes with FM4-64(Figure1C).These results indicate that PIN2-GFP remains both at the plasma membrane and on endosomal compartments,thus supporting the concept of constitutive PIN endocytic cycling.Although single-confocal-section time-lapse microscopy does not deliver a complete picture of the situation,a closer inspection of PIN2-GFP?uorescence at the plasma membrane suggested its sequential inter-nalization as follows:(1)accumulation of PIN2-GFP at speci?c plasma-membrane domains,(2)its enhanced sequestration into a vesicle-like structures,and(3)its subsequent internalization(Figure1B).
To further con?rm the internalization of PIN2from the plasma membrane and its retargeting to the plasma membrane,we engineered a green-to-red photoconver-tible version of PIN2by using the photoconvertible
*Correspondence:jiri.friml@zmbp.uni-tuebingen.de
?uorescent protein EosFP [7].EosFP remained inferior to GFP in terms of sensitivity;therefore,we had to select highly ?uorescent transgenic Arabidopsis lines where PIN2-EosFP exhibits less polarized distribution because of its overexpression.On the other hand,with the ability of UV-induced green-to-red photoconversion of EosFP at the desired location in the cell,we were able to track the translocation of PIN2-EosFP from one place to another.In order to monitor the endocytosis of PIN2-EosFP,we photoconverted it from green-to-red at the selected plasma-membrane area and followed its dy-namics (Figures 1D and 1E).To better visualize the endo-cytosed PIN2-EosFP upon photoconversion,we in-hibited the recycling of proteins from endosomes to the plasma membrane by using Brefeldin A (BFA)[9].Accordingly,photoconverted red PIN2-EosFP,along with its unconverted green form,internalized from the plasma membrane and accumulated in intracellular en-dosomal aggregates—the so-called BFA compartments (Figures 1F and 1G)—thereby directly demonstrating PIN2endocytosis.In a complementary experiment,we allowed PIN2-EosFP to ?rst internalize in response to BFA and then speci?cally photoconverted BFA-com-partment-localized PIN2-EosFP.After BFA removal,photoconverted red PIN2-EosFP disappeared from the intracellular aggregates and appeared back at the plasma membrane (Figures 1H–1K),demonstrating its targeting from the endosomes to the cell surface.In summary,these results provide in vivo evidence for the traf?cking of PIN2to and from the plasma
membrane.
Figure 1.Real-Time Dynamics of PIN2in Arabidopsis Root Cells
(A)PIN2-GFP overlay of frame 1(green)on frame 2(+6s,red)shows dynamics of PIN2-labeled endosomes.
(B)Presumptive internalization of the PIN2-GFP-labeled vesicle from the plasma membrane.White arrows show sequential internalization events.
(C)Colocalization (yellow arrows in merged channel)of PIN2-GFP (green arrows in green channel)and endocytic tracer FM4-64(red arrows in red channel).
(D–G)Tracking of PIN2-EosFP internalization from the plasma membrane to endosomes by UV-induced green-to-red photoconversion;inter-nalization induced by BFA treatment.
(H–K)Tracking of PIN2-EosFP recycling from endosomes to the plasma membrane by UV-induced green-to-red photoconversion;recycling induced by BFA removal.
Clathrin-Mediated Endocytosis in Plants 521
Clathrin Is Localized at Different Stages
of Coated-Vesicle Formation at the Plasma Membrane in Arabidopsis
The detection of constitutive PIN2-GFP internalization raises the question about the mechanism by which PIN proteins are internalized.No mechanism of endocytosis has been identi?ed in plants so far,but several different types have been documented in yeast and animals,all of which involve the regulated formation of vesicles at the plasma membrane[1].The major and best characterized is clathrin-mediated endocytosis,which utilizes the geo-metric constraints resulting from the binding of clathrin triskelions to the plasma membrane at the site of endo-cytic-vesicle formation[20,21].In plants,coated-like pits have been detected at the plasma membrane on many occasions[22],and internalization of a typical clathrin-speci?c cargo—the human transferrin receptor (hTfR)—has recently been demonstrated in plant proto-plasts[23].Plant genomes also contain homologs of components of the clathrin-related machinery,including clathrin heavy and light chains,adaptins,and possible accessory proteins[4,24].
To address the possible role of clathrin in PIN internal-ization,we performed immunogold electron microscopy on Arabidopsis root cells by using an antiserum raised against a plant clathrin heavy-chain peptide[25].In western blots from bacterial extracts,this antibody de-tected a single band of45kDa representing the His-tagged clathrin peptide,whereas in the plant extracts it detected a band of190kDa corresponding to the pre-dicted size of plant clathrin heavy chain(Figure2G).On the basis of the typical morphology of clathrin-coated structures,we detected all of the morphological inter-mediates of clathrin-coated pit formation at the plasma membrane from their initiation through vesicle excision, internalization,and loss of clathrin coat(Figure2A).The sizes of the clathrin-coated vesicles in Arabidopsis were typically smaller(30nm in diameter without the clathrin coat)than those in mammalian cells(approximately 100nm)[1].Using a novel protocol for ultrastructural lo-calization(see Supplemental Experimental Procedures), we found clathrin to be mainly present on internalizing vesicles at the plasma membrane(see arrows in Fig-ure2B),but also at the trans side of the Golgi apparatus (Figure2C).Closer observation clearly revealed the as-sociation of immunogold in a semicircular or circular manner reminiscent of the coming together of individual clathrin triskelions to form clathrin-coated vesicles at the plasma membrane(Figures2D and2E).This locali-zation pattern was further con?rmed by immuno?uores-cence observations,which detected clathrin at the plasma membrane,in the cytosol,and at the trans-Golgi-related structures(Figure2H).The extent of cla-thrin at the plasma membrane was weaker as compared to its intracellular signal,a result that may re?ect the shorter resident time at the plasma membrane as com-pared to the intracellular structures.At the plasma mem-brane,clathrin partially colocalized with PINs(Figures2I and2J).The intracellular clathrin signal partially colocal-ized with the tran s-Golgi marker ST-YFP(Figure2K)[26] and slightly less with the tran s-Golgi network(TGN) marker V-ATPase subunit VHA-a1(a1)[27](Figure2L). This con?rms the presence of clathrin in Arabidopsis root cells at intracellular locations similar to those in mammalian cells[3],emphasizing its prominent locali-zation to coated pits at the plasma membrane.Thus, clathrin is perfectly positioned to mediate internalization of plant plasma-membrane proteins including PINs. Genetic Interference with the Clathrin Machinery Impairs PIN Internalization and Endocytosis
in General
We next addressed whether functionally competent clathrin is required for the internalization of PIN proteins. Studies in mammals have shown that the overexpres-sion of the C-terminal part of clathrin heavy chain (termed‘‘clathrin Hub’’)binds to and titers away the light chains,thus making them unavailable for clathrin cage formation[28].This leads to strong dominant-negative effects on clathrin-mediated processes.We employed the same strategy to examine the functional role of cla-thrin in Arabidopsis.Therefore,we prepared GFP-and RFP-tagged Arabidopsis clathrin Hub1constructs and overexpressed them under the control of a strong viral 35S promoter in Arabidopsis protoplasts.To test for an effect on endocytosis,we monitored the endocytic uptake of FM4-64[19,29,30]in protoplasts showing an observable amount of cytoplasm,in which it is easier to follow endocytosis.Around80%of the protoplasts (n=52)were viable and showed FM4-64uptake into endosomal compartments(Figure3A)that aggregated after application of BFA(Figure3B).After expression of GFP-Hub1,which as expected was localized in the cytosol(green in Figure3D),inhibition of FM4-64inter-nalization was observed both in the absence(Figure3C) and in the presence of BFA(Figure3D,compare FM4-64 distribution with Figure3B)in about75%of the proto-plasts(n=46),indicating a defect in endocytosis.These results point to the existence of clathrin-mediated endo-cytosis in plants.They also show that the uptake of general endocytic tracers, e.g.,FM4-64,largely de-pends on clathrin,suggesting that clathrin-dependent endocytosis accounts for most,if not all,of the inter-nalization at the plant plasma membrane.
To test whether PINs are cargoes for clathrin-medi-ated endocytosis,we monitored the internalization of the PIN1and PIN2in Arabidopsis protoplasts.Consis-tent with the results from Arabidopsis root cells,the localization of PIN1-GFP was detected both at the plasma membrane and as intracellular punctae(Fig-ure3E)in approximately78%protoplasts(n=48). Among the protoplasts bearing intracellular PIN1-GFP punctae,82%of the intracellular PIN1-GFP signal colo-calized with FM4-64(Figure3F),con?rming its localiza-tion along the endocytic pathway.BFA treatment resulted,as expected,in the internalization of PIN1-GFP from the plasma membrane and its accumulation in BFA compartments(Figure3G)in80%of protoplasts (n=40).However,after overexpression of RFP-Hub1 (red in Figure3H),both the incidence of PIN1-GFP in endocytic compartments and its BFA-induced aggrega-tion were impaired(Figure3H,compare PIN1-GFP dis-tribution with Figure3G)in about76%of protoplasts (n=42).Similar results were also obtained with PIN2(Fig-ures3I–3L)and another plasma-membrane protein—the water channel PIP2[31](Figures3M–3P).It seems that Hub1expression affects preferentially PIN endocy-tosis and not so much the exocytosis,because the
Current Biology 522
photobleached PIN2-EosFP recovered at the plasma membrane after Hub1expression too (see Figures S1B and S1E).These ?ndings underline the importance of the clathrin-mediated endocytic pathway for the inter-nalization of plasma-membrane-localized proteins in plants in general,and they identify PIN proteins as prom-inent endogenous substrates of clathrin-dependent en-docytosis.
Pharmacological Interference with Cargo Recruitment into the Clathrin Pathway Inhibits PIN Internalization
In yeast and animals,an established way to inhibit the clathrin-dependent internalization of cell-surface cargo molecules is the pharmacological interference with endocytic-cargo recruitment.The tyrosine analog Tyr-phostin A23is a well-characterized inhibitor of the re-cruitment of endocytic cargo into the clathrin-mediated pathway both in vivo and in vitro [32].It speci?cally pre-vents the interaction between the m 2subunit of the clathrin-binding AP-2adaptor complex and endocytic motifs present in the cytoplasmic domain of plasma-membrane proteins destined for endocytosis such as the hTfR [32].A close structural analog,Tyrphostin A51,does not elicit these effects and is routinely used as a negative control [32].Despite the fact that the molecular target of Tyrphostin A23in plants has not been unequivocally demonstrated,a recent
report
Figure 2.Visualization of clathrin and cla-thrin-coated pits at the plasma membrane of Arabidopsis cells
(A)Transmission electron microscope (TEM)images of subsequent stages of clathrin coated-pit formation through vesicle ?ssion and,?nally,the loss of clathrin coat from the internalized vesicle (yellow arrow).(Sam-ples were high-pressure frozen,freeze substituted,and embedded in epoxy resin.)The scale bar represents 100nm.
(B–F)Immunogold-TEM analysis of root epi-dermis cells with ultrathin thawed cryo-sec-tions.Clathrin labeling with silver-enhanced Nanogold reveals localization of clathrin-coated vesicles both at the plasma mem-brane (B,D,and E)and at the trans side of the Golgi apparatus (C).The plasma membrane is shown by ‘‘PM’’and the Golgi apparatus by ‘‘G,’’and yellow arrows show coated vesicles.For clarity,the forming coated pit (red half circle in [D])and coated vesicles (red circles in [E])are marked by the dotted lines.Control shows no gold label-ing in the absence of primary antibody (F).Scale bars represent 500nm (B),250nm (C),and 100nm (D and E).
(G)Western blot showing the speci?city of the clathrin antibody (1:Hisx6-Clathrin,Coo-massie staining;2:Hisx6-Clathrin,clathrin antibody;3:Arabidopsis extracts,clathrin an-tibody;and 4:Arabidopsis extracts,control serum).
(H–L)Immunolocalization of clathrin (red)with PIN1-GFP (green in [I]),PIN2-GFP (green in [J]),ST-YFP (green in [K]),and a1-GFP (green in [L]).The insets in (H)and (I)and white arrowheads in (H)–(J)and (L)show localization of clathrin at the plasma mem-brane where it shows partial colocalization with plasma-membrane proteins such as PIN1(I)and PIN2(J).Root epidermis and cortex (H,J,K,and L)and stele (I)cells are shown.DAPI-stained nuclei are in blue.
Clathrin-Mediated Endocytosis in Plants 523
shows that Tyrphostin A23ef?ciently inhibited interac-tion between hTfR and a m -adaptin subunit from Arabi-dopsis cytosol and blocked the internalization of the hTfR in Arabidopsis protoplasts [23].We therefore tested the effects of Tyrphostin A23and A51on the internalization of PIN proteins in Arabidopsis root cells.We visualized constitutive PIN2cycling by detecting the PIN2-GFP protein both at the plasma membrane and at the endocytic compartments (Figures 4A and 4B).When applied to Arabidopsis seedlings,
Tyrphostin
Figure 3.Dominant-Negative Clathrin Hub1Expression Inhibits Endocytosis in Arabidopsis Protoplasts
(A and B)Uptake of endocytic tracer FM4-64into the endocytic pathway (A)and its accumulation in BFA compartments after BFA treatment (B).(C and D)Expression of Hub1(C)or GFP-Hub1(green in [D])impairs FM4-64uptake (C)and its accumulation in BFA compartments (D).
(E and F)Localization of PIN1-GFP (green)at the plasma membrane and at the endosomal compartments colocalizing with FM4-64(red).See the inset in (F)for the magni?ed view.
(G and H)Internalization of PIN1-GFP (green)into BFA compartments following BFA treatment (G)is impaired by expression of RFP-Hub1(red)(H).(I and J)Localization of PIN2-EosFP (green)at the plasma membrane and at the endosomal compartments colabeled with FM4-64(red).
(K and L)Internalization of PIN2-EosFP (green)into BFA compartments following BFA treatment (K)is inhibited by expression of RFP-Hub1(red)(L).(M)Localization of PIP2-GFP at the plasma membrane and at the intracellular compartments.
(N and O)Internalization of PIP2-GFP (green)into BFA compartments following BFA treatment (N)is impaired by expression of RFP-Hub1(red)(O).(P)DIC image showing the area occupied by the cytosol (yellow arrow)and vacuole (v).The scale bar represents 20m m.
Current Biology 524
A23(Figures 4C and 4D),but not A51(Figure S2A),ef?-ciently inhibited the intracellular appearance of PIN2but still allowed detectable FM4-64uptake (Figures 4C and 4D).This is consistent with the idea that the interac-tion between a clathrin adaptor complex and cargo molecules at the plasma membrane is required for the sorting of cargo proteins into clathrin-coated vesicles,but is not essential for clathrin-coated vesicle formation per se [33].Indeed,Tyrphostin A23disrupted the recruit-ment of PIN2but nevertheless allowed endocytic vesi-cles (still incorporating FM4-64-labeled membrane)to form and internalize (Figures 4C and
4D).
Figure 4.Tyrphostin A23Inhibits Internalization of Cargoes from the Plasma Membrane of Arabidopsis Root Cells
(A–D)(A)and (B)show PIN2-GFP (green)at the plasma membrane and at the intracellular compartments colocalizing with FM4-64(red).(C and D)Tyrphostin A23inhibits appearance of intracellular PIN2-GFP (green)but allows FM4-64(red)uptake.Note that the inset in (B)shows a magni?ed view of cointernalization of PIN2-GFP with FM4-64to endocytic compartment and the inset in (D)shows internalization of only FM4-64but not that of PIN2-GFP.
(E and F)Internalization of PIN2-GFP (green)into FM4-64-labeled (red)BFA compartments after BFA treatment.
(G and H)Tyrphostin A23inhibits PIN2-GFP (green)BFA-dependent internalization but allows FM4-64(red)uptake into BFA compartments.(I–K)Polar localization of PIN1(red)and staining of TGN by a1(green)in control (I).Internalization of PIN1into a1-labeled BFA compartments following BFA treatment (J)is inhibited by Tyrphostin A23(K).
(L)Tyrphostin A23does not interfere with recovery of PIN1(red)at the plasma membrane and recovery of normal a1-labeled TGN (green)distribution after removal of BFA.
(M and N)PIN2(red)internalization into GNOM-labeled (green)BFA compartments following BFA treatment (M)is inhibited by Tyrphostin A23(N).(O and P)Internalization of PM-ATPase (green)into ARF1-labeled (red)BFA compartments following BFA treatment (O)is inhibited by Tyrphostin A23(P).
(Q and R)Internalization of PIP2-GFP into BFA compartments following BFA treatment (Q)is inhibited by Tyrphostin A23(R).(S and T)Internalization of LTi6b-GFP into BFA compartments following BFA treatment (S)is inhibited by Tyrphostin A23(T).(U)Tyrphostin A23does not interfere with recovery of LTi6b-GFP at the plasma membrane after removal of BFA.DAPI-stained nuclei are in blue.
Clathrin-Mediated Endocytosis in Plants 525
The inhibition of PIN2internalization by Tyrphostin A23was even more apparent in the presence of BFA.In the control situation,BFA caused PIN2to accumulate into the BFA compartments,where it colocalized with FM4-64(Figures4E and4F).In the presence of Tyrphos-tin A23,only FM4-64accumulated in BFA compart-ments,whereas PIN2was retained at the plasma mem-brane(Figures4G and4H).Similar results were also obtained for other plasma-membrane-resident proteins, which were previously suggested to undergo BFA-sensi-tive endocytic cycling,such as PM-ATPase,PIP2water channel,and the low-temperature-induced plasma-membrane protein Lti6b[19,26,34].Tyrphostin A23, but not A51,inhibited the BFA-induced internalization of all these proteins(Figures4O–4T and Figures S2B–S2D).The action of Tyrphostin A23in Arabidopsis was reversible(Figures S3A–S3C)and appeared to be spe-ci?c for endocytic-cargo recruitment at the plasma membrane because it did not show any apparent effects on other subcellular traf?cking processes at concentra-tions that already ef?ciently inhibited cargo internaliza-tion at the plasma membrane.For example,after Tyr-phostin A23treatment,the appearance of the TGN/ endosomes(as visualized by a1[27],GNOM[35],and ARF1[15]markers)was largely unchanged,and in the presence of BFA these markers accumulated into the BFA compartments(Figures4I–4P).In addition,when Tyrphostin A23was washed away with BFA,both plasma-membrane-localized proteins and TGN/endo-somes accumulated into the BFA compartments similar to the ones observed in case of BFA treatment alone(Fig-ures S3A–S3C).In addition,Tyrphostin A23did not visi-bly interfere with exocytosis because,after BFA washout in the presence of Tyrphostin A23,endosomes and the Golgi apparatus recovered and plasma-membrane pro-teins,including PINs,were recycled from the BFA com-partments back to the plasma membrane(Figures4L and4U and Figure S3D).In addition,in the presence of Tyrphostin A23,recovery of Lti6B-GFP[26]was ob-served at the plasma membrane when it was photo-bleached both from the cytosol and from the plasma membrane(see Figure S4),indicating that Tyrphostin A23does not completely inhibit the forward traf?c toward the plasma membrane.It cannot be entirely excluded that Tyrphostin A23may also affect other processes;however,its strong effects on internalization of PINs and other cargoes implicate the importance of clathrin-dependent endocytosis for the internalization of many plasma-membrane-resident proteins in plants. Conclusions
Endocytosis is of paramount importance for the function of both unicellular and multicellular organisms.Despite numerous recent demonstrations that various plant plasma-membrane proteins are internalized from the cell surface[4,6,9,19,26,30,34],a molecular mecha-nism for endocytosis in plants has not been demon-strated.Through several approaches including live-cell imaging,electron microscopy,and genetic and pharma-cological studies,we show here that an endocytic mechanism utilizing the coat protein clathrin is opera-tional in plants.In addition,we have identi?ed several endogenous cargoes for clathrin-dependent endocy-tosis,prominent among them the plant-speci?c,constitutively cycling PIN protein family of auxin ef?ux carriers.Interference with clathrin-dependent endocyto-sis impairs the internalization of many plant plasma-membrane proteins and inhibits endocytosis in general, suggesting that the clathrin-dependent internalization machinery is the major endocytic mechanism in plant cells.We feel that an important topic for future studies will be,in addition to the identi?cation of ancillary,regu-latory elements of the internalization machinery,a full appreciation of the diverse functions of endocytosis in plants.In particular,elucidating the role of clathrin-dependent PIN internalization in regulating auxin transport will be of fundamental importance to our understanding of how this evolutionarily conserved mechanism is utilized in mediating plant-speci?c physi-ological and developmental roles.
Supplemental Data
Supplemental Data include Experimental Procedures and four ?gures and are available with this article online at:http://www. https://www.doczj.com/doc/406453837.html,/cgi/content/full/17/6/520/DC1/. Acknowledgments
We thank J.Wiedenmann,G.Ju¨rgens,C.Luschnig,B.Scheres,M. Grebe,W.Michalke,D.Ehrhardt,and K.Schumacher for sharing published material.We thank C.Brancato for assistance with Arabi-dopsis protoplast transformations.This work was supported by a Volkswagenstiftung(P.D.J.F.,J.M.),EMBO Young Investigator Program(J.F.),the Deutsche Forschungsgemeinschaft(D.G.R.), SFB446(Y.-D.S.),and the Ministerio de Educacio′n y Ciencia,grant BFU2005-00071(F.A.).
Received:November21,2006
Revised:January18,2007
Accepted:January18,2007
Published online:February15,2007
References
1.Alberts,B.,Johnson,A.,Lewis,J.,Raff,M.,Roberts,K.,and
Walter,P.(2002).Molecular Biology of the Cell,4th Edition (New York:Garland Science).
2.Conner,S.D.,and Schmid,S.L.(2003).Regulated portals of
entry into the cell.Nature422,37–44.
3.Kirchhausen,T.(2000).Clathrin.Annu.Rev.Biochem.69,699–
727.
4.Murphy,A.S.,Bandyopadhyay,A.,Holstein,S.E.,and Peer,W.A.
(2005).Endocytotic cycling of PM proteins.Annu.Rev.Plant Biol.56,221–251.
5.Russinova,E.,and de Vries,S.C.(2005).Receptor-mediated
endocytosis in plants.In Plant Endocytosis,Volume1,J.Samaj,
F.Baluska,and D.Menzel,eds.(Heidelberg:Springer Verlag),
pp.103–115.
6.Robatzek,S.,Chinchilla, D.,and Boller,T.(2006).Ligand-
induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis.Genes Dev.20,537–542.
7.Wiedenmann,J.,Ivanchenko,S.,Oswald, F.,Schmitt, F.,
Rocker,C.,Salih,A.,Spindler,K.D.,and Nienhaus,G.U.(2004).
EosFP,a?uorescent marker protein with UV-inducible green-to-red?uorescence https://www.doczj.com/doc/406453837.html,A 101,15905–15910.
8.Petrasek,J.,Mravec,J.,Bouchard,R.,Blakeslee,J.J.,Abas,M.,
Seifertova,D.,Wisniewska,J.,Tadele,Z.,Kubes,M.,Covanova, M.,et al.(2006).PIN proteins perform a rate-limiting function in cellular auxin ef?ux.Science312,914–918.
9.Geldner,N.,Friml,J.,Stierhof,Y.D.,Jurgens,G.,and Palme,K.
(2001).Auxin transport inhibitors block PIN1cycling and vesicle traf?cking.Nature413,425–428.
10.Friml,J.(2003).Auxin transport-shaping the plant.Curr.Opin.
Plant Biol.6,7–12.
Current Biology 526
11.Tanaka,H.,Dhonukshe,P.,Brewer,P.B.,and Friml,J.(2006).
Spatiotemporal asymmetric auxin distribution:A means to coor-dinate plant development.Cell.Mol.Life Sci.63,2738–2754. 12.Friml,J.,Wisniewska,J.,Benkova,E.,Mendgen,K.,and Palme,
K.(2002).Lateral relocation of auxin ef?ux regulator PIN3medi-ates tropism in Arabidopsis.Nature415,806–809.
13.Friml,J.,Vieten,A.,Sauer,M.,Weijers,D.,Schwarz,H.,Hamann,
T.,Offringa,R.,and Jurgens,G.(2003).Ef?ux-dependent auxin gradients establish the apical-basal axis of Arabidopsis.Nature 426,147–153.
14.Benkova,E.,Michniewicz,M.,Sauer,M.,Teichmann,T.,Seifer-
tova,D.,Jurgens,G.,and Friml,J.(2003).Local,ef?ux-depen-dent auxin gradients as a common module for plant organ formation.Cell115,591–602.
15.Xu,J.,and Scheres,B.(2005).Dissection of Arabidopsis ADP-
RIBOSYLATION FACTOR1function in epidermal cell polarity.
Plant Cell17,525–536.
16.Abas,L.,Benjamins,R.,Malenica,N.,Paciorek,T.,Wisniewska,
J.,Moulinier-Anzola,J.C.,Sieberer,T.,Friml,J.,and Luschnig,
C.(2006).Intracellular traf?cking and proteolysis of the Arabi-
dopsis auxin-ef?ux facilitator PIN2are involved in root gravi-tropism.Nat.Cell Biol.8,249–256.
17.Ueda,T.,Yamaguchi,M.,Uchimiya,H.,and Nakano,A.(2001).
Ara6,a plant-unique novel type Rab GTPase,functions in the endocytic pathway of Arabidopsis thaliana.EMBO J.20,4730–4741.
18.Emans,N.,Zimmermann,S.,and Fischer,R.(2002).Uptake of
a?uorescent marker in plant cells is sensitive to brefeldin A and wortmannin.Plant Cell14,71–86.
19.Dhonukshe,P.,Baluska,F.,Schlicht,M.,Hlavacka,A.,Samaj,J.,
Friml,J.,and Gadella,T.W.,Jr.(2006).Endocytosis of cell surface material mediates cell plate formation during plant cytokinesis.Dev.Cell10,137–150.
20.Marsh,M.,and McMahon,H.T.(1999).The structural era of
endocytosis.Science285,215–220.
21.Kirchhausen,T.(1999).Adaptors for clathrin-mediated traf?c.
Annu.Rev.Cell Dev.Biol.15,705–732.
22.Robinson,D.G.,and Hillmer,S.(1990).Endocytosis in plants.
Physiol.Plant.79,96–104.
23.Ortiz-Zapater, E.,Soriano-Ortega, E.,Marcote,M.J.,Ortiz-
Masia′,D.,and Aniento,F.(2006).Traf?cking of the human trans-ferrin receptor in cells:Effects of tyrphostin A23and brefeldin A.
Plant J.48,757–770.
24.Holstein,S.E.(2005).Molecular dissection of the clathrin-endo-
cytosis machinery in plants.In Plant Endocytosis,Volume1, J.Samaj,F.Baluska,and D.Menzel,eds.(Heidelberg:Springer Verlag),pp.83–101.
25.Kim,Y.W.,Park,D.S.,Park,S.C.,Kim,S.H.,Cheong,G.W.,and
Hwang,I.(2001).Arabidopsis dynamin-like2that binds speci?-cally to phosphatidylinositol4-phosphate assembles into
a high-molecular weight complex in vivo and in vitro.Plant Phys-
iol.127,1243–1255.
26.Grebe,M.,Xu,J.,Mobius,W.,Ueda,T.,Nakano,A.,Geuze,H.J.,
Rook,M.B.,and Scheres,B.(2003).Arabidopsis sterol endocy-tosis involves actin-mediated traf?cking via ARA6-positive early endosomes.Curr.Biol.13,1378–1387.
27.Dettmer,J.,Hong-Hermesdorf,A.,Stierhof,Y.D.,and Schu-
macher,K.(2006).Vacuolar H+-ATPase activity is required for endocytic and secretory traf?cking in Arabidopsis.Plant Cell 18,715–730.
28.Liu,S.H.,Wong,M.L.,Craik,C.S.,and Brodsky,F.M.(1995).
Regulation of clathrin assembly and trimerization de?ned using recombinant triskelion hubs.Cell83,257–267.
29.Ueda,T.,and Nakano,A.(2002).Vesicular traf?c:An integral part
of plant life.Curr.Opin.Plant Biol.5,513–517.
30.Russinova,E.,Borst,J.W.,Kwaaitaal,M.,Cano-Delgado,A.,Yin,
Y.,Chory,J.,and de Vries,S.C.(2004).Heterodimerization and endocytosis of Arabidopsis brassinosteroid receptors BRI1 and AtSERK3(BAK1).Plant Cell16,3216–3229.
31.Cutler,S.R.,Ehrhardt,D.W.,Grif?tts,J.S.,and Somerville,C.R.
(2000).Random GFP::cDNA fusions enable visualization of sub-cellular structures in cells of Arabidopsis at a high frequency.
https://www.doczj.com/doc/406453837.html,A97,3718–3723.32.Banbury,D.N.,Oakley,J.D.,Sessions,R.B.,and Banting,G.
(2003).Tyrphostin A23inhibits internalization of the transferrin receptor by perturbing the interaction between tyrosine motifs and the medium chain subunit of the AP-2adaptor complex.
J.Biol.Chem.278,12022–12028.
33.Motley,A.,Bright,N.A.,Seaman,M.N.,and Robinson,M.S.
(2003).Clathrin-mediated endocytosis in AP-2-depleted cells.
J.Cell Biol.162,909–918.
34.Paciorek,T.,Zazimalova,E.,Ruthardt,N.,Petrasek,J.,Stierhof,
Y.D.,Kleine-Vehn,J.,Morris,D.A.,Emans,N.,Jurgens,G.,Geld-ner,N.,et al.(2005).Auxin inhibits endocytosis and promotes its own ef?ux from cells.Nature435,1251–1256.
35.Geldner,N.,Anders,N.,Wolters,H.,Keicher,J.,Kornberger,W.,
Muller,P.,Delbarre,A.,Ueda,T.,Nakano,A.,and Jurgens,G.
(2003).The Arabidopsis GNOM ARF-GEF mediates endosomal recycling,auxin transport,and auxin-dependent plant growth.
Cell112,219–230.
Clathrin-Mediated Endocytosis in Plants 527