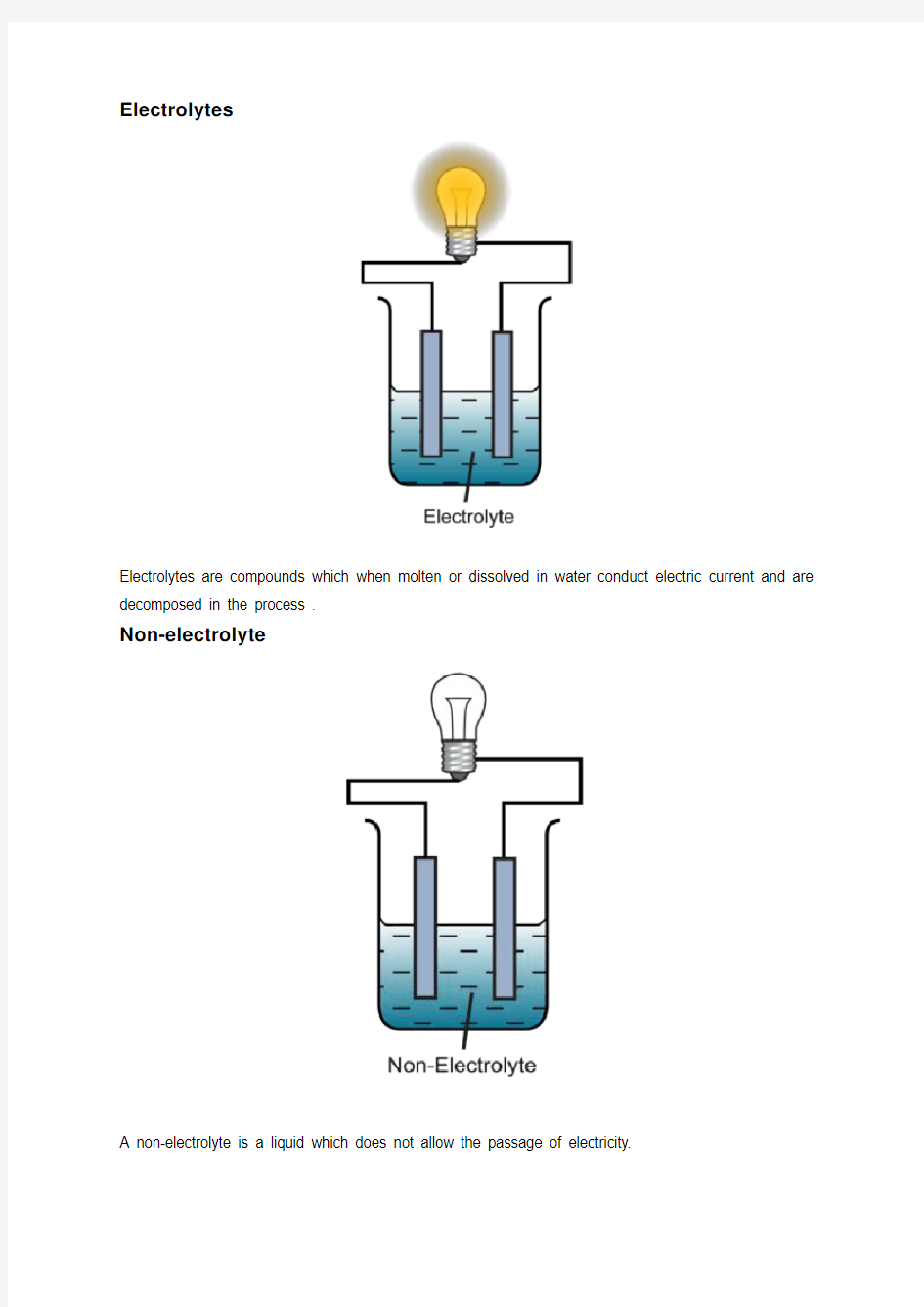

Electrolytes
Electrolytes are compounds which when molten or dissolved in water conduct electric current and are decomposed in the process .
Non-electrolyte
A non-electrolyte is a liquid which does not allow the passage of electricity.
Molten Solution
?This is composed of lead(II) ions, Pb2 + , and bromide ions, Br-. Its chemical formula is therefore PbBr2.
? A suitable apparatus which could be used to carry out this electrolysis is shown in Figure above. ?The bulb helps to show when electricity is flowing in the circuit, and until the lead(II) bromide is completely molten, the bulb does not light up . This confirms that electrolytes have to be molten
?In summary, the lead(II) bromide is split into its component elements :
PbBr2 ---> Pb + Br2
Electrolysis of Aqueous Sulphuric Acid
?As sulphuric acid is aqueous, it is composed not only of hydrogen ions (H+ ) and sulphate ions
(SO42-), but also of hydroxide ions (OH-) from the water.
H2SO4 + H2O --> 2H+ + SO42- + H+ + OH-
?The apparatus used to carry out this electrolysis and collect the gases given off is shown in Figure
9 .8 .
?When we have more than one type of ion moving to an electrode, selective discharge (or preferential discharge) takes place.
?This means that the ion which can lose or gain electrons with the greatest ease is discharged, and the other ions, which are harder to discharge, remain in solution .
?
Extraction of Metal
?The extraction of metals from their ores, in particular aluminium and sodium, is important industrial uses of electrolysis.
?The diagram below shows the methods of extraction for different metals.
?We can see that those metals which are less reactive than carbon in reactivity series are extracted from their ore by displacement reaction using carbon. This will be discussed in detail in chapter 3, form 5, Oxidation and Reduction.
?Copper and mercury can be extracted from their ore by burning directly in air.
?Silver (Ag) and gold (Au) need no extraction because they exist as element in nature.
?Those metals which are more reactive than carbon are extracted by electrolysis.
Extraction of Aluminium
?Aluminium is the most abundant metal found in the earth's crust. It makes up about 8% by weight of the Earth’s solid surface.
?It is also a very useful metal due to its low density and ability to resist corrosion.
?The main source of aluminium is bauxite ore (Aluminium Oxide).
?In industry, aluminium is extracted by electrolysis from bauxite ore.
Adding Cryolite
?In electrolysis, molten aluminium oxide must be used to extract aluminium. Aluminium oxide decompose to form aluminium and oxide ions when melted.
Al2O3 ---> 2Al3+ + 3O2-
?However, the melting point of aluminium oxide is very high (over 2 000°C), so another aluminium compound called cryolite (Na3AIF6) is added to lower down the melting point
(about 980o C).
?The diagram above shows how aluminium is extracted from molten aluminium oxide by electrolysis.
?Graphite is used as the anode and cathode.
?During electrolysis, the aluminium ions are attracted towards the graphite cathode.
?The ions is discharged and become molten aluminium metal.
?The partial equation of this reaction is as follow:
Al3+ + 3e ---> Al
?At the anode, oxygen gas which also has commercial value is collected. The partial equation of this reaction is as follow:
2O2- ---> O2 + 4e
?At the temperature of 980 °C, the oxygen burns the carbon anode. Therefore the anode has to be replaced periodically.
?Also, this cell uses large quantities of electricity, and therefore needs cheap sources of power.
Extraction of sodium chloride
?In industry, sodium is extracted from molten sodium chloride. Molten sodium chloride is put into the apparatus as showing in the diagram above.
?When sodium chloride is melted, the sodium and chloride ions disassociate to become freely move ions, as shown in the chemical equation below.
NaCl ---> Na+ + Cl-
?In this electrolytic cell, graphite was used as anode while iron is used as cathode.
?The negative chloride ions are attracted to the anode and then discharged to form chlorine gas.
2Cl- ---> Cl2 + 2e
?Since chlorine gas is also significant in industry, it is collected and stored.
?In cathode, the sodium ions are discharged to form sodium atom.
Na+ + e ---> Na
?Due to high temperature, the sodium metal formed is in molten form.
?Metal sodium have lower density. Therefore it moves upward and been collected.
Purification Of Copper
?In the refining or purification of copper, the impure copper is made the anode and a thin, pure copper plate is used as a cathode.
?The electrolyte is usually acidified copper(II) sulphate solution.
?When electricity flows, the copper dissolves from the impure anode and goes into solution as copper ions.
?Impurities in the copper do not dissolve, and instead fall off the anode as anode sludge. At the cathode, the copper ions are deposited as pure copper metal.
?Reaction in anode (impure copper)
In anode, the copper atoms from the electrode are ionised to form copper(II) ions.
Cu ---> Cu2+ + 2e
?Reaction in cathode (pure copper)
Cu2+Cu ---> Cu + 2e
Electroplating
Electroplating: Coating with a Thin Protective Layer of Metal
? A very common use of electrolysis is to form a thin protective coating of a metal on the surface of another which is likely to corrode.
?The diagram above illustrate the electroplating of a key with copper.
?In this process, we need to make the cathode the object for plating (the key.
?The anode is then made of the metal we wish to plate with (copper), and the electrolyte needs to be a solution of a salt of this metal (copper(II) sulphate).
Anode
?In anode, the copper atoms from the electrode are ionised to form copper(II) ions.
Cu ---> Cu2+ + 2e
Cathode
?In cathode, the copper ions are discharged to form copper atom and then deposit on the surface of the key
Cu2+ ---> Cu + 2e
Cells and Batteries
? A device which converts chemical energy into electrical energy is called a cell or battery. Battery is a collection of cells.
? A cell consists of a pair of dissimilar metals in an electrolyte.
?Figure above shows an example of a simple voltaic cell consist of a magnesium electrode and a copper electrode immerse in magnesium sulphate solution.
?When chemical reaction happens, the more reactive metal, magnesium, dissolves in the magnesium sulphate solution and become magnesium ions, thereby producing electrons, as shown in the half equation below:
Mg ---> Mg2+ + 2e
?As electrons are produced, the magnesium acts as the negative electrode.
?These electrons then travel to the copper electrode.
?The hydrogen ions around the copper electrode receive the electrons and are discharged to produce bubbles of hydrogen gas:
2H+ + 2e ---> H2
?As electrons are taken in, the copper is the positive electrode.
?This production and movement of electrons is electricity, so electrical energy has been generated and the galvanometer is deflected. *Overall, the chemical reaction can be
represented by the ionic equation:
Mg + 2H+ ---> Mg2+ + H2
?In voltaic cell, the negative electrode is the anode whereas the positive electrode is the cathode, which is the opposite of the electrolytic cell.