
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
1. Host materials for phosphorescent OLEDs
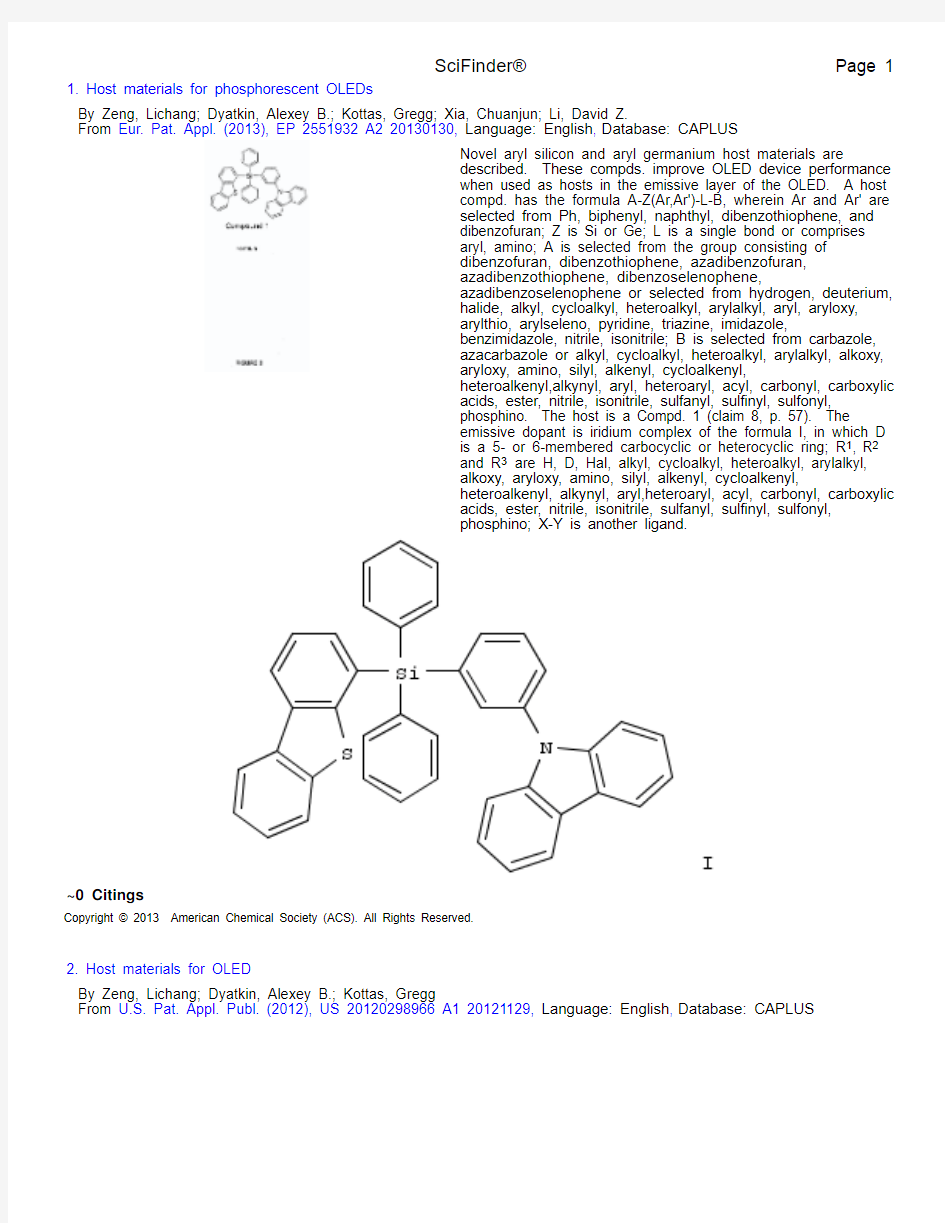
By Zeng, Lichang; Dyatkin, Alexey B.; Kottas, Gregg; Xia, Chuanjun; Li, David Z.From Eur. Pat. Appl. (2013), EP 2551932 A2 20130130, Language: English ,
Database: CAPLUS Novel aryl silicon and aryl germanium host materials are described. These compds. improve OLED device performance when used as hosts in the emissive layer of the OLED. A host compd. has the formula A-Z(Ar,Ar')-L-B, wherein Ar and Ar' are selected from Ph, biphenyl, naphthyl, dibenzothiophene, and dibenzofuran; Z is Si or Ge; L is a single bond or comprises aryl, amino; A is selected from the group consisting of dibenzofuran, dibenzothiophene, azadibenzofuran,azadibenzothiophene, dibenzoselenophene,azadibenzoselenophene or selected from hydrogen, deuterium,halide, alkyl, cycloalkyl, heteroalkyl, arylalkyl, aryl, aryloxy,arylthio, arylseleno, pyridine, triazine, imidazole,benzimidazole, nitrile, isonitrile; B is selected from carbazole,azacarbazole or alkyl, cycloalkyl, heteroalkyl, arylalkyl, alkoxy,aryloxy, amino, silyl, alkenyl, cycloalkenyl,heteroalkenyl,alkynyl, aryl, heteroaryl, acyl, carbonyl, carboxylic acids, ester, nitrile, isonitrile, sulfanyl, sulfinyl, sulfonyl,phosphino. The host is a Compd. 1 (claim 8, p. 57). The emissive dopant is iridium complex of the formula I, in which D is a 5- or 6-membered carbocyclic or heterocyclic ring; R 1, R 2
and R 3 are H, D, Hal, alkyl, cycloalkyl, heteroalkyl, arylalkyl,alkoxy, aryloxy, amino, silyl, alkenyl, cycloalkenyl,heteroalkenyl, alkynyl, aryl,heteroaryl, acyl, carbonyl, carboxylic acids, ester, nitrile, isonitrile, sulfanyl, sulfinyl, sulfonyl,phosphino; X-Y is another ligand.
~0 Citings
2. Host materials for OLED
By Zeng, Lichang; Dyatkin, Alexey B.; Kottas, Gregg From U.S. Pat. Appl. Publ. (2012), US 20120298966 A1 20121129, Language: English , Database: CAPLUS
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
Novel aryl silicon and aryl germanium host materials are described. These compds. improve OLED device performance when used as hosts in the emissive layer of the OLED.
~0 Citings
3. Host materials for OLEDs
By Zeng, Lichang; Dyatkin, Alexey B.; Kottas, Gregg From PCT Int. Appl. (2012), WO 2012162325 A1 20121129, Language: English , Database: CAPLUS
Novel aryl silicon and aryl germanium host materials are described. These compds. improve OLED device performance when used as hosts in the emissive layer of the OLED.
~0 Citings
4. Carbazole/oligocarbazoles substituted silanes as wide bandgap host materials for solution-processable electrophosphorescent devices
By Hu, Dehua; Cheng, Gang; Liu, He; Lv, Ying; Lu, Ping; Ma, Yuguang From Organic Electronics (2012), 13(12), 2825-2831. Language: English , Database: CAPLUS,DOI:10.1016/https://www.doczj.com/doc/0513699576.html,el.2012.09.010
We have designed and synthesized a series of org. wide bandgap materials, namely DCzSiCz, DDCzSi and DTCzSi, by incorporating carbazole/oligocarbazoles via a silicon-bridged linkage mode. All the materials show good thermal stability and excellent soln.-processibility. Their HOMOs and LUMOs could be tuned to facilitate the efficient carriers injection by the incorporated carbazole/oligocarbazoles, while their singlet and triplet energy levels still maintain high levels, all above 3.44 eV and 2.87 eV, resp. High efficient blue electrophosphorescent devices with low turn-on voltage are realized using DCzSiCz, DDCzSi and DTCzSi as hosts for FIrpic through soln.-processable method. Among them, DCzSiCz-based device demonstrates the best performance, showing a max. brightness of 6600 cd m -2 at 11 V and max. luminous efficiency of 8.40 cd A -1 at 5 V.
~0 Citings
5. Preparation of 4H-imidazo[1,2-a]imidazoles for organic electronic applications
By Schaefer, Thomas; Figueira Duarte, Teresa Marina; Schildknecht, Christian; Langer, Nicolle; Heinemeyer, Ute;Wolleb, Heinz; Watanabe, Soichi; Lennartz, Christian; Wagenblast, Gerhard; Wolleb, Annemarie; et al From PCT Int. Appl. (2012), WO 2012130709 A1 20121004, Language: English , Database: CAPLUS
The present invention relates to 4H-imidazo[1,2-a]imidazole compds. [I; X7 = N; X6 = NR1; R1 = A1-(A2)p-(A3)q-(A4)r-R6; p,q, r = 0, or 1; A1, A2, A3, A4 = independently each (un)substituted C6-24 arylene or C2-30 heteroarylene which may be interrupted by one, or more groups of (SiR7R8); R2, R3, R4, R5 = independently H, each (un)substituted a C1-25 alkyl, C6-24 aryl, or C2-30 heteroaryl; R6 = H, (SiR2OR21R22), each
(un)substituted C6-24 aryl or C2-30 heteroaryl; R7, R8 = each (un)substituted C1-25 alkyl or C6-24 aryl ; X1 = N or CR9; X2 = N or CR10; R9, R10 = independently H, each (un)substituted C1-25 alkyl, C6-24 aryl, or C2-30 heteroaryl; or R9 and R10 together form (un)substituted ring; R20, R21, R22 = independently each (un)substituted C1-25 alkyl or C6-24 aryl], a process for their prodn. and their use in electronic devices, esp. electroluminescent devices. When used as host material for phosphorescent emitters in electroluminescent devices, the compds. I may provide improved efficiency, stability, manufacturability, or spectral characteristics of electroluminescent devices. Thus, 1.98 g 9-[8-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)dibenzofuran-2-
yl]carbazole, 4.02 g potassium phosphate tribasic monohydrate, 15 mL dioxane, 60 mL toluene, and 12 mL water were added to 1.50 g 5-(8-bromodibenzofuran-2-
yl)benzimidazolo[1,2-a]benzimidazole. The resulting mixt. was degassed with argon, following by adding 81 mg 2-dicyclohexylphosphino-2',6'-dimethoxybiphenyl (SPhos) and 74 mg palladium(II) acetate, and the reaction mixt. was degassed with argon and stirred for 4.5 h at 100° under argon to give compd. (II). An org. electroluminescent device with a layer of iridium complexes and II showed external quantum efficiency (EQE) of 14.7% and life time of 125 h at 3.6 V.
~0 Citings
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
6. Preparation of 4H-imidazo[1,2-a]imidazole derivatives for the use of electroluminescent devices
By Schaefer, Thomas; Figueira Duarte, Teresa Marina; Schildknecht, Christian; Langer, Nicolle; Heinemeyer, Ute; Wolleb, Heinz; Watanabe, Soichi; Lennartz, Christian; Wagenblast, Gerhard; Wolleb, Annemarie; et al
From U.S. Pat. Appl. Publ. (2012), US 20120241681 A1 20120927, Language: English, Database: CAPLUS
Title compds. I [X6 = N and X7 = NR1, or X7 = N and X6 = NR1; R1 = A1-(A2)p-(A3)q-(A4)m-R6; p, q and m = independently 0 or 1; A1, A2, A3 and A4 = independently (un)substituted arylen; R2, R3, R4 and R5 = independently H, (un)substituted alkyl, aryl or heteroaryl; R6 = H, SiR20R21R22, (un)substituted aryl, or heteroaryl; R20, R21 and R22 = independently (un)substituted alkyl, or aryl; X1 = N, or CR9; X2 = N, or CR10; R9 and R10 = independently H, (un)substituted alkyl or ring when taken together], are prepd. for the use of electroluminescent devices. Thus, e.g., compd. II was prepd. by boronylation of 9-(8-bromodibenzofuran-2-yl)carbazole followed by coupling with 5-(8-bromodibenzofuran-2-
yl)benzimidazo[l,2-a]benzimidazole which was prepd. by ring-closure of 3-(2-aminophenyl)-lH-benzimidazol-2-one and coupling with 2-bromo-8-iodo-dibenzofuran. The compds. of the invention may provide improved efficiency, stability, manufacturability or spectral characteristics for electroluminescent devices. The invention compds. are useful for electronic devices, esp. electroluminescent devices.
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
~0 Citings
7. High-efficiency organic light-emitting diodes utilizing thermally activated delayed fluorescence from triazine-based donor-acceptor hybrid molecules
By Lee, Sae Youn; Yasuda, Takuma; Nomura, Hiroko; Adachi, Chihaya From Applied Physics Letters (2012), 101(9), 093306/1-093306/4. Language: English , Database: CAPLUS,DOI:10.1063/1.4749285
A high-efficiency purely org. luminescent material, 2,4-bis{3-(9H-carbazol-9-yl)-9H-carbazol-9-yl}-6-phenyl-1,3,5-triazine (CC2TA) comprising the bicarbazole donor and phenyltriazine acceptor units, which is capable of emitting thermally activated delayed fluorescence, was designed and synthesized. The mol. design of CC2TA allows spatial sepn. of HOMO and LUMO on the donor and acceptor fragments, resp., leading to an exceptionally small singlet-triplet exchange energy (?E ST = 0.06 eV) together with a high triplet energy. A high external electroluminescence quantum efficiency ≤11% ± 1% was achieved in the sky-blue org. LEDs employing CC2TA as an emitter. (c) 2012 American Institute of Physics.
~0 Citings
8. Dicarbazyl-containing dye special for dye sensitized solar cells and preparation method thereof
By Wang, Kezhi; Chen, Yanmin; Ju, Chuanchuan; Fan, Suhua
From Faming Zhuanli Shenqing (2012), CN 102604412 A 20120725, Language: Chinese, Database: CAPLUS
The invention provides an org. micromol. solar cell dye sensitizer (I). The invention also provides a ligand for prepg. the solar cell dye sensitizer (II, R-ipphen). The invention further provides a Ru-based solar cell dye sensitizer represented by the formula [RuR-ipphen(H x dcbpy)(NCS)2]x-2(M)y2-x, H x dcbpy is 4,4'-dicarboxyl-2,2'-bipyridyl ligand, 0<=x<=2, M is pos. univalent, divalent or trivalent inorg. or org. counter ion. The invention further provides a method for prepn. of the org. micromol. solar cell dye sensitizer, which comprises carrying out condensation reaction of carbazole to obtain dicarbazyl, reacting with p-fluorobenzaldehyde in the presence of K tert-butoxide under N2 atmosphere to obtain dicarbazyl benzaldehyde, and reacting with cyanoacetic acid. The invention further provides a method for prepn. of the Ru-based solar cell dye sensitizer, which comprises carrying out condensation reaction of dicarbazyl benzaldehyde with o-phenanthroline-5,6-dione in the presence of ammonium acetate to obtain the ligand R-ipphen, reacting with [(Me-p-C6H4-i Pr)RuCl2]2, adding 4,4'-dicarboxy-2,2'-bipyridyl, reacting under lucifuge, adding excess ammonium thiocyanate, reacting under lucifuge to obtain crude product. The crude product is then purified by chromatog. The invention further relates to the application of the org. micromol. solar cell dye sensitizer or the Ru-based solar cell dye sensitizer in prepg. inorg. oxide nanocrystal solar cells, with low cost and energy consumption, improved power conversion efficiency by >8%, no use of precious metal and less environmental pollution. The invention also describes a solar cell, which consists of the inorg. oxide nanocrystal film as photoanode, and a layer of photosensitizer specific dye on the inorg. oxide nanocrystal film.
~0 Citings
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
9. Compositions for organic electroluminescent (EL) devices, blue-emitting organic EL devices, and display devices and lighting apparatus using them
By Iida, Koichiro; Ishibashi, Koichi; Li, Yanjun; Endo, Kyoko; Migita, Akira From Jpn. Kokai Tokkyo Koho (2012), JP 2012119471 A 20120621, Language: Japanese , Database: CAPLUS
The title compns. contain charge-transporting materials, luminescent materials contg. I [X = N, C; Y = C when X = N; Y =N when X = C; R 1-R 7 = H, halo, (un)substituted hydrocarbon group, (un)substituted alkoxy, (un)substituted arom.heterocyclic group; R 1-R 7 may form ring with adjacent group; L = monovalent bidentate ligand; m = 1-3], and solvents,wherein the charge-transporting materials and the luminescent materials contain compds. with T g ≤140° individually. The title org. EL devices have org. layers formed from the compns. using wet process between anodes and cathodes. The display devices and the lighting app. using the org. EL devices are also claimed. The org. EL devices have high luminescent efficiency.
~1 Citing
10. A Highly Efficient, Blue-Phosphorescent Device Based on a Wide-Bandgap Host/FIrpic: Rational Design of the Carbazole and Phosphine Oxide Moieties on Tetraphenylsilane
By Liu, He; Cheng, Gang; Hu, Dehua; Shen, Fangzhong; Lv, Ying; Sun, Guannan; Yang, Bing; Lu, Ping; Ma, Yuguang From Advanced Functional Materials (2012), 22(13), 2830-2836, S2830/1-S2830/11. Language: English , Database:CAPLUS, DOI:10.1002/adfm.201103126
A new series of wide-bandgap materials, 4-dipenylphosphine oxide-4'-9H-carbazol-9-yl-tetraphenylsilane (CSPO), 4-diphenylphosphine oxide-4',4''-di(9H-carbazol-9-yl)-tetraphenylsilane (pDCSPO), 4-diphenylphosphine oxide -4'-[3-(9H-carbazol-9-yl)-carbazole-9-yl]-tetraphenylsilane (DCSPO), 4-diphenylphosphine oxide-4',4'',4'''-tri(9H-carbazol-9-yl)-tetraphenylsilane (pTCSPO) and 4-diphenylphosphine oxide -4'-[3,6-di(9H-carbazol-9-yl)-9H-carbazol-9-yl]-tetraphenylsilane (TCSPO), contg. different ratios and linking fashions of p-type carbazole units and n-type phosphine oxide units, are designed and obtained. DCSPO is the best host in FIrpic-doped devices for this series of compds. By utilizing DCzSi and DPOSi as hole- and electron-transporting layers, a high EQE of 27.5% and a max. current efficiency of 49.4 cd A -1 are achieved in the DCSPO/FIrpic doped device. Even at 10 000 cd m -2, the efficiencies still remain 41.2cd A -1 and 23.0%, resp.
~9 Citings
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
11. Novel 3, 9-linked oligocarbazole-based hosts containing DBT and DBF fragments, separated by aromatic spacers
By Dyatkin, Alexey From U.S. Pat. Appl. Publ. (2012), US 20120086329 A1 20120412, Language: English ,
Database: CAPLUS Compds. comprising a 3,9-linked oligocarbazole moiety and a dibenzothiophene, dibenzofuran, dibenzoselenophene, aza-dibenzothiophene, aza-dibenzofuran, or aza-dibenzoselenophene are provided. The 3,9-linked oligocarbazole and dibenzo or aza-dibenzo moiety are sepd. by an arom. spacer. The compds. may be used as non-emissive materials for phosphorescent OLEDs to provide devise having improved performance. Compds. comprising a 3, 9-linked oligocarbazole moiety and a dibenzothiophene, dibenzofuran,dibenzoselenophene, aza-dibenzothiophene, aza-dibenzofuran, or aza-dibenzoselenophene are [I: R'1-'2, R a,b
(mono, di, tri, tetra-substituent) = H, D, alkyl, alkoxy, amino,alkenyl, alkynyl, arylkyl, aryl, heteroaryl; X = aryl, heteroaryl; Y = (aza)dibenzothiophene, (aza)dibenzofuran,(aza)dibenzoselenophene; n =1-20 int.]. The 3, 9-linked oligocarbazole and dibenzo or aza-dibenzo moiety are sepd. by an arom. spacer. The compds. may be used as non-emissive materials for phosphorescent OLEDs to provide devise having improved performance.
~0 Citings
12. Novel 3, 9-linked oligocarbazole-based hosts containing DBT and DBF fragments, separated by aromatic spacers By Dyatkin, Alexey From PCT Int. Appl. (2012), WO 2012048266 A1 20120412, Language: English , Database: CAPLUS
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
Compds. comprising a 3, 9-linked oligocarbazole moiety and a dibenzothiophene, dibenzofuran, dibenzoselenophene, aza-dibenzothiophene, aza-dibenzofuran, or aza-dibenzoselenophene are [I: R'1-'2, R a,b (mono, di, tri, tetra-substituent) = H, D, alkyl, alkoxy, amino, alkenyl, alkynyl,arylkyl, aryl, heteroaryl; X = aryl, heteroaryl; Y =(aza)dibenzothiophene, (aza)dibenzofuran,(aza)dibenzoselenophene; n =1-20 int.]. The 3, 9-linked oligocarbazole and dibenzo or aza-dibenzo moiety are sepd. by an arom. spacer. The compds. may be used as non-emissive materials for phosphorescent OLEDs to provide devise having improved performance.
~0 Citings
13. Compounds for host materials in organic electroluminescent devices for high luminous efficiency, reduced driving voltage, high thermal resistance, and long lifetime, and organic electroluminescent devices comprising the luminescent compounds
By Balaganesan, Banumathy; Fu, Yi-Huan; Guo, Huang-Ming From U.S. Pat. Appl. Publ. (2011), US 20110309345 A1 20111222, Language: English , Database: CAPLUS
Compds. for host materials in org. electroluminescent devices for high luminous efficiency, reduced driving voltage, high thermal resistance, and long lifetime, and org. electroluminescent devices comprising the luminescent compds. are discussed. The compds. are represented by formula I for org. electroluminescent devices: where X and Y are each independently selected for the group consisting of an alkyl substituted, aryl substituted or unsubstituted carbazole, indolocarbazole, triphenylsilyl and diphenylphosphine oxide represented by formula II, III, IV, V or VI, in which R1, R2, and R3 are each independently selected from the group consisting of a hydrogen, an alkyl having 1 to 15 carbons atoms, an aryl group having 6 to 15 carbons atoms, an alkyl substituted, an aryl substituted or unsubstituted triphenylsilyl, and a diphenylphosphine oxide represented by the formula V or VI; m and n are each independently 0 or 1, provided that m+n is 1 or more; and Ar1 and Ar2 are each independently selected from the group consisting of an alkyl substituted, aryl substituted or unsubstituted Ph, tolyl, naphthyl, fluorenyl, anthracenyl, and phenanthryl.
~1 Citing
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
14. Template directed selective deposition of organic luminescent molecules
By Lin, Li; Wei, Shi-Gang; Li, Hui; Hao, Juan-Yuan; Lu, Nan
From Gaodeng Xuexiao Huaxue Xuebao (2011), 32(10), 2349-2352. Language: Chinese, Database: CAPLUS
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
Micro- and nano-scale fluorescent patterns over large area were fabricated by template directed selective deposition of org. luminescent mols. The template was fabricated by combination of nanoimprint lithog. (NIL) and metal evapn. and the following 'lift-off' process. In the template, the org. mols. have larger binding energies on the patterned area in comparison with the substrate, so the site-selective deposition of org. mols. can be obtained by choosing proper template. It is a fast means to fabricate large-area fluorescent pattern, which provides the possibility to increase the extn.efficiency of org. light emitting diodes (OLEDs).
~0 Citings
15. Bridged triarylamines and -phosphines and -phosphine oxides as materials for electronic devices and their preparation and use
By Parham, Amir Hossain; Pflumm, Christof From PCT Int. Appl. (2011), WO 2011128017 A1 20111020, Language: German ,
Database: CAPLUS
The title bridged compds. are described by the general formula I (X = N, P, or P:O; T, Y = independently selected at each occurrence from C(R 1)2, C:O, C:NV, O, S, SO, SO 2, PR 1,POR 1, NAr, NR 1, or a single bond with the restriction that ≥1 Y is a single bond; A = Ar 3 or X(Ar 4)2, with the bond between A and T coming from an arom. ring atom of Ar 3 or Ar 4 and the 2Ar 4 groups of the X(Ar 4)2 unit may joined by T; Ar, Ar 1-4 =independently selected at each occurrence from (hetero)aryl groups with 5-30 ring atoms optionally substituted with ≥1 R 2
group; R 1-2 = independently selected at each occurrence from H, D, halo, CHO, straight chain alkyl, etc.; m = 0 or 1 at each occurrence with the restriction that the total of all m is ≥ 1; and n = 0 or 1 at each occurrence with the restriction that the total of all n is ≥ 1). Oligomers, polymers, or dendrimers incorporating the compds., and solns. of the compds. or oligomers, polymers, or dendrimers incorporating them are described. Methods for producing the compds. are described which entail synthesizing an unbridged precursor that can be used to produce the desired compd. by a ring closing reaction.The use of the compds. in electronic devices and devices employing the compds. (e.g., org. field-effect transistors, org.thin-film transistors, org. light-emitting transistors, org.integrated circuits, org. solar cells, org. field quenching devices,light-emitting electrochem. cells, org. laser diodes, org.photoreceptors, org. optical detectors, and, esp., org.electroluminescent devices) are also described. In particular,the compds. may be used as a matrix material in the emitting layer in electroluminescent devices, as a hole transport material in a hole-transporting or hole-injecting layer, or as an emitting material in an emitting layer.
~2 Citings
16. Aromatic organic electroluminescent materials for light emitting device
By Kim, Bok Yeong; Ahn, Jung Bok; Lee, Jae Seong; Jin, Seong Min; Kang, Ji Seung; Ahn, Do Hwan; Park, No Gil;Han, Geun Hui; Si, Sang Man; Lee, Dae Gyun From Repub. Korean Kongkae Taeho Kongbo (2011), KR 2011079402 A 20110707, Language: Korean , Database:CAPLUS
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
The title org. light emitting device comprises a first electrode, a second electrode and at least an org. film. The org. film contains org. light emitting compd. with chem. formula shown as formula I (A 1, A 2, A 2', A 3, A 4 and A 4' are independent or correlative with each other, and are H, substituted or unsubstituted C6-C50 aryl, substituted or unsubstituted C2-C50hetero aryl, substituted or unsubstituted C2-C50 cycloalkyl, substituted or unsubstituted C2-C50 heterocyclic alkyl, or substituted or unsubstituted satd. or unsatd. hydrocarbon, resp.; Ar is H, substituted or unsubstituted C6-C50 aryl,substituted or unsubstituted C2-C50 hetero aryl, substituted or unsubstituted C2-C50 cycloalkyl, or substituted or unsubstituted C2-C50 heterocyclic alkyl; X is N, O or S; m is 1-10 integer). The org. light emitting device has high luminous efficiency, luminous brightness and color purity, and long luminescent lifetime.
~4 Citings
17. Organic electroluminescent element
By Ogawa, Junya; Kai, Takahiro; Yamamoto, Toshihiro; Matsumoto, Megumi From PCT Int. Appl. (2011), WO 2011081061 A1 20110707, Language: Japanese , Database: CAPLUS
Disclosed is an org. electroluminescent element (org. EL element) which has a simple structure and improved luminous efficiency, while achieving sufficient operational stability. Specifically disclosed is an org. EL element, which comprises a light emitting layer between a pos. electrode and a neg. electrode that are arranged on a substrate, and which was characterized in that the light emitting layer has a light emitting layer that contains a phosphorescent dopant and a 1,9-substituted carbazole compd. that serves as a host material. Examples of the 1,9-substituted carbazole compd. I [Ar,L =arom. hydrocarbon or arom. heterocycle; R 1-3= H, alkyl, cycloalkyl, arom. hydrocarbon or arom. heterocycle; n = integer 1 - 3.].
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
~2 Citings
18. High-Resolution Triple-Color Patterns Based on the Liquid Behavior of Organic Molecules
By Wang, Wenchong; Du, Chuan; Wang, Chenguang; Hirtz, Michael; Li, Liqiang; Hao, Juanyuan; Wu, Qiong; Lu, Ran;Lu, Nan; Wang, Yue; et al From Small (2011), 7(10), 1403-1406. Language: English , Database: CAPLUS, DOI:10.1002/smll.201002210Small-mol. org. semiconductors may behave like liqs. on patterned substrates. The liq. can be controlled on a scale down to sub-hundred nanometers with good stability. The ready functionality and lithog.-compatible processing make them attractive in micro/nanofluidics. Taking advantage of both the energy barrier for morphol. transition and diffusion of mols. on a surface, the authors can suppress the formation of bulges and achieve heteropatterning of org.semiconductors by using hierarchical patterned substrates, thus enabling triple-color patterns with 2 mols. The technique opens up a simple but efficient and scalable way to fabricate high-resoln., full-color org. patterns. The authors believe the technique is not limited to the DtCDQA/NPB system. Both mols. can be replaced to tune or optimize the photoluminescence spectra for specific applications, for example, a mol. with liq. state at a certain temp. for DtCDQA and an emissive mol. for NPB. Although electron-beam lithog. defined Au-patterned SiO 2 substrates were used the authors would like to emphasize that the technique can be extended to other patterning techniques, such as photolithog. and microcontact printing, as well as different substrates such as ITO modified with self-assembled org. monolayers.~3 Citings
19. Selective adsorption of organic light-emitting molecule on PANI microstructure
By Tian, Lu; Bu, Feng-quan; Huang, Chun-yu; Lu, Nan From Gaodeng Xuexiao Huaxue Xuebao (2010), 31(8), 1485-1487. Language: Chinese , Database: CAPLUS
We developed a method for patterning org. light-emitting mols. with polyaniline (PANI) microstructure as a template. Its microstructure was characterized with at. force microscopy (AFM). PANI microstructure was treated with O 2 plasma at low power and short time to get the hydroxyl groups on the silicon substrate, meanwhile, which can minimize the impact on PANI structure. We modified the structure with fluoro-silane reagent-through self-assembly to make substrate bearing different surface energies on different areas. Finally, we deposited dyes on substrate by evapn. The results show that the dyes on the fluoro-silane monolayer can move toward the PANI structure during storage duration and form the structure of org. light emitting mols.
~0 Citings
20. Theoretical investigation on the changes of structures and properties caused by the different link form in bicarbazoles By Wang, Hui-Ping; Bai, Fu-Quan; Zheng, Qing-Chuan; Zhao, Zeng-Xia; Zhang, Hong-Xing From Gaodeng Xuexiao Huaxue Xuebao (2009), 30(12), 2434-2438. Language: Chinese , Database: CAPLUS
D. functional theory (DFT) and CI with single excitations (CIS) method were used to optimize the ground state and excited state structures of carbazole and 14 bicarbazole isomers on the 6-31G (d,p) basis set level, resp. On the base of the geometry structures, the absorption and emission wavelengths and charge transfer characters were calcd. with the time-dependent DFT (TD-DFT) method on the same basis set level. In this paper, we discussed the relationship between different mol. structures and properties, detailedly, the calcd. data are in agreement with the reported corresponding exptl. results. On the base of the varied frontier MO energies, ionization potentials (IP), electron affinities (EA) and reorganization energies (λ) for bicabazoles, the dissimilar applications are studied all round. The studies can provide some help in designing and synthesizing more and greater mol. materials.
~0 Citings
21. Carbazole-containing materials in phosphorescent light emitting diodes
By Lin, Chun; Dyatkin, Alexey Borisovich; Elshenawy, Zeinab From PCT Int. Appl. (2009), WO 2009086028 A2 20090709, Language: English , Database: CAPLUS
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
The invention refers to oligocarbazole-contg. compds. I [a = 1 - 20, b = 0 - 20, m,n = 0 - 2, m + n ≥ 1; X = biphenyl,terphenyl, naphthalene, triphenylene, phenanthrene, fluorene, chrysene, dibenzothiophene, dibenzofuran, benzofuran,benzothiophene, pyrazole, imidazole, triazole, oxazole, thiazole, oxadiazole, oxatriazole, dioxazole, thiadiazole, pyridine,pyridazine, pyrimidine, pyrazine, triazine, indole, benzimidazole, indazole, benzoxazole, benzisoxazole, benzothiazole,quinoline, isoquinoline, cinnoline, quinazoline, quinoxaline, quinoxaline, naphthyridine, phthalazine, pteridine, xanthene,phenothiazine, phenoxazine, benzofuropyridine, furodipyridine, benzothienopyridine or thienodipyridine; R = H, alkyl,heteroalkyl, benzene, biphenyl, terphenyl, naphthalene, phenalene, phenanthrene, fluorene, chrysene,dibenzothiophene, dibenzofuran, benzofuran, benzothiophene, pyrazole, imidazole, triazole, oxazole, thiazole,oxadiazole, oxatriazole, dioxazole, thiadiazole, pyridine, pyridazine, pyrimidine, pyrazine, triazine, indole, benzimidazole,indazole, benzoxazole, benzisoxazole, benzothiazole, quinoline, isoquinoline, cinnoline, quinazoline, quinoxaline,naphthyridine, phthalazine, pteridine, xanthene, phenothiazine, phenoxazine, benzofuropyridine, furodipyridine,benzothienopyridine or thienodipyridine] having an unsym. structure which may be useful in org. light emitting devices, in particular as hosts in the emissive layer of such devices. A method of fabricating an org. light emitting device comprising the compds. in emissive layer or in hole transport layer is also described.
~7 Citings
22. Carbazole-containing materials in phosphorescent light emitting diodes
By Lin, Chun; Dyatkin, Alexey; Elshenawy, Zeinab From U.S. Pat. Appl. Publ. (2009), US 20090153034 A1 20090618, Language: English , Database: CAPLUS
The invention refers to oligocarbazole-contg. compds. I [a = 1 - 20, b = 0 - 20, m,n = 0 - 2, m + n = ≥ 1; X = biphenyl,terphenyl, naphthalene, triphenylene, phenanthrene, fluorene, chrysene, dibenzothiophene, dibenzofuran, benzofuran,benzothiophene, pyrazole, imidazole, triazole, oxazole, thiazole, oxadiazole, oxatriazole, dioxazole, thiadiazole, pyridine,pyridazine, pyrimidine, pyrazine, triazine, indole, benzimidazole, indazole, benzoxazole, benzisoxazole, benzothiazole,quinoline, isoquinoline, cinnoline, quinazoline, quinoxaline, quinoxaline, naphthyridine, phthalazine, pteridine, xanthene,phenothiazine, phenoxazine, benzofuropyridine, furodipyridine, benzothienopyridine or thienodipyridine; R = H, alkyl,heteroalkyl, benzene, biphenyl, terphenyl, naphthalene, phenalene, phenanthrene, fluorene, chrysene,dibenzothiophene, dibenzofuran, benzofuran, benzothiophene, pyrazole, imidazole, triazole, oxazole, thiazole,oxadiazole, oxatriazole, dioxazole, thiadiazole, pyridine, pyridazine, pyrimidine, pyrazine, triazine, indole, benzimidazole,indazole, benzoxazole, benzisoxazole, benzothiazole, quinoline, isoquinoline, cinnoline, quinazoline, quinoxaline,naphthyridine, phthalazine, pteridine, xanthene, phenothiazine, phenoxazine, benzofuropyridine, furodipyridine,benzothienopyridine or thienodipyridine] having an unsym. structure which may be useful in org. light emitting devices, in particular as hosts in the emissive layer of such devices.
~2 Citings
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
23. Carbazole-containing materials in phosphorescent light emitting diodes
By Lin, Chun; Dyatkin, Alexey; Elshenawy, Zeinab
From U.S. Pat. Appl. Publ. (2009), US 20090134784 A1 20090528, Language: English, Database: CAPLUS Carbazole-contg. compds. I [a = 1 - 20, b = 0 - 20, m = 0 - 2, n = 0 - 2 and m + n ≥ 1; X = biphenyl, terphenyl, naphthalene, triphenylene, phenanthrene, fluorene, chrysene, dibenzothiophene, dibenzofuran, benzofuran, benzothiophene, pyrazole, imidazole, triazole, oxazole, thiazole, oxadiazole, oxatriazole, dioxazole, thiadiazole, pyridine, pyridazine, pyrimidine, pyrazine, triazine, indole, benzimidazole, indazole, benzoxazole, benzisoxazole, benzothiazole, quinoline, isoquinoline, cinnoline, quinazoline, quinoxaline, naphthyrimidine, phthalazine, pteridine, xanthene, phenothiazine, phenoxazine, benzofuropyridine, furodipyridine, benzothienopyridine and thienodipyridine; R = H, alkyl, heteroalkyl, benzene, biphenyl, terphenyl, chrysene, dibenzothiophene, dibenzofuran, benzofuran, benzothiophene, pyrazole, imidazole, triazole, oxazole, thiazole, oxadiazole, oxatriazole, dioxazole, thiadiazole, pyridine, pyridazine, pyrimidine, pyrazine, triazine, indole, benzimidazole, indazole, benzoxazole, benzisoxazole, benzothiazole, quinoline, isoquinoline, cinnoline, quinazoline, quinoxaline, naphthyrimidine, phthalazine, pteridine, xanthene, phenothiazine, phenoxazine, benzofuropyridine, furodipyridine, benzothienopyridine and thienodipyridine] having an unsym. structure are provided. The compds. may be useful in org. light emitting devices, in particular as hosts in the emissive layer of such devices.
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
~10 Citings
24. Subporphyrins with monodisperse oligocarbazole arms
By Liu, Xingliang; Lu, Ran; Xu, Tinghua; Xu, Defang; Zhan, Yong; Chen, Peng; Qiu, Xianping; Zhao, Yingying From European Journal of Organic Chemistry (2009), (1), 53-60. Language: English , Database: CAPLUS,DOI:10.1002/ejoc.200800646
Novel star-shaped subporphyrins with monodisperse oligocarbazole arms (I, n = 1-7) were prepd. by using pyridine-tri-N-pyrrolylborane as a template. Photoinduced energy transfer took place from the oligocarbazole arms to the subporphyrin core, and the energy transfer efficiency decreased with an increase in the no. of carbazole units in the arms. These subporphyrins could emit intense yellow-green light when they were excited at 295 nm.
~0 Citings
25. Porphyrins with Four Monodisperse Oligocarbazole Arms: Facile Synthesis and Photophysical Properties
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
By Xu, Tinghua; Lu, Ran; Liu, Xingliang; Chen, Peng; Qiu, Xianping; Zhao, Yingying From Journal of Organic Chemistry (2008), 73(5), 1809-1817. Language: English , Database: CAPLUS,
DOI:10.1021/jo702426r A series of novel monodisperse, well-defined, star-shaped mols. T(OCAn)Ps (n = 2-6) with a central porphyrin core and four oligocarbazole arms are synthesized from the corresponding formyl-substituted oligocarbazoles via Adler reaction. The obtained star-shaped porphyrins are intrinsically two-dimensional nanosized mols., and the diam. of compd.T(OCA6)P is 7.4 nm, representing one of the largest known star-shaped conjugated systems. Their photophys. properties have been investigated by absorption and steady-state fluorescence spectroscopy, together with the corresponding monodisperse oligocarbazole aldehyde precursors. It is found that the light-harvesting capability of T(OCAn)Ps increases with the increasing length of the arms and reaches the max. when n = 6. A selective excitation of the oligocarbazole arms leads to the typical emission from the porphyrin cores, indicating occurrence of photoinduced intramol. energy transfer, and the energy transfer efficiency decreases from T(OCA2)P to T(OCA6)P owing to the Foerster energy-transfer process.Accordingly, the longest effective distance for Foerster energy transfer is estd. to be ca. 3 nm in our system. Such star-shaped porphyrins may find applications in photonic devices,with respect to their intense emission of red light. Notably, the monodisperse oligocarbazole aldehyde precursors give twisted intramol. charge-transfer (TICT) excited states and luminescence in polar solvents with large Stokes shifts.
~12 Citings
26. Novel carbazole-based organogels modulated by tert-butyl moieties
By Yang, Xinchun; Lu, Ran; Xu, Tinghua; Xue, Pengchong; Liu, Xingliang; Zhao, Yingying From Chemical Communications (Cambridge, United Kingdom) (2008), (4), 453-455. Language: English , Database:CAPLUS, DOI:10.1039/b713734f
Tert-Bu groups can modulate the self-assembling properties of carbazole derivs.; organogel fibers with a bright blue emission are generated, directed by the cooperation of hydrogen bonding as well as π-π interactions.
~0 Citings
27. Theoretical studies on the ground state and excited state of poly(3,9-carbazole)
By Bo, Dong-Sheng; Ren, Ai-Min; Feng, Ji-Kang; Yang, Li From Gaodeng Xuexiao Huaxue Xuebao (2007), 28(5), 955-959. Language: Chinese , Database: CAPLUS
The geometries of the oligomers of (3,9-carbazole)n (n = 1, 2, 3, 4, 6, 8) were optimized with the d. functional theory/B3LYP. From the optimized geometries, the ionization potentials (IP), electron affinities (EA), and other related energies were calcd. The absorption spectra were obtained using time-dependent d. functional theory (TD-DFT) and ZINDO. The properties of poly(3,9-carbazole) were extrapolated by the relations between the properties of oligomers and the polymg. chain length n. The results are studied compared to those of poly(2,7-carbazole) and similar polymers.The conjugations of poly(3,9-carbazole) are destroyed partly and absorption spectra blue-shift. The IP value of poly(3,9-carbazole) was similar to that of polyfluorene, and poly(3,9-carbazole) can be used as hole transport materials in multilayer electroluminescent devices. The excited geometries of carbazole and its dimer were calcd. by CIS/6-31G and the emission spectra by TD-DFT and ZINDO.
~0 Citings
28. Facile synthesis of novel monodisperse linear 3,9-linked oligocarbazoles
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
By Xu, Tinghua; Lu, Ran; Jin, Ming; Qiu, Xianping; Xue, Pengchong; Bao, Chunyan; Zhao, Yingying From Tetrahedron Letters (2005), 46(40), 6883-6886. Language: English , Database: CAPLUS,DOI:10.1016/j.tetlet.2005.08.012Novel monodisperse linear 3,9-linked oligocarbazoles (OCAs) were prepd. stepwise using Ullmann coupling reaction in seal-tubes. The resulting OCAs were sol. in common org. solvents. The UV-vis spectra of OCAs exhibited small red shift and their intensities increased linearly with the increase of the carbazole moieties, suggesting that no intramol. π-interactions appeared at ≤ 8-mer. All the OCAs gave strong fluorescence and it was found that the conjugated degree of linear OCAs would be satd. when the no. of carbazole units reaches four. Supplementary data are available via the Internet.
~12 Citings
29. Structure of compounds formed in thermal treatment of technical anthracene
By Vymetal, Jan; Chvatal, Ivan; Simanek, Vilim; Dolejs, Ladislav From Chemicky Prumysl (1983), 33(12), 649-52. Language: Czech , Database: CAPLUS
Heating tech. anthracene, phenanthrene, carbazole, fluorene, and mixts. of anthracene with phenanthrene, carbazole, or fluorene for 96-650 h at 250-290° gave products such as 1,9'-bicarbazole, 4,4'-bicarbazole, 3,9'-bicarbazole,tercarbazole, 9-(9-anthryl)carbazole, 9,10-anthrylene-9,9'-bicarbazole, 9-fluorenone, 9,9'-bifluorenyl, and bifluorenylidene. Anthracene itself was thermally stable except during reaction with carbazole. Phenanthrene was also reasonably stable.
~0 Citings
30. One-electron photooxidation of carbazole in the presence of carbon tetrachloride. Part II. Carbon tetrachloride as a reaction medium. Use of ammonia after irradiation and during irradiation
By Zelent, Bogumil; Durocher, Gilles From Canadian Journal of Chemistry (1982), 60(19), 2442-50. Language: English , Database: CAPLUS,DOI:10.1139/v82-353
The influence of ammonia, used after and during irradn., on the mechanism of secondary transformation and the formation of thermodn. stable products in the title photooxidn. was studied. Such compds. as N-cyanocarbazole, 1-cyanocarbazole, and 3-cyanocarbazole have been formed as the main products during neutralization of the photolyte soln. by ammonia gas. The mechanism of formation of these compds. has been explained by the chem. reaction of ammonia with cations I and II. If ammonia is present in the soln. of carbazole in CCl 4 during irradn., such products as N,N'-dicarbazyl and N-cyanocarbazole are mainly formed along with 3-(N-carbazyl)carbazole, 3,9-di-N-carbazylcarbazole, and N-cyano-3-(N-carbazyl)carbazole. In such a case, reactions of radicals III are the detg. factors in the secondary photochem. transformations. III are formed by the reaction involving ammonia with radical cations of carbazole.
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
Copyright ? 2013 American Chemical Society (ACS). All Rights Reserved.
~7 Citings
31. Reaction of 2,3-diphenylindole with permanganate. Rearrangements of N,N'-dimers
By Dave, Vinod From Canadian Journal of Chemistry (1972), 50(20), 3397-401. Language: English , Database: CAPLUS,DOI:10.1139/v72-547
The dimeric structure I is assigned to the major product from the reaction of 2,3-diphenylindole and KMnO4 from spectroscopic and chem. evidence. Thermal and acid-catalyzed rearrangements of N,N'-dimers I and II were studied.~0 Citings
32. Organic photoconductors
By Fox, Charles J.From Fr. (1969), FR 1556265 19690207, Language: French , Database: CAPLUS
Tetrasubstituted hydrazine derivs. such as tetra-α-naphthylhydrazine, tetra-(3-methyl-4-hydroxyphenyl)hydrazine, tetra-4-tolylhydrazine (I), etc . and N,N-bicarbazyl compds. substituted or not such as cyclotetrakis(3,9-carbazolylene) or 6-(3-carbazolyl)cyclotetrakis(3,9-carbazolylene) are improved photoconductors that give stable, transparent electrophotographic products with higher sensitivity. Thus, to a soln. of 9.8 g di-4-tolylamine in 250 ml Me2CO was added under stirring for 2 hr at 25°, 8 g powd. KMnO4. The stirring was continued an addnl. 6 hr and the mixt. kept overnight. The brown ppt. was filtered and the liq. was concd. to 100 ml and cooled in ice, yielding 5.4 g yellow cryst. I (yield 55 mole %, m.p. 138-139°). An electrophotographic product was then prepd. with I as the photoconductor to which was added a small amt. of a pyrylium sensitizer (Fr. 1,359,095). The mixt. was incorporated in a film-forming polymeric binder (U.S. 231,019, coated on a suitable electrophotographic support, exposed to a W light source (3400°K, 214 lx) in contact with a positive, and developed to give a visible image.
~0 Citings
33. Oxidations with nickel peroxide. V. Reaction of carbazoles with nickel peroxide
By Sugita, Jitsuo From Nippon Kagaku Zasshi (1967), 88(6), 659-67. Language: Japanese , Database: CAPLUS,DOI:10.1246/nikkashi1948.88.6_659
Carbazole (I) in 300 ml. Et2O was treated with 1.5 equivs. Ni peroxide (II) to give 1.5 g. 9,3:9,9-tercarbazolyl (III), m. 267-9°, 2.4 g. 9,9'-bicarbazolyl (IV), m. 219-21°, and polymers. Heating 0.2 g. III and 2 ml. concd. HI gave 0.07 g. I and 0.05g. 3,9'-bicarbazolyl (V), m. 212-15°. V was also prepd. according to Perkin and Tucker (1921) but the following modifications gave better yields of intermediates. 9-(p-Nitrophenyl)carbazole (24 g.) in AcOH and 50 g. Zn was refluxed with gradual addn. of 30 ml. concd. HCl and then heated 6 hrs. to give 13 g. 9-(p-aminophenyl)carbazole, 0.5 g. of which in 10 ml. AcOH was treated with 3 ml. concd. HCl and 0.3 g. NaNO2 to yield 9-[p-(1-benzotriazolyl)phenyl]carbazole, m.163-5°. Oxidn. of I by the Perkin-Tucker method was reexamd. and it was found that "dicarbazyl B" corresponded to III and the "dicarbazyl C" to a mixt. of III, IV, and polymer. Similar oxidn. of carbazole derivs. with II gave the following results (substituents and products given): 3-Cl, 43.2% 3,3'-dichloro-9,9'-bicarbazolyl, m. 208-9°, 13.5% 3,6',3"-trichloro-9,3':9',9"-tercarbazolyl, m. 260-2°, 27.2% polymer; 3,6-Cl2, 96% 3,3',6,6'-tetrachloro-9,9'-bicarbazolyl (VI), m. 243.5-45°;9-Ac, 0.8% III, 0.8% IV, 75% recoverded; 3,6,9-Cl2Ac, 57.1% VI, 28.6% recovered; 9-Me, 94% recovered; 3,6,9-Cl2Me,1% VI, 65.2% recovered; 3,6-(NO2)2, 97% recovered; 9-(9-carbazolyl), 96% recovered; 3-(9-carbazolyl), bis[(3-(9-carbazolyl)carbazol-9-yl], m. 290-3°. Thus the reaction must proceed via formation of a 9-carbazolyl radical, followed by attack at the 3 or 6 position or coupling. N.M.R. spectra were discussed.
~1 Citing
34. Bicarbazyls. V. Synthesis of 3,9'-bicarbazyl
By Nelmes, Margaretta C.; Tucker, S. H.From Journal of the Chemical Society (1933), 1523-5. Language: Unavailable , Database: CAPLUS